 Open Access Article
Open Access ArticleDesign strategies for liquid-phase synthesis of sodium-based quaternary solid-state electrolytes†
Saeed Ahmadi
Vaselabadi
*,
Brynn
Benham
and
Colin A.
Wolden

Chemical and Biological Engineering, Colorado School of Mines, Golden, Colorado 80401, USA. E-mail: cwolden@mines.edu
First published on 2nd July 2025
Abstract
The cost-effective and scalable production of solid-state electrolytes is essential for advancing all-solid-state sodium batteries as a safer, lower-cost alternative to Li-ion batteries. Herein, we explored liquid-phase synthesis methods for quaternary chalcogenide solid-state electrolytes (SSEs) with the formula Na11Sn2PnCh12 (Pn = P, Sb, Ch = S, Se). We outline the key design principles, including reagent selection, solvent chemistry, and thermal treatment, while elucidating reaction mechanisms through systematic studies using XRD, Raman, and FTIR spectroscopy. We demonstrate that acetonitrile, an aprotic solvent with high polarity, enables the synthesis of Na11Sn2PS12via a simple solution process followed by heat-treatment, achieving high ionic conductivity (σNa+ = 0.2 mS cm−1). The antimony analog, Na11Sn2SbS12, can be produced in an aqueous solution facilitated by reactive bisulfide intermediates, yielding a highly crystalline chalcogenide at low temperatures with excellent transport properties (σNa+ = 0.4 mS cm−1). For selenide analogs (e.g. Na11Sn2SbSe12), alkahest amine–thiol solvents facilitate synthesis from elemental and binary precursors, marking the first report of its kind. Additionally, we identified Na11Sn2SbS12 as the most promising candidate for utilization in sodium all-solid-state batteries owing to its wide electrochemical stability and strong compatibility with Na–Sn alloy anodes.
Introduction
Solid-state sodium-ion batteries are attractive candidates to facilitate renewable energy integration due to sodium's abundance, low cost, and reduced environmental impact relative to their high-performance lithium counterparts. Sodium's relatively low atomic weight and its similar electrochemical potential make it a promising target for “post-Li” chemistry. Moreover, substitution of solid-state electrolytes for conventional organic liquid electrolytes can enhance energy density and specific capacity of the cell while mitigating flammability safety concerns. Sodium-based chalcogenides are promising candidates to realize sodium all-solid-state batteries owing to their high ionic conductivity (>0.1 mS cm−1), favorable interfacial contact with electrodes based on their deformability, and mild processing requirements.1–5Synthesis of cubic Na3PS4 with high ionic conductivity (0.2 mS cm−1) ignited interest in Na-based multinary chalcogenides.6 Since then, extensive research has led to the development of a variety of ternary and quaternary compounds such as Na3PnCh4 (Pn = P, Sb, As, Ch = S, Se),7–13 Na11Sn2PnCh12,14–16 Na3−xPn1−xWxS4,17etc. Quaternary chalcogenides are typically formed through cation/anion doping of parent ternary compounds. The first reports of experimental and theoretical investigation of quaternary compounds in the Na–Sn–P–S system were inspired by highly conductive Li quaternary with Li10MP2S12 (M = Si, Ge, Sn) composition.18,19 These efforts resulted in the synthesis of Na11Sn2PS12 (NSnPS), a slightly different stoichiometry than the original assumption, as the phase pure product of the Na–Sn–P–S system by different groups.14,15 NSnPS possessed a newly explored tetragonal phase with I41/acd space group and achieved high ionic conductivity (1.4–3.7 mS cm−2) owing to the presence of vacancies in the crystal structure and three-dimensional network of Na+ migration pathways.
Similar to ternary Na3PS4, the substitution of the phosphorous central atom with other pnictogens was attempted to address the air and moisture sensitivity of NSnPS according to the “hard and soft acid–base” (HSAB) theory. Using “softer” Sb resulted in an isostructural Na11Sn2SbS12 (NSnSbS) with different sodium site occupations and vacancy, resulting in a slightly lower ionic conductivity (0.56 mS cm−2). The difference in conductivity is attributed to changes in local bonding (P–S vs. Sb–S) and the lower electronegativity of Sb which leads to stronger Na–S coulombic attractions in the Sb quaternary.20 In related work, Heo et al.21 also reported Na4−xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) electrolytes derived through systematic doping of Na4SnS4 with Sb atoms. Moreover, the substitution of S atoms with Se was designed to improve the transport properties of quaternary chalcogenides through lattice softening resulting in Na11.1Sn2.1PSe12 composition.22 However, structure-transport studies of Na11Sn2SbS12−xSex (x = 1, 6, and 12), solid-solution showed that the ionic conductivity decreases as the Se content is increased due to the change in the prefactor of the Arrhenius equation.3
In the majority of studies to date the quaternary chalcogenides were synthesized through high-temperature reactions between solid precursors in quartz ampoules, which is energy and time-intensive, and not amenable for industrial scale-up. This has prompted the development of liquid-based approaches which mitigate mass transfer limitations and enhance contact between reacting phases. In addition, the size and morphology of SSEs can be tuned by controlling liquid phase parameters such as solvent type, reagents chemistry, and reaction temperature. Hence, the choice of solvent becomes pertinent since aspects such as solubility and stability of reactants in the solvent and possible solvent complexes formation need to be considered.23,24
The liquid-phase reaction of chalcogenide SSEs can be divided into two categories: “suspension” and “solution”. In the “suspension” approach, binary/elemental precursors are dispersed in organic solvents that facilitate the desired reaction by forming soluble reactive complexes. Following the reaction, the SSE precursor is isolated, and post-synthetic processing like annealing is typically required to induce crystallization or phase transformation. In contrast, the “solution” approach is built on the full dissolution of precursors and simultaneous formation of SSEs in polar protic solvents (i.e. water, EtOH) or amine–thiol co-solvents. The latter provides a homogenous solution that is useful in composite cathode processing. Also, crystallization obtained at low temperatures eliminates the need for further heat treatment.25,26
In this context, Yoon group attempted to extend their aqueous synthesis route of Na3SbS4 to Na3.75Sn0.75Sb0.25S4 (or Na11.25Sn2.25Sb0.75S12). As the stoichiometry is slightly different than the more stable 11-1-2-12 phase, a major phase of Na3SbS4 was yielded at 200 °C while a higher annealing temperature was required to induce the quaternary phase.27 Hartmann and coworkers also explored the Sb doping in the Na4SnS4 compound through co-decomposition of the hydrated phase of Na4SnS4 and Na3SbS4 at 170 °C. Both Na4SnS4·14H2O and Na3SbS4·9H2O were crystallized from aqueous solutions of respective precursors.28 In the case of Na11Sn2PS12, Weng et al. reported the first liquid-phase synthesis by utilizing 1,2-dimethoxyethane (DME) that achieved 0.173 mS cm−1 conductivity. Though the samples sintered at high temperatures (>400 °C) showed the formation of tetragonal Na11Sn2PS12, the as-recovered powder from the liquid phase reaction still contained a significant quantity of unreacted raw materials (i.e., Na2S and SnS2) after a long reaction time. This observance along with the formation of unknown phases in the sintered sample indicates that the quaternary formation mainly occurred through sintering rather than liquid phase reaction.29 In another pioneering study, the Yoon group extended the use of alkahest solvents (amine–thiol mixture) to a series of Li and Na SSEs including NSnPS using ethylenediamine (EDA) and 1,2-ethanedithiol (EDT) co-solvents. The as-synthesized NSnPS contained significant carbonaceous products upon high-temperature annealing due to the high boiling point of dithiols. Consequently, the obtained NSnPS had higher electronic conductivity than ionic conductivity.25 Even though this approach was shown to be useful in producing composite cathodes, it is deemed unsuitable for the synthesis of pure NSnPS. The number of reports on the solution synthesis of quaternary chalcogenides is very limited and selenide quaternaries, in particular, have not yet been synthesized in the liquid phase. In addition, there is a lack of understanding about the underlying mechanism of these approaches. This inspired us to investigate the impacts of the various liquid-phase synthesis routes for a series of quaternary chalcogenides.
In this work, we first demonstrate the synthesis of phase pure SnCh2 (Ch = S, Se) via a simple metathesis reaction between hydrated tin chloride and sodium chalcogenide solutions. The resulting SnCh2 is then used as a precursor in the synthesis of Na11Sn2PnCh12 (Pn = P, Sb, Ch = S, Se) electrolytes through liquid-phase reactions. We compare the efficacy of “suspension” and “solution” routes using aprotic, protic, and alkahest solvents in the context of these compounds. For each material, we outline the design principles and examine how key parameters such as reagent choice, solvent chemistry, and thermal post-treatment influence the formation mechanism, using a series of complementary characterization techniques such as XRD, FTIR, and Raman spectroscopy. Finally, the transport properties and electrochemical stability are probed through electrochemical impedance spectroscopy, DC polarization, and linear sweep voltammetry. Our findings identify Na11Sn2SbS12 as the fastest ionic conductor and most stable quaternary composition for use in sodium all-solid-state batteries. This study demonstrates that Na-based quaternary chalcogenides can be designed and produced systematically through solvent-assisted approaches, further advancing the scalable production of solid-state electrolytes for Na ASSBs.
Experimental methods
Materials
Anhydrous Na2S and highly pure Sb2Ch3 (Ch = S, Se) were produced according to previously reported works.11,30,31 Phosphorus pentasulfide (P2S5, 99%, Sigma Aldrich), and elemental sulfur (S, Sigma Aldrich, 99.998% trace metal basis), sodium borohydride (NaBH4, >98%, Sigma-Aldrich), selenium (Se, 99.99%, UMC), sodium (Na, Sigma-Aldrich), tin powder (Sn, Alfa Aesar, −325 mesh, 99.8%), tin(IV) chloride pentahydrate (SnCl4·5H2O, 98+%, extra pure, Thermo Scientific Chemicals), sodium carbonate (Na2CO3, Alfa-Aesar, anhydrous ACS, 99.5% min), manganese(III) oxide (Mn2O3, Strem, 99%), iron(III) oxide (Fe2O3, Sigma Aldrich, 99%), acetonitrile (ACN, anhydrous, 99.8+%, Thermo Scientific Chemicals), ethanol (EtOH, Sigma-Aldrich, anhydrous, ≥99.5%), 1,2-ethylenediamine (EDA, Alfa Aesar, 99%), 1-propanethiol (PT, Thermo Scientific Chemicals, 98%), and UHP grade argon (Ar, 99.999%, General Air) were used as received without purification. Se pellets were further ground using a mortar and pestle to reduce particle size. All procedures were conducted in an Ar-filled glovebox (<5 ppm of H2O) except otherwise stated.Synthesis
Sb2Ch3 and Na3SbCh4 (Ch = Se, Se) were prepared as described in the literature.11,31Aqueous reaction. First, NaHSe aqueous solution was prepared as follows: 454.2 mg (12 mmol) of NaBH4 in 5 ml of water was slowly added to 395 mg (5 mmol) of Se at RT to prepare the aqueous NaHSe in a 25 ml flask. 20% excess NaBH4 was used to ensure the full reduction of Se powder. After 1 h of reaction, the solution was cooled down to 4 °C and centrifuged to collect and discard the precipitate. Next, 364 mg (0.81 mmol) of Sb2Se3, 181.9 mg (4.55 mmol) of NaOH, 457.5 mg (1.65 mmol) of SnSe2, and 65.3 mg (0.83 mmol) of Se were added to the NaHS solution. The solution was stirred at RT for two days. To collect the selenide precipitate, 8 ml of acetone was added to the solution and cooled at 4 °C for 1 day. After centrifugation and excess washing with acetone, the precipitate was dried overnight under vacuum at RT (yield = 42.6%) to obtain NSnSbSe powder. 2× NaOH was used to ensure a complete reaction.
Ethanolic reaction. Ethanolic NaHSe was prepared by first dissolving 124.8 mg (3.30 mmol) of NaBH4 in 10 ml of EtOH and slowly adding to 217.1 mg (2.75 mmol) of Se powder in a 25 ml flask. Next, 276.6 mg (1 mmol) of SnSe2, 120.1 mg (0.25 mmol) of Sb2Se3, 220 mg (5.5 mmol) of NaOH, and 39.5 mg (0.5 mmol) of Se were added to the ethanolic NaHSe. The solution was stirred at 50 °C for two days, decanted, and washed with EtOH twice. The precipitate was dried overnight under vacuum at RT (yield = 48.1%).
EDA-PT reaction. 109.2 mg (0.227 mmol) of Sb2Se3, 251.5 mg (0.91 mmol) of SnSe2, 233.3 mg (2.99 mmol) of Se, and 115 mg (5 mmol) of Na were dissolved and reacted in 10 ml of EDA-PT (4-1) at RT inside the glovebox. After one day, the EDA-ET solution was centrifuged, and the collected supernatant was dried at 150 °C for 2 h under Ar flow in a tube furnace (yield = 73.3%). Recovered NSnSbSe was pelletized and further heat-treated at various temperatures for 1 h under vacuum in a custom-built quartz tube. Na4SnSe4 was obtained by reacting 276.6 mg (1 mmol) of SnSe2, 157.9 mg (2 mmol) of Se, and 92 mg (4 mmol) of Na in 5 ml of EDA-PT (4-1) at RT. A similar recovery procedure was employed to obtain powder Na4SnSe4 (yield = 77.3%).
Materials characterization
Thermogravimetric analysis and differential scanning calorimetry (TGA/DSC) were performed on a TA Instruments SDT-Q600 model. For a typical run, 10 mg of sample was loaded into a pre-cleaned alumina pan and heated under flowing Ar from RT to 600 °C at 10 °C min−1 rate and then cooled down naturally. X-ray diffraction (XRD) was performed with a Philips X’Pert X-ray diffractometer with Cu Kα radiation (λ = 0.15405 nm) between 10 and 60° at a scan rate of 5° min−1. Samples were prepared on a glass slide with a protective tape covering the material to prevent undesired reactions with ambient air. XRD background subtraction was performed using HighScore software. Raman spectroscopy was conducted with a WiTec alpha 300 M confocal microscope/Raman spectrometer employing a 100 mW 532 nm laser. Samples were mounted on a glass slide and sealed under a 0.1 mm quartz cover slip. The laser was focused through the coverslip onto the sample using a 20× objective, and spectra were collected using a CCD detector (Andor Technologies) at −60 °C. HighScore software was used to subtract the Raman background.Field emission scanning electron microscopy (FESEM) images were collected on a JEOL JSM-7000F FESEM instrument equipped with energy-dispersive X-ray spectroscopy (EDX) for compositional analysis. To prepare the samples for SEM and EDX measurements, powder samples were immobilized onto an aluminum stub using double-sided carbon tape. An accelerating voltage of 5 kV was used for the SEM image, while a higher voltage of 15–20 V was employed for EDX spectra collection. All sample preparation was done in an Ar glovebox.
Electrochemical characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio in a mortar to form the working electrode. The separator layer was formed by pressing 100 mg of the electrolyte at 150 MPa (5 min) in a 12 mm PEEK cell. Next, 20 mg of the working electrode was dispersed on the separator carefully and pressed at 275 MPa for 5 min. Lastly, the reference/counter electrode was formed by adding 60 mg Na–Sn on the other side of the separator layer and pressing the system at 275 MPa for 5 min. The stacking pressure of 50 MPa was applied during measurements. The cathodic and anodic scans were measured on the ranges open circuit voltage (OCV) −5 V and OCV −0 V at a scanning rate of 0.1 mV s−1, respectively.
9 ratio in a mortar to form the working electrode. The separator layer was formed by pressing 100 mg of the electrolyte at 150 MPa (5 min) in a 12 mm PEEK cell. Next, 20 mg of the working electrode was dispersed on the separator carefully and pressed at 275 MPa for 5 min. Lastly, the reference/counter electrode was formed by adding 60 mg Na–Sn on the other side of the separator layer and pressing the system at 275 MPa for 5 min. The stacking pressure of 50 MPa was applied during measurements. The cathodic and anodic scans were measured on the ranges open circuit voltage (OCV) −5 V and OCV −0 V at a scanning rate of 0.1 mV s−1, respectively.
Results and discussion
SnCh2 metathesis
One key challenge in developing Na-based solid-state electrolytes (SSEs) is the limited accessibility of their binary precursors. Binary chalcogenides, such as Na2Ch, Sb2Ch3, and SnCh2, are essential components and significant cost drivers for various components of Na all-solid-state batteries (ASSBs). However, their synthesis is typically expensive and primarily available for research purposes.11,31,32 SnCh2 compounds, in particular, feature layered structures held together by van der Waals forces, which impart excellent optical and electronic properties. Wet chemical methods, including solvothermal33–36 and colloidal approaches,37–40 have been reported for synthesizing SnCh2. Solvothermal synthesis typically requires autoclaves capable of withstanding high pressure and temperature, while the colloidal method uses high boiling point solvents at elevated temperatures. Although these techniques yield size-controlled and highly pure tin chalcogenides, their complex reaction mechanisms, specialized equipment, and toxic precursors hinder their viability for large-scale production.In this context, we employed simple metathesis reactions in polar solvents (H2O and EtOH) followed by mild heat treatment to produce the SnCh2 required for quaternary synthesis. Metathesis involves the exchange of counterions between two chemical species, often resulting in the precipitation of one product, which offers favorable energetics and facile separation. The salts used in this process are generally low-cost and readily available.41,42 The reactions used for the synthesis of SnS2 and SnSe2 in this work are outlined in eqn (1) and (2), respectively.
| 2Na2S(aq) + SnCl4·5H2O(aq) → SnS2(s) + 4NaCl(aq) + 5H2O(l) | (1) |
| 2NaHSe(sol) + SnCl4·5H2O(sol) → SnSe2(s) + 2NaCl(sol) + 2HCl(sol) + 5H2O(l) | (2) |
Fig. 1a shows the XRD patterns of SnCh2 compounds obtained from their respective reactions. For SnS2, the reaction was performed in H2O, and the resulting precipitate was thoroughly washed with excess water to remove the NaCl by-product. The sulfide was then recovered by vacuum drying at room temperature. Since the reduction of Se in water tends to produce persistent borax impurities, the SnSe2 reaction was instead conducted in ethanol, following a similar recovery process as for SnS2.31,43 The amorphous SnCh2 powders obtained at room temperature were further heat-treated to enhance their crystallinity (Fig. S1 and S2, ESI†). Thermogravimetric analysis (TGA) revealed that a temperature of 300 °C was necessary to completely remove the solvate complexes in SnS2 (Fig. S1a, ESI†).
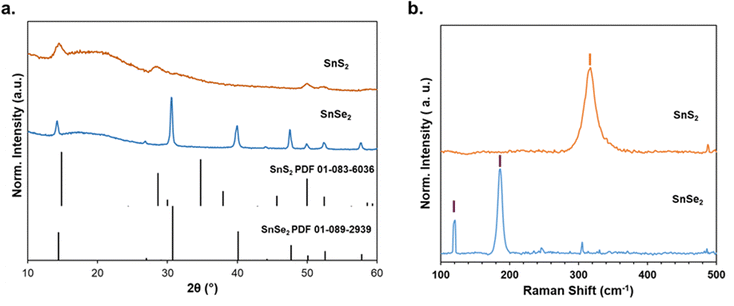 | ||
| Fig. 1 (a) XRD and (b) Raman spectra of SnCh2 (Ch = S, Se) recovered from metathesis reactions after annealing at 300 °C. | ||
The annealed SnS2 exhibited a cubic structure (space group Fd3m), with the presence of pure cubic NaCl confirmed in the supernatant (Fig. S1b, ESI†). Additionally, the Raman spectra of the annealed SnS2 showed the characteristic SnS2 peak at 313 cm−1, verifying its phase purity (Fig. 1b).44 The annealed SnSe2 sample displayed a CdI2-type hexagonal layered structure with P3m1 symmetry. Raman analysis also confirmed the presence of SnSe2, with peaks at 187 cm−1 and 117 cm−1 corresponding to the A1g and Eg modes of SnSe2, respectively.35,40 In addition to SnCh2 compounds developed here, other binaries precursors, such as Sb2Ch3, were synthesized using the metathesis method as described in detail in our previous works.11,31
Synthesis of Na11Sn2PS12
The quaternary reaction to synthesize NSnPS was performed with Na2S, SnS2, and P2S5 as precursors according to the following stoichiometry (eqn (3)):| 11Na2S + 4SnS2 + P2S5 → 2Na11Sn2PS12 | (3) |
The choice of solvents for chalcogenide synthesis depends on factors like dielectric constant, polarity, and molecular composition, which influence the solubility and stability of reagents in the solvent. The dielectric constant (εr) reflects the solvent's ability to dissolve precursors. Polar solvents, such as water (H2O) and ethanol (EtOH), have high dielectric constants due to hydrogen bonding. However, they are unsuitable for P-containing chalcogenides like Na3PS4 and Na11Sn2PS12, as they have been shown to decompose P2S5 and PS43− moieties (Table S1, ESI†).45 In contrast, aprotic solvents such as DME, diethyl ether (DEE), ethyl propionate (EP), ethyl acetate (EA), and ACN have been used in the Na2S–P2S5 system.46–49 These solvents are capable of forming reactive complexes without hydrolyzing the P2S5 precursor. ACN, in particular, offers both a high dielectric constant and easy solvent removal due to its low boiling point. Additionally, reagents like P2S5 and SnS2 are shown to be stable and well-dispersed in non-polar ACN, promoting uniform mixing.
Building on these findings, we selected ACN as the reaction medium for the “suspension” synthesis of Na11Sn2PS12. In this experiment, stoichiometric amounts of Na2S, P2S5, and freshly prepared SnS2 were dispersed in ACN and reacted at 50 °C for 2 days. The resulting precipitate was recovered and washed with excess ACN before drying at room temperature under vacuum. The obtained powder was then heat-treated at various temperatures under vacuum to promote the solid-state reaction and enhance crystallinity. XRD and Raman analyses were conducted to assess the phase purity of the synthesized NSnPS materials and to investigate the formation mechanisms of the ternary and quaternary phases (Fig. 2a and b). The XRD pattern of the sample recovered at room temperature shows the presence of unreacted Na2S; however, the Raman and FTIR spectra (Fig. S3, ESI†) indicate the formation of PS43−, P2S74−, and P2S64− polyanions along with small traces of Na2S and SnS2, S–S bonds. The deconvoluted Raman spectra are presented in Fig. S4 (ESI†). Annealing at 200 °C did not cause any significant change in the XRD pattern of the NSnPS sample, but the Raman peaks attributed to P–S bonds indicated a potential shift in the intensity of [PS44−] and [P2S74−] polyhedra, suggesting a possible transition from amorphous Na3PS4 to Na4P2S7.50–54 This reaction, which involves the release of Na2S, can be represented as follows:
| 2Na3PS4 → Na4P2S7 + Na2S | (4) |
 | ||
| Fig. 2 (a) XRD, and (b) Raman spectra of NSnPS recovered from ACN and heat-treated at different temperatures. | ||
Further increasing the temperature stabilizes the quaternary phase, with minor precipitation of the Na4SnS4 phase. Raman analysis also confirms the consumption of [PS43−] and [P2S74−] polyanions, likely through a solid-state reaction with available Na4SnS4 and Na2S to generate the quaternary phase (eqn (5) and (6)). At this stage, all unreacted SnS2 is converted into ternary and quaternary phases, indicating the consumption of all binary precursors. The peak at 375 cm−1 could be attributed to the characteristic P–P stretching mode of [P2S64−] polyanion, which is likely generated as a side product of high-temperature annealing through the release of S atoms from Na4P2S7.51
| Na2S + SnS2 → Na4SnS4 | (5) |
 | (6) |
The ionic transport properties of NSnPS recovered from ACN were investigated over a wide range of temperatures and frequencies using EIS. Fig. 3a shows the Nyquist plots of NSnPS heat-treated at different temperatures, while the extracted total conductivity values from equivalent circuit fitting are presented in Fig. 3b. The spectra were fitted to the equivalent circuit that consisted of a parallel combination of a resistor and constant phase element (CPE), which describes the suppressed semicircle, in series with another CPE that corresponds to electrode polarization (inset of Fig. 3a). Upon annealing from 300 °C to 550 °C, we observed an improvement in the ionic conductivity, correlating with the reduction in the concentration of Na4SnS4, highlighting the importance of phase purity on ionic transport in quaternary NSnPS. The highest ionic conductivity of 0.2 mS cm−1 was achieved at 550 °C, which is in good agreement with the other reports of NSnPS synthesized through liquid phase routes (Table 1).25,29 Even though the obtained conductivity is not explicitly superior to the other studies due to the presence of a low-conductive Na4SnS4 phase, the detailed investigation into the solvent influence and identification of potential impurities in this system is highly valuable and could serve as a roadmap for the synthetic design of similar materials.
 | ||
| Fig. 3 (a) Nyquist plots, (b) corresponding ionic conductivity, and (c) Arrhenius plots of NSnPS synthesized in ACN as a function of annealing temperature. | ||
| Quaternary | Synthesis method | Precursors | Heat-treatment (°C) | σ Na+ (mS cm−1) | E a (eV) | Electrochemical window vs. Na+/Na (V) | Ref. |
|---|---|---|---|---|---|---|---|
| Na11Sn2PS12 | Solid-state (700 °C, 5 h) | Na2S, P2S5, SnS2 | — | 1.4 | 0.25 | — | 14 |
| Na11Sn2PS12 | Solid-state (600 °C) | Na3PS4, Na4SnS4 | — | 3.7 | 0.39 | — | 15 |
| Na11Sn2PS12 | Suspension (DME) | Na2S, P2S5, SnS2 | 460 | 0.17 | 0.33 | 0.3–3.2 | 29 |
| Na11Sn2SbS12 | Solid-state (700 °C, 5 h) | Na2S, Sb2S3, SnS2, S | — | 0.56 | 0.34 | — | 20 |
| Na4−xSn1−xSbxS12 (0.02 ≤ x ≤ 0.33) | Solid-state (450–550 °C, 12 h) | Na2S, Sb2S3, SnS2, S | 550 | 0.51 (x = 0.25) | 0.39 (x = 0.25) | ∼0.4–4.5 | 21 |
| Na11.1Sn2.1PSe12 | Solid-state (600 °C, 12 h) | Na2Se, Sn, P, Se | 600 | 1.7 | 0.30 | — | 22 |
| Na11Sn2PSe12 | Solid-state (900 °C, 48 h) | Na2Se, Sn, P, Se | 900 | 2.15 | 0.28 | 1.22–2.65 | 58 |
| Na11Sn2PSe12 | Milling (400 rpm, 15 h), annealing (600 °C) | Na, Sn, P, Se | 550 | 0.13 | — | — | 59 |
| Na11Sn2SbSe12 | Milling (33 h, 400 rpm) solid-state (750 °C, 5 h) | Na2Se, Sb2Se3, SnSe2, Se | 750 | 0.15 | 0.39 | — | 3 |
| Na11Sn2PS12 | Suspension (ACN) | Na2S, P2S5, SnS2 | 550 | 0.2 | 0.19 | 0.95–1.7 V | This work |
| Na11Sn2SbS12 | Solution (H2O) | Na2S, Sb2S3, SnS2, S | 550 | 0.42 | 0.14 | 1.15–3.1 V | |
| Na11Sn2SbSe12 | Solution (EDA-PT) | Na, Sb2Se3, SnSe2, Se | 550 | 0.07 | 0.22 | 0.85–2.0 V |
The kinetics of Na+ transport were investigated through temperature-dependent EIS. Fig. 3c presents the Arrhenius plots for the corresponding annealed NSnPS, with activation energies extracted in the temperature range of 25–100 °C. The obtained activation energy values (0.18–0.20 eV) are slightly lower than those reported from other groups, potentially due to variations in experimental setups, off-stoichiometry, and the presence of impurity phases such as Na4SnS4 in the quaternary compounds (Table 1). The electronic conductivity of NSnPS compounds was measured using DC polarization in a symmetric cell configuration (Ti|SSE|Ti), similar to the EIS experiments. For the sample annealed at 550 °C, an electronic conductivity value of σe = 5.7 × 10−6 mS cm−2 was obtained which was on the same order of magnitude as NSnPS derived from DME solvent in the report by Weng and coworkers.29
We also explored the alkahest amine–thiol approach for the NSnPS reaction. For this, we selected a mixture of ethylenediamine (EDA) and propanethiol (PT) due to their combined lower boiling point compared to diamine–dithiol co-solvents. Lee et al.25 previously demonstrated that P2S5 fully dissolves in EDA-ethanethiol (ET) solvents without any noticeable hydrolysis. Our attempt with EDA-PT yielded a compound with an XRD pattern similar to what was reported in their study. A closer examination of the XRD pattern, coupled with Raman analysis (Fig. S6, ESI†), indicated the formation of ternary Na4SnS4 with a tetragonal phase (space group I41/acd), rather than the expected Na11Sn2PS12 in the diamine–monothiol solvents. Interestingly, Hartmann et al.28 recently identified this new phase of Na4SnS4, which is isostructural to NSnPS and NSnSbS compounds, through controlled dehydration of Na4SnS4·14H2O crystals.
Based on these observations, we can infer that the formation of Na11Sn2PS12 in EDA-EDT as described by Yoon group,25 but not in EDA-PT, is closely related to the specific nature of P2S5 dissolution and the thiolate species produced in these systems. A recent study on chalcogen dissolution in amine–thiol solutions found that the type of amine and thiol can significantly impact the reactive intermediates formed, which, in turn, influences subsequent reactions.60 Further investigations are needed to elucidate the specific reaction pathway that facilitates phosphorus incorporation to form NSnPS in diamine–dithiol solvents. In conclusion, the suspension synthesis of NSnPS with ACN proves to be the optimal choice over the diamine–dithiol approach owing to its less complicated synthesis procedure, lower electronic conductivity, and use of benign and environment-friendly solvent. ACN offers a safer alternative compared to the relatively toxic amine–thiol cosolvents.
Synthesis of Na11Sn2SbS12
Na11Sn2SbS12 enhances the air stability relative to its isostructural analog, Na11Sn2PS12. Sb-based and Sn-based chalcogenides such as Na3SbCh4 and Na4SnCh4 (Ch = S, Se) can be synthesized in polar solvents like H2O, MeOH, and EtOH. For example, the aqueous solution synthesis of Na4SnS4 has been reported with Na2S·9H2O, SnCl4, or SnS2 precursors.61,62 We recently investigated different synthetic protocols for Na3SbCh4 compounds in EtOH and demonstrated that the formation of reactive Na chalcogenides is a critical step in producing highly pure Na3SbCh4.11,31,43 In these compounds, the nucleophilic attack of the chalcogenide ions on the binary chalcogenides, such as Sb2Ch3, promotes the formation of [SbCh3]3− anions, followed by the reduction of elemental chalcogen to produce [SbCh3]4− polyhedral anions. We hypothesized that a similar chemistry would be feasible in the synthesis of Na11Sn2SbS12, utilizing the formation of [SbS4]3− and [SnS4]4− polyanions. Accordingly, the following reaction scheme was attempted in H2O and EtOH:| 11Na2S + Sb2S3 + 4SnS2 + 2S → 2Na11Sn2SbS12 | (7) |
The reaction in EtOH was conducted with a similar stoichiometry. Initial attempts indicated slow kinetics for NSnSbS formation in the less polar EtOH; therefore, the solution was heated to 50 °C for an extended period (2 days) to ensure a complete reaction. Since NSnSbS is insoluble in EtOH, this approach facilitated its direct precipitation at room temperature. The sample recovered from EtOH was further dried at 150 °C to eliminate any remaining solvent and to ensure consistent heat treatment with the aqueous experiment. Additionally, we investigated the applicability of amine–thiol co-solvents for this stoichiometry. Pioneering work by the Butchery group has demonstrated the dissolution and redeposition of binary chalcogenides, such as Sb2Ch3 and SnS2, in amine–thiol co-solvents.63,64 Since all precursors are sufficiently soluble in the EDA-PT mixture, this reaction was performed at room temperature for 1 day, and the powder was recovered by discarding unreacted precipitate and drying the supernatant at 150 °C.
Fig. 4a shows the XRD patterns of compounds obtained from H2O, EtOH, and EDA-PT solvents, with reference patterns for Na4SnS4 and Na3SbS4 included for comparison. Detailed synthesis protocols for Na4SnS4 and Na3SbS4 in aqueous solutions can be found in the Experimental section. The Na11SnSbS12 recovered from all solvents exhibits a tetragonal crystalline phase (I41/acd space group) consistent with the reported structure for this compound.20,21 The NSnSbS obtained from an aqueous solution is highly crystalline highlighting the effectiveness of highly polar solvents in promoting room-temperature crystallization of quaternary chalcogenides. In contrast, the powders recovered from EtOH and EDA-PT solvents exhibited lower crystallinity with small traces of Na3SbS4 detected. Since NSnSbS is isostructural with the Na4SnS4 recovered from the aqueous solution, XRD alone is inconclusive regarding the formation of NSnSbS.28 Therefore, Raman spectroscopy was conducted to provide deeper insights into their molecular order and to assess the influence of Sb doping on the structure.
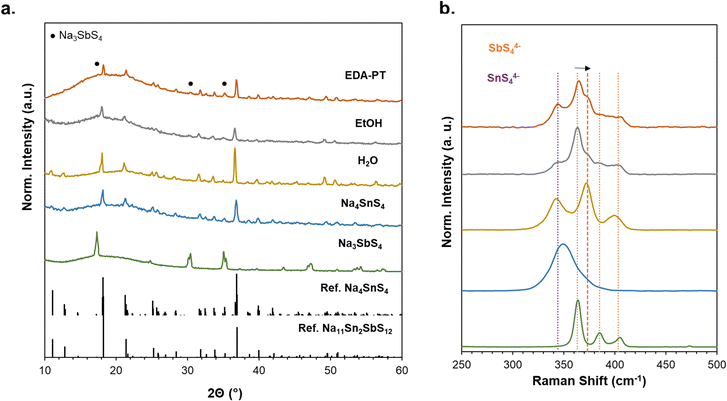 | ||
| Fig. 4 (a) XRD, and (b) Raman of NSnSbS recovered from different solvent systems and dried at 150 °C. | ||
The corresponding Raman spectra are depicted in Fig. 4b. To accurately identify the existing vibrational modes, peak deconvolution was conducted for the Raman peaks of the samples recovered from H2O, EtOH, and EDA-PT (see Fig. S7, ESI†). The NSnSbS obtained from the aqueous solution displays peaks at approximately 341 cm−1 and 363 cm−1, which corresponds to the symmetric vibration (νs) of Sn–S and Sb–S, respectively. For the asymmetric stretching modes of the SbS4 anions, expected peaks at νa = 385 cm−1 and 403 cm−1 were observed in the pure Na3SbS4 sample.65 However, in the H2O-recovered sample, these peaks merged into a broader peak at 399 cm−1, indicating a change in the local structure due to the incorporation of Sn. Additionally, a strong peak at 372 cm−1 can be attributed to the [SbS4] polyhedra within the quaternary lattice framework. The NSnSbS structure features two types of isolated SnS4 (or SbS4) tetrahedra, positioned on distinct Sn-only sites and an Sb mixed site.20
The XRD pattern of NSnSbS obtained from EtOH and EDA-PT shows a strong presence of pure Na3SbS4, which is further emphasized by the high-intensity Sb–S stretching mode at 363 cm−1 in the corresponding Raman spectra. The emergence of a peak at 372 cm−1 as a smaller shoulder supports the direct correlation between the [SbS4] peak signature and the extent of Sb incorporation into the quaternary lattice. Similar to the findings for NSnPS, a blue shift in the Raman peaks of the SbS4 and SnS4 polyanions was observed, likely due to alterations in the local bonding environment compared to the constituent ternary Na3SbS4 and Na4SnS4.56,65 The Raman analysis provides clear evidence for the generation of quaternary Na11Sn2SbS12 compounds; however, higher-resolution X-ray or neutron diffraction studies are necessary to fully elucidate the local crystal structure of the formed quaternary.
To enhance the Na+ transport properties, the NSnSbS powders were annealed at 550 °C under vacuum. Fig. S8 (ESI†) shows the XRD patterns of annealed NSnSbS samples. The aqueous-recovered sample shows no apparent secondary phases, while the ethanolic NSnSbS exhibits traces of NaSbS2, likely formed from the decomposition of the Na3SbS4 phase at high temperatures. The sample recovered from EDA-PT indicates only traces of Na3SbS4. The ionic and electronic conductivities of the samples are summarized in Fig. 5a. As expected, the aqueous-recovered NSnSbS, having the highest crystallinity and phase purity, demonstrates a maximum conductivity of 0.42 mS cm−1 at room temperature – approximately four times higher than the other samples containing NaSbS2 impurities. The electronic conductivity across all samples ranges from 3.8 × 10−7 to 3.1 × 10−6 mS cm−1, rendering them suitable superionic conductors.
 | ||
| Fig. 5 (a) Ionic and electronic conductivity, and (b) Arrhenius plots of NSnSbS obtained from H2O, EtOH, and EDA-PT solvents, and further annealed at 550 °C. | ||
Fig. 5b displays the Arrhenius plots for the NSnSbS samples, indicating a low activation barrier for the aqueous-recovered NSnSbS, attributed to its high crystallinity and phase purity. Additionally, Fig. S9 (ESI†) provides the XRD and Raman spectra from the heat treatment studies conducted on NSnSbS recovered from aqueous solutions. The trend of ionic conductivity as a function of temperature (Fig. S9c, ESI†) illustrates nearly a twofold increase in the ionic conductivity of the quaternary phase as the annealing temperature rises to 550 °C. This enhancement is likely due to the complete incorporation of Sb into the quaternary lattice and the removal of solvated complexes. Overall, these findings highlight the potential of aqueous synthesis methods for developing Sn and Sb-based chalcogenide solid-state electrolytes.
Synthesis of Na11Sn2SbSe12
Lastly, we focused on quaternary selenides with the composition Na11Sn2PnSe12 (where Pn = P, Sb). The common precursors for this class typically include elemental Na, P, Se, or binary selenides such as Na2Se, SnSe2 and Sb2Se3. As for the Sb analog, we explored various activated Se precursors in polar solvents, since aprotic solvents are not suitable due to the insoluble nature of elemental Se. Our attempts to synthesize NSnSbSe using aqueous and ethanolic solutions of NaHSe, following the reaction scheme outlined in eqn (8), led to the formation of Na3SbSe4. However, there was no apparent doping of Sn into the lattice structure of the resultant compound, as evidenced by the data presented in Fig. S10 (ESI†).| 11NaHSe(sol) + Sb2Se3(s) + 4SnSe2(s) + 3Se(s) + 11NaOH(sol) → 2Na11Sn2SbSe12(s) + 11H2O(l) | (8) |
To facilitate Sn incorporation, we turned to the use of amine–thiol solvents. Our previous work has demonstrated that amine–thiol solvents are particularly effective for synthesizing Na3SbSe4 by activating selenium through a redox process. The use of amine–thiol co-solvents allows for the dissolution of highly insoluble selenium at high concentrations, generating homogeneous inks suitable for the solution processing of inorganic semiconductors.66,67 In this process, an amine–thiol mixture produces highly reactive ammonium ions and thiolate species. The thiolate formation reduces Se to its anion form, while the complexation of selenium with ammonium ion yields soluble selenium in the form of [(Se−2)(R-NH3+)2]. Furthermore, this solvent combination has been shown to effectively dissolve a wide range of binary selenides, such as Sb2Se364,68,69 and SnSe.70,71 Inspired by these studies, we attempted the NSnSbSe synthesis in EDA-PT (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) with the following stoichiometry:
1 v/v) with the following stoichiometry:
| 22Na(sol) + Sb2Se3(sol) + 4SnSe2(sol) + 11Se(sol) → 2Na11Sn2SbSe12(sol) | (9) |
Fig. 6a displays the XRD patterns of NSnSbSe recovered from EDA-PT by drying at 150 °C. The predominant phase of NSnSbSe with considerable Na3SbSe4 secondary phase was observed. Upon annealing the sample at 550 °C, we found that the ternary phase incorporates into the quaternary phase through a solid-state reaction. However, attempts to anneal the sample at temperatures exceeding 550 °C led to significant evaporation of selenium species and melting of the material, complicating sample collection. To facilitate high-temperature reactions, melt-quenching techniques using enclosed quartz containers are required.
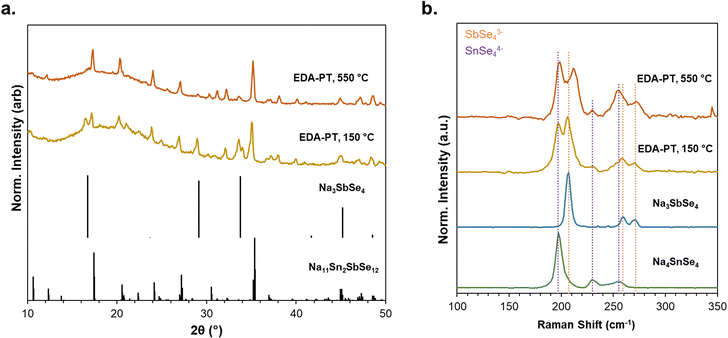 | ||
| Fig. 6 (a) XRD, and (b) Raman of NSnSbSe recovered from amine–thiol solvents after annealing at 150 and 550 °C, respectively. | ||
The Raman spectra of the synthesized selenide at 150 °C and 550 °C are compared with those of the parent ternary compounds, Na4SnSe4 and Na3SbSe4, in Fig. 6b. The Na3SbSe4 is produced through the reaction of Sb2Se3 with sodium and selenium dissolved in EDA-PT solvents, as detailed in our previous work.31 Similarly, the reaction involving SnSe2, sodium, and selenium results in the formation of highly pure Na4SnSe4. The NSnSbSe compound exhibits strong Raman peaks at 197 and 230 cm−1, which correspond to the vibrations of the Sn–Se bond, similar to those observed in other selenostannates such as Ba2SnSe4.72,73 The peaks at 206, 259, and 271 are attributed to the stretching modes of SbSe4 tetrahedra, as seen in the Na3SbSe4 spectrum, which closely resembles other compounds like K3SbSe4.31,74
The samples exhibit all the characteristic peaks associated with [Sn/Sb]Se4 polyhedra, further confirming the successful formation of the quaternary phase. Consistent with the behavior observed in NSnSbS, the signature peak of SbSe4 at 207 cm−1 shifts to a higher wavenumber (∼212 cm−1) upon annealing, indicating effective incorporation of Sb into the lattice structure. This trend is also noted for other vibrational modes of SbSe4, reinforcing the notion of structural modifications upon thermal treatment.
However, the uncorrected full spectrum reveals the presence of D and G bands, which are indicative of carbonaceous species formed during solvent removal, even at low-temperature drying (Fig. S11, ESI†). This presence of carbonaceous material is likely contributing to the elevated electronic conductivity observed for NSnSbSe, as shown in Fig. S12 (ESI†). EIS analysis indicates that the 550 °C sample achieves an ionic conductivity (σNa+) of 0.07 mS cm−1 and an activation energy (Ea) of 0.22 eV, which are considerably lower than those observed in other quaternary phases. This reduction can be attributed to the significant presence of the Na3SbSe4 secondary phase within the material. Previous attempts at the solid-state reaction of Na11Sn2SbSe12 resulted in a substantial impurity phase of NaSbSe2, which negatively impacted its ionic properties, yielding an ionic conductivity of 0.15 mS cm−1.3 Despite the presence of carbonaceous impurities, the current work represents the first liquid-based synthesis of quaternary selenide materials, to our knowledge. This pioneering approach could open new avenues for the development of high-performance solid-state electrolytes. Fig. S12 (ESI†) and Table 1 summarize the preparation conditions and transport properties of Na11PnCh12 (Pn = P, Sb; Ch = S, Se) produced in this work.
For the P-based selenide, the solution approach is highly challenging due to the extreme hygroscopic nature of white phosphorus (P4). While red phosphorus serves as a more stable alternative, it is difficult to activate in organic solvents. Strumolo et al.75 recently reported that red phosphorus can dissolve in an amine–thiol mixture, forming primarily cage-like polyphosphide anions (P162−). However, our attempts to utilize these polyphosphides in EDA-PT were unsuccessful, as significant portion of dissolved phosphorus precipitated out of the solvent mixture. The Raman analysis of the obtained supernatant (Fig. S13, ESI†) closely matched ternary Na4SnSe4, suggesting that phosphorus remained unreactive in these reactions, likely due to steric effects associated with the polyphosphide structure.
Electrochemical performance of quaternary chalcogenides
We assessed the electrochemical stability window as-synthesized quaternary compounds using linear sweep voltammetry (LSV) in a Na–Sn|SSE|SS-SSE cell configuration at room temperature, with a scan rate of 0.1 mV s−1. As shown in Fig. 7a, NSnSbS exhibits a wide electrochemical stability window, with reduction and oxidation processes occurring around 0.95 V and 2.9 V vs. Na–Sn alloy, respectively. A similar potential range was reported by Weng et al. through cyclic voltammetry, though the discrepancy in the low redox potential may stem from differences in establishing faradaic current onset in voltammetry data.76 In contrast, NSnPS and NSnSbSe exhibit narrower stability windows of approximately 0.75–1.5 V and 0.65–1.8 V, respectively. Given the Na–Sn alloy potential of 0.2 V vs. Na+/Na, the updated stability windows are summarized in Table 1. To our knowledge, NSnSbSe's electrochemical stability has not been previously reported. Selenides appear more susceptible to oxidation than their sulfide counterparts at elevated potentials resulting a smaller electrochemical stability as observed in other selenide systems such as selenophosphate-based lithium argyrodites.77We also assembled symmetric Na–Sn|SSE|Na–Sn cells to evaluate the stability of the anode-solid electrolyte interface. As shown in Fig. 7b, NSnSbS exhibited the lowest initial overpotential of 50 mV, which gradually increased to ∼169 mV over 90 hours of cycling. In contrast, NSnPS and NSnSbSe displayed significantly higher overpotentials of ∼400 mV and ∼470 mV, respectively. The transport property measurements (as shown in Fig. S13, ESI† and summarized in Table 1) confirm that aqueous-derived NSnSbS is the most suitable separator for Na all-solid-state batteries, owing to its high ionic conductivity, low electronic conductivity, and low activation barrier.
Conclusions
In conclusion, we presented the rational design of methods for the synthesis of quaternary chalcogenide solid-state electrolytes, Na11Sn2PnCh12 (Pn = P, Sb; Ch = S, Se), using aprotic, protic, and alkahest solvents. The synthesis of P-containing quaternary is more complex due to the limited inventory of phosphorus reagents. Specifically, the high reactivity of P2S5 led us to use acetonitrile (ACN) with a high dielectric constant to ensure intimate mixing of the precursors, forming amorphous thiophosphate moieties, which were then converted to Na11Sn2S12 through annealing. We found that heat-treatment at 300 °C is necessary to initiate the quaternary phase formation in this system, while higher temperatures (550 °C) are required to obtain phase pure Na11Sn2PS12 with minimal Na4SnS4 impurity. For Sb-based analogs, polar solvents such as water, ethanol, and amine–thiol cosolvents facilitated the reaction and crystallization of Na11Sn2SbS12 at a low temperature of 150 °C, with additional annealing needed to further enhance transport properties. The aqueous-recovered sample produced Na-based chalcogenides with performance comparable to that of energy-intensive, high-temperature solid-state reactions. Notably, we achieved the first liquid-phase synthesis of Na11Sn2SbSe12 in this work, despite the presence of minor impurities. Overall, our findings demonstrate the potential of liquid-phase methods for the development of quaternary chalcogenides, offering advantages in achieving high ionic conductivity and phase purity, and paving the way for scalable production of Na-based solid-state electrolytes.Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was supported by the National Science Foundation through Award 2219184. Some of the work was performed in the following core facility, which is a part of Colorado School of Mines’ Shared Instrumentation Facility (X-ray Diffraction & Computed Tomography: RRID: SCR_022053, Scanning Probe and Optical Microscopy: RRID: SCR_022048).References
- H. Jia, L. Peng, C. Yu, L. Dong, S. Cheng and J. Xie, Chalcogenide-based inorganic sodium solid electrolytes, J. Mater. Chem. A, 2021, 9, 5134–5148 RSC.
- Z. Yang, B. Tang, D. Ren, X. Yu, Y. Gao, Y. Wu, Y. Yang, Z. Chen and Z. Zhou, Advancing solid-state sodium batteries: Status quo of sulfide-based solid electrolytes, Mater. Today, 2024, 80, 429–449 CrossRef CAS.
- E. P. Ramos, A. Assoud, L. Zhou, A. Shyamsunder, D. Rettenwander and L. F. Nazar, Structure–transport correlations in Na11Sn2SbSe12 and its sulfide solid solutions, APL Mater., 2023, 11, 011104 CrossRef CAS.
- S. K. Vineeth, M. Tebyetekerwa, H. Liu, C. B. Soni, N. Sungjemmenla, X. S. Zhao and V. Kumar, Progress in the development of solid-state electrolytes for reversible room-temperature sodium-sulfur batteries, Mater. Adv., 2022, 2, 6415–6440 RSC.
- F. Tsuji, A. Nasu, C. Hotehama, A. Sakuda, M. Tatsumisago and A. Hayashi, Preparation and characterization of sodium-ion conductive Na3BS3 glass and glass–ceramic electrolytes, Mater. Adv., 2021, 2, 1676–1682 RSC.
- S. Yubuchi, A. Hayashi and M. Tatsumisago, Sodium-ion conducting Na3PS4 electrolyte synthesized via a liquid-phase process using N-methylformamide, Chem. Lett., 2015, 44, 884–886 CrossRef CAS.
- A. Banerjee, K. H. Park, J. W. Heo, Y. J. Nam, C. K. Moon, S. M. Oh, S.-T. T. Hong and Y. S. Jung, Na3SbS4: A Solution Processable Sodium Superionic Conductor for All-Solid-State Sodium-Ion Batteries, Angew. Chem., Int. Ed., 2016, 55, 9634–9638 CrossRef CAS PubMed.
- L. Zhang, D. Zhang, K. Yang, X. Yan, L. Wang, J. Mi, B. Xu and Y. Li, Vacancy-Contained Tetragonal Na3SbS4 Superionic Conductor, Adv. Sci., 2016, 3, 1600089 CrossRef PubMed.
- D. Zhang, X. Cao, D. Xu, N. Wang, C. Yu, W. Hu, X. Yan, J. Mi, B. Wen, L. Wang and L. Zhang, Synthesis of cubic Na3SbS4 solid electrolyte with enhanced ion transport for all-solid-state sodium-ion batteries, Electrochim. Acta, 2018, 259, 100–109 CrossRef CAS.
- H. Wang, Y. Chen, Z. D. Hood, G. Sahu, A. S. Pandian, J. K. Keum, K. An and C. Liang, An Air-Stable Na3SbS4 Superionic Conductor Prepared by a Rapid and Economic Synthetic Procedure, Angew. Chem., Int. Ed., 2016, 55, 8551–8555 CrossRef CAS PubMed.
- S. A. Vaselabadi, W. H. Smith and C. A. Wolden, Solution Synthesis of Sb2S3 and Na3SbS4 Solid-State Electrolyte, J. Electrochem. Soc., 2021, 168, 110533 CrossRef CAS.
- S. H. Bo, Y. Wang, J. C. Kim, W. D. Richards and G. Ceder, Computational and Experimental Investigations of Na-Ion Conduction in Cubic Na3PSe4, Chem. Mater., 2016, 28, 252–258 CrossRef CAS.
- T. Krauskopf, C. Pompe, M. A. Kraft and W. G. Zeier, Influence of Lattice Dynamics on Na+ Transport in the Solid Electrolyte Na3PS4-xSex, Chem. Mater., 2017, 29, 8859–8869 CrossRef CAS.
- Z. Zhang, E. Ramos, F. Lalère, A. Assoud, K. Kaup, P. Hartman and L. F. Nazar, Na11Sn2PS12: A new solid state sodium superionic conductor, Energy Environ. Sci., 2018, 11, 87–93 RSC.
- M. Duchardt, U. Ruschewitz, S. Adams, S. Dehnen and B. Roling, Vacancy-Controlled Na+ Superion Conduction in Na11Sn2PS12, Angew. Chem., Int. Ed., 2018, 57, 1351–1355 CrossRef CAS PubMed.
- A. Tiwari, S. K. Singh, N. Srivastava, D. Meghnani, R. Mishra, R. K. Tiwari, A. Patel, H. Gupta, V. K. Tiwari and R. K. Singh, Diffusion mechanism in a sodium superionic sulfide-based solid electrolyte: Na11Sn2AsS12, J. Phys. D: Appl. Phys., 2022, 55, 355503 CrossRef CAS.
- T. Fuchs, S. P. Culver, P. Till and W. G. Zeier, Defect-Mediated Conductivity Enhancements in Na3–xPn1–xWxS4 (Pn = P, Sb) Using Aliovalent Substitutions, ACS Energy Lett., 2020, 5, 146–151 CrossRef CAS.
- V. S. Kandagal, M. D. Bharadwaj and U. V. Waghmare, Theoretical prediction of a highly conducting solid electrolyte for sodium batteries: Na10GeP2S12, J. Mater. Chem. A, 2015, 3, 12992–12999 RSC.
- W. D. Richards, T. Tsujimura, L. Miara, Y. Wang, J. C. Kim, S. P. Ong, I. Uechi, N. Suzuki and G. Ceder, Design and synthesis of the superionic conductor Na10SnP2S12-SI, Nat. Commun., 2016, 5, 10–13 Search PubMed.
- E. P. Ramos, Z. Zhang, A. Assoud, K. Kaup, F. Lalère and L. F. Nazar, Correlating Ion Mobility and Single Crystal Structure in Sodium-Ion Chalcogenide-Based Solid State Fast Ion Conductors: Na11Sn2PnS12 (Pn = Sb, P), Chem. Mater., 2018, 30, 7413–7417 CrossRef CAS.
- J. W. Heo, A. Banerjee, K. H. Park, Y. S. Jung and S. T. Hong, New Na-Ion Solid Electrolytes Na4−xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) for All-Solid-State Na-Ion Batteries, Adv. Energy Mater., 2018, 8, 4–9 Search PubMed.
- M. Duchardt, S. Neuberger, U. Ruschewitz, T. Krauskopf, W. G. Zeier, J. Schmedt auf der Günne, S. Adams, B. Roling and S. Dehnen, Superion Conductor Na11.1Sn2.1P0.9Se12: Lowering the Activation Barrier of Na+ Conduction in Quaternary 1–4–5–6 Electrolytes, Chem. Mater., 2018, 30, 4134–4139 CrossRef CAS.
- M. Ghidiu, J. Ruhl, S. P. Culver and W. G. Zeier, Solution-based synthesis of lithium thiophosphate superionic conductors for solid-state batteries: A chemistry perspective, J. Mater. Chem. A, 2019, 7, 17735–17753 RSC.
- J. Xu, L. Liu, N. Yao, F. Wu, H. Li and L. Chen, Liquid-involved synthesis and processing of sulfide-based solid electrolytes, electrodes, and all-solid-state batteries, Mater. Today Nano, 2019, 8, 100048 CrossRef.
- J. E. Lee, K. H. Park, J. C. Kim, T. U. Wi, A. R. Ha, Y. B. Song, D. Y. Oh, J. Woo, S. H. Kweon, S. J. Yeom, W. Cho, K. S. Kim, H. W. Lee, S. K. Kwak and Y. S. Jung, Universal Solution Synthesis of Sulfide Solid Electrolytes Using Alkahest for All-Solid-State Batteries, Adv. Mater., 2022, 2200083, 1–11 Search PubMed.
- Z. Warren and N. C. Rosero-Navarro, Solution-Based Suspension Synthesis of Li2S–P2S5 Glass-Ceramic Systems as Solid-State Electrolytes: A Brief Review of Current Research, ACS Omega, 2024, 9, 31228–31236 CrossRef CAS PubMed.
- T. W. Kim, K. H. Park, Y. E. Choi, J. Y. Lee and Y. S. Jung, Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries, J. Mater. Chem. A, 2018, 6, 840–844 RSC.
- F. Hartmann, A. Benkada, S. Indris, M. Poschmann, H. Lühmann, P. Duchstein, D. Zahn and W. Bensch, Directed dehydratation as synthetic tool for generation of a new Na4SnS4 polymorph: Crystal structure, Na+ conductivity, and influence of Sb-substitution, Angew. Chem., Int. Ed., 2022, 61, e202202182 CrossRef CAS PubMed.
- W. Weng, H. Wan, G. Liu, L. Wu, J. Wu and X. Yao, Liquid-Phase Synthesis of Nanosized Na11Sn2PS12 Solid Electrolytes for Room Temperature All-Solid-State Sodium Batteries, ACS Appl. Energy Mater., 2021, 4, 1467–1473 CrossRef CAS.
- W. H. Smith, J. Birnbaum and C. A. Wolden, Production and purification of anhydrous sodium sulfide, J. Sulfur Chem., 2021, 42, 426–442 CrossRef CAS.
- S. A. Vaselabadi, K. Palmer, W. H. Smith and C. A. Wolden, Scalable Synthesis of Selenide Solid-State Electrolytes for Sodium-Ion Batteries, Inorg. Chem., 2023, 62, 17102–17114 CrossRef CAS PubMed.
- S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng and P. Zhang, Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond, Chem. Soc. Rev., 2019, 48, 4178–4280 RSC.
- J. Yu, C. Y. Xu, F. X. Ma, S. P. Hu, Y. W. Zhang and L. Zhen, Monodisperse SnS2 nanosheets for high-performance photocatalytic hydrogen generation, ACS Appl. Mater. Interfaces, 2014, 6, 22370–22377 CrossRef CAS PubMed.
- W. Fu, J. Wang, S. Zhou, R. Li and T. Peng, Controllable Fabrication of Regular Hexagon-Shaped SnS2 Nanoplates and Their Enhanced Visible-Light-Driven H2 Production Activity, ACS Appl. Nano Mater., 2018, 1, 2923–2933 CrossRef CAS.
- F. Zhang, C. Xia, J. Zhu, B. Ahmed, H. Liang, D. B. Velusamy, U. Schwingenschlögl and H. N. Alshareef, SnSe2 2D Anodes for Advanced Sodium Ion Batteries, Adv. Energy Mater., 2016, 6, 1601188 CrossRef.
- T. Wang, K. Yang, J. Shi, S. Zhou, L. Mi, H. Li and W. Chen, Simple synthesis of sandwich-like SnSe2/rGO as high initial coulombic efficiency and high stability anode for sodium-ion batteries, J. Energy Chem., 2020, 46, 71–77 CrossRef.
- W. Feng, M. Cheng, R. Du, Y. Wang, P. Wang, H. Li, L. Song, X. Wen, J. Yang, X. Li, J. He and J. Shi, Gram-Scale Synthesized Two-Dimensional VSe2 and SnSe2 for Ultrahigh Electrocatalytic Sulfion Recycling, Adv. Mater. Interfaces, 2022, 9, 2200060 CrossRef CAS.
- P. Ramasamy, P. Manivasakan and J. Kim, Phase controlled synthesis of SnSe and SnSe2 hierarchical nanostructures made of single crystalline ultrathin nanosheets, CrystEngComm, 2015, 17, 807–813 RSC.
- J. Choi, J. Jin, I. G. Jung, J. M. Kim, H. J. Kim and S. U. Son, SnSe2 nanoplate–graphene composites as anode materials for lithium ion batteries, Chem. Commun., 2011, 47, 5241–5243 RSC.
- S. Saha, A. Banik and K. Biswas, Few-Layer Nanosheets of n-Type SnSe2, Chem. – Eur. J., 2016, 22, 15634–15638 CrossRef CAS PubMed.
- G. Lindberg, A. Larsson, M. Råberg, D. Boström, R. Backman and A. Nordin, Determination of thermodynamic properties of Na2S using solid-state EMF measurements, J. Chem. Thermodyn., 2007, 39, 44–48 CrossRef CAS.
- H. Gamsjäger, J. Sangster and S. K. Saxena, CHEMICAL THERMODYNAMICS OF TIN Tamás GAJDA Search PubMed.
- S. A. Vaselabadi, B. Benham and C. A. Wolden, Facile one-pot synthesis of high-purity sodium antimony chalcogenides in polar solvents, Inorg. Chem. Front., 2025, 12, 658–671 RSC.
- C. Wang, K. Tang, Q. Y. Aa and Y. Qian, Raman scattering, far infrared spectrum and photoluminescence of SnS2 nanocrystallites, Chem. Phys. Lett., 2002, 357, 371–375 CrossRef CAS.
- S. H. Choi, W. J. Kim, B. H. Lee, S. C. Kim, J. G. Kang and D. W. Kim, Rational design of one-pot solvent-assisted synthesis for multi-functional Sn-substituted superionic Li argyrodite solid electrolytes, J. Mater. Chem. A, 2023, 11, 14690–14704 RSC.
- M. Uematsu, S. Yubuchi, F. Tsuji, A. Sakuda, A. Hayashi and M. Tatsumisago, Suspension synthesis of Na3-xPS4-xCl solid electrolytes, J. Power Sources, 2019, 428, 131–135 CrossRef CAS.
- H. Wan, J. P. Mwizerwa, X. Qi, X. Xu, H. Li, Q. Zhang, L. Cai, Y.-S. Hu and X. Yao, Nanoscaled Na3PS4 Solid Electrolyte for All-Solid-State FeS2/Na Batteries with Ultrahigh Initial Coulombic Efficiency of 95% and Excellent Cyclic Performances, ACS Appl. Mater. Interfaces, 2018, 10, 12300–12304 CrossRef CAS PubMed.
- M. Uematsu, S. Yubuchi, K. Noi, A. Sakuda, A. Hayashi and M. Tatsumisago, Preparation of Na3PS4 electrolyte by liquid-phase process using ether, Solid State Ionics, 2018, 320, 33–37 CrossRef CAS.
- H. Wan, J. P. Mwizerwa, X. Qi, X. Liu, X. Xu, H. Li, Y.-S. Hu and X. Yao, Core–Shell Fe1–xS@Na2.9PS3.95Se0.05 Nanorods for Room Temperature All-Solid-State Sodium Batteries with High Energy Density, ACS Nano, 2018, 12, 2809–2817 CrossRef CAS PubMed.
- M. Olson, J. Wheaton, M. Okkema, N. Oldham and S. W. Martin, Optimized Thin Film Processing of Sodium Mixed Oxy-Sulfide-Nitride Glassy Solid Electrolytes for All-Solid-State Batteries, ACS Appl. Energy Mater., 2023, 6, 5842–5855 CrossRef CAS.
- T. Scholz, C. Schneider, R. Eger, V. Duppel, I. Moudrakovski, A. Schulz, J. Nuss and B. V. Lotsch, Phase formation through synthetic control: polymorphism in the sodium-ion solid electrolyte Na4P2S6, J. Mater. Chem. A, 2021, 9, 8692–8703 RSC.
- T. Famprikis, H. Bouyanfif, P. Canepa, M. Zbiri, J. A. Dawson, E. Suard, F. Fauth, H. Y. Playford, D. Dambournet, O. J. Borkiewicz, M. Courty, O. Clemens, J.-N. Chotard, M. S. Islam and C. Masquelier, Insights into the Rich Polymorphism of the Na+ Ion Conductor Na3PS4 from the Perspective of Variable-Temperature Diffraction and Spectroscopy, Chem. Mater., 2021, 33, 5652–5667 CrossRef CAS PubMed.
- X. Chi, Y. Zhang, F. Hao, S. Kmiec, H. Dong, R. Xu, K. Zhao, Q. Ai, T. Terlier, L. Wang, L. Zhao, L. Guo, J. Lou, H. L. Xin, S. W. Martin and Y. Yao, An electrochemically stable homogeneous glassy electrolyte formed at room temperature for all-solid-state sodium batteries, Nat. Commun., 2022, 13, 2854 CrossRef CAS PubMed.
- C. Dietrich, D. A. Weber, S. Culver, A. Senyshyn, S. J. Sedlmaier, S. Indris, J. Janek and W. G. Zeier, Synthesis, Structural Characterization, and Lithium Ion Conductivity of the Lithium Thiophosphate Li2P2S6, Inorg. Chem., 2017, 56, 6681–6687 CrossRef CAS PubMed.
- A. Benkada, F. Hartmann, M. Poschmann, S. Indris, H. Lühmann and W. Bensch, Directed Dehydration of Na4Sn2S6·5H2O Generates the New Compound Na4Sn2S6: Crystal Structure and Selected Properties, Eur. J. Inorg. Chem., 2023, 6–11 Search PubMed.
- A. Benkada, F. Hartmann, T. A. Engesser, S. Indris, T. Zinkevich, C. Näther, H. Lühmann, H. Reinsch, S. Adams and W. Bensch, Room-Temperature Solid-State Transformation of Na4SnS4·14H2O into Na4Sn2S6·5H2O: An Unusual Epitaxial Reaction Including Bond Formation, Mass Transport, and Ionic Conductivity, Chem. – Eur. J., 2023, 29, e202202318 CrossRef CAS PubMed.
- M. Duchardt, S. Neuberger, U. Ruschewitz, T. Krauskopf, W. G. Zeier, J. Schmedt auf der Günne, S. Adams, B. Roling and S. Dehnen, Superion Conductor Na11.1Sn2.1P0.9Se12: Lowering the Activation Barrier of Na+ Conduction in Quaternary 1–4–5–6 Electrolytes, Chem. Mater., 2018, 30, 4134–4139 CrossRef CAS.
- Z. Yu, S. L. Shang, D. D. Wang, Y. C. Li, H. P. Yennawar, G. Li, H.-T. T. Huang, Y. Gao, T. E. Mallouk, Z.-K. K. Liu and D. D. Wang, Synthesis and understanding of Na11Sn2PSe12 with enhanced ionic conductivity for all-solid-state Na-ion battery, Energy Storage Mater., 2019, 17, 70–77 CrossRef.
- R. P. Rao, X. Zhang, K. C. Phuah and S. Adams, Mechanochemical synthesis of fast sodium ion conductor Na11Sn2PSe12 enables first sodium-selenium all-solid-state battery, J. Mater. Chem. A, 2019, 7, 20790–20798 RSC.
- S. D. Deshmukh, L. F. Easterling, J. M. Manheim, N. J. Libretto, K. G. Weideman, J. T. Miller, H. I. Kenttämaa and R. Agrawal, Analyzing and tuning the chalcogen-amine-thiol complexes for tailoring of chalcogenide syntheses, Inorg. Chem., 2020, 59, 8240–8250 CrossRef CAS PubMed.
- W. Schiwy, S. Pohl and B. Krebs, Darstellung und Struktur von Na4SnS4 - 14 H2O, J. Inorg. Gen. Chem., 1973, 402, 77–86 CAS.
- Y. Oh, S. Bag, C. D. Malliakas and M. G. Kanatzidis, Selective surfaces: High-surface-area zinc tin sulfide chalcogels, Chem. Mater., 2011, 23, 2447–2456 CrossRef CAS.
- P. D. Antunez, D. A. Torelli, F. Yang, F. A. Rabuffetti, N. S. Lewis and R. L. Brutchey, Low Temperature Solution-Phase Deposition of SnS Thin Films, Chem. Mater., 2014, 26, 5444–5446 CrossRef CAS.
- D. H. Webber and R. L. Brutchey, Alkahest for V2VI3 chalcogenides: Dissolution of nine bulk semiconductors in a diamine-dithiol solvent mixture, J. Am. Chem. Soc., 2013, 135, 15722–15725 CrossRef CAS PubMed.
- W. Mikenda and A. Preisinger, Vibrational spectra of Na3SbS4, Na3SbS4·9H2O (Schlippe's salt) and Na3SbS4·9D2O, Spectrochim. Acta, Part A, 1980, 36, 365–370 CrossRef.
- B. C. Walker and R. Agrawal, Contamination-free solutions of selenium in amines for nanoparticle synthesis, Chem. Commun., 2014, 50, 8331–8334 RSC.
- D. H. Webber, J. J. Buckley, P. D. Antunez and R. L. Brutchey, Facile dissolution of selenium and tellurium in a thiol-amine solvent mixture under ambient conditions, Chem. Sci., 2014, 5, 2498–2502 RSC.
- A. Vashishtha, O. Vana and E. Edri, Solvent composition regulates the Se:Sb ratio in antimony selenide nanowires deposited from thiol-amine solvent mixtures, Nanoscale Adv., 2022, 4, 772–781 RSC.
- C. L. McCarthy, D. H. Webber, E. C. Schueller and R. L. Brutchey, Solution-Phase Conversion of Bulk Metal Oxides to Metal Chalcogenides Using a Simple Thiol-Amine Solvent Mixture, Angew. Chem., Int. Ed., 2015, 54, 8378–8381 CrossRef CAS PubMed.
- R. Zhang, S. Cho, D. G. Lim, X. Hu, E. A. Stach, C. A. Handwerker and R. Agrawal, Metal-metal chalcogenide molecular precursors to binary, ternary, and quaternary metal chalcogenide thin films for electronic devices, Chem. Commun., 2016, 52, 5007–5010 RSC.
- C. L. McCarthy and R. L. Brutchey, Preparation of electrocatalysts using a thiol-amine solution processing method, Dalton Trans., 2018, 47, 5137–5143 RSC.
- L. Gao, G. Bian, Y. Yang, B. Zhang, X. Wu and K. Wu, Na4SnS4 and Na4SnSe4 exhibiting multifunctional physicochemical performances as potential infrared nonlinear optical crystals and sodium ion conductors, New J. Chem., 2021, 45, 12362–12366 RSC.
- K. Wu, X. Su, Z. Yang and S. Pan, An investigation of new infrared nonlinear optical material: BaCdSnSe4, and three new related centrosymmetric compounds: Ba2SnSe4, Mg2GeSe4, and Ba2Ge2S6, Dalton Trans., 2015, 44, 19856–19864 RSC.
- N. Ding, Y. Takabayashi, P. L. Solari, K. Prassides, R. J. Pcionek and M. G. Kanatzidis, Cubic gyroid frameworks in mesostructured metal selenides created from tetrahedral Zn2+, Cd2+, and in 3+ ions and the [SbSe4] 3- precursor, Chem. Mater., 2006, 18, 4690–4699 CrossRef CAS.
- M. J. Strumolo, D. B. Eremin, S. Wang, C. Mora Perez, O. V. Prezhdo, J. S. Figueroa and R. L. Brutchey, Formation of a P162- Ink from Elemental Red Phosphorus in a Thiol-Amine Mixture, Inorg. Chem., 2023, 62, 6197–6201 CrossRef CAS PubMed.
- W. Weng, G. Liu, L. Shen and X. Yao, High ionic conductivity and stable phase Na11.5Sn2Sb0.5Ti0.5S12 for all-solid-state sodium batteries, J. Power Sources, 2021, 512, 230485 CrossRef CAS.
- J. Hartel, A. Banik, J. M. Gerdes, B. Wankmiller, B. Helm, C. Li, M. A. Kraft, M. R. Hansen and W. G. Zeier, Understanding Lithium-Ion Transport in Selenophosphate-Based Lithium Argyrodites and Their Limitations in Solid-State Batteries, Chem. Mater., 2023, 35, 4798–4809 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00270b |
| This journal is © The Royal Society of Chemistry 2025 |

