Open Access Article
Open Access ArticleSuperior photocatalytic degradation of Reactive Orange 16 by Ag–AgCl/BiOCl nanocomposites under visible light†
Kamya
Jasuja
a and
Raj Kumar
Das
 *ab
*ab
aDepartment of Chemistry and Biochemistry, Thapar Institute of Engineering & Technology, Patiala-147004, Punjab, India. E-mail: rkdas@thapar.edu
bTIET-Virginia Tech Center of Excellence in Emerging Materials, Thapar Institute of Engineering and Technology, Patiala, 147004, India
First published on 28th April 2025
Abstract
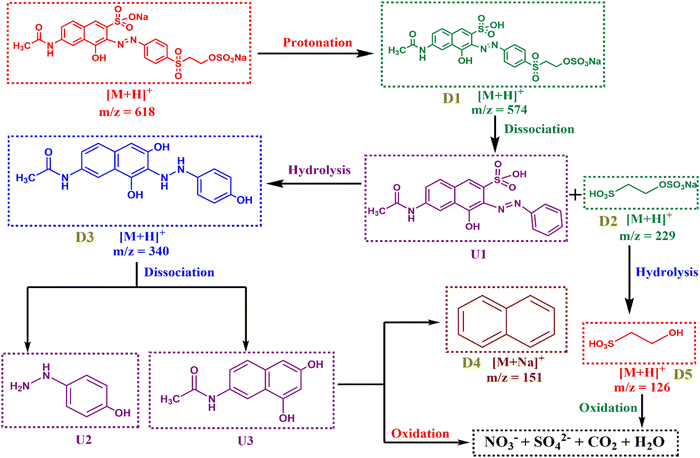
Due to unregulated dumping, the concentration of reactive dyes in wastewater has consistently increased. To mitigate their harmful nature, successful eradication becomes crucial. In this regard, Ag–AgCl–BiOCl nanocomposites were constructed utilizing a simple co-precipitation approach to evaluate the photocatalytic degradation activity towards Reactive Orange 16 in the presence of visible light. The synthesized catalyst was characterized using different methods, including field emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), diffuse reflectance spectroscopy (DRS), etc. Furthermore, XPS spectra show the co-existence of Ag(0) and Ag(+1) in the nanocomposite. The loading of Ag–AgCl results in lowering of electron–hole pair recombination and charge transfer resistance. As a result, AB10 outperformed all other nanocomposites in terms of photocatalytic activity, achieving 92% in 90 minutes. The control experiments suggest that holes act as the reactive species in the photocatalytic reactions, whereas electrons, hydroxyl radicals, and superoxide anions do not participate in the degradation process. Remarkably, Bi(III) acts as an electron scavenger to afford Bi(0). Ag(0), as well as the in situ generated Bi(0), can play an imperative role in improving the photocatalytic degradation efficiency owing to the localized surface plasmon resonance phenomenon. Furthermore, high-resolution mass spectroscopy (HRMS) confirms the presence of smaller fragments, resulting in a more in-depth understanding of the photocatalytic degradation process. The synthesized catalyst is exceptionally stable and recyclable. As a result, the photocatalyst has the potential to eliminate the reactive dye from wastewater.
1. Introduction
The substantial expansion of the textile sector has led to environmental hazards because of the effluents that include strong concentrations of coloring agents—reactive dyes and other organic and inorganic compounds.1–4 A chromophore in reactive dyes possesses a substituent that interacts with the substrate to establish a covalent connection during the dyeing process. They possess excellent wet fastness, providing them an edge over other dyes constrained by physical adsorption or mechanical entrapment.5 Unfortunately, a substantial portion of the dyes does not get attached with the textile during the dyeing process and is discharged directly into the environment. These dyes are xenobiotic compounds that, besides being carcinogenic and genotoxic, can adversely affect water quality due to their coloration, elevated pH, chemical oxygen demand, and limited biodegradability. These dyes can lead to health complications such as asthma, allergic conjunctivitis, hemorrhages, and central nervous system diseases.6–10 Even at minimal concentrations (below 1 ppm), these colours adversely affect aquatic ecosystems. Consequently, the elimination of these harmful pollutants from wastewater is imperative and vital. Adsorption coagulation, flocculation, and biological treatments are widely employed methods for the treatment of industrial effluents to eliminate these pigments.11 Photocatalytic degradation is acknowledged as one of the most efficient techniques for the removal of industrial effluents, as it eradicates them completely instead of merely transferring them from one source to another. Photocatalysis refers to the acceleration of a photoreaction facilitated by a catalyst. The tetragonal matlockite structure of BiOCl is characterized by [Bi2O2] slabs that are interspersed with double halogen slabs. The multilayered structure of BiOX results in inherent static electric fields, which provide adequate space to generate atom and orbital polarization. Due to its chemical stability, anti-corrosive properties, and nontoxicity, BiOCl functions as an efficient photocatalyst.12,13 BiOCl possesses a wide band gap. So, it predominantly absorbs in the ultraviolet region. Moreover, the rapid electron–hole pair recombination limits the activity of bare BiOCl as a photocatalyst. Consequently, loading with other metals and nonmetals and constructing heterojunctions with other semiconductors can enhance its reactivity.14 Nowadays, silver halides have emerged as a new generation of semiconductors due to their various properties, such as high photosensitivity and the ability to absorb visible light, making the material more efficient in degrading the target pollutant.14,15 Moreover, the synthesis of silver halides often leads to the formation of metallic silver (Ag) via in situ reduction. The presence of Ag can be highly beneficial for photocatalytic reactions, due to the localized surface plasmon resonance (LSPR) effect.15,16 The LSPR effect enhances the visible light sensitivity of the materials and produces high-energy excitons that dissipate through the phonons, thereby resulting in high lattice temperature.17–19 Such effects can lead to substantial improvement in the photocatalytic degradation efficiency.15,16 The synergistic impact of Ag and AgCl, in which Ag nanoparticles work as an electron donor and AgCl promotes electron–hole pair separation and light absorption, boosts the photocatalytic degradation efficiency towards the target pollutant.20,21 Zhou and coworkers discovered that Ag/AgCl/BiOCl degrades methyl orange (MO) by up to 97% in 60 minutes under visible light irradiation.22 Zhao and coworkers also demonstrated that Ag/AgCl/BiOCl eliminates Rhodamine B (RhB) dye up to 97% in 50 minutes.23Recent investigations have shown the exceptional photocatalytic degrading efficacy of BiOCl-based composites. Using a hydrothermal technique and in situ preparation, the Ag@ZnO/BiOCl composite was efficiently synthesized to study the photodegradation of tetracycline hydrochloride. In the presence of simulated solar light, the modified sample has a degradation efficiency of 80.4%.24,25
Unfortunately, the complex structure and high durability of reactive dyes make photocatalytic degradation of reactive dyes extremely challenging. The principal goal of this work was to examine the photocatalytic degradation efficiency of a reactive dye (Reactive Orange 16) using a non-toxic photocatalyst that is prepared by using the co-precipitation technique. This study demonstrates the synthesis of different hybrid composites with different loadings of Ag–AgCl on BiOCl and the evaluation of their photocatalytic degradation efficiency for the elimination of Reactive Orange 16.
2. Materials and methods
2.1. Reagents and chemicals used
Reactive Orange 16 (RO16) was procured from Sisco Research Laboratory (SRL), India. Silver nitrate (AgNO3) and glacial acetic acid (CH3COOH, 99.5%) were sourced from Fisher Scientific. Sodium chloride (NaCl) and bismuth nitrate pentahydrate [(Bi(NO3)3·5H2O)] were acquired from Loba Chemie Private Limited. Deionized water (DI) was produced utilizing the Milli-Q, Millipore ultrafiltration system. Absolute ethanol (99%) was purchased from Changshu Hongsheng Fine Chemicals Co. Ltd. Fluorinated tin oxide (FTO) glasses (resistance < 10 Ω) were procured from Vritra Technologies. Carbon black powder (CB) and polyvinylidene fluoride (PVDF) were bought from Nanoshell and Sigma Aldrich, respectively.2.2. Preparation of BiOCl
The co-precipitation method was employed to synthesize bismuth oxychloride (BiOCl). Initially, 3 mmol (1.45 g) of Bi (NO3)3·5H2O was dissolved in 10 mL of glacial acetic acid. Solution A was produced by adding 30 mL of deionized water to the solution and stirring it for 1 hour after complete dissolution. To prepare solution B, 30 mL of DI water was combined with 3 mmol (0.175 g) of NaCl, stirring it for 1 hour. Solution B was subsequently added to solution A in a gradual, dropwise manner, resulting in the formation of white precipitates. After that, the suspension was subjected to stirring for 12 hours. The suspension was subsequently centrifuged. The desired BiOCl product was obtained by washing the precipitate thrice with DI water and ethanol and subsequently dried at 60 °C.262.3. Preparation of Ag–AgCl loaded BiOCl
Using a coprecipitation technique, Ag–AgCl/BiOCl was synthesized. For this, 3.25 g of Bi(NO3)3·5H2O was dissolved in 25 mL of glacial acetic acid. After complete dissolution, 75 mL of DI water was added to the above solution, and this solution was marked as A. Moreover, for solution B, 0.875 g of NaCl was dissolved in 75 mL of DI water. Both the solutions (solution A and B) were left to stir for 1 hour. For synthesizing AB1, 0.0325 g of AgNO3 was added to solution A, and thereafter solution B was gradually poured into solution A to facilitate the precipitate formation. The final reaction mixture was allowed to stir for 16 hours (Scheme S1, ESI†). Afterward, the solid was separated by centrifugation and washed with DI water and ethanol, and dried at 60 °C. Similarly, 0.1625, 0.325, and 0.4875 g of silver nitrate were used to prepare AB5, AB10, and AB15 heterostructures, respectively.272.4. Preparation of thin films
Using the drop-cast method, the working electrodes for electrochemical impedance spectroscopy (EIS) were prepared. A smooth paste was achieved by mixing NMP (40–60 μL) and PVDF (1 mg). Additionally, 1 mg of CB was added and then blended for 15–20 min. Following this, 8 mg of the relevant material (BiOCl and AB10) was incorporated, resulting in a homogenous mixture. Afterward, the homogeneous paste was then drop-cast onto fresh FTO glass films and subsequently dried for 12 hours at 80 °C.2.5. Characterization
A variety of characterization methods were employed to examine the crystallinity, composition, and surface morphology, along with other physicochemical properties of the synthesized photocatalyst. The crystallographic properties were evaluated using X-ray diffraction (XRD). The apparatus employed was an X'Pert pro24 Cu-Kα (1.54 Å) operated at 45 kV, with a diffraction angle configured at 2θ (5–90°). Energy dispersive spectroscopy (EDS) and field emission scanning electron microscopy (Carl-Zeiss Sigma 500, FESEM) were employed to analyse the structural morphology. Moreover, high-resolution transmission electron microscopy (JEOL JEM 2100 plus, HRTEM) was used to analyse the crystallinity and morphology. The oxidation states and electrical properties of the material were assessed using X-ray photoelectron spectroscopy (XPS). A UV-visible spectrophotometer (Shimadzu UV-2600) was employed to determine the diffused reflectance spectra (DRS). A mass spectrometer (Waters QTOF), equipped with XEVO G2 XS UHPLC and integrated APCI and ESI ionisation sources, was utilised for high-resolution mass spectrometry (HRMS) to detect the intermediates. Electrochemical impedance spectroscopy (EIS) tests were conducted in the dark at 0.55 V vs. RHE using a Biologic VSP300 potentiostat, within a frequency range of 1–105 Hz. The system comprised three electrodes: sample loaded on fluorine-doped tin (FTO) glasses, standard calomel electrode and platinum (Pt), which functioned as the working electrode, reference electrode and counter electrode, respectively, with 0.1 M sodium sulphate (Na2SO4) solution.2.6. Photodegradation studies
The degradation efficiencies of the synthesized catalysts towards photodegradation of RO16 were assessed in the presence of visible light. Initially, test tubes containing 10 mL of reactive dye solution were added with 7.5 mg of photocatalyst. Afterwards, the mixture was stirred for 30 minutes in the dark to ensure adsorption–desorption equilibrium. Subsequently, the reaction mixtures were irradiated with visible light using aWipro Garnet B22-50 W LED (wavelength of greater than 360 nm and light intensity of 5000 lumens) for 90 minutes. Then the mixture was subsequently centrifuged to isolate the catalyst. The concentration of the reactive dyes was determined using a Shimadzu UV-2600 spectrometer for UV-visible spectroscopy by monitoring the absorbance at 490 nm.The photodegradation efficiencies were computed by the following eqn (1)
 | (1) |
3. Results and discussion
3.1. DRS studies
UV-visible diffuse reflectance spectroscopy was used to study the optical properties of the prepared BiOCl and its composites. The absorption edge of BiOCl was measured within the region of 270–305 nm. Moreover, the electronic spectrum of AB10 exhibits a significant absorbance in the range of 400–800 nm, attributed to the LSPR effect of Ag–AgCl as illustrated in Fig. 1a, which can enhance the degradation efficiency considerably.28,29 The bare BiOCl has a band position of 3.24 eV. The composites exhibit a gradual decrease in band gap from 3.24 eV to 3.14 eV, as illustrated in Fig. 1b. The band gap decreased to 3.14 eV when Ag–AgCl loading was increased to 10. The band gap values at the absorption edge were found to be in the range of 3.36 to 3.42 eV (Fig. 1c). | ||
| Fig. 1 (a) UV-visible DRS of BiOCl and hybrid-composites and Tauc plot showing (b) band gap and (c) band gap values at the adsorption edge. | ||
3.2. XRD studies
XRD analysis was executed to ascertain the phase purity and crystallinity of the prepared sample. Peaks were observed at 12.26°, 25.94°, 32.61°, 33.54°, 34.91°, 36.51°, 40.90°, 46.81°, 48.43°, 49.81°, 53.27°, 54.24°, 55.18°, 68.27°, 75.65°, and 77.72°, respectively. These 2θ values corresponded to the (001), (002), (101), (110), (102), (003), (112), (201), (113), (202), (211), (104), (212), (114), (214), and (310) planes, indicating tetragonal symmetry, which was well matched with the tetragonal BiOCl (ICDD no: 85-0861).16 According to ICDD no. 31-1238, the planes (111), (200), (220), and (420) were seen at 2θ values of 27.9°, 32.2°, 46.3°, and 76.9°, respectively, which correlated to the formation of AgCl (Fig. 2).30,313.3. Morphology
FESEM was employed to examine the surface morphology of pure BiOCl and its composites with Ag–AgCl, the sample labelled AB10, as illustrated in Fig. 3. Pure BiOCl exhibited an irregular, plate-like morphology, (Fig. 3a and b). Furthermore, (Fig. 3c and d), a flower-like shape is observed for Ag–AgCl–BiOCl. HRTEM was employed to assess the morphology and interior structures in greater detail (Fig. 4a–d). The HRTEM images clearly demonstrate the proper incorporation of pure BiOCl and Ag–AgCl particles. Additionally, it was found that the fringe width measured 0.28 nm (Fig. 4e),28,32 which is characteristic of the crystallographic (110) plane of BiOCl, suggesting the retention of crystallinity in pure BiOCl within the composite. The SAED pattern displays concentric rings with luminous spots, corresponding to the specific crystallographic planes (001), (102), and (110), which align with the tetragonal symmetry of BiOCl. This additionally confirms the development and crystallinity of the nanocomposite, as depicted in Fig. 4f. EDS mapping was utilized to determine the elemental composition of the synthesized catalyst. All components in Fig. S1 (ESI†) were uniformly distributed across the surface, demonstrating the absence of additional elements in the produced sample.3.4. XPS studies
XPS studies were performed to study the atomic composition and oxidation states in AB10 (Fig. 5). All the constituent elements were in the sample based on the XPS survey spectrum, as shown in Fig. 5a. The existence of Bi3+ in the composite was confirmed by two distinct peaks, located at 159.3 eV and 164.6 eV, which are associated with Bi 4f7/2 and Bi 4f5/2, respectively33,34 (Fig. 5b). Additionally, the O 1s spectrum (Fig. 5c) has four binding energies at 530.1 eV and 530.6 eV, which confirms the presence of Bi–O and the O–Cl linkages, respectively.35 Moreover, the peaks at 533.1 eV and 532.3 eV are characteristic of the physisorbed and chemisorbed water molecules, respectively (Fig. 5d). The Cl 2p signals at 198 eV and 199.4 eV are due to Cl 2p3/2 and Cl 2p1/2, respectively; moreover, Ag 3d3/2 and Ag 3d5/2 signals (Fig. 5e) at 368.1 eV and 374.1 eV, respectively, confirm the presence of monovalent Ag. Furthermore, the analogous peaks at 375.4 eV and 369.5 eV indicate the presence of zerovalent silver in the composite.36 | ||
| Fig. 5 XPS spectra of AB10 showing (a) survey spectrum, (b) Bi 4f, (c) O 1s, (d) Cl 2p and (e) Ag 3d patterns. | ||
3.5. Photoluminescence spectra
The variation in electron–hole pair recombination in the synthesized catalysts was investigated using photoluminescence (PL) spectroscopy (Fig. 6). It was observed that the AB10 composite has much lower emission intensity. Such observation confirms that loading Ag–AgCl over the BiOCl surface reduces the charge carrier recombination rate.37 Therefore, the AB10 heterostructure can act as a more efficient photocatalyst than the bare BiOCl.3.6. EIS studies
EIS studies were performed in the dark on BiOCl and AB10 to investigate the parameters of charge separation. The Nyquist plot of AB10 has a smaller radius of AB10, indicating a more facile charge transfer in it compared to pristine BiOCl. Furthermore, these findings imply that the loading of Ag–AgCl facilitates the transfer of charge from BiOCl to its surface, thereby lowering the resistance.38 As a result, AB10 can work as an efficient catalyst (Fig. 7).3.7. Photodegradation studies
To evaluate the photocatalytic degradation of pure BiOCl and its composites (AB1, AB5, AB10, and AB15) in the presence of visible light. Initially, the mixture was stirred in the dark for half an hour to achieve adsorption–desorption equilibrium. Moreover, visible light was exposed to the solution for 90 minutes. The degrading efficiency of pure BiOCl is quite low (Fig. 8a and b). This is explained by its limited capacity to reduce electron–hole pair recombination, which can restrict the number of active sites and, consequently, lower the degradation efficiency. However, from AB1 to AB10, the degradation efficiency steadily increases as the loading of Ag–AgCl on BiOCl increases due to less electron–hole pair recombination that allows charge separation, which increases the degradation efficiency to a higher degree (92%). Furthermore, due to the reduced active sites, a further increase in Ag–AgCl loading has no discernible effect on the photodegradation efficiency (Fig. S3, ESI† and Fig. 8b). All the photocatalytic reactions obey the pseudo-zero order kinetic model (eqn (2)),39 (Fig. 8c and Table S1, ESI†).| Ct = C0 − kt | (2) |
Furthermore, it was observed that AB10 has the greatest rate constant value of 0.438(2) mol L−1 min−1, as demonstrated in Fig. 8d. Additionally, the values of half-life were found to vary linearly with initial concentration, hence confirming the validity of the pseudo zero order kinetics further (Fig. S4, ESI†).
To optimize catalyst utilization, the amount of catalyst used for the degradation of Reactive Orange 16 was adjusted, and a difference in degradation efficiency was noticed. As the amount of catalyst increased, the photocatalytic degradation efficiency dropped, as illustrated in Fig. 9. There were more active sites up to 7.5 mg, resulting in the highest photocatalytic degradation efficiency possible. As the concentration increased, particles accumulated, thereby clogging the active sites and reducing the degradation efficiency. As a result, a dosage of 7.5 mg of the catalyst and pollutant concentrations of 40 ppm were used for subsequent studies.40
3.8. Mechanistic details
| EVB = χ − Ee + 0.5Eg | (3) |
| ECB = EVB − Eg | (4) |
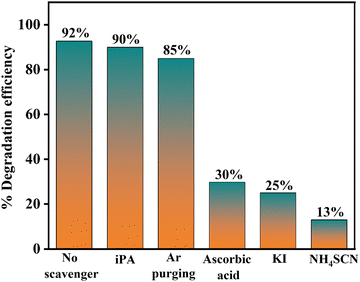
The EVB values at the absorption edge for BiOCl and AgCl are +3.46 eV and +2.44 eV, respectively, whereas the ECB value for BiOCl is +0.04 eV and −0.47 eV for AgCl. Therefore, BiOCl possesses a more positive electrode potential as compared to AgCl.41–45 The proposed mechanism involves electrons being excited from the VB to the CB in order to produce an electron–hole pair (Fig. 1c and 10), followed by the electron transfer from the CB of AgCl to the CB of BiOCl and the hole transfer from the VB of BiOCl to the VB of AgCl. The CB edge potential of BiOCl (+0.04 eV) is less negative than the superoxide radical (−0.046 eV), whereas the VB edge potential of AgCl (+2.44) is less positive than ˙OH/H2O (+2.68 eV). As a result, the oxidation of H2O to hydroxyl radicals and the reduction of dissolved oxygen to superoxide anions are not feasible. The dye (Reactive Orange 16) can get oxidized by holes only, while metal cations can act as an electron scavenger.
3.9. Reusability and stability of the catalyst
To investigate potential future uses, the stability and reusability of the photocatalyst are crucial. At the end of the reaction, the catalyst was recovered from the reaction mixture and utilized further. It was found that the photodegradation efficiency of AB10 declined to only 4% (Fig. 13) from the first to fourth cycle. Such observation suggests that the AB10 catalyst has excellent reusability towards the elimination of RO16. The minor decrease in the removal efficiency can be ascribed to catalyst loss during recovery.In order to determine the stability of the synthesized catalyst (AB10), FESEM studies were performed. It has been observed that there are no significant changes in the morphology of the as-prepared catalyst before and after the reaction (Fig. 14). These observations confirm the high stability of the photocatalysts.
3.10. Comparison of the degradation efficiencies
The synthesized photocatalyst (AB10) has been compared with the existing literature (Table 1). It has to be noted that apart from a few catalysts, our catalyst shows comparable or better photocatalytic efficiency. However, most of these either need a larger dosage of catalyst,47,49 higher light intensity,47,49–51 or longer reaction time.49,51 Such observation shows that compared to the reported literature the AB10 composite shows a superior photocatalytic activity.| S. no. | Catalyst | Reactive dyes | Catalyst dosage (mg L−1) | Intensity of light (W m−2) | Degradation efficiency (%) | Light source | Reaction time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | gC3N4-MU | RO16 | 1000 | 55 | 95 | Visible light | 60 | 50 |
| 2 | MgO/gC3N4 | RO16 | 400 | 39.6 | 82 | Visible light | 30 | 51 |
| 3 | Ag/AgVO4/AgVO3 | RO16 | 1000 | 300 | 97 | Visible light | 200 | 52 |
| 4 | CeO2/ZnO | RO16 | 2000 | 300 | 90 | Visible light | 180 | 53 |
| 5 | S,N, co-doped TiO2/graphene | RO16 | 333.33 | 500 | 96 | Visible light | 120 | 54 |
| 6 | Ag–AgCl/BiOCl | RO16 | 750 | 50 | 92 | Visible light | 90 | This work |
4. Conclusions
In conclusion, this study demonstrates that Ag–AgCl–BiOCl was synthesized utilizing a straightforward co-precipitation technique for the removal of RO16 in the presence of visible light. The controlled studies indicate that the holes were involved in the entire mechanism. The HRMS tests show smaller fragments for photocatalytic degradation of pollutants, which provides further information on the mechanism. During the procedure, Bi (+3) was reduced to Bi(0), causing the LSPR effect as observed in the DRS of the recovered photocatalyst. Furthermore, the photocatalyst produced is very recyclable and stable. Owing to its low cost, low toxicity, and high catalytic activity, the synthesized catalyst could be useful for eliminating reactive dyes from wastewater.AI tools
The grammatical mistakes have been checked and amended using the Grammarly tool.Author contributions
Kamya Jasuja: writing the original draft, visualization, validation, investigation, formal analysis, data curation, and conceptualization. Raj Kumar Das: writing – review & editing, visualization, validation, supervision, resources, funding acquisition, and conceptualization.Data availability
The supporting data have been uploaded as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported by the TIET VT CEEMS Research grant. The authors express gratitude to Sai Labs, TIET, for conducting the XRD measurements. The authors express profound gratitude to the Department of Physics and Material Science for providing SEM-EDS measurements and sprint testing solutions for XPS and HRTEM studies. The authors express gratitude to DST-FIST (Infrastructure Grant number: SR/FST/CS-II/2018/69) for the HRMS facility. The authors express their gratitude to Prof. Bonamali Pal and Prof. Satnam Singh for providing access to their laboratory facilities. Also, the authors are thankful to Dr Banibrata Maity and Ms Malika Phull for the PL analysis. The authors express their gratitude to Prof. B. N. Chudasama and Ms Neha Saini for their contributions to the DRS characterization. The authors express their gratitude to Mr Samarjit Mandal for supplying the ICDD data. The authors express profound gratitude to Prof. O. P. Pandey and Mr Abhishek Chandel for their contributions to EIS characterization.References
- H. A. Kiwaan, T. M. Atwee, E. A. Azab and A. A. El-Bindary, Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide, J. Mol. Struct., 2020, 1200, 127115 CrossRef CAS.
- D. K. Bhatt and U. D. Patel, Photocatalytic degradation of Reactive Black 5 using Ag3PO4 under visible light, J. Phys. Chem. Solids, 2021, 149, 109768 CrossRef CAS.
- Y. Wu, M. Ruan, C. Wang, T. Zhong and Z. Liu, Construction of 2D/2D BiOIO3/Bi2O2CO3 composite structures with face–to–face contacts can facilitate carrier transfer via a built-in electric field and a polar field for pyro-photo-electric catalysis, J. Mater. Chem. C, 2024, 12, 13010–13020 RSC.
- Y. Wu, M. Ruan, Z. Guo, C. Wang and Z. Liu, Optimization of the IO3 polar group of BiOIO3 by bulk phase doping amplifies pyroelectric polarization to enhance carrier separation and improve the pyro-photo-electric catalytic performance, Appl. Catal., B, 2023, 339, 123169 CrossRef CAS.
- Y. G. Alghamdi, B. Krishnakumar, M. A. Malik and S. Alhayyani, Design and Preparation of Biomass-Derived Activated Carbon Loaded TiO2 Photocatalyst for Photocatalytic Degradation of Reactive Red 120 and Ofloxacin, Polymers, 2022, 14, 880 CrossRef CAS PubMed.
- M. F. Lanjwani, M. Tuzen, M. Y. Khuhawar and T. A. Saleh, Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review, Inorg. Chem. Commun., 2024, 159, 11613 CrossRef.
- R. Frei, K. Heye and C. Roduit, Environmental influences on childhood allergies and asthma—The Farm effect, Pediatr. Allergy Immunol., 2022, 33, 13807 CrossRef.
- Ö. F. Ertuğrul and M. F. Akıl, Detecting hemorrhage types and bounding box of hemorrhage by deep learning, Biomed. Signal Process. Control, 2022, 71, 103085 CrossRef.
- J. S. D. Santos, J. P. G. Cirino, P. D. O. Carvalho and M. M. Ortega, The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review, Front. Pharmacol., 2021, 11, 2020 Search PubMed.
- B. A. Labib and D. I. Chigbu, Therapeutic Targets in Allergic Conjunctivitis, Pharmaceuticals, 2022, 15, 547 CrossRef CAS PubMed.
- E. Erusappan, S. Thiripuranthagan, R. Radhakrishnan, M. Durai, S. Kumaravel, T. Vembuli and N. J. Kaleekkal, Fabrication of mesoporous TiO2/PVDF photocatalytic membranes for efficient photocatalytic degradation of synthetic dyes, J. Environ. Chem. Eng., 2021, 9, 105776 CrossRef CAS.
- T. Song, X. Yu, N. Tian and H. W. Huang, Preparation, structure and application of g-C3N4/BiOX composite photocatalyst, Int. J. Hydrogen Energy, 2021, 46, 1857–1878 CrossRef CAS.
- T. Alapi, B. Veres, M. Náfrádi, L. Farkas, Z. Pap and A. Covic, Application of BiOX Photocatalyst to Activate Peroxydisulfate Ion-Investigation of a Combined Process for the Removal of Organic Pollutants from Water, Catalysts, 2023, 13, 513 CrossRef CAS.
- D. O. Adenuga, S. M. Tichapondwa and E. M. N. Chirwa, Facile synthesis of a Ag/AgCl/BiOCl composite photocatalyst for visible-light-driven pollutant removal, J. Photochem. Photobiol., A, 2020, 401, 112747 CrossRef CAS.
- W. Zhu, J. Song, X. Wang, Y. Lu, G. Hu and J. Yang, The fast degradation for tetracycline over the Ag/AgBr/BiOBr photocatalyst under visible light, J. Mater. Sci.: Mater. Electron., 2021, 32, 26465 CrossRef CAS.
- Y. Wu, T. Zhong, M. Ruan, C. Wang and Z. Liu, Optimization of carrier transfer kinetics of BiOIO3 using TFA·/TFA- reversible redox pairs of TFA molecules as co-catalysts for efficient photoelectrochemical water splitting system, Int. J. Hydrogen Energy, 2024, 88, 571–578 CrossRef CAS.
- R. Li, X. Wang and M. Chen, Non-Noble Metal and Nonmetallic Plasmonic Nanomaterials with Located Surface Plasmon Resonance Effects: Photocatalytic Performance and Applications, Catalysts, 2023, 13, 940 CrossRef CAS.
- Z. Zhang, C. Zhang, H. Zheng and H. Xu, Plasmon-Driven Catalysis on Molecules and Nanomaterials, Acc. Chem. Res., 2019, 52, 2506–2515 CrossRef CAS PubMed.
- Z. Zheng, W. Xie, B. Huang and Y. Dai, Plasmon-Enhanced Solar Water Splitting on Metal-Semiconductor Photocatalysts, Chem. – Eur. J., 2018, 24, 18322–18333, DOI:10.1002/chem.201803705.
- Z. Xu and S. Y. Lin, Construction of AgCl/Ag/BiOCl with a concave-rhombicuboctahedron core–shell hierarchitecture and enhanced photocatalytic activity, RSC Adv., 2016, 6, 84738–84747 RSC.
- Y. Huang, S. Kang, Y. Yang, H. Qin, Z. Ni, S. Yang and X. Li, Facile synthesis of Bi/Bi2WO6 nanocomposite with enhanced photocatalytic activity under visible light, Appl. Catal., B, 2016, 196, 89–99 CrossRef CAS.
- S. Zou, X. Mao, L. Wu, Y. Li, J. Li, J. Yang and X. Fan, Deposition of Ag@AgCl onto Flower-Like BiOCl for Promoting the Degradation of Methyl Orange: The Narrow Bandgap and Surface Plasmon Resonance Effect, Korean J. Chem. Eng., 2024, 41, 2705–2715 CrossRef CAS.
- S. Zhao, Y. Zhang, Y. Zhou, K. Qiu, C. Zhang, J. Fang and X. Sheng, Reactable polyelectrolyte-assisted preparation of flower-like Ag/AgCl/BiOCl composite with enhanced photocatalytic activity, J. Photochem. Photobiol., A, 2018, 350, 94–102 CrossRef CAS.
- Z. Zhang, A. Zada, N. Cui, N. Liu, M. Liu, Y. Yang, D. Jiang, J. Jiang and S. Liu, Synthesis of ag loaded ZnO/BiOCl with high photocatalytic performance for the removal of antibiotic pollutants, Crystals, 2021, 11, 981 CrossRef CAS.
- X. Zhang, Y. Huo, M. Shakeel, B. Li, L. Wang, J. Liu and S. Zuo, Fabrication of BiOCl/ZnO/CN Nanocomposite for Visible-Light Photocatalytic Degradation of Dyes, ChemistrySelect, 2020, 5, 1640–1647 CrossRef CAS.
- J. Wang and Z. Zhang, Co-precipitation synthesis and photocatalytic properties of BiOCl microflowers, Optik, 2020, 204, 164149 CrossRef CAS.
- M. Anwar, K. S. Alghamdi, S. Zulfiqar, M. F. Warsi, M. Waqas and M. Hasan, Ag-decorated BiOCl anchored onto the g-C3N4 sheets for boosted photocatalytic and antimicrobial activities, Opt. Mater., 2023, 135, 113336 CrossRef CAS.
- L. Liang, Y. Lv, Z. Yu, R. Wu, Q. Shi, H. Chen and J. Wang, Surface plasmon resonance effect of Ag@BiOCl and its enhanced visible light-photodegradation of acid red B, J. Mater. Sci.: Mater. Electron., 2021, 32, 18646–18656 CrossRef CAS.
- D. Wei, F. Tian, Z. Lu, H. Yang and R. Chen, Facile synthesis of Ag/AgCl/BiOCl ternary nanocomposites for photocatalytic inactivation of: S. aureus under visible light, RSC Adv., 2016, 6, 52264–52270 RSC.
- W. Zhang, X. Dong, Y. Liang, Y. Sun and F. Dong, Ag/AgCl nanoparticles assembled on BiOCl/Bi12 O17Cl2 nanosheets: Enhanced plasmonic visible light photocatalysis and in situ DRIFTS investigation, Appl. Surf. Sci., 2018, 455, 236–243 CrossRef CAS.
- S. Kota, P. Dumpala, R. K. Anantha, M. K. Verma and S. Kandepu, Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa, Sci. Rep., 2017, 7, 11566 CrossRef PubMed.
- L. Wang, D. Lv, Z. Yue, H. Zhu, L. Wang, D. Wang, X. Xu, W. Hao, S. X. Dou and Y. Du, Promoting photoreduction properties via synergetic utilization between plasmonic effect and highly active facet of BiOCl, Nano Energy, 2019, 57, 398–404 CrossRef CAS.
- P. Rohilla, B. Pal and R. K. Das, Bi-doped g-C3N4/Bi2WO6 ternary composites for superior photocatalytic degradation of reactive orange 16 under visible light irradiation, J. Ind. Eng. Chem., 2024, 57, 398 Search PubMed.
- P. Rohilla, B. Pal and R. K. Das, Improved photocatalytic degradation of rhodamine B by g-C3N4 loaded BiVO4 nanocomposites, Heliyon, 2023, 9, e21900 CrossRef CAS.
- A. Phuruangrat, T. Thongtem and S. Thongtem, Microwave-assisted deposition synthesis, characterization and photocatalytic activities of UV-light-driven Ag/BiOCl nanocomposites, Inorg. Nano-Met. Chem., 2021, 51, 1813–1821 CrossRef CAS.
- J. Wang, C. An, M. Zhang, C. Qin, X. Ming and Q. Zhang, Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes, Can. J. Chem., 2012, 90, 858–864 CrossRef CAS.
- X. Xu, Q. Yan, X. Gu and Y. Luo, The preparation and photocatalytic performance of BiOCl@Ag, a visible-light responsive catalyst, J. Mater. Sci.: Mater. Electron., 2019, 30, 8892–8902 CrossRef CAS.
- A. Zulkiflee, M. M. Khan, A. Khan, M. Y. Khan, H. D. M. Dafalla and M. H. Harunsani, Sn-doped BiOCl for photoelectrochemical activities and photocatalytic dye degradation under visible light, Heliyon, 2023, 9, e21270 CrossRef CAS PubMed.
- M. Anjum, R. Kumar, S. M. Abdelbasir and M. A. Barakat, Carbon nitride/titania nanotubes composite for photocatalytic degradation of organics in water and sludge: Pre-treatment of sludge, anaerobic digestion and biogas production, J. Environ. Manage., 2018, 223, 495–502 CrossRef CAS PubMed.
- M. Bansal, K. Jasuja and R. K. Das, Photocatalytic degradation of reactive dyes over Ni–Al layered double hydroxide, Catal. Commun., 2024, 187, 106879 CrossRef CAS.
- L. Cai, Enhanced visible light photocatalytic activity of BiOCl by compositing with g-C3N4, Mater. Res. Innovations, 2015, 19, 392–396 CrossRef CAS.
- N. Chang, Y. R. Chen, F. Xie, Y. P. Liu and H. T. Wang, Facile construction of Z-scheme AgCl/Ag-doped-ZIF-8 heterojunction with narrow band gaps for efficient visible-light photocatalysis, Colloids Surf., A, 2021, 616, 126351 CrossRef CAS.
- A. Kundu, S. Sharma and S. Basu, Modulated BiOCl nanoplates with porous g-C3N4 nanosheets for photocatalytic degradation of color/colorless pollutants in natural sunlight, J. Phys. Chem. Solids, 2021, 154, 110064 CrossRef CAS.
- Y. Chen, G. Zhu, Y. Liu, J. Gao, C. Wang, R. Zhu and P. Liu, Preparation of hollow Ag/AgCl/BiOCl microspheres with enhanced photocatalytic activity for methyl orange under LED light irradiation, J. Mater. Sci.: Mater. Electron., 2017, 28, 2859–2866 CrossRef CAS.
- Y. Li, Z. Liu, Z. Guo, M. Ruan, X. Li and Y. Liu, Efficient WO3 Photoanode Modified by Pt Layer and Plasmonic Ag for Enhanced Charge Separation and Transfer to Promote Photoelectrochemical Performances, ACS Sustainable Chem. Eng., 2019, 7, 12582–12590 CAS.
- Z. Zhao, W. Zhang, Y. Sun, J. Yu, Y. Zhang, H. Wang, F. Dong and Z. Wu, Bi Cocatalyst/Bi2MoO6 Microspheres Nanohybrid with SPR-Promoted Visible-Light Photocatalysis, J. Phys. Chem. C, 2016, 120, 11889–11898 CrossRef CAS.
- S. Tao, S. Sun, T. Zhao, J. Cui, M. Yang, X. Yu, Q. Yang, X. Zhang and S. Liang, One-pot construction of Ta-doped BiOCl/Bi heterostructures toward simultaneously promoting visible light harvesting and charge separation for highly enhanced photocatalytic activity, Appl. Surf. Sci., 2021, 543, 148798 CrossRef CAS.
- P. Kumari, R. K. Das and B. Pal, Enhanced photocatalytic degradation of eco-toxic pharmaceutical waste diclofenac sodium by anion loaded Cu-Al LDH·Bi2O3 composites, J. Taiwan Inst. Chem. Eng., 2021, 129, 227–236 CrossRef CAS.
- R. A. Rather, S. Singh and B. Pal, A Cu+1/Cu0–TiO2 mesoporous nanocomposite exhibits improved H2 production from H2O under direct solar irradiation, J. Catal., 2017, 346, 1–9 CrossRef CAS.
- M. Solehudin, U. Sirimahachai, G. A. M. Ali, K. F. Chong and S. Wongnawa, One-pot synthesis of isotype heterojunction g-C3N4-MU photocatalyst for effective tetracycline hydrochloride antibiotic and reactive orange 16 dye removal, Adv. Powder Technol., 2020, 31, 1891–1902 CrossRef CAS.
- E. Fathi, F. Derakhshanfard, P. Gharbani and Z. G. Tabatabaei, Facile Synthesis of MgO/C3N4 Nanocomposite for Removal of Reactive Orange 16 Under Visible Light, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2234–2240 CrossRef CAS.
- B. H. Fard, R. R. Khojasteh and P. Gharbani, Preparation and Characterization of Visible-Light Sensitive Nano Ag/Ag3VO4/AgVO3 Modified by Graphene Oxide for Photodegradation of Reactive Orange 16 Dye, J. Inorg. Organomet. Polym. Mater., 2018, 28, 1149–1157 CrossRef CAS.
- B. Simović, Ž. Radovanović, G. Branković and A. Dapčević, Hydrothermally synthesized CeO2/ZnO nanocomposite photocatalysts for the enhanced degradation of Reactive Orange 16 dye, Mater. Sci. Semicond. Process., 2023, 162, 107542 CrossRef.
- A. Brindha and T. Sivakumar, Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes, J. Photochem. Photobiol., A, 2017, 340, 146–156 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00105f |
| This journal is © The Royal Society of Chemistry 2025 |