Open Access Article
Open Access ArticleDesign and synthesis of PANI/GO/MoS2 nanocomposites via oxidative polymerization for efficient photocatalytic applications: organic pollutant degradation and hydrogen generation†
Pritam
Hait
a,
Rajeev
Mehta
b and
Soumen
Basu
 *a
*a
aDepartment of Chemistry and Biochemistry, TIET-Virginia Tech Center of Excellence in Emerging Materials, Thapar Institute of Engineering & Technology, Patiala, 147004, India. E-mail: soumen.basu@thapar.edu
bDepartment of Chemical Engineering, TIET-Virginia Tech Center of Excellence in Emerging Materials, Thapar Institute of Engineering & Technology, Patiala, 147004, India
First published on 13th March 2025
Abstract
This study focused on preparing a ternary nanocomposite (PANI/GO/MoS2) using an oxidative polymerization technique. The composite incorporated polyaniline (PANI), graphene oxide (GO), and molybdenum disulfide (MoS2) in different weight ratios. Comprehensive characterization studies were performed including UV-vis-DRS, PL, XRD, XPS, BET, and EDS to evaluate the material's crystallinity, purity, porosity, and optical properties. FESEM imaging revealed the porous nature of PANI, the exfoliated structure of GO, and the nanosphere morphology of MoS2 (35–55 nm in diameter). This composite was tested for its effectiveness in degrading methyl orange (MO) dye and generating green hydrogen via visible-light-driven water splitting. Within 120 minutes, it achieved around 81.23% detoxification and 99% removal of MO dye. The degradation process adhered to first-order kinetics with a rate constant 7.1 times higher than that of pure PANI, 22 times higher than that of GO, 6.35 times higher than that of MoS2, and 9.26 times greater than that of the commercial TiO2-P25 photocatalyst, indicating strong synergy among the components. The study also examined the impact of various reaction parameters like pH, illumination area, catalyst dosage, and scavengers on the degradation process. The reusability of the photocatalyst was assessed over six cycles, maintaining 80% stability, as confirmed by XRD analysis. GC-MS identified the intermediates and final degradation products. The nanocomposite achieved hydrogen production with an apparent quantum efficiency (AQE) of 26% using CH3OH as a sacrificial agent, and AQEs of 22%, 19%, and 15% under acidic, basic, and neutral conditions, respectively. This research highlights the potential of ternary nanocomposites for diverse applications beyond dye degradation, including various solar-driven technologies.
1. Introduction
Semiconductor-based photocatalysis has gained prominence for solar fuel production and pollutant degradation, driven by urgent energy and environmental challenges.1–3 The discharge of dye waste from industries like paper, leather, and nylon production significantly affects aquatic life by causing water pollution. Reducing the coloration of these dyes is a crucial step in wastewater treatment prior to environmental discharge due to their synthetic origin, intricate chemical composition, and potential toxicity, as well as their carcinogenic or explosive properties.4 The rapid exhaustion of fossil fuels like coal, natural gas, and oil, along with rising political and environmental concerns, has heightened the need for renewable energy from sustainable sources for long-term solutions.5 This has led to numerous efforts in investigating innovative approaches to advancing renewable energy technologies. In recent years, transforming solar energy into chemical energy as solar fuels like hydrogen, methanol, and methane has emerged as a promising solution.6 This approach aims to lessen our heavy reliance on depleting fossil resources and address future energy and environmental challenges. Hydrogen (H2) plays a vital role as a superior energy carrier essential for promoting a low-carbon emission economy. While hydrogen (H2) may pose challenges related to safe transportation, it possesses numerous significant advantages: (i) in addition to its environmental friendliness, hydrogen (H2) is also non-toxic, setting it apart from most other fuel sources; (ii) with its abundant resources, hydrogen (H2) is the most plentiful element obtainable from a diverse array of sources such as alcohol and biomass; (iii) the primary benefit of using hydrogen energy is that it produces almost no harmful emissions when burned; and (iv) hydrogen energy is a highly efficient fuel source, outperforming traditional energy sources by producing greater energy per unit of fuel.7 Following the initial report by Honda and Fujishima on TiO2 electrodes, significant research has focused on photocatalytic and photoelectrochemical (PEC) water splitting to enhance hydrogen fuel production and support the hydrogen economy.6 Although TiO2 has been the most extensively studied photocatalyst to date, its large energy gap of 3.2 eV has limited its ability to effectively utilize sunlight.6 Given these intrinsic benefits, further research should focus on developing PANI-based composites to enhance photocatalytic materials. Polyaniline (PANI) is a conductive polymer characterized by a delocalized π–π conjugated structure.8 Its benzenoid and quinonoid units exhibit multiple redox states along with various other intriguing properties. Furthermore, PANI is highly suitable for large-scale applications because of its excellent conductivity, outstanding environmental stability, and ease of preparation.9 A stable matrix can enhance the durability of the resulting composite.On the other hand, graphene oxide, with its extensive surface area and unique optical, transport, mechanical, and electronic properties, has become a strong candidate for various applications, including hydrogen production and catalytic activities.10 It provides a two-dimensional (2D) surface for catalyst deposition and enhances the dye adsorption capacity through π–π interactions between the dye and the aromatic regions of graphene. Moreover, PANI serves as an electron donor and a hole conductor, while graphene functions as an electron acceptor when exposed to visible or UV light.11 Thus, integrating them with inorganic materials, particularly transition metal oxides or sulfides, enhances the degradation rate by leveraging synergistic effects that reduce recombination losses. The photocatalytic properties of various transition-metal oxides, such as NiO, BiOCl, ZnO, MnO2, TiO2, MoO3, and Cu2O, have been analyzed.12–14 An RGO/PANI/Cu2O hydrogel synthesized by Miao et al. demonstrated Congo red degradation within 20 minutes, reflecting an improvement in the composite's performance.15 Zhang et al. showed that a composite of poly(3,4-ethylenedioxythiophene) combined with graphene and MnO2 exhibited catalytic activity, as it successfully degraded methylene blue (MB) after 7 hours.16 Pendiselvi et al. explored the use of graphitic carbon nitride as a mechanical support, achieving methylene blue (MB) degradation in 80 minutes.17 Moreover, in situ polymerization of aniline with graphene and ZnFe2O4 results in the formation of a catalyst that greatly enhances the degradation efficiency of rhodamine B.18 Additionally, the incorporation of gold nanoparticles into a ternary composite with graphene and TiO2 has been investigated, demonstrating complete methylene blue (MB) degradation after 250 minutes.19 Additionally, many of these photocatalysts face challenges such as the limited photo response of TiO2 to visible light, the high expense of Ag or Au used as key components in the composites, and prolonged degradation times. Nevertheless, MoS2 has a layered configuration characterized by a hexagonal pattern of Mo and S atoms, resulting in S–Mo–S bonding.20 Furthermore, the layered structure of bulk MoS2 allows easy intercalation of foreign atoms between its layers.21 In summary, the combined effect of the ternary composite enhances the nanocomposite's photocatalytic activity, establishing it as a leading photocatalyst.
This study focuses on developing a new ternary composite of polyaniline (PANI), molybdenum disulfide (MoS2), and graphene oxide (GO) to evaluate its effectiveness in degrading methyl orange (MO) dye through photocatalysis under an LED light. The synthesized composites were thoroughly characterized using XRD, XPS, FTIR, FESEM, and UV-DRS techniques. Various photocatalytic factors, including the catalyst dosage, illuminated area, solution pH, and effect of scavengers, were investigated. Degradation intermediates and final products were analyzed using GC/MS, and possible mechanisms were explored for better understanding. Additionally, the photocatalyst's potential for green hydrogen generation via photocatalytic water splitting was assessed under various conditions—sacrificial agent presence and acidic, basic, and neutral environments. The apparent quantum yield (AQE%) for water splitting was also calculated, demonstrating the catalyst's ability to absorb photons and drive chemical reactions that degrade organic pollutants and dissociate water into H2 and O2.
2. Experimental section
The chemicals used in the synthesis process are outlined in the ESI† (S1).2.1. Synthesis of MoS2 and a ternary nanocomposite
Polyaniline (PANI) and graphene oxide (GO) are synthesized using the oxidative polymerization method and modified Hummers’ method, respectively, as outlined in our prior research.22 For the synthesis of MoS2, ammonium molybdate tetrahydrate and thiourea were initially dissolved in 20 ml of ethylene glycol; then the mixture was stirred for one hour. The mixture was then sonicated for one hour to ensure complete dispersion of the precursor. Then the prepared solution was heated for 30 minutes at 180 °C temperature in a microwave synthesizer (Biotage initiator +).A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GO/MoS2 (wt/wt%) composite was first synthesized using the MoS2 precursor through an in situ method. Next, different weight percentages of the 1
1 GO/MoS2 (wt/wt%) composite was first synthesized using the MoS2 precursor through an in situ method. Next, different weight percentages of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GO/MoS2 composite were used to form a ternary composite via oxidative polymerization. Initially, 1
1 GO/MoS2 composite were used to form a ternary composite via oxidative polymerization. Initially, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GO/MoS2 and aniline were combined in a 2 M HCl solution, stirred for one hour, and then sonicated for another hour. Next, ammonium persulfate (APS) was added gradually and the mixture was stirred overnight to facilitate polymerization. The resulting solution was rinsed with 2 M HCl and water and then dried at 60 °C for 24 hours. Thus, the PANI/GO/MoS2 composites were prepared in different weight ratios (1, 2.5, and 4) and were designated as 1PGMS, 2.5PGMS, and 4PGMS, respectively. Fig. S1 (ESI†) illustrates the schematic of the synthesis procedure.
1 GO/MoS2 and aniline were combined in a 2 M HCl solution, stirred for one hour, and then sonicated for another hour. Next, ammonium persulfate (APS) was added gradually and the mixture was stirred overnight to facilitate polymerization. The resulting solution was rinsed with 2 M HCl and water and then dried at 60 °C for 24 hours. Thus, the PANI/GO/MoS2 composites were prepared in different weight ratios (1, 2.5, and 4) and were designated as 1PGMS, 2.5PGMS, and 4PGMS, respectively. Fig. S1 (ESI†) illustrates the schematic of the synthesis procedure.
2.2. Techniques for characterization
Full details of characterization and the instruments used are found in the ESI† (S2).2.3. Photocatalysis experiments
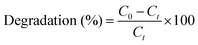 | (1) |
At the start of the experiment, the concentration of MO is denoted as C0, while Ct represents the concentration of MO after it has been subjected to light irradiation for a specified period, ‘t’.
The degree of mineralization was calculated using the following equation:
 | (2) |
In this context, “TOCinitial” refers to the initial concentration of total organic carbon (TOC) in MO, measured in mg L−1, before the degradation process begins. “TOCfinal” indicates the TOC concentration in MO after 120 minutes of degradation.
A GC-MS system identified the degradation products of MO, using m/z values to deduce their chemical structures, where ‘m’ is the molecular mass and ‘z’ is the charge number. The analysis used aqueous solutions with 0.1% dichloromethane (DCM) as the mobile phase.
 | (3) |
In this formula, the variables are defined as follows: n represents the moles of hydrogen generated per unit volume (mole per liter); ΔG denotes the total Gibbs free energy for water splitting (237 kJ per mole); W indicates the intensity of light radiation (637 watts per square meter); S refers to the surface region of the reaction vessel (in square meters); and T signifies the reaction time (in seconds).
3. Results and discussion
3.1. Characterization of the synthesized photocatalyst
 | (4) |
 | ||
| Fig. 1 (a) XRD pattern, (b) UV-vis DRS spectra, (c) Tauc plot, and (d) PL spectra of GO, PANI, MS, 1PGMS, 2.5PGMS, and 4PGMS. | ||
The Scherrer equation is used to calculate the crystallite size (D) and depends on several parameters: the Scherrer constant (k), the X-ray source wavelength (λ, usually 0.15406 nm), the full-width half-maxima (β) in radians, and the peak position (θ) also in radians. Using the Scherrer equation with these parameters, the crystallite sizes (D) are determined to be 40.4 nm for MoS2, 132.21 nm for PANI, 101.6 nm for g-GO, and 108.32 nm for 2.5PGMO.
X-ray photoelectron spectroscopy (XPS) was used to examine the chemical composition and phase state of the synthesized ternary nanocomposite (2.5PGMS). Fig. 2(a) shows the XPS survey spectrum, conclusively confirming the presence of elements Mo, C, N, S, and O within the ternary composite. Gaussian fitting was employed to deconvolute the elemental spectra of Mo 3d, C 1s, N 1s, S 2p, and O 1s, allowing for a more detailed examination of the elemental composition and chemical states. Fig. 2(b) shows the C 1s spectrum with four distinct binding energies. The peak at 283.90 eV corresponds to non-oxygenated carbon, including C–H and C–C bonds. The 284.7 eV peak signifies the presence of graphitic carbon. The 285.5 eV peak indicates nitrogenous carbon, with bonds like C–N. The peak at 288.1 eV corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.25Fig. 2(d) displays three distinct peaks in the N 1s spectrum, each corresponding to different nitrogen environments. The peak at 398.8 eV represents quinoid amine, the 399.1 eV peak is linked to benzenoid amine, and the 399.3 eV peak indicates the presence of a nitrogen cationic radical (N+˙).26Fig. 2(c) shows the O 1s spectrum with two main peaks at 531 eV and 532.3 eV. These peaks correspond to the Mo–O bonds in the MoO3 and hydroxyl groups on the composite's surface, respectively.27 In the Mo 3d spectrum, two peaks are observed at 232.2 eV and 235.6 eV (Fig. 2(e)). The binding energy 232.2 eV for Mo4+ 3d3/2 and the binding energy 235.6 eV is for the Mo6+ oxidation state.28 The S 2p spectra at 159 eV and 164 eV correspond to S2−2p3/2 and S2−2p1/2, respectively (Fig. 2(f)). The peak at 159.2 eV is attributed to C–S–H, while the peak at 164.4 eV is assigned to the N–S–H bond.29 Additionally, a peak at 168.6 eV indicates an S–O bond, indicating partial oxidation of the S edges in MoS2.28 From the peak of the S–O bond, we can conclude the formation of a bond between oxygenated graphene oxide and MoS2. This highlights oxygen's crucial role in the composite's bonding network, contributing to interactions and structural stability.
O bond.25Fig. 2(d) displays three distinct peaks in the N 1s spectrum, each corresponding to different nitrogen environments. The peak at 398.8 eV represents quinoid amine, the 399.1 eV peak is linked to benzenoid amine, and the 399.3 eV peak indicates the presence of a nitrogen cationic radical (N+˙).26Fig. 2(c) shows the O 1s spectrum with two main peaks at 531 eV and 532.3 eV. These peaks correspond to the Mo–O bonds in the MoO3 and hydroxyl groups on the composite's surface, respectively.27 In the Mo 3d spectrum, two peaks are observed at 232.2 eV and 235.6 eV (Fig. 2(e)). The binding energy 232.2 eV for Mo4+ 3d3/2 and the binding energy 235.6 eV is for the Mo6+ oxidation state.28 The S 2p spectra at 159 eV and 164 eV correspond to S2−2p3/2 and S2−2p1/2, respectively (Fig. 2(f)). The peak at 159.2 eV is attributed to C–S–H, while the peak at 164.4 eV is assigned to the N–S–H bond.29 Additionally, a peak at 168.6 eV indicates an S–O bond, indicating partial oxidation of the S edges in MoS2.28 From the peak of the S–O bond, we can conclude the formation of a bond between oxygenated graphene oxide and MoS2. This highlights oxygen's crucial role in the composite's bonding network, contributing to interactions and structural stability.
 | ||
| Fig. 2 (a) XPS survey spectrum of 2.5PGMS and core level spectrum of (b) C 1s, (c) O 1s, (d) N 1s, (e) Mo 3d, and (f) S 2p. | ||
FTIR analysis identifies distinct absorption peaks linked to specific vibrational modes and chemical groups (Fig. S1, ESI†). The 1536 cm−1 peak corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the benzenoid ring, while the 1390 cm−1 peak indicates the C–N bond stretching. The 1265 cm−1 signal reveals the presence of a protonated C–N group.30,31 Additionally, a peak near 776 cm−1 suggests electron delocalization in the polymer matrix. In the graphene oxide (GO) spectrum, distinctive peaks appear. The peak around 3500 cm−1 corresponds to O–H bond stretching, while the 1717 cm−1 peak indicates C
C stretching in the benzenoid ring, while the 1390 cm−1 peak indicates the C–N bond stretching. The 1265 cm−1 signal reveals the presence of a protonated C–N group.30,31 Additionally, a peak near 776 cm−1 suggests electron delocalization in the polymer matrix. In the graphene oxide (GO) spectrum, distinctive peaks appear. The peak around 3500 cm−1 corresponds to O–H bond stretching, while the 1717 cm−1 peak indicates C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in carboxylic groups. The 1615 cm−1 peak represents O–H bending and aromatic C
O stretching in carboxylic groups. The 1615 cm−1 peak represents O–H bending and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, and the 1385 cm−1 peak suggests the presence of tertiary C–OH bonds.32–34 Finally, the 1220 cm−1 peak confirms the existence of epoxy C–O groups. Broad absorption bands at 501 cm−1, 695 cm−1, and 1623 cm−1 are associated with MoS2.35 Peaks at 501 cm−1 correspond to S–S bonds.36 Additionally, the band around 3100 cm−1 is associated with O–H group vibrations.
C stretching, and the 1385 cm−1 peak suggests the presence of tertiary C–OH bonds.32–34 Finally, the 1220 cm−1 peak confirms the existence of epoxy C–O groups. Broad absorption bands at 501 cm−1, 695 cm−1, and 1623 cm−1 are associated with MoS2.35 Peaks at 501 cm−1 correspond to S–S bonds.36 Additionally, the band around 3100 cm−1 is associated with O–H group vibrations.
A photocatalyst's light absorption capability is essential for its photocatalytic activity. Fig. 1(b) shows the UV-vis absorption spectra of the different as-synthesized photocatalysts. MoS2 nanospheres absorb strongly in the UV range (250–400 nm), whereas the ternary nanocomposites (PGMS) exhibit wider absorption across the visible spectra. The broader absorption is due to PANI, which sensitizes the photocatalysts to visible light. Additionally, the color change from black in MoS2 to dark green in the ternary nanocomposites indicates successful PANI integration and enhanced visible light absorption (Fig. 1(b)). Tauc's formula, (αhν)(1/n) = A(hν – Eg), calculates a semiconductor's energy gap. In this formula, hν is the photon energy, Eg is the bandgap, A is a constant, and α is the coefficient of light absorption. The exponent “n” reflects the electronic transition type, where n = 1/2 signifies a direct transition. In this study, the bandgap energy (Eg) was determined from plots of (αhν)1/2versus Eg. As shown in Fig. 1(c), the Eg values are 1.53 eV for MoS2, 2.18 eV for 2.5PGMS, 2.21 eV for 1PGMS, and 2.20 eV for 4PGMS.
Photoluminescence (PL) spectra are commonly used to evaluate processes like carrier capture, movement, transport, isolation, and recombination. Fig. 1(d) shows the PL emission spectra for MoS2, PANI, GO, and PGMS composites, highlighting a luminescent peak around 452 nm. Notably, the PL emission intensity of PGMS composites is significantly lower compared to pure MoS2, PANI, and GO. This reduced fluorescence indicates the potent isolation of light-generated pairs of electrons and holes within the composite structure. Efficient management of electron–hole pairs is crucial for improving photocatalytic degradation. Effective separation of these pairs prevents recombination, allowing for better utilization in photocatalytic reactions.
The specific surface area of the synthesized nanocomposites was evaluated to analyze their surface characteristics in detail. Fig. S4 and S5 (ESI†) present the nitrogen adsorption–desorption isotherms, which exhibit a type IV Langmuir isotherm with distinct H1 hysteresis loops for all the composites, as well as for individual components such as MoS2, PANI, and GO. The presence of these hysteresis loops confirms the mesoporous nature of the materials, indicating well-defined pore structures.
The pore size distribution was determined using the Barrett–Joyner–Halenda (BJH) method, as depicted in Fig. S4 and S5 (ESI†). Table 1 provides a comparative analysis of surface area and pore volume, showing that pure PANI possesses a relatively high surface area and pore volume. However, the synthesized composites exhibit even higher surface areas than pure PANI, GO, and MoS2, highlighting their enhanced porosity and surface characteristics.
| Photocatalyst | Specific surface area (m2 g−1) | Total pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| PANI | 18.3 | 0.0203 | 4.5 |
| GO | 10.6 | 0.034 | 5.3 |
| MS | 7.3 | 0.0073 | 4.2 |
| 1PGMS | 35.1 | 0.044 | 5.0 |
| 2.5PGMS | 52.6 | 0.0841 | 6.4 |
| 4PGMS | 43 | 0.052 | 4.8 |
Notably, the PGMS composites follow an increasing trend in surface area, with values recorded as 35.1 m2 g−1 for 1PGMS, 43 m2 g−1 for 4PGMS, and 52.6 m2 g−1 for 2.5PGMS. The superior photocatalytic performance of the 2.5PGMS composite is attributed to its higher surface area, which enhances pollutant adsorption and provides more active sites for catalytic reactions. This increased active surface area plays a crucial role in improving hydrogen production through water splitting, further confirming the composite's effectiveness in photocatalytic applications.
3.2. Removal of the organic pollutant methyl orange (MO) through photocatalysis
Fig. 4(d) illustrates the limited effectiveness of photolysis alone for removing methyl orange (MO), showing only about a 4% decrease in absorbance after 120 minutes of LED light exposure. This minimal reduction indicates that MO is highly resistant to light-induced degradation. In the dark, after 120 minutes of reaching adsorption–desorption equilibrium, 2.5PGMS adsorbed approximately 32% of MO. In comparison, other materials showed lower adsorption: bare PANI (21%), GO (26%), molybdenum trioxide (9%), 1PG (1 wt% of GO) (12%), 11GMS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of GO and MoS2) (11%), 1PMS (1 wt% of MoS2) (10%), 1PGMS (28%), 4PGMS (29%), and TiO2 (8%) (Fig. 4(d)). The enhanced adsorption capacity of 2.5PGMS is because of the increased surface area and a higher count of adsorption areas for the anionic dye, achieved by incorporating 2.5 wt% GO/MS into PANI. Upon illumination with an LED light for 120 minutes, the photocatalytic efficiency improved as follows: PANI (30%) < GO (32%) < MoS2 (33%) < TiO2 (34%) < 1PMS (46%) < 1PG (53%) < 11GMS (74%) < 1PGMS (87%) < 4PGMS (85%) < 2.5PGMS (99%) (Fig. 4(d)). This indicates that bare MoS2's limited photodegradation capability is due to its restricted photo-response spectrum, inadequate surface area, and rapid recombination of photo-induced charge carriers. PANI and GO improve performance, with 1PMS, 1PG, and 11GMS showing enhanced photocatalytic activity compared to single-component materials. However, increasing 11GMS beyond 2.5 wt% slightly decreased the MO removal efficiency. This reduction is attributed to inefficient charge distribution and potential blocking of active sites by excess 11GMS, which limits light penetration and photocatalytic efficiency. Thus, an optimal 11GMS load maximizes the synergy between PANI, GO, and MoS2, enhancing photocatalytic activity through a smaller band gap, better light absorption, and reduced recombination of electron–hole pairs. Furthermore, the kinetics of MO photocatalytic degradation were quantitatively analyzed using a pseudo-first-order rate equation on the experimental results:
1 of GO and MoS2) (11%), 1PMS (1 wt% of MoS2) (10%), 1PGMS (28%), 4PGMS (29%), and TiO2 (8%) (Fig. 4(d)). The enhanced adsorption capacity of 2.5PGMS is because of the increased surface area and a higher count of adsorption areas for the anionic dye, achieved by incorporating 2.5 wt% GO/MS into PANI. Upon illumination with an LED light for 120 minutes, the photocatalytic efficiency improved as follows: PANI (30%) < GO (32%) < MoS2 (33%) < TiO2 (34%) < 1PMS (46%) < 1PG (53%) < 11GMS (74%) < 1PGMS (87%) < 4PGMS (85%) < 2.5PGMS (99%) (Fig. 4(d)). This indicates that bare MoS2's limited photodegradation capability is due to its restricted photo-response spectrum, inadequate surface area, and rapid recombination of photo-induced charge carriers. PANI and GO improve performance, with 1PMS, 1PG, and 11GMS showing enhanced photocatalytic activity compared to single-component materials. However, increasing 11GMS beyond 2.5 wt% slightly decreased the MO removal efficiency. This reduction is attributed to inefficient charge distribution and potential blocking of active sites by excess 11GMS, which limits light penetration and photocatalytic efficiency. Thus, an optimal 11GMS load maximizes the synergy between PANI, GO, and MoS2, enhancing photocatalytic activity through a smaller band gap, better light absorption, and reduced recombination of electron–hole pairs. Furthermore, the kinetics of MO photocatalytic degradation were quantitatively analyzed using a pseudo-first-order rate equation on the experimental results:| ln(Ct/C0) = −kt | (5) |
 | ||
| Fig. 4 (a) Effect of catalyst concentration, (b) pzc studies, (c) effect of pH, (d) kinetic studies, (e) impact of illuminated surface area, and (f) scavenger studies. | ||
In this analysis, C0 and Ct denote the MO concentrations at the start and after t minutes of irradiation, respectively, while k represents the reaction rate constant (min−1). Fig. S7 (ESI†) illustrates the linear correlation between ln(Ct/C0) and the reaction time for different catalysts. The degradation rate constants are as follows: 1PGMS (0.02353 min−1), 2.5PGMS (0.03 min−1), 4PGMS (0.02523 min−1), 1PG (0.01241 min−1), 1PMS (0.00512 min−1), 11GMS (0.0123 min−1), PANI (0.00422 min−1), GO (0.00135 min−1), MoS2 (0.00472 min−1), and TiO2 (0.00324 min−1). The synergy from combining PANI, GO, and MoS2 was measured using the synergy factor (R), determined by the equation:
 | (6) |
The rate constants for photodegradation of the PANI/GO/MoS2 composite and individual components (PANI, GO, and MoS2) are denoted as K(PANI+GO+MoS2), KPANI, KGO, and KMoS2, respectively. The synergy factors calculated for 1PGMS, 2.5PGMS, 4PGMS, 1PG, 1PMS, and 11GMS are 1.9, 2.6, 2.3, 1.6, 0.5, and 1.8, respectively. Among these, 2.5PGMS exhibited the highest synergy factor, leading to its most effective photocatalytic degradation with a rate constant of 0.03 min−1.
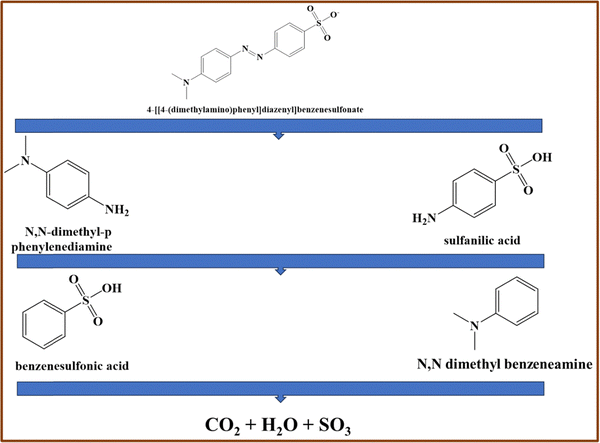
The degradation products of MO using the 2.5PGMS nanocomposite were identified through GC-MS analysis. S3 (ESI†) presents the mass spectra for MO degradation by 2.5PGMS. The characteristic mass spectrum of the MO dye is at m/z 306. After 120 minutes of degradation, the spectra show peaks at m/z 121, 136, 157, and 172, corresponding to intermediate products. These include sulfanilic acid at m/z 172, which breaks down into benzenesulfonic acid at m/z 157 after losing an amino group (NH2). Similarly, N,N-dimethyl-p-phenylenediamine at m/z 136 degrades into N,N-dimethyl benzenenamine at m/z 121, also after losing an amino group. The results suggest that MO degradation involves cleavage of the azo bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), producing sulfanilic acid and N,N-dimethyl-p-phenylenediamine.38 The proposed degradation mechanism is shown in Fig. 5, with further breakdown potentially leading to CO2 and H2O.
N–), producing sulfanilic acid and N,N-dimethyl-p-phenylenediamine.38 The proposed degradation mechanism is shown in Fig. 5, with further breakdown potentially leading to CO2 and H2O.
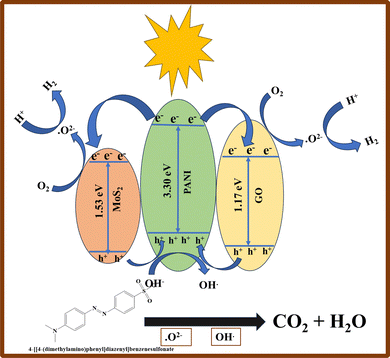
| PANI + hν → eVB− (PANI) + hCB+ (PANI) | (7) |
| GO + hν → eCB− (GO) + hVB+ (GO) | (8) |
| MoS2 + hν → eCB− (MoS2) + hVB+ (MoO3) | (9) |
| eVB− (PANI) + O2 → O2˙− (PANI) | (10) |
| hVB + (PANI/GO/MoS2) + OH− → OH˙ (PANI/GO/MoS2) | (11) |
| O2˙− (PANI), OH˙ (PANI/GO/MoS2) + MO → Degraded products | (12) |
Under acidic conditions:
| Oxidation: 2H2O (l) + 4h+ → O2 (g) + 4H+ (aq) E0 (oxidation) = −1.23 V | (13) |
| Reduction: 2H+ + 2e− → O2 (g) + 2H2 (g) E0 reduction = 0 V. | (14) |
Under basic conditions:
| Oxidation: 2OH− (aq) → 1/2 O2 (g) + H2O (l) + 2e− | (15) |
| Reduction: 2H2O (l) + 2e− → H2 (g) + 2OH− | (16) |
4. Conclusion
Ternary nanocomposites (PANI/GO/MoS2) were prepared through in situ oxidative polymerization, varying the ratios of GO to MoS2. Characterization validated the successful synthesis of pure PANI, GO, MoS2, and their corresponding composites. These materials were utilized for the mineralization of MO and photocatalytic water splitting, employing a sacrificial agent under acidic and basic conditions, as well as with the catalyst on its own. The composite's photocatalytic efficiency under visible light was significantly enhanced by the synergistic interaction among MoS2, PANI, and GO, with 2.5PGMS exhibiting the greatest catalytic activity. Its first-order rate constant (0.03 min−1) was 7.1 times higher than that of PANI, 22 times higher than that of GO, 6.35 times higher than that of MoS2, and 9.26 times higher than that of TiO2-P25. Key parameters like the catalyst concentration (0.2 g L−1), illuminated area (15 cm2), and solution pH (7.6) were investigated for their impact on photodegradation, highlighting the importance of h+, OH˙, and O2˙ in the process. The findings demonstrate that a small amount of photocatalyst (0.2 g L−1) achieves 83.21% detoxification of the MO solution. The apparent quantum efficiency (AQE) for hydrogen production was 26% with CH3OH as the sacrificial agent, 22% under acidic conditions, 19% under basic conditions, and 15% using only the catalyst. This research showcases PANI/GO/MoS2 as a potential ternary composite suitable for a range of solar-powered applications.Author contributions
Pritam Hait: visualization and writing – original draft. Rajeev Mehta: supervision. Soumen Basu: supervision.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors have disclosed that they have no conflicts of interest regarding the conduct of this study and the publication of its findings.Acknowledgements
We gratefully acknowledge the fellowship funding provided by the TIET-Virginia Tech Center of Excellence in Emerging Materials, India. We also extend our gratitude to DCBC and DPMS TIET for providing us characterization facility, and we are also thankful to Dr Akansha Mehta for XPS characterization. We also want to acknowledge Prof. O. P. Pandey and Mr Abhishek Chandel from DPMS, TIET, Patiala, Punjab for EIS analysis.References
- J. Willkomm, K. L. Orchard, A. Reynal, E. Pastor, J. R. Durrant and E. Reisner, Chem. Soc. Rev., 2016, 45, 9–23 Search PubMed.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- S. Wang, C. Liu, K. Dai, P. Cai, H. Chen, C. Yang and Q. Huang, J. Mater. Chem. A, 2015, 3, 21090–21098 Search PubMed.
- J. Abdi, M. Vossoughi, N. M. Mahmoodi and I. Alemzadeh, Chem. Eng. J., 2017, 326, 1145–1158 CrossRef CAS.
- M. J. Kang and Y. S. Kang, MRS Adv., 2018, 3, 3271–3280 CrossRef CAS.
- K. Yan and G. Wu, ACS Sustainable Chem. Eng., 2015, 3, 779–791 CrossRef CAS.
- M.-Y. Qi, M. Conte, M. Anpo, Z.-R. Tang and Y.-J. Xu, Chem. Rev., 2021, 121, 13051–13085 CrossRef CAS PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- K. Vinodgopal, D. E. Wynkoop and P. V. Kamat, Environ. Sci. Technol., 1996, 30, 1660–1666 CrossRef CAS.
- P. Ahuja, S. K. Ujjain, I. Arora and M. Samim, ACS Omega, 2018, 3, 7846–7855 CrossRef CAS PubMed.
- H. Yang, X. Liu, S. Sun, Y. Nie, H. Wu, T. Yang, S. Zheng and S. Lin, Mater. Res. Bull., 2016, 78, 112–118 Search PubMed.
- W. Zhang, X. Li, Z. Yang, X. Tang, Y. Ma, M. Li, N. Hu, H. Wei and Y. Zhang, Nanotechnology, 2016, 27, 265703 CrossRef PubMed.
- E. Pajootan, M. Arami and M. Rahimdokht, Ind. Eng. Chem. Res., 2014, 53, 16261–16269 CAS.
- P. Raizada, P. Singh, A. Kumar, G. Sharma, B. Pare, S. B. Jonnalagadda and P. Thakur, Appl. Catal., A, 2014, 486, 159–169 Search PubMed.
- J. Miao, A. Xie, S. Li, F. Huang, J. Cao and Y. Shen, Appl. Surf. Sci., 2016, 360, 594–600 CrossRef CAS.
- N. B. Grimm, S. H. Faeth, N. E. Golubiewski, C. L. Redman, J. Wu, X. Bai and J. M. Briggs, Science, 2008, 319, 756–760 CAS.
- K. Pandiselvi, H. Fang, X. Huang, J. Wang, X. Xu and T. Li, J. Hazard. Mater., 2016, 314, 67–77 CAS.
- J. Feng, Y. Hou, X. Wang, W. Quan, J. Zhang, Y. Wang and L. Li, J. Alloys Compd., 2016, 681, 157–166 CAS.
- Y. Yang, Z. Ma, L. Xu, H. Wang and N. Fu, Appl. Surf. Sci., 2016, 369, 576–583 CrossRef CAS.
- N. Huo, Y. Yang, Y.-N. Wu, X.-G. Zhang, S. T. Pantelides and G. Konstantatos, Nanoscale, 2018, 10, 15071–15077 CAS.
- T. H. M. Lau, X. Lu, J. Kulhavý, S. Wu, L. Lu, T.-S. Wu, R. Kato, J. S. Foord, Y.-L. Soo, K. Suenaga and S. C. E. Tsang, Chem. Sci., 2018, 9, 4769–4776 CAS.
- P. Hait, R. Mehta and S. Basu, J. Cleaner Prod., 2023, 424, 138851 CAS.
- H. Shin, K. K. Kim, A. Benayad, S. Yoon, H. K. Park, I. Jung, M. H. Jin, H. Jeong, J. M. Kim, J. Choi and Y. H. Lee, Adv. Funct. Mater., 2009, 19, 1987–1992 CAS.
- H. Neelamkodan, U. Megha and P. M. Binitha, Process. Appl. Ceram., 2023, 17, 149–156 CAS.
- H. Liu, Y. Su, Z. Chen, Z. Jin and Y. Wang, J. Hazard. Mater., 2014, 266, 75–83 CAS.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392–1401 Search PubMed.
- Z. Liu, W. Xu, J. Fang, X. Xu, S. Wu, X. Zhu and Z. Chen, Appl. Surf. Sci., 2012, 259, 441–447 Search PubMed.
- B. Li, L. Jiang, X. Li, P. Ran, P. Zuo, A. Wang, L. Qu, Y. Zhao, Z. Cheng and Y. Lu, Sci. Rep., 2017, 7, 11182 Search PubMed.
- H. Wang, M. Thangamuthu, Z. Wu, J. Yang, H. Yuan, M. K. Bayazit and J. Tang, Chem. Eng. J., 2022, 445, 136790 CAS.
- A. Abdolahi, E. Hamzah, Z. Ibrahim and S. Hashim, Materials, 2012, 5, 1487–1494 CAS.
- G. Gaikwad, P. Patil, D. Patil and J. Naik, Mater. Sci. Eng. B, 2017, 218, 14–22 CAS.
- T. Szabó, E. Tombácz, E. Illés and I. Dékány, Carbon, 2006, 44, 537–545 CrossRef.
- Y. Fu and X. Wang, Ind. Eng. Chem. Res., 2011, 50, 7210–7218 CrossRef CAS.
- Q. Wang, J. Hui, J. Li, Y. Cai, S. Yin, F. Wang and B. Su, Appl. Surf. Sci., 2013, 283, 577–583 CrossRef CAS.
- W. Feng, L. Chen, M. Qin, X. Zhou, Q. Zhang, Y. Miao, K. Qiu, Y. Zhang and C. He, Sci. Rep., 2015, 5, 17422 CAS.
- S. Reshmi, M. V. Akshaya, B. Satpati, A. Roy, P. Kumar Basu and K. Bhattacharjee, Mater. Res. Express, 2017, 4, 115012 Search PubMed.
- A. Kumar, R. Kumar and G. Pandey, Macromol. Symp., 2018, 379(1), 1600192 CrossRef.
- M. Kgatle, K. Sikhwivhilu, G. Ndlovu and N. Moloto, Catalysts, 2021, 11, 428 CAS.
- A. Kundu, S. Sharma and S. Basu, J. Phys. Chem. Solids, 2021, 154, 110064 CAS.
- S. Singla, S. Sharma and S. Basu, J. Cleaner Prod., 2021, 324, 129290 CAS.
- H. Alamgholiloo, E. Asgari, S. Nazari, A. Sheikhmohammadi, N. Noroozi Pesyan and B. Hashemzadeh, Sep. Purif. Technol., 2022, 300, 121911 CAS.
- T. Guo, L. Wang, D. G. Evans and W. Yang, J. Phys. Chem. C, 2010, 114, 4765–4772 CAS.
- T. Lv, L. Pan, X. Liu, T. Lu, G. Zhu and Z. Sun, J. Alloys Compd., 2011, 509, 10086–10091 CAS.
- P. S. Rao and E. Hayon, J. Phys. Chem., 1975, 79, 397–402 CrossRef CAS.
- M. Mitra, S. T. Ahamed, A. Ghosh, A. Mondal, K. Kargupta, S. Ganguly and D. Banerjee, ACS Omega, 2019, 4, 1623–1635 CrossRef CAS PubMed.
- T. Jafari, E. Moharreri, A. Amin, R. Miao, W. Song and S. Suib, Molecules, 2016, 21, 900 CrossRef PubMed.
- A. A. Murashkina, T. V. Bakiev, Y. M. Artemev, A. V. Rudakova, A. V. Emeline and D. W. Bahnemann, Catalysts, 2019, 9, 999 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical details, information about the instruments, EDS, FTIR, BET-BJH plots, EIS analysis, Mott–Schottky plot, GC-TCD results of hydrogen generation, GC-MS data, and a comparison table. See DOI: https://doi.org/10.1039/d4ma01249f |
| This journal is © The Royal Society of Chemistry 2025 |