Open Access Article
Open Access ArticleEnvironmental materials: CO2-adsorbing clays for enhancing soil fertility and agricultural sustainability†
Faizah
Altaf
a,
Shakeel
Ahmed
 b,
Shahid
Ali
a,
Muhammad
Mansha
a and
Safyan Akram
Khan
*a
b,
Shahid
Ali
a,
Muhammad
Mansha
a and
Safyan Akram
Khan
*a
aInterdisciplinary Research Center for Hydrogen and Energy Storage (IRC-HES), King Fahd University of Petroleum & Minerals (KFUPM), Dhahran 31261, Saudi Arabia. E-mail: safyan@kfupm.edu.sa
bSchool of Material Science and Engineering, Henan University of Technology, Zhengzhou 450001, Henan, China
First published on 17th April 2025
Abstract
To combat desertification and climate change, innovative solutions are crucial for restoring the fertility of desert lands and mitigating the adverse effects of global warming. One promising approach involves utilizing carbon dioxide (CO2), a major greenhouse gas, as a resource to enhance soil fertility. This study explores the transformation of desert sands into fertile land using CO2 adsorbed on clays, offering a novel solution to combat desertification and mitigate climate change. We developed CO2-enriched fertilizers using the solgel method, and the process involved purifying raw kaolinite followed by CTAB intercalation and impregnation with varying concentrations of polyethyleneimine (PEI) (30 and 50%). The prepared fertilizers were analyzed using FTIR, XRD, SEM, and TEM to assess their structural and morphological properties. Furthermore, these adsorbents were evaluated for CO2 uptake potential. The highest CO2 adsorption capacity of 167.1 mg g−1 was obtained with CKP-50 much higher than unmodified kaolinite (0.901 mg g−1). FTIR analysis confirmed that CO2 adsorption on the prepared fertilizers occurred via chemical interaction with amine groups. The CO2-enriched clays were mixed with sand in appropriate concentrations to support plant growth in desert lands. The plant growth trial showed significant improvements with PEI impregnated samples, S-CKP-30 and S-CKP-50, which supported taller and healthier plants compared to pure kaolinite (S-PK) and CTAB-modified kaolinite (S-CKP-0). By day 30, plants with S-CKP-50 reached 27.1 cm in height demonstrating enhanced plant growth, especially in arid conditions, by improving moisture retention, nutrient availability, and increased CO2 adsorption. These results showed that our prepared fertilizers, especially S-CKP-50, proved to be the most effective material for CO2 mitigation and promoting plant growth, hence offering a promising approach to desert reclamation and CO2 sequestration simultaneously.
1. Introduction
Elevated carbon dioxide (CO2) concentrations contribute to climate change, global warming, and extreme weather events. CO2 levels above 4% can also harm human health, causing fatigue, headaches, and asphyxiation risks.1 To address this, carbon capture and utilization (CCU) technologies are being developed to capture CO2 emissions from industrial processes and power generation.2 These include post-combustion capture, pre-combustion capture, and direct air capture (DAC),3 with CO2 being utilized in applications such as enhanced oil recovery (EOR), the production of synthetic fuels, chemicals, and building materials, and even in agricultural practices like enhancing soil fertility. These technologies help to reduce atmospheric CO2 levels, mitigate health risks, and contribute to global climate change efforts.4,5Desertification is one of the most pressing environmental challenges faced by the world today, with vast areas of the Earth's surface increasingly becoming barren and unproductive.6,7 Land degradation threatens livelihoods, with 20 million hectares lost yearly, potentially reducing global food production by 12%.8 One-third of agricultural land has become barren in the last 40 years. Degraded soil reduces crop productivity, and global food production could drop by 12% in the next 25 years, raising food prices by 30%. The annual cost of land degradation is 3.4 trillion euros, driven by factors like population growth, biofuel demand, poverty, and climate change. In sub-Saharan Africa, 50% of agricultural land has lost productivity, and 80% of rangelands show signs of degradation. Globally, 56% of degraded land results from water erosion, 28% from wind erosion, and 12% from chemical degradation. About 3.6 million hectares of drylands are at risk of degradation.8 The loss of soil fertility is particularly critical in desert regions, where natural conditions already limit agricultural productivity.
Conventional methods such as irrigation and chemical fertilizers9 have been used to address these issues, but they are often unsustainable in the long term due to resource constraints, high costs, and environmental harm.10,11 Additionally, traditional fertilizers often lead to nutrient imbalances in the soil, negatively affecting microbial communities and overall soil health.12,13 Overuse can lead to soil degradation, depletion of essential minerals, water pollution,14,15 health risks (blue baby syndrome, cancer etc.),16 greenhouse gas emissions, and contamination with heavy metals (as seen in the Gulf of Mexico's “dead zone”) and radionuclides like U238, Th232, and Po210.17,18 Many studies have been conducted worldwide to analyze the side effects of conventional fertilizers;19 for example, Abebe et al. discuss how Ethiopia's over-use of fertilizers has resulted in soil fertility depletion and low crop yields.20 Long-term fertilizer use in India has led to increased heavy metal accumulation in soil, posing potential long-term risks to crop growth and environmental health.21 Guo & Wang analyzed NH3 emissions from synthetic nitrogen fertilizers in China from 1991 to 2013, showing an increase from 3.20 to 5.21 Tg NH3 per year.22 Another study identified excessive fertilizer and pesticide use in China (cities like Shandong and Qinghai) as a key factor contributing to total phosphorus loss, and driving water eutrophication.23 Ghanem et al. examined how air and soil pollution affect social development in Saudi Arabia, showing that increased fertilizer and pesticide use, and CO2 emissions raise hospitalizations.24 Al-Busaidi et al. (1993–2020) found that long-term farming caused soil salinization, reduced pH, and elevated phosphorus and ion levels.25 Sulaiman et al. analyzed toxic metals in fertilizers and their accumulation in soil and date palms in the UAE.26 Al-Taani et al. analyzed heavy metal pollution in Liwa, UAE, finding Cd, Ni, Zn, and Cr enriched due to agrochemicals. The pollution load index indicated low to moderate contamination, with Cd posing a high ecological risk.27 These worldwide studies show the requirement for alternative, eco-friendly fertilizers that provide essential nutrients without these negative consequences.
Traditionally seen as harmful to the environment, CO2 can be captured and transformed into a beneficial substance.28,29 CO2 utilization is a growing area of research, with several innovative applications in different30 fields, especially in agriculture, where CO2 can be harnessed as a valuable resource to improve soil fertility, enhance crop yields, and combat desertification. By capturing CO2 and using it to improve soil quality, particularly in arid regions like deserts, it is possible to transform infertile land into fertile, productive soil. One such method involves the adsorption of CO2 onto adsorbents, a process that can provide long-term benefits for soil fertility.31Fig. 1(b) illustrates the possible interactions of CO2 in the soil. CO2 in soil undergoes several important chemical reactions that affect soil health and carbon cycling. When CO2 enters soil moisture, it reacts with water to form carbonic acid (H2CO3), which helps in mineral weathering and nutrient release, such as calcium and magnesium. This acid dissociates into bicarbonate (HCO3−), which can alter soil pH and contribute to carbonate formation, effectively sequestering carbon. Soil microorganisms also play a vital role in converting organic carbon into stable soil organic matter (SOM), which improves soil fertility. In anaerobic conditions, some microbes transform CO2 into methane (CH4), while others release CO2 during respiration. These processes are crucial for nutrient cycling, carbon sequestration, and overall soil health.
 | ||
| Fig. 1 (a) Synthesis of PEI-impregnated clay-based CO2-enriched fertilizers, and (b) illustration of possible interactions of CO2 in soil. | ||
Various materials have been explored for their ability to effectively adsorb carbon dioxide (CO2) due to their high surface area, porosity, and chemical properties, such as zeolites,32,33 metal–organic frameworks (MOFs),34,35 carbon nanotubes (CNTs)36–38 metal oxides,36,39 biochar,40,41 carbon-based materials42–45 and silica-based adsorbents.46
Clay minerals abundant in desert regions have long been recognized for their beneficial properties in soil enhancement. These minerals have a large surface area and high cation-exchange capacity, making them excellent candidates for improving soil structure, water retention, and nutrient availability.47 The adsorption of CO2 onto clay including kaolinite,48 montmorillonite,49 and bentonite50 has been extensively studied in recent years due to their potential for carbon sequestration as well as soil enhancement. CO2 adsorbed onto clay minerals forms stable complexes that release essential nutrients like potassium, calcium, and magnesium, improving soil fertility and structure. This process improves soil structure, boosts microbial activity, and increases cation-exchange capacity.
Kaolinite, a naturally abundant clay mineral, has been investigated as a potential adsorbent for CO2 capture and storage applications. Kaolinite is a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered aluminosilicate with a relatively low cost, high surface area, and chemical stability, making it an attractive candidate for adsorption-based CO2 capture.2,3 However, the native kaolinite exhibits very low CO2 adsorption capacity, typically around 3 mg CO2 per g or less under ambient conditions.4,5 To improve its CO2 adsorption performance various modification techniques are employed such as acid activation leading to enhanced CO2 adsorption capacities of up to 3.4 mg CO2 per g.48 A study found that substituting Al in kaolinite with elements like Mn, Fe, Co, Ni, or Si enhances CO2 adsorption by altering its electronic structure and surface properties, as shown by DFT calculations.51 ZSM-5 molecular sieve was prepared from kaolin and modified with Na, Ni, and Ni–Na to create Ni–Na/ZSM-5 (1.5Ni–0.6Na), which exhibited the best CO2 adsorption capacity of 1.97 mmol g−1.52 Kaolin-derived 4A-zeolite was modified with TEPA and DEA to optimize CO2 adsorption. Optimal 4A-DEA achieved 579 mg g−1 capacity.53 Activated carbon (AC), kaolinite (KAO), and kaolinite-activated carbon composite (KAC) were evaluated as CO2 adsorbents, with maximum capacities of 28.97 mg CO2 per g for AC, 18.54 mg CO2 per g for KAC, and 12.98 mg CO2 per g for KAO.54
1 layered aluminosilicate with a relatively low cost, high surface area, and chemical stability, making it an attractive candidate for adsorption-based CO2 capture.2,3 However, the native kaolinite exhibits very low CO2 adsorption capacity, typically around 3 mg CO2 per g or less under ambient conditions.4,5 To improve its CO2 adsorption performance various modification techniques are employed such as acid activation leading to enhanced CO2 adsorption capacities of up to 3.4 mg CO2 per g.48 A study found that substituting Al in kaolinite with elements like Mn, Fe, Co, Ni, or Si enhances CO2 adsorption by altering its electronic structure and surface properties, as shown by DFT calculations.51 ZSM-5 molecular sieve was prepared from kaolin and modified with Na, Ni, and Ni–Na to create Ni–Na/ZSM-5 (1.5Ni–0.6Na), which exhibited the best CO2 adsorption capacity of 1.97 mmol g−1.52 Kaolin-derived 4A-zeolite was modified with TEPA and DEA to optimize CO2 adsorption. Optimal 4A-DEA achieved 579 mg g−1 capacity.53 Activated carbon (AC), kaolinite (KAO), and kaolinite-activated carbon composite (KAC) were evaluated as CO2 adsorbents, with maximum capacities of 28.97 mg CO2 per g for AC, 18.54 mg CO2 per g for KAC, and 12.98 mg CO2 per g for KAO.54
The cationic surfactant CTAB (cetyltrimethylammonium bromide) has been used to modify clays, introducing positively charged organic cations onto the surface, changing hypoploidy and surface charge.55 Introducing amine functional groups onto the kaolinite surface can improve the affinity towards CO2 through chemisorption. Amine modification significantly enhanced kaolinite's low native CO2 adsorption capacity.56 PEI (polyethyleneimine) enhances CO2 adsorption in various solid adsorbents. For example, protonated titanate nanotubes (PTNTs) modified with 50 wt% PEI demonstrated a high CO2 capacity of 130.8 mg g−1 at 100 °C.57 Similarly, a study examines CO2 adsorption on polyethyleneimine (PEI) loaded onto porous carbon (PC) with varying pore sizes. The highest CO2 adsorption was observed in PC with a 3.63 nm pore size at 50 °C.58
Desert sand presents significant challenges for plant growth due to nutrient deficiency, poor water retention, and unfavorable soil structure. To overcome these issues, this study aimed to develop and evaluate CO2-based fertilizers as a solution to enhance desert soils. These fertilizers can improve soil fertility, enhance water retention, and promote better soil structure by reducing compaction and balancing pH. Additionally, they contribute to carbon sequestration, which helps mitigate climate change. The study hypothesized that CO2-based fertilizers would not only enhance desert soil health but also promote resilient agriculture in arid regions. To test this, kaolinite clay was selected, modified with CTAB, and impregnated with PEI. The materials were characterized using XRD, BET, FTIR, SEM, and TEM. CO2 adsorption capacity was evaluated for each sample, and the CO2-enriched fertilizers were mixed with desert sand to assess their impact on plant growth in a controlled trial. The study's objective was to demonstrate that low concentrations of these fertilizers can improve soil fertility, water retention, and structure without disrupting the desert ecosystem, offering a sustainable solution for agriculture and ecological restoration in arid regions.
2. Materials and methods
The raw kaolinite was purchased from Fluka. The hydrochloric acid (HCl, 36%), sodium chloride (NaCl, 60%), sodium hydroxide (NaOH, 97%), cetyltrimethylammonium bromide (CTAB), polyethyleneimine (PEI), toluene, and methanol (MeOH) were used for the purification and modification of kaolinite and purchased from Sigma Aldrich. Distilled water was used throughout the experiment.2.1. Preparation of CO2 enriched fertilizer
Herein we developed a nanocomposite adsorbent for dual function purposes by employing a solgel method. This technique involves the transition of a liquid precursor into a gel-like network, which is then heated to form a solid material. It is widely used for producing highly uniform materials with specific properties, such as high surface area and porosity. In the context of this study, the sol–gel method was used to modify kaolinite by incorporating surfactants (CTAB) and amines (PEI), enhancing its CO2 adsorption capacity and improving its potential for soil modification. This process allows for precise control over the material's chemical structure and properties, making it ideal for creating CO2-enriched fertilizers. The schematic for the synthesis of CTAB-modified kaolinite impregnated with PEI fertilizers and the CO2 adsorption process is depicted in Fig. 1(b), and a detailed description is given as:• Purification of kaolinite: the purification of kaolinite using water, also known as beneficiation, is a crucial process for enhancing the quality of kaolin clay. This method primarily relies on physical separation techniques to remove impurities and improve the clay's properties. Initially, 10 grams of raw kaolinite (Kao) were dispersed in 1000 mL of deionized water to ensure the complete distribution of the clay in the aqueous medium followed by stirring for 24 hours to ensure the complete and uniform distribution of the kaolinite throughout the aqueous medium. After stirring, the resulting suspension was filtered to separate the purified kaolinite from any coarse particles or contaminants. The filtered clay was then subjected to vacuum drying at 100 °C, which removed the water content and left behind a dry, powdery form of kaolinite. The purified kaolinite, free from impurities, was subsequently coded as “PK” and is now ready for further modification or use in various applications. This method effectively produces a clean and homogeneous kaolin sample suitable for advanced use.
• CTAB intercalation: the synthesis of cetyltrimethylammonium bromide (CTAB) modified kaolinite was conducted using a specific procedure. The process began by dispersing 1 g of pure kaolinite (PK) in 100 ml of distilled water. This mixture was then stirred using a magnetic stirrer for 24 hours at room temperature. The purpose of this step was to allow the kaolinite to swell and reach a homogeneous state. After the dispersion and swelling process, the desired amount of CTAB was slowly added to the dispersed PK. The amount of CTAB used was equivalent to 1.0 times the cation exchange capacity (CEC) of the kaolinite. Following the addition of CTAB, the reaction mixture was stirred for 5 hours. This stirring facilitated the interaction between the CTAB and the kaolinite, resulting in the formation of the CTAB-modified kaolinite, also known as organoclay. Once the stirring was complete, the resultant organoclay was filtered. The filtered organoclay was then washed with distilled water several times to remove any excess salts that may have been present. After the washing step, the organoclay was dried in an oven at a temperature of 90 °C. Finally, the dried organoclay was ground and sieved using standard sieves to obtain the chemically modified adsorbent material. This comprehensive procedure ensured the successful synthesis of CTAB-modified kaolinite (CK or CKP-0), which can be used as an effective adsorbent material for various applications.
• PEI-modification: the exfoliation technique was employed to attach amine groups to the surface of CTAB-intercalated kaolinite. The synthesis of polyethyleneimine (PEI)-impregnated CK was carried out using 30% and 50% wt PEI loadings. The process began by preparing a PEI solution in a 100 mL round-bottom flask, where the measured amount of PEI was dissolved in 40 mL of methanol (MeOH) and the solution was then stirred at room temperature for 30 minutes to ensure complete dissolution of the PEI.
In a separate beaker, approximately 0.5 g of CTAB-modified kaolinite (CK) was added into 5 mL of toluene. The mixture was sonicated in an ultrasonic bath for 5 hours at room temperature. Once prepared, the CK solution was added to the previously prepared PEI solution. The mixture was then stirred for an additional 3 hours to facilitate the interaction between the PEI and the CTAB-modified kaolinite (CK), allowing the PEI to be effectively incorporated onto the surface of the CK. Once stirring was completed, the stir bar was removed, and the clay was allowed to settle at the bottom of the flask. The methanol layer on top was decanted, and the wet clay was dried in a vacuum oven at 75 °C for 12 hours. This procedure was repeated with increasing concentrations of PEI, resulting in two adsorbents (CKP-30 and CKP-50, which refer to CTAB-modified kaolinite samples with 30% and 50% PEI, respectively). The resulting CKP clay materials were characterized using several analytical techniques, including FTIR, EDX, and TEM, providing detailed information on the properties and composition of the PEI-treated CK. Table S1 (ESI†) shows the code and composition of the prepared samples. The percentage yield for each step was calculated and given in the ESI.†
3. Characterization techniques
3.1. Fourier transform infrared spectroscopy (FTIR)
Fourier-transform infrared (FTIR) analysis is a valuable procedure used to investigate the chemical composition and molecular structure of materials. The FTIR of all prepared samples was carried out using a Nicolet 550, to identify the structural changes after CTAB interaction in kaolinite and PEI impregnation. The FTIR spectra were recorded by directly contacting the samples with an ATR (attenuated total reflectance) tip and spectra were obtained by conducting 64 scans in the range of 4000–400 cm−1.3.2. X-Ray diffractometer (XRD)
The crystallography of the synthesized samples was investigated through X-ray diffraction (XRD) analysis. The XRD patterns were attained at room temperature using an X-ray powder diffractometer, specifically a D/Max-3A Rigaku, with Cu Kα radiation (λ = 1.5418 Å), a voltage of 35 kV, and a current of 30 mA. The data was obtained over an angular range from 0 to 100° in increments of 0.02°, employing an X-ray generator.3.3. Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to characterize surface morphologies of all prepared samples. The LEO 1530 scanning electron microscope, USA, is utilized for this purpose. The instrument is coupled with an energy dispersive X-ray spectrometer (EDS) detector to facilitate elemental analysis. The spatial resolution of the detected elements is contingent on the energy of the primary electron beam, the atomic number of the constituent elements, and the sample material's thickness. SEM provides high-resolution images, while EDS enables the identification and quantification of elements present in the samples, including mapping the spatial distribution of elements across the surfaces. This comprehensive analysis aids in understanding the structural and elemental properties crucial for optimizing the performance of these composite membranes.3.4. Field emission transmission electron microscope (FETEM)
The field emission transmission electron microscope (FETEM) is a multipurpose, 200 kV FE (field emission) analytical electron microscope that was used to analyze all prepared samples; pure kaolinite (PK), CTAB modified kaolinite (CK or CKP-0), CTAB modified kaolinite with 30 and 50% PEI (CKP-30, CKP-50).3.5. Brunauer–Emmett–Teller (BET)
The surface area and total pore volume of all prepared fertilizers were determined using a Quantachrome QuadraWin (version 6.0) instrument through nitrogen adsorption at liquid nitrogen temperature. Sample preparation involved degassing overnight for 2 hours at 130 °C under vacuum. The Brunauer–Emmett–Teller (BET) method was employed. This analysis provides precise evaluation of specific surface area through nitrogen multilayer adsorption measured as a function of relative pressure. The BET method allows for the determination of surface area in m2 g−1, offering valuable insights into surface porosity.4. Results and discussion
4.1. Fourier transform infrared spectroscopy (FTIR)
Infrared spectroscopy is used to identify the presence of organic molecules within the clay layers. To confirm the intercalation of quaternary alkylammonium cations into the silicate structure, FTIR spectra of pure kaolinite (PK) and CTAB-modified kaolinite (CKP-O) were recorded and compared. From Fig. 2, PK shows four characteristic OH-stretching bands,59 the band at 3698 relates to inner-surface OH in-phase stretching, those at 3671 and 3655 cm−1 are inner-surface OH anti-phase stretching and that at 3621 cm−1 is due to the stretching of inner stretching OH groups.60–62 In CKP-0, peaks at 2860 cm−1 and 2981 cm−1 correspond to the symmetric and asymmetric stretching vibrations of the methyl (CH3) and methylene (CH2) groups from the aliphatic chain of the surfactant, with these peaks appearing more prominently63 in CKP-0. The presence of these peaks indicates the incorporation of surfactants into PK. The other typical absorption bands of PK at 3667 cm−1 and 3607 cm−1 are attributed to the inner OH groups bonded to Al or O. The stretching peak at 908 cm−1 is associated with the Si–O or Si–O–Al bonds.60 The peak observed at 1394 cm−1 corresponds to the C–N group of the organic modifier, confirming the intercalation of surfactant molecules between the silicates. The characteristic bands of the C–N bond, typically found between 910 and 1000 cm−1 (particularly strong for compounds like R–N + (CH3)3), are obscured in the complex inorganic-clay structure due to the deformation vibrations of Al–OH at 926 cm−1.64In the spectra of PEI-based composite adsorbents (CKP-30 and CKP-50), the bands at 2850 and 2925 cm−1 can be attributed to the stretching vibrations of –CH2 groups in the PEI chain.65 Two bands at 1590 and 1447 cm−1 are attributed to the symmetric and asymmetric bending vibrations of –NH2, while another band at 1660 cm−1 corresponds to the bending vibration of –N(R)H in PEI. Notably, as the PEI loading increased, the intensity of these bands grew, indicating that more PEI was dispersed inside the pores and/or on the inner surface of PK. Additionally, these peaks showed a slight shift from their original positions, suggesting a weak interaction between PEI and the internal surface of the clay.
4.2. X-Ray diffraction (XRD)
Fig. 3 shows the XRD pattern of PK, CKP-0, CKP-30 and CKP50. The structure of pure kaolinite (PK) before and after modification with CTAB was compared, for PK, the peaks at 12.18°, 20.2°, 21.23°, 23.3°, 24.78°, 35.89°, 38.83°, and 55.99° can be indexed to the planes corresponding to kaolinite (001), (110), (111), (002), (131), (202), and (133), respectively.66,67 In the case of CKP-0, the characteristic (001) diffraction peak of PK shifts from 12.1895° to 12.2721° after treatment with CTAB, indicating the intercalation of CTAB into the kaolinite layers. The d-spacing of kaolinite at (001) increases from 7.13411 to 7.15163 Å, further supporting the intercalation of CTAB. The addition of the surfactant increases the basal spacing of the resulting organoclay, suggesting the placement of CTA+ ions between the clay layers and leading to a decrease in hydration water content. As a result, the surface properties change from hydrophilic to hydrophobic.64 XRD analysis was also conducted to examine the impregnation of PEI into CTAB modified kaolinite. The results showed an increase in d-spacing in the CKP composites from 1.36 to 1.92 nm after reaction with PEI, indicating the successful synthesis of intercalated PEI nanocomposites.4.3. Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) images of pure kaolinite (PK) in Fig. 4(a) reveal its characteristic morphology, typically appearing as hexagonal or pseudo-hexagonal platelets. These platelets can be observed in both face-on and edge-on orientations, providing insights into the mineral's structural arrangement. These images also highlight the stacking order of the layers, where well-ordered PK shows regular, parallel fringes, while disordered samples may exhibit wavy or discontinuous patterns. Additionally, TEM analysis reveals structural defects like stacking faults and intergrowths with other clay minerals. TEM images of CTAB-modified kaolinite, i.e. CKP-0 (Fig. 4(b)), reveal significant structural changes and enhanced properties resulting from the modification process. The images show successful intercalation of CTAB molecules into the interlayer spacing of PK, leading to a slight expansion of the basal spacing. Despite this modification, the characteristic hexagonal or pseudo-hexagonal platelet structure of PK is preserved, indicating that its inherent properties remain intact. TEM analysis also demonstrates a homogeneous distribution of CTAB throughout the PK material, contributing to a more porous surface morphology that enhances adsorption capabilities. Additionally, the modification significantly reduces particle agglomeration compared to unmodified kaolinite. It is worth noticing that the structural integrity of kaolinite is maintained during the modification process.68TEM photographs of PEI-impregnated CTB modified kaolinite with 30 and 50% PEI loading (CKP-30 and CKP-50) presented in Fig. 4(c) and (d) showed that PEI is impregnated homogeneously into the clay layers on the nanoscale, although some particles of clay were agglomerated. As polyethyleneimine (PEI) loading increased, the intercalation of PEI molecules between the clay sheets also increased, hence leading to greater adhesion with the clay layers, resulting in a more complex structure. Such interactions improve various properties of the resulting nanocomposites, such as mechanical strength, thermal stability, and gas barrier properties.69
4.4. Scanning electron microscopy (SEM)
SEM analysis was conducted to assess the surface morphology of both pure and modified kaolinite clays, with the results shown in Fig. 5. The surface morphologies of pure kaolinite (PK) and CTAB-modified kaolinite (CKP-0) differ significantly. The PK surface is characterized by stacked pseudo-hexagonal platelets with diameters ranging from 0.1 to 1.5 μm and thicknesses of approximately 30–100 nm (Fig. 5(a)). These platelets form “booklet” structures several micrometers in size, especially noticeable in CKP-0, where a high degree of stacking exposes the edge surfaces. Both PK and CKP-0 have uneven structures with non-uniform size distributions, with kaolinite displaying a more aggregated morphology and occasional large flakes. After modification, the surface of PK was transformed into a non-aggregated morphology, featuring numerous small, crumpled flakes. Additionally, the surface of CKP-0 expanded due to the chemical intercalation of CTAB. The expanded interlayer created by the CTAB modification of kaolinite provides a favourable site for chemical reactions such as interlayer exchange and adsorption. It has been shown that CTAB-modified kaolinite has a larger pore size compared to PK (Fig. 5(b)).SEM images of the PEI/CTAB-kaolinite composites (CKP30 and CKP50) reveal minimal PEI on the external surfaces, suggesting that the PEI is dispersed within the pores of the CTAB-modified kaolinite (Fig. 5(c) and (d)). The images indicate excellent adhesion between the CTAB-kaolinite particles and the PEI polymer matrix, with no voids observed, likely due to the high compatibility between the particles and the polymer, which is facilitated by ammonium ion treatment. For CKP30 (30 wt% PEI), only a small amount of PEI was observed on the external surfaces, while some PEI was coated on the clay particles, leading to partial agglomeration. At PEI loadings below 50 wt%, most of the PEI was found within the pores of the samples.
For CKP50, which had a higher PEI loading (50 wt%), a significant portion of the PEI was present on the external surfaces, acting as linkages between the clay particles. The amine groups in PEI likely bond with the Si–OH and Al–OH groups on the surface of the modified kaolinite, increasing the PEI loading. Based on the SEM observations, it was found that composites with PEI content up to 50 wt% remained as powders. These findings suggest that the optimal PEI loading for preparing effective composite sorbents is approximately 50 wt%, which aligns with the observed CO2 sorption capacity trends of the sorbents at different loadings.
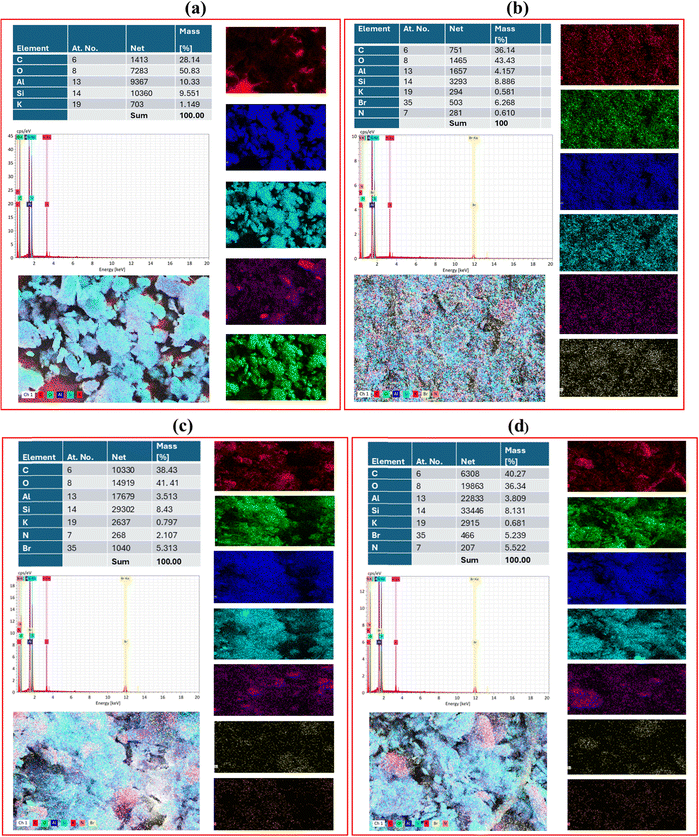
4.5. Energy dispersive spectroscopy (EDS)
The EDS analysis of PK, CKP-0, CKP-30 and CKP-50 provides important insights into the elemental makeup of these materials. In the case of PK, the EDX spectrum typically reveals dominant peaks for silicon (Si), aluminum (Al), and oxygen (O), which are the key elements that make up the clay mineral. These elements are representative of kaolinite's characteristic layered silicate structure (Fig. 6(a)). Upon modification with cetyltrimethylammonium bromide (CTAB), the EDX spectrum of CKP-0 would display additional peaks for carbon (C), nitrogen (N), and bromine (Br). The presence of these elements confirms the successful incorporation of CTAB into the kaolinite structure, which enhances the material's properties for a range of applications. The carbon peak is particularly notable, as it corresponds to the alkyl chains in CTAB, while the nitrogen and bromine peaks are associated with the ammonium group and bromide ion, respectively (Fig. 6(b)). For the PEI-impregnated samples (CKP-30 and CKP-50), EDX analysis would show stronger carbon and nitrogen peaks compared to the CTAB-modified kaolinite. This increase is due to the inclusion of polyethyleneimine (PEI), which has a high content of carbon and nitrogen due to its polymeric structure. The intensity of these peaks would be more pronounced in the 50 wt% sample compared to the 30 wt% sample, indicating a higher amount of PEI loading in the former (Fig. 6(c) and (d)).In summary, EDX analysis is a valuable technique for verifying the successful modification of kaolinite with CTAB and PEI. It provides quantitative data on the elemental composition of the nanocomposite materials, which correlates with their enhanced properties.
4.6. Brunauer–Emmett–Teller (BET)
Table 1 presents the BET surface area, and total pore volume of PK, CKP-0, CKP-30, and CKP-50. The analysis showed that the total surface area of kaolin clay significantly decreased from 20.57 to 5.03 m2 g−1 after modification with the CTAB surfactant. In contrast, the total pore volume increased from 0.305 to 0.591 cm3 g−1 and pore diameter 9.75 to 21.09 nm. These results align with previous studies on surfactant-modified clays.70,71 The reduction in surface area, along with the increase in pore volume and pore diameter can be attributed to the intercalation of surfactants, which filled much of the gallery space in the clay, causing the interlayer to expand and thus increasing the pore volume. For the CKP-30 and CKP-50 samples, the surface areas were 3.97 and 3.31 m2 g−1 and the pore volume was 0.06 and 0.04 cm3 g−1, respectively, while the pore diameter was 23.09 and 23.15 nm, respectively, after the introduction of PEI into the CTAB-modified kaolinite.| Samples | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | CO2 capacity (mg g−1) |
|---|---|---|---|
| PK | 20.57 | 0.305 | 0.901 |
| CKP-0 | 5.03 | 0.591 | 3.44 |
| CKP-30 | 3.97 | 0.06 | 150.0 |
| CKP-50 | 3.31 | 0.04 | 167.1 |
The amount of N2 adsorption and pore volume decreased with increasing PEI loading, as the PEI filled the pores and channels between the clay layers. This led to a reduction in pore volume with increasing PEI content. It can be observed that the amine impregnated sample has a lower surface area and pore volume. This is because amine molecules entered the surface pores of the kaolinite and blocked them, thereby reducing the textural properties of the amine-loaded CK sample. The surface area of the CKP-30 sample (with 30% PEI loadings) decreased to 3.39 m2 g−1, and the pore volume also diminished, and a further increase up to 50% PEI loading led to the filling of the pores and channels in the CK layers and caused further reduction in these surface feature. These results are in agreement with the SEM and TEM findings.
The CKP-50 sample exhibited the highest CO2 adsorption capacity compared to the other samples (Table 1). This suggests that the CO2 adsorption capacity improves with higher amine loading. The increase in capacity is attributed to the interaction between amine groups and CO2 molecules. A higher weight ratio of amines results in more amine groups being incorporated, thereby enhancing the material's adsorption capability. Interestingly, the CO2 adsorption capacity did not correlate directly with the BET surface area or pore volume, suggesting that these factors were not the primary contributors to the CO2 adsorption performance. Instead, the amount of PEI loading and the availability of reaction sites on the adsorbent played a more significant role. While an optimal amount of PEI is necessary, excessive PEI loading can block the internal and inter-particle pores of the sample, potentially decreasing CO2 uptake.72 Based on the results, the optimal PEI loading in the current study was maintained in the range of 50–60 wt%.
4.7. CO2 uptake study
The CO2 adsorption experiments were conducted at 298 K using ultra-high pure CO2 (99.99%) gas with a 20 mL min−1 flow rate. The PK sample has low CO2 uptake due to several structural and chemical limitations. Its specific surface area is high, but it has no fictional groups that can chemically bind CO2, which restricts the available sites for CO2 adsorption. Additionally, kaolinite has a minimal pore volume of about 0.035 cc g−1 and lacks a microporous structure, leading to adsorption primarily on particle surfaces and in larger macropores. The weak interactions between CO2 and the –OH groups on its surface further limit its capacity. Moreover, the tight layered structure of kaolinite prevents CO2 from intercalating between the layers. As a result, the CO2 adsorption capacity of raw kaolinite is extremely low, making it ineffective for CO2 capture applications.The chemical structure of cetyltrimethylammonium bromide (CTAB), a cationic surfactant, significantly influences its interaction with kaolinite clay. CTAB consists of a long hydrophobic alkyl chain (a cetyl group) and a positively charged quaternary ammonium head. This unique structure allows CTAB to interact effectively with the negatively charged surfaces of clay. When CTAB is introduced to a suspension of kaolinite, the hydrophobic tail of CTAB tends to adsorb onto the clay surface, while the positively charged head group interacts with the negatively charged sites on the kaolinite. This interaction leads to a reduction in the surface tension and increases the hydrophobicity of the clay particles.
Hydrophobic modification can improve the interaction between clay and CO2, as the more hydrophobic surface can better accommodate non-polar molecules like CO2. CTAB forms organoclays, which have unique properties compared to their unmodified counterparts. These organoclays can have improved pore volume for enhanced CO2 adsorption. The structural changes induced by CTAB can lead to a more favorable arrangement for CO2 molecules to be trapped within the clay matrix. The presence of CTAB can enhance the physical and chemical adsorption mechanisms of CO2 on the clay surface. The positively charged ammonium groups in CTAB can interact with the electron-rich regions of CO2, promoting chemisorption. Additionally, the increased surface area and porosity allow for greater physisorption of CO2 molecules.
Polyethyleneimine (PEI) significantly enhances the ability of kaolinite to adsorb CO2 through several mechanisms related to its chemical structure and properties. PEI is a branched polyamine that contains multiple amine functional groups, which play a crucial role in the adsorption process. When PEI is incorporated into CTAB-modified kaolinite, it increases the number of active sites available for CO2 interaction, thereby improving the overall adsorption capacity. The primary mechanism by which PEI aids in CO2 adsorption is through chemical interaction. The amine groups in PEI can react with CO2 to form carbamate species, which enhances the binding of CO2 to the kaolinite surface. This reaction occurs primarily at the primary and secondary amine sites of PEI, allowing for the effective capture of CO2 molecules from the surrounding environment. Additionally, the incorporation of PEI into kaolinite modifies the surface properties of the clay. This modification can increase the hydrophilicity of the kaolinite, which helps in the formation of bicarbonate ions in the presence of moisture, further facilitating CO2 capture. The presence of water can enhance the adsorption process as it promotes the formation of bicarbonate through the reaction of CO2 with hydroxyl ions, which can be generated from the interaction of water with the amine groups of PEI. Moreover, the loading percentage of PEI is a critical factor influencing CO2 adsorption performance. Optimal amine loading ensures that there are sufficient active sites for CO2 interaction without causing steric hindrance that could reduce accessibility.
The CKP-50 sample exhibited the highest CO2 adsorption capacity compared to the other samples (Table 1). Interestingly, the CO2 adsorption capacity did not correlate directly with the BET surface area or pore volume, suggesting that these factors were not the primary contributors to the CO2 adsorption performance. Instead, the amount of PEI loading and the availability of reaction sites on the adsorbent played a more significant role. While an optimal amount of PEI is necessary, excessive PEI loading can block the internal and inter-particle pores of the sample, potentially decreasing CO2 uptake.72
The PEI-impregnated approach significantly enhances the CO2 adsorption capacity of various solid adsorbents. Generally, higher amine loading results in an increased CO2 adsorption capacity, as the incorporation of PEI introduces more active sites, facilitating CO2 adsorption onto the solid adsorbent surfaces. For example, Cheng et al. studied the effect of amine loading (20–60 wt%) on Zn/Co zeolitic imidazolate frameworks (Zn/Co ZIFs) under pure CO2 gas flow. They found that a 20% PEI loading increased the CO2 adsorption capacity from 1.07 mmol g−1 to 1.27 mmol g−1, and it continued to rise up to 1.82 mmol g−1 at 40 wt%. However, when the PEI loading was further increased to 60 wt%, the CO2 uptake capacity decreased to 1.35 mmol g−1. This reduction was likely due to the excess PEI blocking the main active sites on the adsorbent.73 Based on these findings, it can be concluded that excessive amine content negatively impacts the CO2 uptake capacity and adsorption rate of PEI-modified adsorbents. This is due to the increased resistance to CO2 diffusion from thicker PEI layers at high amine loadings. Additionally, excess PEI can the block pores completely, reducing the surface area and pore volume, and decreasing the availability of effective active sites on the adsorbent. Therefore, identifying the optimal amine loading for a given support material is essential to maximize CO2 adsorption. Taking these factors into account, the 30 wt% and 50 wt% PEI loadings were chosen in the current study to achieve the highest CO2 adsorption capacity. The accumulation of CO2 onto the amine-modified kaolinites may be through chemical adsorption. The possible adsorption mechanism is in agreement with previous studies.31,32
The FTIR spectrum of the PEI impregnated CK after CO2 adsorption, shown in Fig. 7(b), reveals a decrease or disappearance of the NH2 bending bands at 1568 cm−1 and 1490 cm−1, as well as the CH2 stretching modes at 2947 cm−1 and 2870 cm−1. Additionally, a new absorption peak appears at 1456 cm−1, corresponding to the C–N vibration band, and another new peak appears at 1636 cm−1, attributed to the RNHCOO− (carbamate species). These changes suggest a chemical reaction between the amine groups and CO2 molecules, implying that CO2 accumulation on the amine-modified kaolinites likely occurs through chemical adsorption. The adsorption mechanism is consistent with the findings of Gray et al. and Wang et al.
| Si–OH(kaolinite) + RNH2(amine) → Si–O–N+N3R |
CO2 + 2RNH2 → ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) RNHCOO− + RNH3+ RNHCOO− + RNH3+ |
4.8. Concept of CO2-based fertilizer
Desert sand poses significant challenges for plant growth due to its lack of essential nutrients, poor water retention, and unfavorable soil structure. These factors, combined with the harsh environmental conditions of desert regions, make it extremely difficult for plants to establish and thrive. The approach of using small portions of the fertilizer is particularly noteworthy, as it allows for the improvement of growing conditions while minimally altering the overall desert ecosystem. This innovative fertilizer solution has the potential to transform arid regions, making them more suitable for agriculture or reforestation efforts. By addressing the fundamental limitations of desert sand, it opens up new possibilities for sustainable plant growth in challenging environments, potentially contributing to food security, land reclamation, and ecological restoration in arid and semi-arid regions around the world.| S. No. | Samples | Code | Composition |
|---|---|---|---|
| 1 | Pure kaolinite + sand | S-PK | PK (1 g) + sand (9 g) |
| 2 | CTAB modified kaolinite + sand | S-CKP-0 | CKP-0 (1 g) + sand (9 g) |
| 3 | PEI (30) impregnated/CTAB modified kaolinite + sand | S-CKP-30 | CKP30 (1 g) + sand (9 g) |
| 4 | PEI (50) impregnated/CTAB modified kaolinite + sand | S-CKP-50 | CKP50 (1 g) + sand (9 g) |
Once the mixtures were prepared, they were transferred into cone-like containers made from plastic bottles. These containers served as planting vessels for the coriander seeds, which were selected for the seedling trials. The container seeds were then placed inside a hydroponic apparatus Z216 (17 pods) (Product Model: Z216) providing a controlled environment for the seedlings. The hydroponic system was designed to simulate desert conditions while providing precise control over environmental factors. The apparatus was closed to maintain a controlled environment (Fig. 8(c)), with temperature monitored using a calibrated thermometer (Fig. 8(d)). Light intensity within the system was measured accurately using a digital lux meter (Fig. 8(e)), ensuring optimal illumination for plant growth.
To create a more realistic desert-like substrate, a sand bed was incorporated into the hydroponic system. This sand layer served multiple purposes:
• It provided ample space for root penetration and growth.
• It simulated the natural environment of desert plants.
• It offered support and stability for the developing root systems.
The seed containers were specially designed with open bottoms, allowing them to be partially submerged in the sand bed. This configuration enabled direct root contact with the substrate, mimicking natural growing conditions more closely. To maintain consistent moisture levels crucial for plant development, a precise irrigation schedule was implemented. Each container received 2 mL of water at regular intervals over the course of the 50-day experiment. This controlled watering regimen ensured that the plants received adequate hydration while still experiencing conditions like their native arid environments.
| Time | Adsorbent for plant growth height (cm) | |||
|---|---|---|---|---|
| Days | S-PK | S-CKP-0 | S-CKP-30 | S-CKP-50 |
| 0 | 0 | 0 | 0 | 0 |
| 5 | 1.1 | 1.3 | 2.1 | 3.1 |
| 10 | 3.2 | 4.3 | 6.3 | 7.24 |
| 15 | 6.1 | 8.5 | 10.3 | 12.2 |
| 20 | 7.2 | 9.3 | 14.2 | 16.2 |
| 25 | 8.3 | 11.2 | 20.1 | 22.1 |
| 30 | 9.1 | 13.1 | 25.1 | 27.1 |
 | ||
| Fig. 9 (a–j) Plant growth experiment in hydroponic apparatus over a 55-day period, illustrating controlled growth stages from germination to maturity. | ||
The height of plant growth for each adsorbent was noted for the period of 30 days as after that the height become constant. The height for each fertilizer was measured after each 5 days and the results are presented in Fig. 10 for (a) S-PK, (b) S-CKP-0, (c) S-CKP-30 and (d) S-CKP-50. While comparison of height for each sample is given in Fig. 10(e). The height for each adsorbent after 5 days was as follows: S-PK (1.1 cm), S-CKP-0 (1.3 cm), S-CKP-30 (2.1 cm), and S-CKP-50 (3.1 cm). Significant growth was observed after 10 days: S-PK (3.2 cm), S-CKP-0 (4.3 cm), S-CKP-30 (6.3 cm), and S-CKP-50 (7.24 cm). By day 20 the plants reached: S-PK (7.2 cm), S-CKP-0 (9.3 cm), S-CKP-30 (14.2 cm), and S-CKP-50 (16.2 cm). The growth continued, with the following heights by day 25: S-PK (8.3 cm), S-CKP-0 (11.2 cm), S-CKP-30 (20.1 cm), and S-CKP-50 (22.1 cm). The maximum height was achieved by day 30 with the final heights recorded as: S-PK (9.1 cm), S-CKP-0 (13.1 cm), S-CKP-30 (25.1 cm), and S-CKP-50 (27.1 cm). The maximum height for each adsorbent is given in Fig. 10(f). These results showed that the maximum height was achieved by day 30 by S-CKP-50 representing the full growth of the plant.
CTAB-modified kaolinite (CK) with sand offers several advantages that enhance plant growth compared to unmodified kaolinite. The modification with cetyltrimethylammonium bromide (CTAB) improves the clay's properties, making it more suitable for agricultural applications. CK modification increases the CEC of kaolinite, alters its surface characteristics, and promotes better water retention and moisture availability. The enhanced pore structure improves aeration and drainage, both beneficial for healthy root development.
The structural integrity of CK also helps prevent soil compaction, allowing better root penetration and overall plant stability. Furthermore, the CO2 adsorption capacity of CK is higher than that of PK, increasing soil fertility to some extent and supporting plant growth. The introduction of PEI at a 30% loading level into kaolinite (CKP30) and use with sand brought some benefits, improving plant growth compared to S-PK and S-CKP-0. The 30% PEI loading enhanced the surface characteristics and water retention properties of kaolinite, improving moisture availability for the plants. The CO2 adsorption capacity of CKP30 is higher than that of PK and CK, leading to better plant growth as the increased CO2 boosts fertility and provides essential nutrients. The addition of 30 wt% PEI enhances the adsorption capacity of kaolinite, potentially improving nutrient and water retention in the soil, which directly benefits the growth of coriander plants. The higher concentration of PEI further enhances the interaction between the adsorbent and the plant roots, improving the soil's moisture retention, nutrient availability, and overall growth environment. The 50% PEI loading in CKP50 and use with sand resulted in the most significant improvements in plant growth among all the tested materials. With this higher PEI concentration, kaolinite exhibited optimal water retention, nutrient retention, and aeration properties. The enhanced moisture retention and improved soil structure provided ideal conditions for root development and overall plant growth. S-CKP50 also displayed superior ability to prevent soil compaction, allowing better root penetration and stability. Most notably, CKP50's higher CO2 adsorption capacity further increased soil fertility, providing additional nutrients to support healthy plant growth, making it the most effective material for promoting plant growth. This indicates that 50 wt% PEI provides the most effective modification for optimal coriander plant growth.
In conclusion, the results show that PEI-modified kaolinites (S-CKP-30 and S-CKP-50) significantly improve coriander plant growth compared to pure kaolinite and CTAB-modified kaolinite. The higher the PEI content, the greater the enhancement in plant height, with S-CKP-50 yielding the best results at 27.1 cm. These findings suggest that PEI-modified clay adsorbents are highly effective in improving plant growth, particularly by enhancing the soil's ability to retain moisture and nutrients, thus providing a promising solution for agricultural applications.
5. Conclusion
The transformation of desert sands into fertile land using CO2 adsorbed on clays offers a promising and innovative solution to combat desertification and mitigate climate change. In this study, we prepared a CO2-enriched fertilizer with the potential to combat desertification and CO2 adsorption. Initially, kaolinite was modified using cetyltrimethylammonium bromide (CTAB) and then impregnated with varying concentrations of polyethyleneimine (PEI). The prepared adsorbents were thoroughly analysed using various characterization techniques, including Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). These methods helped in assessing the structural properties and morphology of the modified kaolinite. The adsorbents were tested for CO2 uptake capacity as well as their potential to support plant growth. The results demonstrated that CO2-enriched clays offer a cost-effective and environmentally sustainable solution for CO2 adsorption while significantly enhancing plant growth compared to pure kaolinite clay. Among all the adsorbents, those with amine loadings, particularly S-CKP30 (30% PEI) and S-CKP50 (50% PEI), showed substantial improvements in plant growth relative to both pure kaolinite and CTAB-modified kaolinite. Notably, S-CKP50, produced the most favorable results. These results showed that CO2-enriched clays, especially those modified with PEI, represent an effective and sustainable approach to both CO2 capture and desert reclamation. CKP50, in particular, stands out as an ideal candidate for improving soil conditions, supporting plant growth, and offering long-term environmental benefits, making it a promising solution for combating desertification and addressing the global challenges of climate change.Data availability
The datasets generated and analysed during this study are available from the corresponding author upon reasonable request. Additional information and materials related to the research are provided in the ESI,† where applicable.Conflicts of interest
There are no conflicts to declare.References
- H. W. Ku,
et al., “Active” hydroponic greenhouse system to kick-start and augment reforestation program through carbon sequestration–an experimental and theoretical feasibility study, J. Cleaner Prod., 2016, 129, 637–646 CrossRef
.
- E. Kawai, A. Ozawa and B. D. Leibowicz, Role of carbon capture and utilization (CCU) for decarbonization of industrial sector: A case study of Japan, Appl. Energy, 2022, 328, 120183 CrossRef CAS
.
- M. Yusuf and H. Ibrahim, A comprehensive review on recent trends in carbon capture, utilization, and storage techniques, J. Environ. Chem. Eng., 2023, 111393 CrossRef CAS
.
- J. Podder,
et al., A review of carbon capture and valorization technologies, Energies, 2023, 16(6), 2589 CrossRef CAS
.
- K. Zhao,
et al., Recent advances and future perspectives in carbon capture, transportation, utilization, and storage (CCTUS) technologies: A comprehensive review, Fuel, 2023, 351, 128913 CrossRef CAS
.
- U. S. D'Ettorre, I. S. Liso and M. Parise, Desertification in karst areas: A review, Earth-Sci. Rev., 2024, 104786 CrossRef
.
- S. Zhang,
et al., Research Progress on Ecological carrying Capacity and Ecological Security, and its inspiration on the forest ecosystem in the Karst Desertification Control, Forests, 2024, 15(9), 1632 CrossRef
.
- M. A. AbdelRahman, An overview of land degradation, desertification and sustainable land management using GIS and remote sensing applications, Rend. Lincei, Sci. Fis. Nat., 2023, 34(3), 767–808 CrossRef
.
- D. Shanmugavel,
et al., Sustainable SMART fertilizers in agriculture systems: A review on fundamentals to in-field applications, Sci. Total Environ., 2023, 904, 166729 CrossRef CAS PubMed
.
- S. Liu,
et al., Simultaneously tackling ecological degradation and poverty challenges: Evidence from desertified areas in northern China, Sci. Total Environ., 2022, 815, 152927 CrossRef CAS PubMed
.
- A. Yussupov and R. Z. Suleimenova, Use of remote sensing data for environmental monitoring of desertification, Evergreen: Jt. J. Novel Carbon Resour. Sci. Green Asia Strategy, 2023, 300–307 CrossRef
.
- M. Pizzol, J. C. Smart and M. Thomsen, External costs of cadmium emissions to soil: a drawback of phosphorus fertilizers, J. Cleaner Prod., 2014, 84, 475–483 CrossRef CAS
.
- T. Gomiero, D. Pimentel and M. G. Paoletti, Environmental impact of different agricultural management practices: conventional vs. organic agriculture, Crit. Rev. Plant Sci., 2011, 30(1–2), 95–124 CrossRef
.
-
N. Mushtaq, et al., Freshwater contamination: sources and hazards to aquatic biota, Fresh water pollution dynamics and remediation, 2020, pp. 27–50 Search PubMed
.
-
M. A. Dervash, et al., Biotechnological intervention as an aquatic clean up tool, Fresh water pollution dynamics and remediation, 2020, pp. 183–196 Search PubMed
.
-
G. H. Dar, et al., Microbiota and biofertilizers, Springer, 2021, vol. 2 Search PubMed
.
- R. Dinesh,
et al., Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)], Bioresour. Technol., 2010, 101(12), 4697–4702 CrossRef CAS PubMed
.
- G. Dar,
et al., Characterization of Aeromonas sobria isolated from fish Rohu (Labeo rohita) collected from polluted pond, J. Bacteriol. Parasitol., 2016, 7(3), 1–5 Search PubMed
.
- G. M. Shah,
et al., Toxicity of ZnO and Fe2O3 nano-agro-chemicals to soil microbial activities, nitrogen utilization, and associated human health risks, Environ. Sci. Eur., 2022, 34(1), 106 CrossRef CAS
.
- T. G. Abebe,
et al., Growing use and impacts of chemical fertilizers and assessing alternative organic fertilizer sources in Ethiopia, Appl. Environ. Soil Sci., 2022, 2022(1), 4738416 Search PubMed
.
- A. K. Shukla,
et al., Fertilizer use in Indian agriculture and its impact on human health and environment, Indian J. Fert., 2022, 18(3), 218–237 Search PubMed
.
- Y. Guo and J. Wang, Spatiotemporal changes of chemical fertilizer application and its environmental risks in China from 2000 to 2019, Int. J. Environ. Res. Public Health, 2021, 18(22), 11911 CrossRef PubMed
.
- W. Ouyang,
et al., Increased ammonia emissions from synthetic fertilizers and land degradation associated with reduction in arable land area in China, Land Degrad. Dev., 2018, 29(11), 3928–3939 CrossRef
.
- A. M. Ghanem,
et al., Measuring impact of air and agricultural soil pollution on social development in Saudi Arabia, J. Exp. Biol. Agric. Sci., 2022, 575–583 CAS
.
- W. Al-Busaidi,
et al., Impact of long-term agricultural farming on soil and
water chemical properties: A case study from Al-Batinah regions (Oman), J. Saudi Soc. Agric. Sci., 2022, 21(6), 397–403 CrossRef
.
- M. Sulaiman,
et al., Accumulation of toxic elements in soil and date palm (Phoenix dactylifera L.) through fertilizer application, J. Plant Nutr., 2021, 44(7), 958–969 CrossRef CAS
.
- A. A. Al-Taani,
et al., Contamination assessment of heavy metals in agricultural soil, in the Liwa area (UAE), Toxics, 2021, 9(3), 53 CrossRef CAS PubMed
.
- S. Jain,
et al., Global-scale water security and desertification management amidst climate change, Environ. Sci. Pollut. Res., 2024, 1–25 Search PubMed
.
- S. Velioglu and S. Keskin, Revealing the effect of structure curations on the simulated CO2 separation performances of MOFs, Mater. Adv., 2020, 1(3), 341–353 RSC
.
- S. Samanta and R. Srivastava, Catalytic conversion of CO2 to chemicals and fuels: the collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride, Mater. Adv., 2020, 1(6), 1506–1545 RSC
.
- M. J. Nazir,
et al., Harnessing soil carbon sequestration to address climate change challenges in agriculture, Soil Tillage Res., 2024, 237, 105959 CrossRef
.
- M. Vinoba,
et al., Exploring Enthalpies of CO2 and N2 Adsorption on Zn-and Co-Based Zeolitic Frameworks at Varying Temperatures and Pressures, Results Eng., 2024, 103582 CrossRef CAS
.
- J. D. Souza-Filho,
et al., Zeolite A grown on fiberglass: A prominent CO2 adsorbent for CO2/CH4 separation, Colloids Surf., A, 2024, 683, 132952 CrossRef CAS
.
- B. Mohan,
et al., Carbon dioxide capturing activities of porous metal-organic frameworks (MOFs), Microporous Mesoporous Mater., 2024, 366, 112932 CrossRef CAS
.
- P. Anantharamu,
et al., Improved metal-organic frameworks (MOFs) and their application in catalytic CO2 reduction: A review, Mater. Today Sustainability, 2024, 100745 Search PubMed
.
- N. H. Khdary,
et al., Metal oxides as catalyst/supporter for CO2 capture and conversion, review, Catalysts, 2022, 12(3), 300 CrossRef CAS
.
- F. Fathalian,
et al., Efficient CO2 adsorption using chitosan, graphene oxide, and zinc oxide composite, Sci. Rep., 2024, 14(1), 3186 CrossRef CAS PubMed
.
- S. Wang,
et al., Recent advances in capture of carbon dioxide using alkali-metal-based oxides, Energy Environ. Sci., 2011, 4(10), 3805–3819 RSC
.
- H. Zeng,
et al., Porous adsorption materials for carbon dioxide capture in industrial flue gas, Front. Chem., 2022, 10, 939701 CrossRef CAS PubMed
.
- B. Dziejarski, J. Serafin and K. Andersson, CO2 capture materials: a review of current trends and future challenges, Mater. Today Sustainability, 2023, 100483 CrossRef
.
- B. Dziejarski,
et al., Activated carbons—Preparation, characterization and their application in CO2 capture: A review, Environ. Sci. Pollut. Res., 2023, 31(28), 40008–40062 CrossRef PubMed
.
- N. A. Rashidi and S. Yusup, Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption, J. Cleaner Prod., 2017, 168, 474–486 CrossRef CAS
.
- M. Danish, V. Parthasarthy and M. K. Al Mesfer, CO2 capture by low-cost date pits-based activated carbon and silica gel, Materials, 2021, 14(14), 3885 CrossRef CAS PubMed
.
- A. E. Ogungbenro,
et al., Physical synthesis and characterization of activated carbon from date seeds for CO2 capture, J. Environ. Chem. Eng., 2018, 6(4), 4245–4252 CrossRef CAS
.
- A. S. Ello,
et al., Coconut shell-based microporous carbons for CO2 capture, Microporous Mesoporous Mater., 2013, 180, 280–283 CrossRef CAS
.
- R. Sanz,
et al., CO2 adsorption on branched polyethyleneimine-impregnated mesoporous silica SBA-15, Appl. Surf. Sci., 2010, 256(17), 5323–5328 CrossRef CAS
.
- H. Zhu,
et al., A highly effective and low-cost sepiolite-based solid amine adsorbent for CO2 capture in post-combustion, Sep. Purif. Technol., 2023, 306, 122627 CrossRef CAS
.
- Y.-H. Chen and D.-L. Lu, CO2 capture by kaolinite and its adsorption mechanism, Appl. Clay Sci., 2015, 104, 221–228 CrossRef CAS
.
- L. Wang,
et al., Amine-modified ordered mesoporous silica: The effect of pore size on CO2 capture performance, Appl. Surf. Sci., 2015, 324, 286–292 CrossRef CAS
.
- A. E. Elkhalifah,
et al., Carbon dioxide retention on bentonite clay adsorbents modified by mono-, di-and triethanolamine compounds, Adv. Mater. Res., 2014, 917, 115–122 CAS
.
- J. Hou,
et al., Regulating the effect of element doping on the CO2 capture performance of kaolinite: A density functional theory study, Appl. Surf. Sci., 2020, 512, 145642 CrossRef CAS
.
- Q. Liu,
et al., Preparation of ZSM-5 molecular sieve modified by kaolin and its CO2 adsorption performance investigation, Microporous Mesoporous Mater., 2023, 360, 112678 CrossRef CAS
.
- F. Bahmanzadegan, M. A. Pordsari and A. Ghaemi, Improving the efficiency of 4A-zeolite synthesized from kaolin by amine functionalization for CO2 capture, Sci. Rep., 2023, 13(1), 12533 CrossRef CAS PubMed
.
- S. O. Akpasi and Y. M. Isa, Effect of operating variables on CO2 adsorption capacity of activated carbon, kaolinite, and activated carbon–Kaolinite composite adsorbent, Water-Energy Nexus, 2022, 5, 21–28 CrossRef CAS
.
- L. Stevens,
et al., Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route, Chem. Eng. J., 2013, 215, 699–708 CrossRef
.
- Y.-H. Chen and D.-L. Lu, Amine modification on kaolinites to enhance CO2 adsorption, J. Colloid Interface Sci., 2014, 436, 47–51 CrossRef CAS PubMed
.
- J. Liu,
et al., Polyethyleneimine functionalized protonated titanate nanotubes as superior carbon dioxide adsorbents, J. Colloid Interface Sci., 2012, 386(1), 392–397 CrossRef CAS PubMed
.
- W. Pi,
et al., Carbon dioxide adsorption capacity of porous carbon after polyamine impregnation under different pore structures and temperatures, Chem. Eng. J., 2024, 485, 150025 CrossRef CAS
.
- M. S. Salman,
et al., Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent, J. Mol. Struct., 2023, 1282, 135259 CrossRef CAS
.
- A. S. Chong, M. A. Manan and A. K. Idris, Sodium lignosulfonate as sacrificial agent and effectiveness in reducing CTAB cationic adsorption onto kaolinite, J. King Saud Univ., Eng. Sci., 2021, 33(8), 539–546 Search PubMed
.
- R. Duarte-Silva,
et al., Structural, textural and protein adsorption properties of kaolinite and surface modified kaolinite adsorbents, Appl. Clay Sci., 2014, 90, 73–80 CrossRef CAS
.
-
V. C. Khang, M. V. Korovkin and L. G. Ananyeva, Identification of clay minerals in reservoir rocks by FTIR spectroscopy, IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2016 Search PubMed
.
- Y. Li,
et al., Effective removal of emulsified oil from oily wastewater using surfactant-modified sepiolite, Appl. Clay Sci., 2018, 157, 227–236 CrossRef CAS
.
- N. Belachew and H. Hinsene, Preparation of cationic surfactant-modified kaolin for enhanced adsorption of hexavalent chromium from aqueous solution, Appl. Water Sci., 2020, 10(1), 1–8 CrossRef
.
- Z. Li, Z. Pan and Y. Wang, Mechanochemical preparation of ternary polyethyleneimine modified magnetic illite/smectite nanocomposite for removal of Cr(VI) in aqueous solution, Appl. Clay Sci., 2020, 198, 105832 CrossRef CAS
.
- M. S. Żbik,
et al., Kaolinite platelet orientation for XRD and AFM applications, Appl. Clay Sci., 2010, 50(3), 299–304 CrossRef
.
- A. Kumar and P. Lingfa, Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD, Mater. Today: Proc., 2020, 22, 737–742 CAS
.
- M. Shaban,
et al., Adsorption behavior of inorganic-and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies, J. Sol-Gel Sci. Technol., 2018, 87, 427–441 CrossRef CAS
.
- H. Ahmadizadegan and S. Esmaielzadeh, Organically modified clay as an enhancement filler in novel polyimide mixed matrix membranes for gas separation, Polym. Bull., 2024, 81(4), 3273–3296 CrossRef CAS
.
- L. Zhu and R. Zhu, Simultaneous sorption of organic compounds and phosphate to inorganic–organic bentonites from water, Sep. Purif. Technol., 2007, 54(1), 71–76 CrossRef CAS
.
- S. M. Lee and D. Tiwari, Porous hybrid materials in the remediation of water contaminated with As(III) and As(V), Chem. Eng. J., 2015, 270, 496–507 CrossRef CAS
.
- J. Ouyang,
et al., Polyethyleneimine (PEI) loaded MgO-SiO2 nanofibers from sepiolite minerals for reusable CO2 capture/release applications, Appl. Clay Sci., 2018, 152, 267–275 CrossRef CAS
.
- J. Cheng,
et al., Polyethyleneimine entwine thermally-treated Zn/Co zeolitic imidazolate frameworks to enhance CO2 adsorption, Chem. Eng. J., 2019, 364, 530–540 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma01246a |
| This journal is © The Royal Society of Chemistry 2025 |