 Open Access Article
Open Access ArticleExploring the effects of zirconium doping on barium titanate ceramics: structural, electrical, and optical properties
Suravi
Islam
 *a,
Mohammad Robel
Molla
a,
Nazia
Khatun
*a,
Mohammad Robel
Molla
a,
Nazia
Khatun
 a,
Nazmul Islam
Tanvir
a,
Mahmuda
Hakim
b and
Md. Saidul
Islam
a,
Nazmul Islam
Tanvir
a,
Mahmuda
Hakim
b and
Md. Saidul
Islam
 a
a
aIndustrial Physics Division, BCSIR Dhaka Laboratories, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh. E-mail: suravii@yahoo.com; suraviislambcsir@gmail.com
bBiomedical and Toxicology Research Institute (BTRI), BCSIR, Dhaka 1205, Bangladesh
First published on 24th January 2025
Abstract
In this study, polycrystalline BaZrxTi1−xO3 (x = 0.00, 0.05, 0.10, 0.15, and 0.20) ceramics were synthesized through a solid-state reaction technique. The effect of zirconium doping on the properties of barium titanate (BaTiO3) was investigated by various characterization tools. The structural and morphological properties of the synthesized materials were studied by X-ray diffraction (XRD), Raman spectroscopy, and field emission scanning electron microscopy (FE-SEM). The electrical properties of the Zr-doped BaTiO3 (BZT) materials were examined by impedance spectroscopy and the optical properties were investigated using UV-Vis-NIR spectroscopy. The XRD analyses of the prepared BZT materials revealed a single-phase tetragonal structure. The inclusion of Zr4+ in the BT matrix did not significantly affect the Raman-active modes, suggesting that the tetragonal crystal structure was retained in the as-synthesized samples. FE-SEM analyses revealed that the grain size initially increased from 49.36 nm to 53.24 nm for x = 0.05 wt% and then decreased gradually for higher concentrations up to x = 0.15 wt% (26.99 nm). The dielectric constant, dielectric loss, conductivity, and quality factor determined from the impedance data demonstrated that Zr4+ addition significantly influenced the electrical properties of BT. The band gap energy, Eg, of the synthesized samples were found in the range of 3.19–3.37 eV, which was calculated from the diffuse reflection data. The experimental results suggest that the BZT ceramic materials are suitable for energy storage device applications.
1. Introduction
Fabrication of lead (Pb)-free ceramic materials has attracted increasing attention from researchers over the last few decades. A lot of research is being conducted on lead-based ceramic materials for energy storage, piezoelectric actuators, ferroelectric random access memory, etc. owing to their outstanding performances.1,2 However, Pb-constituted materials are toxic and harmful to the environment. Barium titanate (BaTiO3, BT) is an excellent ferroelectric, dielectric, and piezoelectric material,3 which has numerous applications in multilayer ceramic capacitors (MLCCs), thermistors, transducers, thermal sensors, medical diagnostic transducers, and electroluminescent panels.4 Perovskite-type oxide materials are generally expressed as ABO3, among which BT has attracted great attention as a model perovskite material.5 BT can be synthesized by various routes, such as sol–gel synthesis,6 direct synthesis,7 hydrothermal synthesis,8 solid-state synthesis,9 and a sono-chemical process.10 Among other methods, high-energy ball-milling is a well-known affordable, eco-friendly, and convenient technique for the preparation of BT powders from low-cost raw substances. Since BT ceramics are traditionally obtained via sintering, they does not cover all the requirements for very thin dielectric layers owing to their irregular grain size and morphology, imperfect homogeneity and dispersion.11 Developing high-quality nano-sized BT materials, which are free of by-products, is still a challenging task for researchers.4 The electrical properties of BT ceramics can be increased by introducing rare earth metals, which are actually amphoteric dopants occupying two sites, A and B, following an A/B ratio, where oxygen is incorporated in the structure.12 It has been reported that doping a metal at the A and B sites leads to them acting as donors and acceptors, respectively, which can enhance or reduce the reliability of various devices.13 Kim reported that rare earth metals can dominantly and easily erect better shell and core–shell structures when they are inserted as dopants in to BT.14 Typically, the A site (Ba2+) of BT is normally substituted by Sr2+, Ca2+, Mg2+, etc.15–18 while the B site (Ti4+) is commonly substituted by Zr4+, Sn4+, Nb5+, etc.15,17 The ionic radius of chemically stable Zr4+ (0.72 Å) is comparatively larger than that of Ti4+ (0.605 Å), prompting zirconium to easily expand inside the lattice configuration18 and facilitating the substitution of Ti4+ by Zr4+. Our current research was focused on the solid-state synthesis of BT, the impacts of zirconium doping on BT, and investigation of the structural, electrical, and optical properties of the synthesized materials. In ceramics materials, rare earth elements have been shown to have significant stabilizing effects on the temperature dependence of the relative dielectric constant and lower dissipation capabilities of ceramics.5 Since BZT has less dielectric loss along with a larger dielectric non-linearity than Sr-doped BT, we chose the Z element to improve the dielectric performance of BT materials.There are many reports on Zr-doped BaTiO3, and most of these articles highlighted the dielectric temperature dependence.1,15,17 However, frequency-dependent dielectric studies, and the piezoelectric properties and optical properties of BZT have received less attention. Consequently, we paid special attention to the analysis of these properties for our synthesized samples. Our present study reports the synthesis of BT and Zr-doped BT with different compositions of Zr and starting constituents, which were pre-activated by ball-milling to obtain fine and by-product-free BT. The structural transformation from tetragonal to cubic, ferroelectric to paraelectric phase transformation, dielectric loss factor, dielectric constant, and bandgap energy were examined at different concentrations of Zr-doped BT ceramics, and the findings show that they may be suitable for optoelectronic devices.
2. Experimental
2.1. Materials and method
Barium carbonate; BaCO3 (CAS no. 513-77-9, 99% purity, China), titanium oxide; TiO2 (CAS no. 13463-67-7, 99% purity, Sigma-Aldrich, Germany), zirconium oxide; ZrO2 (CAS no. 1314-23-4, 99% purity, BDH, UK), and polyvinyl alcohol (CAS no. 9002-89-5, 98% purity, BDH, UK) were utilized without further purification. Pure and zirconium-doped barium titanate (BaTi1−xZrxO3; x = 0.00, 0.05, 0.10, 0.15, and 0.20) BT ceramic materials were developed through a solid-state reaction method utilizing a high-energy planetary ball-mill. Initially, three starting materials, namely barium carbonate, titanium oxide, and zirconium oxide, were pre-activated by ball-milling for 4 h at 150 rpm and room temperature. After pre-activation, the raw powders were weighed in certain proportions in line with the above-mentioned chemical formula, in which the ball (10 nm diameter) to powder weight ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The measured samples were mixed thoroughly and ball-milled for 4 h at a fixed 150 rpm, at room temperature. Then, the powder samples were pre-sintered at 700 °C for 3 h and milled for 30 min by hand. Subsequently, 4% polyvinyl alcohol aqueous solution was blended with the samples as a binder to form pellets. Specifically, they were pressed into disk-like pellets by applying a pressure of 2 ± 0.5 MPa for 1 min. Finally, the pellets were sintered at 1300 °C for 3 h in the air in a programmable muffle furnace (Nabertherm, Germany).
1. The measured samples were mixed thoroughly and ball-milled for 4 h at a fixed 150 rpm, at room temperature. Then, the powder samples were pre-sintered at 700 °C for 3 h and milled for 30 min by hand. Subsequently, 4% polyvinyl alcohol aqueous solution was blended with the samples as a binder to form pellets. Specifically, they were pressed into disk-like pellets by applying a pressure of 2 ± 0.5 MPa for 1 min. Finally, the pellets were sintered at 1300 °C for 3 h in the air in a programmable muffle furnace (Nabertherm, Germany).
2.2. Characterization
The synthesized samples BT and Zr-doped BT (BZT) were investigated by different characterization techniques. For structural analysis, X-ray diffraction (XRD) (Rigaku Smart Lab, Japan), with Cu-Kα radiation (λ = 1.5406 Å), a scanning rate of 10° min−1, 40 kV power at 40 mA, and 0.02° steps, was used to confirm the crystallinity of the synthesized samples at room temperature. The optical properties were observed using an ultraviolet-visible-near-infrared (UV-vis-NIR) spectrophotometer (UV-2600, Shimadzu, Japan) with a wavelength range of 220–1400 nm. Field emission scanning electron microscope (FE-SEM) was performed on a JSM-7610F system (Japan). Also, energy-dispersive X-ray spectroscopy (EDX) was utilized to analyze the surface morphology and identify the elemental compositions of the BT and Zr-doped BT materials. The average grain sizes of the synthesized samples were explored using the linear intercept method. ImageJ and Origin Pro software were used to attain the grain-size distribution. Raman spectroscopy (Macro RAM, Japan) was used for studying the structural fingerprints. The piezoelectric constant, d33, was determined using a quasi-static piezoelectric constant testing meter (ZJ-3A, China). Silver paste (D. Leipziger Str. 10, Germany) was painted as a conductive layer on the surface of the pellet samples to be used as electrodes for measuring the dielectric properties. Impedance analysis (Agilent 4294A, 40 kHz to 120 MHz) was used to determine the electrical parameters.3. Results and discussion
3.1. Structural properties
X-Ray diffraction (XRD) analysis was conducted to derive the crystallinity and phase purity of the zirconium-doped BT (BaZrxTi1−xO3) at different doping concentrations, i.e., at x = 0.00, 0.05, 0.10, 0.15, and 0.20. The XRD patterns of the pure and doped barium titanate ceramics obtained between scan angles of 20° and 80° at room temperature are shown in Fig. 1. These reveal that all the samples were crystallized into a single-phase perovskite structure with the tetragonal space group (P4mm) made by one molecule per unit cell. Moreover, the pattern of the synthesized samples well-matched JCPDS card no. # 05-0626 showing they followed a phase structure of the typical perovskite (ABO3), and indicating Zr4+ had diffused successfully into the lattice of the BaTiO3 matrix to form a stable solid solution. Moreover, Zr-doped BT can have tetragonal, rhombohedral, or an orthorhombic structure at room temperature dependent on the concentration of Zr, as reported in earlier studies.19,20The diffraction peaks observed at 2θ = 22.22°, 31.48°, 38.88°, 45.36°/44.88°, 50.97°, 56.25°, and 65.73° corresponding to the Bragg reflection planes (100), (110), (111), (002)/(200), (210), (211), (220), respectively, matched with those previously reported.20,21
The lattice parameters were calculated using the following equation:
The average crystalline sizes (t) of the samples were measured for the (002) and (200) reflections using the Debye–Scherrer equation,22 as follows:
| Samples BaZrxTi1−xO3 | Lattice parameter “a = b” (Å) | Lattice parameter “c” (Å) | Tetragonality (c/a) | Cell volume V (Å3) | Crystalline size, D (nm) | Strain, ε | ||
|---|---|---|---|---|---|---|---|---|
| Debye–Scherrer method | Williamson–Hall method | Debye–Scherrer method (×10−3) | Williamson–Hall method (×10−3) | |||||
| BaTiO3 | 3.9954 | 4.0359 | 1.0101 | 64.426 | 56.50 | 44.27 | 2.262 | 0.60 |
| BaZr0.05Ti0.95O3 | 4.0039 | 4.0224 | 1.0046 | 64.484 | 49.30 | 12.59 | 2.589 | 3.45 |
| BaZr0.10Ti0.90O3 | 4.0139 | 4.0241 | 1.0025 | 64.834 | 39.22 | 68.93 | 3.263 | 2.98 |
| BaZr0.15Ti0.85O3 | 4.0224 | 4.0309 | 1.0021 | 65.219 | 45.39 | 167.50 | 2.827 | 3.12 |
| BaZr0.20Ti0.80O3 | 4.03838 | 4.0376 | 0.9998 | 65.845 | 29.43 | 28.19 | 4.366 | 3.25 |
The magnified XRD pattern at around 2θ = 31° in Fig. 1(b) shows that the (110) peaks were shifted to lower theta values with the higher concentration of zirconium doping. In Fig. 1(c), the splitting of a certain peak into (002) and (200) planes at around 45° provides strong indication of the tetragonal structure of pure BT.22 The peaks were shifted to lower angles with increasing the Zr content and showed the transformation from the tetragonal phase to the cubic phase.21
The lattice parameters a and c of tetragonal BT increased with the Zr doping, owing to the substitution of the smaller Ti4+ (radius = 0.605 Å) by the larger radius (0.72 Å) Zr4+. Therefore, the Zr atom's ionic and atomic sizes are greater than those of titanium atoms due to its electronic configuration and electron cloud density. The interplanar spacing of the crystal may be increased with the increment in the lattice parameters, so there would be a rise in the unit cell volume.22 Indeed, the unit cell volume increased with the rise in the content of Zr because of the increase in the lattice parameters, and hence θ (the angle) decreased. Consequently, a shift towards lower 2θ was obtained for the most intense peak, as illustrated in Fig. 1(b), which also matched with the findings reported in ref. 22. In Fig. 1(c), it can be inferred that the (002) peak tended to shrink as the zirconium concentration rose, which increased the tetragonality since Ti4+ has a smaller radius than Zr4+.
Fig. 1(a)–(c) demonstrate that, as the Zr4+ concentration increased, the lattice parameter (a) increased linearly in accordance with Vegard's law, while the lattice parameter (c) increased through a different trend. In addition, the tetragonal symmetry (c/a) was >1 for all the samples, indicating that the elongation of the (TiO6) octahedron occurred along the Z-axis, where the symmetry remained the same.11,12 The XRD patterns analysis indicated that the splitting in the (001) and (100) reflection declined with the increase in the Zr4+ ions content, indicating the steady phase transition from the tetragonal to pseudo-cubic structure. This effect also agreed with the report of Yao et al., where, beside XRD confirmation, it was also confirmed from the Raman spectra; whereby the Raman intensity of BZT decreased gradually and the peaks turned into weak and wider, revealing that there was a slight structural reordering, like phase transition from tetragonal to cubic.6 Furthermore, it is evident from Table 1 that the crystallite size showed a decreasing trend with increasing the Zr4+ ions content.
The Williamson–Hall method was utilized to calculate the crystallite sizes (D) of the samples, also considering the X-ray diffraction peak widening βhkl (FWHM), which can be given by the following:23
| βhkl = βsize + βstrain |
This relation can be modified by
or,
where λ is the wavelength of Cu-Kα radiation, D is the crystallite size, βhkl is the full width at half maximum, and
 denotes the micro-strain of the synthesized samples. The W–H plots of βhkl
denotes the micro-strain of the synthesized samples. The W–H plots of βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ against 4
θ against 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ for the BZT samples are demonstrated in Fig. 2. The average D and
θ for the BZT samples are demonstrated in Fig. 2. The average D and  values were calculated based on the linear line's slope and y-intercept, and the results are summarized in Table 1. The crystallite size and micro-strain of all the synthesized samples followed the same pattern when compared to the Debye–Scherrer and W–H analyses,24 and were associated with peak broadening. The Goldschmidt tolerance factor, t, is a geometrical factor that has received widespread acceptance to measure the formation of perovskite structures and indicates their stability. It extends the range of what is predicted to be appropriate for A site cations inside cavities.
values were calculated based on the linear line's slope and y-intercept, and the results are summarized in Table 1. The crystallite size and micro-strain of all the synthesized samples followed the same pattern when compared to the Debye–Scherrer and W–H analyses,24 and were associated with peak broadening. The Goldschmidt tolerance factor, t, is a geometrical factor that has received widespread acceptance to measure the formation of perovskite structures and indicates their stability. It extends the range of what is predicted to be appropriate for A site cations inside cavities.
 | ||
| Fig. 2 Williamson–Hall plots of the BaZrxTi1−xO3 ceramics for varied concentrations, x = 0.00, 0.05, 0.10, 0.15, and 0.20. | ||
The Goldschmidt's tolerance factor of the synthesized samples was measured conforming to the following general principle:24
| Sample BaZrxTi1−xO3 | Molecular weight (g) | Cell volume (cm3) ×10−23 | Tolerance factor (t) | d-spacing (Å) | Average grain size (nm) | Piezoelectric coefficient, d33 (pC N−1) | Bandgap energy, Eg (eV) |
|---|---|---|---|---|---|---|---|
| x = 0.00 | 233.192 | 6.4426 | 1.0763 | 2.8396 | 383 ± 100 | 124 | 3.20 |
| x = 0.05 | 235.360 | 6.4484 | 1.0731 | 2.8369 | 367 ± 114 | 174 | 3.19 |
| x = 0.10 | 237.528 | 6.4834 | 1.0699 | 2.8477 | 220 ± 35 | 130 | 3.33 |
| x = 0.15 | 239.696 | 6.5219 | 1.0667 | 2.8507 | 109 ± 58 | 139 | 3.32 |
| x = 0.20 | 241.864 | 6.5845 | 1.0636 | 2.8587 | 425 ± 95 | 136 | 3.38 |
3.2. Raman analysis
Raman spectroscopy is a potent technique to elucidate the structural information and chemical fingerprint of a material. In order to gain clear insights into BT and doped BZT, we performed Raman analysis. Fig. 3 outlines the Raman spectra of BaTi1−xZrxO3 (x = 0.00, 0.05, 0.10, 0.15, and 0.20) sintered at 1300 °C for 3 h. Three remarkable peaks could be observed at 306 cm−1 [B1, E (TO + LO)], 517 cm−1 [A1 (TO), E (TO),] and 716 cm−1 [A1 (LO), E (LO)], indicating the formation of tetragonal BT.26,27 BT has one molecule (5 atoms) per unit cell in both the paraelectric and ferroelectric phases, resulting in 12(3 × 5 − 3) long-wavelength optical modes.The optical modes changed in tandem with 3F1u + F2u during the paraelectric phase (Pm3m symmetry). Since the F1u modes in the paraelectric phase of BT were only infrared (IR) active and the F2u mode was mute, there was no Raman-active mode in this phase. Every F1u mode split into an A1 mode (nondegenerate) and an E mode (doubly degenerate) in the ferroelectric phase with the tetragonal structure (P4mm symmetry), while the F2u mode split into E and B1 modes. In addition, the B1 mode was exclusively Raman active, while the A1 and E modes were both Raman and infrared active. Furthermore, because of the long-range electrostatic interactions associated with lattice ionicity, the A1 and E optical modes further split into longitudinal (LO) and transverse (TO) modes.27
It was observed that above 716 cm−1, the intensities of the peaks gradually declined with the enhancement of x (x > 0.05), indicating the phase transition from the quadratic to pseudo-cubic phase.28 The peaks at 716 cm−1 were broadened gradually and shifted to lower λ as well. The band intensity at 306 cm−1 was reduced with increasing the doping percentage, and it was estimated that the replacement of Ti by Zr did not entirely destroy the long-range order of BT. Again, Raman shifts towards lower wavelength were observed for higher concentrations of Zr in BT. The small peak at 262 cm−1 [A1 (TO), E (TO)] for x = 0.00 suggested the quadratic phase of BT.29 No remarkable peak was observed at 195 cm−1 for pure BT, but for x = 0.05 and x = 0.20, sharp and broad peaks were detected, clearly demonstrating the impact of zirconium. This was also indicated by the formation of small crystals. Anti-resonance was observed by the characteristic band at ∼306 cm−1 of ferroelectric BT, which suggested that the local structure of the BO6 octahedron departed from a proper cubic symmetry.
3.3. Micro-structural analysis
The FE-SEM images of BZT ceramics with various compositions, x = 0.00, 0.05 0.10 0.15, and 0.20, are displayed in Fig. 4(a)–(e), respectively. The surface morphology and the microstructure of all the samples illustrated in the micrographs showed homogenous morphologies without any chemical contrast among the grains. It could also be observed that the grain boundaries were vibrant, while the full formation of the grain structure indicated that crystals had formed. | ||
| Fig. 4 FE-SEM micrographs of the synthesized BZT ceramics with different compositions: (a) x = 0.00, (b) x = 0.05, (c) x = 0.10, (d) x = 0.15, and (e) x = 0.20. | ||
The average grain size of each sample was analyzed by imageJ software and the measured sizes are given in Table 2. The average grain size of BT was ∼383 nm, with some small pores, as shown in Fig. 4(a). However, the average grain size was decreased substantially because of doping zirconium ions at a certain function of x.
The aggregation of the grains and migration of the grain boundary enabled the BZT sample to develop rapidly in the sintering temperature zone, resulting in greater-sized grains. As a result, defects in the grain growth process were more likely to occur.29Fig. 4(c) shows the formation of tiny pores on the surface of the crystal grains. As the Zr content increased, the average grain distribution became more uniform and compact, and the ceramic sample structure became denser, which might have been a result of the liquid-phase sintering effect.
Fig. 5 show the grain-size distribution of each composition for x = 0.00, 0.05, 0.10, 0.15, and 0.20 respectively. After loading Zr ions, the particle size reduction may be the reason for the accumulation of Zr4+ to approximately the grain edge, which occurred gradually for the compositions up to x = 0.15. Afterwards, the average grain size increased again for x = 0.20, as shown in Fig. 4(e), may have been due to the coagulation of Zr ions and a mass-transfer process, which was partially responsible for the grain growth during the sintering process.30
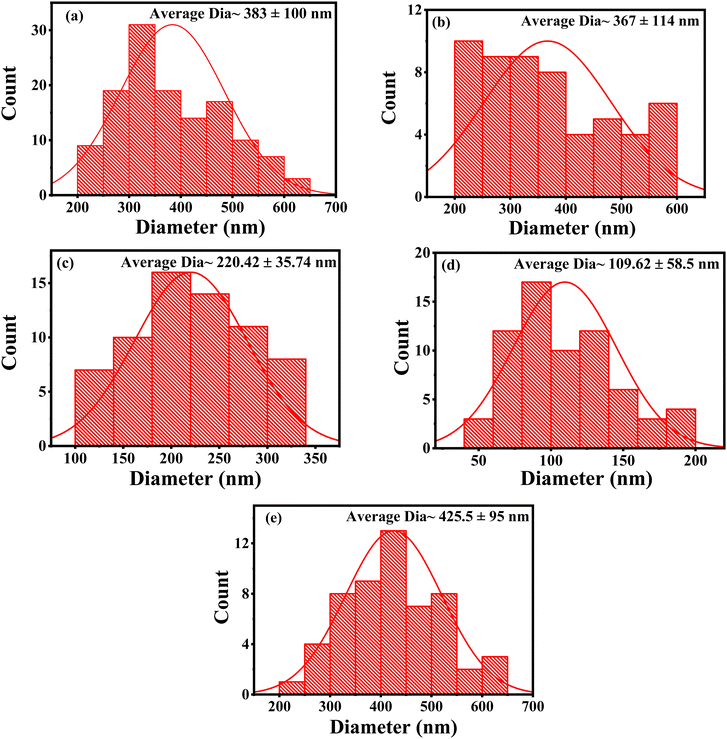 | ||
| Fig. 5 Grain size distribution of synthesized BZT ceramics with different compositions (a) x = 0.00; (b) x = 0.05; (c) x = 0.10; (d) x = 0.15; and (e) x = 0.20 from FE-SEM images. | ||
3.4. Electrical properties
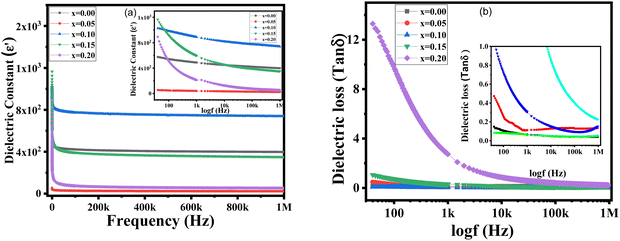 | ||
Fig. 6 Frequency-dependent dielectric properties of BaZrxTi1−xO3 ceramics: (a) dielectric constant; (b) dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ). The inset shows the enlarged view. δ). The inset shows the enlarged view. | ||
Fig. 6(b) displays the arcs for the frequency-dependent dielectric loss of the synthesized BZT at room temperature. A high dielectric constant value could be observed at low frequencies, which decreased as the frequency increased for all the synthesized samples. This occurred due to polarization in the dielectric material, which is caused by ionic, dipolar, electronic, and space charge contributions.32 It is known that a lower frequencies, all forms of polarization play a role in polarization, but as the frequency rises, the influence of the different types of polarization diminishes. At higher frequencies, dipolar and electronic polarization are the main contributors to polarization. Grain boundaries are the only places where space charge accumulation is possible at low frequencies.33 In addition, the dielectric constant falls as the frequency increases because the space charges are less cable to follow the direction of the field. Only the inherent characteristics of the material will be seen when the space charges fail at a specific high frequency. Thus, it was evident here that the space charge-induced polarization was not effective above 1 MHz frequency and that all the space charges were capable of relaxing.
Moreover, there was a non-polar effect at room temperature, such that ε′ also decreased with the doping concentration of zirconium ions (x = 0.05, 0.15, and 0.2), but it showed a maximum increase at x = 0.10, which may have been because of the lower Curie temperature.31 In the lower frequency range in Fig. 6(a), it can be seen that the value of the dielectric constant was a maximum for the x = 0.10 concentration. However, the dielectric change appeared to be negligible with the increase in frequency and was reduces by only a very small amount. The greater ionic radius pf Zr4+ substituted by Ti4+ could be affected the arrangement of the B-sites in the oxygen octahedron. This is why, the B–O bond may become weaker, and as a result the phase-transition temperature would drop. The dielectric constant is known to be influenced by the density, tetragonality, and particle size distribution. The difference in grain-size distribution could account for the modest change seen in the dielectric constant.34Fig. 6(b) displays the dielectric loss of the examined samples at room temperature, showing that the loss generally decreased at higher frequencies and there was negligible change. Moreover, the larger ionic radius of Zr4+ widened the lattice, caused electronic hopping to produce oxygen vacancies, and dielectric loss, thus reducing the conductivity of BZT. It was also observed that the permittivity gradually rose as the Zr content was increased, whereas, the tetragonality declined as the higher zirconium content put more mechanical stress on the structure.35 This resulted in the formation of oxygen vacancies, porosity, and an irregular structure, thus reducing the dielectric characteristics and increasing the dielectric loss tangent. As seen in Fig. 6(b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ experienced a declining trend with increasing frequency.
δ experienced a declining trend with increasing frequency.
 | ||
| Fig. 7 Frequency versus (a) conductivity and (b) impedance of the synthesized BZT samples; insets show magnified views of the respective plots. | ||
The effect of Zr doping on the real component of impedance varied with the frequency dependency of the BZT samples, as shown in Fig. 7(b). It was observed that the real part of the impedance monotonically declined until the value became constant. The tendency toward merging at higher frequencies, regardless of temperature, was caused through the discharge of the space charge or by the continuous decrease in the barrier characteristics.25,37
3.5. Optical properties
Fig. 8 displays the UV-Vis absorbance spectra of the pure and zirconium-doped BT samples versus the incident photons’ wavelength. The absorption band was observed in the wavelength range from 220 nm to ∼380 nm. Even though all the samples exhibited maximum absorption at approximately 330 nm wavelength, the absorbance increased with the increase in the Zr4+ content in the structure, with pure barium titanate achieving the lowest absorption. | ||
| Fig. 8 UV-Vis absorption spectra of BaTi1−xZrxO3 ceramics versus the wavelength of incident photons. | ||
The optical band gap energy (Eg) of BT and BZT were measured from the Tauc plot of the UV-Vis-diffuse reflectance spectroscopy data, using the Kubelka–Munk (K–M) approach, which can be denoted by the following formula:40
where hν, Eg, and n are the photon energy, band gap energy, and type of band transition, respectively. In this current work, Tauc's plots for the samples were drawn and the results are presented in Table 2.
The band gap energies of BT and BT were evaluated from the intercept of the photon energy axis achieved through extrapolating the plot on the energy axis [photon energy versus (αhν)2] depicted in Fig. 9. The value of n = 2 and n = 1/2 were used for the indirect and direct Eg. Fig. 9 shows that the increase in Zr content in the BT structure caused a rise in the band gap energy, except for x = 0.05, 3.190 eV, whereby a value of 3.20 eV was attained for pure BaTiO3. The band gap for the x = 0.05 sample was shifted to lower energy, which might be a result of the stress induced by the introduction of zirconium into the structure of the barium titanate. The symmetry is broken in the structure owing to the presence of [ZrO6] clusters.42 However, for the samples x = 0.10, x = 0.15, and x = 0.20, the Eg values were shifted to higher energies (3.33, 3.32, and 3.38 eV, respectively) compared to pure BT. Increasing the doping concentrations led to an increase in Eg, which may have been caused by a variety of reasons, one of which being the contribution of Zr ions’ third orbitals, an increase in interatomic spacing that coincided with the lattice volume growth, or an increase in oxygen vacancies, which in turn would produce an increase in electrons residing in the minimum states near the edge of the conduction band.43 It is suggested that different factors, such as the crystalline size, structural parameter, and presence of impurities, may responsible for the change in Eg; however, the increase in Eg here may be ascribed to the smaller crystallite size with the increase in Zr4+ content in BT.
 | ||
| Fig. 9 (a) UV-Visible spectra of BZT powders with different compositions and (b)–(f) Tauc plots for extraction of the optical bandgap from the reflectance data. | ||
4. Conclusions
In this research, BaZrxTi1−xO3 (x = 0.00, 0.05, 0.10, 0.15, and 0.20) materials were successfully synthesized by a solid-state reaction method and the effect of Zr4+ doping on the properties of BaTiO3 was investigated. The substitution of Zr4+ into the B-sites of BaTiO3 resulted in an increase in the lattice parameters due to the larger ionic radii of Zr4+ (0.72 Å) compared to the ionic radii of Ti4+ (0.6 Å). In addition, upon Zr doping, the peak at 45.5° was shifted to a lower angle of 45°, owing to tetragonal to cubic structural phase transition. Furthermore, Raman analysis demonstrated that doping resulted in an increase in the line width and a decrease in the magnitudes of the distinctive peaks. The peak at 305 cm−1 associated with the tetragonal structure declined, owing to the phase transition from tetragonal to cubic and this was confirmed by the XRD patterns as well. FE-SEM revealed the grains were uniformly distributed and that the grain size decreased until the x = 0.20 composition. The dielectric properties were enhanced, and were found to be a maximum for x = 0.10. The energy band gap increased with respect to the doping rate. For the sample BaTi0.8Zr0.2O3, the greatest absorption was noted and the band gap energy was improved with increasing the Zr4+ ion doping in the prepared perovskite materials, exhibiting an indirect allowed transition. It was also observed that the loss tangent values were relatively minimal while the dielectric constant was rather high. According to the conductivity tests, the sample was primarily insulating. Studies on impedance were effectively used to highlight the conduction mechanism in a sample, and it was determined that the sample was a suitable dielectric material that can be used in electronic device applications for energy-storage applications, or in multilayer ceramic capacitors.Data availability
The data supporting this study have been included in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bangladesh Council of Science and Industrial Research (BCSIR), Dr Qudrat-I-Khuda Road, Dhanmondi, Dhaka-1205, Bangladesh and Ministry of Science and Technology, Dhaka, Bangladesh.References
- Z. Sun, L. Li, H. Zheng, S. Yu and D. Xu, Effects of sintering temperature on the microstructure and dielectric properties of BaZr0.2Ti0.8O3 ceramics, Ceram. Int., 2015, 41, 12158–12163, DOI:10.1016/j.ceramint.2015.06.035.
- S. Islam, S. A. Satter, N. Khatun, M. S. Hossain, S. F. U. Farhad, P. Bala, S. Tabassum and A. Siddika, Investigation of Structural, Dielectric and Electrical Properties of Barium Titanate Ceramics Co-Doped with Bismuth and Yttrium, J. Mol. Eng. Mater., 2019, 1–9, DOI:10.1111/j.1151-2916.1999.tb01921.x.
- S. Sharma, K. Shamim, A. Ranjan, R. Rai, P. Kumari and S. Sinha, Impedance and modulus spectroscopy characterization of lead free barium titanate ferroelectric ceramics, Ceram. Int., 2015, 41, 7713e7722, DOI:10.1016/j.ceramint.2015.02.102.
- R. Ashiri, On the solid-state formation of BaTiO3 nanocrystals from mechanically activated BaCO3 and TiO2 powders: innovative mechanochemical processing, the mechanism involved, and phase and nanostructure evolutions, RSC Adv., 2016, 6, 17138, 10.1039/C5RA22942A.
- T. Badapand, V. Senthil, S. Panigrahi and S. Anwar, Diffuse phase transition behavior of dysprosium doped barium titanate ceramic, J. Electroceram., 2013, 31, 55–60, DOI:10.1007/s10832-013-9808-x.
- S. Yoon, S. Baik, M. G. Kim, N. Shin and I. Kim, Synthesis of tetragonal barium titanate nanoparticles via Alkoxide-Hydroxide sol-precipitation: effect of water addition, J. Am. Ceram. Soc., 2007, 90, 311e314, DOI:10.1111/j.1551-2916.2006.01361.x.
- J. Q. Qi, T. Peng, Y. M. Hu, L. Sun, Y. Wang, W. P. Chen, L. T. Li, C. W. Nan and H. L. W. Chan, Direct synthesis of ultrafine tetragonal BaTiO3 nanoparticles at room temperature, Nanoscale Res. Lett., 2011, 6, 466, DOI:10.1186/1556-276X-6-466.
- X. Zhu, J. Zhu, Sh Zhou, Zh Liu and N. Ming, Hydrothermal synthesis of nanocrystalline BaTiO3 particles and structural characterization by high-resolution transmission electron microscopy, J. Cryst. Growth, 2008, 310, 434–441, DOI:10.1016/j.jcrysgro.2007.10.076.
- S. Islam, A. Siddika, N. A. Ahmed, N. Khatun and S. N. Rahman, Synthesis and Characterization of Bismuth doped Barium Titanate, Am. Int. J. Res. Sci., Technol. Eng. Math., 2016, 13(1), 28–32 Search PubMed.
- K. Yasui, T. Tuziuti and K. Kato, Numerical simulations of sonochemical production of BaTiO3 nanoparticles, Ultrason. Sonochem., 2011, 18(5), 1211–1217, DOI:10.1016/j.ultsonch.2011.03.006.
- R. Ashiri, Analysis and Characterization of Phase Evolution of Nanosized BaTiO3 Powder Synthesized Through a Chemically Modified Sol–Gel Process, Metall. Mater. Trans. A, 2012, 43, 4414–4426, DOI:10.1007/s11661-012-1242-1.
- Y. Tsur, A. Hitomi, I. Scrymgeour and C. Randall, Site Occupancy of Rare-Earth Cations in BaTiO3, Jpn. J. Appl. Phys., 2001, 40(1R), 255, DOI:10.1143/JJAP.40.255.
- D.-H. Kuo, C.-H. Wang and W.-P. Tsai, Donor- and acceptor-co substituted BaTiO3 for nonreducible multilayer ceramic capacitors, Ceram. Int., 2006, 32(1), 1–5, DOI:10.1016/j.ceramint.2004.11.015.
- C. H. Kim, K. J. Park, Y. J. Yoon, M. H. Hong, J. O. Hong and K. H. Hur, Role of Yttrium and Magnesium in the Formation of Core–Shell Struc-ture of BaTiO3 Grains in MLCC, J. Eur. Ceram. Soc., 2008, 28(6), 1213–1219, DOI:10.1016/j.jeurceramsoc.2007.09.042.
- Q. Xu and Z. Li, Dielectric and ferroelectric behavior of Zr-doped BaTiO3 perovskites, Process. Appl. Ceram, 2020, 14(3), 188–194, DOI:10.2298/PAC2003188X.
- B. W. Ricketts, G. Triani and A. D. Hilton, Dielectric energy storage densities in Ba1−xSrxTi1−yZryO3 ceramics, J. Mater. Sci. Mater. Electron., 2000, 11, 513–517, DOI:10.1023/A:1008924703491.
- P. Julphunthong, S. Chootin and T. Bongkarn, Phase formation and electrical properties of Ba(ZrxTi1−x)O3 ceramics synthesized through a novel combustion technique, Ceram. Int., 2013, 39(1), S415–S419, DOI:10.1016/j.ceramint.2012.10.105.
- S. Islam, A. Siddika, N. A. Ahmed, N. Khatun and S. N. Rahman, Structural, Dielectric and Electric Properties of Manganese-Doped Barium Titanate, Int. J. Nanoelectron. Mater., 2018, 11, 419–426 Search PubMed.
- A. Mandal, D. Yadav, S. K. Mittal, U. Jamwal, D. Kaneria, A. Khokhar, M. Jakhar and K. L. Yadav, Investigation of structural, morphological, dielectric, optical, and ferroelectric-energy storage properties of [(1 − x)BCT−(x)BZT] electroceramics, J. Alloys Compd., 2024, 995, 174817, DOI:10.1016/j.jallcom.2024.174817.
- E. H. Yahakoub, A. Bendahhou, K. Chourti, F. Chaou, I. Jalafi, S. E. Barkany, Z. Bahari and M. A. Salama, Structural, electrical, and dielectric study of the influence of 3.4% lanthanide (Ln3+ = Sm3+ and La3+) insertion in the A-site of perovskite Ba0.95Ln0.034Ti0.99Zr0.01O3, RSC Adv., 2022, 12, 33124, 10.1039/d2ra06758g.
- V. S. Puil, D. K. Pradhan, B. C. Riggs, S. Adireddy, R. S. Katiyar and D. B. Chrisey, Synthesis and characterization of lead-free ternary component BST-BCT-BZT ceramic capacitor, J. Adv. Dielectr., 2014, 4(2), 14500143, DOI:10.1142/S2010135X14500143.
- S. Islam, N. Khatun, M. S. Habib, S. F. U. Farhad, N. I. Tanvir, M. A. A. Shaikh, S. Tabassum, D. Islam, M. S. Hossain and A. Siddika, Effects of yttrium doping on structural, electrical and optical properties of barium titanate ceramics, Heliyon, 2022, 8(9), E10529, DOI:10.1016/j.heliyon.2022e10529.
- E. Hannachi, M. A. Almessiere, Y. Slimani, R. B. Alshamrani, G. Yasin and F. Ben Azzouz, Preparation and characterization of High-Tc (YBa2Cu3O7−δ)1−x/(CNTs)x superconductors with highly boosted superconducting performances, Ceram. Int., 2020, 47(16), 23539–23548, DOI:10.1016/j.ceramint.2021.05.071.
- A. Nfissi, Y. Ababou, M. Belhajji, S. Sayouri and T. Lamcharfi, Structural and dielectric properties of sol–gel processed Ce-doped BaTi0.97Y0.03O3 ceramics, J. Adv. Dielectr., 2021, 11(1), 2150003, DOI:10.1142/S2010135X2150003X.
- P. Arpita and K. Rajesh Kumar, Hafnium (Hf) doped BaTiO3: A study of structural and electrical properties for device application feasibility, Int. J. Novel Res. Dev., 2024, 9(4), b604–b616 Search PubMed.
- H. Z. Akbas, Z. Aydin, F. Guder and S. Turgut, Accelerated formation of BaTiO3 ceramics with mechano-chemical processing in different liquids, J. Alloys Compd., 2017, 699, 87e91, DOI:10.1016/j.jallcom.2016.12.369.
- N. K. Karan, R. S. Katiyar, T. Maiti, R. Guo and A. S. Bhalla, Raman spectral studies of Zr4+-rich BaZrxTi1−xO3 (0.5 ≤ x ≤ 1.00) phase diagram, J. Raman Spectrosc., 2009, 40(4), 370–375, DOI:10.1002/jrs.2134.
- A. El ghandouri, S. Sayouri, T. Lamcharfi and L. Hajji, Effect of strontium on the structural and piezoelectric properties of the sol gel processed barium titanate, J. Mater. Environ. Sci., 2017, 8(S), 4945–4962 Search PubMed.
- C. Q. Wang, C. Shu and D. Y. Zheng, et al., Effects of Li and Y co-doping on electrical properties and dielectric relaxation behavior of BCZT ceramics, J. Mater. Sci.: Mater. Electron., 2022, 33, 3822–3834, DOI:10.1007/s10854-021-07573-z.
- K. Xi, Y. Li, Z. Zheng, L. Zhang, Y. Liu and Y. Mi, Microstructure, dielectric properties, relaxation behavior, and ferroelectric properties of Gd-doped lead-free BZT ceramics by sol–gel process, J. Mater. Sci.: Mater. Electron., 2020, 24, 23044–23051, DOI:10.1007/s10854-020-04832-3.
- M. Graca, M. A. Valente and M. G. Ferreira da Silva, Electrical properties of lithium niobium silicate glasses, J. Non-Cryst. Solids, 2003, 325(1–3), 267–274, DOI:10.1016/S0022-3093(03)00314-4.
- P. Bergo, W. M. Pontuschka and J. M. Prison, Dielectric properties of P2O5-Ni2O–Li2 glasses containing WO3, CoO or Fe2O3, Solid State Commun., 2007, 141(10), 545–547, DOI:10.1016/j.ssc.2006.12.024.
- R. Tomar, R. Pandey, N. B. Singh, M. Kumar Gupta and P. Gupta, Electrical properties of barium titanate in presence of Sn2+ dopant, SN Appl. Sci., 2020, 2, 226, DOI:10.1007/s42452-020-2017-8.
- N. Gouitaa, L. Taj-Dine, A. Farid and A. Fatima Zahra, Structural, Dielectric and Electrical Properties of Modified BaTi0.80Fe0.20O3 Ceramics by Zr Addition in Ti Site at X = 0.00 to 0.10, Iran. J. Mater. Sci. Eng., 2021, 18(3), 1–12 Search PubMed.
- L. Xu and Y. Xu, Effect of Zr4+ content on crystal structure, micromorphology, ferroelectric and dielectric properties of Ba(ZrxTi1−x) O3 ceramics, J. Mater. Sci.: Mater. Electron., 2020, 31, 5492–5498, DOI:10.1007/s10854-020-03114-2.
- I. Ullah, M. Ur Rehman, A. Manan, M. T. Lanagan, R. Wali Khan, A. Ullah, S. Wali Ullah and M. Uzair, Structural, dielectric, electrical, and energy storage properties of Mn-doped Ba0.55Sr0.45TiO3 ceramic, Int. J. Appl. Ceram. Technol., 2024, 21(5), 1–9, DOI:10.1111/ijac.14788.
- S. Mahajan, O. P. Thakur, P. Chandra and K. Sreenivas, Effect of Zr on dielectric, ferroelectric and impedance properties of BaTiO3 ceramic, Bull. Mater. Sci., 2011, 34, 1483–1489, DOI:10.1007/s12034-011-0347-2.
- T. Badapanda, S. Sarangi, B. Behera, S. Anwar, T. P. Sinha, R. Ranjan, G. E. Luz Jr., E. Longo and L. S. Cavalcante, Structural refinement, optical and electrical properties of [Ba1−xSm2x/3] (Zr0.05Ti0.95)O3 ceramics, J. Mater. Sci.: Mater. Electron., 2014, 25, 3427–3439, DOI:10.1007/s10854-014-2035-7.
- M. M. Salem, M. A. Darwish, A. M. Altarawneh and Y. A. Alibwaini, et al., Investigation of the structure and dielectric properties of doped barium titanates, RSC Adv., 2024, 14, 3335–3345, 10.1039/D3RA05885A.
- N. Khatun, S. Ahmed, M. S. Hossain, S. F. U. Farhad, M. A. Mamun, M. S. Alam, M. H. A. Begum, N. I. Tanvir, M. Hakim and S. Islam, Influence of Y3+and La3+ ions on the structural, magnetic, electrical, and optical properties of cobalt ferrite nanoparticles, Heliyon, 2023, 9, e13019, DOI:10.1016/j.heliyon.2023.e13019.
- T. Chen, J. Meng, S. Wu, J. Pei, Q. Lin, J. Wei, X. Wei, J. Li and Z. Zhang, Room temperature synthesized BaTiO3 for photocatalytic hydrogen evolution, J. Alloys Compd., 2018, 754, 184, DOI:10.1016/j.jallcom.2018.04.300.
- L. S. Cavalcante, V. M. Longo, M. Zampieri, J. W. M. Espinosa, P. S. Pizani, J. R. Sambrano, J. A. Varela, E. Longo, M. L. Simões and C. A. Paskocimas, Experimental and theoretical correlation of very intense visible green photoluminescence in BaZrO3 powders, J. Appl. Phys., 2008, 103, 063527 CrossRef.
- M. Reda, E. E. Ateia, S. I. El-Dek and M. M. Arman, New insights into optical properties, and applications of Zr-doped BaTiO3, Appl. Phys. A: Mater. Sci. Process., 2024, 130, 240, DOI:10.1007/s00339-024-07381-2.
| This journal is © The Royal Society of Chemistry 2025 |










