Open Access Article
Open Access ArticleAnalysis of Pb(II) in wheat grain, cow urine and squid samples using modified novel TSDB incorporated MWCNTs
Jayagopi
Gayathri
 *a,
Sivakumar
Sivalingam
*a,
Sivakumar
Sivalingam
 a and
Kumar
Sangeetha Selvan
a and
Kumar
Sangeetha Selvan
 *b
*b
aDepartment of Chemistry, VelTech Rangarajan Dr. Sagunthala R & D Institute of Science and Technology, Avadi, Chennai, Tamil Nadu 600 062, India. E-mail: drgayathrij@veltech.edu.in
bDepartment of Chemistry, Anna Adharsh College for Women, Anna Nagar, Chennai, Tamil Nadu 600040, India. E-mail: msc.sangi@gmail.com
First published on 2nd December 2024
Abstract
To fabricate a selective lead (Pb(II)) ion sensor, a slurry of the synthesized N,N′,N′′,N′′′-tetrasalicylidene-3,3′-diaminobenzidine (TSDB) ligand was deposited on multiwalled carbon nanotubes (MWCNTs)/paraffin graphite electrode (PGE). The ligand (octadentate) was easily synthesized using 3,3′-diaminobenzidine and salicylaldehyde. The TSDB ligand that was synthesized was verified using Fourier transform infrared (FT-IR) spectroscopy, proton nuclear magnetic resonance (1H-NMR) spectroscopy and carbon-13 nuclear magnetic resonance (13C-NMR) spectroscopy. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDAX) were used to analyze the surface morphology of Pb(II)-TSDB/MWCNTs/PGE, TSDB/MWCNTs/PGE, MWCNTs/PGE, and PGE. TSDB/MWCNTs/PGE, MWCNTs/PGE, and PGE were confirmed for conductivity using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Pb(II) was examined using square wave anodic stripping voltammetry (SWASV) on TSDB/MWCNTs/PGE, MWCNTs/PGE, and PGE. Pb(II) stripping voltammetry was carried out using the TSDB/MWCNTs/PGE at varying concentrations (0.8–222 μg L−1). It was found that the lowest detection limit was 0.15 μg L−1. During sensing performances, the Pb(II) sensor with active TSDB exhibits stability, perfect reproducible results, interference, and stability. Above all, its successive applicability to the detection of squid, cow urine and wheat grain samples was demonstrated. Atomic absorption spectroscopy (AAS) measurements are correlated with those of the real samples.
1. Introduction
Ever-increasing amounts of toxic heavy metals are released into the environment, posing a threat to human health as they accumulate in soil and human beings. They are also a major problem since they are contaminants in garbage that is disposed of in outflows.1 Pb(II) and its congeners are difficult to separate from one another and remain stable in soil for extended periods of time, which causes bioaccumulation in agricultural products and trophic transmission to humans.2Even while plants take up a considerable amount of lead ions from the soil, only a small amount of the Pb(II) load moves from the roots to the stems and leaves.3 Although heavy metals are present in large quantities in the Earth's crust, human activities like mining, smelting, burning coal, storing battery effluents, car exhaust, metal plating, tanning leather, finishing operations, and adding fertilizers, pesticides, and additives to paint, gasoline, and other products can enrich these substances in food and cause their contents to exceed safe limits.4 As a result, the extensive use of Pb(II) in many regions of the world has given rise to many environmental issues as well as threats to human health.5,6
Vegetable and wheat plants are occasionally used to treat metal-contaminated soil and groundwater because they are efficient at removing metals from the Earth.7 In commercial vegetable plant production, the application of metal-containing fertilizers, herbicides, lead arsenate, and other pesticides raises the metal Pb(II) content in cabbage leaves, which have a non-negligible Pb(II) concentration. Furthermore, Pb(II) is a well-known toxin that has been connected to numerous issues with human health.8 Pb(II), for example, exhibits an impact on the neurological, reproductive, hematological, cardiovascular, and renal systems.9–11
There is a great tendency to develop selective and sensitive detection methods for controlling toxic heavy metal ions as a result of their significant hazard effect.12 Many techniques, including atomic absorption/emission spectrometry,13 X-ray fluorescence spectrometry (XFS)14 and inductively coupled plasma mass spectrometry (ICP-MS)15 are stable and therefore commonly used to determine Pb(II). However, the disadvantages of these techniques, including complex analytical procedures, expensive instruments, and lengthy sample processing, hinder their practical application. The exceptional sensitivity of square wave anodic stripping voltammetry (SWASV), a traditional electrochemical technique, has drawn a lot of interest for the simultaneous study of several heavy metal ions.16,17
Currently, the development of improved electrode materials is a major factor influencing the sensitivity of SWASV. Over the past ten years, a variety of materials have been utilized as modifiers in the preparation of electrochemical sensors, including complexes,18 nanomaterials,19 carbon-based compounds,20–24 and so forth. These modifiers help increase sensitivity, specific surface area, and conductivity, which make SWASV an attractive substitute for conventional analytical methods with a lower detection limit.25–28
Schiff bases are chemical compounds formed when a primary amine reacts with an aldehyde or a ketone to form an imine. Schiff bases can be easily altered by selecting the right amine and substituting a carbonyl molecule. The utility of ligands containing N and O donors lies in their rigidity, coplanarity, and bite angle, resulting in a wide range of structural diversity. Schiff bases are widely used in electrochemical applications owing to their characteristic structure and their ability to bind to electrode surfaces.29
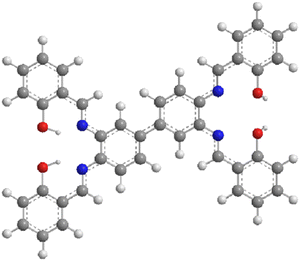
The synthesis of TSDB, modification of the TSDB/MWCNTs/PGE, and evaluation of the electrochemical behavior of the TSDB/MWCNTs/PGE for Pb(II) sensing were carried out in this research work. The present work deals with the synthesis and characterization of an octadentae ligand, N,N′,N′′,N′′′-tetrasalicylidene-3,3′-diaminobenzidine (TSDB, Scheme 1), which bears two sets of N2O2 donor sites separated by a biphenylene group. To the best of our knowledge, no one has yet demonstrated the TSDB/MWCNTs/PGE for Pb(II) measurement. H1-NMR, C13-NMR and FTIR spectroscopy were used to study the TSDB ligand characteristics. The electrodes modified with PGE, MWCNTs/PGE and TSDB/MWCNTs/PGE were evaluated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Finally, Pb(II) was analysed in wheat grain, cow urine and squid samples using square wave anodic stripping voltammetry (SWASV) with TSDB/MWCNTs/PGE in acetate buffer solution. This was the electrochemical application of the sensor. Atomic absorption spectroscopy (AAS) was used to validate the results of SWASV of the samples.
2. Experimental section
2.1. Reagents
Each reagent used was from the explanatory review, and doubly distilled water was used for all the experiments. Salicylaldehyde, 3,3′-diaminobenzidine, 2-aminobenzyl alcohol (assay > 97.5%), lead(II) acetate trihydrate (pure 99%), cadmium acetate dihydrate (extra pure AR, 99%), acetic acid (min. 99.9%), and sodium acetate anhydrous (extra pure AR, 99%) were procured from SRL PVT. Ltd., India. MWCNTs (98% purity) and graphite rods (dia 3 mm) were purchased from Sigma-Aldrich. All the above chemicals and reagents were of analytical grade and were used without further purification.2.2. Instrumentation or characterization apparatus
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) were performed using a Hitachi S3400N (JOEL JSM 6360, with a potential range of 0.3 to 30 kV) instrument. The 1H and 13C NMR spectra were recorded in CDCl3 solution on a Bruker Avance 600 MHz spectrometer. Infrared spectra were recorded on a 650 FT-IR spectrometer (Agilent Technologies). SWASV measurement was carried out using a CHI 660B Analyzer (CH Instruments, USA model). TSDB/MWCNTs/PGE-modified working electrodes were used in conventional three-electrode systems.2.3. Preparation of the ligand (TSDB)
The N′,N′′,N′′′-tetrasalicylidene-3,3′-diaminobenzidine (TSDB) ligand (Scheme 2) was produced in the same way as previously reported,29 with a little change. An ethanolic solution (25 mL) of 3,3′-diaminobenzidine (0.1 mmol) was mixed with salicylaldehyde (0.4 mmol) drop by drop and then refluxed for 3 hours at 60 °C. After cooling the reaction mixture, the yellow compound was filtered, extensively washed with ethanol, recrystallized with methanol, and dried under vacuum.292.4. TSDB/MWCNT fabrication of paraffin graphite electrodes (PGE)
The fabrication of paraffin graphite electrodes (PGE) is highly sensitive, and the PGE's quality affects the effectiveness of the electrochemical responses as well.30 First, 0.1 mg of MWCNT material was added to 1 mL of ethanol medium, and the mixture was sonicated for 10 minutes to prepare 0.1 mg mL−1 MWCNT dispersion. After that, the PGE (bare electrode) surface was covered with 5 μL of MWCNT suspension, and it was left to dry for 10 minutes. Next, 5 μL of the 1 × 10−3 M ligand was deposited on the MWCNT electrode and allowed to dry for 5 minutes in the air after the TSDB ligand had been dissolved in 0.001 M acetone.2.5. Voltametric determination of Pb(II)
Pb(II) analysis for the TSDB/MWCNTs/PGE has been examined using SWASV. The TSDB/MWCNTs/PGE was dipped in 0.1 M acetate buffer (pH = 5.5) solution and preconcentrated at 20 μg L−1 Pb(II) for 180 s while being mechanically stirred in order to perform the Pb(II) detection. After the modified electrode was placed in a freshly prepared 0.1 M acetate buffer (pH = 5.5), the metal ions on the electrode surface were reduced for 90 s at −0.2 V. From a potential range of −0.2 to 0.6 V, the reduced metals on the surface of the TSDB/MWCNTs/PGE were oxidized. The modified electrode was regenerated using 0.01 M ethylene diaminetetraacetic acid (EDTA) for 240 seconds. An explanation is provided for Pb(II)-TSDB/MWCNTs/PGE.2.6. Preparation of standard solutions
A stock solution of 1 mM Pb(II) was dissolved in pH-5.5 acetate buffer. The stock solution was prepared by successive dilution using 10 mM solutions of Pb(II) in acetate buffer. Pb(II) in the quantity of 0.05 μL was added to known volumes of acetate buffer solutions.2.7. Real sample preparation
Samples of wheat grains (sample A), cow urine (sample B) and squid (sample C) were brought in from India. The squid samples (sample-C) were first thoroughly cleaned with DD water and then dried at 60 °C in an oven before being ground into a powder. Samples A, B, and C were all mixed with 0.1 mM Pb(II) to prepare stock solutions for the subsequent experiment. Then, SWASV is utilized to analyze Pb(II) in these samples, and the results are compared to those of AAS.3. Results and discussion
3.1. Characterization of the TSDB ligand
The N,N′,N′′,N′′′-tetrasalicylidene-3,3′-diaminobenzidine (TSDB) ligand was confirmed by Fourier transform infrared (FT-IR) spectroscopy, proton- nuclear magnetic resonance (H1-NMR) spectroscopy and C13-nuclear magnetic resonance (13C-NMR) spectroscopy.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O groups, respectively. The results revealed the presence of hydroxyl and imino nitrogen groups in the synthesized ligand, and the production of the ligand was validated by the FT-IR spectra. These results are also consistent with earlier studies.29
N and C–O groups, respectively. The results revealed the presence of hydroxyl and imino nitrogen groups in the synthesized ligand, and the production of the ligand was validated by the FT-IR spectra. These results are also consistent with earlier studies.29
3.2. PGE, MWCNTs/PGE, and TSDB/MWCNTs/PGE characterization
| Characterization | Terms | PGE | MWCNTs | TSDB/MWCNTs |
|---|---|---|---|---|
| CV | I P | 0.020 cm2 | 0.090 cm2 | 0.150 cm2 |
| I pa (μA) | 58 μA | 83 μA | 208 μA | |
| ΔEP | 230 mV | 180 mV | 140 mV | |
| EIS | R s | 52.36 Ω | 38.16 Ω | 23.4 Ω |
| R ct | 7490 Ω | 917.2 Ω | 194.83 Ω | |
| σ | 165 × 10−5 S cm−1 | 232 × 10−5 S cm−1 | 404.3 × 10−5 S cm−1 | |
| θ | 42.3 | 35.1° | 27.0° |
Additionally, by adding MWCNTs to bare PGE, the peak current (Ipa) was raised from 58 μA to 83 μA. According to the above, a strong and well-defined peak rises at 208 μA for TSDB/MWCNTs/PGE. The much improved Ipa values (peak current) of TSDB/MWCNTs/PGE and lower peak potential, or ΔEp of TSDB/MWCNTs/PGE. The reason for this could be the combination of TSDB/MWCNTs/PGE, which raises the surface area and facilitates electron transport between [Fe(CN)6]3−/4− and TSDB/MWCNTs/PGE, suggesting a higher level of electrochemical activity.
The square root of the scan rate (v1/2) was used to measure the linear increase in the CV peak current in 0.1 M ABS solution for the TSDB/MWCNTs/PGE. This was due to the electron migration reaction at the modified (TSDB/MWCNTs/PGE) electrode, which is controlled by diffusion.
Using the Randles–Sevcik equation, the active surface area of different electrodes in an irreversible process can be studied.
| Ip = 2.69 × 105n3/2AD1/2v1/2C | (1) |
The (σ) electrode conductance (2) was calculated for both the TSDB/MWCNTs/PGE modified and the MWCNTs/PGE modified electrodes in eqn (2).
 | (2) |
Using eqn (3), the phase degree was determined.
 | (3) |
According to the equation, the PGE (bare electrode) phase degree (ϕ) was 42.3°, that of the MWCNTs/PGE was 35.1°, and that of the estimated TSDB/MWCNTs/PGE was 27.0°. Phase angle (ϕ) is directly proportional to (Rct) in accordance with eqn (3). It has improved the electron transfer kinetics at the electrode interface and led to a decrease in the phase angles at the TSDB/MWCNTs/PGE modified electrode. This modified electrode for TSDB/MWCNTs/PGE was therefore appropriate for stripping analysis.
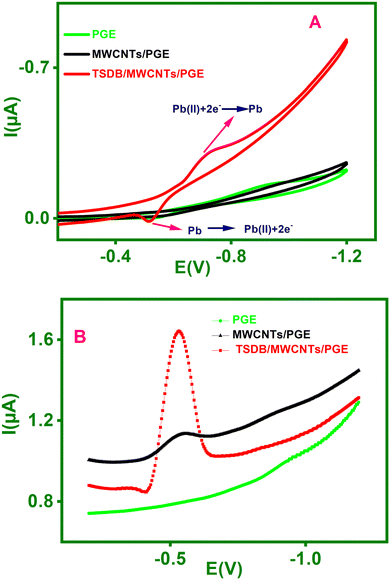
Fig. 6B displays an investigation of the various electrodes of PGE, MWCNTs/PGE and TSDB/MWCNTs/PGE for Pb(II) measurement using square wave anodic stripping voltammetry (SWASV). Preconcentration step-1, a 20 μg L−1 of Pb(II) applied by the dipping the electrode into a 0.1 M acetate buffer solution (pH = 5.5) while it was stirred for 180 s owing to the formation of a metal complex. Subsequently, reduction step-2 proves that the metal Pb(II) reduced to Pb(0) at a potential of −1.2 V for 90 s. In stripping step-3, Pb(0) was oxidized to Pb(II) and was stripped off the electrode into the solution by applying a positive direction potential between −1.2 and −0.2 V. As a result, the stripping peak current for Pb(II) was higher on the TSDB/MWCNTs/PGE modified electrode than it was on the MWCNTs/PGE. It was therefore established that the TSDB ligand, present on the surface of the MWCNTs/PGE and used to stabilize the electrode, absorbed metal ions from the preconcentrated medium. The TSDB ligand is composed of four nitrogen groups and a hydroxyl group that are coordinated with metal ions. As a result, TSDB/MWCNTs/PGE increased Pb(II) sensitivity. Scheme 3 shows the mechanism of the Pb(II)-TSDB/MWCNTs/PGE modified electrode.
The stripping voltammogram for Pb(II) was examined after being preconcentrated from different media in 0.1 M acetate buffer (ABS), KNO3, NH4NO3, and NaNO3 solutions. Fig. 7A presents the measured findings for 25 μg L−1 of Pb(II) for various electrolytes. In an acetate buffer medium, a greater stripping current response was found. As a result, Pb(II) was determined using 0.1 M acetate buffer medium in the following experiments.
The analysis examined the effect of pH on the anodic stripping current during the preconcentration of 25 μg L−1 Pb(II) in 0.1 M acetate buffer. Fig. 7B shows the changes in the stripping current for Pb(II) at pH values ranging from 4.0 to 6.5. It was found that the ideal pH is 5.5 since this pH has a higher peak current for the Pb(II) ion. For this reason, the following measurements were done at pH 5.5.
SWASV was used to examine the effect of preconcentration on Pb(II) electrochemical sensing. Fig. 7C displays the fluctuation in stripping peak currents of 25 μg L−1 of Pb(II) in 0.1 M acetate buffer at various interval times ranging from 60 to 300 s. It is evident from the figure that the stripping peak current increased dramatically for 180 s before somewhat decreasing for 300 seconds. Therefore, it was determined that 180 s was the ideal period to detect Pb(II).
 | ||
| Fig. 8 Determination of Pb(II) by TSDB/MWCNTs/PGE using (A) SWASV corresponding calibration plots for Pb(II) (B) calculated using SWASV. | ||
| Modified electrode | Linear range (μg L−1) | Sensitivity (μA μg L−1 cm−2) | Detection limit (LOD) (μg L−1) | Ref. |
|---|---|---|---|---|
| GSH/AuNPs/NH2-rGO/GCE | 1–120 | 0.507 | 0.38 | 31 |
| ZnO_L-cys/GCE | 10 to 140 | 0.3058 | 0.397 | 32 |
| Glu-h-ZnO/GCE | 200–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0457 | 42 | 33 |
| rGO/MoS2/CS/GCE | 5–50 | 0.311 | 1.6 | 34 |
| ZFO/GCE | 100–2000 | 0.1443 | 5.11 | 35 |
| (PA/PPy)/ZIF-8@ZIF-67/GCE | 20–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.755 | 2.9 | 36 |
| Nb4C3Tx/GCE | 25–500 | 0.584 | 12 | 37 |
| (BiNCs@AB) composite/GCE | 3.0 to 1000 | 0.91 | 1.0 | 38 |
| TSDB/MWCNTs/PGE | 0.8–222 | 0.027 | 0.15 | Our work |
By storing the modified electrode made of TSDB and MWCNTs at room temperature and analyzing 50 μg L−1 of Pb(II) using SWASV over one week, the stability of the electrode was evaluated (Fig. 9B). With a standard variation of 1.0% and 99.1% of its initial peak current response, the electrode recorded a significantly reduced stripping peak current of Pb(II). The outcome verified the suggested electrodes’ long-term stability for electrochemical use.
 | (4) |
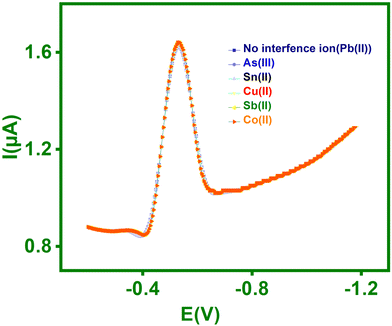 | ||
| Fig. 10 SWASV curves of the TSDB/MWCNTs/PGE in pH-5.5 ABS containing 50 μg L−1 Pb(II) in the presence of 25 μg L−1 As(III), Sn(II), Cu(II), Sb(II), and Co(II). | ||
The RSD values were calculated using the formulae and are displayed in Table 3. The data shown in Table 3 demonstrated that peak currents of Pb(II) had a minor decrease in RSD values by 1.2, 1.8, 3.6, 3.0, and 2.4% when interfering metals such as As(III), Sn(II), Cu(II), Sb(II), and Co(II) were present. As a result, the newly proposed electrode for Pb(II) detection shows superior interference studies.
| Interference ions | Pb(II) | |
|---|---|---|
| Peak current (μA) | Relative signal changes (%) | |
| No interference ions | 1.64 | — |
| As(III) | 1.62 | −1.2 |
| Sn(II) | 1.61 | −1.8 |
| Cu(II) | 1.58 | −3.6 |
| Sb(II) | 1.60 | −3.0 |
| Co(II) | 1.59 | −2.4 |
| Samples | Metal ions | Square wave anodic stripping voltammetry (SWASV) | Atomic absorption spectroscopy (AAS) | ||||
|---|---|---|---|---|---|---|---|
| Added (μg L−1) | Found (μg L−1) | RSD (%) | Recovery (%) | Found (μg L−1) | Recovery (%) | ||
| Sample-A | Pb(II) | 25.0 | 24.8 | 1.3 | 99.4 | 30.0 | 100.0 |
| (Wheat grains) | 50.0 | 56.0 | 1.7 | 102.0 | 60.0 | 100.0 | |
| Sample-B | Pb(II) | 25.0 | 25.3 | 2.0 | 101.0 | 27.0 | 102.0 |
| (Cow urine) | 50.0 | 56.3 | 2.1 | 102.3 | 55.0 | 103.0 | |
| Sample-C | Pb(II) | 25.0 | 27.0 | 2.5 | 108.0 | 33.0 | 105.0 |
| (Squid) | 50.0 | 55.0 | 2.7 | 110.0 | 65.0 | 107.0 | |
4. Conclusion
Utilizing SWASV, the suggested SDA/MWCNTs/PGE approach analyzes Pb(II). Using SWASV, Pb(II) in wheat grain, cow urine and squid samples was evaluated by TSDB/MWCNTs/PGE. Pb(II) showed a robust recovery, rising from 99.2 to 110%. AAS results and these values were associated. Pb(II) stripping voltammetry observations ranged from 0.8–222 μg L−1, with correlation coefficients (R2) of 0.99, LODs of 0.15 μg L−1, and sensitivity values of 1.141 μA μg−1 L−1 cm−2. The stripping current peak for Pb(II) was stable, and the stability of the TSDB/MWCNTs/PGE was recorded for both the new electrode and the original electrode. According to anti-interference tests, the stripping peak of Pb(II) with less than ±5% inaccuracy does not alter when compared to other metal ions that have been measured. Ultimately, it was shown that a newly synthesized TSDB/MWCNTs/PGE significantly increased the sensitivity of Pb(II).Data availability
The data used to support the findings of this study are included within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are very thankful to the Dean R & D and University Management Committee Members of Vel Tech Rangarajan Dr Sagunthala R & D Institute of Science and Technology, Avadi, Chennai, for providing the necessary instrumental facilities to carry out the research work.References
- S. Palisoc, E. T. Lee, M. Natividad and L. Racines, Silver nanoparticle modified graphene paste electrode for the electrochemical detection of lead, cadmium and copper, Int. J. Electrochem. Sci., 2018, 13(9), 8854–8866 CrossRef.
- M. O. C. Ogwuegbu and W. Muhanga, Investigation of lead concentration in the blood of people in the copper belt province of Zambia, J. Environ., 2005, 1, 66–75 Search PubMed.
- M. Wińska-Krysiak, K. Koropacka and S. Gawroński, Determination of the tolerance of sunflower to lead-induced stress, J. Elem., 2015, 20(2), 491–502 Search PubMed.
- S. Kumar, R. Islam, P. B. Akash, M. H. R. Khan, R. Proshad, J. Karmoker and G. R. MacFarlane, Lead (Pb) contamination in agricultural products and human health risk assessment in Bangladesh, Water, Air, Soil Pollut., 2022, 233(7), 257 CrossRef.
- A. Kushwaha, N. Hans, S. Kumar and R. Rani, A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies, Ecotoxicol. Environ. Saf., 2018, 147, 1035–1045 CrossRef.
- S. Xiong, B. Yang, D. Cai, G. Qiu and Z. Wu, 2015. Individual and simultaneous stripping voltammetric and mutual interference analysis of Cd2+, Pb2+ and Hg2+ with reduced graphene oxide-Fe3O4 nanocomposites, Electrochim. Acta, 2015, 185, 52–61 CrossRef.
- T. E. Novotny, S. A. Bialous, L. Burt, C. Curtis, V. L. D. Costa, S. U. Iqtidar, Y. Liu, S. Pujari and E. Tursan d’Espaignet, The environmental and health impacts of tobacco agriculture, cigarette manufacture and consumption, Bull. W. H. O., 2015, 93, 877–880 CrossRef.
- D. C. Bellinger, The protean toxicities of lead: new chapters in a familiar story, Int. J. Environ. Res. Public Health, 2011, 8(7), 2593–2628 CrossRef.
- G. Flora, D. Gupta and A. Tiwari, Toxicity of lead: a review with recent updates, Interdiscip. toxicol., 2012, 5(2), 47–58 Search PubMed.
- Z. Fang, J. Růžička and E. H. Hansen, An efficient flow-injection system with on-line ion-exchange preconcentration for the determination of trace amounts of heavy metals by atomic absorption spectrometry, Anal. Chim. Acta, 1984, 164, 23–39 CrossRef CAS.
- P. P. Gao, X. M. Zhang, P. Y. Xue, J. W. Dong, Y. Dong, Q. L. Zhao, L. P. Geng, Y. Lu, J. J. Zhao and W. J. Liu, Mechanism of Pb accumulation in Chinese cabbage leaves: Stomata and trichomes regulate foliar uptake of Pb in atmospheric PM2.5, Environ. Pollut., 2022, 293, 118585 CrossRef PubMed.
- K. Kocot and R. Sitko, Trace and ultratrace determination of heavy metal ions by energy-dispersive X-ray fluorescence spectrometry using graphene as solid sorbent in dispersive micro solid-phase extraction, Spectrochim. Acta, Part B, 2014, 94, 7–13 CrossRef.
- M. A. Habila, Z. A. ALOthman, A. M. El-Toni and M. Soylak, Combination of syringe–solid phase extraction with inductively coupled plasma mass spectrometry for efficient heavy metals detection, CLEAN:Soil, Air, Water, 2016, 44(6), 720–727 Search PubMed.
- D. Manivannan and V. M. Biju, Determination of toxic heavy metals in sea water by FAAS after preconcentration with a novel chelating resin, Water Sci. Technol., 2011, 64(4), 803–808 CrossRef.
- AOAC International, E. W. Sydenham, G. S. Shephard, P. G. Thiel, S. Stockenström, P. W. Snijman, D. J. Van Schalkwyk and Collaborators, J. AOAC Int., 1995, 78, 688–696 Search PubMed.
- T. Priya, N. Dhanalakshmi and N. Thinakaran, Electrochemical behavior of Pb (II) on a heparin modified chitosan/graphene nanocomposite film coated glassy carbon electrode and its sensitive detection, Int. J. Biol. Macromol., 2017, 104, 672–680 CrossRef.
- S. Xiong, S. Ye, X. Hu and F. Xie, Electrochemical detection of ultra-trace Cu (II) and interaction mechanism analysis between amine-groups functionalized CoFe2O4/reduced graphene oxide composites and metal ion, Electrochim. Acta, 2016, 217, 24–33 CrossRef.
- H. Ge and X. Fan, Adsorption of Pb2+ and Cd2+ onto a Novel Activated Carbon-Chitosan Complex, Chem. Eng. Technol., 2011, 34(10), 1745–1752 CrossRef.
- T. Yan, Z. Wang and Z. J. Pan, Flexible strain sensors fabricated using carbon-based nanomaterials: A review, Curr. Opin. Solid State Mater. Sci., 2018, 22(6), 213–228 CrossRef.
- A. Sinha, Dhanjai, R. Jain, H. Zhao, P. Karolia and N. Jadon, Voltammetric sensing based on the use of advanced carbonaceous nanomaterials: a review, Microchim. Acta, 2018, 185, 1–30 CrossRef PubMed.
- J. Xu, Z. Cao, Y. Zhang, Z. Yuan, Z. Lou, X. Xu and X. Wang, A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism, Chemosphere, 2018, 195, 351–364 CrossRef CAS.
- C. Guo, C. Wang, H. Sun, D. Dai and H. Gao, A simple electrochemical sensor based on rGO/MoS2/CS modified GCE for highly sensitive detection of Pb (ii) in tobacco leaves, RSC Adv., 2021, 11(47), 29590–29597 RSC.
- S. Tajik, A. Lohrasbi-Nejad, P. Mohammadzadeh Jahani, M. B. Askari, P. Salarizadeh and H. Beitollahi, Co-detection of carmoisine and tartrazine by carbon paste electrode modified with ionic liquid and MoO3/WO3 nanocomposite, J. Food Meas. Charact., 2022, 1–9 Search PubMed.
- H. Mahmoudi Moghaddam, H. Beitollahi, S. Tajik, I. Sheikhshoaie and P. Biparva, Fabrication of novel TiO2 nanoparticles/Mn(III) salen doped carbon paste electrode: application as electrochemical sensor for the determination of hydrazine in the presence of phenol, Environ. Monit. Assess., 2015, 187, 1–12 CrossRef CAS PubMed.
- H. Beitollahi, M. Shahsavari, I. Sheikhshoaie, S. Tajik, P. M. Jahani, S. Z. Mohammadi and A. A. Afshar, Amplified electrochemical sensor employing screen-printed electrode modified with Ni-ZIF-67 nanocomposite for high sensitive analysis of Sudan I in present bisphenol A, Food Chem. Toxicol., 2022, 161, 112824 CrossRef PubMed.
- S. Tajik, M. A. Taher and H. Beitollahi, The first electrochemical sensor for determination of mangiferin based on an ionic liquid–graphene nanosheets paste electrode, Ionics, 2014, 20, 1155–1161 CrossRef.
- S. Tajik, Z. Dourandish, F. G. Nejad, H. Beitollahi, P. M. Jahani and A. Di Bartolomeo, Transition metal dichalcogenides: Synthesis and use in the development of electrochemical sensors and biosensors, Biosens. Bioelectron., 2022, 216, 114674 CrossRef PubMed.
- A. S. Ahmed, M. B. I. Mohamed, M. A. Bedair, A. A. El-Zomrawy and M. F. Bakr, A new Schiff base-fabricated pencil lead electrode for the efficient detection of copper, lead, and cadmium ions in aqueous media, RSC Adv., 2023, 13(23), 15651–15666 RSC.
- Lallan Mishra, Kumari Bindu and Subrato Bhattacharya, Indian J. Chem., 2004, 43, 315–319 Search PubMed.
- Jayagopi Gayathri, Sivakumar Sivalingam and Sanglimuthu Sriman Narayanan, Determination of Pb2+ and Cd2+ ions in raw milk, honey and groundnut shell using TSAB/MWCNT, Mater. Adv., 2023, 4(250), 2502–2511 RSC.
- Jinbang Mei, et al., A sensitive and selective electrochemical sensor for the simultaneous determination of trace Cd2+ and Pb2+, Chem. Pap., 2020, 74, 1027–1037 CrossRef.
- Vitor H. B. Oliveira, et al., A sensitive electrochemical sensor for Pb2+ ions based on ZnO nanofibers functionalized by L-cysteine, J. Mol. Liq., 2020, 309, 113041 CrossRef.
- Lateef Ahmad Malik, et al., Studies on a glutathione coated hollow ZnO modified glassy carbon electrode; a novel Pb(II) selective electrochemical sensor, RSC Adv., 2021, 11(30), 18270–18278 RSC.
- Chuanen Guo, et al., A simple electrochemical sensor based on rGO/MoS2/CS modified GCE for highly sensitive detection of Pb(II) in tobacco leaves, RSC Adv., 2021, 11(47), 29590–29597 RSC.
- Changchun Fan, et al., ZnFe2O4 nanoparticles for electrochemical determination of trace Hg(II), Pb(II), Cu(II), and glucose, ACS Appl. Nano Mater., 2021, 4(4), 4026–4036 CrossRef.
- Wanqing Zhang, et al., Electrochemical determination of lead(II) and copper(II) by using phytic acid and polypyrrole functionalized metal-organic frameworks, Microchim. Acta, 2020, 187, 1–9 CrossRef PubMed.
- P. Abdul Rasheed, et al., Large interlayer spacing Nb4C3Tx (MXene) promotes the ultrasensitive electrochemical detection of Pb2+ on glassy carbon electrodes, RSC Adv., 2020, 10(41), 24697–24704 RSC.
- Jin Zou, et al., Bismuth nanoclusters/porous carbon composite: A facile ratiometric electrochemical sensing platform for Pb2+ detection with high sensitivity and selectivity, ACS Omega, 2021, 7(1), 1132–1138 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |