Open Access Article
Open Access ArticleSmart inhibitor systems towards anti-corrosion: design and applications
Danping
Li
a,
Ziwen
Zhang
a,
Bilegsaikhan
Sukhbat
a,
Xuejie
Wang
a,
Xue
Zhang
b,
Jing
Yan
*a,
Junping
Zhang
a,
Qiuyu
Zhang
 a,
Yan
Li
c,
Hao
Wang
d and
Yi
Yan
a,
Yan
Li
c,
Hao
Wang
d and
Yi
Yan
 *a
*a
aDepartment of Chemistry, School of Chemistry and Chemical Engineering, Key Laboratory of Special Functional and Smart Polymer Materials of Ministry of Industry and Information Technology, Northwestern Polytechnical University, Xi'an, Shaanxi 710129, China. E-mail: yanyi@nwpu.edu.cn; yanjing@nwpu.edu.cn
bChengdu Aircraft industrial (Group) Co., Ltd., China
cPetroleum Technology Research Institute, Tarim Oilfield Company, Petrochina, Korla, Xinjiang 841000, China
dThe First Gas Production Plant, Changqing Oilfield Company, Yulin, Shaanxi 718500, China
First published on 12th February 2025
Abstract
Smart corrosion inhibitor systems have been a hot research topic in recent years. Compared with conventional corrosion inhibitors or coatings, corrosion inhibitors can be released according to specific tasks to achieve more precise corrosion protection for metals. Such smart inhibitor systems are generally based on environmental stimuli such as pH, light, ions, etc., which save the cost of corrosion inhibitors and additives, and therefore improve the effectiveness of corrosion inhibition applications. Herein, the research progress on smart corrosion inhibitors in the past decade will be summarized, including the design, synthesis, smart release, and applications of different smart inhibitor systems.
1 Introduction
Corrosion inhibitors, as one of the important solutions for industrial anti-corrosion, have received broad attention in both industry and academia.1–3 They offer the advantages of simple implementation, quick effectiveness, and high cost-efficiency. Organic corrosion inhibitors primarily form protective films through either physical or chemical adsorption, whereas inorganic corrosion inhibitors mainly create precipitation films and oxide films. Compared with anti-corrosion measures such as corrosion-resistant materials and coatings, the use of corrosion inhibitors is consumable and requires a continuous supply, which increases the cost and time associated with manual operations.4–7 Since conventional corrosion inhibitors cannot smartly respond to changing corrosion environments, there is a need to develop a smart response corrosion inhibitor system that can target specific areas and enhance protection, thereby improving the utilization rate and effectiveness of the inhibitor,8,9 which is referred to as a smart inhibitor in this paper. Meanwhile, the synergistic effect between corrosion inhibitors and coatings has also been a focus of research in recent years. The use of corrosion inhibitors to repair damaged areas of coatings and form self-healing coatings addresses the problem of endless monitoring and manual repairs following coating failures such as blistering and peeling, which are time-consuming and resource-intensive.10 However, in different coating systems, conventional corrosion inhibitors that are loaded directly have several drawbacks, such as: (i) a slow or uncontrollable release rate; (ii) the high solubility of corrosion inhibitors, which can lead to blistering and delamination of protective coatings; and (iii) the semi-permeable membrane, which allows for the transportation of water, causing damage to the barrier layer. If the corrosion inhibitor is coated with environmentally sensitive materials11–17 or carried by a carrier,8,16,18–20 and then loaded into the coating, when local corrosive environmental conditions, such as pH, ion exchange, or temperature, change,16,21 the corrosion inhibitor will automatically release,22–24 inhibiting or slowing down the occurrence of corrosion. It achieves the purpose of in-service repair of the coating, while improving the utilization rate of the corrosion inhibitor and reducing the manufacturing and maintenance costs of the coating.25,26Different host materials can be used to construct smart inhibitor systems. For example, mesoporous silica nanocarriers (MSN) were used to encapsulate benzotriazole (BTA) to create a multifunctional smart coating known as ACR-MSN-BTA-Ag. This coating has a dual anti-corrosion mechanism under aggressive conditions involving Cl−: a passive mechanism based on a pH-responsive BTA-Ag complex that triggers the release of BTA to form a protective layer adsorbed on the metal surface. Another active mechanism is attributed to the ability of silver ions to trap chloride ions. In pH 1.5 hydrochloric acid vapor, the ACR-MSN-BTA-Ag smart coating showed an excellent anti-corrosion effect compared with the ACR coating and the ACR-BTA coating.27 pH change can stimulate the release of Ce(III) from a PANI−Ce(III) complex. Najibzad et al.28 prepared praseodymium cation and PANI composites, which were then added to silane coatings applied to magnesium alloys. They found that coatings containing praseodymium cation and PANI composites exhibited better corrosion resistance and self-healing properties than those containing only praseodymium nitrate. Introducing TiO2/PANI-MoO42−/PDA nanocontainers into smart waterborne epoxy (WEP) coatings, the charge transfer resistance increases by a factor of 3.32 × 105 compared with that of WEP coatings.29 The inhibitor was encapsulated into HNTs using a vacuum method, and another inhibitor, dodecylamine (DDA), was intercalated into the polyelectrolyte multilayer film using a layer technique to form HHNTs. The HHNTs (3 wt%) were then sufficiently dispersed into the epoxy resin matrix to develop a smart blend self-healing polymer coating known as a blend coating. It exhibits active release in both acidic and alkaline media, making it suitable for protecting steel in the early stages of coating damage, while DDA is effectively released in acidic media, which may help prevent corrosion activity in the later stages of coating deterioration.30 Polydopamine (PDA) is used as a pH-sensitive gating material that exhibits rapid release in acidic environments. The on-demand release of PDA and La3+ corrosion inhibitors at the scratch site increased the total corrosion resistance (RT) of the carbon nanofiber (CNF) coating containing modified PDA-La(III) composite by 117% in brine compared with the blank epoxy.31 Multifunctional dopamine-based gating materials not only have potential applications in smart self-healing anti-corrosion coatings, but they can also be used in drug delivery, antimicrobial protection, and other fields.32
Metal–organic frameworks (MOFs) can also be used as host materials. For example, stimulus-responsive zeolite imidazole framework was developed and subsequently applied to sense local corrosion and restore the protective effect of polymer coating systems. The change in local pH value and the occurrence of metal corrosion due to coating micro-defects can be sensed and indicated in real-time by the released tannic acid, which provides an obvious color signal. In addition, the decomposition of the ZIF-7@PEG-TA nanosensor produces abundant corrosion inhibitors, allowing the formation of an inhibition layer that can significantly reduce the spread of interface corrosion reactions and actively contribute to anti-corrosion functions.33 Triple-functional microcarriers with corrosion sensing, self-healing, and anti-corrosion capabilities were developed and embedded in the coating to detect color changes and the release of corrosion inhibitors when the local pH near the metal surface changes due to the formation of hydroxide ions during the corrosion process. It significantly reduces the corrosion rate of the coated metal.34 The anodic peak corresponding to the corrosion point of the mechanical defect on the surface of the photosensitive coating prepared with TiO2 disappeared immediately after irradiation with a layer of polyethylene imide (PEI) and polystyrene sodium sulfonate (PSS).35,36 After soaking in a 0.5 M NaCl solution for 2 weeks, the total impedance of the PVB coating decreased from 1 mΩ cm2 to 20 kΩ cm2, while the coating containing the Zn2Fe(CN)6 ion-exchange smart corrosion inhibitor remained basically unchanged at 15 mΩ cm2, demonstrating excellent anti-corrosion properties.37 When polyurea formaldehyde was embedded in BTA and magnetic multi-wall carbon nanotubes and combined with the coating, the corrosion inhibition rate reached 91.2%, and the self-healing efficiency was 6.4 times greater than that of non-magnetic self-healing coatings.38 By adding pH-responsive thiourea-containing nanovessels to the ethylcellulose coating, the rate of thiourea release is increased once stimulated by CO2. The response structure significantly improves the corrosion resistance of the coating in 3.5 wt% NaCl solution with and without CO2.39 In summary, the smart repair of coatings depends on the use of smart corrosion inhibitors.
Some pioneers in this field have already summarized the progress of smart corrosion inhibitor systems. Abu-Thabit et al.40 reviewed the research progress in using polyelectrolyte multilayer films as stimulus-responsive materials to prepare coatings with smart self-healing function in recent years. The novelty of PEMs lies in their ability to provide responsive actions based on various triggering mechanisms of the surrounding environment. The advantage of using PEMs lies in the versatility of layer-by-layer (LbL) assembly, which can be used to prepare PEMs for various planar, non-planar, and granular substrates. Cai et al.10 reviewed the research progress of smart anti-corrosion coatings based on stimulus-responsive micro-nano-containers, introduced the latest design and manufacturing technologies of micro-nano-containers, and provided detailed examples. M. Samadzadeh et al.41 reviewed the effective parameters for the synthesis of micro/nanocapsules, several methods for preparing self-healing coatings based on micro/nanocapsules, and the drawbacks of embedding micro/nanocapsules in coating matrices. Rowsell et al.42 reviewed the synthesis, structure, and properties of metal–organic frameworks (MOFs), as well as their selective absorption of small molecules and optical or magnetic response to objects. Guo et al.43 reviewed pH-responsive and ion exchange-based smart corrosion inhibitors. By introducing cobaltocenium groups to the polymer, we designed a series of metallo-polyelectrolytes and metallo-surfactants, which exhibited robust anti-corrosion performance.44–46 Based on our previous review article on the polymeric inhibitor,47 we herein are trying to summarize the most recent research progress on the response mechanism and smart response structure of smart corrosion inhibitor systems, including pH, light, ion, magnetic, temperature and redox responsive as well as multiple-responsive systems.
2 General mechanisms of smart inhibitor systems
To achieve the controlled release of inhibitors under different conditions, several smart inhibitor systems have been developed. In this section, the general mechanisms of smart inhibitor systems will be discussed, including pH-responsive, photoresponsive, ion-responsive, and other systems.2.1 Mechanism of pH-responsive inhibitor systems
Generally, electrochemical corrosion consists of anodic and cathodic reactions, resulting in an acidic environment in the anode region and alkaline condition in the cathode region. Therefore, a pH-responsive corrosion inhibitor system presents an optimal strategy for mitigating electrochemical corrosion. In principle, these pH-responsive inhibitor systems are designed to release inhibitors in response to pH changes, effectively inhibiting both anodic and cathodic corrosion to achieve the desired anti-corrosion performance. As shown in Fig. 1, there are mainly four types of pH-responsive inhibitor system, namely inhibitors encapsulated in polyelectrolyte capsules, pH-sensitive capsule wall, nanovalve type, and carrier grafting via chemical bonds.A polyelectrolyte microcapsule is usually fabricated via a core-template approach, wherein polyelectrolytes with opposite charges are sequentially deposited on the core. These microcapsules can be categorized into two types according to the different core: (i) a sacrificial core, such as a CaCO3 particle, which can be dissolved after the formation of the microcapsules; and (ii) a mesoporous core that can be used to encapsulate corrosion inhibitors, such as silica, cerium oxide, carbon hollow spheres (CHSs), and halloysite nanotubes (HNTs). The inhibitors may either be encapsulated in the microcapsules or dispersed between the polyelectrolyte layers. Upon corrosion, the pH changes in either the anode or the cathode region may disrupt the overall charge equilibrium and swell the polyelectrolyte shell to release corrosion inhibitors into the solution. In most case, such a process is reversible.
In the case of type 2, the shell of the microcapsule is doped with pH-sensitive components, which may undergo chemical reactions upon a pH change, leading to the formation of pores in the shell, and therefore, the release of the corrosion inhibitor. The chemical reactions used in this system involve ether bonds, ester bonds, disulfide groups, sulfhydryl groups, and so on.48
Besides microcapsules, the nanovalve system can also be used to construct smart inhibitor systems. The nanovalve system is usually composed of a micro-container for inhibitors and a pH-responsive cap. For example, macrocycles such as cucurbituril and its analogues can be used as a cap, while porous materials such as mesoporous silica, halloysite nanotubes and carbon hollow spheres are typically used as micro-containers.
Another method for preparing a pH-responsive inhibitor system involves grafting inhibitors onto the carrier surface via covalent bonds. In this case, 2D nanomaterials such as MXenes and 3D metal–organic frameworks (MOFs) can serve as the carriers. The pH response is achieved by functionalizing the surface of the carrier. For instance, the modification of the surface of MXene with –NH2 and subsequent protonation under acidic conditions will result in the release of corrosion inhibitors.49,50 Additionally, acid-sensitive spacers can be used to graft inhibitors onto the polymer framework. The acid-sensitive linker used in this system include orthoesters, acetals, imines, cis-ketoacyls, vinyl ethers, silyl ethers, hydrazine groups, and β-thiopropionate groups.51
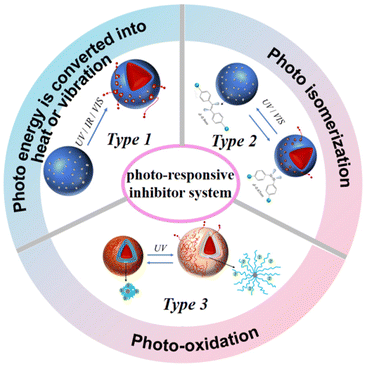
2.2 Mechanism of photoresponsive inhibitor systems
Owing to its green characteristic and remote control capabilities, a photoresponsive system can also be used to achieve the controlled release of corrosion inhibitors through the following strategies: photothermal effect from metal oxide/noble metal nanoparticles, photoisomerization or photooxidation, star polyelectrolytes, and photosensitive dyes (Fig. 2).Metal oxide and noble metal nanoparticles, such as TiO2,20,52 silver,53 gold,54,55 and gold-sulfide clusters,56 possess the capacity to absorb light and convert it to thermal energy. By incorporating these nanoparticles into microcapsules, the photothermal effect can disrupt the shell of the microcapsule and lead to the release of the corrosion inhibitor (Fig. 2 Type 1). For example, in the case of TiO2-containing microcapsules, the electron density of TiO2 can be altered through UV irradiation, while the decomposition of polyelectrolyte capsules is facilitated by the photocatalysis of TiO2 in the inorganic layer. The potential toxicity of metal nanoparticles, coupled with their elevated cost, may constrain their application in certain domains.
Microcapsules with photoisomerization units57 can also be used in the controlled release of inhibitors. Owing to its abundant chemistry and reversible photoisomerization, azobenzene is widely used in the design of photoresponsive microcapsules (Fig. 2 Type 2). Under UV stimulation, the permeability of microcapsules can change from the stable trans form to the unstable cis form, allowing for the release of the encapsulated corrosion inhibitor.
Units that undergo photochemistry, such as photo-oxidation,58 can also be used to construct photoresponsive inhibitor systems (Fig. 2 Type 3). For example, the photoaquation reaction of hexacyanocobaltate(III) ions provides a reversible change between Co(CN)63− and Co(CN)5(H2O)2−, resulting in the collapse and swelling of the microcapsule, respectively. Such reversible transition can be used to control the release of inhibitors.59 Moreover, specific dyes that absorb or emit light within the visible spectrum can be engineered into photosensitive microcapsules. These capsules incorporate elements such as fluorescent and functional dyes,60 which react to either visible light or infrared radiation. Upon exposure to suitable light, alterations occur in the permeability of the capsule shell, facilitating the release of corrosion inhibitors.
2.3 Mechanism of ion-responsive inhibitor systems
Besides the in situ photoaquation reaction of hexacyanocobaltate(III), ions in the corrosion system can also be used to change the state of the microcapsule. In general, the ions doped into the shell of the microcapsule may interact with the ions in the surrounding environment to form a precipitate. Such interaction results in structural disruption of the capsule shell, leading to the subsequent release of the encapsulated inhibitor (Fig. 3).Ion-exchange between the ions in the shell and the corrosive ions in the external environment can also be used to release the encapsulated corrosion inhibitors in the microcapsule. Such ion exchange processes markedly diminish the corrosion rate of metals. Depending on the electronegativity of stimulus-responsive ions, ion-exchange inhibitor systems can be categorized into cationic and anionic types. Currently, most cationic exchange inhibitor systems use layered silicates (such as zeolite, montmorillonite, and kaolinite) as carriers. Owing to the isomorphous substitution of Si4+ and Al3+ in silicates, the negative charge in the framework is insufficient, facilitating the exchange of cations within the framework with environmental cations. Furthermore, organic ion exchange resin, ferricyanide, and zirconium orthophosphate are also candidates for shell materials in cation exchange inhibitor systems. Layered double hydroxides (LDH), such as hydrotalcite,61–66 are among the most popular anion exchange inhibitor systems. LDHs have been investigated as storage media for functional anions,63,67–70 including anionic corrosion inhibitors such as chromate,71,72 vanadate,61 molybdate,73 quinate,74 2-mercaptobenzothiazolate,74 tungstate,75–77 and other organic anions. Chloride in the corrosion medium can efficiently initiate the release of these inhibitory anions, which can subsequently be used as corrosion inhibitors for metals.
2.4 Other types of smart inhibitor system
Besides the aforementioned smart inhibitor systems, magneto-responsive, temperature-responsive, and redox-responsive inhibitor systems have also been developed.Magneto-responsive microcapsules primarily consist of a capsule shell containing magnetic particles, including iron oxides such as magnetic (Fe3O4) and magnetite (γ-Fe2O3), as well as pure metals (such as Fe, Co, and Ni), alloys (such as CoPt3 and FePt) and spinel-type ferromagnets (such as NiFe2O4, MgFe2O, and CoFe2O4). The permeability of the capsule shell can be altered under a magnetic field, facilitating the release of the corrosion inhibitor. Furthermore, the magnetic field provides control over the migration of the microcapsule in the corrosion medium or coating, enabling targeted release.78
Microcapsules with intrinsic temperature-responsive units can be used as smart inhibitor systems. For instance, poly(N-isopropyl acrylamide) (PNIPAm) exhibits typical lower critical solution temperature (LCST) behaviour. At low temperatures, the interaction between PNIPAm and water predominantly involves hydrogen bonding, which results in a solvated layer around the macromolecular chain and causes the macromolecule to adopt a stretched nematic structure. As the temperature increases, there is a sudden shift in the interaction parameters between PNIPAm and water, leading to the disruption of some hydrogen bonds. Consequently, the solvation layer in the hydrophobic region of the macromolecular chain is destroyed, prompting the macromolecule to transition from a loose nematic structure to a compact gelatinous granular structure. Such a temperature-responsive aggregated structure can also be used in smart inhibitor systems.
With regard to electrochemical corrosion, one of the most suitable stimuli is electrochemical redox, owing to its merits such as in situ generation, uniform distribution, robust reduction, and consistent decrease during corrosion. In comparison with barrier-type polymers, intrinsically conductive polymers (ICPs), such as polypyrrole (PPy) and polyaniline (PANI), possess inherent oxidation capabilities. Disulfide bonds are relatively stable under mild oxidation conditions but can be readily broken under reductive conditions. Consequently, employing these substances to design and construct smart corrosion inhibitors presents a highly suitable approach to directly respond to the electrochemical corrosion process.74,79
3 Different types of smart corrosion inhibition system and their applications
3.1 Design and application of pH-responsive inhibitor systems
The alteration in pH serves as one of the most effective triggers for smart corrosion inhibitors. These inhibitors can respond to local acid–base fluctuations within the microanode/cathode region by releasing corrosion inhibitors, thereby halting the progression of corrosion. Smart corrosion inhibitors are often integrated with coatings to create complex anti-corrosive layers. Depending on the pH range of the reaction, these smart inhibitors can be categorized as acidic-sensitive, alkaline-sensitive, and mixed-sensitive. Furthermore, based on the assembly method, they can be classified into the following types: polyelectrolyte type, doped pH-sensitive component type, valve type, and grafting via covalent bond type.As shown in Fig. 4, acid-sensitive polyelectrolytes, such as Eudragit E100, begin to dissolve when the pH is below 5 and the dissolution rate increases as the acidity increases.80 PSS–PAH polyelectrolyte is usually used as a capsule wall because macromolecules can penetrate the capsule at low pH (<6), while they are excluded at high pH (>8), and this transition is reversible. The integration of carbon hollow spheres, melamine, formaldehyde, and PTT is a common structure for developing smart corrosion inhibitors. This is based on the observation that ester groups hydrolyze more effectively in alkaline conditions. Therefore, these microcapsules exhibit higher corrosion inhibitor release rates in alkaline environments.81–84
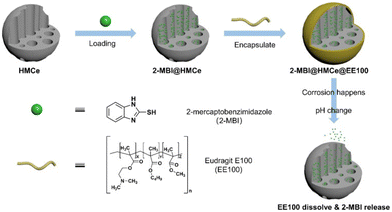 | ||
| Fig. 4 Schematic illustration of the pH-responsive inhibitor system with Eudragit E100. Reproduced with permission80 from Elsevier. | ||
The introduction of corrosion inhibitors such as cerium nitrate or 8-hydroxyquinoline (8-HQ) into microcapsules can provide a self-healing function. 8-HQ is released to form complexes with magnesium ions, which can precipitate and block the corrosion site. Under ultraviolet light, Mg(8-HQ)2 also exhibits fluorescent characteristics in scratched areas.83 Certain polyelectrolyte microcapsules demonstrate responsive release under both acidic and alkaline conditions. This phenomenon is attributed to the increased concentration of cations or anions as the pH value decreases or increases, respectively. The additional positive or negative charge preferentially adsorbs to the negatively or positively charged polyelectrolytes, disrupting the electrostatic interaction balance between polyelectrolytes with opposite charges. This increase in electrostatic repulsion between the polyelectrolyte chains elongates their mutual distance, leading to the swelling of the shell. For instance, a corrosion inhibitor microcapsule composed of DDAC/SPSS/(BTA/SPSS)2/PDDAC/SPSS maintains stability in near-neutral pH environments (5–9). In this instance, the electrostatic forces between BTA, SPSS, and PDDAC, which have opposite charges, reach equilibrium. This results in a contracted state of the multilayer film while storing the BTA inhibitor within the shell. However, when the solution pH is either below 2 or above 11, the polyelectrolyte membrane expands and BTA is released, achieving corrosion inhibition rates of 64% and 71%, respectively.85
Deprotonation of polyelectrolytes serves as an alternative method to achieve pH responsiveness. The microcapsules are composed of poly(acrylic acid) (PAA) and polymethacrylic acid (PMA), which dissolve at a pH above 11.5 or below 2.5, respectively (Fig. 5). As the pH decreases, the carboxyl groups of PMA progressively become protonated, leaving only a minimal number of ionic pairs between PAH and PMA. This process results in an uncompensated ammonium group within the polycyclic aromatic hydrocarbon. The electrostatic repulsion between the positive charges causes the entire structure to swell. Furthermore, the attraction of the polycyclic aromatic hydrocarbon for counterions can increase the local osmotic pressure, thereby enhancing the swelling effect.86
 | ||
| Fig. 5 Swelling model of (PAH/PMA)2 capsules under acidic conditions. Reproduced with permission86 from Wiley. | ||
The application of polyelectrolytes, in conjunction with coatings, can facilitate the self-healing of these coatings. For instance, when the PEI/PSS + 8-HQ smart microcapsule structure is incorporated into a coating, no anodic activity or corrosion products are detected upon the emergence of local defects on the coating surface. This suggests that the release of 8-HQ serves to protect the substrate.87 Nanocontainers with a SiO2/PEI/PSS/BTA/PSS/BTA layered structure were used in combination with zirconium silicate-based hybrid films as anticorrosive coatings.88 The formation of SiO2–Al bonds between the silica and the substrate improved the adhesion between the coating and the metal substrate. Artificial defects were introduced into the coating, and after 24 hours of immersion, significant cathodic activity was observed at the induced defects for undoped ZrOx/SiOx thin film coatings. The activity increased with increasing immersion time. In contrast, doped ZrOx/SiOx film coatings exhibited significant cathodic activity only after 24 hours but repassivated again after 2 hours and remained healed after 48 hours. The corrosion inhibitor imidazole was encapsulated in halloysite nanotubes (HNTs) using vacuum sealing technology. Another inhibitor, dodecylamine, was added to a multilayer polyelectrolyte consisting of polyethyleneimine and sulfonated polyetherketone, and HNTs were formed using the LbL technique. These HNTs (3 wt%) were fully dispersed in an epoxy resin matrix to form a smart hybrid self-healing polymeric coating. In comparison with the control coating, the modified coating and the hybrid coating showed corrosion inhibition efficiencies of 92% and 99.8%, respectively.26
Silk fibroin ionic microcapsules were successfully synthesized using the method of ion pairing and covalent cross-linking. These microcapsules demonstrated significant, highly reversible pH-responsive behavior, with a maximum volume swelling ratio of 800% under conditions where the pH was either lower than 2.0 or higher than 11.0. When the pH value decreased below 2.5, the zeta potential increased to positive due to the carboxylic groups on SF-PG being protonated below their pKa. Conversely, when the pH exceeded 9, the zeta potential of the microcapsule became excessively negative due to the amino groups on SF-PL being deprotonated. Moreover, these silk fibroin ionic microcapsules exhibit pH-triggered permeability, thereby facilitating pH-controlled encapsulation and release.48 By integrating ester groups and disulfide bonds, acid–base-sensitive smart responsive microcapsules can be designed and prepared, which hydrolyze into a shell matrix under both alkaline and acidic conditions.89 Lignin was utilized to construct a smart responsive corrosion inhibitor, LMS@BTA. Under alkaline conditions, the deprotonation of BTA accelerated the dissolution and solubilization of lignin and disrupted the hydrogen bonds between lignin and BTA, resulting in rapid BTA release. Interestingly, although BTA is a neutral molecule, under acidic conditions, BTA becomes protonated cations carrying a large number of positive charges, causing BTA molecules to repel each other and be released from LMS@BTA. Additionally, the concentration difference of BTA inside and outside the microspheres facilitates rapid release followed by slow release.
By creating artificial scratches on the surfaces of the WEP coating and the LMS@BTA/WEP coating, the self-repairing properties of the coatings are tested. After soaking for 48 hours, the Z0.01Hz value of LMS@BTA/WEP is six times that of the WEP coating, indicating that it possesses good anti-corrosion and self-healing properties.90 Resin, primarily composed of resorcinol, exhibits alkaline sensitivity. By utilizing this resin as a wall material and NaNO2 as an inhibitor, pH-sensitive microcapsules can be prepared. When the pH exceeds the pKa value of 7.2 for the resin acid, it facilitates the dissociation of the resin acid in its deprotonated state. At an elevated pH of 12.6 and 9.1, there is a greater release of the microcapsules, with a rate of 44% over a span of 350 hours. As shown in Fig. 6, the OH− present in the solution bind to and dissolve portions of the shell layer, thereby increasing the porosity of the microcapsules and enhancing the diffusion of the encapsulated substances.91
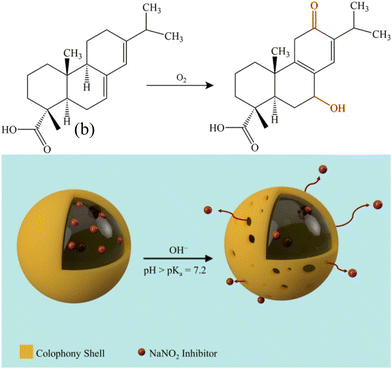 | ||
| Fig. 6 Chemical structure of rosinic acid oxidation in solution and schematic illustration of the controlled release mechanism of resin microcapsules containing NaNO2 inhibitor via OH−-induced porosity of the microcapsules. Reproduced with permission91 from ACS. | ||
The nanovalve-type smart corrosion inhibitor system is usually composed of an encapsulating substance, a corrosion inhibitor, and a carrier. The carrier's role is to load the corrosion inhibitor, typically nanotubes or mesoporous materials, while the encapsulating substance seals the carrier to prevent leakage of the corrosion inhibitor. When the pH changes, the encapsulating substance dissolves, releasing the corrosion inhibitor, for instance, by integrating 8-HQ corrosion inhibitor into halloysite nanotubes, encapsulating Cu-8-HQ coordination fillers, and subsequently blending Cu-8-HQ@HNTs with epoxy resin to create a Cu-8-HQ@HNTs/epoxy coating (Fig. 7). At a pH of 3, the cumulative release amount of 8-HQ in Cu-8-HQ@HNTs is approximately 80%. The self-healing ability of the Cu-8-HQ@HNTs/epoxy coating is due to the dissolution of the carbon nanotube end plugs, triggered by acidification in the coating edge area, which is followed by the release of 8-HQ from the carbon nanotubes.92 Cu-BTA-Na2MoO4-HNTs93 and Cu-BTA-MBT-HNTs can also be prepared in accordance with this principle.94
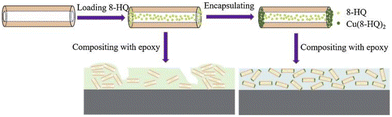 | ||
| Fig. 7 Schematic illustration of the preparation of Cu-8-HQ@HNTs and Cu-8HQ@HNTs/epoxy coatings. Reproduced with permission92 from Elsevier. | ||
A similar structure was achieved by using hollow mesoporous silica spheres (HMSs) as carriers, with benzotriazole (BTA) incorporated as a corrosion inhibitor. As shown in Fig. 8, the HMSs surface was modified with 3-chloropropyltriethoxysilane and 1,4-butanediamine, which can form a host–guest system with CB[6] macrocyles on the HMSs’ surface, resulting in capped functionalized HMSs. In a neutral solution, the 1,4-butanediamine units of the stems exist in a protonated form, allowing them to form highly stable inclusion complexes with CB[6]. The deprotonation degree of the amino group from the stems significantly influenced the initial release rate. The maximum release rate of microcapsules was observed at the most alkaline condition, due to the enhanced extraction of hydrogen ions in alkaline solutions.95
 | ||
| Fig. 8 Schematic illustration of the nanovalve-type smart responsive corrosion inhibitor with CB[6] macrocycle. Reproduced with permission95 from IOP. | ||
The integration of corrosion inhibitors with carriers featuring unique bonds constitutes another method for creating smart corrosion inhibitors. These special bonds are designed to disintegrate under environmental stimuli, triggering the release of the corrosion inhibitor. Mesoporous or layered structures serve as optimal carriers for these inhibitors. Besides mesoporous silica and ceria, other carrier materials such as MOF, graphene-like structured MXenes, hydroxyapatite, and halloysite nanotubes have also been extensively studied.
The release of certain smart corrosion inhibitors is governed by the principle of electrostatic repulsion.18,96–98 Hollow mesoporous zirconium dioxide was synthesized using MBT corrosion inhibitor and silica nanoparticles as a template. Under acidic conditions, both the zirconium dioxide particles and the MBT surfaces possess positive charges. Conversely, they exhibit negative charges under alkaline conditions.98 Nanotube hybrid responsive microcapsules, such as the smart inhibitor nanocontainer, were fabricated using halloysite nanotubes and reduced graphene oxide (rGO). Subsequently, L-histidine (L-His) was adsorbed onto HNTs-rGO via electrostatic adsorption, resulting in a ternary nanocomposite material with pH-responsive properties. L-His and HNTs-rGO carried positive charges within the pH range of 1.82 to 5.5. However, between pH values of 7.59 and 9.17, they both carried negative charges, leading to mutual repulsion. Within the pH range of 5.5 to 7.59, L-His and HNTs-rGO had opposite charges and attracted each other. Consequently, in the release test, even at pH 9, 90.0% of the adsorbed L-His was released. This structure has the potential to extend the lifespan of coatings.99 The incorporation of Fe3+ (FeCl3) into functionalized mesoporous silica activates the cation during the adsorption of corrosion inhibitor ions. Under alkaline conditions, the mesoporous silica post-adsorption of the corrosion inhibitor (MSInh) becomes negatively charged, which facilitates electrostatic repulsion between MSInh and molybdate ions. This results in a more rapid release of these ions under such conditions. Conversely, in acidic environments, manganate and molybdate possess opposing charges. Consequently, their mutual attraction leads to a decreased release of molybdate from MSInh. Therefore, employing MSInh nanocontainers in near-neutral and alkaline media is an effective strategy for enhancing the release of MSInh in corrosive environments (Fig. 9).18 A novel graphene structure based on carbon hollow spheres (CHSs) was also used as microcapsules.100
 | ||
| Fig. 9 Schematic illustration for (a) cylindrical mesoporous silica, (b) mesoporous silica functionalization, (c) FeCl3 compound adsorption, and (d) the corrosion inhibitor adsorption process. Reproduced with permission18 from Elsevier. | ||
MOF structures can release bound corrosion inhibitors in an acidic environment. This is due to the binding of protons, acting as Lewis acids, to the oxygen atoms of the MOF. This interaction disrupts the linkage between the carboxylic group and the metal center of the MOF, leading to the release of BTA. Furthermore, the stable protonated form of the corrosion inhibitor BTA (BTAH2+) in an acidic solution also disrupts the bond between the BTA carboxylic group and the MOF.101,102
When such a structural corrosion inhibitor is used in conjunction with organic tannic acid (TA) and inorganic praseodymium (Pr), an ion exchange process is triggered. This process releases the trapped inhibitor from ZIF8 due to the corrosive action of Na+, Cl−, H+, and OH−. At high pH conditions, Pr3+ and Zn2+ react with OH− to generate a protective hydroxide layer at the cathodic zone. Conversely, at low pH conditions, the formation of Pr-tannic acid-Fe, Zn-tannic acid-Fe, and/or Pr-MI-Fe complexes in a ferrous medium can lead to the formation of an inhibition film at the anodic region.103
MXenes, a type of smart responsive corrosion inhibitor, are exemplified by the Ti3AlC2 substrate with interlayer Ti3C2 MXene sheets formed through HF etching. These thin sheets were subsequently modified with 3-aminopropyltriethoxysilane (APTES) to graft –NH2 groups and coated with cerium ions (Ce3+), serving as an eco-friendly corrosion inhibitor. This was accomplished by utilizing the superior adsorption and intercalation ability of Ti3C2Tx for cations (Fig. 10). The increased release under acidic conditions is ascribed to the protonation of surface functional groups (–NH2, –F, –O, and –OH) of MXene. EIS results showed that after 24 hours of exposure to a normal saline solution, |Z|0.01Hz was approximately 8.9 kΩ cm2 for the epoxy resin coating and approximately 32.1 kΩ cm2 for the MXene-Ce3+@EP coating, indicating a significant improvement in the corrosion resistance of the coatings.49,50
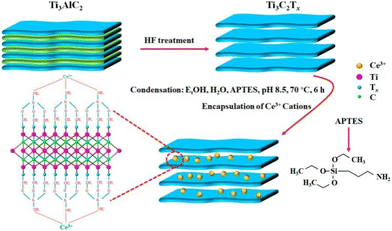 | ||
| Fig. 10 Schematic illustration of the preparation, surface modification, encapsulation principle and inhibition mechanism of Ti3C2 MXene-Ce3+ coating. Reproduced with permission50 from ACS. | ||
Hydroxyapatite (HAP) can be used as a reservoir for various inhibitors, including Ce(III), La(III), salicylaldoxime (Sal), and 8-hydroxyquinoline. Inorganic cations, particularly Ce3+ and La3+, can permeate HAP via an ion exchange mechanism. Organic inhibitors such as Sal and 8-HQ may be adsorbed, while 8-HQ forms a stable complex with Ca2+. The dissolution of HAP by inorganic inhibitors commences at pH values below 6 and is further accelerated at pH below 3.1. However, this strategy does not apply to organic inhibitors (Sal and 8-HQ). Their release begins at microalkaline levels and concludes at pH 2, when HAP is completely dissolved.104
Utilizing the reversibility of Schiff base bonds, recyclable smart corrosion inhibitors were developed. Three representatives, namely the amino acids glycine, alanine, and leucine, were immobilized onto microspheres via the Schiff base reaction. In an acidic environment, glycine was almost entirely released. Subsequent to the release of these amino acids, formaldehyde groups were regenerated, facilitating the recycling of the microspheres. These recycled microspheres exhibited a corrosion inhibition rate of 74.7%.105 By employing an acid-labile β-thiopropionic acid bond, polymerizable derivatives of 8-HQ were synthesized under acidic conditions (pH = 3.5). Notably, these polymer nanoparticles released over 95% of the 8-HQ within a time range of 14 days. Conversely, under neutral conditions (pH = 7.0), the release rate was markedly slower, achieving only 15% in the same time range.51
3.2 Design and application of photoresponsive inhibitor systems
Gold nanoparticles predominantly absorb visible light around 520 nm, while the primary absorption region of silver nanoparticles spans the wavelength range of 380–430 nm. The location of maximum absorption is significantly influenced by particle size and shape.106,107 Upon photoabsorption, nanoparticles generate heat, which subsequently destroys the capsule wall and releases the encapsulated substance. Skirtach et al. pioneered the use of noble metal nanoparticles for optically induced changes in microcapsule shells by integrating silver nanoparticles into PAH/PSS microshells to examine their photoresponsive behavior.53 In recent years, there has been a significant increase in research on doping metal oxide nanoparticles (such as TiO2, ZnO, Fe2O3, Fe3O4) and noble metal nanoparticles (such as AgNPs, AuNPs) onto two-dimensional and three-dimensional LbL multilayer structures.108,109 These photosensitive microcapsule structures are often cored with materials like CaCO3,110,111 and the capsule walls can be composed of polyelectrolytes, liposomes,112 or hydrogels.113Microcapsules of polyelectrolyte structures, such as the (PDADMAC/Au/PSS)4 microcapsule structure, can achieve reversibility. This is achieved by increasing the temperature slightly above the glass transition temperature (Tg) of the polyelectrolyte complex. At elevated temperature, NPs absorb the generated heat, causing the polymer network surrounding the NPs to melt locally, thereby increasing the permeability of the membrane. When the laser is turned off, the melted polyelectrolytes cool below Tg, prompting the nanofilm to self-seal. Under a laser power of 60 mW, 100% of the capsules were found to open.55 Gold nanoparticles do not inherently absorb near-infrared (NIR) light. However, they can be aggregated into larger structures by adding salt ions or other chemicals, resulting in an NIR absorption band. For instance, AuNP aggregates are synthesized using a NaCl solution and hollow shells are constructed with citrate and functionalized polyelectrolytes PDADMAC and PSS. These polyelectrolytes can be functionalized in both homogeneous (non-aggregated) and aggregated (one peak in the NIR spectrum) states. Capsules containing gold particle aggregates exhibit a release of encapsulated materials under low-power NIR laser irradiation, while those without gold particle aggregates show no release at all.108 Laser and pH dual-responsive micro/nano-reservoirs can also be prepared by doping noble metal particles into the polyelectrolyte shell. Compared with pH-stimulated release, laser-stimulated release of corrosion inhibitors is three times faster, which can effectively give better anti-corrosion performance.36
The photoresponsive mechanism of titanium dioxide differs from that of metal nanoparticles, utilizing photocatalytic properties.52 As shown in Fig. 11, polyelectrolyte doped with TiO2 is employed to encapsulate the corrosion inhibitor by forming microcapsules. Upon exposure to ultraviolet light, the TiO2 within the microcapsule becomes excited, generating electron–hole pairs. The holes situated at the valence band of TiO2 exhibit pronounced oxidative activity. Functional groups such as –NH and –SO3−, PSS, and PEI are highly sensitive to variations in the density of the surrounding electrons (or ions). Any alteration in the chemical state of these functional groups can disrupt this relatively weak interaction, ultimately leading to the release of the corrosion inhibitor. Mesoporous TiO2 loaded with BTA is encapsulated by PEI and PSS to form a photoresponsive microcapsule. When this microcapsule is integrated with the coating, it significantly repairs coating defects under ultraviolet light irradiation, and the encapsulated corrosion inhibitor markedly reduces the corrosion process.36 Similar structures have been tested electrochemically, demonstrating that after UV irradiation, the Nyquist diagram successfully reverted to its original shape. The corrosion inhibitor played a supportive role, while a pure TiO2 NPs coating could not restore the corroded surface to its original state.114 Polyelectrolytes with aromatic groups can also be constructed into photoresponsive microcapsules. It has been found that capsules composed of polyelectrolytes containing aromatic groups, such as PSS, undergo significant shrinkage under ultraviolet light irradiation. For instance, upon exposure to 20 mW cm−2 for 60 minutes, the size of five double-layer PDDA/PSS capsules decreased by approximately 80%. Ultraviolet light irradiation triggers chemical changes in aromatic polyelectrolytes, evidenced by the disappearance of aromatic bands and the generation of by-products such as SO42−. These ultraviolet-induced changes in the chemical composition of capsules lead to structural reconstruction and shrinkage.58
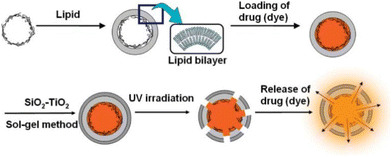 | ||
| Fig. 11 Preparation procedure of drug (dye) controlled release UV-responsive (PSS/PDDA)5/PSS/DDAB/SiO2–TiO2 capsules. Reproduced with permission52 from ACS. | ||
In addition to noble metal particles, infrared dyes and porphyrins can also induce photoabsorption. Silver nanoparticles or an infrared dye were integrated into the polyelectrolyte shell of PAH/PSS capsules. For those doped with an infrared dye, where the dye is only adsorbed on the outer layer, a higher intensity was necessary to activate these capsules using a laser compared with those containing silver nanoparticles. The observed effect for Ag nanoparticle-doped capsules was due to the direct local heating of the nanoparticles, whereas IR-806 dye-doped capsules ruptured due to heating induced by electron–vibration–phonon energy conversion.53
Photosensitive microcapsules can be constructed from photoisomerizable or photo-oxidized substances. Azobenzene (azo), a class of molecules that react to both near-ultraviolet and visible light, is composed of two phenyl groups linked by an N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. The ultraviolet absorption reaction of azobenzene occurs through a reversible internal rearrangement known as cis–trans isomerization. This isomerization process is typically reversible. Recent studies have demonstrated that lipid vesicles containing azo groups can germinate into multiple vesicles under ultraviolet light and fuse with each other under green light. Diazo resin (DZR) forms covalent bonds with polyacrylic acid or PSS in LbL films under ultraviolet light. Similarly, the composite shell of DZR and PSS can also polymerize under ultraviolet light. The resulting crosslinked DZR/PSS microcapsules exhibit superior mechanical stability compared with those not exposed to light. Taking advantage of this property, photoresponsive microcapsules with a structure of (PAH/PAZO)3PAH/PVS were prepared. After 10 hours of light exposure, their diameter decreased to approximately 45% of the original size.57,110
N double bond. The ultraviolet absorption reaction of azobenzene occurs through a reversible internal rearrangement known as cis–trans isomerization. This isomerization process is typically reversible. Recent studies have demonstrated that lipid vesicles containing azo groups can germinate into multiple vesicles under ultraviolet light and fuse with each other under green light. Diazo resin (DZR) forms covalent bonds with polyacrylic acid or PSS in LbL films under ultraviolet light. Similarly, the composite shell of DZR and PSS can also polymerize under ultraviolet light. The resulting crosslinked DZR/PSS microcapsules exhibit superior mechanical stability compared with those not exposed to light. Taking advantage of this property, photoresponsive microcapsules with a structure of (PAH/PAZO)3PAH/PVS were prepared. After 10 hours of light exposure, their diameter decreased to approximately 45% of the original size.57,110
Photoresponsive microcapsules can also be fabricated using the salt-sensitive star polyelectrolyte. The K3[Co(CN)6] salt possesses the capability to decompose into monovalent and divalent ions when subjected to UV light irradiation (eqn (1)). (PSS/PMETAI18)n photosensitive microcapsules were synthesized for which permeability can be adjustable. Introducing a minor quantity of K3[Co(CN)6] salt markedly diminished the permeability of the (PSS/PMETAI18)n shell, leading to a notable reduction in pore size. The presence of trivalent hexacyanocobaltate(III) ions induced the collapse of the PMETAI star polyelectrolyte at low concentrations, potentially due to the combined effects of arm chain collapse and inter-star attraction between PMETAI18 stars. Subsequently, the decomposition of K3[Co(CN)6] salt into monovalent and divalent ions under UV light restored the permeability and size of the (PSS/PMETAI18)n microcapsules. This photoinduced alteration in microcapsule permeability is entirely reversible.59
 | (1) |
3.3 Design and application of ion-responsive inhibitor systems
Cationic type predominantly exchange with Na+ and K+. For instance, in the Zn2Fe(CN)6 structure, Zn2+ is replaced by K+ and Na+ in solution. As shown in eqn (2), the Zn2+ liberated from Zn2Fe(CN)6 forms a protective layer on the cathode, thereby mitigating localized corrosion.117,118 After two weeks of immersion, the impedance of the coating containing Zn2Fe(CN)6 remained largely unchanged, indicating superior anticorrosion performance. In contrast, the impedance of the coating devoid of Zn2Fe(CN)6 decreased by 50 times.37 Numerous researchers have successfully modified montmorillonite to construct Ce(III)-MMT ion-responsive structures. At shorter immersion durations (1 h), the low-frequency impedance increased by two orders of magnitude compared with unmodified silanes.119 Similarly, sodium montmorillonite (Na-MMT) can serve as a nanocontainer (Fig. 12) to load Zn2+ and imidazole inhibitors, thereby enhancing the protective properties of epoxy resin coatings.120 Natural bentonite clay minerals can also be employed as novel cation exchange carriers.121 Ca2+ can function as exchange ions, as evidenced by their exchange with bituminous clay minerals. When the Ca2+-BDT sample was immersed in a 0.1 M NaCl solution, nearly all of the Ca2+ was released after 15 h. Even after soaking in the same solution for 20 h, Ca2+ release persisted. After interacting with a 0.1 M NaCl solution for 20 h, the inhibition efficiency of Ca2+-BDT reached 91%.122
| 2Zn2Fe(CN)6 + 2R+ + xH2O ⇌ R2Zn3[Fe(CN)6]2·xH2O + Zn2+ | (2) |
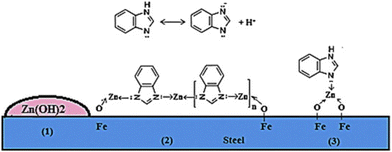 | ||
| Fig. 12 Adsorption model of inhibitors released by Zn-MMT + BIA-MMT within artificial defects on steel surfaces. Reproduced with permission120 from Elsevier. | ||
The efficacy of bentonite pigments with exchangeable Zn2+ in inhibiting corrosion is markedly enhanced. In contrast, smart-release bentonite pigments containing exchangeable cerium(III) and yttrium(III) cations demonstrate negligible effectiveness in decelerating the propagation rate of filiform corrosion (FFC) induced by chloride.63 Nonetheless, anion-exchanged hydrotalcite (HT)-based pigments emerge as potent inhibitors for FFC. In a NaCl solution, magnesium aluminum layered double hydroxide laden with tungstate anion, LDH-WO42−, adsorbs corrosive chloride ions while simultaneously releasing interlayer corrosion inhibitor WO42− anions, achieving an inhibition rate of up to 97%. A structurally analogous system features a reservoir composed of nanostructured Mg/Al and Zn/Al layered double hydroxides, with the interlayer region housing isocyanate anions. These nanocrystalline layered double hydroxides can regulate the release of vanadate ions, exhibiting commendable corrosion inhibition properties.65 Furthermore, Zn/Al doped LDH nanocontainer (Fig. 13) coatings demonstrate notable self-healing capabilities.61,123 Amphoteric exchangeable ions have also been extensively researched. For instance, it has been determined that amphoteric Zn–Al-[V10O28]6− hydrotalcite ion exchange compounds can facilitate the exchange of vanadate with Cl− and Zn2+ with Na+ when subjected to NaCl solutions ranging from 0.0001 M to 1.0 M.124
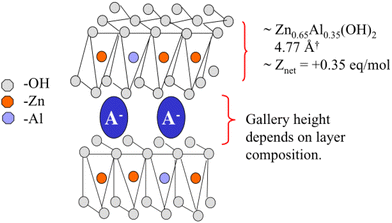 | ||
| Fig. 13 Schematic illustration of the layered structure of a hydrotalcite compound. The structure consists of alternating layers of positively charged mixed metal hydroxide sheets and anions. Reproduced with permission61 from Elsevier. | ||
3.4 Design and application of magnetic-responsive inhibitor systems
Magnetic nanoparticles (NPs) hold potential for applications in magnetic fluids, catalysis, biotechnology/biomedicine, magnetic resonance imaging (MRI), data storage, and environmental remediation.78,125,126 However, their intrinsic instability over extended periods and propensity to aggregate constrain their utility. Coating the surface of magnetic NPs with polymers, silica, or other materials can prevent aggregation and facilitate functionalization. Among these coating materials, silica and carbon are particularly appealing due to their high biocompatibility and stability, low toxicity, and straightforward functionality.127 The discovery of supramolecular self-assemblies of mesoporous materials has sparked significant interest across various research fields.127–130 Magnetic mesoporous materials have been the focus of attention for integrating mesoporous structures with magnetic properties to achieve a magnetic response, high pore volume, large surface area, and ease of functionalization.38,131–139 Magnetic nanocomposites with mesoporous structures can be removed after use by applying a magnetic field, or targeted delivery can be achieved using an external magnetic field. A new class of self-healing magnetic microcapsules was designed and synthesized from poly(urea formaldehyde) encapsulated in BTA and magnetic multi-wall carbon nanotubes (MWCNTs).38 The magnetic targeted microcapsule coating (SMG) was prepared using the microcapsules. The magnetic microcapsules could migrate rapidly through the coating solution by applying a magnetic field. The SMG coating not only shortened the migration route of the released BTA, but also rapidly accelerated the formation of passivation film and hindered the corrosion of copper after mechanical damage of the coating. The corrosion inhibition rate of the magnetic self-healing coating could reach 91.2% within 4 h of immersion. Its self-healing efficiency was 6.4 times higher than that of the non-magnetic self-healing coating. Ferromagnetic gold-coated cobalt nanoparticles (Co@Au) embedded within the polyelectrolyte capsule wall can form magnetically sensitive microcapsules. (PSS/PAH)4(PSS/Co@Au)1(PSS/PAH)6 microcapsules were 99% filled with FITC-dextran after 30 min of an alternating magnetic field, indicating that permeability of the microcapsules was caused by the low-frequency alternating magnetic field, allowing macromolecules normally excluded from the capsules to penetrate into the capsules. At frequencies less than 300 Hz, the lower the frequency, the faster the diffusion of FITC-dextran. The increase in frequency appeared to diminish the stirring effect of the magnetic field on the Co@Au nanoparticles embedded in the capsule wall, thus reducing the permeability of the magnetic capsule131 (Fig. 14). Magnetic fluids can also be composed of polyelectrolyte-stabilized magnetite nanoparticles. This new magnetic fluid has potential for in vivo MRI diagnostics.132 | ||
| Fig. 14 Scheme of Co@Au nanoparticle microcapsule assembly and magnetic permeability test under an oscillating magnetic field. Reproduced with permission131 from ACS. | ||
3.5 Design and application of temperature-responsive inhibitor systems
Smart nanocontainers can be engineered using stimulus-responsive polymers that exhibit temperature sensitivity. Capsules composed of the robust polyelectrolytes PDADMAC and PSS (with PDADMAC forming the first layer) display intriguing temperature-dependent behavior. Shells with an even number of layers, denoted as (PDADMAC/PSS)x where x ranges from 1 to 3, contract upon heating due to increasing wall thickness until they solidify into spheres. Conversely, shells with an odd number of layers, represented by (PDADMAC/PSS)x, expand up to five times their original size before rupturing above 55 °C. The swelling of PDADMAC/PSS microcapsules is attributed to the presence of a large number of positively charged monomers in PDADMAC end-sealed capsules. The inclination to reduce surface tension is counterbalanced by electrostatic forces, specifically repulsion between excess charges, resulting in swelling. In contrast, PSS end-sealed capsules maintain near charge balance. The primary force driving the contraction of charge-balanced capsules is the minimization of the water–polymer interfacial energy.133 Silicon dioxide/polymer double-walled hybrid nanocapsules have been synthesized. Smart containers with controlled release capabilities are achieved by modulating the polymer shell. For instance, these nanocontainers can exhibit thermal responsiveness by incorporating poly(N isopropylacrylamide-co-methylene bisacrylamide) shells. At 25 °C, PNIPAM shells tend to expand, while at 50 °C, they contract, effectively sealing BTA molecules within the lumen and inhibiting their diffusion out of the shell.1343.6 Design and application of redox-responsive inhibitor systems
The most dependable stimulus is the alteration in electrochemical potential, as it is generated in situ, uniformly distributed and highly consistent with the actual situation. In contrast to barrier polymers, intrinsically conductive polymers, such as polypyrrole (PPy) and polyaniline (PANI), possess an inherent oxidizing capacity. Redox-sensitive PANI can be employed as a shell material to fabricate redox-responsive microencapsulated capsules.135 The microcapsules were constructed with the corrosion inhibitor 3-nitrosalicylic acid (3-NisA) as the core material and modified with gold nanoparticles to prevent direct contact between the metal and CP. When no potential was applied (simulating a complete coating), the polyaniline shell layer functioned as a gatekeeper to inhibit the release of the corrosion inhibitor from the core. The reduction of PANI (applying a low potential to simulate the onset of corrosion) resulted in an increase in the shell's permeability and the release of the inhibitor. Conversely, re-oxidation (application of a high potential to simulate metal passivation) led to a decrease in permeability and further release was prevented. Disulfide bonds are relatively stable under mildly oxidizing conditions but tend to break under reducing conditions. Microcapsules containing disulfide bonds were designed and developed. TBP was used as a reducing agent to break the disulfide bonds. The nanocapsules successfully released approximately 90% of the corrosion inhibitor MBT after 5 hours. In the absence of TBP triggering, no release of MBT was detected.79 A sophisticated microcapsule, termed CP-SNCs, has been engineered to exhibit a responsiveness to corrosion potential stimuli and an expedited positioning capability.74 This structure incorporates a supramolecular component, specifically a water-soluble bipyridine column aryl hydrocarbon, onto the external surface of a magnetic nanocarrier (Fe3O4@mSiO2), which is connected via a disulfide linkage. Upon application of the magnesium alloy's corrosion potential (−1.5 V vs. SHE), 8-HQ is immediately released due to the disintegration of the disulfide linker and the removal of the supramolecular assemblies. These CP-SNCs were integrated into an organic–inorganic hybrid sol–gel coating to create corrosion potential-stimulated feedback anticorrosion coating (CP-SFAC), which was subsequently applied to the magnesium alloy AZ31B. In the presence of a magnetic field, the CP-SNCs aggregate near the AZ31B surface. The CP-SFAC demonstrates superior anti-corrosion properties and, notably, possesses a rapid self-healing function when localized corrosion occurs by a microzone electrochemical technique.3.7 Design and application of multiple-responsive inhibitor systems
Smart response structures with dual or multiple responses are designed with the idea of assembling different response substances. For example, polyelectrolyte is combined with benzophenone (BP) moieties and gold nanoparticles to form a pH–light dual response. Polyelectrolyte is combined with disulfide bonds, polyaniline, polypyridine, HMON and other materials to form pH–redox double responsive system and so on. In addition, some substances exhibit dual responses simultaneously, such as Cu-BTA complex nanovalves that respond to both pH and S2−.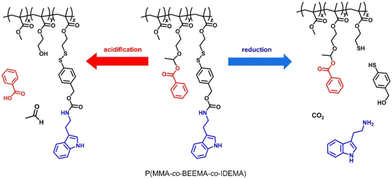 | ||
| Fig. 15 Selective release of the corrosion inhibitors benzoic acid (red) and tryptamine (blue) from a doubly reactive terpolymer by acidification and reduction. Reproduced with permission137 from ACS. | ||
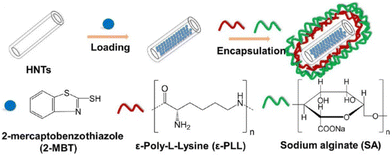 | ||
| Fig. 16 Schematic illustration of the fabrication process of smart microcapsules (MCs). Reproduced with permission147 from ACS. | ||
3.8 Other types
CO2 is a universally corrosive gas, so a smart corrosion inhibitor sensitive to carbon dioxide is very necessary. Since nitrogen atoms of tertiary amine groups can reversibly bind to CO2, a number of polymers containing tertiary amine groups have been developed, such as poly(diethylaminoethyl methacrylate) (PDEAEMA) and poly(dimethylaminoethyl methacrylate) (PDMAEMA), which are the most studied CO2-responsive polymers. Jixing Wang et al.39 successfully polymerized DMAEMA and developed an ethylcellulose-based coating with pH-responsive self-healing properties (Fig. 17). In a 3.5 wt% NaCl solution without CO2, the coating loaded with thiourea@PHMs slowly releases the preservative, thereby improving the corrosion resistance of the scratch coating. What is more, in a 3.5 wt% NaCl solution with CO2, which is more corrosive than one without CO2, the corrosion resistance of scratch coatings with thioure@PHMs added can be significantly improved because the stimulation of CO2 increases the rate of thiourea release. Much research has focused on biomedicine and controllable oil/water separation. Certain researchers have employed PDDA, PAH, and PSS as the walls of the capsule, with PAH serving as the third layer to enhance the fluorescence quantity of PSS–rhodamine. During the assembly process, oligoamine II was integrated into the capsule wall as a cationic compound. The study found that the microcapsules were initially opaque to the dye in the absence of CO2 and N2. However, after 20 minutes’ exposure to CO2, nearly all the microcapsules became transparent to the dye. In contrast, only partial effects were observed following 5 or 10 minutes of CO2 exposure.149 | ||
| Fig. 17 Mechanism of the CO2 stimulus response. Reproduced with permission39 from Elsevier. | ||
A novel pH-responsive luminescent microcapsule utilizing quantum dots is designed. Utilizing dihydrolipoic acid (DHLA)-stabilized quantum dots (QDs) as templates, LbL modification of the QDs was conducted with poly(acrylamine hydride) (PAH), poly(ethylenimine) (PEI) cationic polyelectrolyte, and poly(diallyldimethylammonium chloride) (PDADMAC). Additionally, anionic polyelectrolytes such as poly(acrylic acid) (PAA), poly(sodium-4-styrenesulfonate) (PSS), and polyvinyl sulfonic acid (PVSA) were employed to form PAH-DHLA-QDs. These exhibited pH-dependent luminescence in a 50 mM Tris buffer ranging from pH 3 to 9 (Fig. 18).150
 | ||
| Fig. 18 (A) TOPO-QDs in chloroform; (B) anionic water-soluble DHLA-QDs; (C) cationic polyelectrolyte-coated quantum dots; (D) anionic polyelectrolyte-coated quantum dots. Reproduced with permission150 from ACS. | ||
4 Conclusions and perspectives
Smart corrosion inhibitor systems present a milestone in the development of corrosion inhibitor anti-corrosion, overcoming the shortcomings of conventional corrosion inhibitors and conventional coating anti-corrosion. In this review, we summarized recent progress on the design and application of smart corrosion inhibitor systems based on pH, light, ion, magnetism, temperature, redox reactions, and multiple responses.There are several advantages of applying smart responsive corrosion inhibitor systems: (i) compared with ordinary corrosion inhibitors, it can achieve a targeted response, avoiding the waste of chemicals caused by aimless release; (ii) the smart corrosion inhibitor can be combined with the coating for anti-corrosion, which saves the labour cost brought by filling compared with the corrosion inhibitor, and makes up for the increased maintenance cost and the transportation loss caused by the shutdown caused by the damaged coating; and (iii) personalized customization can be carried out based on the corrosion characteristics of the corrosion conditions.
In the future, smart corrosion inhibitors will have broad applications in marine engineering, aerospace, military equipment, oil and gas exploitation, water treatment, targeted transport and release of medical drugs, and so on.
Although the development prospects of smart corrosion inhibitor systems are very broad, there are still some limitations or challenges that limit their further development and practical applications, and scholars need to conduct further research. Firstly, considering the release speed and capability of smart corrosion inhibitors, they are currently ideal for use in closed systems. If the released corrosion inhibitor is continuously discharged, the concentration of the inhibitor cannot meet the requirements for forming a dense film layer, which is a limiting factor for application in open systems. Secondly, when the smart corrosion inhibitor/smart corrosion inhibitor + carrier is combined with the coating, it needs to be highly compatible and cannot affect the barrier effect of the coating itself. Thirdly, current research on the performance of smart corrosion inhibitors only considers the influence of simple factors, while in practical applications, specific working conditions such as high temperature, high pressure, and concentrated salt environments need to be considered for their performance impact. Finally, in the construction of smart systems, it is necessary to consider simple, stable, and safe synthesis methods as well as economic issues. Moreover, it should become popular to incorporate artificial intelligence to the design of smart inhibitor system.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the support by the NSFC (No. 21975205, 21504068), Key Research and Development Program of Shaanxi (2024GX-YBXM-383), Shaanxi Academy of Fundamental Sciences (Chemistry and Biology, 23JHQ086) and Open Project of State Key Laboratory of Supramolecular Structure and Materials (sklssm2024033). We also appreciate the funding support from the College Students’ Innovative Entrepreneurial Training Plan Program.References
- A. A. Al-Amiery, W. Isahak and W. K. Al-Azzawi, Lubricants, 2023, 11, 174 CrossRef CAS.
- M. Wasim and M. B. B. Djukic, J. Nat. Gas Sci. Eng., 2022, 100, 104467 CrossRef CAS.
- Y. Ding, X. W. Ye, H. Zhang and X. S. Zhang, Steel Compos. Struct., 2024, 50, 363–374 Search PubMed.
- M. Finsgar and J. Jackson, Corros. Sci., 2014, 86, 17–41 CrossRef CAS.
- X. Y. Wang, S. Liu, J. Yan, J. P. Zhang, Q. Y. Zhang and Y. Yan, Materials, 2023, 16, 2954 CrossRef CAS PubMed.
- S. Liu, J. Yan, J. Q. Shi, X. Z. Li, J. P. Zhang, X. Y. Wang, N. J. Cai, Q. H. Fang, Q. Y. Zhang and Y. Yan, Polym. Chem., 2023, 14, 330–342 RSC.
- J. Yan, X. Z. Li, X. Zhang, S. Liu, F. L. Zhong, J. P. Zhang, Q. Y. Zhang and Y. Yan, ACS Appl. Polym. Mater., 2022, 4, 3844–3854 CrossRef CAS.
- M. F. Montemor, Surf. Coat. Technol., 2014, 258, 17–37 CrossRef CAS.
- F. Zhang, P. F. Ju, M. Q. Pan, D. W. Zhang, Y. Huang, G. L. Li and X. G. Li, Corros. Sci., 2018, 144, 74–88 CrossRef CAS.
- H. Cai, P. Wang and D. Zhang, J. Oceanol. Limnol., 2020, 38, 1045–1063 CrossRef CAS.
- A. Stankiewicz, I. Szczygieł and B. Szczygieł, J. Mater. Sci., 2013, 48, 8041–8051 CrossRef CAS.
- Y. Le, P. Hou, J. Wang and J.-F. Chen, Mater. Chem. Phys., 2010, 120, 351–355 CrossRef CAS.
- E. Abdullayev, R. Price, D. Shchukin and Y. Lvov, ACS Appl. Mater. Interfaces, 2009, 1, 1437–1443 CrossRef CAS PubMed.
- Z. Zheng, X. Huang, M. Schenderlein, D. Borisova, R. Cao, H. Möhwald and D. Shchukin, Adv. Funct. Mater., 2013, 23, 3307–3314 CrossRef CAS.
- A. H. Jafari, S. M. A. Hosseini and E. Jamalizadeh, Electrochim. Acta, 2010, 55, 9004–9009 CrossRef CAS.
- A. Khajouei, E. Jamalizadeh and S. M. A. Hosseini, Anti-Corros. Methods Mater., 2015, 62, 88–94 CrossRef CAS.
- J. Y. Yap, S. Mat Yaakob, N. E. Rabat, M. R. Shamsuddin and Z. Man, RSC Adv., 2020, 10, 13174–13184 RSC.
- M. Yeganeh, M. Saremi and H. Rezaeyan, Prog. Org. Coat., 2014, 77, 1428–1435 CrossRef CAS.
- M. Yeganeh and M. Saremi, Prog. Org. Coat., 2015, 79, 25–30 CrossRef CAS.
- C. J. Ward, M. DeWitt and E. W. Davis, in Nanomaterials for Biomedicine, 2012, pp. 209–238, DOI:10.1021/bk-2012-1119.ch010.
- M. L. Zheludkevich, J. Tedim and M. G. S. Ferreira, Electrochim. Acta, 2012, 82, 314–323 CrossRef CAS.
- A. B. M. da Cunha, D. A. Leal, L. R. L. Santos, I. C. Riegel-Vidotti and C. E. B. Marino, Surf. Coat. Technol., 2020, 402, 126338 CrossRef CAS.
- T. Matsuda, K. B. Kashi, K. Fushimi and V. J. Gelling, Corros. Sci., 2019, 148, 188–197 CrossRef CAS.
- K. E. Broaders, S. J. Pastine, S. Grandhe and J. M. Frechet, Chem. Commun., 2011, 47, 665–667 RSC.
- D. I. Njoku, M. Cui, H. Xiao, B. Shang and Y. Li, Sci. Rep., 2017, 7, 15597 CrossRef PubMed.
- A. Khan, A. Hassanein, S. Habib, M. Nawaz, R. A. Shakoor and R. Kahraman, ACS Appl. Mater. Interfaces, 2020, 12, 37571–37584 CrossRef CAS PubMed.
- F. Olivieri, R. Castaldo, M. Cocca, G. Gentile and M. Lavorgna, ACS Appl. Mater. Interfaces, 2021, 13, 48141–48152 CrossRef CAS PubMed.
- A. S. Najibzad, R. Amini, M. Rostami, P. Kardar and M. Fedel, Prog. Org. Coat., 2020, 140, 105504 CrossRef CAS.
- Y. Jin, H. Duan, S. Zhan, J. Tu, T. Yang, W. Zhang, L. Ma, H. Yu and D. Jia, ACS Appl. Mater. Interfaces, 2023, 15, 52971–52983 CAS.
- A. Khan, A. Hassanein, S. Habib, M. Nawaz, R. A. Shakoor and R. Kahraman, ACS Appl. Mater. Interfaces, 2020, 12, 37571–37584 CrossRef CAS PubMed.
- M. Ghaderi, A. R. SaadatAbadi, M. Mahdavian and S. A. Haddadi, Langmuir, 2022, 38, 11707–11723 CrossRef CAS PubMed.
- B. Qian, Z. Zheng, M. Michailids, N. Fleck, M. Bilton, Y. Song, G. Li and D. Shchukin, ACS Appl. Mater. Interfaces, 2019, 11, 10283–10291 CrossRef CAS PubMed.
- C. Liu, B. Qian, P. Hou and Z. Song, ACS Appl. Mater. Interfaces, 2021, 13, 4429–4441 CrossRef CAS PubMed.
- K. Thongchaivetcharat, S. Salaluk, D. Crespy, H. Therien-Aubin and K. Landfester, ACS Appl. Mater. Interfaces, 2020, 12, 42129–42139 CrossRef CAS PubMed.
- E. V. Skorb, D. V. Sviridov, H. Mohwald and D. G. Shchukin, Chem. Commun., 2009, 6041–6043, 10.1039/b914257f.
- A. G. S. Ekaterina, V. Skorb, D. V. Sviridov, D. G. Shchukin and H. Möhwald, ACS Nano, 2009, 3, 1753–1760 CrossRef PubMed.
- J. Li and R. Buchheit, Prog. Org. Coat., 2017, 104, 210–216 CrossRef CAS.
- W. Wang, W. Li, W. Fan, X. Zhang, L. Song, C. Xiong, X. Gao and X. Liu, Chem. Eng. J., 2018, 332, 658–670 CrossRef CAS.
- J. Wang, J. Tang, H. Zhang, Y. Wang, H. Wang, B. Lin, J. Hou and H. Zhang, Corros. Sci., 2021, 180, 109194 CrossRef CAS.
- N. Y. Abu-Thabit and A. S. Hamdy, Surf. Coat. Technol., 2015, 406–424 Search PubMed.
- M. Samadzadeh, S. H. Boura, M. Peikari, S. M. Kasiriha and A. Ashrafi, Prog. Org. Coat., 2010, 68, 159–164 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS.
- L. Guo, C. Verma and D. Zhang, Eco-Friendly Corrosion Inhibitors: Principles, Designing and Applications, Elsevier, 1st edn, 2022 Search PubMed.
- S. Liu, J. Yan, J. Shi, X. Li, J. Zhang, X. Wang, N. Cai, Q. Fang, Q. Zhang and Y. Yan, Polym. Chem., 2023, 14, 330–342 RSC.
- J. Yan, X. Zhang, P. Xu, J. Zhang, X. Li, F. Zhong, Y. Xu, Q. Zhang, Y. Zhu and Y. Yan, Adv. Mater. Technol., 2024, 2401559, DOI:10.1002/admt.202401559.
- J. Yan, X. Li, X. Zhang, S. Liu, F. Zhong, J. Zhang, Q. Zhang and Y. Yan, ACS Appl. Polym. Mater., 2022, 4, 3844–3854 CrossRef CAS.
- X. Wang, S. Liu, J. Yan, J. Zhang, Q. Zhang and Y. Yan, Materials, 2023, 16, 2954 CrossRef CAS PubMed.
- C. Ye, O. Shchepelina, R. Calabrese, I. Drachuk, D. L. Kaplan and V. V. Tsukruk, Biomacromolecules, 2011, 12, 4319–4325 CrossRef CAS PubMed.
- M. Ghidiu, J. Halim, S. Kota, D. Bish, Y. Gogotsi and M. W. Barsoum, Chem. Mater., 2016, 28, 3507–3514 Search PubMed.
- S. A. Haddadi, S. Hu, S. Ghaderi, A. Ghanbari, M. Ahmadipour, S. Y. Pung, S. Li, M. Feilizadeh and M. Arjmand, ACS Appl. Mater. Interfaces, 2021, 13, 42074–42093 CrossRef CAS PubMed.
- N. Dararatana, F. Seidi and D. Crespy, ACS Appl. Mater. Interfaces, 2018, 10, 20876–20883 CrossRef CAS PubMed.
- K. Katagiri, K. Koumoto, S. Iseya, M. Sakai, A. Matsuda and F. Caruso, Chem. Mater., 2009, 21, 195–197 CrossRef CAS.
- A. G. Skirtach, A. A. Antipov, D. G. Shchukin and G. B. Sukhorukov, Langmuir, 2004, 20, 6988–6992 CrossRef CAS PubMed.
- B. Radt, T. A. Smith and F. Caruso, Adv. Mater., 2004, 16, 2184–2189 CrossRef CAS.
- P. K. Andre, G. Skirtach, M. F. Bédard, G. B. Sukhorukov and H. Möhwald, J. Am. Chem. Soc., 2008, 130, 11572–11573 CrossRef PubMed.
- A. G. Skirtach, C. Dejugnat, D. Braun, A. S. Susha, A. L. Rogach, W. J. Parak, H. Möhwald and G. B. Sukhorukov, Nano Lett., 2005, 5, 1371–1377 CrossRef CAS PubMed.
- M. Bédard, A. G. Skirtach and G. B. Sukhorukov, Macromol. Rapid Commun., 2007, 28, 1517–1521 CrossRef.
- K. Katagiri, A. Matsuda and F. Caruso, Macromolecules, 2006, 39, 8067–8074 CrossRef CAS.
- W. Xu, I. Choi, F. A. Plamper, C. V. Synatschke, A. H. E. Müller and V. V. Tsukruk, ACS Nano, 2012, 7, 598–613 CrossRef PubMed.
- M. F. Bédard, S. Sadasivan, G. B. Sukhorukov and A. Skirtach, J. Mater. Chem., 2009, 19, 2226–2233 RSC.
- R. G. Buchheit, H. Guan, S. Mahajanam and F. Wong, Prog. Org. Coat., 2003, 47, 174–182 CrossRef CAS.
- X. Guo, F. Zhang, D. G. Evans and X. Duan, Chem. Commun., 2010, 46, 5197–5210 RSC.
- G. W. H. N. McMurray, Corros. Sci., 2004, 60, 219–228 CrossRef.
- J. M. Vega, N. Granizo, D. de la Fuente, J. Simancas and M. Morcillo, Prog. Org. Coat., 2011, 70, 213–219 CrossRef CAS.
- M. L. Zheludkevich, S. K. Poznyak, L. M. Rodrigues, D. Raps, T. Hack, L. F. Dick, T. Nunes and M. G. S. Ferreira, Corros. Sci., 2010, 52, 602–611 CrossRef CAS.
- J. Tedim, S. K. Poznyak, A. Kuznetsova, D. Raps, T. Hack, M. L. Zheludkevich and M. G. Ferreira, ACS Appl. Mater. Interfaces, 2010, 2, 1528–1535 CrossRef CAS PubMed.
- A. R. Aamir, I. Khan, B. Fong, C. Markland, M. O'Brien, G. R. W. Thomas, G. Dunbar and D. O'Hare, Ind. Eng. Chem. Res., 2009, 48, 10196–10205 CrossRef.
- A. N. Ay, B. Zumreoglu-Karan, A. Temel and V. Rives, Inorg. Chem., 2009, 48, 8871–8877 CrossRef CAS PubMed.
- P. Gunawan, R. Xu, H. Curtius, C. Walther, K. Dardenne, K. Ufer and T. Fanghanel, J. Phys. Chem. C, 2009, 113, 17206–17214 CrossRef CAS.
- T. Stumpf, H. Curtius, C. Walther, K. Dardenne, K. Ufer and T. Fanghanel, Environ. Sci. Technol., 2007, 41, 3186–3191 CrossRef CAS PubMed.
- S. L. Wang, R. J. Hseu, R. R. Chang, P. N. Chiang, J. H. Chen and Y. M. Tzou, Colloids Surf., A, 2006, 277, 8–14 CrossRef CAS.
- L. C. Hsu, S. L. Wang, Y. M. Tzou, C. F. Lin and J. H. Chen, J. Hazard. Mater., 2007, 142, 242–249 CrossRef CAS PubMed.
- X. Yu, J. Wang, M. Zhang, L. Yang, J. Li, P. Yang and D. Cao, Surf. Coat. Technol., 2008, 203, 250–255 CrossRef CAS.
- C. Ding, J. Xu, L. Tong, G. Gong, W. Jiang and J. Fu, ACS Appl. Mater. Interfaces, 2017, 9, 21034–21047 CrossRef CAS PubMed.
- D. Borisova, H. Möhwald and D. G. Shchukin, ACS Nano, 2011, 5, 1939–1946 CrossRef CAS PubMed.
- D. V. Andreeva, E. V. Skorb and D. G. Shchukin, ACS Appl. Mater. Interfaces, 2010, 2, 1954–1962 CrossRef CAS.
- M. L. Zheludkevich, D. G. Shchukin, K. A. Yasakau, H. Möhwald and M. G. S. Ferreira, Chem. Mater., 2007, 19, 402–411 CrossRef CAS.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q (Max). Lu, Small, 2011, 7, 425–443 CrossRef CAS PubMed.
- Y. Zhao, R. Berger, K. Landfester and D. Crespy, Small, 2015, 11, 2995–2999 CrossRef CAS PubMed.
- S. Gu, H. Shi, C. Zhang, W. Wang, F. Liu and E.-H. Han, Prog. Org. Coat., 2021, 158, 106376 CrossRef CAS.
- R. Ghamsarizade, A. A. Sarabi, Sh. Roshan and H. E. Mohammadloo, Colloids Surf., A, 2022, 644, 128883 CrossRef CAS.
- M. Alizadeh and A. Sarabi, Prog. Org. Coat., 2019, 134, 78–90 CrossRef CAS.
- P. Ghahremani, A. A. Sarabi and S. Roshan, Surf. Coat. Technol., 2021, 427, 127820 CrossRef CAS.
- G. R. Argade, S. Sanders, G. Mohandass, A. Alsaleh, F. D'Souza, T. D. Golden and R. S. Mishra, J. Mater. Eng. Perform., 2019, 28, 852–862 CrossRef CAS.
- Y. Feng, S. Chen and Y. F. Cheng, J. Mater. Sci., 2017, 52, 8576–8590 CrossRef CAS.
- T. Mauser, C. Déjugnat and G. B. Sukhorukov, Macromol. Rapid Commun., 2004, 25, 1781–1785 CrossRef CAS.
- D. V. Andreeva, D. Fix, H. Mohwald and D. G. Shchukin, Adv. Mater., 2008, 20, 2789–2794 CrossRef CAS PubMed.
- M. L. Zheludkevich, D. G. Shchukin, K. A. Yasakau, H. Möhwald and M. G. S. Ferreira, Chem. Mater., 2007, 19, 402–411 CrossRef CAS.
- T. Matsuda, N. Jadhav, K. B. Kashi, M. Jensen and V. J. Gelling, Prog. Org. Coat., 2019, 132, 9–14 CrossRef CAS.
- Z. Tan, S. Wang, Z. Hu, W. Chen, Z. Qu, C. Xu, Q. Zhang, K. Wu, J. Shi and M. Lu, Ind. Eng. Chem. Res., 2020, 59, 2657–2666 CrossRef CAS.
- J. Ress, U. Martin, J. Bosch and D. M. Bastidas, ACS Appl. Mater. Interfaces, 2020, 12, 46686–46700 CrossRef CAS PubMed.
- M. Wang, J. Wang and W. Hu, Prog. Org. Coat., 2020, 139, 105434 CrossRef CAS.
- X. Xing, J. Wang, Q. Li, W. Hu and J. Yuan, Colloids Surf., A, 2018, 553, 295–304 CrossRef CAS.
- X. Xing, X. Xu, J. Wang and W. Hu, Chem. Phys. Lett., 2019, 718, 69–73 CrossRef CAS.
- T. Chen and J. Fu, Nanotechnology, 2012, 23, 235605 CrossRef PubMed.
- F. Olivieri, F. Scherillo, R. Castaldo, M. Cocca, A. Squillace, G. Gentile and M. Lavorgna, ACS Appl. Polym. Mater., 2023, 5, 5917–5925 CrossRef CAS PubMed.
- L. R. L. Santos, C. E. B. Marino and I. C. Riegel-Vidotti, Eur. Polym. J., 2019, 115, 86–98 CrossRef CAS.
- A. Chenan, S. Ramya, R. P. George and U. Kamachi Mudali, Ceram. Int., 2014, 40, 10457–10463 CrossRef CAS.
- Y. Jia, T. Qiu, L. Guo, J. Ye, L. He and X. Li, Appl. Surf. Sci., 2020, 504, 144496 CrossRef CAS.
- S. A. Haddadi, S. A. A. Ramazani, M. Mahdavian, P. Taheri and J. M. C. Mol, Chem. Eng. J., 2018, 352, 909–922 CrossRef CAS.
- Z. Mohammadpour and H. R. Zare, Cryst. Growth Des., 2021, 21, 3954–3966 CrossRef CAS.
- K. Wei, Y. Wei, Y. Zhang, V. Kasneryk, M. Serdechnova, H. Wang, Z. Zhang, Y. Yuan, C. Blawert, M. L. Zheludkevich and F. Chen, Corros. Sci., 2024, 227, 111731 CrossRef CAS.
- M. Motamedi, S. Mohammadkhah, M. Ramezanzadeh, H. E. Mohammadloo and B. Ramezanzadeh, ACS Appl. Mater. Interfaces, 2022, 14, 31170–31193 CrossRef CAS PubMed.
- D. Borisova, H. Möhwald and D. G. Shchukin, ACS Nano, 2011, 5, 1939–1946 CrossRef CAS PubMed.
- J. Bao, H. Zhang, X. Zhao and J. Deng, Chem. Eng. J., 2018, 341, 146–156 CrossRef CAS.
- A. M. G. Catherine, J. Murphy, S. E. Hunyadi and C. J. Orendorff, Inorg. Chem., 2006, 45, 7544–7554 CrossRef PubMed.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed.
- M. F. Bédard, D. Braun, G. B. Sukhorukov and A. G. Skirtach, ACS Nano, 2008, 2, 1807–1816 CrossRef PubMed.
- C. Lu, H. Möhwald and A. Fery, J. Phys. Chem. C, 2007, 111, 10082–10087 CrossRef CAS.
- M. F. Bedard, B. G. De Geest, A. G. Skirtach, H. Mohwald and G. B. Sukhorukov, Adv. Colloid Interface Sci., 2010, 158, 2–14 CrossRef CAS PubMed.
- B. G. De Geest, A. G. Skirtach, T. R. M. De Beer, G. B. Sukhorukov, L. Bracke, W. R. G. Baeyens, J. Demeester and S. C. De Smedt, Macromol. Rapid Commun., 2007, 28, 88–95 CrossRef CAS.
- D. V. Volodkin, A. G. Skirtach and H. Möhwald, Angew. Chem., Int. Ed., 2009, 48, 1807–1809 CrossRef CAS PubMed.
- J. Kim, H. J. Lim, Y. K. Hwang, H. Woo, J. W. Kim and K. Char, Langmuir, 2012, 28, 11899–11905 CrossRef CAS PubMed.
- X. He, C. Chiu, M. J. Esmacher and H. Liang, Surf. Coat. Technol., 2013, 237, 320–327 CrossRef CAS.
- W. Xiong, J. Tang, G. Zhu, N. Han, E. Schlangen, B. Dong, X. Wang and F. Xing, Sci. Rep., 2015, 5, 10866 CrossRef PubMed.
- H. Cai, P. Wang, X. Chen, Y. Wang and D. Zhang, Corros. Sci., 2020, 167, 108534 CrossRef CAS.
- J. Li, N. Birbilis and R. G. Buchheit, Corros. Sci., 2015, 101, 155–164 CrossRef CAS.
- J. Li, B. Hurley and R. Buchheit, Corrosion, 2016, 72, 1281–1291 CrossRef CAS PubMed.
- C. Motte, M. Poelman, A. Roobroeck, M. Fedel, F. Deflorian and M. G. Olivier, Prog. Org. Coat., 2012, 74, 326–333 CrossRef CAS.
- A. Ghazi, E. Ghasemi, M. Mahdavian, B. Ramezanzadeh and M. Rostami, Corros. Sci., 2015, 94, 207–217 CrossRef CAS.
- G. Williams, H. N. McMurray and M. J. Loveridge, Electrochim. Acta, 2010, 55, 1740–1748 CrossRef CAS.
- A. A. Aghzzaf, B. Rhouta, E. Rocca, A. Khalil and J. Steinmetz, Corros. Sci., 2014, 80, 46–52 CrossRef CAS.
- S. P. V. Mahajanam and R. G. Buchheit, Corrosion, 2008, 64, 230–240 CrossRef CAS.
- D. Li, F. Wang, X. Yu, J. Wang, Q. Liu, P. Yang, Y. He, Y. Wang and M. Zhang, Prog. Org. Coat., 2011, 71, 302–309 CrossRef CAS.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- A.-H. Lu and F. Schüth, Adv. Mater., 2006, 18, 1793–1805 CrossRef CAS.
- Y. W. A. D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef PubMed.
- F. Hoffmann and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS PubMed.
- Q. Yang, J. Liu, L. Zhang and C. Li, J. Mater. Chem., 2009, 19, 1945–1955 RSC.
- Z. Lu, M. D. Prouty, Z. Guo, V. O. Golub, C. S. S. R. Kumar and Y. M. Lvov, Langmuir, 2005, 21, 2042–2050 CrossRef CAS PubMed.
- S. A. Corr, Y. K. Gun'ko, R. Tekoriute, C. J. Meledandri and D. F. Brougham, J. Phys. Chem. C, 2008, 112, 13324–13327 CrossRef CAS.
- K. Köhler, H. Möhwald and G. B. Sukhorukov, J. Phys. Chem. B, 2006, 110, 24002–24010 CrossRef PubMed.
- G. L. Li, Z. Zheng, H. Möhwald and D. G. Shchukin, ACS Nano, 2013, 7, 2470–2478 CrossRef CAS PubMed.
- A. Vimalanandan, L. P. Lv, T. H. Tran, K. Landfester, D. Crespy and M. Rohwerder, Adv. Mater., 2013, 25, 6980–6984 CrossRef CAS PubMed.
- Q. Yi, D. Wen and G. B. Sukhorukov, Langmuir, 2012, 28, 10822–10829 CrossRef CAS PubMed.
- P. Srikamut, T. Phakkeeree, F. Seidi, S. Iamsaard and D. Crespy, ACS Appl. Polym. Mater., 2021, 3, 5425–5433 CrossRef CAS.
- Z. Chen, X. Li, B. Gong, N. Scharnagl, M. L. Zheludkevich, H. Ying and W. Yang, ACS Appl. Mater. Interfaces, 2023, 15, 2067–2076 CrossRef CAS PubMed.
- T. Siva, K. Kandhasamy, K. Vaduganathan, S. Sathiyanarayanan and A. Ramadoss, Ind. Eng. Chem. Res., 2023, 62, 3942–3951 CrossRef.
- K. R. L. Castagno, V. Dalmoro and D. S. Azambuja, Mater. Chem. Phys., 2011, 130, 721–726 CrossRef CAS.
- E. N. Jun Ge, T. J. Cahill III, R. E. Beygui and R. N. Zare, ACS Nano, 2012, 6, 227–233 CrossRef PubMed.
- L. P. Lv, Y. Zhao, N. Vilbrandt, M. Gallei, A. Vimalanandan, M. Rohwerder, K. Landfester and D. Crespy, J. Am. Chem. Soc., 2013, 135, 14198–14205 CrossRef CAS PubMed.
- Y. Yu, Y. Zhang, Z. Jiang, X. Zhang, H. Zhang and X. Wang, Langmuir, 2009, 25, 10002–10006 CrossRef CAS PubMed.
- Y. Chen, Q. Meng, M. Wu, S. Wang, P. Xu, H. Chen, Y. Li, L. Zhang, L. Wang and J. Shi, J. Am. Chem. Soc., 2014, 136, 16326–16334 CrossRef CAS PubMed.
- C. Li, X. Zhao, C. Meng, T. Zhang, S. Sun and S. Hu, Prog. Org. Coat., 2021, 159, 106437 CrossRef CAS.
- A. A. Antipov, G. B. Sukhorukov, S. Leporatti, I. L. Radtchenko, E. Donath and H. Möhwald, Colloids Surf., A, 2002, 198–200, 535–541 CrossRef CAS.
- B. Li, D. Njuko, M. Meng, A. Tang and Y. Li, ACS Appl. Mater. Interfaces, 2022, 14, 53370–53379 CrossRef CAS PubMed.
- T. S. Vo, T. T. B. C. Vo, T. T. Tran and N. D. Pham, Prog. Nat. Sci.:Mater., 2022, 32, 54–62 CrossRef CAS.
- L. Hartmann, M. Bedard, H. G. Borner, H. Mohwald, G. B. Sukhorukov and M. Antonietti, Soft Matter, 2008, 4, 534–539 RSC.
- A. S. Ashvin, T. Nagaraja, K. E. Meissner and M. J. McShane, ACS Nano, 2013, 7, 6194–6202 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |