 Open Access Article
Open Access ArticleModeling heterojunctions: a computational chemistry perspective
Mesfin
Eshete
and
Giovanni
Di Liberto
 *
*
Dipartimento di Scienza dei Materiali, Università degli Studi di Milano Bicocca, Via R. Cozzi 55, 20125 Milano, Italy. E-mail: giovanni.diliberto@unimib.it
First published on 10th June 2025
Abstract
The design of heterojunction photocatalysts with enhanced photocatalytic performance is a key challenge. Computational chemistry is a valid strategy to access, with atomistic details, the nature of heterojunction-based materials. In this review, we revise and recall a series of important modeling aspects to account for in the modeling of heterojunctions, such as structural models (including lattice mismatch), band offsets, and interface polarization. Lattice mismatch is essential to be considered to avoid spurious effects. Band offsets determine the relative positioning of the band edges, which in turn indicates the way photogenerated charge carriers prefer to move. The charge polarization has an effect on efficient charge separation which instructs the unidirectional charge migration through the preferential migration path of photogenerated charge carriers. In general, we describe general concepts for designing heterojunction photocatalysts. Drawbacks and potential prospects are discussed to help the field in creating more effective photocatalysts.
1. Introduction
The need for overcoming or at least taming the need for fossil fuels has attracted a lot of attention.1–3 A possible way to contribute to solving this problem is the use of solar light to make reactions with photocatalysts.4–7 Investigating highly efficient and environmentally benign photocatalytic materials to convert water into hydrogen is promising for the creation of renewable energy and environmental cleanup.8–10 In order to accomplish this, current research has focused on thoroughly examining semiconductor photocatalysts with low cost, appropriate band gaps, and high durability to enhance the efficiency of solar-to-hydrogen energy conversion.11–13Semiconductor photocatalysis is a promising and environmentally favorable technology.14–17 During excitation by UV or visible light, electrons are promoted from the valence band maximum (VBM) to the conduction band minimum (CBM), leaving holes in the valence band. The two photo-generated charge carriers can in principle promote reactions of interest. Several different oxides are used, such as TiO2,4,18 ZnO,19,20 Cu2O,21,22 and others.23,24 However, these materials often suffer from performance limitations due to two drawbacks: high band gap and fast charge carrier recombination. The fabrication of heterojunctions has been suggested to tame the limitations of single-phase semiconductors.25–28 In heterojunctions, it is possible to improve the separation of charge carriers by engineering the alignment of the band edges. Also, one component of the system can act as a sensitizer improving the absorption of visible light.14,29–31
Increased rates of charge migration and separation, and the use of a broad spectrum of solar light, lead to the improved photocatalytic efficiency of these devices. Key examples in this regard are TiO2/TiO2 and TiO2/BiVO4 junctions.32,33
Over the past ten years, numerous photocatalysts have been created and applied to a variety of reactions, including water splitting, wastewater treatment, and CO2 reduction.9,11,26,34 To deeply analyze the charge dynamics and mechanism of a heterojunction, one needs to focus on the materials synthesis and characterization techniques and also theoretical modeling.12,13,23 Recently, many techniques have been employed to boost the performance of photocatalytic materials, including doping, nanostructuring, semiconductor heterojunction formation, co-catalyst use, or a mix of these methods.9,12,31,35 For example, the formation of a metal–semiconductor Schottky junction can help the electron–hole separation, while the plasmonic effect of noble metals has been used to increase the light absorption of wide-band gap semiconductors.23,36,37 In general, design of heterojunction photocatalysts must consider: (1) appropriate band edge positioning of the VB and CB, (2) efficient charge separation of photoexcited electron–hole pairs upon light illumination and (3) chemical stability.15,34,38–40
In principle, heterojunctions can be classified as p–n junctions (type-I, type-II and type-III), Fig. 2, Z-scheme (including S-scheme), Fig. 3, and ternary heterojunctions, Fig. 4. In this context, the ability to create and design interfaces between various semiconductors allow a wide range of systems to be investigated. In such investigation, consideration of lattice mismatch with the two or more interface structures, the charge transfer mechanism, interface polarization, the nature of chemical bonds, the band gap and band edge positions are important aspects. Density functional theory (DFT)-based electronic structure computations are crucial in this setting.
The number of applications of heterojunctions is very vast, ranging from energy to environmental remediation. The potential of these systems has stimulated increasing interest, which can be clearly shown by scrutinizing the number of publications related to the field, Fig. 1a. Nowadays, thousands of publications are reported every year, related to CO2 reduction, water splitting, hydrogen generation and photodegradation of pollutants, Fig. 1b.
Quantum chemical calculations allow us to provide atomistic insight into the nature of heterojunction materials. The state-of-the-art approach is based on density functional theory (DFT). This tutorial review focuses on the designs with quantum chemical approaches for different semiconductor heterojunctions. In addition, a detailed emphasis is given to structural and electronic properties at the interface including the band edge position, band edge offsets, built-in electric field and charge polarization at the interface.
2. Different types of heterojunctions
2.1. Semiconductor–semiconductor (S–S) heterojunctions
Heterostructures are typically classified according to the relative alignment of the band edges. One can then refer to type I, type II, and type III heterostructures, Fig. 2. In the type-I junction, the valence band maximum (VBM) and conduction band minimum (CBM) of one component are within the band gap of the second unit composing the system, Fig. 2a. Photogenerated charge carriers will migrate (thermodynamically) toward the first phase. In a type-II junction, the band edges of one component are higher in energy than the second, Fig. 2b. If the VBM of one component is higher in energy than the CBM of the second unit, then a type-III junction is formed, Fig. 2c. In this type, photoinduced charges lose their energy while traveling long distance to reach their neighbor CB/VB. In type I (straddling band gap), high energy electrons and holes transfer to the same semiconductor, preventing photocatalytic activity.41,42 The type-II system is the ideal one for charge carrier separation, as photogenerated holes and electrons will be promoted to spatially separate in the different phases. In addition, the efficiency can also be enhanced by forming ternary heterojunction catalysts by integrating type II heterostructures with noble metals.43,44Table 1 lists a series of possible type-II heterojunctions and their applications.| Model | Type | Application | References |
|---|---|---|---|
| CdS/Cu2O | Type II | H2 generation | 45 |
| α-Fe2O3/ZnO | Type II | H2 production | 46 |
| TiO2/BiOBr | Type II | Degradation of rhodamine B (RhB) and methyl orange (MO) | 47 |
| BaTiO3/CuO | Type I | MO degradation | 48 |
| ZnO/NiO | Type II | Degradation of RhB | 49, 50 |
| SnO2/NiO | Type II | Degradation of RhB | 51 |
| CuCo2O4/TiO2 | Type II | H2 evolution | 52 |
| MoS2/WSe2 | Type II | H2 production | 53, 54 |
| TiO2/Cu2O | Type II | H2 generation | 55–58 |
| Cu3SnS4/BiVO4 | Type II | Degradation of methyl blue (MB) | 59 |
| MoS2/ TiO2 | Type II | H2 generation, degradation of MB and acetone | 60 |
| CdS/CuS | Type II | H2 production | 61 |
| BiFeO3/ZnO | Type II | Photodegradation of 2,4-dichlorophenol and RhB | 62 |
| ZnO/TiO2 | Type II | H2 production | 63 |
| BiOBr/BiVO4 | Type II | Degradation of MB | 64 |
| α-Fe2O3/CuPc | Type II | Photoreduction of CO2 | 65 |
| SnO2/Bi2O3 | Type II | Degradation of RhB | 66 |
| Ag3PO4/AgBr | Type II | Degradation of MB | 67 |
| CdS/LaFeO3 | Type II | Degradation of MB, RhB, and MO | 68 |
| CoP3/Ni2P | Type II | H2 production | 69 |
| ZnO/CuO | Type II | Photodegradation of phenol | 70, 71 |
| CuO/TiO2 | Type II | Degradation of MB | 72 |
| TiO2/CeO2 | Type II | Oxidative degradation of crystal violet (CV) | 73 |
| TiO2/CuS | Type II | H2 generation | 74 |
| Fe2O3/Co3O4 | Type II | Overall water splitting | 75 |
| WO3/MoS2 | Type II | Degradation of Congo red (CR) | 76 |
| SnO2/CuO | Type II | Decomposition of MB | 77 |
| ZnO/SnS | Type II | Degradation of MO and RhB | 78 |
The photocatalytic performance of S–S heterojunctions is limited by some drawbacks, even though better charge separation and enhanced photocatalysis are achieved. In particular, the charge carrier migration is accompanied by a partial loss of energy of the absorbed photons, which lowers the photocatalytic activity.
2.2. Z-scheme heterojunctions
Even though better charge separation is achieved in p–n heterojunctions, most of them fall to straddling band gaps (type I) (Fig. 2a). In such cases, both photogenerated electrons and holes migrate to the narrow band gap semiconductor, which leads to a high rate of recombination. On the other hand, type-II and type-III (Fig. 2b and c) junctions guarantee efficient charge separation onto distinct semiconductors.In recent years, more complex systems have been proposed. Relevant examples are the so-called Z-scheme junctions. The Z-scheme combines two distinct photocatalysts with the help of a suitable shuttle redox couple, such as Fe3+/Fe2+ and IO3/I−. Compared to conventional one-step water splitting systems, visible light can be used more efficiently.79–82
Z-scheme heterostructures are depicted in Fig. 3a, where the three elements composing the system are reported, the two photocatalysts and redox couple in aqueous solution. When a photocatalyst (PS II) is photoexcited, the excited electrons migrate from the lower CB to the redox couple, which in turn transfers the electrons to the other semiconductor (PS I) and the electrons recombine with the holes formed at the VBM of PS I. As a result, holes will concentrate at the photocatalyst with a lower VBM, whereas electrons will accumulate on the photocatalyst with a higher CBM. This strategy allows increasing the redox power of the charge carriers.38,40,83 An alternative solution is to replace the redox couple with a metal.34,79,84,85 A list of Z-scheme heterojunctions reported in the literature is in Table 2.
| Model | Type | Application | References |
|---|---|---|---|
| Fe2O3/ZnSe | Z-scheme | CO2 to CO photoconversion | 86 |
| BiVO4/Cu2O | Z-scheme | CO2 reduction | 87, 88 |
| CsPbBr3/BiOC | Z-scheme | CO2 reduction | 89 |
| ZnIn2S4/BiPO4 | Z-scheme | Cr(VI) removal | 90 |
| BiOBr/Bi2WO6 | Z-scheme | Degradation of tetracycline (TC) | 91 |
| ZnIn2S4/TiO2 | Z-scheme | Overall water splitting | 92 |
| TiO2/NiO | Z-scheme | H2 evolution | 93 |
| Ag2O/Fe–TiO2 | Z-scheme | CO2 conversion into methane | 94 |
| NiSe2/Co-CdS | Z-scheme | H2 evolution | 95 |
| ZnO/Ag/Ag2WO4 | Z-scheme | Photoelectrochemical water oxidation | 96 |
| Bi2WO6/Au/CdS | Z-scheme | CO2 reduction | 97 |
| TiO2/Au/g-C3N4 | Z-scheme | CO2 reduction | 98 |
| g-C3N4/Au/ZnO | Z-scheme | CO2 reduction | 36 |
| g-C3N4/Au/SnS | Z-scheme | CO2 reduction | 99 |
| Ag3PO4/Ag/GdCrO3 | Z-scheme | CO2 reduction | 100 |
| BiVO4(010)/Au/Cu2O | Z-scheme | CO2 reduction | 101 |
| WO3/Ag/GdCrO3 | Z-scheme | Photothermocatalytic toluene degradation and CO2 reduction | 102 |
| TiO2/Cu/CaTiO3 | Z-scheme | H2 evolution | 103 |
| g-C3N4/WO3/Ag | Z-scheme | Degradation of oxytetracycline hydrochloride | 104 |
| BiOBr/Ag3PO4/Rgo | Z-scheme | Degradation of TC | 105 |
| SnS2/RGO/g-C3N4 | Z-scheme | Degradation of organic dye | 106 |
| SnS2/LaNiO3 | Z-scheme | Degradation of TC | 107 |
The efficiency of Z-schemes is highly dependent on the locations to the band positions of the VB of semiconductor 1 and the CB of semiconductor 2 relative to the redox potential of the AD species. This limits the combinations of semiconductors that can produce effective photocatalytic heterojunctions. Another possible approach is S-scheme heterojunctions, without a shuttle redox mediator as discussed here after.
2.3. S-scheme heterojunctions
A very special case of Z-scheme systems are the S-scheme junctions, Fig. 3b. In an S-scheme heterojunction, a critical role is played by the interface polarization. Indeed, if an interface dipole is generated with an appropriate orientation, it is possible to promote the spatial separation of charge carriers opposite to what happens to classical type-II systems. As a result, high energy electrons and holes separate in different semiconductors.39,40,108,109Table 3 summarizes a few S-scheme heterojunctions applied in the literature.| Model | Type | Application | References |
|---|---|---|---|
| ZnCdS/ZnS | S-scheme | H2 evolution | 110 |
| CdS/Mo2C | S-scheme | H2 evolution | 111 |
| α-Fe2O3/CeO2 | S-scheme | H2 evolution | 112 |
| α-Fe2O3/TiO2 | S-scheme | H2 evolution | 113 |
| ZnIn2S4/NiTiO3 | S-scheme | H2 evolution | 114, 115 |
| TiO2/ZnIn2S4 | S-scheme | H2 evolution | 116–118 |
| ZnIn2S4/ZnWO4 | S-scheme | H2 evolution | 119 |
| WO3/BiOBr | S-scheme | Degradation of TC and enrofloxacin (ENR) | 120 |
| BiOI/TiO2 | S-scheme | Degradation of RhB | 121 |
| Bi3TaO7/ZnIn2S4 | S-scheme | H2 evolution | 122 |
| Co9S8/In2O3 | S-scheme | H2 evolution | 123 |
| Co9S8/Bi2S3 | S-scheme | H2 evolution | 124 |
| CdS/BiOIO3 | S-scheme | CO2 reduction | 125 |
| Co3Se4/TiO2 | S-scheme | H2 evolution | 126 |
| BiVO4/CeO2 | S-scheme | CO2 reduction | 127 |
| TiO2/CdS | S-scheme | Overall water splitting | 128 |
| ZnIn2S4/CdIn2S4 | S-scheme | CO2 reduction | 129 |
| Cs2AgBiBr6/Bi2WO6 | S-scheme | CO2 reduction | 130 |
| CsPbBr3/AgBr | S-scheme | CO2 reduction | 131 |
| In4SnS8/Cs3Bi2Br9 | S-scheme | Photoreduction CO2 and CO selectivity | 132 |
| ZnO/WO3 | S-scheme | H2O2 production | 133 |
| WO3/ZnIn2S4 | S-scheme | H2 production | 134 |
| MoS2/BiVO4 | S-scheme | Degradation of RhB | 135 |
| BiVO4/Ag3VO4 | S-scheme | Degradation of MB | 136 |
| Bi2S3/CeVO4 | S-scheme | Degradation for naphthalene (NAP) | 137 |
| Cu2-xS/TiO2 | S-scheme | CH4 production | 138 |
| MoS2/2D PbTiO3 | S-scheme | Degradation of MB | 139 |
| MoS2/Ag3PO4 | S-scheme | Removal of RhB and ofloxacin (OFL) | 140 |
| Bi2WO6/Bi2O3 | S-scheme | H2 production | 141 |
| BiVO4/CsPbBr3 | S-scheme | CO2-to-CO conversion | 142 |
| NiS2/MoSe2 | S-scheme | H2 evolution | 143 |
| LaNiO3/TiO2 | S-scheme | Degradation of MO | 144 |
| CeO2/ZnIn2S4 | S-scheme | H2 production | 145 |
| In2S3/Bi2O2CO3 | S-scheme | Degradation of RhB and TC | 146 |
| Cu3SnS4/L-BiOBr | S-scheme | Ciprofloxacin degradation | 147 |
| TiO2/FePS3 | S-scheme | H2 production | 148 |
| BiOBr/Bi2WO6 | S-scheme | CO2 reduction | 149 |
| ZnIn2S4/WO3 | S-scheme | H2 production | 150 |
| FeS2/ZnIn2S4 | S-scheme | H2 production | 151 |
| AgBr/BiOBr | S-scheme | CO2 reduction and H2 production | 152 |
| FeS2/S-ZnSnO3 | S-scheme | H2 evolution | 153 |
| Bi2O2S/NiFe2O4 | S-scheme | Degradation of TC | 154 |
| Bi2MoO6/BiOI | S-scheme | CO2 reduction | 155 |
| BiFeO3/Bi2Fe4O9 | S-scheme | O-chlorophenol degradation | 156 |
| CuWO4−x/Bi12O17Cl2 | S-scheme | Degradation of TC | 157 |
| Cu2O/ BiOI | S-scheme | CO2 reduction | 158 |
| In2O3/ZnO | S-scheme | CO2 reduction | 159 |
| Ta3N5/BiOCl | S-scheme | Degradation of TC and Cr(VI) | 160 |
| WO3/TiO2 | S-scheme | H2 production | 161 |
| NiCo2S4/ZnIn2S4 | S-scheme | H2 production | 162 |
| Co9S8/ZnSe | S-scheme | H2 production | 163 |
| SnO2/SnS2 | S-scheme | Overall water splitting | 164 |
| WO3/CuBi2O4 | S-scheme | CO2 reduction | 165 |
| BaTiO3/TiO2 | S-scheme | Norfloxacin degradation | 166 |
| WS2/BiYWO6 | S-scheme | Degradation of RhB | 167 |
| Bi12O17Cl2/α-Bi2O3 | S-scheme | Degradation of TC | 168 |
| BiOCl/MoS2 | S-scheme | Degradation of TC | 169 |
| Ag3CuS2/VO2 | S-scheme | MB photodegradation and Cr(VI) photocatalytic reduction | 170 |
| ZnS/CoMoO4 | S-scheme | H2 production | 171 |
| CuCo2O4/CeO2 | S-scheme | CO2 reduction | 172 |
| SnFe2O4/ZnFe2O4 | S-scheme | Degradation of TC | 173 |
| Bi2Sn2O7/Bi2MoO6 | S-scheme | Degradation of TC | 174 |
2.4. Ternary heterojunctions
The discussion was limited to two-phase systems. Nowadays, it is possible to invoke more involved catalytic systems made by three (or more) components. In ternary heterojunction photocatalysts, three semiconductors can be combined to create direct Z-schemes. These photocatalysts are commonly referred to as “ternary Z-schemes” or “dual Z-schemes”. These systems promote spatial separation of electron–hole pairs, which favors reduction and oxidation reactions. In addition, it is possible to increase the absorption of visible light.35,38,175,176 A schematic representation of the alignment of the band edges of ternary heterojunctions is reported in Fig. 4. Typically, ternary heterojunctions are categorized as cascade-type (Fig. 4a), arrow-up (Fig. 4b) and arrow-down (Fig. 4c) Z-scheme systems.35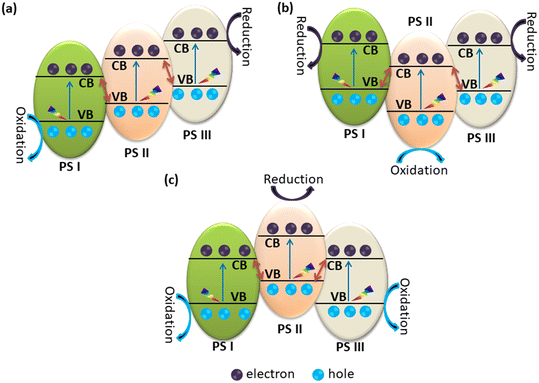 | ||
| Fig. 4 Systematic representation of ternary heterojunctions. (a) Cascade type ternary heterojunction. (b) Arrow-down type ternary heterojunction. (c) Arrow-up type heterojunction. | ||
In the case of the cascade Z-scheme, the excited electrons will concentrate toward the system with the highest CBM. At the same time, holes will follow an opposite path, concentrating to the system with the lowest VBM, Fig. 4a. In an arrow-down system, the holes in PS I and PS III's VBs join with the electrons in PS II's CB, as depicted Fig. 4b. In this ternary Z-scheme, the excited electrons from PS II combine with the holes in both PS III and PS I, thus favoring the oxidation on semiconductor PS II and reduction on PS III and PS I. The other type is an arrow-up Z-scheme ternary heterojunction. In this heterojunction, the excited electrons from the CB of PS I and PS III migrate and combine with the holes at the VB of PS II. Consequently, oxidation can take place on PS III and PS I while reduction on PS II, as depicted in Fig. 4c. A summary of typical ternary heterojunctions is reported in Table 4.
| Model | Type | Application | References |
|---|---|---|---|
| TiO2/Ti3C2/g-C3N4 | Cascade | H2 production | 177 |
| Cu2O/ZnO/Ag3PO4 | Arrow-up | Degradation of MO | 178 |
| ZnS/ZnO/g-C3N4 | Cascade | H2 production | 179 |
| Bi2S3/MoS2/TiO2 | Cascade | Degradation of MB and CO2 reduction | 180, 181 |
| ZnO/CuO/CeO2 | Arrow-down | Degradation of CV and MO | 182 |
| g-C3N4/ZnO/CeO2 | Cascade | Degradation of MB | 183 |
| WO3/g-C3N4/WS2 | Arrow-up | Degradation of RhB and MO | 184 |
| Bi2O3/CeO2/ZnO | Arrow-up | Degradation of RhB | 185 |
| ZnO/NiWO4/Ag2CrO4 | Arrow-down | Degradation of MB, MO, and fuchsine | 186 |
| ZnO/Bi2MoO6/AgBr | Arrow-up | Degradation of RhB | 187 |
| ZnO/CoWO4/Ag3VO4 | Arrow-down | Degradation of RhB | 188 |
| O–g-C3N4/Zn2SnO4N/ZnO | Cascade | Degradation of organic dyes and NO removal | 189 |
| ZnO/ZnS/g-C3N4 | Cascade | H2 production | 190 |
| ZnFe2O4/ZnO/CdS | Cascade | CO2 reduction | 191 |
| CNT/NCDs/Ni2P | Cascade | H2 production | 192 |
| MoP4/Ni3S2/MoO3 | Arrow-up | Overall water splitting | 193 |
| g-C3N/Bi2WO6/AgI | Arrow-up | Removal of tetracycline | 194 |
| g-C3N4/Bi4Ti3O12/Bi4O5I2 | Arrow-up | H2 production and ofloxacin (OFL) removal | 195 |
| BiOCl/BiVO4/N-GQD | Arrow-up | Photodegradation of bisphenol A | 196 |
| Ag3PO4/Co3(PO4)2/g-C3N4 | Arrow-up | Degradation of TC | 197 |
| WSe2/In2S3/ZnIn2S4 | Arrow-up | Degradation of MB | 198 |
| TiO2−x/BiOI/AgBr | Arrow-up | Degradation of RhB | 199 |
| BiOBr/ZnO/BiOI | Arrow-up | Degradation of RhB | 200 |
| Cu2O/S-TiO2/CuO | Arrow-down | CO2 conversion | 201 |
| TiO2/ZnO/SnO2 | Cascade | Degradation of 2,4-dichlorophenol (2,4-DCP) and bisphenol A (BPA) | 202 |
| CdS/1 T-MoS2/TiO2 | Arrow-down | H2 production | 203 |
| TiO2/CdS/MoS2 | Arrow-up | H2 production | 204 |
| Cu2O/WO3/CeO2 | Cascade | CO2 reduction | 205 |
| ZnIn2S4/Ni12P5/g-C3N4 | Cascade | CO2 and H2O2 production | 206 |
| g-C3N4/CuFe2O4/ZnIn2S4 | Cascade | CO2 reduction | 207 |
| Bi2S3/β-Bi2O3/ZnIn2S4 | Arrow-down | H2 production and degradation of TC | 208 |
| Ag2CO3/Bi4O5I2/g-C3N4 | Arrow-down | Degradation of TC | 209 |
| MoS2/Bi2S3/BiVO4 | Arrow-up | Degradation of fluoroquinolones | 210 |
| In2S3/Nb2O5/Nb2C | Arrow-up | H2 production | 211 |
2.5. 2D/2D heterojunctions
It has been shown that ultrathin 2D materials have special physical, chemical, and electronic properties. These properties include high carrier mobility, large specific surface area and unique optical band gaps.212–215Two-dimensional (2D) heterojunctions have benefits for catalysis because of their large surface area, ultrathin thickness and short charge migration distance across the interface. In general, 2D interfaces maximize quantum efficiency, broadening the range of light absorption for improved photocatalytic activity compared to three-dimensional interfaces. Consequently, the creation and application of 2D/2D heterojunctions has quickly emerged as one of the most popular areas of study.216–222
It is possible to generate 2D/2D heterostructures using various contact interfaces oriented laterally or vertically as depicted in Fig. 5. By stacking two or more monolayers of various materials in a vertical direction, 2D/2D heterostructures with a face-to-face interface contact can be created, Fig. 5a and b. Alternatively, both paralleled and patterned connections, like the heterostructures in Fig. 5c and d, can be created in a lateral direction.223–226 A special case in the family of 2D-based heterojunctions is carbon nitride. Carbon nitride is one of the most promising metal-free photocatalysts for efficient utilization of sunlight. A comprehensive summary of typical g-C3N4 heterojunctions is shown in Table 5.
 | ||
| Fig. 5 Methodical representation of 2D/2D heterostructures with various contacts, face-to-face (a and b), lateral and parallel (c and d). Reproduced with permission.223 Copyright 1999–2025 John Wiley & Sons. | ||
| Model | Type | Application | References |
|---|---|---|---|
| g-C3N4/WO3 | Type II | CO2 reduction, Cr(VI) reduction and MB degradation | 227, 228 |
| BiVO4/g-C3N4 | Z-scheme | Overall water splitting | 229, 230 |
| g-C3N4/BiFeO3 | Direct Z-scheme | Overall water splitting | 231 |
| g-C3N4/Bi2WO6 | Z-scheme | Ciprofloxacin photodegradation | 232 |
| α-Fe2O3/g-C3N4 | Direct Z-scheme | Overall water splitting | 233 |
| g-C3N4/TiO2 | Type II | Overall water splitting, degradation of diclofenac | 234–236 |
| AgCl/g-C3N4 | S-scheme | H2 production | 237 |
| MnCo2S4/g-C3N4 | S-scheme | H2 production | 238 |
| BiOIO3/g-C3N4 | Z-scheme | Degradation of NO | 239 |
| W18O49/g-C3N4 | Direct Z-scheme/S-scheme | Overall water splitting | 240–242 |
| Bi4NbO8Cl/g-C3N4 | Direct Z-scheme | H2 evolution | 243 |
| Cu2O/g-C3N4 | Type II | H2 production | 244 |
| K4Nb6O17/g-C3N4 | Z-scheme | Organic pollutant removal and H2 production | 245 |
| Bi2S3/g-C3N4 | Z-scheme | Photoreduction of CO2 to CO | 246 |
| Ag3PO4/ g-C3N4 | S-scheme | O2 production and conversion of Cr(VI) to Cr(III) | 247 |
| g-C3N4/Bi8(CrO4)O11 | S-scheme | Degradation of norfloxacin and BPA | 248 |
| CoFe2O4/ g-C3N4 | S-scheme | Degradation of TC, amoxicillin, ciprofloxacin, and sulfamethoxazole | 249 |
| Bi4V2O11/g-C3N4 | S-scheme | Photocatalytic antibiotic degradation | 250 |
| Fe-g-C3N4/Bi2WO6 | Z-scheme | Degradation of TC | 251 |
| MnCo2O4/g-C3N4 | Type II | H2 production | 252 |
| WS2/g-C3N4 | Type I | H2 production | 253 |
| CuInS2/g-C3N4 | Direct Z-scheme | H2 evolution | 254 |
| Cu3P/g-C3N4 | Type II | H2 evolution | 255 |
| LaCoO3/g-C3N4 | Z-scheme | Phenol degradation | 256 |
| MnIn2S4/g-C3N4 | Direct Z-scheme | H2 evolution | 257 |
| ZnSe/g-C3N4 | Type II | H2 evolution | 258 |
| g-C3N4/SnS | Type II | Reduction of aqueous Cr(VI) | 259 |
| g-C3N4/Ag3PO4 | Z-scheme | Degradation of TC and dye | 260 |
| g-C3N4/BiOBr | Type II | Oxidation of NO and reduction of CO2 | 261 |
| Fe2O3/g-C3N4 | Direct Z-scheme | H2 evolution | 262 |
| O-g-C3N4/B-RGO | Type II | H2 evolution | 263 |
| g-C3N4/Nb2O5 | Type II | Degradation of RhB and phenol | 264 |
| g-C3N4/C-doped BN | Direct Z-scheme | H2 evolution | 265 |
| g-C3N4/MnO2 | Z-scheme | Dye degradation and phenol removal, overall water splitting | 266, 267 |
| CdS/g-C3N4 | Z-scheme | H2 production | 268 |
| CoO/g-C3N4 | Type II | Evolution of H2 and O2 | 269 |
| O–C3N4/SnS2 | S-scheme | H2 evolution | 270 |
| ZnO/g-C3N4 | S-scheme | Degradation of MB | 271 |
| NiSe2/g-C3N4 | Type II | CO2 reduction | 272, 273 |
| TiO2/g-C3N4 | Z-scheme | Degradation of RhB | 274 |
| CoNi2S4/g-C3N4 | Type II | Evolution of H2 and O2 | 275 |
| TiO2/g-C3N4 | Type II | Degradation of MB, decomposition of fluorescein | 276, 277 |
| BiOBr/g-C3N4 | S-scheme | Degradation of RhB | 278, 279 |
| g-C3N4/Bi12O17Cl2 | S-scheme | CO2 reduction | 280 |
| Cu2O/g-C3N4 | S-scheme | Oxidation TC, reduction of Cr(VI) and H2 evolution | 281 |
| CoWO4/g-C3N4 | S-scheme | H2 production | 282 |
| Co3O4/ g-C3N4 | S-scheme | Degradation of TC | 283 |
| NiCo2O4/g-C3N4 | S-scheme | H2 production | 284 |
| Bi3NbO7/g-C3N4 | S-scheme | CO2 reduction | 285 |
| g-C3N4/Bi2MoO6 | S-scheme | Degradation of phenol and H2 evolution | 286 |
| g-C3N4/CoTiO3 | S-scheme | H2 production | 287 |
| Ni5P4/g-C3N4 | S-scheme | H2 production and carbamazepine degradation | 288 |
| g-C3N4/Nb2O5 | S-scheme | CO2 reduction | 289 |
| g-C3N4/MoO3−x | S-scheme | H2 evolution | 290 |
| WO3/g-C3N4 | S-scheme | H2 evolution | 291 |
| CdS/g-C3N4 | S-scheme | Overall water splitting | 292 |
| ZnO/g-C3N4 | S-scheme | H2O2 production | 293 |
| g-C3N4/TiO2 | S-scheme | Degradation of MB and RhB | 294 |
3. How to model an interface
3.1. Importance of structural engineering
The design of suitable interface models is a crucial step in the construction of heterojunctions. When modeling an interface, one needs to accommodate two different material surfaces in the same simulation cell. This introduces an unavoidable lattice mismatch, i.e. the lattice parameters of the interface do not correspond exactly to those of the independent units. Ideally, a well-designed interface should have the smallest possible lattice mismatch. Importantly, the electronic structure of the heterostructure is affected by the strain at the interface that resulted from the lattice mismatch.23,26,295 A good practice always consists of a checking post-process, i.e. after geometry optimization of the interface model that the lattice mismatch of the independent units does not alter their electronic structure, such as the band gap and band edge positioning. Typically, it is considered acceptable if the lattice mismatch induces changes in band edges and the band gap within 0.1 eV. Typically, it is possible to achieve a small lattice mismatch by invoking a rotational angle between the two composing units. For instance, the interface between two stable BiOIO3 surfaces, (010) and (100), can be obtained with a relatively small lattice mismatch, lower than 2% for both a and b lattice parameters with good cation–anion matching achieved by rotating the two surfaces by 90 degrees. The impact of lattice mismatch is very small, about 0.1 eV.296,297The introduction of lattice mismatch comes from the need for designing an a priori suitable working simulation cell for the heterostructure. A better-grounded approach consists of avoiding the need for introducing lattice mismatch. This is possible by invoking unconstrained energy mapping of material interfaces. Among the possible strategies, Fig. 6 reports the case of the “rotating nanodisk” approach. In this framework, disk-shaped nanoparticles of separated units are generated, and they are interfaced by sampling three different degrees of freedom: the in-plane displacement, s = (sx,sy), and the rotational angle, α. In this way, it is possible to sample the energy landscape of heterostructures, and if the size of the disks is sufficiently large, typically a radius of 2.5 nm is a good trade-off; the effect of lattice mismatch is not sizeable. It must be mentioned that free-fitted potential energy surfaces or force fields are needed to sample the energy landscape, and therefore the resulting structures must be used as starting points for more elaborated geometry optimizations with quantum chemical approaches.298
 | ||
| Fig. 6 Systematic approach to exploring disk interface models for the TiO2 anatase (101)/(001) interface. (a) Two disks are cut from the TiO2 (101) and (001) reference slabs. (b) The two disks are orientated with their (101) and (001) surfaces facing each other after being translated by s. (c) Rotation of the (001) disk relative to the (101) disk at angle α. (d) An illustration of a typical anatase disk. Reproduced with permission.298 Copyright 2022. | ||
Another important and more subtle aspect in the modeling of interfaces is the surface termination. When combining two materials and creating an interface, the nature of the interaction strongly depends on the surfaces considered. In some cases, the effect determines the type of alignment of the band edges. The key example is the BiVO4/TiO2 heterojunction. Compelling experimental evidence was reported suggesting that the photocatalytic performances of TiO2/BiVO4 heterojunctions vary upon the exposure of the facet, Fig. 7. More specifically, the TiO2(101)/BiVO4(110) interface outperforms the TiO2(101)/BiVO4(010) one.299 Quantum chemical calculations showed that the BiVO4 band edges are higher than those of TiO2 in the TiO2(101)/BiVO4(110) interface leading to a type II alignment. If BiVO4(010) is considered, then the system is predicted to have a type-I alignment.
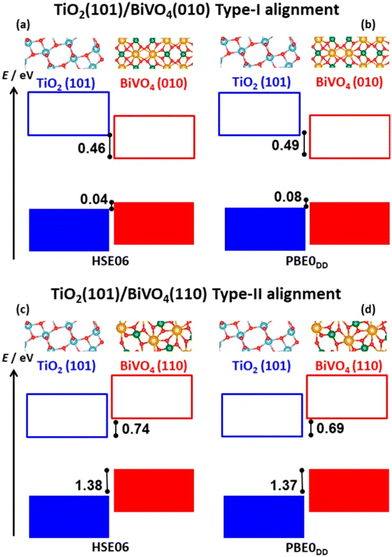 | ||
| Fig. 7 (a) and (b) Schematic representation for the band alignment of TiO2 (101) and BiVO4 (010) with two different hybrid functionals. (c) and (d) Schematic representation for the band alignment of TiO2 (101) and BiVO4 (110). Reproduced with permission.299 Copyright 2020, IOP Publishing Ltd. | ||
Similar theoretical studies have been reported for Si/anatase TiO2,300 GaN/black phosphorus,301 and ZnO/BeCdO heterojunctions.302 The matching of the interface is so important, such that in some cases, the way the two units are terminated determines the properties of the interface. For instance, quantum chemical calculations showed that the BiOIO3(010)/(100) surface junction cannot be made by the direct interaction of the two most stable terminations of the two surfaces.296 This would lead to a band alignment not compatible with experimental evidence, Fig. 8. In this case, the formation of the interface leads to the formation of new chemical bonds, promoting a metastable termination of one surface. This allows reconciling the electronic structure of the material with its photoactivity.
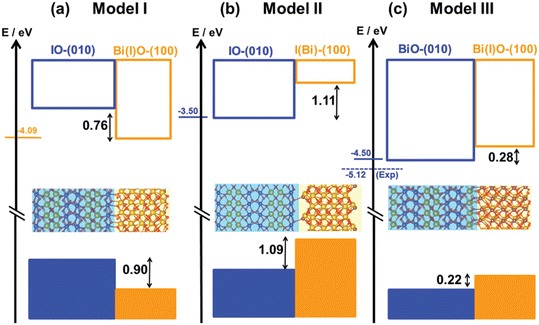 | ||
| Fig. 8 Systematic representation of band alignment for BiOIO3 interfaces with different surface terminations: (a–c) model I, model II and model III interfaces. Labeling of atoms: orange: Bi; red: O; purple: I. The two nanostructures that make up the mixed systems are distinguished by their light blue and orange areas. The experimental CBM is indicated by the horizontal dashed line. Reproduced with permission.296 Copyright 1999–2025 John Wiley & Sons. | ||
Crucially, strain engineering is also employed to adjust and enhance a range of material characteristics in order to control the electrical characteristics and promote charge separation across the heterojunction.303–305 For instance, the calculations of Yang et al. showed a negligible effect (less than 0.1 eV in total energy) of a 5% compressive strain of the MoS2/ZnO heterojunction. The finding also shows that with increasing tensile biaxial strain, the spectrum's absorption edge gradually moves to the infrared region, while compressive biaxial strain causes the spectrum to blue-shift.306 In another study, Quan Li et al. constructed bilayer and trilayer heterojunctions of WS2/C2N, WS2/C2N/WS2, and C2N/WS2/C2N in which biaxial strain can affect the band gap.307
On the other hand, the buffer layer mechanism is also an important strategy to improve photocatalytic activity in heterojunctions.308,309 Recently, Nguyen Dinh Lam et al. proposed a p-Si/p-CuO buffer layer/n-ZnO heterojunction, where the addition of a 250 nm thick CuO buffer layer is beneficial for the activity. In addition, the pseudo-order rate constant (k) was improved by up to 12% in comparison with the p-Si/n-ZnO composite film.310 Similar studies focused on CZTSSe/Zn(O,S),311 ZnO/ZnS/g-C3N4,190 and ZnO/CuO/g-C3N4.312
4. Electronic properties
In the construction of an interface, first-principles investigation plays an important role in determining electronic properties such as charge separation, band offsets and interface polarization.4.1. Band edges and band offsets
In the modeling of an interface, the evaluation of the band offsets is essential. This allows determining the nature of the band edge alignment. The most popular method relies on the electrostatic potential line-up approach, which calculates the plane-averaged electrostatic potential (V) of the heterostructure and separated components. The conduction band minimum (CBM) and valence band maximum (VBM) of the composing units are then aligned using the macroscopic average or stationary points of V as a common reference.308,313,314 An alternative approach uses as a reference core energy levels of specific atoms.315,316More specifically, the VBM and CBM of the bulk composing units (VBM1, CBM1) and (VBM2, CBM2), are aligned using a common reference, which can be taken as the macroscopic average of V, the band offsets are defined as:
VBO = (VBM1 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 1) − (VBM2 − 1) − (VBM2 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 2) − ( 2) − (![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het1 − Het1 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het2) Het2) | (1) |
CBO = (CBM1 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 1) − (CBM2 − 1) − (CBM2 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 2) − ( 2) − (![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het1 − Het1 − ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het2) Het2) | (2) |
![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 1 and
1 and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 2 are the macroscopic averages of the separated components, and
2 are the macroscopic averages of the separated components, and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het1 and
Het1 and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het2 are the same for the composite model. Importantly, the electrostatic potential may display oscillations introducing some uncertainty, and its convergence should be checked, especially in the case of insufficiently thick models.
Het2 are the same for the composite model. Importantly, the electrostatic potential may display oscillations introducing some uncertainty, and its convergence should be checked, especially in the case of insufficiently thick models.
A very similar approach has been proposed by Conesa, and is based on the use of the stationary points of the electrostatic potential.317 This method allows also overcoming the problem of the potential convergence. In this case, one does not need to evaluate the macroscopic average of the electrostatic potential since the stationary points, as the maxima, are directly taken as a reference.317
The band offsets can be calculated also by defining other common references, such as the energy of the core levels, e.g. the 1s orbitals, E1s.315,318 Core level energies are usually adopted as a reference in XPS measurements.315,318–324 In this case, ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 1 and
1 and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) 2 are replaced with E1,1S and E2,1S, and
2 are replaced with E1,1S and E2,1S, and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het1 and
Het1 and ![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) Het2 with EHet1,1S and EHet2,1S. The band offsets become:
Het2 with EHet1,1S and EHet2,1S. The band offsets become:
| VBO = (VBM1 − E1,1S) − (VBM2 − E2,1S) − (EHet1,1S − EHet2,1S) | (3) |
| CBO = (CBM1 − E1,1S) − (CBM2 − E2,1S) − (EHet1,1S − EHet2,1S) | (4) |
Fig. 9 reports the case of the SrTiO3(001)/TiO2(001) interface, where the calculated valence band offset (VBO) changes from 0.28 to 0.37 eV and the conduction band offset (CBO) from 0.03 to 0.14 eV compared to the isolated slab (depicted in Fig. 9a and b).
 | ||
| Fig. 9 Band offsets for the SrTiO3/TiO2 scheme where TiO2(001) is in contact with a SrO layer of SrTiO3(001). (a) Individual slabs. (b) The result obtained from the heterojunction model. Reproduced with permission.319 Copyright 2020 AIP Publishing LLC. | ||
In another combined experimental and theoretical heterojunction investigation on WO3(001)/BiVO4(010), the heterojunction forms a stable interface with favorable band alignment and smooth charge transfer due to small lattice mismatch at the interface, which allows us to attain high efficiency. Similar work has been applied to ZnCoMOF/g-C3N4,328 N-ZnO-g-C3N4,329 and g-C3N4/ZnIn2S4.330
4.2. Interface polarization
In the construction of S-scheme heterojunctions, interface polarization at the junction plays an important role in efficient charge separation. Engineering a semiconductor heterojunction in consideration of an internal electric field allows clearly identifying the route for charge transfer for efficient charge separation to minimize the recombination.331–335The fundamental principle of heterojunction modeling between semiconductors is based on two different semiconductors which are directly in contact. As an example, shown in Fig. 10a, a positive charge is left behind by each electron from the n-type semiconductor that diffuses into the p-type semiconductor; a negative charge is left behind by a hole that migrates from the p-type semiconductor to the n-type semiconductor. Diffusion of electrons and holes persists until the system reaches equilibrium. Consequently, a charged area known as the “internal electric field” develops near the p–n contact. As depicted in Fig. 10b, upon sunlight illumination, the photoinduced electrons migrate from a higher CB to a lower CB and holes transfer from a low VB to a high VB which is facilitated by formation of an internal electric field (IEF) which further keeps the e–h pairs well separated.23,25
 | ||
| Fig. 10 (a) Diagram showing a p–n junction between two semiconductors. (b) Electron–hole separation with an effect of the IEF in a type II p–n junction photocatalyst upon light illumination. | ||
To make an example, Wang et al. reported a study on an S-scheme BiOBr(002)/NiO(200) heterojunction for CO2 photoreduction. NiO nanosheets with hierarchical porous structures result in enhanced light absorption due to their surface area increment. In addition, efficient charge separation is expected with the effect of electric field creation at the interface.
From the first-principles optimization, NiO possesses a band edge higher in energy and can be conceived as a reductive photocatalyst, while BiOBr with a lower band edge can be conceived as an oxidative photocatalyst. Workfunction computation and in situ irradiation X-ray photoelectron spectroscopy results show that the photoexcited electrons migrated from BiOBr to NiO through the S-scheme system, which results in strong redox ability and charge separation. When NiO and BiOBr come into contact, their electrons will move to BiOBr, Fig. 11a and b. At the interface, depletion thus leaves the interface polarized. As a result, a strong IEF that points from NiO to BiOBr is created.
 | ||
| Fig. 11 a) The band structure of BiOBr and NiO. b) Formation of an IEF between BiOBr and NiO. c) Electron transfer system of the BiOBr/NiO junction under visible light illumination. Reproduced with permission.336 Copyright 1999–2025 John Wiley & Sons. | ||
Upon illumination of light, low energy photoexcited electrons in the CB of BiOBr will combine with low energy holes in the VB of NiO while preserving high energy holes and electrons of BiOBr and NiO, respectively, Fig. 11c. On the other hand, the CO2 photoreduction process takes place on the surface of NiO.336 Similar work can be found in GeC/SGaSnP335,337 and others.338 Additional studies on the effects of the IEF and interface polarization can be referenced from BiOCl/TiO2,339 C2N/α-In2Se3,340 CH3NH3PbI3/TiO2,341,342 CdS/WS2,343 BP/Bi2WO6,344 HCa2Nb3O10/g-C3N4,345 and MoO3–Bi4TaO8Cl.346
5. Conclusion and outlook
In this perspective, we presented a tutorial summary on the modeling of heterojunction materials with quantum chemical approaches. We first recalled the main archetypes of heterojunction-based materials by reporting a series of experimental studies. Then, we revised a series of fundamental ingredients to model heterojunctions. First, we discussed the crucial role of the interface model, focusing on the minimization of the lattice mismatch, the role of the surfaces in contact and their termination to the nature of the system including strain engineering and buffer layer creation. Once the importance of the structural model was analysed, we discussed how to determine the band offsets, which in turn determine the type of band edge alignment, the primary actors for improving the separation of charge carriers upon photoexcitation. Finally, we discussed the interface polarization, as the formation (and its direction) of an interface dipole can determine the nature of the heterojunction system.Importantly, hybrid functionals are important to accurately determine the band gap and band edge positions for the individual semiconductors and also the heterojunction. Moreover, the lattice mismatch at the interface, which causes strain in the system, should be carefully taken into account while modeling a heterojunction. Electronic properties, such as band edges and offsets, and charge migration are all directly impacted by the lattice mismatch at the interface. In principle, one should work with the lowest possible lattice mismatch, ideally close to zero. If not possible, it is important to check for the spurious effects induced by the working strain.
Once the heterojunction model is obtained, other aspects like the direction of charge polarization must be considered. It is worth mentioning that the presence of buffer layers in certain systems can be utilized to change the band alignment's nature and charge polarization. In addition, considering strain engineering is important to manage the electrical characteristics and encourage charge space separation across the heterojunction. We finally highlight that the information extracted from heterojunction models is obtained from “nearly” ideal systems. A possible strategy to scale-up the computational models is to invoke multi-scale approaches to reduce the gap between the complexity of experiments and the theoretical models. We hope that this review could help in modeling novel heterojunction materials and existing ones for which atomistic explanation is still missing.
Data availability
The data reported in this manuscript can be found in the mentioned references. No new data were produced in this work.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We thank the COST Action 18234 supported by COST (European Cooperation in Science and Technology).References
- P. A. Owusu and S. Asumadu-Sarkodie, Cogent Eng., 2016, 3(1), 1167990 CrossRef.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8(3), 731–759 RSC.
- S. Li, W. Xu, L. Meng, W. Tian and L. Li, Small Sci., 2022, 2(5), 2100112 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238(5358), 37–38 CrossRef CAS PubMed.
- D. J. Martin, P. J. Reardon, S. J. Moniz and J. Tang, J. Am. Chem. Soc., 2014, 136(36), 12568–12571 CrossRef CAS PubMed.
- X. Yang and D. Wang, ACS Appl. Energy Mater., 2018, 1(12), 6657–6693 CrossRef CAS.
- E. Dupont, R. Koppelaar and H. Jeanmart, Appl. Energy, 2020, 257, 113968 CrossRef.
- M. Melchionna and P. Fornasiero, ACS Catal., 2020, 10(10), 5493–5501 CrossRef CAS.
- X. Zhang, S. Xiong, A. Sathiyaseelan, L. Zhang, Y. Lu, Y. Chen, T. Jin and M. H. Wang, Chemosphere, 2024, 364, 143142 CrossRef CAS PubMed.
- L. Chen, Z. Liu, Z. Guo and X.-J. Huang, J. Mater. Chem. A, 2020, 8(34), 17326–17359 RSC.
- A. Balapure, J. Ray Dutta and R. Ganesan, RSC Appl. Interfaces, 2024, 1(1), 43–69 RSC.
- M. Humayun, C. Wang and W. Luo, Small Methods, 2022, 6(2), e2101395 CrossRef PubMed.
- F. Liu, C. Shi, X. Guo, Z. He, L. Pan, Z. F. Huang, X. Zhang and J. J. Zou, Adv. Sci., 2022, 9(18), e2200307 CrossRef PubMed.
- Y. Wang, H. Suzuki, J. Xie, O. Tomita, D. J. Martin, M. Higashi, D. Kong, R. Abe and J. Tang, Chem. Rev., 2018, 118(10), 5201–5241 CrossRef CAS PubMed.
- N. Goodarzi, Z. Ashrafi-Peyman, E. Khani and A. Z. Moshfegh, Catalysts, 2023, 13(7), 1102 CrossRef CAS.
- H. Mai, D. Chen, Y. Tachibana, H. Suzuki, R. Abe and R. A. Caruso, Chem. Soc. Rev., 2021, 50(24), 13692–13729 RSC.
- A. Rahman and M. M. Khan, New J. Chem., 2021, 45(42), 19622–19635 RSC.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, Adv. Mater., 2019, 31(50), e1901997 CrossRef PubMed.
- R. Saffari, Z. Shariatinia and M. Jourshabani, Environ. Pollut., 2020, 259, 113902 CrossRef CAS PubMed.
- P. Singh, R. Kumar and R. K. Singh, Ind. Eng. Chem. Res., 2019, 58(37), 17130–17163 CrossRef CAS.
- S. Rej, M. Bisetto, A. Naldoni and P. Fornasiero, J. Mater. Chem. A, 2021, 9(10), 5915–5951 RSC.
- B. A. Koiki and O. A. Arotiba, RSC Adv., 2020, 10(60), 36514–36525 RSC.
- S. Bai, J. Jiang, Q. Zhang and Y. Xiong, Chem. Soc. Rev., 2015, 44(10), 2893–2939 RSC.
- X. Shi, L. Mao, P. Yang, H. Zheng, M. Fujitsuka, J. Zhang and T. Majima, Appl. Catal., B, 2020, 265, 118616 CrossRef CAS.
- M. Eshete, X. Li, L. Yang, X. Wang, J. Zhang, L. Xie, L. Deng, G. Zhang and J. Jiang, Small Sci., 2023, 3(3), 2200041 CrossRef CAS PubMed.
- J. Ma, J. Low, J. Zhang and J. Yu, Semicon. Sol. Photocatal., 2021, pp. 71–100 Search PubMed.
- P. Kumari, N. Bahadur, L. Kong, L. A. O'Dell, A. Merenda and L. F. Dumée, Mater. Adv., 2022, 3(5), 2309–2323 RSC.
- A. Wang, S. Wu, J. Dong, R. Wang, J. Wang, J. Zhang, S. Zhong and S. Bai, Chem. Eng. J., 2021, 404, 127145 CrossRef CAS.
- Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48(7), 2109–2125 RSC.
- Q. Xu, S. Wageh, A. A. Al-Ghamdi and X. Li, J. Mater. Sci. Technol., 2022, 124, 171–173 CrossRef.
- Z. Xiong, Q. Liu, Z. Gao, J. Yang, X. Zhang, Q. Yang and C. Hao, Inorg. Chem., 2021, 60(7), 5063–5070 CrossRef CAS PubMed.
- H. Li, H. Yu, X. Quan, S. Chen and H. Zhao, Adv. Funct. Mater., 2015, 25(20), 3074–3080 CrossRef CAS.
- L. Wei, C. Yu, Q. Zhang, H. Liu and Y. Wang, J. Mater. Chem. A, 2018, 6(45), 22411–22436 RSC.
- F. Chen, Y. Wei, M. Ren, S. Sun, S. Ghosh and R. V. Kumar, ChemCatChem, 2024, 16(10), e202301492 CrossRef CAS.
- X. Li, C. Garlisi, Q. Guan, S. Anwer, K. Al-Ali, G. Palmisano and L. Zheng, Mater. Today, 2021, 47, 75–107 CrossRef CAS.
- X. Li, H. Jiang, C. Ma, Z. Zhu, X. Song, H. Wang, P. Huo and X. Li, Appl. Catal., B, 2021, 283, 119638 CrossRef CAS.
- X. Wan, Y. Gao, M. Eshete, M. Hu, R. Pan, H. Wang, L. Liu, J. Liu, J. Jiang, S. Brovelli and J. Zhang, Nano Energy, 2022, 98, 107217 CrossRef CAS.
- L. Schumacher and R. Marschall, Top. Curr. Chem., 2022, 380(6), 53 CrossRef CAS PubMed.
- L. Wang, B. Zhu, J. Zhang, J. B. Ghasemi, M. Mousavi and J. Yu, Matter, 2022, 5(12), 4187–4211 CrossRef CAS.
- M. J. Molaei, J. Am. Ceram. Soc., 2024, 107(9), 5695–5719 CrossRef CAS.
- A. B. Trench, R. Alvarez, V. Teodoro, L. G. da Trindade, T. R. Machado, M. M. Teixeira, D. de Souza, I. M. Pinatti, A. Z. Simões, Y. G. Gobato, J. Andrés and E. Longo, Mater. Chem. Phys., 2022, 280, 125710 CrossRef CAS.
- L. Che, J. Pan, K. Cai, Y. Cong and S.-W. Lv, Sep. Purif. Technol., 2023, 315, 123708 CrossRef CAS.
- H. Lüth, Sol. Surf. Interfaces and Thin Films, 2015, pp. 393–448 Search PubMed.
- J. Tyczkowski and H. Kierzkowska-Pawlak, ACS Appl. Mater. Interfaces, 2024, 16(29), 37339–37345 CrossRef CAS PubMed.
- L. Wang, W. Wang, Y. Chen, L. Yao, X. Zhao, H. Shi, M. Cao and Y. Liang, ACS Appl. Mater. Interfaces, 2018, 10(14), 11652–11662 CrossRef CAS PubMed.
- L. Liccardo, E. Lushaj, L. Dal Compare, E. Moretti and A. Vomiero, Small Sci., 2022, 2(3), 2270006 CrossRef.
- K. Wang, Y. Zhang, L. Liu, N. Lu and Z. Zhang, J. Mater. Sci., 2019, 54(11), 8426–8435 CrossRef CAS.
- S. Joshi, R. K. Canjeevaram Balasubramanyam, S. J. Ippolito, Y. M. Sabri, A. E. Kandjani, S. K. Bhargava and M. V. Sunkara, ACS Appl. Nano Mater., 2018, 1(7), 3375–3388 CrossRef CAS.
- U. Saeed, A. Jilani, J. Iqbal and H. Al-Turaif, Inorg. Chem. Commun., 2022, 142, 109623 CrossRef CAS.
- M. Ding, H. Yang, T. Yan, C. Wang, X. Deng, S. Zhang, J. Huang, M. Shao and X. Xu, Nanoscale Res. Lett., 2018, 13(1), 260 CrossRef PubMed.
- B. Babu, V. V. N. Harish, J. Shim and C. V. Reddy, J. Mater. Sci.: Mater. Electron., 2018, 29(19), 16988–16996 CrossRef CAS.
- C. Xu, C. Jin, W. Chang, X. Hu, H. Deng, E. Liu and J. Fan, Catal. Sci. Technol., 2019, 9(18), 4990–5000 RSC.
- S. Seo, S. Kim, H. Choi, J. Lee, H. Yoon, G. Piao, J.-C. Park, Y. Jung, J. Song, S. Y. Jeong, H. Park and S. Lee, Adv. Sci., 2019, 6(13), 1900301 CrossRef PubMed.
- K. Hernandez Ruiz, Z. Wang, M. Ciprian, M. Zhu, R. Tu, L. Zhang, W. Luo, Y. Fan and W. Jiang, Small Sci., 2022, 2(1), 2100047 CrossRef CAS PubMed.
- P. A. Bharad, A. V. Nikam, F. Thomas and C. S. Gopinath, ChemistrySelect, 2018, 3(43), 12022–12030 CrossRef CAS.
- G. Li, J. Huang, J. Chen, Z. Deng, Q. Huang, Z. Liu, W. Guo and R. Cao, ACS Omega, 2019, 4(2), 3392–3397 CrossRef CAS PubMed.
- V. V. Pham, D. P. Bui, H. H. Tran, M. T. Cao, T. K. Nguyen, Y. S. Kim and V. H. Le, RSC Adv., 2018, 8(22), 12420–12427 RSC.
- L. Xinkun, P. Guo, R. Li, J. Liu and M. Huang, Russ. J. Phys. Chem. A, 2019, 93(3), 515–521 CrossRef.
- F. Liu, C. Chen, Q. Zhang, Z. Zhang and X. Fang, Nanoscale, 2022, 14(32), 11664–11675 RSC.
- Y. Dong, S.-Y. Chen, Y. Lu, Y.-X. Xiao, J. Hu, S.-M. Wu, Z. Deng, G. Tian, G.-G. Chang, J. Li, S. Lenaerts, C. Janiak, X.-Y. Yang and B.-L. Su, Chem. – Asian J., 2018, 13(12), 1609–1615 CrossRef CAS PubMed.
- X. Yang, G. Lu, B. Wang, T. Wang and Y. Wang, RSC Adv., 2019, 9(43), 25142–25150 RSC.
- M. Humayun, Z. Zheng, Q. Fu and W. Luo, Environ. Sci. Pollut. Res., 2019, 26(17), 17696–17706 CrossRef CAS PubMed.
- K. Zhou, P. Li, Y. Zhu, X. Ye, H. Chen, Y. Yang, Y. Dan, Y. Yuan and H. Hou, Catal. Lett., 2021, 151(1), 78–85 CrossRef CAS.
- S. Liu, J. Chen, D. Liu, L. Shan and X. Zhang, J. Nanopart. Res., 2019, 21(8), 191 CrossRef.
- Z. Mu, S. Chen, Y. Wang, Z. Zhang, Z. Li, B. Xin and L. Jing, Small Sci., 2021, 1(10), 2100050 CrossRef CAS PubMed.
- T. Qiu, S. Liu, H. Cai, Y. Zhou, K. Chen, Y. Huang and Q. Feng, J. Mater. Sci.: Mater. Electron., 2018, 29(20), 17463–17472 CrossRef CAS.
- J. Zhuang, J. Liu, Z. Wu, Z. Li, K. Zhu, K. Yan, Y. Xu, Y. Huang and Z. Lin, J. Mater. Sci.: Mater. Electron., 2019, 30(12), 11368–11377 CrossRef CAS.
- A.-A. Hoseini, S. Farhadi, A. Zabardasti and F. Siadatnasab, RSC Adv., 2019, 9(42), 24489–24504 RSC.
- L. Zhang, L. Zhuang, H. Liu, L. Zhang, R. Cai, N. Chen, X. Yang, Z. Zhu, D. Yang and X. Yao, Small Sci., 2021, 1(4), 2000027 CrossRef CAS PubMed.
- L. Zhu, H. Li, Z. Liu, P. Xia, Y. Xie and D. Xiong, J. Phys. Chem. C, 2018, 122(17), 9531–9539 CrossRef CAS.
- Y. T. Prabhu, V. Navakoteswara Rao, M. V. Shankar, B. Sreedhar and U. Pal, New J. Chem., 2019, 43(17), 6794–6805 RSC.
- X. Y. Sun, X. Zhang, X. Sun, C. Liu, N. X. Qian, R. Rao, M. Wang and Y. Q. Ma, Appl. Organomet. Chem., 2019, 33(11), e5173 CrossRef CAS.
- M. Zahoor, A. Arshad, Y. Khan, M. Iqbal, S. Z. Bajwa, R. A. Soomro, I. Ahmad, F. K. Butt, M. Z. Iqbal, A. Wu and W. S. Khan, Appl. Nanosci., 2018, 8(5), 1091–1099 CrossRef CAS.
- M. Chandra, K. Bhunia and D. Pradhan, Inorg. Chem., 2018, 57(8), 4524–4533 CrossRef CAS PubMed.
- S.-S. Yi, B.-R. Wulan, J.-M. Yan and Q. Jiang, Adv. Funct. Mater., 2019, 29(11), 1801902 CrossRef.
- Y. Zeng, N. Guo, H. Li, Q. Wang, X. Xu, Y. Yu, X. Han and H. Yu, Chem. Commun., 2019, 55(5), 683–686 RSC.
- M. Rajesh Kumar, G. Murugadoss, A. N. Pirogov and R. Thangamuthu, J. Mater. Sci.: Mater. Electron., 2018, 29(16), 13508–13515 CrossRef CAS.
- S. Jayswal and R. S. Moirangthem, New J. Chem., 2018, 42(16), 13689–13701 RSC.
- K. Maeda, ACS Catal., 2013, 3(7), 1486–1503 CrossRef CAS.
- H. Yang, Mater. Res. Bull., 2021, 142, 111406 CrossRef CAS.
- J. Wang, B. Wang, W. Zhang, Y. Xiao, H. Xu, Y. Liu, Z. Liu, J. Zhang and Y. Jiang, Appl. Surf. Sci., 2022, 587, 152867 CrossRef CAS.
- Q. Dong, Z. Chen, B. Zhao, Y. Zhang, Z. Lu, X. Wang, J. Li and W. Chen, J. Colloid Interface Sci., 2022, 608(Pt 2), 1951–1959 CrossRef CAS PubMed.
- Z. Xing, S. Shen, M. Wang, F. Ren, Y. Liu, X. Zheng, Y. Liu, X. Xiao, W. Wu and C. Jiang, Appl. Phys. Lett., 2014, 105(14), 143902 CrossRef.
- H. Li, W. Tu, Y. Zhou and Z. Zou, Adv. Sci., 2016, 3(11), 1500389 CrossRef PubMed.
- A. Nakada, R. Kuriki, K. Sekizawa, S. Nishioka, J. J. M. Vequizo, T. Uchiyama, N. Kawakami, D. Lu, A. Yamakata, Y. Uchimoto, O. Ishitani and K. Maeda, ACS Catal., 2018, 8(10), 9744–9754 CrossRef CAS.
- J. Bao, Y. Chen, W. Li, J. Zhang, F. Zeng, L. Liu and G. Tian, J. Mater. Chem. A, 2025, 13, 5365–5373 RSC.
- E. Aguilera-Ruiz, M. de la Garza-Galván, P. Zambrano-Robledo, J. C. Ballesteros-Pacheco, J. Vazquez-Arenas, J. Peral and U. M. García-Pérez, RSC Adv., 2017, 7(73), 45885–45895 RSC.
- N. Khan, F. Stelo, G. H. C. Santos, L. M. Rossi, R. V. Gonçalves and H. Wender, Appl. Surf. Sci. Adv., 2022, 11, 100289 CrossRef.
- S. Zheng, P. Lu, A. M. Idris, B. Chen, X. Jiang, G. Jiang, J. Wang, S. Li and Z. Li, J. Mater. Chem. A, 2025, 13, 5346–5356 RSC.
- X. Li, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, J. Alloys Compd., 2020, 843, 155772 CrossRef CAS.
- C. Lu, F. Guo, Q. Yan, Z. Zhang, D. Li, L. Wang and Y. Zhou, J. Alloys Compd., 2019, 811, 151976 CrossRef CAS.
- G. Zuo, Y. Wang, W. L. Teo, Q. Xian and Y. Zhao, Appl. Catal., B, 2021, 291, 120126 CrossRef CAS.
- Z. Liang, B. Ouyang, T. Wang, X. Liu, H. Huo, D. Liu, H. Feng, J. Ma, K. Deng, A. Li and E. Kan, Int. J. Hydrogen Energy, 2022, 47(20), 10868–10876 CrossRef CAS.
- N. R. Khalid, M. K. Hussain, G. Murtaza, M. Ikram, M. Ahmad and A. Hammad, J. Inorg. Organomet. Polym. Mater., 2019, 29(4), 1288–1296 CrossRef CAS.
- S. Du, C. Li, X. Lin, W. Xu, X. Huang, H. Xu and P. Fang, ChemPlusChem, 2019, 84(7), 999–1010 CrossRef CAS PubMed.
- R. E. Adam, M. Pirhashemi, S. Elhag, X. Liu, A. Habibi-Yangjeh, M. Willander and O. Nur, RSC Adv., 2019, 9(15), 8271–8279 RSC.
- M. Wang, Q. Han, L. Li, L. Tang, H. Li, Y. Zhou and Z. Zou, Nanotechnology, 2017, 28(27), 274002 CrossRef PubMed.
- C. Wang, Y. Zhao, H. Xu, Y. Li, Y. Wei, J. Liu and Z. Zhao, Appl. Catal., B, 2020, 263 Search PubMed.
- M. Liang, T. Borjigin, Y. Zhang, H. Liu, B. Liu and H. Guo, ACS Appl. Mater. Interfaces, 2018, 10(40), 34123–34131 CrossRef CAS PubMed.
- J. Li, X. Yang, C. Ma, Y. Lei, Z. Cheng and Z. Rui, Appl. Catal., B, 2021, 291, 120053 CrossRef CAS.
- C. Zhou, S. Wang, Z. Zhao, Z. Shi, S. Yan and Z. Zou, Adv. Funct. Mater., 2018, 28(31), 1801214 CrossRef.
- J. Li, J. Chen, H. Fang, X. Guo and Z. Rui, Ind. Eng. Chem. Res., 2021, 60(23), 8420–8429 CrossRef CAS.
- J. Yang, C. Shi, Y. Dong, H. Su, H. Sun, Y. Guo and S. Yin, J. Colloid Interface Sci., 2022, 605, 373–384 CrossRef CAS PubMed.
- K. Ouyang, B. Xu, C. Yang, H. Wang, P. Zhan and S. Xie, Mater. Sci. Semicond. Process., 2022, 137, 106168 CrossRef CAS.
- B. Ju, F. Yang, K. Huang and Y. Wang, J. Alloys Compd., 2020, 815, 151886 CrossRef CAS.
- N. Lu, X. Jing, J. Zhang, P. Zhang, Q. Qiao and Z. Zhang, Chem. Eng. J., 2022, 431, 134001 CrossRef CAS.
- S. Yao, R. Zheng, R. Li, Y. Chen, X. Zhou and J. Luo, J. Taiwan Inst. Chem. Eng., 2019, 100, 186–193 CrossRef CAS.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6(7), 1543–1559 CAS.
- F. Li, G. Zhu, J. Jiang, L. Yang, F. Deng and X. Li, J. Mater. Sci. Technol., 2024, 177, 142–180 CrossRef CAS.
- X. Hao, D. Xiang and Z. Jin, ChemCatChem, 2021, 13(22), 4738–4750 CrossRef CAS.
- R. Shen, X. Lu, Q. Zheng, Q. Chen, Y. H. Ng, P. Zhang and X. Li, Sol. RRL, 2021, 5(7), 2100177 CrossRef CAS.
- K. A. Alzahrani and A. A. Ismail, Surf. Interfaces, 2023, 39, 102935 CrossRef CAS.
- W. Bootluck, T. Chittrakarn, K. Techato, P. Jutaporn and W. Khongnakorn, Catal. Lett., 2022, 152(9), 2590–2606 CrossRef CAS.
- J. Zhu, Q. Bi, Y. Tao, W. Guo, J. Fan, Y. Min and G. Li, Adv. Funct. Mater., 2023, 33(15), 2213131 CrossRef CAS.
- Y. Song, J. Zhang, X. Dong and H. Li, Energy Technol., 2021, 9(5), 2100033 CrossRef CAS.
- J. Li, C. Wu, J. Li, B. Dong, L. Zhao and S. Wang, Chin. J. Catal., 2022, 43(2), 339–349 CrossRef CAS.
- X. Wu, G. Chen, L. Li, J. Wang and G. Wang, J. Mater. Sci. Technol., 2023, 167, 184–204 CrossRef CAS.
- G. Zhang, H. Wu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Green Energy Environ., 2022, 7(2), 176–204 CrossRef CAS.
- M. Dai, Z. He, P. Zhang, X. Li and S. Wang, J. Mater. Sci. Technol., 2022, 122, 231–242 CrossRef CAS.
- C. Liu, S. Mao, H. Wang, Y. Wu, F. Wang, M. Xia and Q. Chen, Chem. Eng. J., 2022, 430, 132806 CrossRef CAS.
- Y. Chen, J. Huang, J. Zhong, M. Li, Z. Li and C. Yang, Chem. Phys. Lett., 2021, 780, 138966 CrossRef CAS.
- K. Wang, X. Shao, K. Zhang, J. Wang, X. Wu and H. Wang, Appl. Surf. Sci., 2022, 596, 153444 CrossRef CAS.
- Z. Jin, X. Jiang and X. Guo, Int. J. Hydrogen Energy, 2022, 47(3), 1669–1682 CrossRef CAS.
- J. Li, M. Li and Z. Jin, J. Colloid Interface Sci., 2021, 592, 237–248 CrossRef CAS PubMed.
- D.-E. Lee, N. Mameda, K. P. Reddy, B. M. Abraham, W.-K. Jo and S. Tonda, J. Mater. Sci. Technol., 2023, 161, 74–87 CrossRef CAS.
- W. Huang, W. Xue, X. Hu, J. Fan, C. Tang and E. Liu, Appl. Surf. Sci., 2022, 599, 153900 CrossRef CAS.
- J. Geng, S. Guo, Z. Zou, Z. Yuan, D. Zhang, X. Yan, X. Ning and X. Fan, Fuel, 2023, 333, 126417 CrossRef CAS.
- H. Ge, F. Xu, B. Cheng, J. Yu and W. Ho, ChemCatChem, 2019, 11(24), 6301–6309 CrossRef CAS.
- S. Han, B. Li, L. Huang, H. Xi, Z. Ding and J. Long, Chin. J. Struct. Chem., 2022, 41(1), 2201007–2201013 CAS.
- J. Wang, H. Cheng, D. Wei and Z. Li, Chin. J. Catal., 2022, 43(10), 2606–2614 CrossRef CAS.
- Z. Zhang, Z. Dong, Y. Jiang, Y. Chu and J. Xu, Chem. Eng. J., 2022, 435, 135014 CrossRef CAS.
- Z. Zhang, M. Wang, Z. Chi, W. Li, H. Yu, N. Yang and H. Yu, Appl. Catal., B, 2022, 313, 121426 CrossRef CAS.
- Z. Jiang, B. Cheng, Y. Zhang, S. Wageh, A. A. Al-Ghamdi, J. Yu and L. Wang, J. Mater. Sci. Technol., 2022, 124, 193–201 CrossRef CAS.
- S. Cao, J. Yu, S. Wageh, A. A. Al-Ghamdi, M. Mousavi, J. B. Ghasemi and F. Xu, J. Mater. Chem. A, 2022, 10(33), 17174–17184 RSC.
- A. Xu, W. Tu, S. Shen, Z. Lin, N. Gao and W. Zhong, Appl. Surf. Sci., 2020, 528, 146949 CrossRef CAS.
- L. Liu, T. Hu, K. Dai, J. Zhang and C. Liang, Chin. J. Catal., 2021, 42(1), 46–55 CrossRef CAS.
- J. Huang, B. Zhu, D. Song, B. Wang, L. Chen, L. Lu, Q. Chen, L. Gai, C. Zhai, L. Chen and H. Tao, Chem. Eng. J., 2023, 464, 142784 CrossRef CAS.
- J. Yang, J. Wang, W. Zhao, G. Wang, K. Wang, X. Wu and J. Li, Appl. Surf. Sci., 2023, 613, 156083 CrossRef CAS.
- Y. Qin, K. Xiao, S. Sun, Y. Wang and C. Kang, Appl. Surf. Sci., 2023, 616, 156431 CrossRef CAS.
- J. Piriyanon, T. Chankhanittha, S. Youngme, K. Hemavibool, S. Nijpanich, S. Juabrum, N. Chanlek and S. Nanan, J. Mater. Sci.: Mater. Electron., 2021, 32(14), 19798–19819 CrossRef CAS.
- W. Liu, X. Li, K. Qi, Y. Wang, F. Wen and J. Wang, Appl. Surf. Sci., 2023, 607, 155085 CrossRef CAS.
- X. Yue, L. Cheng, J. Fan and Q. Xiang, Appl. Catal., B, 2022, 304, 120979 CrossRef CAS.
- Y. Liu, X. Hao, H. Hu and Z. Jin, Acta Phys.-Chim. Sin., 2021, 37(6), 2008030 Search PubMed.
- C. Chen, J. Zhou, J. Geng, R. Bao, Z. Wang, J. Xia and H. Li, Appl. Surf. Sci., 2020, 503, 144287 CrossRef CAS.
- X. Lu, L. Quan, H. Hou, J. Qian, Z. Liu and Q. Zhang, J. Alloys Compd., 2022, 925, 166552 CrossRef CAS.
- H. Fan, H. Zhou, W. Li, S. Gu and G. Zhou, Appl. Surf. Sci., 2020, 504, 144351 CrossRef CAS.
- Z. Li, D. Lan, Z. Li, J. Sun, S. Chen, J. Yang, J. Wei, Z. Yu, S. Wang and Y. Hou, Chemosphere, 2022, 301, 134684 CrossRef CAS PubMed.
- B. Xia, B. He, J. Zhang, L. Li, Y. Zhang, J. Yu, J. Ran and S. Z. Qiao, Adv. Energy Mater., 2022, 12(46), 2201449 CrossRef CAS.
- J. Wu, K. Li, S. Yang, C. Song and X. Guo, Chem. Eng. J., 2023, 452, 139493 CrossRef CAS.
- M. Zhao, S. Liu, D. Chen, S. Zhang, S. A. C. Carabineiro and K. Lv, Chin. J. Catal., 2022, 43(10), 2615–2624 CrossRef CAS.
- K. Chen, Y. Shi, P. Shu, Z. Luo, W. Shi and F. Guo, Chem. Eng. J., 2023, 454, 140053 CrossRef CAS.
- Z. Miao, Q. Wang, Y. Zhang, L. Meng and X. Wang, Appl. Catal., B, 2022, 301, 120802 CrossRef CAS.
- S. Zhou, C. Yang, L. Guo, R. Ali Soomro, M. Niu, Z. Yang, R. Du, D. Wang, F. Fu and B. Xu, Appl. Surf. Sci., 2023, 625, 157192 CrossRef CAS.
- S. Pang, Y. Dong, D. Xu, Q. Wang, W. Gao, L. Zhang, K. Wang, G. Zhang, L. Lv, Y. Xia, Z. Ren and P. Wang, J. Mater. Sci. Technol., 2023, 158, 145–155 CrossRef CAS.
- Z. Wang, B. Cheng, L. Zhang, J. Yu, Y. Li, S. Wageh and A. A. Al-Ghamdi, Chin. J. Catal., 2022, 43(7), 1657–1666 CrossRef CAS.
- Y. Wang, Y. Tang, J. Sun, X. Wu, H. Liang, Y. Qu and L. Jing, Appl. Catal., B, 2022, 319, 121893 CrossRef CAS.
- R. Chen, J. Xia, Y. Chen and H. Shi, Acta Phys.-Chim. Sin., 2022, 0(0), 2209012 Search PubMed.
- J. Wang, X. Qiao, W. Shi, J. He, J. Chen and W. Zhang, Acta Phys.-Chim. Sin., 2023, 39(6), 2210003 Search PubMed.
- G. Han, C. Liu, Y. Pan, W. Macyk, S. Wageh, A. A. Al-Ghamdi and F. Xu, Adv. Sustainable Syst., 2023, 7(1), 2200381 CrossRef CAS.
- S. Li, M. Cai, C. Wang, Y. Liu, N. Li, P. Zhang and X. Li, J. Mater. Sci. Technol., 2022, 123, 177–190 CrossRef CAS.
- F. He, A. Meng, B. Cheng, W. Ho and J. Yu, Chin. J. Catal., 2020, 41(1), 9–20 CrossRef CAS.
- Z. Li, J. Xu, Z. Liu, X. Liu, S. Xu and Y. Ma, Int. J. Hydrogen Energy, 2023, 48(9), 3466–3477 CrossRef CAS.
- M. Li, D. Zhang, H. Zhou, K. Sun, X. Ma and M. Dong, Int. J. Hydrogen Energy, 2023, 48(13), 5126–5137 CrossRef CAS.
- J. Mu, F. Teng, H. Miao, Y. Wang and X. Hu, Appl. Surf. Sci., 2020, 501, 143974 CrossRef CAS.
- W. Shi, J.-C. Wang, X. Guo, X. Qiao, F. Liu, R. Li, W. Zhang, Y. Hou and H. Han, Nano Res., 2022, 15(7), 5962–5969 CrossRef CAS.
- Z. Zhao, Q. Ling, Z. Li, K. Yan, C. Ding, P. Chen, L. Yang, Z. Sun and M. Zhang, Sep. Purif. Technol., 2023, 308, 122928 CrossRef CAS.
- T. Bavani, P. Sasikala, S. Arumugam, A. Malathi, P. Praserthdam and J. Madhavan, Environ. Sci. Pollut. Res., 2023, 30(12), 34468–34480 CrossRef CAS PubMed.
- J. Chen, J. Zhong, J. Li and K. Qiu, Appl. Surf. Sci., 2021, 563, 150246 CrossRef CAS.
- M. Liu, P. Ye, M. Wang, L. Wang, C. Wu, J. Xu and Y. Chen, J. Environ. Chem. Eng., 2022, 10(5), 108436 CrossRef CAS.
- A. Shamloufard, S. Hajati, A. A. Youzbashi, K. Dashtian, M. Moradi and J. Toth, Appl. Surf. Sci., 2022, 590, 153118 CrossRef CAS.
- Z. Liu, J. Xu, C. Xiang, Y. Liu, L. Ma and L. Hu, Appl. Surf. Sci., 2021, 569, 150973 CrossRef CAS.
- K. A. Alzahrani, A. A. Ismail and N. Alahmadi, J. Mol. Liq., 2023, 376, 121509 CrossRef CAS.
- J. Wang, Q. Zhang, F. Deng, X. Luo and D. D. Dionysiou, Chem. Eng. J., 2020, 379, 122264 CrossRef CAS.
- S. Li, C. Wang, Y. Liu, M. Cai, Y. Wang, H. Zhang, Y. Guo, W. Zhao, Z. Wang and X. Chen, Chem. Eng. J., 2022, 429, 132519 CrossRef CAS.
- W. Zhao, H. Chen, J. Zhang, P. J. Low and H. Sun, Chem. Sci., 2024, 15(42), 17292–17327 RSC.
- W. Xu, W. Tian, L. Meng, F. Cao and L. Li, Adv. Energy Mater., 2021, 11(8), 2003500 CrossRef CAS.
- V. Q. Hieu, T. C. Lam, A. Khan, T.-T. Thi Vo, T.-Q. Nguyen, V. D. Doan, D. L. Tran, V. T. Le and V. A. Tran, Chemosphere, 2021, 285, 131429 CrossRef CAS PubMed.
- A. M. Taddesse, M. Alemu and T. Kebede, J. Environ. Chem. Eng., 2020, 8(5), 104356 CrossRef CAS.
- A. Bolatov, A. Manjovelo, B. Chouchene, L. Balan, T. Gries, G. Medjahdi, B. Uralbekov and R. Schneider, Mater., 2024, 17(19), 4877 CrossRef CAS PubMed.
- Q. A. Drmosh, A. Hezam, A. H. Y. Hendi, M. Qamar, Z. H. Yamani and K. Byrappa, Appl. Surf. Sci., 2020, 499, 143938 CrossRef CAS.
- K. Alkanad, A. Hezam, Q. A. Drmosh, S. S. Ganganakatte Chandrashekar, A. A. AlObaid, I. Warad, M. A. Bajiri and L. Neratur Krishnappagowda, Sol. RRL, 2021, 5(11), 2100501 CrossRef CAS.
- M. Aadil, T. Kousar, M. Hussain, H. H. Somaily, A. K. Abdulla, E. R. Muhammad, E. A. Al-Abbad, M. A. Salam, S. M. Albukhari, D. F. Baamer and M. Z. Ansari, Ceram. Int., 2023, 49(3), 4846–4854 CrossRef CAS.
- S. Girma, A. M. Taddesse, Y. Bogale and Z. Bezu, J. Photochem. Photobiol., A, 2023, 444, 114963 CrossRef CAS.
- A. Nawaz, S. Goudarzi, H. Zarrin and P. Saravanan, J. Alloys Compd., 2022, 903, 163951 CrossRef CAS.
- A. Hezam, K. Namratha, Q. A. Drmosh, Z. H. Yamani and K. Byrappa, Ceram. Int., 2017, 43(6), 5292–5301 CrossRef CAS.
- M. Pirhashemi and A. Habibi-Yangjeh, Sep. Purif. Technol., 2018, 193, 69–80 CrossRef CAS.
- M. Pirhashemi and A. Habibi-Yangjeh, Mater. Chem. Phys., 2018, 214, 107–119 CrossRef CAS.
- M. Shekofteh-Gohari and A. Habibi-Yangjeh, Solid State Sci., 2017, 74, 24–36 CrossRef CAS.
- M. Wang, G. Tan, H. Ren, A. Xia and Y. Liu, Appl. Surf. Sci., 2019, 492, 690–702 CrossRef CAS.
- Z. Dong, Y. Wu, N. Thirugnanam and G. Li, Appl. Surf. Sci., 2018, 430, 293–300 CrossRef CAS.
- B. Feng, Q. Wang, P. Liu, Z. Yuan, D. Pan, M. Ye, K. Shen and Z. Xin, Nanoscale, 2024, 16(37), 17616–17623 RSC.
- Y. Jiao, Y. Li, J. Wang, Z. He and Z. Li, Appl. Surf. Sci., 2020, 534, 147603 CrossRef CAS.
- Y. Cheng, P. Fu, X. Yang, Y. Zhang, S. Jin, H. Liu, Y. Shen, X. Guo and L. Chen, J. Mater. Chem. A, 2023, 11(45), 24764–24776 RSC.
- W. Xue, D. Huang, J. Li, G. Zeng, R. Deng, Y. Yang, S. Chen, Z. Li, X. Gong and B. Li, Chem. Eng. J., 2019, 373, 1144–1157 CrossRef CAS.
- A. Kumar, G. Sharma, A. Kumari, C. Guo, M. Naushad, D.-V. N. Vo, J. Iqbal and F. J. Stadler, Appl. Catal., B, 2021, 284, 119808 CrossRef CAS.
- M. Zhu, Q. Liu, W. Chen, Y. Yin, L. Ge, H. Li and K. Wang, ACS Appl. Mater. Interfaces, 2017, 9(44), 38832–38841 CrossRef CAS PubMed.
- W. Shi, C. Liu, M. Li, X. Lin, F. Guo and J. Shi, J. Hazard. Mater., 2020, 389, 121907 CrossRef CAS PubMed.
- X. Wang, Q. Lu, Y. Sun, K. Liu, J. Cui, C. Lu and H. Dai, J. Environ. Chem. Eng., 2022, 10(5), 108354 CrossRef CAS.
- N. Sedaghati, A. Habibi-Yangjeh, M. Pirhashemi and S. Vadivel, J. Photochem. Photobiol., A, 2019, 384, 112066 CrossRef CAS.
- S. Zarezadeh, A. Habibi-Yangjeh, M. Mousavi and S. Ghosh, J. Photochem. Photobiol., A, 2020, 389, 112247 CrossRef CAS.
- H. R. Kim, A. Razzaq, C. A. Grimes and S.-I. In, J. CO2 Util., 2017, 20, 91–96 CrossRef CAS.
- W. Ali, H. Ullah, A. Zada, W. Muhammad, S. Ali, S. Shaheen, M. K. Alamgir, M. Z. Ansar, Z. U. Khan, H. Bilal and P.-S. Yap, Sci. Total Environ., 2020, 746, 141291 CrossRef CAS PubMed.
- W. Chen, S. Zhang, G. Wang, G. Huang, Z. Yu, Y. Li and L. Tang, Nanomaterials, 2021, 11, 38 CrossRef CAS PubMed.
- Z. Ai, Y. Shao, B. Chang, B. Huang, Y. Wu and X. Hao, Appl. Catal., B, 2019, 242, 202–208 CrossRef CAS.
- P. K. Prajapati, D. Garg, A. Malik, D. Kumar, V. Amoli and S. L. Jain, J. Environ. Chem. Eng., 2022, 10(4), 108147 CrossRef CAS.
- P. Basyach, P. K. Prajapati, S. S. Rohman, K. Sonowal, L. Kalita, A. Malik, A. K. Guha, S. L. Jain and L. Saikia, ACS Appl. Mater. Interfaces, 2023, 15(1), 914–931 CrossRef CAS PubMed.
- D. Liu, L. Jiang, D. Chen, Z. Hao, B. Deng, Y. Sun, X. Liu, B. Jia, L. Chen and H. Liu, ACS Catal., 2024, 14(7), 5326–5343 CrossRef CAS.
- D. Majhi, K. Das, R. Bariki, S. Padhan, A. Mishra, R. Dhiman, P. Dash, B. Nayak and B. G. Mishra, J. Mater. Chem. A, 2020, 8(41), 21729–21743 RSC.
- Z.-J. Chen, H. Guo, H.-Y. Liu, C.-G. Niu, D.-W. Huang, Y.-Y. Yang, C. Liang, L. Li and J.-C. Li, Chem. Eng. J., 2022, 438, 135471 CrossRef CAS.
- B. Gao, Y. Pan and H. Yang, Appl. Catal., B, 2022, 315, 121580 CrossRef CAS.
- M. Tayyab, Y. Liu, Z. Liu, L. Pan, Z. Xu, W. Yue, L. Zhou, J. Lei and J. Zhang, J. Colloid Interface Sci., 2022, 628, 500–512 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Sci., 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, A. Mishchenko, A. Carvalho and A. H. Castro Neto, Sci., 2016, 353(6298), aac9439 CrossRef CAS PubMed.
- H. Yin, C. Yuan, H. Lv, X. Chen, K. Zhang and Y. Zhang, Sep. Purif. Technol., 2022, 295, 121294 CrossRef CAS.
- K. R. G. Lim, A. D. Handoko, S. K. Nemani, B. Wyatt, H. Y. Jiang, J. Tang, B. Anasori and Z. W. Seh, ACS Nano, 2020, 14(9), 10834–10864 CrossRef CAS PubMed.
- H. Wang, F. Liu, W. Fu, Z. Fang, W. Zhou and Z. Liu, Nanoscale, 2014, 6(21), 12250–12272 RSC.
- C. Tan, P. Yu, Y. Hu, J. Chen, Y. Huang, Y. Cai, Z. Luo, B. Li, Q. Lu, L. Wang, Z. Liu and H. Zhang, J. Am. Chem. Soc., 2015, 137(32), 10430–10436 CrossRef CAS PubMed.
- I. Ahmad, S. Shukrullah, M. Y. Naz, M. Ahmad, E. Ahmed, Y. Liu, A. Hussain, S. Iqbal and S. Ullah, Adv. Colloid Interface Sci., 2022, 304, 102661 CrossRef CAS PubMed.
- H. Wang, X. Liu, P. Niu, S. Wang, J. Shi and L. Li, Matter, 2020, 2(6), 1377–1413 CrossRef.
- M. Xiong, J. Yan, B. Chai, G. Fan and G. Song, J. Mater. Sci. Technol., 2020, 56, 179–188 CrossRef CAS.
- X. Liu, Y. Liu, W. Zhang, Q. Zhong and X. Ma, Mater. Sci. Semicon. Process., 2020, 105, 104734 CrossRef CAS.
- Y. Guo, B. Chang, T. Wen, S. Zhang, M. Zeng, N. Hu, Y. Su, Z. Yang and B. Yang, J. Colloid Interface Sci., 2020, 567, 213–223 CrossRef CAS PubMed.
- J. Su, G. D. Li, X. H. Li and J. S. Chen, Adv. Sci., 2019, 6(7), 1801702 CrossRef PubMed.
- X. Duan, C. Wang, J. C. Shaw, R. Cheng, Y. Chen, H. Li, X. Wu, Y. Tang, Q. Zhang, A. Pan, J. Jiang, R. Yu, Y. Huang and X. Duan, Nat. Nanotechnol., 2014, 9(12), 1024–1030 CrossRef CAS PubMed.
- S. Cheng, Z. Zuo and Y. Li, Mater. Chem. Front., 2024, 8(7), 1835–1843 RSC.
- Y. Zhao, S. Zhang, R. Shi, G. I. N. Waterhouse, J. Tang and T. Zhang, Mater. Today, 2020, 34, 78–91 CrossRef CAS.
- B. Tahir, M. Tahir and M. G. M. Nawawi, J. CO2 Util., 2020, 41, 101270 CrossRef CAS.
- M. Antoniadou, M. K. Arfanis, I. Ibrahim and P. Falaras, Water, 2019, 11(12), 2439 CrossRef CAS.
- W. Chen, M. Liu, X. Li and L. Mao, Appl. Surf. Sci., 2020, 512, 145782 CrossRef CAS.
- M. F. R. Samsudin, H. Ullah, R. Bashiri, N. M. Mohamed, S. Sufian and Y. H. Ng, ACS Sustainable Chem. Eng., 2020, 8(25), 9393–9403 CrossRef CAS.
- H. Sepahvand and S. Sharifnia, Int. J. Hydrogen Energy, 2019, 44(42), 23658–23668 CrossRef CAS.
- J. Mao, B. Hong, J. Wei, J. Xu, Y. Han, H. Jin, D. Jin, X. Peng, J. Li, Y. Yang, J. Gong, H. Ge and X. Wang, ChemistrySelect, 2019, 4(46), 13716–13723 CrossRef CAS.
- X. She, J. W. H. Xu, J. Zhong, Y. Wang, Y. Song, K. Nie, Y. Liu, Y. Yang, M. T. F. Rodrigues, R. Vajtai, J. Lou, D. Du, H. Li and P. M. Ajayan, Adv. Energy Mater., 2017, 7, 1700025 CrossRef.
- P. John, K. Johari, N. Gnanasundaram, A. Appusamy and M. Thanabalan, Environ. Technol. Innovation, 2021, 22, 101412 CrossRef CAS.
- Z. Han, X. Ning, Z. Yin, W. Zhen, G. Lu and B. Su, Int. J. Hydrogen Energy, 2024, 59, 856–865 CrossRef CAS.
- L. Jing, W.-J. Ong, R. Zhang, E. Pickwell-MacPherson and C. Y. Jimmy, Catal. Today, 2018, 315, 103–109 CrossRef CAS.
- Y. Shang, H. Fan, Y. Sun and W. Wang, Sustainable Energy Fuels, 2022, 6(16), 3729–3739 RSC.
- S. Tao, L. Chenxi, B. Yupeng, F. Jun and L. Enzhou, Acta Phys.-Chim. Sin., 2023, 39(6), 2212009 Search PubMed.
- B. Wang, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, J. Colloid Interface Sci., 2020, 576, 426–434 CrossRef CAS PubMed.
- Z. Zhang, J. Huang, Y. Fang, M. Zhang, K. Liu and B. Dong, Adv. Mater., 2017, 29(18), 1606688 CrossRef PubMed.
- Y. Huang, F. Mei, J. Zhang, K. Dai and G. Dawson, Acta Phys.-Chim. Sin., 2021, 0(0), 2108028 Search PubMed.
- Q. Liu, X. He, J. Peng, X. Yu, H. Tang and J. Zhang, Chin. J. Catal., 2021, 42(9), 1478–1487 CrossRef CAS.
- Y. You, S. Wang, K. Xiao, T. Ma, Y. Zhang and H. Huang, ACS Sustainable Chem. Eng., 2018, 6(12), 16219–16227 CrossRef CAS.
- C. Ji, S.-N. Yin, S. Sun and S. Yang, Appl. Surf. Sci., 2018, 434, 1224–1231 CrossRef CAS.
- C. Liu, Y. Feng, Z. Han, Y. Sun, X. Wang, Q. Zhang and Z. Zou, Chin. J. Catal., 2021, 42(1), 164–174 CrossRef CAS.
- R.-T. Guo, X.-Y. Liu, H. Qin, Z.-Y. Wang, X. Shi, W.-G. Pan, Z.-G. Fu, J.-Y. Tang, P.-Y. Jia, Y.-F. Miao and J.-W. Gu, Appl. Surf. Sci., 2020, 500, 144059 CrossRef CAS.
- T. Yang, P. Deng, L. Wang, J. Hu, Q. Liu and H. Tang, Chin. J. Struct. Chem., 2022, 41(6), 2206023–2206030 CAS.
- X. Gu, T. Chen, J. Lei, Y. Yang, X. Zheng, S. Zhang, Q. Zhu, X. Fu, S. Meng and S. Chen, Chin. J. Catal., 2022, 43(10), 2569–2580 CrossRef CAS.
- D. Gogoi, M. R. Das and N. Nath Ghosh, Appl. Surf. Sci., 2023, 619, 156753 CrossRef CAS.
- Z. Liang, L. Yunfeng, Z. Yongkang, Q. Liewei and X. Yan, Acta Phys.-Chim. Sin., 2022, 38(7), 2112027 Search PubMed.
- C. Liu, H. Dai, C. Tan, Q. Pan, F. Hu and X. Peng, Appl. Catal., B, 2022, 310, 121326 CrossRef CAS.
- M. Alhaddad, R. M. Mohamed and M. H. H. Mahmoud, ACS Omega, 2021, 6(12), 8717–8725 CrossRef CAS PubMed.
- J. Li, W. Cao, Y. Li, X. Xu, Y. Jiang and K. Lin, ACS Appl. Energy Mater., 2022, 5(8), 9463–9470 CrossRef CAS.
- X. Li, K. Xie, L. Song, M. Zhao and Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9(29), 24577–24583 CrossRef CAS PubMed.
- R. Shen, J. Xie, X. Lu, X. Chen and X. Li, ACS Sustainable Chem. Eng., 2018, 6(3), 4026–4036 CrossRef CAS.
- Z. Jin, R. Hu, H. Wang, J. Hu and T. Ren, Appl. Surf. Sci., 2019, 491, 432–442 CrossRef CAS.
- W. Chen, Z.-C. He, G.-B. Huang, C.-L. Wu, W.-F. Chen and X.-H. Liu, Chem. Eng. J., 2019, 359, 244–253 CrossRef CAS.
- E. Sitara, H. Nasir, A. Mumtaz, M. F. Ehsan, M. Sohail, S. Iram, S. A. B. Bukhari, S. Ullah, T. Akhtar and A. Iqbal, Int. J. Hydrogen Energy, 2021, 46(50), 25424–25435 CrossRef CAS.
- H. Sun and S.-J. Park, Appl. Surf. Sci., 2020, 531, 147325 CrossRef CAS.
- M. Ding, J. Zhou, H. Yang, R. Cao, S. Zhang, M. Shao and X. Xu, Chin. Chem. Lett., 2020, 31(1), 71–76 CrossRef CAS.
- D. Liu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Angew. Chem., Int. Ed., 2020, 59(11), 4519–4524 CrossRef CAS PubMed.
- Q. Xu, B. Zhu, C. Jiang, B. Cheng and J. Yu, Sol. RRL, 2018, 2(3), 1800006 CrossRef.
- L. K. Putri, B.-J. Ng, W.-J. Ong, H. W. Lee, W. S. Chang and S.-P. Chai, J. Mater. Chem. A, 2018, 6(7), 3181–3194 RSC.
- L. Wang, Y. Li and P. Han, Sci. Rep., 2021, 11(1), 22950 CrossRef CAS PubMed.
- H. Dong, S. Hong, P. Zhang, S. Yu, Y. Wang, S. Yuan, H. Li, J. Sun, G. Chen and C. Li, Chem. Eng. J., 2020, 395, 125150 CrossRef CAS.
- P. Xia, B. Zhu, B. Cheng, J. Yu and J. Xu, Chem. Eng., 2017, 6(1), 965–973 Search PubMed.
- Z. Mo, H. Xu, Z. Chen, X. She, Y. Song, J. Lian, X. Zhu, P. Yan, Y. Lei, S. Yuan and H. Li, Appl. Catal., B, 2019, 241, 452–460 CrossRef CAS.
- L. Chen, Y. Xu and B. Chen, Appl. Catal., B, 2019, 256, 117848 CrossRef CAS.
- F. Guo, W. Shi, C. Zhu, H. Li and Z. Kang, Appl. Catal., B, 2018, 226, 412–420 CrossRef CAS.
- B. Zhu, H. Tan, J. Fan, B. Cheng, J. Yu and W. Ho, J. Materiomics, 2021, 7(5), 988–997 CrossRef.
- S. Shi, M. Jia, M. Li, S. Zhou, Y. Zhao, J. Zhong, D. Dai and J. Qiu, Colloids Surf., A, 2023, 667, 131259 CrossRef CAS.
- J. Jia, Y. Luo, H. Wu, Y. Wang, X. Jia, J. Wan, Y. Dang, G. Liu, H. Xie and Y. Zhang, J. Colloid Interface Sci., 2024, 658, 966–975 CrossRef CAS PubMed.
- S. Wang, P. He, L. Jia, M. He, T. Zhang, F. Dong, M. Liu, H. Liu, Y. Zhang, C. Li, J. Gao and L. Bian, Appl. Catal., B, 2019, 243, 463–469 CrossRef CAS.
- M. Liu, S. Wei, W. Chen, L. Gao, X. Li, L. Mao and H. Dang, J. Chin. Chem. Soc., 2020, 67(2), 246–252 CrossRef CAS.
- R. Zahra, E. Pervaiz, M. M. Baig and O. Rabi, Electrochim. Acta, 2022, 418, 140346 CrossRef CAS.
- D. T. N. Hoa, N. T. T. Tu, H. Q. A. Thinh, L. Van Thanh Son, L. V. T. Son, N. D. V. Quyen, L. L. Son, T. N. Tuyen, P. L. M. Thong, L. H. Diem and D. Q. Khieu, J. Nanomater., 2023, 2023(1), 9967890 Search PubMed.
- A. Alsalme, A. H. Galal, E. F. El-Sherbeny, A. Soltan, M. F. Abdel-Messih and M. A. Ahmed, Diamond Relat. Mater., 2022, 122, 108819 CrossRef CAS.
- T. Zhang, Q. Yang, H. Li, J. Zhong, J. Li and H. Yang, Opt. Mater., 2022, 131, 112649 CrossRef CAS.
- B. Zhang, X. Hu, E. Liu and J. Fan, Chin. J. Catal., 2021, 42(9), 1519–1529 CrossRef CAS.
- Y. Huo, J. Zhang, Z. Wang, K. Dai, C. Pan and C. Liang, J. Colloid Interface Sci., 2021, 585, 684–693 CrossRef CAS PubMed.
- B. Dai, Y. Li, J. Xu, C. Sun, S. Li and W. Zhao, Appl. Surf. Sci., 2022, 592, 153309 CrossRef CAS.
- H. Wang, R. Niu, J. Liu, S. Guo, Y. Yang, Z. Liu and J. Li, Nano Res., 2022, 15(8), 6987–6998 CrossRef CAS.
- T. Ni, H. Zhang, Z. Yang, L. Zhou, L. Pan, C. Li, Z. Yang and D. Liu, J. Colloid Interface Sci., 2022, 625, 466–478 CrossRef CAS PubMed.
- Q. Ding, X. Zou, J. Ke, Y. Dong, Y. Cui, G. Lu and H. Ma, Renewable Energy, 2023, 203, 677–685 CrossRef CAS.
- K. Wang, X. Feng, Y. Shangguan, X. Wu and H. Chen, Chin. J. Catal., 2022, 43(2), 246–254 CrossRef CAS.
- Y. Zhen, C. Yang, H. Shen, W. Xue, C. Gu, J. Feng, Y. Zhang, F. Fu and Y. Liang, Phys. Chem. Chem. Phys., 2020, 22(45), 26278–26288 RSC.
- A. Meng, S. Zhou, D. Wen, P. Han and Y. Su, Chin. J. Catal., 2022, 43(10), 2548–2557 CrossRef CAS.
- X. Lin, A. Kumar, G. Sharma, M. Naushad, G.-P. Alberto and F. J. Stadler, J. Mol. Liq., 2022, 366, 120147 CrossRef CAS.
- F. A. Qaraah, S. A. Mahyoub, A. Hezam, A. Qaraah, F. Xin and G. Xiu, Appl. Catal., B, 2022, 315, 121585 CrossRef CAS.
- Z. Huang, J. Liu, S. Zong, X. Wang, K. Chen, L. Liu and Y. Fang, J. Colloid Interface Sci., 2022, 606(Pt 1), 848–859 CrossRef CAS PubMed.
- J. Fu, Q. Xu, J. Low, C. Jiang and J. Yu, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS.
- D. Ren, W. Zhang, Y. Ding, R. Shen, Z. Jiang, X. Lu and X. Li, Sol. RRL, 2020, 4(8), 1900423 CrossRef CAS.
- B. Liu, C. Bie, Y. Zhang, L. Wang, Y. Li and J. Yu, Langmuir, 2021, 37(48), 14114–14124 CrossRef CAS PubMed.
- M. H. Barzegar, M. M. Sabzehmeidani, M. Ghaedi, V. M. Avargani, Z. Moradi, V. A. L. Roy and H. Heidari, Chem. Eng. Res. Des., 2021, 174, 307–318 CrossRef CAS.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Nanoscale, 2013, 5(18), 8326–8339 RSC.
- G. Di Liberto, S. Tosoni and G. Pacchioni, Adv. Funct. Mater., 2021, 31(20), 2009472 CrossRef CAS.
- G. Di Liberto, S. Tosoni and G. Pacchioni, J. Phys.: Condens. Matter, 2019, 31(43), 434001 CrossRef CAS PubMed.
- G. Di Liberto, A. Morales-Garcia and S. T. Bromley, Nat. Commun., 2022, 13(1), 6236 CrossRef CAS PubMed.
- G. Di Liberto, S. Tosoni and G. Pacchioni, J. Phys.: Condens. Matter, 2021, 33(7), 075001 CrossRef PubMed.
- Y. Chang, J. R. Yates and C. E. Patrick, ACS Omega, 2023, 8(22), 20138–20147 CrossRef CAS PubMed.
- X. Hao, Q. Ma, X. Zhang, J. Wang, D. Yin, S. Ma and B. Xu, J. Appl. Phys., 2024, 136(6), 064303 CrossRef CAS.
- X. Liu, J. Chen, H. Yin, L. Bai, C. Yao, H. Li, H. Yin and Y. Wang, Mater. Res. Lett., 2019, 7(6), 232–238 CrossRef CAS.
- Y. Qi, M. A. Sadi, D. Hu, M. Zheng, Z. Wu, Y. Jiang and Y. P. Chen, Adv. Mater., 2023, 35(12), e2205714 CrossRef PubMed.
- P. A. Vermeulen, J. Mulder, J. Momand and B. J. Kooi, Nanoscale, 2018, 10(3), 1474–1480 RSC.
- T. Huang, W. Wei, X. Chen and N. Dai, Ann. Phys., 2019, 531(4), 1800465 CrossRef CAS.
- J. Yang, X. Li, Y. Yang and R. Dou, J. Phys. Chem. Lett., 2025, 16(11), 2731–2741 CrossRef CAS PubMed.
- Q. Li, C. Pan, J. Wang, L.-L. Wang and X. Zhu, Appl. Surf. Sci., 2022, 605, 154720 CrossRef CAS.
- G. Di Liberto, L. A. Cipriano, S. Tosoni and G. Pacchioni, Chem, 2021, 27(53), 13306–13317 CrossRef CAS PubMed.
- R. Tao, X. Li, X. Li, C. Shao and Y. Liu, Nanoscale, 2020, 12(15), 8320–8329 RSC.
- N. D. Lam, H. Van Thanh, T. D. Thien and T. Nguyen-Tran, Mater. Trans., 2023, 64(2), 578–585 CrossRef CAS.
- J.-M. Delgado-Sanchez, I. Lillo-Bravo, J. A. López-Álvarez and E. Pérez-Aparicio, Sol. RRL, 2022, 6(12), 2200818 CrossRef CAS.
- M. A. Hanif, J. Akter, Y. S. Kim, H. G. Kim, J. R. Hahn and L. K. Kwac, Catal., 2022, 12(2), 151 CAS.
- C. G. Van de Walle and R. M. Martin, Phys. Rev. B: Condens. Matter, 1987, 35(15), 8154–8165 CrossRef CAS PubMed.
- G. Di Liberto, S. Tosoni and G. Pacchioni, Phys. Chem. Chem. Phys., 2019, 21(38), 21497–21505 RSC.
- S.-H. Wei and A. Zunger, Appl. Phys. Lett., 1998, 72(16), 2011–2013 CrossRef CAS.
- S.-H. Wei and A. Zunger, J. Appl. Phys., 1995, 78(6), 3846–3856 CrossRef CAS.
- J. C. Conesa, Nanomaterials, 2021, 11(6), 1581 CrossRef CAS PubMed.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Nat. Mater., 2013, 12(9), 798–801 CrossRef CAS PubMed.
- G. Di Liberto, S. Tosoni, F. Illas and G. Pacchioni, J. Chem. Phys., 2020, 152(18), 184704 CrossRef CAS PubMed.
- R. W. Grant, E. A. Kraut, S. P. Kowalczyk and J. R. Waldrop, J. Vac. Sci. Technol., B: Microelectron. Process. Phenom., 1983, 1(2), 320–327 CrossRef CAS.
- J. R. Waldrop, S. P. Kowalczyk, R. W. Grant, E. A. Kraut and D. L. Miller, J. Vac. Sci. Technol., 1981, 19, 573–575 CrossRef CAS.
- I. E. L. Stephens, A. S. Bondarenko, L. Bech and I. Chorkendorff, ChemCatChem, 2012, 4(3), 341–349 CrossRef CAS.
- E. A. Kraut, R. W. Grant, J. R. Waldrop and S. P. Kowalczyk, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28(4), 1965–1977 CrossRef CAS.
- G. Di Liberto, S. Tosoni and G. Pacchioni, ChemCatChem, 2020, 12(7), 2097–2105 CrossRef CAS.
- N. Pueyo Bellafont, F. Vines, W. Hieringer and F. Illas, J. Comput. Chem., 2017, 38(8), 518–522 CrossRef CAS PubMed.
- F. Vines, C. Sousa and F. Illas, Phys. Chem. Chem. Phys., 2018, 20(13), 8403–8410 RSC.
- I. Grigioni, G. Di Liberto, M. V. Dozzi, S. Tosoni, G. Pacchioni and E. Selli, ACS Appl. Energy Mater., 2021, 4(8), 8421–8431 CrossRef CAS PubMed.
- P. Lv, F. Duan, J. Sheng, S. Lu, H. Zhu, M. Du and M. Chen, Appl. Organomet. Chem., 2020, 35(3), e6124 CrossRef.
- S. Kumar, A. Kumar, A. Kumar, R. Balaji and V. Krishnan, ChemistrySelect, 2018, 3(6), 1919–1932 CrossRef CAS.
- Y. Lu, Z. Zhuang, L. Li, F.-F. Chen, P. Wei and Y. Yu, J. Mater. Chem. A, 2025, 13, 4718–4745 RSC.
- Y. Guo, W. Shi and Y. Zhu, EcoMat, 2019, 1(1), e12007 CrossRef.
- T. Lv, J. Li, N. Arif, L. Qi, J. Lu, Z. Ye and Y.-J. Zeng, Matter, 2022, 5(9), 2685–2721 CrossRef CAS.
- F. Chen, H. Huang, L. Guo, Y. Zhang and T. Ma, Angew. Chem., Int. Ed., 2019, 58(30), 10061–10073 CrossRef CAS PubMed.
- X. Yue, J. Fan and Q. Xiang, Adv. Funct. Mater., 2021, 32(12), 2110258 CrossRef.
- Z. Lou, P. Wang, B. Huang, Y. Dai, X. Qin, X. Zhang, Z. Wang and Y. Liu, ChemPhotoChem., 2017, 1(5), 136–147 CrossRef CAS.
- Z. Wang, B. Cheng, L. Zhang, J. Yu and H. Tan, Sol. RRL, 2021, 6(1), 2100587 CrossRef.
- W. Lou, G. Liu, X. Ma, C. Yang, L. Feng, Y. Liu and X. Gao, J. Mater. Chem. A, 2025, 13(6), 4356–4366 RSC.
- L. Sun, W. Wang, C. Zhang, M. Cheng, Y. Zhou, Y. Yang, H. Luo, D. Qin, C. Huang and Z. Ouyang, Chem. Eng. J., 2022, 446, 137027 CrossRef CAS.
- W. Ouyang, F. Teng and X. Fang, Adv. Funct. Mater., 2018, 28(16), 1707178 CrossRef.
- Y. Zhong, Front. Chem., 2023, 11, 1278370 CrossRef CAS PubMed.
- R. Long, W.-H. Fang and O. V. Prezhdo, J. Phys. Chem. C, 2017, 121(7), 3797–3806 CrossRef CAS.
- Y. Guo, Y. Xue, X. Li, C. Li, H. Song, Y. Niu, H. Liu, X. Mai, J. Zhang and Z. Guo, Nanomaterials, 2019, 9(7), 966 CrossRef CAS PubMed.
- K. Zhang, M. Fujitsuka, Y. Du and T. Majima, ACS Appl. Mater. Interfaces, 2018, 10(24), 20458–20466 CrossRef CAS PubMed.
- J. Hu, D. Chen, Z. Mo, N. Li, Q. Xu, H. Li, J. He, H. Xu and J. Lu, Angew. Chem., Int. Ed., 2019, 58(7), 2073–2077 CrossRef CAS PubMed.
- J. Shi, L. Mao, C. Cai, G. Li, C. Cheng, B. Zheng, Y. Hu, Z. Huang, X. Hu and G. Żyła, Catal. Sci. Technol., 2020, 10(17), 5896–5902 RSC.
- X. Tao, Y. Gao, S. Wang, X. Wang, Y. Liu, Y. Zhao, F. Fan, M. Dupuis, R. Li and C. Li, Adv. Energy Mater., 2019, 9(13), 1803951 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |





