Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functionalizing graphene oxide in polysulfone composite adsorption cartridges through in-flow, in situ treatment†
Angela
Pintus‡
 ab,
Andrea
Trifoglio‡
ab,
Andrea
Trifoglio‡
 bc,
Sara
Khaliha
bc,
Sara
Khaliha
 b,
Sebastiano
Mantovani
b,
Sebastiano
Mantovani
 b,
Davide
Paci
d,
Alessandro
Kovtun
b,
Davide
Paci
d,
Alessandro
Kovtun
 *b,
Letizia
Bocchi
d and
Manuela
Melucci
*b,
Letizia
Bocchi
d and
Manuela
Melucci
 *b
*b
aDipartimento di Fisica, Università di Modena e Reggio Emilia, Via Campi 213/A, 41125 Modena (MO), Italy
bIstituto per la Sintesi Organica e la Fotoreattività (ISOF)-CNR, Via Gobetti 101, 40129 Bologna (BO), Italy. E-mail: manuela.melucci@cnr.it
cDepartment of Chemistry ‘G. Ciamician’, Alma Mater Studiorum – University of Bologna, Via Selmi 2, 40129 Bologna (BO), Italy
dMedica Spa, Via degli Artigiani 7, 41036, Medolla (MO), Italy
First published on 22nd May 2025
Abstract
We present a scalable chemical method to upcycle polysulfone–graphene oxide (PSU–GO) industrial waste into customized adsorbent materials through in situ, in flow L-lysine functionalization of GO embedded in the polymer composite. Enhanced carbamazepine removal from water is demonstrated for PSU–GOLys filled cartridges.
Graphene-based composites have shown significant potential across various applications,1 ranging from sensors and flexible electronics,2 advanced energy storage systems3 and environmental remediation applications.4 Due to its processability, water dispersibility, and superior chemical versatility, graphene oxide (GO) has been widely exploited as a component in composite tailoring.5–7 The presence of a variety of oxygenated functional groups (i.e. hydroxyl, epoxide, and carboxyl groups) on the GO nanosheet surface enables the chemical functionalization through covalent chemistry, allowing for the formulation and synthesis of a wide class of modified GO-based materials with different chemical features.8,9 The surface chemistry tunability of GO nanosheets has recently been widely exploited to tune the adsorption properties toward water organic and metallic contaminants.10–12 In this scenario, several examples of modification strategies have been developed, including the use of polyethyleneimine and triethyl-phosphate for toxic metal adsorption,13,14 polysaccharides and amino acids (AA) for targeting organic pollutants,15–17 and cyclodextrin (CD) for the removal of per- and polyfluoroalkyl substances.18 The adsorption mechanism has been widely explored, demonstrating that the reactivity is related to the balance of different energy contributions, including van der Waals forces (VDW), electrostatic interactions and π–π stacking interactions.19,20 On this line, we recently reported a study on amino acid-modified GO that demonstrates that the shape complementarity between the AA pendant and contaminant molecules enhances the hydrophobic interactions between the core of the contaminant and the amino acid side chains, eventually increasing the adsorption capacity. Among GO modified with AA, L-lysine-modified GO (GO-Lys) showed an effective adsorption of carbamazepine (CBZ), an antiepileptic drug of increasing concern as an emerging water contaminant, with the maximum adsorption capacity two orders of magnitude higher than that of pristine GO.21 As for pristine GO-Lys nanosheets, alginate-GOLys composites, showed excellent adsorption properties for a selection of organic contaminants, including benzophenone-4 (BP4), bisphenol-A (BPA), diclofenac (DCF) and CBZ.22 While a number of GO composites such as foams, aerogels, and membranes have been reported in the last years,23–25 the post-functionalization of GO nanosheets, already embedded in such composites, has been relatively unexplored, with only a few studies reporting successful modification strategies. In this context, S. de León et al. demonstrated that GO incorporated into photosensitive acrylic resins could be functionalized with biomolecules, thereby improving both mechanical and functional properties of the final material.26
Here, we demonstrate L-lysine in situ functionalization of GO embedded in a plastic waste material (Scheme 1), i.e. polysulfone graphene oxide (PSU–GO) granules (Fig. 1). These granules are obtained by grinding the waste of the production of Graphisulfone® membranes27 and have been already exploited as sorbents for drinking water treatment.28 The chemical stability of the PSU polymer requires the use of activating agents and organic solvents for surface functionalization,29,30 making post-production modifications under mild conditions unfeasible and thereby limiting the ability to fine-tune the surface properties and adsorption selectivity of PSU membranes.31,32 Here, we aim at exploiting the GO nanosheets embedded in the polymer structure to tune the adsorption properties of PSU granules. In situ synthesis of GO-Lys in PSU–GO filled cartridges was performed by loop recirculation of an aqueous solution of L-lysine. PSU–GO with different GO % amounts (i.e. PSU–GO-A with 3.5% w/w and PSU–GO-B with 10% w/w GO/PSU ratios) were modified with lysine by an epoxide ring-opening reaction (Scheme 1).9 The reaction was carried out under basic conditions (pH 9.7) to ensure that the alpha-NH2 of lysine was unprotonated and available for reaction as the nucleophilic moiety.21,33 The PSU structure does not include electrophilic groups available for direct amination, so in the exploited conditions the reaction is expected to involve only GO.34 To optimize permeation and maximize the interaction between lysine and GO in the porous structure of the granules, the solution of amino acid was recirculated at a constant flow of 5 mL min−1 for 2 h (Fig. 2). Then, the cartridges were kept at 80 °C overnight, to facilitate the formation of a stable covalent bond between lysine and the GO surface. Finally, the modified granules were washed by passing 2.5 L of ultrapure water through the cartridge to remove unreacted residues and then air-dried before use (Section S2, ESI†). Scanning electron microscopy (SEM) images provide insight into the morphology of the PSU–GO composites before and after functionalization (Fig. S2, ESI†). The granules have a hollow tubular structure (Fig. 3a and b) with surface pores in the range 5–10 μm. The characteristic porous structure of the hollow fiber granules is preserved after lysine functionalization,27,28 indicating that the synthetic process does not significantly alter the overall morphology of the granules.
 | ||
| Scheme 1 In situ lysine functionalization of GO embedded in PSU–GO granules with two different GO amounts (A: 3.5% w/w and B: 10% w/w PSU/GO). | ||
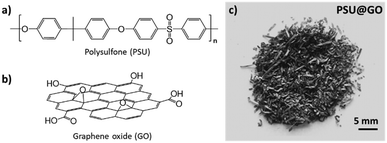 | ||
| Fig. 1 Chemical structures of a) polysulfone (PSU) and b) graphene oxide (GO); c) granules of composite PSU–GO. | ||
 | ||
| Fig. 2 Set up for in situ GO modification. The L-lysine solution is recirculated using a peristaltic pump through the PSU–GO cartridge. | ||
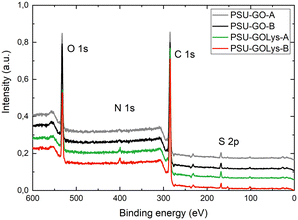
ATR-FTIR spectra of PSU–GOLys composites (Fig. S3, ESI†) were recorded on granules before and after functionalization without highlighting any differences attributable to the lysine grafting as most of the observed signal is ascribed to the PSU polymer. The surface chemical composition and L-lysine loading of the PSU–GO composites were analysed by X-ray photoelectron spectroscopy (XPS). The XPS survey spectra are shown in Fig. 4 while the atomic compositions of the pristine and L-lysine-modified PSU–GO samples are summarized in Table 1. As shown in Scheme 1, the colour of PSU–GO granules within the cartridge darkened after reacting with L-lysine. This change can be attributed to the partial reduction of GO under the reaction conditions (basic nucleophile and thermal treatment),9,35 and it is confirmed by XPS analysis that shows an increase in the C/O ratio from 4.2 to 4.5 for PSU–GO-A and from 3.6 to 4.3 for PSU–GO-B (Table 1). The surface of the materials predominantly consists of carbon, oxygen, sulphur and nitrogen, corresponding to i) the aliphatic C–C, C–S, and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C regions of PSU and GO, ii) the C–O/C–O–C/C
C regions of PSU and GO, ii) the C–O/C–O–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/S
O/S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities from both PSU and GO, iii) the sulfonated groups of PSU and GO, and iv) the nitrogen functional groups (C–N) of L-lysine (Section S4, ESI†). The successful covalent functionalization of GO is confirmed by the N 1s signal, which arises from the bound amino acid. As expected, higher L-lysine loading was achieved for PSU–GOLys-B with a higher GO extent, i.e. 10% w/w GO/PSU, with the nitrogen content increasing from 0.7% to 1.9% after functionalization, corresponding to an L-lysine loading of about 6%. On the other hand, the nitrogen content increased from 0.8% for pristine PSU–GO-A to 1.5% for PSU–GOLys-A, corresponding to a Lys-loading of 3.5%. To confirm the successful lysine modification of PSU–GO composites, we performed adsorption experiments on carbamazepine (CBZ) comparing pristine PSU–GO-A/B with PSU–GOLys-A/B. This choice was supported by both literature reports on its preferential interaction with amino acid-modified GO materials21,22 and by preliminary selectivity tests conducted under batch and flow conditions on a mixture of six emerging organic contaminants (Fig. S5, ESI†). As expected, batch experiments revealed a significantly higher CBZ removal for GO-Lys (65%) compared to unmodified GO (13%) after 15 minutes of contact time (Fig. S6a, ESI†). The same trend is observed in flow-through experiments (Fig. S6b and c, ESI†), carried out with the same configuration used for lysine functionalization (Fig. 5a). PSU–GOLys-A removed 58% of CBZ compared to 35% for PSU–GO-A, while PSU–GOLys-B achieved 84% removal, compared to 60% for PSU–GO-B. The enhanced selectivity toward CBZ adsorption was then used as a functional probe to assess the effectiveness of lysine modification in PSU–GO composite granules. A control experiment was performed using a PSU cartridge (without GO) treated with lysine under the same conditions used for PSU–GO composite functionalization, to exclude non-covalent interactions with the polymer and the possible side-adsorption effect on CBZ. The resulting material, PSU@Lys, showed poor CBZ removal performance similar to pristine PSU, confirming that lysine functionalization requires the presence of GO for effective modification, rather than occurring through nonspecific adsorption on the polymer (Fig. S7, ESI†). Fig. 5b and c present the CBZ removal performance of pristine PSU–GO and modified PSU–GOLys cartridges under continuous flow conditions. The introduction of GO and L-lysine into PSU led to a significant enhancement in CBZ removal efficiency compared to pristine PSU. Specifically, PSU–GOLys-A demonstrated an initial CBZ removal rate of 64%, outperforming PSU–GO-A (41%) and PSU (16%). After filtering 200 mL of CBZ-spiked water, PSU–GOLys-A retained a removal efficiency of around 40%, while that of PSU–GO-A and PSU dropped to 12% and 5%, respectively. After treating 600 mL of the CBZ solution, complete breakthrough occurred for all tested modules, with a total CBZ uptake of 107.2 μg g−1 for PSU–GOLys-A, 27.5 μg g−1 for PSU–GO-A, and 6.6 μg g−1 for PSU (Fig. S8a, ESI†). Similarly, PSU–GOLys-B exhibited the highest initial removal rate among all tested materials, reaching 87% removal efficiency. Within the first 500 mL of treated water, PSU–GOLys-B retained a 60% CBZ removal rate, achieving complete breakthrough after treating 1 L of the solution, with a total adsorption capacity of 259.5 μg g−1, substantially higher than those of PSU–GO-B (66.5 μg g−1) and PSU (Fig. S8b, ESI†).
O functionalities from both PSU and GO, iii) the sulfonated groups of PSU and GO, and iv) the nitrogen functional groups (C–N) of L-lysine (Section S4, ESI†). The successful covalent functionalization of GO is confirmed by the N 1s signal, which arises from the bound amino acid. As expected, higher L-lysine loading was achieved for PSU–GOLys-B with a higher GO extent, i.e. 10% w/w GO/PSU, with the nitrogen content increasing from 0.7% to 1.9% after functionalization, corresponding to an L-lysine loading of about 6%. On the other hand, the nitrogen content increased from 0.8% for pristine PSU–GO-A to 1.5% for PSU–GOLys-A, corresponding to a Lys-loading of 3.5%. To confirm the successful lysine modification of PSU–GO composites, we performed adsorption experiments on carbamazepine (CBZ) comparing pristine PSU–GO-A/B with PSU–GOLys-A/B. This choice was supported by both literature reports on its preferential interaction with amino acid-modified GO materials21,22 and by preliminary selectivity tests conducted under batch and flow conditions on a mixture of six emerging organic contaminants (Fig. S5, ESI†). As expected, batch experiments revealed a significantly higher CBZ removal for GO-Lys (65%) compared to unmodified GO (13%) after 15 minutes of contact time (Fig. S6a, ESI†). The same trend is observed in flow-through experiments (Fig. S6b and c, ESI†), carried out with the same configuration used for lysine functionalization (Fig. 5a). PSU–GOLys-A removed 58% of CBZ compared to 35% for PSU–GO-A, while PSU–GOLys-B achieved 84% removal, compared to 60% for PSU–GO-B. The enhanced selectivity toward CBZ adsorption was then used as a functional probe to assess the effectiveness of lysine modification in PSU–GO composite granules. A control experiment was performed using a PSU cartridge (without GO) treated with lysine under the same conditions used for PSU–GO composite functionalization, to exclude non-covalent interactions with the polymer and the possible side-adsorption effect on CBZ. The resulting material, PSU@Lys, showed poor CBZ removal performance similar to pristine PSU, confirming that lysine functionalization requires the presence of GO for effective modification, rather than occurring through nonspecific adsorption on the polymer (Fig. S7, ESI†). Fig. 5b and c present the CBZ removal performance of pristine PSU–GO and modified PSU–GOLys cartridges under continuous flow conditions. The introduction of GO and L-lysine into PSU led to a significant enhancement in CBZ removal efficiency compared to pristine PSU. Specifically, PSU–GOLys-A demonstrated an initial CBZ removal rate of 64%, outperforming PSU–GO-A (41%) and PSU (16%). After filtering 200 mL of CBZ-spiked water, PSU–GOLys-A retained a removal efficiency of around 40%, while that of PSU–GO-A and PSU dropped to 12% and 5%, respectively. After treating 600 mL of the CBZ solution, complete breakthrough occurred for all tested modules, with a total CBZ uptake of 107.2 μg g−1 for PSU–GOLys-A, 27.5 μg g−1 for PSU–GO-A, and 6.6 μg g−1 for PSU (Fig. S8a, ESI†). Similarly, PSU–GOLys-B exhibited the highest initial removal rate among all tested materials, reaching 87% removal efficiency. Within the first 500 mL of treated water, PSU–GOLys-B retained a 60% CBZ removal rate, achieving complete breakthrough after treating 1 L of the solution, with a total adsorption capacity of 259.5 μg g−1, substantially higher than those of PSU–GO-B (66.5 μg g−1) and PSU (Fig. S8b, ESI†).
| Composites | C (%) | O (%) | N (%) | S (%) | C/O | Loading |
|---|---|---|---|---|---|---|
| PSU–GO-A | 78.4 | 18.5 | 0.8 | 2.3 | 4.1 | — |
| PSU–GO-B | 76.5 | 21.2 | 0.7 | 1.6 | 3.6 | — |
| PSU–GOLys-A | 78.7 | 17.6 | 1.5 | 2.2 | 4.5 | 3.5% |
| PSU–GOLys-B | 78.6 | 18.1 | 1.9 | 1.4 | 4.3 | 6% |
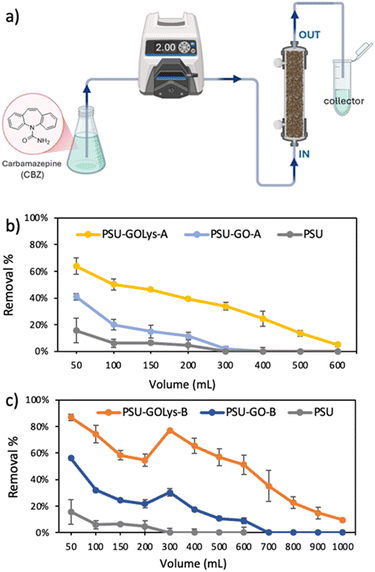 | ||
| Fig. 5 a) Experimental setup used for the adsorption test of CBZ and the removal performance of b) PSU, PSU–GO-A and PSU–GOLys-A and c) PSU, PSU–GO-B and PSU–GOLys-B. | ||
In summary, we have presented a novel approach for the functionalization of GO nanosheets embedded in a polymeric matrix. The proposed protocol, which utilizes water for both synthesis and workup, was specifically developed for the preparation of PSU–GO granular composites derived from industrial plastic waste. Successful in situ functionalization of GO with L-lysine was confirmed by X-ray photoelectron spectroscopy (XPS) and further supported by adsorption tests on CBZ in water. The adsorption test not only validated the successful functionalization but also highlighted the potential of PSU–GOLys cartridges for drinking water treatment, with CBZ adsorption increasing fourfold following chemical modification. The post-production modification of GO-based composites, such as PSU–GO, offers a scalable, cost-effective method to tailor the interface properties of these materials, thereby broadening their range of applications without altering the fabrication processes.
The authors gratefully acknowledge the support of this work by the projects Life-Remembrance, ENV/IT/001001 Life Resource and Environment LIFE20 ‘Give plastic wastes from the production of hollow-fiber membranes a second life’, project PNRR MUR project ECS_00000033_ECOSISTER, and ‘Le materie prime del futuro da fonti non-critiche, residuali e rinnovabili’ (FUTURAW, FOE 2022). The authors gratefully acknowledge the support of Franco Corticelli (CNR-ISMN) for SEM micrograph acquisition.
Data availability
ESI.† Materials, PSU–GO functionalization with lysine, scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), the carbamazepine (CBZ) adsorption test under flow conditions, and experimental conditions for CBZ analysis.Conflicts of interest
There are no conflicts to declare.Notes and references
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- A. Razaq, F. Bibi, X. Zheng, R. Papadakis, S. H. M. Jafri and H. Li, Materials, 2022, 15, 1012 CrossRef CAS PubMed.
- B. Wang, T. Ruan, Y. Chen, F. Jin, L. Peng, Y. Zhou, D. Wang and S. Dou, Energy Storage Mater., 2020, 24, 22–51 CrossRef.
- N. Devi, R. Kumar, S. Singh and R. K. Singh, Crit. Rev. Solid State Mater. Sci., 2024, 49, 72–140 CrossRef CAS.
- M. Antunes and J. I. Velasco, Prog. Polym. Sci., 2014, 39, 486–509 CrossRef CAS.
- W.-H. Liao, S.-Y. Yang, J.-Y. Wang, H.-W. Tien, S.-T. Hsiao, Y.-S. Wang, S.-M. Li, C.-C. M. Ma and Y.-F. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 869–877 CrossRef CAS PubMed.
- J. Phiri, P. Gane and T. C. Maloney, Mater. Sci. Eng., B, 2017, 215, 9–28 CrossRef CAS.
- M.-C. Hsiao, S.-H. Liao, M.-Y. Yen, P.-I. Liu, N.-W. Pu, C.-A. Wang and C.-C. M. Ma, ACS Appl. Mater. Interfaces, 2010, 2, 3092–3099 CrossRef CAS PubMed.
- S. Guo, S. Garaj, A. Bianco and C. Ménard-Moyon, Nat. Rev. Phys., 2022, 4, 1–16 CrossRef.
- X. Wang, Y. Liu, H. Pang, S. Yu, Y. Ai, X. Ma, G. Song, T. Hayat, A. Alsaedi and X. Wang, Chem. Eng. J., 2018, 344, 380–390 CrossRef CAS.
- K. Thakur and B. Kandasubramanian, J. Chem. Eng. Data, 2019, 64, 833–867 CrossRef CAS.
- A. I. A. Sherlala, A. A. A. Raman, M. M. Bello and A. Asghar, Chemosphere, 2018, 193, 1004–1017 CrossRef CAS PubMed.
- S. Mantovani, S. Khaliha, L. Favaretto, C. Bettini, A. Bianchi, A. Kovtun, M. Zambianchi, M. Gazzano, B. Casentini, V. Palermo and M. Melucci, Chem. Commun., 2021, 57, 3765–3768 RSC.
- X. Liu, J. Li, X. Wang, C. Chen and X. Wang, J. Nucl. Mater., 2015, 466, 56–64 CrossRef CAS.
- Y. Qi, M. Yang, W. Xu, S. He and Y. Men, J. Colloid Interface Sci., 2017, 486, 84–96 CrossRef CAS PubMed.
- G. Moro, S. Khaliha, A. Pintus, S. Mantovani, M. Feltracco, A. Gambaro, T. D. Marforio, M. Calvaresi, V. Palermo, M. Melucci and C. Zanardi, Mater. Today Chem., 2024, 36, 101936 CrossRef CAS.
- S. Mantovani, T. D. Marforio, S. Khaliha, A. Pintus, A. Kovtun, F. Tunioli, L. Favaretto, A. Bianchi, M. L. Navacchia, V. Palermo, M. Calvaresi and M. Melucci, Environ. Sci.: Water Res. Technol., 2023, 9, 1030–1040 RSC.
- F. Tunioli, T. D. Marforio, L. Favaretto, S. Mantovani, A. Pintus, A. Bianchi, A. Kovtun, M. Agnes, V. Palermo, M. Calvaresi, M. L. Navacchia and M. Melucci, Chem. – Eur. J., 2023, 29, 202301854 CrossRef PubMed.
- H. Yan, H. Wu, K. Li, Y. Wang, X. Tao, H. Yang, A. Li and R. Cheng, ACS Appl. Mater. Interfaces, 2015, 7, 6690–6697 CrossRef CAS PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- S. Mantovani, S. Khaliha, T. D. Marforio, A. Kovtun, L. Favaretto, F. Tunioli, A. Bianchi, G. Petrone, A. Liscio, V. Palermo, M. Calvaresi, M. L. Navacchia and M. Melucci, Chem. Commun., 2022, 58, 9766–9769 RSC.
- F. Tunioli, S. Khaliha, S. Mantovani, A. Bianchi, A. Kovtun, Z. Xia, M. S. S. Bafqi, B. S. Okan, T. D. Marforio, M. Calvaresi, V. Palermo, M. L. Navacchia and M. Melucci, J. Environ. Chem. Eng., 2023, 11, 109566 CrossRef CAS.
- Y. Ma and Y. Chen, Natl. Sci. Rev., 2014, 2, 40–53 CrossRef.
- G. Nassar, E. Daou, R. Najjar, M. Bassil and R. Habchi, Carbon Trends, 2021, 4, 100065 CrossRef CAS.
- J. Ma, D. Ping and X. Dong, Membranes, 2017, 7, 52 CrossRef PubMed.
- A. S. de León, M. de la Mata, F. J. Delgado and S. I. Molina, Macromol. Mater. Eng., 2022, 307, 2100784 CrossRef.
- M. Zambianchi, S. Khaliha, A. Bianchi, F. Tunioli, A. Kovtun, M. L. Navacchia, A. Salatino, Z. Xia, E. Briñas, E. Vázquez, D. Paci, V. Palermo, L. Bocchi, B. Casentini and M. Melucci, J. Membr. Sci., 2022, 658, 120707 CrossRef CAS.
- S. Khaliha, F. Tunioli, L. Foti, A. Bianchi, A. Kovtun, T. D. Marforio, M. Zambianchi, C. Bettini, E. Briñas, E. Vázquez, L. Bocchi, V. Palermo, M. Calvaresi, M. L. Navacchia and M. Melucci, Environ. Sci.: Water Res. Technol., 2024, 10, 1097–1107 RSC.
- E. Avram, B. Elena, L. Cornelia and I. Druta, J. Macromol. Sci., Part A: Pure Appl. Chem., 1997, 34, 1701–1714 CrossRef.
- N. E. Temnikova and O. V. Stoyanov, Polym. Sci., Ser. D, 2024, 17, 140–148 CrossRef CAS.
- O. S. Serbanescu, S. I. Voicu and V. K. Thakur, Mater. Today Chem., 2020, 17, 100302 CrossRef CAS.
- O. Dumbrava, A. Filimon and L. Marin, Eur. Polym. J., 2023, 196, 112316 CrossRef CAS.
- C. B. Rosen and M. B. Francis, Nat. Chem. Biol., 2017, 13, 697–705 CrossRef CAS PubMed.
- M. D. Guiver, G. P. Robertson and S. Foley, Macromolecules, 1995, 28, 7612–7621 CrossRef CAS.
- N. G. de Barros, A. C. Gonzaga Neto, K. B. Vaccioli, H. R. V. Angulo, L. G. de Andrade e Silva, S. M. Toffoli and T. S. Valera, C, 2023, 9, 73 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials, PSU–GO functionalization with lysine, SEM, XPS, and the carbamazepine (CBZ) adsorption test under flow conditions. See DOI: https://doi.org/10.1039/d5lf00105f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |