Open Access Article
Open Access ArticleTuning the band gap energy of CuxInyS for superior photothermocatalytic CO2 conversion to C2H4†
Longlong Wangab,
Ruirui Wang*b,
Shuang Weib,
Kexin Lib,
Hasnain Nawazb,
Bin Heb,
Mengyue Lib and
Ruixia Liu *abc
*abc
aHenan Institute of Advanced Technology, Zhengzhou University, Zhengzhou, 450001, P. R. China
bBeijing Key Laboratory of Solid State Battery and Energy Storage Process, CAS Key Laboratory of Green Process and Engineering, State Key Laboratory of Mesoscience and Engineering, Institute of Process Engineering, Innovation Academy for Green Manufacture, CAS, Beijing 100190, P. R. China. E-mail: wangruirui@ipe.ac.cn; rxliu@ipe.ac.cn
cLongzihu New Energy Laboratory, Zhengzhou Institute of Emerging Industrial Technology, Henan University, Zhengzhou 450000, P. R. China
First published on 17th April 2025
Abstract
Photothermal catalysis significantly enhances the efficiency of photocatalytic CO2 reduction, offering a promising strategy for accelerated CO2 resource utilization. Herein, a series of CuxInyS photocatalysts were synthesized, exhibiting tunable band gap energy by varying the Cu/In/S atomic ratios for photothermocatalytic CO2 conversion to C2H4. The typical CuInS2 catalyst demonstrates a more negative conduction band, significantly enhancing the electron reduction ability and facilitating the multi-electron reduction of CO2 to C2H4. Additionally, the abundant sulfur vacancies in CuInS2 generate additional active sites, enhance charge separation efficiency, and consequently improve catalytic activity. The generation rate of ethylene reaches 45.7 μmol g−1 h−1 with a selectivity of 79.7%. This study provides a new avenue for producing ethylene in photothermal catalysis, as well as highlighting the superiorities of the CuInS2 catalyst.
Keywords: Photothermal catalysis; CO2 reduction; Ethylene; Metal sulfides; Band gap energy.
1 Introduction
The excessive use of fossil fuels has led to excessive emissions of carbon dioxide (CO2) into the atmosphere, causing severe climate change.1–4 Photocatalytic conversion of CO2 into hydrocarbons, artificial photosynthesis based on H2O as the electron transfer medium and proton source, is a technology with environmental sustainability values.5 Nevertheless, the efficiency of traditional photocatalytic CO2 reduction remains hindered by the sluggish multi-electron transfer kinetics and suboptimal photon utilization efficiency.6 Recent research reveals that the photothermal synergistic catalysis strategy can accelerate the photocatalytic conversion of CO2, significantly enhancing the efficiency of CO2 conversion and product selectivity.7,8Photocatalytic efficiency of CO2 conversion remains unsatisfactory, primarily because of the high thermodynamic stability of CO2 molecules (ΔG 298 K0 = −394.36 kJ mol−1)9 and the limited photon utilization.10 To date, the primary products of CO2 photothermal conversion have been C1 compounds such as carbon monoxide, methane and formic acid.11–14 C2H4, as an important C2 chemical, has widespread applications in the synthesis of fibers, rubbers, plastics, and alcohols.15 C–C coupling to produce C2H4 remains a significant challenge.16 Theoretically, the selective photoreduction of CO2 to C2 products is primarily hindered by the difficulties associated with C–C coupling of key intermediates such as *CO and *COOH, which requires a high kinetic barrier.17 To achieve efficient photothermal catalysis for the reduction of CO2 to C2H4, the catalyst should possess several critical properties: efficient electron transfer and utilization, favorable adsorption of *CO intermediates,18 and low energy barrier for the formation of *OCCO.19,20 Accordingly, various catalytic strategies to enhance the photothermal catalytic CO2 reduction capability have been investigated.
The band gap width is a pivotal factor influencing the performance of photocatalysts. Significant photogenerated electron–hole pairs can only be generated when the photon energy matches or exceeds the band gap energy. Concurrently, the conduction band (CB) potential must be more negative than the surface electron acceptor potential to ensure effective photocatalytic CO2 reduction.21 Bai et al.22 synthesized a series of ZnmIn2S3+m catalysts with tailored bandgap energies and constructed Zn2In2S5/BiVO4 heterojunctions, which improve the separation of charge carriers and the photocatalytic performance. Chai et al.23 synthesized various metal sulfides and demonstrated CuInSnS4 as having the most negative conduction band potential, achieving exceptional photocatalytic activity and selectivity for multi-electron CO2 reduction. Therefore, tuning the band gap provides an effective strategy for enhancing the selectivity of photocatalytic CO2 conversion to C2H4.
Metal sulfides have garnered extensive attention due to their structural tunability and broad light absorption capabilities.24 Among these, Cu-based catalysts are highly efficient for CO2 to C2H4 conversion due to their unique electronic structure and surface properties, which stabilize CO intermediates and promote C–C coupling.25 Furthermore, In-based materials, as high-performance semiconductors, offer a favorable band structure for efficient electron–hole separation, enhancing photothermal catalytic performance. Gao et al.26 reported CuInP2S6 nanosheets achieving 56.4% selectivity for the photocatalytic reduction of CO2 to C2H4. The In sites facilitated the reduction of CO2 to *CO, and the C–C coupling reaction occurred on the Cu sites. Furthermore, sulfur vacancies are frequently employed as a modulation strategy in metal sulfides. These defects significantly influence catalyst reactivity, modulate the electronic structure, facilitate charge transport, and effectively lower the kinetic barrier.27,28 Yan et al.29 designed a Bi2S3@In2S3 catalyst, with the In–Sv–Bi active center composed of adjacent Bi and In sites accompanied by abundant Sv defects, which reduces the energy barrier of CO2 activation and C–C coupling, achieving a C2H4 generation rate of 11.81 μmol g−1 h−1, with a selectivity of approximately 90%. Therefore, regulating the band structure and defect sites of the CuxInyS photocatalyst holds significant prospects for enhancing the reduction of CO2 to C2H4.
Herein, we have successfully synthesized a series of metal sulfides, including CuInS2, CuIn2S4, CuIn5S8, CuS and In2S3 by a simple hydrothermal method. Among these, the CuInS2 catalyst demonstrated superior performance in photothermal catalytic CO2 reduction, utilizing H2O as a proton donor. The generation rate of ethylene reached 45.7 μmol g−1 h−1 with a selectivity of 79.7%. The high performance was attributed to the photothermal effect, the high amount of sulfur vacancies and the narrow band gap of the CuInS2 catalyst, where the abundant sulfur vacancies create additional active sites, promoting CO2 activation, and a more negative conduction band significantly enhancing the electron reduction ability and facilitating the selectivity of CO2 conversion to C2H4.
2 Results and discussion
X-ray diffraction (XRD) analysis was conducted to investigate the crystal structures and compositions of the synthesized samples.30 As illustrated in Fig. 1a, the diffraction peaks observed at 27.9°, 46.5°, and 55.1° correspond to the (112), (220), and (312) crystal planes of the CuInS2 phase, respectively. Additionally, diffraction peaks at 14.3°, 27.7°, 33.5°, 44.0°, and 48.1° are attributable to the (111), (311), (400), (511), and (440) crystal planes of CuIn2S4 and CuIn5S8 phases, respectively. In Fig. 1b, the XRD patterns further reveal the successful synthesis of monometallic sulfides, specifically CuS (PDF#79-2321) and In2S3 (PDF#73-1366). The diffraction peaks at 29.3°, 31.8°, 32.9°, 48.0°, 52.8°, and 59.4° are assigned to the (102), (103), (006), (110), (108), and (116) crystal planes of CuS, respectively. Meanwhile, peaks at 14.2°, 27.4°, 33.2°, 43.6°, and 47.7° correspond to the (103), (109), (00 12), (309), and (22 12) crystal planes of In2S3, respectively.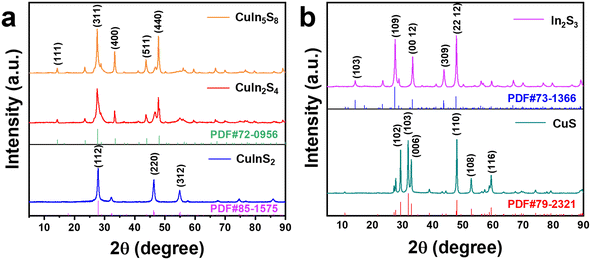 | ||
| Fig. 1 (a) XRD patterns of CuInS2, CuIn2S4 and CuIn5S8 samples; (b) XRD patterns of CuS and In2S3 samples. | ||
The microstructure and structural characteristics of the samples were carefully studied using scanning electron microscopy (SEM). The SEM comparisons of different samples under identical magnifications are shown in Fig. 2a–e. CuS exhibits a basic nanoparticle morphology, whereas CuIn2S4, CuIn5S8, In2S3 and CuInS2 display a microsphere morphology self-assembled by nanosheets. The thicknesses of the catalyst flakes for CuS, CuIn2S4, CuIn5S8, In2S3 and CuInS2 were measured to be 53 nm, 26 nm, 25 nm, 38 nm, and 20 nm, respectively. Notably, CuInS2 demonstrates an ultrathin nanosheet morphology offering significant advantages for photocatalytic applications. The reduced thickness effectively shortens the charge transport distance, thereby minimizing the probability of charge recombination, ultimately enhancing the quantum efficiency of the photocatalytic process.31
 | ||
| Fig. 2 SEM images of (a) CuS, (b) CuIn2S4, (c) CuIn5S8, (d) In2S3 and (e) CuInS2; (f) TEM image, (g) HRTEM image, and (h) EDS mapping of CuInS2; (i) static water contact-angle of CuxInyS. | ||
The TEM analysis confirms that CuInS2 consists of nano-flakes (Fig. 2f). Due to the low surface activity of CuInS2 nanosheets, it is easy to agglomerate together, which forms the microsphere morphology observed in the SEM diagram. The high-resolution TEM image (Fig. 2g) reveals an exposed crystal plane of CuInS2 with a lattice spacing of 0.167 nm, which corresponds to the (312) crystal face. Additionally, the elemental mapping in Fig. 2h demonstrates a uniform distribution of Cu, In, and S elements across the CuInS2 nanoparticles. The composition of the CuInS2 sample was analyzed by energy dispersive spectroscopy (EDS), as presented in Table S1.† The EDS results indicate that the atomic ratio of Cu, In and S is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, closely matching the stoichiometric composition of the CuInS2 compound, indicating the high purity of the CuInS2 nanocrystal. Furthermore, inductively coupled plasma mass spectrometry (ICP-MS) was employed to determine the metal element content in the CuInS2 sample, with the results detailed in Table S2.† The Cu and In atomic ratio was found to be approximately 1
2, closely matching the stoichiometric composition of the CuInS2 compound, indicating the high purity of the CuInS2 nanocrystal. Furthermore, inductively coupled plasma mass spectrometry (ICP-MS) was employed to determine the metal element content in the CuInS2 sample, with the results detailed in Table S2.† The Cu and In atomic ratio was found to be approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corroborating the theoretical stoichiometric value of CuInS2. Collectively, these results confirm the successful preparation of the CuInS2 nanocrystal with high-quality exposed (312) crystal faces. The hydrophilicity and hydrophobicity of the catalyst were examined, as depicted in Fig. 2i, and all the samples demonstrated hydrophilic characteristics, with contact angle measurements following the order: CuS > CuInS2 > CuIn2S4 > CuIn5S8 > In2S3. This implies that a certain degree of hydrophilicity is beneficial to the adsorption of water on their surfaces, hence possibly aiding subsequent proton transfer to participate in the CO2 reduction reactions.32
1, corroborating the theoretical stoichiometric value of CuInS2. Collectively, these results confirm the successful preparation of the CuInS2 nanocrystal with high-quality exposed (312) crystal faces. The hydrophilicity and hydrophobicity of the catalyst were examined, as depicted in Fig. 2i, and all the samples demonstrated hydrophilic characteristics, with contact angle measurements following the order: CuS > CuInS2 > CuIn2S4 > CuIn5S8 > In2S3. This implies that a certain degree of hydrophilicity is beneficial to the adsorption of water on their surfaces, hence possibly aiding subsequent proton transfer to participate in the CO2 reduction reactions.32
X-ray photoelectron spectroscopy (XPS) was employed to investigate the electronic states of the synthesized samples.30 The high-resolution XPS spectra (Fig. 3a) reveal that the Cu 2p1/2 and Cu 2p3/2 binding energies for the CuInS2 sample are 951.6 eV and 931.8 eV, respectively. Importantly, the Cu 2p binding energy of CuInS2 is 0.3 eV lower than that observed for CuS. This shift can be attributed to changes in electron distribution and chemical bond strength arising from the interaction between Cu and In, resulting in a reduction in binding energy.33 Further analysis of the In 3d region (Fig. 3b) shows that the binding energies of In 3d3/2 and In 3d5/2 in the CuInS2 sample are 452.1 eV and 444.6 eV, respectively. Compared with In2S3, the In 3d binding energies of CuInS2 uniformly shift to lower binding energy. This shift is attributed to the distinct coordination environments of In atoms in CuInS2 and In2S3 because the partial In atom in In2S3 exists in the state of [InS4] tetrahedron.34 For S 2p, the binding energies of S 2p1/2 and S 2p3/2 are measured at 162.9 eV and 161.7 eV, respectively (Fig. 3c). Due to the fact that the average bond length between sulfur and metal atoms in CuInS2 is slightly longer than that of monometallic sulfides, the S atom in CuInS2 exhibits the highest binding energy. Notably, an additional peak at 164.5 eV is observed in both CuInS2 and CuS samples, which has been attributed to sulfur vacancies as reported in the literature.35 The higher binding energy of S atoms in CuInS2 relative to monometallic sulfides suggests that the S atoms in CuInS2 exist in an electron-deficient state. To provide further insights into the formation of sulfur vacancies, the g = 2.003 signal observed in the room-temperature EPR spectra was attributed to sulfur defects (Fig. 3d).36 The presence of the sulfur vacancy has the potential to act as a reaction site to enhance the ability of CO2 adsorption and subsequent activation and promote C–C coupling to generate C2H4.23,29 Subsequent photo-thermal catalytic CO2 reduction experiments also confirmed that the catalytic activity is positively correlated with the sulfur vacancy content, with CuInS2, which has the highest sulfur vacancy concentration, exhibiting the best catalytic performance.
The photothermal catalytic performance of the sample for CO2 reduction was evaluated using a flow fixed-bed reactor (Fig. S1†). In this gas–solid reaction system, CO2 and H2O vapor were continuously introduced. Under illumination from a 300 W xenon lamp with full-spectrum output, the reaction temperature was maintained at 130 °C. After 6 hours of reaction, the only detectable gaseous products were C2H4 and CO, while no liquid-phase products were observed. To address the limitation of recombination of electron and hole pairs, triethanolamine (TEOA) was introduced as a sacrificial agent to effectively consume photogenerated holes formed during the photocatalytic reaction. To preliminarily exclude the impact of TEOA on the products of the CO2 reduction reaction, its decomposition behavior was investigated under the N2 atmosphere. As shown in Table S3,† at temperature of 130 °C, neither C2H4 nor CO was detected. Further, the thermal stability of TEOA was evaluated (Fig. S2†). Results show no mass loss at 130 °C, confirming the thermal stability of TEOA at this temperature. These findings collectively indicate that TEOA does not decompose to produce C2H4 or CO at the reaction temperature of 130 °C. Consequently, the influence of TEOA on the photothermal catalytic CO2 reduction products can be preliminarily excluded.
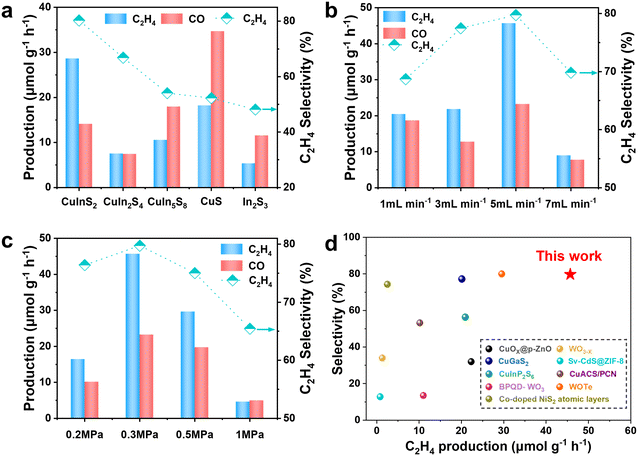
The photothermal catalytic CO2 reduction performance of the sample under reaction conditions of 0.5 MPa and 5 mL min−1 is shown in Fig. 4a. The CuInS2 sample demonstrates exceptional performance in photothermal catalytic CO2 reduction, producing C2H4 and CO as the primary products. The generation rate of C2H4 was 28.6 μmol g−1 h−1, while the CO generation rate was 14.1 μmol g−1 h−1. The selectivity to C2H4 was calculated to be 80.2%, based on the carbon-containing product contents. In contrast, monometallic sulfides such as CuS and In2S3 predominantly produce CO as the main product. This variation in product selectivity suggests that bimetallic sulfides and monometallic sulfides differ in their CO2 reduction mechanisms or exhibit distinct active sites.23 Thermogravimetric analysis (Fig. S3†) was performed to investigate the thermal stability of the sample. The analysis reveals a degree of mass loss at approximately 130 °C, likely attributable to the presence of trace amounts of H2O in the sample. As the temperature increases, the volatilization of H2O results in the observed weight loss. The reaction conditions were further explored. The photothermal catalytic CO2 reduction performance of the CuInS2 sample under 0.3 MPa pressure and varying flow rates is evaluated in Fig. 4b, with superior photothermal catalytic performance demonstrated at a flow rate of 5 mL min−1. This may be because the moderate flow rate enhances the supply of reactants, thereby increasing reaction rates, whereas excessive flow reduces catalyst interaction time, ultimately lowering efficiency.37 The photothermal catalytic CO2 reduction performance of the CuInS2 sample under a flow rate of 5 mL min−1 and varying pressures is evaluated in Fig. 4c. The results demonstrate excellent photothermal catalytic performance at 0.3 MPa. Within a certain range, an increase in pressure allows the active sites on the catalyst surface to interact more effectively with reactants, thereby improving catalyst activity.37 Comparative analysis reveals that at a pressure of 0.3 MPa and a flow rate of 5 mL min−1, the CuInS2 sample exhibits optimal activity. Under these conditions, the rate of C2H4 generation reaches 45.7 μmol g−1 h−1, with a C2H4 selectivity of 79.7%. Notably, compared with previously reported photothermal catalysts, the prepared CuInS2 catalyst demonstrates superior rate and selectivity for C2H4 production (Fig. 4d, Table S4†).
With the increase in reaction time, the rates of C2H4 and CO exhibited a noticeable upward trend. Notably, after 6 hours of reaction, the rate of C2H4 using the CuInS2 catalyst was significantly higher compared to the other samples (Fig. S4 and S5†). The durability and stability of the CuInS2 catalyst were further assessed through cyclic experiments illustrated38 in Fig. 5a, over five consecutive cycles (6 hours per cycle); the C2H4 production rate remained above 85% of the baseline activity, with the activity decay mainly attributed to catalyst poisoning by CO.39–41 The XRD patterns of the CuInS2 catalyst before and after the cyclic reaction (Fig. S6†) revealed no discernible changes in the crystal structure, confirming the structural integrity of the material post-reaction. Furthermore, the XPS results of the CuInS2 catalyst after the reaction, shown in Fig. S7–S9,† indicated that the binding energies of Cu, In, and S in the sample remained relatively unchanged. These findings collectively suggest that the CuInS2 catalyst maintains robust stability during the photothermal catalytic CO2 reduction process. To confirm the occurrence of CO2 reduction on CuInS2, blank control experiments under various conditions were conducted. As shown in Fig. 5b, no product was detected in the absence of the catalyst, indicating its essential role in the reaction. When the reaction was performed at 130 °C without light, the rate of C2H4 generation was only 0.6 μmol g−1 h−1, highlighting the fundamental importance of light as a driving force in the photothermal catalytic system. Similarly, under light irradiation but without external heating, the rate of C2H4 generation was merely 0.5 μmol g−1 h−1. In contrast, under normal photothermal conditions (0.3 MPa, 5 mL min−1, 130 °C, 300 W xenon lamp), the rate of C2H4 generation reached 45.7 μmol g−1 h−1, demonstrating that thermal energy significantly enhances the production of C2H4.42 The efficiency of photothermal conversion of CO2 to C2H4 was notably higher than that achieved by photocatalysis or thermal catalysis alone. This underscores the indispensable roles of both light and heat in the photothermal reaction system. Importantly, the rate of C2H4 increased nearly tenfold upon the addition of the sacrificial agent TEOA. Control experiments conducted with N2 instead of CO2 yielded only trace amounts of C2H4 and CO (Table S5†), directly confirming that the source of the C2H4 and CO products was CO2. To further verify the carbon source of C2H4 and CO in the products, isotope tracer experiments using 13CO2 were performed.43 The products were analyzed by gas chromatography–mass spectrometry (GC–MS). As shown in Fig. 5c and d, the mass spectral fragment ion distributions of C2H4 and CO were systematically characterized. The comparison experiments show that the fragment peaks of C2H4 (m/z = 28 → 30) and CO (m/z = 29 → 30) were obviously shifted when 13CO2 is involved in the reaction. This isotopic shift exclusively confirms that the carbon in C2H4 and CO originates from CO2, rather than carbonaceous components of the catalyst or decomposition of organic sacrificial agents.
The light absorption capacity and charge separation efficiency of catalysts are critical factors influencing the experimental results of photothermal synergistic catalysis. The optical absorption abilities of the CuInS2, CuIn2S4, CuIn5S8, CuS, and In2S3 samples were investigated using UV-vis diffuse reflectance spectroscopy (UV-vis DRS), as shown in Fig. S10.† Notably, the absorption edges of these materials are not distinct, reflecting full-spectrum absorption characteristics. The broad absorption range of these photocatalysts is advantageous for capturing photons, thereby enhancing photocatalytic reactions.44 CuInS2 exhibits strong light absorption across the spectral range, demonstrating promising potential for photocatalytic activity. Photoluminescence spectroscopy (PL) serves as an effective tool to assess the electron–hole recombination capability in photocatalysts. Lower recombination rates enhance charge utilization efficiency, thereby improving reactivity. As shown in Fig. 6a, the CuInS2 sample exhibits the weakest PL signal, indicative of the most effective charge separation. Furthermore, the time-resolved photoluminescence (TRPL) spectrum presented in Fig. 6b reveals that the CuInS2 sample has an average carrier lifetime of 10.98 ns, which is markedly higher than that of the other samples. This extended carrier lifetime suggests a significant suppression of charge recombination. Additional photoelectrochemical tests corroborated these findings, further elucidating the electron–hole pair separation efficiency of the representative photocatalyst samples. These results underscore the importance of the CuInS2 sample's superior charge separation characteristics in promoting photothermal synergistic catalysis.
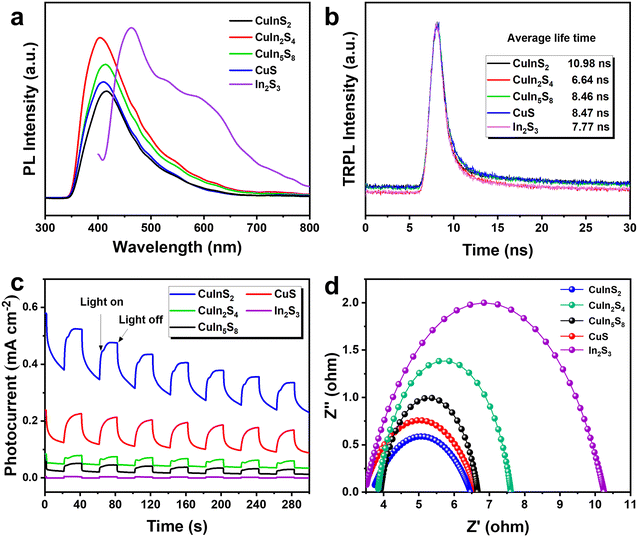 | ||
| Fig. 6 (a) Steady-state fluorescence spectra, (b) transient fluorescence spectra, (c) transient photocurrent response curves and (d) electrochemical impedance spectra of CuxInyS. | ||
Transient photocurrent measurements (Fig. 6c) were conducted to investigate the photocurrent response of the as-prepared samples under optical switching conditions. Among the tested samples, the CuInS2 sample exhibited the highest photocurrent response, signifying superior separation efficiency of photogenerated electron–hole pairs under illumination conditions.45,46 These results are consistent with previous studies. Electrochemical impedance spectroscopy (EIS) was employed to gain deeper insights into the electron transfer capabilities of the samples. As illustrated in Fig. 6d, the Nyquist plot for CuInS2 demonstrates a smaller semicircle radius compared to the other samples, indicating a lower charge transfer resistance and enhanced interfacial charge transfer efficiency.47 Overall, these results suggest that CuInS2 exhibits outstanding activity in photothermal catalytic CO2 reduction reactions due to its excellent charge separation and electron transport properties.
To elucidate the factors governing the divergent selectivity patterns in catalytic reactions, we conducted a comprehensive analysis of the band gap properties for each candidate catalyst. The conduction band (CB) positions of the samples were evaluated through Mott–Schottky (M–S) analysis,48 with CB potentials measured as −1.19 eV, −1.01 eV, −0.96 eV, −1.07 eV, and −0.74 eV (V vs. NHE) for CuInS2, CuIn2S4, CuIn5S8, CuS and In2S3, respectively (Fig. 7a–e). The positive slopes observed in the M–S curves at multiple frequencies (2000, 3000, and 4000 Hz) confirm that CuInS2 is an n-type semiconductor. As revealed by XPS valence band spectra49 (Fig. 7f), the valence band (VB) potentials were calculated to be 0.14 eV, 0.49 eV, 0.45 eV, 0.08 eV, and 1.64 eV (V vs. NHE) for CuInS2, CuIn2S4, CuIn5S8, CuS and In2S3, respectively. Based on the formula Eg = EVB − ECB,44 the Eg values for CuInS2, CuIn2S4, CuIn5S8, CuS and In2S3 were determined to be 1.33 eV, 1.50 eV, 1.41 eV, 1.15 eV, and 2.38 eV, respectively.
 | ||
| Fig. 7 Mott–Schottky curves of (a) CuInS2, (b) CuIn2S4, (c) CuIn5S8, (d) CuS and (e) In2S3; (f) XPS valence band spectra of CuxInyS; (g) the optical band gap energy of CuxInyS. | ||
Based on the aforementioned results, an electronic band diagram relative to the standard hydrogen electrode was constructed (Fig. 7g), which critically determine the driving force for redox reactions. All the samples demonstrated the capability to reduce CO2 to C2H4 and CO. CuInS2, in particular, has a band gap energy that aligns closely with the energy required for CO2 reduction to C2H4, and its more negative CB position endows the electrons with stronger reducing power,23 facilitating multi-electron CO2 reduction pathways to C2H4. These findings underscore the potential of CuInS2 as a highly efficient photocatalyst for CO2 reduction, supported by its advantageous band structure and strong optical absorption properties.
To gain deeper insight into the band gap structure of CuxInyS, projected density of states (PDOS) calculations were performed using density functional theory (DFT),50 as shown in Fig. 8. The results indicate that the band gap energies of CuS, CuInS2 and In2S3 follow the order: CuS < CuInS2 < In2S3. The band gap of CuInS2 lies between that of CuS and In2S3, enabling efficient visible-light absorption while avoiding the rapid carrier recombination caused by the excessively narrow band gap of CuS and the reduced light absorption efficiency resulting from the overly wide band gap of In2S3.51 The PDOS for CuInS2 reveals that the Cu 3d orbitals are the most active, contributing significantly to the electronic states near the Fermi level.30 This suggests that during photoexcitation, electrons from the Cu 3p orbitals transition from the valence band maximum (VBM) to the conduction band minimum (CBM), playing a crucial role in the photocatalytic CO2 reduction reaction.
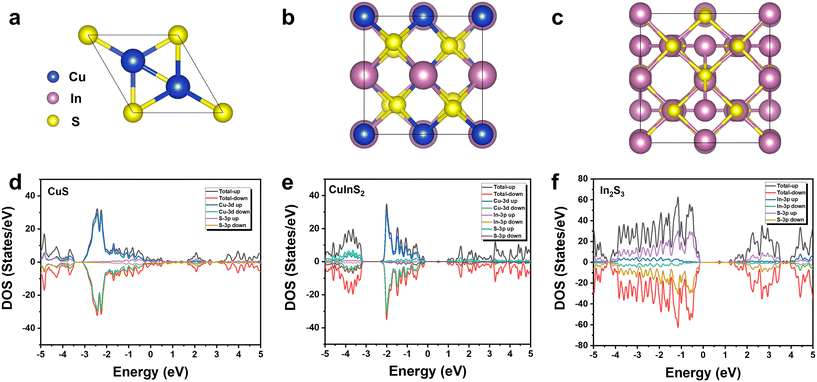 | ||
| Fig. 8 Structural model diagram of (a) CuS, (b) CuInS2 and (c) In2S3; PDOS of (d) CuS, (e) CuInS2 and (f) In2S3. | ||
3 Conclusion
In summary, this study has successfully synthesized a series of CuxInyS photocatalysts modulating the Cu/In/S atomic ratios by a simple hydrothermal method. The CuInS2 catalyst demonstrated a remarkable rate of 45.7 μmol g−1 h−1 for photothermal CO2 reduction with H2O to C2H4, achieving a selectivity of 79.7% and excellent cycle stability. Control experiments conclusively proved the photothermal synergistic catalytic process, which significantly enhanced the efficiency of the reaction, addressing the limitations of single-mode photocatalysis and thermal catalysis. Electrochemical measurements, along with PL and TRPL analyses, confirmed the superior charge transport and separation efficiency demonstrated by the CuInS2 catalyst. Furthermore, the abundant sulfur vacancies create additional active sites, promoting CO2 activation, and the more negative conduction band of the CuInS2 catalyst facilitates the reduction of CO2 to C2H4. This work offers valuable insights into the design of metal sulfide catalysts for the photocatalytic conversion of CO2 into C2+ products.4 Experimental section
4.1 Chemicals and materials
All chemicals were used as received without further purification. Cupric chloride dihydrate (CuCl2·2H2O), indium chloride tetrahydrate (InCl3·4H2O, 99.9%), thioacetamide (TAA, ≥98.0%), deionized water and anhydrous ethanol (C2H5OH, 99.5%) were purchased from Aladdin Reagent Company (Shanghai, China).4.2 Preparation of CuInS2
1.8 mmol of CuCl2·2H2O, 1.8 mmol of InCl3·4H2O and 3.6 mmol of TAA were dissolved in 80 mL deionized water and 80 mL ethanol, and reacted under hydrothermal conditions of 160 °C for 12 hours. After the reaction, the product was collected and washed with deionized water and ethanol, and dried under vacuum at 60 °C for 12 hours; the synthesized sample was denoted as CuInS2.The synthesis methods for CuIn2S4 and CuIn5S8 are consistent with the described procedure, with the only modification being the variation in the molar ratios of CuCl2·2H2O, InCl3·4H2O, and TAA.
4.3 Preparation of CuS
1.8 mmol of CuCl2·2H2O and 3.6 mmol of TAA were dissolved in 80 mL deionized water and 80 mL ethanol, and reacted under hydrothermal conditions of 160 °C for 12 hours. After the reaction, the product was collected and washed with deionized water and ethanol, and dried under vacuum at 60 °C for 12 hours; the synthesized sample was denoted as CuS.4.4 Preparation of In2S3
1.8 mmol of InCl3·4H2O and 3.6 mmol of TAA were dissolved in 80 mL deionized water and 80 mL ethanol, and reacted under hydrothermal conditions of 160 °C for 12 hours. After the reaction, the product was collected and washed with deionized water and ethanol, and dried under vacuum at 60 °C for 12 hours; the synthesized sample was denoted as In2S3.4.5 Photothermal catalytic reduction of CO2
For CO2 photothermal conversion, 0.2 g catalyst and 0.1 mL TEOA were filled into the reaction tube. Subsequently, the flow fixed-bed reactor was filled with high-purity CO2 gas to reach the reaction pressure. In the reaction system, deionized water was used as the proton source and a 300 W xenon lamp was used as the light source (Fig. S1†). The reaction products were analyzed in real time online by gas chromatography (GC 9790 II, FuLi, FID and TCD detector). The carbon-based products of CO and C2H4 were quantified with a flame ionization detector (FID).The photothermocatalytic CO and C2H4 production amounts and the rate could be calculated as follows:
 | (1) |
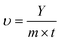 | (2) |
The selectivity for the CO2 reduction products of CO and C2H4 was calculated using the following equation:
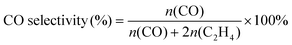 | (3) |
 | (4) |
4.6 Electrochemical measurements
The preparation of the working electrode was as follows. 5 mg photocatalyst was dispersed in a mixed solution of 350 μL C3H8O, 50 μL Nafion and 100 μL H2O, and sonicated for 1 h. Then the mixed liquid was dispersed on FTO glass as a photoelectrode. The transient photocurrent response and electrochemical impedance spectra (EIS) were determined by using a CHI760E electrochemical workstation. 0.5 M Na2SO4 solution was used as the electrolyte and a 300 W xenon lamp was used as the light source. The sample photoelectrode, platinum foil and Ag/AgCl served as the working electrode, counter electrode and reference electrode, respectively.4.7 Characterization
X-ray diffraction (XRD) was carried out using an X-ray diffractometer (Smartlab (9)) with the 2θ range from 5° to 90°. The morphology and sizes were examined by field-emission transmission electron microscopy (TEM) and field-emission scanning electron microscopy (SEM) using a JEOL F200 and JSM-7800 (Prime), respectively. X-ray photoelectron spectroscopy (XPS) analyzing the chemical compositions was conducted with an AXIS SUPRA+. The electron paramagnetic resonance (EPR) was measured on a Bruker EMX-Plus ESR spectrometer. The UV-vis diffuse reflectance spectra (DRS) were obtained with a Hitachi UH4150 spectrophotometer using BaSO4 as the reflectance standard. Steady-state photoluminescence (PL) spectra of the as-prepared samples were detected with a Hitachi F-4700. Time-resolved photoluminescence (TRPL) spectra of the as-prepared samples were detected with an Edinburgh Instruments 980.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Longlong Wang: investigation, confirmation, conceptualization, methodology, data analysis and writing – original draft. Ruirui Wang: supervision, formal analysis, data curation and writing – review & editing. Shuang Wei: investigation, confirmation, conceptualization. Kexin Li: methodology, data analysis. Hasnain Nawaz: conceptualization, methodology. Bin He: writing – review & editing. Mengyue Li: investigation. Ruixia Liu: supervision, resources, project administration, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Note added after first publication
This article replaces the version published on 17th April 2025, which inadvertently omitted the keywords from the Abstract.Acknowledgements
This work was financially supported by the National Science Fund for Excellent Young Scholars (22222813), the National Key Research and Development Program of China (2023YFA1506803), the National Natural Science Foundation of China (22078338), the Young Scientists Fund of the National Natural Science Foundation of China (No.22408376) and the Postdoctoral Fellowship Program of CPSF (GZC20232700).References
- X. Li, J. Yu, M. Jaroniec and X. Chen, Cocatalysts for selective photoreduction of CO2 into solar fuels, Chem. Rev., 2019, 119, 3962–4179 CrossRef CAS PubMed.
- P. Chen, P. Zhang, X. Kang, L. Zheng, G. Mo, R. Wu, J. Tai and B. Han, Efficient electrocatalytic reduction of CO2 to ethane over nitrogen-doped Fe2O3, J. Am. Chem. Soc., 2022, 144, 14769–14777 CrossRef CAS PubMed.
- Y. Li, F. Li, A. Laaksonen, C. Wang, P. Cobden, P. Boden, Y. Liu, X. Zhang and X. Ji, Electrochemical CO2 reduction with ionic liquids: Review and evaluation, Ind. Chem. Mater., 2023, 1, 410–430 RSC.
- X. Jiao, Z. Hu, L. Li, Y. Wu, K. Zheng, Y. Sun and Y. Xie, Progress and perspectives for engineering and recognizing active sites of two-dimensional materials in CO2 electroreduction, Sci. China: Chem., 2022, 65, 428–440 CrossRef CAS.
- L. Zhang, G. Kong, Y. Meng, J. Tian, L. Zhang, S. Wan, J. Lin and Y. Wang, Direct coupling of thermo and photocatalysis for conversion of CO2-H2O into fuels, ChemSusChem, 2017, 10, 4709–4714 CrossRef CAS PubMed.
- Y. Zheng, L. L. Zhang, Y. K. Li, Y. Y. Wang, J. L. Chen, B. Z. Lin, Y. Z. Zheng, L. Cheng, S. B. Wang and Y. L. Chen, Triptycene incorporated carbon nitride based donor-acceptor conjugated polymers with superior visible-light photocatalytic activities, J. Colloid Interface Sci., 2022, 622, 675–689 CrossRef CAS PubMed.
- M. Y. Sun, B. H. Zhao, F. P. Chen, C. B. Liu, S. Y. Lu, Y. F. Yu and B. Zhang, Thermally-assisted photocatalytic CO2 reduction to fuels, Chem. Eng. J., 2021, 408, 127280 CrossRef CAS.
- D. Wu, K. Deng, B. Hu, Q. Lu, G. Liu and X. Hong, Plasmon-assisted photothermal catalysis of low-pressure CO2 hydrogenation to methanol over Pd/ZnO catalyst, ChemCatChem, 2019, 11, 1598–1601 CrossRef CAS.
- X. Chang, T. Wang and J. Gong, CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts, Energy Environ. Sci., 2016, 9, 2177–2196 RSC.
- L. Xue, Q. Y. Fan, Y. Zhao, Y. Liu, H. Zhang, M. Sun, Y. Wang and S. Zeng, Ultralow Ag-assisted carbon-carbon coupling mechanism on Cu-based catalysts for electrocatalytic CO2 reduction, J. Energy Chem., 2023, 82, 414–422 CrossRef CAS.
- S. Akrami, Y. Murakami, M. Watanabe, T. Ishihara, M. Arita, M. Fuji and K. Edalati, Defective high-entropy oxide photocatalyst with high activity for CO2 conversion, Appl. Catal., B, 2022, 303, 120896 CrossRef CAS.
- Q. Zhang, M. Mao, Y. Li, Y. Yang, H. Huang, Z. Jiang, Q. Hu, S. Wu and X. Zhao, Novel photoactivation promoted light-driven CO2 reduction by CH4 on Ni/CeO2 nanocomposite with high light-to-fuel efficiency and enhanced stability, Appl. Catal., B, 2018, 239, 555–564 CrossRef CAS.
- Y. Li, C. Wang, M. Song, D. Li, X. Zhang and Y. Liu, TiO2-x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with H2O steam, Appl. Catal., B, 2019, 243, 760–770 CrossRef CAS.
- J. Xiong, P. Song, J. Di and H. Li, Ultrathin structured photocatalysts: A versatile platform for CO2 reduction, Appl. Catal., B, 2019, 256, 117788 CrossRef CAS.
- M. H. Zhang and Y. Z. Yu, Dehydration of ethanol to ethylene, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 CrossRef CAS.
- Q. Zhang, J. Wang, F. Guo, G. He, X. Yang, W. Li, J. Xu and Z. Yin, Nitrogen cold plasma treatment stabilizes Cu0/Cu+ electrocatalysts to enhance CO2 to C2 conversion, J. Energy Chem., 2023, 84, 321–328 CrossRef CAS.
- K. Das, R. Das, M. Riyaz, A. Parui, D. Bagchi, A. K. Singh, A. K. Singh, C. P. Vinod and S. C. Peter, Intrinsic charge polarization in Bi19S27Cl3 nanorods promotes selective C-C coupling reaction during photoreduction of CO2 to ethanol, Adv. Mater., 2023, 35, 2205994 CrossRef CAS PubMed.
- S. Rozas, F. C. Gennari, M. Atilhan, A. Bol and S. Aparicio, Theoretical investigation of carbon dioxide adsorption on MgH2 with a cobalt catalyst, Ind. Chem. Mater., 2024, 2, 587–599 RSC.
- H. Shi, H. Wang, Y. Zhou, J. Li, P. Zhai, X. Li, G. G. Gurzadyan, J. Hou, H. Yang and X. Guo, Atomically dispersed Indium-copper dual-metal active sites promoting C-C coupling for CO2 photoreduction to ethanol, Angew. Chem., Int. Ed., 2022, 134, e202208904 CrossRef.
- J. Albero, Y. Peng and H. García, Photocatalytic CO2 reduction to C2+ products, ACS Catal., 2020, 10, 5734–5749 CrossRef CAS.
- B. Y. Lan and H. F. Shi, Review of systems for photocatalytic conversion of CO2 to hydrocarbon fuels, Acta Phys.-Chim. Sin., 2014, 30, 2177–2196 CAS.
- P. Bai, P. Wang, Y. Wu, X. Pang, M. Song, C. Du and Y. Su, Junction of ZnmIn2S3+m and bismuth vanadate as Z-scheme photocatalyst for enhanced hydrogen evolution activity: The role of interfacial interactions, J. Colloid Interface Sci., 2022, 628, 488–499 CrossRef CAS.
- Y. Chai, Y. Kong, M. Lin, W. Lin, J. Shen, J. Long, R. Yuan, W. Dai, X. Wang and Z. Zhang, Metal to non-metal sites of metallic sulfides switching products from CO to CH4 for photocatalytic CO2 reduction, Nat. Commun., 2023, 14, 6168 CrossRef CAS.
- Y. Chen, C. L. Tan, J. Y. Li, M. Y. Qi, Z. R. Tang and Y. J. Xu, Cocatalyst-modified In2S3 photocatalysts for C-N coupling of amines integrated with H2 evolution, Ind. Chem. Mater., 2024, 2, 289–299 RSC.
- S. You, J. Xiao, S. Liang, W. Xie, T. Zhang, M. Li, Z. Zhong, Q. Wang and H. He, Doping engineering of Cu-based catalysts for electrocatalytic CO2 reduction to multi-carbon products, Energy Environ. Sci., 2024, 17, 5795–5818 RSC.
- W. Gao, L. Shi, W. T. Hou, C. Ding, Q. Liu, R. Long, H. Q. Chi, Y. C. Zhang, X. Y. Xu, X. Y. Ma, Z. Tang, Y. Yang, X. Y. Wang, Q. Shen, Y. J. Xiong, J. L. Wang, Z. G. Zou and Y. Zhou, Tandem synergistic effect of Cu-In dual sites confined on the edge of monolayer CuInP2S6 toward selective photoreduction of CO2 into multi-carbon solar fuels, Angew. Chem., Int. Ed., 2024, 63, e202317852 CrossRef CAS PubMed.
- X. Pan, M. Q. Yang, X. Fu, N. Zhang and Y. J. Xu, Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications, Nanoscale, 2013, 5, 3601–3614 RSC.
- Y. Ji and Y. Luo, New mechanism for photocatalytic reduction of CO2 on the anatase TiO2(101) surface: The essential role of oxygen vacancy, J. Am. Chem. Soc., 2016, 138, 15896–15902 CrossRef CAS PubMed.
- K. Yan, D. Wu, T. Wang, C. Chen, S. Liu, Y. Hu, C. Gao, H. Chen and B. Li, Highly selective ethylene production from solar-driven CO2 reduction on the Bi2S3@In2S3 catalyst with In-SV-Bi active sites, ACS Catal., 2023, 13, 2302–2312 CrossRef CAS.
- J. Wang, C. Yang, L. Mao, X. Cai, Z. Geng, H. Zhang, J. Zhang, X. Tan, J. Ye and T. Yu, Regulating the metallic Cu-Ga bond by S vacancy for improved photocatalytic CO2 reduction to C2H4, Adv. Funct. Mater., 2023, 33, 2213901 CrossRef CAS.
- C. Bie, J. Fu, B. Cheng and L. Zhang, Ultrathin CdS nanosheets with tunable thickness and efficient photocatalytic hydrogen generation, Appl. Surf. Sci., 2018, 462, 606–614 CrossRef CAS.
- J. R. Adleman, D. A. Boyd, D. G. Goodwin and D. Psaltis, Heterogenous catalysis mediated by plasmon heating, Nano Lett., 2009, 9, 4417–4423 CrossRef CAS.
- Z. Zhang, X. Ma, Y. Li, N. Ma, M. Wang, W. Liu, J. Peng, Y. Liu and Y. Li, Heterovalent metal pair sites on metal-organic framework ordered macropores for multimolecular Co-activation, J. Am. Chem. Soc., 2024, 146, 8425–8434 CrossRef CAS.
- F. Horani and E. Lifshitz, Unraveling the growth mechanism forming stable γ-In2S3 and β-In2S3 colloidal nanoplatelets, Chem. Mater., 2019, 31, 1784–1793 CrossRef CAS.
- L. Fan, S. Lei, H. M. K. Sari, L. Zhong, A. Kakimov, J. Wang, J. Chen, D. Liu, L. Huang, J. Hu, L. Lin and X. Li, Controllable S-vacancies of monolayered Mo-S nanocrystals for highly harvesting lithium storage, Nano Energy, 2020, 78, 105235 CrossRef CAS.
- J. Wang, T. Bo, B. Shao, Y. Zhang, L. Jia, X. Tan, W. Zhou and T. Yu, Effect of S vacancy in Cu3SnS4 on high selectivity and activity of photocatalytic CO2 reduction, Appl. Catal., B, 2021, 297, 120498 CrossRef CAS.
- R. R. Ratnakar, S. Shankar, R. Agrawal and B. Dindoruk, Modeling and experimental study on CO2 adsorption in fixed-bed columns: Applications to carbon capture and utilization, J. Nat. Gas Sci. Eng., 2021, 94, 104111 CrossRef CAS.
- C. Liao, Z. He, F. Wang, Y. Liu and L. Guo, Anti-site defect-induced cascaded sub-band transition in CuInS2 enables infrared light-driven CO2 reduction, ACS Nano, 2024, 18, 35480–35489 CrossRef CAS PubMed.
- L. Falbo, C. G. Visconti, L. Lietti and J. Szanyi, The effect of CO on CO2 methanation over Ru/Al2O3 catalysts: A combined steady-state reactivity and transient DRIFT spectroscopy study, Appl. Catal., B, 2019, 256, 117791 CrossRef CAS.
- K. Sun, C. Shen, R. Zou and C. J. Liu, Highly active Pt/In2O3-ZrO2 catalyst for CO2 hydrogenation to methanol with enhanced CO tolerance: The effects of ZrO2, Appl. Catal., B, 2023, 320, 122018 CrossRef CAS.
- S. Xu, S. Chansai, S. Xu, C. E. Stere, Y. Jiao, S. Yang, C. Hardacre and X. Fan, CO poisoning of Ru catalysts in CO2 hydrogenation under thermal and plasma conditions: A combined kinetic and diffuse reflectance infrared fourier transform spectroscopy-mass spectrometry study, ACS Catal., 2020, 10, 12828–12840 CrossRef CAS.
- X. Zhang, Y. Yang, Y. Hu, L. Xiong, T. Wang, P. Li and J. Shen, Photothermal catalytic C-C coupling to ethylene from CO2 with high efficiency by synergistic cooperation of oxygen vacancy and half-metallic WTe2, J. Energy Chem., 2024, 93, 547–556 CrossRef CAS.
- W. Wang, C. Deng, S. Xie, Y. Li, W. Zhang, H. Sheng, C. Chen and J. Zhao, Photocatalytic C-C coupling from carbon dioxide reduction on copper oxide with mixed-valence Copper(I)/Copper(II), J. Am. Chem. Soc., 2021, 143, 2984–2993 CrossRef CAS PubMed.
- W. Yang, G. Ma, Y. Fu, K. Peng, H. Yang, X. Zhan, W. Yang, L. Wang and H. Hou, Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution, Chem. Eng. J., 2022, 429, 132381 CrossRef CAS.
- N. Xiao, S. Li, X. Li, L. Ge, Y. Gao and N. Li, The roles and mechanism of cocatalysts in photocatalytic water splitting to produce hydrogen, Chin. J. Catal., 2020, 41, 642–671 CrossRef CAS.
- Y. Gao, G. Yang, Y. Dai, X. Li, J. Gao, N. Li, P. Qiu and L. Ge, Electrodeposited Co-substituted LaFeO3 for enhancing the photoelectrochemical activity of BiVO4, ACS Appl. Mater. Interfaces, 2020, 12, 17364–17375 CrossRef CAS PubMed.
- Y. X. Pan, Y. You, S. Xin, Y. T. Li, G. T. Fu, Z. M. Cui, Y. L. Men, F. F. Cao, S. H. Yu and J. B. Goodenough, Photocatalytic CO2 reduction by carbon-coated Indium-oxide nanobelts, J. Am. Chem. Soc., 2017, 139, 4123–4129 CrossRef CAS PubMed.
- Y. Sun, K. Lai, N. Li, Y. Gao and L. Ge, Efficient photocatalytic CO2 reduction to CH4 via electric field-regulated d-band center on Ga2S3/CuS S-type heterojunction interface structures, Appl. Catal., B, 2024, 357, 124302 CrossRef CAS.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- T. Huang, Z. Huang, X. Yang, S. Yang, Q. Gao, X. Cai, Y. Liu, Y. Fang, S. Zhang and S. Zhang, Green and regulable synthesis of CdNCN on CdS semiconductor: Atomic-level heterostructures for enhanced photocatalytic hydrogen evolution, Adv. Powder Mater., 2024, 3, 100242 CrossRef.
- D. Yang, Y. Li, R. Chen, X. Wang, Z. Li, T. Xing, L. Wei, S. Xu, P. Dai and M. Wu, Flower-like superstructure of boron carbon nitride nanosheets with adjustable band gaps for photocatalytic hydrogen peroxide production, J. Mater. Sci. Technol., 2024, 183, 23–31 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5im00015g |
| This journal is © Institute of Process Engineering of CAS 2025 |