DOI:
10.1039/D5IM00009B
(Review Article)
Ind. Chem. Mater., 2025,
3, 412-430
Experimental and theoretical progress on the reduction of Np(VI) with salt-free reagents in the PUREX process
Received
21st January 2025
, Accepted 8th April 2025
First published on 9th April 2025
Abstract
Effectively controlling the oxidation state of neptunium (Np) is crucial for the separation of Np during the advanced plutonium uranium reduction extraction process. The reduction reactions and kinetics of Np(VI) with salt-free reagents were explored by applying experimental and theoretical studies. This review summarizes the reduction reaction, kinetics, mechanism and electronic structures as well as the potential energy surfaces of Np(VI) to Np(V) using salt-free reagents, such as hydrazine, hydroxylamine, aldehydes, oximes, hydroxamic acids and their derivatives. This review will hopefully serve as a useful resource to inspire further research on the reduction of Np(VI) using salt-free reagents.
Keywords: Reduction kinetics; Reduction mechanism; Np(VI); Salt-free reagents; Theoretical simulation.
 Xin Huang | Ms. Xin Huang received her BE degree from Yuncheng University (2021). She is currently an ME candidate at University of South China. During her master's program, she was jointly trained at Institute of High Energy Physics, Chinese Academy of Sciences, primarily studying the reduction of Np(VI) by salt-free reagents. |
 Qun-Yan Wu | Dr. Qun-Yan Wu received her BE and ME degrees from Qufu Normal University (2006) and PhD from Beijing Institute of Technology (2010). She then pursued postdoctoral research at Tsinghua University. She joined Institute of High Energy Physics, Chinese Academy of Sciences in 2013 as a research assistant and is currently an associate professor. Her scientific interests include the theoretical design of lanthanum/actinium separation ligands, study of the reduction mechanism of Np(VI) by a salt-free reductant and study of actinide multiple bonds and oxidation states. |
 Wei-qun Shi | Professor Wei-qun Shi completed his Bachelor of Engineering degree at Hubei University of Technology in 1999, followed by a Master of Engineering from the China Institute of Atomic Energy in 2002 and a Doctor of Philosophy from Tsinghua University in 2007. Subsequently, he joined Institute of High Energy Physics, Chinese Academy of Sciences, where he worked until 2024. Currently, he serves as a full professor at Shanghai Jiao Tong University. His ongoing research interests focus on actinide coordination chemistry, as well as the separation of actinides from lanthanides through solvent extraction and molten salt electrolysis techniques. To date, he has co-authored more than 400 scientific articles published in prestigious international peer-reviewed journals, contributing significantly to the relevant academic fields. |
1 Introduction
The plutonium uranium reduction extraction (PUREX) process is effectively applied in the commercial scale reprocessing of spent nuclear fuel (SNF). In the PUREX process, U and Pu are separated from HNO3 solution to the organic phase using tri-n-butyl phosphate (TBP). The extraction performance of U, Np and Pu is significantly sensitive to their oxidation states in the PUREX process. Generally, hexavalent and tetravalent actinide ions exhibit higher extractability compared with trivalent and pentavalent ones. Therefore, the extractability of these actinides can be changed by oxidation or reduction, which achieves independent separation of U, Np and Pu in the different flowsheets. Actinides are very difficult to separate because they are similar in many respects.1,2 Thus, an advanced PUREX process is desired to achieve comprehensive control of U, Np, and Pu extraction in a single extraction cycle. 237Np has an exceptionally long half-life of 2.14 × 106 years3 and poses significant environmental hazards and radiotoxicity.4 Furthermore, 237Np serves as a key precursor in the production of 238Pu, an essential component in the manufacture of nuclear-powered batteries.5 Therefore, the separation of Np is very crucial and urgent. Np exhibits three oxidation states during the PUREX process, and their extractability by TBP follows the sequence of Np(VI) > Np(IV) ≫ Np(V).6–8 The valence state of Np in aqueous nitric acid depends on the concentration of HNO2.9–11 At low concentrations of HNO2, Np(V) is rapidly oxidized to Np(VI), while at high concentrations of HNO2, Np(VI) is reduced to Np(V). HNO2 exhibits a dual role in equilibrium reactions. At low concentrations, it acts as a catalyst, facilitating the oxidation of Np(V). Conversely, at high concentrations, it acts as a reductant, converting Np(VI) to Np(V). The redox reaction between Np(VI) and Np(V) is quasi-reversible and has simple one-electron transfer characteristic:In addition, Np(V) slowly disproportionates, generating Np(VI) and Np(IV) in highly acidic solution though the following reaction. The extent of the disproportionation is promoted when the acidity of the solution and the concentration of Np(V) are high:| | | 2Np(V) + 4H+ ↔ Np(IV) + Np(VI) + 2H2O | (2) |
The valence state of Np ions in the different stages of the PUREX process is displayed in Fig. 1. The most of Np in the co-decontamination step is oxidized to Np(VI) by nitric acid and is extracted to the organic solvent accompanied with U(vi) and Pu(IV). In the U/Pu partition step, Np(VI) is reduced to Np(IV) or Np(V) by reductants and Np is distributed to the U and the Pu purification cycles. It is essential to isolate Np ions from Pu and U ions before the U/Pu split stream to simplify the PUREX process. In the advanced PUREX, it is necessary to effectively control the oxidation state of Np ions.6,12
 |
| | Fig. 1 Distribution of Np(VI,V,IV) in the PUREX process. | |
Extensive studies have been conducted with the aim of developing an advanced PUREX process involving an efficient precise control of the Np oxidation state. There are several methods to control the Np oxidation state in the flowsheet of the PUREX process: Np can be entirely directed to (1) the high-level liquid waste (HLLW) stream, (2) the Pu purification stage, or (3) the U purification stage before the separation of the U and Pu stream. Np speciation in HNO3 solution is dominated by the Np(VI)/Np(V) equilibrium, which is a very complex process due to the interactions among reaction kinetics, chemical equilibria, acidity, temperature and the presence of other ions.9–11 In particular, the Np(VI)/Np(V) equilibrium is sensitive to the HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO2 ratio and temperature. For example, the concentration of Np(VI) is enhanced at higher HNO3 concentrations, lower HNO2 concentrations and higher temperatures.9 Salt-free reagents are considered to be promising for Np(VI) reduction because they can be decomposed into gases, such as N2, CO2 and H2O.13 The reduction behaviors of Np(VI) with many salt-free reagents have been explored by the groups of Taylor,1,6,14,15 Uchiyama,16,17 Koltunov18,19 and Ye.20,21 A variety of salt-free reductants can effectively reduce Np(VI) to Np(V), such as hydrazine,14,22–25 aldehydes,16,17 hydroxylamine,26–30 oxime,31–34 hydroxamic acid15,35 and their derivatives. Recently, we systematically explored the Np(VI) reduction using hydrazine, hydroxylamine and its derivatives and elucidated their reduction mechanisms by employing density functional theory (DFT).36–45 Koltunov and co-workers reported the reaction kinetics of Np and Pu ions using hydrazine, organic nitriles, hydroxylamine, monoximes, diaziridinylethane and their derivatives.18,20,21 Marchenko et al. described the characteristics of organic reductants that can keep Np and Pu in a specific oxidation state.46 Although much research has focused on Np(VI) reduction by salt-free reagents, there is no review on the subject. Noticeably, some salt-free reagents can reduce Np(VI) to Np(V) and reduce Np(V) to Np(IV). In this review, we mainly elucidate the experimental and theoretical progress on the reduction of Np(VI) to Np(V) by salt-free reagents. We hope that this review will serve as a useful resource to inspire further research on Np(VI) reduction by salt-free reagents in the reprocessing of SNF.
HNO2 ratio and temperature. For example, the concentration of Np(VI) is enhanced at higher HNO3 concentrations, lower HNO2 concentrations and higher temperatures.9 Salt-free reagents are considered to be promising for Np(VI) reduction because they can be decomposed into gases, such as N2, CO2 and H2O.13 The reduction behaviors of Np(VI) with many salt-free reagents have been explored by the groups of Taylor,1,6,14,15 Uchiyama,16,17 Koltunov18,19 and Ye.20,21 A variety of salt-free reductants can effectively reduce Np(VI) to Np(V), such as hydrazine,14,22–25 aldehydes,16,17 hydroxylamine,26–30 oxime,31–34 hydroxamic acid15,35 and their derivatives. Recently, we systematically explored the Np(VI) reduction using hydrazine, hydroxylamine and its derivatives and elucidated their reduction mechanisms by employing density functional theory (DFT).36–45 Koltunov and co-workers reported the reaction kinetics of Np and Pu ions using hydrazine, organic nitriles, hydroxylamine, monoximes, diaziridinylethane and their derivatives.18,20,21 Marchenko et al. described the characteristics of organic reductants that can keep Np and Pu in a specific oxidation state.46 Although much research has focused on Np(VI) reduction by salt-free reagents, there is no review on the subject. Noticeably, some salt-free reagents can reduce Np(VI) to Np(V) and reduce Np(V) to Np(IV). In this review, we mainly elucidate the experimental and theoretical progress on the reduction of Np(VI) to Np(V) by salt-free reagents. We hope that this review will serve as a useful resource to inspire further research on Np(VI) reduction by salt-free reagents in the reprocessing of SNF.
2 Experimental progress on the reduction of Np(VI) to Np(V) by salt-free reagents
2.1 Hydrazine and its derivatives
Hydrazine and its derivatives can reduce Np(VI) to Np(V), which enables the separation of Np in SNF.14,22–25,47 There are about 20 types of hydrazine and its derivatives studied in the experiment presented in Fig. 2, some of which can reduce four Np(VI) ions such as hydrazine, carbohydrazide, and 3,3′-bis(diaziridinyl), while the others only enable the reduction of two Np(VI) ions. The total reaction equation for excess Np(VI) and hydrazine, carbohydrazide and 3,3′-bis(diaziridinyl) is expressed in eqn (3)–(5), respectively.| | | 4Np(VI) + N2H4 → 4Np(V) + N2 + 4H+ | (3) |
| | | 4Np(VI) + CO(N2H3)2 → 4Np(V) + HCOH + 2N2 + 4H+ | (4) |
| | | 4Np(VI) + H2N2HCCHN2H2 → 4Np(V) + N2HCCHN2 + 4H+ | (5) |
Taking hydrazine as an example, the reduction mechanisms for the excess Np(VI) and N2H4 are shown in eqn (6)–(9).22| | | Np(VI) + N2H4 → Np(V) + N2H4+˙ | (6) |
| | | Np(VI) + N2H4+˙ → Np(V) + N2H2 + 2H+ | (7) |
| | | Np(VI) + N2H2 → Np(V) + N2H2+˙ | (8) |
| | | Np(VI) + N2H2+˙ → Np(V) + N2 + 2H+ | (9) |
Hydrazine can react with nitric acid and form hydrazinium nitrate, which is often used as a scavenging agent for nitrous acid (HNO2) in the PUREX process. The reaction rate for the reduction of Np(VI) is enhanced by increasing the hydrazinium nitrate concentrations.24 The kinetics of the reaction of hydrazinium nitrate and HNO2 in eqn (10) is very fast. Moreover, the HN3 product can react with HNO2 in eqn (11) as soon as all the N2H5+ has disappeared.48| | | N2H5+ + HNO2 → HN3 + 2H2O + H+ | (10) |
| | | HN3 + HNO2 → N2O + N2 + H2O | (11) |
The total reaction equation for Np(VI) with excess RN2H3 and R2N2H2 is given in eqn (12) and (13), respectively.| | | 2Np(VI) + RN2H3 → 2Np(V) + RH + N2 + 2H+ | (12) |
| | | 2Np(VI) + R2N2H2 → 2Np(V) + products + 2H+ | (13) |
where R is a substituent group of hydrazine.
 |
| | Fig. 2 Structures of hydrazine and its derivatives for Np(VI) reduction studies. | |
The reaction mechanism of eqn (12) is shown in eqn (14)–(16)
| | | Np(VI) + RN2H3 → Np(V) + RN2H3+˙ | (14) |
| | | Np(VI) + RN2H3+˙ → Np(V) + RN2H + 2H+ | (15) |
The reaction mechanism of
eqn (13) corresponds to
eqn (17)–(19)| | | Np(VI) + R2N2H2 → Np(V) + R2N2H2+˙ | (17) |
| | | Np(VI) + R2N2H2+˙ → Np(V) + R2N2+ 2H+ | (18) |
We summarized temperature, equation of the reaction rate, activation energy (
E) and rate constant for the reaction of Np(
VI) reduction with hydrazine and its derivatives in
Table 1. The oxidation rate of hydrazine and its derivatives in the HNO
3 solution follows the first-order kinetic equation, while its order with respect to H
+ is about −1. These results indicate that the reaction rate is not sensitive to the ionic strength. The activation energy for phenylhydrazine and hydrazinopropionitrile is smaller than that of other hydrazine derivatives.
18,49 Koltunov
et al. first studied the kinetics of Np(
VI) reduction using different hydrazine derivatives,
22,47 and revealed that the reduction by these derivatives exhibits a significant difference. Their reduction ability in HNO
3 solution, judged by the reaction rate, follows the order of CH
3CON
2H
3 < HCON
2H
3 < N
2H
4 < C
2H
5OCON
2H
3 < C
2H
5N
2H
3 < CH
2CHCH
2N
2H
3 < CH
3N
2H
3 < C
6H
5CH
2N
2H
3 < C
2H
5OCOCH
2N
2H
3 < CH
3N
2H
2CH
3 < HOC
2H
4N
2H
3. The reduction rate of Np(
VI) is enhanced by some hydrazine derivatives modified by electron-donating groups, which is ascribed to the increase of the electron density around the N atom by introducing electron-donating groups. Oppositely, some hydrazine derivatives modified by electron-withdrawing groups will make the Np(
VI) reduction more difficult. In the reaction process, the free radical ion RN
2H
3+˙ is an intermediate product of the first Np(
VI) reduction by RN
2H
3, which can result in the second Np(
VI) reduction.
Table 1 Temperature (t), kinetic equation, rate constant (k) and activation energy (E) for the Np(VI) reduction reaction with hydrazine and its derivatives
| Reductants (R) |
t (°C) |

|
k (min−1) |
E (kJ mol−1) |
Ref. |
| N2H4 |
25 |
e = 1, n = 1.24 |
14.0 |
78.7 |
50
|
| CH3N2H3 |
25 |
e = 1, n = 1 |
52.7 |
58.6 |
50
|
| (CH3)2N2H2 |
25 |
e = 1, n = 0.9 |
55.3 (L mol−1)0.1 |
47.2 |
51
|
| CH3N2H2CH3 |
25 |
e = 1, n = 0.8 |
118 |
50.2 |
18, 22 |
| C2H5N2H3 |
25 |
e = 1, n = 1 |
30 |
61.5 |
18, 22 |
| (CH3)2CHN2H3 |
25 |
e = 1, n = 0.9 |
19.3 |
69.4 |
18
|
| (CH3)3CN2H3 |
25 |
e = 0.9, n = 1 |
2.18 ± 0.19 (L mol−1)0.1 |
63.1 ± 2.7 |
14
|
| (CH3)3CN2H3 |
25 |
e = 0.9, n = 0.75 |
5.4 (L mol−1)0.15 |
61.2 |
52
|
| CH2CHCH2N2H3 |
25 |
e = 1, n = 1 |
46 |
63.6 |
18, 22 |
| HO(CH2)2N2H3 |
25 |
e = 1, n = 1 |
391 |
56.6 |
53
|
| HCON2H3 |
25 |
e = 1, n = 1 |
7.95 |
85.2 |
18, 22 |
| CH3CON2H3 |
25 |
e = 1, n = 1.2 |
7.52 |
76.5 |
18, 22 |
| C6H5N2H3 |
25 |
e = 1, n = 0.4 |
3000 |
31.4 |
18
|
| C6H5CH2N2H3 |
25 |
e = 1, n = 1 |
53.2 |
98.2 |
18
|
| C2H5COON2H3 |
25 |
e = 1, n = 1.2 |
19.3 |
65.4 |
18, 22 |
| C2H5COOCH2N2H3 |
25 |
e = 1, n = 1.1 |
97.6 |
70.1 |
18
|
| NCCH2N2H3 |
25 |
e = 1, n = 1 |
920 ± 38 (L mol−1)0.1 |
38.7 ± 1.3 |
49
|
| CHONHNHCHO |
15 |
e = 1.3, n = 1.55 |
10.4 ± 0.3 (L mol−1)0.25 |
85 ± 10 |
54
|
| N2H3(CO)N2H3 |
15 |
e = 1.15, n = 1.35 |
39.7 ± 2.7 (L mol−1)0.2 |
85 ± 20 |
55
|
| H2N2HCCHN2H2 |
35.7 |
e = 1, n = 1 |
132 ± 10 |
73.1 ± 4.6 |
56
|
Koltunov reported the rate constants, mechanisms, activation energies, and kinetic equations for Np(VI) reduction with sixteen kinds of hydrazine derivatives in HNO3 solution.18 Xiao et al. obtained a quantitative correlation between the molecular structures of hydrazine derivatives with the Np(VI) reduction rate.21 They concluded that the reduction rates are mainly related to the molecular dipole moment, the level of LUMO as well as hydrophobic parameters.
Taylor et al.14 investigated the solvent extraction of Np ions by selective reduction of Np(VI) using salt-free reagents and found that tert-butylhydrazine can be a potential candidate for the selective reduction of Np(VI) in certain conditions.57 The reaction kinetics for Np(VI) with tert-butylhydrazine were explored using spectrophotometry in HNO3 solution.52 The reduction rate of Np(VI) by tert-butylhydrazine in TBP/HNO3 solution is similar to that by dibenzylhydrazine.58 Subsequently, Koltunov and coworkers explored the kinetic behavior and mechanisms of Np(VI) reduction by dibenzylhydrazine in TBP/HNO3 solution and revealed the final reaction products, kinetic equation, and activation energy with an excess of the reductant.59
The separation of Np from Pu was explored using hydrazine as a stripping reductant in a single-stage extraction experiment and the Np/Pu separation efficiency is enhanced by elevating the concentrations of hydrazine, nitric acid, and temperature.24 The feasibility of the Np/Pu separation with methylhydrazine was also assessed by a single-stage extraction device, which suggested that the separation ability of Np(VI)/Pu(IV) becomes weaker as the reaction time gets longer, which is similar to hydrazine.24,25 The Np(VI) selective reduction by N,N-dimethylhydrazine within a TBP/n-dodecane HNO3 solution including Pu(IV), U(VI) and Np(VI) was explored by spectrophotometry and numerical simulation.23 The kinetic behavior for the Np(VI) reduction with CH3N2H3 and HOC2H4N2H3 and their application in U/Np separation were investigated by Zhang et al.53,60–62 Yin and coworkers also explored the reduction kinetics of Np(VI) by (CH3)2N2H2, which enables (CH3)2N2H2 as selective reductant of Np(VI) for U/Np separation.51,63 The rate-determining step for Np(VI) reduction by HOC2H4N2H3, (CH3)2N2H2, and CH3N2H3 in the kerosene-30% TBP/HNO3 solution is the back-extraction of Np(V) from the kerosene-30% TBP into the HNO3 solution,64 which is important to separate Np in the SNF reprocessing. Carbohydrazide is an effective reductant of Np(VI) in high-burn-up SNF. Volk et al. found that Np(VI) is quickly reduced to Np(V) using carbohydrazide, but the reduction rate of Np(V) to Np(IV) is very slow.65 Subsequently, Zavalina and coworkers examined the kinetic behavior of Np(VI) reduction to Np(V) using carbohydrazide in HNO3 solution,55 and Shilov and Fedoseev explored the corresponding reduction in HClO4 solution.66 The reduction rate for Np(VI) to Np(V) via carbohydrazide improves with increasing temperature, the concentration of carbohydrazide, and lowering the concentration of HNO3. The activation energy for the corresponding reduction reaction is 85 kJ mol−1 in HNO3 solution at 15 °C and 86 ± 5 kJ mol−1 in HClO4 solution at 25 °C.
2.2 Hydroxylamine and its derivatives
In the PUREX process, a strategy for controlling Np separation includes the extraction of U(VI), Np(VI), and Pu(IV) together and the separation of Pu(III) and Np(V) from U(VI). Effective and rapid reduction of Np(VI) to Np(V) and Pu(IV) to Pu(III) necessitates the application of a suitable reductant. Hydroxylamine (HA) and its derivatives have been extensively explored for this purpose. Notably, HA enables rapid reduction of Np(VI) to Np(V).46,57 HA is further able to reduce Np(V) to Np(IV) with increasing acidity and temperature.1,57 The reduction equations of Np(VI) with HA are followed as eqn (20)–(22):26,67
In excess Np(VI),
| | | 2NpO22+ + 2NH3OH+ → 2NpO2+ + N2 + 2H2O + 4H+ | (20) |
In excess HA,
| | | 4NpO22+ + 2NH3OH+ → 4NpO2+ + N2O + H2O + 6H+ | (21) |
| | | 2NpO2+ + 2NH3OH+ + 4H+ → 2Np4+ + N2 + 6H2O | (22) |
The reduction rate constant of Np(
VI) by HA is 3.5 s
−1 in HNO
3 solution with
μ = 2 at 22 °C.
19 HA is quite stable at low acidity and room temperature. However, it can decompose into N
2O as the HNO
3 concentration and/or temperature increase, which is enhanced under the catalysis of Fe.
68 Additionally, the reduction of Np(
VI) by HA in 0.5 M HNO
3 solutions with a high U concentration of 850 g L
−1 at 60 °C predominantly produces Np(
V), while in 0.33 M HNO
3 solutions under the same temperature and uranium concentration conditions, the reduced products consist of a mixture of Np(
V) and Np(
VI).
20
Most hydroxylamine derivatives exhibit a high reduction rate for Pu(IV) and Np(VI) (Fig. 3), making them suitable for the co-extraction of Np and Pu from U.19,58 The reaction rates of Np(VI) reduction by HA and its derivatives are presented in Table 2. HA derivatives avoid additional stabilizers due to their rapid reactivity with HNO2, which precludes the production of HN3 and NH4NO3.46 At moderate acidity and room temperature, HA derivatives can reduce Np(VI) to Np(V), but they cannot further reduce Np(V) to Np(IV) in the absence of catalysts,30,69 and the reaction products are mainly alcohols and aldehydes.19
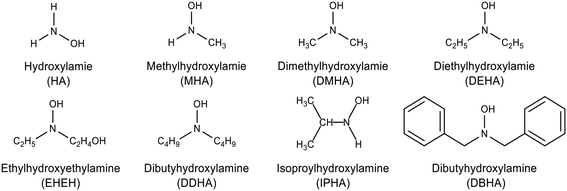 |
| | Fig. 3 Structures of hydroxylamine and its derivatives. | |
Table 2 Temperature (t), kinetic equation, rate constant (k), activation energy (E) for Np(VI) reduction by hydroxylamine and its derivatives at μ = 2
| Reductants (R) |
t (°C) |
The kinetic equation 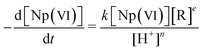
|
k (min−1) |
E (kJ mol−1) |
Ref. |
| NH2OH (HA) |
15 |
e = 1, n = 1 |
92.1 ± 1.0 |
82.00 |
26
|
| CH3NHOH (MHA) |
25 |
e = 0.7, n = 0.4 |
35.0 ± 0.9 (L mol−1)0.3 min |
63.6 ± 3 |
19
|
| (CH3)2NOH (DMHA) |
25 |
e = 1, n = 0.65 |
24.2 ± 11 (L mol−1)0.35 min |
60.03 ± 0.02 |
70
|
| (C2H5)2NOH |
25.2 |
e = 1, n = 1 |
23.0 ± 1.8 |
69.1 |
28
|
| C2H5 (HOC2H4)NOH (EHEH) |
25.6 |
e = 1, n = 0.8 |
334 ± 12 (L mol−1)0.2 min |
42.3 ± 2.7 |
27
|
| CH3CH(CH3)NHOH (IPHA) |
25.8 |
e = 1, n = 0.8 |
41.8 ± 1.0 (L mol−1)0.2 min |
58.20 ± 0.03 |
71
|
| (C4H9)2NOH (DDHA) |
25.5 |
e = 1, n = 1 |
15.6 ± 1.1 |
45.8 ± 0.8 |
72
|
| (C6H5CH2)2NOH (DBHA) |
25 |
e = 1, n = 1 |
1.8 |
— |
58
|
The stoichiometry of the Np(VI) reaction with HA derivatives is determined by the starting concentrations of the reactants. In cases where there is an excess of Np(VI) ions, the stoichiometric coefficient for Np(VI)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HA derivatives is (6–8)
HA derivatives is (6–8)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, the corresponding stoichiometric coefficient decreases to (0.5–2)
1. However, the corresponding stoichiometric coefficient decreases to (0.5–2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 when the HA derivatives are in excess.57
1 when the HA derivatives are in excess.57
The reaction order relative to hydrogen ion ranges from 0.4 to 1.0.18 These reactions occur through different mechanisms, which contain several parallel pathways with R′RNOH and R′RNHOH+.26,70–72 The mechanisms of Np(VI) reduction by mono- and disubstituted hydroxylamines are different. For the disubstituted hydroxylamines, the rate-determining step is the decomposition reaction of the activated complex including the nitroxyl radical, which undergoes further oxidation to nitron, then forms aldehyde and monoalkyl hydroxylamine, or nitrosoalkyl and alcohol. In the case of monosubstituted hydroxylamine, it forms nitroxide radical, which oxidizes to alcohol and nitrosoalkyl, with the nitrosoalkyl further forms HNO3.
The reduction rates of Np(VI) by most hydroxylamine alkyl derivatives are slower compared with that of HA,57,67 except for N,N-ethyl(hydroxyethyl)hydroxylamine (EHEH) and N,N-dimethylhydroxylamine (DMAH). The reduction rate of Np(VI) by EHEH is much faster than that by N,N-diethylhydroxylamine (DEHA).57τ99 for the Np(VI) reduction by HA,26 DMHA,19 DEHA19 and EHEH73 is 0.12, 0.18, 0.15, and 0.14 min in 1.0 M HNO3 and 0.01 M Np(VI) solution at 25 °C, respectively. Koltunov et al.57,67,74 studied the reduction kinetics of Np(VI) by N-methylhydroxylamine (MAH) and DMAH in 2.0 M HNO3 at 22 °C, and the τ99 values are 1.13 and 0.38 min, respectively, while the corresponding τ99 values change to 0.65 min and 0.18 min in 2.0 M HNO3 at 25 °C. These values indicated that the reduction rate of Np(VI) by DMAH is much faster compared with that of MAH under the same conditions.57,67,68,70
Np(VI) is quickly reduced to Np(V) by DEHA in HNO3 solution,28τ99 value for the reaction of DEHA and HNO2 is 0.15 min at 1 M HNO3 and 0.1 M DEHA solution.75 DEHA is a better stabilizer compared with HA, The presence of U(VI) (up to 0.5 M) does not affect Np(VI) reduction by DEHA.19,28 That is, DEHA is a more effective reductant than HA as an antinitrite. Zhang et al. applied DEHA to isolate Np and Pu from U for the U(VI) purification cycle step in the PUREX process and suggested that DEHA can quickly reduce over 99% of Np(VI) to Np(V).29 Moreover, the cascade experiment and the cascade extraction separation demonstrate that DEHA is a promising reductant for the PUREX process. The combination of DEHA with hydrazine derivatives like 2-hydroxyethylhydrazine or 1,1-dimethylhydrazine enables the reprocessing process to be carried out without the presence of salts.76
The kinetics of Np(VI) by extraction mass transfer and reversible redox reaction by DEHA were examined. Zhang et al. discovered that the rate of Np(VI) transfer is obviously faster than that of the redox reaction.76 Li's group further determined that the reduction rate of Np(VI) depends on the rate of Np(V) transfer from the kerosene-30% TBP organic phase to HNO3 solution.7 EHEH is DEHA modified by a hydroxyl group, which can enhance the reduction rate of Np(VI).19 The reaction of Np(VI) with EHEH is:64,73
| | | 4NpO22+ + HOC2H4(C2H5)NHOH+ + 3H2O → 4NpO2+ + 2C2H5OH + HNO3 + 5H+ | (23) |
This rate constant is much larger than that of Np(
VI) reduction by DEHA (
Table 2). EHEH exhibits adequate stability in less than 3–4 M HNO
3 solution, even in the presence of Tc(
VII) ions.
27 Moreover, the reaction of EHEH with HNO
2 is very slow, which eliminates the need for an additional scavenging agent.
19 Therefore, the rapidity of the reduction renders EHEH an ideal candidate for application in the U/Pu split process.
2.3 Aldehyde
Uchiyama et al. introduced an innovative method for separating Np/Pu/U by utilizing n- and iso-butyraldehyde, which are illustrated in Fig. 4, capitalizing on their distinct reduction capabilities.17 Both n- and iso-butyraldehydes are favorable reductants for selective Np(VI) reduction in the presence of Pu(IV) and/or U(VI). Moreover, n-butyraldehyde has much higher selectivity and a much lower reduction rate compared with iso-butyraldehyde. Therefore, n-butyraldehyde can selectively reduce Np(VI), while iso-butyraldehyde is utilized to reduce both Np(VI) and Pu(IV). According to the different reduction abilities of n- and iso-butyraldehyde, the selective Np, Pu and U separation process in the PUREX is proposed (Fig. 5). n-Butyraldehyde can extract and reduce about 99.98% Np in the Np separation process, and iso-butyraldehyde is employed for reducing Pu(IV) during the U/Pu split stage.
 |
| | Fig. 4 Some structures of aldehydes, oxime, hydroxyurea, hydroxamic acid and methylsalicylic acids. | |
 |
| | Fig. 5 U, Np, and Pu separation using n- and iso-butyraldehydes. | |
Subsequently, Uchiyama et al. conducted a more in-depth study on the reduction kinetics of Np(VI) by both iso- and n-butyraldehyde in HNO3 solution.16 Iso-butyraldehyde shows superior performance for reducing Np(VI) compared with n-butyraldehyde. Np(VI) is totally converted to Np(V) in 0.069 M iso-butyraldehyde and 3 M HNO3. This work showed that n-butyraldehyde appears to be a potential reagent for Np separation in the PUREX process. Subsequently, the reduction kinetics of Np(VI) with n-butyraldehyde in n-dodecane/30% TBP solution were examined with spectrophotometry.77 The rate constant is about 10–105 smaller in n-dodecane than that in aqueous solution, suggesting that the Np(VI) reduction process with n-butyraldehyde primarily occurs in aqueous solution at 294 ± 1 K.
2.4 Oxime
Monomimes are known to be weakly acidic and basic, which have strong reducing abilities towards Np(VI) ions owing to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– double bond (Fig. 4). Acetaldoxime is an example of the monoximes, which is considered a valuable reagent for Np/U separation in the advanced PUREX process. Koltunov and coworkers investigated the reduction rate for the reaction of Np(VI) and acetaldoxime in HNO3 solution:
N– double bond (Fig. 4). Acetaldoxime is an example of the monoximes, which is considered a valuable reagent for Np/U separation in the advanced PUREX process. Koltunov and coworkers investigated the reduction rate for the reaction of Np(VI) and acetaldoxime in HNO3 solution:| | | −d[Np(VI)]/dt = k[Np(VI)][CH3CHNOH]/[HNO3] | (24) |
where k = 254 ± 10 min−1 with μ = 2 at 26.0 °C as well as the activation energy of 62.6 ± 2.6 kJ mol−1.31 They also suggest that Np(VI) reduction by acetaldoxime may proceed via an intermediate CH3CHṄO radical and the hydrolysis product. Extraction studies show that Np(VI) is reduced to Np(V), which is stripped from 30% TBP/n-dodecane solution bearing U(VI). After studying the Np(VI) reduction with acetaldoxime, they show the advantages of acetaldoxime in that it reduces Np(VI) to Np(V) but does not form Np(IV).34 Subsequently, Koltunov et al. reported that the reduction rate of Np(VI) by butanal oxime is 230 ± 15 min−1 with μ = 2 at 25.0 °C and the activation energy in 1 M HNO3 is 69.4 ± 12.4 kJ mol−1.33 The results indicated that Np(VI) reduction by butanal oxime and acetaldoxime is a first-order reaction. The reduction kinetics of Np(VI) by butanal oxime were further studied in undiluted TBP in the presence of HNO3. The reaction rate constant is 0.058 ± 0.007 L2.5 mol−2.5 min−1 between 0.01 to 0.27 M HNO3 solution with the activation energy of 79 ± 9 kJ mol−1 at T = 25.0 °C.32 Noticeably, oxime can act as a scavenging agent for HNO2. For example, butanal oxime can react with HNO2 and obtain N2O and N2 as shown in eqn (25)–(27). The NO2+ formed in eqn (27) can be quickly eliminated by reaction with HNO2 as shown in eqn (28).78| | | H+ + HNO2 ↔ H2NO2+ ↔ H2O + NO+ | (25) |
| | | C3H7CHNOH + NO+ → C3H7CHO + N2O + H+ | (26) |
| | | C3H7CHNOH + 2NO+ → C3H7CHO + N2 + NO2+ + H+ | (27) |
| | | NO2+ + HNO2 → N2O4 + H+ | (28) |
2.5 Hydroxyurea
Hydroxyurea (HU) presented in Fig. 4 is also a potential salt-free reagent for U/Np separation. Zhu and coworkers reported that Np(VI) is reduced to Np(V) with HU, in which Np(V) is effectively transferred from TBP to the aqueous phase.79 Noticeably, the effectiveness of Np stripping is weakened in HNO3 solution since the Np(VI) reduction is impeded at higher acidity.
Spectrophotometric analysis was applied to study the Np(VI) reduction by dihydroxyurea (DHU).80 The reduction rate of Np(VI) by DHU is quick, which makes it suitable for the U/Np separation. The rate constant for the above reaction is 1.86 s−1 in 7.5 × 10−2 M DHU and 0.44 M HNO3 solution at 4 °C. Under the given experimental conditions, 98% of Np(VI) was quickly stripped to the aqueous phase. The stripping ability decreases with increasing [HNO3], while it increases with increasing [DHU], so DHU can be used to separate Np in the advanced PUREX process. The reduction rate of Np(VI) by hydroxysemicarbazide (HSC) is also quick with a rate constant of 1037 ± 60 M−1.40 s−1 at 4.0 °C;81 it is positively influenced by increasing [HSC] or temperature and decreasing [HNO3] or ionic strength.
2.6 Hydroxamic acids
Hydroxamic acids with the general formula RCONHOH illustrated in Fig. 4 are excellent chelating reagents, which provides great benefits in separating Np in advanced PUREX. Furthermore, hydroxamic acids are easily decomposed to gases and consume HNO2 without an additional reagent. Formohydroxamic acid (FHA) and acetohydroxamic acid (AHA) are hydrophilic ligands that do not enter into TBP. Hydroxamic acids undergo hydrolysis in less than 3 M HNO3 and result in the formation of hydroxylamine and carboxylic acid. Taylor and co-workers found that Np(VI) is reduced to Np(V) by AHA and FHA in nitric acid.15 They verified that Np(VI) is effectively reduced and removed from the solvent phase bearing U(VI) by AHA and FHA in laboratory scale experiments.35 Subsequently, further investigation of the reduction kinetics of Np(VI) by FHA in HNO3 solution shows that it is rapidly reduced within a few seconds and is first order with respect to Np(VI) by stopped-flow spectrophotometry with a rate constant of 1.17 × 103 M−1 s−1 in 2 M HNO3 solution at 22 °C.67
Chung and Lee investigated the reduction of Np(VI) by AHA in HNO3 solution.82 The reduction reaction is first-order relative to the concentration of Np(VI) and AHA with the rate constant of 191.2 ± 11.2 M−1 s−1 at 25 ± 0.5 °C and 1.0 M HNO3 solution. Investigation of the reduction stripping behavior of Np(VI) by AHA from kerosene/30% TBP organic phase to HNO3 solution83 shows that the stripping rate is dominated by the kinetic process at the interface. In addition, the reduction kinetics of Np(VI) with AHA was studied with infrared spectroscopy in 1 M HClO4 media.84 When AHA is in excess, the reduction rate equation of Np(VI) is shown below:
| | | −d[Np(VI)]/dt = k[Np(VI)][AHA] | (29) |
where
k = 2.57 × 10
3 M
−1 s
−1 at 10 °C.
When Np(VI) is in great excess relative to AHA, the mechanism of Np(VI) reduction is controlled by two different reactions. The reaction rate is 3.7 × 10−4 and 1.0 × 10−3 s−1 for the first order mechanism at 10 °C and 22 °C, respectively.
2.7 Others
Rao and Choppin explored the reduction kinetics of Np(V) with three methylsalicylic acids (MSA) as shown in Fig. 4 using spectrophotometry.85 Their rate constants decrease in the order of 5-MSA > 3-MSA > 4-MSA, which is attributed to the electronic effect of substituents. Humic acid (HA) is one of the significant species in groundwater. The rate constant for Np(VI) reduction by HA is 59 ± 6 M0.1 min−1 at 10 °C and μ = 0.41.86 Additionally, the reduction kinetics of Np(VI) by H2O2 was studied in a perchloric acid–sodium perchlorate solution.87 The stoichiometric equation was established to be 2Np(VI) + H2O2 = 2Np(V) + 2H+ + O2. The respective rate of Np(VI) reduction by H2O2 is (2.19 ± 0.01) × 103 in 0.05 M Na2CO3 at 25 °C.88 In addition, ethylenediaminetetraacetic acid (EDTA) is a common organic chelating reagent that was studied to reduce Np(VI) in low ionic strength media.89 EDTA can effectively reduce Np(VI) to Np(V) in the presence of organic complexes.
3 Theoretical progress on the reduction of Np(VI) to Np(V) by salt-free reagents
Simulation for accurate prediction of electronic structures and properties as well as the reaction mechanics of actinide systems is one of the most challenging issues in computational chemistry. Nevertheless, significant advances were achieved in the last few decades owing to the development of electronic structure methods for actinides and improvement in computational speed. Our group has theoretically explored the extraction behaviors of the actinide systems and the corresponding electronic structures as well as the bonding characters.90–109 Recently, we have focused on exploring the mechanisms of Np(VI) reduction by some salt-free reagents using scalar-relativistic DFT calculations. All calculations of Np(VI) systems were performed using the B3LYP hybrid functional110,111 within the Gaussian 16 program.112 The scalar-relativistic effective core potentials (RECPs)113 and the ECP60MWB-SEG valence basis set114,115 were applied for Np. The solvation effect was considered by implicit solvation models, solvation model density (SMD)116 or the conductor-like polarizable continuum model (CPCM) approach with Klamt's radii.117,118 Transition states (TSs), initial complexes (ICs) and intermediates (INTs) were confirmed by the harmonic frequency calculations. The calculations of the intrinsic reaction coordinate were carried out to verify the ICs and INTs. The bonding change of the reduction process was analyzed using the quantum theory of atoms-in-molecules (QTAIM),119,120 electron localization function (ELF),121–123 and localized molecular orbitals (LMOs)124 using the Multiwfn.125,126 Analysis of spin density uncovered the oxidation state of the metal atom for ICs, TSs, and INTs, which can elucidate the nature of the reduction reaction.
3.1 Introduction to transition state theory and method
The studies on the mechanisms of Np(VI) reduction by salt-free reagents essentially depend on the transition state theory, which has been successfully applied to actinide systems.38,127–131 Here, we briefly introduce transition state theory. The concepts of potential energy surface and saddle point were originally introduced by Pelzer and Wigner.132 Later, Erying and Polanyi proposed the transition state theory (TST) based on quantum mechanics and statistical mechanics when they studied organic reactions from reactants to products in 1935.133,134 TST includes classic and generalized TSTs. Classic TST is a statistical-mechanical theory of chemical reaction rate according to the no-return assumption and the quasi-equilibrium assumption. That is, transition states are in local equilibrium with reactant molecules and do not return to the reaction region once they fall into the product region. The generalized TST defines the transition state as a hypersurface in phase space and no longer localizes the transition state to the saddle point of the reaction pathway.
3.2 Hydrazine and its derivatives
Hydrazine and its derivatives reduce Np(VI) to Np(V) in the PUREX process, while their reduction mechanisms remain unclear. Therefore, studies on their reduction mechanism of Np(VI) to Np(V) using theoretical approaches are needed. The reduction mechanism of Np(VI) by hydrazine was explored theoretically and three reaction pathways were obtained.36 A free radical mechanism is thermodynamically and kinetically feasible based on the results for energy barrier and thermodynamic energy (Fig. 6). Np(VI) reduction by N2H4 accompanies dissociation of the N–H bond and formation of the Oyl–H bond. To investigate the influence of substituent effects of hydrazine on the reducing ability of Np(VI), the mechanisms of Np(VI) by CHON2H3, HOC2H4N2H3 and CH3N2H3 were explored.43 Their reduction mechanisms are similar, based on the potential energy profiles (PEPs), the order of the energy barrier follows HOC2H4N2H3 (15.15) < CH3N2H3 (16.57) < CHON2H3 (25.73 kcal mol−1) for the free radical ion mechanism, which agrees with the rates of reduction observed in the experiments.
 |
| | Fig. 6 PEP and four TS structures for [NpVIO2(H2O)5]2+ reduction by N2H4. | |
Carbohydrazide (CO(N2H3)2) appears significant for potential applications in reducing Np(VI) to Np(V) in high-burn-up SNF. The mechanism of Np(VI) reduction by CO(N2H3)2 is shown in Fig. 7.45 The calculated energy barrier agrees with the experimental activation energy.66 The value of spin density (Fig. 8) shows that the reduction nature is outer-sphere electron transfer and hydrogen transfer.
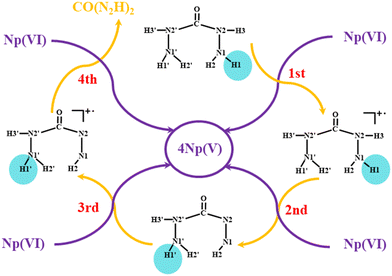 |
| | Fig. 7 Mechanism of Np(VI) reduction by CO(N2H3)2. Reprinted with permission from ref. 45. Copyright 2023, American Chemical Society. | |
 |
| | Fig. 8 Diagrams and value of spin density on the Np atom for the ICs, TSs and INTs of Np(VI) reduction by CO(N2H3)2. Yellow dashed lines depict H-bonds. Reprinted with permission from ref. 45. Copyright 2023, American Chemical Society. | |
Tert-butylhydrazine ((CH3)3CN2H3) has exceptional selectivity in the reduction of Np(VI) in experiments, and the reduction reaction of Np(VI) with (CH3)3CN2H3 was explored using scalar-relativistic DFT.41 During Np(VI) reduction, the free radical ion [(CH3)3CN2H3]+˙ intermediate can achieve another Np(VI) reduction (Fig. 9). The first Np(VI) reduction in pathway I and the second Np(VI) reduction in pathway III is kinetically optimal based on the energy barrier of the pathways. The reduction nature of the first and second Np(VI) reduction for the three pathways is outer-sphere electron transfer and hydrogen transfer, respectively. Moreover, the optimal reduction process from IC2 to TS2 is probably due to the existence of water-mediated proton transfer.
 |
| | Fig. 9 Reduction processes of Np(VI) ions by (CH3)3CN2H3. Reprinted with permission from ref. 41. Copyright 2024, Springer Nature. | |
The short τ99 for the Np(VI) reduction by phenylhydrazine (C6H5N2H3) and hydrazinopropionitrile (NCCH2N2H3) indicates that they quickly reduce Np(VI) to Np(V), which was confirmed by theoretical investigations.37,44 Pathways I and II are the free radical ion mechanism and pathway III is the free radical mechanism for Np(VI) reduction by C6H5N2H3 (Fig. 10(a)). The first Np(VI) reduction by C6H5N2H3 is the rate-determining step with an energy barrier of 12.89 kcal mol–l. LMOs of the structures for pathway II reveal N–H bond dissociation and Oyl–H bond formation. The reduction kinetics of Np(VI) by NCCH2N2H3 was studied. Each NCCH2N2H3 molecule can reduce two Np(VI) ions and the second Np(VI) reduction is the rate-determining step, with an energy barrier of 14.36 kcal mol−1. This is attributed to water-mediated proton transfer.37 The fast reduction kinetics of Np(VI) by NCCH2N2H3 and C6H5N2H3 is attributed to the σ–π hyperconjugation effect and delocalized π-orbital, respectively. The spin density of the Np atom indicates that the reduction of Np(VI) by NCCH2N2H3 arises from outer-sphere electron transfer and hydrogen transfer.
 |
| | Fig. 10 Three PEPs for Np(VI) reduction with C6H5N2H3 and LMOs for the structures of pathway II (a). Reprinted with permission from ref. 45. Copyright 2023, American Chemical Society. PEPs for [NpVIO2(H2O)5]2+ and [NpVIO2(NO3)(H2O)3]+ reduction by CH3N2H3 (b). | |
The stable Np(VI) species are [NpVIO2(H2O)5]2+, [NpVIO2(NO3)(H2O)3]+ and [NpVIO2(NO3)2(H2O)] in 0–14 M HNO3 solution.135 The reduction mechanisms of [NpVIO2(NO3)(H2O)3]+ by CH3N2H3136 and [NpVIO2(NO3)2(H2O)] by N2H4, CHON2H3, and HOC2H4N2H342 were also explored. The reduction of [NpVIO2(H2O)5]2+ by CH3N2H3 has a lower energy barrier compared with that of [NpVIO2(NO3)(H2O)3]+ due to the coordination of nitrate ions (Fig. 10(b)). To further explore the influence of nitrate ions on the energy barrier of Np(VI) reduction, the reactions for [NpVIO2(NO3)2(H2O)] with N2H4, CHON2H3, and HOC2H4N2H3 were also investigated. [NpVIO2(NO3)2(H2O)] reduction by the three reductants needs to overcome the higher energy barrier. Overall, the reduction of Np(VI) becomes more difficult when the nitrate ion coordinates Np(VI).
3.3 Hydroxylamine and its derivatives
Hydroxylamine derivatives are promising salt-free reductants that can reduce Np(VI) to Np(V) in nitric acid solution and their kinetic behavior of Np(VI) to Np(V) were studied experimentally. The reduction mechanism of Np(VI) by diethylhydroxylamine (DEHA) in aqueous solution was explored using scalar-relativistic DFT.39 The whole reduction process of Np(VI) to Np(V) by DEHA occurs via four stages (Fig. 11). For the first stage, the Ho atom of DEHA contacts the Oyl atom of [NpVIO2(H2O)5]2+ and forms Np(V) and free radical (C2H5)2ṄO. Subsequently, the HC atom of free radical (C2H5)2ṄO contacts the Oyl atom of [NpVIO2(H2O)5]2+ and obtains Np(V) and (C2H5)2N(O)C2H4 for the second stage. Then, (C2H5)2N(O)C2H4 is quickly hydrolyzed to mono-substituted C2H5NHOH, which can further reduce Np(VI) via two reaction pathways due to the different participating position of the H atom. The largest energy barrier among the four Np(VI) reduction processes is 12.23 kcal mol−1 for the third stage of pathway I, which indicates that the reduction rate of Np(VI) by DEHA is rapid, agreeing with the experimental observations. The Np–Oyl bond distances and spin density on Np elucidate the reduction essence, including outersphere electron transfer or hydrogen atom transfer.
 |
| | Fig. 11 Reaction of Np(VI) with DEHA. Reprinted with permission from ref. 39. Copyright 2024, The Royal Society of Chemistry. | |
The reduction rates of Np(VI) by hydroxylamine (HA), monomethyl substituted N-methylhydroxylamine (MHA) and dimethyl substituted N,N-dimethylhydroxylamine (DMHA) are different. To evaluate the impact of methyl substitution on the reduction mechanism, the Np(VI) reduction reactions by HA, MHA and DMHA were theoretically investigated (Fig. 12).40 The electron density results indicate that the nature of Np(VI) reduction by HA and the first Np(VI) reduction by methyl-substituted MHA and DMHA are hydrogen atom transfer processes. Meanwhile, the second Np(VI) reduction is outersphere electron transfer, probably due to the electronic effect of methyl substitution. The rate-determining step for MHA and DMHA is the first Np(VI) reduction, Moreover, the energy barrier for the Np(VI) reduction by DMHA is lower than that by MHA, which reflects that the reaction rate of the former is faster than that of the latter. This work provided a kinetic insight into the effect of methyl substitution on the reduction of Np(VI) by hydroxylamine.
 |
| | Fig. 12 Redox process of Np(VI) with HA, MHA and DMHA. Reprinted with permission from ref. 40. Copyright 2025, The Royal Society of Chemistry. | |
3.4 Other related reactions of Np(VI)
As far as we know, there are no theoretical investigations on the Np(VI) reduction by other reductants, except for hydrazine and hydroxylamine and their derivatives as discussed above. Some relevant Np(VI) reactions were explored by applying theoretical approaches, including the water exchange mechanism of neptunyl(VI) aqua ion38,130 and the hydrolysis mechanism of [(CH3)NpVIO2(O2C–CH3)2]−.129 The water exchange of the [NpVIO2(H2O)5]2+ complex can be achieved by dissociative-, associative-, and interchange mechanisms.38 The reaction pathway and the corresponding structures are presented in Fig. 13(a and b). As for the dissociative water exchange mechanism, the water molecule in the second shell gradually deviates from the neptunyl ion in the structure of IC to INT. Concurrently, a water molecule coordinated to Np(VI) shifts out of the initial coordination sphere and forms two hydrogen bonds in the INT with four-coordinated Np(VI) (Fig. 13(a)). As for the associative water exchange mechanism in Fig. 13(b), the six-coordinated Np(VI) complex was formed. The energy barrier for the dissociative water mechanism is 16.73 kcal mol−1, which is obviously larger than that for the associative one (7.17 kcal mol−1), indicating that the latter is more feasible kinetically. The hydrolysis reaction of [(CH3)NpVIO2(O2C–CH3)2]− was investigated in Fig. 13(c).129 [(CH3)NpVIO2(O2C–CH3)2]− with one water molecule forms structure I by two hydrogen bonds, which is characterized as the exothermic reaction with the energy of 9.32 kcal mol−1. Structure I overcomes the energy barrier of 14.10 kcal mol−1 and forms structure II. In this process, the H atom from the H2O molecule transfers to the CH3− fragment coordinated with Np(VI), which leads to the coordination of the hydroxyl group (OH−) with Np(VI) and formation of the methane molecule. Essentially, the hydrolysis reaction of [(CH3)NpVIO2(O2C–CH3)2]− was achieved by the dissociation of the water molecule, leading to the exchange of ligands from the CH3− to OH− group.
 |
| | Fig. 13 Reaction pathway and the corresponding structures for the dissociative (a) and associative (b) water exchange mechanisms for the [NpVIO2(H2O)5]2+ complex. Reprinted with permission from ref. 38. Copyright 2004, American Chemical Society. PEP and the corresponding structures for the [(CH3)NpVIO2(O2C–CH3)2]− hydrolysis reactions (c). Reprinted with permission from ref. 130. Copyright 2018, American Chemical Society. | |
4 Conclusions and outlook
An important aim of the future advanced PUREX process is to create a single cycle reprocessing flowsheet that streamlines plant size, simplifies operations, and minimizes waste. The effective control of the valence state of Np is an important factor in the single cycle design. Although many salt-free reagents have been studied for the Np(VI) reduction, it is not easy to accomplish within the simplified flowsheets favored for an advanced reprocessing plant. Hydrazine and its derivatives, as well as n-butyraldehyde effectively reduce Np(VI) to Np(V) and do not reduce U(VI) and Pu(IV) under specific experimentally conditions, which are suitable for the Np separation. Hydroxylamine and its derivatives, as well as iso-butyraldehyde efficiently and quickly reduce Pu(IV) to Pu(III) and Np(VI) to Np(V), so they are applied to separate Np and Pu from U. The reduction ability of Np(VI) with different salt-free reagents are suitable for different flowsheets, but no single reductant has been applied for the Np(VI) reduction in a single cycle reprocessing flowsheet. Therefore, it is essential to develop effective reductants for the U/Np and Np/Pu separation during SNF reprocessing.
Generally, experimental techniques for the investigation of Np(VI) reduction by salt-free reagents are difficult or dangerous; therefore, theoretical simulation seems to be an effective method to obtain reliable predictions. However, computational approach for accurate prediction of electronic structures and mechanisms of Np(VI) reduction remains a scientific challenge due to their relativistic and electron correlation effects. It requires intensive algorithms and available computational power for large actinide systems. Fortunately, with the rapid development of artificial intelligence, machine learning (ML) and the improvement of computational power, ab initio molecular dynamics (AIMD) and quantum mechanics/molecular mechanics (QM/MM) is applied for Np(VI) reduction by salt-free reagents. ML facilitates high-throughput screening and can obtain the key descriptors between molecular structures and the reduction rate of Np(VI). AIMD can investigate the effect of temperature, solvent and nitric acid concentration on the reduction rate of Np(VI) in the water/organic phase. The QM/MM approach can evaluate local electron transfer between Np(VI) and salt-free reagents. These methods synergistically uncover electronic structure, bonding evolution, thermodynamics and kinetics of Np(VI) reduction by salt-free reagents.
In summary, research on the reduction of Np(VI) by salt-free reagents still faces many challenges. (1) Finding stable efficient salt-free reagents could be achieved by conducting an in-depth study on the reduction mechanism and kinetics of Np(VI) by salt-free reagents, and accurately controlling the amount of salt-free reagents and reaction conditions such as temperature, pH value, reaction time, etc. for quantitative reduction of Np(VI). (2) Np(VI) may form various coordinated complexes in different solutions, which increases the complexity of the Np(VI) reduction process. (3) Np is radioactive: special radioactive analysis techniques and equipment are required. Although the research on the reduction of Np(VI) by salt-free reagents faces many challenges, with the rapid development of science and technology, (1) it is expected to develop more salt-free reagents with excellent performance to achieve accurate and efficient reduction of Np(VI); (2) with the help of advanced spectroscopy technology and actinide quantum chemical calculations, the reaction mechanism of Np(VI) reduction by salt-free reagents is studied in depth, revealing the electron transfer and chemical bond evolution; (3) the environmentally friendly process of Np(VI) reduction by salt-free reagents is optimized to reduce the amount of radioactive substances. In conclusion, the design and development of a new and environmentally friendly process for Np(VI) reduction by salt-free reagents is one of the future directions for the nuclear fuel cycle. In conclusion, the design, synthesis and development of salt-free reagents that specifically reduce Np(VI) is one of the future directions for the nuclear fuel cycle.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Author contributions
Xin Huang and Xiao-Bo Li: writing – original draft. Qun-Yan Wu: writing – original draft, supervision. Wei-Qun Shi: supervision.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22376197, U2441225, 22076188).
References
- R. J. Taylor, I. S. Denniss and A. L. Wallwork, Neptunium control in an advanced Purex process, Nucl. Energy, 1997, 36, 39–46 CAS.
- J. E. Birkett, M. J. Carrott, O. D. Fox, C. J. Jones, C. J. Maher, C. V. Roube, R. J. Taylor and D. A. Woodhead, Recent developments in the Purex process for nuclear fuel reprocessing: Complexant based stripping for uranium/plutonium separation, Chimia, 2005, 59, 898–904 CrossRef CAS.
-
Z. Yoshida, S. G. Johnson, T. Kimura and J. R. Krsul, Neptunium, in The Chemistry of The Actinide and Transactinide Elements, Springer, Dordrecht, 2008, pp. 699–812 Search PubMed.
- R. C. Thompson, Neptunium-The neglected actinide: A review of the biological and environmental literature, Radiat. Res., 1982, 90, 1–32 CrossRef CAS PubMed.
- M. A. Prelas, C. L. Weaver, M. L. Watermann, E. D. Lukosi, R. J. Schott and D. A. Wisniewski, A review of nuclear batteries, Prog. Nucl. Energy, 2014, 75, 117–148 CrossRef CAS.
- R. J. Taylor, C. R. Gregson, M. J. Carrott, C. Mason and M. J. Sarsfield, Progress towards the full recovery of neptunium in an advanced PUREX process, Solvent Extr. Ion Exch., 2013, 31, 442–462 CrossRef CAS.
- H. Zhang, Z. Y. Liu, X. M. Zhou and L. Li, The complex reaction kinetics of neptunium including redox and extraction process in 30% TBP-nitric acid system, J. Radioanal. Nucl. Chem., 2017, 312, 173–180 CrossRef CAS.
- P. K. Verma and P. K. Mohapatra, Fate of neptunium in nuclear fuel cycle streams: State-of-the art on separation strategies, Radiochim. Acta, 2022, 110, 527–548 CrossRef CAS.
- C. Gregson, C. Boxall, M. Carrott, S. Edwards, M. Sarsfield, R. Taylor and D. Woodhead, Neptunium (V) oxidation by nitrous acid in nitric acid, Procedia Chem., 2012, 7, 398–403 CrossRef CAS.
- B. J. Mincher, M. Precek, S. P. Mezyk, G. Elias, L. R. Martin and A. Paulenova, The redox chemistry of neptunium in γ-irradiated aqueous nitric acid, Radiochim. Acta, 2013, 101, 259–266 CrossRef CAS.
- H. Chen, R. J. Taylor, M. Jobson, D. A. Woodhead, C. Boxall, A. J. Masters and S. Edwards, Simulation of neptunium extraction in an advanced PUREX process—Model improvement, Solvent Extr. Ion Exch., 2017, 35, 1–18 CrossRef CAS.
- V. A. Drake, Predicting the behavior of neptunium during nuclear-fuel reprocessing, Nucl. Energy, 1987, 26, 253–258 CAS.
- S. Li, Y. Gao and Y. Ouyang, A review of the kinetics of oxidation and reduction of sale-free reagents in the U/Pu separation process, Nucl. Tech., 2012, 35, 929–935 CAS.
- R. J. Taylor, I. May, V. S. Koltunov, S. M. Baranov, V. I. Marchenko, E. A. Mezhov, V. G. Pastuschak, G. I. Zhuravleva and O. A. Savilova, Kinetic and solvent extraction studies of the selective reduction of Np(VI) by new salt-free reducing agents, Radiochim. Acta, 1998, 81, 149–156 CrossRef CAS.
- R. J. Taylor, I. May, A. L. Wallwork, I. S. Denniss, N. J. Hill, B. Y. Galkin, B. Y. Zilberman and Y. S. Fedorov, The applications of formo- and aceto-hydroxamic acids in nuclear fuel reprocessing, J. Alloys Compd., 1998, 271, 534–537 CrossRef.
- G. Uchiyama, S. Hotoku, S. Fujine and M. Maeda, Reduction of neptunium(VI) by butyraldehyde isomers in nitric acid solution, Nucl. Technol., 1998, 122, 222–227 CrossRef CAS.
- G. Uchiyama, S. Fujine, S. Hotoku and M. Maeda, New separation process for neptunium, plutonium, and uranium using butyraldehydes as reductants in reprocessing, Nucl. Technol., 1993, 102, 341–352 CrossRef CAS.
- V. S. Koltunov, Kinetics and mechanism of redox reactions of Np and Pu Ions with several organic reductants, J. Nucl. Sci. Technol., 2002, 39, 347–350 CrossRef.
- V. S. Koltunov and S. M. Baranov, Organic derivatives of hydrazine and hydroxylamine in future technology of spent nuclear fuel reprocessing, Trans. Am. Nucl. Soc., 1993, 67, 1 Search PubMed.
- X. M. Zhou, G. A. Ye, H. Zhang, L. Li, F. X. Luo and Z. K. Meng, Chemical behavior of neptunium in the presence of technetium in nitric acid media, Radiochim. Acta, 2014, 102, 111–116 CrossRef CAS.
- S. T. Xiao, G. A. Ye, L. Li, X. C. Liu, H. Yang, H. B. Li and Z. W. Yuan, The investigation of the quantitative structure-activity relationships between the structure of hydrazine derivatives and the reduction of Np(VI), J. Radioanal. Nucl. Chem., 2017, 311, 1565–1575 CrossRef CAS.
- V. S. Koltunov and S. M. Baranov, Kinetics and mechanism of Np and Pu reactions with organic derivatives of hydrazine, Inorg. Chim. Acta, 1987, 140, 31–34 CrossRef CAS.
- Y. Ban, T. Asakura and Y. Morita, Reduction kinetics of Pu(IV) and Np(VI) by N,N-dimethylhydrazine, and its potential application in nuclear fuel reprocessing, J. Radioanal. Nucl. Chem., 2009, 279, 423–429 CrossRef CAS.
- H. Yang, H. Zhang, L. Li, X. H. Huang and R. T. Wang, Separation Np from Pu based on reduction-stripping by using hydrazine nitrate as reductant, Nucl. Tech., 2016, 39, 1–8 Search PubMed.
- Z. Y. Liu, H. Zhang, R. T. Wang and Y. Z. Ning, Separation Np from Pu based on reduction-stripping by using methyl hydrazine as reductant, Nucl. Tech., 2017, 40, 1–7 Search PubMed.
- V. S. Koltunov and M. F. Tikhonov, Reduction kinetics of actinoids with hydroxylamine. I. Neptunium (VI) reduction in nitric acid solutions, Radiochemistry, 1977, 19, 611–619 CAS.
- V. S. Koltunov, R. J. Taylor, S. M. Baranov, E. A. Mezhov and I. May, The reduction of plutonium (IV) and neptunium (VI) ions by N,N-ethyl (hydroxyethyl) hydroxylamine in nitric acid, Radiochim. Acta, 1999, 86, 115–122 CrossRef CAS.
- A. Y. Zhang and Y. Liu, Hydroxylamine derivative in Purex process III. The kinetics of oxidation-reduction reaction between N,N-diethylhydroxylamine and neptunium(VI), J. Radioanal. Nucl. Chem., 2000, 245, 357–361 CrossRef CAS.
- A. Y. Zhang, J. X. Hu, X. Y. Zhang and F. D. Wang, Hydroxylamine derivative in the Purex process – Part V. The single-stage reduction extraction and back extraction of neptunium with N,N-diethylhydroxylamine, J. Radioanal. Nucl. Chem., 2002, 253, 107–113 CrossRef CAS.
- Z. Anyun, H. Jingxin, Z. Xianye and W. Fangding, Hydroxylamine derivative in Purex process, J. Radioanal. Nucl. Chem., 2002, 252, 565–571 CrossRef.
- V. S. Koltunov, R. J. Taylor, S. M. Baranov, E. A. Mezhov, V. G. Pastuschak and I. May, The reduction of plutonium and neptunium ions by acetaldoxime in nitric acid, Radiochim. Acta, 2000, 88, 65–70 CrossRef CAS.
- V. S. Koltunov, V. I. Marchenko, G. I. Zhuravleva and O. A. Savilova, Kinetics of redox reactions of U, Pu, and Np in TBP solutions: VII. kinetics of reduction of Pu(IV) and Np(VI) with butanal oxime in undiluted TBP, Radiochemistry, 2001, 43, 334–337 CrossRef CAS.
- V. S. Koltunov, E. A. Mezhov and S. M. Baranov, Kinetics of Np(VI) reduction with butanal oxime, Radiochemistry, 2001, 43, 342–345 CrossRef CAS.
- V. S. Koltunov, R. J. Taylor, S. M. Baranov, E. A. Mezhov, V. G. Pastuschak and G. V. Koltunov, Oxidation reactions of acetaldoxime in nitric acid, J. Nucl. Sci. Technol., 2014, 39, 878–881 CrossRef.
- R. J. Taylor and I. May, The reduction of actinide ions by hydroxamic acids, Czech. J. Phys., 1999, 49, 617–621 CrossRef CAS.
- Z. P. Cheng, Q. Y. Wu, Y. H. Liu, J. H. Lan, C. Z. Wang, Z.
F. Chai and W. Q. Shi, The redox mechanism of NpVI with hydrazine: A DFT study, RSC Adv., 2016, 6, 109045–109053 RSC.
- X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, M. Zhang, J. K. Gibson, Z. F. Chai and W. Q. Shi, Reduction of Np(VI) with hydrazinopropionitrile via water-mediated proton transfer, Phys. Chem. Chem. Phys., 2022, 24, 17782–17791 RSC.
- V. Vallet, T. Privalov, U. Wahlgren and I. Grenthe, The mechanism of water exchange in AmO2(H2O)52+ and in the isoelectronic UO2(H2O)5+ and NpO2(H2O)52+ complexes as studied by quantum chemical methods, J. Am. Chem. Soc., 2004, 126, 7766–7767 CrossRef CAS PubMed.
- X. Huang, X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, H. Q. Wang and W. Q. Shi, Uncovering the reduction mechanism of Np(VI) with N, N-diethyl hydroxylamine: a scalar-relativistic DFT investigation, Phys. Chem. Chem. Phys., 2024, 26, 27395–27405 RSC.
- X. Huang, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, H.-Q. Wang and W.-Q. Shi, Theoretical study on the kinetic behavior of Np(VI) reduction by hydroxylamine and its derivatives: substituent effect, Phys. Chem. Chem. Phys., 2025, 27, 6014–6023 RSC.
- X.-B. Li, M. Zhang, C.-Z. Wang, J.-H. Lan and Q.-Y. Wu, Theoretical insights into the reduction reaction of Np(VI) by tert-butylhydrazine, J. Radioanal. Nucl. Chem., 2025, 334, 995–1006 CAS.
- Z. P. Cheng, X. B. Li, Q. Y. Wu, Z. F. Chai and W. Q. Shi, Theoretical insights into the reduction mechanism of neptunyl nitrate by hydrazine derivatives, Radiochim. Acta, 2022, 110, 471–480 CrossRef CAS.
- X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, S. Y. Ning, Y. Z. Wei, Z. F. Chai and W. Q. Shi, Theoretical study on the reduction mechanism of Np(VI) by hydrazine derivatives, J. Phys. Chem. A, 2020, 124, 3720–3729 CrossRef CAS PubMed.
- X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, M. Zhang, Z. F. Chai and W. Q. Shi, Theoretical insights into the reduction mechanism of Np(VI) with phenylhydrazine, J. Phys. Chem. A, 2021, 125, 6180–6188 CrossRef CAS PubMed.
- X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, M. Zhang, Z. F. Chai and W. Q. Shi, Insights into the reduction mechanisms of Np(VI) to Np(V) by carbohydrazide, J. Phys. Chem. A, 2023, 127, 4259–4268 CrossRef CAS PubMed.
- V. I. Marchenko, K. N. Dvoeglazov and V. I. Volk, Use of redox reagents for stabilization of Pu and Np valence forms in aqueous reprocessing of spent nuclear fuel: Chemical and technological aspects, Radiochemistry, 2009, 51, 329–344 CrossRef CAS.
- V. S. Koltunov, G. I. Zhuravleva, V. I. Marchenko and M. F. Tikhonov, Features of kinetics and mechanism of some oxidation-reduction reactions of Np, Pu and U, J. Inorg. Nucl. Chem., 1976, 189–196 CAS.
- L. Venault, P. Moisy, P. Blanc and C. Madic, Kinetics of hydrazinium nitrate decomposition in nitric acid solutions under the effect of power ultrasound, Ultrason. Sonochem., 2001, 8, 359–366 CrossRef CAS PubMed.
- V. S. Koltunov, S. M. Baranov, E. A. Mezhov and V. G. Pastushchak, Kinetics of reactions of Np and Pu ions with hydrazine derivatives. 14. Hydrazinopropionitrile, Radiokhimiya, 2000, 42, 117–120 Search PubMed.
- A. Y. Zhang, J. X. Hu, X. Y. Zhang and F. D. Wang, Progress of reaction kinetics between organic reductant and Np(VI), Pu(IV), At. Energy Sci. Technol., 2001, 35, 83–90 Search PubMed.
- D. G. Yin, X. Y. Zhang and J. X. Hu, Kinetic study of Np(VI) reduction with 1,1-dimethylhydrazine, He Huaxue Yu Fangshe Huaxue, 1997, 19, 23–27 CAS.
- W. Q. Shi, H. B. Tang, Y. X. Ye, J. X. Hu and X. Y. Zhang, Kinetic study of the Np (VI) reduction by tert-butyl hydrazine, He Huaxue Yu Fangshe Huaxue, 2002, 24, 134–137 CAS.
- X. Y. Zhang, Z. L. Huang, S. T. Xiao and J. X. Hu, Reduction of Np (VI) with 2-hydroxyethylhydrazine I. Studies on the reaction kinetics, At. Energy Sci. Technol., 1997, 32, 433–437 Search PubMed.
- K. N. Dvoeglazov, E. Y. Pavlyukevich and P. V. Mitrikas, Kinetics of Np(VI) reduction with diformylhydrazine in nitric acid, Radiochemistry, 2018, 60, 581–587 CrossRef CAS.
- O. A. Zavalina, K. N. Dvoeglazov, E. Y. Pavlyukevich and S. I. Stepanov, Kinetics of Np(VI) reduction with carbohydrazide in nitric acid, Radiochemistry, 2017, 59, 453–457 CrossRef CAS.
- V. S. Koltunov, E. A. Mezhov and S. M. Baranov, Kinetics of reaction between neptunium (VI) and 3, 3′-bis (diaziridinyl), Radiochemistry, 2000, 42, 65–68 CAS.
- V. I. Marchenko, V. N. Alekseenko and K. N. Dvoeglazov, Organic reductants of Pu and Np ions in wet technology for spent nuclear fuel reprocessing, Radiochemistry, 2015, 57, 366–377 CrossRef CAS.
- V. I. Marchenko, V. S. Koltunov, O. A. Savilova and G. I. Zhuravleva, Redox kinetics of U, Pu, and Np in TBP solutions VI. Reactions of neptunium and plutonium with organic reducing agents determination of the final oxidation states and reaction rates, Radiochemistry, 2001, 43, 276–283 CrossRef CAS.
- V. S. Koltunov, K. M. Frolov and Y. V. Isaev, Kinetics of redox reactions of U, Np, and Pu in TBP solutions: IX. Reduction of Np(VI) with dibenzylhydrazine, Radiochemistry, 2002, 44, 121–126 CrossRef CAS.
- Z. X. Ye, Y. G. An, X. S. Tao, Y. D. Guang and H. J. Xin, Reduction of Np(VI) with monomethylhydrazine I. Studies on reaction kinetics, At. Energy Sci. Technol., 1997, 31, 193–198 Search PubMed.
- Z. X. Ye, Y. G. An, X. S. Tao, Y. D. Guang and H. J. Xin, Reduction of Np(VI) with monomethyhydrazine II. Studies on partition of U-Np in Purex process, At. Energy Sci. Technol., 1997, 31, 315–320 Search PubMed.
- X. Y. Zhang, Z. L. Huang, S. T. Xiao and J. X. Hu, Reduction of Np (VI) with 2-hydroxyethylhydrazine II. Studies on separation of U-Np in Purex process, At. Energy Sci. Technol., 1999, 33, 8–11 CAS.
- D. G. Yin, X. Y. Zhang, J. X. Hu and S. T. Xiao, Separation of neptunium from uranium in contactor 1A with 1,1-dimethylhydrazine, He Huaxue Yu Fangshe Huaxue, 1998, 20, 146–151 CAS.
- C. Li, T. Yan, S. Xiao and W. Zheng, Rate determining step of reduction Np(VI) by organic reagents from 30%TBP/Kerosene to nitrate solution, Radiochim. Acta, 2020, 108, 839–846 CrossRef CAS.
- V. I. Volk, V. I. Marchenko, K. N. Dvoeglazov, V. N. Alekseenko, S. I. Bychkov, E. Y. Pavlyukevich, V. V. Bondin and A. S. D'yachenko, Reduction of Pu (IV) and Np (VI) with carbohydrazide in nitric acid solution, Radiochemistry, 2012, 54, 143–148 CrossRef CAS.
- V. P. Shilov and A. M. Fedoseev, Reduction of Np(VI) with carbohydrazide in a perchloric acid solution, Radiochemistry, 2019, 61, 309–311 CrossRef CAS.
- B. J. Colston, G. R. Choppin and R. J. Taylor, A preliminary study of the reduction of Np(VI) by formohydroxamic acid using stopped-flow near-infrared spectrophotometry, Radiochim. Acta, 2000, 88, 329–334 CrossRef CAS.
- V. I. Marchenko, G. I. Zhuravleva, K. N. Dvoeglazov and O. A. Savilova, Behaviors of plutonium and neptunium in nitric acid solutions containing hydrazine and technetium ions, Theor. Found. Chem. Eng., 2008, 42, 733–739 CrossRef CAS.
- V. S. Koltunov and S. M. Baranov, Organic derivatives of hydrazine and hydroxylamine and future reprocessing of irradiated nuclear-fuel, Radiochemistry, 1993, 35, 622–630 Search PubMed.
- V. S. Koltunov, S. M. Baranov, T. P. Zharova and E. V. Abramina, Reaction-kinetics of Np and Pu ions with hydroxylamine derivatives. 2. Reaction
of Np(VI) with N, N-dimethydroxylamine, Radiochemistry, 1993, 35, 408–412 Search PubMed.
- V. S. Koltunov, S. M. Baranov and T. P. Zharova, Reaction-kinetics of Np and Pu ions with hydroxylamine derivatives. 1. Reaction of Np(VI) with isopropylhydroxylamine, Radiochemistry, 1993, 35, 402–407 Search PubMed.
- V. S. Koltunov and S. M. Baranov, Kinetics of reactions of Np and Pu ions with hydroxylamine derivatives: VIII. Reduction of Np(VI) with N, N-dibutylhydroxylamine, Radiochemistry, 2000, 42, 236–241 CAS.
-
S. M. Baranov, V. S. Koltunov, R. J. Taylor and I. May, Nuclear fuel reprocessing using hydrophilic substituted hydroxylamines, U. S. Pat., US6444182B1, 2002 Search PubMed.
- V. S. Koltunov, S. M. Baranov, R. J. Taylor and T. P. Zharova, Kinetics of reactions of Np and Pu ions with hydroxylamine derivatives. V. Reaction of Np(VI) with N-methyldroxylamine, Radiochemistry, 1993, 35, 71–78 CAS.
- A. Y. Zhang, H. X. Hu, Z. Y. Zhang and F. D. Wang, Hydroxylamine derivatives in Purex Process I. Study on the kinetics of redox reaction between N,N-diethylhydroxylamine and nitrous acid, J. Radioanal. Nucl. Chem., 1998, 230, 235–239 CrossRef CAS.
- A. Y. Zhang, J. X. Hu, X. Y. Zhang and F. D. Wang, Hydroxylamine derivative in Purex process. VI. Study on the partition of uranium/neptunium and uranium/plutonium with N,N-diethylhydroxylamine in the purification cycle of uranium contactor, Solvent Extr. Ion Exch., 2001, 19, 965–979 CrossRef CAS.
- Y. Ban, T. Asakura and Y. Morita, Reduction kinetics of Np(VI) by n-butyraldehyde in tributyl phosphate diluted with n-dodecane, Radiochim. Acta, 2004, 92, 883–887 CrossRef CAS.
- A. Costagliola, L. Venault, A. Deroche, G. Garaix, J. Vermeulen, R. Omnee, F. Duval, G. Blain, J. Vandenborre, M. Fattahi-Vanani and N. Vigier, Radiation chemical behavior of aqueous butanal oxime solutions irradiated with helium ion beams, Radiat. Phys. Chem., 2016, 119, 186–193 CrossRef CAS.
- Z. W. Zhu, J. Y. He, Z. F. Zhang, V. Zhang, J. M. Zhu and W. F. Zhen, Uranium/plutonium and uranium/neptunium separation by the Purex process using hydroxyurea, J. Radioanal. Nucl. Chem., 2004, 262, 707–711 CrossRef CAS.
- T. H. Yan, W. F. Zheng, C. Zuo, L. Xian, Y. Zhang, X. Y. Bian, R. X. Li and Y. Di, The reduction of Np(VI) and Np(V) by tit dihydroxyurea and its application to the U/Np separation in the PUREX process, Radiochim. Acta, 2010, 98, 35–38 CAS.
- Y. Wang, S. Xiao, T. Lan, X. Liu, Y. Ouyang and H. Li, Kinetics of reaction between Np(VI) and hydroxysemicarbazide in nitric acid solution, He Huaxue Yu Fangshe Huaxue, 2018, 40, 359–365 CAS.
- D. Y. Chung and E. H. Lee, The reduction of Np(VI) by acetohydroxamic acid in nitric acid solution, Bull. Korean Chem. Soc., 2005, 26, 1692–1694 CrossRef CAS.
- C. Li, T. Yan, C. Zuo and W. Zheng, Kinetics of reductive stripping of Np(VI) by acetohydroxamic acid from 30% TBP/kerosene to nitrate medium using a high-speed stirred cell, Radiochim. Acta, 2015, 103, 627–634 CrossRef CAS.
-
B. S. Matteson, M. Precek and A. Paulenova, Presented in part at the IOP Conference Series: Materials Science and Engineering, San Francisco, CA, Jul 12–17, 2010 Search PubMed.
- L. F. Rao and G. R. Choppin, Kinetics and mechanism of the reduction of neptunium(VI) by methylsalicylic acids, Radiochim. Acta, 1991, 54, 21–24 CrossRef CAS.
- Y. Z. Chen, B. M. Tan and Z. J. Lin, A kinetic-study of the reduction of Np(VI) with humic-acid, Radiochim. Acta, 1993, 62, 199–201 CrossRef CAS.
- A. J. Zielen, J. C. Sullivan, D. Cohen and J. C. Hindman, A kinetic study of the reduction of neptunium (VI) by hydrogen peroxide, J. Am. Chem. Soc., 1958, 80, 5632–5635 CrossRef CAS.
- M. E. Thompson, K. L. Nash and J. C. Sullivan, Complexes of hydrogen-peroxide with dioxoactinide(VI) species in aqueous carbonate and bicarbonate media-formation of An(VI)-H2O2 Complexes, Isr. J. Chem., 1985, 25, 155–158 CrossRef CAS.
- D. T. Reed, D. G. Wygmans, S. B. Aase and J. E. Banaszak, Reduction of Np(VI) and Pu(VI) by organic chelating agents, Radiochim. Acta, 1998, 82, 109–114 CrossRef CAS.
- J. H. Lan, W. Q. Shi, L. Y. Yuan, Y. L. Zhao, J. Li and Z. F. Chai, Trivalent actinide and lanthanide separations by tetradentate nitrogen ligands: A quantum chemistry study, Inorg. Chem., 2011, 50, 9230–9237 CrossRef CAS PubMed.
- C.-Z. Wang, J.-H. Lan, Y.-L. Zhao, Z.-F. Chai, Y.-Z. Wei and W.-Q. Shi, Density functional theory studies of UO22+ and NpO2+ complexes with carbamoylmethylphosphine oxide ligands, Inorg. Chem., 2013, 52, 196–203 CrossRef CAS PubMed.
- Q.-Y. Wu, Y.-T. Song, L. Ji, C.-Z. Wang, Z.-F. Chai and W.-Q. Shi, Theoretically unraveling the separation of Am(III)/Eu(III): insights from mixed N,O-donor ligands with variations of central heterocyclic moieties, Phys. Chem. Chem. Phys., 2017, 19, 26969–26979 RSC.
- X.-W. Chi, Q.-Y. Wu, C.-Z. Wang, J.-P. Yu, K. Liu, R.-A. Chi, Z.-F. Chai and W.-Q. Shi, A theoretical study of unsupported uranium–ruthenium bonds based on tripodal ligands, Organometallics, 2022, 41, 1304–1313 CrossRef CAS.
- J.-H. Lan, W.-Q. Shi, L.-Y. Yuan, J. Li, Y.-L. Zhao and Z.-F. Chai, Recent advances in computational modeling and simulations on the An(III)/Ln(III) separation process, Coord. Chem. Rev., 2012, 256, 1406–1417 CrossRef CAS.
- Q.-Y. Wu, J.-H. Lan, C.-Z. Wang, C.-L. Xiao, Y.-L. Zhao, Y.-Z. Wei, Z.-F. Chai and W.-Q. Shi, Understanding the bonding nature of uranyl ion and functionalized graphene: A theoretical study, J. Phys. Chem. A, 2014, 118, 2149–2158 CrossRef CAS PubMed.
- Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, C.-L. Xiao, X.-K. Wang, Y.-L. Zhao, Z.-F. Chai and W.-Q. Shi, Theoretical investigation on multiple bonds in terminal actinide nitride complexes, Inorg. Chem., 2014, 53, 9607–9614 CrossRef CAS PubMed.
- Q.-Y. Wu, J.-H. Lan, C.-Z. Wang, Y.-L. Zhao, Z.-F. Chai and W.-Q. Shi, Terminal U≡E (E = N, P, As, Sb, and Bi) bonds in uranium complexes: A theoretical perspective, J. Phys. Chem. A, 2015, 119, 922–930 CrossRef CAS PubMed.
- H. Wu, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-R. Liu, Z.-F. Chai and W.-Q. Shi, New insights into the selectivity of four 1,10-phenanthroline-derived ligands toward the separation of trivalent actinides and lanthanides: A DFT based comparison study, Dalton Trans., 2016, 45, 8107–8117 RSC.
- Q. Y. Wu, J. H. Lan, C. Z. Wang, Z. P. Cheng, Z. F. Chai, J. K. Gibson and W. Q. Shi, Paving the way for the synthesis of a series of divalent actinide complexes: A theoretical perspective, Dalton Trans., 2016, 45, 3102–3110 RSC.
- X.-W. Chi, Q.-Y. Wu, Q. Hao, J.-H. Lan, C.-Z. Wang, Q. Zhang, Z.-F. Chai and W.-Q. Shi, Theoretical study on unsupported uranium–metal bonding in uranium–group 8 complexes, Organometallics, 2018, 37, 3678–3686 CrossRef CAS.
- X.-H. Kong, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-F. Chai, C.-M. Nie and W.-Q. Shi, Insight into the extraction mechanism of americium(III) over europium(III) with pyridylpyrazole: A relativistic quantum chemistry study, J. Phys. Chem. A, 2018, 122, 4499–4507 CrossRef CAS PubMed.
- C.-Z. Wang, T. Bo, J.-H. Lan, Q.-Y. Wu, Z.-F. Chai, J. K. Gibson and W.-Q. Shi, Ultrastable actinide endohedral borospherenes, Chem. Commun., 2018, 54, 2248–2251 RSC.
- Q.-Y. Wu, Z.-P. Cheng, J.-H. Lan, C.-Z. Wang, Z.-F. Chai, J. K. Gibson and W.-Q. Shi, Insight into the nature of M–C bonding in the lanthanide/actinide-biscarbene complexes: A theoretical perspective, Dalton Trans., 2018, 47, 12718–12725 RSC.
- X.-W. Chi, Q.-Y. Wu, J.-H. Lan, C.-Z. Wang, Q. Zhang, Z.-F. Chai and W.-Q. Shi, A theoretical study on divalent heavier group 14 complexes as promising donor ligands for building uranium–metal bonds, Organometallics, 2019, 38, 1963–1972 CrossRef CAS.
- C. Wang, Q.-Y. Wu, X.-H. Kong, C.-Z. Wang, J.-H. Lan, C.-M. Nie, Z.-F. Chai and W.-Q. Shi, Theoretical insights into the selective extraction of americium(III) over europium(III) with dithioamide-based ligands, Inorg. Chem., 2019, 58, 10047–10056 CrossRef CAS PubMed.
- Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-F. Chai and W.-Q. Shi, Electronic structures and bonding of the actinide halides An(TRENTIPS)X (An = Th–Pu; X = F–I): A theoretical perspective, Dalton Trans., 2020, 49, 15895–15902 RSC.
- Z.-R. Ye, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-F. Chai, H.-Q. Wang and W.-Q. Shi, Theoretical insights into the separation of Am(III)/Eu(III) by hydrophilic sulfonated ligands, Inorg. Chem., 2021, 60, 16409–16419 CrossRef CAS PubMed.
- X.-F. Luan, C.-Z. Wang, Q.-Y. Wu, J.-H. Lan, Z.-F. Chai, L.-S. Xia and W.-Q. Shi, Theoretical insights on improving amidoxime selectivity for potential uranium extraction from seawater, J. Phys. Chem. A, 2022, 126, 406–415 CrossRef CAS PubMed.
- X.-P. Lei, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-F. Chai, C.-M. Nie and W.-Q. Shi, Theoretical insights into the substitution effect of phenanthroline derivatives on Am(III)/Eu(III) separation, Inorg. Chem., 2023, 62, 2705–2714 CrossRef CAS PubMed.
- C. T. Lee, W. T. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, F. Ding Williams, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr , J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. B.01., Gaussian Inc., Wallingford, CT, 2016 Search PubMed.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, Energy-adjusted pseudopotentials for the actinides-parameter sets and test calculations for thorium and thorium monoxide, J. Chem. Phys., 1994, 100, 7535–7542 CrossRef.
- X. Cao, M. Dolg and H. Stoll, Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials, J. Chem. Phys., 2003, 118, 487–496 CrossRef CAS.
- X. Y. Cao and M. Dolg, Segmented contraction scheme for small-core actinide pseudopotential basis sets, J. Mol. Struct.:THEOCHEM, 2004, 673, 203–209 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed.
- V. Barone and M. Cossi, Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- J. Cioslowski and S. T. Mixon, Covalent bond orders in the topological theory of atoms in molecules, J. Am. Chem. Soc., 1991, 113, 4142–4145 CrossRef CAS.
- R. F. W. Bader, Atoms in molecules, Acc. Chem. Res., 1985, 18, 9–15 CrossRef CAS.
- A. Savin, R. Nesper, S. Wengert and T. F. Fässler, ELF: The electron localization function, Angew. Chem., Int. Ed. Engl., 1997, 36, 1808–1832 CrossRef CAS.
- A. D. Becke and K. E. Edgecombe, A simple measure of electron localization in atomic and molecular systems, J. Chem. Phys., 1990, 92, 5397–5403 CrossRef CAS.
- B. Silvi and A. Savin, Classification of chemical bonds based on topological analysis of electron localization functions, Nature, 1994, 371, 683–686 CrossRef CAS.
- J. Pipek and P. G. Mezey, A fast intrinsic localization procedure applicable for ab initio and semiempirical linear combination of atomic orbital wave functions, J. Chem. Phys., 1989, 90, 4916–4926 CrossRef CAS.
- T. Lu and F. W. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu, A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn, J. Chem. Phys., 2024, 161, 082503 CrossRef CAS PubMed.
- L. Castro, A. Yahia and L. Maron, A DFT study of the reactivity of actinidocenes (U, Np and Pu) with pyridine and pyridine N-oxide derivatives, Dalton Trans., 2010, 39, 6682–6692 RSC.
- M. Sundararajan, R. S. Assary, I. H. Hillier and D. J. Vaughan, The mechanism of the reduction of [AnO2]2+ (An = U, Np, Pu) in aqueous solution, and by Fe(II) containing proteins and mineral surfaces, probed by DFT calculations, Dalton Trans., 2011, 40, 11156–11163 RSC.
- P. D. Dau, D. Rios, Y. Gong, M. C. Michelini, J. Marçalo, D. K. Shuh, M. Mogannam, M. J. Van Stipdonk, T. A. Corcovilos, J. K. Martens, G. Berden, J. Oomens, B. Redlich and J. K. Gibson, Synthesis and hydrolysis of uranyl, neptunyl, and plutonyl gas-phase complexes exhibiting discrete actinide–carbon bonds, Organometallics, 2016, 35, 1228–1240 CrossRef CAS.
- F. P. Rotzinger, Quantum chemical study of the water exchange mechanism of the
neptunyl(VI) and -(V) aqua ions, Inorg. Chem., 2018, 57, 2425–2431 CrossRef CAS PubMed.
- N. Shan, Q. Wang, H. Xiao, L. Wan and T. Gao, Ab initio molecular dynamics study, the reaction mechanism and topological properties of the microscopic interaction of PuO2 and H2O, ChemistrySelect, 2022, 7, 202104589 CrossRef.
- H. Pelzer and E. Wigner, Über die Geschwindigkeitskonstante von Austauschreaktionen, Z. Phys. Chem., Abt. B, 1932, 15, 445–471 Search PubMed.
- H. Eyring, The activated complex and the absolute rate of chemical reactions, Chem. Rev., 1935, 17, 65–77 CrossRef CAS.
- M. G. Evans and M. Polanyi, Some applications of the transition state method to the calculation of reaction velocities, especially in solution, Trans. Faraday Soc., 1935, 31, 0875–0893 RSC.
- P. Lindqvist-Reis, C. Apostolidis, O. Walter, R. Marsac, N. L. Banik, M. Y. Skripkin, J. Rothe and A. Morgenstern, Structure and spectroscopy of hydrated neptunyl(VI) nitrate complexes, Dalton Trans., 2013, 42, 15275–15279 RSC.
- X. B. Li, Q. Y. Wu, C. Z. Wang, J. H. Lan, M. Zhang and W. Q. Shi, Theoretical perspectives on the reduction of Pu(IV) and Np(VI) by methylhydrazine in HNO3 solution: Implications for Np/Pu separation, Chin. Chem. Lett., 2024, 35, 109359 CrossRef CAS.
Footnote |
| † Xin Huang and Xiao-Bo Li contribute equally to this work. |
|
| This journal is © Institute of Process Engineering of CAS 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a and
Wei-Qun
Shi
*a and
Wei-Qun
Shi
 *ab
*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO2 ratio and temperature. For example, the concentration of Np(VI) is enhanced at higher HNO3 concentrations, lower HNO2 concentrations and higher temperatures.9 Salt-free reagents are considered to be promising for Np(VI) reduction because they can be decomposed into gases, such as N2, CO2 and H2O.13 The reduction behaviors of Np(VI) with many salt-free reagents have been explored by the groups of Taylor,1,6,14,15 Uchiyama,16,17 Koltunov18,19 and Ye.20,21 A variety of salt-free reductants can effectively reduce Np(VI) to Np(V), such as hydrazine,14,22–25 aldehydes,16,17 hydroxylamine,26–30 oxime,31–34 hydroxamic acid15,35 and their derivatives. Recently, we systematically explored the Np(VI) reduction using hydrazine, hydroxylamine and its derivatives and elucidated their reduction mechanisms by employing density functional theory (DFT).36–45 Koltunov and co-workers reported the reaction kinetics of Np and Pu ions using hydrazine, organic nitriles, hydroxylamine, monoximes, diaziridinylethane and their derivatives.18,20,21 Marchenko et al. described the characteristics of organic reductants that can keep Np and Pu in a specific oxidation state.46 Although much research has focused on Np(VI) reduction by salt-free reagents, there is no review on the subject. Noticeably, some salt-free reagents can reduce Np(VI) to Np(V) and reduce Np(V) to Np(IV). In this review, we mainly elucidate the experimental and theoretical progress on the reduction of Np(VI) to Np(V) by salt-free reagents. We hope that this review will serve as a useful resource to inspire further research on Np(VI) reduction by salt-free reagents in the reprocessing of SNF.
HNO2 ratio and temperature. For example, the concentration of Np(VI) is enhanced at higher HNO3 concentrations, lower HNO2 concentrations and higher temperatures.9 Salt-free reagents are considered to be promising for Np(VI) reduction because they can be decomposed into gases, such as N2, CO2 and H2O.13 The reduction behaviors of Np(VI) with many salt-free reagents have been explored by the groups of Taylor,1,6,14,15 Uchiyama,16,17 Koltunov18,19 and Ye.20,21 A variety of salt-free reductants can effectively reduce Np(VI) to Np(V), such as hydrazine,14,22–25 aldehydes,16,17 hydroxylamine,26–30 oxime,31–34 hydroxamic acid15,35 and their derivatives. Recently, we systematically explored the Np(VI) reduction using hydrazine, hydroxylamine and its derivatives and elucidated their reduction mechanisms by employing density functional theory (DFT).36–45 Koltunov and co-workers reported the reaction kinetics of Np and Pu ions using hydrazine, organic nitriles, hydroxylamine, monoximes, diaziridinylethane and their derivatives.18,20,21 Marchenko et al. described the characteristics of organic reductants that can keep Np and Pu in a specific oxidation state.46 Although much research has focused on Np(VI) reduction by salt-free reagents, there is no review on the subject. Noticeably, some salt-free reagents can reduce Np(VI) to Np(V) and reduce Np(V) to Np(IV). In this review, we mainly elucidate the experimental and theoretical progress on the reduction of Np(VI) to Np(V) by salt-free reagents. We hope that this review will serve as a useful resource to inspire further research on Np(VI) reduction by salt-free reagents in the reprocessing of SNF.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HA derivatives is (6–8)
HA derivatives is (6–8)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, the corresponding stoichiometric coefficient decreases to (0.5–2)
1. However, the corresponding stoichiometric coefficient decreases to (0.5–2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 when the HA derivatives are in excess.57
1 when the HA derivatives are in excess.57![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– double bond (Fig. 4). Acetaldoxime is an example of the monoximes, which is considered a valuable reagent for Np/U separation in the advanced PUREX process. Koltunov and coworkers investigated the reduction rate for the reaction of Np(VI) and acetaldoxime in HNO3 solution:
N– double bond (Fig. 4). Acetaldoxime is an example of the monoximes, which is considered a valuable reagent for Np/U separation in the advanced PUREX process. Koltunov and coworkers investigated the reduction rate for the reaction of Np(VI) and acetaldoxime in HNO3 solution: