Open Access Article
Open Access ArticleEvaluation of three solvent-based recycling pathways for circular polypropylene†
Benjamin
Caudle
 *,
Thuy T. H.
Nguyen
*,
Thuy T. H.
Nguyen
 and
Sho
Kataoka
and
Sho
Kataoka

National Institute of Advanced Industrial Science and Technology, Japan 305-8565. E-mail: ben-caudle@aist.go.jp
First published on 10th December 2024
Abstract
Solvent-based methods for recycling polyolefin plastic waste have caught increasing attention, as they can produce recycled plastic of significantly higher quality than currently employed techniques. In this study, to demonstrate the development of plastic recycling systems for a circular economy, three solvent-based processes used for recycling polypropylene (PP), one of the most widely used plastic materials in Japan, were rigorously modeled and analyzed in terms of economic performance and CO2 emissions. A cradle-to-gate life cycle assessment-based method was applied to quantify all sources of CO2 emissions comprehensively. The most common solvent-based recycling method, in which the polymer is dissolved in a solvent and precipitated with an antisolvent, had the lowest economic performance and produced the highest CO2 emissions: 1.30 kg of CO2-equivalent per kg rPP. A more recently developed process in which the temperature of the solvent is manipulated to effect dissolution and precipitation had lower CO2 emissions, at 0.92 kg kg−1 rPP, and the most promising economic performance. A novel process using supercritical propane as the solvent achieved the lowest emissions of 0.32 kg kg−1 rPP with similar economic performance to the temperature-dependent separation process. The environmental competitiveness (in terms of CO2 emissions) of these recycling processes is further investigated by comparison with alternative state-of-the-art methods of plastic waste disposal, including mechanical recycling, gasification, and incineration with thermal recovery. Sensitivity studies were carried out to explore the effect of the waste plastic feed composition resulting from different preparation (sorting) methods on the economic and environmental performance of the three solvent-based recycling processes. The results obtained from this study are expected to provide valuable insights for constructing a green and cost-effective PP recycling process toward a circular economy.
1. Introduction
The nature and benefits of plastic materials are well known to society and the scientific community, as are the problems of plastic disposal and leakage into the environment. To address these problems, governments around the world are slowly moving to restrict the ways in which plastic waste is disposed of.1 In Japan, only 6% of plastic waste collected in 2022 was disposed of in landfills.2 However, two thirds of plastic waste is incinerated, and although a large percentage of energy is used for power or heat co-generation, the incineration process produces detrimental emissions and fails to achieve the resource circulation target.3 It has become particularly relevant, then, to find methods of disposal that eliminate the flow of plastic waste into landfills, reduce the amount of “virgin” plastics being manufactured from fossil resources, and reduce the amount of planet-warming greenhouse gases being released. This work focuses on polypropylene (PP), the polymer that comprises the largest single fraction of post-consumer plastic waste in Japan.2 Many recycling methods are available that contribute to the three goals described above, but rigorous analyses that address product circularity, carbon footprint, and economic feasibility are rare.Mechanical recycling of PP is technically feasible and may be practical for certain types of waste streams of homogeneous PP, such as scraps from industrial production.4 In the post-consumer recycling process, targeted polymers are physically separated into ostensibly homogeneous streams, using relatively small amounts of chemicals and energy.5 However, with the notable exception of clear bottles made of polyethylene terephthalate (PET), it has proved to be prohibitively difficult to collect and sort acceptably pure streams of post-consumer waste plastic.6 PP found in post-consumer waste includes a multitude of unidentified additives that are undesirable in a virgin polymer7 and is often combined with other polymers and non-polymers in products that make them impossible to separate mechanically (i.e. multilayer packaging).8 Even with state-of-the-art optical sorting technology, it is not possible to physically separate a significant portion of post-consumer PP waste into a stream that will produce a product of equal quality to the virgin polymer.9 As a result, the products of mechanical recycling are always, to some degree, of lower quality than the original polymer and must be “downcycled” to less valuable uses.10 Larrain et al. have recently performed a detailed technoeconomic analysis on mechanical recycling of various polymers, including PP, and determined that none are economically feasible without government assistance or a drastic rise in oil prices to make them competitive with conventionally produced polymers.5
In contrast to mechanical recycling, chemical recycling breaks down polymers to their building blocks so that they can be inserted into the conventional polymerization route. There are two broad categories: the first breaks a single type of plastic into its constituent monomers via solvolysis. With some exceptions, such as PET, polyurethane, and nylon, effective chemicals for depolymerization have yet to be found for most plastics.11 Some recent work indicates that supercritical water, CO2, or ionic liquids may be suitable for depolymerization of polypropylene, but the information available at these early stages is insufficient to design an industrial process based on this technology.12 The second category of chemical recycling is pyrolysis or gasification. These methods have the advantage of accepting a wide range of plastic waste with a high level of contamination and converting them into chemical feedstock.13 This is effective at limiting waste but does not contribute directly to circularity, as the products of pyrolysis have been found to be unsuitable for polyolefin production,14 and the process to convert gasification products back to polymers is energy-intensive and has a low overall yield.15 The feed must also be carefully sorted to remove polyvinyl chloride (PVC) and PET, reducing the advantages of the relative insensitivity to feed composition.13b Energy-efficient methods to reduce polymers into their monomers via chemical means are still in their investigative stages. For example, Dong et al. have proposed a process in which PP is broken down into monomers via electrified heating.16 This process has a higher rate of conversion from polypropylene to propene monomers, at 36 wt%, than that of catalyzed pyrolysis, at 10–25 wt%, but requires a high-purity feed and is not suitable for scale-up as it depends on the area of the electrical heating medium. In summary, while chemical recycling of polypropylene shows promise as a method for generating circular polymers in the future, the current state of the art is better suited to making value-added chemicals, such as methanol,15b than returning a polymer to its original state.
Solvent-based recycling, technically a form of mechanical recycling, has many similarities to chemical recycling. It involves the dissolution of waste polymers but does not break them into monomers, so the overall polymeric structure of the plastic is not altered. Solvents can be chosen to dissolve a certain polymer at a given temperature, even if multiple polymers are physically attached, allowing a mixed feed to be separated into relatively pure product streams.8,17 Since the polymers are separated at the molecular level, most additives may also be extracted or left out of the extraction, resulting in a product with near-virgin purity.18 There are several proposed methods within the overall category of solvent-based recycling, and this work examines the three most studied methods that differ in terms of how they control the solubility of polymers within the solvents. The solvent–antisolvent (SA) method uses a solvent at a favorable temperature to dissolve the plastic, then adds an antisolvent and lowers the temperature to induce precipitation.19 The benefit of this method is the facile separation of solid and liquid phases, but the need to separate the solvent and antisolvent for reuse is a major drawback.20 The temperature-swing (TS) method uses a solvent, similar to the SA method, but induces precipitation through lowering the temperature alone.8,17b,21 This requires more energy to change the temperature than the SA method but uses much less solvent and is simpler overall. The supercritical solvent (SS) method utilizes a solvent above its critical temperature and pressure to dissolve the polymer and then lowers the temperature and/or pressure to induce precipitation.18,22 As long as the bulk of the solvent is maintained in the supercritical state, the heating and energy demands are not excessive, and the volatile solvent is easily removed from the product when it returns to near-atmospheric pressure before extrusion.
Alternative solvent-based methods, such as those using switchable-hydrophobicity solvents23 or ionic liquids,12b have been proposed in the literature but are insufficiently studied to build a robust process model for design and analysis.
Many efforts to compare polymer recycling methods have been made recently, including a thorough comparison of several plastics and methods by Uekert et al.24 Such reviews are useful in their broad scope but fail to take into account variations within classes of recycling methods – such as the three different solvent-based methods discussed here. Most studies concerning solvent-based recycling methods are based on experiments concerning one or a few solvents and focus on proof of concept.17,19,25 Little interest is paid to how these solvents would be utilized in an industrial setting, and measuring recovery is prioritized over solubility or process efficiency. Other reviews focus on the design and optimization of a single method, comparing it to one or a few alternatives.13a An example is the modeling effort of Nordahl et al., which thoroughly assesses the greenhouse gas emissions of solvent–antisolvent and mechanical recycling but neglects economic factors and alternative recycling methods.26
This work employs detailed process simulations to generate data for technoeconomic and environmental analysis of three solvent-based alternatives for recycling waste PP. Beginning with a stream of visually sorted post-consumer plastic waste typical of Japan,2 the input can be varied based on the degree of pre-sorting performed.27 Subsequently, the detailed model includes selective dissolution, precipitation, and recovery of the plastic and recovery/purification of the solvent(s). The analysis includes carbon footprint and economic factors, which indicate the environmental impact and commercial viability of these processes for circular polypropylene production.
2. Methodology
Three processes for solvent-based recycling of PP were modeled using the process simulator Aspen Plus.28 The feed of each model is an identical mixture of sorted plastic waste, based on a study of optical sorting of typical municipal waste in Japan carried out by Kawai et al.27a The functional unit of product is a mass of recycled PP (rPP) that has been extruded into pellets for export from the plant boundary.2.1 Process description
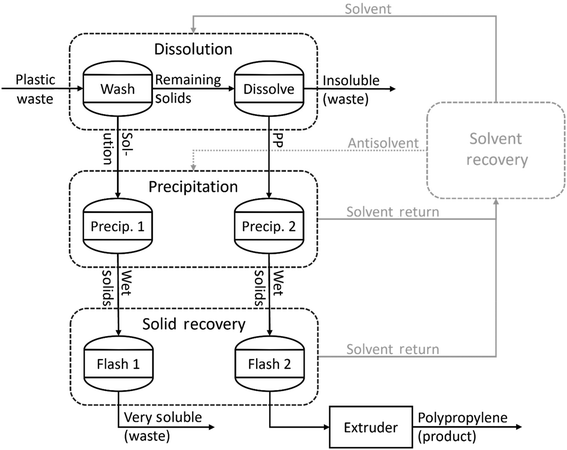
The three processes are based on accounts of laboratory and pilot scale experiments,8,17b,19c,19d,22 as well as patents,29 and are described by the generic process flow diagram in Fig. 1. In the base case, the nominal PP waste stream is expected to contain approximately 79 wt% PP, 10 wt% polyethylene (PE), and 11 wt% others (including plastics and non-plastics) after undergoing physical sorting.27a The mixed PP waste is fed into one of three vessels that cycle through the dissolution process, shown as two separate steps in the process diagram. First, the solids are washed for 30 minutes with a hot solvent at a temperature that will not dissolve PP, removing soluble plastics other than PP and contaminants such as additives. Second, the remaining solids are brought into contact with the hot solvent for 30 minutes at a higher temperature that will dissolve PP but not the other plastics or contaminants. The vessel is then emptied and prepared for the next batch of plastic feed.The solutions from the washing and dissolution steps are conveyed to the precipitation section, which consists of two pairs of vessels that alternate between precipitation and solid removal. In the first vessel, the conditions of the washing solution are altered to precipitate as many of the soluble materials collected in the wash step as possible, which are filtered out and collected. In the second vessel, the conditions of the dissolution solution are altered to precipitate PP, which is filtered out and collected. The recovered solids are transferred to the solid recovery step, where the soluble solids and PP are flashed under various conditions to remove excess solvent. The conditions of the PP recovery are carefully controlled to recover 99 wt% PP with less than 1 wt% solvent. The solvent streams from the recovery section are combined and sent to the solvent recovery section, where they are prepared to be reused. The flash step heats the PP product to 221 °C, well above the melting point, so that it can be sent directly to the extruder, where it is pelletized for export from the plant boundary.
In the solvent–antisolvent (SA) and temperature swing (TS) processes, the washing fluid is xylene at 100 °C and the dissolution fluid is xylene at 140 °C. This allows PE and soluble contaminants to be extracted in the wash step and leaves various insoluble plastics and contaminants. In the supercritical solvent (SS) process the washing fluid is propane at 110 °C and 250 bar and the dissolution fluid is propane at 140 °C and 250 bar. This extracts various soluble contaminants in the wash step and leaves PE and other insoluble contaminants. In the SA process, precipitation is achieved by mixing the wash and dissolution solutions with an antisolvent (hexane) at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane
1 hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylene volume ratio and lowering the temperature to 40 °C. The solvent and antisolvent are then separated via distillation, while the solvent remaining in the PP product is removed via vacuum flash. The operating conditions of the solvent recovery column for the SA process are manipulated so that the recovered xylene and hexane each have a purity of at least 97 wt%. In the TS process, precipitation is achieved by lowering the temperature of the wash and dissolution solutions to 30 °C. The xylene remaining in the PP product is removed via vacuum flash. The solvent is simply filtered before returning to the dissolution section. In the SS process, precipitation is achieved by reducing the pressure of the solutions to 200 bar and reducing the temperature of the wash solution to 80 °C and the dissolution solution to 100 °C. All outlet streams are flashed to atmospheric pressure, effectively removing propane from the PP product stream and soluble/insoluble waste streams. The propane is recompressed to be returned to the dissolution section. Table 1 summarizes the differences between the three processes, and detailed process flow diagrams are included in the ESI.†
xylene volume ratio and lowering the temperature to 40 °C. The solvent and antisolvent are then separated via distillation, while the solvent remaining in the PP product is removed via vacuum flash. The operating conditions of the solvent recovery column for the SA process are manipulated so that the recovered xylene and hexane each have a purity of at least 97 wt%. In the TS process, precipitation is achieved by lowering the temperature of the wash and dissolution solutions to 30 °C. The xylene remaining in the PP product is removed via vacuum flash. The solvent is simply filtered before returning to the dissolution section. In the SS process, precipitation is achieved by reducing the pressure of the solutions to 200 bar and reducing the temperature of the wash solution to 80 °C and the dissolution solution to 100 °C. All outlet streams are flashed to atmospheric pressure, effectively removing propane from the PP product stream and soluble/insoluble waste streams. The propane is recompressed to be returned to the dissolution section. Table 1 summarizes the differences between the three processes, and detailed process flow diagrams are included in the ESI.†
| Solvent–antisolvent | Temperature swing | Supercritical solvent | ||
|---|---|---|---|---|
| Dissolution | Wash | Xylene, 100 °C | Xylene, 100 °C | Propane, 110 °C, 250 bar |
| Dissolution | Xylene, 140 °C | Xylene, 140 °C | Propane, 140 °C, 250 bar | |
| Precipitation | Waste | Add hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), 40 °C 1 ratio), 40 °C |
Reduce temp., 30 °C | Lower T & P to 80 °C, 200 bar |
| PP | Add hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), 40 °C 1 ratio), 40 °C |
Reduce temp., 30 °C | Lower T & P to 100 °C, 200 bar | |
| Solvent recovery | Distillation | Recompression | ||
| PP purification | Vacuum flash | Vacuum flash | Atmospheric flash | |
2.2 Modeling methodology
The processes were modeled in Aspen Plus. Solubility and liquid entrainment were calculated for each wash, dissolution, and precipitation unit operation block based on literature values for PP and PE in xylene19c and supercritical propane.30 Since the exact slopes of the solubility curves are not known, the solubility of PE in xylene was set to 15 wt% at temperatures of 100 °C and above, and the solubility of PP in xylene was set to 18 wt% at 140 °C and above. The maximum solubilities are higher than these, but beyond this point the solution becomes excessively viscous for fluid handling equipment. The solubility of ‘soluble materials’ in propane was set to 15 wt% at 250 bar and 100 °C and above, and the solubility of PP in propane was set to 20 wt% at 250 bar and 140 °C and above. In all processes, the solubility of components outside of those conditions was set to 0.1 wt%, reflecting a low level of dissolved solids that remain circulating within the process. The amount of solvent used in a given step was a further 25% in excess of the amount needed to achieve complete dissolution of the target solid, to account for process fluctuations and uncertainty in the solubility measurements. The process is robust enough to handle polymers of various molecular weights, as the dissolution and precipitation measurements were carried out on polymers (including post-use waste) with molecular weights that varied over multiple orders of magnitude.19 For expediency, the polymers were approximated by oligomers made up of 100 monomer units.Liquid entrainment with completely insoluble solids leaving the dissolution section is assumed to be 5 wt%. The amount of xylene–hexane antisolvent mixture and supercritical propane entrained with solids leaving the precipitation section is assumed to be 20 wt%. Due to swelling of the polymer, the solids leaving the TS precipitation section are assumed to have a large solvent retention, 60 wt% xylene before vacuum devolatilization. Since solvent entrainment is typically not discussed in the literature, these are considered to be safe overestimates based on the available data and unpublished experiments.8,20,22 A large amount of solvent loss is avoided by repressurizing the solvent from the devolatilization stage and returning it to the process. The amount of solvent remaining in the product and waste streams after devolatilization is calculated based on the vapor–liquid equilibrium of the solvent and polymer, as the high temperatures involved cause the polymer to melt.
The conventional solvent models use the Redlich–Kwong (RK) equation of state for the vapor phase and polymer-NRTL (p-NRTL) for the condensed phase. The supercritical solvent model uses the polymer Soave–Redlich–Kwong (p-SRK) equation of state for the fluid phases. These models are widely used in academia and industry for polymers and hydrocarbons, in case of p-NRTL with RK, and such systems at high temperatures and pressures, in the case of p-SRK.
2.3 Evaluation methodology
The processes were compared based on their carbon footprint, measured in CO2-equivalent emissions, and the potential economic benefits of operating a commercial-scale facility. The facility is designed to process 5 tons of plastic waste per hour, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year with downtime included. The process boundary, illustrating the inputs and outputs to the process model, is shown in Fig. 2. Sorting is indicated as a separate step, as its associated emissions were calculated based on the literature (multiple sources), but it was not modeled in this work.
000 tons per year with downtime included. The process boundary, illustrating the inputs and outputs to the process model, is shown in Fig. 2. Sorting is indicated as a separate step, as its associated emissions were calculated based on the literature (multiple sources), but it was not modeled in this work.
The CO2-equivalent emissions of each recycling process were calculated by multiplying the amounts of material and energy that pass across the process boundary by the CO2-equivalent emission factors given in Table 2. These factors include the emissions from the production of raw materials, power generation, and transportation. The emission factor for electricity, 0.439 kg of CO2-equivalent per kWh, is based on the most recent data published by the Japan Electric Power Information Center (JEPIC). The emission factor for collection and preliminary sorting of plastic waste, 179 CO2-equivalent per kW h, is taken from a study by Uekert et al.24 and the (proprietary) emissions factors for other raw materials produced in Japan are from the IDEA database.31 The factors for cooling water and steam are calculated from the processes used to create them, namely cooling tower systems that rely on electricity for circulating pumps and natural gas-fired steam boilers. The CO2 emissions due to plastic waste collection and sorting are obtained from literature sources, namely the collection data compiled by Uekert et al.24 and the mechanical sorting process designed by Larrain et al.5
| Input/output (unit) | Price ($ per unit) | Emission factor (kg CO2 per unit) |
|---|---|---|
| a From the METI energy report.32 b From the UN Comtrade database.33 c From Turton et al.34 d From Uekert et al.24 e From the METI commodities report.35 f From the JEPIC annual report.36 g From the IDEA database (proprietary).31 h From Tonegawa Sangyo Co., Ltd.37 | ||
| Electricity (kW h) | 0.12a | 0.439f |
| Steam (GJ) | 10.82 | 84.5 |
| Cooling water (GJ) | 0.354c | 1.3 |
| Plastic waste (ton) | 330h | 179d |
| Xylene (ton) | 368e | |
| Hexane (ton) | 543e | |
| Liquid propane (ton) | 870b | |
The results of three alternative PP disposal methods – mechanical recycling, gasification followed by reforming PP via methanol synthesis, and thermal recovery – were compared to the results of the solvent-based recycling methods. Lifecycle inventory data were taken from literature studies of these processes, and the common CO2 emission factors shown in Table 2 were applied for the evaluation. The process inventory for mechanical recycling was described by Uekert et al.24 based on an industry survey performed by Franklin Associates.38 The process inventory for gasification/reformation was put together based on the waste polyolefin to methanol process designed by Prifti et al.15b and the methanol to polyolefin process designed by Kuusela et al.15a The process inventory for the manufacture of virgin PP, used to calculate avoided emissions from the production of rPP, was based on a study of polymer production in Japan by Narita et al.39 The net emissions of thermal recovery include the emissions from collecting and burning waste plastic without sorting and the avoided emissions from replacing natural gas as a source of energy.
The quality of recycled PP reported in the literature varies widely from source to source. For the accounting of avoided emissions, an estimate is needed for the mass of virgin PP that can be replaced by rPP. All sources agree that mechanical recycling of PP results in a product of lower quality, and the replacement ratio of 0.7 (kg of rPP per kg of virgin PP) used in this study was based on the relative quality estimated by Uekert et al.24 and matches the lower end of the substitution ratio reported in the ESI of Nordahl et al.26 Several sources report that the products of solvent recycling are of near-virgin quality.17b,19a,20,40 As such, the solvent-recycled PP is given a replacement factor of 0.99. The gasification/reformation method produces virgin polymer, which gives it a 1.0 replacement ratio.
The economic comparison of the three solvent-based processes is based on the net present value (NPV) and the minimum sale price (MSP) of the produced rPP. NPV is a function of the capital expenses (CAPEX), operating expenses (OPEX), operating income from the sale of rPP (OPIN), and economic factors (such as internal rate of return, depreciation structure, tax rate, etc.) (ECON), over the number of years of operation, n:
| NPV = NPV(CAPEX, OPEX, OPIN, and ECON)n |
The MSP is the price of rPP needed to achieve the break-even NPV at the end of the final year of plant operation, N:
| NPV(CAPEX, OPEX, OPIN(MSP), and ECON)N = 0 |
More detailed equations for calculating NPV and intermediate variables can be found in the ESI† and section II of Turton et al.34 The capital expenses (CAPEX) are calculated based on the sizing information of the major equipment (i.e., pressure vessels, heat exchangers, pumps, compressors, and columns), which are obtained from the process simulation. The equipment costs were updated to reflect prices in 2022, the most recent year for which price data are consistently available, with a Chemical Engineering Plant Cost Index (CEPCI) value of 816.41
The operating expenses (OPEX) are calculated mainly based on the costs of raw materials and utilities. The price of xylene and hexane are estimated based on the most recent industry statistics published by the Ministry of Economy, Trade and Industry of Japan (METI),35 while the price of liquefied propane is estimated from information in the UN Comtrade Database,33 based on the average value per unit of imports from the largest sources of propane in 2022. The cost of steam is calculated based on burning natural gas to provide the heat energy and a small amount of electricity for running equipment. The cost of cooling water is taken from Turton et al.34 and was not updated, since the cost is very low and the prices have not changed drastically enough for an accurate adjustment to be deemed worthwhile. The cost of electricity is the nationwide average from the most recent data published by METI.32
For the base case calculation of NPV, the price of rPP is fixed at the MSP for the lowest-performing process (893 $ per ton). It is compared to the price of virgin PP, roughly 1500 $ per ton.35 The waste stream containing mostly PE was treated as “plastic waste”, and assumed to be passed on or resold for the same price as the plastic waste feed (recouping some of the cost of collection).
Based on the number of large pieces of equipment and solid handling sections, labor was estimated to include 6 operators per shift. The boundary was placed at the plant gate, thus excluding the costs and emissions from the transport, storage, and use of the product. The NPV and MSP were calculated based on a typical process lifespan of 15 years. The capital expenses are invested over 3 years up to “year zero”, and then depreciated linearly over the first 5 years of operation. Additional financial considerations for base case evaluation are a discount rate of 10%, typical for a process that has been well-studied with some commercial implementation, and an annual tax rate of 40%.
3. Results
3.1 Base case comparison
Each process achieves the desired throughput and purity, but the energy and material requirements vary considerably between the processes. A comparison of significant energy and mass flows can be seen in Table 3.| Metric | Solvent–antisolvent | Temperature swing | Supercritical solvent |
|---|---|---|---|
| Feed rate | 5000 kg h−1 waste | 5000 kg h−1 waste | 5000 kg h−1 waste |
| Makeup solvent | 26 kg h−1 xylene | 58 kg h−1 xylene | 10 kg h−1 propane |
| 5 kg h−1 hexane | |||
| Product rate | 3977 kg h−1 | 3988 kg h−1 | 3978 kg h−1 |
| 99.1 wt% PP | 98.9 wt% PP | 99.1 wt% PP | |
| (Wet) solid waste | 1054 kg h−1 | 1070 kg h−1 | 1032 kg h−1 |
| Process heating | 1.36 GJ h−1 | 5.07 GJ h−1 | 1.89 GJ h−1 |
| Product melting | 2.58 GJ h−1 | 6.12 GJ h−1 | 1.22 GJ h−1 |
| Distillation column | 45.30 GJ h−1 | — | — |
| Solvent pumps | 0.5 kW | 0.1 kW | 218 kW |
| Compressors | 52 kW | 273 kW | 117 kW |
The main source of solvent loss in the SA process is the retentate of the dissolution step (the insoluble components), which is almost entirely xylene. The other two boundary-crossing streams, for very soluble (PE) components and PP, have lower rates of entrainment in the polymer product, thanks to the addition of the antisolvent. After undergoing flash devolatilization little solvent is lost, so the amount of makeup hexane is less than its amount of circulation in the system would suggest. On the other hand, without an antisolvent to reduce polymer swelling, a large amount of xylene is retained in the soluble (PE) and PP streams of the TS process. This leads to more solvent loss, especially via the less rigorously separated soluble (PE) stream, and more energy required to maintain the vacuum as a proportionally large amount of solvent is recovered. The SS process can recover almost all of the entrained solvent due to the high volatility of propane at elevated temperature and atmospheric pressure.
All three processes require a significant amount of electrical energy, 600 kW, to wash and sort the plastic waste prior to feeding it to the solvent recycling process. The SA process requires large amounts of medium-pressure steam to operate the column reboiler and low-pressure steam to change the temperature of the large amounts of solvent and antisolvent involved, 11.7 MJ kg−1 rPP in total. The TS process has much lower medium- and low-pressure steam requirements, 6.1 MJ kg−1 rPP, reflecting only the need to heat and cool xylene. Both conventional solvent processes use vacuum devolatilization to separate solvent from the PP product, which requires high pressure steam to heat the mixture to a temperature that allows for separation at a reasonable pressure. Because the TS process has much higher solvent entrainment in the solid products, it requires considerably more high-pressure steam to heat the product for flash devolatilization, 1.5 MJ kg−1 rPP as opposed to 0.6 MJ kg−1 rPP for the SA process, and uses much more electrical energy, 0.068 kW h kg−1 rPP as opposed to 0.013 kW h kg−1 rPP, to maintain the vacuum.
The SS process uses the least steam, 0.8 MJ kg−1 rPP, as the temperature changes within the process are smaller than those in the conventional processes. The total power draw of the compressor and pumps is higher than those of conventional solvent processes, at 0.084 kW h kg−1 rPP, although it is within the same order of magnitude. While the solvent circulation pumps require much more energy to cycle between 200 and 250 bar within the process, the amount of energy required by the compressors in the devolatilization section is similar between the processes. The TS process has the highest compressor duty because of the large amount of entrained solvent, and the power required to compress propane from atmospheric pressure to 250 bar is only over twice that needed to compress xylene from 0.02 bar to atmospheric pressure (as in the SA process).
Fig. 3 summarizes the CO2-equivalent emissions of the three processes operating under the base case conditions. The CO2 emissions from steam generation far outweigh those of the electricity for the two conventional processes. This is primarily because so much more energy is needed to change the temperature of the solvent than is needed to transport it, even when vacuum recompression is considered. The other major sources of emissions are the collection of post-consumer waste and pre-sorting of the plastic waste feed. These are consistent factors in any polymer waste disposal methods, as can been seen in the comparison in the following section.
Fig. 4 compares the capital and operating costs of the three processes under base case conditions. Excluding contingency and fees, 41% of the cost of the SA process comes from the equipment associated with the solvent recovery column, and 19% comes from heat exchangers due to the need to heat and cool large amounts of solvent and antisolvent. Pumps and compressors make up 31% of the cost of the TS process because of the large amount of vacuum needed to remove the entrained solvent from the solid product. Both conventional solvent processes have a large expenditure related to vessels, which include the dissolution and precipitation vessels, and all must be made of stainless steel to handle xylene and hexane. The TS process requires larger, more expensive vessels and compressors to handle the vacuum flash of a large amount of solvent. The SS process has the highest vessel cost, due entirely to the high pressures involved. Other equipment costs are low due to the low demands for heating and relatively small pressure changes within the process.
Operating costs are dominated by the cost of raw materials, that is, the collection and preliminary sorting of plastic waste, making up 72–91% of the total. While electricity is more expensive per unit of energy, the small amount used results in a cost similar to that of steam, decreasing relatively for the heat-intensive SA process and increasing for the more electricity-intensive SS process. Maintenance, which is directly related to capital costs, is a larger factor for the SS process.
The NPV calculations for the base case processes can be seen in Fig. 5; using an assumed sales price of 893 $ per ton of rPP, the MSP for the worst-performing (SA) process. For reference, the base-case MSP of the TS process is 788 $ per ton of rPP while that of the SS process is 792 $ per ton of rPP. Since all processes produce rPP at the same rate and the same quality, the NPV depends on the capital and operating expenses. Even though the SA process has the lowest capital expenses, the higher operating expenses cause it to be the least profitable of the base case processes. The SS process has the highest capital expenses, but slightly lower operating expenses result in a NPV at the end of plant operation of $14.6 million, similar to the value of $15.4 million for the relatively cheaper TS process. As the assumed sales price of rPP is well under the approximately 1500 $ per ton price of virgin PP, and the quality should be nearly equivalent, each of these processes should be competitive with the manufacture of PP from fossil resources.
3.2 Comparison of emissions with those of other recycling methods
The emissions of the solvent-based recycling processes in their base case configuration were compared to those of literature data for mechanical recycling,24 gasification/reformation,15 and thermal recovery.39Fig. 6 presents the CO2-equivalent emissions of each process on the basis of 1 kg of PP waste. Each of these processes produces a product: rPP for the recycling processes, PP for gasification/reformation and thermal energy for thermal recovery. Accordingly, each process is credited with negative emissions to represent the replacement of products produced via conventional means: the fossil fuel route for PP and natural gas for thermal energy.The solvent-based recycling methods all have negative net emissions roughly in line with the value of mechanical recycling, −0.96 kg of CO2-equivalent per kg rPP. The SA process has higher net emissions at −0.69 kg kg−1, while the TS and SS processes are significantly lower at −1.48 kg kg−1 and −1.67 kg kg−1. While mechanical recycling has lower positive emissions than all but the SS process, the lower quality of the rPP produced results in a significantly lower replacement ratio to virgin PP and thus a smaller credit for avoided emissions. The solvent-based recycling methods receive full credit for replacing PP with essentially virgin-quality rPP. The values for positive emissions are in line with the relative magnitudes calculated by a similar study that compared mechanical and conventional solvent (SA) recycling to manufacturing of PP in the United States.26 In that study, the solvent recycling (SA) process had approximately 3 times the emissions of mechanical recycling, but approximately 30% less than the conventional manufacture of PP (2.01 kg CO2-equivalent per kg PP).
Gasification/reformation and thermal recovery both perform poorly in terms of CO2-equivalent emissions. The process of gasification followed by reformation requires significantly more energy and material inputs, such as pure oxygen, but the driving factor of the high emissions is the low conversion of waste PP to recycled PP, around 35% (calculated based on previous studies).15 As a result, the majority of the avoided emissions come from steam and other byproducts rather than replacing PP from fossil resources. In contrast, while thermal recovery of 1 kg of PP waste avoids 2.21 kg of CO2 from burning an energy-equivalent amount of methane, PP releases 3.14 kg of CO2 and emits an additional 0.18 kg of CO2 collecting the waste material. On the other hand, these two disposal methods have the advantage of being able to accept relatively dirty plastic waste streams with a highly variable composition. If PP is not the desired product, and so low yield is not an issue, gasification has potential as a more circular alternative to thermal recovery for streams that are not suitable for other recycling methods.
With the current modeling and evaluation methodologies, the CO2-equivalent emissions of the alternative recycling processes were comprehensively evaluated. The rPP obtained from the solvent-based recycling processes have relatively high quality as indicated by previous studies,17b,20 but further investigation is required to measure the various quality indicators used by plastics manufacturers, including the melt flow index, tensile strength, elongation, etc. to ensure its potential application as a substitute for virgin PP.
3.3 Sensitivity study
The three solvent recycling processes were subjected to a sensitivity study based on the composition of plastic waste supplied to the solvent recycling process. A low-PP case was considered where a typical plastic waste stream was not sorted optically and only subjected to float/sink separation in addition to shredding and washing. This method results in a combined PP/PE stream with approximately 40 wt% PP, 58 wt% PE, and 2 wt% other materials. A high-PP case was also considered, where optically sorted plastics are subjected to float/sink separation to yield a stream with 87 wt% PP, 11 wt% PE, and 2 wt% other materials. Although there is a wide variety of additives (plasticizers, dyes, etc.) included in plastic products, it was assumed that these materials would have either high- or low-solubility behavior, and be found in either the wash or insoluble streams. Thus, these two variants in addition to the base case were considered to be good indicators for a demonstration of the ability of the solvent recycling models to handle varying feeds.Fig. 7 compares the overall CO2-equivalent emissions, MSP, and economic performance of the three processes with three different feed compositions. As the impact of waste collection on CO2 emissions is significant and based on the total mass of plastic waste collected, it follows that having more non-PP components in the feed increases the emissions per kg rPP. The conventional solvent processes require additional solvent to wash out PE, causing further increases in emissions and costs in the low-PP (high PE) case. PE is not soluble in supercritical propane under the process conditions, so not as much solvent is needed for the SS process when the PP content is low. The result is that the separation energy used more closely correlates with the amount of PP in the feed and can be reduced as the feed content decreases. The MSP correlates closely with the operating costs, which in turn are dependent on waste collection and energy usage, and so has a similar trend to the CO2 emissions. Unlike the emissions, however, there are fewer differences between the three methods in terms of MSP. This is mainly due to the outsize effect of waste collection on the operating costs reducing the impact of the differences between the processes, but also the fact that, while heat energy and electricity have a similar cost, the CO2 emissions associated with electricity are much lower than those of direct heating in Japan. The net result is that the MSP of the TS and SS processes are very close when the PP content of the feed is high. It is significant to note that all values of MSP are below the estimated selling price of virgin PP at 1.5 $ per kg, indicating that all cases have high potential to be economically competitive as an alternative to manufacturing from fossil resources. The 40 wt% PP-feed cases are the worst-performing, but might be expanded at relatively low cost to include the production of high-quality recycled PE for an additional income stream. The present analysis assumes that the plastic waste remaining after PP is removed can be sold at roughly the same price as was paid for collection, which is why the MSP of rPP is noticeably lower than the operating costs for the 40 wt% PP cases.
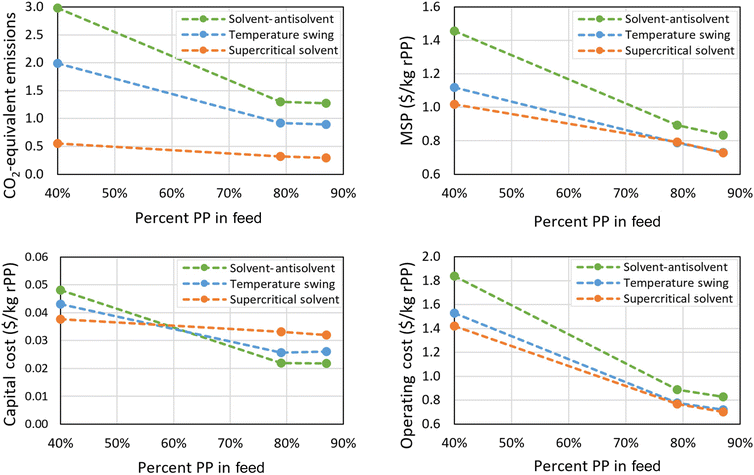 | ||
| Fig. 7 CO2-equivalent emissions, MSP, and distributed CAPEX and OPEX for solvent-based recycling processes with varying feed compositions. | ||
The capital cost also shows noteworthy behavior. The size of the SS plant is closely linked to the amount of PP in the feed, as opposed to the combined PP and PP content, so the cost per kilogram remains fairly constant across the three feed compositions. The conventional solvent processes must allocate equipment to handle PE in the feed, and so the cost per kg of PP produced increases with the amount of PE in the feed. The 79 wt% PP case also stands out: because of the high content of the other components in the feed, the capital costs of the SS process are slightly higher than they would be in order to ensure that any potentially soluble components are removed in the wash. This causes a deviation in what would otherwise be a nearly linear pattern in the capital and operating costs. While the MSP is driven by operating costs, capital costs have the most noticeable effect when the quality of the feed is very low, giving the supercritical solvent process an advantage in terms of both capital and operating expenses for the case with 40 wt% PP in the feed. This advantage would be somewhat negated if PE was targeted as an additional product. PE only dissolves in supercritical propane at very high temperatures and pressures, so the process could only recover pure PE at significant additional expense.
4. Conclusions
The three processes presented here show the potential of solvent-based recycling to address polypropylene waste disposal in a way that reduces overall CO2 emissions, promotes circularity, and is economically viable. All processes were superior to the conventional method of waste PP disposal via thermal recovery in terms of CO2-equivalent emissions (net −1.67 to −0.69, versus 1.11 kg kg−1 PP waste), as well as the competing circular polymer route of gasification/reformation (net 2.13 kg kg−1 PP waste). However, the solvent–antisolvent process was the worst-performing in terms of both CO2 emissions and process economics. It performed worse in terms of CO2-equivalent emissions than estimates for mechanical recycling (net −0.69 vs. −0.96 kg kg−1 PP waste). The supercritical solvent method had the lowest net emissions and good economic performance, similar to that of the single-solvent temperature swing method.The two broad classes of solvent-based recycling processes have benefits and drawbacks with regard to process and product safety. The supercritical solvent process involves a volatile, flammable solvent at high temperature and pressure, which requires extra caution when designing processes for safe operation. On the other hand, xylene has a larger impact on human health and so additional processing may be needed to reduce it to acceptable levels within the product. Propane has less of a health impact and is, as a more volatile molecule, easier to bring within acceptable limits. The operation of commercial supercritical solvent-based plastic recycling facilities, by PureCycle, for example, is evidence that these safety hazards can be acceptably mitigated.
While this study was limited to comparing the CO2 emissions of the six alternative disposal methods, it is important to consider the quality of waste a process can accept, the type of product that it produces, and the cost. Mechanical recycling requires relatively expensive equipment for handling and sorting solids but produces a product with lower quality and thus has low potential for circularity with current state-of-the-art technology. Gasification can accept a stream with high heterogeneity and produce a stream of useful chemicals, including virgin-quality polymers, but their low conversion back into polyolefins makes this method poor in terms of emissions and circularity. The larger process footprint and energy usage compared to solvent-based methods also bode poorly for the cost performance. Thermal recovery can cheaply deal with highly contaminated and mixed plastic waste but produces high net emissions and requires continued production of PP from fossil resources. Further modelling of these processes is needed to better understand the economic characteristics and potential emission reductions when they are applied to a variety of waste streams. For example, it is likely that mechanical recycling will continue to be the optimal method for dealing with homogeneous waste such as industrial cuttings. On the other hand, solvent recycling might be best applied to streams with a few different plastics, such as the light polyolefin stream from float-sink separation. The most irreversibly mixed or contaminated streams may be better subjected to gasification to recover a circular product or thermal recovery to cheaply dispose of the waste.
The most significant unknown for this study is the quality of the rPP product. Experimental and commercial rPP processes report “near-virgin quality”, but there is an enormous variety in the composition of plastic waste a process could encounter. This work attempts to reduce uncertainty with the two-step dissolution process targeting PP, which separates out common polymers and metals, but there is a possibility that some additives will have the same solubility characteristics as PP. There are further characteristics, such as the melt flow index, tensile strength, and elongation at break, with relevance and target values that vary depending on the user. These characteristics are inherent to the original waste polymer and, according to the literature, are virtually unchanged by solvent-based recycling, but will need to be studied in subsequent research to verify these claims and find a more cohesive set of desired qualities. Rather than tailoring a recycling process to specifically separate out plastics based on varying characteristics, the combination of physically attached plastics, and the types of additives, it would likely be more cost-effective to regulate the plastic characteristics, combinations, and additives allowed in plastic of a given type. In pursuit of a circular economy, some small limitations on functionality may be necessary to achieve fully recyclable plastic waste streams. An ongoing dialogue between plastic manufacturers, existing recycling companies, and government entities, a part of this ongoing project, should steer the direction of research and policy for circular polymers going into the future.
Data availability
This article utilized data from the published literature cited therein, and data generated by models are included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Council for Science, Technology and Innovation (CSTI), the Cross-ministerial Strategic Innovation Promotion Program (SIP), and the third period of the SIP “Circular Economy System” (Funding agency: Environmental Restoration and Conservation Agency) (JPJ012290).References
- P. J. Landrigan, H. Raps, M. Cropper, C. Bald, M. Brunner, E. M. Canonizado, D. Charles, T. C. Chiles, M. J. Donohue, J. Enck, P. Fenichel, L. E. Fleming, C. Ferrier-Pages, R. Fordham, A. Gozt, C. Griffin, M. E. Hahn, B. Haryanto, R. Hixson, H. Ianelli, B. D. James, P. Kumar, A. Laborde, K. L. Law, K. Martin, J. Mu, Y. Mulders, A. Mustapha, J. Niu, S. Pahl, Y. Park, M. L. Pedrotti, J. A. Pitt, M. Ruchirawat, B. J. Seewoo, M. Spring, J. J. Stegeman, W. Suk, C. Symeonides, H. Takada, R. C. Thompson, A. Vicini, Z. Wang, E. Whitman, D. Wirth, M. Wolff, A. K. Yousuf and S. Dunlop, Ann. Glob. Health, 2023, 89, 23 CrossRef PubMed.
- Plastic Waste Management Institute (PWMI), Plastic Products, Plastic Waste and Resource Recovery, 2024, vol. 53 Search PubMed.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Green Chem., 2022, 24, 8899–9002 RSC.
- M. Baker, M. Stewert, S. Padibjo, C. Stavropoulos, R. Campbell and T. Boseley, An investigation into the technical and market feasibility of recycling post-consumer polypropylene, Polysearch Pty., Ltd, 2005 Search PubMed.
- M. Larrain, S. Van Passel, G. Thomassen, B. Van Gorp, T. T. Nhu, S. Huysveld, K. M. Van Geem, S. De Meester and P. Billen, Resour., Conserv. Recycl., 2021, 170, 105607 CrossRef CAS.
- F. Welle, Resour., Conserv. Recycl., 2011, 55, 865–875 CrossRef.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef.
- M. Xanthos, Science, 2012, 337, 700–702 CrossRef CAS PubMed.
- S. A. Stoian, A. R. Gabor, A.-M. Albu, C. A. Nicolae, V. Raditoiu and D. M. Panaitescu, J. Therm. Anal. Calorim., 2019, 138, 2469–2480 CrossRef CAS.
- (a) M. Goto, J. Jpn. Pet. Inst., 2016, 59, 254–258 CrossRef CAS; (b) K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS; (c) M. Hong and E. Y. X. Chen, Green Chem., 2017, 19, 3692–3706 RSC.
- (a) T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS; (b) M. Mohan, J. D. Keasling, B. A. Simmons and S. Singh, Green Chem., 2022, 24, 4140–4152 RSC.
- (a) R. R. Bora, R. Wang and F. You, ACS Sustainable Chem. Eng., 2020, 8, 16350–16363 CrossRef CAS; (b) S. Kumagai, J. Nakatani, Y. Saito, Y. Fukushima and T. Yoshioka, J. Jpn. Pet. Inst., 2020, 63, 345–364 CrossRef CAS.
- B. Erkmen, A. Ozdogan, A. Ezdesir and G. Celik, Polymers, 2023, 15, 859 CrossRef CAS PubMed.
- (a) K. Kuusela, V. Uusitalo, J. Ahola and J. Levänen, J. CO2 Util., 2021, 52, 101672 CrossRef CAS; (b) K. Prifti, A. Galeazzi and F. Manenti, Ind. Eng. Chem. Res., 2023, 62, 6167–6175 CrossRef CAS.
- Q. Dong, A. D. Lele, X. Zhao, S. Li, S. Cheng, Y. Wang, M. Cui, M. Guo, A. H. Brozena, Y. Lin, T. Li, L. Xu, A. Qi, I. G. Kevrekidis, J. Mei, X. Pan, D. Liu, Y. Ju and L. Hu, Nature, 2023, 616, 488–494 CrossRef CAS PubMed.
- (a) R. C. Gorre and T. P. Tumolva, IOP Conf. Ser. Earth Environ. Sci., 2020, 463, 012070 CrossRef; (b) J. Yu, A. d. C. Munguía-López, V. S. Cecon, K. L. Sánchez-Rivera, K. Nelson, J. Wu, S. Kolapkar, V. M. Zavala, G. W. Curtzwiler, K. L. Vorst, E. Bar-Ziv and G. W. Huber, Green Chem., 2023, 25, 4723–4734 RSC.
- S. Ugduler, K. M. Van Geem, M. Roosen, E. I. P. Delbeke and S. De Meester, Waste Manage., 2020, 104, 148–182 CrossRef CAS.
- (a) J. G. Poulakis and C. D. Papaspyrides, Resour., Conserv. Recycl., 1997, 20, 31–41 CrossRef; (b) G. Pappa, C. Boukouvalas, C. Giannaris, N. Ntaras, V. Zografos, K. Magoulas, A. Lygeros and D. Tassios, Resour., Conserv. Recycl., 2001, 34, 33–44 CrossRef; (c) D. S. Achilias, C. Roupakias, P. Megalokonomos, A. A. Lappas and E. V. Antonakou, J. Hazard. Mater., 2007, 149, 536–542 CrossRef CAS PubMed; (d) D. S. Achilias, A. Giannoulis and G. Z. Papageorgiou, Polym. Bull., 2009, 63, 449–465 CrossRef CAS.
- Y. B. Zhao, X. D. Lv and H. G. Ni, Chemosphere, 2018, 209, 707–720 CrossRef CAS PubMed.
- P. Zhou, J. Yu, K. L. Sánchez-Rivera, G. W. Huber and R. C. Van Lehn, Green Chem., 2023, 25, 4402–4414 RSC.
- R. E. Martini, E. A. Brignole and S. E. Barbosa, Polym. Eng. Sci., 2009, 49, 602–612 CrossRef CAS.
- T. Mumladze, S. Yousef, M. Tatariants, R. Kriūkienė, V. Makarevicius, S.-I. Lukošiūtė, R. Bendikiene and G. Denafas, Green Chem., 2018, 20, 3604–3618 RSC.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- A. J. Hadi, G. F. Najmuldeen and K. B. Yusoh, J. Polym. Eng., 2013, 33, 471–481 CAS.
- S. L. Nordahl, N. R. Baral, B. A. Helms and C. D. Scown, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2306902120 CrossRef CAS PubMed.
- (a) M. Kawai, J. Nakatani, K. Kurisu and Y. Moriguchi, Resour., Conserv. Recycl., 2022, 180, 106162 CrossRef CAS; (b) J. Lim, Y. Ahn, H. Cho and J. Kim, Process Saf. Environ. Prot., 2022, 165, 420–430 CrossRef CAS.
- Aspen Plus V14 (40.0.0.359), Aspen Technology, Inc., 2022 Search PubMed.
- (a) E. B. Nauman and J. C. Lynch, Polymer recycling by selective dissolution, US Pat., US5278282, 1994 Search PubMed; (b) J. M. Layman, D. I. Collias, H. Schonemann and K. Williams, Method for purifying reclaimed polypropylene, 246015 A1, 2019 Search PubMed.
- (a) P. D. Condo, E. J. Colman and P. Ehrlich, Macromolecules, 1992, 25, 750–753 CrossRef CAS; (b) P. D. Whaley, H. H. Winter and P. Ehrlich, Macromolecules, 1997, 30, 4882–4886 CrossRef CAS.
- National Institute of Advanced Industrial Science and Technology, Inventory Database for Environmental Analysis (IDEA), 2024, https://idea-lca.com/ Search PubMed.
- Agency for Natural Resources and Energy, 4. Trends in Secondary Energy, 2022 Search PubMed.
- United Nations, 2022, vol. 2022, https://comtradeplus.un.org/.
- R. Turton, R. C. Bailie, W. B. Whiting, J. A. Sheaiwitz and D. Bhattacharyya, Analysis, Synthesis, and Design of Chemical Processes, Prentice Hall, Tokyo, 4th edn, 2012 Search PubMed.
- Research and Statistics Department, Ministry of Economy, Trade and Industry (METI), 2020.
- D. Ukita, R. Sato, M. Nabeshima, M. Hirano, Y. Masaki, S. Sakamoto, T. Watanabe, T. Okamura, T. Yamamoto, K. Kodama and K. Takahashi, The Electric Power Industry in Japan, Japan Electric Power Information Center, Inc., 2022 Search PubMed.
- Y. Tonegawa, Industry Information, Tonegawa Sangyo Co./Blog, 2023, https://www.tonegawa-s.co.jp/blog/industry/waste-plastic-kg/#rtoc-10 Search PubMed.
- F. Associates, The Association of Plastic Recyclers, 2018 Search PubMed.
- N. Narita, M. Sagisaka and A. Inaba, Int. J. Life Cycle Assess., 2002, 7, 277–282 CrossRef CAS.
- (a) E. Feick, B. Heller, T. Kaiser, S. Ruf, R. Schleich, B. Bungert, A. Thiele and G. Gorski, Method for separating at least one selected polymer from a mixture of polymers, United States, C08K 5/17 edn, 2004 Search PubMed; (b) J. M. Layman, M. Gunnerson, E. B. Bond, H. Schonemann and K. Williams, Reclaimed polypropylene composition, The Proctor & Gamble Company, United States, C08F 110/06 edn, 2017/0002119 A1, 2017 Search PubMed.
- CEPCI, Chemical Engineering, 2023 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc02646b |
| This journal is © The Royal Society of Chemistry 2025 |