Association between fatty acids and female infertility: dual evidence from a cross-sectional study and Mendelian randomization analysis†
Qiaorui
Yang
 ab,
Jing
Tao
c,
Shengxiao
Jia
c and
Zhenliang
Fan
*de
ab,
Jing
Tao
c,
Shengxiao
Jia
c and
Zhenliang
Fan
*de
aDepartment of Gynecology, Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
bGuanghua Clinical Medical College, Shanghai University of Traditional Chinese Medicine, Shanghai, China
cHeilongjiang University of Chinese Medicine, Harbin, China
dNephrology Department, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
eAcademy of Chinese Medical Science, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China. E-mail: fanmlov@sina.cn
First published on 2nd December 2024
Abstract
Background: Infertility poses a considerable threat to female reproductive health on a global scale. Dietary pattern, as a modifiable lifestyle factor, is frequently recommended as an important intervention for infertility-related diseases. Fatty acids play a crucial role in maintaining the health of the female reproductive system. However, the available evidence on the specific relationship between various types of fatty acids and infertility remains insufficient and controversial. Methods: Initially, a cross-sectional study was conducted utilizing the National Health and Nutrition Examination Survey (NHANES) database to collect data from women aged 18–45 years who met the inclusion criteria across the 2013–2020 cycles. Infertility was defined based on information gleaned from reproductive questionnaires. Fatty acid intake was determined by analyzing two 24 hour dietary recall interviews. Weighted logistic regression and weighted restricted cubic spline (RCS) analyses, incorporating covariate adjustments, were employed to preliminarily delineate the association between various types of fatty acids and proportions of fatty acid intake and female infertility risk. Model performance evaluation was carried out through receiver operating characteristic (ROC) curve analysis, complemented by the utilization of a nomogram diagram to gauge the infertility risk attributed to covariates. Genetic instrumental variables pertinent to diverse fatty acid profiles and female infertility were sourced from genome-wide association studies (GWAS). Mendelian randomization (MR), multivariable MR (MVMR) and reverse MR analyses were subsequently used to ascertain causality and reverse causality between distinct fatty acids and infertility, concurrently assessing for heterogeneity and horizontal pleiotropy. Results: In our NHANES analysis, a total of 3159 women were enrolled in the study, with a self-reported infertility prevalence of 11.49%. Infertile women exhibited significantly elevated intake of total omega-6 and omega-6/total fatty acids (TFA) compared to the controls. Weighted logistic regression models confirmed positive correlations between total omega-6 (continuous) and omega-6/TFA (categorical) and infertility risk, while omega-3 (continuous) intake demonstrated a negative correlation. Model 2, post rigorous multivariate covariate adjustment, showed improved predictive performance according to ROC curve analysis. Subgroup analysis suggested that the positive correlation between omega-6/TFA (continuous) and female infertility risk was not affected by stratification. Total omega-6 (continuous) emerged as a risk factor for infertile women aged 18–34 years. However, total saturated fatty acids (TSFAs, continuous), total omega-3 (continuous) and total polyunsaturated fatty acids (PUFAs, categorical) were protective factors only in the infertile women with a BMI ≥ 25 kg m−2. The positive associations between total omega-6 (Q4) and omega-6/TFA (continuous and Q3–Q4) and infertility risk were consistent across all BMI subgroups. MR analysis employing inverse variance weighted (IVW) as the primary method and Bonferroni correction revealed that genetically predicted TSFAs, monounsaturated fatty acids (MUFAs), omega-6 and MUFA/TFA were positively associated with female infertility risk, whereas PUFA/TFA showed a negative association. Importantly, the positive associations between MUFAs and omega-6 and infertility risk remained robust even after adjusting for potential confounders using MVMR analyses. Reverse MR analysis did not provide any evidence for reverse causality. The MR-Egger regression intercept and Cochran's Q test did not detect any heterogeneity or horizontal pleiotropy. Conclusions: This study presents compelling evidence to substantiate the link between diverse fatty acids, particularly omega-6 PUFAs, and the risk of female infertility. However, to fully comprehend the potential mechanisms and impact of distinct fatty acids and their compositional ratios on female infertility, extensive future research spanning fundamental and large-scale clinical inquiries is imperative.
1 Introduction
Infertility, defined as the inability to achieve a clinical pregnancy outcome following a minimum of 12 months of regular and unprotected intercourse, or 6 months for women aged 35 and older,1,2 affects an estimated 8–12% of reproductive-age couples globally and represents a significant public health concern.3 The etiology of infertility is multifaceted and intricate, encompassing unilateral or bilateral factors in both sexes.4 Of these factors, female-related causes, such as polycystic ovary syndrome (PCOS), pelvic inflammatory disease (PID), endometriosis, and obstructed fallopian tubes, contribute to approximately 43% of cases of infertility.5,6 In the USA, about 11% of married women exhibit impaired fecundability.7 Disturbingly, global statistics show that the age-standardized prevalence of female infertility increased by 14.962% from 1990 to 2017, with an annual change of 0.377%.8 The harm of infertility is not only reflected in the huge psychological and social distress it causes, but also extends to the substantial economic burdens placed on patients and healthcare systems. Therefore, it is crucial to reduce the social costs and public health burden associated with infertility, as well as to identify preventable and modifiable risk factors, which hold significant clinical implications and guidance.As an adjustable factor in daily life, dietary pattern plays an important role in maintaining the normal function of the reproductive system.9 Numerous previous studies have highlighted the link between abnormal consumption of certain foods, such as fatty acids (FAs), whole milk, animal protein, carbohydrate-rich diets, alcohol and caffeine, and impaired fertility.10–13 These dietary choices and their associated nutrients may impact female fertility and ovulation by disrupting endocrine and metabolic balance, inducing oxidative stress, promoting chronic inflammation, and affecting carbohydrate metabolism.13 Among them, FAs have garnered much attention due to their significant pathophysiological effects on the reproductive system. FAs belong to carboxylic acids composed of a long carbon chain and at least one carboxyl group. According to the degree of saturation in their hydrocarbon chains, FAs can be categorized into saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs).14 PUFAs can be further classified into omega-3 PUFAs and omega-6 PUFAs. The former primarily encompasses α-linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), while the latter mainly includes linoleic acid (LA) and arachidonic acid (AA).
A previous prospective study indicated that a high intake of ALA was effective in increasing baseline oestradiol (E2) levels in women undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI).15 Conversely, a high intake of EPA and DHA resulted in decreased E2 response and follicle number after ovarian stimulation.15 In particular, supplementation with omega-3 PUFAs, especially ALA and DHA, was beneficial for embryonic morphology.15 Similarly, omega-3 PUFA supplementation was also noticed to significantly reduce follicle-stimulating hormone (FSH) levels and FSH response to gonadotropin-releasing hormone (GnRH) in normal weight women, while in obese women, it led to lower serum levels of interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α).16 Furthermore, maternal preconception intake of PUFAs, particularly omega-6 PUFAs and LA, was associated with improved pregnancy rates in overweight and obese women undergoing IVF.17 In contrast, a prospective cohort study suggested that MUFAs are linked to increased fertility or a shorter duration of pregnancy, while PUFAs are associated with decreased fertility or a longer duration of pregnancy in normal-weight women.18 No significant associations were found between fertility and SFAs, trans FAs, omega-3 PUFAs and LA.18
Despite the inconsistent findings across these studies, it is undeniable that FAs play a crucial role in maintaining the reproductive health of women. To shed light on this matter, we first conducted a cross-sectional study utilizing data from the National Health and Nutrition Examination Survey (NHANES), in order to investigate the association between the intake of various FAs and the risk of infertility in women of childbearing age. Next, we employed Mendelian randomization (MR) analysis, an emerging epidemiological method that can effectively address the limitations of traditional observational studies, such as unknown confounding factors and reverse causality. Through this approach, we aimed to further explore the causal relationship between genetically predicted FA levels and different FA proportions and the likelihood of female infertility. Our study mainly identified that MUFAs and omega-6 PUFAs were associated with an elevated risk of female infertility, whereas omega-3 PUFAs exhibited a protective effect on fertility. Although these findings are generally consistent with previous reports, they also suggest that the underlying biological mechanisms through which these FAs influence the reproductive system may vary depending on specific fertility-related conditions. Additionally, the subclassifications of these FAs also appeared to exert distinct effects on reproductive health. We conducted an in-depth discussion on the effects and mechanisms of these FAs, endeavouring to provide valuable evidence for enhancing clinical prevention and treatment of infertility through lifestyle improvement.
2 Methods
This research comprised of two components. The initial segment involved the utilization of publicly accessible data from the NHANES (https://www.cdc.gov/nchs/nhanes/index.htm) to investigate the correlation between various FA intake levels and the risk of female infertility, while accounting for data weighting and adjusting for potential confounding variables. The subsequent segment involved the execution of MR, MVMR and reverse MR analyses using aggregated genome-wide association studies (GWAS) data to further assess the causal impact of genetically predicted FA levels on female infertility.2.1 Observational study
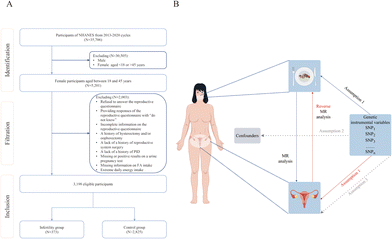
The data utilized in this cross-sectional study were sourced from three cycles of the NHANES database spanning the years 2013 to 2020, encompassing five distinct categories: demographic data, dietary data, examination data, laboratory data and questionnaire data. This particular time frame was selected due to the fact that the infertility assessment was exclusively conducted within this timeframe. In particular, we focused on the 18 to 45 age range as this represents the prime reproductive period for women, during which fertility gradually declines, with a sharp decrease after 45, eventually leading to the end of natural fertility.19,20 Initially, data were gathered for a total of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 participants across the three cycles. Then, the study population was excluded according to the following criteria: (1) male individuals; (2) individuals outside the age range of 18 to 45 years; (3) individuals declining to respond to the reproductive questionnaire, providing responses of “do not know”, or having incomplete information on it; (4) individuals with a history of hysterectomy and/or oophorectomy, or lacking a history of reproductive system surgery; (5) individuals lacking a history of PID; (6) individuals with missing or positive results on a urine pregnancy test; (7) individuals without information on FA intake; and (8) individuals with extreme daily energy intake (600 kilocalories < total daily energy intake < 6000 kilocalories).21 A total of 3159 participants were enrolled in our study, with 363 individuals in the infertility group and 2796 in the control group. The comprehensive screening process is illustrated in Fig. 1A.
706 participants across the three cycles. Then, the study population was excluded according to the following criteria: (1) male individuals; (2) individuals outside the age range of 18 to 45 years; (3) individuals declining to respond to the reproductive questionnaire, providing responses of “do not know”, or having incomplete information on it; (4) individuals with a history of hysterectomy and/or oophorectomy, or lacking a history of reproductive system surgery; (5) individuals lacking a history of PID; (6) individuals with missing or positive results on a urine pregnancy test; (7) individuals without information on FA intake; and (8) individuals with extreme daily energy intake (600 kilocalories < total daily energy intake < 6000 kilocalories).21 A total of 3159 participants were enrolled in our study, with 363 individuals in the infertility group and 2796 in the control group. The comprehensive screening process is illustrated in Fig. 1A.
This study analysed the average daily intake of various types of fatty acids, including total SFAs (TSFAs), total MUFAs, total PUFAs, total omega-3 PUFAs and total omega-6 PUFAs, for each participant. Specifically, omega-3 PUFAs encompassed ALA, DHA, docosapentaenoic acid (DPA), EPA and stearidonic acid (SDA), while omega-6 PUFAs included AA and LA. Additionally, the ratios of omega-3 to total fatty acids (omega-3/TFA), omega-6 to total fatty acids (omega-6/TFA), monounsaturated fatty acids to total fatty acids (MUFA/TFA) and polyunsaturated fatty acids to total fatty acids (PUFA/TFA) were also calculated.
The covariates were derived from multiple data modules, namely demographic data, dietary data, examination data, laboratory data and questionnaire data. Firstly, the covariates from the demographic data module consisted of age, race, educational level, marital status, and poverty income ratio (PIR). Race was categorized into six groups: “Mexican American”, “Other Hispanic”, “Non-Hispanic White”, “Non-Hispanic Black”, “Non-Hispanic Asian” and “Other/Multiracial”. Educational level was divided into three categories: “less than high school”, “high school” and “more than high school”. Marital status was categorized as “married/living with partner”, “widowed/divorced/separated” and “never married”. Age and PIR were treated as continuous variables for analysis.
Secondly, covariates obtained from the dietary data module comprised the mean daily calorie intake, which was a continuous variable. The examination data module sourced covariate contained categorical data on body mass index (BMI), which was classified as “underweight (<18.5 kg m−2)”, “normal (18.5 to <25 kg m−2)”, “overweight (25 to <30 kg m−2)” and “obese (30 or greater kg m−2)”. The covariate in the laboratory data module included the continuous variable serum cotinine levels. Finally, the questionnaire data module encompassed covariates pertaining to drinking status, smoking status, history of PID, regular periods, depression, sleeplessness, history of diabetes, history of hypertension, history of hypercholesteremia, physical activity and sedentary behaviour, all of which were treated as categorical variables. Physical activity was quantified using the metabolic equivalent (MET) recommended by NHANES multiplied by the duration of the specific activity, and categorized as sufficient or insufficient based on a threshold of 450 MET-min per week.36 Sedentary behaviour was assessed by self-reported daily sedentary time and categorized as either present or absent using a cut-off of 360 minutes per day.36
2.2 Mendelian randomization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 participants involving 12
999 participants involving 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 321
321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 SNP sites. The GWAS dataset for female infertility was obtained from the FinnGen biobank (GWAS ID: finn-b-N14_FEMALEINFERT, 6481 cases and 68
875 SNP sites. The GWAS dataset for female infertility was obtained from the FinnGen biobank (GWAS ID: finn-b-N14_FEMALEINFERT, 6481 cases and 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969 controls involving 16
969 controls involving 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377
377![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 038 SNP sites). No sample overlap was observed between the datasets in this study, as the source populations for all the GWAS data were distinctly diverse.
038 SNP sites). No sample overlap was observed between the datasets in this study, as the source populations for all the GWAS data were distinctly diverse.
The detailed procedure for screening IVs is outlined below. Firstly, SNPs associated with FA levels were extracted as IVs at a genome-wide significant level (P < 5 × 10−8). Secondly, SNPs with linkage disequilibrium (LD, clumping r2 = 0.001 and window size = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kilobases) were excluded. We then calculated the F-statistic for each remaining SNP to quantify the strength of SNP exposure and eliminated weak exposures with an F-statistic <10, aiming to minimize weak instrument bias. The F-statistic was calculated using the formula: F = R2 × (N − 2)/(1 − R2), where N represents the sample size of the exposure factor and R2 represents the proportion of variance explained by IVs.37,38R2 can be calculated as follows: R2 = 2 × EAF × (1 − EAF) × β2/[(2 × EAF × (1 − EAF) × β2) + (2 × EAF × (1 − EAF) × N × SE2)], where EAF is the SNP effect allele frequency, β is the estimated effect size of the SNP on the exposure factor, and SE is the standard error of the genetic effect, representing the degree of variation.38,39 Ambiguous and palindromic SNPs were then removed during the coordination process. Finally, PhenoScanner (https://www.phenoscanner.medschl.cam.ac.uk/) was visited to obtain the phenotype information for each of the SNPs, and any related phenotypes that can affect outcomes were manually ruled out.
000 kilobases) were excluded. We then calculated the F-statistic for each remaining SNP to quantify the strength of SNP exposure and eliminated weak exposures with an F-statistic <10, aiming to minimize weak instrument bias. The F-statistic was calculated using the formula: F = R2 × (N − 2)/(1 − R2), where N represents the sample size of the exposure factor and R2 represents the proportion of variance explained by IVs.37,38R2 can be calculated as follows: R2 = 2 × EAF × (1 − EAF) × β2/[(2 × EAF × (1 − EAF) × β2) + (2 × EAF × (1 − EAF) × N × SE2)], where EAF is the SNP effect allele frequency, β is the estimated effect size of the SNP on the exposure factor, and SE is the standard error of the genetic effect, representing the degree of variation.38,39 Ambiguous and palindromic SNPs were then removed during the coordination process. Finally, PhenoScanner (https://www.phenoscanner.medschl.cam.ac.uk/) was visited to obtain the phenotype information for each of the SNPs, and any related phenotypes that can affect outcomes were manually ruled out.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 samples involving 9
434 samples involving 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851
851![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 SNP sites), alcohol consumption (GWAS ID: ukb-a-25, 336
867 SNP sites), alcohol consumption (GWAS ID: ukb-a-25, 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 samples involving 10
965 samples involving 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894
894![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 596 SNP sites), BMI (GWAS ID: ukb-a-248, 336
596 SNP sites), BMI (GWAS ID: ukb-a-248, 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 samples involving 10
107 samples involving 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894
894![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 596 SNP sites), mood disorders (GWAS ID: ukb-b-18336, 53
596 SNP sites), mood disorders (GWAS ID: ukb-b-18336, 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414 cases and 407
414 cases and 407![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 controls involving 9
288 controls involving 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851
851![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 SNP sites) and insomnia (GWAS ID: ukb-a-13, 336
867 SNP sites) and insomnia (GWAS ID: ukb-a-13, 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 samples involving 10
965 samples involving 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894
894![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 596 SNP sites) were acquired from the Neale Lab consortium or the MRC-IEU consortium. In the MVMR analysis, we applied the same stringent quality-control step (clumping r2 = 0.001, window size = 10
596 SNP sites) were acquired from the Neale Lab consortium or the MRC-IEU consortium. In the MVMR analysis, we applied the same stringent quality-control step (clumping r2 = 0.001, window size = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kb) to select independent SNPs that reached the genome-wide significant level (P < 5 × 10−8) as the exposure factors. The details are shown in Table S1.†
000 kb) to select independent SNPs that reached the genome-wide significant level (P < 5 × 10−8) as the exposure factors. The details are shown in Table S1.†
In order to address the concerns regarding reverse causality, we conducted a reverse MR analysis to assess the impact of genetically predicted female infertility on the levels of various fatty acids. This analysis utilized a similar methodology to that described above, with female infertility as the exposure factor and various FA levels as the outcome factors.
2.3 Statistical analysis
Due to the intricate multistage and stratified sampling methodology utilized in NHANES, we adhered to the sample weights provided in the NHANES database during our analysis to make the sample nationally representative. Initially, we analysed the baseline characteristics of the participants. Descriptive statistics on the features of the study population are presented as weighted means with standard deviation (SD) for continuous variables and as unweighted sample size (frequency) for categorical variables. Differences between the infertility and control subjects were assessed by a weighted t-test, a Wilcoxon rank-sum test or a chi-square test with Rao and Scott's second-order correction. Additionally, taking full account of the special effect of age and BMI on female infertility, we also conducted a stratified analysis of these lamination factors (age: 18–34 years and 35–45 years; BMI: <25 kg m−2 and ≥25 kg m−2) to examine whether the association between FA intake and female infertility risk differs by age and BMI.To further investigate the link between different types of FA intake and the risk of female infertility, multiple weighted logistic regression models were employed, and the odds ratio (OR) and 95% confidence interval (CI) were calculated to determine the effect of each variable on infertility. FA intake was included in the models as a continuous variable and a component variable (Q1–Q4), respectively, where categorical variables were grouped according to quartiles, with the lowest quartile (Q1) serving as the reference group. Missing values were imputed using the mice package in R (version 4.3.0) through a fully conditional chained equations approach for multiple imputation.44 The intake of each fatty acid type was normalized to body weight (g kg−1 day−1) and then incorporated into the multivariate regression models. In addition, a directed acyclic graph was established to identify and account for potential confounding factors that may affect the relationship between FA intake and female infertility.45 Specifically, the crude model did not adjust for any covariates, while model 1 accounted for age, BMI and race. Model 2 was additionally adjusted for educational level, marital status, PIR, drinking status, smoking status, history of PID, regular periods, depression, sleeplessness, serum cotinine, calorie intake, history of diabetes, history of hypertension, history of hypercholesteremia, physical activity and sedentary behaviour.
Moreover, weighted restricted cubic splines (RCS) were applied to assess the dose–response relationship between varying levels of FA intake and the risk of female infertility.46 A nomogram diagram was drawn to quantify the magnitude of the risk of female infertility in relation to covariates. The sensitivity and performance of the models were further assessed through the calculation of the area under the receiver operating characteristic (ROC) curve (AUC).
In the context of MR analysis, SNPs demonstrating a robust association with the exposure and exhibiting independence from LD were chosen as IVs. Inverse variance weighted (IVW), MR-Egger, weighted median, simple mode, and weighted mode approaches were employed to investigate the potential causal relationship between the genetically predicted levels of fatty acids and female infertility. The IVW method, serving as the primary analytical tool in MR analysis, utilizes a meta-analytic framework to amalgamate the Wald estimates from individual SNPs for the derivation of an overall causal estimate.47 The remaining four methodologies were considered as supplementary analyses. Sensitivity analyses were performed to evaluate the presence of heterogeneity and horizontal pleiotropy. As the exposures and outcomes come from distinct populations, conducting the heterogeneity test is essential to assess the consistency of various IVs in estimating the relationship between these exposures and outcomes. Significant heterogeneity frequently indicates potential violations of model assumptions or that certain genetic variants do not conform to the IV assumption.48,49 Horizontal pleiotropy, in which a genetic variant influences the outcome through biological pathways unrelated to the risk factor of interest, contravenes the exclusion restriction assumption fundamental to MR analysis. The presence of horizontal pleiotropy may lead to either a spurious non-causal association or a false negative finding between the genetic predictors of the studied risk factor and the outcome.50 In this study, Cochran's Q test was employed to assess the heterogeneity of the genetic IV effect,48 while the MR-Egger regression intercept was utilized to test horizontal pleiotropy.51P > 0.05 indicates the absence of significant heterogeneity and horizontal pleiotropy, suggesting the robustness of the results. Leave-one-out sensitivity analysis was conducted to determine the impact of individual SNPs on MR estimates, thereby verifying the reliability of the results. MR pleiotropy residual sum and outlier (MR-PRESSO) was employed to detect and eliminate potential outliers.52 Additionally, funnel plots were constructed to provide a preliminary assessment of potential asymmetry and pleiotropy, and scatter plots were used to visually evaluate the direction of effect and the consistency of the association between exposure and outcome.
MVMR and reverse MR analyses were conducted to mitigate potential influences on the correlation between FAs and infertility and to rule out reverse causality. The MVMR-IVW method was utilized as the primary approach for assessing causal effects, with the MVMR-Lasso method serving as a supplementary analysis technique and the MVMR-Egger intercept test utilized to evaluate pleiotropy. The principles underlying reverse MR analysis were consistent with those of MR. Given the bidirectional nature of the MR analysis examining various FA levels and female infertility in our study, Bonferroni correction was applied, with statistical significance defined as a P value less than 0.00556 (0.05/9). However, the significance of certain results was overlooked due to the low P value obtained after adjusting for multiple comparisons. Consequently, findings that were initially deemed significant (P < 0.05) prior to Bonferroni correction but did not meet the adjusted threshold (P > 0.00556) were still considered in our analysis.53,54
Statistical analyses of the cross-sectional study and MR analysis mentioned above were performed using R (version 4.3.0). Furthermore, the MR analysis involved the utilization of R packages such as “TwoSampleMR” and “MR-PRESSO”. A two-tailed P value of less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the participants
In three cycles of NHANES spanning from 2013 to 2020, a total of 3159 women were enrolled in the study, with 363 (11.49%) belonging to the infertility group and 2796 (88.51%) to the control group, representing the national populations of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207
207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 and 38
575 and 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 967
967![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 795, respectively, across the United States. The baseline characteristics of the study subjects are summarized in Table 1. Notably, significant differences were observed between the two groups in terms of age, BMI, marital status, drinking status, history of PID, sleeplessness, history of diabetes, history of hypertension and history of hypercholesteremia. Furthermore, infertile women had significantly higher levels of total omega-6 intake and omega-6/TFA compared to the control group. Race, educational level, PIR, smoking status, regular periods, depression, serum cotinine, calorie intake, physical activity and sedentary behaviour showed no significant differences between the two groups. However, compared with the fertile population, the total intake of omega-3, omega-3/TFA and MUFAs in the infertile population tended to be lower, and the total intake of TSFA and PUFA tended to be higher, but the difference was not statistically significant.
795, respectively, across the United States. The baseline characteristics of the study subjects are summarized in Table 1. Notably, significant differences were observed between the two groups in terms of age, BMI, marital status, drinking status, history of PID, sleeplessness, history of diabetes, history of hypertension and history of hypercholesteremia. Furthermore, infertile women had significantly higher levels of total omega-6 intake and omega-6/TFA compared to the control group. Race, educational level, PIR, smoking status, regular periods, depression, serum cotinine, calorie intake, physical activity and sedentary behaviour showed no significant differences between the two groups. However, compared with the fertile population, the total intake of omega-3, omega-3/TFA and MUFAs in the infertile population tended to be lower, and the total intake of TSFA and PUFA tended to be higher, but the difference was not statistically significant.
| Characteristics | Total (N = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370) 370) |
Control (N = 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 967 967![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 795) 795) |
Infertility (N = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575) 575) |
P-value |
|---|---|---|---|---|
| Abbreviations: BMI: body mass index; PIR: poverty income ratio; PID: pelvic inflammatory disease; TSFAs: total saturated fatty acids; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids; TFA: total fatty acids. | ||||
| Age (years) | 31.42 ± 7.21 | 31.06 ± 7.23 | 34.14 ± 6.42 | <0.001 |
| Race | 0.84 | |||
| Mexican American | 533 (12.46%) | 470 (12.39%) | 63 (12.96%) | |
| Other Hispanic | 339 (8.24%) | 304 (8.34%) | 35 (7.47%) | |
| Non-Hispanic White | 1028 (54.92%) | 901 (54.88%) | 127 (55.29%) | |
| Non-Hispanic Black | 759 (13.78%) | 667 (13.65%) | 92 (14.75%) | |
| Non-Hispanic Asian | 380 (6.79%) | 341 (7.00%) | 39 (5.18%) | |
| Other/Multiracial | 159 (3.81%) | 142 (3.74%) | 17 (4.34%) | |
| BMI | <0.001 | |||
| Underweight (<18.5 kg m−2) | 83 (2.51%) | 78 (2.67%) | 5 (1.31%) | |
| Normal (18.5 to <25 kg m−2) | 1008 (34.51%) | 909 (35.68%) | 99 (25.84%) | |
| Overweight (25 to <30 kg m−2) | 762 (24.10%) | 696 (24.83%) | 66 (18.67%) | |
| Obese (30 or greater kg m−2) | 1331 (38.88%) | 1128 (36.82%) | 203 (54.18%) | |
| Educational level | 0.43 | |||
| Less than high school | 467 (10.29%) | 408 (9.97%) | 59 (12.68%) | |
| High school | 631 (20.53%) | 555 (20.53%) | 76 (20.54%) | |
| More than high school | 2100 (69.18%) | 1862 (69.50%) | 238 (66.78%) | |
| Marital status | <0.001 | |||
| Married/living with partner | 1776 (57.18%) | 1506 (54.70%) | 270 (75.72%) | |
| Widowed/divorced/separated | 309 (9.08%) | 269 (8.83%) | 40 (10.97%) | |
| Never married | 1113 (33.74%) | 1050 (36.47%) | 63 (13.31%) | |
| PIR | 2.67 ± 1.67 | 2.66 ± 1.67 | 2.78 ± 1.60 | 0.19 |
| Drinking status | 0.045 | |||
| Never drinker | 1704 (51.00%) | 1505 (50.77%) | 199 (52.69%) | |
| Former drinker | 835 (23.09%) | 724 (22.42%) | 111 (28.08%) | |
| Current drinker | 659 (25.92%) | 596 (26.81%) | 63 (19.23%) | |
| Smoking status | 0.58 | |||
| Never smoker | 2280 (68.67%) | 2033 (69.09%) | 247 (65.51%) | |
| Former smoker | 349 (12.84%) | 303 (12.61%) | 46 (14.50%) | |
| Current smoker | 567 (18.49%) | 487 (18.29%) | 80 (19.99%) | |
| History of PID | 0.003 | |||
| Yes | 150 (4.46%) | 116 (3.85%) | 34 (9.01%) | |
| No | 3032 (95.54%) | 2695 (96.15%) | 337 (90.99%) | |
| Regular periods | 0.27 | |||
| Yes | 3000 (94.08%) | 2649 (94.31%) | 351 (92.33%) | |
| No | 198 (5.92%) | 176 (5.69%) | 22 (7.67%) | |
| Depression | 0.066 | |||
| Yes | 324 (10.74%) | 269 (10.19%) | 55 (14.91%) | |
| No | 2868 (89.26%) | 2550 (89.81%) | 318 (85.09%) | |
| Sleeplessness | 0.002 | |||
| Yes | 728 (24.73%) | 610 (23.46%) | 118 (34.22%) | |
| No | 2470 (75.27%) | 2215 (76.54%) | 255 (65.78%) | |
| Cotinine (ng mL −1 ) | 40.69 ± 99.07 | 39.90 ± 98.15 | 46.58 ± 105.62 | 0.56 |
| Calorie intake (kcal) | 1860.01 ± 659.45 | 1860.07 ± 656.98 | 1859.60 ± 678.56 | 0.86 |
| Diabetes | <0.001 | |||
| Yes | 190 (4.64%) | 149 (3.85%) | 41 (10.50%) | |
| No | 3008 (95.36%) | 2676 (96.15%) | 332 (89.50%) | |
| Hypertension | 0.022 | |||
| Yes | 455 (11.55%) | 385 (10.96%) | 70 (16.03%) | |
| No | 2743 (88.45%) | 2440 (89.04%) | 303 (83.97%) | |
| Hypercholesteremia | 0.003 | |||
| Yes | 430 (13.10%) | 361 (12.07%) | 69 (20.79%) | |
| No | 2768 (86.90%) | 2464 (87.93%) | 304 (79.21%) | |
| Physical activity | 0.10 | |||
| Insufficient | 938 (24.14%) | 812 (23.57%) | 126 (28.44%) | |
| Sufficient | 2260 (75.86%) | 2013 (76.43%) | 247 (71.56%) | |
| Sedentary behaviour | 0.29 | |||
| Yes | 1572 (48.52%) | 1393 (48.12%) | 179 (51.52%) | |
| No | 1626 (51.48%) | 1432 (51.88%) | 194 (48.48%) | |
| TSFAs (g) | 25.42 ± 11.94 | 25.38 ± 11.95 | 25.70 ± 11.90 | 0.50 |
| Total MUFAs (g) | 17.75 ± 9.21 | 17.75 ± 9.18 | 17.72 ± 9.47 | 0.84 |
| Total PUFAs (g) | 23.94 ± 11.76 | 23.91 ± 11.84 | 24.12 ± 11.18 | 0.51 |
| Total omega-3 (g) | 1.76 ± 1.04 | 1.76 ± 1.04 | 1.70 ± 1.07 | 0.35 |
| Total omega-6 (g) | 15.31 ± 8.60 | 15.11 ± 8.60 | 16.80 ± 8.44 | 0.002 |
| Omega-3/TFA | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.15 |
| Omega-6/TFA | 0.23 ± 0.08 | 0.22 ± 0.07 | 0.25 ± 0.10 | <0.001 |
| MUFA/TFA | 0.38 ± 0.05 | 0.38 ± 0.05 | 0.38 ± 0.04 | 0.80 |
| PUFA/TFA | 0.26 ± 0.07 | 0.26 ± 0.07 | 0.26 ± 0.07 | 0.36 |
3.2 Associations between FA intake and infertility risk
Weighted logistic regression analysis was conducted to further investigate the impact of different types of FA intake on the risk of female infertility (Table 2). Our findings revealed that when TSFA (OR: 0.35, 95% CI: 0.16–0.78, P = 0.011), total MUFAs (OR: 0.40, 95% CI: 0.18–0.89, P = 0.026) and total PUFA (OR: 0.27, 95% CI: 0.09–0.79, P = 0.018) were treated as continuous variables, they displayed a negative correlation with female infertility risk solely in the crude model. Similarly, when considering total omega-3 and total omega-6 as continuous variables, total omega-3 intake exhibited an inverse association with the risk of female infertility in three models (crude model: OR: 0.00, 95% CI: 0.00–0.01, P = 0.008; model 1: OR: 0.00, 95% CI: 0.00–0.13, P = 0.022; model 2: OR: 0.00, 95% CI: 0.00–0.02, P = 0.016), while total omega-6 intake showcased a positive association with the risk of female infertility in both model 1 and model 2 (model 1: OR: 3.54, 95% CI: 1.31–9.57, P = 0.014; model 2: OR = 3.39, 95% CI: 1.28–8.98, P = 0.012). In addition, the third quartile of total omega-3 intake showed a negative association with infertility in all models (crude model: OR: 0.52, 95% CI: 0.34–0.78, P = 0.002; model 1: OR: 0.62, 95% CI: 0.40–0.97, P = 0.038; model 2: OR: 0.55, 95% CI: 0.32–0.93, P = 0.028). Inversely, total omega-6 intake in the third (model 2: OR: 1.75, 95% CI: 1.08–2.84, P = 0.025) and fourth (model 2: OR: 2.22, 95% CI: 1.25–3.96, P = 0.009) quartiles demonstrated a positive association with infertility risk in the fully adjusted models. When categorizing omega-6/TFA and incorporating it into the weighted logistic regression model, we observed a consistent positive association with an increased risk of female infertility in all models.| Crude model | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Abbreviations: TSFAs: total saturated fatty acids; MUFAs: monounsaturated fatty acids; PUFAs: polyunsaturated fatty acids; TFA: total fatty acids. | ||||||
| TSFAs (g) | ||||||
| Continuous | 0.35 (0.16, 0.78) | 0.011 | 0.84 (0.34, 2.06) | 0.696 | 0.40 (0.10, 1.70) | 0.205 |
| Q1 | Ref. | |||||
| Q2 | 0.93 (0.65, 1.33) | 0.680 | 1.01 (0.69, 1.48) | 0.948 | 0.96 (0.61, 1.52) | 0.856 |
| Q3 | 0.75 (0.48, 1.18) | 0.207 | 0.95 (0.60, 1.51) | 0.820 | 0.85 (0.48, 1.49) | 0.551 |
| Q4 | 0.74 (0.49, 1.11) | 0.142 | 1.09 (0.69, 1.73) | 0.690 | 0.99 (0.51, 1.89) | 0.963 |
| Total MUFAs (g) | ||||||
| Continuous | 0.40 (0.18, 0.89) | 0.026 | 0.95 (0.41, 2.19) | 0.906 | 0.80 (0.21, 3.08) | 0.741 |
| Q1 | Ref. | |||||
| Q2 | 0.73 (0.46, 1.14) | 0.157 | 0.82 (0.52, 1.30) | 0.400 | 0.75 (0.44, 1.28) | 0.278 |
| Q3 | 0.69 (0.46, 1.02) | 0.060 | 0.86 (0.57, 1.30) | 0.471 | 0.83 (0.49, 1.39) | 0.454 |
| Q4 | 0.71 (0.46, 1.10) | 0.120 | 1.14 (0.72, 1.79) | 0.573 | 1.13 (0.55, 2.31) | 0.732 |
| Total PUFAs (g) | ||||||
| Continuous | 0.27 (0.09, 0.79) | 0.018 | 0.77 (0.25, 2.40) | 0.650 | 0.77 (0.17, 3.45) | 0.719 |
| Q1 | Ref. | |||||
| Q2 | 0.66 (0.43, 1.01) | 0.054 | 0.73 (0.48, 1.12) | 0.149 | 0.67 (0.43, 1.06) | 0.087 |
| Q3 | 0.71 (0.48, 1.05) | 0.082 | 0.86 (0.56, 1.32) | 0.482 | 0.76 (0.49, 1.17) | 0.199 |
| Q4 | 0.56 (0.37, 0.84) | 0.006 | 0.81 (0.52, 1.26) | 0.343 | 0.68 (0.37, 1.26) | 0.213 |
| Total omega-3 (g) | ||||||
| Continuous | 0.00 (0.00, 0.01) | 0.008 | 0.00 (0.00, 0.13) | 0.022 | 0.00 (0.00, 0.02) | 0.016 |
| Q1 | Ref. | |||||
| Q2 | 0.76 (0.50, 1.17) | 0.206 | 0.81 (0.52, 1.27) | 0.342 | 0.75 (0.46, 1.23) | 0.245 |
| Q3 | 0.52 (0.34, 0.78) | 0.002 | 0.62 (0.40, 0.97) | 0.038 | 0.55 (0.32, 0.93) | 0.028 |
| Q4 | 0.61 (0.40, 0.93) | 0.021 | 0.84 (0.53, 1.34) | 0.465 | 0.70 (0.38, 1.27) | 0.226 |
| Total omega-6 (g) | ||||||
| Continuous | 1.04 (0.41, 2.64) | 0.927 | 3.54 (1.31, 9.57) | 0.014 | 3.39 (1.28, 8.98) | 0.012 |
| Q1 | Ref. | |||||
| Q2 | 0.90 (0.57, 1.41) | 0.630 | 1.04 (0.66, 1.62) | 0.877 | 1.10 (0.69, 1.75) | 0.674 |
| Q3 | 1.15 (0.74, 1.78) | 0.538 | 1.44 (0.92, 2.27) | 0.110 | 1.75 (1.08, 2.84) | 0.025 |
| Q4 | 1.02 (0.65, 1.59) | 0.937 | 1.59 (1.00, 2.54) | 0.051 | 2.22 (1.25, 3.96) | 0.009 |
| Omega-3/TFA | ||||||
| Continuous | 0.96 (0.45, 2.04) | 0.921 | 0.83 (0.46, 1.50) | 0.521 | 0.98 (0.61, 1.55) | 0.912 |
| Q1 | Ref. | |||||
| Q2 | 0.82 (0.47, 1.42) | 0.468 | 0.81 (0.46, 1.44) | 0.471 | 0.85 (0.46, 1.58) | 0.591 |
| Q3 | 0.87 (0.55, 1.37) | 0.540 | 0.81 (0.50, 1.30) | 0.367 | 0.77 (0.46, 1.29) | 0.307 |
| Q4 | 0.81 (0.49, 1.35) | 0.408 | 0.81 (0.49, 1.34) | 0.410 | 0.86 (0.51, 1.43) | 0.538 |
| Omega-6/TFA | ||||||
| Continuous | 1.96 (0.60, 6.41) | 0.252 | 1.06 (0.57, 1.96) | 0.852 | 1.21 (0.72, 2.06) | 0.454 |
| Q1 | Ref. | |||||
| Q2 | 1.90 (1.17, 3.10) | 0.010 | 1.85 1.10, 3.12 | 0.021 | 1.97 (1.11, 3.49) | 0.022 |
| Q3 | 2.06 (1.31, 3.22) | 0.002 | 2.00 1.27, 3.15 | 0.004 | 2.28 (1.38, 3.75) | 0.002 |
| Q4 | 2.14 (1.28, 3.57) | 0.004 | 2.17 1.30, 3.61 | 0.004 | 2.50 (1.47, 4.27) | 0.002 |
| MUFA/TFA | ||||||
| Continuous | 1.08 (0.33, 3.58) | 0.892 | 1.15 (0.36, 3.71) | 0.802 | 2.15 (0.63, 7.33) | 0.210 |
| Q1 | Ref. | |||||
| Q2 | 1.20 (0.75, 1.92) | 0.451 | 1.13 (0.68, 1.88) | 0.630 | 1.03 (0.55, 1.90) | 0.934 |
| Q3 | 1.35 (0.93, 1.96) | 0.111 | 1.25 (0.84, 1.86) | 0.264 | 1.12 (0.70, 1.79) | 0.630 |
| Q4 | 1.14 (0.73, 1.80) | 0.555 | 1.11 (0.68, 1.81) | 0.674 | 1.03 (0.58, 1.83) | 0.907 |
| PUFA/TFA | ||||||
| Continuous | 0.41 (0.04, 3.83) | 0.430 | 0.46 (0.05, 3.94) | 0.474 | 1.04 (0.12, 8.77) | 0.973 |
| Q1 | Ref. | |||||
| Q2 | 0.93 (0.56, 1.55) | 0.783 | 0.88 (0.52, 1.47) | 0.611 | 0.84 (0.47, 1.50) | 0.547 |
| Q3 | 1.11 (0.73, 1.68) | 0.628 | 1.09 (0.72, 1.65) | 0.681 | 1.21 (0.79, 1.87) | 0.363 |
| Q4 | 0.84 (0.53, 1.33) | 0.447 | 0.86 (0.55, 1.34) | 0.488 | 0.94 (0.59, 1.49) | 0.787 |
In addition to the aforementioned analyses, we also utilized a nomogram diagram to visually represent the relationship between each covariate and the risk of female infertility, as depicted in Fig. S1.† Furthermore, for the purpose of demonstrating the robustness of the results, we generated the ROC curve and calculated the AUC, as shown in Fig. S2.† The AUC for all types of FA intake in the fully adjusted model exceeded 0.65, indicating a substantial predictive capability of the models. Besides, weighted RCS with five knots at the 5th, 35th, 50th, 65th and 95th centiles were employed to flexibly model the non-linear association of different types of fatty acids with the risk of female infertility. Notably, the non-linear model demonstrated a superior ability than a linear model in elucidating the connection between the intake of omega-6, omega-6/TFA, omega-3/TFA and infertility risk after accounting for all covariates (Fig. S3†).
3.3 Subgroup analysis
Taking into account previous literature detailing the specific effects of BMI and age on female infertility, our study aimed to further investigate the relationship between dietary FA intake and infertility through stratified analyses. Upon analyzing the baseline characteristics of the participants, it was noted that the infertility group exhibited significantly higher levels of total omega-6 intake compared to the control group, regardless of age or BMI stratification factors (Tables S2 and S3†). Additionally, a higher omega-6/TFA ratio was only observed in the infertility group with a BMI ≥ 25 kg m−2.Significantly, the results of the weighted logistic regression analysis indicated that, in fully adjusted models, a positive association between total omega-6 intake (as continuous) and the risk of infertility was observed solely within the subgroup of women aged 18 to 34 years. Furthermore, the relationship between omega-6/TFA (as continuous) and infertility risk remained consistent across all subgroups, establishing it as a risk factor for female infertility (Table S4†). Based on model 2 in the BMI stratified analysis, TSFAs, total omega-3 and its third and fourth quantiles, as well as Q2–Q4 of total PUFA were identified as protective factors for infertility in the population with a BMI ≥ 25 kg m−2. However, these factors did not show the same protective effects in individuals with a BMI < 25 kg m−2. In addition, the positive associations between the Q4 of total omega-6, omega-6/TFA and its third and fourth quantiles and infertility risk were consistently observed across all subgroups (Table S5†).
3.4 MR analysis of FA levels with the risk of infertility
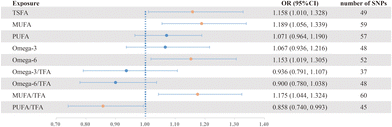
To further clarify the causal relationship between the results in the cross-sectional study, we additionally conducted an MR analysis in order to elucidate the potential causal relationship between FA levels and female infertility. Following the established selection criteria, a total of 49, 59, 57, 48, 52, 37, 48, 60 and 45 SNPs were ultimately selected as IVs to evaluate the causal relationship between TSFA, MUFA, PUFA, omega-3, omega-6, omega-3/TFA, omega-6/TFA, MUFA/TFA, PUFA/TFA and female infertility, respectively. The specific information on SNPs can be found in Tables S6–S14.†In the analyses of genetically predicted FA levels on female infertility using MR methodology (Table S15†), we primarily employed the IVW method to examine the causal association between them, which is visually presented through the forest plot in Fig. 2. Our findings revealed significant associations between the levels of five specific types of fatty acids and female infertility. Specifically, TSFA (ORIVW: 1.158, 95% CI: 1.010–1.328, P = 0.035), MUFA (ORIVW: 1.189, 95% CI: 1.056–1.339, P = 0.004), omega-6 (ORIVW: 1.153, 95% CI: 1.019–1.305, P = 0.024) and MUFA/TFA (ORIVW: 1.175, 95% CI: 1.044–1.324, P = 0.008) showed positive correlations with infertility, with the most significant result being MUFA. Conversely, a negative correlation between PUFA/TFA and infertility (ORIVW: 0.858, 95% CI: 0.740–0.993, P = 0.040) was noted. However, there was no conclusive evidence of causal relationship between PUFA (ORIVW: 1.071, 95% CI: 0.964–1.190, P = 0.199), omega-3 (ORIVW: 1.067, 95% CI: 0.936–1.216, P = 0.334), omega-3/TFA (ORIVW: 0.936, 95% CI: 0.791–1.107, P = 0.439), omega-6/TFA (ORIVW: 0.900, 95% CI: 0.780–1.038, P = 0.146) and female infertility. The visual results can be found in Fig. S4.†
3.5 Sensitivity analysis for MR analysis
According to the results of the MR-Egger intercept test and Cochran's Q test (IVW and MR-Egger method), there was no significant heterogeneity (P > 0.05) and horizontal pleiotropy (P > 0.05) observed in the MR analysis. Furthermore, the MR-PRESSO method successfully identified and eliminated the outlier SNPs. The summary table of the specific results is presented in Table 3. To visually represent the data, funnel plots were also plotted (Fig. S5†). Additionally, the leave-one-out analysis was performed to assess the stability of the results and no significant changes were observed when individual SNPs were excluded singly, indicating the reliability and stability of all analyses (Fig. S6†). The aforementioned sensitivity analyses provided strong evidence to support the robustness of our MR analysis results.| Exposure | Outcome | Pleiotropy test | Heterogeneity test | MR-PRESSO | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger | Inverse-variance weighted | Raw | Outlier-corrected | ||||||||||||
| Intercept | SE | P-value | Q-value | Q-df | Q-pval | Q-value | Q-df | Q-pval | Beta | SD | P-value | Beta | SD | P-value | ||
| Abbreviations: MR-PRESSO: Mendelian randomization pleiotropy residual sum and outlier; SE: standard error; Q-value, the statistics of Cochran's Q test; Q-df: the degree of freedom of Cochran's Q test; Q-pval: the P-value of Cochran's Q test; SD: standard deviation. | ||||||||||||||||
| TSFA | Infertility | 0.001 | 0.007 | 0.832 | 51.123 | 47 | 0.315 | 51.172 | 48 | 0.350 | 0.147 | 0.070 | 0.040 | NA | NA | NA |
| MUFA | 0.005 | 0.006 | 0.393 | 51.183 | 57 | 0.692 | 51.924 | 58 | 0.699 | 0.173 | 0.057 | 0.004 | NA | NA | NA | |
| PUFA | 0.003 | 0.006 | 0.636 | 54.700 | 55 | 0.486 | 54.926 | 56 | 0.516 | 0.069 | 0.053 | 0.200 | NA | NA | NA | |
| Omega-3 | 0.000 | 0.007 | 0.986 | 57.460 | 46 | 0.120 | 57.461 | 47 | 0.141 | 0.064 | 0.067 | 0.339 | NA | NA | NA | |
| Omega-6 | −0.001 | 0.007 | 0.841 | 48.935 | 50 | 0.516 | 48.975 | 51 | 0.554 | 0.142 | 0.062 | 0.026 | NA | NA | NA | |
| Omega-3/TFA | −0.003 | 0.008 | 0.726 | 43.347 | 35 | 0.157 | 43.502 | 36 | 0.182 | −0.066 | 0.086 | 0.444 | NA | NA | NA | |
| Omega-6/TFA | −0.005 | 0.007 | 0.421 | 52.568 | 46 | 0.235 | 53.322 | 47 | 0.244 | −0.106 | 0.073 | 0.153 | NA | NA | NA | |
| MUFA/TFA | 0.011 | 0.006 | 0.061 | 71.321 | 58 | 0.112 | 75.793 | 59 | 0.069 | 0.162 | 0.061 | 0.010 | NA | NA | NA | |
| PUFA/TFA | −0.012 | 0.007 | 0.088 | 48.403 | 43 | 0.264 | 51.840 | 44 | 0.195 | −0.154 | 0.075 | 0.046 | NA | NA | NA | |
| Infertility | TSFA | 0.003 | 0.004 | 0.540 | 4.845 | 8 | 0.774 | 5.254 | 9 | 0.812 | 0.002 | 0.010 | 0.861 | NA | NA | NA |
| MUFA | 0.003 | 0.004 | 0.430 | 6.495 | 8 | 0.592 | 7.186 | 9 | 0.618 | −0.005 | 0.012 | 0.674 | NA | NA | NA | |
| PUFA | 0.004 | 0.004 | 0.341 | 6.548 | 8 | 0.586 | 7.571 | 9 | 0.578 | 0.005 | 0.012 | 0.700 | NA | NA | NA | |
| Omega-3 | 0.007 | 0.004 | 0.127 | 6.276 | 8 | 0.616 | 9.170 | 9 | 0.422 | 0.006 | 0.013 | 0.658 | NA | NA | NA | |
| Omega-6 | 0.003 | 0.004 | 0.489 | 5.276 | 8 | 0.728 | 5.801 | 9 | 0.760 | 0.004 | 0.011 | 0.716 | NA | NA | NA | |
| Omega-3/TFA | 0.006 | 0.004 | 0.165 | 6.376 | 8 | 0.605 | 8.709 | 9 | 0.465 | 0.007 | 0.013 | 0.615 | NA | NA | NA | |
| Omega-6/TFA | −0.004 | 0.004 | 0.390 | 4.906 | 8 | 0.768 | 5.732 | 9 | 0.766 | 0.004 | 0.010 | 0.725 | NA | NA | NA | |
| MUFA/TFA | 0.003 | 0.004 | 0.500 | 6.271 | 8 | 0.617 | 6.771 | 9 | 0.661 | −0.012 | 0.011 | 0.306 | NA | NA | NA | |
| PUFA/TFA | −0.001 | 0.004 | 0.721 | 5.526 | 8 | 0.700 | 5.663 | 9 | 0.773 | 0.004 | 0.010 | 0.726 | NA | NA | NA | |
3.6 MVMR and reverse analysis
In the MVMR framework, we accounted for five potential confounders, namely current smoking, alcohol consumption, BMI, mood disorders and insomnia, in order to verify the direct causal effect of FA levels on female infertility. Our findings revealed that the causal relationship between MUFA and female infertility remained robust after adjusting for any factor. In addition, omega-6 (ORMVMR-IVW: 1.09, 95% CI: 1.02–1.16, P = 0.046) and MUFA/TAF (ORMVMR-IVW: 1.10, 95% CI: 1.00–1.20, P = 0.031) exhibited a significant causal relationship with female infertility after accounting for all confounding factors. The results obtained from the MVMR-Lasso method were consistent with those of the MVMR-IVW approach, further enhancing the reliability of the findings that higher FA levels increases the risk of female infertility, irrespective of confounding factors (Table S16†). Importantly, the P values for the MVMR-Egger intercept test and Cochran's Q test were all above 0.05, hinting the absence of horizontal pleiotropy.In the reverse MR analysis, we also applied MR-Egger, weighted median, IVW, simple mode and weighted mode methods to effectively rule out any possible reverse causality. We did not detect any significant association between genetically predicted female infertility and FA levels (Fig. S7†). Furthermore, sensitivity analyses of reverse MR analysis were also conducted, and the results of the MR-Egger intercept test and Cochran's Q test did not indicate any heterogeneity or horizontal pleiotropy (P > 0.05), thus further reinforcing the reliability of our results, as outlined in Table 3.
4 Discussion
In this study, we aimed to elucidate the intricate correlation between various types of fatty acids and their constituent ratios and female infertility, focusing on the underlying mechanisms and causality. Our study provides compelling evidence that partial fatty acids are important contributors to the development of female infertility with a strong genetic correlation. Specifically, analysis of data from the NHANES cross-sectional study revealed a positive association between both omega-6 PUFAs and omega-6/TFA and the risk of infertility, while omega-3 PUFAs exhibited an inverse association with infertility risk. Through MR analysis, we observed that genetically predicted TSFA, MUFA, omega-6 and MUFA/TFA were positively associated with infertility risk, while PUFA/TFA demonstrated a negative association. Furthermore, after accounting for potential confounders using MVMR analysis, the positive associations between MUFA and omega-6 and infertility risk remained robust. Importantly, our study did not uncover any evidence suggesting heterogeneity, horizontal pleiotropy, or reverse causality. These results indicate that dietary interventions targeting these FAs may be a promising strategy to reduce the risk of female infertility, particularly given the modifiable nature of dietary habits, which offers a non-pharmacological option for women seeking to enhance their fertility prospects.Fatty acids play a crucial role in providing energy for the human body and serving as a vital component of cellular structure, and can be broadly categorized into saturated FAs and unsaturated FAs based on their chemical bond characteristics. While all the PUFAs and other FAs are carried in the bloodstream, only a minor proportion reaches target organs like the ovary in their free state.55 Among them, SFAs and MUFAs are primarily utilized by the oocytes and the surrounding cumulus cells as a source of energy and structural elements. On the other hand, PUFAs play diverse roles in oocyte biology and metabolism.56 Targeted deletion of Δ6-fatty acid desaturase (FADS2), essential for PUFA synthesis, did not impact the viability or lifespan of fads2−/− mice but caused infertility in both sexes.57 Female fads2−/− mice exhibited inadequate ovarian blood supply during the cycle, a lack of granulosa cell syncytium and theca folliculi, poorly developed or absent zona pellucida, and dysmorphic follicles.57 Besides, the gap junction (GJ) channel system, tagged with Cx43, was severely disordered within the ovarian granulosa cell syncytium, with GJs barely detectable in the plasma membranes of adjacent granulosa cells.57 Remarkably, this infertility caused by FADS2 deficiency can be restored through dietary supplementation with specific PUFAs, underscoring the crucial role of PUFAs in intercellular signaling, folliculogenesis, oocyte maturation, and the maintenance of normal reproductive function.
Previous studies have shown that the mean number of total and metaphase II (MII) oocytes is positively correlated with the intake of TFA, SFAs, MUFAs, PUFAs, LA, ALA and, oleic acids (OA), and negatively correlated with the intake of EPA and DHA.58 In addition, higher intakes of TFA, SFAs, PUFAs, MUFAs, LA, and OA were associated with lower fertilization rates.58 In contrast, higher intakes of ALA and EPAs increased fertilization rates, with PUFA intake directly affecting the proportion of high-quality embryos.58 Moreover, in healthy women with regular menstrual cycles, a high intake of PUFAs was correlated with elevated levels of total and free T, while DPA intake was linked to increased progesterone levels during the luteal phase and a reduced risk of anovulation.59 These findings suggest that various subtypes of dietary FA intake may have distinct roles in processes such as hormonal regulation, oogenesis, and fertilization, ultimately influencing female reproductive outcomes. However, two cohort studies involving populations from North America and Denmark identified no significant relationship between dietary intake of total fat, SFAs, MUFAs, PUFAs, or omega-6 PUFAs and fertility.60 Among North American women, a high intake of trans fatty acids was linked to reduced fertility, whereas greater consumption of omega-3 PUFAs was correlated with enhanced fertility.60 These associations were not observed in the Danish cohort, likely due to the generally lower intake of trans fatty acids and higher consumption of omega-3 PUFAs. Interestingly, substituting just 2% of the energy intake of trans fatty acids with MUFAs can reduce the risk of ovulatory infertility by less than half.61 In PCOS patients, the lowest intake of MUFAs was identified as one of the three key predictors of the highest testosterone (T) level,62 while the supplementation of MUFA-rich almonds can significantly reduce the free androgen index,63 suggesting that MUFA intake may be a potential strategy for the management of T levels in PCOS patients, thereby improving hyperandrogenism-related ovulation disorders and infertility. OA, one of the most representative MUFAs, also has a protective effect on oocyte maturation and implantation, which can counteract the adverse effects of higher levels of SFAs and enhance the developmental competence.64 These perspectives are generally consistent with ours, underscoring the notable distinctions in the effects of various types and consumption of FAs on female fertility disorders. Hence, it is crucial to ensure an appropriate intake of FAs in terms of both quantity and quality to prevent fertility disorders.
PUFAs are significant sources of cholesterol in the body and important precursors for prostaglandin synthesis. Cholesterol derived from PUFAs is vital for the synthesis of steroid hormones, while prostaglandins are involved in various physiological processes such as steroidogenesis, follicle maturation, ovulation, implantation and pregnancy.65 Both omega-3 and omega-6 belong to the subgroup of PUFAs, which can be distinguished by the distance between their first double bond and the terminal methyl group in the aliphatic hydrocarbon chain. Studies have demonstrated that women who took omega-3 supplements were 1.51 times more likely to conceive compared to those who did not.66 Additionally, couples who both consumed at least eight servings of omega-3-rich seafood per cycle experienced a 22% increase in sexual frequency and a 61% increase in fertility compared to couples with lower intake.67 The intake of omega-3 can also significantly reduce FSH levels in eumenorrheic women of normal weight, whereas no comparable effect was observed in obese women.16,68 In addition, omega-3 consumption effectively decreased serum levels of the pro-inflammatory cytokines, IL-1β and TNF-α, in obese women, but not in their normal-weight counterparts.16 Notably, omega-3 intake resulted in a slight elevation of the endoplasmic reticulum stress marker CCAAT-enhancer-binding homologous protein (CHOP) in normal-weight women, while it correspondingly reduced CHOP levels in obese women, with diversity in the mean expression of CHOP between the two groups approaching statistical significance.68 These findings suggest that omega-3 may significantly contribute to the delay of ovarian aging in women of normal weight. In contrast, the influence of omega-3 on ovarian function in obese women is more intricate. It appears that obesity may significantly modulate the dietary omega-3 supplementation effects on the reproductive axis, highlighting the need for further investigation into the complex interplay between omega-3, body composition, and reproductive health.
Our study identified omega-3 as a protective factor against female infertility, specifically in women with a BMI ≥ 25 kg m−2. This finding contrasts somewhat with previous research, potentially due to the different characteristics of populations. While our investigation included both overweight and obese groups, prior research predominantly focused on individuals classified as obese (BMI ≥ 30 kg m−2). Overweight and obesity are often linked to complex pathological changes, such as elevated inflammation and more severe metabolic dysfunction, which may impair the bioavailability of omega-3 and diminish the positive effects of it on reproductive health.69,70 These results highlight the need for further investigation into the mechanisms by which omega-3 affects female infertility, especially in populations with diverse characteristics.
In vivo experiments have shown that exposing oocytes to high levels of omega-3 during maturation in the ovary leads to notable changes in the distribution of active mitochondria and calcium levels, as well as an increase in the generation of reactive oxygen species (ROS).71 This exposure also adversely affected the morphological characteristics of embryos during in vivo fertilization and diminished their developmental capacity to the blastocyst stage.71 However, no significant impact of omega-3 supplementation was observed on the number and morphology of matured oocytes retrieved in vivo.71 Conversely, in vitro studies have revealed that omega-3 and L-carnitine can prevent spindle and chromosome damage in bovine oocytes cultured with FF derived from infertile women with minimal to mild endometriosis (FFEI/II), as well as those with moderate to severe endometriosis (FFEIII/IV).72 This dietary intervention also increased the normal MII rate in the FFEI/II and FFEIII/IV groups, while improving the total MII rate in the EIII/IV and endometrioma groups.72 Additionally, transgenic Fat-1 mice, which endogenously produce high levels of omega-3, exhibited reduced levels of immune mediators (interleukin 6 and cyclooxygenase-2) and the marker of epithelial cell proliferation (phosphohistone 3) in endometriosis-like lesions,73 suggesting that omega-3 supplementation may inhibit the early establishment and maintenance of ectopic lesions. Similarly, oral administration of omega-3 significantly lowered the levels of interleukin 3, TNF-α, and vascular endothelial growth factor in the peritoneal fluid of endometriosis model rats and decreased glandular tissue and stromal tissue scores.74 These results further confirm the anti-proliferative and anti-inflammatory properties of omega-3 in the context of endometriosis, indicating its potential to enhance the reproductive health in patients with endometriosis through both indirect mechanisms, such as mitigating the development of endometriosis lesions and alleviating abdominal inflammation, and direct mechanisms by protecting oocytes from meiotic damage.
PCOS is a common cause of infertility due to ovulation failure. Prior research has indicated that omega-3 supplementation can effectively reverse the elevated levels of luteinizing hormone (LH), LH/FSH, E2 and T induced by dehydroepiandrosterone (DHEA), decrease the number of cystic follicles, enhance corpus luteum formation, normalize estrous cycles, and ultimately ameliorate ovarian dysfunction in mouse models of PCOS.75In vitro investigations have additionally shown that omega-3 can notably accelerate the extrusion of the first polar body, diminish the proportion of abnormal spindle distribution, restore the expression of key antioxidant enzymes (Sirt1 and SOD3) as well as DNA damage repair genes (Brca1 and Msh2), and reduce superoxide dismutase (SOD) levels in oocytes of PCOS mice, thus exerting a protective effect.76 Direct evidence comes from a double-blind randomized trial, which suggests that omega-3 intake increases the rate of clinical pregnancy in women with PCOS.77 In contrast to the above evidence, Stanhiser et al. found no association between serum concentrations of both omega-3 and omega-6 PUFAs and spontaneous fertility, the risk of miscarriage, or levels of the anti-Müllerian hormone (AMH) among couples attempting natural pregnancy.78 This incongruous outcome may be ascribed to the demographic composition of the participants enrolled in the survey, specifically, who were between 30 and 44 years old. This aligns with our own observation that, in the case of older women, no discernible correlation between omega-3 and omega-6 (excluding Q4) and the risk of infertility was observed (Table S2†).
Although both omega-3 and omega-6 belong to a subgroup of PUFAs, they serve different functions and cannot be easily substituted for one another. They compete for the same enzyme system to produce longer and more unsaturated products.14,79 Therefore, maintaining a proper balance between omega-6 and omega-3 is essential for the homeostasis and normal development of organisms. It has been proven that lifelong consumption of a diet rich in omega-3 can extend the reproductive function of mice until advanced maternal age, while a diet high in omega-6 is linked to reduced reproductive success rate in advanced maternal age.80 Moreover, even short-term dietary treatment rich in omega-3, initiated during the rapid age-related decline in reproductive function, was connected with improved oocyte quality, while omega-6 had the opposite effect.80 This is consistent with our cross-sectional findings showing the existence of diametrical opposite effects of omega-3 and omega-6 on the reproductive system. Ma et al. discovered that treatment with omega-3/omega-6 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 in PCOS rats resulted in a reduced number of large antral follicles, an increased number of cystic follicles, and a decreased T level accompanied by an increase in FSH and E2, and promoted the expression of hormone-synthesizing enzymes.81 This also attenuated the apoptosis in ovarian cells accompanied by improved proliferative status of granulosa cells, theca cells and oocytes.81 Furthermore, an increase in the ratio of omega-3/omega-6 PUFAs led to an improvement in the ovarian structure, including the thickness of the granular and ovalbumin layers.81 These studies suggest that a balanced omega-6 and omega-3 system is beneficial for improving the reproductive performance of infertile women, including those with PCOS.
15 in PCOS rats resulted in a reduced number of large antral follicles, an increased number of cystic follicles, and a decreased T level accompanied by an increase in FSH and E2, and promoted the expression of hormone-synthesizing enzymes.81 This also attenuated the apoptosis in ovarian cells accompanied by improved proliferative status of granulosa cells, theca cells and oocytes.81 Furthermore, an increase in the ratio of omega-3/omega-6 PUFAs led to an improvement in the ovarian structure, including the thickness of the granular and ovalbumin layers.81 These studies suggest that a balanced omega-6 and omega-3 system is beneficial for improving the reproductive performance of infertile women, including those with PCOS.
ALA and LA are the precursors of omega-3 and omega-6 PUFAs, respectively, and both belong to the essential FAs that must be obtained from food.82 Zhang et al. discovered that LA can activate the endoplasmic reticulum and trigger the transcription of Foxo1 gene by binding to estrogen receptors on granulosa cells.83 This process brought about the accumulation of ROS and an inflammatory response, ultimately inducing apoptosis in granulosa cells.83 In contrast, administering a high LA diet for three months to adult female mice resulted in a notable reduction in ALA levels, an increase in the LA/AL ratio, an extended luteal phase, and an elevated concentration of plasma progesterone.84 This dietary supplementation also led to a higher percentage of cleaved oocytes, positively influencing ovulation rates and oocyte quality, ultimately enhancing overall fecundity.84 Moreover, the administration of Urtica pilulifera L. seed extract, abundant in LA, palmitic acid, and OA, was observed to decrease serum FSH levels, increase LH and E2 levels, enhance serum SOD activity, and reduce malondialdehyde levels in a dose-dependent manner in DOR model mice.85 This intervention resulted in a reduction in the number of primordial and atretic follicles, as well as a decrease in the proportion of apoptotic cells within ovarian tissues,85 suggesting that LA-rich Urtica pilulifera L. seed extract may contribute to the preservation of fecundity in patients with DOR by mitigating oxidative stress, modulating steroid hormone levels, and inhibiting apoptosis. LA is converted by desaturase to synthesize γ-linolenic acid (GLA) and dihomo-γ-linolenic acid (DGLA) in the body, which are subsequently metabolized to AA.86 It is noteworthy that GLA, as an intermediary metabolite, has been proven to enhance reproductive health by reducing ovarian volume, the levels of DHEA, and the degree of neutrophil infiltration in the ovary induced by DHEA through the PPAR-γ pathway.87 Oppositely, DGLA, another intermediary metabolite, was linked to germ cell destruction and infertility.88
Although the conversion rate of LA to AA in humans is less than 1%, numerous studies still highlight the important role of AA in follicular growth and development, and ovulation. One study showed that the concentration of AA in FF of normal-weight PCOS patients was significantly higher compared to that in the normal-weight control group.89 Subsequent in vitro experiments demonstrated that AA markedly decreased the activities of key antioxidant enzymes (SOD, catalase, glutathione peroxidase, and glutathione reductase), upregulated the levels of oxidative stress markers (malondialdehyde, ROS, and superoxide anion), reduced the total antioxidant capacity, and impaired mitochondrial function and endocrine steroid secretion, ultimately promoting apoptosis in KGN cells.89 Li et al. conducted a study to examine the temporal metabolic profiles of mouse oocytes during maturation in vivo,90 indicating that the supplementation of AA leads to a heightened occurrence of meiotic defects in metaphase I oocytes, such as spindle disorganization and chromosome aberrations, which signify changes in the meiotic structure. In contrast, a study revealed that 15 oxylipin metabolites were reduced in the follicular fluid (FF) of infertile women with diminished ovarian reserve (DOR), specifically related to the AA metabolic pathway.91 Notably, the AA oxidative metabolite, 20-COOH-AA, exhibited a significant negative correlation with FSH levels, while showing positive correlations with AMH levels, antral follicle counting, oocyte retrieval, MII oocytes and fertilization.91 Importantly, only 20-COOH-AA was positively correlated with the number of high-quality embryos.91 These results suggest that AA and its metabolites may exert different effects on follicle growth, development, and maturation. Modulating the transformation process between AA and its metabolites can be a potential target for improving reproductive outcomes.
ALA can be metabolized to EPA and DHA in the human body, with a higher conversion rate to EPA (approximately 8%) compared to DHA (less than 1%).92 Recent studies have elucidated a notable connection between the consumption of DHA and EPA and an increased likelihood of successful live births, juxtaposed by an inverse relationship concerning the incidence of both clinical and total pregnancy loss.93 Conversely, the ingestion of total omega-3 and ALA has not demonstrated any discernible correlation with implantation, clinical pregnancy, or live birth probabilities.93 These findings underscore the pivotal role of long-chain omega-3 PUFAs, specifically EPA and DHA, in dietary regimens, emphasizing their profound impact on fertility outcomes, in contrast to their short-chain counterparts such as ALA. In contrast, Broughton et al. reported that very high consumption of ALA in rats correlates with enhanced ovulation, similar to the effects observed with high intake of EPA and DHA.94 Notably, ALA intake significantly increased prostaglandin F synthesis and decreased prostaglandin E synthesis in the ovary, while EPA and DHA did not exert a significant impact on these pathways.94 Moreover, EPA/DHA is more effective than ALA in reducing ovarian AA levels, indicating that ALA and EPA/DHA may facilitate ovulation through the regulation of prostaglandin synthesis and AA, though the underlying mechanisms may differ.94 Besides, the oral administration of ALA-rich flaxseed oil has been shown to significantly improve ovarian response, enhance oocyte and embryo quality, and increase the rates of MII oocyte, fertilization, cleavage, blastocyst formation, and embryo implantation in DOR patients undergoing assisted reproductive technology.95 These findings highlight the need for future research to further explore how ALA affects reproductive processes such as folliculogenesis, fertilization, and implantation across various populations and species. By elucidating its mechanisms of action at different stages of reproduction, it is beneficial for developing evidence-based dietary recommendations.
Furthermore, research has indicated that DHA supplementation exhibits the capacity to ameliorate disrupted estrous cycles, mitigate the reduction in primordial follicle counts, and counteract alterations in ovarian gene expression induced by prolonged exposure to a high-fat diet in murine models.96 Similarly, DHA had beneficial effects on ovarian growing follicles in both sedentary and exhaustive exercise rat models, as evidenced by reduced caspase-3 levels and elevated SOD and catalase activities.97 Additional support for the protective attributes of AA and DHA is gleaned from investigations involving PUFA synthesis-deficient auxotrophic fads2−/− mouse mutants. The study revealed the potential of AA and DHA to mitigate pathological changes associated with ovarian cycle disturbances, disruptions in the granulosa cell layers, disassembly of the gap junction network among follicular granulosa cells, stunted folliculogenesis, atresia of oocytes and infertility arising from PUFA synthesis deficiencies.98 Nevertheless, high concentrations of DHA and EPA have been postulated to exert deleterious effects on oocyte developmental competence in vitro, with observed disparities potentially stemming from variances in experimental models.99,100
In this study, we initially explored the potential association between different fatty acids and the risk of infertility in women. However, given that infertility often involves factors from both male and female, it is equally important to investigate the potential effects of male fatty acid intake on reproductive health. For instance, a cross-sectional study demonstrated that each 1 g increase in SFA consumption was significantly correlated with a 38% reduction in the likelihood of producing more than 1.5 mL of semen volume and a 21% decrease in the likelihood of achieving a sperm concentration greater than 15 million.101 However, there was no significant effect of SFA intake on other semen quality parameters, such as sperm motility and morphology.101 Eslamian et al. also identified a positive association between high TSFA intake and asthenozoospermia.102 These findings align with our preliminary genetic predictive results in the infertile female population, obtained through the MR analysis approach. Furthermore, an increased intake of omega-3 PUFAs and DHA was significantly associated with a reduced risk of asthenozoospermia, with total omega-3 PUFA intake positively correlated with sperm concentration, count, and motility.95,103 These findings suggest that omega-3 supplementation may confer protective benefits to the male reproductive system, paralleling our cross-sectional results observed in infertile women. Additionally, a study demonstrated that omega-6 PUFA intake in the fourth quantile was positively correlated with total sperm motility and normal sperm morphology.104 Interestingly, this finding contradicts the results of our cross-sectional and MR analyses in women, where omega-6 intake was identified as a risk factor.
These observed similarities and differences in the impact of fatty acids on male and female reproductive health highlight the necessity of assessing dietary fatty acid intake in both partners when developing infertility prevention and management strategies. Given the dietary convergences in couples’ food choices, a woman's fatty acid consumption often reflects that of the partner. Thus, fertility-enhancing dietary interventions may need to be designed individually for both parties, as this can enhance fertility. Further research is warranted to explore the intricate dynamics between fatty acid intake and reproductive health in both males and females, offering more holistic guidance for nutritional advice and interventions aimed at boosting fertility and reducing the risk of infertility.
Building on these understandings, it becomes evident that assessing the impact of fatty acid intake on reproductive health requires a more comprehensive framework that considers a broader spectrum of factors potentially influencing fecundability. While this study accounted for a variety of potential confounding variables, it is important to acknowledge that certain unmeasured factors, such as lifestyle factors (e.g., stress and anxiety), dietary patterns, occupational exposures, and environmental conditions, may still impact the female reproductive system and contribute to infertility.105–108 Furthermore, a range of additional dietary components, including herbal extracts and herbal formulae, have been shown to improve reproductive health by enhancing oocyte development, promoting ovulation, improving endometrial receptivity, and restoring hormonal balance.109,110 However, these components may not have been fully captured in the NHANES dataset, representing potential unmeasured confounders that can impact the assessment of the relationship between fatty acid intake and female infertility. Future studies should incorporate a broader range of aspects to provide a clearer picture of the multifaceted influences on fertility.
Our investigation represents a comprehensive exploration of the interplay between diverse fatty acid profiles and the risk of female infertility, employing a fusion of the NHANES database and MR analysis. Nonetheless, it is crucial to acknowledge the restrictions inherent in our study design. Firstly, we cannot exclude the possibility of infertility due to male factors because of the limitations of the NHANES database. This can potentially overemphasize the role of female factors in infertility research, thereby introducing a potential bias. Additionally, the incomplete or missing data from the Sexual Behavior Questionnaire prevented us from effectively excluding individuals who have not had sexual intercourse in the past 12 months or those without sexual experience. Their potential inclusion in the fertility group may have affected the assessment of the link between fatty acid intake and infertility. Secondly, some unmeasured potential confounders were not taken into account, such as occupational and living environment, as well as other dietary components like Chinese herbal medicine, which may introduce confounding bias. Thirdly, within the framework of a cross-sectional study, the evaluation of fatty acid consumption and the characterization of infertility relied upon questionnaire-based methodologies and dietary recall interviews, potentially engendering recall bias. Fourthly, inherent to the nature of a cross-sectional study, we cannot delineate a definitive causal relationship between fatty acid intake and the incidence of infertility. It remains plausible that individuals diagnosed with infertility may alter their dietary habits post-diagnosis, thus introducing potential confounding variables. Furthermore, food constituents extend beyond mere nutritive value to non-nutritive components, including environmental contaminants and synthetic by-products resultant from food processing and packaging. Regrettably, the design of NHANES precludes the exclusion of potential confounding influences stemming from these factors on infertility. In response, we employed MR analysis to deepen our understanding of the nexus between genetically predicted fatty acid levels and infertility risk, aiming to attenuate the impact of confounding variables, which may elucidate the partial disparities observed between the findings of our cross-sectional study and those derived from the MR analysis.
5 Conclusions
In summary, our analysis provides suggestive evidence supporting the nexus between fatty acids and female infertility risk. Particularly noteworthy is the consistently positive correlation between omega-6 PUFAs and infertility risk, evident across both cross-sectional investigations and MR analyses. However, it is imperative to acknowledge that the veracity, robustness and generalizability of these findings necessitate further validation via comprehensive and large-scale clinical inquiries. Subsequent research should probe into the potential mechanisms and impacts of diverse fatty acid intake profiles, their proportional compositions and various metabolites in the origin and development of female infertility, which will lay a pivotal groundwork for the formulation and implementation of effective clinical interventions aimed at preventing and treating infertility.Author contributions
Qiaorui Yang: conceptualization, methodology, software, formal analysis, and writing – original draft. Jing Tao: methodology, software, formal analysis, writing – original draft, and writing – review and editing. Shengxiao Jia: validation, investigation, resources, data curation, and writing – review and editing. Zhenliang Fan: visualization and supervision. All the authors have read and approved the published version of the manuscript.Data availability
This study was carried out using publicly available data from the National Health and Nutrition Examination Survey (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm and the IEU Open GWAS Project at https://gwas.mrcieu.ac.uk/ (6th March 2024).Conflicts of interest
There are no conflicts to declare. The graphical abstract and Fig. 1 were created using Figdraw (https://www.figdraw.com).Acknowledgements
This project was funded by the National Natural Science Foundation of China (No. 82104756) and the Natural Science Foundation of Zhejiang Province (No. LQ22H270002). We gratefully acknowledge the authors and participants of all NHANES and GWAS from which we used the statistical data.References
- F. Zegers-Hochschild, G. D. Adamson, S. Dyer, C. Racowsky, J. de Mouzon, R. Sokol, L. Rienzi, A. Sunde, L. Schmidt, I. D. Cooke, J. L. Simpson and S. van der Poel, The International Glossary on Infertility and Fertility Care, 2017, Fertil. Steril., 2017, 108, 393–406 CrossRef PubMed.
- Practice Committee of the American Society for Reproductive Medicine, Definitions of infertility and recurrent pregnancy loss: a committee opinion, Fertil. Steril., 2020, 113, 533–535 CrossRef PubMed.
- M. Vander Borght and C. Wyns, Fertility and infertility: Definition and epidemiology, Clin. Biochem., 2018, 62, 2–10 CrossRef.
- E. Bouko-Levy, C. Vialaret, C. Sallée, P. Marquet, F. Margueritte, L. Dion, V. Lavoue and T. Gauthier, Estimation of the prevalence of uterine infertility and its different causes in France according to data from a literature review, J. Gynecol. Obstet. Hum. Reprod., 2023, 52, 102684 CrossRef CAS.
- E. Harris, Infertility Affects 1 in 6 People Globally, J. Am. Med. Assoc., 2023, 329, 1443 Search PubMed.
- A. Omidvar-Mehrabadi, F. Ebrahimi, M. Shahbazi and M. Mohammadnia-Afrouzi, Cytokine and chemokine profiles in women with endometriosis, polycystic ovary syndrome, and unexplained infertility, Cytokine, 2024, 178, 156588 CrossRef CAS.
- A. Chandra, C. E. Copen and E. H. Stephen, Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth, Natl. Health Stat. Rep., 2013, 67, 1–18 Search PubMed.
- H. Sun, T. T. Gong, Y. T. Jiang, S. Zhang, Y. H. Zhao and Q. J. Wu, Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017, Aging, 2019, 11, 10952–10991 CrossRef.
- M. Maitin-Shepard, E. F. Werner, L. A. Feig, J. E. Chavarro, S. L. Mumford, B. Wylie, O. J. Rando, A. J. Gaskins, D. Sakkas, M. Arora, R. Kudesia, M. E. Lujan, J. Braun and D. Mozaffarian, Food, nutrition, and fertility: from soil to fork, Am. J. Clin. Nutr., 2024, 119, 578–589 CrossRef CAS PubMed.
- T. Silva, M. Jesus, C. Cagigal and C. Silva, Food with Influence in the Sexual and Reproductive Health, Curr. Pharm. Biotechnol., 2019, 20, 114–122 CAS.
- J. Lyngsø, U. S. Kesmodel, B. Bay, H. J. Ingerslev, A. M. N. Andersen and C. H. Ramlau-Hansen, Impact of female daily coffee consumption on successful fertility treatment: a Danish cohort study, Fertil. Steril., 2019, 112, 120–129 CrossRef PubMed.
- J. Lee, C. W. Choo, K. Y. Moon, S. W. Lyu, H. Kim, J. Y. Lee, J. R. Lee, B. C. Jee, K. Hwang, S. H. Kim and S. K. Park, Risk Factors for Infertility in Korean Women, J. Korean Med. Sci., 2024, 39, e85 CrossRef PubMed.
- J. Jurczewska and D. Szostak-Węgierek, The Influence of Diet on Ovulation Disorders in Women-A Narrative Review, Nutrients, 2022, 14, 1556 CrossRef CAS PubMed.
- K. Smolińska, A. Szopa, J. Sobczyński, A. Serefko and P. Dobrowolski, Nutritional Quality Implications: Exploring the Impact of a Fatty Acid-Rich Diet on Central Nervous System Development, Nutrients, 2024, 16, 1093 CrossRef.
- F. Hammiche, M. Vujkovic, W. Wijburg, J. H. de Vries, N. S. Macklon, J. S. Laven and R. P. Steegers-Theunissen, Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology, Fertil. Steril., 2011, 95, 1820–1823 CrossRef CAS PubMed.
- Z. A. Al-Safi, H. Liu, N. E. Carlson, J. Chosich, M. Harris, A. P. Bradford, C. Robledo, R. H. Eckel and A. J. Polotsky, Omega-3 Fatty Acid Supplementation Lowers Serum FSH in Normal Weight But Not Obese Women, J. Clin. Endocrinol. Metab., 2016, 101, 324–333 CrossRef CAS PubMed.
- L. J. Moran, V. Tsagareli, M. Noakes and R. Norman, Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation, Nutrients, 2016, 8, 10 CrossRef.
- S. L. Mumford, R. W. Browne, K. Kim, C. Nichols, B. Wilcox, R. M. Silver, M. T. Connell, T. L. Holland, D. L. Kuhr, U. R. Omosigho, N. J. Perkins, R. Radin, L. A. Sjaarda and E. F. Schisterman, Preconception Plasma Phospholipid Fatty Acids and Fecundability, J. Clin. Endocrinol. Metab., 2018, 103, 4501–4510 Search PubMed.
- D. García, S. Brazal, A. Rodríguez, A. Prat and R. Vassena, Knowledge of age-related fertility decline in women: A systematic review, Eur. J. Obstet. Gynecol. Reprod. Biol., 2018, 230, 109–118 CrossRef PubMed.
- M. J. Eijkemans, F. van Poppel, D. F. Habbema, K. R. Smith, H. Leridon and E. R. te Velde, Too old to have children? Lessons from natural fertility populations, Hum. Reprod., 2014, 29, 1304–1312 CrossRef.
- R. Wang, Y. Feng, J. Chen, Y. Chen and F. Ma, Association between polyunsaturated fatty acid intake and infertility among American women aged 20-44 years, Front. Public Health, 2022, 10, 938343 CrossRef PubMed.
- H. Xu, Q. Wen, X. Xing, Y. Chen, Q. Zhu, M. Tan, M. Zhang, T. Pan and S. Wu, High Dietary Inflammatory Index increases the risk of female infertility: An analysis of NHANES 2013-2018, Nutr. Res., 2024, 125, 50–60 CrossRef CAS.
- Y. Chen, H. Xu, J. Yan, Q. Wen, M. Ma, N. Xu, H. Zou, X. Xing, Y. Wang and S. Wu, Inflammatory markers are associated with infertility prevalence: a cross-sectional analysis of the NHANES 2013-2020, BMC Public Health, 2024, 24, 221 CrossRef CAS.
- J. Lu, J. Tang, Y. Zou, R. Wu, H. Chen and W. Wang, Association between dietary inflammatory index and self-reported female infertility from the National Health and Nutrition Examination Survey 2013-2020, J. Hum. Nutr. Diet., 2024, 37, 354–364 CrossRef PubMed.
- J. Tang, Y. Xu, Z. Wang, X. Ji, Q. Qiu, Z. Mai, J. Huang, N. Ouyang and H. Chen, Association between metabolic healthy obesity and female infertility: the national health and nutrition examination survey, 2013-2020, BMC Public Health, 2023, 23, 1524 CrossRef PubMed.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee, Female age-related fertility decline. Committee Opinion No. 589, Fertil. Steril., 2014, 101, 633–634 CrossRef.
- M. A. Emokpae and S. I. Brown, Effects of lifestyle factors on fertility: practical recommendations for modification, Reprod. Fertil., 2021, 2, R13–R26 Search PubMed.
- B. V. Rossi, M. Abusief and S. A. Missmer, Modifiable Risk Factors and Infertility: What are the Connections?, Am. J. Lifestyle Med., 2014, 10, 220–231 CrossRef PubMed.
- J. D. Kloss, M. L. Perlis, J. A. Zamzow, E. J. Culnan and C. R. Gracia, Sleep, sleep disturbance, and fertility in women, Sleep Med. Rev., 2015, 22, 78–87 CrossRef.
- S. Untaaveesup, B. Chongthanadon, C. Kositamongkol, P. Phisalprapa, K. Panyakhamlerd and V. Titapant, Economic evaluation of lifestyle interventions in infertility management: A systematic review, PLoS One, 2024, 19, e0306419 CrossRef CAS.
- M. D. Jørgensen, E. M. Mikkelsen, E. E. Hatch, K. J. Rothman, L. A. Wise, H. T. Sørensen and A. S. D. Laursen, Socioeconomic status and fecundability in a Danish preconception cohort, Hum. Reprod., 2023, 38, 1183–1193 CrossRef.
- N. Andlib, S. Prabha and S. C. Thakur, Unraveling the molecular pathogenesis of Type 2 Diabetes and its impact on female infertility: A bioinformatics and systems biology approach, Comput. Biol. Med., 2024, 180, 108987 CrossRef CAS PubMed.
- W. Xiong, L. Han, X. Tang, R. Li, W. Chen, X. Liu, H. Nie, W. Qin and L. Ling, Maternal Hypertension and Fecundability: A Population-Based Cohort Study, Hypertension, 2024, 81, e173–e184 CAS.
- T. Yang, J. Zhao, F. Liu and Y. Li, Lipid metabolism and endometrial receptivity, Hum. Reprod. Update, 2022, 28, 858–889 CrossRef CAS PubMed.
- S. Hunt and B. Vollenhoven, Pelvic inflammatory disease and infertility, Aust. J. Gen. Pract., 2023, 52, 215–218 CrossRef.
- Y. Zhang and X. Liu, Effects of physical activity and sedentary behaviors on cardiovascular disease and the risk of all-cause mortality in overweight or obese middle-aged and older adults, Front. Public Health, 2024, 12, 1302783 CrossRef.
- Q. Wang, Q. Shi, J. Lu, Z. Wang and J. Hou, Causal relationships between inflammatory factors and multiple myeloma: A bidirectional Mendelian randomization study, Int. J. Cancer, 2022, 151, 1750–1759 CrossRef CAS PubMed.
- N. Papadimitriou, N. Dimou, K. K. Tsilidis, B. Banbury, R. M. Martin, S. J. Lewis, N. Kazmi, T. M. Robinson, D. Albanes, K. Aleksandrova, S. I. Berndt, D. T. Bishop, H. Brenner, D. D. Buchanan, B. Bueno-de-Mesquita, P. T. Campbell, S. Castellví-Bel, A. T. Chan, J. Chang-Claude, M. Ellingjord-Dale, J. C. Figueiredo, S. J. Gallinger, G. G. Giles, E. Giovannucci, S. B. Gruber, A. Gsur, J. Hampe, H. Hampel, S. Harlid, T. A. Harrison, M. Hoffmeister, J. L. Hopper, L. Hsu, J. M. Huerta, J. R. Huyghe, M. A. Jenkins, T. O. Keku, T. Kühn, C. La Vecchia, L. Le Marchand, C. I. Li, L. Li, A. Lindblom, N. M. Lindor, B. Lynch, S. D. Markowitz, G. Masala, A. M. May, R. Milne, E. Monninkhof, L. Moreno, V. Moreno, P. A. Newcomb, K. Offit, V. Perduca, P. D. P. Pharoah, E. A. Platz, J. D. Potter, G. Rennert, E. Riboli, M. J. Sánchez, S. L. Schmit, R. E. Schoen, G. Severi, S. Sieri, M. L. Slattery, M. Song, C. M. Tangen, S. N. Thibodeau, R. C. Travis, A. Trichopoulou, C. M. Ulrich, F. J. B. van Duijnhoven, B. Van Guelpen, P. Vodicka, E. White, A. Wolk, M. O. Woods, A. H. Wu, U. Peters, M. J. Gunter and N. Murphy, Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis, Nat. Commun., 2020, 11, 597 CrossRef CAS PubMed.
- X. Liang, L. Liang and Y. Fan, Two-sample mendelian randomization analysis investigates ambient fine particulate matter's impact on cardiovascular disease development, Sci. Rep., 2023, 13, 20129 CrossRef CAS PubMed.
- E. Sanderson, Multivariable Mendelian Randomization and Mediation, Cold Spring Harbor Perspect. Med., 2021, 11, a038984 CrossRef CAS PubMed.
- L. Peng, X. Luo, B. Cao and X. Wang, Unraveling the link: environmental tobacco smoke exposure and its impact on infertility among American women (18-50 years), Front. Public Health, 2024, 12, 1358290 CrossRef.
- W. Xu, Y. You, T. Yu and J. Li, Insights into Modifiable Risk Factors of Infertility: A Mendelian Randomization Study, Nutrients, 2022, 14, 4042 CrossRef CAS.
- N. Salari, F. Babajani, A. Hosseinian-Far, R. Hasheminezhad, N. Abdoli, P. Haydarisharaf and M. Mohammadi, Global prevalence of major depressive disorder, generalized anxiety, stress, and depression among infertile women: a systematic review and meta-analysis, Arch. Gynecol. Obstet., 2024, 5, 1833–1846 CrossRef.
- S. van Buuren and K. Groothuis-Oudshoorn, mice: Multivariate Imputation by Chained Equations in R, J. Stat. Softw., 2011, 45, 1–67 Search PubMed.
- N. Sasaki, L. E. Jones and D. O. Carpenter, Fish consumption and omega-3 polyunsaturated fatty acids from diet are positively associated with cognitive function in older adults even in the presence of exposure to lead, cadmium, selenium, and methylmercury: a cross-sectional study using NHANES 2011-2014 data, Am. J. Clin. Nutr., 2024, 119, 283–293 CrossRef CAS.
- D. H. Lee, N. Keum, F. B. Hu, E. J. Orav, E. B. Rimm, W. C. Willett and E. L. Giovannucci, Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study, Br. Med. J., 2018, 362, k2575 CrossRef.
- S. Burgess, F. Dudbridge and S. G. Thompson, Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods, Stat. Med., 2016, 35, 1880–1906 CrossRef PubMed.
- J. Bowden, M. F. Del Greco, C. Minelli, Q. Zhao, D. A. Lawlor, N. A. Sheehan, J. Thompson and G. D. Smith, Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption, Int. J. Epidemiol., 2019, 48, 728–742 CrossRef PubMed.
- M. F. Greco, C. Minelli, N. A. Sheehan and J. R. Thompson, Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome, Stat. Med., 2015, 34, 2926–2940 CrossRef PubMed.
- S. C. Larsson, A. S. Butterworth and S. Burgess, Mendelian randomization for cardiovascular diseases: principles and applications, Eur. Heart J., 2023, 44, 4913–4924 CrossRef PubMed.
- J. Bowden, G. D. Smith and S. Burgess, Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression, Int. J. Epidemiol., 2015, 44, 512–525 CrossRef.
- M. Verbanck, C. Y. Chen, B. Neale and R. Do, Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases, Nat. Genet., 2018, 50, 693–698 CrossRef CAS PubMed.
- P. Zhao, F. Han, X. Liang, L. Meng, B. Yu, X. Liu and J. Tian, Causal Effects of Basal Metabolic Rate on Cardiovascular Disease: A Bidirectional Mendelian Randomization Study, J. Am. Heart Assoc., 2024, 13, e031447 CrossRef.
- X. Chen, S. Zhang, X. Wu, Y. Lei, B. Lei and Z. Zhao, Inflammatory cytokines and oral lichen planus: a Mendelian randomization study, Front. Immunol., 2024, 15, 1332317 CrossRef CAS.
- M. Abodi, V. De Cosmi, F. Parazzini and C. Agostoni, Omega-3 fatty acids dietary intake for oocyte quality in women undergoing assisted reproductive techniques: A systematic review, Eur. J. Obstet. Gynecol. Reprod. Biol., 2022, 275, 97–105 CrossRef CAS PubMed.
- R. Zarezadeh, A. Mehdizadeh, J. Leroy, M. Nouri, S. Fayezi and M. Darabi, Action mechanisms of n-3 polyunsaturated fatty acids on the oocyte maturation and developmental competence: Potential advantages and disadvantages, J. Cell. Physiol., 2019, 234, 1016–1029 CrossRef CAS PubMed.
- W. Stoffel, B. Holz, B. Jenke, E. Binczek, R. H. Günter, C. Kiss, I. Karakesisoglou, M. Thevis, A. A. Weber, S. Arnhold and K. Addicks, Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids, EMBO J., 2008, 27, 2281–2292 CrossRef CAS PubMed.
- M. Jahangirifar, M. Taebi, M. H. Nasr-Esfahani, M. Heidari-Beni and G. H. Asgari, Dietary Fatty Acid Intakes and the Outcomes of Assisted Reproductive Technique in Infertile Women, J. Reprod. Infertil., 2021, 22, 173–183 Search PubMed.
- S. L. Mumford, J. E. Chavarro, C. Zhang, N. J. Perkins, L. A. Sjaarda, A. Z. Pollack, K. C. Schliep, K. A. Michels, S. M. Zarek, T. C. Plowden, R. G. Radin, L. C. Messer, R. A. Frankel and J. Wactawski-Wende, Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women, Am. J. Clin. Nutr., 2016, 103, 868–877 CrossRef CAS PubMed.
- L. A. Wise, A. K. Wesselink, K. L. Tucker, S. Saklani, E. M. Mikkelsen, H. Cueto, A. H. Riis, E. Trolle, C. J. McKinnon, K. A. Hahn, K. J. Rothman, H. T. Sørensen and E. E. Hatch, Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies, Am. J. Epidemiol., 2018, 187, 60–74 CrossRef PubMed.
- J. E. Chavarro, J. W. Rich-Edwards, B. A. Rosner and W. C. Willett, Dietary fatty acid intakes and the risk of ovulatory infertility, Am. J. Clin. Nutr., 2007, 85, 231–237 CrossRef CAS.
- L. Barrea, A. Arnone, G. Annunziata, G. Muscogiuri, D. Laudisio, C. Salzano, G. Pugliese, A. Colao and S. Savastano, Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS), Nutrients, 2019, 11, 2278 CrossRef CAS.
- S. Kalgaonkar, R. U. Almario, D. Gurusinghe, E. M. Garamendi, W. Buchan, K. Kim and S. E. Karakas, Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS, Eur. J. Clin. Nutr., 2011, 65, 386–393 CrossRef CAS.
- P. Mirabi, M. J. Chaichi, S. Esmaeilzadeh, S. G. A. Jorsaraei, A. Bijani, M. Ehsani and S. F. H. Karooee, The role of fatty acids on ICSI outcomes: a prospective cohort study, Lipids Health Dis., 2017, 16, 18 CrossRef CAS.
- D. C. Wathes, D. R. Abayasekara and R. J. Aitken, Polyunsaturated fatty acids in male and female reproduction, Biol. Reprod., 2007, 77, 190–201 CrossRef CAS.
- J. Stanhiser, A. M. Z. Jukic, D. R. McConnaughey and A. Z. Steiner, Omega-3 fatty acid supplementation and fecundability, Hum. Reprod., 2022, 37, 1037–1046 CrossRef CAS PubMed.
- A. J. Gaskins, R. Sundaram, G. M. B. Louis and J. E. Chavarro, Seafood Intake, Sexual Activity, and Time to Pregnancy, J. Clin. Endocrinol. Metab., 2018, 103, 2680–2688 CrossRef PubMed.
- J. L. Bauer, K. Kuhn, A. P. Bradford, Z. A. Al-Safi, M. A. Harris, R. H. Eckel, C. Y. Robledo, A. Malkhasyan, J. Johnson, N. R. Gee and A. J. Polotsky, Reduction in FSH Throughout the Menstrual Cycle After Omega-3 Fatty Acid Supplementation in Young Normal Weight but not Obese Women, Reprod. Sci., 2019, 26, 1025–1033 CrossRef CAS.
- S. Punia, K. S. Sandhu, A. K. Siroha and S. B. Dhull, Omega 3-metabolism, absorption, bioavailability and health benefits–A review, PharmaNutrition, 2019, 10, 100162 CrossRef.
- K. Albracht-Schulte, N. S. Kalupahana, L. Ramalingam, S. Wang, S. M. Rahman, J. Robert-McComb and N. Moustaid-Moussa, Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update, J. Nutr. Biochem., 2018, 58, 1–16 CrossRef CAS PubMed.
- S. L. Wakefield, M. Lane, S. J. Schulz, M. L. Hebart, J. G. Thompson and M. Mitchell, Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse, Am. J. Physiol.: Endocrinol. Metab., 2008, 294, E425–E434 CrossRef CAS.
- V. S. I. Giorgi, R. A. Ferriani and P. A. Navarro, Omega-3 fatty acids and L-carnitine prevent meiotic oocyte damage induced by follicular fluid from infertile women with endometriosis: an experimental study, JBRA Assist. Reprod., 2023, 27, 610–618 Search PubMed.
- J. A. Attaman, A. K. Stanic, M. Kim, M. P. Lynch, B. R. Rueda and A. K. Styer, The anti-inflammatory impact of omega-3 polyunsaturated Fatty acids during the establishment of endometriosis-like lesions, Am. J. Reprod. Immunol., 2014, 72, 392–402 CrossRef CAS PubMed.
- A. Akyol, M. Şimşek, R. İlhan, B. Can, M. Baspinar, H. Akyol, H. F. Gül, F. Gürsu, B. Kavak and M. Akın, Efficacies of vitamin D and omega-3 polyunsaturated fatty acids on experimental endometriosis, Taiwan J. Obstet. Gynecol., 2016, 55, 835–839 CrossRef.
- H. Zhang, L. Zheng, C. Li, J. Jing, Z. Li, S. Sun, T. Xue, K. Zhang, M. Xue, C. Cao, L. Ouyang, Z. Qian, R. Xu, Z. He, R. Ma, L. Chen and B. Yao, Effects of gut microbiota on omega-3-mediated ovary and metabolic benefits in polycystic ovary syndrome mice, J. Ovarian Res., 2023, 16, 138 CrossRef CAS.
- R. Ma, S. Wang, M. Xue, H. Zhang, Z. He, K. Jueraitetibaike, X. Ge, L. Chen and B. Yao, Effects of n-3 PUFA supplementation on oocyte in vitro maturation in mice with polycystic ovary syndrome, J. Ovarian Res., 2023, 16, 87 CrossRef CAS PubMed.
- S. Trop-Steinberg, E. M. Heifetz, Y. Azar, I. Kafka, A. Weintraub and M. Gal, Omega-3 Intake Improves Clinical Pregnancy Rate in Polycystic Ovary Syndrome Patients: A Double-Blind, Randomized Study, Isr. Med. Assoc. J., 2023, 25, 131–136 Search PubMed.
- J. Stanhiser, A. M. Z. Jukic and A. Z. Steiner, Serum omega-3 and omega-6 fatty acid concentrations and natural fertility, Hum. Reprod., 2020, 35, 950–957 CrossRef CAS PubMed.
- S. Bianconi, M. E. Santillán, M. D. R. Solís, A. C. Martini, M. F. Ponzio, L. M. Vincenti, H. B. Schiöth, V. P. Carlini and G. Stutz, Effects of dietary omega-3 PUFAs on growth and development: Somatic, neurobiological and reproductive functions in a murine model, J. Nutr. Biochem., 2018, 61, 82–90 CrossRef CAS PubMed.
- D. Nehra, H. D. Le, E. M. Fallon, S. J. Carlson, D. Woods, Y. A. White, A. H. Pan, L. Guo, S. J. Rodig, J. L. Tilly, B. R. Rueda and M. Puder, Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids, Aging Cell, 2012, 11, 1046–1054 CrossRef CAS.
- X. Ma, X. Weng, X. Hu, Q. Wang, Y. Tian, Y. Ding and C. Zhang, Roles of different n-3/n-6 PUFA ratios in ovarian cell development and steroidogenesis in PCOS rats, Food Funct., 2019, 10, 7397–7406 RSC.
- R. K. Saini and Y. S. Keum, Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review, Life Sci., 2018, 203, 255–267 CrossRef CAS.
- W. Zhang and F. Wu, Linoleic acid induces human ovarian granulosa cell inflammation and apoptosis through the ER-FOXO1-ROS-NFκB pathway, Sci. Rep., 2024, 14, 6392 CrossRef CAS PubMed.
- L. L. Oliva, M. E. Santillán, L. C. Ryan, S. Bianconi, L. M. Vincenti, A. C. Martini, M. F. Ponzio and G. Stutz, Mouse plasma progesterone levels are affected by different dietary ω6/ω3 ratios, Horm. Metab. Res., 2014, 46, 120–125 CAS.
- S. Hekmat, M. Sharifzadeh, T. Toliyat, R. S. Kouzehkonan, M. M. Ardestani, M. Tabarrai and S. N. S. Lamardi, Urtica pilulifera L. seed extract promotes folliculogenesis and alleviates the diminished ovarian reserve in the Balb/c mice model: An experimental study, Int. J. Reprod. Biomed., 2024, 22, 111–126 CAS.
- J. K. Innes and P. C. Calder, Omega-6 fatty acids and inflammation, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2018, 132, 41–48 CrossRef CAS.
- Y. D. Prabhu and A. V. Gopalakrishnan, γ-Linolenic acid ameliorates DHEA induced pro-inflammatory response in polycystic ovary syndrome via PPAR-γ signaling in rats, Reprod. Biol., 2020, 20, 348–356 CrossRef.
- C. M. Webster, M. L. Deline and J. L. Watts, Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans, Dev. Biol., 2013, 373, 14–25 CrossRef CAS PubMed.
- Y. Ma, L. Zheng, Y. Wang, Y. Gao and Y. Xu, Arachidonic Acid in Follicular Fluid of PCOS Induces Oxidative Stress in a Human Ovarian Granulosa Tumor Cell Line (KGN) and Upregulates GDF15 Expression as a Response, Front. Endocrinol., 2022, 13, 865748 CrossRef.
- L. Li, S. Zhu, W. Shu, Y. Guo, Y. Guan, J. Zeng, H. Wang, L. Han, J. Zhang, X. Liu, C. Li, X. Hou, M. Gao, J. Ge, C. Ren, H. Zhang, T. Schedl, X. Guo, M. Chen and Q. Wang, Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation, Mol. Cell, 2020, 80, 525–540 CrossRef PubMed.
- C. Liang, X. Zhang, C. Qi, H. Hu, Q. Zhang, X. Zhu and Y. Fu, UHPLC-MS-MS analysis of oxylipins metabolomics components of follicular fluid in infertile individuals with diminished ovarian reserve, Reprod. Biol. Endocrinol., 2021, 19, 143 CrossRef CAS PubMed.
- M. Plourde and S. C. Cunnane, Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements, Appl. Physiol., Nutr., Metab., 2007, 32, 619–634 CrossRef CAS PubMed.
- A. Salas-Huetos, M. Arvizu, L. Mínguez-Alarcón, M. Mitsunami, J. Ribas-Maynou, M. Yeste, J. B. Ford, I. Souter and J. E. Chavarro, Women's and men's intake of omega-3 fatty acids and their food sources and assisted reproductive technology outcomes, Am. J. Obstet. Gynecol., 2022, 227, 246 CrossRef.
- K. S. Broughton, J. Bayes and B. Culver, High α-linolenic acid and fish oil ingestion promotes ovulation to the same extent in rats, Nutr. Res., 2010, 30, 731–738 CrossRef CAS PubMed.
- Q. Chu, Y. X. Yu, J. Z. Zhang, Y. T. Zhang and J. P. Yu, Effects of flaxseed oil supplementation on metaphase II oocyte rates in IVF cycles with decreased ovarian reserve: a randomized controlled trial, Front. Endocrinol., 2024, 15, 1280760 CrossRef PubMed.
- N. M. Hohos, E. M. Elliott, K. J. Cho, I. S. Lin, M. C. Rudolph and M. E. Skaznik-Wikiel, High-fat diet-induced dysregulation of ovarian gene expression is restored with chronic omega-3 fatty acid supplementation, Mol. Cell. Endocrinol., 2020, 499, 110615 CrossRef CAS.
- A. F. Mostafa, S. M. Samir and R. M. Nagib, Omega-3 polyunsaturated fatty acid docosahexaenoic acid and its role in exhaustive-exercise-induced changes in female rat ovulatory cycle, Can. J. Physiol. Pharmacol., 2018, 96, 395–403 CrossRef CAS.
- W. Stoffel, I. Schmidt-Soltau, E. Binczek, A. Thomas, M. Thevis and I. Wegner, Dietary ω3-and ω6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice, Mol. Metab., 2020, 36, 100974 CrossRef CAS.
- M. Oseikria, S. Elis, V. Maillard, E. Corbin and S. Uzbekova, N-3 polyunsaturated fatty acid DHA during IVM affected oocyte developmental competence in cattle, Theriogenology, 2016, 85, 1625–1634 CrossRef CAS.
- N. Nikoloff, A. Campagna, C. Luchetti, A. C. Carranza-Martín, A. M. Pascua, J. M. Anchordoquy, J. P. Anchordoquy, D. M. Lombardo, A. Seoane and C. C. Furnus, Effects of EPA on bovine oocytes matured in vitro with antioxidants: Impact on the lipid content of oocytes and early embryo development, Theriogenology, 2020, 146, 152–161 CrossRef CAS PubMed.
- H. Dadkhah, A. Kazemi, M. H. Nasr-Isfahani and S. Ehsanpour, The Relationship between the Amount of Saturated Fat Intake and Semen Quality in Men, Iran. J. Nurs. Midwifery Res., 2017, 22, 46–50 CrossRef.
- G. Eslamian, N. Amirjannati, B. Rashidkhani, M. R. Sadeghi, A. R. Baghestani and A. Hekmatdoost, Dietary fatty acid intakes and asthenozoospermia: a case-control study, Fertil. Steril., 2015, 103, 190–198 CrossRef CAS PubMed.
- C. González-Ravina, M. Aguirre-Lipperheide, F. Pinto, D. Martín-Lozano, M. Fernández-Sánchez, V. Blasco, E. Santamaría-López and L. Candenas, Effect of dietary supplementation with a highly pure and concentrated docosahexaenoic acid (DHA) supplement on human sperm function, Reprod. Biol., 2018, 18, 282–288 CrossRef.
- F. Haeri, M. Nouri, O. Sadrmanesh, M. Shirani and R. Ghiasvand, The relationship between the intake of dietary fatty acids and minerals with sperm parameters in infertile men, Clin. Nutr. ESPEN, 2023, 58, 201–207 CrossRef PubMed.
- A. Salas-Huetos, M. Mitsunami, S. Wang, L. Mínguez-Alarcón, J. Ribas-Maynou, M. Yeste, I. Souter and J. E. Chavarro, Women's Adherence to Healthy Dietary Patterns and Outcomes of Infertility Treatment, JAMA Netw. Open, 2023, 6, e2329982 CrossRef.
- K. S. Hougaard, H. Hannerz, H. Feveile and J. P. Bonde, Increased incidence of infertility treatment among women working in the plastics industry, Reprod. Toxicol., 2009, 27, 186–189 CrossRef CAS PubMed.
- F. Y. Koochaksaraei, M. Simbar, M. Khoshnoodifar, M. Faramarzi and M. Nasiri, Interventions promoting mental health dimensions in infertile women: a systematic review, BMC Psychol., 2023, 11, 254 CrossRef PubMed.
- M. Sørensen, A. H. Poulsen, B. Nøhr, J. Khan, M. Ketzel, J. Brandt, O. Raaschou-Nielsen and A. Jensen, Long term exposure to road traffic noise and air pollution and risk of infertility in men and women: nationwide Danish cohort study, Br. Med. J., 2024, 386, e080664 CrossRef.
- J. F. Xia, Y. Inagaki, J. F. Zhang, L. Wang and P. P. Song, Chinese medicine as complementary therapy for female infertility, Chin. J. Integr. Med., 2017, 23, 245–252 CrossRef PubMed.
- M. Masjedi, Y. Izadi, T. Montahaei, R. Mohammadi, M. A. Helforoush and K. R. Rad, An illustrated review on herbal medicine used for the treatment of female infertility, Eur. J. Obstet. Gynecol. Reprod. Biol., 2024, 302, 273–282 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04020a |
| This journal is © The Royal Society of Chemistry 2025 |