Effects of UV-C irradiation on the physicochemical properties of freeze-dried beetroot (Beta vulgaris) powder†
Received
31st March 2025
, Accepted 26th June 2025
First published on 1st July 2025
Abstract
UV-C irradiation, known for its germicidal properties, is an effective postharvest food preservation method that enhances bioactive compounds in fresh produce. However, many of these bioactive components are susceptible to thermal degradation, meaning that conventional thermal-based preservation methods may not retain the UV-C induced bioactivity. This study aimed to investigate the effect of UV-C irradiation (1.25 × 10−3 J cm−2) on freeze-dried beetroot to determine whether similar enhancements in bioactive components occur. Compared to the untreated sample, the UV-C treated beetroot powder demonstrated significantly higher loose bulk density, higher tapped bulk density, lower hygroscopicity (p < 0.05), improved flowability, and reduced compressibility. Additionally, the treated powder exhibited significantly lower ash content, and increased levels of total betalains (by 3.9%), total flavonoids (by 1.9%), and ascorbic acid (by 6.6%). These results suggest that UV-C treatment positively impacted several physical characteristics of the beetroot powder, particularly in terms of compactness, flowability, and moisture stability without affecting its solubility or solubility rate. UV-C can serve as an effective processing step to enhance certain bioactive compounds while influencing the mineral content and carbohydrate content of the beetroot freeze-dried powders, potentially offering increased health benefits.
Sustainability spotlight
The application of UV-C irradiation directly to freeze-dried beetroot powder significantly enhances the concentration of the health-promoting bioactive compounds of the powder by increasing the content of betalains, ascorbic acid and flavonoids present. The application of UV-C irradiation in this way leads to a more sustainable utilization of perishable fresh produce as the already shelf stable freeze-dried powder is converted to a version that possesses an enhanced level of bioactivity which can lead to improved public health. Furthermore, as no form of thermal processing is utilized throughout the process, these health-promoting changes occur without compromising the nutritional integrity of the thermally labile compounds present in the produce, thereby supporting UN SDG 3 (good health and well-being).
|
Introduction
In recent years, consumer demand has favoured minimally processed, natural foods free from chemical preservatives. This trend has spurred the development of biological alternatives in the food industry, including antimicrobial essential oils, bioactive edible films and coatings, and innovative preservation technologies.1,2 At the same time, interest in functional foods, which contain health promoting bioactive compounds has grown.3,4 Fresh fruits and vegetables rich in antioxidants, vitamins, minerals, and phytochemicals, are key sources of these compounds.5 The health benefits of a diet rich in fresh produce are well-documented, with numerous studies supporting their positive impact when consumed regularly.6,7
UV-C irradiation, while widely recognized in the food industry for its germicidal properties, has also been shown to enhance bioactive components in fresh produce.8,9 For example, Valerga et al. (2024) reported that postharvest UV-C treatment of carrots led to a nearly threefold increase in phenolic compounds and antioxidant activity, while Menaka et al. (2024) observed increases in total phenolic content, ascorbic acid, and overall antioxidant capacity in guava fruits.10,11 Although thermal preservation methods can extend the shelf life of perishable produce, they often degrade thermally sensitive bioactive compounds, potentially diminishing the benefits of UV-C-induced enhancements.12 Non-thermal technologies like freeze-drying offer a promising alternative, effectively inhibiting metabolic and microbial activity without the use of heat.13 While recent research has demonstrated that UV-C irradiation helps maintain the quality of freeze-dried goat milk powder, the combined application of UV-C treatment and freeze-drying in fresh produce remains largely underexplored, highlighting a critical gap in the current body of research.14
This study aimed to investigate the effect of UV-C irradiation as a post-treatment for freeze-dried produce, using beetroot (Beta vulgaris) as a model. As shown in Fig. 1, beetroot is considered a superfood due to its significant phytochemical content, including bioactive polyphenols and flavonoids.15 Additionally, betalains, the compounds responsible for beetroot's deep red colour, have been shown to possess potent anti-inflammatory, antioxidant, anti-hypertensive, antidiabetic and cholesterol-lowering properties.16 In this study, fresh beetroot was obtained from a local farmers' market, freeze-dried and converted into powder. The powder was then divided into two batches, with one batch subjected to UV-C irradiation, while the other served as the control. The two batches were compared based on their physicochemical properties and nutrient profiles to assess the impact of UV-C treatment on both the quality of the beetroot powder and its bioactive components.
 |
| | Fig. 1 Summary of the phytochemical components of red beetroot (Beta vulgaris). | |
Materials and methods
Chemical reagents
All chemical reagents used in the study were of analytical grade and sourced from Sigma Aldrich (St. Louis, Missouri, USA).
Raw material preparation
Freshly harvested beetroot was purchased from a local farmer's market in central Trinidad. The beets were selected based on uniform size and the absence of any visual defects or deformations. After purchase, they were washed under running tap water, peeled with a vegetable peeler, and sliced into uniform 5 mm pieces with a mechanical slicer (Hobart Corporation, Troy, OH, USA).
Freeze drying
The sliced beets were placed in a freeze dryer (Infitek, LYO60B-1S, Shandong, China) for lyophilization over a period of 48 h. The freeze-dried beet slices were then blended into a fine powder, which was divided into two 40 g batches and labelled as FP (freeze-dried beetroot powder) and UVFP (UV-C treated freeze-dried beetroot powder).
Screening of UV-C treatment
UV-C radiation was provided by 15 W fluorescent germicidal lamps (Cole-Palmer Instrument Co., Chicago, IL, USA) with a peak emission at 254 nm. Irradiation was conducted immediately after freeze drying, and the radiation dose was calculated using the measured radiation intensity (5.2 μW cm−2) and the exposure time (eqn (1)), as measured by a portable digital radiometer (Analytik Jena, Upland, CA, USA). To determine the optimal UV-C treatment for freeze-dried beetroot powder, 1 g samples were exposed to the UV-C radiation for 60, 120, 240, 360, and 600 seconds, corresponding to energy doses of 3.12 × 10−4, 6.24 × 10−4, 1.25 × 10−3, 1.87 × 10−3, and 3.12 × 10−3 J cm−2, respectively. After treatment, the samples were evaluated for betalain content and antioxidant capacity. The energy dose that produced the highest values for both parameters was selected as the optimal treatment condition for subsequent analyses.| | | UVC dose (J cm−2) = UVC intensity (W cm−2) × exposure time (s) | (1) |
UV-C treatment
Based on initial screening results, the sample treated with a UV-C dose of 1.25 × 10−3 J cm−2 exhibited the highest betalain content and antioxidant capacity. As a result, this dose was selected as the optimal treatment for the UV-C-treated freeze-dried beetroot powder (UVFP). The treatment was carried out in a UV-C chamber (Fig. 2), with an exposure time of 240 s to achieve the selected treatment dose. Following UV-C irradiation, the UVFP sample was vacuum-sealed and stored in a desiccator until further analysis.
 |
| | Fig. 2 UVFP samples placed in the UV-C chamber for treatment with UV-C radiation. | |
Physical assessment of powders
Yield.
The powder yield obtained from freeze-dried beetroot was calculated using the following equation (eqn (2)).| |  | (2) |
Colour.
The colour parameters of the powders were measured using a Chroma meter (Konica Minolta, CR-400, Tokyo, Japan). Measurements were taken within the CIE Lab colour space, where L* represents lightness, a* represents the red-green and b* represents the yellow–blue axis. Chroma values were determined (eqn (3)), as well as the total colour difference (ΔE) between day 0 and day 20 (eqn (4)).| |  | (3) |
| |  | (4) |
where L1, a1, b1 represent readings on day 0 and L2, a2, b2 represent readings on day 20.
Bulk density and flowability.
Bulk density and flowability of the freeze-dried powders were determined following the methods outlined by Kapoor et al. (2021).17 Briefly, 10 g of sample was carefully added to a measuring cylinder, and the initial volume of the loose powder was recorded. The cylinder was then tapped on a rubber mat until the volume of the loose powder became stable. The final volume of the powder sample was noted, and both the tapped and loose bulk densities were calculated using eqn (5) and (6). The flowability of the powders was evaluated using the Hausner ratio18 and Carr's compressibility index19 (eqn (7) and (8)).| | 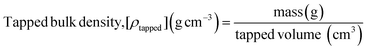 | (5) |
| |  | (6) |
| |  | (7) |
| |  | (8) |
Hygroscopicity.
The hygroscopicity of the powders was determined using a method adapted from O'Donoghue et al. (2019).20 A mass of 1 g of each sample was placed on a pre-weighed watch glass, which was then placed in a desiccator containing a saturated sodium chloride solution. After one week, the watch glasses were re-weighed and the hygroscopicity was calculated using eqn (9).| |  | (9) |
where Wf = final sample weight (g), Wi = initial sample weight (g)
Solubility.
Solubility was determined by transferring 1 g of each sample into to a test tube containing 20 mL of distilled water, following the method of Yousefi et al. (2022).21 The mixtures were vortexed until the samples dissolved and then transferred to labelled 50 mL centrifuge tubes. The samples were then centrifuged at 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g for 15 min. After centrifugation, the sediments were resuspended in 20 mL distilled water, vortexed, and centrifuged again. The resulting sediment was placed on a watch glass and dried in an oven at 105 °C for 4 h. After drying, the sediments were weighed, and the solubility was then calculated (eqn (10)).
g for 15 min. After centrifugation, the sediments were resuspended in 20 mL distilled water, vortexed, and centrifuged again. The resulting sediment was placed on a watch glass and dried in an oven at 105 °C for 4 h. After drying, the sediments were weighed, and the solubility was then calculated (eqn (10)).| | 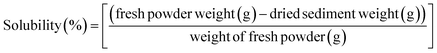 | (10) |
Wettability.
Wettability was assessed following the method described by Kapoor et al. (2021) with minor modifications.17 A 250 mL beaker was filled with 200 mL distilled water at room temperature. A 0.5 g of powder was placed onto the water surface. The wettability was determined by measuring the time taken (s) for the powder to fully submerge.
Particle morphology.
The morphology of the powder particles was examined using scanning electron microscopy (SEM). The samples were first fixed onto a sample holder using double-sided carbon tape and then sputter-coated with a thin layer of gold powder under vacuum. SEM images were recorded using a scanning electron microscope (SEM 515, Philips, Holland) at accelerating voltages of 20 and 30 kV, with magnifications of 110×, 655× and 885×.
Quality parameters of powders
Proximate analysis.
Carbohydrate content was determined using the phenol-sulphuric method, with glucose as the standard as described by Tamboli et al. (2020).22 Crude fibre, moisture and ash contents of the extracted SCP were quantified using AOAC methods.23
Water activity.
The water activity (aw) of the powder was measured according to Todorović et al. (2022) using the Aqua Lab CX-2 water activity meter (Decagon Devices Inc., Pullman, WA, USA).24
Total betalain content (TBC).
The TBC was determined based on the method described by Prieto-Santiago et al. (2020) with minor modifications.25 To a centrifuge tube containing 10 mL of distilled water, 1 g of powdered sample was added and centrifuged at 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g for 15 min. The supernatant was carefully extracted, mixed with 4 mL of distilled water and vortexed. Absorbance was measured using a UV-VIS spectrophotometer (Thermo Scientific Evolution S/N, Madison, USA) at 476 nm and 538 nm for betaxanthins and betacyanins respectively. TBC was calculated using eqn (11).
g for 15 min. The supernatant was carefully extracted, mixed with 4 mL of distilled water and vortexed. Absorbance was measured using a UV-VIS spectrophotometer (Thermo Scientific Evolution S/N, Madison, USA) at 476 nm and 538 nm for betaxanthins and betacyanins respectively. TBC was calculated using eqn (11).| | 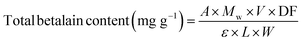 | (11) |
where A = absorbance reading at 538 nm (betacyanins) or 476 nm (betaxanthins), Mw = molecular weight (550 g mol−1 for betacyanins and 308 g mol−1 for betaxanthins), V = extract volume (mL), DF = dilution factor, ε = molar extraction coefficient (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for betacyanins and 48
000 L mol−1 cm−1 for betacyanins and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for betaxanthins), L = cuvette path length (1 cm), W = sample mass (g).
000 L mol−1 cm−1 for betaxanthins), L = cuvette path length (1 cm), W = sample mass (g).
Total phenolic content (TPC) and total flavonoid content (TFC).
TPC was determined using the Folin–Ciocalteu colourimetric method, with gallic acid as the standard. Absorbance was measured at 765 nm, and TPC was expressed as mg of gallic acid equivalents (GAE) per mL of sample. TFC was determined using the aluminium chloride colorimetric method, with quercetin as the standard. Absorbance was measured at 415 nm, and the results were expressed as mg of quercetin equivalents (QE) per mL of sample.
Ascorbic acid content and antioxidant properties.
The ascorbic acid content of the powders was determined using the spectrophotometric method outlined by Papoutis et al. (2016), with L-ascorbic acid as the standard.26 Antioxidant properties were evaluated using the DPPH method as described by Farhan et al. (2024).27 Solutions of 1 g L−1 were prepared from the freeze-dried powders, and 0.1 mL of each solution was mixed with 3.9 mL of a prepared 0.1 mM DPPH solution. After incubation in the dark for 30 min at room temperature, absorbance at 517 nm was measured, and the DPPH scavenging rate was calculated using eqn (12).| |  | (12) |
where A0 = absorbance of the powder/DPPH solution. A1 = absorbance of the powder sample alone. A2 = absorbance of the DPPH solution alone.
Statistical analysis
The qualitative analyses were conducted in triplicate and the results are presented as the average value ± standard deviation. Using Minitab software (version 21.4.10), significant differences were determined using one-way ANOVA, with post-hoc comparisons made using Dunnett's multiple comparison test and Tukey's multiple range test, with a significance level set at p < 0.05.
Results and discussion
Powder physical characteristics
The yield of the freeze-dried beetroot powder was 18.86% which represents an improvement over the previously reported 10–12% reported by Bunkar et al. (2020).28 The physical characteristics of the obtained beetroot powders obtained are summarized in Table 1. The results indicate that the UV-C treated freeze-dried beetroot powder (UVFP) exhibited significantly higher values (p < 0.05) for both loose and tapped bulk density parameters compared to the untreated freeze-dried beetroot powder (FP). Bulk density is a critical parameter for powders as it directly influences both the fluidity and solubility of the product.29 Powders with lower bulk densities typically have more air spaces between particles, which can affect their stability. The presence of these air spaces makes powders more susceptible to oxidation.30 Since the UVFP had higher bulk densities than the FP, it suggests that the UV-C treatment may have altered the structure and size of the beetroot powder particles, causing them to pack more tightly and thus reducing the amount of air spaces. SEM images shown in Fig. 3a–d, support this observation, as UVFP particles appeared smaller and more shrunken compared to FP particles.
Table 1 Characteristics of freeze-dried beetroot powdersa
| Analysis performed |
FPb |
UVFPb |
|
FP = untreated freeze-dried beetroot powder, UVFP = UV-C treated freeze-dried beetroot powder, and different superscript letters in a column indicate significant differences (p < 0.05).
Values are reported as the average ± standard deviation.
|
| Loose bulk density [g cm−3] |
0.31 ± 0.01a |
0.40 ± 0.02b |
| Tapped bulk density [g cm−3] |
0.38 ± 0.01a |
0.48 ± 0.02b |
| Hausner ratio |
1.21 ± 0.02a |
1.19 ± 0.01b |
| Carr's compressibility index |
17.52 ± 0.01a |
16.02 ± 0.01a |
| Hygroscopicity [%] |
14.76 ± 1.0c |
1.92 ± 0.0b |
| Solubility [%] |
80.52 ± 0.19a |
81.69 ± 0.45a |
| Wettability [s] |
15.36 ± 1.54a |
7.47 ± 0.52b |
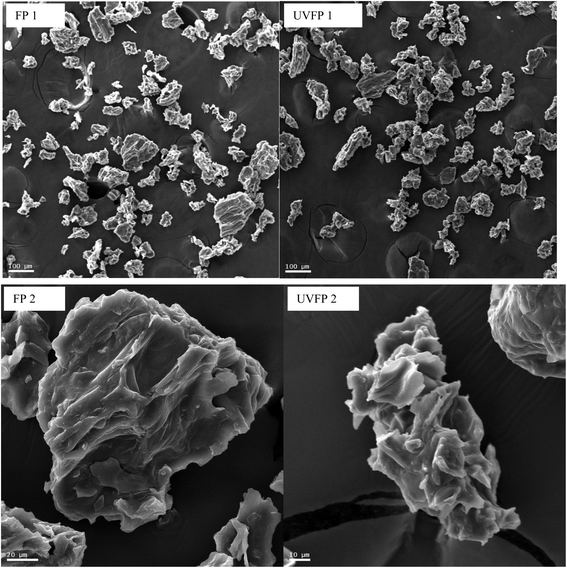 |
| | Fig. 3 Scanning electron microscope images of FP (1 and 2 at 110× and 655× respectively) and UVFP (1 and 2 at 110× and 885× respectively). | |
The flowability of powdered products is an important indicator of how easily particles move or flow. The Hausner ratio is a key indicator of flowability; powders with a ratio below 1.25 are considered to have adequate flowability, while ratios above 1.25 indicate poor flowability.31 Both FP and UVFP demonstrated adequate flowability of 1.21 and 1.19, respectively. However, the UVFP exhibited a significantly lower Hausner ratio than the FP (p < 0.05), indicating improved flowability. Additionally, Carr's compressibility index (CI), another measure of flowability, was lower in the UVFP (16.02) than in the FP (17.52). The CI measures the resistance and stability of powder particles in contact with each other, providing insight into powder flowability. Powders with a CI greater than 25 are considered to have low flowability, while those with a CI below 25 are considered to have acceptable flowability.20 Although the difference in CI was not significant (p > 0.05), the UVFP exhibited better flowability and lower compressibility compared to the FP powder. Although the differences were marginal, the UVFP appeared to be more flowable than the FP, despite its higher density which contrasts with what is typically observed in the literature. Particle size plays a crucial role in determining the flowability of a powder, with a decrease in particle size typically leading to increased cohesion and reduced flowability.32 As shown in Fig. 3, the UVFP particles were smaller than those of the FP. This would suggest that the flowability of the UVFP should have been lower than that of the FP; however, this was not the case.
A possible explanation for this observation is that UV-C treatment altered the surface chemistry of the powdered particles, reducing cohesion and agglomeration despite the small size. Wu and Li (2024) reported similar findings in UV-irradiated Al2O3 powder, where irradiation reduced agglomeration.33 They attributed this to surface oxidation caused by high-energy photons, which generated oxygen vacancies through the oxidation of surface hydroxyl and carboxyl groups. This reduced the polarity of the powder particles and weakened inter-particle hydrogen bonding, leading to less agglomeration and improved flowability.
Another explanation for the observed difference in flowability between the freeze-dried powders may be attributed to their varying hygroscopic properties. Hygroscopicity refers to a powder's ability to absorb moisture from the air, depending on its physical and chemical structure.34 Typically, an increase in water content correlates with a decrease in powder flowability due to the caking and agglomeration of particles.34 As shown in Table 1, the FP exhibited significantly higher (p < 0.05) hygroscopicity (14.76%) compared to the UVFP (1.92%). This substantial difference could explain the variation in flowability between the two powders. The FP, with its greater affinity for ambient moisture, was more susceptible to humidity, likely leading to particle agglomeration and reduced flowability. In its natural state, beetroot contains high moisture content and soluble components, which upon freeze drying, readily absorb moisture from the surrounding environment. On the other hand, UV-C treatment alters the structure of intramembranous proteins, affecting their ability to interact with moisture.35 This structural change likely explains why the UVFP exhibited reduced hygroscopicity and consequently improved flowability. The significantly lower hygroscopicity shown with the UVFP made it more stable and less prone to moisture uptake and may result in better storage stability, as lower moisture absorption can prevent clumping and dehydration.
The dissolving properties of the powders were assessed through solubility and wettability tests. Solubility is a key factor in how easily powders can be rehydrated, which is particularly importance for consumer use, as it influences how well the powders integrate into various food applications. In this study, the solubility of both FP (80.52%) and UVFP (81.69%) was not significantly different (p > 0.05) and was higher than that of similar freeze-dried fruit and vegetable powders reported in the literature. For example, freeze-dried mango powder has a solubility of 45.90%, while dragon fruit powder, shows a solubility of around 60%.36,37
While solubility values were similar, a significant difference (p < 0.05) was observed in the wettability of the powders. Wettability refers to a powder's ability to overcome the surface tension between its particles and a liquid, allowing it to submerge.38 Particle size is often a good indicator of the wettability, as larger particles typically have more space between them, making it easier for water to penetrate these gaps.38 The SEM images indicated that the FP particles appeared slightly larger than the UVFP particles, so it was expected that the FP would have a faster wettability time. However, this was not the case. As shown in Table 1, the UVFP had a significantly shorter wettability time it can be seen that the wettability time (7.47 s) compared to the FP (15.36 s), indicating that the UVFP had much better wettability, possibly due to changes in particle structure or surface characteristics that make the powder more hydrophilic. This is a beneficial characteristic, especially in applications where rapid dispersion of the powder in liquids is required and is an advantageous trait for applications in liquid formulations. This observation aligns with the findings Taskin et al. (2021) who studied the wettability of freeze-dried cranberry bush powder at different particle sizes. Their results indicated that the powder with the smallest particle size had the fastest wettability time.39
Quality assessment of powders
Colour stability.
The colour parameters of both freeze-dried beetroot powders over the 20 day storage period are presented in Fig. 4a–e. The results indicate that both powders darkened during storage, as evidenced by the slightly lower L* values at day 20 (Fig. 4a). Additionally, the UV-C treated freeze-dried powder (UVFP) consistently exhibited lower L* values than the untreated freeze-dried powder (FP) throughout the storage period, with a slightly greater overall decrease observed by day 20 compared to the FP (2.56 vs. 2.25, respectively). This finding aligns with the results of Ramirez-Fajardo et al. (2024) who reported a reduction in L* values in dehydrated tomatoes following UV-C treatment.40
 |
| | Fig. 4 Comparison of colour parameters of the freeze-dried beetroot powders over a 20 day period using L*a*b* values (a–c), chroma values (d) and the total colour difference, (ΔE) between day 0 and day 20 (e). FP = untreated freeze-dried beetroot powder, UVFP = UV-C treated freeze-dried beetroot powder. *Values are reported as average ± standard deviation and different superscript letters in a column indicate values are significantly different (p < 0.05). | |
Regarding the a* values, both powders had similar values at day 0 (p > 0.05). However, by day 20, the UVFP exhibited a significant decrease, with values significantly lower (p < 0.05) than the FP (Fig. 4b). A similar trend was observed with for the b* values. Although the UVFP had a significantly higher b* value at day 0 (p < 0.05), by day 20, it was significantly lower than the FP (p < 0.05) (Fig. 4c). Furthermore, although no significant difference in chroma values was observed at day 0 (p > 0.05), by day 20, the FP had significantly higher chroma values than the UVFP (p < 0.05) (Fig. 4d). Chroma values represent the saturation or intensity of colour in a sample.41 Betalains, the pigments responsible for the red-purple colour of beets, contain aromatic rings and multiple double bonds that can degrade upon UV exposure.42,43 Thus, the UV-C treatment applied to the UVFP in this study likely induced structural changes in betalain compounds, resulting in lower chroma values compared to the untreated FP.
Consistent with these observations, the total colour difference (ΔE) between day 0 and day 20 (Fig. 4e), was significantly greater in the UVFP, at ΔE = 4.30, compared to ΔE = 1.97 in the FP. This nearly two-fold increase suggests that UV-C irradiation substantially reduced colour stability during storage. Given that colour retention is critical for the use of beetroot powder as a natural colorant, particularly in the food and beverage applications, this decline in visual quality may limit the commercial viability of UVFP in its current form. To mitigate this issue, strategies such as encapsulation and modified atmosphere packaging (MAP) may be effective. Encapsulation of betalains using carriers like maltodextrin and other biopolymers has been shown to improve pigment stability and extend shelf life, potentially preserving the initial colour characteristics of UV-C treated powders during storage.44 Similarly, reducing oxygen exposure through MAP can help minimize oxidative degradation, further enhancing colour retention and overall product consistency.
Proximate analysis of powder.
The results presented in Table 2 show that both the FP and UVFP had similar moisture content and water activity levels, with no significant differences observed (p > 0.05). These findings were expected, as UV-C treatment is a non-thermal form of irradiation and would not be anticipated to cause moisture loss in the samples. No significant difference was observed in the crude fibre content of the powders. However, regarding the ash content, the UVFP exhibited a significantly lower value than the FP, suggesting that the UV-C treatment negatively affected the total mineral content of the beetroot powder. This finding is consistent with the study by Adetuyi et al. (2019) which reported that UV-C exposure as a post-harvest treatment to Clerodendrum volubile leaves caused notable change in the mineral composition.45 Their results showed that minerals such as phosphorous, manganese, sodium and calcium increased following UV-C exposure, while potassium levels decreased. This is of particular interest, as potassium is the most abundant mineral present in beetroot (260–407 mg/100 g).46 Thus, it is possible that the UV-C exposure in this study led to a reduction in potassium levels of the UVFP. Since potassium is typically the predominant mineral in beetroot, this decrease could explain the lower mineral content observed in UVFP compared to the untreated FP.
Table 2 Analytical assessment results of freeze-dried beetroot powdersa
| Analysis performed |
FPb |
UVFPb |
|
FP = untreated freeze-dried beetroot powder, UVFP = UV-C treated freeze-dried beetroot powder, and different superscript letters in a column indicate values are significantly different (p < 0.05).
Values are reported as average ± standard deviation.
|
| Powder yield [%] |
18.86 ± 0.02 |
— |
| Water activity |
0.31 ± 0.01a |
0.31 ± 0.01a |
| Moisture content [%] |
3.88 ± 0.76a |
4.86 ± 0.04a |
| Crude fibre content [%] |
4.44 ± 0.18a |
4.68 ± 0.20a |
| Ash/Mineral content [%] |
6.96 ± 0.03a |
5.81 ± 0.02b |
| Total carbohydrate content [%] |
21.05 ± 0.02a |
33.89 ± 0.02b |
| Total soluble solids (°Brix) |
35.67 ± 0.6a |
34.33 ± 0.6b |
| pH |
6.25 ± 0.02a |
6.05 ± 0.02b |
| Betaxanthin content [mg g−1 DM] |
16.03 ± 0.06a |
18.11 ± 0.05b |
| Betacyanin content [mg g−1 DM] |
49.13 ± 0.06a |
49.08 ± 0.05a |
| Total betalain content [mg g−1 DM] |
62.81 ± 0.35a |
65.23 ± 0.32b |
| Total phenolic content [%] |
12.78 ± 0.01a |
12.96 ± 0.02a |
| Total flavonoid content [%] |
7.69 ± 0.01a |
9.58 ± 0.01b |
| Ascorbic acid content [%] |
31.85 ± 0.02a |
38.49 ± 0.02b |
| Total antioxidant capacity [%] |
70.11 ± 0.05a |
70.45 ± 0.06a |
Additionally, the effect of the UV-C treatment was evident in the carbohydrate content of the powders. The UVFP exhibited a significantly higher carbohydrate content (33.89%) compared to the FP (21.05%) (p < 0.05), suggesting that UV-C exposure enhanced the availability of total carbohydrates. Similar findings were reported by Djaoud et al. (2024), who observed that UV-C irradiation increased the soluble carbohydrate content in dates (Phoenix dactylifera L.) depending on the exposure time.47 The observed increase in carbohydrate content in the UVFP can be attributed to the UV-C-induced breakdown of complex polysaccharides in the beetroot powder. Rodríguez-Rodríguez et al. (2019) reported a similar effect, where UV-C irradiation hydrolyzed polysaccharides and oligosaccharides in Aloe vera gel, leading to an increase measurable glucose content.48 Since the total carbohydrate content in this study was measured using the phenol-sulfuric acid method with glucose as the standard, the enhanced release of glucose subunits likely contributed to the significantly higher carbohydrate values in the UVFP compared to the untreated FP. The UV-C treatment also led to a significantly lower (p < 0.05) total soluble solid content in the UVFP (34.33 ± 0.06) compared to the FP (35.67 ± 0.06). This trend was also reported by Pelaic et al. (2022) who showed that increasing UV-C exposure, led to a decrease in total soluble solids in potato.49 They suggested that the degradative effects of UV-C irradiation on the conjugated bonds in some of the soluble solids led to an alteration of their chemical composition, resulting in a lower total soluble solid content.
Bioactive compounds.
Betalains, consisting of betacyanins and betaxanthins are water-soluble nitrogenous pigments derived from betalamic acid that give beetroot its characteristic deep purple-red colour. These pigments are primary responsible for the bioactive properties of beetroot, as research has shown they are directly linked to a reduction of oxidative stress, a decrease in bad cholesterol, and the protection of DNA from damage.50 The total betalain content of a sample represents the sum of both of the betacyanin and betaxanthin contents. In this study, although there was no significant difference (p > 0.05) in the betacyanin contents, the betaxanthin content of the UVFP was significantly higher (p < 0.05) than the FP. This resulted in the UVFP displaying a significantly higher total betalain content (65.23 mg per g DM) compared to the FP (62.81 mg per g DM) (p < 0.05). While some studies have indicated that photons from UV-C exposure can be absorbed by the aromatic rings of betalains, potentially causing structural changes to the molecule, other studies suggest that controlled UV-C exposure can actually enhance betalain content.51 It was found that while extended UV-C irradiation (30 min) decreased betalain content in fresh beetroot, a 10 minutes exposure resulted in the highest betalain levels.52 Previous research has also shown that postharvest UV-C irradiation can increase the total phenolic and total flavonoid content of produce.53 It was proposed that this effect is likely due to the increased activity of the phenylalanine ammonia lyase (PAL) enzyme.26 PAL is a key enzyme in the phenolic compound synthesis pathway, catalysing the conversion of L-phenylalanine to trans-cinnamic acid.41 Furthermore, betalains are more stable at lower, acidic pH levels, as they can become hydrolysed at higher, more alkaline pH levels.54 Although the values were still relatively close, the pH levels of the FP were significantly higher than the UVFP, which may explain why the betalain content was higher in the UVFP than in the FP.
In this study, UV-C treatment had a more pronounced effect on the total flavonoid content (TFC) than on the total phenolic content (TPC). Although the difference was not statistically significant (p > 0.05), the UVFP exhibited a slightly higher total phenolic content (TPC) than the untreated FP. This marginal increase may be attributed to the UV-C-induced reduction in particle size (Fig. 3), which could enhance the release and extractability of phenolic compounds. Since phenolics are often bound to cell wall components such as cellulose, pectin, and structural proteins, disruption of cellular structures could increase their accessibility.55 Supporting this, Yu et al. (2024) reported that microstructural preservation in ultrasonic osmotic dehydrated lotus root was key to retaining phenolic content, implying that structural alterations observed in the UVFP, may facilitate greater phenolic release.56 However, the lack of statistical significance suggests that the UV-C exposure applied in this study may not have been sufficient to induce a substantial release of phenolic compounds from the cell wall.
Nevertheless, the TFC of the UVFP was significantly higher (p < 0.05) than that of the FP showing a 1.9% increase. This indicated that UV-C treatment promoted the synthesis of flavonoid compounds in the beetroot powder. Flavonoids are synthesized in plants as a response to various stresses, including UV exposure, heat, and water. Studies have shown that UV-C exposure can enhance the activity of key flavonoid-synthesis enzymes such as phenylalanine ammonia lyase (PAL) and chalcone synthase.57 Although enzymatic activity typically decreases during drying, PAL activity was retained in freeze-dried wheat seedlings even after three months of storage.58 Given that flavonoids are well known for their antioxidant, anti-inflammatory and antiviral properties, the increased TFC in the UVFP suggests that UV-C radiation may be an effective processing step for producing more bioactive powders.
Regarding ascorbic acid content, the UVFP exhibited a significantly higher value (38.49%) compared to the FP (31.85%) (p < 0.05). UV-C irradiation stimulates the L-galactono-1,4-lactone dehydrogenase (GalDH) enzyme, which catalyses the final step of ascorbic acid synthesis.59 It has been reported that UV-C treatment minimized the breakdown of ascorbic acid during storage in acerola fruit.59 In contrast, while another study found no effect of UV-C on ascorbic acid content in lemon pomace powder, this study showed that UV-C treatment significantly increased ascorbic acid content in freeze-dried beetroot powder.26 Numerous studies have shown that UV-C irradiation, by increasing antioxidant compounds like phenols and flavonoids, also enhances the overall antioxidant capacity of produce.60 A recent study reported that antioxidant enzymes such as superoxide dismutase, remain active even after freeze-drying. Similarly, another study found that moderate UV-C treatment of lemon pomace powder led to a significant increase in antioxidant activity.26,61 Despite the higher ascorbic acid content and flavonoid content in the UVFP, no significant differences were observed between the antioxidant capacities of the UVFP and FP in this study, which contradicts some literature findings. One possible explanation is that the DPPH antioxidant assay used in this study may not have been sensitive enough to detect differences. In support of this, Zhou et al. (2024) demonstrated that the DPPH assay exhibited lower detection efficiency and responsiveness compared to the ABTS assay in the antioxidant evaluation of Manuka honey.62 Furthermore, similar findings were reported by Papoutsis et al. (2016) where UV-C treated lemon pomace powder showed significantly improved antioxidant properties with the FRAP and CUPRAC assays, but no significant effect in the DPPH assay.26
Conclusions
This study investigated the effect of UV-C irradiation as a post-processing treatment on freeze-dried produce, specifically freeze-dried beetroot powder. The results showed that UV-C treatment did not cause significant moisture loss, as expected from a non-thermal treatment nor did it significantly affect the solubility of the freeze-dried beetroot powder. On the other hand, UV-C treated powder exhibited significantly higher wettability, loose and tapped bulk densities than untreated powder, most likely due to differences in particle size, making the powder more hydrophilic, dense and less airy. Additionally, UV-C treatment significantly reduced hygroscopicity (p < 0.05), which contributed to slightly improved flowability, despite its higher density and reduced compressibility compared to the untreated powder. The significantly lower hygroscopicity shown with the UVFP made it more stable and less prone to moisture uptake which is important for product shelf life. UV-C treatment also led to a significant reduction (p < 0.05) in ash content and an increase in carbohydrate content in the treated powder. Regarding bioactive compounds in the freeze-dried beetroot powder, UV-C exposure significantly increased (p < 0.05) the total betalain content by 3.9%, total flavonoid content by 1.9% and ascorbic acid content by 6.6%. These findings highlight the potential of UV-C irradiation to enhance the bioactive profile of freeze-dried powders. Since freeze-dried powders are stable when stored properly and can be easily reconstituted, the results of this study suggest that perishable fresh produce can be converted into stable powders with enhanced bioactivity through a combination of freeze-drying and UV-C treatment. However, given the limited scope of this study, further research is warranted to explore the potential applications of UV-C treated freeze-dried produce powders in functional food formulations. In particular, as this study employed only the DPPH assay to assess antioxidant capacity, future investigations should incorporate a broader range of antioxidant assays to comprehensively evaluate the enhanced antioxidant properties of these powders.
Data availability
All the data is presented within the manuscript itself.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: Karishma Dhowtal and Rohanie Maharaj; formal analysis and investigation: Karishma Dhowtal; draft manuscript preparation: Che John; review and editing: Che John and Rohanie Maharaj; supervision: Rohanie Maharaj. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the technical assistance of the laboratory personnel in the Food Science and Technology unit, Chemistry and Physics Departments at the University of the West Indies, St. Augustine Campus, Trinidad. The study was supported by The UWI CRP.3.NOV21.01 grant awarded to Rohanie Maharaj.
References
- E. L. Chávez-Delgado and D. A. Jacobo-Velázquez, Essential oils: recent advances on their dual role as food preservatives and nutraceuticals against the metabolic syndrome, Foods, 2023, 12, 1–27 CrossRef PubMed.
- E. Teshome, S. F. Forsido, H. P. Rupasinghe and E. O. Keyata, Potentials of natural preservatives to enhance food safety and shelf life: a review, Sci. World J., 2022, 2022, 1–11 CrossRef PubMed.
- F. Sgroi, C. Sciortino, A. Baviera-Puig and F. Modica, Analyzing consumer trends in functional foods: a cluster analysis approach, J. Agric. Food Res., 2024, 15, 1–8 Search PubMed.
- M. M. Abedin, R. Chourasia, L. C. Phukon, P. Sarkar and R. C. Ray,
et al.
, Lactic acid bacteria in the functional food industry: biotechnological properties and potential applications, Crit. Rev. Food Sci. Nutr., 2024, 64(29), 10730–10748 CrossRef CAS PubMed.
-
A. Srivastav, A. K. Shukla, M. Kumar, S. Kumar and R. K. Mishr, Nutraceutical bioactive compounds in fruits and vegetables, in Plant Metabolites and Vegetables as Nutraceuticals, ed. R. K. Keservani, B. T. Tung, R. K. Kersharwani and E. D. Ahire, Apple Academic Press, 2024, vol. 1, pp. 111–142 Search PubMed.
- A. R. S. Mateus, A. Pena and A. Sanches-Silva, Unveiling the potential of bioactive compounds in vegetable and fruit by-products: exploring phytochemical properties, health benefits, and industrial opportunities, Curr. Opin. Green Sustainable Chem., 2024, 48, 1–9 Search PubMed.
- S. Boulaajine and H. Hajjaj, Lycopene extracted from tomato – A review, Food Sci. Technol., 2024, 12, 1–14 CAS.
- M. G. Gąstoł and U. Błaszczyk, Effect of magnetic field and UV-C radiation on postharvest fruit properties, Agriculture, 2024, 14, 1–25 CrossRef.
- A. B. Bilgin and G. Güneş, Effects of ultraviolet-C treatment on postharvest physiologies and decay of berries: a review, ITU J. Food Sci. Technol., 2024, 2, 85–100 Search PubMed.
- L. Valerga, R. E. González, M. Mauricci, A. Concellón and P. F. Cavagnaro, Use of UVC radiation as a postharvest stressor to increase phenolic compounds concentration and antioxidant status in purple, orange, and white carrots, Postharvest Biol. Technol., 2024, 211, 112817 CrossRef CAS.
- M. Menaka, R. Asrey, B. R. Vinod, S. Ahamad and N. K. Meena,
et al.
, UV-C irradiation enhances the quality and shelf-life of stored guava fruit via boosting the antioxidant systems and defense responses, Food Bioprocess Technol., 2024, 17, 3704–3715 CrossRef CAS.
- J. S. Chacha, L. Zhang, C. E. Ofoedu, R. A. Suleiman and J. M. Dotto,
et al.
, Revisiting non-thermal food processing and preservation methods—action mechanisms, pros and cons: a technological update (2016–2021), Foods, 2021, 10, 1430 CrossRef CAS PubMed.
- J. Yao, W. Chen and K. Fan, Novel efficient physical technologies for enhancing freeze drying of fruits and vegetables: a review, Foods, 2023, 12, 1–27 Search PubMed.
- Z. Yu, S. Fu, L. Li and Y. Liu, Quality characteristics of goat milk powder produced by freeze drying followed by UV-C radiation sterilization, Food Chem.: X, 2024, 22, 101495 CAS.
- M. Thiruvengadam, I. Chung, R. Samynathan, S. R. H. Chandar and B. Venkidasamy,
et al.
, A comprehensive review of beetroot (Beta vulgaris L.) bioactive components in the food and pharmaceutical industries, Crit. Rev. Food Sci. Nutr., 2022, 64, 708–739 CrossRef PubMed.
- R. M. Martinez, C. P. Melo, I. C. Pinto, S. Mendes-Pierotti and J. A. Vignoli,
et al.
, Betalains: a narrative review on pharmacological mechanisms supporting the nutraceutical potential towards health benefits, Foods, 2024, 13, 3909 CrossRef CAS PubMed.
- N. Kapoor, A. M. Mohite, N. Sharma and D. Sharma, Comparative analysis of freeze dried and spray dried beetroot powder according to physico-chemical, functional and color properties, Bull. Transilvania Univ. Brasov, Ser. II, 2021, 14, 153–162 Search PubMed.
- A. Saker, M. G. Cares-Pacheco, P. Marchal and V. Falk, Powders flowability assessment in granular compaction: what about the consistency of Hausner ratio?, Powder Technol., 2019, 354, 52–63 CrossRef CAS.
- M. M. Camacho, M. A. Silva-Espinoza and N. Martínez-Navarrete, Flowability, rehydration behaviour and bioactive compounds of an orange powder product as affected by particle size, Food Bioprocess Technol., 2022, 15, 683–692 CrossRef CAS.
- L. T. O'Donoghue, M. K. Haque, D. Kennedy, F. R. Laffir and S. A. Hogan,
et al.
, Influence of particle size on the physicochemical properties and stickiness of dairy powders, Int. Dairy J., 2019, 98, 54–63 CrossRef.
- S. Yousefi, M. Kavyanirad, M. Aminifar, W. Weisany and A. M. Mousavi Khaneghah, Yogurt fortification by microencapsulation of beetroot extract (Beta vulgaris L.) using maltodextrin, gum arabic, and whey protein isolate, Food Sci. Nutr., 2022, 10, 1875–1887 CrossRef CAS PubMed.
- F. A. Tamboli, H. N. More, S. S. Bhandugare, A. S. Patil, N. R. Jadhav and S. G. Killedar, Estimation of total carbohydrate content by phenol sulphuric acid method from Eichhornia crassipes (Mart.) solms, Asian J. Res. Chem., 2020, 13, 357–359 CrossRef.
-
AOAC, Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, AOAC, Washington DC, 21st edn, 2019 Search PubMed.
- A. Todorović, L. Šturm, A. Salević-Jelić, S. Lević and I. G. O. Crnivec,
et al.
, Encapsulation of bilberry extract with maltodextrin and gum arabic by freeze-drying: formulation, characterization, and storage stability, Processes, 2022, 10, 1991 CrossRef.
- V. Prieto-Santiago, M. M. Cavia, S. R. Alonso-Torre and C. Carrillo, Relationship between color and betalain content in different thermally treated beetroot products, J. Food Sci. Technol., 2020, 57, 3305–3313 CrossRef CAS PubMed.
- K. Papoutsis, Q. Vuong, P. Pristijono, J. B. Golding and M. C. Bowyer,
et al.
, Enhancing the total phenolic content and antioxidants of lemon pomace aqueous extracts by applying UV-C irradiation to the dried powder, Foods, 2016, 5, 1–10 CrossRef PubMed.
- M. Farhan, Z. Ahmad, M. Waseem, T. Mehmood and M. R. Javed,
et al.
, Assessment of beetroot powder as nutritional, antioxidant, and sensory evaluation in candies, J. Agric. Food Res., 2024, 15, 1–8 Search PubMed.
- D. S. Bunkar, A. Anand, K. Kumar, M. Meena, S. K. Goyal and V. K. Paswan, Development of production technology for preparation of beetroot powder using different drying methods, Ann. Phytomed., 2020, 9, 1–9 Search PubMed.
- E. E. Özdemir, A. Görgüç, E. Gençdağ and F. M. Yılmaz, Physicochemical, functional and emulsifying properties of plant protein powder from industrial sesame processing waste as affected by spray and freeze drying, LWT, 2022, 154, 1–9 Search PubMed.
- H. Pashazadeh, O. Zannou, M. Ghellam, I. Koca, C. M. Galanakis and T. M. Aldawoud, Optimization and encapsulation of phenolic compounds extracted from maize waste by freeze-drying, spray-drying, and microwave-drying using maltodextrin, Foods, 2021, 10, 1–19 CrossRef PubMed.
- C. Lima, M. R. Afonso, S. Rodrigues and A. C. Aquino, Flowability of spray-dried sapodilla pulp powder, J. Food Process Eng., 2022, 45, 1–10 Search PubMed.
- R. Suhag, A. Kellil and M. Razem, Factors influencing food powder flowability, Powders, 2024, 3, 65–76 CrossRef.
- H. Wu and W. Li, Effect of UV irradiation on the densification behavior, microstructure, and microwave dielectric properties of low temperature sintering Al2O3 ceramics, Heliyon, 2024, 10(4), 1–6 Search PubMed.
- E. Juarez-Enriquez, G. I. Olivas, P. B. Zamudio-Flores, S. Perrez-Vega and I. Salmeron,
et al.
, A review on the influence of water on food powder flowability, J. Food Process Eng., 2022, 45, 1–12 Search PubMed.
- Kumar, R. Nayak, S. R. Purohit and P. S. Rao, Impact of UV-C irradiation on solubility of Osborne protein fractions in wheat flour, Food Hydrocolloids, 2021, 110, 105845 CrossRef CAS.
- G. W. Shuen, L. Y. Yi, T. S. Ying, G. C. Y. Von and Y. A. B. Yusof,
et al.
, Effects of drying methods on the physicochemical properties and antioxidant capacity of kuini powder, Braz. J. Food Technol., 2021, 24, 1–14 Search PubMed.
- V. C. Oliveira, M. T. Lourenço, M. J. Paiva, D. J. Guerra and C. W. Silva,
et al.
, Freeze-dried dragon fruit powder: characterization and incorporation in plant-based drink, Observ. La Econ. Latinoam., 2024, 22, 1–18 Search PubMed.
- C. W. Seo, Improved flowability and wettability of whey protein-fortified skim milk powder via fluidized bed agglomeration, Food Sci. Anim. Resour., 2022, 42, 915–927 CrossRef PubMed.
- O. Taskin, G. Izli and N. Izli, Physicochemical and morphological properties of European cranberrybush powder manufactured by freeze drying, Int. J. Fruit Sci., 2021, 21, 1008–1017 CrossRef.
- F. Ramírez-Fajardo, C. Martín-Vizcaíno, I. Rodríguez-García and J. L. Guil-Guerrero, Vegetables treated before drying with natural antioxidants plus UV-C improve color and bioactive compounds, AgriEngineering, 2024, 6, 3635–3651 CrossRef.
- M. M. Zin and S. Bánvölgyi, Emerging technology approach for extractability and stability of betalains from the peel of beetroot (Beta vulgaris L.), Biomass Convers. Biorefinery, 2021, 13, 10759–10769 CrossRef.
- I. Sadowska-Bartosz and G. Bartosz, Biological properties and applications of betalains, Molecules, 2021, 26, 2520 CrossRef CAS PubMed.
- M. Kida and S. Ziembowicz, The influence of ultraviolet radiation, ozonation, and ultrasonic field on the effectiveness of dye removal from aqueous solutions, Appl. Sci., 2025, 15, 2373 CrossRef CAS.
- S. J. Calva-Estrada, M. Jiménez-Fernández and E. Lugo-Cervantes, Betalains and their applications in food: The current state of processing, stability, and future opportunities in the industry, Food Chem.: Mol. Sci., 2022, 3, 1–11 Search PubMed.
- F. O. Adetuyi, K. O. Karigidi, E. S. Akintmehinm and T. F. Fajembola, Effect of postharvest UV-C irradiation as physical elicitor on anti-nutritional factor, B-vitamins and mineral profile of Clerodendrum volubile leaves, Croat. J. Food Technol. Biotechnol. Nutr., 2019, 14, 113–120 Search PubMed.
-
D. Székely and M. Máté, in Red Beetroot (Beta vulgaris L.), ed. P. Kaushik, United Kingdom, IntechOpen, 2023 Search PubMed.
- K. Djaoud, R. De la Peña-Armada, A. García-Alonso, V. Correcher and L. Boulekbache-Makhlouf,
et al.
, UV-C treatment impact on the availability of water-soluble carbohydrates, polyphenols, and antioxidant capacity of an Algerian underutilized date fruit (Phoenix dactylifera L.), Foods, 2024, 13, 1–18 CrossRef PubMed.
- M. Z. Rodríguez-Rodríguez, C. O. Meléndez-Pizarro and J. C. Espinoza-Hicks, Effects of UV-C irradiation and traditional thermal processing on acemannan contained in aloe vera gel blends, Carbohydr. Polym., 2019, 222, 114998 CrossRef PubMed.
- Z. Pelaić, Z. Čošić and M. Repajić,
et al.
, Effect of UV-C irradiation on the shelf-life of fresh-cut potato and its sensory properties after cooking, Food Technol. Biotechnol., 2022, 60(2), 166–177 Search PubMed.
- S. P. Bangar, A. Singh, V. Chaudhary, N. Sharma and J. M. Lorenzo, Beetroot as a novel ingredient for its versatile food applications, Crit. Rev. Food Sci. Nutr., 2022, 63, 8403–8427 CrossRef PubMed.
- K. Bodhipadma, S. Noichinda, P. Wachirabongkoth, A. Thongruang and K. Nathalang,
et al.
, Callus induction and influence of UV-C on betalain content in the callus of an amaranth, Rom. Biotechnol. Lett., 2017, 22, 1–9 Search PubMed.
- A. E. El-Ashry, M. K. El-Bahr and A. M. M. Gabr, Effect of light quality on betalain content of red beet (Beta vulgaris L.) cultured in vitro, Egpt. Pharm. J., 2020, 19, 143–148 Search PubMed.
- Q. Zhang, W. Yang, J. Liu, H. Liu and Z. Lv,
et al.
, Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid
pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.), Postharvest Biol. Technol., 2021, 175, 111490 CrossRef CAS.
- D. M. Lukitasari, R. Indrawati, R. D. Chandra, H. Shioi and T. H. Botosudarmo, pH-dependent stability of major betalains in the encapsulated beetroot extracts (Beta vulgaris L.), J. Food Sci., 2024, 89, 2761–2773 CrossRef CAS PubMed.
- F. Shahidi and A. Hossain, Importance of insoluble-bound phenolics to the antioxidant potential is dictated by source material, Antioxidants, 2023, 12(1), 1–28 CrossRef PubMed.
- Y. Yu, Y. Chen, Y. Wang, X. Sun, Y. Guo, D. Su and H. Xu, Unlocking new drying potential for Lotus root: ultrasonic osmotic dehydration and microwave hot air drying based on phenolic retention and microstructure, Innovative Food Sci. Emerging Technol., 2024, 97, 1–14 Search PubMed.
- E. Solovchenko and M. N. Merzlyak, Screening of visible and UV radiation as a photoprotective mechanism in plants, Russ. J. Plant Physiol., 2008, 55, 719–737 CrossRef.
- K. Sheng, S. Shui, L. Yan, C. Liu and L. Zheng, Effect of postharvest UV-B or UV-C irradiation on phenolic compounds and their transcription of phenolic biosynthetic genes of table grapes, J. Food Sci. Technol., 2018, 55, 3292–3302 CrossRef CAS PubMed.
- M. C. Rabelo, W. Y. Bang, V. Nair, R. E. Alves and D. A. Jacobo-Velazquez,
et al.
, UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production, Sci. Rep., 2020, 10, 1–14 CrossRef PubMed.
- J. González-Villagra, M. Reyes-Díaz, M. Alberdi, M. L. Mora and E. M. Ulloa-Inostroza,
et al.
, Impact of cold-storage and UV-C irradiation postharvest treatments on quality and antioxidant properties of fruits from blueberry cultivars grown in southern Chile, J. Soil Sci. Plant Nutr., 2020, 20, 1751–1758 CrossRef.
- D. Martysiak-Żurowska, P. Rożek and M. Puta, The effect of freeze-drying and storage on lysozyme activity, lactoferrin content, superoxide dismutase activity, total antioxidant capacity and fatty acid profile of freeze-dried human milk, Dry Technol., 2020, 40, 615–625 CrossRef.
- X. Zhou, W. Zhao, Z. Ye, J. Tang and Y. Zhang, Optimizing the methodology for antioxidant activity analysis of Manuka honey, Foods, 2025, 14(8), 1–14 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article * and
Rohanie
Maharaj
* and
Rohanie
Maharaj



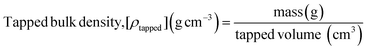




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g for 15 min. After centrifugation, the sediments were resuspended in 20 mL distilled water, vortexed, and centrifuged again. The resulting sediment was placed on a watch glass and dried in an oven at 105 °C for 4 h. After drying, the sediments were weighed, and the solubility was then calculated (eqn (10)).
g for 15 min. After centrifugation, the sediments were resuspended in 20 mL distilled water, vortexed, and centrifuged again. The resulting sediment was placed on a watch glass and dried in an oven at 105 °C for 4 h. After drying, the sediments were weighed, and the solubility was then calculated (eqn (10)).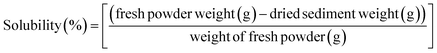
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g for 15 min. The supernatant was carefully extracted, mixed with 4 mL of distilled water and vortexed. Absorbance was measured using a UV-VIS spectrophotometer (Thermo Scientific Evolution S/N, Madison, USA) at 476 nm and 538 nm for betaxanthins and betacyanins respectively. TBC was calculated using eqn (11).
g for 15 min. The supernatant was carefully extracted, mixed with 4 mL of distilled water and vortexed. Absorbance was measured using a UV-VIS spectrophotometer (Thermo Scientific Evolution S/N, Madison, USA) at 476 nm and 538 nm for betaxanthins and betacyanins respectively. TBC was calculated using eqn (11).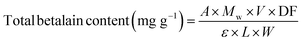
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for betacyanins and 48
000 L mol−1 cm−1 for betacyanins and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for betaxanthins), L = cuvette path length (1 cm), W = sample mass (g).
000 L mol−1 cm−1 for betaxanthins), L = cuvette path length (1 cm), W = sample mass (g).





