Open Access Article
Open Access ArticleVanillin reinforced cationic starch/poly(vinyl alcohol) based antimicrobial and antioxidant bioactive films: sustainable food packaging materials†
Lingaraj Kariyappa
Kurabetta
a,
Saraswati P.
Masti
 *a,
Manjushree Nagaraj
Gunaki
a,
Ajitkumar Appayya
Hunashyal
a,
Ravindra B.
Chougale
b,
Nagarjuna Prakash
Dalbanjan
c and
S. K.
Praveen Kumar
*a,
Manjushree Nagaraj
Gunaki
a,
Ajitkumar Appayya
Hunashyal
a,
Ravindra B.
Chougale
b,
Nagarjuna Prakash
Dalbanjan
c and
S. K.
Praveen Kumar
 c
c
aDepartment of Chemistry, Karnatak Science College, Dharwad-580 001, India. E-mail: dr.saraswatimasti@yahoo.com
bP. G. Department of Studies in Chemistry, Karnatak University, Dharwad-580 003, India
cDepartment of Biochemistry, Karnatak University, Dharwad-580 003, India
First published on 24th June 2025
Abstract
The growing demand for eco-friendly and sustainable packaging solutions has accelerated the development of biodegradable materials for fresh food preservation. In this study, bioactive films were developed using cationic starch (CT) and poly(vinyl alcohol) (PVA), functionalized with varying concentrations of vanillin (VN) via a solvent casting method. The resulting CT/PVA/VN (CPVN) films were systematically characterized to evaluate their functional properties. Notably, films with 3 wt% VN exhibited excellent UV-blocking ability and a remarkable enhancement in tensile strength (∼49.44%) compared to the pristine CT/PVA blend. The incorporation of VN also significantly improved the barrier properties of the films, reducing water vapor permeability (∼60.01%) and moisture adsorption (∼30.54%). Furthermore, the phenolic structure of VN contributed to a substantial increase in antioxidant activity (∼81.33%) and imparted potent antimicrobial activity against foodborne pathogens such as Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans. The CPVN films also demonstrated promising environmental compatibility, achieving over 40% biodegradation in soil within 30 days. These findings highlight VN's multifunctional role in enhancing the structural, barrier, and bioactive properties of CT/PVA films, making CPVN films strong candidates for sustainable food packaging applications.
Sustainability spotlightThe demand for eco-friendly and sustainable packaging films to preserve fresh food products has surged, driven by environmental concerns and the push for greener alternatives. Herein, we developed bioactive films using cationic starch (CT) and poly(vinyl alcohol) (PVA), functionalized with varying concentrations of vanillin (VN) through a solvent casting technique. These CPVN bioactive films showcased notable physico-mechanical, antimicrobial and antioxidant properties, which make CPVN bioactive films a promising choice for sustainable food packaging, addressing environmental pollution concerns by offering an eco-friendly alternative. |
1 Introduction
Biopolymers are naturally occurring polymers produced by living organisms.1 They include a wide range of materials, such as proteins, nucleic acids, and polysaccharides, each serving critical functions in biological systems. Unlike synthetic polymers derived from petroleum, biopolymers are typically biodegradable and can be synthesized sustainably from renewable resources.2 This makes them an alternative option for reducing environmental impact and supporting the development of green technologies. The unique properties and diverse applications of biopolymers have garnered significant interest in the field of food packaging, in order to replace conventional plastic materials, which are the major contributors to environmental trash issues. Biopolymers derived from renewable resources such as starch, cellulose, proteins, and polysaccharides are biodegradable and can significantly reduce the environmental footprint associated with food packaging waste.3,4 Their application in food packaging is driven by their ability to form films and coatings that provide effective barriers against moisture, gases, and contaminants, thereby preserving food quality and extending shelf life. Additionally, biopolymer based packaging can be engineered to possess antimicrobial and antioxidant properties, further enhancing food safety.5–7 As consumer demand for eco-friendly packaging solutions grows and regulatory pressures increase, biopolymers are poised to play a crucial role in transforming the food packaging industry toward more sustainable practices.8Starch is considered to be a prime candidate for packaging material solutions primarily due to its abundance. It can be derived from common carbohydrate sources found in staple foods such as corn, wheat, potato, rice, and tapioca.9,10 The advantages of starch include its low cost, renewability, sustainable manufacturing, and biodegradability. Besides, it also has some limitations, such as brittleness and a highly hydrophilic nature. To overcome these drawbacks, various strategies can be employed to enhance the properties of starch, including chemical modifications. Cationic starch (CT) is a modified form of starch obtained by treating starch with 2-diethylaminoethyl chloride and 2,3-(epoxypropyl) trimethylammonium chloride.11 This modification introduces positive charges onto the starch molecule chain, creating electrostatic links that enhance its interaction. This electrostatic attraction improves the starch's functionality and makes it a highly effective binding agent for various industrial applications,12,13 which plays a key role in this study in order to improvise the intermolecular interactions among the polymers, thereby strengthening the structural integrity within the polymer matrix.
Polyvinyl alcohol (PVA) is a biodegradable synthetic polymer known for its unique properties and wide range of applications. PVA is a water-soluble polymer, exhibits excellent film-forming, emulsifying and adhesive characteristics, making it a valuable component in various industries, especially in the food packaging field. However, PVA alone does not fulfil all the criteria for effective packaging materials, particularly in terms of remarkable tensile strength and water barrier properties. These shortcomings can be addressed by blending PVA with a compatible polymer such as cationic starch (CT). This CT/PVA combination enhances the mechanical properties and improves its water resistance, positioning it as a promising option for food packaging applications. In this context, the previous study by Amalia Cano et al. fabricated PVA/starch based food packaging films infused with ZnO NPs, which demonstrated notable physical and antimicrobial properties.14 Another work by Xugang Dang et al. investigated the antimicrobial properties of oxidized corn starch based nonionic biopolymer (OCSI) and gelatin (Gel) films, which showcased promising characteristics in order to replace petroleum based polymers.15
Vanillin (VN) (4-hydroxy-3-methoxybenzaldehyde) is the primary component of vanilla extract, is being explored for its use in food packaging due to its antimicrobial and antioxidant properties.16,17 The rationale for adding vanillin into packaging materials lies in its ability to extend the shelf life of food products by inhibiting microbial growth and oxidative degradation.18 Its natural origin and pleasant aroma further enhance the value of the packaging, offering notable functional characteristics. Moreover, vanillin is approved by the Food and Drug Administration (FDA) and is Generally Recognized As Safe (GRAS) for use in food and medical applications, making it a safe and effective choice for active packaging systems.19,20 The previous reports by Westlake et al., prepared vanillin crosslinked chitosan films and revealed their potential as promising degradable food packaging materials.21 Another work by Campardelli et al., demonstrated the antimicrobial activity of vanillin loaded zein submicron electro-spun fibres for food packaging applications.22 Based on these observations, it is concluded that the use of VN as an active agent significantly enhances the functional properties of polymeric films. The innovative approach of the present study lies in the use of chemically modified starch, specifically cationic starch (CT), to develop vanillin incorporated CPVN bioactive films through a sustainable and cost-effective solvent casting method. In addition to enhancing the functional properties of the films, this study considers the practical and economic factors essential for future industrial adoption. By systematically evaluating the impact of vanillin incorporation on the CT/PVA polymer matrix, this work investigates key attributes such as mechanical properties, optical characteristics, surface morphology, as well as antimicrobial and antioxidant activities.
With growing consumer demand for sustainable and functional packaging solutions, bioactive films like CPVN offer a promising alternative to conventional plastics. The use of inexpensive, renewable raw materials and an easily scalable fabrication process positions these films as attractive candidates for packaging applications across various food sectors. Their potential to extend shelf life, ensure product safety and meet regulatory standards highlights their relevance for both emerging and established markets. Overall, the development of CPVN bioactive films supports the advancement of market ready, bio-based packaging materials that are commercially viable, environmentally sustainable and aligned with the future of green packaging technologies.
2 Materials and methods
2.1. Materials
Cationic starch (CT) (CAS No.: 56780-58-6) was supplied by Millennium Starch Pvt. Ltd, Athani, Karnataka. Poly(vinyl alcohol) (PVA) (CAS No.: 9002-89-5, Mw ≈ 125000 g mol−1) was procured from Thomas Baker Pvt. Ltd Mumbai, vanillin (VN) (CAS No.: 121-33-5, Mw = 152.15 g mol−1), ascorbic acid (CAS No.: 50-81-7, Mw = 176.12 g mol−1) and Mueller–Hinton agar (MHA) were sourced from Himedia, India. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (CAS No.: 1898-66-4, Mw = 394.32 g mol−1) was provided by SRL Chemicals, India. All reagents used in this study were of analytical grade and the experiments were conducted using Milli-Q water.2.2. Preparation of bioactive films
CPVN bioactive films were developed by implementing a feasible solvent casting approach. In brief, 1 g of CT was dissolved in 50 mL of Milli-Q water at 50 ± 2 °C on a magnetic stirrer for 5 h. Likewise, 2 g of PVA was dispersed in another 50 mL of Milli-Q water at 60 ± 2 °C. Furthermore, CT/PVA film forming solution was prepared by mechanical blending of CT and PVA on a until the formation of a homogeneous solution. Furthermore, VN (0%, 1%, 2% and 3% on dry weight of polymers) was dissolved in 10 mL of ethyl alcohol and blended with CT/PVA film forming solution and labelled as CPVN-0, CPVN-1, CPVN-2 and CPVN-3 respectively as illustrated in Table 1. Afterwards, all the homogeneous solutions were casted on Petri dish and dried at room temperature (27 ± 2 °C). Finally, all the control CPVN and bioactive films were removed from the Petri dish and preserved in a desiccator until further studies.| Sample code | CT (g) | PVA (g) | VN (%) |
|---|---|---|---|
| CPVN-0 | 1 | 2 | 0% |
| CPVN-1 | 1 | 2 | 1% |
| CPVN-2 | 1 | 2 | 2% |
| CPVN-3 | 1 | 2 | 3% |
2.3. Characterization of bioactive films
The optical properties of control CPVN and bioactive films were assessed by the use of UV-vis spectrophotometer (JASCO V670) over UV-vis radiation range of 200–800 nm. Universal testing machine (UTM, DAK System) was employed to evaluate the mechanical parameters of control CPVN and bioactive films in accordance with ASTM D882-91.23 Further, ATR-FTIR spectroscopy was used to study molecular interaction between VN and CT/PVA polymer matrix. Scanning electron microscope (JSM-IT500) and atomic force microscopy (Nanosurf Easyscan 2, Switzerland) were used to examine the surface morphology of prepared films. The water contact angle (WCA) of control CPVN and bioactive films was measured via sessile drop method by utilizing a WCA analyser (DMs-401). Likewise, water barrier properties such as water solubility (WS), moisture adsorption capacity (MAC) and water vapor transmission rate (WVTR) was analysed as per prior methodologies.24–27 The functional properties including antimicrobial, antioxidant and biodegradability of CPVN and bioactive films were studied according to previous reports.28–30 The detailed characterization techniques and analysed methods employed to study control CPVN and bioactive films were given in ESI file.†3 Results and discussion
3.1. Thickness and mechanical studies
The thickness of a film is a vital factor in assessing its physical characteristics. The prepared control CPVN and bioactive films were found to be of uniform thickness (ranging from 0.08 to 0.1 mm) and exhibited smoother surfaces throughout the composition ensuring homogeneity among the VN and CT/PVA polymer matrix.The mechanical parameters such as tensile strength (TS), elongation at break (EB), modulus of elasticity (EM) and stress–strain curve of control CPVN and bioactive films were depicted in Fig. 1(a–d). The control CPVN film exhibited 16.84 ± 1.22 MPa of TS and 219.12 ± 10.14 MPa of EM. It was found that the addition of VN into the CT/PVA polymer matrix showed a synergistic influence on the TS and EM of CPVN bioactive films i.e., CPVN-3 film demonstrated 33.31 ± 0.48 MPa of TS and 398.44 ± 7.16 MPa of EM respectively. This improvement in TS is due to the cross-linking network formed between the functional groups of VN and the CT/PVA polymer matrix. Similar outcomes were reported by Roy et al.,31 suggested that the incorporation of grape seed extract into poly(vinyl alcohol) matrix subsequently boosted the tensile strength. Furthermore, the elongation at break of CPVN bioactive films was reduced from 119.27 ± 2.48% to 53.25 ± 2.01%. This result might be a reason of hydrogen bond formation between hydroxyl groups of CT/PVA and active agent VN, which reduced the chain mobility of CPVN bioactive films. On the other hand, enhanced physical cross-linking caused by the dispersal of VN in the CT/PVA polymer matrix could have increased TS and reduced EB.32,33
 | ||
| Fig. 1 (a) Tensile strength, (b) elongation at break, (c) elastic modulus and (d) stress–strain curve of control CPVN and active films. | ||
3.2. % Transmittance, transparency and opacity
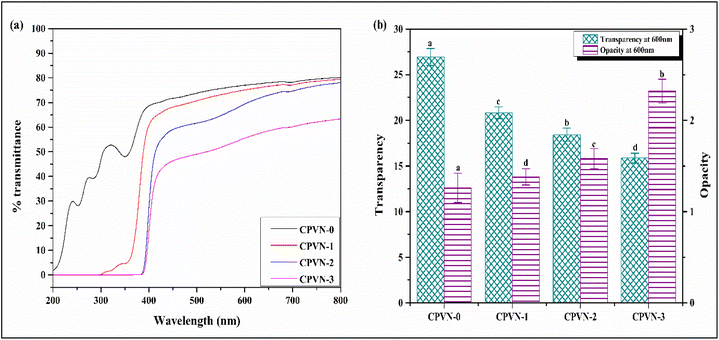
One of the desired properties of packaging material is to shield food from the adverse effects of light, particularly UV radiation, which can cause oxidation, impact the flavour of food and reduces nutritional quality.34Fig. 2(a) demonstrates the % transmittance of control CPVN and bioactive films over a range of 200–800 nm. As shown in Fig. 2(a), CPVN bioactive films exhibit negligible UV light transmission (200–400 nm) compared to control CPVN films, indicating superior UV screening capability. The minimal UV transmission in CPVN bioactive films is likely due to the presence of the aromatic –OH group in VN, which enhances the absorbance in the 200–400 nm range.35,36 These findings suggest that CPVN bioactive films are highly effective as UV barriers.Further, the transparency of bioactive films is calculated by measuring absorbance at 600 nm and the results are shown in Fig. 2(b). It was observed that the transparency of CPVN bioactive films was reduced with the incorporation of VN in the CT/PVA polymer matrix and increased the opacity of bioactive films. This may be attributed to the contraction of the film matrix, which reduced the inter-chain spacing of the polymer and allowed less light to pass through the film.37,38
3.3. FTIR and XRD studies
FTIR spectroscopy is a valuable tool for examining the intermolecular interactions between the active agents and polymer matrix. Fig. 3(a) illustrates the FTIR spectra of active agent VN, control CPVN and active films. Control CPVN film exhibited characteristic peaks at 3265 cm−1 for –OH stretching and 2916 cm−1 for N–H stretching.11 Peaks at 1625 cm−1 and 1547 cm−1 correlates to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and N–H bending respectively. 1017 cm−1 and 861 cm−1 represents stretching of alkoxy –C–O and –C–O–C.39,40 The FTIR spectrum of pure vanillin displays distinct characteristic peaks at 785, 1518 and 1650 cm−1, which are associated with the stretching absorption bands of the benzene ring. The peak at 1660 cm−1 corresponds to the stretching vibration of the C
O stretching and N–H bending respectively. 1017 cm−1 and 861 cm−1 represents stretching of alkoxy –C–O and –C–O–C.39,40 The FTIR spectrum of pure vanillin displays distinct characteristic peaks at 785, 1518 and 1650 cm−1, which are associated with the stretching absorption bands of the benzene ring. The peak at 1660 cm−1 corresponds to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the aldehyde group. Additionally, the peak observed at 1150 cm−1 represent –C–O–C– bond, indicating the presence of ether groups in active agent VN.41
O bond in the aldehyde group. Additionally, the peak observed at 1150 cm−1 represent –C–O–C– bond, indicating the presence of ether groups in active agent VN.41
However, the addition of active agent VN into the CT/PVA polymer matrix led to notable changes in the spectral peaks of CPVN active films. The –OH stretching peak at 3265 cm−1 (for control CPVN) has been shifted to higher wavenumber (3270–3284 cm−1), suggesting the formation of intermolecular hydrogen bonding among the hydroxyl groups of VN and CT/PVA polymer matrix.42 In addition, the peak at 1547 cm−1 shifted to 1550–1568 cm−1. This shift in spectral peak is attributed to cross-linking network formation between –NH2 and –OH of active agent VN and CT/PVA polymer matrix.43 This cross-linking likely improves the structural integrity and stability of the polymer matrix, as the interactions between VN and the CT/PVA create a more interconnected and cohesive molecular structure. These spectral shifts indicate significant changes in the molecular structure of the films due to the incorporation of VN, suggesting alterations in the material's characteristics that could impact its functional properties.
The XRD spectra of active agent VN, control CPVN and active films are shown in Fig. 3(b). The control CPVN film exhibited a crystallinity of 41.42 ± 1.36%, which can be attributed to the inherent crystalline nature of PVA,44,45 a key component of the film. The incorporation of VN into the CPVN active films significantly increased their crystallinity to 54.73 ± 1.71%. This notable rise in crystallinity suggests that there is a substantial interaction between VN and the CT/PVA polymer matrix.46 The increased crystallinity likely results from the formation of a more ordered structure within the matrix,47 driven by the incorporation of VN, which interacts with the CT and PVA components, enhancing the overall crystalline alignment of the polymer chains. This increased crystallinity could influence the physical and mechanical properties of the films, potentially improving their tensile strength and other functional properties, making them more suitable for specific applications.
3.4. Surface morphology
Scanning electron microscopy (SEM) and atomic force microscopy (AFM) are the remarkable imaging techniques used to study the surface morphology of polymeric materials at a high resolution. The SEM micrographs of prepared films are presented in Fig. 4(a). The control CPVN film displayed a surface with noticeable roughness. This roughness is indicative of heterogeneity within the film, suggesting poor compatibility between the CT and PVA components. Such observations are consistent with findings reported in various earlier studies,43,48,49 where similar systems have exhibited rough and uneven surfaces, signifying the challenges in achieving a homogeneous blend between CT and PVA.However, the incorporation of VN into the CT/PVA polymer matrix, the resulting films demonstrated a significantly more compact, dense and homogeneous surface. This transformation in surface morphology suggests that the addition of VN facilitated better interaction between the CT and PVA components. The more uniform surface observed in the SEM images implies that VN acted as a compatibilizer, enhancing the miscibility of the blend components. This improvement is likely due to the formation of intermolecular hydrogen bonds among the functional groups of VN and the polymer matrix, specifically between the hydroxyl groups in CT and PVA with VN. Such interactions can lead to a more uniform polymer network, reducing phase separation and resulting in a smoother surface morphology.50 Furthermore, at higher concentrations of VN, specifically in the CPVN-3 bioactive film, the SEM images reveal the presence of white spots on the surface. These spots are attributed to undissolved particles of VN, which may not have fully integrated into the polymer matrix due to the limited solubility of VN. These observations are in line with previous studies,11,51 where similar phenomena have been reported, indicating that there is a critical concentration of VN beyond which the material's homogeneity is compromised by the presence of residual particles.
Fig. 4(b) presents AFM micrographs of the control CPVN and active films. It was observed that, the control CPVN film exhibited a rougher surface, indicating lower compatibility between PVA and CT. In contrast, the surface roughness decreased with the addition of VN into the CT/PVA polymer matrix, which is attributed due to enhanced intermolecular interactions between the active agent and the polymer matrix leading to the formation of more homogeneous and compact structures. The AFM results are consistent with results of SEM.
3.5. Water contact angle (WCA) measurements
The water contact angle (WCA) is a measure of how water interacts with a surface polymer film, specifically the angle formed between the tangent to the water droplet and the surface at the point of contact. In the packaging field, the water contact angle provides insight into the film's surface properties, such as its ability to resist or absorb moisture. A lower contact angle suggests better wettability and moisture absorption, while a higher contact angle indicates greater water resistance. The WCA results of control CPVN and active films are displayed in Fig. 5.The control CPVN film demonstrated a WCA of 66.4 ± 0.48°, due to the presence of polar groups on the surface of CPVN-0 film. Subsequently, the addition of active agent VN into the CT/PVA polymer matrix enhanced the hydrophobicity of CPVN bioactive films. This remarkable increment in WCA is due to intermolecular hydrogen bond formation between the active agent and polymer matrix and reduced availability of surface polar groups, which hinders the spreading ability of water droplets. These results are consistent with previous findings.52,53
3.6. Moisture adsorption capacity (MAC)
Moisture adsorption capacity (MAC) refers to the phenomenon by which a material attracts and holds moisture from its surrounding environment. This can affect the performance and stability of food packaging films. In the context of packaging, low moisture adsorption is desirable to ensure that the packaging maintains its integrity and effectively protects the food from environmental humidity, which can otherwise lead to spoilage or degradation. The MAC of control CPVN and bioactive films are enlisted in Table 2. It was observed that the control CPVN film showed 16.42 ± 0.48% moisture adsorption capacity. The incorporation of VN into CT/PVA polymer matrix reduced the MAC (%) of CPVN bioactive films to 7.54 ± 0.43%. This significant decrease in MAC (%) is attributed to the formation cross linking network between the functional groups of VN and hydroxyl groups of the CT/PVA polymer matrix. These findings are correlated with WCA results of the present study.| Sample code | Crystallinity (%) | MAC (%) | WS (%) | WVTR (g m−2 h−1) | SBT (%) |
|---|---|---|---|---|---|
| a a–d denotes significant differences (n = 3, p < 0.05). | |||||
| CPVN-0 | 41.42 ± 1.36b | 16.42 ± 0.48b | 28.42 ± 1.63a | 27.48 ± 1.22c | 64.48 ± 2.61a |
| CPVN-1 | 45.38 ± 0.45d | 10.17 ± 0.32d | 20.57 ± 0.71d | 24.36 ± 1.95a | 52.19 ± 0.78d |
| CPVN-2 | 48.54 ± 0.93c | 8.14 ± 0.76a | 18.19 ± 1.12c | 15.76 ± 0.76d | 50.41 ± 1.04c |
| CPVN-3 | 54.73 ± 1.71a | 7.54 ± 0.43c | 12.11 ± 1.19b | 10.99 ± 1.47b | 44.32 ± 1.71b |
3.7. Water solubility (WS)
Water solubility (WS) is the ability of a substance to dissolve in water. In the context of packaging films, water solubility is an important property that influences the material's functionality and durability. For packaging applications, low water solubility is often preferred because it helps to maintain the integrity of the packaging and prevents it from disintegrating or becoming weakened when the packaging film comes in contact with water. This ensures that the packaging can effectively protect the food product and prolong its shelf life.Table 2 consists of water solubility findings of control CPVN and bioactive films. The control CPVN film exhibited 28.42 ± 1.63% of WS due to the availability of free hydroxyl groups on the surface of the film. However, the addition of VN dropped the water solubility to 12.11 ± 1.19%. This might be due to the reduction of free hydroxyl groups on the surface as increasing the content of VN, which limits the interaction of CT/PVA polymer matrix with water molecules and remarkably hinders the water solubility.
3.8. Water vapor transmission rate (WVTR)
The Water vapor transmission rate (WVTR) quantifies the rate at which water vapor passes through a polymer material. It is a key metric for assessing the material's ability to act as a barrier against moisture. A lower WVTR indicates better moisture resistance, meaning the material can more effectively prevent water vapor from penetrating and potentially causing spoilage or degradation of the packaged contents.54 WVTR is crucial in packaging applications where maintaining optimal moisture levels is essential for preserving product quality and extending shelf life.The WVTR results of control CPVN and active films were presented in Table 2. The control CPVN film exhibited a water vapor transmission rate (WVTR) of 27.48 ± 1.22 g m−2 h−1. In contrast, the CPVN-3 active film demonstrated a WVTR of 10.99 ± 1.47 g m−2 h−1. This represents a 60% reduction in the WVTR compared to the control CPVN film. This decrease suggests an improvement in the film's barrier properties against water vapor transmission, which is beneficial for extending the shelf life of packed products or enhancing the performance of the films in humid environments. This observed reduction in WVTR is consistent with the trends reported in other studies.55,56 The decrease in WVTR of the CPVN-3 active films can be attributed to the uniform dispersion of the active agent VN within the polymer matrix. VN interacts with the polymer chains of CT (chitosan) and PVA effectively filling the structural gaps within the matrix. These gaps or voids in the polymer network are potential pathways for water vapor transmission. By occupying these spaces, VN reduces the free volume within the matrix, thereby closing the channels through which water vapor could otherwise pass. The improved dispersion and integration of VN into the CT and PVA polymer matrix likely result from favourable interactions, such as hydrogen bonding between VN and the polymer chains. These interactions lead to a more cohesive and dense polymer network, which in turn enhances the film's ability to resist water vapor permeation.57
3.9. Biodegradation study
Biodegradability refers to the capacity of a substance to break down naturally by the action of microorganisms into simpler, non-toxic components over time. This process typically occurs in the presence of environmental factors like moisture, heat, and oxygen. In the case of packaging polymers, biodegradability is an important characteristic as it determines how quickly and safely the material will decompose after use, reducing environmental impact and waste. Biodegradable materials are often preferred for their sustainability benefits, as they help to minimize landfill accumulation and reduce pollution compared to non-degradable or persistent materials.The biodegradability of control CPVN and bioactive films was determined by employing a soil burial test (SBT) for 30 days and the outcomes are tabulated in Table 2. The control CPVN film demonstrated a degradation rate of 64.48 ± 2.61%. This faster breakdown of control CPVN film is due to the high tendency of starch-based films to be prone to microbial attack.58 Whereas the VN incorporated films had degradation rates ranging from 44.32 ± 1.71% to 52.19 ± 0.78%. Compared to the control film, the bioactive films showed a slower rate of deterioration. This delay can be attributed to the dense structure, reduced solubility and water resistant properties of the CPVN bioactive films, as supported by SEM micrographs, water solubility studies and WVTR measurements. On the other hand, the slower rate of degradation of CPVN bioactive films is a consequence of the inherent antimicrobial properties of VN. These findings are consistent with antimicrobial results. However, all the films showed more than 40% degradation within 20 days, suggesting that CPVN bioactive films are better alternatives to non-degradable plastics.
3.10. Antimicrobial properties
The antimicrobial property of packaging materials enables them to prevent the growth of microorganisms that could lead to food spoilage. This innovative feature is increasingly being integrated into the food packaging field to enhance food safety, extend shelf life, and reduce waste. The incorporation of active agents into packaging materials inhibits microbial growth to protect the food. This approach not only preserves the quality and freshness of the food but also meets the growing consumer demand for safer, longer-lasting products. The antimicrobial mechanism typically unfolds in two key steps such as disruption of the microbial membrane and intracellular targeting. During membrane disruption, antimicrobial agents breach the microbial membrane, creating pores that cause cellular contents to leak out, ultimately resulting in cell death. The second step, intracellular targeting, involves the entry of these agents into microbial cells, where they interact with specific intracellular targets, leading to cellular dysfunction and the eventual inhibition or destruction of the microorganism.59,60 As per the Swiss norm (SN) 195920 and ASTM E2149-01 standards, substances exhibiting an inhibition zone greater than 1 mm are known as effective antimicrobial agents.61In this study, the antimicrobial efficacy of control CPVN and active films was assessed against various foodborne microbes, including Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), and Candida albicans (C. albicans). The antimicrobial activity of the control CPVN and active films is demonstrated in Fig. 6, showcasing their effectiveness against these foodborne pathogens. The inhibition zones corresponding to this activity are shown in Table 3. The control CPVN film exhibited inhibitory zones of 5.44 mm, 5.28 mm, 7.29 mm, 8.15 mm, and 6.76 mm against B. subtilis, S. aureus, P. aeruginosa, E. coli, and C. albicans respectively. This could be due to the antimicrobial properties of cationic starch. Comparable results have been reported by Venkataraman et al. (2019) and Liu et al. (2017).62,63 However, the incorporation of VN led to a significant increase in inhibition zones against food borne pathogens under investigation. This clearly demonstrates that the active agents VN can penetrate more uniformly into the surrounding medium. This enhanced penetration disrupts the media needed for microbial growth, effectively limiting the proliferation of the pathogens. As a result, the area of microbial inhibition is expanded, leading to a broader inhibition zone that highlights the increased antimicrobial efficacy of the CPVN active films.64,65
 | ||
| Fig. 6 Visual representation of zone of inhibition of control CPVN and active films against different food borne pathogens. | ||
| Sample code | Diameter of growth of inhibition zone (mm) | ||||
|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | P. aeruginosa | C. albicans | |
| a a–d denotes significant differences (n = 3, p < 0.05). | |||||
| CPVN-0 | 5.44 ± 1.21a | 5.28 ± 1.12c | 8.15 ± 1.29b | 7.29 ± 1.14b | 6.76 ± 1.15d |
| CPVN-1 | 8.59 ± 0.96b | 9.18 ± 1.49b | 11.58 ± 0.68d | 11.55 ± 0.94c | 8.66 ± 1.28c |
| CPVN-2 | 10.20 ± 0.64c | 10.31 ± 0.51d | 12.91 ± 1.48a | 13.95 ± 1.22a | 9.63 ± 1.39b |
| CPVN-3 | 11.72 ± 0.29d | 10.92 ± 1.71a | 15.04 ± 1.04c | 15.50 ± 0.73d | 11.97 ± 1.77a |
3.11. Antioxidant study
In food packaging, antioxidant activity plays a crucial role in extending the shelf life and preserving the quality of food products. Antioxidants are incorporated into packaging materials to protect food from oxidative damage caused by exposure to oxygen, light, and other environmental factors by means of reactive oxygen species (ROS) and free radicals. This helps to prevent spoilage, rancidity, and nutrient degradation, ensuring that food remains fresh for longer periods. The antioxidant activity control CPVN and active films were examined by DPPH radical scavenging activity. DPPH˙ is a stable radical that can capture hydrogen from antioxidant compounds, converting itself into DPPH-H molecule.66 Therefore, the DPPH radical scavenging method was chosen to evaluate the antioxidant activity in the present study.The DPPH free radical activity (%) of control CPVN and active films are shown in Fig. 7. The control CPVN film demonstrated 10.85% antioxidant activity at 100 μg mL−1 concentration. It was observed that as increasing concentrations of VN in the CT/PVA polymer matrix led to a significant improvement in the antioxidant activity. For instance, the CPVN-3 film (at 3% concentration of VN) demonstrated a DPPH radical scavenging activity of 81.33%. This marked improvement suggests that VN plays a crucial role in enhancing the antioxidant properties of the film. The enhanced antioxidant activity can be attributed to the phenolic groups of VN, which are known for their ability to donate hydrogen atoms. When these hydrogen atoms are donated to free radicals like DPPH, they neutralize the radicals by converting them into stable molecules, thereby reducing the oxidative damage. This mechanism is supported by previous studies,67,68 which have shown that phenolic compounds are effective free radical scavengers. These outcomes indicate that CPVN active films are highly effective in preventing food spoilage caused by oxidation, suggesting that CPVN active films could be particularly useful in the food packaging industry, where they could serve as protective layers that extend the shelf life of food products by minimizing oxidative damage. This not only has potential health benefits by preserving the nutritional quality of the food but also has economic implications by reducing food waste (Table 4).
| Polymer composition | Active agent/plasticizers | Tensile strength (MPa) | Zone of inhibition (mm) | Antioxidant activity (%) | References | ||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | |||||
| a PVA: poly(vinyl alcohol), CT: cationic starch, ZnO NPs: zinc oxide nanoparticles. | |||||||
| Strach/PVA | ZnO NPs | 9.38 | — | — | — | — | 69 |
| Starch/PVA | Glycerol | 16.96 | — | — | — | — | 70 |
| Starch/PVA | Barley husk | 13.38 | — | — | — | — | 71 |
| Starch/PVA | Citric acid | 8.24 | — | — | — | 72 | |
| Corn starch/PVA | Pineapple peel extract | 17.12 ± 1.68 | — | — | — | 42 | 73 |
| Corn starch/PVA | Curcumin loaded pickering emulsion | 2.13 ± 0.51 | 3.40 ± 0.3 | 1.9 ± 0.17 | — | ∼55 | 74 |
| Starch/PVA | Nano titania | 2.201 ± 0.0015 | — | 4.5 | — | — | 75 |
| CT/PVA | Vanillin | 33.31 ±0.48 | 10.92 ± 1.71 | 15.04 ± 1.04 | 11.97 ± 1.77 | 81.33 | Present work |
4 Conclusions
The research focused on developing cationic starch/poly(vinyl alcohol) (CT/PVA) based bioactive films enhanced with vanillin (VN) as a sustainable and eco-friendly packaging solution for food preservation. The resulting cationic starch/poly(vinyl alcohol)/vanillin (CPVN) bioactive were thoroughly characterized using various instrumental techniques. ATR-FTIR spectroscopy confirmed the presence of secondary molecular interactions between CT, PVA, and VN. Scanning electron microscopy (SEM) analysis revealed that VN incorporation led to a smoother and compact film surface. The addition of VN significantly improved the film's UV light barrier, tensile properties, and moisture adsorption capacity, while also reducing transparency due to the intermolecular interactions within the CT/PVA film matrices. The water vapor transmission rate (WVTR) of the CPVN films was notably enhanced, along with surface wettability. Furthermore, the CPVN bioactive films demonstrated strong antimicrobial activity against foodborne pathogens, including Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans. Additionally, films containing 3 wt% VN exhibited excellent antioxidant activity, as evidenced by their significant DPPH free radical scavenging capabilities.From a commercial perspective, these CPVN films present a highly promising, inexpensive solution for sustainable food packaging applications. The use of reasonable and renewable raw materials like starch, combined with the simple and scalable solvent casting process, ensures economic viability for large-scale production. Moreover, the incorporation of FDA approved and GRAS listed vanillin not only enhances functionality but also meets regulatory requirements, making these films ready for market deployment. Their multifunctionality combining mechanical integrity, barrier protection, and bioactivity adds substantial market value, particularly for food sectors targeting biodegradable and remarkable performance packaging. These outcomes suggest CPVN bioactive films as a promising solution for sustainable food packaging, providing an eco-friendly alternative that effectively addresses environmental pollution concerns.
Data availability
The data is made available on request.Author contributions
Lingaraj Kariyappa Kurabetta: conceptualization, data interpretation, formal analysis, writing – original manuscript. Saraswati P. Masti: supervision, corresponding author, review, formal correction. Manjushree Nagaraj Gunaki: formal analysis, design of study, data curation, investigation. Ajitkumar Appayya Hunashyal: data curation, formal analysis, Ravindra B. Chougale: revising manuscript. Nagarjuna Prakash Dalbanjan: analysis of data (antimicrobial and antioxidant studies). Praveen Kumar S. K.: review and editing (antimicrobial and antioxidant studies).Conflicts of interest
All authors declare no conflict of interest.Acknowledgements
Authors are thankful to SERB for providing instrumental facilities under SERB project sanctioned to Dr Saraswati P. Masti, principal investigator, SERB (Sanction letter No. SB/EMEQ-213/2014 dated: 29-01-2016). Authors would like to express gratitude the Sophisticated Analytical Instrument Facilities (SAIF), University Scientific and Instrumentation Centre (USIC) and DST-PURSE Phase II Program [Grant No. SR/PURSE PHASE-2/13(G)] Karnatak University, Dharwad, for rendering the necessary instrumentation amenities. One of the authors, Mr Lingaraj Kariyappa Kurabetta would like to thank Karnatak University, Dharwad, Karnataka, India, for providing University Research Studentship.References
- A. George, M. R. Sanjay, R. Srisuk, J. Parameswaranpillai and S. Siengchin, Int. J. Biol. Macromol., 2020, 154, 329–338 CrossRef PubMed.
- D. L. Kaplan, in Biopolymers from Renewable Resources, Springer Berlin Heidelberg, Berlin, Heidelberg, 1998, pp. 1–29 Search PubMed.
- Roohi, P. Srivastava, K. Bano, M. R. Zaheer and M. Kuddus, in Bio-based Materials for Food Packaging, Springer Singapore, Singapore, 2018, pp. 197–216 Search PubMed.
- O. A. Adeyeye, E. R. Sadiku, A. Babu Reddy, A. S. Ndamase, G. Makgatho, P. S. Sellamuthu, A. B. Perumal, R. B. Nambiar, V. O. Fasiku, I. D. Ibrahim, O. Agboola, W. K. Kupolati, O. O. Daramola, M. J. Machane and T. Jamiru, Green Biopolymers and their Nanocomposites, 2019, pp. 137–158 Search PubMed.
- K. Gautam, R. Vishvakarma, P. Sharma, A. Singh, V. Kumar Gaur, S. Varjani and J. Kumar Srivastava, Bioresour. Technol., 2022, 361, 127650 CrossRef.
- X. Dang, S. Han, Y. Du, Y. Fei, B. Guo and X. Wang, Food Chem., 2025, 466, 142192 CrossRef.
- X. Dang, Y. Cai and X. Wang, Food Chem., 2025, 480, 143922 CrossRef PubMed.
- S. Liang, X. Wang, C. Wei, L. Xie and X. Dang, ACS Sustain. Chem. Eng., 2025, 13, 3066–3077 CrossRef.
- H. Samsudin and N. M. Hani, in Starch-Based Materials in Food Packaging, Elsevier, 2017, pp. 229–256 Search PubMed.
- V. D. Hiremani, T. Gasti, S. Sataraddi, V. N. Vanjeri, N. Goudar, S. P. Masti and R. B. Chougale, Chem. Data Collect., 2020, 28, 100416 CrossRef.
- L. K. Kurabetta, S. P. Masti, M. P. Eelager, M. N. Gunaki, S. Madihalli, A. A. Hunashyal, R. B. Chougale, P. Kumar S.K. and A. J. Kadapure, Int. J. Biol. Macromol., 2023, 253, 127552 CrossRef CAS.
- M. Avella, J. J. De Vlieger, M. E. Errico, S. Fischer, P. Vacca and M. G. Volpe, Food Chem., 2005, 93, 467–474 CrossRef.
- S. P. Bangar, W. S. Whiteside, A. O. Ashogbon and M. Kumar, Food Packag. Shelf Life, 2021, 30, 100743 CrossRef.
- A. Cano, M. Cháfer, A. Chiralt and C. González-Martínez, Food Packag. Shelf Life, 2016, 10, 16–24 CrossRef.
- X. Dang, Y. Du and X. Wang, Food Chem., 2024, 439, 138119 CrossRef PubMed.
- M. Stroescu, A. Stoica-Guzun, G. Isopencu, S. I. Jinga, O. Parvulescu, T. Dobre and M. Vasilescu, Food Hydrocolloids, 2015, 48, 62–71 CrossRef.
- I. Mourtzinos, S. Konteles, N. Kalogeropoulos and V. T. Karathanos, Food Chem., 2009, 114, 791–797 CrossRef.
- M. Ngarmsak, P. Delaquis, P. Toivonen, T. Ngarmsak, B. Ooraikul and G. Mazza, J. Food Prot., 2006, 69, 1724–1727 CrossRef.
- Y. Liu, L. Sun, Y.-X. Huo and S. Guo, Microb. Cell Fact., 2023, 22, 147 CrossRef PubMed.
- N. J. Gallage and B. L. Møller, Mol. Plant, 2015, 8, 40–57 CrossRef PubMed.
- J. R. Westlake, M. Laabei, Y. Jiang, W. C. Yew, D. L. Smith, A. D. Burrows and M. Xie, ACS Food Sci. Technol., 2023, 3, 1680–1693 CrossRef PubMed.
- R. Campardelli, M. Pettinato, E. Drago and P. Perego, Chem. Eng. Technol., 2021, 44, 1390–1396 CrossRef.
- J. P. Pinto, O. J. D’souza, C. Chavan, R. F. Bhajanthri, S. P. Masti and R. B. Chougale, Diamond Relat. Mater., 2023, 140, 110483 CrossRef.
- L. K. Kurabetta, S. P. Masti, M. N. Gunaki, A. A. Hunashyal, M. P. Eelager, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Int. J. Biol. Macromol., 2024, 134191 CrossRef.
- M. N. Gunaki, S. P. Masti, O. J. D’souza, M. P. Eelager, L. K. Kurabetta, R. B. Chougale, A. J. Kadapure and S. K. Praveen Kumar, Food Hydrocolloids, 2024, 152, 109937 CrossRef.
- M. P. Eelager, S. P. Masti, N. P. Dalbanjan, S. Madihalli, M. N. Gunaki, L. K. Kurbetta, P. Kumar S.K. and R. B. Chougale, Prog. Org. Coat., 2024, 192, 108510 CrossRef.
- M. N. Gunaki, S. P. Masti, L. K. Kurabetta, J. P. Pinto, A. A. Hunashyal, N. P. Dalbhanjan, R. B. Chougale and S. K. Praveen Kumar, Int. J. Biol. Macromol., 2025, 299, 140029 CrossRef PubMed.
- L. K. Kurabetta, S. P. Masti, M. N. Gunaki, A. A. Hunashyal, R. B. Chougale, N. P. Dalbanjan and S. K. Praveen Kumar, Food Biosci., 2024, 61, 104869 CrossRef.
- M. P. Eelager, S. P. Masti, S. Madihalli, N. Gouda, L. K. Kurbetta, M. N. Gunaki, A. A. Hunashyal and R. B. Chougale, J. Environ. Chem. Eng., 2025, 13, 116029 CrossRef.
- S. Madihalli, S. P. Masti, M. P. Eelager, R. B. Chougale, L. K. Kurabetta, A. A. Hunashyal, N. P. Dalbanjan and S. K. Praveen Kumar, Food Biosci., 2024, 62, 105492 CrossRef.
- S. Roy and J.-W. Rhim, J. Environ. Chem. Eng., 2021, 9, 104694 CrossRef.
- J. Huang, Y. Cheng, Y. Wu, X. Shi, Y. Du and H. Deng, Int. J. Biol. Macromol., 2019, 139, 191–198 CrossRef PubMed.
- J. P. Pinto, O. J. D’souza, V. D. Hiremani, N. P. Dalbanjan, S. K. Praveen Kumar, S. S. Narasagoudr, S. P. Masti and R. B. Chougale, Food Biosci., 2023, 56, 103340 CrossRef.
- A. R. Khoirunnisa, I. M. Joni, C. Panatarani, E. Rochima and D. Praseptiangga, AIP Conf. Proc., 2018, 030041 CrossRef.
- J. Li, Z. Wu, C. Huang, H. Liu, R. Huang and L. Li, Compos. Sci. Technol., 2014, 90, 166–173 CrossRef.
- D. Piñeros-Hernandez, C. Medina-Jaramillo, A. López-Córdoba and S. Goyanes, Food Hydrocolloids, 2017, 63, 488–495 CrossRef.
- J. Ghaderi, S. F. Hosseini, N. Keyvani and M. C. Gómez-Guillén, Food Hydrocolloids, 2019, 95, 122–132 CrossRef.
- J. P. Pinto, M. H. Anandalli, A. A. Hunashyal, Priyadarshini, S. P. Masti, R. B. Chougale, V. Gudihal and R. F. Bhajantri, J. Mater. Sci.: Mater. Electron., 2025, 36, 1035 CrossRef.
- A. Abu Krorra, W. Noshy, A. Oun and M. Abu Elleif, Adv. Res. Conserv. Sci., 2021, 2, 21–30 CrossRef.
- S. A. A. K. M. Hamed and M. L. Hassan, J. Cult. Herit., 2019, 35, 140–144 CrossRef.
- E. Shekarforoush, A. Mendes, V. Baj, S. Beeren and I. Chronakis, Molecules, 2017, 22, 1708 CrossRef PubMed.
- Y. Wu, Y. Ying, Y. Liu, H. Zhang and J. Huang, Int. J. Biol. Macromol., 2018, 118, 2131–2137 CrossRef PubMed.
- S. S. Narasagoudr, V. G. Hegde, V. N. Vanjeri, R. B. Chougale and S. P. Masti, Carbohydr. Polym., 2020, 236, 116049 CrossRef.
- R. Sabarish and G. Unnikrishnan, Carbohydr. Polym., 2018, 199, 129–140 CrossRef CAS.
- T. Wang, Y. Li, S. Geng, C. Zhou, X. Jia, F. Yang, L. Zhang, X. Ren and H. Yang, RSC Adv., 2015, 5, 88958–88964 RSC.
- S. Huang, Y. Xiong, Y. Zou, Q. Dong, F. Ding, X. Liu and H. Li, Food Hydrocolloids, 2019, 90, 198–205 CrossRef CAS.
- P. Kohn, Z. Rong, K. H. Scherer, A. Sepe, M. Sommer, P. Müller-Buschbaum, R. H. Friend, U. Steiner and S. Hüttner, Macromolecules, 2013, 46, 4002–4013 CrossRef CAS.
- S. S. Narasagoudr, V. G. Hegde, R. B. Chougale, S. P. Masti and S. Dixit, Int. J. Biol. Macromol., 2020, 154, 48–61 CrossRef CAS PubMed.
- S. S. Narasagoudr, V. G. Hegde, R. B. Chougale, S. P. Masti and S. Dixit, Int. J. Biol. Macromol., 2020, 154, 48–61 CrossRef CAS PubMed.
- Z.-H. Zhang, Z. Han, X.-A. Zeng, X.-Y. Xiong and Y.-J. Liu, Int. J. Biol. Macromol., 2015, 81, 638–643 CrossRef CAS.
- X. Sun, Z. Wang, H. Kadouh and K. Zhou, LWT-Food Sci. Technol., 2014, 57, 83–89 CrossRef CAS.
- K. Zheng, W. Li, B. Fu, M. Fu, Q. Ren, F. Yang and C. Qin, Int. J. Biol. Macromol., 2018, 118, 707–715 CrossRef CAS.
- K. Rambabu, G. Bharath, F. Banat, P. L. Show and H. H. Cocoletzi, Int. J. Biol. Macromol., 2019, 126, 1234–1243 CrossRef CAS.
- F. Wu, M. Misra and A. K. Mohanty, Prog. Polym. Sci., 2021, 117, 101395 CrossRef CAS.
- X. Sun, Z. Wang, H. Kadouh and K. Zhou, LWT–Food Sci. Technol., 2014, 57, 83–89 CrossRef CAS.
- H. E. Salama, M. S. Abdel Aziz and M. Alsehli, Int. J. Biol. Macromol., 2019, 139, 614–620 CrossRef CAS PubMed.
- U. Siripatrawan and B. R. Harte, Food Hydrocolloids, 2010, 24, 770–775 CrossRef CAS.
- V. D. Hiremani, T. Gasti, S. P. Masti, R. B. Malabadi and R. B. Chougale, Iran. Polym. J., 2022, 31, 503–518 CrossRef CAS.
- R. M. Epand, C. Walker, R. F. Epand and N. A. Magarvey, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 980–987 CrossRef CAS PubMed.
- M. P. Stewart, R. Langer and K. F. Jensen, Chem. Rev., 2018, 118, 7409–7531 CrossRef CAS PubMed.
- J. Jung, G. Kasi and J. Seo, Int. J. Biol. Macromol., 2018, 112, 530–536 CrossRef CAS.
- Z. Liu, M. Huang, A. Li and H. Yang, Water Res., 2017, 119, 57–66 CrossRef CAS PubMed.
- S. Venkataraman, A. L. Z. Lee, J. P. K. Tan, Y. C. Ng, A. L. Y. Lin, J. Y. K. Yong, G. Yi, Y. Zhang, I. J. Lim, T. T. Phan and Y. Y. Yang, Polym. Chem., 2019, 10, 412–423 RSC.
- A. Bahrami and R. Fattahi, Food Sci. Nutr., 2021, 9, 4974–4985 CrossRef CAS.
- J. P. Pinto, V. D. Hiremani, O. J. D’souza, S. Khanapure, S. S. Narasagoudr, N. Goudar, S. K. Vootla, S. P. Masti and R. B. Chougale, Food Humanit., 2023, 1, 378–390 CrossRef.
- Y. Jing, Y. Diao and X. Yu, React. Funct. Polym., 2019, 135, 16–22 CrossRef CAS.
- A. R. Garrett, E. G. Weagel, A. D. Martinez, M. Heaton, R. A. Robison and K. L. O'Neill, Food Chem., 2014, 158, 490–496 CrossRef CAS.
- V. P. Romani, V. G. Martins and J. M. Goddard, Food Control, 2020, 109, 106946 CrossRef CAS.
- S. Yari, J. Mohammadi-Rovshandeh and M. Shahrousvand, J. Polym. Environ., 2022, 30, 1502–1517 CrossRef CAS.
- C. Sen and M. Das, J. Polym. Environ., 2018, 26, 4331–4337 CrossRef CAS.
- A. Mittal, S. Garg and S. Bajpai, Mater. Today Proc., 2020, 21, 1577–1582 CrossRef CAS.
- N. Gürler, S. Paşa, Ö. Erdoğan and O. Cevik, Starch Stärke, 2023, 75(3–4), 2100074 CrossRef.
- P. Kumar, R. Tanwar, V. Gupta, A. Upadhyay, A. Kumar and K. K. Gaikwad, Int. J. Biol. Macromol., 2021, 187, 223–231 CrossRef CAS PubMed.
- D. Liu, S. Dang, L. Zhang, K. Munsop and X. Li, Int. J. Biol. Macromol., 2021, 188, 974–982 CrossRef CAS PubMed.
- D. Lin, Y. Huang, Y. Liu, T. Luo, B. Xing, Y. Yang, Z. Yang, Z. Wu, H. Chen, Q. Zhang and W. Qin, LWT-Food Sci. Technol., 2018, 96, 704–712 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fb00132c |
| This journal is © The Royal Society of Chemistry 2025 |