Open Access Article
Open Access ArticleExtending the shelf life of red chilies (Capsicum annuum): exploring steam, microwave, and pulsed light treatments under different storage conditions†
Kosana
Pravallika
a and
Snehasis
Chakraborty
 *ab
*ab
aFood Engineering and Technology Department, Institute of Chemical Technology, Matunga, Mumbai – 400019, India. E-mail: fet21k.pravallika@pg.ictmumbai.edu.in; pravafst07@gmail.com; snehasisftbe@gmail.com
bDepartment of Grain Science and Industry, Kansas State University, Manhattan, Kansas 66506, USA. E-mail: snehasis@ksu.edu
First published on 10th April 2025
Abstract
Red chilies face significant challenges in maintaining quality and safety during storage, thus necessitating effective preservation strategies to extend their shelf life under varying environmental conditions. In this study, the effects of steam (ST) (120 °C|300 s), microwave (MW) (540 kJ), and pulsed light (PL) (2.59 J cm−2) conditions on the shelf life of 0.6 and 0.35 aw red chillies were examined while packed in polypropylene (PP) pouches under ambient (28 °C) and refrigerated storage (4 °C) conditions. Post-treatment, steam-treated red chillies retained 26.0% and 22.0% fewer phenolics, 37.4% and 36.2% fewer flavonoids, 13.3% and 9.4% fewer antioxidants, 34.6% and 35.0% fewer ascorbic acid, and 13.7% and 24.1% fewer carotenoids than MW and PL treated chillies, respectively. Based on the microbiological limit of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1, steam-treated red chillies had a shorter shelf life compared to even untreated samples, i.e., 22 days and 59 days for 0.60 and 0.35 aw chillies, whereas untreated chillies had 35 days and 101 days for respective aw under ambient storage. Meanwhile, the shelf life of microwave and pulsed light-treated red chillies was more than 150 days at both aw and storage temperatures. There was a significant colour change in steam-treated samples with an E* value of 7.85 compared to MW (2.15) and PL (2.30). Even after 210 days, PL-treated red chilies retained >80% of their bioactive compounds. The first-order kinetic model confirmed that the retention of bioactive compounds is greater in MW- and PL-treated samples than in steam-treated chillies at both aw and storage temperatures. The red chillies treated with MW showed almost similar shelf life to the PL treated chillies in terms of all the quality attributes, but there was an increase (3.3% to 24.9%) in enzyme activity after PL treatment. Hence, MW and PL can be used as alternative sterilization techniques to extend the shelf life of red chillies.
log10 CFU g−1, steam-treated red chillies had a shorter shelf life compared to even untreated samples, i.e., 22 days and 59 days for 0.60 and 0.35 aw chillies, whereas untreated chillies had 35 days and 101 days for respective aw under ambient storage. Meanwhile, the shelf life of microwave and pulsed light-treated red chillies was more than 150 days at both aw and storage temperatures. There was a significant colour change in steam-treated samples with an E* value of 7.85 compared to MW (2.15) and PL (2.30). Even after 210 days, PL-treated red chilies retained >80% of their bioactive compounds. The first-order kinetic model confirmed that the retention of bioactive compounds is greater in MW- and PL-treated samples than in steam-treated chillies at both aw and storage temperatures. The red chillies treated with MW showed almost similar shelf life to the PL treated chillies in terms of all the quality attributes, but there was an increase (3.3% to 24.9%) in enzyme activity after PL treatment. Hence, MW and PL can be used as alternative sterilization techniques to extend the shelf life of red chillies.
Sustainability spotlightFood preservation is crucial for reducing waste and promoting sustainability. While conventional steam sterilization effectively inactivates microorganisms and enzymes, it requires prolonged high-temperature exposure, leading to nutrient loss and diminished sensory quality. Emerging technologies, such as microwave heating (novel-thermal) and pulsed light treatment (non-thermal), are revolutionizing food processing by ensuring microbial safety and enzymatic stability while preserving phytochemical content and sensory appeal. These advanced methods operate with shorter processing times, reducing energy consumption and minimizing thermal damage. With high energy efficiency, quality retention, and minimal maintenance, they support UN Sustainable Development Goal 12 “Responsible Consumption and Production”, which can sustainably enhance the shelf life of fresh produce. Embracing these innovations is key to a sustainable, efficient food supply chain. |
1 Introduction
Chilli has a prominent place amongst the major spices across the globe with the cultivation of over 400 different varieties. Red chillies (Capsicum annuum), belonging to the family Solanaceae, are classified as fruit-vegetables and are widely valued for their exceptionally high content of vitamin C, phenolics, and antioxidants, surpassing many other vegetables in nutritional richness.1 These are used in a variety of foods in several forms due to their unique flavour (heat), aroma and colour, which result from the presence of capsaicinoids and carotenoids, respectively.2 Spices have been used not only for seasoning but also as medicines, cosmetics, and perfumes. Therefore, the market for spices has been increasing tremendously. In 2022–23, the global production of dried red chillies was 5.06 mT, which costs $1.3 billion. The top producer and exporter countries of chillies were India (2.78 mT), Bangladesh (0.63 mT), and Ethiopia (0.33 mT) in 2022–23.3 The largest exported spices from India in 2023–24 were chilli (0.6 mT), cumin (0.17 mT), turmeric (0.16 mT), and ginger (0.06 mT) products. In 2022, India exported 1.53 mT, marking a 9.63% increase in volume and a 12.49% rise in annual revenue.4 Spice exporters face challenges in assuring consumers of the safety and hygiene of their products.Freshly harvested red chillies have a limited shelf life of 3 to 5 days under ambient conditions due to their high moisture content and respiration rates, making them highly susceptible to microbial spoilage and quality deterioration.5 Post-harvest losses of chillies contribute to 25–35% of agricultural produce, posing significant economic and sustainability challenges by reducing farmer incomes, disrupting market efficiency, and increasing costs for consumers. To mitigate these losses, advanced storage techniques, improved processing methods, and effective preservation strategies are essential.6 Drying is one of the most effective techniques for extending the shelf life of chillies by reducing moisture content, thereby inhibiting microbial growth and enzymatic degradation. However, dried chillies stored at ambient temperatures (25–30 °C) deteriorate rapidly, experiencing substantial weight loss, discolouration, and loss of bioactive compounds.7,8 The application of preservation technologies, controlled storage conditions, and modified packaging can further enhance the shelf life of dried chillies.9 While traditional methods like sun drying and air drying are widely used, they are always combined with other hurdles such as low temperatures; chemical treatment such as application of edible coatings, ozone and chlorine dioxide treatment; thermal treatments such as drying, steam sterilization, microwave, infrared, and ohmic heating; non-thermal treatments such as irradiation,5,8 pulsed light,10 ultrasound,11 cold plasma,12 and high hydrostatic pressure.13 The use of chemical treatments and irradiation on spices is not readily accepted by consumers because of the residue presence on the final product, which might be carcinogenic; hence, these are banned in a few countries.14 Among the preservation technologies, the most popular and conventional technique is thermal treatment, which completely inactivates microorganisms and enzymes but causes a huge loss of essential bioactive compounds, but in some cases, it still does not completely inactivate even spoilage microorganisms at 60–80 °C.6 There are some literature studies available on the thermal processing of dried red pepper;6 paprika;15 black peppercorns;16 green and red chillies;17 and the activity of bioactives in chillies.18 Microwave (MW) treatment is a novel thermal technology, which is a form of electromagnetic radiation that lies between infrared and radio waves in the electromagnetic spectrum. In the United States, federal regulations designate two specific frequencies, 915 MHz and 2450 MHz, for industrial applications. Consequently, microwave radiation presents a viable alternative for pasteurizing or even sterilizing food at lower temperatures in a shorter time compared to conventional methods.19 In previous studies, microwave treatment has been used as a pretreatment prior to drying20 and as a drying technology21 but not as a technique alternative to pasteurization or sterilization.
Nonthermal technologies like pulsed light (PL) have shown greater efficiency in inactivating microorganisms and enzymes while preserving the nutritional quality of food. Pulsed light generates short, high-intensity pulses with wavelengths ranging from 100 to 1100 nm, encompassing ultraviolet (UV), visible (Vis), and infrared (IR) light. Several studies have evaluated the effectiveness of PL treatment on spices, including fresh-cut red bell peppers,10 green chillies,22 red chillies,23,24 and red pepper powder.25 These studies highlight that PL technology offers significant potential for producing microbially safe and enzymatically stable chillies, outperforming conventional thermal methods.
The shelf life of spices is crucial for manufacturers and retailers as it impacts product quality, food safety, inventory management, and overall profitability. A thorough evaluation of shelf life is essential to ensure that products align with customer expectations while supporting long-term business success. Besides, no study has examined the quality changes in dehydrated red chillies during storage. The existing literature reveals limited studies on shelf-life extension of chillies through edible coatings1 or irradiation,14 with no research addressing the impact of thermal or microwave processing on their shelf life. Additionally, Rybak et al.10 demonstrated that pulsed light (PL) treatment on fresh-cut red bell peppers effectively preserved bioactive compounds such as phenolics, ascorbic acid, and carotenoids, enhanced antioxidant activity, and significantly reduced microbial load, particularly at fluence levels exceeding 16 J cm−2. Similarly, Rico et al.6 investigated the shelf life of red chilli powder treated with steam and irradiation over six months. Pravallika and Chakraborty23,24 explored the potential of the PL technique to decontaminate whole red chillies. Another study by the same group reported that PL can decontaminate whole and dehydrated green chillies while retaining maximum phytochemicals.22 However, the shelf life of PL-treated samples has not been explored. According to Kalathil et al.26 non-thermal, light-based techniques like UV-C, blue LEDs, and atmospheric plasma effectively reduce aflatoxin contamination in red chilli pods while enhancing their bioactive properties. Short-term red and far-red light irradiation, along with low temperatures, can regulate phytochromes in Capsicum annuum L., enhancing carotenoid and alkaloid biosynthesis.27 Pulsed light plasma (PLP) treatment effectively inactivates microbial contaminants in red pepper flakes without altering their quality.28 Far-infrared radiation-assisted microwave-vacuum drying (FIR-MVD) improved drying efficiency and preserved the quality of red chillies better than microwave-vacuum drying (MVD).29 Microwave rotary drying at 2000 W and a 60° chamber angle optimized red chili drying, enhancing moisture diffusivity, pungency, and vitamin A retention while improving drying efficiency.20 Microwave drying significantly accelerates chilli drying compared to sun drying, preserving quality while achieving higher moisture diffusivity and efficiency.30 However, no prior research has assessed the potential of steam, microwave, or pulsed light treatments in preserving whole dehydrated red chillies, thus fulfilling the critical gap. This research is the first to systematically evaluate the influence of steam treatment (ST), microwave (MW) processing, and pulsed light (PL) processing on the microbiological safety, enzymatic stability, and phytochemical retention of dehydrated red chillies. By directly comparing untreated and treated samples, the study will provide valuable insights into optimizing storage conditions, ultimately benefiting producers, retailers, and consumers by enhancing product stability and quality.
2 Materials and methods
2.1 Experimental design and selection of process conditions
The process conditions of steam (ST), microwave (MW), and pulsed light (PL) treatment used for the red chillies to be stored were chosen based on microbial and enzymatic inactivation. Due to the presence of enzymes inside the chillies, higher process conditions were used to achieve enzymatic stability (achieving >99% inactivation of PPO and POD). The minimum process intensity required to achieve microbial safety and enzymatic stability (99% inactivation of PPO and POD) along with the maximum retention of bioactive compounds was 0.2 MPa/120 °C/300 s (ST), 540 kJ (MW), and 2.59 J cm−2 (PL). The selected process conditions ensured the microbial safety of the red chillies, i.e., >8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycle reduction of all the microorganisms. Full factorial design was employed with three independent variables: two water activity levels (0.60 and 0.35 aw), four treatments (untreated, steam treatment, microwave treatment, and pulsed light treatment), and two different storage temperatures (28 and 4 °C). The storage stability of sixteen samples, for instance, 0.60 aw/UT/28 °C; 0.60 aw/UT/4 °C; 0.60 aw/ST/28 °C; 0.60 aw/ST/4 °C; 0.60 aw/MW/28 °C; 0.60 aw/MW/4 °C; 0.60 aw/PL/28 °C; 0.60 aw/PL/4 °C and 0.35 aw/UT/28 °C; 0.35 aw/UT/4 °C; 0.35 aw/ST/28 °C; 0.35 aw/ST/4 °C; 0.35 aw/MW/28 °C; 0.35 aw/MW/4 °C; 0.35 aw/PL/28 °C; 0.35 aw/PL/4 °C, was evaluated for 210 days6 (Table 1).
log cycle reduction of all the microorganisms. Full factorial design was employed with three independent variables: two water activity levels (0.60 and 0.35 aw), four treatments (untreated, steam treatment, microwave treatment, and pulsed light treatment), and two different storage temperatures (28 and 4 °C). The storage stability of sixteen samples, for instance, 0.60 aw/UT/28 °C; 0.60 aw/UT/4 °C; 0.60 aw/ST/28 °C; 0.60 aw/ST/4 °C; 0.60 aw/MW/28 °C; 0.60 aw/MW/4 °C; 0.60 aw/PL/28 °C; 0.60 aw/PL/4 °C and 0.35 aw/UT/28 °C; 0.35 aw/UT/4 °C; 0.35 aw/ST/28 °C; 0.35 aw/ST/4 °C; 0.35 aw/MW/28 °C; 0.35 aw/MW/4 °C; 0.35 aw/PL/28 °C; 0.35 aw/PL/4 °C, was evaluated for 210 days6 (Table 1).
| Treatment | ST (°C) | Packaging material | Sampling day | Responses | |
|---|---|---|---|---|---|
| 0.60 aw | Control | 28 °C | Propylene pouch | 0, 10, 20, 30, 35, 40 | • Aerobic mesophiles• Yeast and mould count• Polyphenol oxidase activity• Peroxidase activity• Total phenolic content• Total flavonoid content• Antioxidant capacity• Ascorbic acid• Capsaicinoid content• Carotenoid content• Pungency• Total extractable colour• Total colour change• Weight change• Water activity• Moisture content• Texture profile |
| 4 °C | Propylene pouch | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 | |||
| Steam treated (120 °C/300 s) | 28 °C | Propylene pouch | 0, 10, 20, 25 | ||
| 4 °C | Propylene pouch | 0, 10, 20, 30, 35, 40, 45, 50, 55, 60, 65, 70 | |||
| Microwave treated (540 kJ) | 28 °C | Propylene pouch | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 175 | ||
| 4 °C | Propylene pouch | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210 | |||
| Pulsed light treated (2.59 J cm−2) | 28 °C | Propylene pouch | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210 | ||
| 4 °C | Propylene pouch | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210 | |||
| 0.35 aw | Untreated | 28 °C | Propylene pouch | 0, 15, 30, 45, 60, 70, 75 | |
| 4 °C | Propylene pouch | 0, 15, 30, 45, 60, 75, 90, 105, 120, 125, 130 | |||
| Steam treated (120 °C/300 s) | 28 °C | Propylene pouch | 0, 10, 20, 30, 35, 40, 45, 50, 55, 60, 65, 70 | ||
| 4 °C | Propylene pouch | 0, 10, 20, 30, 35, 40, 45, 50, 55, 60, 65, 70 | |||
| Microwave treated (540 kJ) | 28 °C | Propylene pouch | 0, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 195, 210 | ||
| 4 °C | Propylene pouch | 0, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 195, 210 | |||
| Pulsed light treated (2.59 J cm−2) | 28 °C | Propylene pouch | 0, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 195, 210 | ||
| 4 °C | Propylene pouch | 0, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 195, 210 |
| Properties | Fresh red chillies (0.85 aw) | Intermediate moisture chillies (0.60 aw) | Dried red chillies (0.35 aw) |
|---|---|---|---|
| Moisture content (%) | 76.7 ± 3.2 | 31.5 ± 1.2 | 11.2 ± 0.9 |
| Water activity (aw) | 0.85 ± 0.01 | 0.60 ± 0.01 | 0.35 ± 0.01 |
| Colour | Deep red | Maroon to deep red | Deep maroon |
| L* | 38.7 ± 2.3 | 37.6 ± 0.6 | 35.7 ± 0.5 |
| a* | 42.8 ± 0.4 | 41.3 ± 1.1 | 39.3 ± 0.2 |
| b* | 24.2 ± 1.3 | 24.1 ± 1.4 | 22.3 ± 1.26 |
| Shape | Long and slender | Long and slender | Long and curved |
| Length × diameter (cm) | 7.4 × Φ0.8 | 7.1 × Φ0.7 | 6.8 × Φ0.4 |
| Weight | 1.5 to 3 g | 0.8 to 1.5 g | 0.2 to 0.6 g |
| Surface/texture | Smooth and glossy | Slightly wrinkled and rough | Wrinkled and brittle |
2.1.2.1 Steam treatment (ST). The steam treatment was conducted using a portable stainless steel vertical steam autoclave (LSC-05, Labline, Vadodara, Gujarat, India) with a capacity of 20 L and a working pressure of 20 psi. Dehydrated red chillies (1 kg), packed in a sterile polypropylene pouch, were placed in a basket (3.6 × 3.6 m) inside the autoclave and treated at 120 °C for 300 s (pressure was 0.2 MPa with 2706 kJ kg−1 enthalpy).6 The temperature and pressure of the autoclave are controlled to reach the desired temperature of 120 ± 0.1 °C. The steam-treated chillies pouches were kept in a laminar air flow to remove the condensed water on the film surface and then stored at the desired storage temperature. Microbes and quality-degrading enzymes were evaluated under steam treatment conditions of 120 °C for 300 s. The selection of these conditions was based on achieving complete inactivation of the most thermoresistant microorganisms, as well as key quality-degrading enzymes such as polyphenol oxidase (PPO) and peroxidase (POD). The sample, enclosed in pouches, was placed within an autoclave chamber under atmospheric pressure. The steam temperature was monitored and confirmed to reach 120 °C as the internal pressure increased to 15 psig. The duration required for the pressure to rise from 0 to 15 psig was recorded as the come-up time, which is 11.5 min. Preliminary trials validated that these processing parameters effectively ensured microbial inactivation and enzymatic stability, rendering them suitable for subsequent storage stability investigations.
2.1.2.2 Microwave (MW) treatment. A continuous microwave processing unit (4.2 m × 0.65 m × 1.9 m) was used to carry out the treatment of red chillies. The processing unit consists of a MW processing cavity (1.18 m × 0.5 m × 0.4 m) with a conveyor belt made of Teflon and thermocouples at the inlet and outlet. There are two magnetrons, each bearing a power (Po, W) of 1000 W and producing microwaves at a frequency of 2450 Hz.32 Dehydrated red chillies were placed on a conveyor belt that was disinfected with 70% ethanol and were treated at 270 s while maintaining a conveyor belt speed of 11 rpm and a microwave power of 2.0 kW. A digital infrared thermometer (Agilent, USA) was used to measure the surface temperature (±0.1 °C) of the product. Microwave treatments delivered a total microwave intensity (Po, kJ) of 540 kJ (microwave power × treatment duration = 2 kW × 270 s). Hence, the microwave intensity of 540 kJ achieved >8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inactivation of microbes and >99% inactivation of PPO and POD, thus ensuring both microbiological safety and enzyme stability of red chillies. This microwave treatment at 540 kJ was considered for the storage study.
log inactivation of microbes and >99% inactivation of PPO and POD, thus ensuring both microbiological safety and enzyme stability of red chillies. This microwave treatment at 540 kJ was considered for the storage study.
2.1.2.3 Pulsed light (PL) treatment. Pulsed light (PL) processing of red chillies was conducted using a benchtop batch PL system (X-1100, Xenon Corporation, USA). A xenon flash lamp (Φ2.54 × 40.6 cm, model LH-840, mercury-free, B-type) operating at a maximum voltage of 3 kV emitted high-intensity, non-collimated white light with wavelengths ranging from 190 to 1100 nm. The PL setup included a capacitor, a lamp support system, an air blower (to prevent lamp overheating), and a treatment chamber. Prior to treatment, the sample holder was sanitized with 70% ethyl alcohol to avoid environmental contamination.33
Red chillies (1000 g) were placed in an open-lid glass container (100 mm × 15 mm) on the sample holder at a 90° angle to the lamp, maintaining a distance of 3.6 cm. The samples were treated with PL for 360 s at 2.2 kV, delivering a fluence rate of 12.00 ± 0.34 W cm−2 and a total effective fluence of 2.59 J cm−2. These treatment conditions achieved a complete microbial reduction (>6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycle) and enzymatic inactivation (>99%), ensuring microbiological safety and enzymatic stability. The fluence rate per pulse on the sample surface was measured using a pyroelectric energy sensor (PE 50, Ophir Optronics Solutions Ltd, Singapore), while a digital infrared thermometer (Raytek, MT4, USA) monitored the product's surface temperature (±0.1 °C).
log cycle) and enzymatic inactivation (>99%), ensuring microbiological safety and enzymatic stability. The fluence rate per pulse on the sample surface was measured using a pyroelectric energy sensor (PE 50, Ophir Optronics Solutions Ltd, Singapore), while a digital infrared thermometer (Raytek, MT4, USA) monitored the product's surface temperature (±0.1 °C).
For red chillies with water activities of 0.60 and 0.35 aw, the temperature increased by 16.8 ± 0.4 °C and 13.6 ± 0.3 °C, respectively, from an initial temperature of 25.2 ± 0.1 °C. Following steam, microwave, and pulsed light treatments, the 0.60 and 0.35 aw red chilli samples were packed in sterile polypropylene pouches and stored according to the experimental design.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1)34 or experienced excessive browning (ΔE* > 12).35 The samples were discarded upon reaching either of these thresholds. In the case of 0.6 aw chillies, sampling intervals for untreated samples were 10 days initially and have been reduced to 5 days, while treated samples were analysed at intervals of 10 days, depending on the observed changes in key parameters as mentioned in Table 1.
log10 CFU g−1)34 or experienced excessive browning (ΔE* > 12).35 The samples were discarded upon reaching either of these thresholds. In the case of 0.6 aw chillies, sampling intervals for untreated samples were 10 days initially and have been reduced to 5 days, while treated samples were analysed at intervals of 10 days, depending on the observed changes in key parameters as mentioned in Table 1.
2.2 Determination of quality attributes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1.37
log10 CFU g−1.37
 | (1) |
 | (2) |
 | (3) |
3 Results and discussion
3.1 Effect of sequential drying on quality attributes of the red chillies
The fresh red chillies had AM and YM counts of 5.37 ± 0.08 and 5.46 ± 0.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1, respectively. Reducing the water activity to 0.60 and 0.35, there was a 1.87 and 1.44
log10 CFU g−1, respectively. Reducing the water activity to 0.60 and 0.35, there was a 1.87 and 1.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycle reduction in the AM population. On a similar note, there was a 2.65 and 2.39
log cycle reduction in the AM population. On a similar note, there was a 2.65 and 2.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in the YM population in 0.60 and 0.35 aw red chillies. In a study on green and red chilies, Pravallika and Chakraborty22,23 observed a similar reduction in the populations of AM and YM. These findings align with the results reported by Pravallika and Chakraborty.22 In green chillies, lowering the water activity (aw) to 0.60 resulted in a reduction of 1.95
log reduction in the YM population in 0.60 and 0.35 aw red chillies. In a study on green and red chilies, Pravallika and Chakraborty22,23 observed a similar reduction in the populations of AM and YM. These findings align with the results reported by Pravallika and Chakraborty.22 In green chillies, lowering the water activity (aw) to 0.60 resulted in a reduction of 1.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 in AM and 2.10
log10 CFU g−1 in AM and 2.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 in YM. Similarly, a thermal treatment at 100 °C for 960 s in dried red pepper reduced AM by 1.00
log10 CFU g−1 in YM. Similarly, a thermal treatment at 100 °C for 960 s in dried red pepper reduced AM by 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 and YM by 2.66
log10 CFU g−1 and YM by 2.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1.6 After sequential drying, red chillies stored at room temperature (25.9 ± 2.0 °C) for several days exhibited microbial regrowth. After 15 days, AM and YM counts increased to 4.13 ± 0.14
log10 CFU g−1.6 After sequential drying, red chillies stored at room temperature (25.9 ± 2.0 °C) for several days exhibited microbial regrowth. After 15 days, AM and YM counts increased to 4.13 ± 0.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 and 4.50 ± 0.26
log10 CFU g−1 and 4.50 ± 0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 at 0.60 aw, and to 3.72 ± 0.37
log10 CFU g−1 at 0.60 aw, and to 3.72 ± 0.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 and 3.64 ± 0.70
log10 CFU g−1 and 3.64 ± 0.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 at 0.35 aw. The observed microbial growth can be attributed to the tendency of chilies to absorb moisture from the surrounding environment, even though their aw values remained largely unchanged. Microorganisms can reemerge under favourable conditions, posing significant challenges for export, particularly when moisture content exceeds 11%, which heightens the risk of mould proliferation.16 Although dried chillies have low water activity (<0.6), which inhibits bacterial growth, certain fungi can still proliferate under storage conditions, particularly if residual moisture or oxygen is present within the packaging. Studies have reported the presence of mycotoxigenic Aspergillus species in dried spices, posing food safety risks.31 Localized variations in moisture distribution within the food matrix can create microenvironments that support microbial growth. Additionally, packaging materials with high oxygen and water vapor barrier properties may not entirely prevent microbial contamination, as spores can remain dormant and become active due to fluctuations in storage conditions.16 In our previous study by Pravallika and Chakraborty,24 there was significant microbial contamination in whole red chillies post-drying, highlighting the need for improved microbial control strategies. This trend is also supported by reports documenting microbial persistence in dried spices and herbs.34 Thus, further research is needed to assess the effectiveness of processing technologies, such as steam, microwave, and pulsed light treatments, in improving the microbiological safety of dehydrated red chillies. An increase in drying temperature and a decrease in water activity (aw) from 0.85 to 0.60 resulted in a decrease in polyphenol oxidase (PPO) activity to 58.1% and peroxidase (POD) activity to 70.3%. In red chillies with 0.35 aw, the activities of PPO and POD were further reduced to 28.3% and 39.7%, respectively. PPO exhibited slightly greater sensitivity to temperature compared to POD. Similar findings were reported by Castro et al.39 in their study on pepper.
log10 CFU g−1 at 0.35 aw. The observed microbial growth can be attributed to the tendency of chilies to absorb moisture from the surrounding environment, even though their aw values remained largely unchanged. Microorganisms can reemerge under favourable conditions, posing significant challenges for export, particularly when moisture content exceeds 11%, which heightens the risk of mould proliferation.16 Although dried chillies have low water activity (<0.6), which inhibits bacterial growth, certain fungi can still proliferate under storage conditions, particularly if residual moisture or oxygen is present within the packaging. Studies have reported the presence of mycotoxigenic Aspergillus species in dried spices, posing food safety risks.31 Localized variations in moisture distribution within the food matrix can create microenvironments that support microbial growth. Additionally, packaging materials with high oxygen and water vapor barrier properties may not entirely prevent microbial contamination, as spores can remain dormant and become active due to fluctuations in storage conditions.16 In our previous study by Pravallika and Chakraborty,24 there was significant microbial contamination in whole red chillies post-drying, highlighting the need for improved microbial control strategies. This trend is also supported by reports documenting microbial persistence in dried spices and herbs.34 Thus, further research is needed to assess the effectiveness of processing technologies, such as steam, microwave, and pulsed light treatments, in improving the microbiological safety of dehydrated red chillies. An increase in drying temperature and a decrease in water activity (aw) from 0.85 to 0.60 resulted in a decrease in polyphenol oxidase (PPO) activity to 58.1% and peroxidase (POD) activity to 70.3%. In red chillies with 0.35 aw, the activities of PPO and POD were further reduced to 28.3% and 39.7%, respectively. PPO exhibited slightly greater sensitivity to temperature compared to POD. Similar findings were reported by Castro et al.39 in their study on pepper.
The concentrations of TPC, TFC, AOX, and CAP in fresh red chillies prior to drying were 21.71 ± 0.23 mg GAE per g, 4.40 ± 0.03 mg quercetin per g, 17.16 ± 0.47 mg GAEAC per g, and 13.45 ± 0.32 mg g−1 (dry basis), respectively. Sequential drying positively influenced the quality attributes of red chillies. For instance, there was an increase in TPC by 4.9%, TFC by 2.4%, AOX by 3.1%, and CAP by 5.8%, respectively, with a decrease in aw from 0.85 to 0.60. On a similar note, there was an increase of 9.0%, 8.9%, 6.3%, and 14.3% in TPC, TFC, AOX, and CAP in 0.35 aw red chillies, respectively. The observed increase is likely attributed to the thermal depolymerization of cell structures during elevated drying temperatures, which enhances the extractability of phenolic compounds, flavonoids, and capsaicinoids. Similar findings have been reported for red chilli peppers.17,40 The rise in AOX levels may result from the alkylation and glycosylation of phenolic and flavonoid compounds into simpler, more bioavailable molecules.41 Additionally, the capsaicin content, responsible for the pungency of red chillies, increased with lower water activity and it was further enhanced by elevated drying temperatures. This improvement is likely due to the enhanced extractability of capsaicin caused by cell rupture.17 The initial pungency of fresh red chillies, measured as 157.9 ± 2.0 MSHU, increased to 161.6 ± 1.9 MSHU at 0.60 aw and 171.9 ± 2.8 MSHU at 0.35 aw. This improvement in capsaicinoid content is primarily attributed to the enhanced extractability facilitated by cell disruption during drying.2 In contrast, the concentrations of ascorbic acid (AA) and carotenoids decreased as drying temperature increased. Specifically, AA levels decreased by 9.4% and 18.7%, while total carotenoid content (TCC) decreased by 11.3% and 18.7% in red chillies with 0.60 and 0.35 water activity (aw), respectively. Deng et al.40 and Kamal et al.17 observed similar trends, reporting AA losses of 66.6% and 54.7% when red pepper and green chillies were dried at 70 °C. This degradation occurs as AA breaks down into Maillard intermediates, such as furfural and 2-furoic acid, under the influence of heat, light, and oxygen.23 Similarly, carotenoids undergo isomerization and oxidation, leading to a loss of bioactivity. Total extractable colour also declined, decreasing from 66.2 ± 3.3 to 58.5 ± 1.9 and 52.7 ± 3.3 ASTA units as aw was reduced to 0.60 and 0.35, respectively.
The L*, a*, and b* values, which were initially 38.69 ± 2.31, 42.84 ± 0.37, and 24.16 ± 1.26, respectively, also dropped significantly (p < 0.05) with decreasing aw. This indicates a noticeable colour shift from red to maroon, primarily attributed to the Maillard reaction.17 Such changes suggest that the dried chillies become maroon as a result of non-enzymatic browning, consistent with findings by Chitravathi et al.42 and Kamal et al.17 Similar reductions were reported in red peppers dried at 70 °C.40 The decline in carotenoids can be linked to surface heating, which induces oxidation and non-enzymatic degradation. The total colour difference (ΔE*) values recorded were 4.25 ± 1.70 at 0.60 aw and 6.81 ± 2.28 at 0.35 aw. For comparison, drying in a cabinet dryer at 60 °C and a hot air dryer at 70 °C resulted in ΔE* values of 10.8 and 31.7, respectively.31 Additional processing steps are required to ensure a >6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in microbial counts and >99% inactivation of PPO and POD, thereby ensuring microbiological safety and enzymatic stability.
log reduction in microbial counts and >99% inactivation of PPO and POD, thereby ensuring microbiological safety and enzymatic stability.
3.2 Effect of processing techniques on microbiological, enzymatic, and quality attributes of red chillies
Steam treatment of red chillies with water activity (aw) levels of 0.60 and 0.35 at 120 °C for 300 s effectively reduced AM and YM populations to below the detection limit of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1. Comparable results were observed in paprika, where a 2.48 and 1.11
log10 CFU g−1. Comparable results were observed in paprika, where a 2.48 and 1.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in AM and YM populations, respectively, was achieved at 125 °C for 120 s.15 Similarly, a 1.00 and 2.66
log reduction in AM and YM populations, respectively, was achieved at 125 °C for 120 s.15 Similarly, a 1.00 and 2.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in the population of aerobic mesophiles and yeasts and moulds was reported in dried red pepper treated at 100 °C for 960 s.6 Steam treatment is particularly effective due to its volumetric heating mechanism, which disrupts membrane integrity, damages cell walls, and denatures proteins and nucleic acids, causing irreversible loss of cell integrity and microbial death.43 However, microbes in lower aw samples exhibit greater thermal resistance, as reduced water content stabilizes bacterial protein structures and prevents heat-sensitive proteins from denaturing.44
log reduction in the population of aerobic mesophiles and yeasts and moulds was reported in dried red pepper treated at 100 °C for 960 s.6 Steam treatment is particularly effective due to its volumetric heating mechanism, which disrupts membrane integrity, damages cell walls, and denatures proteins and nucleic acids, causing irreversible loss of cell integrity and microbial death.43 However, microbes in lower aw samples exhibit greater thermal resistance, as reduced water content stabilizes bacterial protein structures and prevents heat-sensitive proteins from denaturing.44
Similarly, microwave treatment at 540 kJ (2 kW/270 s) achieved target inactivation levels of AM and YM as the temperature reached 75.7 °C and 68.8 °C in 0.60 and 0.35 aw chillies, respectively. On a similar note, black pepper treated with 497.3 kJ of microwave radiation achieved a 4.31 and 4.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in AM and YM populations, respectively.45 Similarly, paprika powder with an aw of 0.88 treated at 39 kJ achieved a 4.8
log reduction in AM and YM populations, respectively.45 Similarly, paprika powder with an aw of 0.88 treated at 39 kJ achieved a 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in the AM population.19 Microwave treatment exerts both thermal and nonthermal effects on microbial cells, contributing to their inactivation. The deep penetration of microwave energy generates intense heating, leading to the leakage of essential cellular components such as proteins, nucleic acids, purines, and pyrimidines. However, beyond its thermal impact, microwaves also induce nonthermal effects that enhance microbial destruction. The high electromagnetic field strengths generated during microwave exposure disrupt microbial cell membranes, causing alterations in permeability, pore formation, and structural damage. These nonthermal mechanisms compromise the integrity of the cell, leading to irreversible physiological stress and eventual cell death. As a result, microwave processing offers an efficient means of microbial inactivation, leveraging both heat-induced damage and electromagnetic field interactions to enhance food safety and stability.46 However, the microbial inactivation mechanism of microwave radiation is not yet fully understood.
log reduction in the AM population.19 Microwave treatment exerts both thermal and nonthermal effects on microbial cells, contributing to their inactivation. The deep penetration of microwave energy generates intense heating, leading to the leakage of essential cellular components such as proteins, nucleic acids, purines, and pyrimidines. However, beyond its thermal impact, microwaves also induce nonthermal effects that enhance microbial destruction. The high electromagnetic field strengths generated during microwave exposure disrupt microbial cell membranes, causing alterations in permeability, pore formation, and structural damage. These nonthermal mechanisms compromise the integrity of the cell, leading to irreversible physiological stress and eventual cell death. As a result, microwave processing offers an efficient means of microbial inactivation, leveraging both heat-induced damage and electromagnetic field interactions to enhance food safety and stability.46 However, the microbial inactivation mechanism of microwave radiation is not yet fully understood.
Pulsed light (PL) treatment with a fluence of 2.59 J cm−2 (2.2 kV/360 s) caused complete inactivation of AM and YM populations. The temperature increased from 28 °C to 42.1 °C and 38.9 °C in 0.60 and 0.35 aw chillies, respectively, at 2.59 J cm−2. The highest microbial inactivation efficiency is observed for high aw samples, as demonstrated by a 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in the AM population in red pepper powder treated at 9.1 J cm−2.25 For tomatoes, a PL fluence of 4 J cm−2 resulted in a 1
log reduction in the AM population in red pepper powder treated at 9.1 J cm−2.25 For tomatoes, a PL fluence of 4 J cm−2 resulted in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in the AM population.47 The effectiveness of pulsed light (PL) treatment is primarily attributed to UV-C-induced photochemical effects, which trigger conjugated dimer formation in nucleic acids, leading to cell lysis. The combined photochemical and photothermal actions create photophysical changes that ultimately result in microbial cell death. Additionally, repeated pulsations exert mechanical stress on microbial cells, causing internal collapse and further enhancing inactivation. PL treatment, on the other hand, faces challenges in low aw samples due to uneven surfaces and wrinkles, which create shadow effects that hinder light distribution and require high fluence for complete inactivation.24,28
log reduction in the AM population.47 The effectiveness of pulsed light (PL) treatment is primarily attributed to UV-C-induced photochemical effects, which trigger conjugated dimer formation in nucleic acids, leading to cell lysis. The combined photochemical and photothermal actions create photophysical changes that ultimately result in microbial cell death. Additionally, repeated pulsations exert mechanical stress on microbial cells, causing internal collapse and further enhancing inactivation. PL treatment, on the other hand, faces challenges in low aw samples due to uneven surfaces and wrinkles, which create shadow effects that hinder light distribution and require high fluence for complete inactivation.24,28
Enzymes such as polyphenol oxidase (PPO) and peroxidase (POD), which contribute to quality deterioration, are completely inactivated (>99%) in red chillies subjected to steam treatment. Complete PPO inactivation has been observed at 80 °C for 600 s, while 90% POD inactivation was achieved at 100 °C for 300 s in chillies and paprika pods, respectively.48 Similarly, parsley leaves treated at 80 °C for 600 s demonstrated 70% and 80% inactivation of PPO and POD, respectively.49 The inactivation mechanism of these enzymes is primarily attributed to irreversible denaturation caused by steam treatment, leading to changes in apoenzyme conformation, degradation of prosthetic groups, and a loss of enzymatic activity. This process disrupts weaker bonds such as disulfide and hydrogen bonds, resulting in the unfolding of proteins and alterations in their secondary and tertiary structures.50
At 540 kJ microwave intensity, the PPO and POD levels reached target inactivation, i.e., >99%. In red bell peppers treated at 90 kJ, PPO and POD inactivation reached 90.2% and 83.6%, respectively.50 Similarly, in potatoes subjected to a microwave energy of 2160 kJ, 50% PPO inactivation was observed.51 Microwave treatment is particularly effective due to its ability to cause irreversible denaturation by cell decompartmentalization, exposing enzymes to external environments and leading to apoenzyme conformational changes and prosthetic group degradation.50
Similarly, PL treatment (2.59 J cm−2) has also demonstrated substantial enzyme inactivation. For instance, a PL fluence of 128 J cm−2 led to a 95% reduction in peroxidase (POD) activity in horseradish,52 while a 90% reduction in polyphenol oxidase (PPO) was noted in mushrooms treated at 3.96 J cm−2.53 The mechanism for PL-induced enzyme inactivation involves structural deformation caused by disrupting protein conformations and altering enzyme functionality. This may include conformational changes in apoenzymes and degradation of prosthetic groups from holoenzymes. Despite these findings, the precise mechanisms underlying enzyme inactivation in red chillies remain complex and challenging to interpret due to the intricate interplay of structural and functional changes in enzyme systems.54
In steam-treated red chillies, bioactive compound retention varied across different water activity levels. At 0.60 and 0.35 aw, total phenolic content (TPC) was retained at 70.3% and 69.1%, total flavonoid content (TFC) at 55.9% and 56.2%, antioxidant activity (AOX) at 79.5% and 80.6%, and capsaicin (CAP) at 102.4% and 101.9%, respectively. However, there was a loss of 45.5% and 34.5% in ascorbic acid (AA) and 30.3% and 25.1% in total carotenoid content (TCC) at the respective aw levels. These findings align with previous studies. For example, a TPC retention of 56.2% was reported in parsley treated at 100 °C for 300 s.49 In litchi pericarp, TFC degraded by 45.5% at 80 °C for 120 min.55 Red pepper treated at 95 °C for 900 s retained 80% TPC, 69.5% AOX, and 87.5% TCC.56 In serrano red bell peppers, only 17.5% AA was retained after treatment at 150 °C for 20 min, while a 1.2% increase in capsaicin was noted in habanero bell peppers under the same conditions.18 Steam treatment involves high temperatures that provide energy exceeding the activation energy of chemical bonds in bioactive compounds, leading to the breakdown of covalent bonds and the formation of smaller, inactive molecules.56 Temperatures around 120 °C can induce conformational changes, altering the molecular geometry of these bioactives. Additionally, oxidation during steam treatment results in the formation of free radicals, which can polymerize into insoluble and inactive compounds.18 Elevated temperatures, in combination with sugars, can also trigger Maillard reactions, resulting in browning and potential loss of bioactivity. Furthermore, certain bioactive compounds, due to their volatile nature, may be lost along with vapor during the process.57
Microwave treatment resulted in higher retention rates. For 0.60 aw red chillies, TPC, TFC, AOX, AA, CAP, and TCC were retained at 95.0%, 89.3%, 91.7%, 83.4%, 84.3%, and 80.7%, respectively. At 0.35 aw, the retention levels were 96.3%, 94.8%, 96.8%, 82.4%, 95.9%, and 95.8%, respectively. In hot peppers treated at 45 kJ, TPC, TFC, and AOX losses were 11%, 14%, and 7.5%, respectively.58 In the case of microwave treatments, paprika treated at 480 kJ retained 91% AA,15 and chili extracts retained 89% and 92% CAP in water- and oil-based systems, respectively, at 42 kJ.53 A carotenoid retention of 79.5% was observed in red bell peppers treated at 90 kJ (MW).50 Microwave treatment generates heat by inducing dipole rotation and interactions in polar molecules such as water, sugars, and bioactives. This process leads to rapid heating and localized temperature increases, which can cause bond cleavage and molecular breakdown.50 Heat-sensitive compounds, such as ascorbic acid and carotenoids, are particularly prone to oxidative degradation under these conditions. The enhanced movement of water molecules during microwave treatment also promotes hydrolytic breakdown of esters, glycosides, and amides. Additionally, rapid heating triggers Maillard reactions, leading to browning in products like chilies and hot peppers.58 Microwaves can disrupt hydrogen bonds and alter hydrophobic interactions in bioactive compounds, affecting their stability. Furthermore, the uneven distribution of microwave energy often results in localized overheating, degrading bioactives in those zones, while some compounds remain stable in areas of lower energy absorption.53
Pulsed light (PL) treatment also preserved bioactive compounds effectively, with 90.2%, 87.8%, 87.7%, 83.8%, 89.9%, and 91.8% in 0.60 aw; 90.2%, 89.0%, 91.7%, 87.1%, 93.0%, and 93.7% in 0.35 aw red chillies of TPC, TFC, AOX, AA, CAP, and TCC, respectively. Similar trends have been observed in previous studies. For instance, fresh cut red bell peppers exhibited a 28.5%, 5%, and 19.2% reduction in total phenolic content (TPC), antioxidant activity (AOX), and carotenoid levels, respectively, after pulsed light treatment of 32 J cm−2. In contrast, ascorbic acid (AA) content increased by 9.1% under the same treatment conditions.10 In another study, Pataro et al.59 reported only a 5% loss in TPC in tomatoes after exposure to a fluence of 1 J cm−2. Similarly, spinach treated with a fluence of 4 J cm−2 exhibited a decrease in TPC.60 Furthermore, studies on red chilies showed 83.8% and 87.2% retention of ascorbic acid (AA); 87.8% and 91.8% retention of antioxidant capacity (AOX); 89.9% and 93.0% retention of capsaicinoids (CAP); and 91.8% and 93.7% retention of total carotenoid content (TCC) after PLT at 2.59 J cm−2 in red chilies with water activities (aw) of 0.6 and 0.35, respectively.24 The reduction in bioactive compounds during processing is primarily due to adverse photochemical reactions. Structural alterations, such as the repositioning of functional groups within benzoic rings, can decrease their reactivity. Pulsed light (PL) processing disrupts cellular structures, including vacuoles and cell walls, breaking covalent bonds in complex bioactive compounds. This breakdown triggers oxidation and metabolic reactions, further contributing to the decline in bioactive levels.10,40 Increased pulsed light (PL) fluence can lead to partial degradation, oxidative modifications, polymerization, and condensation of certain compounds, resulting in a reduction of antioxidant activity (AOX).10 Bioactive compounds may compromise their functionality due to photo-oxidation and trans–cis isomerization, as the chromophore absorbs visible and UV light. In samples with a water activity of 0.35, reduced light penetration due to the thicker pericarp and limited oxygen interaction (caused by the absence of free water) contributes to the greater retention of bioactives.
The surface colour of red chillies showed notable changes following treatment. Specifically, decreases in L*, a*, and b* values were observed in steam-treated (ST) and pulsed light-treated (PL) samples. For microwave-treated (MW) chillies, there was a decrease in L* and b* values, while the a* value increased at both water activity levels. The total colour change (ΔE*) values were 6.19 ± 0.45 and 4.97 ± 0.7 in steam-treated chillies; 6.84 ± 0.79 and 4.27 ± 0.26 in MW-treated chillies; and 5.54 ± 0.21 and 4.63 ± 0.38 in PL-treated chillies, depending on water activity. Weight loss also varied significantly with treatment. Weight losses of 7.5% and 5.6% were observed in steam-treated samples, 28.1% and 9.2% in MW-treated samples, and 3.3% and 0.02% in PL-treated samples at 0.60 and 0.35 aw, respectively. These results align with previous studies reported by Rico et al.,6 Monisha et al.,36 and Dittrich et al.61
3.3 Effect of steam, microwave and pulsed light treatments on microbiological, enzymatic, and bioactive compounds during storage
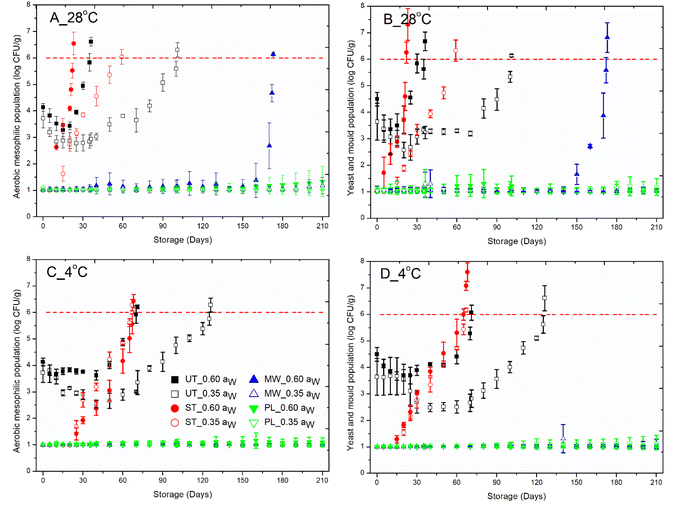
The untreated, steam-treated, microwave-treated, and pulsed light-treated red chilies were stored under both ambient (28 °C) and refrigerated (4 °C) conditions. Significant alterations in microbial growth, enzyme activity, and bioactive concentrations were observed, with the extent of these changes varying according to the treatment method applied. The shelf life of the dehydrated products was assessed using a microbial safety threshold of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 and a colour change of >12, providing essential criteria for evaluating the effectiveness of each treatment in preserving product quality and safety.62
log10 CFU g−1 and a colour change of >12, providing essential criteria for evaluating the effectiveness of each treatment in preserving product quality and safety.62
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1, respectively. Meanwhile, in 0.35 aw red chillies, the AM and YM populations are 3.72 ± 0.37 and 3.64 ± 0.70
log10 CFU g−1, respectively. Meanwhile, in 0.35 aw red chillies, the AM and YM populations are 3.72 ± 0.37 and 3.64 ± 0.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1, respectively. The AM and YM counts increased to 6.61 ± 0.16 and 6.68 ± 0.35 in 0.60 and 0.35 aw red chillies on the 36th day of storage. In 0.35 aw red chillies, the population of AM and YM increased to 6.31 ± 0.27 and 6.14 ± 0.05
log10 CFU g−1, respectively. The AM and YM counts increased to 6.61 ± 0.16 and 6.68 ± 0.35 in 0.60 and 0.35 aw red chillies on the 36th day of storage. In 0.35 aw red chillies, the population of AM and YM increased to 6.31 ± 0.27 and 6.14 ± 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 on the 102nd day of storage, respectively. This leads to spoilage and limits the shelf life of red chilies with 0.60 and 0.35 aw to 35 and 101 days, respectively, when stored under ambient (28 °C) conditions. This shorter shelf life for 0.60 aw samples is likely due to higher moisture content (>12%), which promotes mould growth. On a similar note, the AM and YM counts in refrigerated samples increased to 6.22 ± 0.06 and 6.07 ± 0.29
log10 CFU g−1 on the 102nd day of storage, respectively. This leads to spoilage and limits the shelf life of red chilies with 0.60 and 0.35 aw to 35 and 101 days, respectively, when stored under ambient (28 °C) conditions. This shorter shelf life for 0.60 aw samples is likely due to higher moisture content (>12%), which promotes mould growth. On a similar note, the AM and YM counts in refrigerated samples increased to 6.22 ± 0.06 and 6.07 ± 0.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 (0.60 aw red chillies) on the 71st day; 6.28 ± 0.27 and 6.61 ± 0.47
log10 CFU g−1 (0.60 aw red chillies) on the 71st day; 6.28 ± 0.27 and 6.61 ± 0.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 (0.35 aw red chillies) on the 127th day of storage, respectively. It restricts the shelf life to 70 and 126 days in 0.60 aw and 0.35 aw red chillies, respectively (Table 3). The shelf life of a product depends upon a variety of factors, i.e., temperature, type of packaging material, water activity, etc. According to Rico et al.,6 there was no significant change in microbial growth in untreated and steam treated (100 °C/960 s) red chilli powder with 10% moisture content after six months storage at 4 and 20 °C. The shelf life of dried chillies (10%) packed in LDPE is estimated to be 60 days.63 The shelf life of green chillies increased to 28 days after applying modified atmospheric packaging.42
log10 CFU g−1 (0.35 aw red chillies) on the 127th day of storage, respectively. It restricts the shelf life to 70 and 126 days in 0.60 aw and 0.35 aw red chillies, respectively (Table 3). The shelf life of a product depends upon a variety of factors, i.e., temperature, type of packaging material, water activity, etc. According to Rico et al.,6 there was no significant change in microbial growth in untreated and steam treated (100 °C/960 s) red chilli powder with 10% moisture content after six months storage at 4 and 20 °C. The shelf life of dried chillies (10%) packed in LDPE is estimated to be 60 days.63 The shelf life of green chillies increased to 28 days after applying modified atmospheric packaging.42
| Water activity | Treatment | Storage temperature | Shelf life (days) | AMC (log10 CFU g−1) | YMC (log10 CFU g−1) | PPO (%) | POD (%) |
|---|---|---|---|---|---|---|---|
| a AMC: aerobic mesophilic count; YMC: yeast and mould count; PPO: polyphenol oxidase; POD: peroxidase. Different superscript letters (a, b, and c) within one column indicate significant (p ≤ 0.05) differences among means determined by ANOVA and Tukey's test. | |||||||
| 0.60 | Untreated | 4.1 ± 0.1a | 4.5 ± 0.3a | 58.1 ± 0.9a | 70.3 ± 1.1a | ||
| 28 °C | 35 | 6.6 ± 0.2b | 6.7 ± 0.4c | 74.0 ± 1.8c | 85.3 ± 3.2b | ||
| 4 °C | 70 | 6.2 ± 0.1c | 6.1 ± 0.3b | 67.4 ± 0.9b | 85.5 ± 2.3c | ||
| Steam treated | <DL | <DL | <1 | <1 | |||
| 28 °C | 22 | 6.5 ± 0.4a | 7.3 ± 0.6a | <1 | <1 | ||
| 4 °C | 67 | 6.4 ± 0.3a | 7.6 ± 0.4b | <1 | <1 | ||
| Microwave treated | <DL | <DL | <1 | <1 | |||
| 28 °C | 172 | 6.1 ± 0.1a | 6.8 ± 0.56a | <1 | <1 | ||
| 4 °C | >210 | <DL | <DL | <1 | <1 | ||
| Pulsed light treated | <DL | <DL | <1 | <1 | |||
| 28 °C | >210 | <DL | <DL | 24.9 ± 3.3b | 20.9 ± 2.3b | ||
| 4 °C | >210 | <DL | <DL | 7.7 ± 1.7a | 8.8 ± 0.2a | ||
| 0.35 | Untreated | 3.7 ± 0.4a | 3.6 ± 0.7a | 28.3 ± 2.3a | 39.8 ± 1.3a | ||
| 28 °C | 101 | 6.3 ± 0.3b | 6.1 ± 0.1b | 46.1 ± 0.9c | 53.8 ± 1.5c | ||
| 4 °C | 126 | 6.3 ± 0.3b | 6.6 ± 0.5c | 32.6 ± 1.5b | 50.5 ± 1.4b | ||
| Steam treated | <DL | <DL | <1 | <1 | |||
| 28 °C | 59 | 6.0 ± 0.3a | 6.3 ± 0.4ab | <1 | <1 | ||
| 4 °C | 66 | 6.3 ± 0.2b | 6.2 ± 0.1a | <1 | <1 | ||
| Microwave treated | <DL | <DL | <1 | <1 | |||
| 28 °C | >210 | <DL | <DL | <1 | <1 | ||
| 4 °C | >210 | <DL | <DL | <1 | <1 | ||
| Pulsed light treated | <DL | <DL | <1 | <1 | |||
| 28 °C | >210 | <DL | <DL | 9.0 ± 1.1b | 8.3 ± 0.6b | ||
| 4 °C | >210 | <DL | <DL | 3.7 ± 0.7a | 3.3 ± 1.1a | ||
The populations of aerobic mesophiles (AM) and yeasts and moulds (YM) were below the detection limit (DL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1) immediately after steam, MW, and PL treatments. In steam-treated red chillies, the AM and YM populations started growing from day 10 and exceeded the limit on the 23rd day in 0.60 aw red chillies, while in 0.35 aw red chillies, AM and YM counts started increasing from day 15 and exceeded the limit on the 60th day of storage. Hence, ambient stored steam-treated red chillies have a shelf life of 22 days and 59 days in 0.60 and 0.35 asw red chillies, respectively (Fig. 1). Similarly, refrigerated steam-treated chillies, the AM and YM counts exceeded the limit on the 68th day and 67th day in 0.60 and 0.35 aw red chillies, respectively. Therefore, the shelf life is 67 days and 66 days for 0.60 and 0.35 aw red chillies, respectively. The shelf life of steam treated chillies was comparatively even shorter than that of untreated chillies. Although the chilies were packed, some moisture may still remain trapped inside, providing a conducive environment for microbial growth. The polypropylene (PP) packaging material may permit limited transmission of water vapor and oxygen, which can support microbial growth. Steam treatment also raises the humidity within the packaging. If the packaging does not allow for adequate air exchange, the elevated humidity can persist, creating an ideal environment for microbial growth, particularly moulds.
log10 CFU g−1) immediately after steam, MW, and PL treatments. In steam-treated red chillies, the AM and YM populations started growing from day 10 and exceeded the limit on the 23rd day in 0.60 aw red chillies, while in 0.35 aw red chillies, AM and YM counts started increasing from day 15 and exceeded the limit on the 60th day of storage. Hence, ambient stored steam-treated red chillies have a shelf life of 22 days and 59 days in 0.60 and 0.35 asw red chillies, respectively (Fig. 1). Similarly, refrigerated steam-treated chillies, the AM and YM counts exceeded the limit on the 68th day and 67th day in 0.60 and 0.35 aw red chillies, respectively. Therefore, the shelf life is 67 days and 66 days for 0.60 and 0.35 aw red chillies, respectively. The shelf life of steam treated chillies was comparatively even shorter than that of untreated chillies. Although the chilies were packed, some moisture may still remain trapped inside, providing a conducive environment for microbial growth. The polypropylene (PP) packaging material may permit limited transmission of water vapor and oxygen, which can support microbial growth. Steam treatment also raises the humidity within the packaging. If the packaging does not allow for adequate air exchange, the elevated humidity can persist, creating an ideal environment for microbial growth, particularly moulds.
On the other hand, the AM and YM counts did not start growing till 165 days in MW treated 0.60 aw red chillies stored under ambient conditions. The AM and YM populations exceeded the limit on the 173rd day; therefore, the shelf life is restricted to 172 days. In a similar study, the shelf life of ground nuts increased to 120 days when treated at 5 W g−1 for 60 s and stored in a PP pouch.64 Under both storage conditions, the MW treated 0.35 aw red chillies did not experience microbial growth till 210 days (Fig. 1). It might be the evaporation of water during MW treatment from the red chillies that reduced the moisture content to below 8%, which helps to prevent the growth of microorganisms.
PL preserved 0.60 and 0.35 aw red chillies for >210 days with microbial growth of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1. When fresh cut bell peppers were treated at 32 J cm−2, the AM and YM counts remained at 1
log10 CFU g−1. When fresh cut bell peppers were treated at 32 J cm−2, the AM and YM counts remained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 till 7 days of storage at 4 °C.10 Onion shreds treated at 2208 J cm−2 exceeded the microbial limit after 30 days, 27 days, and 19 days at 25 °C, 30 °C, and 37 °C, respectively. Pulsed light causes irreversible damage to microbial DNA, RNA, and proteins, leading to cellular dysfunction and inhibiting the growth of AMC and YMC. When coupled with unfavourable storage conditions, such as low temperatures, restricted oxygen availability, and limited nutrients, PL treatment effectively enhances microbial inactivation, leaving any surviving cells unable to recover or multiply during storage.10 MW and PL treatments were the most effective in extending the shelf life of dried chillies by maintaining microbial populations below the detection limit (1
log10 CFU g−1 till 7 days of storage at 4 °C.10 Onion shreds treated at 2208 J cm−2 exceeded the microbial limit after 30 days, 27 days, and 19 days at 25 °C, 30 °C, and 37 °C, respectively. Pulsed light causes irreversible damage to microbial DNA, RNA, and proteins, leading to cellular dysfunction and inhibiting the growth of AMC and YMC. When coupled with unfavourable storage conditions, such as low temperatures, restricted oxygen availability, and limited nutrients, PL treatment effectively enhances microbial inactivation, leaving any surviving cells unable to recover or multiply during storage.10 MW and PL treatments were the most effective in extending the shelf life of dried chillies by maintaining microbial populations below the detection limit (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1). Under ambient storage, untreated chillies lasted 35 to 101 days depending on water activity (aw), while MW-treated chillies lasted up to 210 days, and PL-treated chillies had the longest shelf life of 210 days for both 0.60 and 0.35 aw. Refrigeration extended the shelf life of untreated and steam-treated chillies but provided no additional benefit for MW- and PL-treated samples, which remained stable for 210 days.
log10 CFU g−1). Under ambient storage, untreated chillies lasted 35 to 101 days depending on water activity (aw), while MW-treated chillies lasted up to 210 days, and PL-treated chillies had the longest shelf life of 210 days for both 0.60 and 0.35 aw. Refrigeration extended the shelf life of untreated and steam-treated chillies but provided no additional benefit for MW- and PL-treated samples, which remained stable for 210 days.
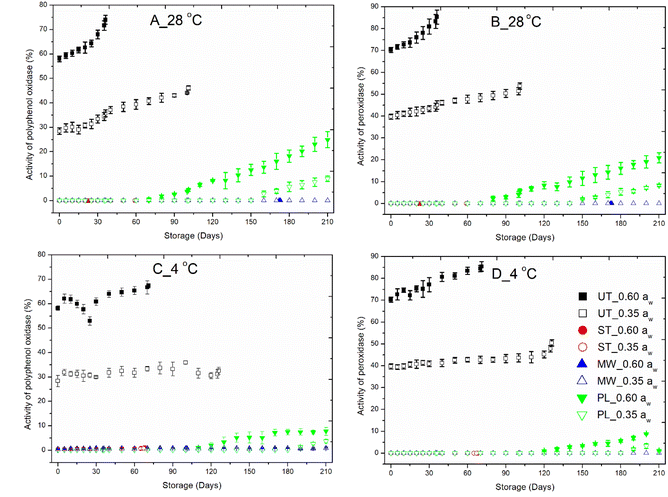
Complete inactivation of these enzymes was observed in red chilies treated with steam, microwaves, and pulsed light at the beginning of storage. During the storage period, the steam treated chillies exhibited no detectable PPO or POD activity. This is because enzymes, being proteins, are exposed to higher temperatures typically above the threshold required for denaturation. It also causes unfolding of enzymes, which might be due to disruption of covalent, hydrogen, and hydrophobic bonds, leading to irreversible denaturation of the enzyme.37 Additionally, steam treatment causes breakdown of substrates that are necessary for enzyme activity.50
Microwave treated red chillies did not show PPO and POD activity throughout the storage of 210 days. The rapid heating during microwave treatment disrupts the three-dimensional structure of these enzymes by damaging their secondary and tertiary structures at the active site, thus rendering them inactive. Additionally, microwave treatment alters the composition of substrates, further limiting the potential for enzyme activity.66 Wang et al.50 reported a reduction in β-sheet content accompanied by an increase in β-turns and random coils, signifying structural reorganization.
In contrast, the activity of PPO and POD increased in PL treated red chilies during storage, suggesting that PL causes reversible denaturation. Structural or chemical changes induced by PL treatment may have reverted over the course of storage.38 In ambient-stored PL-treated samples, PPO and POD activity began to increase on the 80th day for both enzymes at a water activity (aw) of 0.60. At a lower water activity (0.35 aw), the increase was delayed, beginning on the 160th day for PPO and the 150th day for POD. In refrigerated PL-treated samples, PPO and POD activity started increasing later: on the 110th and 130th days, respectively, at 0.60 aw, and on the 190th and 200th days, respectively, at 0.35 aw. Notably, in refrigerated storage, PPO and POD activity remained below 8% throughout the storage period (Fig. 2). These enzymes play a key role in the degradation of phenolic compounds and the formation of brown pigments. In this study, PL treatment completely inactivated these enzymes by causing denaturation and aggregation. PP film, although resistant to moisture penetration, exhibits moderate permeability to oxygen and other gases. This characteristic can enhance enzyme activity by facilitating oxidative and degradative processes. The resulting oxygen transfer and associated oxidation may further impact enzyme stability.
Steam-treated chilies demonstrated 99% enzymatic stability, with a shelf life of 22 days at 0.60 aw and 59 days at 0.35 aw. In contrast, microwave treatment significantly prolonged the shelf life, maintaining stability for 172 days at 0.60 aw and 210 days at 0.35 aw. Pulsed light (PL) treatment resulted in a shelf life of 79 days at 0.60 aw and 149 days at 0.35 aw under ambient storage conditions, while refrigerated PL-treated chilies lasted 109 days at 0.60 aw and 189 days at 0.35 aw. Overall, steam and microwave treatments exhibited superior enzymatic stability across both storage temperatures, whereas PL treatment led to a gradual increase in enzyme activity throughout the storage period.
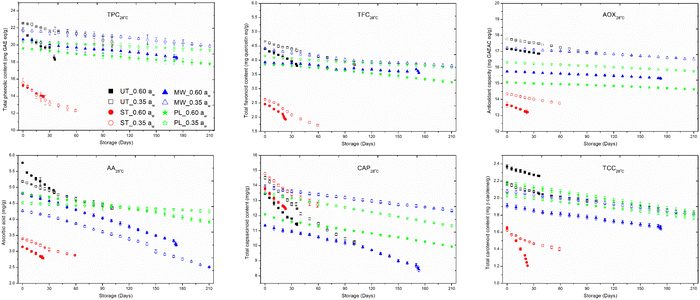
Steam-treated red chillies showed a better retention of 91.6% and 79.0% of TPC; 79.7% and 69.3% of TFC; 96.8% and 96.0% of AOX; 91.0% and 86.0% of CAP in 0.60 and 0.35 aw red chillies, respectively, under ambient storage conditions (Fig. 3). At 4 °C, steam treated red chillies caused a loss of 25.3% and 17.2% TPC; 14.0% and 11.1% TFC; 6.7% and 3.6% AOX; 24.6% and 21.5% CAP in 0.60 and 0.35 aw red chillies, respectively. The antioxidant capacity and capsaicinoid content did not degrade after treatment but decreased during storage.6 There was 92.9% retention of capsaicin in steam treated red pepper powder samples at 4 °C, while at 20 °C, there was 83.9% in steam treated samples after six months.6 Steam treatment triggers degradation processes that persist during storage, potentially causing oxidation, hydrolysis, and isomerization, which reduce the stability of bioactives. The PP film may permit oxygen transmission, allowing it to react with bioactives exposed due to cellular structure disruption.34 Additionally, steam treatment releases sugars, amino acids, and other compounds that can interact with bioactives, leading to the formation of insoluble or inactive complexes.10
Microwave-treated red chillies showed better retention (>85%) of bioactives under both ambient and refrigerated storage, but the CAP content was reduced to 75.1% and 88.5% in 0.60 and 0.35 aw red chillies, respectively. Microwaves may disrupt cellular integrity and expose these bioactives to oxygen, which might lead to oxidative degradation. Microwaves generate reactive oxygen species that degrade sensitive bioactives.67
PL-treated red chillies showed better retention (>85%) of bioactives under both ambient and refrigerated storage, but the CAP content was reduced to 82.3% and 83.8% in 0.60 and 0.35 aw red chillies, respectively. The pungency also decreased, as it is directly correlated to the content of total capsaicinoids.2 The concentration of CAP in PL treated chillies showed >88% retention at both aw levels under refrigerated storage. When fresh cut bell peppers were treated at 16 J cm−2 PL-fluence, they retained 96.5% TPC and 96.2% AOX after 7 days of storage at 4 °C.10 The TPC and TFC were retained at 45.7% and 19.6% on the 30th day; 44.8% and 22.4% on the 27th day; 47.6% and 26.1% on the 19th day of storage at 25, 30, and 37 °C, respectively.34 The infrared (IR) and ultraviolet-C (UV-C) components of pulsed light contribute to the breakdown and photo-oxidation of phenolic compounds. Several bioactive substances possess inherently unstable structures and are highly susceptible to environmental factors such as temperature, oxygen, light, and free radicals. Although unlikely, bioactive compounds may degrade during storage if there is continuous oxygen exposure through the polypropylene (PP) film.34 Limited research exists on the degradation of bioactive compounds, but available studies suggest that phenolics, flavonoids, and antioxidants decrease over time due to the formation of complex polymeric structures with proteins, leading to their precipitation.10
Among all the bioactive compounds, ascorbic acid and carotenoids were the most sensitive to the treatments. The retention of AA and TCC at 28 °C is 84.8% and 95.5% in 0.60 aw (untreated); 84.2% and 90.6% in 0.35 aw (untreated); 89.6% and 74.6% in 0.60 aw (steam-treated); 84.7% and 86.4% in 0.35 aw (steam-treated), respectively. The refrigerated storage caused comparatively better retention of AA and TCC, which was >75% and >90% in untreated samples and >85% and >75% in steam-treated samples, respectively. Similarly, capsanthin retention was 87.2% and 82.6% at 4 °C and capsanthin retention was 61.7% and 63.0% at 20 °C in untreated and steam treated red pepper powder.6
MW treatment preserved 67.2% AA and 86.7% carotenoids in 0.60 aw, while in 0.35 aw, there is 58.9% and 87.3% retention of AA and TCC, respectively, at 28 °C. For instance, in MW treated samples, there was >80% retention of AA and >90% retention of carotenoids at 4 °C. The rapid generation of heat and uneven heating of MW develop hotspots, which disturb the stability of AA, leading to its degradation. Once the disruption of the food matrix occurs, the bioactive molecules are exposed to the external environment such as water, oxygen and light, which promotes oxidation and degradation of ascorbic acid to ascorbyl radicals.62
PL treatment showed better preservation of AA and TCC. For instance, the loss of AA and TCC is only 18.7% and 16.2% in 0.60 aw and 5.9% and 12.9% in 0.35 aw at 28 °C, respectively. It preserved >90% of AA and >80% of carotenoids were preserved at 4 °C. The extractable colour (ASTA) was directly proportional to the concentration of carotenoids. A similar trend has been reported by Jalgaonkar et al.2 (Table 4). When fresh cut bell peppers were treated at 16 J cm−2 fluence, they retained 32.7% AA and 89.2% of TCC after 7 days of storage at 4 °C.10 The ascorbic acid was retained at 13%, 15%, and 24% after 30, 27, and 19 days of storage at 25, 30, and 37 °C, respectively.34 Reactive oxygen species generated in PL treatment can persist and initiate oxidative degradation of ascorbic acid during storage. Carotenoids are more prone to photobleaching when exposed to UV and visible light, both of which are part of PL. The unsaturated bonds of carotenoids were broken down by PL, which leads to structural degradation.23 The generation of reactive oxygen species (ROS) and free radicals, which react with carotenoids, causes the breakdown of conjugated double bonds of carotenoids, limiting their activity.10
| Water activity | Treatment | Storage temperature | Storage days | TPC (mg GAE eq. per g) | TFC (mg quercetin per g) | AOX (mg GAEAC per g) | AA (mg g−1) | CAP (mg g−1) | TCC (mg g−1) | Pungency (MSHU) | Total extractable colour (ASTA units) | CoC (ΔE*) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a TPC: total phenolic content; TFC: total flavonoid content; AOX: antioxidant capacity; AA: ascorbic acid; CAP: capsaicinoids; TCC: total carotenoid content; MSHU: million Scoville heat units; ASTA: American Spice Trade Association; CoC: total colour change. Different superscript letters (a, b, and c) within one column indicate significant (p ≤ 0.05) differences among means determined by ANOVA and Tukey's test. | ||||||||||||
| 0.60 | Untreated | 0 | 21.7 ± 0.2c | 4.4 ± 0.0b | 17.2 ± 0.0c | 5.8 ± 0.0c | 13.5 ± 0.0b | 2.4 ± 0.0c | 215 ± 5.1c | 52.2 ± 0.6c | 0.0 ± 0.0a | |
| 28 °C | 35 | 18.5 ± 0.3a | 3.9 ± 0.0a | 16.9 ± 0.0b | 4.9 ± 0.2b | 11.5 ± 0.0a | 2.3 ± 0.0b | 183.2 ± 2.7b | 49.8 ± 0.2b | 3.1 ± 0.8c | ||
| 4 °C | 70 | 19.4 ± 0.0b | 3.9 ± 0.1a | 16.8 ± 0.0a | 4.6 ± 0.0a | 11.4 ± 0.1a | 2.2 ± 0.0a | 182.8 ± 3.7a | 48.5 ± 0.7a | 2.2 ± 0.6b | ||
| Steam treated | 0 | 15.3 ± 0.1c | 2.5 ± 0.0c | 13.7 ± 0.2c | 3.1 ± 0.0c | 13.8 ± 0.2c | 1.7 ± 0.0c | 220.6 ± 3.4c | 36.2 ± 0.6c | 0.0 ± 0.0a | ||
| 28 °C | 22 | 14.0 ± 0.2b | 2.0 ± 0.0a | 13.2 ± 0.0b | 2.8 ± 0.0b | 12.5 ± 0.1b | 1.2 ± 0.0a | 200.7 ± 5.4b | 27.2 ± 1.0b | 7.9 ± 0.8c | ||
| 4 °C | 67 | 11.4 ± 0.0a | 2.1 ± 0.0ab | 12.7 ± 0.0a | 2.7 ± 0.1a | 10.4 ± 0.1a | 1.3 ± 0.0b | 166.3 ± 2.4a | 28.1 ± 1.9a | 4.5 ± 1.0b | ||
| Microwave treated | 0 | 20.6 ± 0.1c | 3.9 ± 0.0c | 15.8 ± 0.1b | 4.8 ± 0.0c | 11.4 ± 0.0c | 1.9 ± 0.0c | 181.6 ± 0.5c | 42.0 ± 0.6c | 0.0 ± 0.0a | ||
| 28 °C | 172 | 18.5 ± 0.2b | 3.6 ± 0.0a | 15.3 ± 0.0a | 3.2 ± 0.1a | 8.5 ± 0.1a | 1.6 ± 0.0a | 136.4 ± 5.1a | 36.3 ± 0.5a | 2.2 ± 0.2c | ||
| 4 °C | 210 | 17.3 ± 0.1a | 3.7 ± 0.2b | 15.3 ± 0.1a | 3.9 ± 0.1b | 10.0 ± 0.1b | 1.7 ± 0.0b | 160.5 ± 2.4b | 37.8 ± 0.2b | 1.6 ± 0.4b | ||
| Pulsed light treated | 0 | 19.6 ± 0.1c | 3.9 ± 0.0b | 15.1 ± 0.1c | 4.8 ± 0.0c | 12.1 ± 0.0c | 2.2 ± 0.0b | 193.5 ± 5.4c | 47.8 ± 0.6c | 0.0 ± 0.0a | ||
| 28 °C | 210 | 17.8 ± 0.2b | 3.2 ± 0.0a | 14.6 ± 0.0a | 3.9 ± 0.1a | 10.0 ± 0.0a | 1.8 ± 0.0a | 159.2 ± 5.4a | 40.0 ± 0.7b | 2.3 ± 1.3c | ||
| 4 °C | 210 | 16.8 ± 0.4a | 3.2 ± 0.1a | 14.7 ± 0.1b | 4.5 ± 0.1b | 11.0 ± 0.2b | 1.8 ± 0.0a | 176.1 ± 3.4b | 39.8 ± 0.8a | 1.2 ± 0.1b | ||
| 0.35 | Untreated | 0 | 22.5 ± 0.0c | 4.7 ± 0.0c | 17.8 ± 0.1c | 5.2 ± 0.0c | 14.5 ± 0.1c | 2.2 ± 0.0b | 232.2 ± 1.9c | 47.7 ± 0.4c | 0.0 ± 0.0a | |
| 28 °C | 101 | 20.2 ± 0.2a | 3.8 ± 0.0b | 16.9 ± 0.1a | 4.4 ± 0.0b | 10.2 ± 0.1a | 2.0 ± 0.0a | 163.4 ± 12a | 43.2 ± 0.2a | 4.1 ± 1.5c | ||
| 4 °C | 126 | 21.7 ± 0.2b | 3.7 ± 0.2a | 17.2 ± 0.0b | 4.0 ± 0.1a | 12.4 ± 0.1b | 2.0 ± 0.0a | 198.1 ± 10.4b | 44.0 ± 0.4b | 3.3 ± 1.1b | ||
| Steam treated | 0 | 15.6 ± 0.5c | 2.6 ± 0.0c | 14.3 ± 0.1b | 3.4 ± 0.0c | 14.8 ± 0.1c | 1.6 ± 0.0b | 236.7 ± 7.5c | 35.6 ± 0.4c | 0.0 ± 0.0a | ||
| 28 °C | 59 | 12.3 ± 0.2a | 1.7 ± 0.0a | 13.8 ± 0.1a | 2.9 ± 0.0a | 12.7 ± 0.2b | 1.4 ± 0.0a | 203.6 ± 7.5b | 30.6 ± 1.0b | 7.0 ± 0.9c | ||
| 4 °C | 66 | 12.8 ± 0.3b | 2.3 ± 0.1b | 13.8 ± 0.0a | 3.0 ± 0.0b | 11.6 ± 0.1a | 1.4 ± 0.1a | 182.3 ± 15.7a | 29.8 ± 1.3a | 3.5 ± 1.6b | ||
| Microwave treated | 0 | 21.7 ± 0.3c | 4.4 ± 0.0c | 17.2 ± 0.1c | 4.3 ± 0.0c | 13.9 ± 0.1c | 2.1 ± 0.0c | 222.9 ± 2.1c | 45.7 ± 0.5c | 0.0 ± 0.0a | ||
| 28 °C | 210 | 19.9 ± 0.2a | 3.8 ± 0.0a | 16.5 ± 0.1b | 2.5 ± 0.0a | 12.3 ± 0.1a | 1.8 ± 0.0a | 197.3 ± 5.1a | 39.8 ± 0.5a | 2.0 ± 0.3c | ||
| 4 °C | 210 | 20.1 ± 0.7b | 4.3 ± 0.0b | 16.4 ± 0.1a | 3.7 ± 0.1b | 13.1 ± 0.1b | 1.9 ± 0.0b | 209.1 ± 9.0b | 41.7 ± 0.6b | 1.5 ± 0.1b | ||
| Pulsed light treated | 0 | 20.4 ± 0.1c | 4.2 ± 0.0c | 16.3 ± 0.1c | 4.5 ± 0.0b | 13.5 ± 0.0c | 2.0 ± 0.0b | 216.0 ± 5.1c | 44.7 ± 0.6c | 0.0 ± 0.0a | ||
| 28 °C | 210 | 19.4 ± 0.1b | 3.8 ± 0.0a | 15.8 ± 0.0b | 4.2 ± 0.1a | 11.3 ± 0.1a | 1.8 ± 0.0a | 181.0 ± 5.9a | 38.8 ± 0.7a | 1.4 ± 0.5c | ||
| 4 °C | 210 | 18.2 ± 0.4a | 4.0 ± 0.1b | 15.7 ± 0.2a | 4.2 ± 0.1a | 13.2 ± 0.0b | 1.8 ± 0.0a | 211.5 ± 5.9b | 39.7 ± 0.7b | 1.2 ± 0.1b | ||
Temperature and light accelerate the reaction, leading to a greater reduction in bioactive compounds when stored at ambient temperature. As phenolic compounds undergo browning and colour degradation, these changes can serve as indicators of quality loss. The stability of these bioactives in red chillies was compromised by the minimal activity of POD and PPO during storage. However, the loss of bioactives was significantly lower in microwave (MW) and pulsed light (PL) treatments compared to steam treatment. This could be due to the leaching and degradation caused by prolonged exposure to high temperatures.37 Natural antioxidants such as AA, TPC, and CAP might also degrade due to changes in the food matrix caused by treatments that may also expose them to oxygen, light, or other degrading factors, which accelerate oxidative processes.10 Oxygen and moisture migration during storage can create favourable conditions for bioactive degradation, especially because the treated red chillies have a disrupted or compromised barrier system.
The degradation kinetics of bioactives followed first order kinetics with adj R2 > 0.85, which describes the potential fitting of the model. The k values ranged from 0.49 ± 0.01 to 5.02 ± 0.22 × 10−3 d−1, 0.72 ± 0.01 to 12.03 ± 0.05 × 10−3 d−1, 0.20 ± 0.00 to 2.27 ± 0.06 × 10−3 d−1, and 0.13 ± 0.00 to 0.94 ± 0.01 × 10−3 d−1 in untreated, steam-treated, MW-treated, and PL-treated red chillies, respectively, under ambient storage (Table 4). The rate of bioactive degradation highly varied with the treatment performed. For instance, the highest k (× 10−3) value was found for AA, which is 5.02 ± 0.22 d−1, followed by CAP with a k (× 10−3) value of 4.61 ± 0.07 d−1 and TPC with a k (× 10−3) value of 4.29 ± 0.21 d−1 in 0.60 aw/untreated red chilli samples. Meanwhile in 0.35 aw, the highest k (× 10−3) value was found for CAP, which is 3.73 ± 0.07 d−1 (Table 5). Similarly, the k (× 10−3) value for TCC is 12.03 ± 0.05 d−1 followed by TFC with a k (× 10−3) value of 9.16 ± 0.05 d−1 in 0.60 aw/steam-treated red chillis. The rate of degradation of TFC was found to be higher with a k (× 10−3) value of 7.22 ± 0.18 d−1 in 0.35 aw/steam-treated samples. AA was found to be the most sensitive bioactive compound towards microwave treatment with k (× 10−3) values of 2.13 ± 0.03 d−1 and 2.27 ± 0.06 d−1, in 0.60 and 0.35 aw red chillies, respectively. The higher the k value, the greater the degradation of bioactive compounds and vice versa. A similar study was conducted on PL treated onion shreds and the rate of degradation of bioactives followed zero order kinetics.34 In PL treated chillies, the k values for all the bioactive compounds are almost in the homogeneous range, indicating little higher values for capsaicinoids and carotenoids. The higher k values were found in 0.60 aw red chillies and 0.35 aw red chillies irrespective of the treatment due to the presence of PPO and POD. Similarly, the k values of refrigerated samples were lower than those of the samples stored under ambient storage conditions. Polypropylene (PP) is sensitive to oxygen transmission, which affects its ability to allow oxygen to pass through. To mitigate this issue, it can be coextruded with another material to reduce the rate of oxygen transmission through the PP film.
| Treatment | Water activity | Bioactive compounds | Model fitting parameters from the first-order kinetic model for red chillies during storage at 28 °C | Model fitting parameters from the first-order kinetic model for red chillies during storage at 4 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Rate constant k (10−3) d−1 ± CI | Adjusted R2 | Reduced chi-square (10−3) | Rate constant k (10−3) d−1 ± CI | Adjusted R2 | Reduced chi-square | |||
| a TPC: total phenolic content; TFC: total flavonoid content; AOX: antioxidant capacity; AA: ascorbic acid; CAP: capsaicinoids; TCC: total carotenoid content. CI: 95% confidence interval of the mean values. Dissimilar small alphabets (a to f) recognize that the mean k-values (d−1) belong to different statistical subsets across the column at p < 0.05. | ||||||||
| Untreated | 0.60 | TPC | 4.29 ± 0.21d | 0.93 | 0.2098 | 1.75 ± 0.07c | 0.93 | 0.1052 |
| TFC | 3.60 ± 0.10c | 0.98 | 0.0504 | 1.94 ± 0.06d | 0.97 | 0.0638 | ||
| AOX | 0.49 ± 0.01a | 0.99 | 0.0004 | 0.29 ± 0.01a | 0.96 | 0.0019 | ||
| AA | 5.02 ± 0.22f | 0.93 | 0.2262 | 3.48 ± 0.10f | 0.97 | 0.1802 | ||
| CAP | 4.61 ± 0.07e | 0.99 | 0.0212 | 2.57 ± 0.07e | 0.97 | 0.1064 | ||
| TCC | 1.38 ± 0.03b | 0.98 | 0.0046 | 1.07 ± 0.03b | 0.96 | 0.0240 | ||
| 0.35 | TPC | 1.03 ± 0.02b | 0.98 | 0.0226 | 0.33 ± 0.01ab | 0.96 | 0.0061 | |
| TFC | 1.93 ± 0.02e | 0.99 | 0.0160 | 1.79 ± 0.04e | 0.97 | 0.1408 | ||
| AOX | 0.51 ± 0.01a | 0.99 | 0.0018 | 0.29 ± 0.01a | 0.96 | 0.0049 | ||
| AA | 1.76 ± 0.02d | 0.99 | 0.0213 | 2.02 ± 0.04f | 0.98 | 0.1515 | ||
| CAP | 3.73 ± 0.07f | 0.98 | 0.2491 | 1.29 ± 0.03d | 0.97 | 0.0753 | ||
| TCC | 1.13 ± 0.05c | 0.88 | 0.1173 | 0.65 ± 0.02c | 0.97 | 0.0224 | ||
| Steam treated | 0.60 | TPC | 4.06 ± 0.06b | 0.99 | 0.0071 | 4.22 ± 0.08d | 0.99 | 0.1502 |
| TFC | 9.16 ± 0.05e | 0.93 | 0.5693 | 2.31 ± 0.05b | 0.98 | 0.0578 | ||
| AOX | 1.46 ± 0.03a | 0.99 | 0.0019 | 1.07 ± 0.03a | 0.98 | 0.0150 | ||
| AA | 4.96 ± 0.08d | 0.99 | 0.0158 | 2.48 ± 0.05c | 0.98 | 0.0604 | ||
| CAP | 4.37 ± 0.05c | 0.99 | 0.0048 | 4.20 ± 0.08d | 0.99 | 0.1421 | ||
| TCC | 12.03 ± 0.05f | 0.96 | 0.4536 | 3.60 ± 0.07e | 0.98 | 0.1200 | ||
| 0.35 | TPC | 4.58 ± 0.24d | 0.91 | 0.5588 | 3.03 ± 0.08e | 0.98 | 0.1099 | |
| TFC | 7.22 ± 0.18e | 0.99 | 0.3064 | 1.88 ± 0.05b | 0.97 | 0.0448 | ||
| AOX | 0.72 ± 0.01a | 0.99 | 0.0009 | 0.59 ± 0.02a | 0.97 | 0.0045 | ||
| AA | 3.00 ± 0.07c | 0.98 | 0.0512 | 2.05 ± 0.05c | 0.97 | 0.0535 | ||
| CAP | 2.85 ± 0.10b | 0.96 | 0.1044 | 3.94 ± 0.09f | 0.98 | 0.1600 | ||
| TCC | 2.86 ± 0.16b | 0.88 | 0.2671 | 2.64 ± 0.06d | 0.98 | 0.0766 | ||
| Microwave treated | 0.60 | TPC | 0.63 ± 0.01c | 0.97 | 0.0350 | 0.82 ± 0.00e | 0.99 | 0.0027 |
| TFC | 0.54 ± 0.02b | 0.89 | 0.1109 | 0.29 ± 0.00b | 0.99 | 0.0001 | ||
| AOX | 0.15 ± 0.01a | 0.99 | 0.0007 | 0.14 ± 0.00a | 0.99 | 0.00004 | ||
| AA | 2.13 ± 0.03f | 0.98 | 0.2656 | 1.03 ± 0.00f | 0.99 | 0.0080 | ||
| CAP | 1.47 ± 0.04e | 0.95 | 0.4349 | 0.61 ± 0.00d | 0.99 | 0.0012 | ||
| TCC | 0.83 ± 0.01d | 0.99 | 0.0141 | 0.48 ± 0.00c | 0.99 | 0.0004 | ||
| 0.35 | TPC | 0.38 ± 0.01b | 0.98 | 0.0173 | 0.37 ± 0.00d | 0.99 | 0.0001 | |
| TFC | 0.83 ± 0.03e | 0.88 | 0.2431 | 0.16 ± 0.00a | 0.99 | 0.000001 | ||
| AOX | 0.20 ± 0.00a | 0.99 | 0.0005 | 0.23 ± 0.00b | 0.99 | 0.00001 | ||
| AA | 2.27 ± 0.06f | 0.96 | 1.2400 | 0.71 ± 0.00f | 0.99 | 0.0018 | ||
| CAP | 0.58 ± 0.01c | 0.99 | 0.0075 | 0.30 ± 0.00c | 0.99 | 0.0001 | ||
| TCC | 0.63 ± 0.00d | 0.99 | 0.0032 | 0.43 ± 0.00e | 0.99 | 0.0002 | ||
| Pulsed light treated | 0.60 | TPC | 0.47 ± 0.00b | 0.99 | 0.0041 | 0.72 ± 0.00d | 0.99 | 0.0014 |
| TFC | 0.78 ± 0.02c | 0.98 | 0.0805 | 0.86 ± 0.00e | 0.99 | 0.0028 | ||
| AOX | 0.13 ± 0.00a | 0.99 | 0.0008 | 0.11 ± 0.00a | 0.99 | 0.00001 | ||
| AA | 0.94 ± 0.01d | 0.99 | 0.0293 | 0.33 ± 0.00b | 0.99 | 0.0001 | ||
| CAP | 0.93 ± 0.01d | 0.98 | 0.0521 | 0.45 ± 0.00c | 0.99 | 0.0003 | ||
| TCC | 0.82 ± 0.00c | 0.99 | 0.0033 | 0.92 ± 0.02f | 0.93 | 0.1766 | ||
| 0.35 | TPC | 0.22 ± 0.00c | 0.99 | 0.0024 | 0.52 ± 0.00c | 0.99 | 0.0010 | |
| TFC | 0.14 ± 0.00a | 0.99 | 0.00001 | 0.14 ± 0.00a | 0.99 | 0.00002 | ||
| AOX | 0.16 ± 0.00b | 0.99 | 0.0003 | 0.17 ± 0.00ab | 0.99 | 0.00001 | ||
| AA | 0.28 ± 0.00d | 0.99 | 0.0008 | 0.34 ± 0.00b | 0.99 | 0.0001 | ||
| CAP | 0.83 ± 0.00f | 0.99 | 0.0016 | 0.10 ± 0.00a | 0.99 | 0.000001 | ||
| TCC | 0.65 ± 0.00e | 0.99 | 0.0013 | 0.55 ± 0.00d | 0.99 | 0.0010 | ||
As per the categorization by Cserhalmi et al.35 the total colour change (ΔE*) can be unnoticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well-visible (3.0–6.0) and greatly visible (6.0–12.0). The shift of colour in MW and PL treated chillies was in the “slightly noticeable to noticeable range” or close to it. The untreated and steam treated chillies fall into the categories of “well-visible” and “greatly visible” respectively at the end of the storage. The total colour change (CoC) > 12 can be considered as completely unacceptable.34,35 In a similar study, there was a total colour change of 0.19 and 0.03 in untreated and steam treated red chillies stored at 4 °C, while there was a total colour change of 1.05 and 0.84 in untreated and steam treated red chillies stored at 20 °C after six months.10 On a similar note, the total colour change of 50.1 was observed in garlic after microwave treatment at 800 W.64 PL treatment of 2208 J cm−2 dehydrated onion shreds with a water activity of 0.6 resulted in total colour changes of 5.8, 7.2 and 8.6 on the 30th, 27th, and 19th days of storage at 25 °C, 30 °C, and 37 °C.29 The colour of treated red chillies might be lost due to the conversion of carotenoids to their cis form, apocarotenoids, ionone, epoxides, carbonyl compounds, alcohols, etc.23 The decline in the L* value over the storage period led to an increase in ΔE* for the steam-treated samples. The colour change of red chillies may arise from various factors, such as the reduction of AA and TCC, the breakdown of phenolics, antioxidants, and the partial reactivation (9–10%) of the PPO and POD enzymes.67 The high levels of bioactives retained in red chilies treated with PL suggest that the reduced browning in these samples is due to the preservation of these compounds. However, the permeability of water vapour and oxygen through polypropylene (PP) packaging contributes to losses in carotenoid content, along with degradation of natural antioxidants such as phenolics, antioxidants, and ascorbic acid, leading to more significant colour loss.34 Most PL studies do not extensively address the shelf-life impacts on the colour of red chillies. For optimal retention of phenolics, antioxidants, and colour in red chilies, microwave MW and PL treatments are promising approaches.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 or higher was considered unsafe for consumption, as it indicated significant microbial contamination and potential spoilage.62 In untreated red chillies, the shelf life is increased to 35 days and 101 days in 0.60 and 0.35 aw, respectively. Steam treated, microwave treated, and pulsed light treated red chillies preserved chillies for 22 days and 59 days; 173 days and 210 days; and 210 days and 210 days, at 0.60 and 0.35 aw, respectively, under ambient storage. Under refrigerated storage, the shelf life increased to 67 days and 66 days; 210 days and 210 days in 0.60 and 0.35 aw red chillies after steam treatment, microwave treatment, and pulsed light treatment, respectively (Tables 3 and 4). There is very limited literature on the estimation of the shelf life of red chillies. The shelf life of fresh cut red bell peppers increased to 7 days after PL treatment at 16 J cm−2, where AM and YM counts exceeded the limit.10 Although the chillies were microbiologically safe, their sensory appeal could pose a challenge. The sample's storage limit was defined by a minimum total colour change score of >12.35 However, the colour of red chillies did not exceed the limit under all treatment conditions during storage (Table 4). As a result, the physical appearance and microbial safety of the red chillies were in alignment.
log10 CFU g−1 or higher was considered unsafe for consumption, as it indicated significant microbial contamination and potential spoilage.62 In untreated red chillies, the shelf life is increased to 35 days and 101 days in 0.60 and 0.35 aw, respectively. Steam treated, microwave treated, and pulsed light treated red chillies preserved chillies for 22 days and 59 days; 173 days and 210 days; and 210 days and 210 days, at 0.60 and 0.35 aw, respectively, under ambient storage. Under refrigerated storage, the shelf life increased to 67 days and 66 days; 210 days and 210 days in 0.60 and 0.35 aw red chillies after steam treatment, microwave treatment, and pulsed light treatment, respectively (Tables 3 and 4). There is very limited literature on the estimation of the shelf life of red chillies. The shelf life of fresh cut red bell peppers increased to 7 days after PL treatment at 16 J cm−2, where AM and YM counts exceeded the limit.10 Although the chillies were microbiologically safe, their sensory appeal could pose a challenge. The sample's storage limit was defined by a minimum total colour change score of >12.35 However, the colour of red chillies did not exceed the limit under all treatment conditions during storage (Table 4). As a result, the physical appearance and microbial safety of the red chillies were in alignment.
The two principal components (PC1 and PC2) accounted for more than 80% and 85% of the total variability in the dataset at 28 °C and 4 °C, respectively. At 28 °C, PC1 explained 65.3% of the variability, while PC2 accounted for 15.0%. In contrast, at 4 °C, PC1 explained 70.3% of the variability, and PC2 explained 14.2%.
From the loading plot at 28 °C (Fig. 5), it is evident that CAP, AOX, and TCC aligned positively with both PC1 and PC2, while TPC, TFC, and AA aligned positively with PC1 but negatively with PC2. In contrast, CoC aligned negatively with both PC1 and PC2. This indicates that CAP, AOX, and TCC exhibit behaviour opposite to other bioactives. During storage at 4 °C (Fig. 6), TPC, AOX, and CAP aligned positively with both PC1 and PC2, whereas TFC, TCC, and AA aligned positively with PC1 but negatively with PC2. CoC again aligned negatively with both PC1 and PC2.
The correlation values of these quality attributes are presented in Table 6. Phenolic content, flavonoid content, and antioxidant capacity showed strong correlations of over 90% and 95% at 28 °C and 4 °C, respectively, indicating a slower rate of degradation during storage. Additionally, AA and TCC were positively correlated, with correlation values of 81% and 89% at 28 °C and 4 °C, respectively. These compounds are heat, light, and oxygen-sensitive, resulting in a faster rate of degradation compared to other bioactives during storage. The negative correlation between total colour change (CoC) and other bioactive compounds suggests that the extent of colour change is dependent on the degradation of phenolics, flavonoids, antioxidants, ascorbic acid, capsaicinoids, and carotenoids. These findings align with previous studies on pulsed light-treated pomegranate juice, as reported by Pravallika, Shaik, and Chakraborty.37
| Storage temperature | Bioactive compounds | Correlation coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC | TFC | AOX | AA | CAP | TCC | CoC | ||
| a TPC: total phenolic content; TFC: total flavonoid content; AOX: antioxidant capacity; AA: ascorbic acid; CAP: capsaicinoids; TCC: total carotenoid content; CoC: total colour change. | ||||||||
| 28 °C | TPC | 1.00 | ||||||
| TFC | 0.98 | 1.00 | ||||||
| AOX | 0.90 | 0.89 | 1.00 | |||||
| AA | 0.60 | 0.67 | 0.51 | 1.00 | ||||
| CAP | −0.02 | −0.03 | 0.19 | −0.04 | 1.00 | |||
| TCC | 0.79 | 0.85 | 0.75 | 0.81 | 0.07 | 1.00 | ||
| CoC | −0.64 | −0.67 | −0.41 | −0.48 | −0.11 | −0.59 | 1.00 | |
| 4 °C | TPC | 1.00 | ||||||
| TFC | 0.95 | 1.00 | ||||||
| AOX | 0.95 | 0.92 | 1.00 | |||||
| AA | 0.72 | 0.74 | 0.67 | 1.00 | ||||
| CAP | 0.41 | 0.35 | 0.47 | 0.06 | 1.00 | |||
| TCC | 0.88 | 0.86 | 0.85 | 0.89 | 0.31 | 1.00 | ||
| CoC | −0.54 | −0.57 | −0.38 | −0.61 | −0.39 | −0.56 | 1.00 | |
4 Conclusions
The shelf life of 0.35 aw red chillies treated with steam (120 °C/300 s), microwaves (540 kJ), and pulsed light (2.59 J cm−2) was 66 days, 210 days, and 210 days at 4 °C. Steam treated red chillies with 0.60 aw had a shelf life of 22 days at 28 °C with inferior bioactive compounds and huge colour loss, limiting its appeal and consumer acceptance. Weight loss was not significant (p > 0.05) in any of the samples during storage. MW treated red chillies preserved 83.8% phenolics, 94.00% flavonoids, 97.0% antioxidants, 80.1% ascorbic acid, 88.4% capsaicinoids, and 90.2% carotenoids in 0.60 aw red chillies at 4 °C. While PL preserved 85.7%, 83.2%, 97.7%, 93.3%, 91.0%, and 83.4% of phenolics, flavonoids, antioxidants, ascorbic acid, capsaicinoids, and carotenoids, respectively. The rate of bioactive degradation followed a first-order kinetic model (adj R2 > 0.9). MW and PL treated chillies are 3.1 to 9.5 times better preserved than steam-treated chillies. The shelf life of untreated, ST treated, MW treated and PL treated 0.60 and 0.35 aw red chillies at 4 °C was 70 and 126 days, 67 and 66 days, 210 and 210 days, and 210 and 210 days, respectively. Microwave and pulsed light treatments exhibited superior efficacy in preserving bioactive compounds and extending the shelf life of red chillies compared to steam treatment, particularly at lower water activity (0.35 aw). This highlights the importance of selecting appropriate post-drying treatments to ensure both microbial safety and bioactive retention. The substantial retention of phenolics, flavonoids, antioxidants, and carotenoids in MW and PL-treated samples underscores their potential in maintaining both nutritional and sensory attributes during storage. An optimized strategy involves drying to 0.35 aw, applying microwaves or pulsed light for microbial and enzymatic inactivation, and storing under refrigeration with high-barrier packaging to ensure long-term stability and quality. The degradation kinetics of bioactive compounds followed a first-order model (adj R2 > 0.9), emphasizing the need for further optimization to enhance long-term stability. Future research should explore the scalability of these methods, validate their efficacy in commercial settings, and include comprehensive sensory evaluations to assess consumer acceptance and industrial feasibility.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Kosana Pravallika: methodology, formal analysis, investigation, validation, resources, and writing – original draft. Snehasis Chakraborty: conceptualization, visualization, supervision, and writing – reviewing and editing.Conflicts of interest
The authors have declared no conflicts of interest for this article.Acknowledgements
Kosana Pravallika would like to thank the All India Council for Technical Education (AICTE) for providing the fellowship through the ADF scheme 2021.References
- K. Chitravathi, O. P. Chauhan and P. S. Raju, Shelf life extension of green chillies (Capsicum annuum L.) using shellac-based surface coating in combination with modified atmosphere packaging, J. Food Sci. Technol., 2016, 53, 3320–3328, DOI:10.1007/s11483-016-9490-x.
- K. Jalgaonkar, M. K. Mahawar, S. Girijal and G. Hp, Post-harvest profile, processing and value addition of dried red chillies (Capsicum annuum L.), J. Food Sci. Technol., 2024, 61(2), 201–219, DOI:10.1007/s11483-024-08597-w.
- Grand View Research, Seasoning & Spices Market Size, Share & Growth Report, 2030, https://www.grandviewresearch.com Search PubMed.
- Spice Board of India, Trade Information and Statistics, Review of Export Performance of Spices during 2023-24, https://www.indianspices.com/sites/default/files/majoritemwiseexport2024web.pdf Search PubMed.
- K. Chitravathi, O. P. Chauhan and P. S. Raju, Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.), Food Packag. Shelf Life, 2015, 4, 1–9, DOI:10.1016/j.fpsl.2015.06.003.
- C. W. Rico, J. J. Ahn, H. K. Kim, M. Furuta and J. H. Kwon, The comparative effect of steaming and irradiation on the physicochemical and microbiological properties of dried red pepper (Capsicum annuum L.), Food Chem., 2010, 119(3), 1012–1016, DOI:10.1016/j.foodchem.2009.07.073.
- V. O. Edusei, J. Ofosu-Anim, P. N. T. Johnson and E. W. Cornelius, Extending postharvest life of green chilli pepper fruits with modified atmosphere packaging, Ghana J. Horticult., 2012, 10, 131–140 Search PubMed.
- K. Chitravathi, O. P. Chauhan and J. Kizhakkedath, Shelf life extension of green chillies (Capsicum annuum L.) using passive modified atmosphere packaging and gamma irradiation, J. Food Process. Preserv., 2020, 44(8), e14622, DOI:10.1111/jfpp.14622.
- S. Singh, S. N. S. Chaurasia, I. Prasad, P. Khemariya and T. Alam, Nutritional quality and shelf life extension of capsicum (Capsicum annuum) in expanded polyethylene biopolymer, Asian J. Dairy Food Res., 2020, 39(1), 40–48, DOI:10.18805/AJDFR-2907.
- K. Rybak, A. Wiktor, K. Pobiega, D. Witrowa-Rajchert and M. Nowacka, Impact of pulsed light treatment on the quality properties and microbiological aspects of red bell pepper fresh-cuts, LWT--Food Sci. Technol., 2021, 149, 111906, DOI:10.1016/j.lwt.2021.111906.
- X. Nian, J. Wang, M. Wang, Y. Wang, S. Liu and Y. Cao, Influence of ultrasonic pretreatment on the quality attributes and pectin structure of chili peppers (Capsicum spp.), Ultrason. Sonochem., 2024, 110, 107041, DOI:10.1016/j.ultsonch.2024.107041.
- S. Rajendran, G. A. Annor, M. Kumar and S. Ganapathy, Effect of Radio Frequency Low Pressure Cold Plasma on Total Phenol, Antioxidant Activity and Colour Degradation Kinetics of Red Chilli Pepper Powder, J. Agric. Eng., 2021, 58(3), 241–249, DOI:10.5958/0974-2626.2021.00056.1.
- M. Hernández-Carrión, I. Hernando and A. Quiles, High hydrostatic pressure treatment as an alternative to pasteurization to maintain bioactive compound content and texture in red sweet pepper, Innovative Food Sci. Emerging Technol., 2014, 26, 76–85, DOI:10.1016/j.ifset.2014.04.002.
- N. Balakrishnan, S. M. Yusop, I. A. Rahman, E. Dauqan and A. Abdullah, Efficacy of gamma irradiation in improving the microbial and physical quality properties of dried chillies (Capsicum annuum L.): a review, Foods, 2021, 11(1), 91, DOI:10.3390/foods11010091.
- H. Molnár, I. Bata-Vidács, E. Baka, Z. Cserhalmi, S. Ferenczi, R. Tömösközi-Farkas, N. Adányi and A. Székács, The effect of different decontamination methods on the microbial load, bioactive components, aroma and colour of spice paprika, Food Control, 2018, 83, 131–140, DOI:10.1016/j.foodcont.2017.08.018.
- Z. Zhou, S. Zuber, M. Campagnoli, T. Putallaz, F. Devlieghere and M. Uyttendaele, Effect of mild steaming treatment on the inactivation of Salmonella, Listeria monocytogenes, Escherichia coli O157:H7 and their surrogates on black peppercorns, Food Control, 2019, 106, 106726, DOI:10.1016/j.foodcont.2019.106726.
- M. M. Kamal, M. R. Ali, M. M. Rahman, M. R. I. Shishir, S. Yasmin and M. S. H. Sarker, Effects of processing techniques on drying characteristics, physicochemical properties and functional compounds of green and red chilli (Capsicum annuum L.) powder, J. Food Sci. Technol., 2019, 56, 3185–3194, DOI:10.1007/s11483-019-01430-6.
- M. Hamed, D. Kalita, M. E. Bartolo and S. S. Jayanty, Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: comparative study of the effect of ripening stage and cooking methods, Antioxidants, 2019, 8(9), 364, DOI:10.3390/antiox8090364.
- L. Eliasson, S. Isaksson, M. Lövenklev and L. Ahrné, A comparative study of infrared and microwave heating for microbial decontamination of paprika powder, Front. Microbiol., 2015, 6, 1071, DOI:10.3389/fmicb.2015.01071.
- T. Pradechboon, R. Ramaraj, N. Dussadee and S. Chindaraksa, Advances application of a newly developed microwave rotary dryer for drying agricultural products of red chili pepper, Biomass Convers. Biorefin., 2024, 14(8), 9187–9196, DOI:10.1007/s13399-024-03018-9.
- A. Krzykowski, S. Rudy, R. Polak, B. Biernacka, A. Krajewska, E. Janiszewska-Turak, I. Kowalska, J. Żuchowski, B. Skalski and D. Dziki, Drying of Red Chili Pepper (Capsicum annuum L.): Process Kinetics, Colour Changes, Carotenoid Content and Phenolic Profile, Molecules, 2024, 29(21), 5164, DOI:10.3390/molecules29215164.
- K. Pravallika and S. Chakraborty, Effect of pulsed light technology on microbial quality, enzyme activity and physicochemical attributes of green chilies (Capsicum annuum var. longum) at different levels of water activity, J. Food Meas. Charact., 2024, 18, 5982–5999, DOI:10.1007/s11483-024-07663-7.
- K. Pravallika and S. Chakraborty, Effect of pulsed light on enzyme inactivation, colour, firmness, surface morphology, and bioactive compounds in fresh and dried whole red chilies (Capsicum annuum var. longum), Food Bioprod. Process., 2024, 148, 377–391, DOI:10.1016/j.fbp.2023.12.005.
- K. Pravallika and S. Chakraborty, Pulsed Light Decontamination of Red Chilies (Capsicum annuum var. longum), J. Food Saf., 2024, 44(5), e13168, DOI:10.1111/jfs.13168.
- H. W. Woldemariam, H. Harmeling, S. A. Emire, P. G. Teshome, S. Toepfl and K. Aganovic, Pulsed light treatment reduces microorganisms and mycotoxins naturally present in red pepper (Capsicum annuum L.) powder, J. Food Process Eng., 2022, 45(2), e13948, DOI:10.1111/jfpe.13948.
- N. Kalathil, N. Thirunavookarasu, K. Lakshmipathy, D. V. Chidanand, M. Radhakrishnan and N. Baskaran, Application of light-based, non-thermal techniques to determine physico-chemical characteristics, pungency and aflatoxin levels of dried red chilli pods (Capsicum annuum), J. Agric. Food Res., 2023, 13, 100648 Search PubMed.
- P. Pashkovskiy, N. Sleptsov, M. Vereschagin, V. Kreslavski, N. Rudometova, P. Sorokoumov and V. Kuznetsov, Post-harvest red- and far-red-light irradiation and low temperature induce the accumulation of carotenoids, capsaicinoids, and ascorbic acid in Capsicum annuum L. green pepper fruit, Foods, 2023, 12(8), 1715 CrossRef PubMed.
- S. Y. Lee, H. H. Park and S. C. Min, Pulsed light plasma treatment for the inactivation of Aspergillus flavus spores, Bacillus pumilus spores, and Escherichia coli O157:H7 in red pepper flakes, Food Control, 2020, 118, 107401, DOI:10.1016/j.foodcont.2020.107401.
- R. Saengrayap, A. Tansakul and G. S. Mittal, Effect of far-infrared radiation-assisted microwave-vacuum drying on drying characteristics and quality of red chilli, J. Food Sci. Technol., 2015, 52, 2610–2621 CrossRef PubMed.
- S. U. Handayani, I. Mujiarto, A. P. Siswanto, D. Ariwibowo, I. S. Atmanto and M. Mustikaningrum, Drying kinetics of chilli under sun and microwave drying, Mater. Today: Proc., 2022, 63, S153–S158 Search PubMed.
- A. E. Alamu, B. I. O. Ade-Omowaye, B. A. Akinwande, O. E. Dudu and F. O. Obori, Pigments and colour characteristics: influence of drying methods on Nigerian pepper (Capsicum frutescence), J. Agric. Food Res., 2023, 14, 100760, DOI:10.1016/j.jafr.2023.100760.
- R. Dhar and S. Chakraborty, Bael (Aegle marmelos) beverage pasteurization by non-isothermal heating with continuous microwave to achieve microbial safety, Innovative Food Sci. Emerging Technol., 2024, 97, 103801, DOI:10.1016/j.ifset.2024.103801.
- R. Dhar and S. Chakraborty, Enhancing shelf-life of bael beverage through various pasteurization techniques: a comparative study at 25 °C and 4 °C with thermal, pulsed light, and microwave treatments, J. Food Process Eng., 2024, 47(11), e14776, DOI:10.1111/jfpe.14776.
- S. Savitha, S. Chakraborty and B. N. Thorat, Changes in quality attributes of pulsed light treated dehydrated onion shreds during storage, J. Agric. Food Res., 2023, 13, 100650, DOI:10.1016/j.jafr.2023.100650.
- Z. Cserhalmi, A. Sass-Kiss, M. Tóth-Markus and N. Lechner, Study of pulsed electric field treated citrus juices, Innovative Food Sci. Emerging Technol., 2006, 7(1–2), 49–54, DOI:10.1016/j.ifset.2005.11.002.
- S. Monisha, A. Niveditha, D. Pavithra, D. Chidanand, C. Sunil and A. Rawson, Effect of microwave treatment on colour of turmeric (Curcuma longa L.), Int. J. Sci. Environ. Technol., 2016, 5, 2062–2070 Search PubMed.
- K. Pravallika, L. Shaik and S. Chakraborty, Changes in the quality attributes of pulsed light and thermally pasteurized pomegranate (Punica granatum) juice stored at refrigerated condition (4 °C), J. Food Meas. Charact., 2023, 17(6), 6620–6638, DOI:10.1007/s11483-023-09756-9.
- A. Dhawan and S. Chakraborty, Pulsed light treatment of whole white button mushroom (Agaricus bisporus): kinetics and mechanism of microbial inactivation and storage study, J. Food Sci., 2024, 89(9), 5319–5334, DOI:10.1111/1750-3841.17750.
- S. M. Castro, J. A. Saraiva, J. A. Lopes-da-Silva, I. Delgadillo, A. Van Loey, C. Smout and M. Hendrickx, Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.), Food Chem., 2008, 107(4), 1436–1449, DOI:10.1016/j.foodchem.2007.09.015.
- L. Z. Deng, X. H. Yang, A. S. Mujumdar, J. H. Zhao, D. Wang, Q. Zhang, J. Wang, Z. J. Gao and H. W. Xiao, Red pepper (Capsicum annuum L.) drying: effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure, Drying Technol., 2018, 36(8), 893–907, DOI:10.1080/07373937.2018.1444076.
- N. Shaheen, S. Imam, S. Abidi, R. A. Sultan, I. Azhar and Z. A. Mahmood, Comparative pharmacognostic evaluation and standardization of Capsicum annuum L. (red chili), Int. J. Pharm. Sci. Res., 2018, 9(7), 2807–2817 CAS.
- K. Chitravathi, O. P. Chauhan, P. S. Raju and N. Madhukar, Efficacy of aqueous ozone and chlorine in combination with passive modified atmosphere packaging on the postharvest shelf-life extension of green chillies (Capsicum annuum L.), Food Bioprocess Technol., 2015, 8, 1386–1392, DOI:10.1007/s11947-015-1626-7.
- S. H. Kim, S. H. Park and D. H. Kang, Inactivation of Bacillus cereus endospores on black pepper by pulsed superheated steam system, Food Res. Int., 2023, 167, 112649, DOI:10.1016/j.foodres.2023.112649.
- H. W. Park, J. Xu, V. M. Balasubramaniam and A. B. Snyder, The effect of water activity and temperature on the inactivation of Enterococcus faecium in peanut butter during superheated steam sanitation treatment, Food Control, 2021, 125, 107942, DOI:10.1016/j.foodcont.2021.107942.
- G. C. Jeevitha, H. B. Sowbhagya and H. U. Hebbar, Application of microwaves for microbial load reduction in black pepper (Piper nigrum L.), J. Sci. Food Agric., 2016, 96(12), 4243–4249, DOI:10.1002/jsfa.7756.
- M. T. Kubo, E. S. Siguemoto, E. S. Funcia, P. E. Augusto, S. Curet, L. Boillereaux, S. K. Sastry and J. A. Gut, Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: a critical review, Curr. Opin. Food Sci., 2020, 35, 36–48, DOI:10.1016/j.cofs.2020.02.004.
- I. Aguiló-Aguayo, F. Charles, C. M. Renard, D. Page and F. Carlin, Pulsed light effects on surface decontamination, physical qualities and nutritional composition of tomato fruit, Postharvest Biol. Technol., 2013, 86, 29–36, DOI:10.1016/j.postharvbio.2013.06.011.
- U. Schweiggert, A. Schieber and R. Carle, Inactivation of peroxidase, polyphenoloxidase, and lipoxygenase in paprika and chili powder after immediate thermal treatment of the plant material, Innovative Food Sci. Emerging Technol., 2005, 6(4), 403–411, DOI:10.1016/j.ifset.2005.06.001.
- A. Kaiser, M. Brinkmann, R. Carle and D. R. Kammerer, Influence of thermal treatment on color, enzyme activities, and antioxidant capacity of innovative pastelike parsley products, J. Agric. Food Chem., 2012, 60(12), 3291–3301, DOI:10.1021/jf3001183.
- J. Wang, X. M. Fang, A. S. Mujumdar, J. Y. Qian, Q. Zhang, X. H. Yang, Y. H. Liu, Z. J. Gao and H. W. Xiao, Effect of high-humidity hot air impingement blanching (HHAIB) on drying and quality of red pepper (Capsicum annuum L.), Food Chem., 2017, 220, 145–152, DOI:10.1016/j.foodchem.2016.09.101.
- J. H. Kang, S. H. Roh and S. C. Min, Inactivation of potato polyphenol oxidase using microwave cold plasma treatment, J. Food Sci., 2019, 84(5), 1122–1128, DOI:10.1111/1750-3841.14532.
- J. A. Pellicer and V. M. Gómez-López, Pulsed light inactivation of horseradish peroxidase and associated structural changes, Food Chem., 2017, 237, 632–637, DOI:10.1016/j.foodchem.2017.05.139.
- R. Zhang, G. Chen, B. Yang, Y. Wu, M. Du and J. Kan, Insights into the stability of carotenoids and capsaicinoids in water-based or oil-based chili systems at different processing treatments, Food Chem., 2021, 342, 128308, DOI:10.1016/j.foodchem.2020.128308.
- J. A. Pellicer, P. Navarro and V. M. Gómez-López, Pulsed light inactivation of mushroom polyphenol oxidase: a fluorometric and spectrophotometric study, Food Bioprocess Technol., 2018, 11, 603–609, DOI:10.1007/s11947-018-2167-1.
- H. N. Kessy, Z. Hu, L. Zhao and M. Zhou, Effect of steam blanching and drying on phenolic compounds of litchi pericarp, Molecules, 2016, 21(6), 729 CrossRef PubMed.
- I. G. Hwang, Y. J. Shin, S. Lee, J. Lee and S. M. Yoo, Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.), Prev. Nutr. Food Sci., 2012, 17(4), 286, DOI:10.3746/pnf.2012.17.4.286.
- S. Damodaran, K. L. Parkin and O. R. Fennema, Fennema's Food Chemistry, CRC Press, 2007 Search PubMed.
- A. B. Hassan, S. M. Ahmed, K. A. Sir Elkhatim, T. S. Abdelhalim, S. O. Fawale, O. Q. Adiamo and I. A. Mohamed Ahmed, Effect of gamma irradiation and microwave heating treatments on microbial load and antioxidant potentials in cinnamon, fennel and hot pepper, J. Food Meas. Charact., 2019, 13, 1130–1138, DOI:10.1007/s11483-019-09691-0.
- G. Pataro, M. Sinik, M. M. Capitoli, G. Donsì and G. Ferrari, The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage, Innovative Food Sci. Emerging Technol., 2015, 30, 103–111, DOI:10.1016/j.ifset.2015.03.003.
- M. V. Agüero, R. J. Jagus, O. Martín-Belloso and R. Soliva-Fortuny, Surface decontamination of spinach by intense pulsed light treatments: impact on quality attributes, Postharvest Biol. Technol., 2016, 121, 118–125, DOI:10.1016/j.postharvbio.2016.05.004.
- A. J. Dittrich, M. Ludewig, S. Rodewald, P. G. Braun and C. Wiacek, Pulsed-light treatment of dried parsley: reduction of artificially inoculated Salmonella and impact in given quality parameters, J. Food Prot., 2021, 84(8), 1421–1432, DOI:10.4315/JFP-21-046.
- S. S. Alam, S. Akther, M. R. Islam, M. R. Islam and M. Akhtaruzzaman, Effects of ultrasound, microwave, and their combined treatments on the shelf life and quality characteristics of fresh litchi juice, Future Foods, 2023, 8, 100254, DOI:10.1016/j.fufo.2023.100254.
- A. Anjaneyulu and A. B. Sharangi, Effect of different packaging materials on the shelf life of dried red pepper (Capsicum annuum. L.) stored in ambient conditions, J. Food Sci. Technol., 2022, 18(1), 1536, DOI:10.22271/09746315.2022.v18.i1.1536.
- S. Misra, K. Rayaguru, S. K. Dash, S. Mohanty and C. Panigrahi, Efficacy of microwave irradiation in enhancing the shelf life of groundnut (Arachis hypogaea L.), J. Stored Prod. Res., 2022, 97, 101957, DOI:10.1016/j.jspr.2022.101957.
- A. A. Kader, Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, 2002 Search PubMed.
- Y. Huang, J. Sheng, F. Yang and Q. Hu, Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea, J. Food Eng., 2007, 78(2), 687–692, DOI:10.1016/j.jfoodeng.2005.11.029.
- M. Benlloch-Tinoco, M. Igual, D. Rodrigo and N. Martínez-Navarrete, Superiority of microwaves over conventional heating to preserve shelf-life and quality of kiwifruit puree, Food Control, 2015, 50, 620–629, DOI:10.1016/j.foodcont.2014.08.010.
- I. R. Kubra and L. J. Rao, Effect of microwave drying on the phytochemical composition of volatiles of ginger, Int. J. Food Sci. Technol., 2012, 47(1), 53–60, DOI:10.1111/j.1365-2621.2011.02850.x.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00380b |
| This journal is © The Royal Society of Chemistry 2025 |