Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Digestibility and enteric release achieved with microencapsulates made from emulsion-templated plant proteins†
Luke Wayne
Browning
 *a,
Huafu
Wang
a,
James Ward
Taylor
*a,
Huafu
Wang
a,
James Ward
Taylor
 a,
Pete
Wilde
b,
Marc
Rodriguez-Garcia
a,
Lynette Anne Makins
Holland
a and
Tuomas P. J.
Knowles
a,
Pete
Wilde
b,
Marc
Rodriguez-Garcia
a,
Lynette Anne Makins
Holland
a and
Tuomas P. J.
Knowles
 ac
ac
aXampla Ltd, Bioinnovation Centre, 25 Cambridge Science Park Road, Cambridge, CB4 0FW, UK
bQuadram Institute Bioscience, Rosalind Franklin Road, Norwich Research Park, Norwich, NR4 7UQ, UK
cYusuf Hamied Department of Chemistry, Lensfield Road, Cambridge CB2 1EW, UK
First published on 26th February 2025
Abstract
Microencapsulation of functional ingredients in food and drinks can improve their stability through manufacture, shelf-life, and digestion. A key challenge is to discover materials and approaches that allow cargo to be protected under gastric digestion conditions, yet provide subsequently effective release in the intestine where many actives are most effectively absorbed. Here, we address this challenge by developing a robust plant protein microcapsule with ability to retain oil-based cargo during simulated digestive conditions. To generate the capsule, pea protein isolate was exposed to aqueous organic acid and high shear to form a stable colloidal dispersion. The aqueous dispersion was subsequently emulsified with a test cargo (vitamin D2) dissolved in a solid lipid phase and spray dried to produce microcapsules with a D50 of 19 μm. This process yielded microcapsules with smooth, continuous surfaces and effective internal encapsulation. The stability of microcapsules and release of vitamin D2 cargo was characterised by a static in vitro digestion model following the INFOGEST protocol. The results show that the processing conditions of the pea protein did not negatively impact digestibility. Crucially, our results further show that microcapsules are resilient to gastric conditions but highly susceptible to intestinal conditions, supporting an enteric release profile for vitamin D2 cargo. This study provides a model for encapsulation of oil-soluble cargoes and inspires the development of other encapsulates that would benefit from protective and controlled release mechanisms in food and beverage matrices.
Sustainability spotlightSingle-use plastics are a major problem in the global plastic pollution crisis. Their poor biodegradation and difficult recyclability increases the presence of microplastics in natural environments, which harms biota. Currently, many encapsulation techniques rely on use of synthetic polymers to form protective shells around active ingredients, which contributes to the formation of microplastics. We have developed encapsulates made with natural plant polymers, which are biodegradable, renewable and edible. Our work aligns with UN SDGs on multiple fronts: our encapsulation technology can be used to fortify food & beverages with nutrients that support good health and well-being; and replacing plastic with natural, biodegradable polymers supports life on land and below water. |
1. Introduction
Microencapsulation involves embedding active ingredients in a microscale matrix, offering physical protection from external conditions and providing a means to release cargo at an advantageous time. Often, active ingredients are highly sensitive to environmental challenges, so encapsulation provides crucial improvements in shelf life in product formulations and prevents premature activation. Common environmental challenges encountered during product manufacture, shelf life and consumer storage include light, acidic or alkaline pH, temperature, ionic strength, humidity and mechanical disruption. All of these factors may cause degradation of active ingredients and leakage from within capsule cores. A further important challenge with active components is their controlled delivery to the optimum site within the human body for absorption. Commonly for oral administration routes, this implies delivery to the intestine where many small molecules, such as vitamins, can be absorbed effectively. To reach the intestine, capsules have to survive the highly degradation-promoting conditions in oral cavity and the stomach, yet be able to release their cargo effectively once they reach the intestine.Currently, many encapsulation techniques rely on use of synthetic polymers to form protective shells around active ingredients.1 However, synthetic encapsulates may not be suitable for pharmaceutical or food applications and lead to formation of microplastics, which are harmful to biota.2–4 Encapsulates made with natural polymers, such as proteins and polysaccharides from animal or plant sources, are advantageous because they are biodegradable, renewable and edible. There is growing interest in encapsulation of various actives to enhance nutritional or sensory value of food and beverage products. In light of global deficiencies of critical nutrients, such as vitamin D, fortification of ‘convenience’ food and beverages with nutrient encapsulates has become an attractive prospect.5,6
Proteins and polysaccharides already have diverse functionalities in food and beverage applications but development of discrete particles with mechanical and chemical robustness is challenging. The solubility and colloidal stability of microcapsules in solution is determined by formulation and processing conditions. The most common techniques for production of microcapsules from natural polymers are complex coacervation and spray drying, each with respective advantages and disadvantages. Complex coacervates can produce larger particles in aqueous systems with potentially high active loading but particles are often mechanically weak; on the other hand spray dried particles tend to be smaller with lower active loading but their production is simple, highly scalable and they can be more mechanically robust depending on polymer response to dehydration and processing parameters, such as concentration, temperature, pH and combinations with adjuncts.7,8 Strengthening of microcapsules by covalent (and non-covalent) crosslinking can be achieved but extensive chemical modification may drift too far from consumer expectations of ‘natural’ foods.
The film forming and emulsification properties of proteins and polysaccharides have been demonstrated for production of various oil-based encapsulates by spray drying. The benefits of minimising odour and oxidation of high-value polyunsaturated fatty acids (PUFAs) and protection of other functional oils has been well-studied.8,9 However, presence of polysaccharides can increase solubility of microcapsules, leading to fast dissolution in aqueous systems and so inadequate protection of actives over useful timeframes.10 Therefore, the qualities of current encapsulates may not alleviate existing wasteful practices in industry, such as ingredient overages and increased cost.11,12
We have developed a novel pea protein microcapsule system13 and for this study have focussed on elucidating the relationship between plant processing and digestibility, and characterised the controlled release of the active throughout simulated digestive conditions (Fig. 1). Samples under digestibility analysis followed different stages of processing the microcapsules and include the raw starting material pea protein isolate (PPI), processed acid-treated pea protein dispersion (ATD) and spray dried encapsulates (microcapsule) containing vitamin D2 as a test cargo.
 | ||
| Fig. 1 Outline of work in this study. We are investigating the digestibility of PPI as a raw material, as a processed dispersion, and as the final spray dried vitamin D2 encapsulate. | ||
Our work addresses the need for robust microcapsules made from natural, biodegradable materials with several useful properties, such as: encapsulation of oil-soluble cargo; microscopic sizing; dispersibility in aqueous systems; resilience to gastric digestion and enteric-release profiles.
2. Materials and methods
2.1 Material suppliers
Acetic acid (glacial 99.9%), methanol (analytical grade) and ethanol (analytical grade) were purchased from Fisher Scientific, UK. Vitamin D2 oil (1 MIU in sunflower oil) was purchased from Nutraceuticals Group, UK. Vitamin D2 powder (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 IU g−1; gum acacia based) was purchased from Prinova, UK. Vitamin D2 standard (ergocalciferol #95220) was purchased from Sigma, UK. Softisan 100 was purchased from IOI Oleochemical GmbH, Germany. PPI (ProEarth 80% P16109) was purchased from Cambridge Commodities, UK. Potassium hydroxide and sodium ascorbate were purchased from Thermo Fisher Scientific, UK. Enzymes and other digestive reagents used for in vitro digestion were purchased from Sigma-Aldrich, UK, and corresponded to the specific products recommended by the INFOGEST network.14
000 IU g−1; gum acacia based) was purchased from Prinova, UK. Vitamin D2 standard (ergocalciferol #95220) was purchased from Sigma, UK. Softisan 100 was purchased from IOI Oleochemical GmbH, Germany. PPI (ProEarth 80% P16109) was purchased from Cambridge Commodities, UK. Potassium hydroxide and sodium ascorbate were purchased from Thermo Fisher Scientific, UK. Enzymes and other digestive reagents used for in vitro digestion were purchased from Sigma-Aldrich, UK, and corresponded to the specific products recommended by the INFOGEST network.14
2.2 Lab scale production of samples
Percentage solids content of the final dispersion (abbreviated to ‘ATD’; typically 10% w/w) was determined by measuring mass remaining after drying at 120 °C for 2 h and dispersions were stored at 4 °C until used.
Microcapsules were typically dried to 8.5% w/w moisture and stored in the dark until used.
2.3 Pilot scale production of samples
Samples investigated in this study for digestibility include the raw starting material PPI, processed acid-treated pea protein dispersion (ATD) and spray dried vitamin D2 encapsulates (microcapsule) made on larger spray drying equipment. These samples were chosen because they reflect key stages of the microcapsule manufacturing process and when intermittent (dispersion) and significant (spray drying) processing is involved.Microcapsules were typically dried to 8.5% w/w moisture and stored dark until used.
2.4 Light and scanning electron microscopy (SEM)
Light microscopy images were obtained using an open Frame microscope equipped with a CellCam 200CR camera, Aura Pro phase contrast illuminator and universal plan fluorite objectives at 4×, 10× and 20×. Samples were prepared for imaging by adding microcapsules suspended in RO water to glass slides with cover slips. For SEM, samples were prepared by pipetting microcapsules suspended in RO water onto double-sided carbon adhesive and left to dry overnight. The samples were sputtered with 10 nm platinum using a Quorum Technologies Q150T ES sputter coater. SEM images were acquired using Tescan Mira3 operating at 5 kV and a 6 mm working distance.2.5 Particle size analysis
Particle size was measured with an Anton Paar laser diffraction particle size analyser PSA 1190. Measurements were carried out by diluting microcapsules in an aqueous solution with 2% w/v acetic acid. The test material was diluted to the required concentration to prevent microcapsule aggregation and to have the desired optical density (2–8% obscuration) for the measurement. The D50 quoted is for the volume distribution, as calculated via a general analysis using Mie theory within Anton Paar's Kalliope™ software (Anton Paar, Germany).2.6 Sample preparation for digestion
The solids content of all samples was equalised for a fairer comparison by in vitro digestion. Samples were baselined against ATD because the solids content of this sample could not be manipulated once made. Since the percentage solids content of ATD was measured as 10.4% w/w, all other samples were prepared as 10.4% w/w slurries using RO water (for microcapsules) or 7.2% w/w acetic acid (for PPI) as diluents. Samples were mixed to homogeneity before addition to oral digesta.2.7 In vitro digestions
Samples were digested using a static in vitro digestion model following the INFOGEST protocol14 Starting with a simulated oral phase, also known as a model “chew”, followed by simulated gastric and duodenal digestions.Briefly, each sample (5 g) was subjected to an oral phase of digestion in the presence of salivary amylase (Sigma, 75 U ml−1) for 2 min at 37 °C, pH 7.0, followed by gastric digestion in the presence of pepsin (Sigma, 2000 U ml−1) and gastric lipase (Lipolytech RGE25, 60 U ml−1) for 2 h at 37 °C, pH 3.0, and a duodenal digestion in the presence of pancreatin (Sigma, 100 U ml−1) and bile solution (Sigma, 10 mM) for 2 h at 37 °C, pH 7.0. Individual enzymes trypsin (100 U mL−1, Merck, T0303) and chymotrypsin (25 U mL−1, Merck, C4129) were used in place of pancreatin for the PPI and the ATD samples in order to understand the fate of individual proteins.
The digestion study was in two stages. Firstly, we wanted to understand the fate of individual proteins in the PPI and ATD sample to determine the effect of processing on the PPI sample, so for the PPI and ATD samples we used individual enzymes (trypsin, chymotrypsin and lipase) during the intestinal phase. For the microcapsules, the main aim was to understand the overall digestibility and vitamin D2 release in a more realistic simulation, so the pancreatin extract was used as the enzyme source during the intestinal phase for the microcapsule sample.
Aliquots (100 μL) taken at different time points (baseline, post oral digestion, after 2, 5, 30, 60 and 120 min gastric digestion, after 2, 5, 30, 60 and 120 min duodenal digestion) were stored at −20 °C for further analysis. Due to the difficulties in consistently sampling microcapsules, separate runs for each time point (3 time points in the gastric phase and 4 time points in the intestinal phase) were performed. After centrifugation (5000g for 10 min), both the supernatant and pellet were separated for further analysis. Digesta samples were centrifuged prior to protein analysis as described below.
2.8 Protein digestibility
Protein digestibility was investigated using two separate techniques. Aliquots (100 μL) of the digesta were taken and centrifuged (5000g for 10 min) to separate the insoluble material (pellet) from the soluble, bioaccessible material (supernatant) and subjected to subsequent analyses.2.9 Vitamin D2 quantification by high-performance liquid chromatography (HPLC)
Methods for the saponification of vitamin D2 prior to HPLC were based on Upreti et al.16 (2002) and international standard method EN 12821:2009.17 HPLC analysis was performed on an Agilent 1200 Infinity with a reverse phase column (Phenomenex Kinetex 5 μm EVO-C18 150 × 4.6 mm) using methanol/water (95/5, w/w) as eluent, and UV detection at 265 nm. Flow rate: 1.0 ml min−1; injection volume: 10 μl; run time: 10 min.Vitamin D2 standard (ergocalciferol, Sigma-Aldrich, #95220) was typically made into a stock solution of 2 mg g−1 in absolute ethanol. Stock solution was diluted further in 50% KOH/ethanol (40/60, w/w) for preparing a series of calibrants ranging from 20 to 0.02 μg g−1. An average response factor across the calibrants and masses of samples were used to calculate concentration of vitamin D2 in samples.
The masses of various samples (dry powder, supernatants and pellets) were recorded for mass balance and calculation of vitamin D2 concentrations.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min and supernatant containing vitamin D2 was transferred into amber HPLC vials for injection.
000g for 15 min and supernatant containing vitamin D2 was transferred into amber HPLC vials for injection.
2.10 Statistical analyses
Experimental samples were prepared in triplicate where stated and significance differences between samples means were evaluated by one-way ANOVA with significance level (p < 0.05) and pairwise comparisons of multiple means were evaluated using Tukey Honest Significant Difference (HSD) test (p < 0.05).3. Results & discussion
3.1 Comparative leakage test of microcapsules made at lab scale
Whilst the intake of vitamin D is acceptable as D2 or D3, we decided to encapsulate vitamin D2 as the test cargo for this study because it can be sourced synthetically and avoids ethical concerns of using vitamin D3, which is often sourced from animals. As an early indication on the robustness of spray dried microcapsules made at lab scale from acid and non-acid dispersions, we quantified the retention and leakage of vitamin D2 cargo after exposure of the microcapsules to boiling water and vortex mixing for 2 min. These stresses would approximate pasteurisation conditions typically encountered during food and beverage processing. Microcapsules made from acid-based pea protein dispersion (ATD) had no detectable leakage of vitamin D2. Conversely, microcapsules made from a comparative non-acid dispersion leaked 25% of vitamin D2 and were clearly not suitable for further experiments and long-term stability in food & beverage matrices (Table 1; absolute data with statistical analysis in Fig. 1, ESI†). Percentage release from microcapsules is expressed as the amount of vitamin D2 released into sample supernatants as a portion of total vitamin D2 recovered from both sample supernatants and pellets. These findings corroborate previous investigations into the stability of encapsulates and other structured materials when organic acids are included in dispersion formulations to lower pH and cause gelation of plant proteins.13,15 Since only the acid-based dispersion effectively encapsulated vitamin D2, our study focussed on this formulation for subsequent experiments.| Sample | Vitamin D2 | ||
|---|---|---|---|
| Retained (% of detected) | Released (% of detected) | Recovery (% of added) | |
| Acid-based dispersion (ATD) | 100 | ND | 88 |
| Comparative non-acid dispersion | 75 | 25 | 94 |
The average loading of active was determined by microcapsule disintegration and quantification by HPLC. Microcapsules contained 943 μg g−1 and 1363 μg g−1 vitamin D2 when made from acid-based and non-acid dispersions, respectively.
3.2 Characterisation of spray dried vitamin D2 microcapsules made at pilot scale
Emulsion from ATD spray dried at pilot scale formed discrete microscopic particles, resembling collapsed wrinkled spheres. To confirm the robustness of microcapsules spray dried at pilot scale, as was evident at lab scale, leakage of vitamin D2 was also not detected after challenging microcapsules with the boiling water test mentioned above (Table 1, ESI†). Typical microcapsule morphologies under light and SEM microscopy are shown in Fig. 2 and 3, respectively. Particles formed smooth, continuous surfaces and showed no obvious partitioning of oil-vitamin phase into larger pockets, showing good stability of oil droplets in emulsion and prevention of coalescence during spray drying. General morphologies under SEM are similar to those observed by others spray drying oil emulsions with plant or animal proteins.10,18–20 Surface depressions caused by collapse during drying particularly resembled particles made by Di Giorgio et al.,21 who encapsulated fish oil with soy protein, whose gelling and film-forming behaviour of major storage proteins are highly similar to those of pea. Although broken particles were not apparent for the specific batches of microcapsules made herein, we have seen in other batches that the particle shell forms a homogenous network of oil-vitamin phase and protein hydrogel, and the particle interior is largely hollow (Fig. 2, ESI†). Such morphologies were also distinct features of microcapsules made from a fish oil and whey protein isolate.18The average loading of active was determined by microcapsule disintegration and quantification by HPLC. Microcapsules contained 1035 μg g−1 vitamin D2. Particle size analysis by laser diffraction showed microcapsules had a D50 of 19 μm. The sizes of particles are typical of spray dried particles and are largely influenced by the spray dryer nozzle parameters and sample viscosity.
3.3 Digestive profiles of microcapsules
| Sample | PPI | ATD | Microcapsule |
|---|---|---|---|
| Baseline | 11.3 | 12.1 | 10.5 |
| End oral | 12.2 | 12.1 | 9.9 |
| End gastric | 8.3 | 8.0 | 8.9 |
| End intestinal | 2.6 | 1.2 | ND |
| Protein digestibility (%) | 79 | 90 | 100 |
The apparent digestibility of PPI in our investigation (79%) is similar to that reported by others.22,23 Nosworthy et al. reported 67–75% digestibility scores according to PDCAAS (Protein Digestibility-Corrected Amino Acid Score) and DIAAS (Digestible Indispensable Amino Acid Score) methods, where the extent of digestibility was moderately affected by pea cultivar type and processing conditions, such as cooking, baking and extrusion. These authors also showed a strong correlation between rat in vivo digestion with in vitro PDCAAS/DIAAS digestion, suggesting that in vitro models are sometimes a good proxy for in vivo analysis. Jiménez-Munoz et al.23 characterised digestion by degree of hydrolysis (DH) and reported >63% DH in INFOGEST intestinal digestates of different commercial PPI sources. Overall, the extent of digestibility in our results is similar to the measurements of others and shows that processing does not have a negative effect on digestibility. Untreated PPI, which is the starting material for all other samples herein and which is consumed regularly in diverse food and beverage matrices, is less digestible than the processed samples. Whilst we acknowledge the limitations of the INFOGEST method in representing physiological ratios of sample (food) to final digestates, the main purpose of our study was to test the protein encapsulation system as a carrier for oil-soluble bioactive molecules, using vitamin D2 as an example and to compare its relative digestibility to the pea protein starting material.
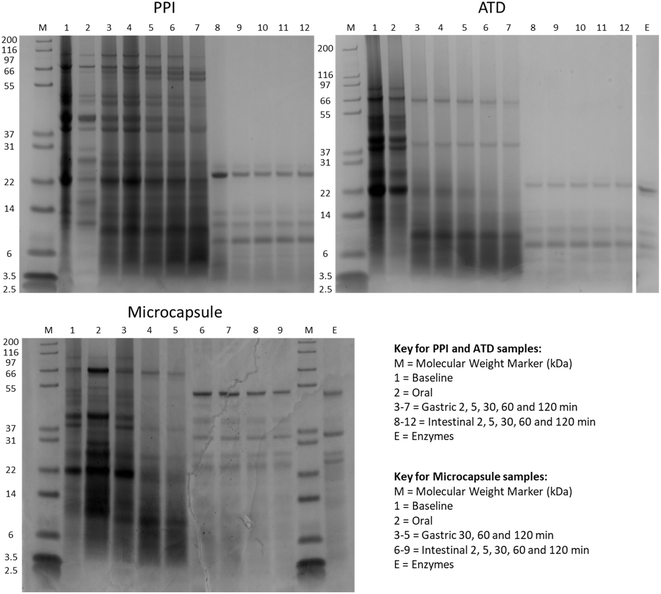
SDS-PAGE gels are shown in Fig. 4. At oral phase, some soluble proteins were released from PPI and ATD but the bands were less prominent than the baselines. Proteins released at oral phase were likely derived from loosely associated aggregates and could not be digestive products due to lack of proteolytic enzymes in oral digesta.
During gastric phase, an approximately equal quantity of proteins from PPI and ATD were liberated into soluble phase according to nitrogen measurements (∼30% loss from insoluble phase). However, SDS-PAGE shows that proteins released from PPI were more resistant to digestion than proteins released from ATD. This resistance may be due to differences in structural elements and aggregation state of proteins in PPI over proteins in the ATD, which could resist pepsin action. Also, the thermal and physical processes encountered by ATD may have resulted in some unfolding and denaturation, rendering them more susceptible to proteolysis.24,25 Proteins were also liberated from microcapsules but to a lesser extent than its respective dispersion, and were also resistant to pepsin action. During intestinal phase, all samples behaved similarly in that all remaining protein was quickly and fully or near-fully released into soluble phase and digested.
Overall, gastric phase differentiated the digestive profile of samples, suggesting the structure and degree of processing of samples influenced the amount of protein released during gastric phase but not during intestinal phase. The migration of protein bands across gels matched expected molecular weights of major storage proteins in pea, such as fragments of legumin, vicilin and convicilin.26–28
After intestinal phase, some aggregates remained for PPI and ATD samples whereas microcapsules were fully digested, in which only debris from the crude pancreatic extract could be seen (bottom right, Fig. 5). These observations agree with and are complimentary to the nitrogen measurements and SDS-PAGE gels herein.
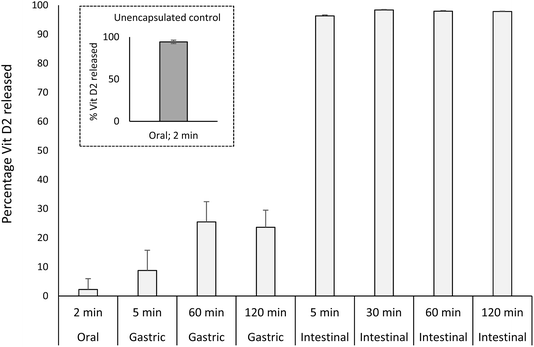 | ||
| Fig. 6 Vitamin D2 release profile of microcapsules throughout digestion. Data points are an average of three replicate samples and error around the means is represented as propagated standard error. | ||
The high resilience of microcapsules to gastric conditions is influenced by conditions around formulation and processing. In the production of microcapsules, PPI was exposed to a relatively large amount of organic acid, which caused protein gelation and necessitated a washing step to reduce acid content and a high shear step to form a homogeneous slurry. The relatively large exposure of PPI to organic acid during production may have affected protein structure and aggregation state, reducing susceptibility of proteins to pepsin hydrolysis. Their resilience to gastric digestion is also evident despite their small sizing (D50 = 19 μm), which may have influenced kinetics of digestion by effect of increased surface area. Finally, the triglyceride content of microcapsules will affect particle hydrophobicity and likely the orientation and aggregation state of proteins within and on the surface of capsules.
This work provides a novel example of a robust plant protein microcapsule produced by spray drying with ability to retain oil-based cargo during simulated gastric digestive conditions with release of cargo under intestinal conditions. Xue et al.29 assessed stability of solid lipid nanoparticles (SLNs) containing curcumin under simulated gastrointestinal conditions and found that particle sizes and polydispersity indices remained relatively stable throughout challenges in pH and ionic strength. However, gastric and intestinal conditions were studied independently and not sequentially, and leakage of curcumin was not assessed. A spray dried curcumin encapsulate made by other researchers demonstrated some protection of the active under in vitro digestive conditions.30 Their encapsulates contained additional shell materials, such as alginate and shellac, and were reported to reduce the degradation of encapsulated curcumin vs. free, unencapsulated curcumin during processing and digestion. However, the kinetics of cargo release and morphologies of particle disintegration throughout digestive stages were not reported and therefore controlled enteric release was not demonstrated.
While our work serves as a model for encapsulation of oil-soluble cargoes, it could be useful for nutrients that would benefit from a protein shielding mechanism, such as iron, whose absorption in the intestine is hindered by antinutritive factors, including common metal chelators phytate and polyphenols.31–34 Our work may also extend to encapsulation of probiotics, whose viability suffer from hostile gastric conditions and whose targeted delivery to the intestines in significant viable quantities is thought to confer their beneficial health effects.35–38
4. Conclusions
This study reports a novel example of a robust plant protein microcapsule produced by spray drying with ability to retain oil-based cargo during simulated digestive conditions with selective release in the enteric phase. The results show that the processing conditions of PPI did not negatively impact digestibility. Furthermore, the results show that microcapsules are resilient to gastric conditions but are highly susceptible to intestinal conditions, supporting an enteric-controlled release profile for vitamin D2 cargo. This study provides a model for encapsulation of oil-soluble cargoes and inspires the development of other encapsulates that would benefit from protective and controlled release mechanisms in food and beverage matrices.The scalability of our approach to microencapsulation is supported by industrial spray drying equipment – the pilot-scale spray dryer used herein was designed to be directly scalable to a full-scale commercial dryer. In future work, it will be important to assess microcapsule stabilities and retention of cargoes in various food and beverage matrices before or concurrent with simulated digestion conditions, and in quantities that are closer to physiologically relevant portions of food. We plan to investigate the effect of microencapsulation on reducing exposure of sensitive nutrients to UV light, such as vitamin D2/D3, or in reducing oxidation of polyunsaturated fatty acids, such as DHA. Such protections could greatly extend the shelf life of active ingredients in fortified products and so enhance nutritional opportunities for everyday consumers.
Data availability
Data supporting this study are available within the article and its ESI.†Author contributions
Luke Wayne Browning: data curation, formal analysis, investigation, project administration, writing – original draft, writing – review & editing. Huafu Wang: investigation, methodology. James Ward Taylor: writing – original draft, writing – review & editing. Pete Wilde: supervision, project administration, writing – original draft, writing – review & editing. Marc Rodriguez-Garcia: conceptualisation. Lynette Anne Makins Holland: writing – review & editing. Tuomas P. J. Knowles: conceptualisation, writing – review & editing. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
Xampla is a company that produces plant protein-based materials for packaging and microencapsulation, including a microencapsulated vitamin D powder for food and beverage applications.Acknowledgements
PW declares that this work was entirely funded by Xampla through QIB Extra (a subsidiary of Quadram Institute Bioscience) which provides short and long-term high-quality strategic and applied research and consulting services. We would like to thank Gareth Hennighan, Stephen Roe, Terez Kolonics and James Jarratt for making dispersions; Polly Keen and Scott Stevens for spray drying vitamin D2 emulsions; Yasin Gee for vitamin D2 analysis; Ayaka Kamada for help with designing graphics and Dan Hawksley for help with data analysis. This work was supported in part by Innovate UK 10015915 for investigating the digestibility of Xampla materials.References
- L. He, J. Hu and W. Deng, Preparation and application of flavor and fragrance capsules, Polym. Chem., 2018, 9, 4926–4946 RSC.
- A. Hasan Anik, S. Hossain, M. Alam, M. Binte Sultan, M. T. Hasnine and M. M. Rahman, Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100530 Search PubMed.
- A. Ashrafy, A. A. Liza, M. N. Islam, M. M. Billah, S. T. Arafat, M. M. Rahman and S. M. Rahman, Microplastics Pollution: A Brief Review of Its Source and Abundance in Different Aquatic Ecosystems, J. Hazard. Mater. Adv., 2023, 9, 100215 Search PubMed.
- G. Lamichhane, A. Acharya, R. Marahatha, B. Modi, R. Paudel, A. Adhikari, B. K. Raut, S. Aryal and N. Parajuli, Microplastics in environment: global concern, challenges, and controlling measures, Int. J. Environ. Sci. Technol., 2023, 20, 4673–4694 CrossRef PubMed.
- M. Haham, S. Ish-Shalom, M. Nodelman, I. Duek, E. Segal, M. Kustanovich and Y. D. Livney, Stability and bioavailability of vitamin D nanoencapsulated in casein micelles, Food Funct., 2012, 3, 737–744 RSC.
- R. E. Grossmann and V. Tangpricha, Evaluation of vehicle substances on vitamin D bioavailability: A systematic review, Mol. Nutr. Food Res., 2010, 54, 1055–1061 CrossRef PubMed.
- A. Nesterenko, I. Alric, F. Silvestre and V. Durrieu, Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness, Ind. Crops Prod., 2013, 42, 469–479 CrossRef.
- C. I. Piñón-Balderrama, C. Leyva-Porras, Y. Terán-Figueroa, V. Espinosa-Solís, C. Álvarez-Salas and M. Z. Saavedra-Leos, Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies, Processes, 2020, 8, 889 CrossRef.
- M. Geranpour, E. Assadpour and S. M. Jafari, Recent advances in the spray drying encapsulation of essential fatty acids and functional oils, Trends Food Sci. Technol., 2020, 102, 71–90 CrossRef.
- C. Bustos-Garza, J. Yáñez-Fernández and B. E. Barragán-Huerta, Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials, Food Res. Int., 2013, 54, 641–649 CrossRef.
- L. T. Abe-Matsumoto, G. R. Sampaio and D. H. M. Bastos, Do the labels of vitamin A, C, and E supplements reflect actual vitamin content in commercial supplements?, J. Food Compos. Anal., 2018, 72, 141–149 CrossRef.
- A. Flynn, L. Kehoe, Á. Hennessy and J. Walton, Estimating safe maximum levels of vitamins and minerals in fortified foods and food supplements, Eur. J. Nutr., 2017, 56, 2529–2539 CrossRef PubMed.
- H. L. A. Makins, R. G. Marc, S. R. N. Patrick, K. P. H. Ruth, T. J. Ward, D. J. M. Caroline and S. S. Edward, Worldwide Pat., WO2023/170156A1, 2023 Search PubMed.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14, 991–1014 CrossRef.
- A. Kamada, M. Rodriguez-Garcia, F. S. Ruggeri, Y. Shen, A. Levin and T. P. J. Knowles, Controlled self-assembly of plant proteins into high-performance multifunctional nanostructured films, Nat. Commun., 2021, 12, 1–10 CrossRef.
- P. Upreti, V. V. Mistry and J. J. Warthesen, Estimation and fortification of vitamin D3 in pasteurized process cheese, J. Dairy Sci., 2002, 85, 3173–3181 CrossRef PubMed.
- EN 12821:2009 – Foodstuffs – Determination of vitamin D by high performance liquid chromatography – Measurement of cholecalciferol (D3) or ergocalciferol (D2), 2009 Search PubMed.
- Y. Wang, W. Liu, X. D. Chen and C. Selomulya, Micro-encapsulation and stabilization of DHA containing fish oil in protein-based emulsion through mono-disperse droplet spray dryer, J. Food Eng., 2016, 175, 74–84 CrossRef.
- L. Le Priol, A. Dagmey, S. Morandat, K. Saleh, K. El Kirat and A. Nesterenko, Comparative study of plant protein extracts as wall materials for the improvement of the oxidative stability of sunflower oil by microencapsulation, Food Hydrocolloids, 2019, 95, 105–115 CrossRef.
- A. P. Pierucci, L. Andrade, M. Farina, C. Pedrosa and M. H. Rocha-Leão, Comparison of α-tocopherol microparticles produced with different wall materials: Pea protein a new interesting alternative, J. Microencapsulation, 2007, 24, 201–213 Search PubMed.
- L. Di Giorgio, P. R. Salgado and A. N. Mauri, Encapsulation of fish oil in soybean protein particles by emulsification and spray drying, Food Hydrocolloids, 2019, 87, 891–901 CrossRef.
- M. G. Nosworthy, A. J. Franczyk, G. Medina, J. Neufeld, P. Appah, A. Utioh, P. Frohlich and J. D. House, Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum), J. Agric. Food Chem., 2017, 65, 7790–7796 CrossRef PubMed.
- L. Jiménez-Munoz, M. Torp Nielsen, L. Roman and M. Corredig, Variation of in vitro digestibility of pea protein powder dispersions from commercially available sources, Food Chem., 2023, 401, 134178 CrossRef.
- A. E. Hall and C. I. Moraru, Comparative effects of high pressure processing and heat treatment on in vitro digestibility of pea protein and starch, npj Sci. Food., 2022, 6, 2 CrossRef PubMed.
- A. Rivera del Rio, A. C. Möller, R. M. Boom and A. E. M. Janssen, In vitro gastro-small intestinal digestion of conventional and mildly processed pea protein ingredients, Food Chem., 2022, 387, 132894 CrossRef PubMed.
- M. Barac, S. Cabrilo, M. Pesic, S. Stanojevic, S. Zilic, O. Macej and N. Ristic, Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes, Int. J. Mol. Sci., 2010, 11, 4973–4990 CrossRef PubMed.
- M. Griga, J. Horáček and H. Klenotičová, Protein patterns associated with Pisum sativum somatic embryogenesis, Biol. Plant., 2007, 51, 201–211 CrossRef.
- X. D. Sun and S. D. Arntfield, Gelation properties of salt-extracted pea protein isolate catalyzed by microbial transglutaminase cross-linking, Food Hydrocolloids, 2011, 25, 25–31 CrossRef.
- J. Xue, T. Wang, Q. Hu, M. Zhou and Y. Luo, Insight into natural biopolymer-emulsified solid lipid nanoparticles for encapsulation of curcumin: Effect of loading methods, Food Hydrocolloids, 2018, 79, 110–116 CrossRef.
- B. Sinead, K. Robert and M. M. Olga, Worldwide Pat., WO2023/170156A1, 2020 Search PubMed.
- K. L. Moore, I. Rodríguez-Ramiro, E. R. Jones, E. J. Jones, J. Rodríguez-Celma, K. Halsey, C. Domoney, P. R. Shewry, S. Fairweather-Tait and J. Balk, The stage of seed development influences iron bioavailability in pea (Pisum sativum L.), Sci. Rep., 2018, 8, 1–11 Search PubMed.
- S. Bejjani, R. Pullakhandam, R. Punjal and K. Madhavan Nair, Gastric digestion of pea ferritin and modulation of its iron bioavailability by ascorbic and phytic acids in caco-2 cells, World J. Gastroenterol., 2007, 13, 2083–2088 CrossRef.
- A. Perfecto, I. Rodriguez-Ramiro, J. Rodriguez-Celma, P. Sharp, J. Balk and S. Fairweather-Tait, Pea ferritin stability under gastric pH conditions determines the mechanism of iron uptake in Caco-2 cells, J. Nutr., 2018, 148, 1229–1235 CrossRef.
- N. Abbaspour, R. Hurrell and R. Kelishadi, Review on iron and its importance for human health, J. Res. Med. Sci., 2014, 19, 3–11 Search PubMed.
- S. Huang, M. L. Vignolles, X. D. Chen, Y. Le Loir, G. Jan, P. Schuck and R. Jeantet, Spray drying of probiotics and other food-grade bacteria: A review, Trends Food Sci. Technol., 2017, 63, 1–17 CrossRef.
- M. Afzaal, F. Saeed, Y. A. Shah, M. Hussain, R. Rabail, C. T. Socol, A. Hassoun, M. Pateiro, J. M. Lorenzo, A. V. Rusu and R. M. Aadil, Human gut microbiota in health and disease: Unveiling the relationship, Front. Microbiol., 2022, 13, 1–14 Search PubMed.
- S. Lebeer, P. A. Bron, M. L. Marco, J. P. Van Pijkeren, M. O'Connell Motherway, C. Hill, B. Pot, S. Roos and T. Klaenhammer, Identification of probiotic effector molecules: present state and future perspectives, Curr. Opin. Biotechnol., 2018, 49, 217–223 CrossRef PubMed.
- K. Hou, Z.-X. Wu, X.-Y. Chen, J.-Q. Wang, D. Zhang, C. Xiao, D. Zhu, J. B. Koya, L. Wei, J. Li and Z.-S. Chen, Microbiota in health and diseases, Signal Transduction Targeted Ther., 2022, 7, 135 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00375f |
| This journal is © The Royal Society of Chemistry 2025 |