 Open Access Article
Open Access ArticleHigh-intensity ultrasound affects the physicochemical, structural and functional properties of proteins recovered from noni (Morinda citrifolia) seeds
Kevin Ulises
López-Mártir
a,
José Armando
Ulloa
 *ab,
Judith Esmeralda
Urías-Silvas
*ab,
Judith Esmeralda
Urías-Silvas
 c,
Petra
Rosas-Ulloa
b and
Blanca Estela
Ulloa-Rangel
d
c,
Petra
Rosas-Ulloa
b and
Blanca Estela
Ulloa-Rangel
d
aDoctorado en Ciencias Biológico Agropecuarias en el Área de Ciencias Agrícolas, Universidad Autónoma de Nayarit, Carretera Tepic-Compostela, Xalisco 63780, Nayarit, Mexico
bCentro de Tecnología de Alimentos, Universidad Autónoma de Nayarit, Ciudad de la Cultura Amado Nervo, Tepic 63155, Nayarit, Mexico. E-mail: arulloa5@gmail.com
cTecnología Alimentaria, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A. C., Avenida Normalistas 800, Colinas de la Normal, Guadalajara 44270, Jalisco, Mexico
dUnidad Académica de Ciencias Químico Biológicas y Farmacéuticas, Universidad Autónoma de Nayarit, Ciudad de la Cultura Amado Nervo, Tepic 63155, Nayarit, Mexico
First published on 28th February 2025
Abstract
Recently, fruit seeds have been considered as an alternative source of protein with the potential to replace those of animal origin in food products. However, such proteins often present some limitations in their functional and nutritional properties. Therefore, the objectives of this study were to prepare a protein concentrate from noni seeds (NSPC) and to evaluate the impact of ultrasound (42 kHz, 130 W, 20 min and 40 min) on their structural, physicochemical, and functional properties. According to the results obtained, ultrasound modified the secondary and tertiary protein structures of NSPC, with changes that were reflected through the parameters of surface hydrophobicity, fluorescence intensity, molecular flexibility, particle size, as well as the contents of sulfhydryls, α-helix, β-sheet, β-turn and random coil. As a consequence of these modifications, the protein purity of NSPC increased by 7.5%, protein solubility by 90.90%, oil holding capacity by 12.0%, emulsifying capacity by 17.1%, emulsion stability by 15.12%, and foaming capacity by 25.7%. Furthermore, protein digestibility and antioxidant capacity were increased by 4.32% and 6.49%, respectively. Therefore, the modification of the physicochemical, functional, biochemical and structural properties of NSPC by the effect of ultrasound enhances its application as a food ingredient. Further research is needed to evaluate the performance of NSPC in specific foods.
Sustainability spotlightFruits are one of the food groups that generate the most waste. Among fruit waste, seeds stand out due to their protein content. This research proposes the use of noni seeds for the production of a protein concentrate, as well as their treatment with high-intensity ultrasound to improve their functional properties. The recovery of nutrients such as proteins from plant waste, as well as their treatment with high-intensity ultrasound to improve their properties and their application as food ingredients or in the development of new products, contributes to combating hunger (SDG 2), promoting responsible food production and consumption (SDG 12), and using environmentally friendly technologies (SDG 13). |
1 Introduction
With the relentless growth of the world population, the need for food sources, especially those that supply protein, also increases.1 Although animal proteins are considered to have the highest nutritional quality, issues regarding sustainability and safety pose challenges for finding alternative sources of dietary proteins.2 Among the alternative sources to animal proteins, those of plant origin stand out because they are cheaper, more environmentally sustainable and have a wide acceptance.3 The main sources of plant proteins include pulses, cereals, pseudocereals, seeds, nuts, and others.4 Other available plant protein resources are protein concentrates or isolates, which are produced mainly from the by-products of oil extraction from oilseeds that are used as protein supplements or as techno-functional ingredients of food products.5Additionally, much research has also been done to recover proteins from by-products of food processing, such as fruits. Some recent studies on proteins recovered as isolates or concentrates are those obtained from tamarind,6 orange,7 soursop,8 guamuchil,9 and bitter melon seeds.10 To recover proteins, either as concentrates or isolates, there are various methods available which involve the extraction, concentration and drying of these polymers, using chemical substances such as acids, bases or salts, heat and other types of non-thermal energy. Such conditions generally limit the functional properties of proteins, so it is desirable to restore or improve these characteristics.11
Luckily, there are several physical (high pressure, heat treatment and high intensity ultrasound), chemical (Maillard reaction, acylation and pH-shifting), and biological (enzymatic proteolysis) treatments that can enhance the physicochemical, nutritional and rheological properties of proteins. However, among the physical treatments to improve protein quality, HI-U stands out for its beneficial impact on the techno-functional properties of proteins, its ease of application, and its environmentally friendly nature.9,12 This technology has been successfully applied using an ultrasonic probe or an ultrasonic bath.13,14
During the application of HI-U, the phenomenon of cavitation occurs, which causes high temperatures (up to 5000 K) and pressures (up to 1000 atm), shear forces, shock waves, turbulence and reactive radicals in the liquid medium due to the sudden generation and collapse of small gas bubbles.15 All these effects due to acoustic cavitation modify the structure of proteins by breaking of electrostatic interactions (van der Waals forces and hydrogen bonds) and disulfide bonds, changes in particle size, and the balance of hydrophilic and hydrophobic groups on the surface, and consequently their functional properties.16
In a recently published study, the results of valorization of noni seeds (Morinda citrifolia) as a source of protein were presented for the first time.17 According to these results, noni seed protein concentrate (NSPC, 76.59% as protein) prepared by alkaline extraction and isoelectric precipitation showed good functional properties, so it could be used as an ingredient in bread, soups, salad dressings, mayonnaise, and processed meat products.
However, there are no studies yet on the impact of high-intensity ultrasound treatment on the properties of noni seed proteins, which could determine other possible applications in the food industry. Considering the above, the present study was carried out with the purpose of evaluating the effect of HI-U on the physicochemical, functional, and biochemical properties of noni seed protein concentrate and its relationship with structural changes, thereby expanding its application as a food ingredient.
2 Materials and methods
2.1 Materials
NSPC from the previous study on noni seed valorization was used in this investigation.17 Chemicals and solvents employed in the experiment were of analytical grade and acquired from Sigma-Aldrich Corp (St. Louis, Missouri, USA) and Avantor (Radnor, PA, USA).2.2 HI-U treatment
HI-U treatment was applied to the samples according to the procedure described by Espinosa-Murillo et al.18 Briefly, 10% protein dispersions of NSPC were prepared in 500 mL Erlenmeyer flasks by adjusting the pH to 7 with 0.1 M NaOH. The protein dispersions were then placed at the center of an ultrasonic bath (Branson Ultrasonic Corp., USA) model MTH3510 (tank capacity of 5 L; internal dimensions of 290 × 150 × 150 mm; acoustic density of 0.026 W cm−3) for 20 min and 40 min. The water temperature in the ultrasonic bath for the treatment of NSPC protein dispersions was naturally maintained in the range of 25–33 °C. In addition, a control sample was prepared by magnetic stirring at 350 rpm. Finally, both the sonicated samples and the control were lyophilized in 50 mL plastic tubes in a FreeZone model 12 L unit (Labconco, USA) for 72 h at −46 °C and 0.2 Mbar. The NSPC powders were stored in hermetically sealed glass jars and at room temperature for further analysis.2.3 Structural characterization
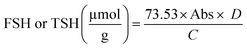 | (1) |
2.4 Physicochemical characterization
 | (2) |
 | (3) |
 | (4) |
2.5 Techno-functional characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Then, the samples were centrifuged at 1200 rpm for 5 min. The ECA was determined with eqn (5), using the volume of the emulsified phase (VE) and the total volume (VT).
000 rpm for 1 min. Then, the samples were centrifuged at 1200 rpm for 5 min. The ECA was determined with eqn (5), using the volume of the emulsified phase (VE) and the total volume (VT). | (5) |
For EST, the samples were heated at 80 °C for 30 min, cooled to 25 °C and centrifuged again. EST was expressed as the percentage of ECA remaining after heating.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The generated foam was transferred to a test tube to measure its initial volume (V0) and then was measured again after 20 min (V20). Foaming capacity (FCA) and foam stability (FST) were calculated using eqn (6) and (7), respectively.
000 rpm. The generated foam was transferred to a test tube to measure its initial volume (V0) and then was measured again after 20 min (V20). Foaming capacity (FCA) and foam stability (FST) were calculated using eqn (6) and (7), respectively. | (6) |
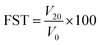 | (7) |
2.7 Biochemical characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v), respectively, by shaking for 60 min at 700 rpm and centrifuged at 11
20 (w/v), respectively, by shaking for 60 min at 700 rpm and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 15 min.35 Finally, protein quantification in each extracted fraction was performed using the Kjeldahl method (N × 6.25).
000×g for 15 min.35 Finally, protein quantification in each extracted fraction was performed using the Kjeldahl method (N × 6.25).
| IVPD (%) = 10.464 − 18.103 × X | (8) |
 | (9) |
2.8 Statistical analysis
The results were presented as means ± standard deviations of triplicates. One-way analysis of variance using Statgraphics Centurion XV (Statgraphics Technologies, Inc., USA) software was used for statistical analysis. Tukey's test identified significant differences between treatments (p < 0.05). Additionally, a Pearson correlation analysis was performed to examine relationships between structural, functional, and biochemical properties, with statistical significance at p < 0.01 and p < 0.05 levels.3 Results and discussion
3.1 Structural characteristics
HI-U causes partial denaturation of proteins, allowing intermolecular bonds to form between them. This protects their hydrophobic regions and consequently reduces SH.38,39 In this study, it was observed that the reduction of SH increased the PSOL of NSPC (see section 3.5.1) because such conditions favor a greater interaction of the hydrophilic groups with the aqueous environment. Similarly, in a study on soursop seed proteins, HI-U (200 W, 30 min) reduced 68% SH,8 while in a protein isolate from guamuchil seeds, under the same conditions, an increase of 53% was observed.9
HI-U unfolds or refolds protein molecules, which can cause an increase or decrease in the exposure of chromophores such as tryptophan, resulting in a modification in fluorescence intensity.41,42 Furthermore, changes in the tertiary structure of proteins are associated with the type of protein source and its intrinsic properties, as well as the ultrasonic processing conditions.13 The modification of the tertiary structure of NSPC by HI-U was related to the improvement of its PSOL, OHCA, ECA and FCA (see sections 3.5.1–3.5.4).8,9,13 Similar results to those obtained in this study on the behavior of fluorescence intensity because of HI-U were observed in an almond protein isolate.43
| Properties | NSPC-0 min (control) | Ultrasound treatment | |
|---|---|---|---|
| NSPC-20 min | NSPC-40 min | ||
| a The results were obtained in triplicate and are expressed as mean ± standard deviation. Different letters indicate significant differences between treatments (p < 0.05). For each treatment the second term represents the exposure time to ultrasound in min. | |||
| Molecular flexibility (abs) | 0.73 ± 0.01b | 0.63 ± 0.02c | 0.79 ± 0.01a |
| Particle size (nm) | 248 ± 3.51a | 237 ± 4.61b | 200 ± 1.56c |
| Total sulfhydryl (μmol g−1) | 10.05 ± 0.15a | 8.26 ± 0.12b | 8.41 ± 0.13b |
| Free sulfhydryl (μmol g−1) | 1.39 ± 0.25b | 2.03 ± 0.23b | 3.35 ± 0.30a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Secondary structure (%) | |||
| β-Sheet | 23.82 ± 0.20a | 21.36 ± 0.28b | 19.66 ± 0.40c |
| Random coil | 14.95 ± 0.36a | 12.20 ± 0.14b | 11.50 ± 0.31b |
| α-Helix | 17.09 ± 0.55a | 8.42 ± 0.31b | 16.70 ± 0.07a |
| β-Turn | 44.14 ± 0.33c | 58.01 ± 0.41a | 52.13 ± 0.35b |
According to Kang et al.,24 HI-U provokes changes in hydrophobic interactions and in hydrogen and disulfide bonds between protein molecules, which modify their MFx.24,46 The modification in MFx of NSPC in this study was associated with the improvement of NSPC techno-functional properties, such as PSOL and FST (see subsections 3.5.1 and 3.5.4). In a study with proteins obtained from soursop seeds,8 HI-U caused a reduction of up to 37% of MFx, similar to this investigation when ultrasound was applied for 20 min.
The reduction in TSH content could be related to the formation of disulfide bonds through the interaction of reactive sulfhydryl groups, while the increase in FSH content with the unburying of these same groups and their exposure on the surface of the protein, due to the unfolding of the polypeptide chain by the effect of ultrasound.52 The increased exposure of the FSH groups of the NSPC by HI-U was reflected in the improvement of some of its functional properties, including those of PSOL, ECA and FCA (subsections 3.5.1, 3.5.3, and 3.5.4). In studies on protein isolates from chickpea24 and melon seeds,51 HI-U decreased the TSH and FSH contents, respectively, consistent with the result obtained for NSPC in this study.
According to Gul et al.,19 ultrasonic cavitation induces changes in the secondary structure by breaking the intermolecular forces that hold hydrogen bonds in proteins. Although it has been demonstrated that both the intrinsic properties of proteins and the HI-U conditions influence the modification of the structural characteristics of proteins, these changes do not follow a predictable behavior.8 In this study with NSPC, it was found that the reduction of their β-sheet content by HI-U increased PSOL and FCA (see sections 3.5.1 and 3.5.4). In a study on kiwifruit proteins,56 HI-U modified their secondary structure, reducing the α-helix content and increasing the β-sheet content, which benefited their functional properties.
 | ||
| Fig. 2 Modification of the microstructure of noni seed protein concentrate (NSPC) by high-intensity ultrasound. The term following NSPC represents the exposure time to ultrasound in min. | ||
The larger microstructures of HI-U-treated proteins may be a consequence of the interactions of sulfhydryl groups on the surface of the proteins.58 Furthermore, ultrasonic cavitation generates micropores on the surface of the protein matrix, which favors water uptake and consequently an improvement in functional properties due to better solubilization.59 In this investigation, the alteration of the microstructure manifested by pore formation on the surface of the protein matrix and its consequent benefit in protein solubility, improved OHCA, ECA, ESA and FCA of the NSPC treated by HI-U (see subsections 3.3.1–3.3.4). A similar effect about the impact of HI-U on the microstructure of NSPC in this study was observed for a protein isolate obtained from passion fruit seeds sonicated at 100 W for 15 min and 30 min.18
3.2 Physicochemical characteristics
| Components (%) | NSPC-0 minA (control) | Ultrasound treatment | |
|---|---|---|---|
| NSPC-20 min | NSPC-40 min | ||
| a The results were obtained in triplicate and are expressed as mean ± standard deviation. Different letters indicate significant differences between treatments (p < 0.05). For each treatment the second term represents the exposure time to ultrasound in min. AHernández-Ramírez et al.17 | |||
| Protein (N × 6.25) | 76.59 ± 0.3c | 77.87 ± 0.04b | 82.36 ± 0.81a |
| Moisture | 7.67 ± 0.07a | 5.31 ± 0.15b | 3.34 ± 0.04c |
| Ash | 6.99 ± 0.05a | 6.86 ± 0.12b | 6.97 ± 0.23a |
| Lipids | 8.44 ± 0.12a | 6.85 ± 0.10b | 3.41 ± 0.28c |
| Nitrogen-free extract | 0.29 ± 0.47c | 3.10 ± 0.16b | 4.23 ± 0.75a |
A similar effect of HI-U on the variation of the chemical composition of the NSPC in this study was reported for protein isolates from soursop,8 guamuchil,9 passion fruit,18 and gourd seeds.14
The reduction in WA by HI-U is due to sound waves passing through the food material, which facilitate the rate of heat and mass transfer and therefore water removal,9 while the decrease in BD is a consequence of the formation of larger and more heterogeneous structures of protein concentrates or isolates treated-HI-U.64 The reduction in TU of protein solutions treated with HI-U is produced by the high-energy sound waves and turbulence. The decrease in TU of protein solutions by HI-U is due to the fact that these proteins are violently agitated by high shear energy waves and turbulence, which causes the diameter of the protein particles to become smaller.65 The depletions in WA, BD, and TU by HI-U, as were observed in this study with NSPC, were also reported for a protein isolate from guamuchil seeds.9
| Properties | NSPC-0 min (control) | Ultrasound treatment | |
|---|---|---|---|
| NSPC-20 min | NSPC-40 min | ||
| a The results were obtained in triplicate and are expressed as mean ± standard deviation. Different letters indicate significant differences between treatments (p < 0.05). AHernández-Ramírez et al.17 | |||
| Water activity | 0.397 ± 0.01bA | 0.406 ± 0.01a | 0.358 ± 0.01c |
| Bulk density (g cm−3) | 0.056 ± 0.01bA | 0.071 ± 0.03a | 0.039 ± 0.04c |
| Turbidity | 0.34a ± 0.03a | 0.14 ± 0.01c | 0.17 ± 0.01b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Color | |||
| Lightness (L*) | 55.32 ± 0.08aA | 53.06 ± 0.07b | 55.24 ± 0.10a |
| +Redness, −greenness (a*) | 4.60 ± 0.02aA | 4.44 ± 0.03b | 4.44 ± 0.03b |
| +Yellowness, −blueness (b*) | 13.09 ± 0.01bA | 12.58 ± 0.05c | 13.51 ± 0.03a |
| Difference color (ΔE) | 61.46 ± 0.11bA | 64.64 ± 0.10a | 61.64 ± 0.14b |
| Chroma (C*) | 13.87 ± 0.02bA | 13.34 ± 0.06c | 14.30 ± 0.03a |
| Hue angle (H*) | 70.61 ± 0.06bA | 70.66 ± 0.06b | 70.99 ± 0.09a |
The color modification of protein isolates or concentrates by the effect of HI-U could be related to changes in the protein structure or pigment degradation, depending on the ultrasound conditions.67 In a study by Naik et al. with a protein isolate from melon seeds,10 it was reported that HI-U produced a less luminous and yellowish product, with more reddish tones, as found for the NSPC in this study.
3.3 Techno-functional characteristics
| Properties | NSPC-0 min (control) | Ultrasound treatment | |
|---|---|---|---|
| NSPC-20 min | NSPC-40 min | ||
| a The results were obtained in triplicate and are expressed as mean ± standard deviation. Different letters indicate significant differences between treatments (p < 0.05). WHCA = water holding capacity, OHCA = oil holding capacity, and Act-DPPH = DPPH radical scavenging activity. AHernández-Ramírez et al.17 | |||
| Functional | |||
| WHCA (g H20 g−1 protein) | 4.36 ± 0.24aA | 4.39 ± 0.16a | 4.45 ± 0.18a |
| OHCA (g oil g−1 protein) | 11.69 ± 0.50bA | 11.14 ± 0.14b | 13.09 ± 0.30a |
| Emulsifying capacity (%) | 29.20 ± 0.79bA | 30.60 ± 0.91b | 34.19 ± 0.95a |
| Emulsifying stability (%) | 50.00 ± 1.00bA | 50.76 ± 0.87b | 57.56 ± 1.00a |
| Foaming capacity (%) | 180.33 ± 0.57cA | 200.33 ± 0.57b | 226.60 ± 0.01a |
| Foaming stability (%) | 94.60 ± 0.34aA | 90.33 ± 0.57b | 90.66 ± 0.57b |
| Least gelation concentration (%) | 4.00 ± 0.00aA | 4.00 ± 0.00a | 4.00 ± 0.00a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Biochemical (%) | |||
| In vitro protein digestibility | 78.45 ± 0.01cA | 81.15 ± 0.01b | 81.84 ± 0.01b |
| Act-DPPH | 22.19 ± 0.51d | 27.89 ± 0.06b | 23.63 ± 0.13c |
Cavitation by HI-U creates a mechanical shear stress that exposes the hydrophobic side chains of amino acids, facilitating protein adsorption at the oil–water interface and improving emulsifying properties.76 Additionally, the increase in FSH group content, as well as the decrease in Ps and SH of NSPC, were correlated with the increase in ECA (see Table 5). In research on a protein isolate from guamuchil seeds the improvement of emulsifying properties due to HI-U was also reported,9 consistent with the results obtained for NSPC in this study.
| PSOL | WHCA | ECA | FCA | FST | Prolamin | Glutelin | FSH | TSH | MFx | Ps | SH | β-Sheet | Rand-C | IVPD | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a *Significance at the probability level of p < 0.05; **significance at the probability level of p < 0.01. PSOL: protein solubility; WHCA: water holding capacity; ECA: emulsifying capacity; FCA: foaming capacity; FST: foaming stability; FSH−: free sulfhydryl; TSH−: total sulfhydryl; MFx: molecular flexibility; Ps: particle size; SH: surface hydrophobicity; Rand-C: random coil; IVPD: in vitro protein digestibility; DPPH: DPPH activity. | ||||||||||||||||
| PSOL | 1 | 0.965 | 0.945 | 0.980 | −0.874 | −0.317 | 0.839 | 0.959 | −0.870 | −0.971 | −1.000* | −0.925 | −1.000* | −0.971 | 0.971 | 0.321 |
| WHCA | 0.965* | 1 | 0.997* | 0.998* | −0.717 | −0.059 | 0.668 | 1.000* | −0.711 | −0.874 | −0.971 | −0.992 | −0.961 | −0.874 | 0.874 | 0.063 |
| ECA | 0.945 | 0.997** | 1 | 0.991 | −0.666 | 0.012 | 0.613 | 0.999* | −0.659 | −0.838 | −0.952 | −0.998* | −0.938 | −0.838 | 0.838 | −0.008 |
| FCA | 0.980* | 0.998** | 0.991* | 1 | −0.761 | −0.124 | 0.715 | 0.996 | −0.755 | −0.904 | −0.985 | −0.982 | −0.977 | −0.904 | 0.904 | 0.128 |
| FST | −0.874 | −0.717 | −0.666 | −0.761 | 1 | 0.738 | −0.998* | −0.702 | 1.000** | 0.965 | 0.862 | 0.624 | 0.883 | 0.965 | −0.965 | −0.741 |
| Prolamin | −0.317 | −0.059 | 0.012 | −0.124 | 0.738 | 1 | −0.783 | −0.037 | 0.744 | 0.536 | 0.295 | −0.066 | 0.334 | 0.536 | −0.536 | −1.000** |
| Glutelin | 0.839 | 0.668 | 0.613 | 0.715 | −0.998** | −0.783 | 1 | 0.651 | −0.998* | −0.945 | −0.825 | −0.569 | −0.848 | −0.945 | 0.945 | 0.785 |
| FSH | 0.959* | 1.000** | 0.999** | 0.996* | −0.702 | −0.037 | 0.651 | 1 | −0.695 | −0.863 | −0.966 | −0.995 | −0.954 | −0.863 | 0.863 | 0.041 |
| TSH | −0.870 | −0.711 | −0.659 | −0.755 | 1.000** | 0.744 | −0.998** | −0.695 | 1 | 0.963 | 0.858 | 0.617 | 0.878 | 0.963 | −0.963 | −0.747 |
| MFx | −0.971* | −0.874 | −0.838 | −0.904 | 0.965* | 0.536 | −0.945 | −0.863 | 0.963* | 1 | 0.965 | 0.806 | 0.975 | 1.000** | −1.000** | −0.540 |
| Ps | −1.000** | −0.971* | −0.952* | −0.985* | 0.862 | 0.295 | −0.825 | −0.966* | 0.858 | 0.965* | 1 | 0.934 | 0.999* | 0.965 | −0.965 | −0.298 |
| SH | −0.925 | −0.992* | −0.998** | −0.982* | 0.624 | −0.066 | −0.569 | −0.995* | 0.617 | 0.806 | 0.934 | 1 | 0.918 | 0.807 | −0.806 | 0.063 |
| β-Sheet | −1.000** | −0.961* | −0.938 | −0.977* | 0.883 | 0.334 | −0.848 | −0.954* | 0.878 | 0.975* | 0.999** | 0.918 | 1 | 0.975 | −0.975 | −0.338 |
| Rand-C | −0.971* | −0.874 | −0.838 | −0.904 | 0.965* | 0.536 | −0.945 | −0.863 | 0.963* | 1.000** | 0.965* | 0.807 | 0.975* | 1 | −1.000** | −0.539 |
| IVPD | 0.971* | 0.874 | 0.838 | 0.904 | −0.965* | −0.536 | 0.945 | 0.863 | −0.963* | −1.000** | −0.965* | −0.806 | −0.975* | −1.000** | 1 | 0.540 |
| DPPH | 0.321 | 0.063 | −0.008 | 0.128 | −0.741 | −1.000** | 0.785 | 0.041 | −0.747 | −0.540 | −0.298 | 0.063 | −0.338 | −0.539 | 0.540 | 1 |
HI-U improves the FCA of proteins by reducing the particle size, allowing for greater contact at the protein–air interface.78 However, prolonged HI-U times reduce FST by exposing hydrophobic and sulfhydryl groups, altering the hydrophobic–hydrophilic equilibrium on protein surfaces.79 Proteins from pumpkin seed exposed to HI-U also showed an increase in FCA but a reduction in FST,80 as was observed in this study.
The cleavage of covalent and non-covalent bonds by HI-U, with the consequent partial unfolding of the protein structure to change SH, can give rise to new intermolecular and intramolecular protein interactions to improve gelation properties.83 A study with melon seed proteins treated with HI-U revealed benefits in their gelling properties,10 while others with proteins from soursop,8 and jackfruit30 seeds did not detect changes in such properties.
3.4 Biochemical characteristics
In this investigation, the decrease in TSH content in NSPC due to HI-U treatment was associated with an increase in glutelin content (see Table 5). According to various studies, HI-U alters the solubility pattern of proteins in different solvents due to changes in the structure and the balance of hydrophobic–hydrophilic groups on their surface.7–9,18
The improvement in digestibility by HI-U is due to the modification of the secondary structure, which promotes greater contact of the enzymes with the proteins.87 In this study, HI-U modified the secondary structure of NSPC by decreasing the random coil content, which was correlated with the increase in IVPD (Table 5). In other investigations, with proteins from bean88 and watermelon seeds,89 a beneficial effect of HI-U on digestibility was also observed, as occurred with the NSPC proteins in this study.
HI-U enhances the antioxidant capacity by unfolding proteins and exposing amino acid residues and side chains with antioxidant properties, which are normally hidden in their three-dimensional structure.20 The decrease in NSPC prolamin content due to HI-U treatment was associated with the improvement of Act-DPPH (Table 5). In a study with soy proteins, HI-U increased their antioxidant capacity,55 consistent with the findings of this research.
3.5 Pearson correlation analysis
The Pearson correlation coefficient, which takes values between 0 ± 1, is a statistical measure that assesses the strength and direction of the relation between two variables.91Table 5 shows the Pearson correlation coefficients among structural, functional, and biochemical properties of NSPC treated with HI-U and the control. Most of the functional, structural, and biochemical properties of NSPC are dependent on each other. However, the structural properties of NSPC that had a highly significant (p < 0.01) positive influence on the properties were FSH/WHCA (r2 = 1.000), FSH/ECA (r2 = 0.999), and TSH/FST (r2 = 1.000), while those that had a negative influence were Ps/PSOL (r2 = −1.000), β-sheet/PSOL (r2 = −1.000), and SH/ECA (r2 = −0.998).Other studies on proteins from peanut,92 soybean,93 soursop,8 and jackfruit30 treated with HI-U also reported high dependence between their structural and functional properties, as was observed in this study with NSPC.
4 Conclusions
HI-U improved the structural, physicochemical, functional, and biochemical properties of NSPC obtained by alkaline extraction and isoelectric precipitation. Ultrasonic cavitation caused modifications in both the tertiary structure (SH, fluorescence intensity, MFx, Ps, TSH and FSH) and secondary structure (α-helix, β-sheet, β-turn and random coil) of NSPC proteins, in addition to their microstructure, which showed a more porous and homogeneous matrix. Structural changes of NSPC affected the solubility pattern of proteins, which changed the contents of albumins, globulins, prolamins, and glutelins while improving the functional properties of PSOL, OHCA, ECA, EST, FCA, IVPD, and Act-DPPH. Therefore, HI-U enhanced the potential of NSPC as a food ingredient. However, further studies on other characteristics of NSPC, such as rheological and nutritional properties, as well as its evaluation in foods or the development of new products through specific assays, are recommended to determine its feasibility and industrial application.Abbreviations
| NSPC | Noni seed protein concentrate |
| HI-U | High-intensity ultrasound |
| SPB | Sodium phosphate buffer |
| ANS | 1-Anilino-8-naphthalene sulfonate |
| SH | Surface hydrophobicity |
| MFx | Molecular flexibility |
| Ps | Particle size |
| FSH | Free sulfhydryl |
| TSH | Total sulfhydryl |
| WA | Water activity |
| BD | Bulk density |
| TU | Turbidity |
| L* | Lightness |
| a* | +Redness, −greenness |
| b* | +Yellowness, −blueness |
| C* | Chroma |
| H* | Hue angle |
| ΔE | Color difference |
| PSOL | Protein solubility |
| WHCA | Water-holding capacity |
| OHCA | Oil-holding capacity |
| ECA | Emulsifying capacity |
| EST | Emulsifying stability |
| FCA | Foaming capacity |
| FST | Foaming stability |
| LGC | Least gelation concentration |
| IVPD | In vitro protein digestibility |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| Act-DPPH | DPPH activity |
Data availability
The data supporting the findings of this research are available in the article.Author contributions
Kevin Ulises López-Mártir: conceptualization; investigation; methodological development; use of software; and writing of the original draft. José Armando Ulloa: conceptualization; data management; obtaining funding; investigation; provision of resources; writing of the original draft; and review and editing of the manuscript. Judith Esmeralda Urías-Silvas: investigation; provision of resources; and supervision. Petra Rosas-Ulloa: conceptualization; supervision; and guidance. Blanca Estela Ulloa Rangel: conceptualization; supervision; and guidance.Conflicts of interest
The authors affirm they have no financial interests or personal relationships that could have influenced the work presented in this paper.Acknowledgements
The authors would like to thank the Secretariat of Science, Humanities, Technology and Innovation (SECIHTI) of Mexico and the Council of Science and Technology of the State of Nayarit (COCYTEN) for the scholarship awarded (1182335) and for supporting the payment of tuition fees for Kevin Ulises López-Mártir, MSc, the Board of Trustees for the Administration of the Special Tax Destined for the Autonomous University of Nayarit (UAN) for the financial support and Nancy Dinorah Ruelas Hernández, MSc, for her invaluable help in preparing and capturing the SEM images.References
- Q. Zhao, T. Xie, X. Hong, Y. Zhou, L. Fan, Y. Liu and J. Li, Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion, Food Chem., 2022, 368, e130848, DOI:10.1016/j.foodchem.2021.130848.
- F. Higa and M. T. Nickerson, Plant protein–carbohydrate conjugates: a review of their production, functionality and nutritional attributes, Food Rev. Int., 2023, 30, 750–771, DOI:10.1080/87559129.2021.1926485.
- N. Ravindran, S. K. Singh and P. Singha, A comprehensive review on the recent trends in extractions, pretreatments and modifications of plant-based proteins, Food Res. Int., 2024, 190, e114575, DOI:10.1016/j.foodres.2024.114575.
- S. Thakur, A. K. Pandey, K. Verma, A. Shrivastava and N. Singh, Plant-based protein as an alternative to animal proteins: a review of sources, extraction methods and applications, Int. J. Food Sci. Technol., 2024, 59, 488–497, DOI:10.1111/ijfs.16663.
- M. Meganaharshini, V. Sudhakar, N. Dhivya Bharathi and S. Deepak, Review on recent trends in the application of protein concentrates and isolates – a food industry perspective, Food and Humanity, 2023, 1, 308–325, DOI:10.1016/j.foohum.2023.05.022.
- A. Singh and N. Sit, Dual modification of manila tamarind protein isolate by ultrasonication and autoclaving and their characterization, Food Bioprocess Technol., 2023, 16, 2947–2960, DOI:10.1007/s11947-023-03100-6.
- P. Rosas Ulloa, J. A. Ulloa, B. E. Ulloa Rangel and K. U. López Mártir, Protein isolate from orange (Citrus sinensis L.) seeds: Effect of high-intensity ultrasound on its physicochemical and functional properties, Food Bioprocess Technol., 2023, 16, 589–602, DOI:10.1007/s11947-022-02956-4.
- K. U. López-Mártir, J. A. Ulloa, J. E. Urías-Silvas, P. Rosas-Ulloa, J. C. Ramírez-Ramírez and J. A. Resendiz-Vazquez, Modification of the physicochemical, functional, biochemical and structural properties of a soursop seed (Annona muricata L.) protein isolate treated with high-intensity ultrasound, Ultrason. Sonochem., 2024, 105, e106870, DOI:10.1016/j.ultsonch.2024.106870.
- N. T. Flores-Jiménez, J. A. Ulloa, J. E. Urías-Silvas, J. C. Ramírez-Ramírez, P. U. Bautista-Rosales and R. Gutiérrez-Leyva, Influence of high-intensity ultrasound on physicochemical and functional properties of a guamuchil Pithecellobium dulce (Roxb.) seed protein isolate, Ultrason. Sonochem., 2022, 84, e105976, DOI:10.1016/j.ultsonch.2022.105976.
- M. Naik, V. Natarajan, N. Modupalli, S. Thangaraj and A. Rawson, Pulsed ultrasound assisted extraction of protein from defatted bitter melon seeds (Momardica charantia L.) meal: kinetics and quality measurements, LWT–Food Sci. Technol., 155, e112997, DOI:10.1016/j.lwt.2021.112997.
- A. Hewage, O. O. Olatunde, C. Nimalaratne, M. Malalgoda, R. E. Aluko and N. Bandara, Novel extraction technologies for developing plant protein ingredients with improved functionality, Trends Food Sci. Technol., 2022, 129, 492–511, DOI:10.1016/j.tifs.2022.10.016.
- N. Bhargava, R. S. Mor, K. Kumar and V. S. Sharanagat, Advances in application of ultrasound in food processing: a review, Ultrason. Sonochem., 2021, 70, e105293, DOI:10.1016/j.ultsonch.2020.105293.
- J. A. Ulloa, M. C. Villalobos-Barbosa, J. A. Resendiz-Vazquez, P. Rosas-Ulloa, J. C. Ramírez-Ramírez, Y. Silva-Carrillo and L. González-Torres, Production, physico-chemical and functional characterization of a protein isolate from jackfruit (Artocarpus heterophyllus) seeds, CyTA - J. Food, 2017, 15, 497–507, DOI:10.1080/19476337.2017.1301554.
- Y. Silva-Carrillo, J. A. Ulloa, J. E. Urías-Silvas, J. C. Ramírez-Ramírez and R. Gutiérrez-Leyva, Physicochemical and functional characteristics of a gourd (Cucurbita argyrosperma Huber) seed protein isolate subjected to high-intensity ultrasound, Heliyon, 2024, 10, e32225, DOI:10.1016/j.heliyon.2024.e32225.
- D. Y. Hoo, Z. L. Low, D. Y. S. Low, S. Y. Tang, S. Manickam, K. W. Tan and Z. H. Ban, Ultrasonic cavitation: an effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose, Ultrason. Sonochem., 2022, 90, e106176, DOI:10.1016/j.ultsonch.2022.106176.
- W. Chen, H. Ma and Y. Y. Wang, Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: potential mechanisms, combination with other methods, equipment innovations and future directions, Ultrason. Sonochem., 2022, 85, e105993, DOI:10.1016/j.ultsonch.2022.105993.
- J. A. Hernández Ramírez, J. A. Ulloa, B. E. Ulloa Rangel and P. Rosas Ulloa, Valorization of the noni (Morinda citrifolia) seeds as source of a protein concentrate and its physicochemical, functional, and structural characterization, Waste Biomass Valorization, 2024, 15, 2033–2043, DOI:10.1007/s12649-023-02270-w.
- N. C. Espinosa-Murillo, J. A. Ulloa, J. E. Urias-Silvas, P. Rosas-Ulloa, J. C. Ramírez-Ramírez, R. Gutiérrez-Leyva and B. E. Ulloa-Rangel, Impact of high-intensity ultrasound on the physicochemical and functional properties of a protein isolate from passion fruit (Passiflora edulis) seeds, Int. J. Food Eng., 2021, 17, 609–618, DOI:10.1515/ijfe-2021-0050.
- O. Gul, F. T. Saricaoglu, I. Atalar, L. B. Gul, F. Tornuk and S. Simsek, Structural characterization, technofunctional and rheological properties of sesame proteins treated by high-intensity ultrasound, Foods, 2023, 12, e1791, DOI:10.3390/foods12091791.
- A. Hu and L. Li, Effects of ultrasound pretreatment on functional property, antioxidant activity, and digestibility of soy protein isolate nanofibrils, Ultrason. Sonochem., 2022, 90, e106193, DOI:10.1016/j.ultsonch.2022.106193.
- S. Yan, J. Xu, S. Zhang and L. Li, Effects of flexibility and surface hydrophobicity on emulsifying properties: ultrasound-treated soybean protein isolate, LWT–Food Sci. Technol., 2021, 142, e110881, DOI:10.1016/j.lwt.2021.110881.
- N. Wang, X. Zhou, W. Wang, L. Wang, L. Jiang, T. Liu and D. Yu, Effect of high intensity ultrasound on the structure and solubility of soy protein isolate-pectin complex, Ultrason. Sonochem., 2021, 80, e105808, DOI:10.1016/j.ultsonch.2021.105808.
- F. Alavi, L. Chen and Z. Enam-Djomeh, Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein, Food Chem., 2021, 354, e129494, DOI:10.1016/j.foodchem.2021.129494.
- S. Kang, J. Zhang, H. Guo, Y. Lei and M. Yang, Effects of ultrasonic treatment on the structure, functional properties of chickpea protein isolate and its digestibility in vitro, Foods, 2022, 11, e880, DOI:10.3390/foods11060880.
- R. Rawat and C. S. Saini, High-intensity ultrasound (HIUS) treatment of sunnhemp protein isolate (Crotalaria juncea L.): modification of functional, structural, and microstructural properties, Food Bioprocess Technol., 2023, 16, 1464–1477, DOI:10.1007/s11947-023-03011-6.
- AOAC, Official Methods of Analysis of AOAC International, AOAC International, Gaithersburg, MD, 21st edn, 2019 Search PubMed.
- S. H. Budnimath, G. Bhuvaneshwari, V. M. Ganiger, S. L. Jagadeesh, G. Goudar, N. S. Patil and V. M. Chandrashekar, Physical, reconstitution and phenolic properties of instant drink mix prepared with Moringa oleifera leaf, raw banana and whey protein concentrate, Measurement: Food, 2023, 11, e100108, DOI:10.1016/j.meafoo.2023.100108.
- M. Gu, J. Shi, B. Zhang, X. Wang and X. Wang, Covalent modification of soy protein isolates by hydroxytyrosol: effects on structural and functional properties of adducts, LWT–Food Sci. Technol., 2024, 198, e116041, DOI:10.1016/j.lwt.2024.116041.
- A. Mykhalevych, M. Buniowska-Olejnik, G. Polishchuk, C. Puchalski, A. Kamińska-Dwórznicka and A. Berthold-Pluta, The influence of whey protein isolate on the quality indicators of acidophilic ice cream based on liquid concentrates of demineralized whey, Foods, 2024, 13, e170, DOI:10.3390/foods13010170.
- J. A. Resendiz-Vazquez, J. A. Ulloa, J. E. Urías-Silvas, P. U. Bautista-Rosales, J. C. Ramírez- Ramírez, P. Rosas-Ulloa and L. González-Torres, Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate, Ultrason. Sonochem., 2017, 37, 436–444, DOI:10.1016/j.ultsonch.2017.01.042.
- Ö. Coşkun and I. Gülseren, Aqueous extraction and functionality of protein concentrates manufactured from cold press meals of pumpkin, pomegranate, and grape seeds, Nutrire, 2020, 45, 1–12, DOI:10.1186/s41110-020-00114-4.
- G. Ozkan, P. Tataroglu, S. Gulec and E. Capanoglu, Modification of pea protein isolates by high-intensity ultrasonication: functional, structural and nutritional properties, Food Chem. Adv, 2024, 5, e100793, DOI:10.1016/j.focha.2024.100793.
- M. Ye, Z. Wang, X. Yan, Z. Zeng, T. Peng, J. Xia, J. Zhao, W. Wang, D. Gong and P. Yu, Effects of drying methods on the physicochemical and functional properties of Cinnamomum camphora seed kernel protein isolate, Foods, 2024, 13, e968, DOI:10.3390/foods13060968.
- K. A. Illingworth, Y. Y. Lee and L. F. Siow, Functional properties of Moringa oleifera protein isolates as influenced by different isolation techniques, pH, and ionic strength, Food Bioprocess Technol., 2024, 17, 3060–3073, DOI:10.1007/s11947-023-03279-8.
- R. Nunes, P. Ferreira-Santos, C. Moreira, J. A. Teixeira and C. M. Rocha, Tuning the extraction methodology targeting protein-enriched fractions from red algae, Future Foods, 2024, 9, e100335, DOI:10.1016/j.fufo.2024.100335.
- L. H. Lim and D. H. Kim, Effects of roasting conditions on physicochemical properties and antioxidant activities in Ginkgo biloba seeds, Food Sci. Biotechnol., 2018, 27, 1057–1066, DOI:10.1007/s10068-018-0348-7.
- R. Zhao, W. Fu, D. Li, C. Dong, Z. Bao and C. Wang, Structure and functionality of whey protein, pea protein, and mixed whey and pea proteins treated by pH shift or high-intensity ultrasound, J. Dairy Sci., 2024, 107, 726–741, DOI:10.3168/jds.2023-23742.
- S. A. Vargas, R. J. Delgado-Macuil, H. Ruiz-Espinosa, M. Rojas-López and G. G. Amador-Espejo, High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: evaluation of selected functional properties, Ultrason. Sonochem., 2021, 70, e105340, DOI:10.1016/j.ultsonch.2020.105340.
- X. Zhao, X. Fan, X. Shao, M. Cheng, C. Wang, H. Jiang, X. Zhang and C. Yuan, Modifying the physicochemical properties, solubility and foaming capacity of milk proteins by ultrasound-assisted alkaline pH-shifting treatment, Ultrason. Sonochem., 2022, 88, e106089, DOI:10.1016/j.ultsonch.2022.106089.
- J. Yan, S. Zhao, X. Xu and F. Liu, Enhancing pea protein isolate functionality: a comparative study of high-pressure homogenization, ultrasonic treatment, and combined processing techniques, Curr. Res. Food Sci., 2024, 8, e100653, DOI:10.1016/j.crfs.2023.100653.
- D. Li, M. Yao, Y. Yang, B. Wang, D. Zhang and N. Zhang, Changes of structure and functional properties of rice protein in the fresh edible rice during the seed development, Food Sci. Hum. Wellness, 2023, 12, 1850–1860, DOI:10.1016/j.fshw.2023.02.049.
- J. Mijalković, N. Šekuljica, S. Jakovetić-Tanasković, P. Petrović, B. Balanč, M. Korićanac, A. Conić, J. Bakrač, V. Đorđević, B. Bugarski and Z. Knežević-Jugović, Ultrasound as green technology for the valorization of pumpkin leaves: intensification of protein recovery, Molecules, 2024, 29, e4027, DOI:10.3390/molecules29174027.
- L. Tian, X. You, S. Zhang, Z. Zhu, J. Yi and G. Jin, Enhancing functional properties and protein structure of almond protein isolate using high-power ultrasound treatment, Molecules, 2024, 29, e3590, DOI:10.3390/molecules29153590.
- M. Wang, S. Yang, N. Sun, T. Zhu, Z. Lian, S. Dai, J. Xu, X. Tong, H. Wang and L. Jiang, Soybean isolate protein complexes with different concentrations of inulin by ultrasound treatment: structural and functional properties, Ultrason. Sonochem., 2024, 105, e106864, DOI:10.1016/j.ultsonch.2024.106864.
- Q. Cui, A. Zhang, R. Li, X. Wang, L. Sun and L. Jiang, Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates, Food Biosci., 2020, 38, e100747, DOI:10.1016/j.fbio.2020.100747.
- Á. E. Rodríguez-Rivera, J. A. Ulloa, J. E. Urias-Silvas, J. C. Ramírez- Ramírez and J. A. Rsendiz-Vazquez, Physicochemical, techno-functional, biochemical and structural characterization of a protein isolate from groundnut (Arachis hypogaea L.) paste treated with high-intensity ultrasound, Food Chem., 2025, 464, 141848, DOI:10.1016/j.foodchem.2024.141848.
- H. Li, Y. Hu, X. Zhao, W. Wan, X. Du, B. Kong and X. Xia, Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad, LWT–Food Sci. Technol., 2021, 137, e110403, DOI:10.1016/j.lwt.2020.110403.
- F. Xue, C. Zhu, F. Liu, S. Wang, H. Liu and C. Li, Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate, J. Sci. Food Agric., 2018, 98, 5690–5699, DOI:10.1002/jsfa.9116.
- L. Jiang, J. Wang, Y. Li, Z. Wang, J. Liang, R. Wang, Y. Chen, W. Maa, B. Qi and M. Zhang, Effects of ultrasound on the structure and physical properties of black bean protein isolates, Food Res. Int., 2014, 62, 595–601, DOI:10.1016/j.foodres.2014.04.022.
- A. Amiri, P. Sharifian and N. Soltanizadeh, Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins, Int. J. Biol. Macromol., 2018, 111, 139–147, DOI:10.1016/j.ijbiomac.2017.12.167.
- G. Fu, M. Zhao, X. Wang, Z. Zheng, S. Shen, J. Yan, Q. Li, C. Gao, X. Dong, J. Xiao and L. Liu, Effect of ultrasound-assisted pH-shifting treatment on the physicochemical properties of melon seed protein, Ultrason. Sonochem., 2024, 110, e107039, DOI:10.1016/j.ultsonch.2024.107039.
- Z. Yu, L. Ma, B. Liu, W. Wang, Z. Shang, H. Dang and C. Liu, Improvement of foaming properties of ovalbumin: insights into the synergistic effect of preheating and high-intensity ultrasound on physicochemical properties and structure analysis, Ultrason. Sonochem., 2023, 101, e106672, DOI:10.1016/j.ultsonch.2023.10667.
- B. Dong, Z. Liu, D. Xu, C. Hou, N. Niu and G. Wang, Impact of multi-factor features on protein secondary structure prediction, Biomolecules, 2024, 14, e1155, DOI:10.3390/biom14091155.
- H. Chen, Z. Guo, Z. Wang, B. Yang, X. Chen, L. Wen, Q. Yang and J. Kan, Structural and physicochemical properties of the different ultrasound frequency modified Qingke protein, Ultrason. Sonochem., 2023, 94, e106338, DOI:10.1016/j.ultsonch.2023.106338.
- R. Tian, G. Zhu, J. Feng, B. Tian, Y. Zhang, L. Jiang and X. Sui, Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates, Ultrason. Sonochem., 2020, 68, e105202, DOI:10.1016/j.ultsonch.2020.10520.
- J. Wang, J. Wang, S. K. Vanga and V. Raghavan, Influence of high-intensity ultrasound on the IgE binding capacity of Act d 2 allergen, secondary structure, and in vitro digestibility of kiwifruit proteins, Ultrason. Sonochem., 2021, 71, e105409, DOI:10.1016/j.ultsonch.2020.105409.
- H. Cao, R. Sun, J. Shi, M. Li, X. Guan, J. Liu, K. Huang and Y. Zhang, Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates, Ultrason. Sonochem., 2021, 77, e105685, DOI:10.1016/j.ultsonch.2021.105685.
- M. M. Rahman and B. P. Lamsal, Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1457–1480, DOI:10.1111/1541-4337.12709.
- J. Jin, O. D. Okagu, A. E. A. Yagoub and C. C. Udenigwe, Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates, Ultrason. Sonochem., 2021, 70, e105348, DOI:10.1016/j.ultsonch.2020.10534.
- M. R. Pérez-Saucedo, J. A. Ulloa, P. Rosas-Ulloa, J. C. Ramírez-Ramírez, Y. Silva-Carrillo and B. E. Ulloa-Rangel, Caracterización tecno-funcional de un concentrado proteínico obtenido de la semilla de mango (Mangifera indica L.), Biotecnia, 2021, 23, 120–126, DOI:10.18633/biotecnia.v23i1.1306.
- E. Maltini, D. Torreggiani, E. Venir and G. Bertolo, Water activity and the preservation of plant foods, Food Chem., 2003, 82, 79–86, DOI:10.1016/S0308-8146(02)00581-2.
- Z. Zukryandry, S. Nurdjanah and M. Murhadi, Physicochemical characteristics, amylography profile and infrared spectroscopy of stored product insects (SPI)-attacked rice powder, Univers. J. Agric. Res., 2024, 12, 13–23, DOI:10.13189/ujar.2024.120102.
- S. Martini, R. Potter and M. K. Walsh, Optimizing the use of power ultrasound to decrease turbidity in whey protein suspensions, Int. Food Res. J., 2010, 43, 2444–2451, DOI:10.1016/j.foodres.2010.09.018.
- M. R. Zúniga-Salcedo, J. A. Ulloa, P. U. Bautista-Rosales, P. Rosas Ulloa, J. C. Ramírez-Ramírez, Y. Silva Carrillo, L. Gutiérrez and C. Hernandez, Effect of ultrasound treatment on physicochemical, functional and nutritional properties of a safflower (Carthamus tinctorius L.) protein isolate, Ital. J. Food Sci., 2019, 31, 591, DOI:10.14674/IJFS-1440.
- S. Lv, A. Taha, H. Hu, Q. Lu and S. Pan, Effects of ultrasonic-assisted extraction on the physicochemical properties of different walnut proteins, Molecules, 2019, 2, e4260, DOI:10.3390/molecules24234260.
- J. Cheng and L. Cui, Effects of high-intensity ultrasound on the structural, optical, mechanical and physicochemical properties of pea protein isolate-based edible film, Ultrason. Sonochem., 2021, 80, e105809, DOI:10.1016/j.ultsonch.2021.105809.
- N. A. Mir, C. S. Riar and S. Singh, Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed, Food Hydrocolloids, 2019, 96, 433–441, DOI:10.1016/j.foodhyd.2019.05.052.
- L. Grossmann and D. J. McClements, Current insights into protein solubility: a review of its importance for alternative proteins, Food Hydrocolloids, 2023, 137, e108416, DOI:10.1016/j.foodhyd.2022.108416.
- N. Guo, S. Ye, G. Zhou, Y. Zhang, F. Zhang, J. Xu, S. Pan, G. Zhu and Z. Wang, Effect of ultrasound treatment on interactions of whey protein isolate with rutin, Ultrason. Sonochem., 2023, 95, e106387, DOI:10.1016/j.ultsonch.2023.106387.
- D. Tawalbeh, W. A. N. Wan Ahmad and N. M. Sarbon, Effect of ultrasound pretreatment on the functional and bioactive properties of legumes protein hydrolysates and peptides: a comprehensive review, Food Rev. Int., 2022, 39, 5423–5445, DOI:10.1080/87559129.2022.206925.
- M. M. Rahman and B. P. Lamsal, Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1457–1480, DOI:10.1111/1541-4337.12709.
- K. Jahan, A. Ashfaq, K. Younis, O. Yousuf and R. U. Islam, A review of the effects of ultrasound-assisted extraction factors on plant protein yield and functional properties, J. Food Meas. Charact., 2022, 16, 2875–2883, DOI:10.1007/s11694-022-01390-6.
- S. M. Gadalkar and V. K. Rathod, Extraction of watermelon seed proteins with enhanced functional properties using ultrasound, Prep. Biochem. Biotechnol., 2020, 50, 133–140, DOI:10.1080/10826068.2019.1679173.
- B. Biswas and N. Sit, Effect of ultrasonication on functional properties of tamarind seed protein isolates, J. Food Sci. Technol., 2020, 57, 2070–2078, DOI:10.1007/s13197-020-04241-8.
- A. S. Chandran, P. Kashyap and M. Thakur, Effect of extraction methods on functional properties of plant proteins: a review, eFood, 2024, 5, e151, DOI:10.1002/efd2.151.
- J. Xu, S. Yan, J. Xu and B. Qi, Ultrasound-assisted modification of soybean protein isolate with l-histidine: relationship between structure and function, Ultrason. Sonochem., 2024, 107, e106934, DOI:10.1016/j.ultsonch.2024.106934.
- T. M. Ho, T. H. A. Le, A. Yan, B. R. Bhandari and N. Bansal, Foaming properties and foam structure of milk during storage, Food Res. Int., 2019, 116, 379–386, DOI:10.1016/j.foodres.2018.08.051.
- R. Morales, K. D. Martínez, V. M. P. Ruiz-Henestrosa and A. M. R. Pilosof, Modification of foaming properties of soy protein isolate by high ultrasound intensity: particle size effect, Ultrason. Sonochem., 2015, 26, 48–55, DOI:10.1016/j.ultsonch.2015.01.011.
- Z. Wang, H. Zhao, H. Tao, B. Yu, B. Cui and Y. Wang, Ultrasound improves the physicochemical and foam properties of whey protein microgel, Front. Nutr., 2023, 10, e1140737, DOI:10.3389/fnut.2023.1140737.
- H. Du, J. Zhang, S. Wang, A. Manyande and J. Wang, Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata duch.)-seed protein isolates, LWT–Food Sci. Technol., 2022, 155, e112952, DOI:10.1016/j.lwt.2021.112952.
- S. W. Janssen, L. Pouvreau and R. J. de Vries, Commercial plant protein isolates: the effect of insoluble particles on gelation properties, Food Hydrocolloids, 2024, 154, e110049, DOI:10.1016/j.foodhyd.2024.110049.
- M. Khalesi, K. Glenn-Davi, N. Mohammadi and R. J. FitzGerald, Key factors influencing gelation in plant vs. animal proteins: a comparative mini-review, Gels, 2024, 10, e575, DOI:10.3390/gels10090575.
- S. P. Bangar, O. J. Esua, N. Sharma and R. Thirumdas, Ultrasound-assisted modification of gelation properties of proteins: a review, J. Texture Stud., 2023, 53, 763–774, DOI:10.1111/jtxs.12674.
- A. K. Stone, D. Shi, C. P. Marinangeli, J. Carlin and M. T. Nickerson, Current review of faba bean protein fractionation and its value-added utilization in foods, Sustainable Food Proteins, 2024, 2, 101–124, DOI:10.1002/sfp2.1028.
- N. Sachdev, S. Goomer, L. R. Singh, V. M. Pathak, D. Aggarwal and R. K. Chowhan, Current status of millet seed proteins and its applications: a comprehensive review, Appl. Food Res., 2023, 3, e100288, DOI:10.1016/j.afres.2023.100288.
- G. Santos-Sánchez, B. Miralles, A. Brodkorb, D. Dupont, L. Egger and I. Recio, Current advances for in vitro protein digestibility, Front. Nutr., 2024, 11, e1404538, DOI:10.3389/fnut.2024.1404538.
- F. Aghababaei, D. J. McClement and M. Hadidi, Ultrasound processing for enhanced digestibility of plant proteins, Food Hydrocolloids, 2024, 155, e110188, DOI:10.1016/j.foodhyd.2024.110188.
- B. A. Narale, A. Mounika and A. Shanmugam, Modifications of physicochemical, functional, structural, and nutritional properties of a field bean protein isolate obtained using batch and continuous ultrasound systems, Sustainable Food Technol., 2024, 2, 470–484, 10.1039/d3fb00243h.
- S. M. Gadalkar and V. K. Rathod, Extraction of watermelon seed proteins with enhanced functional properties using ultrasound, Prep. Biochem. Biotech., 2020, 50, 133–140, DOI:10.1080/10826068.2019.1679173.
- R. J. Elias, S. S. Kellerby and E. A. Decker, Antioxidant activity of proteins and peptides, Crit. Rev. Food Sci. Nutr., 2008, 48, 430–441, DOI:10.1080/10408390701425615.
- P. Schober, C. Boer and L. A. Schwarte, Correlation coefficients: appropriate use and interpretation, Anesth. Analg., 2018, 126, 1763–1768, DOI:10.1213/ANE.0000000000002864.
- Q. T. Zhang, Z. C. Tu, H. Xiao, H. Wang, X. Q. Huang, G. X. Liu, Y. Shi, L. L. Fan and D. R. Lin, Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate, Food Bioprod. Process., 2014, 92, 30–37, DOI:10.1016/j.fbp.2013.07.006.
- R. Li, Q. Cui, G. Wang, J. Liu, S. Chen, X. Wang, X. Wang and L. Jiang, Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates, Food Hydrocolloids, 2019, 95, 349–357, DOI:10.1016/j.foodhyd.2019.04.030.
| This journal is © The Royal Society of Chemistry 2025 |



