Open Access Article
Open Access ArticleUltrasonication-assisted polyol-osmosed persimmon candies: effect of ultrasonication and drying techniques on product quality†
Ranjani
M.
a,
Shalini Gaur
Rudra
 *a,
Radha Mohan
Sharma
b,
Arun
T.
c,
Gautam
Chawla
d,
Sukanta
Dash
e and
Dinesh
Kumar
a
*a,
Radha Mohan
Sharma
b,
Arun
T.
c,
Gautam
Chawla
d,
Sukanta
Dash
e and
Dinesh
Kumar
a
aDivision of Food Science and Postharvest Technology, ICAR-Indian Agricultural Research Institute, New Delhi, India. E-mail: gaurshalini@gmail.com; shalini.rudra@icar.gov.in
bDivision of Fruits and Horticultural Technology, ICAR-Indian Agricultural Research Institute, New Delhi, India
cDivision of Agricultural Engineering, ICAR-Indian Agricultural Research Institute, New Delhi, India
dDivision of Nematology, ICAR-Indian Agricultural Research Institute, New Delhi, India
eDivision of Design of Experiments, ICAR-Indian Agricultural Statistics Research Institute, New Delhi, 110012, India
First published on 13th December 2024
Abstract
Persimmon is a widely cultivated fruit known for its sweet and rich flavour and nutrition. However, this climacteric seasonal fruit is underutilized and scarcely processed. Persimmon has good potential for conversion into dehydrated fruit candies. To override the high calorific value associated with sugar-osmosed fruit candies, xylitol and erythritol were used as osmotic agents. The long processing duration for osmotic dehydration (OD) was aimed to be shortened through ultrasonication (US) for enhancing the mass transfer rate and product quality. The moisture ratio, weight loss, solid gain with osmotic agents, sucrose, erythritol and xylitol at 30–60% concentrations and 50–70 °C were modelled using non-linear models. The logistic model was found to be most appropriate to describe the kinetics of the OD process. Rates of OD varied in the following order: xylitol > erythritol > sucrose. US significantly affected the rate of polyol-osmosed dehydration and greatly improved textural and sensory qualities. To offer convenience attribute to the fruit, the osmosed fruits were converted into dry candies using a tray dryer and infra-red dryer and compared for nutritional, textural and sensory attributes. SEM images and lower bite force confirmed that US-OD followed by IR drying yielded much better quality of candies than conventional methods for all sweeteners. The highest carotenoid retention, L* values, and ascorbic acid retention were recorded for persimmon candies osmosed in 60% erythritol. This study provides valuable insights into the application of ultrasonication and polyols for the effective utilization of nutritious persimmon and caters to the needs of faster processing times, higher nutrient retention and low calorific load in fruit candies.
Sustainability spotlightThis research work on the development of low-calorie candies from persimmon aligns to two SDG goals: Goal 3Ensure healthy lives and promoting well-being for all at all ages: and specifically relates to reduction of mortality from non-communicable diseases (NCDs). Calorie management in the age of sedentary lifestyle is a major challenge globally. Diabetes and healthy weight management account for more than 70% of world population concerns, with NCDs accounting for 63% of total deaths. In this respect, development of fruit candies with low calorific load and their comparison to conventional sugar-osmosed candies aim to provide fruit snacks with lesser concerns of calorific load. Goal 12Responsible production and consumption: Persimmon is emerging as a popular crop in temperate regions. However, it is a climacteric fruit that rapidly undergoes softening (few hours) soon after ripening initiates. This results in a glut situation in the market and high postharvest losses. Currently, these are hardly processed for any value-added product. Low-cost and simple processing techniques to process the ripe fruit on farm will prevent wastage of fruits and their preservation for use in off-season as a healthy snack. This activity will bolster farmers' incomes and provide incentives for traditional osmodehydration activity since conventional sugar-osmosed fruits are losing customers in the health-conscious society. The products thus prepared shall meet the requirements of modern-day consumers. |
Introduction
Persimmon (Diospyros kaki) is a highly nutritious fruit rich in vitamins; minerals such as potassium, sodium, iron, and calcium; and antioxidants.1 Its production area is increasing rapidly in India owing to the development of attractive, non-astringent varieties, low plant maintenance and increasing popularity amongst consumers. However, its seasonality, highly climacteric nature and rapid perishability mandate the development of low-cost, on-farm technologies to process it into shelf-stable forms. It is worth noting that despite their acknowledged health benefits and abundance of bioactive components, persimmons remain underutilized. Moreover, sufficient studies on their potential applications are lacking.2Dehydration is the most convenient, economic and widely used method to preserve fruits on farm while reducing their volume and weight, thereby reducing the cost of packaging, storage and transport. There is a growing consumer preference for dehydrated fruit as a healthy and convenient snack option with an extended shelf life.3 Osmotic dehydration (OD) using sucrose, fructose, and common salt is the most common practice for fruit and vegetable dehydration. OD is a form of non-thermal dehydration method that involves counter-current transfer of mass from a hypertonic solution into the fruit tissue, while moisture is extracted from the interior of the fruit into the hypertonic solution, thereby causing moisture reduction and solute gain by fruits. The conventional process however suffers from drawbacks of a highly time-consuming process and production of high-calorie or salt-rich, syrupy products. Considering the requirement of today's industry and consumer preferences, technological innovations such as an improved rate of mass transfer, higher retention of nutrients, lesser shrinkage, better mouthfeel in the OD process using more efficient methods such as ultrasound, promoting longer shelf life and waste utilization of persimmon are imminent. As dried persimmon is popular in Japan and Korea, the development of low-calorie polyol-based persimmon candies will create more ventures.
Persimmons are traditionally sundried in Japan and Korea, retaining valuable dietary fibers, minerals, and antioxidants, despite some loss of bioactive compounds. Drying enhances their flavor, color, and nutritional value by concentrating sugars and phytochemicals, while also extending the shelf life. Dried persimmons are lightweight, efficient for storage and shipping, and helpful to reduce food waste during surplus seasons. Traditional drying methods such as solar and air drying are cost-effective and eco-friendly but can be time-consuming and susceptible to contamination. Weather-dependent and prone to inconsistent results, they may also lead to nutrient loss. Modern techniques such as tray drying and infrared drying offer improved efficiency and quality control. Tray drying provides consistent results but can lead to fruit shrinkage and uneven drying. Infrared drying addresses these issues by offering faster, uniform heating that preserves color and nutrients while minimizing the drying time. Although initial costs are higher, it typically results in lower energy consumption and a more appealing texture for consumers.
Ultrasonication (US) has been employed by several researchers4–7 to reduce the processing time for mass transfer. Being residue-free, effective and environmentally friendly, various forms of US are used for pre-treatment, extraction and dispersion in food processing. Ultrasound-assisted osmotic dehydration (USOD) has a highly positive influence on the dehydration parameters for reducing the drying time, minimizing costs and improving quality. Bozkir et al.8 highlighted that USOD increased water reduction and sugar gain in persimmon samples, reducing the overall drying time by 46%. The reduction of drying time for pineapples, increased water diffusivity and sucrose incorporation have been reported through use of ultrasound.9
The problem of high calorific value in osmodehydrated fruits may be resolved by use of sugar-alcohols or polyols (xylitol, erythritol, maltitol, sorbitol, etc.) as osmotic agents, which have good dehydration capacity. Polyols have a lower caloric value (0.2 to 2.7 kcal g−1) than sucrose (4 kcal g−1). Erythritol (E968) and xylitol (E967) both are GRAS food additives and are routinely used in a number of food products such as toothpaste and chewing gums. Erythritol provides 0.2 kcal g−1 compared to sucrose that provides 4 kcal g−1, while xylitol provides 2.4 kcal g−1. Both of these have no after taste, rather erythritol enhances mouthfeel and effectively masks unwanted aftertastes, such as astringency. Its use is allowed up to 45% by weight in fruit leathers, candies, and fruit novelty snacks. Erythritol creates a notable cooling effect when dissolved, attributed to its high negative heat of solution. These characteristics make erythritol a valuable ingredient in food and beverage applications.10 As these polyols are not as humectant (like sorbitol) or expensive (maltitol), instead of more expensive sweeteners, less viscous erythritol (1.3 cP) and xylitol (2.3 cP) are used, which are preferred for value addition of persimmon at the farmgate level. Moreover, the consumption of products containing sugar alcohols does not induce an increase in blood glucose or insulin secretion, and thus, such products are recommended for diabetes11 or weight management. Mäkinen12 has recommended a safe daily dose of 20 to 70 g xylitol per day, while Wölnerhanssen et al.13 have recommended that the largest safe dose for a single bolus lies around 0.6–0.8 g kg−1 bw. Jacqz-Aigrain et al.14 reported good tolerance for rapid ingestion of up to 15 g (corresponding to 0.73 g kg−1 bw) of erythritol by 184 children aged four-six years. Lowe and Anthony15 have suggested recommendation of 71 to 143 mg kg−1 BW per day of xylitol to reach plaque-reducing therapeutic levels of xylitol after conducting clinical trials on dogs. EFSA Panel on Food Additives and Flavourings16 have recommended an acceptable daily intake (ADI) of 0.5g kg−1 body weight for erythritol considering protection for the immediate laxative effect as well as potential chronic effects, secondary to diarrhoea.
Cichowska-Bogusz et al.4 reported that sugar alcohols enabled shorter drying times for apples, and ultrasound during osmotic dehydration did not significantly affect sensory attributes. Though this domain is less explored, the area seems promising and has wide industrial acceptance since global market for candied and osmodehydrated fruits was valued at USD 42,159.0 million in 2022, with projections of growth at a CAGR of 4.03% through 2028, reaching 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 436 million USD. Consumption trends suggest a rising trajectory, with anticipated annual growth rates of 1.5% in volume and 3.5% in value from 2022 to 2030. India secured the third position in this market segment, contributing 57
436 million USD. Consumption trends suggest a rising trajectory, with anticipated annual growth rates of 1.5% in volume and 3.5% in value from 2022 to 2030. India secured the third position in this market segment, contributing 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons with a 7.1% share.17,18
000 tons with a 7.1% share.17,18
In the above context, this study aimed to determine the effect of temperature, osmotic agent (sucrose, erythritol, and xylitol), concentration and ultrasonication on the mass transfer kinetics during the osmodehydration of persimmon fruit ‘Fuyu’. Further the effect of tray drying and infrared drying on the quality of candy has been analyzed. This is the first report that deals with ultrasonication-assisted osmodehydration of persimmon with polyol solutions and their conversion to fruit candies.
Materials and methods
The red fleshed variety ‘Fuyu’ persimmon fruits were procured from Azadpur mandi, Delhi. Sucrose, erythritol (Herboveda, India), and xylitol (Herboveda, India) with >99% purity were obtained from the local market. Reagents and chemicals including ethanol, dichlorophenol indophenol dye, petroleum ether, lead acetate, potassium acetate, Fehling's solution, methylene blue, potassium oxalate, phenolphthalein, NaOH, sodium bicarbonate, sodium carbonate, metaphosphoric acid, ascorbic acid, Folin–Ciocalteu reagent, copper chloride, neocuproine, ammonium acetate buffer, sodium anhydrous, glucose, phenol, HCL, H2SO4, acetone, glutaraldehyde, sodium phosphate buffer, and phenol red were sourced from SRL, Mumbai, and Merck, India.Ultrasonication treatment
Ultrasonication treatments were administered using a probe sonicator assembly (PRO650, Labman Scientific Instruments, India) at a power rate of 10%. The probe-type sonicator was used at 10 watts equivalent to 0.6 kHz in the pulse mode (2 s Off and 2 s On). The time duration of 90 minutes was standardized based on preliminary trials and used for modelling and quality parameters.Preparation of fruits
Persimmon fruits were peeled, and cubes of 0.7 × 0.7 × 0.7 cm3 size were prepared using a molded cutter. These cubes were weighed and immersed in an osmotic solution with a sample-to-solution ratio of 1/10 (w/v).Osmotic dehydration process
The cubes were treated with different concentrations of sucrose, xylitol, and erythritol solutions ranging from 30% to 60%. Citric acid at 0.7% concentration was added to enhance the taste. Osmotic solutions were maintained at temperatures of 50, 60, and 70 °C using a water bath. Samples were withdrawn at time intervals of 0, 10, 20, 30, 40, 50, 60, 90, and 120 min for analysis. For USOD treatments, similar conditions were provided to persimmon cubes, with the application of ultrasound at 10% power rate. This power intensity was standardized to ensure effective treatment without compromising the structural integrity of the fruit tissue.Preparation of osmodehydrated persimmon candies
Peeled persimmon fruits were sliced into 0.7 cm thick slices. Osmotic dehydration was conducted for 90 min in sucrose (60%), erythritol (40%, 50%, and 60%), and xylitol (40%, 50%, and 60%) solutions containing 0.7% citric acid at 60 °C (Fig. 1). The osmotically dehydrated persimmon slices were further dried in a tray dryer (60 °C for 4 to 5 h) and an infrared dryer (25–30 °C for 120 to 140 min) until a constant moisture level was attained. The persimmon candies were then sealed in aluminum laminates until analysis within 15 days.Quality evaluation studies
Physico-chemical parameters including fruit firmness, moisture content, titratable acidity, total soluble solids (TSS), ascorbic acid, total carbohydrates, total sugars, reducing sugars, carotenoids, total phenolic content, antioxidant activity, and tannin content of fresh persimmon were evaluated. The candies obtained from the above-mentioned experiments were analyzed for moisture content, ascorbic acid, total carbohydrates, carotenoids, total phenolic content, antioxidant activity, and bite force. All processes and the quality measurements were conducted in triplicates.The moisture content of the cubes was determined in a hot air oven maintained at 60 °C for 8 h until attainment of constant weight. The moisture ratio was determined as follows:
 | (1) |
The firmness of persimmon fruits (N) was measured using a texture analyzer (TAxT2, Stable Micro Systems, UK) with a 2 mm cylindrical SS probe. The test speed was 0.5 mm s−1, respectively, until a distance of 10 mm. The bite force for the candied persimmon discs was measured using a texture analyzer with a knife blade attachment probe, and expressed in Newton (N).
Total soluble solids (TSS) was measured with a hand refractometer (Atago, Tokyo). The reducing sugars were estimated by titrating the sample filtrate against Fehling's solution and using methylene blue as an indicator. Similar to reducing sugars, total sugars were determined by acid hydrolysis, neutralization, and titration against Fehling's solution. Ascorbic acid was measured by titrating the sample against a dichloroindophenol solution and expressed in mg/100 g.19 The total phenolic content was determined using the Folin–Ciocalteu reagent, the absorbance was measured at 750 nm and expressed in milligram of Gallic Acid Equivalents (GAE) per 100 g. The antioxidant activity was expressed in μmol Trolox equivalents per gram. Total carbohydrates were estimated by the phenol-sulfuric acid method, and the absorbance was measured at 490 nm against a blank. The titratable acidity was determined by titrating the sample with 0.1 N NaOH using a phenolphthalein indicator. Total Carotenoids were measured using acetone and petroleum ether extraction, with absorbance measurement at 452 nm. Scanning Electron Microscopy (SEM): samples were fixed, dehydrated, and observed at 500–10000× magnification using an SEM machine (VEGA3, TESCAN).
The browning index of candies was measured by extracting 1 g of sample in 100 mL ethanol (67%) and determining absorbance at 420 nm. The colour values were measured using the rapid tables software and expressed as L* (lightness), a* (redness), and b* (yellowness).
 | (2) |
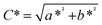 | (3) |
Sensory evaluation
The persimmon candies were evaluated using a 9-point hedonic scale by a panel of 20 members comprising 10 females and 10 males aged between 21 and 26 years. The panel was apprised and informed about the samples prior to the evaluation.Data analysis
At each time interval during osmodehydration (OD) up to 120 min, three cubes were taken out and the surface of the OD cubes was cleaned gently with the help of blotting paper. The samples were weighed using a digital balance (±0.01 g). Water loss (WL) and solid gain (SG) were determined according to eqn (4) and (5).7 | (4) |
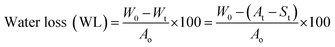 | (5) |
The goodness of fit of models was based on the degree of fit as determined using variance explained, coefficient of determination (R2), root mean square error (RMSE), mean absolute error (MAE), mean absolute percentage error (MAPE) and mean squared error (MSE) based on comparison of observed and predicted MR as per models. The higher value of coefficient of determination (R2) and the lower root mean square error (RMSE) indicate the accuracy of the fit.20
The Arrhenius equation introduces an exponential factor that demonstrates how a reduction in activation energy leads to a significant exponential increase in the rate constant of a reaction. As the rate of a reaction is directly linked to its rate constant, this exponential growth also applies to the reaction rate itself.
The Arrhenius equation is as follows:
| K = A × exp−Ea/RT | (6) |
The experimental data for osmodehydration and candying of persimmon were organized using a full factorial design at a significance level of p = 0.05. Statistical analysis was performed using the SAS (Statistical Analysis System) software. Each treatment was replicated to ensure the reliability and accuracy of the results.
Results and discussion
Composition of persimmon fruit var. Fuyu
The persimmon fruits of Fuyu variety were bright orange-reddish in colour and weighed between 200 and 300 g. These attractive fruits were firm fleshed (158–203 N) and had shelf-life of 6 weeks in refrigerated storage and approximately 7 days under ambient conditions. Once the fruit entered the climacteric phase, it underwent rapid softening, leading to the development of unmarketable fruits with a jelly-like texture within just a day.The moisture content of the fruits was 82.62%. Mature fresh persimmon fruits were rich in sugars (16.17%) with a reducing sugar content of 6.9% and total soluble solids of 21.3 °Brix. The water activity samples were observed as 0.56. The TSS varied considerably with maturity with values ranging from 12 to 23 °B2. Our findings are close to those of Smrke21 who reported persimmon (Fuyu cv) to have a carbohydrate content of 14.30%, primarily in the form of glucose (7.50 g per 100 g) and fructose (6.40 g per 100 g) and a calorific value of persimmon between 102.90 and 274.40 kcal. The fruits had low acidity (0.34%), which gave a sweet bland taste on consumption. Persimmon was found rich in total phenolics (163.09 ± 55.88 mg/100 g) and antioxidants (907.00 ± 0.32 μmol TEAC/100 g). Persimmon fruits were found to have 8.3 μg g−1 total carotenoids (Table 1), while the tannin content was below the measurable range. β-Carotene has been reported to be most abundant in ripe persimmons, especially in the peel of the Hana-Fuyu variety (8.75 mg kg−1 (ref. 22)). González et al.23 reported Rojo Brillante variety of persimmon to contain β-carotene and other carotenoids, including β-cryptoxanthin, violaxanthin, zeaxanthin, and lutein. Ascorbic acid in the mature Fuyu persimmons was found to be 14.34 mg/100 g in concurrence to Preethika et al.2 who has reported persimmon fruits to contain vitamin C from 7.5 to 29.6 mg/100 g. Persimmons are known to possess high antioxidant capacity over other fruits due to their rich content of phenolic compounds, carotenoids, and water-soluble vitamins such as vitamin C. The fruits were found to have a total phenolic content of 163.09 mg GAE/100 g. Giordani et al.24 have reported a similar amount of vitamin C, total phenolic compounds, and flavonoids in Fuyu peel as 139.91, 169.36, and 60.79 mg%, respectively. They identified the polyphenols to be catechin, epigallocatechin, p-coumaric acid, epicatechin, and proanthocyanidin. The astringency of persimmons varies among different cultivars based on their tannin content. The higher the tannin content, the more astringent the fruit is. The astringency of persimmons is also influenced by their ripeness. Young fruits are rich in proanthocyanidins, which contribute to their astringent taste. However, certain cultivars like ‘Fuyu’ have low or undetectable tannin content, making them non-astringent when ripe. The tannin concentration was reported by Altuntaş et al.25 in Fuyu as 9–27%. In the studied ripened ‘Fuyu’ variety of persimmon fruits, tannin content was not detectable.
| Attribute | Value |
|---|---|
| a Different superscripts indicate difference in the level of significance (p < 0.05). | |
| Moisture content (%) | 82.62a ± 1.42 |
| Titratable acidity (%) | 0.34a ± 0.07 |
| TSS (°Brix) | 21.3a ± 0.17 |
| Ascorbic acid (mg/100 g) | 14.95a ± 0.01 |
| Reducing sugars (%) | 6.93a ± 0.64 |
| Total carbohydrates (%) | 18.51b ± 0.03 |
| Total sugars (%) | 16.17a ± 0.16 |
| Carotenoids (μg per g) | 17.85a ± 0.03 |
| Total phenols (mg GAE/100 g) | 163.09a ± 55.88 |
| Antioxidant activity (μmol TEAC per g) | 9.07a ± 0.32 |
Osmodehydration with polyols
The impact of ultrasound during osmotic dehydration on water loss (WL) and solid gain (SG) varied depending on the type of osmoactive substance used (erythritol, sorbitol, and sucrose), with the most significant effects observed in sucrose. However, the highest dehydration, along with a simultaneous increase in SG, was observed when using sugar alcohols. In our study, USOD resulted in a reduction in OD duration only for samples treated with sugar alcohols. Our findings are in variation with Salehi et al.7 who reported that for the USOD, the apple slices gained 12.5% of sucrose after 60 min and in NSOD, the apple slices gained 9.1% of sucrose in the 60 min.Osmotic dehydration conducted in low-molecular-weight polyols presents an advantageous blend of processes that facilitate a higher osmotic driving force in osmo-dehydrated products without the presence of simple sugars, all while conserving energy—a boon for sustainable technologies. The preference for low-molecular-mass saccharides such as sorbitol, fructose, and glucose lies in their swift penetration due to high velocity.26 Cichowska et al.27 found that OD in a 40% solution concentration of erythritol and xylitol surpassed the effectiveness of the dehydration process in a sucrose solution, attributed to the lower molecular weights of erythritol (122.12 g mol−1) and xylitol (152.15 g mol−1). Huang et al.28 also proposed that sugar alcohols with lower molecular weights, such as xylitol, can intensify the osmotic pressure and enhance the mass transfer.
The superiority of xylitol and erythritol solutions in generating larger osmotic effects, compared to sucrose, stems from the significantly higher molecular weight of sucrose and the ensuing lower osmotic pressure.27 Substances with higher molecular weights exhibit lower osmotic pressures, resulting in diminished kinetic parameters and reduced penetration into the material.29 The strategic use of xylitol as an osmotic substance during dehydration of apples notably lowered the water activity in dried apple samples.4
Effect of ultrasonication on osmodehydration process
The application of ultrasound during the process of OD greatly helped in maximizing the rate of water loss and solid gain. Ultrasonication had significant effects on the rate and extent of mass transfer during OD in all sweeteners. Fig. S1–S3† depict the decline in moisture ratio during the osmodehydration of persimmon cubes in non-sonicated (NSOD) and (b) ultrasonication-assisted OD processes (USOD). A steeper decline is visible in most USOD samples, especially in the case of xylitol and erythritol. As the concentration of sweeteners increased, the MR value decreased. As the concentration of sucrose increased from 30 to 60%, the MR values decreased from 1 to 0.83 following 120 min of OD. Moreover, the effect of increased sucrose concentration on lowering the MR became more evident at higher temperatures. Similarly, for xylitol and erythritol, the MR values decreased from 1–0.76 to 1–0.79, respectively, as the concentration enhanced from 30 to 60%. As the temperature increased, the slope of curves increased. As anticipated, the lower MR (0.7073–0.77) was recorded at 70 °C with USOD at higher concentrations of xylitol (50–60%) followed by sucrose and erythritol. Moisture removal was directly proportional to temperature during USOD in sucrose solutions, with a faster mass transfer (moisture removal) and reduction in drying time. Xylitol and erythritol showed the effects of increased temperature during USOD mainly during the first 30–60 min.With ultrasonication, lower MR values could be obtained. A clear distinction between the final MR of persimmon cubes processed with USOD (for 120 min at 60 °C) was observed for 60% sucrose (0.78), followed by 60% xylitol (0.81) and 60% erythritol (0.84). For persimmon cubes to attain an MR of 0.4, higher time was recorded in erythritol dipped cubes (90 min) compared to sucrose (61 min) and xylitol (57.5 min) at 60 °C. Comparatively lower ranges of MR from 0.74 to 0.79 were recorded for USOD of persimmon cubes immersed in xylitol solutions at all concentrations (30–60%) over erythritol and sucrose. The lowest MR was recorded for sonicated persimmon cubes immersed in 50% xylitol solutions at 70 °C (0.7073). Significantly lower MR was recorded for USOD treatments of persimmon cubes. Sucrose USOD cubes showed a lower MR than erythritol cubes, in general.
Similarly, in erythritol solutions, the rate of decrease in MR increased with the use of ultrasonication at all concentrations. Lower MR values were realized in erythritol solutions of same concentration upon application of US at all temperatures. This effect of US in decreasing MR effectively can be clearly seen from Fig. S1–S3† and 2. The final MR of persimmon cubes was also lesser in USOD treatment for all the three sweeteners.
Mathematical modeling of the moisture ratio
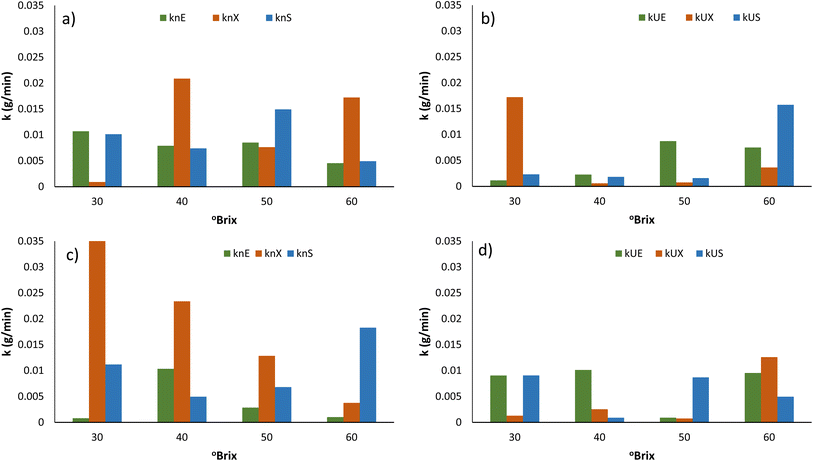
The experimental moisture data obtained during OD was fit to non-linear models. Though the Henderson-Pabis and simplified Fick model showed a reasonable degree of fit, the best prediction of our experimental data was by a logistic model. The use of logarithmic transformation and complex models such as Henderson-Pabis has however been discouraged by researchers.30 A logistic model with the highest R2 and the lowest RMSE, MAE, MAPE and MSE values exhibited the highest goodness of fit. The logistic model is among the most popular models used by researchers to explain the sigmoidal pattern of data. Logistic regression's dependent variable obeys the ‘Bernoulli distribution’ and the estimation is based on ‘maximum likelihood method’. The logistic regression does not evaluate the coefficient of determination (R2), as observed in linear regression. Instead, the model's fitness is assessed through a concordance, which can be seen clearly in the model fit curves. In logistic-type regression, the logit transformation shows the impact of independent variables on the variation of the dependent variable's natural logarithm of the odds.The parameters of logistic models are B, A and k, indicating the intercepts and drying rate constant. The rate parameter, k, holds most significance to compare the effect of temperature, ultrasonication and sweetener on the OD process. In general, the k values were higher for the USOD process than for the OD process for all three sweeteners. In particular, for xylitol and sucrose at 60 °C, the USOD k values were higher over the NSOD process by 22.24% and 57.26%, and at 70 °C, the rates of USOD treatments were higher by 247% and 37.89%, respectively.
As expected, the k values were higher for xylitol (overall average 0.0372 min−1) followed by sucrose (0.0225 min−1) and erythritol (0.0149 min−1). With the increase in the concentration of sucrose, increased k values were obtained for both USOD and NSOD. A 0.8-fold increase was recorded for the averaged k values for increase in sucrose from 50 to 70%. For USOD samples, the increase in the concentration of xylitol from 50 to 60% leads to an increase in the k value by 0.68–fold, while in NSOD, 0.92-fold. In NSOD treatment with erythritol, as the concentration was increased from 50 to 60%, a 5.88-fold increase in k (rate of mass transfer) was observed (Fig. 2). At 70 °C, xylitol showed a 2.47-fold increase in the k value for the non-sonicated sample compared to the ultrasonicated sample. Similarly, at 60 °C, erythritol exhibited a 1.975-fold increase in the k value for the NSOD sample over the ultrasonicated sample. Overall, the k values increased 0.625 folds as the concentrations of sweeteners increased from 30% to 60%. As expected, USOD treatment showed 1.36 folds increase in k values over the same concentration range.
The quantifiable effect was also evident from the rate parameters of the mathematical model (Logistic) used to predict the variation of moisture ratio with time for each sweetener: concentration and temperature combination. Overall, the k values were 0.48 folds for USOD in sucrose, 1.025 folds for erythritol and 0.47 folds for xylitol compared to the NSOD process. In particular for 60% xylitol at 70 °C, the k values of USOD were 11.84 times higher than that of the NSOD process. For sucrose, the k value was 25% higher under same conditions. For erythritol, the k USOD was 1.8 times that of NSOD. The USOD process was found to be more effective for accelerating the OD process for sucrose and erythritol than xylitol. However, USOD in xylitol at 70 °C was found beneficial for enhancing the rate of OD at 30–50%. These findings are in agreement with those of Fadil et al.6 and Salehi et al.,7 who have reported increased mass transfer during OD and reduction in the drying time of kiwifruit and apple.
Under USOD, the highest k values among all sweeteners were observed for 30% xylitol solutions maintained at 60 °C. Xu et al.31 reported that strawberry slices subjected to ultrasonic treatment with different frequency modes exhibited significantly higher WL and SG values (p < 0.05). They attributed this to the “sponge effect” generated by ultrasonic treatment, which led to the formation of micro pores in the strawberry tissue and facilitated the discharge of water. They concluded that ultrasonic pretreatment, particularly with dual-frequency ultrasound, could enhance the water removal during the osmotic dehydration of strawberry slices, offering potential benefits for improving the dehydration process and preserving the quality of the fruit. Li et al.32 reported that Sanhua plum treated with ultrasound-assisted osmotic dehydration showed reduced drying time, improved drying rate, and increased effective water diffusivity.
Briki et al.33 applied a modified logistic model for modelling the drying kinetics of pomegranate arils in an infrared and tray dryer. Chin et al.34 have shown that for convective drying of kiwi slices at different temperatures, the best fit was obtained using simplified Fick's second law of diffusion.
The coefficient of determination (R2) was in the range between 0.998 and 0.999, RMSE from 0.0052 to 0.0255 and χ2 ranged from 9.275 × 10−5 to 6.72 × 10−4. The correlation coefficient R2 was found to be 0.67 between the predicted and experimental values of effective diffusion coefficient. The effective diffusion coefficient was empirically correlated with the concentration and temperature using the Arrhenius-type equation. The Arrhenius model was applied to determine the temperature dependence of k for MR for each sweetener for USOD treatment data. The activation energy was found to be 0.021 J mol−1 (xylitol); 0.304 J mol−1 (erythritol) and 0.358 J mol−1 (sucrose). Lower activation energy facilitates a quicker transition to the reaction's transition state, resulting in a more rapid progression than the reactions with a higher activation energy. The Arrhenius model has been applied by several researchers35,36 for OD of fruits such as guava, apricots, aonla and apples in sucrose. This is however the first report on polyols under ultrasonicated conditions.
Effect of ultrasonication on the quality characteristics of candied persimmon
Though the difference was non-significant, higher values of browning index were recorded for NSOD candies (0.3948) than those for USOD (0.3857). Rahaman et al.39 reported a significant decrease in lightness, a*, b* and chroma parameters of USOD plum, which were explained by increased Maillard reactions. The higher L* and NEB may be attributed to the release of bound sugars contributing to browning reactions during dehydration.
The shrinkage of persimmon slices was much lower in USOD samples (2.72%) than the 8.9% in NSOD candies, thus making the USOD persimmon candy more attractive in appearance. Similar findings have been reported by Prithani and Dash40 for US-assisted OD kiwifruit.
| Sweeteners | Concentration | Drying technique | NSOD | USOD | ||||
|---|---|---|---|---|---|---|---|---|
| Carotenoids (μg per g) | Ascorbic acid (mg/100 g db) | Antioxidant activity (μmol TEAC per g) | Carotenoids (μg per g) | Ascorbic acid (mg/100 g db) | Antioxidant activity (μmol TEAC per g) | |||
| a Different letters across individual columns show significant differences (p < 0.05) in the mean values of triplicates. | ||||||||
| Sucrose | 60% | Tray | 0.35 ± 0.04a | 178.40 ± 0.07d | 2.96 ± 0.12c | 0.23 ± 0.01a | 120.37 ± 0.27f | 3.35 ± 0.03b |
| Infrared | 1.78 ± 0.02f | 73.40 ± 0.40b | 2.36 ± 0.06c | 0.37 ± 0.07b | 24.29 ± 0.29a | 1.31 ± 0.03d | ||
| Erythritol | 50% | Tray | 0.77 ± 0.03e | 200.19 ± 0.47e | 1.31 ± 0.08b | 0.37 ± 0.09b | 78.47 ± 0.60d | 2.78 ± 0.10f |
| Infrared | 0.75 ± 0.01e | 36.86 ± 0.14a | 1.21 ± 0.06b | 0.21 ± 0.08a | 60.42 ± 0.44c | 4.25 ± 0.48f | ||
| 60% | Tray | 0.70 ± 0.04d | 66.94 ± 0.93b | 5.35 ± 0.10e | 0.90 ± 0.03e | 105.88 ± 0.21e | 7.58 ± 0.09a | |
| Infrared | 0.64 ± 0.01c | 60.69 ± 0.35b | 1.26 ± 0.02b | 0.32 ± 0.03b | 36.78 ± 0.22a | 0.26 ± 0.03c | ||
| Xylitol | 50% | Tray | 1.21 ± 0.04f | 179.25 ± 0.43d | 0.22 ± 0.04a | 0.48 ± 0.01c | 101.08 ± 0.54e | 0.57 ± 0.06f |
| Infrared | 0.54 ± 0.04b | 40.10 ± 0.12a | 0.20 ± 0.01a | 0.37 ± 0.02b | 49.07 ± 0.80b | 1.45 ± 0.01f | ||
| 60% | Tray | 0.60 ± 0.02c | 99.34 ± 0.57c | 8.83 ± 0.17f | 0.57 ± 0.03d | 128.34 ± 0.46f | 0.16 ± 0.03a | |
| Infrared | 0.52 ± 0.04b | 86.84 ± 0.15c | 3.15 ± 0.04d | 0.83 ± 0.05e | 73.00 ± 0.06d | 0.47 ± 0.03f | ||
Ultrasonication caused a reduction in the total phenolic content of persimmon candies. NSOD candies contained higher phenolics (118.95 mg/100 g) than USOD candies (80.83 mg/100 g). However, the antioxidant activity was not affected by US treatment. Non-significant but higher antioxidant values were estimated for the NSOD samples (27.739 μmol TEAC per g) over USOD candies (23.307 μmol TEAC per gram).
Chandra et al.41 also reported that the sample pre-treated with 33 kHz ultrasound and osmodehydrated with 35 °Brix sucrose resulted in a lower moisture content (<12% w.b.) and water activity (<0.41), higher phenolic content (88.5 mg GAE mL−1), higher β-carotene content (184.54 mg g−1) and antioxidant capacity (48.3%), and preserved color after drying. They recommended the pre-treatment of papaya slices with ultrasound and osmotic dehydration before drying. Li et al.34 also reported that Sanhua plum with low ultrasound intensity treatment exhibited a higher antioxidant capacity and total phenolic retention.
Overall, the ascorbic acid content in NSOD candies was higher (126.20 mg/100 g) than USOD candies (110.95 mg/100 g; Table 2). The highest ascorbic acid retention of 49.62% was found for the 60% erythritol USOD candied persimmon slices and dried in an IR dryer (60E USIRD), and the remarkable ascorbic acid retention suggests that erythritol may play a protective role. Erythritol is known for its low hygroscopicity and ability to stabilize the moisture content during drying, which can help maintain the integrity of heat-sensitive compounds such as ascorbic acid. This contrasts with traditional sugar drying methods, where higher sugar concentrations can create a more hostile environment for nutrient retention; followed by 60% sucrose USIRD (43.24%) and 50% xylitol USIRD (39.94%), ultasonication while effective for certain textural improvements may lead to nutrient loss due to the intense energy and pressure changes that can destabilize certain vitamins.
Ultrasonication had significant effects on lowering the bite force of candied persimmon slices. The normal bite force for chocolate candies ranges from 191 to 275 N. The lowest bite force was recorded for 50× USIRD (109 N) and the highest for 60S TD (478.43 N; Table 3). The 60S TD sample indicates that traditional drying methods without ultrasonication can lead to denser and tougher textures. This aligns with the findings of Li et al.,34 which showed that ultrasound treatment effectively softens fruit by altering its physical properties, thereby enhancing consumer acceptance. In the 50× USIRD samples, ultrasound treatment not only improved the texture but also resulted in a more appealing product with a desirable mouthfeel. Chandra et al.43 also reported more desirable texture (hardness) of the USOD papaya from an industrial standpoint, which was further validated using SEM micrographs that showed a more porous structure.
| Sweeteners | Concentration | Drying technique | Non-ultrasonicated | Sonicated | ||
|---|---|---|---|---|---|---|
| Bite force (N) | Overall acceptability | Bite force (N) | Overall acceptability | |||
| a Different letters across individual columns show significant differences (p < 0.05) in the mean values of triplicates. | ||||||
| Sucrose | 60% | Tray | 478.43 ± 7.82d | 7.91 ± 0.08d | 117.15 ± 3.55a | 8.00 ± 0.04b |
| Infrared | 262.72 ± 8.87b | 7.89 ± 0.09c | 226.87 ± 9.42b | 8.00 ± 0.05b | ||
| Erythritol | 50% | Tray | 293.1 ± 3.34b | 7.97 ± 0.04d | 213.52 ± 3.15b | 8.26 ± 0.06d |
| Infrared | 323.77 ± 9.28c | 7.84 ± 0.01c | 244.01 ± 5.16b | 8.35 ± 0.07e | ||
| 60% | Tray | 349.98 ± 6.05c | 7.84 ± 0.07c | 208.71 ± 1.99b | 8.15 ± 0.09c | |
| Infrared | 293.5 ± 4.29b | 7.66 ± 0.08b | 208.59 ± 8.58b | 8.34 ± 0.04e | ||
| Xylitol | 50% | Tray | 447.35 ± 2.47c | 7.93 ± 0.07d | 334.01 ± 15.84c | 8.05 ± 0.04b |
| Infrared | 315 ± 1.69a | 8.41 ± 0.04e | 109.64 ± 3.64a | 8.69 ± 0.05f | ||
| 60% | Tray | 435.41 ± 6.68b | 7.34 ± 0.02a | 242.53 ± 10.69c | 7.75 ± 0.08a | |
| Infrared | 437.84 ± 6.76d | 7.16 ± 0.04a | 213.36 ± 3.28b | 7.89 ± 0.09a | ||
Regarding the bite force, the substantial reduction observed in the ultrasonicated samples can be explained by the effect of ultrasonication on the cellular structure of the persimmons. Ultrasonication generates high-frequency sound waves that create cavitation bubbles in the liquid surrounding the fruit. When these bubbles collapse, they produce micro-jets that can disrupt the cell walls of the fruit. This breakdown in structure can lead to a softer texture, making the candies easier to chew. Overall, the importance of selecting appropriate drying and treatment methods to optimize both nutritional content and textural characteristics in fruit processing ultimately enhances the quality and marketability of the final product.
The better sensory experience of USOD could be due to the lesser force required for mastication and better release of flavour due to disrupted tissue structure caused by ultrasonication. Higher total solid retention in USOD candies might also enhance the flavour and taste appeal of consumers. Sensory evaluation results clearly indicated significantly higher overall acceptability for USOD candies (7.35) over NSOD (6.98; Table 3). Thus, ultrasonication played an important role in enhancing the mouthfeel and appearance, ultimately resulting in very good overall acceptability. The highest overall acceptability scores were those of erythritol followed by xylitol and sucrose.
Conclusion
Our study on the composition of the Fuyu persimmon variety revealed its rich nutritional profile, with high moisture content (82.62%), sugars (16.17%), total soluble solids (21.3 °Brix), and notable levels of carotenoids and phenolics. The effect of ultrasonication on osmodehydration showed significant improvements in water loss, solid gain, and drying kinetics across different sweeteners, particularly xylitol and erythritol. Ultrasonication-assisted osmodehydration (USOD) enhanced the quality of candied persimmons, resulting in better color retention, texture, and overall sensory acceptability compared to non-sonicated samples. The logistic model provided the best fit for predicting moisture ratio changes during drying. Overall, ultrasonication proved to be a beneficial pre-treatment for improving the efficiency and quality of persimmon osmodehydration. The study conclusively demonstrated that ultrasonication, when combined with specific sweeteners and infra-red dehydration technique, had a substantial impact on the physical, chemical, and sensory attributes of persimmon candies. The findings suggest that ultrasonication holds great potential for the valorization of persimmons, opening avenues for further research in optimizing process parameters and exploring its applicability in other fruits and food products. Based on the study, further research avenues could include investigating the scalability of ultrasonication-assisted osmodehydration for industrial applications. Additionally, exploring the economic feasibility and environmental impact of this novel technique would be valuable for commercial adoption.Abbreviations
| OD | Osmodehydration |
| US | Ultrasonication |
| MR | Moisture ratio |
| NSOD | Non-sonicated osmodehydration |
| USOD | Ultrasonication-assisted osmodehydration |
Data availability
The data shall be made available upon reasonable request.Conflicts of interest
The authors report no conflict of interest.Acknowledgements
This work is based on master's dissertation research. The authors received no funding for this research. The authors acknowledge ICAR-Indian Agricultural Research Institute, New Delhi for providing the necessary resources and support for conducting this research. We acknowledge The Graduate School, IARI for providing fellowship support to Ranjani M.References
- M. S. Butt, M. T. Sultan, M. Aziz, A. Naz, W. Ahmed, N. Kumar and M. Imran, Excli J., 2015, 14, 542–561 Search PubMed.
- P. Murali, H. Hamid, R. Shams and A. Dar, Food Chem. Adv., 2023, 3, 100431 CrossRef.
- P. Tarancón, P. Fernández-Serrano and C. Besada, Food Res. Int., 2021, 140, 110000 CrossRef PubMed.
- J. Cichowska-Bogusz, A. Figiel, A. A. Carbonell-Barrachina, M. Pasławska and D. Witrowa-Rajchert, Molecules, 2020, 25(5), 1078 CrossRef CAS PubMed.
- J. Kroehnke, J. Szadzińska, E. Radziejewska-Kubzdela, R. Biegańska-Marecik, G. Musielak and D. Mierzwa, Ultrason. Sonochem., 2021, 71, 105377 CrossRef CAS PubMed.
- I. N. Fadil, W. M. F. Wan Mokhtar, W. A. F. Wan Mohamad and I. Ismail, J. Agrobiotechnol., 2021, 12(1S), 74–82 CrossRef.
- F. Salehi, R. Cheraghi and M. Rasouli, Sci. Rep., 2022, 12(1), 15392 CrossRef CAS PubMed.
- H. Bozkir, A. R. RaymanErgün, E. Serdar, G. Metin and T. Baysal, Ultrason. Sonochem., 2019, 54, 135–141 CrossRef CAS PubMed.
- F. A. N. Fernandes, M. I. Gallão and S. Rodrigues, LWT--Food Sci. Technol., 2008, 41(4), 604–610 CrossRef CAS.
- T. A. Mazi and K. L. Stanhope, Nutrients, 2023, 15(1), 204 CrossRef CAS PubMed.
- M. Grembecka, J. Grembecki and J. Szpunar, J. Diabetes Res., 2015, 260805 Search PubMed.
- K. K. Mäkinen, Int. J. Dent., 2016, 5967907 Search PubMed.
- B. K. Wölnerhanssen, A. C. Meyer-Gerspach, C. Beglinger and M. S. Islam, Crit. Rev. Food Sci. Nutr., 2019, 59, 1–13 CrossRef PubMed.
- E. Jacqz-Aigrain, B. Kassai, C. Cornu, J.-M. Cazaubiel, B. Housez, M. Cazaubiel, J.-M. Prével, M. Bell, A. Boileau and P. De Cock, Eur. J. Clin. Nutr., 2015, 69(6), 746–751 CrossRef CAS PubMed.
- C. Lowe and J. Anthony, Can. Vet. J., 2020, 61(1), 63–68 CAS.
- EFSA FAF Panel, M. Younes, G. Aquilina, L. Castle, G. Degen, K.-H. Engel, P. J. Fowler, M. J. Frutos Fernandez, P. Fürst, U. Gundert-Remy, R. Gürtler, T. Husøy, M. Manco, W. Mennes, P. Moldeus, S. Passamonti, R. Shah, I. Waalkens-Berendsen, M. Wright, et al.EFSA J., 2023, 21(12), e8430 Search PubMed.
- https://www.indexbox.io/, accessed on July 2, 2024.
- https://www.marketreportsworld.com/, accessed on July, 2, 2024.
- S. Ranganna, Handbook of Analysis and Quality Control for Fruit and Vegetable Products, Tata McGraw-Hill Publishing Company Limited, New Delhi, 1999 Search PubMed.
- N. Nurhasmanina and N. Norhadi, J. Environ. Manage., 2020, 256, 109967 CrossRef PubMed.
- T. Smrke, M. Persic, R. Veberic, H. Sircelj and J. Jakopic, Sci. Rep., 2019, 9(1), 19069 CrossRef CAS PubMed.
- R. Veberic, J. Jurhar, M. Mikulic-Petkovsek, F. Stampar and V. Schmitzer, Food Chem., 2010, 119, 477–483 CrossRef CAS.
- C. M. González, A. L. García, E. Llorca, I. Hernando, P. Atienzar and A. Bermejo, et al. , Lebensm.-Wiss.-Technol., 2021, 142, 111007 CrossRef.
- E. Giordani, S. Doumett, S. Nin and M. Del Bubba, Food Res. Int., 2011, 44(7), 1752–1767 CrossRef CAS.
- E. Altuntaş, E. Özgöz and Ö. F. Taşer, J. Food Eng., 2011, 71(1), 37–43 CrossRef.
- U. D. Chavan and R. Amarowicz, J. Food Res., 2012, 1(2), 202–209 Search PubMed.
- J. Cichowska, J. Żubernik, J. Czyżewski, H. Kowalska and D. Witrowa-Rajchert, Molecules, 2018, 23(2), 446 CrossRef PubMed.
- Y. W. Huang, M. Zhang, R. H. Ju, C. L. Law, D. C. Fan, G. V. Semenov and Z. J. Luo, Drying Technol., 2023, 41(10), 1636–1650 CrossRef CAS.
- N. Phisut, Int. Food Res. J., 2012, 19(1), 7–18 CAS.
- S. Buzrul, Processes, 2022, 10(1), 118 CrossRef.
- B. Xu, J. Chen, E. Sylvain Tiliwa, W. Yan, S. M. RoknulAzam, J. Yuan, B. Wei, C. Zhou and H. Ma, Ultrason. Sonochem., 2021, 78, 105714 CrossRef CAS PubMed.
- L. Li, Y. Yu, Y. Xu, J. Wu, Y. Yu, J. Peng, K. An, B. Zou and W. Yang, Lebensm.-Wiss.-Technol., 2021, 138, 110653 CrossRef CAS.
- S. Briki, B. Zitouni, B. Bechaa and M. Amiali, Heat Mass Transfer, 2019, 55(11), 3189–3199 CrossRef CAS.
- S. K. Chin, E. S. Siew and W. L. Soon, Int. Food Res. J., 2015, 22, 2188–2195 CAS.
- F. R. Assis, R. M. Morais and A. M. Morais, J. Food Process. Preserv., 2017, 41(3), e12895 CrossRef.
- M. C. Giannakourou, K. P. Saltaouras and N. G. Stoforos, J. Food Sci., 2021, 86(6), 2172–2193 CrossRef CAS PubMed.
- M. Nowacka, U. Tylewicz, S. Romani, M. Dalla Rosa and D. Witrowa-Rajchert, Innovative Food Sci. Emerging Technol., 2017, 41, 71–78 CrossRef.
- Z. Allahdad, M. Nasiri, M. Varidi and M. J. Varidi, J. Food Eng., 2019, 244, 202–211 CrossRef CAS.
- A. Rahaman, X. A. Zeng, A. Kumari, M. Rafiq, A. Siddeeg, M. F. Manzoor, Z. Baloch and Z. Ahmed, Ultrason. Sonochem., 2019, 58, 104643 CrossRef CAS PubMed.
- R. Prithani and K. K. Dash, Innovative Food Sci. Emerging Technol., 2020, 64, 102407 CrossRef CAS.
- A. Chandra, S. Kumar, S. Kumar and P. Nema, Syst. Microbiol. Biomanuf., 2022, 3, 615–626 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00253a |
| This journal is © The Royal Society of Chemistry 2025 |