GC/MS analysis & biological activities of mulberry leaf extract and formulation of instant freeze-dried functional beverages following encapsulation in protein-rich skim milk powder
Received
4th July 2024
, Accepted 9th December 2024
First published on 13th December 2024
Abstract
This study examines the volatile constituents and bioactive potential of mulberry leaf extract (MLE). Gas chromatography-mass spectrometry (GC-MS) identified key compounds such as gibberellic acid, 9,12,15-octadecatrienoic acid, and 5,7-dihydroxy isoflavone (mefenamic acid). MLE was then encapsulated in skim milk powder, and enhanced with rose flavour and sea-buckthorn anthocyanins, to create an instant freeze-dried beverage. The powder was evaluated for physical, structural, and biological properties, including antioxidant, antidiabetic, and anti-obesity activities under simulated gastrointestinal conditions. Scanning electron microscopy (SEM) revealed microcapsules with enclosed particles, while Fourier-transform infrared spectroscopy (FTIR) indicated phenolic compounds via increased –OH stretching in the 3000–3500 cm−1 range. The beverage powder showed 75–81 mgQE per g phenolic content, 40–61% antidiabetic activity and 45–67% anti-obesity activity. Both bioactivity and consumer acceptability were increased with the addition of rose flavour and anthocyanins. This beverage powder could be considered a sustainable healthy food to manage the lifestyles of diabetic and obese groups of the population.
Sustainability spotlight
This research is a step towards the zero hunger goal to achieve food security, nutrition and sustainable agriculture. In this study, mulberry leaves were valorised to a develop sustainable functional food. The leaves are packed with essential nutrients like vitamins and minerals (potassium, phosphorus, zinc, calcium, vitamin C, iron, and magnesium) and phenolic acids (gallic, caffeic, vanillic, chlorogenic, protocatechuic, syringic, p-coumaric, p-hydroxybenzoic, ferulic and m-coumaric acids). Imino sugar alkaloid 1-deoxynojirimycin (DNJ), is one of the main bioactive compounds and an inhibitor of α-glycosidase and controls type 2 diabetes. The mulberry leaf extract was utilized to form a functional beverage using encapsulation in skim-milk powder. This beverage could be consumed for management of healthy life-styles particularly by elderly, diabetic and obese groups of the population.
|
1. Introduction
Mulberries are diverse species of deciduous trees belonging to the genus Morus of the family Moraceae. They grow wild and spread extensively ranging from the tropics in the Southern Hemisphere to temperate to subtropical regions in the Northern Hemisphere.1 Being flourishing and perennial, the woody tree species of the family has an origin in the Himalayan foothills of India and China. Generally, the genus consists of 64 recognized species, and the most notable species are white mulberry (Morus alba), black mulberry (M. nigra) and red mulberry (M. rubra).2 The majority of mulberry-growing countries, like China and India, use the leaves of the plant as food for silkworms (Bombyx mori L.). In these countries, mulberry breeding has concentrated on increasing foliage yield. Mulberry fruits have several medicinal uses, including worming, treating dysentery, laxative, odontalgic, anthelmintic, expectorant, hypoglycemic, and emetic.3 Deeply coloured fruits, particularly black and red mulberry fruits are considered to be better for human health by the locals.4 Leaves from mulberries (Morus alba L.), which are frequently fed to silkworms, have been utilized in traditional Chinese medicine (TCM). They contain compounds such as flavonoids, alkaloids, and polysaccharides, which are believed to contribute to their therapeutic properties. Mulberry leaves have been traditionally used to alleviate symptoms associated with conditions like the common cold and diabetes.5 The leaves are rich in antioxidants and polyphenols, which provide numerous health benefits. Phenolic acids in mulberry leaves were identified as gallic, caffeic, vanillic, chlorogenic, protocatechuic, syringic, p-coumaric, p-hydroxybenzoic, ferulic and m-coumaric acids.6 In addition, the identified flavonol compounds in mulberry leaves include isoquercitrin (quercetin 3-β-D-glucoside), astragalin (kaempferol 3-β-D-glucopyranoside) and rutin (3-O-rutinoside quercetin).7 These components have numerous health benefits, including anti-inflammatory, anti-obesity, anti-atherosclerosis, antioxidant and antidiabetic activities.8,9 Moreover, the presence of vitamins and minerals like potassium, phosphorus, zinc, calcium, vitamin C, iron, and magnesium further enhances the nutritional profile of mulberry leaves. These nutrients play crucial roles in various body functions, including supporting bone health, boosting the immune system, aiding energy metabolism, and maintaining electrolyte balance.10 Imino sugar alkaloid 1-deoxynojirimycin (DNJ), is one of the main bioactive compounds found in mulberry leaves, and is a potent inhibitor of α-glycosidases and has shown potential therapeutic effects on many diseases, markedly type II diabetes. DNJ content in young leaves was found to range from 30 to 170 mg/100 g of dry leaves and it varied among mulberry varieties.11 In the digestive tract, this alpha glycosidase inhibitor competitively blocks the active site of polysaccharide-degrading enzymes. When the enzymes are inhibited, digestion and absorption of dietary carbohydrates are eventually diminished, thus proving helpful in lowering blood glucose levels without enteric side effects. The evidence from in vitro and in vivo studies as well as some clinical trials is well reported in the literature, and demonstrates the potential of mulberry leaves to manage lifestyle-related non-communicable diseases like type II diabetes, cardiovascular diseases, cancer, obesity and kidney failure.1,10,12 Therefore, it will be interesting to utilize mulberry leaves to formulate bioactive foods and to meet the sustainable goals for future food development. However, there are several limitations for their utilization in food products as phytochemicals may impart bitterness and off-flavour to the final product; secondly, the extraction of phytochemicals from the leaf matrix is quite tedious; third, these phytochemicals are highly unstable and may get degraded upon exposure to external conditions like light and heat. Therefore, we aimed to improve the extraction and stability and to mask bitterness of phytochemicals of mulberry leaves. To improve the extraction efficiency of phytochemicals from mulberry leaves, we reduced the size of dried mulberry leaves by ball milling, and the small-sized particles have a larger surface area and allow easy and faster release of compounds from the plant matrix. The phytochemical extracts obtained from the leaves after ball milling were studied for various biological activities & phytochemical content and compared with the native extract. The volatile constituent was examined by using GC/MS, which could help to understand the identification of untargeted volatiles including aroma/flavour compounds and pesticides as well. Subsequently, to improve the stability and to mask the bitterness, the phytochemical extracts were co-encapsulated in skim milk powder with the addition of natural rose flavour and the natural colourant anthocyanin, which were previously extracted from the sea buckthorn; besides this, they can also add more biological value to the product. The skim milk powder is rich in proteins and widely preferred for direct consumption, and milk proteins could interact with mulberry polyphenols and can further reduce the bitterness of the final product. This formulation was freeze-dried and the powder was studied for various physico-chemical properties and biological properties like antioxidant, antidiabetic and anti-obesity activities after being subjected to simulated gastrointestinal conditions. This instant freeze-dried beverage powder was later remixed with water to carry out sensory and consumer acceptability tests.
2. Materials and methods
Mulberry leaves (M. nigra) were collected and washed thoroughly to remove contamination and then air dried to wipe off excess moisture before the final drying stage in an oven. All the required chemicals were of analytical grade and purchased from Sigma Aldrich India. Pvt. Ltd. The natural rose flavour was purchased from the local market and natural colourant anthocyanin was available in the lab, which was previously extracted from sea buckthorn.
2.1. Drying and size reduction of mulberry leaf powder
The leaves were dried in an oven at 70 °C overnight. The leaves were then separated into two parts: crushed mulberry leaves (CML); crushed using a motor and pestle, and ball milled mulberry leaves (BML). The size reduction of mulberry leaf powder was done using a planetry ball mill (PM100, Retsch, India). The milling was continued for 5 hours with a 10 min break after each hour. 5 zirconium balls of 8 mm diameter were used and the rotation speed was maintained at 600 rpm.
2.2. Extraction method
The powdered mulberry leaves CML and BML were first treated with a 50% ethyl alcohol solution in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixtures were then magnetically stirred at 2000 rpm for 4 hours at 40 °C. The concentration was kept constant by adding an equal amount of 50% ethanol removed by the process of heating during extraction. The supernatant, or Mulberry Extracts (ME), was then collected after centrifugation and stored at 5 °C for further analysis.
10. The mixtures were then magnetically stirred at 2000 rpm for 4 hours at 40 °C. The concentration was kept constant by adding an equal amount of 50% ethanol removed by the process of heating during extraction. The supernatant, or Mulberry Extracts (ME), was then collected after centrifugation and stored at 5 °C for further analysis.
2.3. GC-MS analysis
GC-MS analysis of the plant extract of mulberry leaves (M. nigra) was conducted using a GC-MS/MS Agilent 7000D. The analysis utilized a DB-5MS Capillary Standard non-polar column with dimensions of 30 m × 0.25 mm ID × 0.25 μm film thickness. Helium was employed as the carrier gas at a flow rate of 1.0 mL min−1. The injector temperature was set to 250 °C, and the oven temperature was programmed to start at 60 °C for 15 minutes, gradually increasing to 280 °C in 3 minutes. Compounds were identified by matching their mass spectra with those in the NIST and Wiley libraries attached to the GC-MS instrument.
2.4. Total flavonoid content
Total flavonoid content was determined based on the method described by13 Zhishen et al. (1999). The already diluted sample was filtered through Whatman's filter paper, in a 10 ml test tube, and 300 microlitre sample extract was added in 3.4 ml of 30% methanol, 150 microliters of 0.5 M NaNo2, and 150 microlitres of 0.3 M AlCl3·6H2O followed by 5 min incubation and the addition of 1 ml NaOH 1 M. Absorbance was measured at 510 nm with a spectrophotometer. The standard curve was made using quercetin standard solution 1 mg ml−1 using the same above-mentioned protocol. The total flavonoid content was shown as milligrams of quercetin equivalents per gram of dry matter extract.
2.5. Free radical scavenging activity by DPPH (1,1-diphenyl-2-picrylhydrazyl)
DPPH (1,1-diphenyl-2-picrylhydrazyl) inhibition activity of the sample was calculated according to the method suggested by14 Matthäus, (2002). Briefly, methanol (2720 μL) was added to the respective samples/extract (80 μL) before adding the DPPH solution (50 mg/10 mL, 200 μL) and mixed thoroughly in a vortex shaker. The reaction mixture was incubated for 30 minutes in the dark at ambient temperature before measuring the absorbance at 515 nm. The results for the DPPH scavenging effect were reported as the inhibition percentage, calculated using the following equation:| |  | (1) |
2.6. Reducing power
The reducing power of the sample was determined following the method outlined by Gani et al. (2015)15 with minor adaptations. Initially, 0.1 ml of the extract/sample was combined with 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) aqueous potassium ferricyanide. This mixture was incubated at 50 °C for 20 minutes. Subsequently, 2.5 ml of 10% (w/v) trichloroacetic acid was added, followed by centrifugation at 3000 rpm for 10 minutes. After centrifugation, 2.5 ml of the supernatant was retrieved, diluted with 2.5 ml of distilled water, and then mixed with 0.5 ml of 0.1% (w/v) ferric chloride. The absorbance of this solution was measured at 700 nm against distilled water and compared to a standard solution of gallic acid. A higher absorbance value indicates greater reducing power. The higher absorbance indicates a higher reducing power and was computed by using the given formula:| | 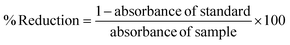 | (2) |
2.7. Encapsulation of mulberry leaf extract and formulation of instant functional beverage powder
The 10% of skimmed milk powder (SMP) was dissolved in distilled water, using a magnetic stirrer at 1500 rpm for 20 minutes at 60 °C, and mulberry extract (10 ml) obtained from ball-milled leaves was added dropwise into it under continuous stirring (10 min) followed by which tween 40 (1–2) drops were also added into the solutions along with few drops of vegetable oil. During the process, different formulations were prepared; for sample 1. Ready to mix encapsulated mulberry leaf extract (RML), sample 2 was prepared in the same manner but with an addition of 2 ml rose flavour and hence called ready to mix encapsulated mulberry leaf extract with rose flavour added (RMLF), and sample 3 was produced following the same protocol with an addition of 2 ml rose flavour and 1 ml colourant (anthocyanin extract with 12.5% Brix) and labelled as RMLAF. The suspension was sonicated for 10 min at 60 kHz for 5 s “onset” and 3 s “offset” at 28 °C to further reduce the particle size. All the samples were then homogenized (Witeg, Germany) for 10 min at maximum speed and then frozen at −18 °C and lyophilized using a freeze dryer (Lyovapour™ 200, Buchi; Switzerland) to produce the dried formulation powders. The prepared encapsulated powders were stored at 4 °C for further analysis.
10 ml of the beverage was mixed with 50% ethanol in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after vortexing for 10 minutes, the samples were centrifuged at 3000 rpm for 15 minutes and the supernatant was collected and stored under refrigerator conditions for bioactive analysis.
1 after vortexing for 10 minutes, the samples were centrifuged at 3000 rpm for 15 minutes and the supernatant was collected and stored under refrigerator conditions for bioactive analysis.
2.8. Bioactive properties of beverage powder under the simulated gastrointestinal conditions
A steady model that simulates digestion in the stomach and intestine was established. The method for stimulated gastric juice (SGJ) and simulated intestinal juice (SIJ) was prepared by Ahmad et al. (2019).16 The simulated gastric juice (SGJ) was prepared by dissolving 3 g per L pepsin in sterile NaCl solution (9 g L−1) and the pH was adjusted to 2.0 with 1.0 mol per L HCl. The simulated intestinal juice (SIJ) was prepared by dissolving 10 g per L bile salts and 3 g per L pancreatin in phosphate-buffered saline, pH was maintained at 8 with 0.1 M NaOH solution.
50 mg of the instant freeze-dried functional beverage was mixed with 5 ml of SGJ and incubated at 37 °C while stirring/mixing for 30 min. To this reaction mixture, 5 ml of SIJ was added and the reaction was continued for another 1 hour. The reaction was stopped by heating the sample at 90 °C for 10 min. This was later mixed with 50% ethanol in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vortexed for 10 minutes and then centrifuged at 3000 rpm for 15 minutes. The supernatant was collected and stored under refrigerator conditions for bioactive analysis.
1, vortexed for 10 minutes and then centrifuged at 3000 rpm for 15 minutes. The supernatant was collected and stored under refrigerator conditions for bioactive analysis.
2.8.1. Total phenolic content and antioxidant activities.
Total phenolic content was estimated following the procedure of17 Ahmad et al. (2015) with a slight modification. Specifically, 500 microliters of the 1/100 diluted sample were added to 2.5 ml of FC (Folin Ciocalteu reagent) which was made by diluting it with 10 volumes of water. Two millilitres of 7.5% sodium carbonate were added after five minutes, and the mixture was allowed to stand at room temperature for two hours. The absorbance was obtained at 760 nm using a UV spectrophotometer. The results were represented as micrograms of gallic acid equivalents per milligram of a sample (μg GAE per mg of the sample) and the measurements were performed in duplicate. Gallic acid was used to obtain the calibration curve.
The antioxidant activities like DPPH inhibition and reducing power activities were tested as per the method described above.
2.8.2. Antidiabetic activity by α-glucosidase inhibition assay.
α-Glucosidase inhibitory activity was measured using the protocol outlined by Zhang et al. (2014)18 Precisely, 50 μL of the sample was combined with 50 μL of 0.2 U per ml yeast α-glucosidase enzyme solution and 50 μL of 4 mM 4-nitrophenyl-β-D-glucopyranoside (pNPG) solution (dissolved in 0.1 M phosphate buffer, pH 6.8), and thoroughly mixed in a 96 well plate. The amount of p-nitrophenol released from pNPG was measured at 405 nm after the plates were incubated for 30 minutes at 37 °C. The following equation was used to compute the % inhibition:| |  | (3) |
where A (control) is the absorbance in the presence of an enzyme and without the test sample. B (control blank) is the absorbance without the enzyme and test sample. C (reaction) is the absorbance with the enzyme and test sample. D (reaction blank) is the absorbance with the test sample but without the enzyme.
2.8.3. Anti-obesity activity by cholesterol esterase (CE) inhibition assay.
The inhibitory activity of cholesterol esterase (CE) was measured with slight adjustments utilising a photometric approach described by19 Ahmad et al. (2021). The analysis involved a mixture containing 25 μL of pNPB (p-nitrophenyl butyrate) solution at a concentration of 10 mM, 50 μL of the samples being tested, and 25 μL of cholesterol esterase enzyme solution at a concentration of 10 μg mL−1. This mixture was prepared in sodium phosphate buffer (0.2 M; pH 7.2) and then incubated for 30 minutes at 37 °C. During the incubation period, the cholesterol esterase enzyme catalyzed the hydrolysis of pNPB to release p-nitrophenol (pNP), which could be quantified spectrophotometrically at 405 nm. The CE % inhibition can be computed using the equation below| |  | (4) |
where A (control) is the absorbance in the presence of the enzyme and without a test sample. B (control blank) is the absorbance without the enzyme and test sample. C (reaction) is the absorbance with the enzyme and test sample. D (reaction blank) is the absorbance with the test sample but without the enzyme.
2.9. Scanning electron microscopy (SEM)
The morphology of the encapsulated powders was examined using Scanning Electron Microscopy (SEM) (Zeiss, EVO 50) under vacuum conditions. Before analysis, a nanolayer of gold was coated onto the surface of the sample using sputtering which involves the ejection of gold atoms from the target to be deposited on the poorly conducting specimen and was then affixed to the adhesive tape on circular aluminium specimen stubs. Subsequently, SEM imaging was conducted at an acceleration potential of 5 kV.
2.10. Particle size analysis
The dynamic light scattering technique (Anton Paar, Litessizer-500) was used to determine the size distribution and average diameter of the microparticles. To prepare the sample for analysis, it was dissolved in deionized Milli-Q water at a concentration of 1.0 mg mL−1. Before measurement, the sample suspension was kept in a sonicator bath for 40 minutes at 20 kHz to ensure complete dispersion of the polymer particles in the solution.
2.11. Physical properties of instant functional mulberry beverage powder
The bulk densities of powders were determined by pouring a fixed weight of the sample into a 10 ml graduated cylinder, and the volume occupied by the powder was recorded. The bulk density was calculated by dividing the mass of the powder by the volume it occupied in the measuring cylinder. The tapped density was determined by measuring the volume occupied after tapping the sample at least 20 times. The bulk density and the tapped density were computed by using the given formula:| |  | (5) |
| | 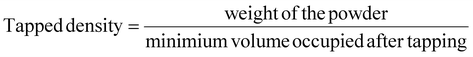 | (6) |
2.12. Fourier transform infrared (FTIR) spectroscopy
Using an FTIR spectrophotometer (Agilent, Cary630), the infrared spectra of the lyophilized encapsulated mulberry powder molecules were measured. The sample was examined within the range of 4000 cm−1 to 650 cm−1 and the system software was utilised to collect and analyse the spectra for each sample.
2.13. Sensory analysis
Using a 9-point hedonic scale, the sensory analysis was conducted in accordance with the methodology suggested by Ahmad et al. (2015).17 A nine-point hedonic scale was used, where 1 represented extreme dislike, 5 neither like nor dislike, and 9 represented extreme like. A panel consisting of 15–20 semi-trained personnel from the Department of Food Science and Technology at the University of Kashmir examined the samples for colour, flavour, taste, and aftertaste. The samples, which included both the experimental and control groups, were presented to the panellists in ceramic glasses with the appropriate coding. In addition, potable water was available for mouthwash following sample analysis.
3. Statistical analysis
The commercial statistical software SPSS.10.1 (USA) and Primer of Biostatistics (version 4.0) were used for all statistical analyses. Analysis of variance (ANOVA), Duncan's multiple range test, and paired t-test were used to evaluate the data at a significance level of 5%. The means ± standard deviation of the data are displayed. Every experiment was carried out three times.
4. Results and discussion
4.1. Total flavonoid content and antioxidant analysis of mulberry leaf extract
The flavonoid content and antioxidant analysis (DPPH and reducing power) of mulberry leaf extract are shown in Table 1. Flavonoids, a group of plant secondary metabolites that include flavanols, flavones, and condensed tannins are known for their health benefits. Consuming fruits and vegetables rich in flavonoids has been associated with a reduced risk of cancer and heart disease20 according to Juan et al. (2010). It has been revealed from the results that the ball-milled mulberry leaves showed significantly (p < 0.05) higher antioxidant and total flavonoid contents of about 6.37 mgQE per g than crushed mulberry leaves (5.07 mgQE per g). The reason might be attributed to the fact that ball milling (BML) subjects the mulberry leaves to intense mechanical forces, leading to the disruption of cell walls and release of bioactive compounds trapped within the cells. The polyphenols/flavonoids have a low molecular weight that allows them to get released easily from the disrupted tissues. Therefore, mechanical action may be more efficient in extracting flavonoids compared to the simple crushing method (CML). A similar increase in bioactive compound content was also observed for ball milling of persimmon by-product powder by Ramachandraiah et al. (2016).21 The antioxidant and polyphenolic contents were also observed to be higher after ball milling of mushroom powder by Wang et al. (2016).22
Table 1 Total flavonoid content and antioxidant properties of mulberry extracta
| Sample |
Parameters |
| TFC mgQE per g |
DPPH % |
Reducing power % |
|
Values represent means ± SD of triplicates. Values with different superscripts in a column vary significantly (P < 0.05) TFC; total flavonoid content, BML = ball milled mulberry leaves, and CML = crushed mulberry leaves.
|
| CML |
5.07 ± 0.43a |
51.09 ± 0.40a |
21.02 ± 1.05a |
| BML |
6.37 ± 0.98b |
62.10 ± 0.22b |
26.63 ± 0.75b |
The potential of DPPH as an antioxidant is determined by its ability to readily diminish its stable free radical in the presence of a proton-donating sample. The absorbance at 517 nm is used to measure the radical-scavenging effects by measuring the colour change from purple to light yellow.23 The results revealed that the scavenging ability of ball-milled mulberry leaves (BML) is higher (62.10%) as compared to the crushed mulberry leaves (61.09%) as shown in Table 1. This could be explained by the fact that ball-milled mulberry leaves have smaller particles than crushed mulberry leaves. Because finer particles have a larger surface area, there may be more interaction between the DPPH radical and the antioxidant compounds in mulberry leaves, which could result in higher DPPH values. A similar increase in antioxidant potential found for nanoreduction of starch from underutilized millets was observed by Jhan et al. (2020).24
The reducing power of a substance is evaluated based on its ability to donate hydrogen atoms or electrons to a suitable electron acceptor, such as Fe3+ (ferric ion). Fe3+ converts into Fe2+ in the presence of a reducing agent, producing a ferric–ferrous complex. Using spectrophotometry this process can be observed by measuring the complex's absorbance at a certain wavelength, usually at around 700 nm.24 The results revealed a significant difference (p < 0.05) between the ball milled mulberry leaves (BML) and crushed mulberry leaves (CML). Since reducing power is directly correlated with antioxidant activity, it was found that ball-milled mulberry leaves had the highest potential for reducing power (26.63%) when compared to simple crushing (24.02%). This indicates that the leaves have a high ability to inhibit the free radical chain reaction, which may indicate their potential as antioxidants. The increasing % of reducing power was also supported by Ramachandraiah et al. (2016).21 The presence of compounds like gibberellic acid, 9,12,15-octadecatrienoic acid, and 5,7-dihydroxyisoflavone (mefenamic acid) identified by GC/MS analysis is also reported to imply good antioxidant, anti-inflammatory, antidiabetic and anti-obesity properties.25
4.2. GC-MS analysis
GC-MS analysis of mulberry extract with higher flavonols and antioxidant activity i.e. BML was conducted and is shown in Table 2. The components of the extract were identified using gas chromatography, and the decomposition products were characterized with a mass analyzer detector (GC/MS). GC-MS analysis revealed the presence of the main compounds: gibberellic acid, 9,12,15-octadecatrienoic acid, and 5,7-dihydroxyisoflavone (mefenamic acid). Gibberellic acid is abundantly found in mulberry extract and plays a crucial role in promoting plant growth and leaf enlargement.26 The major proportion of chemical constituents of the extract has been identified as gibberellic acid by27 Emniyet et al. (2014) in Morus alba L. leaves. GC-MS analysis identified 9,12,15-octadecatrienoic acid in Vitis sitosa. This compound is known for its diverse biological activities, including anti-inflammatory, hypocholesterolemic, cancer preventive, hepatoprotective, and antimicrobial properties.28 5,7-Dihydroxyisoflavone, also known as mefenamic acid, possesses anti-inflammatory properties, inhibits prostaglandin synthesis, and acts as an analgesic.29
Table 2 Major phytochemical compounds present in mulberry leaves identified by GC/MS
| Phytochemical content |
RT |
Area |
| Gibberallic acid |
28.709 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969.129596 969.129596 |
| 9,12,15-Octadecatrienoic acid |
18.801 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 093![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.1835332 963.1835332 |
| 5,7-Dihydroxyisoflavone (mefenamic acid) |
17.425 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163.5932868 163.5932868 |
| 3-Methyl-1-adamantyl isothiocyanate |
16.902 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 659.8149976 659.8149976 |
| 7-[Beta-d-ribofuranosyl]imidazo[4,5-d][1,2,3]-triazin-4-one(2-azainosine) |
14.310 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968 968![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455.3265524 455.3265524 |
| 4′,5,7-Trihydroxy isoflavone (genistein) |
13.357 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135.4806299 135.4806299 |
| 8-Glycosyl apigenin (vitexin) |
13.128 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608.954995 608.954995 |
| 2-Azido-2,4,4,6,6,8,8,-heptamethylnonane |
12.440 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 371 371![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106.4253073 106.4253073 |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-(apigenin) |
12.263 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.3374415 794.3374415 |
| 9-Borabicyclo[3.3.1]nonan-9-amine,n-methyl |
11.872 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 349![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452.3972489 452.3972489 |
| Diethyl(1-aminocyclohexyl) phosphonate |
11.433 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510.942413 510.942413 |
| Isophorone diisocyanate |
7.497 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 994![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021.7725115 021.7725115 |
4.3. Biological properties of encapsulated powders under simulated conditions
4.3.1. Antioxidant properties and total phenolic content.
Antioxidant properties encompass a spectrum of functions important for cellular health and disease prevention. These include total phenolic content, scavenging free radicals, reducing power, protection against DNA damage, inhibition of lipid peroxidation, and preservation of the integrity of cellular structures. Furthermore, antioxidants are essential for reducing inflammation, enhancing the immune system, and maybe lowering the risk of chronic illnesses like cancer, neurological diseases, and cardiovascular problems.30Table 3 presents the antioxidant activity of encapsulated mulberry extract formulated with ready to mix encapsulated mulberry leaves extract powder (RML), ready to mix encapsulated mulberry leaf extract powder with rose flavour added (RMLF), and ready to mix encapsulated mulberry leaf extract powder with added rose flavour and anthocyanin as the colourant (RMLAF) respectively. It has been revealed from the results that RMLAF exhibited significantly higher (p < 0.05) total phenolic content (82.07 ± 0.62%) followed by a non-significant difference in RML (74.98 ± 0.44%) and RMLF (72.75 ± 0.62%) respectively. The reason might be attributed to the addition of anthocyanins to mulberry extract in the encapsulated form which resulted in increases in its total phenolic content because of the synergistic impact between these components. They scavenge free radicals, preventing oxidative damage to cellular structures, which in turn preserves other phenolic compounds. A cumulative rise in the extract's total phenolic content as a result of this synergistic interaction results in increased antioxidant activity and possible health benefits. A similar finding was observed by Gungor et al. (2008),31 for white mulberry (Morus alba L.) fruits32 and anthocyanin-rich Morus nigra and Morus rubra fruits showed increased total phenolic content.
Table 3 Total phenolic content, DPPH, reducing power, and antidiabetic and antiobesity properties of functional beveragesa
| Sample |
TPC mgQE per g |
DPPH% |
Reducing power % |
α-Glucosidase inhibition % |
Cholesterol esterase inhibition % |
|
The results are the means of three replications ± standard deviations. Values with distinct superscripts are significantly different (p < 0.05) in the same row and columns, where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn.
|
| RML |
74.98 ± 0.44a |
18.28 ± 0.13ab |
53.84 ± 0.17a |
40.5 ± 0.57a |
45.2 ± 0.06a |
| RMLF |
72.75 ± 0.62a |
17.85 ± 0.9a |
52.54 ± 0.43a |
52.06 ± 0.005b |
59.1 ± 0.20b |
| RMLAF |
82.07 ± 0.62b |
18.94 ± 0.26b |
54.62 ± 0.40a |
61.03 ± 0.002c |
67.02 ± 0.009c |
The DPPH and reducing power of the samples were observed to be significantly high (p < 0.05), in RMLAF (18.94 ± 0.26%, 54.62 ± 0.40), followed by RMLF (17.85 ± 0.9, 54.62 ± 0.40%) and RML (18.28 ± 0.13, 53.84 ± 0.17%). The reason for the high antioxidant activity in RMLAF may be attributed to the increased number of hydroxyl groups because of the addition of anthocyanin. Anthocyanins are reported to exert antioxidant activity by scavenging free radicles as reported by Cheng et al. (2023).33 Similar findings were reported by Wu et al. (2011)34 for mulberry (Morus atropurpurea) anthocyanins.
4.3.2. Antidiabetic activity (α-glucosidase inhibition assay).
Mulberry leaves contain bioactive compounds, such as flavonoids and alkaloids, that have been shown to inhibit α-glucosidase enzymes. α-Glucosidase is an enzyme responsible for breaking down complex carbohydrates into simple sugars (glucose) in the digestive tract. By inhibiting α-glucosidase, mulberry leaf extract can slow down the digestion and absorption of carbohydrates, leading to lower postprandial (after-meal) blood glucose levels.35 In individuals with type 2 diabetes mellitus in particular, the inhibition of the enzyme α-glucosidase is useful in treating hyperglycemia.36 Disaccharides are broken down into monosaccharides by the enzyme α-glucosidase, and inhibiting this enzyme can lower blood glucose levels and, in turn, lower postprandial plasma glucose levels in diabetic individuals. The percentage inhibition values in the present research were determined, and the outcomes are displayed in Table 3. The results have indicated that RMLAF and RMLF demonstrated a significantly higher α-glucosidase inhibitory effect compared to RML. The inhibition values ranged from 40.5% to 61.03%. It was found that the inhibition of α-glucosidase was more in encapsulated mulberry extract incorporated with anthocyanin as a colourant and rose as a flavour than RML. The reason could be attributed to the synergistic interaction between the anthocyanin and other bioactives in the mulberry leaves. Because of their strong antioxidant qualities, anthocyanins can stabilise and preserve the integrity of other active components in the extract, increasing its total bioactivity. The combined bioactive ingredients operate together to block α-glucosidase more effectively, which is likely the result of this synergistic action. These results suggest that the incorporation of anthocyanins and rose flavour not only enhances the palatability of the extracts but also potentially boosts their therapeutic efficacy. There are several studies which have shown the α-glucosidase activity of mulberry extract.8,9,37 Imino sugar alkaloid 1-deoxynojirimycin (DNJ) is one of the main bioactive compounds found in mulberry leaves, and is a potent inhibitor of α-glycosidases, besides gibberellic acid, 9,12,15-octadecatrienoic acid, and 5,7-dihydroxy isoflavone (mefenamic acid) as identified by GC/MS analysis25 (Olivia, Goodness & Obinna, 2021).
4.3.3. Antiobesity activity (cholesterol esterase inhibition assay).
The enzyme cholesterol esterase (CE) is linked to the breakdown of numerous dietary lipids. By slowing the breakdown of fat and reducing its absorption in the small intestine, CE inhibition may be effective in the fight against obesity.16 Cholesterol esterase (CE) is an enzyme involved in the hydrolysis of various dietary lipids. It plays a key role in the breakdown of cholesterol esters into free cholesterol and fatty acids in the small intestine. By reducing the amount of fat absorbed in the small intestine and delaying its breakdown, CE inhibition may be effective in the fight against obesity.16 The percentage inhibition of CE by encapsulated mulberry powders is presented in Table 3. It has been revealed from the results that RMLAF and RMLF showed better inhibition percentages than RML. The percentage inhibition values were found to be 59.1%, and 67.2% by RMLF and RMLAF respectively, while RML showed a comparatively lower inhibition of 45.02% (Table 3).
Our findings showed that good bioactivity after the in vitro digestion process may be due to the protective effect and sustainable release enabled by the encapsulation system. Previous studies have demonstrated that encapsulated compounds exhibit better bioactivity potential than non-encapsulated compounds, which has been supported by our previous studies, for example nano-encapsulated resveratrol and catechin showed better retention of anti-diabetic and anti-obesity properties in comparison to non-encapsulated compounds of similar form and quantity.16,19
4.4. Physical properties of mulberry leaf extracts and encapsulated powders
Bulk and tapped densities are important indicators for monitoring the quality of powdered food products. The significant increase in bulk and tapped densities from 0.137 g cm−3 and 0.184 g cm−3 for crushed mulberry leaves powder to 0.592 g cm−3 and 0.719 g cm−3 respectively (Table 4) for ball milled mulberry leaves (BML) can be attributed to the reduction in particle size during the ball milling process. Smaller particle sizes lead to decreased pore spaces between particles, resulting in tighter packing and higher bulk and tapped densities.38 Similar findings were reported by Chen et al. (2015)39 for mulberry leaf powder; and Jiang et al. (2020)40 for onion peel respectively. Upon encapsulation of mulberry extract incorporated with anthocyanin as a colourant, it has been revealed from the results that RMLAF exhibited significantly higher bulk and true densities (0.680 g cm−3 and 0.698 g cm−3) followed by RMLF (0.671 g cm−3 and 0.695 g cm−3) and RML (0.523 g cm−3 and 0.526 g cm−3). The reason for the high bulk and tapped densities in RMLAF can be attributed to the encapsulation process and the presence of anthocyanin. A protective shell that forms around the active substance during encapsulation usually results in a denser and more compact structure. Further improvement in the cohesiveness and packing effectiveness of the encapsulated particles may be due to the addition of anthocyanin, which is recognised for its colouring and molecular characteristics. Similar findings were reported by Shi et al. (2024) for mulberry anthocyanin and silk fibroin.41
Table 4 Bulk density and tapped density of dried mulberry leaf powder and the encapsulated powdera
| Densities |
CML |
BML |
RML |
RMLF |
RMLAF |
|
The results are the means of three replications ± standard deviations. Values with distinct superscripts are significantly different (p < 0.05) in the same row and columns, where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn.
|
| Bulk density (g cm−3) |
0.137 ± 0.004a |
0.592 ± 0.006b |
0.523 ± 0.19b |
0.671 ± 0.02a |
0.680 ± 0.01b |
| Tapped density (g cm−3) |
0.184 ± 0.004a |
0.719 ± 0.009b |
0.526 ± 0.00a |
0.695 ± 0.01a |
0.698 ± 0.04a |
4.5. Scanning electron microscopy
The micrographs of RML, RMLF, and RMLAF observed under scanning electron microscopy are shown in Fig. 1. The morphological characteristics of encapsulated particles revealed a distinctive pattern wherein certain particles appeared embedded within the hollow spheres of the wall materials. This observation suggests a unique encapsulation structure, wherein the particles are effectively entrapped within the confines of the wall material's cavity. Similar results were observed by Ahmad et al. (2018), during microencapsulation of saffron anthocyanin.42 Furthermore, our study revealed that the sample RML exhibited a compact cell wall structure resembling hexagonal shapes with rough surfaces, whereas the encapsulated samples displayed smooth surfaces without any fractures as reported by Ahmad et al. (2018).42
 |
| | Fig. 1 Micrographs of instant functional beverage powder. Where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn. | |
4.6. Particle size distribution
The hydrodynamic particle diameter and polydispersity index of RML, RMLF, and RMLAF respectively are shown in Table 5. It has been noted from the findings that there was a non-significant (p < 0.05) decrease in the average hydrodynamic diameter of RMLF and RMLAF, found to be 362.19 nm and 348.66 nm respectively as compared to the RML (376.51 nm). The observed reduction in particle size upon the addition of anthocyanins could potentially be attributed to their intrinsic smaller molecular size when compared to other polyphenols found in the extract. Smaller particle sizes were found to improve granules' adsorption capacity and moisture penetration by increasing the contact area by43 Huang et al. (2019). Similar findings were reported by Zahed et al. (2023) for anthocyanin of pomegranate peel.44 Polydispersity indices refer to a measure of the distribution of particle sizes within a sample. It has been observed from the results that RML had non-significantly higher (26.93%) PDI values as compared to the RMLF (26.62%) and RMLAF (18.73%). The observed decrease in polydispersity indices (PDI) after the addition of anthocyanin to the mulberry extract can be attributed to the encapsulation process.45 The anthocyanin acts as a stabilizing agent during encapsulation, resulting in more consistent particle sizes and narrower size distributions in the encapsulated mulberry extract (RMLAF) compared to the unencapsulated mulberry extract (RML). Similar findings were reported by Moura et al. (2018).46
Table 5 Hydrodynamic diameters and polydispersity indicesa
| Sample |
RML |
RMLF |
RMLAF |
|
The results are the means of three replications ± standard deviations. Values with distinct superscripts are significantly different (p < 0.05) in the same row and columns, where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn.
|
| Hydrodynamic diameter (nm) |
376.51 ± 35.70a |
362.19 ± 27.49a |
348.66 ± 14.78a |
| Polydispersity index (%) |
26.93 ± 2.93a |
26.62 ± 2.97a |
18.73 ± 8.93a |
4.7. Molecular fingerprinting by ATR-FTIR
The FTIR spectra of all samples are presented in Fig. 2. The mulberry extract and anthocyanins interacted as demonstrated by the ATR-FTIR study revealing the characteristic spectrum alterations suggestive of molecular binding. Based on the spectra, the peak infrared bands were found in all samples to be connected to the stretched hydroxyl groups (OH) in mulberry extract and ranged from 3200 to 3300 cm−1. In particular, changes in peak intensities and shifts in absorption bands were noted, which may indicate the occurrence of hydrogen bonds or other non-covalent interactions between the two chemicals. Anthocyanin vibrational bands overlapped with the characteristic band from RMLAF and RML in the 1700 cm−1 area, which supports the presence of anthocyanins in the sample. FTIR showed a prominent increase in the 3000–3500 peak area associated with the phenolic –OH stretching suggesting an increase in the overall phenolic content due to the addition of anthocyanin in RMLAF. When encapsulated anthocyanins are compared to non-encapsulated samples, it was found that the bands from the former are narrower, suggesting that the integration of anthocyanins alters the molecular order. A similar finding was reported by Ahmad et al. (2018).42 This change in the spectra towards a broader range of wavelengths suggests a compatible non-covalent interaction of anthocyanin within the formulation. The overall spectra of the samples were however identical and this similarity in the pattern of spectra demonstrates the stability of the compounds within the encapsulated system. However, the additional peaks that might be attributed to the presence of anthocyanins and mulberry polyphenols were not visible in the spectra due to overlapping by the encapsulation process.
 |
| | Fig. 2 FTIR spectra of instant functional beverage powder, where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn. | |
4.8. Sensory analysis of functional beverages
The organoleptic characteristics of functional beverages including color, aroma, taste, and overall acceptability are presented in Table 6. The beverages were prepared using water as the solvent at a concentration of 6% for each respective powder. Among the three drinks made from freeze-dried powders, RMLF and RMLAF were most favoured due to the incorporation of rose extract. The addition of rose extract served as a flavouring agent, contributing to a pleasant taste in the drink.47 Meanwhile, the presence of anthocyanin not only provides an appealing colour but also potentially offers additional health benefits and antioxidant properties.48 This dual combination of flavour enhancement and visual appeal highlights the importance of utilizing multiple functional ingredients to develop beverages that are both appealing and enjoyable to consumers. The overall acceptability score for RMLAF and RMLF was non-significantly high as compared to the RML sample.
Table 6 Sensory evaluation of functional mulberry beveragesa
|
|
RML (6%) |
RMLF (6%) |
RMLAF (6%) |
|
The results are the means of three replications ± standard deviations. Values with distinct superscripts are significantly different (p < 0.05) in the same row and columns where RML = ready to mix encapsulated mulberry leaf extract powder, RMLF = ready to mix encapsulated mulberry leaf extract powder with rose flavour, and RMLAF = ready to mix encapsulated mulberry leaf extract powder with rose flavour and anthocyanin as the colourant obtained from sea buckthorn.
|
| Color |
7.00 ± 0.57a |
8.34 ± 0.0.76b |
8.66 ± 0.57b |
| Aroma |
6.33 ± 0.57a |
8.00 ± 1.00b |
8.83 ± 0.28b |
| Taste |
6.66 ± 0.57a |
7.31 ± 0.57a |
8.72 ± 0.34b |
| Overall acceptability |
6.67 ± 0.57a |
7.94 ± 0.96ab |
8.72 ± 0.57b |
5. Conclusion
The research underscores the potential of Morus (mulberry) leaves as a rich source of bioactive compounds, particularly polyphenols, which exhibit significant health-promoting properties, such as antioxidant, anti-inflammatory, and anti-diabetic activities. The study suggests mulberry leaves as a valuable ingredient for the development of sustainable functional foods. Among various extraction methods, ball milling has emerged as a superior technique for isolating polyphenolic compounds from mulberry leaves, offering enhanced yield and quality compared to traditional crushing methods. This approach increases the surface area of the leaves, facilitating better solvent penetration and resulting in more efficient extraction of bioactive compounds.
Furthermore, encapsulating mulberry leaf extracts in a matrix of skimmed milk powder has shown promise in improving their bioactivity and stability throughout the gastrointestinal (GI) tract. The encapsulation technique shields the bioactive compounds and improved the bioactive properties of the final product. The incorporation of rose extracts and anthocyanins not only improved consumer acceptance by contributing a pleasant flavour and aromatic profile to the final beverage but also enhanced the antidiabetic, antiobesity and overall antioxidant capacity of the formulation.
In conclusion, mulberry leaves are a rich source of bioactive compounds as revealed by GC/MS analysis. These extracts can be incorporated into healthy ready-to-mix drinks, and their bioactivity can be further enhanced through the encapsulation addition of flavour and colourants. This integrated approach not only maximizes the health benefits but could also improve the sensory properties and consumer acceptance of the final product.
Data availability
The data used to support the findings of this study are included in the article.
Conflicts of interest
All authors declare that they have no conflict of interest.
Acknowledgements
Mudasir Ahmad is highly thankful to the Jawharlal Nehru centre for Advanced Scientific Research and Department of Science and Technology (DST), Government of India, for the award of the National Postdoctoral Fellowship (JNC/AO/A.1707(18)/24-OW-713).
References
- P. Wen, T. G. Hu, J. R. Linhardt, T. S. Liao, H. Wu and X. Y. Zou, Mulberry: A review of bioactive compounds and advanced processing technology, Trends Food Sci. Technol., 2019, 83, 138–158 CrossRef CAS.
- D. Yigit, F. Akar, E. Baydas and M. Buyukyildiz, Elemental composition of various mulberry species, Asian J. Chem., 2010, 22(5), 3554 CAS.
- S. Ercisli and E. Orhan, Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits, Food Chem., 2007, 103(4), 1380–1384 CrossRef CAS.
-
S. Ercisli and E. Orhan, Natural mulberry (Morus spp.) production in Erzurum Region in Turkey, in Proceedings of the International Scientific Conference: Environmentally Friendly Fruit Growing, Tartu University Press, Polli, EstoniaI, 2005, pp. 129–135 Search PubMed.
- Y. Yu, H. Li, B. Zhang, J. Wang, X. Shi, J. Huang and Z. Deng, Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials, Int. J. Food Prop., 2018, 21(1), 1495–1507 CrossRef CAS.
- A. Gryn-Rynko, G. Bazylak and D. Olszewska-Slonina, New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves, Biomed. Pharmacother., 2016, 84, 628–636 CrossRef CAS PubMed.
- E. Flaczyk, J. Kobus-Cisowska, M. Przeor, J. Korczak, M. Remiszewski, E. Korbas and M. Buchowski, Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes, Agric. Sci., 2013, 4(05), 141 Search PubMed.
- S. Shivangi, D. Dorairaj, S. P. Negi and N. P. Shetty, Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components, Food Hydrocolloids, 2021, 121, 107046 CrossRef CAS.
- I. Liguori, G. Russo, F. Curcio, G. Bulli, L. Aran, D. Della-Morte, G. Gargiulo, G. Testa, F. Cacciatore, D. Bonaduce and P. Abete, Oxidative stress, aging, and diseases, Clin. Interventions Aging, 2018, 13, 757, DOI:10.2147/CIA.S158513.
- G. Ma, X. Chai, G. Hou, F. Zhao and Q. Meng, Phytochemistry, bioactivities and future prospects of mulberry leaves: A review, Food Chem., 2022, 372, 131335 CrossRef CAS PubMed.
- C. Vichasilp, K. Nakagawa, P. Sookwong, O. Higuchi, S. Luemunkong and T. Miyazawa, Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction, LWT – Food Sci. Technol., 2012, 45(2), 226–232, DOI:10.1016/j.lwt.2011.09.008.
- C. Tang, T. Bao, Q. Zhang, H. Qi, Y. Huang, B. Zhang and X. Tong, Clinical potential and mechanistic insights of mulberry (Morus alba L.) leaves in managing type 2 diabetes mellitus: Focusing on gut microbiota, inflammation, and metabolism, J. Ethnopharmacol., 2023, 306, 116143 CrossRef PubMed.
- J. Zhishen, T. Mengcheng and W. Jianming, The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem., 1999, 64(4), 555–559, DOI:10.1016/S0308-8146(98)00102-2.
- B. Matthäus, Antioxidant activity of extracts obtained from residues of different oilseeds, J. Agric. Food Chem., 2002, 50(12), 3444–3452 CrossRef PubMed.
- A. Gani, N. Rasool, A. Shah, M. Ahmad, A. Gani, T. A. Wani and F. A. Masoodi, DNA scission inhibition, antioxidant, and antiproliferative activities of water chestnut (Trapa natans) extracted in different solvents, CyTA – J. Food, 2015, 13(3), 415–419 CrossRef CAS.
- M. Ahmad, P. Mudgil, A. Gani, F. Hamed, F. A. Masoodi and S. Maqsood, Nano-encapsulation
of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion, Food Chem., 2019, 270, 95–104 CrossRef CAS PubMed.
- M. Ahmad, W. N. Baba, A. Gani, T. A. Wani, A. Gani and F. A. Masoodi, Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS, Cogent Food Agric., 2015, 1, 1106387 CrossRef.
- L. L. Zhang, Y. L. Bai, S. L. Shu, D. W. Qian, Z. Ou-yang, L. Liu and J. A. Duan, Simultaneous quantitation of nucleosides, nucleobases, amino acids, and alkaloids in mulberry leaf by ultra high performance liquid chromatography with triple quadrupole tandem mass spectrometry, J. Separ. Sci., 2014, 37(11), 1265–1275 CrossRef CAS PubMed.
- M. Ahmad and A. Gani, Ultrasonicated resveratrol loaded starch nanocapsules: Characterization, bioactivity and release behaviour under in-vitro digestion, Carbohydr. Polym., 2021, 251, 117111 CrossRef CAS PubMed.
- M. Y. Juan and C. Chou, Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715, Food Microbiol., 2010, 27(5), 586–591 CrossRef CAS PubMed.
- K. Ramachandraiah and K. B. Chin, Evaluation of ball-milling time on the physicochemical and antioxidant properties of persimmon by-products powder, Innovat. Food Sci. Emerg. Technol., 2016, 37, 115–124 CrossRef CAS.
- J. Wang, C. Wang, W. Li, Y. Pan, G. Yuan and H. Chen, Ball milling improves extractability and antioxidant properties of the active constituents of mushroom Inonotus obliquus powders, Int. J. Food Sci. Technol., 2016, 51(10), 2193–2200, DOI:10.1111/ijfs.13180.
- A. A. Khan, A. Gani, F. A. Masoodi, U. Mushtaq and S. A. Naik, Structural, rheological, antioxidant, and functional properties of β–glucan extracted from edible mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus attrimentarius, Bioact. Carbohydr. Diet. Fibre, 2017, 11, 67–74 CrossRef.
- F. Jhan, A. Shah, A. Gani, M. Ahmad and N. Noor, Nano-reduction of starch from underutilised millets: Effect on structural, thermal, morphological and nutraceutical properties, Int. J. Biol. Macromol., 2020, 159, 1113–1121 CrossRef CAS PubMed.
- N. U. Olivia, C. U. Goodness and O. M. Obinna, Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves, Futur. J. Pharm. Sci., 2021, 7, 59, DOI:10.1186/s43094-021-00208-4.
- C. Matthew, W. A. Hofmann and M. A. Osborne, Pasture response to gibberellins: a review and recommendations, N. Z. J. Agric. Res., 2009, 52(2), 213–225 CrossRef CAS.
- A. A. Emniyet, E. Avci, B. Ozcelik, G. A. Avci and D. A. Kose, Antioxidant and antimicrobial activities with GC/MS analysis of the Morus alba L. leaves, Hittite j. sci. eng., 2014, 1(1), 37–41 CrossRef.
- R. Gobalakrishnan, P. Manikandan and R. Bhuvaneswari, Antimicrobial potential and bioactive constituents from aerial parts of Vitis setosa wall, J. Med. Plants Res., 2014, 8(11), 454–460 CrossRef CAS.
- Y. Kavitha and A. Geetha, Anti-inflammatory and preventive activity of white mulberry root bark extract in an experimental model of pancreatitis, J. Tradit. Complementary Med., 2018, 8(4), 497–505 CrossRef PubMed.
- P. Maisuthisakul, R. Pongsawatmanit and M. H. Gordon, Characterization of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer), Food Chem., 2007, 100(4), 1620–1629 CrossRef CAS.
- N. Gungor and M. Sengul, Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits, Int. J. Food Prop., 2008, 11(1), 44–52 CrossRef CAS.
- M. Özgen, S. Serçe and C. Kaya, Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits, Sci. Hortic., 2009, 119(3), 275–279 CrossRef.
- Y. Cheng, J. Liu, L. Li, J. Ren, J. Lu and F. Luo, Advances in embedding techniques of anthocyanins: Improving stability, bioactivity and bioavailability, Food Chem., 2023, X, 100983 Search PubMed.
- X. Wu, L. Liang, Y. Zou, T. Zhao, J. Zhao, F. Li and L. Yang, Aqueous two-phase extraction, identification and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.), Food Chem., 2011, 129(2), 443–453 CrossRef CAS PubMed.
- H. Nakanishi, S. Onose, E. Kitahara, S. Chumchuen, M. Takasaki, H. Konishi and R. Kanekatsu, Effect of environmental conditions on the α-glucosidase inhibitory activity of mulberry leaves, Biosci., Biotechnol., Biochem., 2011, 75(12), 2293–2296 CrossRef CAS PubMed.
- H. Bischoff, The mechanism of alpha-glucosidase inhibition in the management of diabetes, Clin. Invest. Med., 1995, 18(4), 303–311 CAS.
- A. Abudurexiti, R. Zhang, Y. Zhong, H. Tan, J. Yan, S. Bake and X. Ma, Identification of α-glucosidase inhibitors from Mulberry using UF-UPLC-QTOF-MS/MS and molecular docking, J. Funct. Foods, 2023, 101, 105362 CrossRef CAS.
- X. Zhao, Z. Yang, G. Gai and Y. Yang, Effect of superfine grinding on properties of ginger powder, J. Food Eng., 2009, 91(2), 217–222 CrossRef.
- G. Chen, L. Wang, F. Zhang, C. Li and J. Kan, Effect of superfine grinding on physicochemical properties of mulberry leaf powder, Trans. Chin. Soc. Agric. Eng., 2015, 31(24), 307–314 Search PubMed.
- G. Jiang, K. Ramachandraiah, Z. Wu, S. Li and J. B. Eun, Impact of ball-milling time on the physical properties, bioactive compounds, and structural characteristics of onion peel powder, Food Biosci., 2020, 36, 100630 CrossRef CAS.
- Y. Shi, X. Chen, Q. Gao, Y. Zou, D. Xing, R. Chen and Q. Li, Preparation and chemical properties of microencapsulation developed with mulberry anthocyanins and silk fibroin, Ind. Crops Prod., 2024, 212, 118383 CrossRef CAS.
- M. Ahmad, B. Ashraf, A. Gani and A. Gani, Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion, Int. J. Biol. Macromol., 2018, 109, 435–442 Search PubMed.
- F. Huang, H. Liu, R. Zhang, L. Dong, L. Liu, Y. Ma and M. Zhang, Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques, Carbohydr. Polym., 2019, 206, 344–351 CrossRef CAS PubMed.
- N. Zahed, R. Kenari Esmaeilzadeh and R. Farahmandfar, Effect of different extraction methods on antioxidant properties and encapsulation efficiency of anthocyanin of pomegranate peel, Food Sci. Nutr., 2023, 11(7), 3780–3787 CrossRef CAS PubMed.
- A. Gharsallaoui, G. Roudaut, O. Chambin, A. Voilley and R. Saurel, Applications of spray-drying in microencapsulation of food ingredients: An overview, Food Res. Int., 2007, 40(9), 1107–1121 CrossRef CAS.
- S. C. de Moura, C. L. Berling, S. P. Germer, D. I. Alvim and M. D. Hubinger, Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles, Food Chem., 2018, 241, 317–327 CrossRef CAS PubMed.
- A. Kumar, A. Kaur, V. Tomer, K. Gupta and K. Kaur, Effect of rose syrup and marigold powder on the physicochemical, phytochemical, sensorial and storage properties of nutricereals and milk-based functional beverage, J. Am. Coll. Nutr., 2021, 40(2), 133–140 CrossRef CAS PubMed.
- H. E. Khoo, A. Azlan, T. S. Tang and S. M. Lim, Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits, Food Nutr. Res., 2017, 61(1), 1361779, DOI:10.1080/16546628.2017.1361779.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab,
Asir
Gani
c,
Iqra
Qureshi
a and
Kouser
Parveen
d
*ab,
Asir
Gani
c,
Iqra
Qureshi
a and
Kouser
Parveen
d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixtures were then magnetically stirred at 2000 rpm for 4 hours at 40 °C. The concentration was kept constant by adding an equal amount of 50% ethanol removed by the process of heating during extraction. The supernatant, or Mulberry Extracts (ME), was then collected after centrifugation and stored at 5 °C for further analysis.
10. The mixtures were then magnetically stirred at 2000 rpm for 4 hours at 40 °C. The concentration was kept constant by adding an equal amount of 50% ethanol removed by the process of heating during extraction. The supernatant, or Mulberry Extracts (ME), was then collected after centrifugation and stored at 5 °C for further analysis.

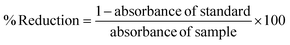
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after vortexing for 10 minutes, the samples were centrifuged at 3000 rpm for 15 minutes and the supernatant was collected and stored under refrigerator conditions for bioactive analysis.
1 after vortexing for 10 minutes, the samples were centrifuged at 3000 rpm for 15 minutes and the supernatant was collected and stored under refrigerator conditions for bioactive analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vortexed for 10 minutes and then centrifuged at 3000 rpm for 15 minutes. The supernatant was collected and stored under refrigerator conditions for bioactive analysis.
1, vortexed for 10 minutes and then centrifuged at 3000 rpm for 15 minutes. The supernatant was collected and stored under refrigerator conditions for bioactive analysis.


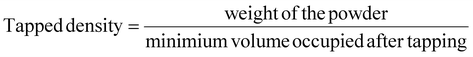
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255
255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969.129596
969.129596![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093
093![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.1835332
963.1835332![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527
527![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163.5932868
163.5932868![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147
147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 659.8149976
659.8149976![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968
968![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455.3265524
455.3265524![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279
279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135.4806299
135.4806299![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223
223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608.954995
608.954995![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 371
371![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106.4253073
106.4253073![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174
174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.3374415
794.3374415![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349
349![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452.3972489
452.3972489![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308
308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510.942413
510.942413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994
994![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021.7725115
021.7725115

