Open Access Article
Open Access ArticleOptimization of ultrasonic-assisted extraction of dietary fiber from bhimkol (Musa balbisiana) peel using central composite design: physicochemical, functional, and thermal properties
Laxmi Kant
Rawat
and
Tabli
Ghosh
 *
*
Department of Food Engineering and Technology, School of Engineering, Tezpur University, Assam 784028, India. E-mail: tablighosh1@gmail.com
First published on 10th October 2024
Abstract
Bhimkol is a seeded banana found in northeastern and southern India, and its peel is a good source of dietary fiber (DF) and can be utilized for various food applications. Considering this, in this study, the optimization of ultrasonic-assisted extraction (UAE) of bhimkol (Musa balbisiana) peel powder (BPP)-based DF was carried out. Proximate analysis of the prepared BPP was performed for factors such as moisture (1.40%), fat (2.22%), protein (7.30%), crude fiber (23.39%), and ash (10.47%) content as well as physicochemical, hydration, and thermal properties. The optimization of UAE of DF was carried out considering three independent variables, namely, processing time (20 to 60 min), solvent-to-solid ratio (30 to 70 mL g−1), temperature (40 to 80 °C), and one dependent variable, viz. yield (%). The highest extraction yield of DF (49.58 ± 0.88%) was obtained from UAE at a time, solvent/solid ratio, and temperature of 60 min, 30 mL g−1, and 40 °C, respectively. The UAE of DF at optimized conditions was compared to the hot water extraction method (HEM). The obtained DF from BPP under optimized conditions of UAE and HEM was analyzed and compared for physicochemical properties, functional properties and thermal properties. In the food sector, DF can be possibly used in processed food, bakery products, and dairy products for improving food quality and properties.
Sustainability spotlightBhimkol peel can be transformed into valuable resources, thus reducing environmental impact and promoting sustainability by creating eco-friendly products such as food products and packaging materials. Bhimkol peel is a good source of dietary fibre, which can be extracted using green technologies. Ultrasonic-assisted extraction technique can enhance the quantity and quality of dietary fibre obtained from plant-based sources. Ultrasonic extraction is more effective and faster than conventional techniques, utilizing less chemicals and time. This green approach assists the development and comparison of dietary fibre obtained from waste for sustainable food technologies. |
Introduction
Bhimkol (Musa balbisiana) belongs to the Musaceae family, which is native to the eastern South region and northern southeast region of Asia;1 its domestication started approximately 7000 years ago in southeast Asia.2 Bhimkol is a famous variety of banana in northeast India and is abundant in nutrients such as proteins, fats, carbohydrates (dietary fiber) and micronutrients such as Mg, Zn, K, Se, and amino acids.3 Thus, being a rich source of minerals, it is widely used for developing functional foods.Dietary fiber (DF) is classified based on its water solubility into insoluble dietary fiber (IDF) and soluble dietary fiber (SDF). IDF includes cellulose, hemicellulose and lignin, while SDF comprises pectin, mucilage and β-glucans.4,5 The structure and composition of DF include oligosaccharides and polysaccharides such as cellulose, hemicellulose, resistant starch, gums, pectin, waxes, lignin, mucilage, and glucans, which may have great physiological effects on humans.6 However, although DF may be partially or fully fermented in the large intestine, it remains resistant to absorption and digestion in the small intestine.7 DF has various health beneficial properties; for instance, it improves gut health, reduces blood cholesterol, reduces the risk of obesity, reduces the risk of cardiovascular disease, controls blood sugar, and reduces constipation.4,8–13
The essential nutrient DF is widely obtained from fruits and vegetables, oat bran, barley bran, wheat bran, cereals, and nuts.14,15 However, considerable loss may occur during the pre- and post-harvest processing of various food items. DF is also extracted from the fruit, vegetable, and agriculture waste such as papaya peel, kiwifruit, lemon seeds, orange seeds, grapefruit seeds, coconut flour, potato residue, okara, and mango peel.4,5,12,16–19 Fruit, vegetable and agricultural wastes consist of fats, proteins, SDF, IDF, and other components.14,20 Thus, the above-mentioned sources can be used to extract various types of DF.
Many different methods can be used to extract DF, including drying, chemical methods, enzymatic methods, ultrasound-assisted extraction, microwave-assisted extraction, high hydrostatic pressure, and subcritical water extraction.18,19,21,22 Ultrasonic-assisted extraction (UAE) is a potent method for separating bioactive substances from different plant sources. UAE uses high-frequency sound waves to enhance the extraction process and provide significant yields that are effective.19 Phytochemicals such as polyphenols, vitamins, and polysaccharides are examples of bioactive compounds. Because ultrasonic irradiation greatly improves mass transfer processes, thus, it is a recommended technique for producing high-quality extracts.23,24 UAE has been previously used to extract DF from various sources such as soybean residues,25 papaya peel,12 defatted coconut flour,19 banana bracts,13 and mango peel.19 DF is used in several food products, cosmetic, and pharmaceutical sector. Additionally, it can improve the taste of food products such as meatballs, baked goods, pasta, and ice cream.26–29
Based on the above discussion, the current study focused on optimizing the processing condition of DF obtained from BPP using the UAE method based on the yield. Additionally, the UAE method DF extraction was compared with the hot water bath extraction method (HEM). In order to ensure the highest yield of DF, the amounts of the preparation factors were optimized using central composite design (CCD). The properties of DF were compared based on the structural and morphological characteristics and physical properties (bulk density, tapped density, angle of repose, and density). Additionally, the physicochemical properties of DF such as WHC (water holding capacity), OHC (oil holding capacity), and thermal properties and GAC (glucose absorption capacity) were also compared.
Experimental
Raw material
Fresh and mature northeast varieties of bhimkol banana were purchased from a local market of Tezpur, Napaam, Assam, India. For the study, sodium hydroxide and selenium dioxide were procured from Thermo Fisher Scientific India Pvt. Ltd, Mumbai. Copper sulphate, potassium sulphate, and sulfuric acid were obtained from Avantor Performance Materials India Ltd, Maharashtra. Hydrochloric acid and boric acid were supplied by Merck Specialities Private Ltd, Mumbai. Petroleum benzene was received from Merck Life Science Private Ltd, Mumbai. Hexane was obtained from Sisco Research Laboratories Pvt. Ltd, Mumbai. Further, 3,5-dinitrosalicylic acid was obtained from Loba Chemie Pvt. Ltd, Mumbai. Sodium hydrogen phosphate, sodium potassium tartrate, and glucose were supplied by Himedia Laboratories Pvt. Ltd, Maharashtra. All the chemicals were used without any further purification.Extraction of dietary fiber from bhimkol peel using different techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 w/v for 72 h under ambient temperature to remove the fat content with slight modification to the procedure followed earlier by Wachirasiri et al., 2009; Wu et al., 2020.30,31 After the treatment, hexane was evaporated using a hot air oven at 50 °C and stored for further treatment.
2.5 w/v for 72 h under ambient temperature to remove the fat content with slight modification to the procedure followed earlier by Wachirasiri et al., 2009; Wu et al., 2020.30,31 After the treatment, hexane was evaporated using a hot air oven at 50 °C and stored for further treatment.
| Independent variables | Symbols | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| a Where X1 represents time (min), X2 represents solvent/solid ratio (mL g−1), and X3 represents temperature (°C). | ||||
| Time (min) | X 1 | 20 | 40 | 60 |
| Solvent/solid ratio (mL g−1) | X 2 | 30 | 50 | 70 |
| Temperature (°C) | X 3 | 40 | 60 | 80 |
The extraction yield (%) of DF was measured using eqn (1).
 | (1) |
Optimization of dietary fiber using central composite design (CCD)
CCD design was applied for optimizing DF extraction. Three independent variables such as (1) time (20 to 60 min) (X1), (2) solvent–solid ratio (30 to 70 mL g−1) (X2), and (3) temperature (40 to 80 °C) (X3) and one dependent variable, viz., yield (%), were taken for the optimization. There were 20 experiments in CCD, including 6 centre points, 6 axial points, and 8 factorial points. The obtained experimental data were fitted into a quadratic model, as represented in eqn (2) below. | (2) |
Characterization of dietary fiber
Bulk density. The bulk density (ρ bulk) of the samples (UT-BPP, HEM-BPP & UAE-BPP) was determined according to the method followed by Savlak et al., 2016 (ref. 35) with minor modifications. The samples were weighed (3 g) and their volume measured using a (10 mL) graduated cylinder. The ratio of the mass of the extracted samples to the volume inside the cylinder provides the value of bulk density, as represented in the eqn (3) below.
 | (3) |
Tapped density. For measuring the tapped density, a constant volume was taken in a 10 mL graduated cylinder and a glass rod was used to tap to a constant volume.35 The tapped density was calculated following eqn (4) below.
 | (4) |
Angle of repose. A funnel, which was placed at a certain height (2 cm), was gradually moved upwards when the samples (UT-BPP, HEM-BPP & UAE-BPP) fall on a flat surface (graph paper), forming a heap. This helped to keep the powder tip and funnel at a constant height and created a pile powder. As the powder touched the tip of the funnel, the funnel was removed and a circular mark was plotted against the perimeter of the powder heap, measuring the diameter and height of the heap, using a ruler and plotting it against the given formula (5).
 | (5) |
Density & volume. Gas pycnometry (Make # Porous Materials, inc., USA Model # PYC-100A serial no. #01262016-3269) is a non-destructive method that is used to measure the volume by the gas displacement method, which makes it perfect for determining the real density. For the analysis, samples (UT-BPP, HEM-BPP & UAE-BPP) of known weight (5 g) were sealed inside a compartment maintained at a constant temperature. The system was then filled with helium for measuring the volume and density.
Water holding capacity (WHC). WHC was determined according to the method used by Wang et al., 2021 and Moczkowska et al., 2019 (ref. 4 and 32) with minor modification. Firstly, 20 mL of distilled water was used individually to dissolve 0.5 g of the samples (UT-BPP, HEM-BPP & UAE-BPP) (W1). The samples were kept for 24 h at the ambient temperature, after which they were centrifuged for 15 min using a centrifuge (Make # Remi Elektrotechnik Ltd, India Model # R-8C PLUS serial no. #ZILN-46961) at 6700 rpm and 25 °C. After centrifugation, the weight (W2) was measured and the residues were immediately removed. At the end, the WHC was calculated using the following formula (6).
 | (6) |
Oil holding capacity (OHC). OHC was measured by following the procedure used by Zhang et al., 2017 with minor modification.12 For the analysis, 0.5 g of the samples (UT-BPP, HEM-BPP & UAE-BPP) (W1) were added in 5 mL of refined soybean oil and kept for 1 h at 5 °C. They were then centrifuged (Make # Remi Elektrotechnik Ltd, India Model # R-8C PLUS serial no. #ZILN-46961) at 6700 rpm for 15 min at 25 °C, followed by the removal of the sediments, and the weight (W2) was again recorded.4,30 OHC was determined using eqn (7).
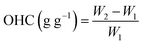 | (7) |
Glucose absorption capacity. The GAC was analyzed according to the method described by Gan et al., 2020; Ma & Mu 2016, Wang et al., 2021, with some modifications.4,36,37 The extracted samples (UT-BPP, HEM-BPP & UAE-BPP) (0.25 g) were mixed with 25 mL of glucose (50 and 100 mmol L−1) in a centrifuge tube and was kept in an incubator (Make # Scigenics Biotech Pvt. Ltd India Model # ORBITEK LETTD serial no. #2012 400 106) at 37 °C for 6 h. After centrifuging (Make # Remi Elektrotechnik Ltd, India Model # R-8C PLUS serial no. #ZILN-46961) the samples at 4800 rpm for 10 min, 0.5 mL of the supernatant was transferred into a glass tube, where distilled water was added to the tube until the volume reached 3 mL, followed by mixing with 2 mL of 3,5-dinitro-salicylic acid (DNS), a color development reagent. The mixture was then incubated in a water bath (Make # JEIO TECH, Korea Model # BW-20 G serial no. #T039511) at 95 °C for 10 min. After the solution temperature dropped to room temperature, it was shaken using a vortex, and the residual concentration of glucose was measured at 520 nm using a spectrophotometer (Make # Thermo Fisher Scientific Model # AQUAMATE 8100 serial no. #9A8Z246013 Verna Rd. Madison, WI 53711, USA, assembled in China) and quantified based on the standard curve.
| GAC (mmol g−1) = Ci − CS × V/S | (8) |
Thermogravimetric analysis (TGA). Thermogravimetric analysis (TGA) of the samples (UT-BPP, UAE-BPP & HEM-BPP) was carried out using a thermogravimetric analyzer (Make # Netzsch, Germany Model # TG 209 F1 LIBRA).19,36 For the analysis, the samples were kept in a TGA crucible and heated from 25 to 600 °C at 10 °C min−1 with a gas flow rate of 20 mL min−1 under a high-purity N2 atmosphere.
Differential scanning calorimetry (DSC). For the DSC analysis, the DSC (Model # DSC 214 POLYMA, Make # Netzsch, Germany) apparatus was calibrated utilizing an empty aluminium pan as a point of reference. The characterization was done at a rate of 20 °C min−1, a gas flow rate of 40 mL min−1 was selected, and the selected temperature range for the analysis was from 20 to 450 °C. An aluminium pan was filled with the sample (15 mg) and covered with an hermetically sealed aluminium lid. The peak temperature (Tp) and thermal transition (ΔH) were determined. Additionally, melting temperature, glass transition temperature, crystalline temperature, and latent and specific heats were also measured.
X-ray diffraction (XRD). An X-ray diffractometer operated with Cu radiation at 40 kV/125 mA of incident current was used to perform X-ray diffraction (Make # BRUKER, Germany Model # D8 FOCUS) of the samples. An angle of diffraction between 10° and 80° was selected for the characterization with a step size of 0.5°. The crystallinity index (CI) of the DF samples was obtained using the diffraction intensity data in accordance with Ma & Mu 2016; Gan et al., 2020.36,37
Fourier transform infrared (FTIR) spectroscopy. For the analysis, the extracted DFs were thoroughly mixed with KBr in a ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and pelletized. The FTIR spectra of the DFs obtained by three different extraction methods (UT-BPP, UAE-BPP & HEM-BPP) were recorded using an FTIR spectrometer (Make # BRUKER, Germany Model # D8 FOCUS) in the wavelength range from 400 to 4000 cm−1 with 32 scans and a resolution of 4 cm.
3 and pelletized. The FTIR spectra of the DFs obtained by three different extraction methods (UT-BPP, UAE-BPP & HEM-BPP) were recorded using an FTIR spectrometer (Make # BRUKER, Germany Model # D8 FOCUS) in the wavelength range from 400 to 4000 cm−1 with 32 scans and a resolution of 4 cm.
Statistical analysis
The statistical analysis was performed using the Design Expert software version 13. The mean ± standard deviation (SD) of three parallel measurements was used to represent the results, and a significance level of p < 0.05 was used to evaluate mean differences using analysis of variance (ANOVA) polynomial quadratic regression model.Results and discussion
Proximate compositions of bhimkol peel powder
The agro-industrial waste bhimkol peel is considered to be a remarkable resource of DF.38,39 The BPP obtained after cutting, blanching, drying, and sieving was analyzed for the proximate composition as represented in Table 2. The crude fiber content of BPP was found to be around 23.39 ± 1.10%, which is found to be in line with the results from banana peel (19.0%) as reported by Pyar & Peh 2018.38 Additionally, the other proximate compositions of BPP were found as 7.30%, 2.22%, 1.40%, and 10.47% for protein, fat, moisture content, and ash content, respectively. Similar proximate compositions of banana peels have previously been reported in several research study.30,40,41 Considering this, bhimkol peels are a good resource of crude fiber. Further, the various extraction techniques are employed to extract DF from BPP such as UAE, and hot water bath extraction.Optimization of extraction of dietary fiber from bhimkol peel powder
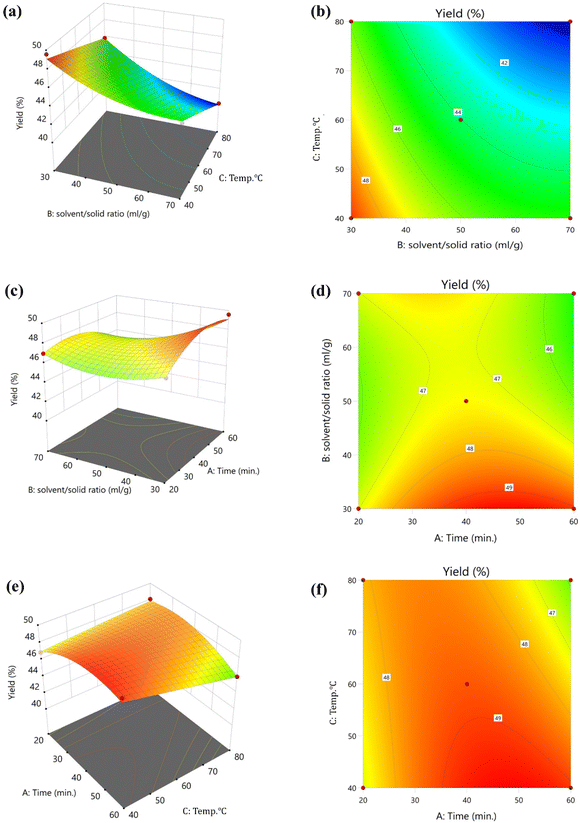
The optimization of processing conditions to extract bhimkol (Musa balbisiana) peel powder (BPP)-based DF was done using response surface methodology. The factors and level of the central composite design (CCD) for the extraction such as independent variables including time (20 to 60 min) (X1), solvent–solid ratio (30 to 70 mL g−1) (X2), and temperature (40 to 80 °C) (X3) and one dependent variable such as yield (%) have been represented in Fig. 1 and Table 3. The entire experimental design representing the actual, predicted value, and coded values of the independent variables along with the obtained yield of DF factors has been represented in Fig. 2 and Table 3.| Run | Coded variable levels | Yielda (%) | |||
|---|---|---|---|---|---|
| Factor 1 A: time (min) | Factor 2 B: solvent/solid ratio (mL g−1) | Factor 3 C: temperature (°C) | Actual value | Predicated value | |
| a Mean = 3 ± standard deviation. | |||||
| 1 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 46.60 ± 1.56 | 46.28 |
| 2 | 40 (0.00) | 30 (−1.00) | 60 (0.00) | 48.09 ± 0.53 | 48.79 |
| 3 | 20 (−1.00) | 50 (0.00) | 60 (0.00) | 46.16 ± 0.14 | 45.93 |
| 4 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 46.12 ± 1.02 | 46.28 |
| 5 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 45.45 ± 0.48 | 46.28 |
| 6 | 60 (1.00) | 30 (−1.00) | 80 (1.00) | 45.91 ± 0.76 | 45.88 |
| 7 | 60 (1.00) | 30 (−1.00) | 40 (−1.00) | 49.58 ± 0.88 | 49.09 |
| 8 | 40 (0.00) | 70 (1.00) | 60 (0.00) | 46.44 ± 1.19 | 45.92 |
| 9 | 20 (−1.00) | 30 (−1.00) | 40 (−1.00) | 46.91 ± 0.88 | 46.98 |
| 10 | 40 (0.00) | 50 (0.00) | 40 (−1.00) | 47.05 ± 0.41 | 47.27 |
| 11 | 60 (1.00) | 70 (1.00) | 80 (1.00) | 40.25 ± 2.01 | 40.14 |
| 12 | 20 (−1.00) | 30 (−1.00) | 80 (1.00) | 48.15 ± 0.22 | 47.89 |
| 13 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 46.51 ± 0.69 | 46.28 |
| 14 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 46.65 ± 1.07 | 46.28 |
| 15 | 20 (−1.00) | 70 (1.00) | 80 (1.00) | 45.61 ± 0.05 | 46.05 |
| 16 | 40 (0.00) | 50 (0.00) | 60 (0.00) | 46.68 ± 1.05 | 46.28 |
| 17 | 60 (1.00) | 70 (1.00) | 40 (−1.00) | 44.97 ± 1.62 | 45.18 |
| 18 | 40 (0.00) | 50 (0.00) | 80 (1.00) | 45.25 ± 1.09 | 45.21 |
| 19 | 20 (−1.00) | 70 (1.00) | 40 (−1.00) | 46.99 ± 0.05 | 46.97 |
| 20 | 60 (1.00) | 50 (0.00) | 60 (0.00) | 43.62 ± 1.54 | 44.03 |
 | ||
| Fig. 2 Effect of optimization of independent variables (actual and predicted value) on the extraction yield of DF. | ||
Various processing conditions such as time, solvent-to-solid ratio, and processing temperature influence the yield of DF from BPP. Furthermore, the processing time influences the yield of DF and if the extraction time increases, it may cause thermal degradation in the DF. Based on the responses, the highest processing time for extracting DF was 60 min. In the present study, the extraction process was carried out at different times (20, 40, and 60 min), and the highest yield was obtained at 60 min, as shown in Fig. 1a and b. The effect of temperature on the DF yield was determined in the range from 40 to 80 °C. The highest yield of DF was obtained at 40 °C, and the lowest yield was obtained at the highest selected temperatures. According to the study conducted by Zhang et al., 2017,12 the yield of DF increased at 1% NaOH concentration, and temperature also plays a significant role in determining the optimum DF production temperature at 40 °C (Fig. 1c and d). Furthermore, a subsequent increase in temperature resulted in a rapid reduction in the yield of DF from 49.58 ± 0.88% to 40.25 ± 2.01%. The yield of DF was also increased at solvent/solid ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as shown in Fig. 1e and f; similar trends have been observed in the research reported by Sun et al. 2018.25 Considering this, as represented in Table 4, the results of 20 experiments obtained using a CCD design of RSM to optimize the processing conditions have been represented. The outcomes of single factor experiments were used as basis for these investigations. Polynomial regression model analysis and significance test were done to study the interaction of various independent variables on the yield (Table 4). The extraction yield can be explained by the following quadratic regression model represented in eqn (9).
1, as shown in Fig. 1e and f; similar trends have been observed in the research reported by Sun et al. 2018.25 Considering this, as represented in Table 4, the results of 20 experiments obtained using a CCD design of RSM to optimize the processing conditions have been represented. The outcomes of single factor experiments were used as basis for these investigations. Polynomial regression model analysis and significance test were done to study the interaction of various independent variables on the yield (Table 4). The extraction yield can be explained by the following quadratic regression model represented in eqn (9).
| Y = 42.19 + 0.52X1 − 0.21X2 + 0.11X3 − 0.002X1X2 − 0.002X1X3 − 0.0009X2X3 − 0.003X12 + 0.003X22 − 0.0001X32 | (9) |
| Source | Sum of squares | DF | MSS | F-Value | P-Value | |
|---|---|---|---|---|---|---|
| a R 2 = 0.9585, adjusted R2 = 0.9212, predicated R2 = 0.7213, CV% = 1.14, adequate precision = 24.0604. b df, degrees of freedom; MSS, mean sum of square, CV = coefficient of variation. | ||||||
| Model | 64.05 | 9 | 7.12 | 25.68 | <0.0001 | Significant |
| A-Time (X1) | 9.01 | 1 | 9.01 | 32.49 | 0.0002 | |
| B-Solvent/solid ratio (X2) | 20.68 | 1 | 20.68 | 74.61 | <0.0001 | |
| C-Temp. (X3) | 10.67 | 1 | 10.67 | 38.50 | 0.0001 | |
| AB (X1X2) | 7.62 | 1 | 7.62 | 27.51 | 0.0004 | |
| AC (X1X3) | 8.51 | 1 | 8.51 | 30.70 | 0.0002 | |
| BC (X2X3) | 1.68 | 1 | 1.68 | 6.07 | 0.0334 | |
| A 2 | 4.62 | 1 | 4.62 | 16.65 | 0.0022 | |
| B 2 | 3.20 | 1 | 3.20 | 11.56 | 0.0068 | |
| C 2 | 0.0035 | 1 | 0.0035 | 0.0125 | 0.9133 | |
| Residual | 2.77 | 10 | 0.2772 | |||
| Lack of fit | 1.62 | 5 | 0.3246 | 1.41 | 0.3568 | Not significant |
| Pure error | 1.15 | 5 | 0.2297 | |||
| Cor total | 66.82 | 19 | ||||
The results of ANOVA that was used to determine and sum up the effects of significant variables in both quadratic forms and linear are compiled in Table 4. The model has a P-value of <0.0001 and an F-value of 25.68. Further, the ANOVA quadratic regression model (p < 0.0001) and the R2 value (0.9585) were significant in the extraction of DF. The lack-of-fit was non-significant (p > 0.05), indicating that the yield of DF can be accurately predicted using the quadratic model. In this case, the coefficients X1, X2, X3, X1X2, X1X3, X2X3, X12, and X22 were found to be significant model terms, and the other coefficients were insignificant (p > 0.05).
The lack-of-fit was not significant relative to the pure error because the corresponding F-value was 1.41. In addition, the value of the determination coefficient (R2) was 0.9585, which implies that 95.85% of the variation could be explained by the model. The relatively low values of the coefficient of variation (1.14%) and adequate precision (24.0604) indicate a good model fit.
Extraction of dietary fiber using UAE and hot water extraction method
In this research, the yield of extraction of DF from BPP was compared using two different methods such as UAE and hot water bath extraction method (HEM). The highest yield of DF obtained using the UAE method was 49.19 ± 1.39%, whereas for the HEM method, it was 45.54 ± 2.32%. The highest yield of DF by the ultrasonic bath method was obtained due to the involved frequency of the ultrasonication, which resulted in the breakdown of the cell wall of the sample to extract DF, leading to the increased yield of DF when compared to HEM.12,25 Additionally, the temperature also influenced the yield of DF along with the frequency of ultrasonication.12| Properties | UT-BPP | HEM-BPP | UAE-BPP |
|---|---|---|---|
| Data sets are represented as mean ± SD. Different letters (a, b, c) define the significant differences between samples (p < 0.05). | |||
| Bulk density (g mL−1) | 0.55 ± 0.02a | 0.39 ± 0.01b | 0.31 ± 0.03c |
| Tapped density (g mL−1) | 0.69 ± 0.03a | 0.5 ± 0.02b | 0.38 ± 0.01c |
| Angle of repose (°) | 31.95 ± 1.46a,b | 32.93 ± 1.49a | 29.62 ± 1.29c |
| Density (g cm−3) | 1.61 ± 0.01a | 1.55 ± 0.01c | 1.57 ± 0.01b |
| Volume (cm3) | 1.84 ± 0.01b | 1.91 ± 0.01a | 1.91 ± 0.01a |
 | ||
| Fig. 3 Hydration and functional properties of BPP dietary fiber: (a) oil holding capacity and water holding capacity, (b) glucose absorption capacity, (c) FTIR spectra, and (d) XRD patterns. | ||
The DF for OHC play a critical role in food applications like reducing fat loss during cooking or removing excess fat from human body.4,12 OHC depends on the hydrocolloid, surface properties, hydrophobic characteristics and total electrical charge density.4,44 BPP-based DF has an OHC value ranging from 1.84 to 2.72 g g−1. As shown in Fig. 3a, HEM-BPP had higher ability to retain oil (2.71 ± 0.11 g g−1), whereas DF obtained by UT-BPP has an OHC of 1.84 ± 0.07 g g−1. According to SEM, the partially soft texture produced by ultrasonic treatment may be the source of the higher OHC of BPP-DF.
Additionally, the digestive juice contains DF, which binds to glucose, lowering postprandial blood glucose. Consequently, another major functional characteristic of DF is GAC. As shown in Fig. 3b, we found that samples of DF had a significant effect on glucose binding at different glucose concentrations of 50 mmol g−1 and 100 mmol g−1 and depends upon how the glucose solution will bind with the sample and its reaction.
The GAC glucose concentration of UT-BPP-based DF at 100 mmol g−1 concentration was found to be higher than that at 50 mmol g−1 concentration, as shown in Fig. 3b. Wang et al., 2021 observed that IDF absorbs more glucose as compared to SDF.4 This finding suggest that DF extraction methods may have an impact on GAC.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) might be the characteristic asymmetric and symmetric absorption; similar results were obtained by Gan et al., 2020.36 The C–O stretching vibration, which may be the bending vibration of primary alcohols or C–O–C and C–O, was specifically responsible for the distribution of peaks at 1230–1040 cm−1. The ether stretching vibration peak is at 1205 cm−1 for C–O–C, and the C–O peak is at the 1063 cm−1.24
O) might be the characteristic asymmetric and symmetric absorption; similar results were obtained by Gan et al., 2020.36 The C–O stretching vibration, which may be the bending vibration of primary alcohols or C–O–C and C–O, was specifically responsible for the distribution of peaks at 1230–1040 cm−1. The ether stretching vibration peak is at 1205 cm−1 for C–O–C, and the C–O peak is at the 1063 cm−1.24
Thermal properties
 | ||
| Fig. 4 (a) Thermogravimetric and differential scanning calorimetry curves of BPP dietary fiber: (b) UT-BPP, (c) HEM-BPP, and (d) UAE-BPP. | ||
| Samples | T 10 (°C) | T 50 (°C) |
|---|---|---|
| UT-BPP | 162.30 | 317.26 |
| HEM-BPP | 69.75 | 304.74 |
| UAE-BPP | 117.30 | 324.78 |
| Sample | Endothermic reaction | Exothermic reaction | ||||
|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | End set (°C) | Onset (°C) | Peak (°C) | End set (°C) | |
| UT-BPP | 43.7 | 74.9 | 111.8 | 268.2 | 300.3 | 323.1 |
| HEM-BPP | 42.8 | 76.6 | 115.0 | 249.9 | 290.0 | 332.0 |
| UAE-BPP | 57.2 | 96.5 | 134.8 | 250.0 | 327.1 | 365.6 |
Conclusions
In this study, the optimization of DF extraction from BPP using the UAE method was done through RSM (CCD). The extracted DF was analyzed for its physicochemical, hydration, functional, and thermal properties and compared with the conventional extraction method. The results show that using an alkaline solvent for UAE gave a higher yield of DF (49.19%) compared to the hot water extraction method (45.54%). UAE-based DF extraction methods often yield DF with superior properties compared to traditional hot water extraction. Furthermore, the DF extracted using UAE-BPP exhibited enhanced WHC, OHC, crystallinity, and thermal properties, indicating improved functional properties. The obtained DF can be utilized in food sectors, such as in bakery, dairy products, and processed foods for improved food properties and qualities.Data availability
The data can be obtained upon request to the authors.Author contributions
Laxmikant Rawat: original draft writing, review and editing. Tabli Ghosh: conceptualization, supervision, validation, final review and editing.Conflicts of interest
The authors declare no conflict of interest related to this research.Acknowledgements
The authors are thankful to the Sustainable Food Lab at the Department of Food Engineering and Technology, School of Engineering, Tezpur University, Assam, India, for the funding.References
- T. Ghosh and K. K. Dash, Engineering in Agriculture, Environment and Food, 2018, 11, 186–195 CrossRef.
- M. B. Gogoi, I. Chetia, B. K. Sarmah, T. Nath, S. Bhowal, K. Dey and P. Bhorali, Int. J. Curr. Microbiol. Appl. Sci., 2020, 9, 2555–2565 CrossRef CAS.
- R. B. Watharkar, S. Chakraborty, P. P. Srivastav and B. Srivastava, J. Food Meas. Char., 2021, 15, 3336–3349 CrossRef.
- K. Wang, M. Li, Y. Wang, Z. Liu and Y. Ni, Food Hydrocolloids, 2021, 110, 106162 CrossRef CAS.
- E. Karaman, E. Yılmaz and N. B. Tuncel, Bioact. Carbohydr. Diet. Fibre, 2017, 11, 9–17 CrossRef CAS.
- L. Cheng, X. Zhang, Y. Hong, Z. Li, C. Li and Z. Gu, Int. J. Biol. Macromol., 2017, 101, 1004–1011 CrossRef CAS PubMed.
- K. S. Poutanen, S. Fiszman, C. F. Marsaux, S. P. Pentikainen, R. E. Steinert and D. J. Mela, Am. J. Clin. Nutr., 2018, 108, 437–444 CrossRef PubMed.
- L. R. Ferguson, R. R. Chavan and P. J. Harris, Nutr. Cancer, 2001, 39, 155–169 CrossRef CAS.
- A. Kurhade, S. Patil, S. K. Sonawane, J. S. Waghmare and S. S. Arya, J. Food Meas. Char., 2016, 10, 32–41 CrossRef.
- Y. A. McKenzie, R. K. Bowyer, H. Leach, P. Gulia, J. Horobin, N. A. O'Sullivan and IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association, J. Hum. Nutr. Diet., 2016, 29, 549–575 CrossRef CAS.
- A. Ren, L. Chen, W. Zhao, S. Shan, Z. Li and Z. Tang, J. Funct. Foods, 2023, 107, 105659 CrossRef CAS.
- W. Zhang, G. Zeng, Y. Pan, W. Chen, W. Huang, H. Chen and Y. Li, Carbohydr. Polym., 2017, 172, 102–112 CrossRef CAS PubMed.
- Y. A. Begum and S. C. Deka, J. Food Process. Preserv., 2019, 43, e14256 CAS.
- V. V. Khanpit, S. P. Tajane and S. A. Mandavgane, Biomass Convers. Biorefin., 2021, 1–20 Search PubMed.
- D. Dhingra, M. Michael, H. Rajput and R. T. Patil, J. Food Sci. Technol., 2012, 49, 255–266 CrossRef CAS PubMed.
- Q. Ma, W. Wang, Z. Ma, Y. Liu, J. Mu, J. Wang and X. Hui, J. Funct. Foods, 2021, 84, 104606 CrossRef CAS.
- X. Du, L. Wang, X. Huang, H. Jing, X. Ye, W. Gao and H. Wang, LWT, 2021, 143, 111031 CrossRef CAS.
- E. Perez-Lopez, I. Mateos-Aparicio and P. Ruperez, J. Food Sci. Technol., 2017, 54, 1333–1339 CrossRef CAS PubMed.
- B. Kaur, P. S. Panesar and A. Thakur, Biomass Convers. Biorefin., 2021, 1–10 CAS.
- I. Buljeta, D. Subaric, J. Babic, A. Pichler, J. Simunovic and M. Kopjar, Appl. Sci., 2023, 13, 9309 CrossRef CAS.
- Y. Y. Yang, S. Ma, X. X. Wang and X. L. Zheng, J. Chem., 2017, 9340427 Search PubMed.
- V. Tejada-Ortigoza, T. Garcia-Cayuela, J. Welti-Chanes, M. P. Cano and J. A. Torres, Science and Technology of Fibers in Food Systems, 2020, pp. 363–381 Search PubMed.
- H. Fooladi, S. A. Mortazavi, A. Rajaei, D. Salar Bashi and S. Savabi Sani Kargar, Optimize the extraction of phenolic compounds of jujube (Ziziphus Jujube) using ultrasound-assisted extraction method, 2013, https://d1wqtxts1xzle7.cloudfront.net/77283724/271_1-libre.pdf?1640368532=&response-contentdisposition=inline%3B+filename%3DOptimize_the_extraction_of_phenolic_comp.pdf&Expires=1730182109&Signature=C4Sbpr9c58bX5v4ARYimUO~whZgzpjmM0W9umUApJJ4xeE4bAT5~nSuLfIm~Xlo4C0XgtBzhqvlp7jj-B1sZxSe4NGyz261YupZyU23u0kSjt3R7BrhFjc46VsB4YZhQePvtVElPIIBOwejNFoVvvUEf6VkouC3BoqaT62mnUHcB4jhqfB4j~Fb1XjdE83UqQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Search PubMed.
- C. Chen, L. J. You, A. M. Abbasi, X. Fu and R. H. Liu, Carbohydr. Polym., 2015, 130, 122–132 CrossRef CAS PubMed.
- J. Sun, Z. Zhang, F. Xiao, Q. Wei and Z. Jing, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 392, 052005 Search PubMed.
- S. Adapa, H. Dingeldein, K. A. Schmidt and T. J. Herald, J. Dairy Sci., 2000, 83, 2224–2229 CrossRef CAS.
- Y. Niu, N. Li, Q. Xia, Y. Hou and G. Xu, LWT, 2018, 88, 56–63 CrossRef CAS.
- N. Aravind, M. Sissons and C. M. Fellows, Food Chem., 2012, 131, 893–900 CrossRef CAS.
- S. Martinez-Cervera, A. Salvador, B. Muguerza, L. Moulay and S. M. Fiszman, LWT, 2011, 44, 729–736 CrossRef CAS.
- P. Wachirasiri, S. Julakarangka and S. Wanlapa, Songklanakarin J. Sci. Technol., 2009, 31, 605–611 Search PubMed.
- C. Wu, F. Teng, D. J. McClements, S. Zhang, Y. Li and Z. Wang, Food Res. Int., 2020, 134, 109251 CrossRef CAS.
- M. Moczkowska, S. Karp, Y. Niu and M. A. Kurek, Food Hydrocolloids, 2019, 90, 105–112 CrossRef CAS.
- M. A. Kurek, S. Karp, J. Wyrwisz and Y. Niu, Food Hydrocolloids, 2018, 85, 321–330 CrossRef CAS.
- Q. Yuan, S. Lin, Y. Fu, X. R. Nie, W. Liu, Y. Su and D. T. Wu, Int. J. Biol. Macromol., 2019, 127, 178–186 CrossRef CAS.
- N. Savlak, B. Turker and N. Yeşilkanat, Food Chem., 2016, 213, 180–186 CrossRef CAS.
- J. Gan, Z. Huang, Q. Yu, G. Peng, Y. Chen, J. Xie and M. Xie, Food Hydrocolloids, 2020, 101, 105549 CrossRef CAS.
- M. M. Ma and T. H. Mu, Food Chem., 2016, 194, 237–246 CrossRef CAS.
- H. Pyar and K. K. Peh, Res. J. Chem. Environ., 2018, 22, 108–111 Search PubMed.
- M. K. Saeed, N. Zahra, A. Saeed, S. Y. E. D. Quratulain and S. H. I. ABIDI, Acta Pharm. Sci., 2024, 62, 89–103 CAS.
- E. H. Lee, H. J. Yeom, M. S. Ha and D. H. Bae, Food Sci. Biotechnol., 2010, 19, 449–455 CrossRef CAS.
- A. N. Tsado, N. R. Okoli, A. G. Jiya, D. Gana, B. Saidu, R. Zubairu and I. Z. Salihu, BIOMED Natural and Applied Science, 2021, 1, 032–042 CrossRef.
- J. Ahmed, A. Taher, M. Z. Mulla, A. Al-Hazza and G. Luciano, J. Food Eng., 6186, 201, 34–41 Search PubMed.
- M. S. Anuar, S. M. Tahir, M. I. Najeeb and S. B. Ahmad, Adv. Mater. Processes Technol., 2019, 5, 181–190 Search PubMed.
- M. Jia, J. Chen, X. Liu, M. Xie, S. Nie, Y. Chen and Q. Yu, Food Hydrocolloids, 2019, 94, 468–474 CrossRef CAS.
- M. C. Rouhou, S. Abdelmoumen, S. Thomas, H. Attia and D. Ghorbel, Int. J. Biol. Macromol., 2018, 116, 901–910 CrossRef.
- S. Wang, Y. Fang, Y. Xu, B. Zhu, J. Piao, L. Zhu and J. Wu, J. Funct. Foods, 2022, 93, 105081 CrossRef CAS.
- D. Mudgil, S. Barak and B. S. Khatkar, Int. J. Biol. Macromol., 2012, 50, 1035–1039 CrossRef CAS.
- Y. Wang, W. Wang, Y. Wu, J. JiLiu, X. Hu, M. Wei and L. Cao, Front. Nutr., 2023, 10, 1157015 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |