In vitro digestibility and physicochemical analysis of heat-moisture treated finger millet flour and starch†
Received
14th August 2024
, Accepted 16th October 2024
First published on 12th November 2024
Abstract
Heat-moisture treatment (HMT) at 110 °C for 6 h (25% moisture content) of finger millet flour (FMF) and starch (FMS) was conducted to assess the effect on their functional, physico-chemical, and in vitro digestibility properties. Water activity (aw) and pH decreased significantly (p < 0.05) from 0.31 to 0.25 and 6.7 to 6.3 respectively for HMT samples. The oil absorption capacity (OAC), water absorption index (WAI), water solubility index (WSI), and swelling power (SP) also significantly (p < 0.05) increased due to heat-moisture treatments of the samples. The values for the OAC, WAI, WSI, and SP were in the ranges of 1.9–2.5 g g−1, 2.1–10.7 g g−1, 0.14–0.44%, and 3.4–18.4 g g−1, respectively. X-ray diffractometry (XRD) revealed that the HMT-modified samples showed a significant decrease in the relative crystallinity. Scanning electron microscopy (SEM) showed that the FMF sample became clumpier, and the surface of FMS showed more porosity and cracks due to the HMT process. Fourier Transform Infrared (FTIR) spectroscopy indicated the presence of hydroxyl (–OH), alkane (–CH), amine (–NH), carbonyl (–COH), and alkene (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) functional groups in the regions of 3300–3250, 2930–2850, 1750–1630, 1180–1070, and 930–860 cm−1, respectively. There were no significant changes observed in the number of peaks of the samples due to the HMT process. The rapidly digestible and slowly digestible starch fractions increased significantly (p < 0.05), while the resistant starch fraction decreased due to the HMT process. RDS, SDS, and RS values were in the ranges of 14.3–22.4%, 28.0–60.9%, and 30.9–55.7%, respectively. This study provides a new way of utilizing this starch source for the development of food products and can reduce dependence on other starch sources such as rice and corn.
CH) functional groups in the regions of 3300–3250, 2930–2850, 1750–1630, 1180–1070, and 930–860 cm−1, respectively. There were no significant changes observed in the number of peaks of the samples due to the HMT process. The rapidly digestible and slowly digestible starch fractions increased significantly (p < 0.05), while the resistant starch fraction decreased due to the HMT process. RDS, SDS, and RS values were in the ranges of 14.3–22.4%, 28.0–60.9%, and 30.9–55.7%, respectively. This study provides a new way of utilizing this starch source for the development of food products and can reduce dependence on other starch sources such as rice and corn.
1 Introduction
Finger millet is a semi-arid crop cultivated in Sri Lanka, the Himalayan regions of the Indus Valley, Bhutan, Nepal, China, Japan, and Africa.1–3 Finger millet is a gluten-free, low glycemic index crop, good for people with diabetes and celiac disease.4,5 The finger millet grain consists of carbohydrates, protein, dietary fiber, calcium, and iron in high concentrations.6 The major component of finger millet is starch, consisting of 58–85% of total grain weight,7 which influences its textural and rheological properties.8 However, despite the high amount of starch, starch from millets is rarely used in the food industry due to its poor water solubility, high tendency to retrograde, low paste clarity and shear resistance, and high sensitivity to pH, shear, and heat due to its semi-crystalline structure.9–12 Due to these challenges, the need has increased for chemical and physical modification of starch, which can provide desired attributes to starch. Chemically modified starches are used for industrial purposes, while physically modified starches are favored in the food and pharmaceutical sectors.13 To manufacture natural food constituents, there is a focus on altering the characteristics of starches without using chemicals.14 Starch modification by physical treatments such as radiation, pulsed electric field, shear, osmotic pressure, annealing, heat-moisture treatment (HMT), and microwaves has been widely accepted in the food industry because these processes can alter the physico-chemical and functional properties of samples.12,15,16
HMT is a low moisture (<35%) and high temperature (up to 120 °C) technique applied from 15 min to 16 h.15 HMT influences granular swelling, amylose leaching, pasting properties, gelatinization behavior, molecular structure, crystalline structure, and in vitro digestibility of starch17,18 with the least influence on the granular structure.19 HMT encourages the interaction between amylopectin and amylose chains20 by disrupting the helical and crystalline structures. Subsequently, the re-association of the disturbed crystals and the rearrangement of the amorphous structure provide a new order to double helices.15,21,22 HMT induces changes in both crystalline and amorphous regions of starch granules.23 Due to the structural reorganization of starch, granular crystallinity, swelling, amylose leaching, gelatinization, thermal stability, paste stability, and retrogradation changes occur.18,23 Due to the HMT of starch, improvements in the adhesiveness, hardness, and shear stability of starch gel,24 an increase in the gelatinization temperature and decrease in the gelatinization temperature range,23 and an increase in the shear resistance and starch stability25,26 have been reported. HMT does not obliterate the starch granule structure but alters the hydration, crystallinity, gelatinization, and viscosity characteristics, so heat-moisture treated starch can be applied to several products27 including confections, retort foods, salad dressings, batter products,28 gluten free bakery products,27 gluten free porridges, steam-cooked and boiled products, and alcoholic and non-alcoholic beverages.29–31
Many studies have been conducted to evaluate the influence of HMT on starch and flour of different sources including proso millet,32 white finger millet,33 pearl millet,28 finger millet,23 rice,34 potato,35 maize,36 cassava,37 and wheat.38 However, the investigation of the influence of HMT on the physico-chemical, functional, rheological, thermal, pasting, and digestibility properties of finger millet starch (FMS) and its flour (FMF) and relative analysis are sparse. Thus, the objective of this study was to overcome these challenges and evaluate the effect of HMT on the physico-chemical, functional, pasting, gelatinization, morphological, and digestibility properties of FMF and FMS. This study discussed the detailed mechanism of HMT, which can provide a new way of starch utilization from finger millet to reduce dependence on other starch sources such as rice, corn, and cassava.
2 Materials and methods
2.1 Materials
Ragi finger millet (Eleusine coracana) was procured from Darich Green Co., Ltd. Bangkok, Thailand. All other reagents were analytical grade, including phosphate buffer, NaOH, HCl, 3-5-dinitro salicylic acid, pancreatic amylase, distilled water (DW), and trypsin, and were purchased from CTI Science Co. Ltd, Thailand. The total starch assay kits were purchased from Megazyme International, Ireland.
2.2 Preparation of finger millet flour
Finger millet grains (200 g) were pulverized to powder utilizing a high-speed milling machine (DXM-400, DXFILL, Thailand) at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3–5 min. This finger millet flour (FMF) was sifted through a 70-mesh sieve to remove the bigger particles and was stored in zip-locked high-density polyethylene (HDPE) bags at 4 °C for further analysis.
000 rpm for 3–5 min. This finger millet flour (FMF) was sifted through a 70-mesh sieve to remove the bigger particles and was stored in zip-locked high-density polyethylene (HDPE) bags at 4 °C for further analysis.
2.3 Starch isolation
Finger millet starch (FMS) was extracted using an alkaline steeping method.39 A grain sample of 200 g was soaked in NaOH (1000 mL, 0.2% (w/v)) at 4 °C for 24 h. The samples were decolorized by washing with tap water and ground using a high-speed mixer (IKA, Germany). The slurry was passed through a 200-mesh sieve and centrifuged for 15 min at 2400 × g using a centrifuge (EBA 8S, Hettich, Germany). Supernatants were removed, and the rest was blended again in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/v DW for 30 min and centrifuged for 15 min at 2400 × g using a centrifuge (EBA 8S, Hettich, Germany). This washing and centrifugation step was repeated until only white color starch was achieved at the bottom of the test tube. The samples were further washed for 15 min using ethanol (100%) and centrifuged to discard the supernatant. The samples were neutralized (pH 6.5) by adding HCl (1 M), while pH was monitored using a pH meter (Hanna, USA). The samples were dried in a hot air oven (Memmert, UN, Schwabach, Germany) at 45 °C and 50% relative humidity for 6 h. The dried samples were powdered using a hammer mill (Perten, 120, Finland) and passed across a 100-mesh sieve. The starch powders were stored in HDPE bags at 4 °C for further analysis.
3 w/v DW for 30 min and centrifuged for 15 min at 2400 × g using a centrifuge (EBA 8S, Hettich, Germany). This washing and centrifugation step was repeated until only white color starch was achieved at the bottom of the test tube. The samples were further washed for 15 min using ethanol (100%) and centrifuged to discard the supernatant. The samples were neutralized (pH 6.5) by adding HCl (1 M), while pH was monitored using a pH meter (Hanna, USA). The samples were dried in a hot air oven (Memmert, UN, Schwabach, Germany) at 45 °C and 50% relative humidity for 6 h. The dried samples were powdered using a hammer mill (Perten, 120, Finland) and passed across a 100-mesh sieve. The starch powders were stored in HDPE bags at 4 °C for further analysis.
2.4 Heat-moisture treatment (HMT) of samples
The heating time and temperature were selected based on a preliminary study indicating the highest values for functional properties. The weight of water added to the dried grains was estimated using eqn (1). 100 g of samples (FMF and FMS) was stored in a fridge (4 °C) for 24 hours to achieve moisture balance. The initial and final moisture of the samples was measured using a microwave moisture analyzer (DFMC Liaoning, China) and dry mass (Wd) was calculated using eqn (1), while the amount of water was estimated by subtracting the dry mass from the total sample weight (Wt).| | | Wd = Wt × (1 − percentage moisture) | (1) |
The amount of water (Ww) to be added was calculated according to the required moisture content of 25% (M1) using eqn (2).
| | | Ww = Wd × 100/(100 − M1) | (2) |
The moist samples were sealed in glass bottles and placed in a hot air oven (Memmert, UN, Schwabach, Germany) to heat at 110 °C for 6 h. The samples were manually shaken every 20 min and turned upside down. After this treatment, the samples were spread in a tray and moisture was removed from the samples using a hot air oven (Memmert, UN, Schwabach, Germany) at 40 °C and 50% relative humidity for 6 h. The samples were cooled to 25 °C and crushed into a fine powder using a hammer mill (Perten, 120, Finland). The processed samples of FMF and FMS were powdered using a hammer mill (Perten, 120, Finland) and passed through 70 and 100-mesh sieves, respectively. The samples were kept in HDPE bags at 4 °C for further analysis.33
2.5 Physico-chemical properties
The carbohydrate, ash, fat, and protein contents were estimated according to AOAC standard protocols.40 The starch content was estimated according to the method described by McCleary et al. (2019) using a starch assay kit (Megazyme International, Ireland).41 The pH was observed using a Hanna pH meter (Hanna, USA) and the water activity (aw) was estimated using a water activity meter at 25 °C (EZ-200, Freund, Japan).42 The color parameters (L*, a*, and b*) of the samples were evaluated through a Hunter lab colorimeter (CX-1075, Colorflex, USA). A pure starch sample (native millet starch) was studied as a standard (ref. 43).| | | WI = 100 − [(100 − L*)2 + a*2 + b*2]1/2 | (3) |
| | | ΔE = [ΔL*2 + Δa*2 + Δb*2]1/2 | (4) |
2.6 Functional characteristics of samples
2.6.1 Oil absorption index.
Oil absorption capacity (OAC) was measured by mixing 1 g of samples with 10 mL of soybean oil in a centrifuged tube. The samples were mixed for 30 s and incubated at 25 °C for 30 min. After incubation, the samples were centrifuged for 20 min at 3000 rpm using a centrifuge (EBA 8S, Hettich, Germany) at room temperature. The excess oil was decanted and the tubes were inverted for 20 min. The final weight of the sample was measured to estimate the OAC.33| | 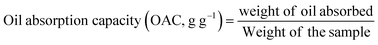 | (5) |
2.6.2 Hydration characteristics of samples.
Water absorption and solubility indices were determined according to the method described by Yousf et al. (2017) with minor modifications.44 The sample (2.5 g) was added in 30 mL of DW to cook the samples at 90 °C for 15 min using a water bath. The samples were centrifuged (EBA-8S, Hettich, Germany) for 10 min at 3000 rpm and the supernatant was shifted in a dish. The weight of the residue was measured, and the weight of the dried supernatant was estimated by drying the sample at 110 °C until a constant weight was attained.| | 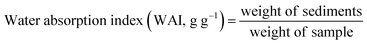 | (6) |
| |  | (7) |
2.7 Pasting properties
The pasting characteristics were estimated according to the method described by Tangsrianugul et al. (2019) with some modifications.39 2.5 g of each sample was added to a container with 25 mL of DW and stirred well. The canister was fitted into a Rapid Visco-Analyzer (RVA-4C, Newport Scientific. Co., Australia) and heated according to a prespecified heat cycle. The samples were maintained at 50 °C for the initial 1 min and heated to 95 °C with a uniform heating rate over 5 min. The samples were held at 95 °C for 3 min. The samples were cooled down to 50 °C in the next 3 min and maintained at that temperature for 1 min. The viscosities were estimated through thermocline software.
2.8 Thermal denaturation analysis of powders
2 mg of sample was sealed hermetically in an aluminum pan and placed at 25 °C to attain moisture equilibrium. The weights of pans before and after sample addition were recorded carefully. The samples were heated from 25–150 °C at a heating rate of 10 °C min−1. The gelatinization temperatures were monitored using a DSC instrument (Model-214, NETZSCH, Germany). The onset, peak, conclusion temperatures, and enthalpy (ΔH, J g−1) were recorded. An empty aluminum pan was used as a reference (ref. 45).
2.9 X-ray diffractometry (XRD)
1–2 g of starch sample was poured and spread evenly on the sample plate of an X-ray diffractometer and extra sample was removed to form a flat surface. The analysis of the structure was carried out to measure the crystallinity using the X-ray diffractometer (Model 10190376, Bruker, Germany) at 25 °C. 2–3 g of sample was evenly spread on the instrument sample plate and locked on the stub. The instrument was controlled at 40 mA and 40 kV with Cu Kα radiation (k = 1.5406 Å). The samples were scanned from 4–40° two theta angles with a step size of 0.05 and a step time of 1.0 s.42
2.10 Fourier transform infrared spectroscopy (FTIR)
The structural changes in powder samples were evaluated using FTIR to highlight the effect on the functional properties of powders. 2–3 mg of powder samples were pressed on the surface of an optical crystal cell at 25 °C. The spectra of the samples were recorded from 4000 to 500 cm−1 using a spectrometer (Nicolet iS50, Thermo Scientific, USA).46
2.11 Scanning electron microscopy (SEM).
A SEM equipment (JSM-7800F, JEOL Ltd, Japan) was used to examine the morphological properties of samples. The samples were coated on adhesive tape (double-sided carbon) connected to the specimen stub. The extra sample was removed using nitrogen on the adhesive tape. A gold coating was smeared on the sample for 45 s, with a sputter coater (E-1010 Ion Sputter, HITACHI, Japan) at a sputter current of 23 mA. The observations were recorded at 2000× magnification.45
2.12
In Vitro starch digestibility (IVSD)
Samples (50 mg mL−1 in 0.2 M phosphate buffer) at pH 6.9 were added with pancreatic amylase (0.5 mL, 40 U mg−1) solution (1.5 mg mL−1 in 0.2 M phosphate buffer, pH 6.9) and incubated for 2 h at room temperature.47 2 mL of 3, 5-dini-trosalicylic acid was poured into the suspension. The volume was adjusted to 25 mL with DW, and the samples were boiled for 5 min. Aliquot samples (0.25 mL) were taken at 20 and 120 min of incubation and mixed with 10 mL of ethanol (70% v/v) to stop the enzyme activity. The aliquots were centrifuged (EBA 8S, Hettich, Germany) for 10 min at 1500 × g and filtered to remove the large particles. These aliquots were used to estimate the concentration of glucose in the sample liberated during 20 (G20) and 120 min (G120) with a GOPOD assay kit. The starch fractions including rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) were estimated using eqn (8)–(10) respectively.| | | % SDS = (% G120 − % G20) × 0.9 | (9) |
| | | % RS = % TS − (% RDS + % SDS) | (10) |
2.13 Statistical analysis
The statistical software SPSS-16 (Chicago, USA) was employed to conduct the statistical analysis of triplicate readings. One-way ANOVA was applied with post hoc Tukey's test to observe the significant differences among the samples with a confidence interval of 95%.
3 Results and discussion
3.1 Effect of HMT on the composition and physico-chemical properties of samples
The nutritional values of native and modified samples were estimated and are listed in Table 1. A higher amount of carbohydrate content was observed in FMF and FMS. The HMT exerted a non-significant (p > 0.05) effect on the nutritional values of both FMF and FMS. The higher amount of carbohydrates in the samples demonstrates the purity of starch, which serves as an energy and starch source.48 The carbohydrate content was in the range of 78.5 to 92.4 for both native and modified samples. Similarly, no changes were observed in hydrothermally treated starch, ash, fat, and carbohydrates of proso millet.49 The starch, protein, fat, and ash contents were in the ranges of 59.0 to 96.2%, 0.5 to 8.6%, 0.6 to 4.6%, and 0.4 to 2.3%, respectively. These values were consistent with the values obtained in previous studies for finger millet.50,51
Table 1 Nutritional composition (%, w/w) and physico-chemical properties of native and HMT samplesa
| Sample |
Total carbs |
Starch |
Protein |
Fat |
Ash |
pH |
a
w
|
WI |
ΔE |
|
Different superscripts in the same column indicate significant differences at the confidence interval of 95%.
|
| FMF |
79.0 ± 2.4a |
59.0 ± 3.1a |
8.6 ± 0.3b |
4.6 ± 0.4b |
2.3 ± 0.2b |
6.7 ± 0.01b |
0.31 ± 0.01b |
69.4 ± 0.3b |
— |
| HMT-FMF |
78.5 ± 1.7a |
58.3 ± 1.1a |
8.4 ± 1.3b |
4.4 ± 0.5b |
2.2 ± 0.7b |
6.4 ± 0.03a |
0.25 ± 0.01a |
63.4 ± 0.4a |
3.4 ± 0.03 |
| FMS |
91.1 ± 2.1b |
96.2 ± 1.2b |
0.6 ± 0.9a |
0.7 ± 1.1a |
0.4 ± 0.3a |
6.8 ± 0.02b |
0.31 ± 0.01b |
92.2 ± 0.2c |
— |
| HMT-FMS |
92.4 ± 1.2b |
95.5 ± 1.4b |
0.5 ± 1.1a |
0.6 ± 0.3a |
0.4 ± 0.6a |
6.3 ± 0.01a |
0.27 ± 0.01a |
91.1 ± 0.4c |
1.2 ± 0.02 |
Water activity (aw) and pH decreased significantly (p < 0.05) for modified samples (Table 1) as compared to native samples. The aw of FMF and FMS was in the range of 0.25 to 0.31 and pH values were in the range of 6.3 to 6.8 for modified and native samples (Table 1). The oxidative species generated during processing of starch may generate bonding with starch molecules to produce acidic constituents, which ultimately reduce the pH of samples.52 The pH reduction of samples depends on several intrinsic factors such as starch type, presence of other constituents, and processing time and temperature.53 The decrease in the moisture content of starches greatly influences the pH of starch samples and starch solutions with low pH show improved water sorption capabilities and tensile strength in flexible films.54
The change in color due to processing provides valuable information about enzymatic or browning reactions including Maillard, caramelization, and degree of cooking.19 The color of samples highly depends on the chemical composition. Since the starch is different from flour in chemical composition, the FMF sample was significantly darker than the FMS with a lower WI value (69.4) (Table 1). The WI values were 69.4 and 63.4 for native and modified FMF, respectively, while the WI for native and modified starch was 92.2 and 91.21. The HMT decreased the WI significantly (p < 0.05) for FMF, while it remained almost unchanged for FMS. The WI for FMS showed no change after HMT owing to low protein content, which did not allow the Maillard reaction to occur.43 The HMT can also induce the browning reaction (non-enzymatic), which subsequently contributes to the darkening of color.55 The starch samples were brighter even after heat-moisture treatment. The chroma (ΔE) value was also lower (1.2) for HMT-FMS as compared to native and modified FMF (3.4) (Table 1). HMT-induced changes in color can be related to non-enzymatic reactions, the Maillard reaction between reducing sugars and amino groups of proteins, and variations in the composition of FMF and FMS.43,56
3.2 Effect of HMT on the functional characteristics of samples
The oil absorption capacity (OAC) is a crucial parameter in the food matrix that correlates with the flavor, mouthfeel, and other sensory and functional properties of food formulation.33 The effects of HMT on hydration properties (WAI, WSI, and SP) and OAC are shown in Table 2. The values for OAC for FMF and FMS were in the range of 1.9–2.5 g g−1 (Table 2). The OAC was significantly (p < 0.05) increased for modified samples as compared to native samples. This change in OAC due to HMT can be because of an improvement in the starch's ability to absorb oil due to a larger number of hydrophobic amino acids. The aw and moisture content of the sample also play a significant role in OAC. The decrease in the aw of samples due to HMT can be responsible for the increase in the OAC of samples.57
Table 2 Functional characteristics of native and HMT samplesa
| Sample |
OAC |
WAI (g g−1) |
WSI (%) |
SP(g g−1) |
| 70 °C |
80 °C |
90 °C |
70 °C |
80 °C |
90 °C |
70 °C |
80 °C |
90 °C |
|
Different superscripts in the same column indicate significant differences at the confidence interval of 95%.
|
| FMF |
1.9 ± 0.2a |
2.1 ± 0.1a |
3.4 ± 0.3a |
4.8 ± 0.3a |
0.18 ± 0.01b |
0.22 ± 0.01a |
0.27 ± 0.1a |
3.4 ± 0.1a |
5.6 ± 0.1a |
7.7 ± 0.1a |
| HMT-FMF |
2.2 ± 0.4b |
2.7 ± 0.2b |
4.7 ± 0.1b |
6.7 ± 1.1b |
0.21 ± 0.01c |
0.31 ± 0.01b |
0.43 ± 0.9c |
4.2 ± 0.1b |
6.7 ± 0.2b |
9.2 ± 0.2b |
| FMS |
2.1 ± 0.2b |
2.8 ± 0.1b |
5.7 ± 0.2c |
7.8 ± 0.1c |
0.14 ± 0.01a |
0.24 ± 0.01a |
0.35 ± 0.1b |
4.4 ± 0.2b |
8.8 ± 0.3c |
12.3 ± 0.1c |
| HMT-FMS |
2.5 ± 0.4c |
3.3 ± 0.1c |
6.2 ± 0.3d |
10.7 ± 0.5d |
0.27 ± 0.01d |
0.34 ± 0.01c |
0.44 ± 0.2c |
4.9 ± 0.2c |
13.4 ± 0.4d |
18.4 ± 0.1d |
The water activity index (WAI) of FMF and FMS was in the range of 2.1–10.7 g g−1 (Table 2). The WAI increased significantly (p < 0.05) due to the increase in the temperature of the samples. The WAI for FMS was highly influenced at the highest temperature as compared to lower temperatures. The WAI of samples is related to the hydrophilicity of macromolecules such as proteins and starches.58 Different samples have varied affinity to water molecules due to differences in their polar amino acid residues of proteins.59 The protein denaturation due to heat treatment initiates protein unfolding, which increases the number of polar side chains and improves the WAI of samples. Similarly, in a previous study of finger millet, hydrothermal treatment enhanced the water absorption capacity of samples.23 The modification of the WAI can significantly influence the gelatinization properties of starch, which can help in the development of new textures of food products.60 The gelatinization of starch in water occurs when heated between 60 and 80 °C, which results in significant changes in the functional properties of starch. In gelatinization, the crystallites melt, the granule loses its molecular order and structure and solubilization occurs.61 During heating, water enters the amorphous regions and alters the disruptive interaction in the crystalline regions. Due to this water movement, amylopectin and amylose recrystallize (retrogradation), which affects the food texture. The higher firmness, staling, loss of crispness, flavor, and aroma of bakery products are the effects of this phenomenon.62
The water solubility index (WSI) also increased significantly with increasing temperature of samples. The WSI of FMF and FMS was in the range of 0.14–0.44% (Table 2). The maximum WSI (0.44) was obtained for HMT-FMS at 90 °C. The results of HMT samples also indicated an increase in the WSI, confirming the effect of heat treatment.63 In the HMT, the starch granules undergo structural reorientation, which improves the interaction between amylopectin and amylose side chains, improving the WSI of starch molecules.64 Ananthu et al. (2023) also observed an improvement in the WSI due to microwave treatment of white finger millet.65 The microwave treatment changes the degree of depolymerization and weakens the starch structure, thereby improving the WSI.
The SP of samples also increased due to an increase in temperature from 70 to 90 °C and increased significantly due to HMT. The SP of FMF and FMS was in the range of 3.4–18.4 g g−1 (Table 2). This increase in SP was due to surface modification and damage of starch granules through HMT, which allowed the swelling of amylopectin and more water molecules to penetrate easily. However, at 80 and 90 °C, the SP was much higher than that at 70 °C for FMF and FMS because these temperatures were higher than the gelatinization temperatures of finger millet.23 The hydration characteristics of starch gels depend on the modifying treatment.9 Moreover, SP increase can also be due to an increase in starch granule size due to HMT. The amylopectin undergoes structural changes and exposes a larger number of hydroxyl groups that increase the SP of the starch samples.63 Similar results for the hydration properties of proso millet starch,32 white finger millet starch,33 and finger millet starch23 have been reported previously.
3.3 Pasting properties of samples
Pasting characteristics depend on the stiffness of starch particles in the sample, which affect the swelling power of granules and amylose seeping ability in the solution. It is crucial for the selection of binders and thickeners for food products.66 Peak (PV), final (FV), setback (SBV), and breakdown (BDV) viscosities were in the ranges of 45.0–345.4, 67.2–364.4, 19.0–122.4, and 6.9–213.1 RVU, respectively, for all samples (Table 3). There was a significant difference (p < 0.05) in the pasting viscosities of FMF and FMS, indicating the purity of starch as compared to FMF with a high amount of protein, fat, and fiber. Significantly (p < 0.05) higher values of PV, FV, TV, SBV, and BDV were observed for FMS as compared to FMF. The HMT significantly (p < 0.05) reduced the pasting viscosities of FMF and FMS.
Table 3 Pasting properties measured in RVU of samples before and after heat-moisture treatmenta
| Samples |
PV |
TV |
BDV |
FV |
SBV |
Peak T (min) |
Pasting T (°C) |
|
PV: peak viscosity, TV: viscosity at trough, FV: final viscosity, BDV: breakdown viscosity, and SBV: setback viscosity. Different superscripts in the same column indicate significant differences at the confidence interval of 95%.
|
| FMF |
122.3 ± 1.8b |
114.5 ± 2.1b |
7.8 ± 0.2b |
244.7 ± 1.1b |
122.4 ± 0.2c |
6.4 ± 0.4b |
84.5 ± 2.4b |
| HMT-FMF |
45.3 ± 2.1a |
38.4 ± 2.2a |
6.9 ± 1.4a |
67.2 ± 1.2 a |
21.9 ± 1.1a |
6.8 ± 0.5b |
89.4 ± 3.2c |
| FMS |
345.4 ± 3.1d |
142.3 ± 2.1d |
213.1 ± 0.8d |
364.4 ± 2.3d |
19.0 ± 0.3a |
5.4 ± 0.6a |
78.3 ± 3.5a |
| HMT-FMS |
230.5 ± 2.3c |
128.7 ± 3.1c |
101.8 ± 1.5c |
297.2 ± 2.2c |
66.7 ± 1.2b |
6.3 ± 0.7b |
83.2 ± 1.7b |
Due to HMT, structural changes occur in the proteins and carbohydrates of flour samples.67 Less engorged starch particles have a good propensity to be disrupted causing higher BDV, which also reduces the complexes of amylose to lipids and proteins causing a decrease in peak viscosities.39 The starch's past inclination to retrograde is indicated by SBV and susceptibility to mechanical and thermal shear is defined by BDV. The decrease in BDV for proso millet was observed due to microwave treatment, which altered the interactions and hydrogen bonding between starch chains.68 The decrease in PV, BDV, and SBV was also observed in sorghum flour due to HMT.13 Due to this lower SBV of modified samples, the retrogradation process will be slower, indicating stability and a cold starch paste-like aging trend.69 HMT-FMF showed a significantly (p < 0.05) higher decline in pasting viscosity than HMT-FMS, indicating the higher effect of HMT on FMF. This can be related to protein denaturation (FMF contains a higher amount of protein) due to heat treatment, resulting in interactions between starch and denatured proteins causing differences in pasting characteristics.70 The higher lipid content in FMF can generate an insoluble matrix with amylose due to HMT, inhibiting the starch granule swelling and causing a decrease in pasting viscosity and an increase in pasting temperature. Similar trends for the pasting properties of pearl millet due to HMT were observed by Sharma et al. (2015).71 However, the pasting temperature for FMF was higher (p < 0.05) than that of FMS, which increased due to HMT for both samples. The increase in pasting temperature could be because of the generation of double-helical extremely structured clusters of amylopectin.72 This trend for pasting temperature was also observed for heat treatment of white finger, where protein is denatured causing many interactions with starch.29
3.4 Gelatinization properties of samples
The gelatinization properties of samples were observed using a DSC and are presented in Table 4. The To, Tp, and Tc values for all samples were in the ranges of 64.4–77.2 °C, 71.8–83.3 °C, and 74.2–86.9 °C, respectively. The maximum values of To, Tp, and Tc were obtained for FMF. However, the HMT of the samples increased To, Tp, and Tc values both for FMF and FMS, while decreasing the ΔH values. Since the ΔH value specifies the double helical structure content, heat treatment can decrease the ΔH value due to the gelatinization and rearrangement of starch granules, increasing the ideal double helical order and gelatinization temperature.73 The increase in gelatinization temperatures indicates the melting of crystallites formed due to amylopectin–amylose and amylose–amylose interactions along the starch chains. These interactions are stronger in modified flour and starch, which hindered gelatinization by suppressing the swelling of starch granule.23 During HMT, the gelatinization temperature of starch increased because of melting of unstable crystallites which left behind more stable crystallites. The breakdown of starch crystallite increases the amorphous portions and decreases the water accessibility to crystallites. The lower ΔH value due to HMT indicated that melting a smaller amount of crystallite required less quantity.66 The higher Tc – To values due to HMT show a higher discrepancy in amylopectin and amylose structures of starch granules.70 These findings were in agreement with previous studies on starch and flour from finger millet23 and wheat.72
Table 4 Gelatinization temperatures and relative crystallinity of samples before and after HMTa
| Samples |
T
o (°C) |
T
p (°C) |
T
c (°C) |
T
c − To (°C) |
ΔH (J g−1) |
Relative crystallinity (%) |
|
T
o: onset temperature, Tp: peak temperature, Tc: conclusion temperature, and ΔH: change in enthalpy. Different superscripts in the same column indicate significant differences at the confidence interval of 95%.
|
| FMF |
72.4 ± 0.4c |
76.5 ± 0.1c |
78.3 ± 0.3b |
6.9 ± 0.2a |
1.85 ± 0.01c |
51.9 |
| HMT-FMF |
77.2 ± 0.3d |
83.3 ± 0.2d |
86.9 ± 0.2c |
9.7 ± 0.1b |
1.16 ± 0.01a |
51.2 |
| FMS |
66.2 ± 0.2b |
71.8 ± 0.2a |
74.2 ± 0.4a |
11.0 ± 0.3c |
1.61 ± 0.01b |
54.4 |
| HMT-FMS |
64.9 ± 0.4a |
73.7 ± 0.2 ab |
78.4 ± 0.3b |
13.5 ± 0.4d |
1.17 ± 0.01a |
52.2 |
3.5 X-ray diffraction analysis
The effect of HMT on the structure of native and modified samples was evaluated using X-ray diffraction techniques and is presented in Fig. 1. All samples showed diffraction single peaks at 15.2°, 20.1°, and 23.2° (2θ) and a double diffraction peak between 17.2 and 18.3° (2θ), which shows the typical X-ray pattern of millet starch (A-type). However, the relative crystallinity and intensity peaks of FMF were slightly lower than those of FMS. This difference could be due to protein, lipids, and fiber molecules inhibiting the interactions between starch molecules. These molecules weaken the strength of crystalline structure peaks.74 HMT-FMS showed no changes in the pattern, while HMT-FMF showed a peak at a 2θ value of 13°. The peak at the 2θ value of 13° indicates the complex formation of amylose and lipids in HMT-FMF.72 The HMT-modified samples showed a significant decrease in the relative crystallinity, while FMS showed the highest relative crystallinity (54.4) due to the higher realignment of the structure (Table 4). The HMT caused some alterations in the double-helical structures and gelatinization of starch granules, which decreased the relative crystallinity.33 These changes in the structure could be due to the disruption of crystallite or double helical reorientation. This decline in relative crystallinity is consistent with the previous results of finger millet starch due to HMT.33,75
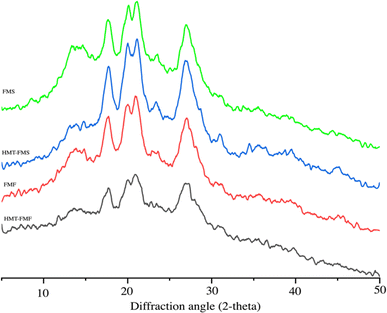 |
| | Fig. 1 The X-ray diffractogram of samples before and after heat-moisture treatment. | |
3.6 Morphological observations
The morphology of samples before and after HMT was studied using a scanning electron microscope and the micrographs are shown in Fig. 2. The FMF has an irregular gritty surface due to protein and other molecules over the starch granule surface. The FMS displayed an irregular indented surface with spherical and polygonal shapes containing some pores. FMF also comprised some damaged granules of starch, which could be because of physical damage to millet grains through the grinding process. The porous starch granule surface could be due to the elimination of protein and other molecules through the starch isolation procedure.69 Similar types of micrographs were also observed for pearl millet starch,10 finger millet flour,76 finger millet starch,23 and white finger millet.33 Slight changes in the morphology of the samples were observed after HMT, and the FMF sample became clumpier due to protein molecules.73,77 The heat removed the surface water during HMT instigating granule enlargement due to sticking with each other and with protein particles. Upon completion of the HMT process, the drop in temperature collapses the outer structure of the granule and creates cracks.78 After HMT, the surface of FMS showed more porosity and cracks. These changes after HMT can be associated with higher water levels on the starch granule surface, which could not be absorbed by granules during HMT.79 These findings were consistent with previous work showing the effect of the HMT process on white finger millet starch,33 wheat,72 and rice70 flour.
 |
| | Fig. 2 The morphological observation of samples before and after heat-moisture treatment at 2000×. | |
3.7 Structural changes in samples
FTIR examination was conducted using functional group identification to observe the effect of HMT on FMF and FMS samples. Major absorption peaks were observed in the regions of 3300–3250, 2930–2850, 1750–1630, 1180–1070, and 930–860 cm−1 (Fig. 3). These peak regions indicated the presence of carbonyl (–COH), hydroxyl (–OH), alkane (–CH), amine (–NH), and alkene (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) functional groups respectively.67,80 There was no significant change observed in the number of peaks of the samples due to HMT but there were changes in the intensity of absorption peaks. The wide and strong absorption peak in the region of 3300–3250 cm−1 was observed due to –OH stretching from the glucose unit, protein–starch interactions and carboxylic acid.33 Due to HMT, a variation in the peak intensity was observed, which indicated hydrogen bond formation.81 The absolute peaks indicated that the heating affects the absorption intensity due to the hydration reaction during the gelatinization of starch. The hydration of the sample generates alterations and hydrogen bonding in inter- and intra-molecular structures, which cause these variations in the spectra.82 The alkene group (–CH) caused the asymmetric stretching of the bond at 2924 cm−1, which changed in the modified starch sample.9 The peak regions of 1700–1500 cm−1 and 1200–900 cm−1 represent proteins in the sample. The region of amide I (1700–1600 cm−1) mainly consists of the C–O stretching of polypeptides from the backbone and the region of amid II (1600–1500 cm−1) consists of the N–H bending and C–N stretching of proteins.83 The absorption peak at 1638.8 cm−1 indicated the presence of water molecules bound to starch.13 In HMT-FMS, an early Maillard reaction caused a variation in the intensity of this peak due to heat.84 These results indicate that there were no major changes in the molecular composition and no new functional group formation in modified FMF and FMS, which elucidates the effect of these processing methods with improved functional and physicochemical characteristics.
CH) functional groups respectively.67,80 There was no significant change observed in the number of peaks of the samples due to HMT but there were changes in the intensity of absorption peaks. The wide and strong absorption peak in the region of 3300–3250 cm−1 was observed due to –OH stretching from the glucose unit, protein–starch interactions and carboxylic acid.33 Due to HMT, a variation in the peak intensity was observed, which indicated hydrogen bond formation.81 The absolute peaks indicated that the heating affects the absorption intensity due to the hydration reaction during the gelatinization of starch. The hydration of the sample generates alterations and hydrogen bonding in inter- and intra-molecular structures, which cause these variations in the spectra.82 The alkene group (–CH) caused the asymmetric stretching of the bond at 2924 cm−1, which changed in the modified starch sample.9 The peak regions of 1700–1500 cm−1 and 1200–900 cm−1 represent proteins in the sample. The region of amide I (1700–1600 cm−1) mainly consists of the C–O stretching of polypeptides from the backbone and the region of amid II (1600–1500 cm−1) consists of the N–H bending and C–N stretching of proteins.83 The absorption peak at 1638.8 cm−1 indicated the presence of water molecules bound to starch.13 In HMT-FMS, an early Maillard reaction caused a variation in the intensity of this peak due to heat.84 These results indicate that there were no major changes in the molecular composition and no new functional group formation in modified FMF and FMS, which elucidates the effect of these processing methods with improved functional and physicochemical characteristics.
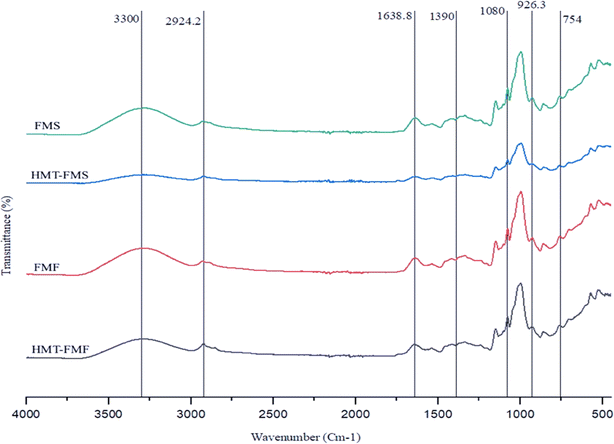 |
| | Fig. 3 The structural changes observed in the samples before and after heat-moisture treatment. | |
3.8
In vitro starch digestibility (IVSD)
Starch digestion is a crucial nutritional parameter for millets, indicating the product value and health benefits. Higher fractions of RS and SDS provide many health benefits, such as a low glycemic index, reduction in fat and cholesterol levels, and cancer prevention in the colon.85 The digestibility of starch fractions was estimated in FMF and FMS before and after HMT (Fig. 4). RDS, SDS, and RS values were in the ranges of 14.3–22.4%, 28.0–60.9%, and 30.9–55.7%, respectively. RDS and SDS fractions increased significantly (p < 0.05) in both starch and flour, while the RS fraction decreased significantly (p > 0.05) due to the HMT process. Similar results for the digestibility of flour and starches of buckwheat77 and Chinese Yam74 due to the HMT process were observed in previous studies. The influence of HMT on the enzymatic digestibility of starch is influenced by the starch source, moisture content, temperature and duration during HMT and amylose–lipid interactions.86 The HMT process can increase or decrease the digestibility of starch, which depends on the millet type, processing operation, lipids, protein fractions, amylose content, and amylose lipid complexes in the sample.87 It has been described in several studies that the HMT process can increase the RDS and SDS content, which is good for health.88 After HMT, the surface of FMS showed more porosity and cracks as observed in Fig. 2. This implies that the increase in the porosity of starch enables the entry of the enzyme into the granule, increasing the enzymatic susceptibility of samples. Due to increased enzymatic activity, the RDS and SDS fractions of modified FMF and FMS increased.86
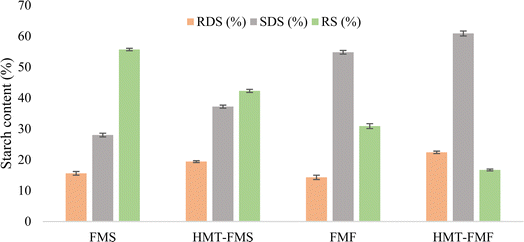 |
| | Fig. 4 Starch digestion of samples before and after heat-moisture treatment. Rapidly digestible starch: RDS, slowly digestible starch: SDS, and resistant starch: RS. | |
4 Conclusion
Heat-moisture treatment (HMT) showed a significant influence on the structural characteristics of finger millet starch. These structural changes influenced the physico-chemical and functional characteristics of modified flour and starch samples. The nutritional composition of the samples remained unchanged due to the HMT process of the samples. Functional properties including OAC, WSI, WAI, and SP were improved due to structural breakdown of the complex molecule and an increase in the concentration of hydrophilic components and protein. The pasting viscosity decreased due to HMT, indicating the improved SP of starch granules. The gelatinization temperatures were improved in both FMF and FMS samples and relative crystallinity decreased due to the HMT of the samples. FTIR spectra confirmed that there were no changes in the functional groups of the samples due to HMT. In vitro, digestibility results indicated that the starch digestibility was improved for all samples due to HMT. Increases in the RDS and SDS fractions of native starch and flour were observed. This processing technique can be used to improve the functional, pasting and digestibility properties of millet starch, which have great potential in the food industry for new product formulations such as confections, retort foods, salad dressings, batter products, gluten free bakery products, porridges, and steam-cooked and boiled products.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- I. A. Jideani,
et al., Digitaria exilis (acha/fonio), Digitaria iburua (iburu/fonio) and Eluesine coracana (tamba/finger millet)--non-conventional cereal grains with potentials, Sci. Res. Essays, 2012, 7(45), 3834–3843 Search PubMed.
- A. Gull, R. Jan, G. A. Nayik, K. Prasad and P. Kumar, Significance of finger millet in nutrition, health and value added products: a review, Magnesium, 2014, 130(32), 120 Search PubMed.
-
P. N. Mathur, Global Strategy for the Ex Situ Conservation of Finger Millet and its Wild Relatives, Biodiversity International, Sub-regional Office for South Asia, NASC Complex, Pusa Campus, New Delhi, India, 2012, pp. 1–62 Search PubMed.
- M. Muthamilarasan, A. Dhaka, R. Yadav and M. Prasad, Exploration of millet models for developing nutrient rich graminaceous crops, Plant Sci., 2016, 242, 89–97 CrossRef CAS PubMed.
- I. A. Jideani and V. A. Jideani, Developments on the cereal grains Digitaria exilis (acha) and Digitaria iburua (iburu), J. Food Sci. Technol., 2011, 48, 251–259 CrossRef CAS.
- S. Sood, A. Kumar, B. K. Babu, V. S. Gaur, D. Pandey and L. Kant,
et al., Gene discovery and advances in finger millet [Eleusine coracana (L.) Gaertn.] genomics—an important nutri-cereal of future, Front. Plant Sci., 2016, 7, 219694 Search PubMed.
- N. Gautam, S. Garg and S. Yadav, Underutilized finger millet crop for starch extraction, characterization, and utilization in the development of flexible thin film, J. Food Sci. Technol., 2021, 58, 4411–4419 CrossRef CAS PubMed.
- M. Piecyk and K. Domian, Effects of heat--moisture treatment conditions on the physicochemical properties and digestibility of field bean starch (Vicia faba var. minor), Int. J. Biol. Macromol., 2021, 182, 425–433 CrossRef CAS PubMed.
- A. S. Babu, R. J. Mohan and R. Parimalavalli, Effect of single and dual-modifications on stability and structural characteristics of foxtail millet starch, Food Chem., 2019, 271, 457–465 CrossRef CAS PubMed.
- A. K. Siroha, K. S. Sandhu, M. Kaur and V. Kaur, Physicochemical, rheological, morphological and in vitro digestibility properties of pearl millet starch modified at varying levels of acetylation, Int. J. Biol. Macromol., 2019, 131, 1077–1083 CrossRef CAS PubMed.
-
S. Thangaraju, M. Shankar, M. Buvaneshwaran and V. Natarajan, Effect of processing on the functional potential of bioactive components, in Bioactive Components: A Sustainable System for Good Health and Well-Being, Springer, 2022, p. 183–207 Search PubMed.
- K. Schafranski, V. C. Ito and L. G. Lacerda, Impacts and potential applications: a review of the modification of starches by heat-moisture treatment (HMT), Food Hydrocolloids, 2021, 117, 106690 CrossRef CAS.
- Q. Sun, M. Gong, Y. Li and L. Xiong, Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch, Carbohydr. Polym., 2014, 110, 128–134 CrossRef CAS.
- F. E. Ortega-Ojeda and A.-C. Eliasson, Gelatinisation and retrogradation behaviour of some starch mixtures, Starch/Staerke, 2001, 53(10), 520–529 CrossRef CAS.
- E. da Rosa Zavareze and A. R. G. Dias, Impact of heat-moisture treatment and annealing in starches: a review, Carbohydr. Polym., 2011, 83(2), 317–328 CrossRef.
- H.-J. Chung, R. Hoover and Q. Liu, The impact of single and dual hydrothermal modifications on the molecular structure and physicochemical properties of normal corn starch, Int. J. Biol. Macromol., 2009, 44(2), 203–210 CrossRef CAS PubMed.
- L. Bian and H.-J. Chung, Molecular structure and physicochemical properties of starch isolated from hydrothermally treated brown rice flour, Food Hydrocolloids, 2016, 60, 345–352 CrossRef CAS.
- H.-J. Chung, Q. Liu and R. Hoover, Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches, Food Res. Int., 2010, 43(2), 501–508 CrossRef CAS.
- T. Thilagavathi, P. Banumathi, S. Kanchana and M. Ilamaran, Effect of heat moisture treatment on functional and phytochemical properties of native and modified millet flours, Plant Arch., 2015, 15, 15–21 Search PubMed.
- M. S. Amarnath, A. Muhammed, A. K. Antony, B. Malini and C. K. Sunil, White finger millet starch: physical modification (annealing and ultrasound), and its impact on physicochemical, functional, thermal and structural properties, Food Humanity, 2023, 1, 599–606 CrossRef.
- M. Pratiwi, D. N. Faridah and H. N. Lioe, Structural changes to starch after acid hydrolysis, debranching, autoclaving-cooling cycles, and heat moisture treatment (HMT): a review, Starch/Staerke, 2018, 70(1–2), 1700028 CrossRef.
- B. Zheng, H. Wang, W. Shang, F. Xie, X. Li and L. Chen,
et al., Understanding the digestibility and nutritional functions of rice starch subjected to heat-moisture treatment, J. Funct. Foods, 2018, 45, 165–172 CrossRef CAS.
- K. O. Adebowale, T. A. Afolabi and B. I. Olu-Owolabi, Hydrothermal treatments of Finger millet (Eleusine coracana) starch, Food Hydrocolloids, 2005, 19(6), 974–983 CrossRef CAS.
- L. S. Collado and H. Corke, Heat-moisture treatment effects on sweetpotato starches differing in amylose content, Food Chem., 1999, 65(3), 339–346 CrossRef CAS.
- R. Hoover, The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources, Crit. Rev. Food Sci. Nutr., 2010, 50(9), 835–847 CrossRef CAS.
- M. Kaur and S. Singh, Influence of heat-moisture treatment (HMT) on physicochemical and functional properties of starches from different Indian oat (Avena sativa L.) cultivars, Int. J. Biol. Macromol., 2019, 122, 312–319 CrossRef CAS.
- B. Fathi, M. Aalami, M. Kashaninejad and A. Sadeghi Mahoonak, Utilization of heat-moisture treated proso millet flour in production of gluten-free pound cake, J. Food Qual., 2016, 39(6), 611–619 CrossRef CAS.
- K. S. Sandhu, A. K. Siroha, S. Punia and M. Nehra, Effect of heat moisture treatment on rheological and in vitro digestibility properties of pearl millet starches, Carbohydr. Polym. Technol. Appl., 2020, 1, 100002 Search PubMed.
- I. Amadou, M. E. Gounga, Y.-H. Shi and G.-W. Le, Fermentation and heat-moisture treatment induced changes on the physicochemical properties of foxtail millet (Setaria italica) flour, Food Bioprod. Process., 2014, 92(1), 38–45 CrossRef CAS.
- I. Amadou, O. S. Gbadamosi and G.-W. Le, Millet-based traditional processed foods and beverages—a review, Cereal Foods World, 2011, 56(3), 115 Search PubMed.
- A. Kalaisekar and P. G. Padmaja, Insect Pests of Millets and Their Host Plant Relations, Millets Sorghum Biol. Genet. Improv., 2017, 267–290 Search PubMed.
- M. Zheng, Y. Xiao, S. Yang, H. Liu, M. Liu and S. Yaqoob,
et al., Effects of heat--moisture, autoclaving, and microwave treatments on physicochemical properties of proso millet starch, Food Sci. Nutr., 2020, 8(2), 735–743 CrossRef CAS.
- M. Balakumaran, K. G. Nath, B. Giridharan, K. Dhinesh, A. K. Dharunbalaji and B. Malini,
et al., White finger millet starch: hydrothermal and microwave modification and its characterisation, Int. J. Biol. Macromol., 2023, 242, 124619 CrossRef CAS PubMed.
- P. Van Hung, V. T. Binh, P. H. Y. Nhi and N. T. L. Phi, Effect of heat-moisture treatment of unpolished red rice on its starch properties and in vitro and in vivo digestibility, Int. J. Biol. Macromol., 2020, 154, 1–8 CrossRef PubMed.
- R. Colussi, D. Kringel, L. Kaur, E. da Rosa Zavareze, A. R. G. Dias and J. Singh, Dual modification of potato starch: effects of heat-moisture and high pressure treatments on starch structure and functionalities, Food Chem., 2020, 318, 126475 CrossRef CAS.
- R. Chang, Y. Tian, H. Lu, C. Sun and Z. Jin, Effects of fractionation and heat-moisture treatment on structural changes and digestibility of debranched waxy maize starch, Food Hydrocolloids, 2020, 101, 105488 CrossRef CAS.
- C. Chatpapamon, Y. Wandee, D. Uttapap, C. Puttanlek and V. Rungsardthong, Pasting properties of cassava starch modified by heat-moisture treatment under acidic and alkaline pH environments, Carbohydr. Polym., 2019, 215, 338–347 CrossRef CAS PubMed.
- M.-N. Li, Y. Xie, H.-Q. Chen and B. Zhang, Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A-and B-type starch granules, Food Chem., 2019, 272, 523–529 CrossRef CAS.
- N. Tangsrianugul, R. Wongsagonsup and M. Suphantharika, Physicochemical and rheological properties of flour and starch from Thai pigmented rice cultivars, Int. J. Biol. Macromol., 2019, 137, 666–675 CrossRef CAS PubMed.
- N. Thiex, Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles, J. AOAC Int., 2009, 92(1), 61–73 CrossRef CAS.
- B. V. McCleary, L. M. J. Charmier and V. A. McKie, Measurement of starch: critical evaluation of current methodology, Starch/Staerke, 2019, 71(1–2), 1800146 CrossRef.
- M. Umar, U. R. Ruktanonchai, D. Makararpong and A. K. Anal, Compositional and functional analysis of freeze-dried bovine skim colostrum powders, J. Food Meas. Char., 2023, 1–11 CAS.
- Z. Li, D. Guo, X. Li, Z. Tang, X. Ling and T. Zhou,
et al., Heat-Moisture treatment further reduces in vitro digestibility and enhances resistant starch content of a high-resistant starch and low-glutelin rice, Foods, 2021, 10(11), 2562 CrossRef CAS PubMed.
- N. Yousf, F. Nazir, R. Salim, H. Ahsan and A. Sirwal, Water solubility index and water absorption index of extruded product from rice and carrot blend, J. Pharmacogn. Phytochem., 2017, 6(6), 2165–2168 CAS.
- M. Umar, U. R. Ruktanonchai, D. Makararpong and A. K. Anal, Effects of pH and concentrations of colostrum whey and caseinate on fabrication of nanoparticles and evaluation of their techno-functionalities and in vitro digestibility, J. Food Meas. Char., 2023, 1–12 CAS.
- R. P. Bebartta, M. Umar, U. R. Ruktanonchai and A. K. Anal, Development of cryo-desiccated whey and soy protein conglomerates; effect of maltodextrin on their functionality and digestibility, J. Food Process. Eng., 2023, 46(7), e14350 CrossRef CAS.
- R. Wongsagonsup, T. Nateelerdpaisan, C. Gross, M. Suphantharika, P. D. Belur and E. M. G. Agoo,
et al., Physicochemical properties and in vitro digestibility of flours and starches from taro cultivated in different regions of Thailand, Int. J. Food Sci. Technol., 2021, 56(5), 2395–2406 CrossRef CAS.
- P. Kaushal, V. Kumar and H. K. Sharma, Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeonpea (Cajanus cajan) flour and their blends, LWT--Food Sci. Technol., 2012, 48(1), 59–68 CrossRef CAS.
- M. Singh and A. A. Adedeji, Characterization of hydrothermal and acid modified proso millet starch, LWT--Food Sci. Technol., 2017, 79, 21–26 CrossRef CAS.
- M. Mutshinyani, M. E. Mashau and A. I. O. Jideani, Bioactive compounds, antioxidant activity and consumer acceptability of porridges of finger millet (Eleusine coracana) flours: effects of spontaneous fermentation, Int. J. Food Prop., 2020, 23(1), 1692–1710 CrossRef CAS.
- S. O. Azeez, C. E. Chinma, S. O. Bassey, U. R. Eze, A. F. Makinde and A. A. Sakariyah,
et al., Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours, LWT--Food Sci. Technol., 2022, 154, 112734 CrossRef CAS.
- B. Klein, N. L. Vanier, K. Moomand, V. Z. Pinto, R. Colussi and E. da Rosa Zavareze,
et al., Ozone oxidation of cassava starch in aqueous solution at different pH, Food Chem., 2014, 155, 167–173 CrossRef CAS PubMed.
- F. Zhu, Plasma modification of starch, Food Chem., 2017, 232, 476–486 CrossRef CAS.
- R. G. T. Kalaivendan, A. Mishra, G. Eazhumalai and U. S. Annapure, Effect of atmospheric pressure non-thermal pin to plate plasma on the functional, rheological, thermal, and morphological properties of mango seed kernel starch, Int. J. Biol. Macromol., 2022, 196, 63–71 CrossRef CAS PubMed.
- N. Navyashree, A. S. Sengar, C. K. Sunil and N. Venkatachalapathy, White Finger Millet (KMR-340): a comparative study to determine the effect of processing and their characterisation, Food Chem., 2022, 374, 131665 CrossRef CAS PubMed.
-
Q. Wang, L. Li, C. Liu and X. Zheng. Heat-moisture modified blue wheat starch: physicochemical properties modulated by its multi-scale structure. Food Chem., 2022, 386, 132771, Available from: https://www.sciencedirect.com/science/article/pii/S0308814622007336 Search PubMed.
- H. Marta, Y. Cahyana, H. R. Arifin and L. Khairani, Comparing the effect of four different thermal modifications on physicochemical and pasting properties of breadfruit (Artocarpus altilis) starch, Int. Food Res. J., 2019, 26(1), 269–276 CAS.
- K. Maninder, K. S. Sandhu and N. Singh, Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars, Food Chem., 2007, 104(1), 259–267 CrossRef CAS.
-
S. Damodaran and K. L. Parkin, Amino acids, peptides, and proteins, in Fennema's Food Chemistry, CRC Press, 2017, p. 235–356 Search PubMed.
- J. Ye, X. Hu, S. Luo, W. Liu, J. Chen and Z. Zeng,
et al., Properties of starch after extrusion: a review, Starch/Staerke, 2018, 70(11–12), 1700110 CrossRef.
-
D. Donmez, L. Pinho, B. Patel, P. Desam, O. H. Campanella. Characterization of starch–water interactions and their effects on two key functional properties: starch gelatinization and retrogradation. Curr. Opin. Food Sci., 2021, 39, 103–109, Available from: https://www.sciencedirect.com/science/article/pii/S2214799321000011 Search PubMed.
- J. A. Gray and J. N. Bemiller, Bread staling: molecular basis and control, Compr. Rev. Food Sci. Food Saf., 2003, 2(1), 1–21 CrossRef CAS.
- D. Deka and N. Sit, Dual modification of taro starch by microwave and other heat moisture treatments, Int. J. Biol. Macromol., 2016, 92, 416–422 CrossRef CAS.
- I. Bharti, S. Singh and D. C. Saxena, Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars, LWT--Food Sci. Technol., 2019, 110, 197–206 CrossRef CAS.
- R. Ananthu, M. Buvaneswaran, L. Meena and C. K. Sunil, Microwave treatment effect on functional and pasting properties, and storage stability of white finger millet, J. Food Process. Eng., 2023, 46(7), e14341 CrossRef CAS.
- X. Liu, S. Huang, C. Chao, J. Yu, L. Copeland and S. Wang, Changes of starch during thermal processing of foods: current status and future directions, Trends Food Sci. Technol., 2022, 119, 320–337 CrossRef CAS.
- I. A. Wani, H. Hamid, A. M. Hamdani, A. Gani and B. A. Ashwar, Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting, J. Adv. Res., 2017, 8(4), 399–405 CrossRef CAS PubMed.
- Y. Li, A. Hu, X. Wang and J. Zheng, Physicochemical and in vitro digestion of millet starch: effect of moisture content in microwave, Int. J. Biol. Macromol., 2019, 134, 308–315 CrossRef CAS.
- F. Uzizerimana, K. Dang, Q. Yang, M. S. Hossain, S. Gao and P. Bahati,
et al., Physicochemical properties and in vitro digestibility of tartary buckwheat starch modified by heat moisture treatment: a comparative study, NFS J., 2021, 25, 12–20 CrossRef CAS.
- S. Puncha-arnon and D. Uttapap, Rice starch vs. rice flour: differences in their properties when modified by heat--moisture treatment, Carbohydr. Polym., 2013, 91(1), 85–91 CrossRef CAS.
- M. Sharma, D. N. Yadav, A. K. Singh and S. K. Tomar, Rheological and functional properties of heat moisture treated pearl millet starch, J. Food Sci. Technol., 2015, 52, 6502–6510 CrossRef CAS.
- X. Chen, X. He, X. Fu and Q. Huang, In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture
treatment, J. Cereal. Sci., 2015, 63, 109–115 CrossRef CAS.
- T.-T. Huang, D.-N. Zhou, Z.-Y. Jin, X.-M. Xu and H.-Q. Chen, Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch, Food Hydrocolloids, 2016, 54, 202–210 CrossRef CAS.
- B. Yu, J. Li, H. Tao, H. Zhao, P. Liu and B. Cui, Physicochemical properties and in vitro digestibility of hydrothermal treated Chinese yam (Dioscorea opposita Thunb.) starch and flour, Int. J. Biol. Macromol., 2021, 176, 177–185 CrossRef CAS.
- U. Dharmaraj, P. Parameswara, R. Somashekar and N. G. Malleshi, Effect of processing on the microstructure of finger millet by X-ray diffraction and scanning electron microscopy, J. Food Sci. Technol., 2014, 51, 494–502 CrossRef CAS.
- S. E. Ramashia, E. T. Gwata, S. Meddows-Taylor, T. A. Anyasi and A. I. O. Jideani, Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa, Food Res. Int., 2018, 104, 110–118 CrossRef CAS PubMed.
- Y. Xiao, H. Liu, T. Wei, J. Shen and M. Wang, Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment, LWT--Food Sci. Technol., 2017, 86, 285–292 CrossRef CAS.
- J. Dong, L. Huang, W. Chen, Y. Zhu, B. Dun and R. Shen, Effect of heat-moisture treatments on digestibility and physicochemical property of whole quinoa flour, Foods, 2021, 10(12), 3042 CrossRef CAS.
- B. A. Alimi and T. S. Workneh, Structural and physicochemical properties of heat moisture treated and citric acid modified acha and iburu starches, Food Hydrocolloids, 2018, 81, 449–455 CrossRef CAS.
- Z. Yang, H. Hao, Y. Wu, Y. Liu and J. Ouyang, Influence of moisture and amylose on the physicochemical properties of rice starch during heat treatment, Int. J. Biol. Macromol., 2021, 168, 656–662 CrossRef CAS.
- Q. Sun, Z. Han, L. Wang and L. Xiong, Physicochemical differences between sorghum starch and sorghum flour modified by heat-moisture treatment, Food Chem., 2014, 145, 756–764 CrossRef CAS PubMed.
- D. Fan, W. Ma, L. Wang, J. Huang, J. Zhao and H. Zhang,
et al., Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy, Starch/Staerke, 2012, 64(8), 598–606 CrossRef CAS.
- M. Umar, U. R. Ruktanonchai, D. Makararpong, A. Panya and A. K. Anal, Fabrication of biopolymeric nanoparticles of colostrum whey-caseinate, characterization, and in vitro digestibility, J. Food Eng., 2024, 111933 CrossRef CAS.
- V. S. Sharanagat, R. Suhag, P. Anand, G. Deswal, R. Kumar and A. Chaudhary,
et al., Physico-functional, thermo-pasting and antioxidant properties of microwave roasted sorghum [Sorghum bicolor (L.) Moench], J. Cereal. Sci., 2019, 85, 111–119 CrossRef CAS.
- Y. D. Livney, Milk proteins as vehicles for bioactives. Vol. 15, Curr. Opin. Colloid. Interface Sci., 2010, 73–83 CrossRef CAS.
- F. Zeng, F. Ma, F. Kong, Q. Gao and S. Yu, Physicochemical properties and digestibility of hydrothermally treated waxy rice starch, Food Chem., 2015, 172, 92–98 CrossRef CAS.
- R. Souilah, B. Belhadi, N. Boudries, D. Djabali and B. Nadjemi, Pretreatments Effect of Sorghum (Bicolor (L.) Moench) and Millet (Pennisetum Glaucum) Flours on the In Vitro Starch Digestibility, J. Univ. Chem. Technol. Metallurgy, 2012, 47(1), 63–68 CAS.
- L. M. Fonseca, S. L. M. El Halal, A. R. G. Dias and Z. E. da Rosa, Physical modification of starch by heat-moisture treatment and annealing and their applications: a review, Carbohydr. Polym., 2021, 274, 118665 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Muhammad
Hassan
b and
Natthakan
Rungraeng
a,
Muhammad
Hassan
b and
Natthakan
Rungraeng
 *cd
*cd
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) functional groups in the regions of 3300–3250, 2930–2850, 1750–1630, 1180–1070, and 930–860 cm−1, respectively. There were no significant changes observed in the number of peaks of the samples due to the HMT process. The rapidly digestible and slowly digestible starch fractions increased significantly (p < 0.05), while the resistant starch fraction decreased due to the HMT process. RDS, SDS, and RS values were in the ranges of 14.3–22.4%, 28.0–60.9%, and 30.9–55.7%, respectively. This study provides a new way of utilizing this starch source for the development of food products and can reduce dependence on other starch sources such as rice and corn.
CH) functional groups in the regions of 3300–3250, 2930–2850, 1750–1630, 1180–1070, and 930–860 cm−1, respectively. There were no significant changes observed in the number of peaks of the samples due to the HMT process. The rapidly digestible and slowly digestible starch fractions increased significantly (p < 0.05), while the resistant starch fraction decreased due to the HMT process. RDS, SDS, and RS values were in the ranges of 14.3–22.4%, 28.0–60.9%, and 30.9–55.7%, respectively. This study provides a new way of utilizing this starch source for the development of food products and can reduce dependence on other starch sources such as rice and corn.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3–5 min. This finger millet flour (FMF) was sifted through a 70-mesh sieve to remove the bigger particles and was stored in zip-locked high-density polyethylene (HDPE) bags at 4 °C for further analysis.
000 rpm for 3–5 min. This finger millet flour (FMF) was sifted through a 70-mesh sieve to remove the bigger particles and was stored in zip-locked high-density polyethylene (HDPE) bags at 4 °C for further analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/v DW for 30 min and centrifuged for 15 min at 2400 × g using a centrifuge (EBA 8S, Hettich, Germany). This washing and centrifugation step was repeated until only white color starch was achieved at the bottom of the test tube. The samples were further washed for 15 min using ethanol (100%) and centrifuged to discard the supernatant. The samples were neutralized (pH 6.5) by adding HCl (1 M), while pH was monitored using a pH meter (Hanna, USA). The samples were dried in a hot air oven (Memmert, UN, Schwabach, Germany) at 45 °C and 50% relative humidity for 6 h. The dried samples were powdered using a hammer mill (Perten, 120, Finland) and passed across a 100-mesh sieve. The starch powders were stored in HDPE bags at 4 °C for further analysis.
3 w/v DW for 30 min and centrifuged for 15 min at 2400 × g using a centrifuge (EBA 8S, Hettich, Germany). This washing and centrifugation step was repeated until only white color starch was achieved at the bottom of the test tube. The samples were further washed for 15 min using ethanol (100%) and centrifuged to discard the supernatant. The samples were neutralized (pH 6.5) by adding HCl (1 M), while pH was monitored using a pH meter (Hanna, USA). The samples were dried in a hot air oven (Memmert, UN, Schwabach, Germany) at 45 °C and 50% relative humidity for 6 h. The dried samples were powdered using a hammer mill (Perten, 120, Finland) and passed across a 100-mesh sieve. The starch powders were stored in HDPE bags at 4 °C for further analysis.
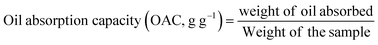
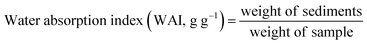

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) functional groups respectively.67,80 There was no significant change observed in the number of peaks of the samples due to HMT but there were changes in the intensity of absorption peaks. The wide and strong absorption peak in the region of 3300–3250 cm−1 was observed due to –OH stretching from the glucose unit, protein–starch interactions and carboxylic acid.33 Due to HMT, a variation in the peak intensity was observed, which indicated hydrogen bond formation.81 The absolute peaks indicated that the heating affects the absorption intensity due to the hydration reaction during the gelatinization of starch. The hydration of the sample generates alterations and hydrogen bonding in inter- and intra-molecular structures, which cause these variations in the spectra.82 The alkene group (–CH) caused the asymmetric stretching of the bond at 2924 cm−1, which changed in the modified starch sample.9 The peak regions of 1700–1500 cm−1 and 1200–900 cm−1 represent proteins in the sample. The region of amide I (1700–1600 cm−1) mainly consists of the C–O stretching of polypeptides from the backbone and the region of amid II (1600–1500 cm−1) consists of the N–H bending and C–N stretching of proteins.83 The absorption peak at 1638.8 cm−1 indicated the presence of water molecules bound to starch.13 In HMT-FMS, an early Maillard reaction caused a variation in the intensity of this peak due to heat.84 These results indicate that there were no major changes in the molecular composition and no new functional group formation in modified FMF and FMS, which elucidates the effect of these processing methods with improved functional and physicochemical characteristics.
CH) functional groups respectively.67,80 There was no significant change observed in the number of peaks of the samples due to HMT but there were changes in the intensity of absorption peaks. The wide and strong absorption peak in the region of 3300–3250 cm−1 was observed due to –OH stretching from the glucose unit, protein–starch interactions and carboxylic acid.33 Due to HMT, a variation in the peak intensity was observed, which indicated hydrogen bond formation.81 The absolute peaks indicated that the heating affects the absorption intensity due to the hydration reaction during the gelatinization of starch. The hydration of the sample generates alterations and hydrogen bonding in inter- and intra-molecular structures, which cause these variations in the spectra.82 The alkene group (–CH) caused the asymmetric stretching of the bond at 2924 cm−1, which changed in the modified starch sample.9 The peak regions of 1700–1500 cm−1 and 1200–900 cm−1 represent proteins in the sample. The region of amide I (1700–1600 cm−1) mainly consists of the C–O stretching of polypeptides from the backbone and the region of amid II (1600–1500 cm−1) consists of the N–H bending and C–N stretching of proteins.83 The absorption peak at 1638.8 cm−1 indicated the presence of water molecules bound to starch.13 In HMT-FMS, an early Maillard reaction caused a variation in the intensity of this peak due to heat.84 These results indicate that there were no major changes in the molecular composition and no new functional group formation in modified FMF and FMS, which elucidates the effect of these processing methods with improved functional and physicochemical characteristics.




