Sustainable nanocarriers fabricated via a dipeptide-based co-assembly approach for enhancing the delivery and translocation of herbicides†
Zirui
Zheng
,
Ziyun
Yang
,
Zehua
Meng
,
Siyang
Liu
,
Tianyue
Wu
,
Chengyi
He
,
Chenhui
Zhang
,
Chen
Ma
*,
Yuxia
Gao
* and
Fengpei
Du
 *
*
Department of Applied Chemistry, College of Science, China Agricultural University, Beijing 100193, China. E-mail: machen@cau.edu.cn; gaoyuxia@cau.edu.cn; dufp@cau.edu.cn
First published on 9th October 2024
Abstract
Pesticide delivery platforms are an effective means to improve the control efficiency of pesticides through multiple functionalities. However, the prevalent use of pesticide carriers is often hindered by complex synthetic pathways, environmentally unfriendly components, and limited loading capacities, which restrict their practical applications in agriculture. In this work, we developed sustainable pesticide nanocarriers using a straightforward supramolecular co-assembly approach, employing bio-based diphenylalanine (FF) as the assembling molecule and the herbicide fluroxypyr (FP) as the active ingredient. Driven by the synergistic effects of hydrogen bonding, electrostatic interactions, π–π stacking, hydrophobic interactions and van der Waals forces, FP and FF co-assembled into stable assemblies with high loading capacity. Notably, the co-assemblies with different compositions exhibited tunable microstructures, including wormlike micelles, micelle-arranged coils, and vesicles. Additionally, these nanocarriers demonstrated sensitive release properties based on the FP/FF ratio and changes in pH value; nearly 97.78% of FP could be released by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-assemblies at a pH value of 5. Furthermore, the alteration in size and microscopic morphology after co-assembly facilitated the translocation of FP in the target weed, ultimately enhancing herbicidal activity. Overall, this work proposes a promising approach for developing green and simplified pesticide carriers, offering novel insights and strategies for sustainable agricultural development.
1 co-assemblies at a pH value of 5. Furthermore, the alteration in size and microscopic morphology after co-assembly facilitated the translocation of FP in the target weed, ultimately enhancing herbicidal activity. Overall, this work proposes a promising approach for developing green and simplified pesticide carriers, offering novel insights and strategies for sustainable agricultural development.
Environmental significancePesticide delivery platforms are essential for improving pesticide utilization and reducing environmental pollution. However, most current carriers involve complex synthetic pathways, environmentally unfriendly components and lower loading capacity. In this work, sustainable herbicide-loaded nanocarriers were fabricated using a simple supramolecular co-assembly strategy. Bio-based diphenylalanine (FF) serves as the assembling molecule, while fluroxypyr (FP) is the active ingredient. These nano pesticide delivery platforms consist solely of the active ingredient, the assembling molecule and water, without the use of organic solvents or undegradable materials. Driven by non-covalent interactions, stable assemblies are formed with high loading capacity and tunable microstructures, resulting in excellent and adjustable application performance. Thus, our study provides an effective strategy for developing a green and simplified pesticide delivery system for sustainable agricultural development. |
Introduction
Pesticides are indispensable chemical substances that protect crops from diseases, pests and weeds, playing an irreplaceable role in agricultural production.1 The latest data showed that 3.5 billion kg of synthetic pesticides are utilized annually worldwide, comprising 47.5% herbicides, 29.5% insecticides, 17.5% fungicides and 5.5% other pesticides.2–4 However, due to the unavoidable pesticide losses during application process, such as volatilization, spray drift, run-off, degradation under environmental conditions, etc., less than 0.1% of the applied pesticide is absorbed by the biological target.5–8 This extremely low utilization rate results in waste and overuse of pesticides, leading to serious environmental pollution and potential risks to human health.9–13 Therefore, developing an efficient strategy to address these issues is crucial for promoting agricultural production and green sustainable development.Pesticide delivery platforms, formed by active ingredients in carrier materials through physical or chemical means, offer significant advantages over traditional pesticide formulations. With their excellent stability, efficient targeted delivery, high interfacial adhesion, and stimuli-responsiveness, numerous pesticide delivery systems have been developed to improve pesticide utilization.14–16 For example, Zhao et al. prepared a pH-responsive core–shell nanocarrier using ZnO nanosphere and ZIF-8, which effectively loaded the bactericide berberine and demonstrated good antibacterial activity against tomato bacterial wilt disease.17 Cao et al. constructed a spinosad-loaded insect gut microenvironment nano-response system consisting of aminated mesoporous silica nanoparticles and polylactic acid, exhibiting superior efficacy in controlling O. furnacalis and excellent transportability in maize plants.18 Our group prepared a temperature-responsive nanogel formulation for endowing non-systemic pesticides with the ability to translocate upward.19 As stated above, although the reported delivery systems have improved the utilization efficiency of pesticides by 10–50% or even more,14,15 some issues still remain. First, owing to the major mass fraction of carrier materials, the loading capacity is normally low.18,20 Second, the preparation of carriers often involves complex organic reactions, complicated processes, high costs and large consumption of energy.21–23 Finally, this process also requires the use of organic solvents, and the materials are usually difficult to degrade in the environment, posing a potential threat to the environment and human health.24 As a result, high loading capacity, simple processes and environmental friendliness are the development directions for pesticide delivery systems.
Supramolecular co-assembly is a process in which two or more molecules spontaneously assemble to form ordered assemblies with specific geometric structures via non-covalent interactions such as hydrogen bonding, electrostatic interactions, hydrophobic interactions, π–π stacking interaction and van der Waals forces.25–28 Therefore, active ingredients with an amino group, carboxyl group, or other groups that may give rise to a non-covalent interaction system can find suitable assembling molecules to form a co-assembled system. The delivery systems formed by the co-assembly of active ingredients with assembling molecules have received widespread attention due to their simple preparation process and high loading capacity.29–31 Yao et al. prepared a nano-transformer by assembling doxorubicin, tannic acid, and indocyanine green, which resulted in prolonged circulation time of nanodrugs in blood, enhanced cellular uptake, rapid lysosomal escape, precise drug release and increased tumor penetration during drug delivery.32 Cao et al. constructed self-assembled nanoparticles based on fungicide fenhexamid (FHA) and polyhexamethylene biguanide (PHMB), which exhibited desirable physicochemical properties and synergistic antimicrobial effect. The inhibitory activity against Botrytis cinerea and Sclerotinia sclerotiorum was 53.03% and 94.56%, respectively, which was higher than that of FHA and PHMB alone.33 Liu et al. used polyethylene glycol and 4,4-methylenediphenyl diisocyanate assembled with λ-cyhalothrine to prepare pesticide-loaded nanogels, which showed great improvements in washing resistance, pesticide retention, and safety to aquatic organisms. Compared with the neat pesticide, the nanogels increased the foliar protection area by 2.21 times, the washing resistance by 80 times, and the security by 9.33 times.34 It can be seen that supramolecular co-assembly is a promising strategy to overcome the shortcomings of the traditional delivery systems; however, most of the current studies adopted synthetic petroleum-based molecules as assembly elements, which still pose potential risks to environment. To address this issue, we innovatively propose the use of a bio-based small-molecule dipeptide as a small-molecule pesticide carrier to load pesticides via supramolecular co-assembly, and this system is speculated to own the advantages of simple preparation process, high loading capacity and environmental friendliness.
Fluroxypyr (4-amino-3,5-dichloro-6-fluoro-2-pyridyloxyacetic acid, FP), a herbicide in the class of synthetic auxins, is widely applied to control broadleaf weeds in agricultural production.35,36 The hydrophobicity of FP makes it commonly used as an organic solvent-based formulation such as emulsifiable concentrate (EC), posing a greater threat to the environment, while traditional means are difficult to process FP into water-based formulations. Furthermore, diphenylalanine (FF) is an environmentally friendly bio-based dipeptide featuring good assembly properties. Self-assemblies of FF with different morphologies such as nanotubes, nanowires, microtubes, vesicles, and nanofibers can be obtained by adjusting the solvent type, FF concentration and preparation conditions.37–39 For example, Li et al. have prepared FF microrods as active optical waveguide materials, allowing locally excited photoluminescence to propagate along the length of microrods with coupling out at the microrod tips.39 Notably, the current research on FF mainly focuses on theoretical studies and materials science, and its use in agriculture is still rare. Considering the unique molecular structures of FF and FP, they may form supramolecular structures through the non-covalent interactions to improve the solubility and utilization of FP. Herein, FF was selected as the assembly molecule and co-assembled with FP to fabricate a sustainable nano delivery system via a simple process, which only consists of the active ingredient, assembly molecule and water. Co-assembled nanocarriers with different microscopic morphologies were obtained by adjusting the ratio of FP/FF. These nanocarriers showed the characteristics of high loading capacity and pH-responsive release. Compared with FP alone, co-assembly with FF significantly improved its translocation in weed plants, which, in turn, enhanced the herbicidal activity (Scheme 1). Overall, this study prepared a sustainable nano delivery system for FP using environmentally friendly materials via a simple process, and this approach can be applied to carrier design for other active ingredients, providing ideas and strategies for green and sustainable development in agriculture.
Experimental section
Materials
Diphenylalanine (FF, 98%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Fluroxypyr (FP, 95%) was provided by Qingdao Jiner Agrochemicals (Group) R&D Co., and ethanol (EtOH, AR), methanol (MeOH, AR), NaOH (AR), and HCl (AR) were provided by Beijing Chemical Works. 7-Amino-4-trifluoromethylcoumarin (AT, 98%) was purchased from Aladdin Co., Ltd. Silwet 408 was purchased from Momentive Performance Materials Co., Ltd. Deionized water was used for aqueous solutions. The dialysis bags (35 mm and 77 mm width to accommodate different-sized containers; MW 500 for the preparation and release experiment of FP-loaded nanocarriers, while MW 200 for the preparation of individual FP suspensions) were purchased from Beijing Biotopped Science & Technology Co., Ltd. The hydrophobic surface poly(tetrafluoroethylene) (PTFE) was bought from Haining Kono Filter Equipment Co., Ltd.Preparation of FP-loaded nanocarriers
The FP-loaded nanocarriers were fabricated by the supramolecular co-assembly of FF and FP via a simple mixing and dialysis procedure. FP and FF were dissolved in EtOH at a concentration of 0.005 mol L−1, respectively. Then the obtained solutions were mixed in volume ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and deionized water was added drop by drop, which was 1/3 of the volume of the mixed solution. Subsequently, the solutions were placed in dialysis bags to dialyze against deionized water for 24 h (water was changed every 6 h) to remove EtOH and unassembled FP and FF. Finally, the FP/FF co-assembled suspension was prepared. All of the above-mentioned steps were performed at room temperature.
1, and deionized water was added drop by drop, which was 1/3 of the volume of the mixed solution. Subsequently, the solutions were placed in dialysis bags to dialyze against deionized water for 24 h (water was changed every 6 h) to remove EtOH and unassembled FP and FF. Finally, the FP/FF co-assembled suspension was prepared. All of the above-mentioned steps were performed at room temperature.
Sample characterization
Stability was characterized by digital photography and using an MS 20 stability analyzer (Data-Physics). The freshly prepared FP/FF co-assembled suspension was placed at room temperature for 48 h, recording digital photos every 12 h. At the same time, the suspension was also placed in the MS 20 stability analyzer and tested at a constant temperature of 25 °C for 48 h, with the instrument scanning at 0.5 h interval. The average transmission intensity and stability index (SI) over 48 h were analyzed using the MSC software to evaluate the stability of the suspensions.Transmission electron microscopic (TEM) images were obtained using a Hitachi HT7700 TEM at an accelerating voltage of 80 kV. FTIR spectra were recorded using a Fourier transform infrared spectroscopy (Nicolet 560). UV spectra were recorded using a UV-visible spectrophotometer (TU-1810PC). The loading capacity and release performance were measured by high-performance liquid chromatography (HPLC) using an Agilent TC-C18 column (4.6 mm × 250 mm, 5 μm; Agilent, CA, USA). The mobile phases were methyl alcohol and water (v/v = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at a flow rate of 0.80 mL min−1. The column temperature was kept at 25 °C. The injection volume was 10 μL. The detection wavelength was set as 210 nm. The confocal laser scanning microscopic (CLSM) images were obtained using a Leica-TCS SP8.
10) at a flow rate of 0.80 mL min−1. The column temperature was kept at 25 °C. The injection volume was 10 μL. The detection wavelength was set as 210 nm. The confocal laser scanning microscopic (CLSM) images were obtained using a Leica-TCS SP8.
Molecular dynamics simulations
The molecular dynamics (MD) simulations were carried out using the GROMACS 2020.3 software. The general Amber force field (GAFF) was used to generate the parameter and topology of ligands, respectively. The simulation box size was optimized with the distance between each atom of the ligands and the box greater than 1.0 nm. Then, fill the box with water molecules based on a density of 1. To make the simulation system electrically neutral, the water molecules were replaced with Cl− and Na+ ions. Following the steepest descent method, energy optimization of 5.0 × 104 steps was performed to minimize the energy consumption of the entire system, and finally to reduce the unreasonable contact or atom overlap in the entire system. After energy minimization, first-phase equilibration was performed with the NVT ensemble at 300 K for 100 ps to stabilize the temperature of the system. Second-phase equilibration was simulated with the NPT ensemble at 1 bar and 100 ps. All MD simulations were performed for 50 ns under an isothermal and isostatic ensemble with a temperature of 300 K and a pressure of 1 atmosphere. The temperature and pressure were controlled by the V-rescale and Parrinello–Rahman methods, respectively, and the temperature and pressure coupling constants were 0.1 and 0.5 ps, respectively. The Lennard-Jones function was used to calculate the van der Waals force, and the nonbond truncation distance was set to 1.4 nm. The bond length of all atoms was constrained by the LINCS algorithm. The long-range electrostatic interaction was calculated by the particle mesh-Ewald method with a Fourier spacing of 0.16 nm.Loading capacity and release performance of FP-loaded nanocarriers
The prepared FP/FF co-assembled suspension was lyophilized. Then, 50 mg of the dried FP-loaded nanocarriers were dissolved in 50 mL of MeOH and treated under ultrasonication for 10 min to accelerate pesticide release. After that, the mixture was incubated at room temperature for 24 h to ensure complete release of the pesticide. The pesticide concentration was determined by HPLC and the loading capacity (LC) was calculated using eqn (1).| LC (%) = MFP in nanocarriers/MNanocarriers × 100% | (1) |
The release behaviors of FP from the nanocarriers were investigated by a dialysis method. The assembled suspension (4 mL) was packed in a dialysis bag (MW 500) and placed into 46 mL of mixed solvent (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 1
deionized water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with pH 5, 7, and 9 (pH was adjusted using NaOH and HCl). The release system was placed at room temperature with stirring at 80 rpm. When sampling, 1 mL of dialysate was removed from the test suspension, and 1 mL of mixed solvent (MeOH
1, v/v) with pH 5, 7, and 9 (pH was adjusted using NaOH and HCl). The release system was placed at room temperature with stirring at 80 rpm. When sampling, 1 mL of dialysate was removed from the test suspension, and 1 mL of mixed solvent (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 1
deionized water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, the relative pH is adjusted with NaOH and HCl) was injected as a supplement simultaneously to ensure the constant volume of 50 mL at different times (0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12 and 14 h). The pesticide concentration of each dialysate was measured by HPLC. The cumulative release ratio (CRR) was calculated using eqn (2):19,21
1, v/v, the relative pH is adjusted with NaOH and HCl) was injected as a supplement simultaneously to ensure the constant volume of 50 mL at different times (0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12 and 14 h). The pesticide concentration of each dialysate was measured by HPLC. The cumulative release ratio (CRR) was calculated using eqn (2):19,21
 | (2) |
Moreover, four mathematical models were used to analyze the release mechanism of FP from the nanocarriers, namely, zero-order, first-order, Higuchi and Ritger–Peppas models, according to eqn (3)–(6):
| zero-order model: Mt/M∞ = kt | (3) |
| first-order model: ln(1 − Mt/M∞) = −kt | (4) |
| Higuchi model: Mt/M∞ = kt0.5 | (5) |
| Ritger–Peppas model: Mt/M∞ = ktn | (6) |
Interfacial behavior of FF, FP, and FP/FF co-assembled suspensions
Surface tension was measured by the Wilhelmy method using a DCAT 21 automatic surface tensiometer (Data-Physics) with a platinum piece at 25 °C, and each sample was analyzed at least three times. The contact angle was measured using an OCA 50 (Data-Physics) by the sessile drop method, and a droplet (2.5 μL) was moved to a PTFE surface directly using an automatic injector. Each sample was tested five times.Translocation behavior of FP-loaded nanocarriers
Chenopodium album L. (C. album) is a widely distributed annual broadleaf weed. Using C. album as a target weed, the translocation behavior of FP-loaded nanocarriers in the plant was investigated. All C. album plants were collected from the West Campus of China Agricultural University. The collected C. album plants were cultivated indoors for 3 days, and then the plants with good and similar growth conditions were selected for the experiment. 7-Amino-4-trifluoromethylcoumarin (AT, an oil-soluble fluorescent molecule), which is similar to FP in molecular size and structure, was added at 4 wt% as an assembly factor to the assembly system to facilitate better observation of FP translocation. At the same time, Silwet 408, a silicone surfactant, was added at 0.1 wt% to promote droplet wetting and penetration on the leaf surface, while ensuring that the droplet will not drop onto the bottom leaves through gravity. A drop (5 μL) of the FP/FF co-assembled suspension diluted from the lyophilized FP-loaded nanocarriers containing the fluorescent molecules and surfactant was applied to the top leaves of C. album plant using a pipette gun, ensuring that the amount of FP in each sample was 10 wt%, while the amount of FF in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 samples was 10.02 wt%, 6.21 wt%, and 3.82 wt%, respectively (calculated from the experimental values of the loading capacity experimental values). In addition, after 20 min, the bottom leaves, approximately 10 cm long from the application site, were sectioned and observed using CLSM. All CLSM images were computationally processed using the Image J software to obtain the average fluorescence density.
1 samples was 10.02 wt%, 6.21 wt%, and 3.82 wt%, respectively (calculated from the experimental values of the loading capacity experimental values). In addition, after 20 min, the bottom leaves, approximately 10 cm long from the application site, were sectioned and observed using CLSM. All CLSM images were computationally processed using the Image J software to obtain the average fluorescence density.
Greenhouse pesticide activity
C. album plants were sprayed using the standard FF nozzles ST110-03 (Lechler GmbH Ltd., Germany) under a pressure of 300 kPa applying the FP/FF co-assembled suspension diluted from the lyophilized FP-loaded nanocarriers, ensuring that the amount of FP in each sample was 10 wt%, and the amount of surfactant Silwet 408 was 0.1 wt%. The height of C. album plants was measured at 0, 1, 4, 7, 10, and 14 days after treatment,40,41 respectively. The control efficacy was defined as the plant height inhibition rate, which was calculated using eqn (7): | (7) |
Results and discussion
Fabrication and stability of FP-loaded nanocarriers
FP-loaded nanocarriers were prepared by a simple solvent substitution method based on supramolecular co-assembly (Fig. 1A). FF and FP molecules were first dissolved in EtOH, respectively, and their EtOH solutions were mixed, allowing them to come into full contact through molecular thermal motion. Deionized water was then added to the mixture, causing the molecules to aggregate as a result of the hydrophobic interactions. Finally, EtOH was removed from the system via a gentle dialysis process, during which the unassembled monomers were removed (Fig. S1†) and the closely interacting FP/FF assemblies were precipitated to form a homogeneous suspension. Considering the effect of assembly structure on the pesticide delivery process, in this work, different co-assemblies were fabricated by adjusting the ratio of FP/FF. As shown in Fig. 1B, the FP-loaded nanocarriers were prepared with FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form translucent suspensions. The suspensions of FP-loaded nanocarriers were basically stable within 12 h, and then appeared to be noticeably settling over time, but they could be evenly dispersed after shaking and no obvious precipitates were observed. Overall, the FP/FF co-assembled suspensions were found to be relatively stable.
1 to form translucent suspensions. The suspensions of FP-loaded nanocarriers were basically stable within 12 h, and then appeared to be noticeably settling over time, but they could be evenly dispersed after shaking and no obvious precipitates were observed. Overall, the FP/FF co-assembled suspensions were found to be relatively stable.
To further characterize the stability of the nanocarriers, their changes in transmission were monitored over a period of 48 h using an MS 20 stability analyzer. For a specific location, an increase in transmission indicated a decrease in particle concentration, while a decrease in transmission indicated an increase in particle concentration.42 As shown in Fig. 1C–F, with the extension of time, the transmission increased in varying degrees from red lines to purple lines, exhibiting the sedimentation. The greater the relative distance between the purple and red lines, the more significant the sedimentation. It can be seen that for the four samples, the variation in transmission at different height positions is essentially homogeneous, that is, there are no particular variations at specific positions. Therefore, to quantitatively compare the difference in transmission changes, the mean transmission of these suspensions was compared in Fig. 1G. For the whole monitoring period, the transmission of the suspension with an FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was the highest, and the transmission of the suspension with FP alone was the lowest, showing that the order of sedimentation was 1
2 was the highest, and the transmission of the suspension with FP alone was the lowest, showing that the order of sedimentation was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 > 1
2 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > 2
1 > 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > FP. Besides, the stability index (SI, the smaller this value represents the more stable system) was also compared in Fig. 1H and the stability difference was demonstrated, showing that the stability follows the order FP > 2
1 > FP. Besides, the stability index (SI, the smaller this value represents the more stable system) was also compared in Fig. 1H and the stability difference was demonstrated, showing that the stability follows the order FP > 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > 1
1 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > 1
1 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.43,44 That is, the increase in FF proportion accelerated the sedimentation rate, which might due to the strong assembling ability of FF. The participation of FF molecules would form more compact assemblies with FP under the non-covalent interactions, resulting in a greater density difference between the assembled particles and the continuous phase, and therefore a faster sedimentation rate.45
2.43,44 That is, the increase in FF proportion accelerated the sedimentation rate, which might due to the strong assembling ability of FF. The participation of FF molecules would form more compact assemblies with FP under the non-covalent interactions, resulting in a greater density difference between the assembled particles and the continuous phase, and therefore a faster sedimentation rate.45
Microscopic morphology of FP-loaded nanocarriers
Since the plant leaf surfaces are composed of hierarchical micro-/nanostructures, the topological effect between their micro-/nanostructures and the delivery system will significantly affect the utilization rate of pesticides.46 In detail, the delivery systems with specific morphology can be embedded into the micro-/nanostructure of the leaf surface to promote the deposition of the pesticide on the surface, thereby improving the effectiveness of prevention.14,21 In this regard, it is important to investigate the morphology of the pesticide nanocarriers. In this work, transmission electron microscopy (TEM) was employed to reveal the morphologies of the FF, FP, and co-assemblies. As shown in Fig. 2A and B, FF self-assembled into one-dimensional rod-like assemblies with a diameter ranging from 500 nm to 2 μm, while the individual FP just aggregated into irregular agglomerates. Notably, the co-assemblies with different FP/FF ratios exhibited various microscopic morphologies. Flexible wormlike micelles with a length in the range of about 200–900 nm and a diameter around 20–100 nm were obtained at a FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 2C and D). Staggered coils formed by micelles in the range of 50–350 nm were obtained at a ratio of 1
2 (Fig. 2C and D). Staggered coils formed by micelles in the range of 50–350 nm were obtained at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2E and F). In addition, at a ratio of 2
1 (Fig. 2E and F). In addition, at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the vesicles with a diameter of about 0.5–1.5 μm and a wall thickness in the range of 50–120 nm were observed (Fig. 2G and H). Besides, the fusion process of vesicles was also captured (red arrows in Fig. 2G).
1, the vesicles with a diameter of about 0.5–1.5 μm and a wall thickness in the range of 50–120 nm were observed (Fig. 2G and H). Besides, the fusion process of vesicles was also captured (red arrows in Fig. 2G).
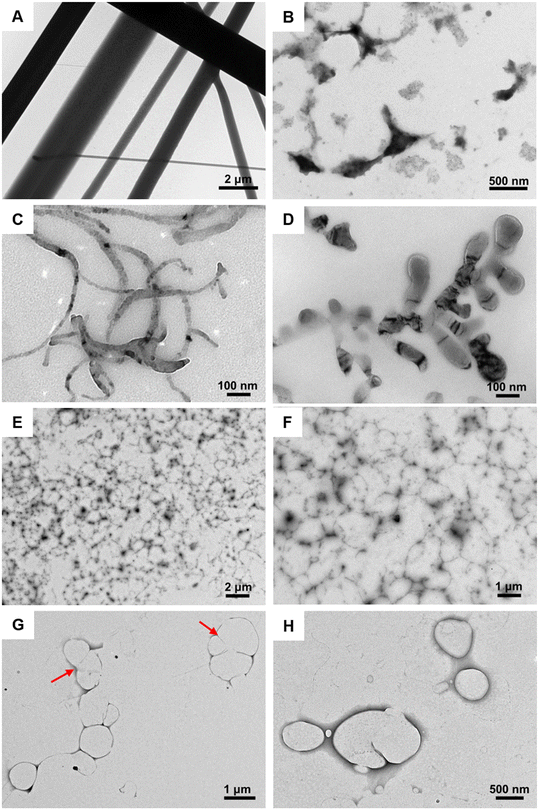 | ||
Fig. 2 TEM images of assemblies formed by: (A) FF, (B) FP, (C and D) FP/FF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (E and F) FP/FF = 1 2, (E and F) FP/FF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and (G and H) FP/FF = 2 1, and (G and H) FP/FF = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
It can be seen that adjusting the molar ratio of FP and FF can yield co-assemblies with different microscopic morphologies, which may be attributed to the differences in their intermolecular forces. Individual FFs had excellent assembly capabilities, forming rigid rod-like assemblies due to their very strong intermolecular interactions such as hydrogen bonding, electrostatic interactions and π–π stacking.47 On the contrary, individual FP could only form irregular agglomerates due to its weak assembling ability without strong intermolecular forces. Moreover, when FF was introduced to co-assemble with FP, the assembly became regular because of the newly generated non-covalent interactions between FF and FP molecules. The different molecular ratios of FP and FF lead to different intermolecular forces, thus arranging in different patterns and forming different assemblies.
Formation mechanism of FP/FF co-assembled nanocarriers
After understanding the co-assembly morphologies of FP/FF, their assembly mechanism was further explored. UV-vis and FTIR spectroscopy were employed to investigate the driving forces between FP and FF, and their co-assembly process was also revealed by MD simulations. In Fig. 3A, FF shows a strong absorption peak at 192 nm and a weak absorption band at 206 nm, while FP shows a strong absorption peak at 212 nm and a weak absorption band at 236 nm. After co-assembly, the characteristic peaks of both FF and FP were merged and red-shifted to 214 nm, and the intensity was also decreased. Meanwhile, the band peak at 236 nm was also red-shifted to 240 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 systems. Notably, new broad absorption peaks at 287 nm and 311 nm appeared for 1
1 systems. Notably, new broad absorption peaks at 287 nm and 311 nm appeared for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 systems, respectively. This may be due to the different degrees of π–π stacking between FP and FF with different molar ratios during the process of FP and FF co-assembly, forming different degrees of conjugated systems, which resulted in the shifting of absorption peaks to the long-wavelength direction.
1 systems, respectively. This may be due to the different degrees of π–π stacking between FP and FF with different molar ratios during the process of FP and FF co-assembly, forming different degrees of conjugated systems, which resulted in the shifting of absorption peaks to the long-wavelength direction.
Fig. 3B shows the FTIR spectra of FP, FF and the co-assembled nanocarriers. FF exhibited stretching vibration peaks of the amino group and carboxyl group at 3248 cm−1, and the peak at 2937 cm−1 was ascribed to the stretching vibration of methylene. The stretching vibration of the N–H bond of the amino group (3488 cm−1) and the stretching vibration of the O–H bond of the carboxyl group (3363 cm−1) could be observed in FP, as well as the stretching vibration of the C–H bond of methylene (2931, 2858 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1746 cm−1). The changes in the IR spectra after the co-assembly of FP and FF were mainly the shift of the peak at 3488 cm−1 to 3478 cm−1, the shift of the peak at 3363 cm−1 to 3354, 3353 and 3352 cm−1 (corresponding to the FP/FF ratios of 1
O bond (1746 cm−1). The changes in the IR spectra after the co-assembly of FP and FF were mainly the shift of the peak at 3488 cm−1 to 3478 cm−1, the shift of the peak at 3363 cm−1 to 3354, 3353 and 3352 cm−1 (corresponding to the FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively), the shift of the peak at 2931 and 2858 cm−1 to the lower wavenumber region, and the shift of the peak at 1746 cm−1 to 1737 and 1736 cm−1 (corresponding to FP/FF ratios of 1
1, respectively), the shift of the peak at 2931 and 2858 cm−1 to the lower wavenumber region, and the shift of the peak at 1746 cm−1 to 1737 and 1736 cm−1 (corresponding to FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively). The shifting of these characteristic peaks proved the occurrence of non-covalent interactions between FP and FF. Generally, the formation of intermolecular hydrogen bonds and conjugated systems cause the characteristic absorption peaks to shift in the direction of low wavenumbers.20,31 The shifts of the characteristic peaks of the co-assemblies suggest that hydrogen bonding and electrostatic interactions between the amino and carboxyl groups of the two molecules occurred, as well as the presence of van der Waals forces between the molecules. Considering the result of UV-vis and IR spectra, it is reasonable to speculate that electrostatic interactions, hydrogen bonding, van der Waals force, and π–π stacking mainly occur between FF and FP during the co-assembly process.
1, respectively). The shifting of these characteristic peaks proved the occurrence of non-covalent interactions between FP and FF. Generally, the formation of intermolecular hydrogen bonds and conjugated systems cause the characteristic absorption peaks to shift in the direction of low wavenumbers.20,31 The shifts of the characteristic peaks of the co-assemblies suggest that hydrogen bonding and electrostatic interactions between the amino and carboxyl groups of the two molecules occurred, as well as the presence of van der Waals forces between the molecules. Considering the result of UV-vis and IR spectra, it is reasonable to speculate that electrostatic interactions, hydrogen bonding, van der Waals force, and π–π stacking mainly occur between FF and FP during the co-assembly process.
To further investigate the co-assembly process of FP with FF, MD simulations were performed. As shown in Fig. 3C, FP/FF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with water molecules were distributed inside the simulated box to observe their state changes within 100 ns. The root mean square deviation (RMSD) of the whole simulation process fluctuated slightly and tended to equilibrate after 10 ns (Fig. S2A†), indicating that the whole simulation process was stable and reliable. The solvent-accessible surface areas (SASAs) were calculated from van der Waals forces interacting with solvent molecules, which decreased with the increase in molecular compactness. As shown in Fig. 3D, the SASA during the simulation decreased rapidly in the first 40 ns and then reached a steady state, indicating that the tightness between the FP and FF molecules increased and stabilized. In this process, the hydrophobic interaction drove the two molecules to reduce their exposure to aqueous solvents, which, in turn, resulted in the formation of a tighter assembly. Meanwhile, there were hydrogen bonding interactions during the co-assembly process, and the average number of hydrogen bonds between the two molecules is 15.4 (Fig. 3E). Fig. S2B† shows the change in binding energy during co-assembly, where Coul-SR is the binding energy for van der Waals forces and LJ-SR is the binding energy for electrostatic interactions. During the assembly process, the van der Waals force and the electrostatic interaction between the two molecules increased and stabilized over time, and the main driving force for the binding of the two molecules was the electrostatic interaction. In summary, using UV-vis, FTIR spectroscopy and MD simulations, the driving forces for the co-assembly between FP and FF were shown to be hydrogen bonding, electrostatic interactions, π–π stacking, hydrophobic interactions and van der Waals forces.
1) with water molecules were distributed inside the simulated box to observe their state changes within 100 ns. The root mean square deviation (RMSD) of the whole simulation process fluctuated slightly and tended to equilibrate after 10 ns (Fig. S2A†), indicating that the whole simulation process was stable and reliable. The solvent-accessible surface areas (SASAs) were calculated from van der Waals forces interacting with solvent molecules, which decreased with the increase in molecular compactness. As shown in Fig. 3D, the SASA during the simulation decreased rapidly in the first 40 ns and then reached a steady state, indicating that the tightness between the FP and FF molecules increased and stabilized. In this process, the hydrophobic interaction drove the two molecules to reduce their exposure to aqueous solvents, which, in turn, resulted in the formation of a tighter assembly. Meanwhile, there were hydrogen bonding interactions during the co-assembly process, and the average number of hydrogen bonds between the two molecules is 15.4 (Fig. 3E). Fig. S2B† shows the change in binding energy during co-assembly, where Coul-SR is the binding energy for van der Waals forces and LJ-SR is the binding energy for electrostatic interactions. During the assembly process, the van der Waals force and the electrostatic interaction between the two molecules increased and stabilized over time, and the main driving force for the binding of the two molecules was the electrostatic interaction. In summary, using UV-vis, FTIR spectroscopy and MD simulations, the driving forces for the co-assembly between FP and FF were shown to be hydrogen bonding, electrostatic interactions, π–π stacking, hydrophobic interactions and van der Waals forces.
Loading capacity and pH-responsive release properties of FP-loaded nanocarriers
To better understand the application potential of FP-loaded nanocarriers, the loading capacity (LC) and release performance were further investigated. The LCs of the lyophilized nanocarriers were determined by high-performance liquid chromatography (HPLC). As depicted in Table S1,† the LCs of the FP-loaded nanocarriers with FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were 49.96%, 61.68% and 72.34%, respectively, exhibiting much higher LCs in comparison with conventional pesticide carriers. (Table S2†) This is mainly due to the fact that the complex composition of the traditional carrier structure occupies the main mass of the loading system, making it difficult to further increase the loading capacity. In contrast, co-assembled molecular nanocarriers consist only of the active ingredient and the assembled molecules, leading to a significant increase in the loading capacity as a result of the simple composition, which demonstrates the advantages of using supramolecular co-assembly approaches in pesticide loading. In addition, theoretical calculations based on the molar ratios of FP and FF showed that the theoretical LC values of the nanocarriers with FP/FF ratios of 1
1 were 49.96%, 61.68% and 72.34%, respectively, exhibiting much higher LCs in comparison with conventional pesticide carriers. (Table S2†) This is mainly due to the fact that the complex composition of the traditional carrier structure occupies the main mass of the loading system, making it difficult to further increase the loading capacity. In contrast, co-assembled molecular nanocarriers consist only of the active ingredient and the assembled molecules, leading to a significant increase in the loading capacity as a result of the simple composition, which demonstrates the advantages of using supramolecular co-assembly approaches in pesticide loading. In addition, theoretical calculations based on the molar ratios of FP and FF showed that the theoretical LC values of the nanocarriers with FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 should be 28.99%, 44.90%, and 62.02%, respectively, which are significantly lower than the experimental values. The reason may be that some of the FF molecules were not involved in the assembly and left the system during the dialysis process, resulting in significantly higher LC values.
1 should be 28.99%, 44.90%, and 62.02%, respectively, which are significantly lower than the experimental values. The reason may be that some of the FF molecules were not involved in the assembly and left the system during the dialysis process, resulting in significantly higher LC values.
In agricultural production, the pH differences between target and non-target, in different climates and environments, etc., make the pH-responsive release function a very important application potential. The release performances of FP-loaded nanocarriers were studied by the release curves in the mixed solvent (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 1
deionized water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) of pH 5, 7 and 9. As shown in Fig. 4A–C, the nanocarriers with different FP/FF ratios presented different release rates and release amounts under different pH conditions. For the FP/FF ratio of 1
1, v/v) of pH 5, 7 and 9. As shown in Fig. 4A–C, the nanocarriers with different FP/FF ratios presented different release rates and release amounts under different pH conditions. For the FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, when the nanocarrier was in a neutral solvent environment, it could be basically released within about 5 h, and the release amount was 63.24% (Fig. 4A). While in acidic or alkaline environment, the rate of FP release would be significantly increased, and the release amount could reach 73.41% and 71.49%, respectively, reflecting the release performance of the nanocarriers in response to both acidic and alkaline conditions. For the nanocarriers with an FP/FF ratio of 1
2, when the nanocarrier was in a neutral solvent environment, it could be basically released within about 5 h, and the release amount was 63.24% (Fig. 4A). While in acidic or alkaline environment, the rate of FP release would be significantly increased, and the release amount could reach 73.41% and 71.49%, respectively, reflecting the release performance of the nanocarriers in response to both acidic and alkaline conditions. For the nanocarriers with an FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the release characteristics under different pH conditions showed significant differences (Fig. 4B). Under neutral conditions at pH 7, the release rate of the nanocarriers was similar to that of the 1
1, the release characteristics under different pH conditions showed significant differences (Fig. 4B). Under neutral conditions at pH 7, the release rate of the nanocarriers was similar to that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 assemblies, which were able to release only 58.58% of FP. The release rate and amount of the nanocarriers were significantly increased under alkaline and acidic conditions, which can reach 72.41% and 97.78%, respectively. For the nanocarriers with an FP/FF ratio of 2
2 assemblies, which were able to release only 58.58% of FP. The release rate and amount of the nanocarriers were significantly increased under alkaline and acidic conditions, which can reach 72.41% and 97.78%, respectively. For the nanocarriers with an FP/FF ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it exhibited similar release rates at pH 7 and pH 9, being able to reach 71.12% and 75.61%, respectively. However, the acidic environment significantly inhibited the release of FP at pH 5, reaching only 47.93% (Fig. 4C). It can be concluded that the FP-loaded nanocarriers own different pH-responsive release behaviors.
1, it exhibited similar release rates at pH 7 and pH 9, being able to reach 71.12% and 75.61%, respectively. However, the acidic environment significantly inhibited the release of FP at pH 5, reaching only 47.93% (Fig. 4C). It can be concluded that the FP-loaded nanocarriers own different pH-responsive release behaviors.
 | ||
Fig. 4 Release performance of FP-loaded nanocarriers in different FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (A), 1 2 (A), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (B), and 2 1 (B), and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (C); and plot on pH 5 (D), pH 7 (E), and pH 9 (F). 1 (C); and plot on pH 5 (D), pH 7 (E), and pH 9 (F). | ||
The charges of the FP and FF molecules under different pH conditions affected their electrostatic interactions, and determined intermolecular binding, which was the main reason for the pH-responsive release behavior of the FP-loaded nanocarriers. Both FP and FF molecules possess amino and carboxyl groups, making it necessary to consider the ionization of all these groups when analyzing the electrostatic interactions of the molecules. FP is a strong acid that exhibits a negative charge under the three selected pH conditions.48 In addition, the dipeptide FF has an isoelectric point of 5.48, which means that it is positively charged when the pH is less than 5.48 and negatively charged when the pH is greater than 5.48.38 In an acidic environment of pH 5, the nanocarriers with an FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was the fastest and most released, followed by the 1
1 was the fastest and most released, followed by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 systems, respectively (Fig. 4D). The reason may be that FP and FF exhibit opposite charges at pH 5, resulting in electrostatic attraction between them. When the FP/FF ratio was 1
1 systems, respectively (Fig. 4D). The reason may be that FP and FF exhibit opposite charges at pH 5, resulting in electrostatic attraction between them. When the FP/FF ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the attractive force was small, so the release was the fastest; and when the FF ratio was increased to 1
1, the attractive force was small, so the release was the fastest; and when the FF ratio was increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the more attractive force provided by FF made the non-covalent binding of the two tighter, slowing down the release of FP; however, when the FP/FF ratio was 2
2, the more attractive force provided by FF made the non-covalent binding of the two tighter, slowing down the release of FP; however, when the FP/FF ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the excessive FP molecules interacted with each other and agglomerated together themselves, making it more difficult to be released, and thus the release was the slowest and least. When the environment was neutral or alkaline (pH 7 or 9), the release rates of the three co-assembled nanocarriers did not differ significantly, with the greatest release from the nanocarriers with an FP/FF of 2
1, the excessive FP molecules interacted with each other and agglomerated together themselves, making it more difficult to be released, and thus the release was the slowest and least. When the environment was neutral or alkaline (pH 7 or 9), the release rates of the three co-assembled nanocarriers did not differ significantly, with the greatest release from the nanocarriers with an FP/FF of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4E and F). The possible reason was that when the pH was 7 or 9, both FP and FF have the same negative charge and they were electrostatic repulsive. When the ratio of FP to FF was 2
1 (Fig. 4E and F). The possible reason was that when the pH was 7 or 9, both FP and FF have the same negative charge and they were electrostatic repulsive. When the ratio of FP to FF was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the relative charges were matched, and the electrostatic repulsion was strong, which promoted the release of FP, and therefore the most release. When the ratios were 1
1, the relative charges were matched, and the electrostatic repulsion was strong, which promoted the release of FP, and therefore the most release. When the ratios were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the increase in the proportion of FF molecules made the electrostatic repulsive force decrease, slowing down the release of FP.
2, the increase in the proportion of FF molecules made the electrostatic repulsive force decrease, slowing down the release of FP.
Based on the above-mentioned results, four kinetic models, namely, zero-order, first-order, Higuchi, and Ritger–Peppas models were carried out to investigate the release mechanisms of FP-loaded nanocarriers at different ratios and pH values. As shown in Fig. S3† and Table 1, the release performance of the nanocarriers followed the Higuchi model well when FP/FF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, with squared correlation coefficients (R2) of 0.992, 0.992 and 0.995 and fitting parameters k of 0.383, 0.320 and 0.341 for pH 5, 7, and 9, respectively. While for the 1
2, with squared correlation coefficients (R2) of 0.992, 0.992 and 0.995 and fitting parameters k of 0.383, 0.320 and 0.341 for pH 5, 7, and 9, respectively. While for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 assembly, the release performance followed the first-order model well, with R2 values of 0.993, 0.994 and 0.994, and fitting parameters k of 0.041, 0.280 and 0.377 for pH 5, 7, and 9, respectively. For the nanocarriers with an FP/FF ratio of 2
1 assembly, the release performance followed the first-order model well, with R2 values of 0.993, 0.994 and 0.994, and fitting parameters k of 0.041, 0.280 and 0.377 for pH 5, 7, and 9, respectively. For the nanocarriers with an FP/FF ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it also followed the first-order model well, with R2 values of 0.997, 0.987 and 0.994, and fitting parameters k of 0.205, 0.239 and 0.311 for pH 5, 7, and 9, respectively. Both release models belonged to Fickian diffusion, and simple diffusion appeared to dominate for FP-loaded nanocarriers.49 FP gradually separated from the attraction of FF molecules and entered the external environment via diffusion.
1, it also followed the first-order model well, with R2 values of 0.997, 0.987 and 0.994, and fitting parameters k of 0.205, 0.239 and 0.311 for pH 5, 7, and 9, respectively. Both release models belonged to Fickian diffusion, and simple diffusion appeared to dominate for FP-loaded nanocarriers.49 FP gradually separated from the attraction of FF molecules and entered the external environment via diffusion.
| Conditions | Zero-order model | First-order model | Higuchi model | Ritger–Peppas model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | R 2 | k | R 2 | k | R 2 | k | n | R 2 | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
pH 5 | 0.229 | 0.944 | 0.372 | 0.982 | 0.383 | 0.992 | 0.371 | 0.536 | 0.923 |
| pH 7 | 0.192 | 0.948 | 0.277 | 0.975 | 0.320 | 0.992 | 0.307 | 0.548 | 0.931 | |
| pH 9 | 0.205 | 0.957 | 0.310 | 0.989 | 0.341 | 0.995 | 0.321 | 0.569 | 0.963 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 5 | 0.387 | 0.955 | 0.041 | 0.993 | 0.580 | 0.993 | 0.563 | 0.542 | 0.920 |
| pH 7 | 0.205 | 0.983 | 0.280 | 0.994 | 0.302 | 0.981 | 0.255 | 0.739 | 0.955 | |
| pH 9 | 0.251 | 0.976 | 0.377 | 0.994 | 0.371 | 0.986 | 0.326 | 0.681 | 0.944 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 5 | 0.162 | 0.991 | 0.205 | 0.997 | 0.237 | 0.974 | 0.188 | 0.821 | 0.974 |
| pH 7 | 0.161 | 0.957 | 0.239 | 0.987 | 0.296 | 0.993 | 0.257 | 0.660 | 0.976 | |
| pH 9 | 0.205 | 0.968 | 0.311 | 0.994 | 0.338 | 0.990 | 0.299 | 0.634 | 0.963 | |
Translocation of FP-loaded nanocarriers in C. album
In order to evaluate the translocation behaviors of FP-loaded nanocarriers in target plants, 7-amino-4-trifluoromethylcoumarin (AT), an oil-soluble fluorescent molecule with structural similarity to FP, was chosen as a tracer agent to participate in the entire assembly process and to be loaded into the co-assembled nanocarriers. Chenopodium album L. (C. album), a widely distributed annual broadleaf weed, is one of the control plants of FP. Besides, since the surface activity of the co-assembled suspensions was not sufficient to wet the hydrophobic plant surface (Fig. S4†), 0.1 wt% Silwet 408 was added to promote droplet wetting and penetration on the leaf surface. The fluorescent assembled suspension was applied to the top leaves of C. album, and after 20 min, sections were taken from the bottom leaves to about 10 cm from the application site and observed using CLSM. As shown in Fig. 5A1–E1 and A2–E2, after 20 min, the fluorescence intensity in the bottom leaves of the applied FP/FF co-assembled nanocarriers was significantly higher than that of FP and FF alone, demonstrating that the co-assemblies indeed enhanced FP translocation in the target plants, whereas, among the three ratios of FP-loaded nanocarriers, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-assembled nanocarriers resulted in a significantly higher fluorescence intensity than that of the other two, and that of 1
1 co-assembled nanocarriers resulted in a significantly higher fluorescence intensity than that of the other two, and that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was slightly higher than that of the 1
1 was slightly higher than that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 co-assembly. Quantitative statistics on the translocation is shown in Fig. 5F, where “integrated density” means the total fluorescence intensity in images.
2 co-assembly. Quantitative statistics on the translocation is shown in Fig. 5F, where “integrated density” means the total fluorescence intensity in images.
After FP was applied to the leaves, it was transported in the weed mainly through the vascular system-phloem transport pathways.50,51 In this process, the size and morphology of aggregates have the greatest influence on the transport capacity and efficiency. For FF and FP, either the rigid microrods or the larger irregular aggregates made it difficult to transport through the phloem, so fewer molecules could reach the bottom leaves.51 When FP and FF were assembled into co-assemblies with smaller sizes and different morphologies, their translocation process in plants was greatly facilitated. For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 system, the flexible wormlike micelles with diameters around 20–200 nm allowed for easy translocation through the vascular system and the phloem, so that more molecules could reach the bottom leaves. Similarly, for the flexible coils arranged by micelles (50–350 nm) at an FP/FF ratio of 1
2 system, the flexible wormlike micelles with diameters around 20–200 nm allowed for easy translocation through the vascular system and the phloem, so that more molecules could reach the bottom leaves. Similarly, for the flexible coils arranged by micelles (50–350 nm) at an FP/FF ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the transport capacity in the plant was significantly enhanced. In addition, the vesicles in the 2
1, the transport capacity in the plant was significantly enhanced. In addition, the vesicles in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system were able to deform or rupture during transportation, which significantly facilitated the pesticide transport process in the plant, showing better translocation ability than others. It can be seen that nanocarriers enhanced the transport capacity and efficiency of the original pesticide molecules in the plant, which was consistent with the conclusions drawn from previous work.19,52 The use of nanocarriers for pesticide could facilitate the faster downward transport of nanoparticles through vascular bundles. Generally speaking, the smaller and more flexible the nanocarriers, the better the transport capacity within the plants. In addition to the particle size and charge, this study has demonstrated that the morphology of the carrier may also influence its transport efficiency within the plant, which needs to be further explored in the future work.
1 system were able to deform or rupture during transportation, which significantly facilitated the pesticide transport process in the plant, showing better translocation ability than others. It can be seen that nanocarriers enhanced the transport capacity and efficiency of the original pesticide molecules in the plant, which was consistent with the conclusions drawn from previous work.19,52 The use of nanocarriers for pesticide could facilitate the faster downward transport of nanoparticles through vascular bundles. Generally speaking, the smaller and more flexible the nanocarriers, the better the transport capacity within the plants. In addition to the particle size and charge, this study has demonstrated that the morphology of the carrier may also influence its transport efficiency within the plant, which needs to be further explored in the future work.
Herbicidal activity of FP-loaded nanocarriers
To further investigate the control effect of FP-loaded nanocarriers on the target weeds, C. album was selected as the model target for indoor bioassay experiments. C. album plants with similar and good growth conditions were treated with FF, FP, and assembled FP/FF suspensions, respectively, and their growth states were recorded on days 0, 1, 4, 7, 10, and 14 days, respectively. As shown in Fig. 6A, weed plants treated with pure FF grew well for 14 days without any signs of wilting or yellowing, which proved that FF had no inhibitory effect on the growth of weed plants. FP is a herbicide in the class of synthetic auxins, which enters the plant through the foliage and replaces natural auxins at binding sites, disrupting the normal growth process and making it misshapen, distorted and ultimately dead.48 The weeds treated with pure FP and three different FP-loaded nanocarriers displayed varying degrees of wilting and deformity on the first day after treatment and stopped absorbing nutrients, leading to further wilting and eventual death, which was consistent with the action mode of FP. | ||
| Fig. 6 (A) Photographs of C. album after treatments for 0, 1, 4, 7, 10, and 14 days. (B) Plant height inhibition over 14 days. | ||
The control efficacy was defined as the plant height inhibition rate, which is shown in Fig. 6B and S5.† Statistical data showed that FF did not inhibit weed growth, but it had some growth promoting effects, and hence, the 14 day high inhibition performance was −9.35% ± 8.56%. The height inhibition rate of FP alone was 21.90% ± 9.02%, and after co-assembly with FF, the assemblies with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 showed a slight decrease in height inhibition rate, exhibiting 17.05% ± 10.80%, whereas both 1
2 showed a slight decrease in height inhibition rate, exhibiting 17.05% ± 10.80%, whereas both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 assembled nanocarriers further improved the control efficacy on the basis of pure FP, with inhibitory rates of 23.14% ± 12.68% and 25.26% ± 12.86%, respectively. That is, specific ratios of assembled nanocarriers can promote the action of FP with binding sites in the plant to enhance the control efficacy of FP, which is consistent with their release and translocation behaviors. The 2
1 assembled nanocarriers further improved the control efficacy on the basis of pure FP, with inhibitory rates of 23.14% ± 12.68% and 25.26% ± 12.86%, respectively. That is, specific ratios of assembled nanocarriers can promote the action of FP with binding sites in the plant to enhance the control efficacy of FP, which is consistent with their release and translocation behaviors. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-assembled nanocarriers released the most FP under neutral conditions (Fig. 4E) and also showed the best transport capacity in the target weed (Fig. 5), allowing more FP molecules to be better distributed in all parts of the weed plant and promoting the herbicidal activity of FP. In comparison, the 1
1 co-assembled nanocarriers released the most FP under neutral conditions (Fig. 4E) and also showed the best transport capacity in the target weed (Fig. 5), allowing more FP molecules to be better distributed in all parts of the weed plant and promoting the herbicidal activity of FP. In comparison, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-assembled nanocarriers released less FP under neutral conditions (Fig. 4E) and showed weaker transport capacity (Fig. 5); therefore, they showed weaker herbicidal activity. These results indicate that the herbicidal activity of the co-assembled nanocarriers was not only dependent on the content of the active ingredient, but also affected by a combination of release ability and translocation in the plant. This has been demonstrated in previous studies, where the nanogels with good translocation and enrichment capacity in plants showed better on-target bioactivity, and at the same time, a longer sustained release for the pesticide delivery system to exert the bioactivity of the holding period.19
1 co-assembled nanocarriers released less FP under neutral conditions (Fig. 4E) and showed weaker transport capacity (Fig. 5); therefore, they showed weaker herbicidal activity. These results indicate that the herbicidal activity of the co-assembled nanocarriers was not only dependent on the content of the active ingredient, but also affected by a combination of release ability and translocation in the plant. This has been demonstrated in previous studies, where the nanogels with good translocation and enrichment capacity in plants showed better on-target bioactivity, and at the same time, a longer sustained release for the pesticide delivery system to exert the bioactivity of the holding period.19
Conclusions
In summary, we have developed a strategy to produce environmentally friendly molecular carriers for pesticides via supramolecular co-assembly. FP-loaded nanocarriers were prepared by a simple mixing and dialysis procedure, which consisted of only the active ingredient FP, the dipeptide FF, and water, reducing the potential threat to the environment. The co-assembled suspension showed white translucent appearance, good stability and higher loading capacity (up to 72.34%). Besides, the microscopic morphology of the assembled nanocarriers can be regulated according to the composition ratio, which formed wormlike micelles, coils, and vesicles at FP/FF ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The combination of UV-vis, IR, and MD simulation demonstrated that FP-loaded nanocarriers were formed by the driven forces of hydrogen bonding, electrostatic interactions, π–π stacking, hydrophobic interactions and van der Waals forces. Moreover, the nanocarriers with different FP/FF ratios had adjustable pH-responsive release behaviors and good translocation ability in C. album. Furthermore, specific ratios of FP/FF assembled nanocarriers could also enhance the efficacy against the target weeds. This work provides new ideas and methods for developing green and simple pesticide carriers, and the results have important reference values. However, the interface properties of the prepared nanocarriers need to be improved, which requires the selection of surface-active assembly molecules to replace FF or further compounding. Our strategy of supramolecular co-assembly has a wider scope of application due to its simple principle, and is not limited to the molecules mentioned in this work. In the future, we will further investigate better assembly molecules and methods to obtain more efficient and safe molecular carriers for pesticides.
1, respectively. The combination of UV-vis, IR, and MD simulation demonstrated that FP-loaded nanocarriers were formed by the driven forces of hydrogen bonding, electrostatic interactions, π–π stacking, hydrophobic interactions and van der Waals forces. Moreover, the nanocarriers with different FP/FF ratios had adjustable pH-responsive release behaviors and good translocation ability in C. album. Furthermore, specific ratios of FP/FF assembled nanocarriers could also enhance the efficacy against the target weeds. This work provides new ideas and methods for developing green and simple pesticide carriers, and the results have important reference values. However, the interface properties of the prepared nanocarriers need to be improved, which requires the selection of surface-active assembly molecules to replace FF or further compounding. Our strategy of supramolecular co-assembly has a wider scope of application due to its simple principle, and is not limited to the molecules mentioned in this work. In the future, we will further investigate better assembly molecules and methods to obtain more efficient and safe molecular carriers for pesticides.
Data availability
The data supporting this article are included in the ESI.†Author contributions
Zirui Zheng: data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, writing-original draft preparation; Ziyun Yang: investigation, validation, visualization; Zehua Meng: investigation, validation, visualization; Siyang Liu: visualization, software; Tianyue Wu: methodology, investigation; Chengyi He: investigation, resources; Chenhui Zhang: software; Chen Ma: supervision, validation; Yuxia Gao: data curation, formal analysis, funding acquisition, methodology, project administration, supervision, writing-reviewing and editing; Fengpei Du: data curation, funding acquisition, project administration, supervision, validation.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the National Key R&D Program of China (No. 2022YFD1700502) and the Ministry of Science and Technology of China (2021YFA0716700).References
- J. R. Rohr, C. B. Barrett, D. J. Civitello, M. E. Craft, B. Delius, G. A. DeLeo, P. J. Hudson, N. Jouanard, K. H. Nguyen, R. S. Ostfeld, J. V. Remais, G. Riveau, S. H. Sokolow and D. Tilman, Emerging human infectious diseases and the links to global food production, Nat. Sustain., 2019, 2, 445–456 CrossRef PubMed.
- A. Sharma, V. Kumar, B. Shahzad, M. Tanveer, S. G. P. Sidhu, N. Handa, K. S. Kohli, P. Yadav, A. S. Bali, R. D. Parihar, O. I. Dar, K. Singh, S. Jasrotia, P. Bakshi, M. Ramakrishnan, S. Kumar, R. Bhardwaj and A. K. Thukral, Worldwide pesticide usage and its impacts on ecosystem, SN Appl. Sci., 2019, 1, 1446 CrossRef CAS.
- L. Bondareva and N. Fedorova, Pesticides: behavior in agricultural soil and plants, Molecules, 2021, 26, 5370 CrossRef CAS.
- J. Pretty, Intensification for redesigned and sustainable agricultural systems, Science, 2018, 362, 908 CrossRef CAS PubMed.
- T. Wu, K. Zhao, C. Zhang, T. Zhong, Z. Li, Z. Bao, Y. Gao and F. Du, Promising delivery platform for smart pest control with high water-retaining capacity, ACS Appl. Mater. Interfaces, 2022, 14, 55062–55074 CrossRef CAS PubMed.
- X. Zhao, H. Cui, Y. Wang, C. Sun, B. Cui and Z. Zeng, Development strategies and prospects of nano-based smart pesticide formulation, J. Agric. Food Chem., 2018, 66, 6504–6512 CrossRef CAS.
- M. Nuruzzaman, M. M. Rahman, Y. Liu and R. Naidu, Nanoencapsulation, nano-guard for pesticides: a new window for safe application, J. Agric. Food Chem., 2016, 64, 1447–1483 CrossRef CAS.
- K. P. Kong, B. H. Zhang and J. Wang, Multiple roles of mesoporous silica in safe pesticide application by nanotechnology: a review, J. Agric. Food Chem., 2021, 69, 6735–6754 CrossRef.
- M. Yamamuro, T. Komuro, H. Kamiya, T. Kato, H. Hasegawa and Y. Kameda, Neonicotinoids disrupt aquatic food webs and decrease fishery yields, Science, 2019, 366, 620–623 CrossRef CAS PubMed.
- R. Schulz, S. Bub, L. L. Petschick, S. Stehle and J. Wolfram, Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops, Science, 2021, 372, 81–84 CrossRef CAS.
- V. Bueno, P. Wang, O. Harrisson, S. Bayen and S. Ghoshal, Impacts of a porous hollow silica nanoparticle-encapsulated pesticide applied to soils on plant growth and soil microbial community, Environ. Sci.: Nano, 2022, 9, 1476–1488 RSC.
- H. Siviter, E. J. Bailes, C. D. Martin, T. R. Oliver, J. Koricheva, E. Leadbeater and M. F. Brown, Agrochemicals interact synergistically to increase bee mortality, Nature, 2021, 596, 389–392 CrossRef CAS.
- S. Mostafalou and M. Abdollahi, Pesticides: an update of human exposure and toxicity, Arch. Toxicol., 2017, 91, 549–599 CrossRef CAS PubMed.
- K. Zhao, B. Wang, C. Zhang, Y. Guo, Y. Ma, Z. Li, T. Wu, Z. Bao, Y. Gao and F. Du, Catechol functionalized hat-shape carriers for prolonging pesticide retention and flush resistance on foliage, Chem. Eng. J., 2021, 420, 127689 CrossRef CAS.
- S. Sharma, O. P. Setter, H. A. Hamad and E. Segal, Multifunctional halloysite nanotube-polydopamine agro-carriers for controlling bacterial soft rot disease, Environ. Sci.: Nano, 2024, 11, 1114 RSC.
- M. Wang, Z. Hu, T. Yang, H. Pei and F. Zhang, A dual pesticide-fertilizer silicon-base nanocomposite to synergistically control fungal disease and provide nutrition, Environ. Sci.: Nano, 2023, 10, 3462 RSC.
- W. Liang, J. Cheng, J. Zhang, Q. Xiong, M. Jin and J. Zhao, pH-Responsive on-demand alkaloids release from core-shell ZnO@ZIF-8 nanosphere for synergistic control of bacterial wilt disease, ACS Nano, 2022, 16, 2762–2773 CrossRef CAS PubMed.
- C. Wang, K. Qiao, Y. Ding, Y. Liu, J. Niu and H. Cao, Enhanced control efficacy of spinosad on corn borer using polylactic acid encapsulated mesoporous silica nanoparticles as a smart delivery system, Int. J. Biol. Macromol., 2023, 253, 126425 CrossRef CAS PubMed.
- T. Wu, K. Zhao, S. Liu, Z. Bao, C. Zhang, Y. Wu, R. Song, Y. Gu, Y. Gao and F. Du, Promising nanocarriers endowing non-systemic pesticides with upward translocation ability and microbial community enrichment effects in soil, Chem. Eng. J., 2023, 474, 145570 CrossRef CAS.
- Y. Tian, G. Tang, Y. Li, Z. Zhou, X. Chen, Y. Gao, J. Niu, J. Yang, J. Tang, Y. Zhang, X. Zhang and Y. Cao, A simple preparation process for an efficient nano-formulation: small molecule self-assembly based on spinosad and sulfamic acid, Green Chem., 2021, 23, 4882–4891 RSC.
- K. Zhao, J. Hu, Y. Ma, T. Wu, Y. Gao and F. Du, Topology-regulated pesticide retention on plant leaves through concave Janus carriers, ACS Sustainable Chem. Eng., 2019, 7, 13148–13156 CrossRef CAS.
- X. Jia, W. Sheng, W. Li, Y. Tong, Z. Liu and F. Zhou, Adhesive polydopamine coated avermectin microcapsules for prolonging foliar pesticide retention, ACS Appl. Mater. Interfaces, 2014, 6, 19552–19558 CrossRef CAS.
- J. Liang, M. Yu, L. Guo, B. Cui, X. Zhao, C. Sun, Y. Wang, G. Liu, H. Cui and Z. Zeng, Bioinspired development of P(St-MAA)-avermectin nanoparticles with high affinity for foliage to enhance folia retention, J. Agric. Food Chem., 2018, 66, 6578–6584 CrossRef CAS.
- M. F. Hochella, D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Natural, incidental, and engineered nanomaterials and their impacts on the Earth system, Science, 2019, 363, 1414 CrossRef.
- H. Yamagishi, H. Sato, A. Hori, Y. Sato, R. Matsuda, K. Kato and Y. Aida, Self-assembly of lattices with high structural complexity from a geometrically simple molecule, Science, 2018, 361, 1242–1246 CrossRef CAS PubMed.
- Z. Xu, S. Jia, W. Wang, Z. Yuan, B. J. Ravoo and D. S. Guo, Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation, Nat. Chem., 2019, 11, 86–93 CrossRef CAS PubMed.
- P. Makam and E. Gazit, Minimalistic peptide supramolecular co-assembly: expanding the conformational space for nanotechnology, Chem. Soc. Rev., 2018, 47, 3406–3420 RSC.
- S. Fleming and R. V. Ulijn, Design of nanostructures based on aromatic peptide amphiphiles, Chem. Soc. Rev., 2014, 43, 8150–8177 RSC.
- S. Fu, G. Li, W. Zang, X. Zhou, K. Shi and Y. Zhai, Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy, Acta Pharm. Sin. B, 2022, 12, 92–106 CrossRef CAS.
- H. Xiao, Y. Guo, H. Liu, Y. Liu, Y. Wang, C. Li, J. Císařa, D. Škoda, I. Kuřitka, L. Guo and V. Sedlařík, Structure-based design of charge-conversional drug self-delivery systems for better targeted cancer therapy, Biomaterials, 2020, 232, 119701 CrossRef CAS PubMed.
- Y. Shen, H. Lin, M. Yang, X. Gong, B. Guan, Y. Han, S. Wang and Y. Wang, Hierarchical superstructure of plant polyphenol and arginine surfactant for long-lasting and target-selective antimicrobial application, Adv. Mater., 2023, 35, 2210936 CrossRef CAS PubMed.
- H. Xiong, Z. Wang, C. Wang and J. Yao, Transforming complexity to simplicity: protein-like nanotransformer for improving tumor drug delivery programmatically, Nano Lett., 2020, 20, 1781–1790 CrossRef CAS.
- G. Tang, Y. Tian, J. Niu, J. Tang, J. Yang, Y. Gao, X. Chen, X. Li, H. Wang and Y. Cao, Development of carrier-free self-assembled nanoparticles based on fenhexamid and polyhexamethylene biguanide for sustainable plant disease management, Green Chem., 2021, 23, 2531–2540 RSC.
- J. Luo, Y. Gao, Y. Liu, X. Huang, D. Zhang, H. Cao, T. Jing, F. Liu and B. Li, Self-assembled degradable nanogels provide foliar affinity and pinning for pesticide delivery by flexibility and adhesiveness adjustment, ACS Nano, 2021, 15, 14598–14609 CrossRef CAS.
- L. Tao and H. Yang, Fluroxypyr biodegradation in soils by multiple factors, Environ. Monit. Assess., 2011, 175, 227–238 CrossRef CAS PubMed.
- L. Wang, J. Xu, P. Zhao and C. Pan, Dissipation and residues of fluroxypyr-meptyl in rice and environment, Bull. Environ. Contam. Toxicol., 2011, 86, 449–453 CrossRef CAS PubMed.
- M. Reches and E. Gazit, Casting metal nanowires within discrete self-assembled peptide nanotubes, Science, 2003, 300, 625–627 CrossRef CAS PubMed.
- T. H. Han, J. Kim, J. S. Park, C. B. Park, H. Ihee and S. O. Kim, Liquid crystalline peptide nanowires, Adv. Mater., 2007, 19, 3924–3927 CrossRef CAS.
- Q. Li, Y. Jia, L. Dai, Y. Yang and J. Li, Controlled rod nanostructured assembly of diphenylalanine and their optical waveguide properties, ACS Nano, 2015, 9, 2689–2695 CrossRef CAS PubMed.
- Y. Gao, Z. Zhou, X. Chen, Y. Tian, Y. Li, H. Wang, X. Li, X. Yu and Y. Cao, Controlled release of herbicides by 2,4-D-, MCPA-, and bromoxynil-intercalated hydrotalcite nanosheets, Green Chem., 2021, 23, 4560 RSC.
- C. An, B. Huang, J. Jiang, X. Wang, N. Li, H. Liu, Y. Shen, C. Sun, S. Zhan, X. Li, C. Wang, Z. Zeng, H. Cui, Q. Wu, Y. Zhang, Z. Guo, P. Zhang, I. Lynch, J. Gao and Y. Wang, Design and synthesis of a water-based nanodelivery pesticide system for improved efficacy and safety, ACS Nano, 2024, 18, 662–679 CrossRef CAS.
- W. Wu, J. Ma, J. Xu and Z. Wang, Mechanistic insights into chemical conditioning by polyacrylamide with different charge densities and its impacts on sludge dewaterability, Chem. Eng. J., 2021, 410, 128425 CrossRef CAS.
- J. Liu, L. Zhong, T. Hao, L. Ren and Y. Liu, A collaborative emulsification system capable of forming stable small droplets of oil-in-water emulsions for enhancing heavy oil recovery, J. Mol. Liq., 2022, 355, 118970 CrossRef CAS.
- G. Zhang, Y. Chen, X. Sui, M. Kang, Y. Feng and H. Yin, Nonionic surfactant stabilized polytetrafluoroethylene dispersion: Effect of molecular structure and topology, J. Mol. Liq., 2022, 345, 116988 CrossRef CAS.
- S. Zhang, Z. Bao, Y. Wu, Y. Wang, R. Liu, Y. Gao, X. Zhao, C. Zhang and F. Du, Enhancing the stability and effectivity of multiple pesticide formulation mixtures by adding an eco-friendly adjuvant, ACS Sustainable Chem. Eng., 2023, 11, 15385–15396 CrossRef CAS.
- B. Wang, J. Wang, C. Yu, S. Luo, J. Peng, N. Li, T. Wang, L. Jiang, Z. Dong and Y. Wang, Sustained agricultural spraying: from leaf wettability to dynamic droplet impact behavior, Global Chall., 2023, 7, 2300007 CrossRef.
- Y. Song, S. R. Challa, C. J. Medforth, Y. Qiu, R. K. Watt, D. Peña, J. E. Miller, F. Swolab and J. A. Shelnutt, Synthesis of peptide-nanotube platinum-nanoparticle composites, Chem. Commun., 2004, 1044–1045 RSC.
- T. R. Wright, G. Shan, T. A. Walsh, J. M. Lira, C. Cui, P. Song, M. Zhuang, N. L. Arnold, G. Lin, K. Yau, S. M. Russell, R. M. Cicchillo, M. A. Peterson, D. M. Simpson, N. Zhou, J. Ponsamuel and Z. Zhan, Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoatedioxygenase transgenes, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20240–20245 CrossRef CAS.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release, Chem. Rev., 2016, 116, 2602–2663 CrossRef CAS.
- P. Bussières, Estimating the number and size of phloem sieve plate pores using longitudinal views and geometric reconstruction, Sci. Rep., 2014, 4, 4929 CrossRef.
- X. Wang, H. Xie, P. Wang and H. Yin, Nanoparticles in plants: uptake, transport and physiological activity in leaf and root, Materials, 2023, 16, 3097 CrossRef CAS PubMed.
- Q. Xiong, W. Liang, W. Shang, Z. Xie, J. Cheng, B. Yu, Y. Fang, L. Sun and J. Zhao, Bidirectional uptake, transfer, and transport of dextran-based nanoparticles in plants for multidimensional enhancement of pesticide utilization, Small, 2023, 20, 2305693 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00696h |
| This journal is © The Royal Society of Chemistry 2025 |




