Facet-dependent oxysulfidation of Cu2O nanomaterials: implications for improving the efficacy of nanopesticides†
Tingting
Du
 a,
Wenyu
Guan
b,
Zhanhua
Zhang
b,
Chuanjia
Jiang
a,
Wenyu
Guan
b,
Zhanhua
Zhang
b,
Chuanjia
Jiang
 b,
Pedro J. J.
Alvarez
b,
Pedro J. J.
Alvarez
 c,
Wei
Chen
c,
Wei
Chen
 b and
Tong
Zhang
b and
Tong
Zhang
 *b
*b
aInformation Materials and Intelligent Sensing Laboratory of Anhui Province, Institutes of Physical Science and Information Technology, Anhui University, Hefei 230601, China
bCollege of Environmental Science and Engineering, Ministry of Education Key Laboratory of Pollution Processes and Environmental Criteria, Tianjin Key Laboratory of Environmental Remediation and Pollution Control, Nankai University, Tianjin 300350, China. E-mail: zhangtong@nankai.edu.cn
cDepartment of Civil and Environmental Engineering, Rice University, 6100 Main Street, Houston, TX 77005, USA
First published on 14th October 2024
Abstract
Copper (hydr)oxide nanomaterials are an important class of nanomaterials with various applications, including next-generation pesticides. The efficacy of these materials is largely affected by oxysulfidation, one of the most important transformation processes in the environment. Here, we show that the extent and route of oxysulfidation are facet-dependent for these materials. Specifically, oxysulfidation of Cu2O_{100} and Cu2O_{111}—two Cu2O nanomaterials with predominantly exposed {100} and {111} facets—is fast and complete, with only a hollow shell left at the end of the experiment. In comparison, oxysulfidation of Cu2O_{110}, a nanomaterial with {110} facet, is much less complete, in that, the end-product exhibits a yolk–shell structure with a large cuprite core. The varied degrees of oxysulfidation are attributable to the facet-dependent adsorption affinities of Cu2O for both oxygen and sulfide ions, leading to the formation of different initial oxysulfidation products. Unlike the porous coatings of yarrowite on Cu2O_{111} and a mixture of yarrowite and covellite on Cu2O_{100}, the condensed layer of djurleite formed on Cu2O_{110} passivates the material by sealing the surface of Cu2O, hindering subsequent copper dissolution. Consequently, Cu ions released from Cu2O_{100} and Cu2O_{111} are 2.2 and 2.4 times higher than that from Cu2O_{110}. These findings underline the important role of exposed facets in dictating the interfacial processes of soft metal-based nanomaterials, and have important implications for improving the efficiency of nanopesticides in redox dynamic rhizospheres to minimize the environmental impacts associated with the overuse of conventional pesticides.
Environmental significanceWith the increasing production and application of soft metal-based nanomaterials, their environmental release is inevitable. The fate, transport, and effects of these nanomaterials in the environment are largely dependent on their transformation processes, particularly, oxysulfidation in sulfur-rich environments such as wastewater treatment plants, sediments, and rhizosphere soils. The critical role of exposed facets in determining the route and propensity of oxysulfidation as demonstrated in this study underlines the importance in further understanding the structure–activity relationship governing the interfacial processes of soft metal-based nanomaterials. The findings also have important implications for the design of next-generation agricultural chemicals to minimize the environmental impacts associated with the overuse of conventional pesticides and fertilizers. |
1. Introduction
Copper (hydr)oxide nanomaterials are an important class of nanomaterials that have shown great promise in a variety of applications, such as photocatalysis, gas sensing, and electrode materials for lithium-ion batteries, to mention a few.1–5 The projected production of copper (hydr)oxide nanomaterials is 1900 metric tons by 2031.1 Under such circumstances, environmental release of these nanomaterials during production, transportation, use and disposal is inevitable.6–8 Understanding the environmental behavior and fate of these nanomaterials is crucial for the assessment of their potential risks and for the improvement of their environmental compatibility.9–12 Oxysulfidation is one of the most important transformation processes of soft metal-based nanomaterials, and is induced by dissolved oxygen due to redox fluctuation as well as sulfide species that are ubiquitous as a result of microbial activities,13,14 particularly in wastewater treatment plants,15–17 sediments,18 and rhizosphere soils.19 Oxysulfidation results in (partial) transformation of the nanomaterials into metal sulfides, and consequently, can significantly alter their bioavailability and risks.20–24 Note that understanding the oxysulfidation processes of copper (hydr)oxide also sheds light on nano-enabled agriculture, as these nanomaterials are considered next-generation pesticides for the prevention of root rot diseases of crops,25 owing to their excellent antimicrobial activities.26,27Previous research has shown that the extent of oxysulfidation of copper (hydr)oxide nanomaterials is closely related to their physicochemical properties (e.g., size, shape and surface coating).28–32 One structural property likely of particular importance is exposed facet – by dictating surface atomic arrangement of crystalline nanomaterials, exposed facets largely control the surface reactivities of nanocrystals, such as solubility and adsorption affinity.33–38 For example, both experimental studies and theoretical calculations have shown that Cu2O nanocrystals with the exposed {111} facet and those with the {100} facet exhibit different affinities for molecular O2, leading to different extents of Cu2+ release and surface oxidation.39–42 Since the primary oxysulfidation pathway of copper (hydr)oxides is oxidative dissolution–reprecipitation that involves surface reactions with both dissolved oxygen and sulfide species (H2S(aq), HS− and S2−),22,43,44 we postulate that exposed facets can determine the kinetics and extent of oxysulfidation of copper (hydr)oxide nanomaterials, by regulating the adsorption affinities for oxygen and sulfide, and possibly, the specific reaction pathways.
In this study, we used three Cu2O nanomaterials—with predominantly exposed {100}, {111}, and {110} facets, respectively—as model copper (hydr)oxide nanomaterials to understand how facets control oxysulfidation propensity. A range of microscopic and spectroscopic analyses, including scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS), were conducted to compare the physicochemical properties of each pristine Cu2O nanomaterial and its oxysulfidized counterparts. Density function theory calculations were carried out to assess the affinities of different facets for the precursors of oxysulfidation reactions. The findings have important implications for improving the performance and durability of nanopesticides in redox dynamic rhizospheres to minimize environmental impacts associated with overuse of conventional pesticides. The conceptual model demonstrated herein likely can be extended to improve the efficacy of nanofertilizers to decrease the negative environmental effects due to the overuse of conventional fertilizers. Likely, the findings also inform the design of antimicrobial nanomaterials for sustained release of antimicrobial ions. Moreover, the significant effects of facet-dependent reaction intermediates on the specific oxysulfidation pathways highlight the critical importance to understand the time-dependent evolution of mineral formation and transformation.
2. Experimental section
2.1. Synthesis of Cu2O nanomaterials with different exposed facets
Three types of Cu2O nanomaterials with predominantly exposed {100}, {111}, and {110} facets (referred to as Cu2O_{100}, Cu2O_{111}, and Cu2O_{110}, respectively) were prepared using previously reported methods.35,45 For Cu2O_{100} and Cu2O_{111}, 50 mL of CuCl2·2H2O aqueous solution (0.01 M) was prepared with (for Cu2O_{111}) or without (for Cu2O_{100}) the addition of 4.44 g of PVP. Then, 5.0 mL of NaOH solution (2.0 M) was added slowly into the above light green solution. After stirring for 30 min, 5.0 mL of ascorbic acid solution (0.60 M) was added slowly to the above dark brown mixture. Next, the resulting turbid red suspension was constantly stirred for different times (5 h for Cu2O_{100} and 3 h for Cu2O_{111}) at 55 °C. The brick-red precipitate was centrifuged and washed three times with deionized (DI) water and absolute ethanol and finally dried in a vacuum drying oven at 40 °C for 24 h. For Cu2O_{110}, 5 mL of oleic acid and 20 mL of absolute ethanol were added in succession into 40 mL of CuSO4 aqueous solution (0.025 M) under vigorous stirring. Then, the mixture was heated to 100 °C, and 10 mL of NaOH solution (0.80 M) was added dropwise into the solution. After 5 min, 30 mL of D-(+)-glucose solution (0.63 M) was added to the resulting deep blue solution. After further stirring for 60 min, the resulting brick-red precipitate was centrifuged and washed with absolute ethanol and cyclohexane and dried in a vacuum drying oven at 40 °C for 24 h. Detailed information on the chemicals used is given in the ESI.†2.2. Oxysulfidation of Cu2O nanomaterials
Oxysulfidation experiments were carried out in 250 mL amber glass bottles at room temperature (25 ± 1 °C), using a literature method.19,20 4-(2-Hydroxyethyl)-1-piperazinepropanesulfonic acid (HEPPS) was selected as the buffer for the oxysulfidation experiments because it would not induce metal complexation.46 First, a 25 mM HEPPS buffer solution was prepared in DI water with 8.0 ± 0.2 mg L−1 dissolved oxygen and adjusted to pH 8.0 ± 0.1 with NaOH solution. Next, 18 mg of Cu2O nanomaterial was added and dispersed by bath sonication for 30 min. The resultant Cu2O nanomaterial suspensions (with 0.5 mM Cu2O) were amended with a Na2S stock solution to yield an aqueous mixture with 1.2 mM Na2S. At the reaction pH 8.0, HS− is the predominant dissolved S(−II) species, accounting for 90.5% of the total dissolved sulfide (Fig. S1†). Each suspension was kept open to the atmosphere with no limitation of oxygen exchange. After incubation on a shaker at 150 rpm for 10 min or 1 day, the resulting suspensions were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min, and the pellets were washed three times with DI water to remove residual sulfide, collected, and allowed to dry in a vacuum freeze dryer. The oxidation of Cu2O nanomaterials was also investigated to explore the different reactivities of the Cu2O nanomaterials toward oxygen (see ESI† for details).
000g for 15 min, and the pellets were washed three times with DI water to remove residual sulfide, collected, and allowed to dry in a vacuum freeze dryer. The oxidation of Cu2O nanomaterials was also investigated to explore the different reactivities of the Cu2O nanomaterials toward oxygen (see ESI† for details).
2.3. Characterization of pristine and oxysulfidized Cu2O nanomaterials
The morphology of the Cu2O nanomaterials before and after oxysulfidation was observed by SEM (JSM-7800F, JEOL Ltd.) and TEM (JEM-2800, JEOL Ltd.). The crystal phases were determined using MDI Jade 6 software based on the XRD patterns collected on an Ultima IV diffractometer (Rigaku) with Cu Kα radiation (λ = 1.5418 Å). The chemical compositions of the oxysulfidized Cu2O nanomaterials were characterized by elemental mapping of the samples, collected with the JEM-2800 TEM system, and EDS with the JSM-7800F SEM system. The surface proportion of Cu(II) in the initial oxysulfidized Cu2O nanomaterials was examined by XPS (K-Alpha, Thermo Fisher Scientific). The ζ potential of pristine Cu2O nanomaterials in HEPPS buffer solution was measured on a Litesizer 500 particle size analyzer (Anton Paar GmbH) at room temperature (25 ± 1 °C).2.4. Release of Cu ions from Cu2O nanomaterials during oxysulfidation
Release of copper ions from Cu2O nanomaterials during oxysulfidation was determined with the 2,9-dimethyl-1,10-phenanthroline (neocuproine) spectrophotometric method.47,48 Briefly, 5 μL of concentrated sulfuric acid was added to 0.75 mL of reaction solution passing through a 0.22 μm membrane at selected time intervals. Then, 75 μL of 100 g L−1 hydroxylamine hydrochloride solution or 75 μL of DI water was added to measure total Cu and Cu+, respectively. An additional 150 μL of 375 g L−1 sodium citrate solution and 150 μL of sodium acetate buffer (pH 5.7) were added. After thorough mixing, 75 μL of 1.0 g L−1 neocuproine reagent was spiked and the absorbance values at 457 nm were measured using a Cary 100 UV-vis spectrophotometer (Agilent Technologies). The Cu2+ concentration was obtained by subtracting the Cu+ concentration from the total Cu ion concentration. Each test group included three replicates, and statistical differences among the three groups were analyzed by one-way analysis of variance (ANOVA) with Dunnett's test; a p-value of less than 0.05 is considered statistically significant.2.5. Density functional theory (DFT) calculations
To estimate the adsorption energies (Eads) of O2 and HS− on different facets of Cu2O nanomaterials (the optimized geometric structures are given in Fig. S2†), DFT calculations were performed using the Vienna Ab initio Simulation Package (VASP 5.3.5) code with the Perdew–Burke–Ernzerhof generalized gradient approximation and the projected augmented wave method. The cutoff energy for the plane-wave basis set was set to 400 eV. The Brillouin zone of the bulk and surface unit cell was sampled using Monkhorst–Pack (MP) grids for optimization of the Cu2O structure. The {100}, {110}, and {111} surfaces were determined using 3 × 3 × 1, 2 × 2 × 1, and 3 × 3 × 1 MP grids. The convergence criteria for the electronic self-consistent iteration and force were set to 10−5 eV and 0.01 eV Å−1, respectively. A vacuum layer of 12 Å was introduced to avoid interactions between periodic images. The adsorption energy was calculated as:| Eads = Etotal − Esurface − Especies | (1) |
3. Results and discussion
3.1. Characteristics of different faceted Cu2O nanomaterials
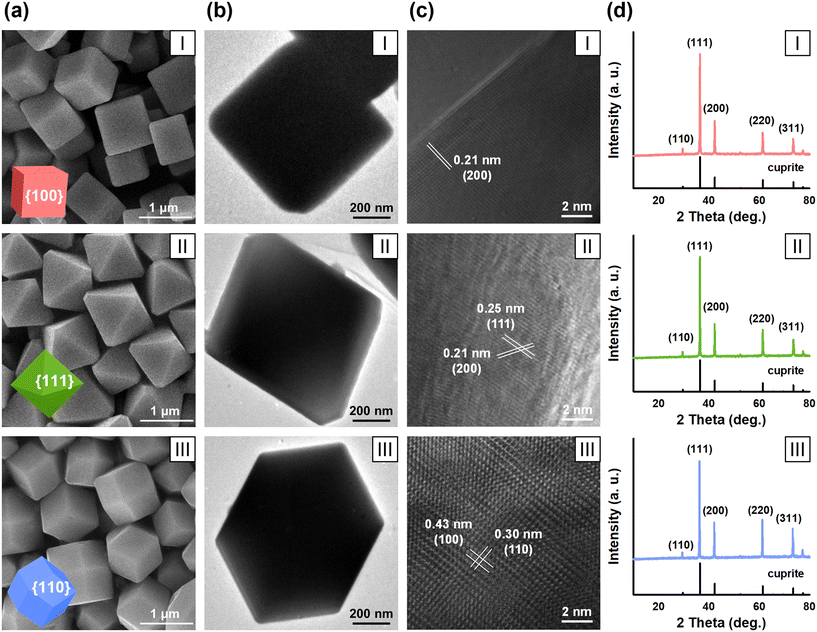
The three as-prepared Cu2O nanomaterials were similar in dimension, and exhibited well-defined exposed facets that differed among the three materials. The SEM images (Fig. 1a) show that the three nanomaterials had uniform cubic, octahedral, and rhombic dodecahedral morphologies, respectively. The side lengths of the three materials were 887 ± 135, 860 ± 97, and 509 ± 80 nm, respectively (Table S1†). The TEM images confirm the morphologies of the Cu2O nanomaterials (Fig. 1b). The high-resolution TEM (HRTEM) images reveal the single-crystal structure of the three nanomaterials and further identify their predominantly exposed crystal facets (Fig. 1c). The HRTEM image of Cu2O_{100} shows a lattice spacing of 0.21 nm, which can be indexed to the (200) plane of Cu2O, indicating that the cubic material predominantly exposed {100} facets.49 Cu2O_{111} exhibited two sets of lattice fringes of 0.21 nm and 0.25 nm, corresponding to the (200) and (111) planes of Cu2O, suggesting that the octahedral material was enclosed by eight {111} facets.50 The two sets of lattice spacings observed for Cu2O_{110} were 0.30 nm and 0.43 nm, corresponding to the (100) and (110) planes, showing that the rhombic dodecahedral material predominantly exposed {110} facets.51 The XRD patterns (Fig. 1d) of the three Cu2O nanomaterials display sharp diffraction peaks that are characteristic of the cuprite phase (JCPDS No. 05-0667), with little impurities (e.g., CuO or Cu) detected, confirming that the samples were of good crystallinity and high purity.3.2. Transformation products upon nano-Cu2O oxysulfidation are facet-dependent
The oxysulfidation products of the three Cu2O nanomaterials differed significantly, with two products taking the hollow–shell form and one exhibiting a yolk–shell structure. The morphological features of all three Cu2O nanomaterials changed upon exposure to sulfide, in the presence of O2. The surfaces of the materials became less smooth, and the shapes less well defined (Fig. 2a). Nonetheless, the changes were more pronounced for Cu2O_{100} and Cu2O_{111}, in that, nanoflakes were observed on the surfaces of these two materials and the appearance of the materials became nearly spherical (Fig. 2a). The changes of Cu2O_{110} were more subtle, with the surfaces becoming slightly wrinkled while the rhombic dodecahedral morphology remaining largely intact (Fig. 2a). The TEM images of the transformation products corroborate these morphological changes (Fig. 2b). Remarkably, TEM images show that oxysulfidation of Cu2O_{100} and Cu2O_{111} led to the formation of hollow shells (Fig. 2b), consisting mainly of Cu and S, with only a trace amount of O, based on the elemental mapping results (Fig. 2c). In contrast, the transformation product of Cu2O_{110} exhibited a yolk–shell structure, with the core consisting of Cu and O and the shell consisting of Cu, S, and a trace amount of O (Fig. 2b and c), indicating a much lower extent of transformation. The S/Cu ratios of the transformed Cu2O_{100} and Cu2O_{111}, calculated based on the EDS results, were close to the stoichiometric S/Cu ratio of covellite (i.e., 1), whereas that of Cu2O_{110} was only 0.67 (Table S2†), consistent with the existence of the Cu2O core. The formation of the hollow and yolk–shell structures suggested that the oxysulfidation process was in accordance with the Kirkendall effect.45,52,53 That is, the reaction between Cu ions and HS− ions generated a layer of CuxSy on the surface of the Cu2O nanomaterials. Due to the smaller effective diameters of Cu ions (1.54 Å for Cu+ and 1.46 Å for Cu2+) than that of HS− (3.68 Å),54,55 the outward diffusion of Cu ions from the Cu2O core to the CuxSy shell would prevail over the inward diffusion of HS− ions, leading to the formation of the hollow and yolk–shell structures.The crystalline phase compositions of the oxysulfidation products were also facet-dependent. Multiple new diffraction peaks were observed in the XRD patterns of all three Cu2O nanomaterials upon oxysulfidation. For Cu2O_{100} and Cu2O_{111}, the diffraction peaks were identified as covellite (CuS; JCPDS No. 06-0464), with no visible characteristic diffraction peaks of Cu2O left (Fig. 2d and Table S3†), indicating that the two materials were completely sulfidized. In comparison, the XRD pattern of Cu2O_{110} shows that this material was only partially sulfidized, consisting of both covellite and cuprite (Fig. 2d and Table S3†). The HRTEM images of the three Cu2O nanomaterials show the lattice fringes with spacings of 0.30, 0.28, 0.23, 0.27, and 0.32 nm, corresponding to the (102), (103), (105), (006), and (101) planes of covellite (Fig. 2e), showing that even though transformation of Cu2O_{110} was incomplete, the shell of this material consisted of the same crystalline phase as Cu2O_{100} and Cu2O_{111}.
The extent of copper dissolution during the course of oxysulfidation also differed among the three Cu2O nanomaterials. At any given time interval, the measured total concentration of copper ions, including both Cu2+ and Cu+, was significantly greater for Cu2O_{100} and Cu2O_{111} than that for Cu2O_{110} (Fig. 3a; the copper dissolution patterns were similar between Cu2O_{100} and Cu2O_{111}). At the end of the 24 h reaction, the total amounts of copper ions released from Cu2O_{100} and Cu2O_{111} were 7.2 μM and 8.0 μM, respectively, 2.2 to 2.4 times higher than that from Cu2O_{110} (3.3 μM) (Fig. 3a). The difference was mainly attributable to the different extents of Cu2+ release – Cu2+ released from Cu2O_{100} and Cu2O_{111} accounted for 79.2% and 68.8% of total copper ions, respectively, compared with 33.3% from Cu2O_{110} (Fig. 3b). The measured concentrations of Cu+ were 1.5 μM for Cu2O_{100}, 2.4 μM for Cu2O_{111}, and 2.2 μM for Cu2O_{110} (Fig. 3c). These observations are in line with the different morphological and compositional changes mentioned above, as Cu2O_{110} appeared passivated during the oxysulfidation process (thus hindering subsequent copper dissolution), whereas the other two materials continued to release copper ions until the majority of Cu2O was consumed. Thus, one explanation for the facet-dependent transformation of the Cu2O nanomaterials is that a relatively condensed CuxSy shell formed on the surface of Cu2O_{110} during the initial stage of oxysulfidation, which impeded the outward diffusion of Cu ions (and possibly the inward diffusion of oxygen and HS−), whereas the nanoflakes on the surfaces of Cu2O_{100} and Cu2O_{111} left enough spaces for mass transfer. Previous studies on the formation of copper sulfides from Cu2O also showed that the copper sulfide layer formed on the surface of Cu2O nanomaterials may prevent the internal copper ions from reaching the external sulfur ions, and further reactions depend on how efficient the diffusion of copper and sulfur ions through the interface.45,52,53
3.3. Exposed facets determine the initial oxysulfidation products of Cu2O nanomaterials by regulating adsorption affinities for O2 and HS−
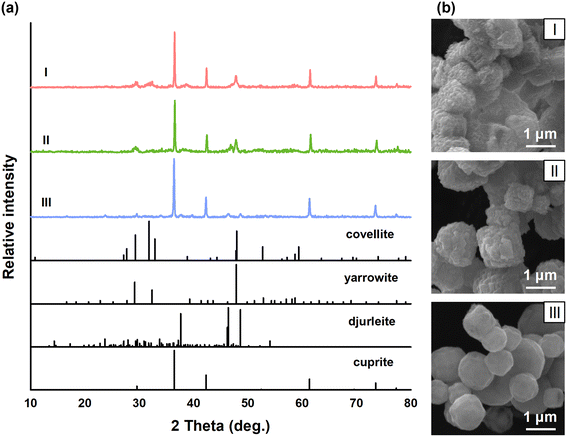
To characterize the evolution of the CuxSy shells during the transformation process, we examined the initial oxysulfidation products of the Cu2O nanomaterials with XRD. After a reaction time of 10 min, multiple diffraction peaks appeared on the XRD patterns of all three materials, indicating the formation of CuxSy species, while the characteristic diffraction peaks of cuprite remained (Fig. 4a). Interestingly, the initial CuxSy species formed on the three Cu2O nanomaterials differed, with both yarrowite (Cu9S8; JCPDS No. 36-0379) and covellite (CuS; JCPDS No. 06-0464) observed on Cu2O_{100}, yarrowite (Cu9S8; JCPDS No. 36-0379) on Cu2O_{111}, and djurleite (Cu31S16; JCPDS No. 34-0660) on Cu2O_{110} (Fig. 4a and Table S3†). Djurleite is composed of an ordered superlattice of copper in distorted hexagonal-close-packed sulfur layers (Fig. S3a†),56 and the tiny tunnels between CuS3 triangles and distorted CuS4 tetrahedra in the crystal structure are not conducive to mass transport.57,58 In comparison, covelline consists of an ordered structure of alternating CuS3 triangles and CuS4 tetrahedral layers connected by S–S bonds,59 leaving large spaces between the two CuS4–CuS3–CuS4 triple layers (Fig. S3b†). The layer spacing of covelline is 2.07 Å,60 larger than the diameters of the Cu+ (1.54 Å) and Cu2+ (1.46 Å) ions, yet the pores of djurleite are much narrower. It has been reported that yarrowite has a similar structure to covelline.61 Thus, surface coverage by djurleite would inhibit the diffusion of Cu ions more significantly, compared with surfaces covered by yarrowite and covellite. The inward diffusion of O2 would be hampered more by djurleite in a similar manner. The SEM images show that even at this very early stage of oxysulfidation, the morphological features differed substantially between Cu2O_{110} and the other two nanomaterials (Fig. 4b).It is also noteworthy that the initial CuxSy species on Cu2O_{110} were predominantly Cu(I), whereas those on Cu2O_{100} and Cu2O_{111} consisted mainly of Cu(II) (Table S3†). The XPS results of the initial oxysulfidation products were in general consistent with the XRD data, as indicated by the proportion of Cu(II) on the surface of the Cu2O nanomaterials, calculated based on the deconvoluted Cu 2p XPS spectra (Fig. S4†). This indicates that oxidation dissolution of Cu2O_{110} was hampered, likely because this specific facet had a lower affinity for O2. Indeed, the Eads values calculated based on DFT follow the order of Cu2O_{100} > Cu2O_{111} > Cu2O_{110} (Fig. 5a–d). Thus, the weakest adsorption energy of O2 on Cu2O_{110} corroborates the overall lower valence of the CuxSy species formed on Cu2O_{110}. Moreover, the higher adsorption energy of Cu2O_{100} than Cu2O_{111} is also consistent with the different CuxSy speciation between the two materials. The reactivity of the different faceted Cu2O nanomaterials towards O2 was further verified with a supplementary experiment, in which the suspensions of the three materials were treated with dissolved O2 in the absence of sulfide. The XRD patterns (Fig. S5a†) show that tenorite (CuO; JCPDS No. 48-1548) was generated upon the oxidation of the materials, and the intensity ratio of the highest diffraction peak of tenorite (2θ at 38.7°) to the highest diffraction peak of Cu2O (2θ at 36.4°) followed the order of Cu2O_{100} > Cu2O_{111} > Cu2O_{110} (Fig. S5b†). Overall, these results collectively supported that the formation of djurleite, a lower-valence CuxSy species, on Cu2O_{110} was due to the low affinity of the {110} facet for dissolved O2.
The DFT results corroborate that adsorption affinity of sulfide to Cu2O nanomaterials is facet-dependent. Clearly, this would also affect the speciation of CuxSy on the surfaces of Cu2O. The stable binding configuration of HS− on the {111} and {100} facets is one S-atom binding to two Cu-atoms, while on the {110} facet one S-atom binds to one Cu atom (Fig. 5e–g). This difference in binding configuration results in a more negative adsorption energy of HS− on the {111} and {100} facets than that on the {110} facet (Fig. 5h). Thus adsorption of HS− to Cu2O_{111} and Cu2O_{100} is energetically more favorable than that to Cu2O_{110}. Moreover, the {111} facet has a higher affinity for HS− than the {100} facet, a reverse trend from their relative affinities for O2. The different abilities of the two facets to bind HS− and O2 were likely the reason that different initial CuxSy species formed on these two materials. This was consistent with the literature finding that the generation of different stoichiometric copper sulfides from Cu2O may be modulated by controlling the concentrations of sulfur source and the O2 content in the reaction system.32 The measured ζ potential values of the Cu2O nanomaterials were consistent with the theoretical calculation results, in that, Cu2O_{110} was more negatively charged than Cu2O_{100} and Cu2O_{111} (Fig. S6†), making the material less favorable for the binding of the anionic HS−. This is consistent with the higher degree of sulfidation of Cu2O_{100} and Cu2O_{111}. Overall, it is concluded that the facet-dependent affinities for both oxygen and sulfide would regulate the formation of the specific CuxSy species in the very early stage of the oxysulfidation process, resulting in varied ion diffusion potentials and oxysulfidation propensities among different faceted Cu2O nanomaterials.
4. Conclusions
In this study, we demonstrate that oxysulfidation of Cu2O nanomaterials is facet-dependent, as Cu2O nanomaterials with different exposed facets undergo different transformation routes, resulting in different extents of copper ion release. This is attributed to the facet-dependent adsorption affinities of Cu2O for both oxygen and sulfide ions, which leads to the formation of different initial oxysulfidation products on Cu2O, and the structural properties of these products control the subsequent copper dissolution and propensity of sulfidation. Such facet-dependent effects have important implications for the design of high-performance nanopesticides. Indeed, a common challenge for the application of nano-enabled agrichemicals is maintaining the performance of these nanomaterials in the dynamic environment of rhizosphere soils. The findings show that facet engineering can be exploited to ensure sustained release of active antimicrobial ingredients (i.e., copper ions), by mitigating the passivation of nanopesticides from surface precipitation of copper sulfides. Since the initial products formed on the two Cu2O nanomaterials with predominantly exposed {100} and {111} facets are relatively loose in structure, these materials are less prone to passivation and better suitable as nanopesticides. The conceptual model demonstrated herein likely can be extended to improve the efficacy of nanofertilizers (e.g., apatite and hydroxyapatite nanoparticles), which are designed to achieve controlled release of nutrients to decrease the negative environmental effects due to the overuse of conventional fertilizers.The findings of this study can also inform the design of antimicrobial nanomaterials such as Ag, ZnO and CuO nanoparticles, which are used as alternative antibiotics for preventing the development of antibiotic resistance.62 The performance of these nanomaterials relies on the sustained release of antimicrobial ions (e.g., Ag+), but is prone to passivation due to aging. Moreover, the significant effects of facet-dependent reaction intermediates on the specific oxysulfidation pathways highlight the critical importance to understand the time-dependent evolution of mineral formation and transformation. This is particularly important for the dynamic reactions occurring in relatively short time scales, in that, even subtle differences in the initial stage of the reactions can lead to markedly different environmental consequences. For such dynamic systems, attention should be paid to fully understand the mechanisms and kinetics of nano-scale intermediate formation.
Data availability
The data supporting the findings of this study are available within the article and/or its ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This project was supported by the National Natural Science Foundation of China (22125603, 22020102004), Tianjin Municipal Science and Technology Bureau (21JCJQJC00060), the Anhui Provincial Natural Science Foundation (2108085QB55), Fundamental Research Funds for the Central Universities (63233056), and China–US Center for Environmental Remediation and Sustainable Development.References
- A. A. Keller, A. Ehrens, Y. F. Zheng and B. Nowack, Developing trends in nanomaterials and their environmental implications, Nat. Nanotechnol., 2023, 18, 834–837 CrossRef CAS.
- S. Kumar, A. Bhawna, R. Gupta, A. Kumar, A. Kumar Bharti and V. Kumar, New insights into Cu/Cu2O/CuO nanocomposite heterojunction facilitating photocatalytic generation of green fuel and detoxification of organic pollutants, J. Phys. Chem. C, 2023, 127, 7095–7106 CrossRef CAS.
- L. Y. Zhu, L. X. Ou, L. W. Mao, X. Y. Wu, Y. P. Liu and H. L. Lu, Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: Overview, Nano-Micro Lett., 2023, 15, 89 CrossRef CAS PubMed.
- Y. X. Liu, C. Zhou, D. N. Li, M. K. Xu, J. E. Lu, E. R. Xu, S. M. Yang, L. P. Zeng, J. L. Zhang and X. Chen, Large-scale synthesis of highly porous CuO/Cu2O/Cu/carbon derived from aerogels for lithium-ion battery anodes, Langmuir, 2024, 40, 8608–8616 CrossRef CAS.
- E. Semenzin, V. Subramanian, L. Pizzol, A. Zabeo, W. Fransman, C. Oksel, D. Hristozov and A. Marcomini, Controlling the risks of nano-enabled products through the life cycle: The case of nano copper oxide paint for wood protection and nano-pigments used in the automotive industry, Environ. Int., 2019, 131, 104901 CrossRef CAS PubMed.
- M. F. Hochella, D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Natural, incidental, and engineered nanomaterials and their impacts on the Earth system, Science, 2019, 363, eaau8299 CrossRef PubMed.
- N. Z. Jankovic and D. L. Plata, Engineered nanomaterials in the context of global element cycles, Environ. Sci.: Nano, 2019, 6, 2697–2711 RSC.
- A. A. Keller, A. S. Adeleye, J. R. Conway, K. L. Garner, L. J. Zhao, G. N. Cherr, J. Hong, J. L. Gardea-Torresdey, H. A. Godwin, S. Hanna, Z. X. Ji, C. Kaweeteerawat, S. J. Lin, H. S. Lenihan, R. J. Miller, A. E. Nel, J. R. Peralta-Videa, S. L. Walker, A. A. Taylor, C. Torres-Duarte, J. I. Zink and N. Zuverza-Mena, Comparative environmental fate and toxicity of copper nanomaterials, NanoImpact, 2017, 7, 28–40 CrossRef.
- Q. Abbas, B. Yousaf, M. U. Amina, M. A. M. Ali, A. Munir, J. Rinklebe El-Naggar and M. Naushad, Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review, Environ. Int., 2020, 138, 105646 CrossRef CAS PubMed.
- P. Zhang, Z. L. Guo, Z. Y. Zhang, H. L. Fu, J. C. White and I. Lynch, Nanomaterial transformation in the soil-plant system: Implications for food safety and application in agriculture, Small, 2020, 16, 2000705 CrossRef CAS.
- Q. Liu, X. M. Ding, Y. T. Pang, Y. N. Cao, J. L. Lei, J. W. Wu and T. Zhang, New insights into the safety assessment of quantum dots: Potential release pathways, environmental transformations, and health risks, Environ. Sci.: Nano, 2022, 9, 3277–3311 RSC.
- M. Kah, D. Navarro, W. Schenkeveld, R. S. Kookana, J. K. Kirby, S. Santra and A. Ozcan, Fate of copper in soil: Effect of agrochemical (nano)formulations and soil properties, Environ. Sci.: Nano, 2022, 9, 653–662 RSC.
- W. E. Connell and W. H. Patrick, Jr., Sulfate reduction in soil: Effects of redox potential and pH, Science, 1968, 159, 86–87 CrossRef CAS.
- G. R. Gibson, Physiology and ecology of the sulphate-reducing bacteria, J. Appl. Bacteriol., 1990, 69, 769–797 CrossRef CAS PubMed.
- R. Ma, C. Levard, J. D. Judy, J. M. Unrine, M. Durenkamp, B. Martin, B. Jefferson and G. V. Lowry, Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids, Environ. Sci. Technol., 2014, 48, 104–112 CrossRef CAS.
- R. D. Kent, J. G. Oser and P. J. Vikesland, Controlled evaluation of silver nanoparticle sulfidation in a full-scale wastewater treatment plant, Environ. Sci. Technol., 2014, 48, 8564–8572 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- A. L. Dale, G. V. Lowry and E. A. Casman, Modeling nanosilver transformations in freshwater sediments, Environ. Sci. Technol., 2013, 47, 12920–12928 CrossRef CAS.
- H. M. Su, X. T. Qian, Z. H. Gu, Z. L. Xu, H. J. Lou, X. Y. Bian, T. Zeng, D. H. Lin, J. Filser and L. X. Y. Li, Cu(OH)2 nanorods undergo sulfidation in water: In situ formation of CuO nanorods as intermediates and enhanced toxicity to Escherichia coli, Environ. Chem. Lett., 2020, 18, 1737–1744 CrossRef CAS.
- C. Levard, B. C. Reinsch, F. M. Michel, C. Oumahi, G. V. Lowry and G. E. Brown, Jr., Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate, Environ. Sci. Technol., 2011, 45, 5260–5266 CrossRef CAS.
- C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner and G. V. Lowry, Sulfidation of silver nanoparticles: Natural antidote to their toxicity, Environ. Sci. Technol., 2013, 47, 13440–13448 CrossRef CAS PubMed.
- Z. Y. Wang, A. von dem Bussche, P. K. Kabadi, A. B. Kane and R. H. Hurt, Biological and environmental transformations of copper-based nanomaterials, ACS Nano, 2013, 7, 8715–8727 CrossRef CAS PubMed.
- G. Lee, B. Lee and K. T. Kim, Mechanisms and effects of zinc oxide nanoparticle transformations on toxicity to zebrafish embryos, Environ. Sci.: Nano, 2021, 8, 1690–1700 RSC.
- Z. Z. Zhang, Y. Zhang, Y. F. Cheng and R. C. Jin, Sulfidation attenuates the adverse impacts of metallic nanoparticles on anammox from the perspective of chronic exposure, Environ. Sci.: Nano, 2020, 7, 1681–1691 RSC.
- S. M. Kamel, S. F. Elgobashy, R. I. Omara, A. S. Derbalah, M. Abdelfatah, A. El-Shaer, A. A. Al-Askar, A. Abdelkhalek, K. A. Abd-Elsalam, T. Essa, M. Kamran and M. M. Elsharkawy, Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber, J. Fungi, 2022, 8, 911 CrossRef CAS PubMed.
- L. A. Herrnida-Montero, N. Pariona, A. I. Mtz-Enriquez, G. Carrion, F. Paraguay-Delgado and G. Rosas-Saito, Aqueous-phase synthesis of nanoparticles of copper/copper oxides and their antifungal effect against Fusariurn oxysporum, J. Hazard. Mater., 2019, 380, 120850 CrossRef.
- S. Rtimi and J. Kiwi, Recent advances on sputtered films with Cu in ppm concentrations leading to an acceleration of the bacterial inactivation, Catal. Today, 2020, 340, 347–362 CrossRef CAS.
- S. Chakraborty, A. Nair, M. Paliwal, A. Dybowska and S. K. Misra, Exposure media a critical factor for controlling dissolution of CuO nanoparticles, J. Nanopart. Res., 2018, 20, 331 CrossRef.
- M. L. Xu, Y. S. Wang, Z. T. Mu, S. W. Li and H. L. Li, Dissolution of copper oxide nanoparticles is controlled by soil solution pH, dissolved organic matter, and particle specific surface area, Sci. Total Environ., 2021, 772, 145477 CrossRef CAS PubMed.
- S. Ortelli, A. L. Costa, M. Blosi, A. Brunelli, E. Badetti, A. Bonetto, D. Hristozov and A. Marcomini, Colloidal characterization of CuO nanoparticles in biological and environmental media, Environ. Sci.: Nano, 2017, 4, 1264–1272 RSC.
- Z. H. Yang, D. P. Zhang, W. X. Zhang and M. Chen, Controlled synthesis of cuprous oxide nanospheres and copper sulfide hollow nanospheres, J. Phys. Chem. Solids, 2009, 70, 840–846 CrossRef CAS.
- S. H. Jiao, L. F. Xu, K. Jiang and D. S. Xu, Well-defined non-spherical copper sulfide mesocages with single-crystalline shells by shape-controlled Cu2O crystal templating, Adv. Mater., 2006, 18, 1174–1177 CrossRef CAS.
- K. Chanda, S. Rej and M. H. Huang, Facet-dependent catalytic activity of Cu2O nanocrystals in the one-pot synthesis of 1,2,3-triazoles by multicomponent click reactions, Chem. – Eur. J., 2013, 19, 16036–16043 CrossRef CAS PubMed.
- W. C. Huang, L. M. Lyu, Y. C. Yang and M. H. Huang, Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS.
- D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han and Z. Zhang, Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability, J. Mater. Chem., 2009, 19, 5220–5225 RSC.
- C. S. Tan, S. C. Hsu, W. H. Ke, L. J. Chen and M. H. Huang, Facet-dependent electrical conductivity properties of Cu2O crystals, Nano Lett., 2015, 15, 2155–2160 CrossRef CAS.
- J. Ren, W. Z. Wang, S. M. Sun, L. Zhang, L. Wang and J. Chang, Crystallography facet-dependent antibacterial activity: The case of Cu2O, Ind. Eng. Chem. Res., 2011, 50, 10366–10369 CrossRef CAS.
- X. R. Wang, T. F. Hung, F. R. Chen and W. X. Wang, In situ tracking of crystal-surface-dependent Cu2O nanoparticle dissolution in an aqueous environment, Environ. Sci. Technol., 2023, 57, 1006–1016 CrossRef CAS PubMed.
- W. H. Fan, Z. W. Shi, X. P. Yang, M. M. Cui, X. L. Wang, D. F. Zhang, H. Liu and L. Guo, Bioaccumulation and biomarker responses of cubic and octahedral Cu2O micro/nanocrystals in Daphnia magna, Water Res., 2012, 46, 5981–5988 CrossRef CAS.
- W. H. Fan, X. L. Wang, M. M. Cui, D. F. Zhang, Y. Zhang, T. Yu and L. Guo, Differential oxidative stress of octahedral and cubic Cu2O micro/nanocrystals to Daphnia magna, Environ. Sci. Technol., 2012, 46, 10255–10262 CrossRef CAS.
- C. L. Wang, H. Tissot, C. Escudero, V. Pérez-Dieste, D. Stacchiola and J. Weissenrieder, Redox properties of Cu2O(100) and (111) surfaces, J. Phys. Chem. C, 2018, 122, 28684–28691 CrossRef CAS.
- X. H. Yu, X. M. Zhang, X. X. Tian, S. G. Wang and G. Feng, Density functional theory calculations on oxygen adsorption on the Cu2O surfaces, Appl. Surf. Sci., 2015, 324, 53–60 CrossRef CAS.
- A. Gogos, B. Thalmann, A. Voegelin and R. Kaegi, Sulfidation kinetics of copper oxide nanoparticles, Environ. Sci.: Nano, 2017, 4, 1733–1741 RSC.
- R. Ma, J. Stegemeier, C. Levard, J. G. Dale, C. W. Noack, T. Yang, G. E. Brown, Jr. and G. V. Lowry, Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide, Environ. Sci.: Nano, 2014, 1, 347–357 RSC.
- X. D. Liang, L. Gao, S. W. Yang and J. Sun, Facile synthesis and shape evolution of single-crystal cuprous oxide, Adv. Mater., 2009, 21, 2068–2071 CrossRef CAS.
- C. M. H. Ferreira, I. S. S. Pinto, E. V. Soares and H. M. V. M. Soares, (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions - a review, RSC Adv., 2015, 5, 30989–31003 RSC.
- L. H. Wang, H. D. Xu, N. Jiang, Z. M. Wang, J. Jiang and T. Zhang, Trace cupric species triggered decomposition of peroxymonosulfate and degradation of organic pollutants: Cu(III) being the primary and selective intermediate oxidant, Environ. Sci. Technol., 2020, 54, 4686–4694 CrossRef CAS PubMed.
- Y. Luo, H. Li, H. L. Yang, Z. M. Yang, C. Li, S. H. Liu, Q. Chen, W. H. Xu, W. Zhang and X. F. Tan, Critical role of dissolved oxygen and iron-copper synergy in dual-metal/char catalyst systems, Environ. Sci.: Nano, 2024, 11, 2091–2102 RSC.
- S. J. Yang, Y. K. Lin, Y. C. Pu and Y. J. Hsu, Crystal facet dependent energy band structures of polyhedral Cu2O nanocrystals and their application in solar fuel production, J. Phys. Chem. Lett., 2022, 13, 6298–6305 CrossRef CAS.
- Z. G. Liu, Y. F. Sun, W. K. Chen, Y. Kong, Z. Jin, X. Chen, X. Zheng, J. H. Liu, X. J. Huang and S. H. Yu, Facet-dependent stripping behavior of Cu2O microcrystals toward lead ions: A rational design for the determination of lead ions, Small, 2015, 11, 2493–2498 CrossRef CAS PubMed.
- J. Lin, W. Hao, Y. Shang, X. T. Wang, D. L. Qiu, G. S. Ma, C. Chen, S. Z. Li and L. Guo, Direct experimental observation of facet-dependent SERS of Cu2O polyhedra, Small, 2018, 14, 1703274 CrossRef.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Formation of hollow nanocrystals through the nanoscale Kirkendall effect, Science, 2004, 304, 711–714 CrossRef CAS PubMed.
- H. L. Cao, X. F. Qian, C. Wang, X. D. Ma, J. Yin and Z. K. Zhu, High symmetric 18-facet polyhedron nanocrystals of Cu7S4 with a hollow nanocage, J. Am. Chem. Soc., 2005, 127, 16024–16025 CrossRef CAS.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- C. C. Vidyasagar, G. Hosamani, P. Kariyajjanavar, R. O. Yathisha and Y. A. Nayaka, One-pot microwave synthesis and effect of Cu2+ ions on structural properties of Cu-ZnO nano crystals, Mater. Today: Proc., 2018, 5, 22171–22180 CAS.
- T. D. Sands, J. Washburn and R. Gronsky, High resolution observations of copper vacancy ordering in chalcocite (Cu2S) and the transformation to djurleite (Cu1.97 to 1.94 S), Phys. Status Solidi A, 1982, 72, 551–559 CrossRef CAS.
- H. T. Evans, Jr., Djurleite (Cu1.94S) and low chalcocite (Cu2S): New crystal structure studies, Science, 1979, 203, 356–358 CrossRef.
- Y. F. Yuan, K. He and J. Lu, Structure-property interplay within microporous manganese dioxide tunnels for sustainable energy storage, Angew. Chem., Int. Ed., 2024, 63, e202316055 CrossRef CAS PubMed.
- S. Arora, K. Kabra, K. B. Joshi, B. K. Sharma and G. Sharma, Structural, elastic, thermodynamic and electronic properties of covellite, CuS, Phys. B, 2020, 582, 311142 CrossRef CAS.
- A. Morales-García, A. L. Soares, E. C. Dos Santos, H. A. de Abreu and H. A. Duarte, First-principles calculations and electron density topological analysis of covellite (CuS), J. Phys. Chem. A, 2014, 118, 5823–5831 CrossRef.
- R. J. Goble, The relationship between crystal structure, bonding and cell dimensions in the copper sulfides, Can. Mineral., 1985, 23, 61–76 CAS.
- Y. Wang, Y. N. Yang, Y. R. Shi, H. Song and C. Z. Yu, Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives, Adv. Mater., 2020, 32, 1904106 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00545g |
| This journal is © The Royal Society of Chemistry 2025 |