Manganese-based nanozyme enabled efficient mitigation of Huanglongbing-induced oxidative damage in Citrus†
Received
7th June 2024
, Accepted 19th September 2024
First published on 30th September 2024
Abstract
Huanglongbing (HLB) is a severe disease in Citrus caused by the infection of Candidatus Liberibacter asiaticus (CLas), which has brought about substantial economic losses in the global Citrus industry. Recently, HLB has been recognized as a plant immune-mediated disease resulting from the CLas-colonization stimulated immune responses and the accumulation of excessive reactive oxygen species (ROS) in Citrus tissues. Here, we report a manganese oxide nanozyme (MONPs) based strategy to scavenge and regulate the ROS metabolism in HLB infected Citrus, thereby protecting leaf tissues against oxidative stress. Transmission electron microscopy (TEM) imaging revealed that MONPs enable efficient delivery into the intercellular space by spraying dispersion. ROS detection indicated the direct ROS scavenging ability of MONPs with high efficiency in HLB-Citrus with about 60% of ROS decreasing. Enzyme activation and gene expression analysis exhibited that the MONP treatment could regulate the ROS metabolism gene in Citrus to alleviate oxidative stress. Current research demonstrated that the HLB-Citrus sprayed with MONPs showed a noticeable protective effect within 22 days to alleviate the blotchy mottle symptoms. Furthermore, various physiological indexes such as MDA, starch, total soluble sugar, carotenoid and chlorophyll in HLB-Citrus leaves exhibited significant improvement post-MONP treatment. This MONP-based approach provides a promising alternative strategy to modulate and mitigate oxidative stress in HLB-Citrus, thereby serving the Citrus industry.
Environmental significance
Citrus Huanglongbing (HLB) is regarded as an immune-mediated disease caused by the infection of Candidatus Liberibacter asiaticus (CLas), which creates various responses in infected citrus plants including, the production of reactive oxygen species (ROS) in response to pathogen invasion. CLas-triggered ROS production results in systemic cell death of related phloem tissues in citrus leaves, leading to economic losses amounting to billions of dollars. Herein, we report a manganese oxide nanozyme (MONP) based strategy to relieve the oxidative stress in HLB-Citrus. We found that the manganese oxide nanozyme (MONPs) could be efficiently internalized into Citrus leaf tissues via a foliar spray. Treatment of MONPs could significantly regulate ROS metabolism in HLB-Citrus to alleviate oxidative stress caused by HLB. This work provides a versatile, stable, and an easy batch-prepared strategy for protecting HLB-Citrus.
|
1. Introduction
Citrus, a principal source of vitamin C in the human diet worldwide faces a significant threat from Citrus Huanglongbing (HLB), a disease induced by the bacterium Candidatus Liberibacter asiaticus (CLas). This pathogen has substantially declined the Citrus industry within pivotal production areas, including the Guangxi province of China and Florida in the United States. Citrus trees affected by HLB experience a substantial decrease in production, with reductions ranging from 50% to 90% in some regions. The economic implications of HLB are profound, which necessitates immediate and sustained research efforts to develop effective management strategies aimed at controlling the disease spread and mitigating its impact on Citrus cultivation.1,2
CLas infection can cause the yellowing of leaf tissues, a decrease in fruit production, and ultimately, the death of trees, which bring about economic losses of billions of dollars and numerous job losses.3–5 Unfortunately, there is currently no effective approach to cure HLB-Citrus due to the nature of the internally colonized bacterium.6CLas is the most prevalent strain and has been commonly studied. Exploring the battle strategies between CLas and Citrus plants is critical for the establishment of control remedies. A recent study reported that HLB is an immune-mediated disease and the CLas infection can stimulate systemic and chronic immune responses in phloem tissue.7 This results in multiple responses in infected Citrus, including phloem blockage, rise in the level of salicylic acid, and the production of reactive oxygen species (ROS) against the invasion of the pathogen.8–10CLas-triggered continuous ROS production localizes in phloem-enriched tissue and is followed by widespread cell death in phloem tissues and other areas, affecting various plant functions such as phloem activity, hormone production and transport, and metabolic processes in Citrus.7 Meanwhile, ROS have been shown to promote callose deposition and inhibit root development that affect Citrus growth.11–13 Cell death in the phloem tissue can lead to diminished transport of photosynthates, which, combined with the inhibition of root growth by reactive oxygen species (ROS), might cause root decay. The possible stems with stunted growth are the direct impact of ROS and the reduced movement of carbohydrates and hormones. It is highly desirable to find novel potential materials with high antioxidant efficiency and good biocompatibility targeted at combating CLas-induced oxidative stress in HLB-Citrus.
Currently, some artificial metal nanomaterials are being studied for their natural enzyme-like activities, such as peroxidase (POD), catalase (CAT), oxidase (OXD), and superoxide dismutase (SOD) to regulate the environmental ROS.14,15 Among them, the manganese oxide-based nanozymes have recently been reported as biocompatible nanozymes with several enzyme-like activities, inhibiting ROS-induced damage under physiological conditions.16,17 With the development of nanomaterials as ideal alternatives to native enzymes used in plants for mitigating different types of ROS stress from temperature variation, drought, salinity stress, irradiance, and pollutant stress, an opportunity arises to enhance plant tolerance, improve crop production as nano-fertilizers, and minimize pathogen attacks.14,18–22 In the previous report, a hybrid solution of silver nanoparticles and chitosan oligomers could be used for citrus greening disease treatment.23 Additionally, Mn elements employ crucial roles in activating a variety of physiological processes in plants.24 However, a few studies have focused on elaborating on the practical application, uptake, and transport of the Mn-based nanozyme in economic crops so far. Plant leaves, especially the existence of surface waxes in Citrus, are known to repel foliar-sprayed-based nanoparticle delivery.25,26 Meanwhile, the nanoscale translocation of nanozymes for Citrus tissues and the subsequent fate is still to be determined.
In this study, we report a manganese oxide nanozyme (MONPs)-based strategy to relieve oxidative stress in HLB Citrus. We found that MONPs could be efficiently internalized into Citrus leaf tissues via the foliar spray pathway, which mainly surrounds the plant cell wall under transmission electron microscopy (TEM) imaging. MONPs showed a notable ability to scavenge multiple types of ROS both in vitro and in HLB-Citrus. MONP treatment could bring about positive effects and changes in a series of physiological and biochemical indexes in HLB-Citrus. Furthermore, we demonstrated that foliar spray of HLB-Citrus with MONPs enables significant mitigation of oxidative damage in Citrus caused by HLB and improves the Citrus growth to alleviate the HLB symptoms. MONPs provide an alternative method serving as an antioxidant with the advantages of versatility, high stability, and easy batch preparation, showing potential for optimization and application in the field of Citrus cultivation and industry.
2. Materials and methods
2.1 Materials and reagents
Potassium permanganate (KMnO4), oleic acid (OA), 3,3′,5,5′-tetramethylbenzidine (TMB) were obtained from Sigma-Aldrich. H2O2 and enzyme activity detection kits were purchased from Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China, A064-1 for H2O2, A084-3 for POD, A001-1 for SOD and A007-1 for CAT). 2× SYBR Green qPCR Mix (Low ROX) was bought from Aidlab Biotechnologies (Beijing). Rapid Purification Universal Plant Total RNA Isolation kit and TaKaRa MiniBEST Plant Genomic DNA Extraction kit were obtained from Vazyme (Nanjing). PrimeScript™ II 1st Strand cDNA Synthesis kit was bought from TaKaRa Biotechnology.
2.2 Synthesis of MONPs
KMnO4 (0.2 g) was added to a conical flask containing deionized water (100 mL) and stirred well for 30 min to achieve complete dissolution of KMnO4. Subsequently, 2 mL oleic acid was added, and vigorous stirring was continued for 5 h at room temperature. MONPs were synthesized when the liquid turned brownish-black, according to a previous study.27 MONPs were subsequently isolated through centrifugation at 8000 rpm and subjected to three successive washes with ethanol and deionized water. The resulting product was then subjected to vacuum drying at 50 °C and subsequently stored at room temperature.
2.3 Peroxidase-like activity of MONPs and kinetic assay
Briefly, 0.5 mL 2 mg mL−1 3,3′,5,5′-tetramethylbenzidine (TMB) was mixed with 50 mM H2O2 (0.5 mL) and 0.3 mL MONPs (final concentrations of 0.005, 0.0075, 0.01, 0.015, 0.02, 0.025, 0.05, 0.075, 0.1, 0.15, 0.2, 0.25, 0.75 and 1 mg mL−1), vortexed and shocked. After 10 min of the reaction, the mixture was centrifuged, and a calibration curve was obtained by measuring the absorbance at 652 nm. Following the approach outlined by the previous report.28 TMB was utilized as a substrate to measure the peroxidase (POD)-like activity of MONPs. Different concentrations of TMB solution (0.0625, 0.125, 0.187, 0.25, 0.312, 0.5, 1, and 2 mg mL−1), 50 mM H2O2, and 0.1 mL 0.1 mg mL−1 MONPs were mixed. The variation in absorbance (at 652 nm) throughout the reaction was monitored, and the Michaelis–Menten equation was employed to perform a kinetic analysis of MONPs.
2.4 Catalase-like activity of MONPs and kinetic assay
Ammonium molybdate undergoes a complex formation with H2O2, and the quantification of H2O2 can be achieved by measuring its absorbance at 405 nm.29,30 The catalase (CAT)-like enzyme activity of MONPs in scavenging H2O2 was assessed as follows: 0.2 mL H2O2 was combined with 0.2 mL MONPs (at concentrations of 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, and 1 mg mL−1) and allowed to react for 5 min. Subsequently, 0.4 mL of ammonium molybdate (200 mM) was added to terminate the reaction and initiate the color development. After centrifugation at 8000 rpm, the absorbance at 405 nm of the supernatant was determined. To construct a Michaelis–Menten kinetics curve for the CAT-like activity of MONPs, experiments were carried out using varying concentrations of H2O2 solutions (10, 20, 40, 60, 80, 100, 150, and 200 mM) as the substrate, with ammonium molybdate employed as a chromogenic reagent.
2.5 Superoxide dismutase-like activity of MONPs
The elimination of O2˙− followed the procedure outlined in the previous report.31 O2˙− was generated by incubating xanthine (X) and xanthine oxidase (XO) at 37 °C. Subsequently, 0.2 mL MONPs (0.01, 0.05, 0.1, 0.5, 1, and 2 mg mL−1) were added to the solution. After a 30-minute incubation, the mixture was subjected to centrifugation at 8000 rpm for 5 min. The fluorescence intensity was promptly measured following the addition of 0.2 mL of DCFH-DA to the supernatant, with DCFH-DA being excited at 488 nm and emitted at 525 nm.
2.6 Scavenging of hydroxyl radicals by MONPs
In summary, 0.5 mL of FeSO4 (6 mmol L−1), 0.5 mL of H2O2 (6 mmol L−1), and 0.5 mL of MONPs were mixed and reacted for 10 min. Following this, 0.5 mL of salicylic acid (6 mmol L−1) was added, and the mixture was heated in a water bath at 37 °C for 30 min.32,33 The solution's color deepened in proportion to the hydroxyl radicals' concentration. After the reaction, the solution was centrifuged at 8000 rpm for 5 min, and the absorbance was measured at 510 nm.
2.7 Real-time PCR analysis
To analyze the expression of antioxidant enzyme genes, total RNA was extracted from Citrus leaf tissues using the Rapid Purification Universal Plant Total RNA Isolation kit (Vazyme, Nanjing). Genomic contamination in the total RNA was eliminated and the first strand cDNA was synthesized using the AdvanceFast 1st Strand cDNA Synthesis kit (No Dye) (Yeasen Biotechnology, Shanghai). Genomic contamination in the total RNA was eliminated and the first strand cDNA was synthesized using the PrimeScript™ II 1st Strand cDNA Synthesis kit (TaKaRa Biotechnology, Beijing). RT-qPCR was conducted using 2× SYBR Green qPCR Mix (Low ROX) (Aidlab Biotechnologies, Beijing). The PCR cycling conditions were as follows: 10 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 15 s at 60 °C, 10 s at 72 °C, and finally 15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C. The primer sequences used in the expression analysis are listed in Table S1.† The gene expression was further quantified using the comparative 2−ΔΔCT method with respect to actin as reference genes.
2.8 NBT staining
In situ accumulation of O2˙− was assessed through NBT (nitroblue tetrazolium) staining, following the method of the previous report.34,35Citrus leaves were immersed in a freshly prepared NBT solution (50 mL potassium phosphate, pH = 7.8). After 12 h of dark incubation at room temperature, chlorophyll was removed using 75% ethanol in a boiling water bath, and the leaves were subsequently photographed. Color intensity was quantified using ImageJ. To facilitate the staining process, given the thick waxy layer on Citrus leaves, the leaves were first immersed in the staining solution and subjected to vacuum treatment at room temperature for 20 min.
2.9 ROS determination and enzyme activity assay
Citrus tissues were first accurately weighed and then subjected to cryogenic grinding with liquid nitrogen. A homogenate was prepared following kit instructions by adding the appropriate volume of PBS (0.1 M, pH 7.0) and vortexing. Subsequently, the homogenate was centrifuged at 4 °C, and the resulting supernatant was collected for the determination of H2O2, SOD, CAT, and POD levels. The relevant assay kits were utilized according to the manufacturer's instructions (Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China, A064-1 for H2O2, A084-3 for POD, A001-1 for SOD and A007-1 for CAT).36,37
2.10 Determination of malondialdehyde (MDA) content
The determination of malondialdehyde (MDA) content was performed following the protocol outlined by Zhong et al.34 Initially, 0.5 g Citrus tissue was cryogenically ground in liquid nitrogen. Subsequently, 5 mL of 10% trichloroacetic acid (TCA) was added and the mixture was vortexed to create a homogenate. Next, 1 mL of the extraction solution was transferred to a tube and mixed with 1 mL of 0.5% thiobarbituric acid (TBA). The mixture was placed in boiling water for 30 min and then cooled on ice for 20 min. Afterward, centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm was performed for 5 min, and the absorbance of the samples was measured at 450 nm, 532 nm, and 600 nm using an enzyme marker. The MDA concentration in the extraction solution was calculated by the formula:
000 rpm was performed for 5 min, and the absorbance of the samples was measured at 450 nm, 532 nm, and 600 nm using an enzyme marker. The MDA concentration in the extraction solution was calculated by the formula:
| MDA (μmol L−1) = 6.45(A532 − A600) − 0.56A450 |
2.11 Determination of chlorophyll and carotenoid content
For chlorophyll content analysis, 0.1 g of Citrus tissue was weighed and cryogenically ground with liquid nitrogen. Chlorophyll extraction was carried out by adding 8 mL of 80% acetone solution and protecting it from light for 12 h. The absorbance of the resulting extract was measured at 663 nm, 645 nm, and 470 nm.34 Chlorophyll a (Ca), chlorophyll b (Cb), total chlorophyll (CT), and carotenoid (Cc) contents can be calculated using the following formula.
| Ca (mg L−1) = 12.21A663 − 2.81A645 |
| Cb (mg L−1) = 20.13A645 − 5.03A663 |
| Cc (mg L−1) = (1000A470 − 3.27Ca − 104Cb)/229 |
2.12 Determination of the soluble sugar content
The soluble sugar content was determined using the anthrone–sulfuric acid method.38 0.1 g Citrus leaves were weighed and cryogenically ground with liquid nitrogen. They were then mixed with 2 mL PBS and boiled at 95 °C for 15 min. Subsequently, centrifugation at 5000 rpm was conducted for 5 min, and the resulting supernatant was transferred to a clean centrifuge tube. This process was repeated three times, and the final 6 mL of the supernatant was diluted tenfold. 0.4 mL of the diluted solution, 100 μL anthrone solution, and 1 mL of concentrated sulfuric acid were combined and allowed to react for 30 min at room temperature. The absorbance was then measured at 630 nm. The same procedure was employed to construct the standard curve.
2.13 Determination of starch content
The starch content was determined using the iodine–starch colorimetric method.39 For the sample analysis, Citrus tissue was weighed and cryogenically ground with liquid nitrogen. Subsequently, 1 mL of 50% Ca(NO3)2 was added, followed by 10 min of incubation in a boiling water bath. After centrifugation at 5000 rpm for 5 min, 0.8 mL of the supernatant was transferred to a new centrifuge tube. To this, an equal volume of the 5% KI–I2 solution was added, and the mixture was left to stand for 30 min. Following another centrifugation step at 5000 rpm for 10 min, the precipitate was washed twice with the 5% iodine-containing calcium nitrate solution and then dissolved in 0.8 mL NaOH (0.1 M) and diluted appropriately. Finally, 1 mL of the diluted solution was combined with 1 mL HCl (0.1 M), and the absorbance at 590 nm was measured. The same procedure was employed to obtain the standard curve using 0.5 mg mL−1 of starch solution.
2.14 Plant growth
Isolation and inoculation with Citrus Huanglongbing (HLB) through grafting, fertilization and water management were carried out according to the conventional management methods for Citrus seedlings. Regular observations and detections were conducted to monitor plant infection. Branches of the plants that tested positive for Candidatus Liberibacter asiaticus (CLas) through PCR were designated as the experimental treatment group. The experimental residues were autoclaved and treated as hazardous waste. The plant materials used in this study were sweet orange (Citrus sinensis) kept in the greenhouse. To evaluate the effects of MONPs on HLB-Citrus, MONPs (0.1 mg mL−1, 0.5 mL) were applied to the leaves of Citrus using a syringe or spray bottle every three days. Before injection, the back of the leaf was gently scratched with a needle to avoid the leaf vein and piercing through the leaf. Then, using a syringe without the needle, MONPs were injected into the scratched area of the leaf (Fig. 1).
 |
| | Fig. 1 Schematic of the MONPs-mediated protection effect on the HLB-Citrus. | |
2.15 Data analysis of RNA-seq
The total RNA was extracted from Citrus leaves at 22 days post-spray, followed by library constructing and sequencing. The libraries with different indexes were multiplexed and loaded on an Illumina instrument according to the manufacturer's instructions (Azenta Life Sciences, Nanjing). GOSeq (v1.34.1) was used to identify Gene Ontology (GO) terms and the heatmap plots were drawn using R language with FPKM value as the input.
3. Results and discussion
3.1 Characterization of the MONPs nanozyme
The Mn nanozyme was synthesized by reacting oleic acid and potassium permanganate using a one-pot method at room temperature. Transmission electron microscopy (TEM) imaging showed that the MONPs were flower-liked spherical nanoparticles in shape with a uniform size of about 100–200 nm (Fig. 2A). Furthermore, to evaluate the stability and storage of MONPs, MONPs stored for 1 month were analyzed by dynamic light scattering (DLS). Results shown in Fig. S1† exhibited that the size of MONPs was extremely stable and they could be stored for a long time without sedimentation and aggregation. Meanwhile, the composition of the MONPs was analyzed by energy-dispersive spectrometry (EDS) mapping. As shown in (Fig. 2B), the results exhibited that C, O, and Mn elements were distributed in the same nanoparticle, meaning the uniform deposition of manganese oxide. The hydrodynamic size of MONPs was measured from DLS analysis, revealing good dispersion under an aqueous solution (Fig. 2C). The zeta potential of MONPs was −19 mV (Fig. S2A†). Additionally, the X-ray photoelectron spectroscopy (XPS) also confirmed the presence of Mn and O (Fig. 2D). The 2p3/2 peak can be resolved into three distinct peaks at 641.6, 642.3, and 643.4 eV, corresponding to Mn2+, Mn3+, and Mn4+, respectively (Fig. 2E). Powder X-ray diffraction (XRD) and Raman were employed for further characterization of the crystal lattice of the synthesized nanoflowers (Fig. S2B and C†). The characteristic peaks of the pattern aligned with the previous reports at 644 (cm−1).40,41 These results confirmed the successful fabrication of MONPs.
 |
| | Fig. 2 Characterization of the MONPs. (A) Representative TEM images of the MONPs. (B) Energy-dispersive spectrometry analysis of the MONPs. (C) Dynamic light scattering analysis of MONPs. (D) X-ray photoelectron spectroscopy (XPS) analysis of the MONPs. (E) XPS spectra of Mn 2p3/2 in MONPs. | |
3.2
In vitro ROS scavenging effect of the MONPs
To investigate whether MONPs were efficient for ROS scavenging under a physiological environment, we prepared multiple ROSs mainly containing hydrogen peroxide (H2O2), superoxide anion (O2˙−), and hydroxyl radical (OH·), which were treated with MONPs for evaluating the antioxidant activity. To test the POD activity, different concentrations of MONPs (0 to 1.0 mg mL−1) were co-incubated with H2O2, and this reaction was monitored using 3,3′,5,5′-tetramethylbenzidine (TMB) assay. Results demonstrated that there is an upward trend in the reaction speed as the concentration of MONPs increased from 0 to 0.5 mg mL−1 (Fig. 3A) and the kinetics curve was calculated as shown in Fig. 3B, Km = 1.069 ± 0.3 mM and Vmax = 0.147 ± 0.016 s−1. Next, the OH· scavenging ability was measured using the FENTON colorimetric method, and the results shown in Fig. 3C revealed that OH· degradation can be achieved using MONPs, with a pronounced gradient dependency observed within the range of 0 to 0.5 mg m−1. The reactivity of hydroxyl radicals with TMB is probably greater than the activity of nanozymes in scavenging hydroxyl radicals, leading to colonizing the phloem. Ammonium molybdate was used to test the CAT-like activity, and absorbance analysis revealed that a plateau was attained when the MONP concentration exceeded 0.5 mg mL−1 (Fig. 3D), indicating that the maximum catalytic rate for hydrogen peroxide by MONPs had been reached. The kinetics of CAT-like activity revealed a Michaelis–Menten constant of Km (hydrogen peroxide) = 20.2 ± 1.4 μM (mean kinetics ± SD) and Vmax = 105 μM min−1 (Fig. 3E). To simulate the naturally acidic pH of plant extracellular environment,42 the POD-like activity of MONPs was tested under slightly acidic conditions (pH = 5.5) and the result shown in Fig. 3F indicated that the catalytic activity of pH = 5.5 became more reactive compared to that at pH = 7.4, implying that the MONPs are more suitable for plant systems. In order to measure SOD-like activity, xanthine (X) and xanthine oxidase (XO) were used to generate O2˙−, with DCFH-DA as an indicator. The results of fluorescence spectroscopy revealed that O2˙− could also be degraded by MONPs (Fig. S2D and E†). In short, these results indicated that MONPs nanozyme can efficiently clear multiple ROS (H2O2, O2˙−, and OH·), exhibiting a broad-spectrum scavenging effect.
 |
| | Fig. 3 ROS scavenging activities assay of MONPs in vitro. (A) POD-like activity assay of the MONPs. (B) Kinetic analysis for POD-like activity of the MONPs. (C) Hydroxyl radicals scavenging of the MONPs. (D) CAT-like activity assay of the MONPs, and the inset shows the corresponding colorimetric reaction. (E) Kinetic analysis for CAT-like activity of the MONPs. (F) Absorbance intensity of TMB aqueous solution at 650 nm after exposure to MONPs at pH values of 5.5 and 7.4 (all data means the ± SD, n = 3). | |
3.3 Internalization of MONPs in Citrus tissues
To investigate the internalization and subcellular distribution of MONPs in Citrus leaf tissues, we collected the MONP-treated Citrus leaves after 24 h of MONPs post-spray and infiltration, followed by fixing and preparing for (transmission electron microscopy) TEM imaging. TEM examination revealed the absence of any visible nanostructures on the cell wall in the untreated group. Meanwhile, with arrows marking the MONPs sprayed and infiltrated Citrus leaf tissues both showed an amount of characteristic flower-like nanoparticles located in the extracellular space and surrounding the cell wall (Fig. 4). The extracellular or intracellular spaces were characterized through the cell wall structures and chloroplasts to visually recognize the location of MONPs. These TEM images suggested that MONPs enable effective internalization by the Citrus leaves and have an affinity to plant cell walls. These results indicated that the MONPs enable efficient delivery into Citrus leaf tissues by the simple method of spray or infiltration. Nowadays, the constraints on delivery methods such as antibiotics and plant nutrients within the living plants have hindered the development of techniques for controlling Citrus HLB disease. The dense waxy coating on Citrus leaves prevented the transportation of exogenous drugs into the CLas-colonized phloem or vascular system.43,44 Hence, this MONP-based delivery system has the potential to provide an alternative, effective and eco-friendly method for being crucial as the delivery vehicles into Citrus and other plants.
 |
| | Fig. 4 TEM imaging of the internalization of the MONPs in Citrus tissues. (A) Representative TEM images of the cell wall in the non MONPs-treated Citrus tissues. Scale bar: the zoomed-in image of 500 nm (right) and full size of 5 μm (left). Localization of MONPs in the extracellular space via (B) injection pathway (red arrow). (C) Spray pathway (red arrow). Scale bar: 500 nm (right) and full size of 5 μm (left). CW, cell wall; CM, cell membrane; EC, extracellular; IC, intracellular; V, vacuole; Ch, chloroplast; and Cy, cytoplasm. | |
3.4 Effect of the MONPs in HLB-Citrus
After confirming the plant delivery of the MONPs, we evaluated the scavenging activity of MONPs in HLB-Citrus. Firstly, we used PCR analysis and TEM methods to confirm the CLas-positive plant, and the PCR results; as shown in Fig. S2† the genomic DNA of CLas was present in the Citrus tissues and the TEM image of Fig. S3† shows that CLas could be observed for colonizing in the intercellular space, indicating that all the leaf tissues of the prepared Citrus were infected with CLas. To investigate the ROS location, the DCFH probe was used to detect the ROS level in the leaf tissues of healthy Citrus and HLB-Citrus. As shown in Fig. S4,† the fluorescence signal is evident in both the vein and lamina of HLB-Citrus. This observation is because CLas typically colonizes the phloem or the vascular system within Citrus tissues. To examine the antioxidant effect of the MONPs effect on plants, the leaves of CLas-positive Citrus were treated with MONPs dispersion by the spray and infiltration pathways. After 5 days of post-treatment, the leaf tissues were treated with nitroblue tetrazolium chloride (NBT) staining to measure the production of superoxide ions (O2˙−). The blue signal could track the location of O2˙−, reflecting the ROS accumulation in plant tissues. In contrast to the healthy Citrus, there was no noticeable change in the blue spots of leaves from MONP-infiltrated HLB-Citrus, whereas the non-treated HLB-Citrus exhibited a significant blue region (Fig. 5A). The quantifications of NBT staining intensity are shown in (Fig. 5B). Additionally, the H2O2 level of MONP treated HLB-Citrus showed a continuous decrease approaching that of healthy Citrus (Fig. 5C). These results suggested that the MONP treatment effectively reduced ROS accumulation in HLB-affected Citrus. Usually, plants exhibit the accumulation of multiple biological components to counteract oxidative stress during CLas-infection progress. Hence, at 1, 3, and 5 days of post-spray or infiltration of MONPs, the treated HLB-Citrus were extracted to measure three representative physiological indexes: malondialdehyde (MDA) content, carotenoid levels, and soluble sugars. MDA is a product of lipid peroxidation, and its content serves as an indicator of the extent of oxidative damage.45 As shown in Fig. 5D, the MONP treatment could decrease the content of MDA. Furthermore, the chlorophyll and carotenoid levels of Citrus samples were evaluated using an extraction method. MONP treatment resulted in an increase in chlorophyll and carotenoid levels in the leaf tissues with the prolongation of the post-treatment time (Fig. 5E and S5†). When plants are subjected to oxidative stress, they may increase the synthesis and accumulation of soluble sugars as a physiological response to cope with oxidative stress. After the MONP treatment, there was a decrease in the content of soluble sugars, reflecting the alleviation of oxidative stress levels in HLB-affected Citrus (Fig. 5F). These physiological indices of the MONP-treated HLB-Citrus at 5 days showed improved signals compared to those at day 0 and were closer to those of the healthy Citrus. These results indicated the ROS scavenging activity of MONPs in Citrus tissues, reducing the stress from CLas infection.
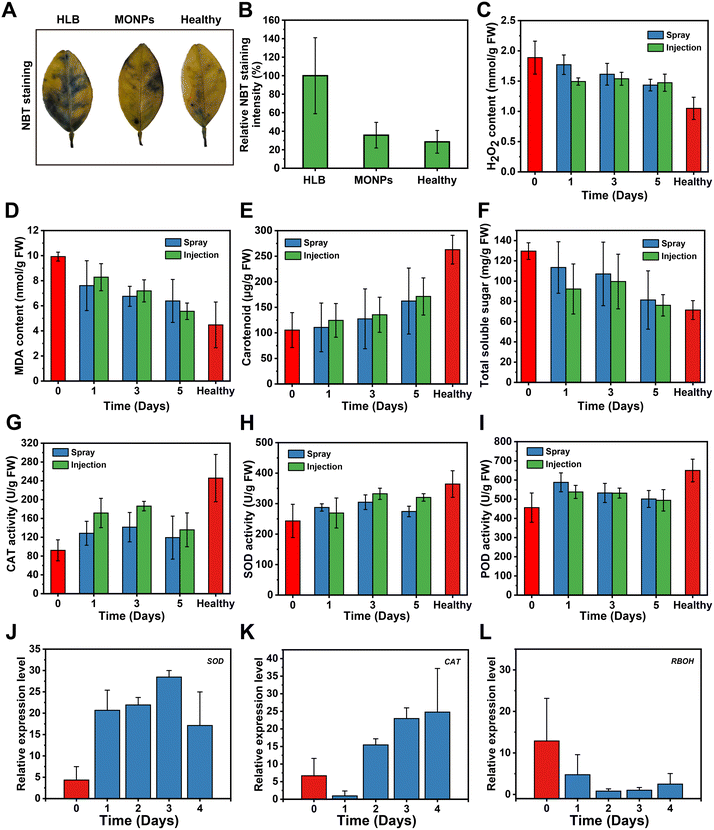 |
| | Fig. 5 Effect of MONPs in the HLB-Citrus. (A) Representative NBT staining of HLB-Citrus leaf, healthy Citrus leaf, and the MONPs-treated HLB-Citrus leaf after 5 days post-infiltration. (B) Quantitative analysis of the NBT staining of Citrus tissue. (C–F) The H2O2 content analysis, MDA content, carotenoid, and total soluble sugar of the HLB-Citrus via the spray and injection pathway for MONPs delivery. (G–I) The analysis of CAT, SOD, and POD activities in the MONPs-treated HLB-Citrus via spray and injection pathway. (J–L) The qPCR analysis of the relative gene expression changes of the SOD gene, CAT gene, and RBOH gene (data are means ± SD, n = 3). | |
3.5 Regulation of the MONPs on ROS relative enzyme activities and transcription levels in HLB-Citrus
After confirming the direct ROS scavenging effect of MONPs in plant tissue, we investigated whether MONPs can regulate antioxidant defense-related gene expression and enzyme activities to enhance the antioxidant ability in HLB-Citrus. Homogenates and total RNA were extracted from HLB-Citrus at 0–5 days after treatment with MONPs nanozyme. Results showed that the CAT activity of MONP-treated HLB-Citrus increased by approximately 29–46% at 5 days of post-spray or infiltration compared to day 0 post-treatment (Fig. 5G). The SOD activity of MONP-treated HLB-Citrus gradually increased within 5 days, reaching levels comparable to those observed in the healthy Citrus (Fig. 5H). The POD activity of MONP-treated HLB-Citrus increased at day 1 post-treatment and slightly decreased at 5 days of post-treatment (Fig. 5I). These results indicate that the MONP treatment in HLB-Citrus can restore ROS-related enzyme activities to counteract oxidative stress. Furthermore, we observed that the enhancement in CAT and SOD activities through the infiltration pathway was slightly superior to that through the spray pathway. This is mainly because infiltration enables MONPs to cross stomata, entering the intercellular space, and facilitating direct contact between MONPs and plant cells. Moreover, qPCR analysis of antioxidant defense-related genes in HLB-Citrus exhibited that the expression levels of SOD and CAT were gradually increased within 4 days of MONP treatment (Fig. 5J and K). Meanwhile, the expression levels of the RBOH gene decreased in HLB-Citrus 4 days after the MONP treatment (Fig. 5L). The expression of the respiratory burst oxidase homologue (RBOH) gene is an omnipresent indicator to reflect biotic or abiotic stress in plants.46,47 Hence, the downregulation of the RBOH gene compared to the untreated HLB-Citrus indicated that the MONP treatment enabled significant CLas-infection caused biotic stress in HLB-Citrus, suggesting that GONs strategy has a good biocompatibility for Citrus tissue. The mechanism by which MONPs alleviate oxidative stress in HLB-Citrus may be related to the fact that MONPs can boost endogenous antioxidant defenses in the plant. Increasing the activity and expression of antioxidant enzymes and related genes, thereby alleviating pathogen-induced oxidative stress has been evidenced by the previous study.48 These results further demonstrated that the foliar-delivered MONPs could bring about positive stimulation of antioxidant systems to regulate the oxygen stress in HLB-Citrus.
3.6 Protection effect of the MONPs against oxygen stress in HLB-Citrus
After confirming the internalization and ROS scavenging activity of MONPs in HLB-Citrus leaf tissues, we attempted to investigate whether MONP treatment enables the prevention and protection of Citrus against HLB infection. As described above, the spray and infiltration-mediated treatment both exhibited the antioxidant effect in HLB-Citrus leaves. Considering the convenient application in agricultural production, we chose the spraying method for MONP treatment to investigate whether MONP spraying can improve the antioxidant defense against the abundant ROS production caused by CLas infection and alleviate the symptoms (Fig. 6). Compared to the MONP-sprayed HLB-Citrus, the HLB-Citrus leaves exhibited obvious etiolation and blotchy mottle phenotypes compared to the healthy Citrus after 22 days. Usually, the death of companion and sieve element cells in the phloem leads to starch accumulation, which manifests as blotchy mottle symptoms on the leaves. The observed hardening of the leaves is likely due to ROS-induced strengthening of cell walls.49 This result indicated that treatment of MONPs enables efficient symptom remission by the antioxidant effect. Meanwhile, we measured the in vitro antibacterial effect by co-incubating MONPs with bacteria, to investigate whether the alleviation of HLB by MONPs was due to ROS scavenging activity or direct killing of CLas. Due to the inability to culture CLas in vitro, Sinorhizobium meliloti Rm1021 (S. meliloti Rm1021), belonging to the same Proteobacteria phylum as CLas, was selected for in vitro antibacterial experiments.50 Different concentrations of MONPs were used to treat S. meliloti Rm1021 to verify its antibacterial activity. As shown in Fig. S6,† the MONPs did not exhibit visible inhibition of the growth of S. meliloti Rm1021, meaning that the phenotypic change mainly relies on the interaction of the MONPs with Citrus cells. Meanwhile, at 22 days after spraying MONPs, a series of physiological indices including H2O2, MDA, starch, total soluble sugar, carotenoid, and chlorophyll content in the treated Citrus leaves were measured to quantify the protective effect of MONPs. The H2O2 level of MONPs sprayed HLB-Citrus showed an obvious decrease (Fig. 7A). The MDA content was measured to evaluate the lipid peroxidation in Citrus tissues, which is usually used to reflect the plant responses to redox signaling and oxidative stresses.45 As shown in Fig. 7B, the MDA level of MONPs-sprayed HLB-Citrus was significantly lower than that in the HLB group. This indicates that the MONPs could reduce the lipid peroxidation stress caused by HLB infection. Additionally, starch accumulation is a representative phenomenon that occurs in CLas-positive Citrus, which results in chloroplast disruption and leaf yellowing. The starch content of the MONP-sprayed HLB-Citrus showed a significant decrease compared to that of the non-treated HLB-Citrus (Fig. 7C). Next, we measured the soluble sugar levels in these Citrus leaves because soluble sugars play a crucial role in regulating osmotic pressure balance within the plant cells, while also serving as an energy source to support the Citrus growth.51 Results of Fig. 7D showed that the sugar levels of the MONP sprayed HLB-Citrus leaves slightly decreased compared to those in the non-treated HLB-Citrus. Next, to investigate whether the method of MONP spraying could affect the photosynthesis in these Citrus leaves, two important photosynthetic indices, carotenoid, and chlorophyll, were extracted from leaf tissues to evaluate the protective effect on photosynthesis in HLB-Citrus. Compared to the untreated HLB-Citrus, the carotenoid and chlorophyll levels in MONP-treated HLB-Citrus showed a pronounced upward trend, approaching that of healthy Citrus (Fig. 7E and F). This implies that MONPs enable the enhancement of photosynthesis under CLas-induced abiotic stress in Citrus. Furthermore, the samples of Citrus leaves were sectioned and stained with histochemical safranin O-fast green to analyze their morphology. The spongy parenchyma of HLB-Citrus exhibited obvious hyperplasia compared to the healthy Citrus, whereas the symptoms of MONP-treated Citrus were significantly alleviated (Fig. S7†).
 |
| | Fig. 6 Progression of the HLB phenotype in the MONPs-treated Citrus and the non-treated Citrus. | |
 |
| | Fig. 7 Physiological indexes of the HLB-Citrus, healthy Citrus, and MONPs-treated HLB-Citrus after 22 days treatment. (A–F) Quantification of the H2O2 content, MDA content, starch content, total soluble sugar, carotenoid, and chlorophyll of the HLB-Citrus, healthy Citrus, and MONPs treated HLB-Citrus. (G) The gene expression changes between HLB-Citrus, healthy Citrus, and the MONPs treated HLB-Citrus. (H–J) Analysis of the ROS-related enzyme activities of CAT, POD, and SOD in the HLB-Citrus, MONPs-treated HLB-Citrus, and healthy Citrus, respectively (data are means ± SD, n = 3). | |
After analyzing the main physiological indexes of metabolites of MONP-treated Citrus at 22 days post-spray, we tested the expression of antioxidant defense-related genes and enzyme activities of these Citrus leaves. The changes in mRNA expression levels were analyzed using qPCR, and the results are shown in Fig. 7G. The gene expression levels of Cs6226SOD, Cs6642SOD, Cs2588SOD (superoxide dismutase), Cs6866CAT, Cs5999CAT (catalase) and Cs0069RBOH (respiratory burst oxidase homolog) displayed varying degrees of upregulation, which indicated that the MONP treatment could regulate the gene expression against oxidative stress in HLB-Citrus. Meanwhile, the results of the enzyme activity assay showed that the CAT and SOD activities in MONP-sprayed HLB-Citrus increased compared to those in the non-treated group at 22 days post-spray (Fig. 7H–J). These results confirmed that the MONP treatment efficiently enhances the endogenous antioxidant activity in Citrus against the abiotic stress from CLas-infection. Finally, we used the Deep Sequencing analysis to compare the transcriptomes of the healthy Citrus, non-treated HLB-Citrus, and the MONP sprayed HLB-Citrus at 22 days post-treatment. Additionally, RNA-Seq analysis showed that MONP spraying enables the up-regulation of the ROS scavenging genes such as the ascorbate peroxidase, catalase, glutathione peroxidase, blue copper protein, and ubiquinol oxidase in HLB-Citrus. Additionally, MONP spray could decrease the expression of thioredoxin and diphosphate reductase (Fig. 8). The GO enrichment analysis categorized HLB symptom-related differentially expressed genes (DEGs) into biological processes, cellular components, and molecular functions based on their roles. Genes within organisms perform biological functions through various interactions. The 30 most significantly enriched GO terms were selected and classified according to GO database annotations. In Contrast to the HLB-Citrus group, DEGs were primarily enriched in defense response pathways after MONP treatment (Fig. S8†). These RNA-Seq analysis suggest that MONP treatment could bring about obvious positive gene regulation, thereby alleviating the oxidative stress caused by CLas infection in Citrus tissues. In recent years, the application of nanomaterials in agriculture has received widespread attention, with nanomaterials emerging as a new auxiliary tool to promote sustainable agricultural development.36,52–56 Our work proposes a nanomaterials approach to protect HLB-Citrus from oxidative stress. This strategy is also expected to be practically applied to reduce oxidative stress in crops resulting from heat, drought, etc.
 |
| | Fig. 8 The expression profiling of genes related to ROS pathway between the healthy Citrus, HLB-Citrus, and the MONPs-treated HLB-Citrus. | |
4. Conclusions
In summary, current research demonstrated that MONPs could be efficiently internalized into Citrus leaf tissues for mitigating oxidative damage via the foliar spray and infiltration pathway. MONP treatment could bring about positive effects in a series of physiological and biochemical indexes to alleviate the HLB symptoms. This study has shown versatility, high stability, and an easily batch-prepared strategy for protecting HLB-Citrus plants against oxidative stress through the delivery of MONP. Our study suggests that the MONPs could provide a promising alternative strategy to modulate and mitigate oxidative stress in HLB-Citrus serving the Citrus industry.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
H. H., Y. L., G. D. and S. L. have filed patent applications related to this work. The other authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFD1400803). The authors are grateful to the financial support of the National Natural Science Foundation of China (22277036), China Postdoctoral Science Foundation (2023M743485), and Science and Technology Major Project of Guangxi (Gui Ke AA18118027). We thank the Core Facilities at the College of Life Science and Technology (Huazhong Agricultural University) and CAS Center for Excellence in Molecular Cell Science with the CLSM support.
References
- S. S. Pandey, C. Hendrich, M. O. Andrade and N. Wang, Candidatus Liberibacter: From Movement, Host Responses, to Symptom Development of Citrus Huanglongbing, Phytopathology, 2022, 112, 55–68 CrossRef CAS.
- J. M. Bove, Huanglongbing: A destructive, newly-emerging, century-old disease of citrus, J. Plant Pathol., 2006, 88, 7–37 Search PubMed.
- A. Singerman and M. E. Rogers, The economic challenges of dealing with Citrus Greening: The case of Florida, J. Integr. Pest Manag., 2020, 11, 3–9 CrossRef.
- N. Wang, The Citrus Huanglongbing Crisis and Potential Solutions, Mol. Plant, 2019, 12, 607–609 CrossRef CAS.
- Z. Zheng, J. Chen and X. Deng, Historical Perspectives, Management, and Current Research of Citrus HLB in Guangdong Province of China, Where the Disease has been Endemic for Over a Hundred Years, Phytopathology, 2018, 108, 1224–1236 CrossRef PubMed.
- A. Sechler, E. L. Schuenzel, P. Cooke, S. Donnua, N. Thaveechai, E. Postnikova, A. L. Stone, W. L. Schneider, V. D. Damsteegt and N. W. Schaad, Cultivation of ‘Candidatus Liberibacter asiaticus’, ‘Ca. L. africanus’, and ‘Ca. L. americanus’ associated with Huanglongbing, Phytopathology, 2009, 99, 480–486 CrossRef CAS.
- W. Ma, Z. Pang, X. Huang, J. Xu, S. S. Pandey, J. Li, D. S. Achor, F. N. C. Vasconcelos, C. Hendrich, Y. Huang, W. Wang, D. Lee, D. Stanton and N. Wang, Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin, Nat. Commun., 2022, 13, 529–541 CrossRef CAS PubMed.
- R. Galletti, C. Denoux, S. Gambetta, J. Dewdney, F. M. Ausubel, G. De Lorenzo and S. Ferrari, The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea, Plant Physiol., 2008, 148, 1695–1706 CrossRef CAS.
- N. Wang and P. Trivedi, Citrus Huanglongbing: A newly relevant disease presents unprecedented challenges, Phytopathology, 2013, 103, 652–665 CrossRef PubMed.
- S. Zhang, X. Wang, J. He, S. Zhang, T. Zhao, S. Fu and C. Zhou, A Sec-dependent effector, CLIBASIA_04425, contributes to virulence in ‘Candidatus Liberibater asiaticus’, Front. Plant Sci., 2023, 14, 1224736–1224745 CrossRef.
- J. Zhang, F. Shao, H. Cui, L. Chen, H. Li, Y. Zou, C. Long, L. Lan, J. Chai, S. Chen, X. Tang and J.-M. Zhou, A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-Induced immunity in plants, Cell Host Microbe, 2007, 1, 175–185 CrossRef CAS.
- C. Dunand, M. Crèvecoeur and C. Penel, Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development:: possible interaction with peroxidases, New Phytol., 2007, 174, 332–341 CrossRef CAS PubMed.
- Y. Jing, N. Shen, X. Zheng, A. Fu, F. Zhao, W. Lan and S. Luan, Danger-associated peptide regulates root immune responses and root growth by affecting ROS formation in arabidopsis, Int. J. Mol. Sci., 2020, 21, 4590–4606 CrossRef CAS.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Intrinsic peroxidase-like activity of ferromagnetic nanoparticles, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- H. Wei and E. Wang, Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- X. F. Zhu, Y. N. Liu, G. L. Yuan, X. Guo, J. Q. Cen, Y. C. Gong, J. Liu and Y. Gang, In situ fabrication of MS@MnO2 hybrid as nanozymes for enhancing ROS-mediated breast cancer therapy, Nanoscale, 2020, 12, 22317–22329 RSC.
- Y. Cheng, C. Q. Cheng, J. Yao, Y. J. Yu, Y. F. Liu, H. Zhang, L. Y. Miao and H. Wei, Mn3O4 nanozyme for inflammatory bowel disease therapy, Adv. Ther., 2021, 4, 2100081–2100089 CrossRef CAS.
- D. Singh, D. Sillu, A. Kumar and S. Agnihotri, Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm, Environ. Sci.: Nano, 2021, 8, 1308–1325 RSC.
- H. Wu, N. Tito and J. P. Giraldo, Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species, ACS Nano, 2017, 11, 11283–11297 CrossRef CAS.
- S. Arora, G. Murmu, K. Mukherjee, S. Saha and D. Maity, A comprehensive overview of nanotechnology in sustainable agriculture, J. Biotechnol., 2022, 355, 21–41 CrossRef CAS.
- K. S. Siddiqi and A. Husen, Plant Response to Engineered Metal Oxide Nanoparticles, Nanoscale Res. Lett., 2017, 12, 92 CrossRef.
- K. Ahmadian, J. Jalilian and A. Pirzad, Nano-fertilizers improved drought tolerance in wheat under deficit irrigation, Agric. Water Manag., 2021, 244, 106544–106556 CrossRef.
- P. Vatcharakajon, A. Sornsaket, K. Choengpanya, C. Susawaengsup, J. Sornsakdanuphap, N. Boonplod, P. Bhuyar and R. Dangtungee, Silver nano chito oligomer hybrid solution for the treatment of Citrus Greening Disease (CGD) and biostimulants in citrus horticulture, Horticulturae, 2023, 9, 725–736 CrossRef.
- R. Millaleo, M. Reyes-Diaz, A. G. Ivanov, M. L. Mora and M. Alberdi, Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms, J. Soil Sci. Plant Nutr., 2010, 10, 470–481 CrossRef.
- H. Wu and Z. Li, Nano-enabled agriculture: How do nanoparticles cross barriers in plants?, Plant Commun., 2022, 3, 100346 CrossRef CAS.
- J. Burkhardt, S. Basi, S. Pariyar and M. Hunsche, Stomatal penetration by aqueous solutions - an update involving leaf surface particles, New Phytol., 2012, 196, 774–787 CrossRef CAS PubMed.
- K. Zhang, Y. Long, Z. Ma, S. Li, Y. Zhao and H. Han, Artificial nanoplatelet regulation of tumor immune microenvironment to inhibit post-surgical tumor recurrence and lung metastasis, Mater. Today, 2023, 67, 68–83 CrossRef CAS.
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu, Y. Tang, X. Xu, M. Liang, B. Jiang, X. Yan and L. Gao, In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy, Nat. Commun., 2018, 9, 1440–1450 CrossRef.
- M. H. Hadwan and H. N. Abed, Data supporting the spectrophotometric method for the estimation of catalase activity, Data Brief, 2016, 6, 194–199 CrossRef PubMed.
- L. Góth, A simple method for determination of serum catalase activity and revision of reference range, Clin. Chim. Acta, 1991, 196, 143–151 CrossRef PubMed.
- J. Ma, Z. Song, J. Yang, Y. Wang and H. Han, Cobalt ferrite nanozyme for efficient symbiotic nitrogen fixation via regulating reactive oxygen metabolism, Environ. Sci.: Nano, 2021, 8, 188–203 RSC.
- Y. Y. Shurui Zhao, M. Zheng and B. Xue, Comparison of detection methods of hydroxyl radicals based on Fenton reaction, Shiyan Jishu Yu Guanli, 2020, 37, 67–71 Search PubMed.
- L. Wang, L. Ding, Y. Wang, Y. Zhang and J. Liu, Isolation and characterisation of in vitro and cellular free radical scavenging peptides from corn peptide fractions, Molecules, 2015, 20, 3221–3237 CrossRef.
- X. Zhong, G. Su, Q. Zeng, G. Li, H. Xu, H. Wu, H. Zhou and X. Zhou, Preparation of salicylic acid-functionalized nanopesticides and their applications in enhancing salt stress resistance, ACS Appl. Mater. Interfaces, 2023, 15, 43282–43293 CrossRef CAS PubMed.
- J. Geng and J.-H. Liu, The transcription factor CsbHLH18 of sweet orange functions in modulation of cold tolerance and homeostasis of reactive oxygen species by regulating the antioxidant gene, J. Exp. Bot., 2018, 69, 2677–2692 CrossRef CAS PubMed.
- L. Chen, Y. Peng, L. Zhu, Y. Huang, Z. Bie and H. Wu, CeO2 nanoparticles improved cucumber salt tolerance is associated with its induced early stimulation on antioxidant system, Chemosphere, 2022, 134474–134486, DOI:10.1016/j.chemosphere.2022.134474.
- Y. Li, J. Liu, C. Fu, M. N. Khan, J. Hu, F. Zhao, H. Wu and Z. Li, CeO2 nanoparticles modulate Cu–Zn superoxide dismutase and lipoxygenase-IV isozyme activities to alleviate membrane oxidative damage to improve rapeseed salt tolerance, Environ. Sci.: Nano, 2022, 1116–1132, 10.1039/d1en00845e.
- H. Gao, J. Niu, X. Yang, D. He and C. Wang, Impacts of powdery mildew on wheat grain sugar metabolism and starch accumulation in developing grains, Starch, 2014, 66, 947–958 CrossRef CAS.
- J. Wang, F. Wang, G. Zhang, Y. Xin, H. Zhang and H. Liu, Comparison of five methods for starch measurement in Tobaccos, Fenzi Zhiwu Yuzhong, 2019, 17, 1673–1678 Search PubMed.
- T. Gao, H. Fjellvåg and P. Norby, A comparison study on Raman scattering properties of α- and β-MnO2, Anal. Chim. Acta, 2009, 648, 235–239 CrossRef CAS PubMed.
- P. Murovhi, D. J. Tarimo, K. O. Oyedotun and N. Manyala, High specific energy asymmetric supercapacitor based on alpha-manganese dioxide/activated expanded graphite composite and activated carbon-polyvinyl alcohol, J. Energy Storage, 2020, 32, 101797–101807 CrossRef.
- L. Liu, W. Song, S. Huang, K. Jiang, Y. Moriwaki, Y. Wang, Y. Men, D. Zhang, X. Wen, Z. Han, J. Chai and H. Guo, Extracellular pH sensing by plant cell-surface peptide-receptor complexes, Cell, 2022, 185, 3341–3355 CrossRef CAS.
- J. Hu, J. Jiang and N. Wang, Control of Citrus Huanglongbing via trunk injection of plant defense activators and antibiotics, Phytopathology, 2018, 108, 186–195 CrossRef CAS.
- J. Hu and J. Hu, Evaluation of the Spatiotemporal Dynamics of Oxytetracycline and Its Control Effect against Citrus Huanglongbing via Trunk Injection, Phytopathology, 2016, 106, 62 Search PubMed.
- M. Morales and S. Munne-Bosch, Malondialdehyde: Facts and Artifacts, Plant Physiol., 2019, 180, 1246–1250 CrossRef CAS.
- N. Nicot, J. F. Hausman, L. Hoffmann and D. Evers, Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress, J. Exp. Bot., 2005, 56, 2907–2914 CrossRef CAS.
- H. Yoshioka, N. Numata, K. Nakajima, S. Katou, K. Kawakita, O. Rowland, J. D. Jones and N. Doke,
Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans, Plant Cell, 2003, 15, 706–718 CrossRef CAS PubMed.
- X. Luo, Z. Wang, C. Wang, L. Yue, M. Tao, W. H. Elmer, J. C. White, X. Cao and B. Xing, Nanomaterial Size and Surface Modification Mediate Disease Resistance Activation in Cucumber (Cucumis sativus), ACS Nano, 2023, 17, 4871–4885 CrossRef CAS.
- M. A. Torres, J. D. G. Jones and J. L. Dangl, Reactive oxygen species signaling in response to pathogens, Plant Physiol., 2006, 141, 373–378 CrossRef CAS PubMed.
- S. Jagoueix, J. M. Bove and M. Garnier, The Phloem-Limited Bacterium Of Greening Disease Of Citrus Is A Member Of The Alpha-Subdivision Of The Proteobacteria, Int. J. Syst. Bacteriol., 1994, 44, 379–386 CrossRef CAS.
- F. J. Sánchez, M. A. Manzanares, E. F. de Andres, J. L. Tenorio and L. Ayerbe, Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress, Field Crops Res., 1998, 59, 225–235 CrossRef.
- C. Su, A. Chen, W. Liang, W. Xie, X. Xu, X. Zhan, W. Zhang and C. Peng, Copper-based nanomaterials: Opportunities for sustainable agriculture, Sci. Total Environ., 2024, 926, 171948–171955 CrossRef CAS PubMed.
- J. W. Gong, Q. Liu, L. L. Cai, Q. Yang, Y. P. Tong, X. Chen, S. Kotha, X. B. Mao and W. W. He, Multimechanism collaborative superior antioxidant CDzymes to alleviate salt stress-induced oxidative damage in plant growth, ACS Sustainable Chem. Eng., 2023, 11, 4237–4247 CrossRef CAS.
- G. Zhao, Y. Zhao, W. Lou, J. Su, S. Wei, X. Yang, R. Wang, R. Guan, H. Pu and W. Shen, Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity, Nanoscale, 2019, 11, 10511–10523 RSC.
- P. Qiu, J. Li, L. Zhang, K. Chen, J. Shao, B. Zheng, H. Yuan, J. Qi, L. Yue, Q. Hu, Y. Ming, S. Liu, L. Long, J. Gu, X. Zhang, K. Lindsey, W. Gao, H. Wu and L. Zhu, Polyethyleneimine-coated MXene quantum dots improve cotton tolerance to Verticillium dahliae by maintaining ROS homeostasis, Nat. Commun., 2023, 14, 7392–7403 CrossRef.
- P. Vatcharakajon, A. Sornsaket, K. Choengpanya, C. Susawaengsup, J. Sornsakdanuphap, N. Boonplod, P. Bhuyar and R. Dangtungee, Silver nano chito oligomer hybrid solution for the treatment of Citrus Greening Disease (CGD) and biostimulants in citrus horticulture, Horticulturae, 2023, 9, 725–736 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1, Fig. S1–S6. Materials and methods, including figures and data. See DOI: https://doi.org/10.1039/d4en00519h |
| ‡ These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  ac,
Yuying
Long‡
b,
Guiyun
Deng
a,
Yinghui
Men
d,
Feifan
Lu
b,
Zihan
Wang
c,
Jiaying
Li
a and
Heyou
Han
ac,
Yuying
Long‡
b,
Guiyun
Deng
a,
Yinghui
Men
d,
Feifan
Lu
b,
Zihan
Wang
c,
Jiaying
Li
a and
Heyou
Han
 *ab
*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm was performed for 5 min, and the absorbance of the samples was measured at 450 nm, 532 nm, and 600 nm using an enzyme marker. The MDA concentration in the extraction solution was calculated by the formula:
000 rpm was performed for 5 min, and the absorbance of the samples was measured at 450 nm, 532 nm, and 600 nm using an enzyme marker. The MDA concentration in the extraction solution was calculated by the formula:







