Open Access Article
Open Access ArticleFollowing the smell: terpene emission profiles through the cannabis life-cycle†
Davi
de Ferreyro Monticelli
 a,
Cynthia
Pham
b,
Sahil
Bhandari
a,
Cynthia
Pham
b,
Sahil
Bhandari
 bc,
Amanda
Giang
bc,
Amanda
Giang
 bc,
Nadine
Borduas-Dedekind
bc,
Nadine
Borduas-Dedekind
 d and
Naomi
Zimmerman
d and
Naomi
Zimmerman
 *ab
*ab
aDepartment of Earth, Ocean and Atmospheric Sciences, University of British Columbia, Vancouver, Canada. E-mail: nzimmerman@mech.ubc.ca
bDepartment of Mechanical Engineering, University of British Columbia, Vancouver, Canada
cInstitute for Resources, Environment and Sustainability, University of British Columbia, Vancouver, Canada
dDepartment of Chemistry, University of British Columbia, Vancouver, Canada
First published on 11th June 2025
Abstract
Cannabis cultivation and processing are emerging sources of air pollutants, particularly malodorous volatile organic compounds (VOCs), yet uncertainties remain regarding their emission rates and chemical composition. Emission rates are typically the starting point for an air quality assessment; not addressing their uncertainty and chemical profile may lead to under/over estimation of impacts. This study aims to quantify terpene emissions from indoor cannabis operations in the Lower Fraser Valley, BC, Canada a region already affected by odorous sources and peak ozone concentrations in the summer due to imbalance of VOC and nitrogen oxides (NOx) emissions. We assessed terpene concentration variability across activities, and evaluated their potential odor impacts during peak summertime. For this, we developed an automated gas chromatography sampling and processing protocol to measure concentrations of 22 key cannabis terpenes in (1) eight rooms of an indoor cultivation facility and (2) six rooms of a processing and extraction facility. Emission rates varied widely, ranging from 1.05 × 10−3 to 3.09 × 10−1 kg h−1, with the highest emissions occurring during trimming (i.e., buds' extraction). We observed substantial temporal variability; individual terpene concentrations fluctuated by up to 1500% depending on activity type and lighting conditions. Pearson correlation analysis revealed non-linear relationships between individual terpenes and total emissions, suggesting shifts in chemical composition during peak emissions. To assess odor implications, we conducted screening dispersion modeling for β-myrcene, a terpene considered a tracer of cannabis emissions. Of the 7560 dispersion scenarios evaluated, 88 exceeded the odor threshold under average emissions, increasing to 241 scenarios during peak trimming emissions. Because emission rates and chemical compositions vary significantly depending on activity type and conditions, and dispersion modeling results showed that average conditions are sufficient to cause odor episodes, it is important to characterize both the temporal and chemical profiles of terpene emissions in cannabis facilities to avoid mis-estimating their air pollution and odor impact. Given the growing industry and the potential for odor complaints and secondary air pollution impacts (e.g., ozone formation), it is crucial to understand these emissions in detail. Policymakers, scientists, and industry stakeholders can use our findings to develop better mitigation strategies and inform environmental regulations.
Environmental significanceWith the recent cannabis legalization and decriminalization in the world, cannabis cultivation and processing facilities surged. Understanding their environmental impact is even more critical than before, including the variation in Biogenic Volatile Organic Compound (BVOC) emissions during the plant life-cycle. These emissions contribute to community complaints due to malodors, and potentially the formation of pollutants like ozone and secondary organic aerosols. This study shows that individual terpene emissions, driven by room conditions and activities, vary non-linearly with total BVOC emissions, challenging the assumption of a proportional chemical profile between emission baseline and emission peak. By introducing time-resolved monitoring methodology, this work can inform emission inventories and dispersion modeling, offering insights applicable to managing malodor impacts and air quality for the cannabis industry. |
1 Introduction
As new jurisdictions have legalized the use and commercialization of cannabis for medical and recreational purposes,1 there has been a surge of interest among researchers and regulators about the potential occupational and environmental impacts of the increased number and size of cannabis production facilities.2 Those include greenhouse gases emissions,3 land cover change and resources use,4 depletion of natural systems,5 and air quality.6Most studies have focused on investigating Biogenic Volatile Organic Compound (BVOC) air emissions from cannabis cultivation7–9 and found that the composition can vary by cannabis strain, life-cycle stage, and between indoor and outdoor environments. The main terpenes found to be emitted by plants are β-myrcene, (+/–)-limonene, terpinolene, α-pinene and β-pinene. Of these, β-myrcene has been identified as a potential tracer due to its higher fraction of the total BVOC emissions from cannabis plant, and its relative absence in ambient air and indoor air of homes.10,11 To a lesser extent, cannabis processing has also been investigated12,13 and it was found that activities that involve manipulation (e.g., trimming and packing) have higher emissions of terpenes and particulate matter. A more detailed overview of these findings can be found at de Ferreyro Monticelli et al.14
The interest in BVOCs is partly motivated by their potential to form secondary organic aerosols (SOA) and ozone (O3),15 which contribute to degraded air quality. While cannabis plants emit more BVOCs per dry weight of plant than many crops,14 modeling studies suggest that the overall low VOC emission rates from the industry relative to other sectors16 contribute to a low impact on O3 and PM2.5 formation.7,8,16
Nevertheless, cannabis emissions can be odorous and can cause annoyance to communities living nearby facilities.17 Although BVOCs are not the only chemicals responsible for the cannabis smell,18–20 they contribute to the mix of odorous emissions.21 Because odor impact is a major cause of complaints towards the cannabis industry,22 it is important to understand the total amount and the chemical content of BVOC emissions to better predict odors downwind of cannabis facilities. Environmental impact assessments typically assume that the emissions from cannabis facilities are constant.23–25 Yet, recent investigations suggest temporal variability in total BVOC emissions.13,16
In a previous review of the cannabis industry's air quality impacts, de Ferreyro Monticelli et al.14 identified sixteen gaps that must be addressed to better understand the environmental impact of cannabis cultivation and processing. Of these include improving sampling methods to enable building more detailed emission inventories. Thus, the goals of this study were to (1) develop an automated GC-FID sampling protocol and (2) apply it in indoor rooms of a cannabis cultivation and a cannabis processing facility, and (3) investigate the temporal concentration profile and emissions of 22 terpenes. We sought to determine: (1) if the speciated-temporal profile varies significantly within a day, (2) what environmental and work factors contribute to any identified variation and (3) if the variation should be accounted for in emission inventories and community exposure investigation studies.
2 Methods
2.1 Study location and relevance
Our study is situated in the Lower Fraser Valley (LFV), BC, Canada, which comprises the districts of Metro Vancouver and the southwestern portion of the Fraser Valley Regional District. Home to ≈3.3 million people,26 the LFV faces a range of air quality issues due to its emissions profile, complex terrain, and meteorology.27–29 Previous research in the region focused on peak tropospheric ozone in the spring/summer (O3),30–38 particulate matter (PM) and secondary organic aerosols (SOA) formation and transport,39–46 and BVOC and anthropogenic VOCs emissions.47–52To a lesser extent, odors have been evaluated by recent studies using inverse dispersion modeling,53 and mobile monitoring and citizen science.54,55 Odors are now the main cause of air quality complaints in the region, and the contribution of the cannabis sector to odor reports has been noticeable.56 For instance, in the municipality of Delta, cannabis-related odors account for 73% of submissions made to the Smell Vancouver initiative during the first year of operation.55
In this context, it is imperative to investigate emissions from cannabis operations in the LFV, as they may increasingly contribute to odor-related concerns and exacerbate air quality challenges in a region already burdened by complex emission sources and atmospheric dynamics.
2.2 Facility recruitment
We recruited facilities by advertising the study at industry conferences (e.g., GROW EXPO 2022), by phone calls, by e-mails, and by referral from project partners (WorkSafe BC). Prospective participating facilities were asked to complete a questionnaire that addressed cultivation practices, facility management, and air emissions control technologies (Fig. S1–S13 of the ESI†). This protocol was approved by the University of British Columbia Office of Research Ethics, ID: H22-00450. We selected two facilities (named CCF for Cannabis Cultivation Facility and CPF for Cannabis Processing Facility) for this study, based on their market influence, type of operation, and their capacity to participate in logistical planning for sampling.2.3 Facility details
The CCF is an approximately 4400 m2 indoor cannabis cultivation facility licensed for cultivation for medical purposes, with a capacity for 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 kg per
500 kg per ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yr of cannabis plants in wet weight, making it a “standard cultivation” in Canada (>800 m2 grow surface area57). We sampled the following cultivation and processing operations/rooms: mother (female plants prolonged in vegetative state), vegetative (3.5weeks ≤ t ≤ 6 weeks old), growing (>6 weeks, we sampled shortly after propagation and when plants were mature), drying (where plants lose most of their water content, we sampled before and after plant introduction), trimming (in this facility performed by machine), packing, and storage. We were not able to sample the harvesting activity because we were not allowed to have our instruments present during the process and during milling because the process had to be rescheduled (Fig. 1).
yr of cannabis plants in wet weight, making it a “standard cultivation” in Canada (>800 m2 grow surface area57). We sampled the following cultivation and processing operations/rooms: mother (female plants prolonged in vegetative state), vegetative (3.5weeks ≤ t ≤ 6 weeks old), growing (>6 weeks, we sampled shortly after propagation and when plants were mature), drying (where plants lose most of their water content, we sampled before and after plant introduction), trimming (in this facility performed by machine), packing, and storage. We were not able to sample the harvesting activity because we were not allowed to have our instruments present during the process and during milling because the process had to be rescheduled (Fig. 1).
The CPF is an approximately 1400 m2 cannabis processing and extraction facility with average annual shipment volumes of 1500 kg of cannabis products (defined as “standard processing”, may possess >2400 kg of dried cannabis each calendar year57). Products include pre-rolls, dried flower, vapes, concentrates and other extraction products. We were able to sample in the pre-roll room (performed by a machine in this facility), packing room, ethanol extraction room (where tetrahydrocannabinolic acid, THCA, and cannabidiolic acid, CDBA, are separated from plant material), distillation room (where crude oil is further refined to isolate the desired cannabinoids), formulation room (where the product flavor is boosted), and hydro extraction room (also known as purging in vacuum ovens) process rooms. We were not able to sample the grinding, winterization (subjecting crude extract mixed with alcohol to freezing temperatures), filtration, solvent recovery (extracting target products from waste or by-product solvents), and storage rooms either due to space availability, or because the room was not in use at time of measurement (Fig. 1).
A detailed description of rooms and operational conditions in each facility, including HVAC schedule, lighting, floor area, space volume, number of plants and/or processing dry weight is available in the Tables S1–S5 of the ESI†.
2.4 Instrument operation
Terpenes were measured via Gas Chromatography coupled with Flame Ionization Detection (GC-FID), using the GC-FID 8610C and Peak Simple software (v4.90) by SRI Instruments. The column used for terpene separation was a 30 m × 0.53 mm I.D. 1.0U MXT-502.2 capillary column (diphenyl/dimethyl polysiloxane phase, range: −20 °C to 320 °C), which is a low-polarity stationary phase and a good match for hydrocarbons such as terpenes.58 The temperature program consisted of: hold at 40 °C for 1 min, then ramp at 10 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min−1 until reaching 280 °C, similar to the one used by Wang et al.8,10 and finally hold at 280 °C for 5 min in order to bake-out any leftovers (30 min total). The only event triggered at the start of the program was zeroing the FID signal (usually 15 mV to 35 mV before each run). The helium carrier gas (He, 99.9999%, Linde Canada Inc.) was set at 11 psi (20 ml min−1) and the hydrogen (H2, 99.9999%, Linde Canada Inc.) used to light the FID flame was set at 20 psi (25 ml min−1). A further Thermo Scientific™Click-On™Inline Gas Filter was used to prevent moisture and oxygen intrusion into the carrier gas. The air necessary for the FID flame was set at 5 psi (250 ml min−1). The flame ignite setting was −752 mV and the temperature of the FID detector in high gain was set at 300 °C to avoid water and eluted peak condensation.59
min−1 until reaching 280 °C, similar to the one used by Wang et al.8,10 and finally hold at 280 °C for 5 min in order to bake-out any leftovers (30 min total). The only event triggered at the start of the program was zeroing the FID signal (usually 15 mV to 35 mV before each run). The helium carrier gas (He, 99.9999%, Linde Canada Inc.) was set at 11 psi (20 ml min−1) and the hydrogen (H2, 99.9999%, Linde Canada Inc.) used to light the FID flame was set at 20 psi (25 ml min−1). A further Thermo Scientific™Click-On™Inline Gas Filter was used to prevent moisture and oxygen intrusion into the carrier gas. The air necessary for the FID flame was set at 5 psi (250 ml min−1). The flame ignite setting was −752 mV and the temperature of the FID detector in high gain was set at 300 °C to avoid water and eluted peak condensation.59
2.5 Calibration
Calibration occurred prior to sampling using two standards solutions from RESTEK, which together comprise 22 terpenes (Table 1). From each original standard (2500 μg ml−1), five diluted solutions were prepared, resulting in calibration concentrations of 1000 μg ml−1, 100 μg ml−1, 10 μg ml−1, 1 μg ml−1, 0.1 μg ml−1. These concentrations were used to build the calibration curve so that we could characterize environments with low terpene concentrations (e.g., vegetative room) and high concentrations (e.g., trimming)14 without the need to recalibrate or use different settings.| Terpenes | Formula | Limit of detection (LoD)a | Retentionb (min) |
|---|---|---|---|
| a Area under the peak converted to μg ml−1. b Average of five samples. | |||
| Cannabis terpenes standard #1, 2500 μg mL −1 , isopropanol, 1 mL per ampul | |||
| α-Pinene | C10H16 | 1.76 | 6.07 |
| Camphene | C10H16 | 1.93 | 6.47 |
| β-Pinene | C10H16 | 2.30 | 7.09 |
| β-Myrcene | C10H16 | 2.58 | 7.33 |
| δ-3-Carene | C10H16 | 2.54 | 7.69 |
| α-Terpinene | C10H16 | 2.66 | 7.87 |
| (+/–)-Limonene | C10H16 | 3.67 | 8.09 |
| p-Cymene | C10H14 | 3.78 | 8.23 |
| Ocimene | C10H16 | 3.02 | 8.48 |
| γ-Terpinene | C10H16 | 3.56 | 8.74 |
| Terpinolene | C10H16 | 4.84 | 9.31 |
| Linalool | C10H18O | 1.94 | 9.60 |
| (–)-Isopulegol | C10H18O | 2.19 | 10.56 |
| Geraniol | C10H18O | 1.57 | 12.10 |
| β-Caryophyllene | C15H24 | 3.32 | 14.75 |
| α-Humulene | C15H24 | 3.01 | 15.30 |
| cis-Nerolidol | C15H26O | 4.06 | 16.34 |
| trans-Nerolidol | C15H26O | 4.96 | 16.78 |
| (–)-Guaiol | C15H26O | 3.60 | 17.47 |
| (–)-α-Bisabolol | C15H26O | 3.38 | 18.58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Cannabis terpenes standard #2, 2500 μg mL −1 , isopropanol, 1 mL per ampul | |||
| 1,8-Cineole | C10H18O | 1.48 | 8.56 |
| (–)-Caryophyllene oxide | C15H24O | 1.56 | 17.78 |
Each injection of 1 μl was made by depositing the sample in the column to avoid boiling point discrimination. The 10 μl syringe used was then washed in acetone three times, dried, and washed one time in isopropanol prior to each loading. Polynomial fit calibration curves had R2 values between 0.879 —0.999 (Fig. S14†). Blanks (i.e., only carrier gas) were run in between each dilution injection showing no contamination (Fig. S16†). We noticed that for the last two solutions of 1 μg ml−1 and 0.1 μg ml−1 the terpene FID signal was undistinguishable from noise. Thus, using repeated samplings of the 10 μg ml−1 and the equations described in Armbruster and Pry60 we estimated the Limit of Detection (LoD) for each terpene (Table 1).
2.6 Sampling protocol
For the operation of the GC-FID we used a combination of procedures.61–63 First, the MTX 502.2 column was baked at 300 °C for six hours one week prior to sampling in each facility. We also cleaned the instrument in between rooms using lint free wipes (Kimwipes®) and isopropanol (70%) to avoid transferring terpenes between rooms and other sources of contamination.The indoor air sampling routine consisted of moving the GC-FID to the selected room, installing the gas connections, and applying the Quality Assurance (QA) and Quality Control (QC) procedures. Those consisted of performing H2 and He leak checks before every sampling. Additionally, we turned on the carrier filter bake-out switch twice, and treated the column at 150 °C for 10 min while baking occurred. Next, the calibration program was loaded, and the oven was returned to 40 °C. Then, up to four column blanks (i.e., only carrier gas) were performed prior to sampling start.
The GC-FID generally sampled between 10 am and 10 am of the following day to capture the workday hours and the overnight profile (i.e., no person or activity performed). Some rooms had restricted operation hours that had to be followed. For instance, grow rooms needed all lights off, including those of our instruments, between 11 am and 11 pm, meaning that any instrument handling had to be performed outside those hours and the screen of the computer and other equipment had to be covered in between, so as to not disturb the plants' night-time metabolism.
Due to the unique challenge of sampling continuously in an uncontrolled environment with restricted access, the protocol adopted was a mix of established procedures such as U.S. EPA Methods TO-15 and TO-17,64,65 NIOSH 1552 (ref. 66) and recent GC-FID autonomous approaches.67
The protocol started with terpenes sampled through PFA (Perfluoroalkoxy) 1/8′′ tubing68,69 of length ≈ 150 cm at a flow rate of 120 ml min−1 and trapped in a Tenax®TA cartridge70–72 for either one min or 11 min. The trapping time reflected the expected concentration of the room. Essentially, in rooms where the concentrations were expected to be high (e.g., drying and trimming), the one min protocol was adopted to avoid saturating the column. The entry port was set at 155 °C to avoid water condensation, and the FID detector was set at 300 °C.
One minute before the trapping ended, the Tenax®TA trap began heating from 35 °C to 200 °C. After trapping concluded, the valve switched from the load to inject position, releasing terpenes into the column. The calibration temperature cycle began immediately after the valve switch, starting at 40 °C. Once the cycle was complete, the valve returned to the load position, and the trap was baked by heating from 200 °C to 250 °C over two minutes, while the vacuum pump removed any residual contents.
After baking, the system cooled to 40 °C, the FID signal was zeroed, and the cycle repeated without sampling the indoor air, ensuring a field blank (i.e., carrier gas) is associated with each sample. The PeakSimple v4.90 software automated these steps, initiating a new run every 15 minutes. Fig. S18† provides an overview of the protocol.
2.7 Post processing and analysis
A series of Python scripts were developed to semi-automate the analysis of the 277 sampling chromatograms we collected. They function by (a) assisting the creation of a log file, (b) adjusting peaks based on fluctuations in the retention time, (c) aggregating multiple samples per room into one spreadsheet (d) exporting multiple chromatograms as .pdfs. Additionally, further Python scripts were developed to generate times series, box-plots, and heat maps of samples. All scripts can be downloaded from Scripts. Chromatograms for each sample are available in Portable Document Format (.pdf) in this repository: Samples. We converted the area under the peak to concentration using eqn (1).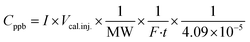 | (1) |
2.8 Emissions estimation
The CPF had a noticeable cannabis smell within its fenceline, and the CCF is surrounded by vegetation and farms, thus we assumed a conservative outdoor BVOC concentration of 1% of the facility's mean value ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) BVOCfacility, which is in-line with other studies7 and terpene odor thresholds.18
BVOCfacility, which is in-line with other studies7 and terpene odor thresholds.18
Froomin = 0.01![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) BVOCfacility × AERroom × Vroom BVOCfacility × AERroom × Vroom | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h−1; AERroom is the air exchange rate, in h−1, and Vroom is the room volume, in m3;
h−1; AERroom is the air exchange rate, in h−1, and Vroom is the room volume, in m3;
The information about the type of HVAC system, including schedule and extraction capacity, were provided by both facilities (Tables S1–S5†). We were not able to measure the actual Air Exchange Rates (AER) of the rooms, but the values calculated (10–40 h−1) using each indoor environment dimensions (Vroom) and HVAC capacity (Qext) match facility averages in the literature.3
| AERroom = Qext × 1/Vroom | (3) |
Loss due to dry deposition (L) was assumed to occur only at indoor environment walls and floor, for simplicity. Thevenet et al.73 quantified the deposition of (+/–)-limonene onto several different surfaces for concentration ranges of 50 ppb to 900 ppb at 23 °C and RH 50% ± 2% - similar conditions as those within the CCF and CPF rooms. Although a partitioning coefficient of ≈K = 0.08–0.19m was found for painted surfaces, about 50% was reversible (i.e., emitted back). Using the room total area, terpene concentration, K = 0.135m and reversibility of 50%, the total removal by dry deposition was estimated to range from 5–11% depending on the room. This value was then accounted for when estimating the removal by chemical reaction (R).
| Lroom = CBVOCroom × Aroom × K × f | (4) |
We also considered terpene losses due to chemical reactions with O3 and OH. We used direct measurements of O3, nitrogen monoxide (NO), and nitrogen dioxide (NO2) from a low-cost sensor co-deployed with the GC-FID (Section S1.7†) and indirect OH determination using eqn (6). Both facilities have no exposure to sunlight (i.e., all light sources are from indoor lamps). We also measured the light spectrum in each room using the OCEAN FLAME Mini-Spectrometer (Section S1.7†) and observed no meaningful contribution in the 300 nm to 400 nm wavelengths that could result in O3 or nitrous acid (HONO) photolysis and OH formation. Thus, we assume that the OH found indoors would be a function of outdoor OH and the balance between OH produced by terpenes reacting with O3 and consumption by OH reacting with NO2 and NO. The rate constants and OH yields used are found in the74 and Section S1.8.†
 | (5) |
 is the ozone concentration, in kg m−3; and
is the ozone concentration, in kg m−3; and  is the BVOC concentration after accounting for the dry deposition, in kg m−3.
is the BVOC concentration after accounting for the dry deposition, in kg m−3.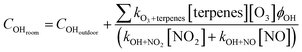 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s−1);75 and [NO] is the rooms' concentration of NO (molecules cm−1). COHdoor is the outdoor OH concentration, assumed to be 2.6 × 105 molecules cm−3 at nighttime (8 pm to 5 am) and 1.9 × 106 molecules cm−3 at daytime (5 am to 8 pm).76 We decided to use Emmerson and Carslaw76 results because they refer to measurements done in a hot summer at a location away (≥40 km) from a major urban center, relatively near (≤4 km) of a major road, in a site surrounded by agricultural land, thus matching in many aspects our facilities location. Furthermore, the values correspond to the concentration range reported in other field investigations made in British Columbia77 and modeling studies for Canada.78
s−1);75 and [NO] is the rooms' concentration of NO (molecules cm−1). COHdoor is the outdoor OH concentration, assumed to be 2.6 × 105 molecules cm−3 at nighttime (8 pm to 5 am) and 1.9 × 106 molecules cm−3 at daytime (5 am to 8 pm).76 We decided to use Emmerson and Carslaw76 results because they refer to measurements done in a hot summer at a location away (≥40 km) from a major urban center, relatively near (≤4 km) of a major road, in a site surrounded by agricultural land, thus matching in many aspects our facilities location. Furthermore, the values correspond to the concentration range reported in other field investigations made in British Columbia77 and modeling studies for Canada.78
Only the CCF had a terpene removal control strategy in place inside the rooms (charcoal filters), rather than in the ventilation path. We assume that the concentration measured corresponds to the equilibrium concentration between plants emission, room ventilation, and control technology, thus the value for removal by treatment (Croom) can be also set to zero.
| Froomout − Froomin = ERroom − Lroom − Croom − Room | (7) |
| ERroom = Froomout − Froomin + Rroom + Lroom(Croom = 0) | (8) |
 | (9) |
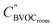 the concentration after dry deposition and chemistry removal in kg m−3.
the concentration after dry deposition and chemistry removal in kg m−3.
 | (10) |
 | (11) |
2.9 Screening dispersion modeling
We conducted screening dispersion modeling to investigate if the short-lived spikes observed in terpene emissions would lead ambient concentrations exceeding odor thresholds and thus detection by nearby communities. We used as input for the dispersion model the cumulative emission rates across rooms for the minimum, average, and maximum emission of β-myrcene, a compound routinely used as a marker of cannabis operations10,11 and which has one of the lowest terpene odor thresholds (13 ppb),18 and (+/–)-Limonene for comparison, since it was the key terpene in cannabis processing.In this screening analysis, we assume that all rooms contribute to the facility output and there are no controls (i.e., losses) between the ventilation that connects the rooms and the stack exit. We refer to this as a screening dispersion model because conducting a full dispersion analysis is outside the scope of this study and many more variables, such as building dimensions, terrain, land use, and local meteorology, would be needed. Instead, we use eqn (12) considering moderately stable (Pasquill–Gifford class F), neutral (Pasquill–Gifford class D), and moderately unstable (Pasquill–Gifford class B) atmospheric conditions.79 The full suite of input variables are provided in the Section S1.9 of the ESI† and are either assumed (e.g., 1.5 m for the receptor height and 5 m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s−1 for stack gas exit velocity) or based on the facilities informed heights (4 m and 16 m) and typical wind speeds at the location obtained from the closest meteorological station (2–20 m
s−1 for stack gas exit velocity) or based on the facilities informed heights (4 m and 16 m) and typical wind speeds at the location obtained from the closest meteorological station (2–20 m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s−1). A sensitivity analysis varying the input variables was also conducted, and in total 7560 dispersion scenarios were investigated. After generating the dispersion model, we compare the concentrations in each scenario at several receptor distances to the β-myrcene and (+/–)-Limonene odor thresholds, 13 ppb (or 72.84 μg m−3) and 38 ppb (or 212.91 μg m−3), respectively.18,80
s−1). A sensitivity analysis varying the input variables was also conducted, and in total 7560 dispersion scenarios were investigated. After generating the dispersion model, we compare the concentrations in each scenario at several receptor distances to the β-myrcene and (+/–)-Limonene odor thresholds, 13 ppb (or 72.84 μg m−3) and 38 ppb (or 212.91 μg m−3), respectively.18,80
 | (12) |
 | (13) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−3); Q is the emission rate (g s−1); Us is the stack height wind speed (m s−1); σy is the lateral dispersion parameter (m); σz is the vertical dispersion parameter (m); y is the crosswind distance (m); z is the elevation of the receiver (m); and Hs is the effective stack height, calculated as hs + ΔH.
m−3); Q is the emission rate (g s−1); Us is the stack height wind speed (m s−1); σy is the lateral dispersion parameter (m); σz is the vertical dispersion parameter (m); y is the crosswind distance (m); z is the elevation of the receiver (m); and Hs is the effective stack height, calculated as hs + ΔH.
3 Results
To study the malodor BVOC time profiles of the cannabis sector we measured concentrations of 22 terpenes for eight rooms of the indoor cannabis cultivation facility (CCF) and six rooms of the processing and extraction facility (Facility P & E). Emissions were then linked to time patterns and rooms' environment conditions (Table 2). Emission factors were also calculated to improve current and future inventories (Table 3).| Cultivation | # of samples | Concentration (ppb) (mean + − std. err.) | Emissions (kg h−1) (mean + − std. err.) | Observations (a ‘%’ is relative to the average) |
|---|---|---|---|---|
| 183 | (2.57 ± 0.24) × 103 | — | — | |
| a Milk powder and hydrogen peroxide at low concentrations. | ||||
| Packing | 16 | (6.21 ± 0.18) × 102 | (2.39 ± 0.07) × 10−2 | Emissions increase 17% during packing |
| Drying | 41 | (3.80 ± 0.05) × 103 | (1.45 ± 0.02) × 10−1 | Steady emissions (±5%) unless door is open |
| Drying (no plants) | 10 | (1.73 ± 0.02) × 102 | (7.28 ± 0.09) × 10−3 | 1800% lower emissions than when in use |
| Grow (1 week propagation) | 15 | (4.66 ± 0.26) × 101 | (3.59 ± 0.20) × 10−3 | Emissions increase 47% with the lights ON |
| Grow (1 week propagation + pest controla) | 11 | (2.85 ± 0.60) × 102 | (2.16 ± 0.46) × 10−2 | Emissions increase 585% when treated with pest controla |
| Grow (mature plants) | 13 | (2.24 ± 0.20) × 103 | (8.56 ± 0.76) × 10−2 | Emissions increase 81% with the lights ON |
| Mother | 15 | (1.37 ± 0.50) × 102 | (1.32 ± 0.04) × 10−2 | Emissions increase 19% with the lights ON |
| Trimming | 27 | (8.00 ± 0.88) × 103 | (3.09 ± 0.34) × 10−1 | Emissions increase 87% during trimming |
| Storage (Vault) | 16 | (3.03 ± 0.08) × 103 | (1.16 ± 0.03) × 10−1 | Steady emissions (±8%) (unless door is open) |
| Vegetative | 13 | (5.00 ± 0.86) × 101 | (4.94 ± 0.82) × 10−3 | Emissions increase 163% with the lights ON |
| 6 | (7.76 ± 0.95) × 101 | (7.39 ± 0.90) × 10−3 | Emissions increase 37% with the lights ON | |
| Processing & Extraction | # of samples | Concentration (ppb) (mean + − std. err.) | Emissions (kg h−1) (mean + − std. err.) | Observations (a ‘%’ is relative to the average) |
|---|---|---|---|---|
| 94 | (4.66 ± 0.66) × 102 | — | — | |
| Packing area | 15 | (5.67 ± 1.12) × 102 | (3.01 ± 0.59) × 10−2 | Higher emissions during work hours (6am–6pm) |
| Distillation | 13 | (2.22 ± 0.51) × 102 | (3.89 ± 0.93) × 10−3 | Clear diurnal (6am–6pm) emission profile |
| Ethanol extraction | 12 | (3.25 ± 1.78) × 102 | (3.46 ± 2.64) × 10−3 | Peak in emissions during extraction |
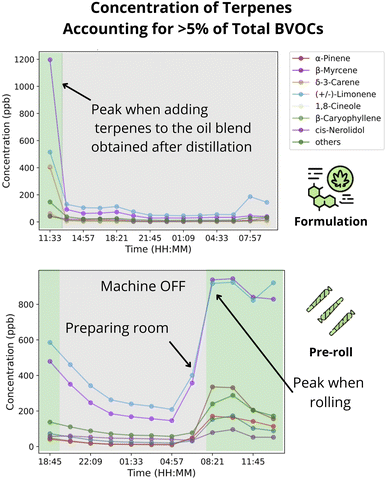
| Formulation | 27 | (3.41 ± 1.02) × 102 | (1.05 ± 0.29) × 10−3 | Adding terpenes to the mixture increase emissions by 630% |
| Hydro extraction | 15 | (1.31 ± 0.14) × 102 | (8.68 ± 0.87) × 10−3 | Peak in emissions during room preparation for extraction |
| Pre-roll | 12 | (1.41 ± 0.27) × 103 | (1.22 ± 0.23) × 10−3 | Emissions increase 114% when machine is in use |
| Grand total | 277 | — | — | — |
| Cultivation | Description | Emission factor (total terpenes) | ||
|---|---|---|---|---|
| (kg h−1) per [factor] | Min | Mean | Max | |
| a Observing the ratio of 5 vegetative to 1 mother plant. b Milk powder and hydrogen peroxide at low concentrations. c The net weight of dried cannabis that is intended to be consumed by means of inhalation in each discrete unit of a cannabis product must not exceed 1.0 g (Canada's Cannabis Regulations, SOR/2018-144). | ||||
| Vegetative | Plant cultivated | 1.96 × 10−7 | 3.08 × 10−7 | 5.84 × 10−7 |
| Mother | Plant cultivateda | 1.72 × 10−5 | 2.20 × 10−5 | 2.56 × 10−5 |
| Grow (1 week propagation) | Plant cultivated | 4.30 × 10−6 | 5.99 × 10−6 | 8.82 × 10−6 |
| Grow (1 week propagation) | Plant cultivated with pesticidesb | 8.74 × 10−6 | 3.61 × 10−5 | 6.33 × 10−5 |
| Grow (mature plants) | Plant cultivated | 2.16 × 10−4 | 2.85 × 10−4 | 5.32 × 10−4 |
| Drying | Plant hung dry | 1.20 × 10−4 | 1.45 × 10−4 | 1.66 × 10−4 |
| Trimming | kilogram trimmed with machine | 1.81 × 10−3 | 6.18 × 10−3 | 1.19 × 10−2 |
| Storage (Vault) | kilogram of product stored | 4.15 × 10−5 | 5.81 × 10−5 | 6.27 × 10−5 |
| Packing | kilogram of product packed | 9.02 × 10−5 | 1.19 × 10−4 | 1.41 × 10−4 |
| Processing & Extraction | Description | Emission factor (total terpenes) | ||
|---|---|---|---|---|
(kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h−1) per [factor] h−1) per [factor] |
Min | Mean | Max | |
| Packing area | Package in discrete unitsc | 1.17 × 10−6 | 3.76 × 10−6 | 1.09 × 10−5 |
| Distillation | kilogram of bulk extract | 2.14 × 10−5 | 7.77 × 10−5 | 1.94 × 10−4 |
| Ethanol extraction | kilogram of mixture of biomass and extract | 1.68 × 10−6 | 2.30 × 10−5 | 2.16 × 10−4 |
| Formulation | kilogram of bulk extract with terpenes added | 2.73 × 10−5 | 1.05 × 10−4 | 8.13 × 10−4 |
| Hydro extraction | kilogram of bulk biomass | 1.25 × 10−4 | 1.93 × 10−4 | 3.51 × 10−4 |
| Pre-roll | 0.5 g or 1.0 g rolled unit | 3.52 × 10−7 | 9.77 × 10−7 | 2.01 × 10−6 |
3.1 Terpene profile from cannabis cultivation
We explored the concentration timeseries of individual terpenes in order to answer the question if a speciated temporal profile of cannabis cultivation emissions is required from inventories. In the CCF, β-myrcene mean concentration varied between 25 ppb to 3200 ppb, and accounted for 39-78% of the total terpene, making it the dominant terpene across rooms. (+/–)-Limonene varied from 12 pbb to 3075 ppb, and accounted for 17-53% of the total terpene, making it the second most dominant. All other 20 terpenes accounted for less than 5% during practically all samples. Thus, the key terpenes that must be accounted in cannabis cultivation emissions profile are β-myrcene and (+/–)-Limonene.Next, we investigated the link of total terpene concentrations and room activity. Concentrations varied across different rooms, with stability observed in storage areas and significant fluctuations in cultivation and processing spaces. The drying and Vault (storage) rooms had steady concentrations (i.e., less than 10% variation across all samples) due to the limited staff activity during sampling. The plants' emission capacity is evident in the drying room, where a ≈ 1800% increase in concentration was observed after the introduction of plants (Fig. 2). Conversely, the rooms with live plants and those with some processing activities such as trimming or packing had fluctuations throughout the day (Section S2.2).†
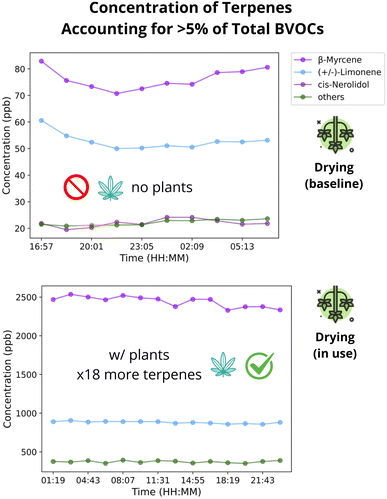 | ||
| Fig. 2 Time series of terpene concentrations in the dry room before and after plant introduction (notice the different y-axis scales). | ||
For the vegetative, grow, and mother rooms sampled, the action of turning the lights ON and OFF had a significant enhancement effect (≈20%–90%) on terpene concentrations (e.g., Fig. 3). Studies support that light spectrum, strain metabolism, and plant positioning affect the production of cannabinoids and terpenoids.81–86 For instance, Ahsan et al.81 found that terpene production generally increases in cannabis plants when growing under blue (430 nm) and red light (600-700 nm). Furthermore, Reichel et al.84 found that buds less exposed to light (e.g., shaded by higher buds) experience a significant decrease (50–77%) in key terpene production, such as β-myrcene and (+/–)-limonene.
 | ||
| Fig. 3 The effect of lights ON on terpene concentrations (increase) during grow operation at different stages of plant life. | ||
Our measurements show that independent of plant age (i.e., newly propagated or close to harvesting), the lights ON condition was associated with an increase in concentration whereas the lights OFF was associated with a decay. The observed increase and decay rate was steeper at earlier stages of plant development, likely due to the differences in strain metabolism and the fact that more blue (420–500 nm), red (700–750 nm), and infra-red (750–900 nm) light is provided to the cannabis plants when newly propagated (see Section S1.8†). For instance, when the lights were OFF the terpene concentration decreased (during one sampling cycle of ≈1 h and 42 min) from 475 ppb to 161 ppb (or −66%) in the early growing stage for “Blue Dream” plants treated with pesticides. Whereas for mature “Girl Scout Cookies” plants, the initial decrease during lights OFF was from 4170 ppb to 3142 ppb (or −24%). In the Grow rooms, We found similar terpene concentrations normalized both by number of plants and by weight as that of other studies(Table S10†).
At last, we compared our findings with the established literature in order to validate our method. We found the concentration per kilogram of plant (3.6–4.6 ppb kg−1) in the Drying room to be in the lower-bound compared to other studies (3.2–13.2 ppb kg−1)13 (Table S11†). In Urso et al.,13 the lowest ratio of 3.2 ppb kg−1 was found for a drying room with the highest plant weight (1827 kg). Additionally, the door was open to a processing room, potentially increasing the total volume, changing the HVAC load, and decreasing concentration as opposed to having the door closed. In our study, the drying room had the door closed at all times, and also had a charcoal filter installed in the room, which contributed to lower concentrations. Furthermore, drying rooms sampled in Urso et al.13 study had multiple strains present, while ours was only one, “Blue Dream”.
For other rooms, the previously published studies consulted lacked production information (e.g., number of plants, dry weight, cultivation area) or had diverging conditions (e.g., strains in the room) for a fair comparison, however in terms of absolute concentrations, the Vegetative room of Urso et al.13 (129.5 ppb) was within range of the CCF (27−136 ppb). The same could be said for the trimming operation: CCF (2326−15521 ppb) vs. Urso et al.13 (4479−7763 ppb) and Silvey et al.12 (6112−8090 ppb). Similar to previous studies,7,9,13 we observed that as the life cycle of the plant progressed the emissions increased, starting at an average of 4.94 × 10−3 kg h−1 in the vegetative stage reaching a peak during the trimming activity, where we observed an average emission of terpenes of 3.09 × 10−1 kg h−1.
3.2 Terpene profile from cannabis processing
Similar to the cultivation sector, we also explored the concentration timeseries of individual terpenes in the processing facility. In the CPF, (+/–)-Limonene mean concentration ranged between 60 ppb to 526 ppb, and accounted for 3452% of the total terpenes, thus being the dominant terpene across rooms. In second position was β-myrcene, with mean concentrations of 23 pbb to 470 ppb, or 15-33% of total terpene. Contrary to the CCF, though, was the fact that more terpenes contributed above 5% of the total concentration.We followed up exploring the activity patterns influence in emissions. The time series of terpene concentrations had a clear diurnal pattern, with low concentrations between 6pm–6am and high concentrations between 6am–6pm (e.g., Fig. 5). Additionally, emissions from the formulation, ethanol extraction, and pre-roll rooms were clearly correlated to room activity. In the formulation room, peak concentrations were associated with the activity of adding terpenes to the oil blend obtained during distillation, which resulted in concentrations ≈730% above the average (e.g., Fig. 4). These peak emissions quickly dissipated within the GC-FID sampling interval (≈90 min).
 | ||
| Fig. 5 Time series showing the reduction in terpene concentration in the distillation and packing rooms when the facility was closed and no work was performed. | ||
As with the CCF, comparing the CPF emission factors, which are useful for building inventories, is challenging due to the lack of available production data for other published studies, such as dry weight processed or number of pre-rolls per batch. However, in terms of absolute concentration our results are comparable to previous work. For instance, in the CPF Pre-roll room (492−2919 ppb) vs. Silvey et al.12 (1115−1980 ppb) and in the CPF Purge (i.e., hydro extraction) room (84−250 ppb) vs. Samburova et al.7 (180 ppb ± 20 ppb).
3.3 Timeseries variability of individual terpenes
In addition to the time variation of total terpene concentrations and emissions, we also assessed the time variation of individual terpenes, and how this variation correlated to total terpene emission variability (Section S2.3†). We observed that individual terpene time variation does not always follow the total terpene time variation.For the cultivation facility, only β-myrcene and (+/–)-limonene, had a strong correlation (r ≥ 0.7) with total terpene concentration for all rooms. The processing facility rooms had a minority of individual terpenes (n = 9), including β-myrcene, which were strongly correlated to total BVOC across all rooms.
Thus, not only is β-myrcene a candidate tracer of cannabis emissions, as highlighted previous studies,10,11 but also the only terpene to have a strong linear relationship with total terpene concentration emitted. Another interpretation is that using a linear regression model to fit an averaged canister composition to a total terpene time series may misrepresent the actual chemical profile, since the majority of terpenes do not increase or decrease proportionally with total terpene concentration over time.
Apart from β-myrcene and (+/–)-Limonene, other terpenes that were notable (≥5%) contributors to total emissions included p-cymene, terpinolene, linalool, and cis-nerolidol, which alternate as the third and second most emitted in each life-cycle stage.
The joint analysis of correlation and emissions revealed that negatively correlated terpenes are either emitted in single bursts (e.g., 1,8-cineole during trimming), or had higher concentrations in the room prior to an activity (e.g., terpinolene in the trimming room). One hypothesis is that the emission of some species is only triggered by phenomena associated with minor total emissions, resulting in a lower profile in more emissive episodes. While it is well-known that terpenes emission is affected by biotic and abiotic factors,82,87,88 to get a better comprehension of the triggers, we would have required a higher temporal resolution in our measurements of BVOCs.
We also found that although the overall emission time variation (in each room) is often more than ±100% for non-dominant terpenes, the absolute time variation is often ±0.1 kg h−1 in the CCF and CPF.
3.4 Relative removal rates are higher in the processing facilities compared to cultivation
Here we investigated the importance of each component of the mass-balance equation used to estimate emissions, and if they varied significantly between cultivation and processing rooms. Between removal rates due to chemistry (10−5 to 10−2 kg h−1), terpene mass flow into the room (10−13 to 10−14 kg h−1), and dry deposition (10−10 to 10−8 kg h−1), (R) was more significant for the emission box model (Section S2.4†). The removal rates (R) at the CCF ranged from 5.69 × 10−5 kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h−1 to 4.58 × 10−2 kg h−1, while at the CPF, R varied between 4.94 × 10−4 kg h−1 and 7.60 × 10−3 kg h−1. These differences align with the higher terpene concentrations typically observed in cultivation rooms. However, the relative removal rate (R), defined as the ratio between emissions rates and removal, was significantly higher during processing and extraction operations than during cultivation (processing: 19.5–72.8% vs. cultivation: 1.7–13.9%). Overall, the total removal rates, along with those attributed solely to reactions with OH (ROH) and O3 (RO3), align with the estimations reported by Urso et al.13.
h−1 to 4.58 × 10−2 kg h−1, while at the CPF, R varied between 4.94 × 10−4 kg h−1 and 7.60 × 10−3 kg h−1. These differences align with the higher terpene concentrations typically observed in cultivation rooms. However, the relative removal rate (R), defined as the ratio between emissions rates and removal, was significantly higher during processing and extraction operations than during cultivation (processing: 19.5–72.8% vs. cultivation: 1.7–13.9%). Overall, the total removal rates, along with those attributed solely to reactions with OH (ROH) and O3 (RO3), align with the estimations reported by Urso et al.13.
3.5 Screening assessment of ambient air odor impacts considering time variation
For a terpene emission to be relevant in an odor community exposure assessment, the downwind concentration of that terpene must surpass the odor threshold.21,89,90Our screening dispersion analysis indicates that out of the 7560 possible dispersion scenarios created for the CCF's average β-myrcene emissions of 0.373 kg h−1, 88 (1.1%) may result in an episode of community-detectable odor event ≤500 m downwind.
Accounting for the increase in emissions during trimming (+0.132 kg h−1), 241 (3.2%) combinations surpassed the β-myrcene odor threshold of 72.84 μg m−3, including 18 under favorable atmospheric stability conditions (moderately unstable).
For the majority of the odor episodes, they occurred during stable atmospheric conditions, and at 2 m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s−1 wind speeds measured at 10 meters height. The meteorological analysis (Section S1.10†) of the nearest station to the CCF suggested that such conditions occurred ≈18.2% of the hours during the year of sampling. Thus, although odor episodes are a small percentage of scenarios tested, they had the potential to occur almost 1/5 of the year.
s−1 wind speeds measured at 10 meters height. The meteorological analysis (Section S1.10†) of the nearest station to the CCF suggested that such conditions occurred ≈18.2% of the hours during the year of sampling. Thus, although odor episodes are a small percentage of scenarios tested, they had the potential to occur almost 1/5 of the year.
For the CPF β-myrcene and (+/–)-Limonene emissions, as well as the (+/–)-limonene emissions of the CCF, no dispersion scenario resulted in concentrations above the odor threshold, suggesting that the combination of emissions above 0.4 kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h−1 and low terpene odor threshold (≤15 ppb) is needed to cause a community-detectable odor event from stack emissions.
h−1 and low terpene odor threshold (≤15 ppb) is needed to cause a community-detectable odor event from stack emissions.
4 Environmental implications
4.1 Key findings
This study shows that individual terpenes have a non-linear relationship with total terpene emissions in most cases. The composition of emissions during cultivation and processing is influenced by the room conditions, routine, and activity performed. Given the complexities in the emissions, we recommend future studies to carefully report on these parameters for more robust comparisons going forward.For occupational exposure, the emissions variation may be of less importance, since, thus far, studies (included ours) have not shown total or individual terpene concentrations surpassing local exposure limits.14 For community exposure (when emissions leave the facility's premises and interact with outdoor air), understanding the timing of increased emissions of compounds like β-myrcene is crucial, since this terpene have a low odor threshold, and is highly correlated to total emissions throughout the plant life-cycle, meaning that an environmental impact assessment could underestimate odor impacts if temporal variations in emission composition are not considered.
The temporal variation of β-myrcene (and (+/–)-Limonene) might also be important when considering each terpene's reactivity with atmospheric oxidants,15 which can lead to the formation of ozone (O3), ultrafine particles (PM<0.1μm) in the form of secondary organic aerosols (SOA). Such knowledge might assist with sensitivity analysis of modeling studies (e.g.,7,9,16), helping explore the range of potential concentrations modeled at sensitive receptors and concentrations used in model validation.
Although driven by a few assumptions, our results support that only a small percentage (3.2%) of dispersion scenarios would lead to odor in a nearby community (≤500 m away). Nevertheless, the frequency of the meteorological conditions required to cause an impact during the sampling year was 18.2% of the hours. Therefore, future investigation concerned with this type of impact, as well as the need for odor control technology, should aim for evaluating facilities with an indoor cultivation capacity above 25 ton![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) per year and exported products capacity above 1.5 ton
per year and exported products capacity above 1.5 ton![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) per year, and be accompanied by meteorological analysis. Additionally, we only investigated stack emissions impact, which could differ from area and volume emissions from greenhouse and outdoor cultivation or those emissions in indoor facilities with a leakier building envelope or inappropriate outdoor waste disposal practices.
per year, and be accompanied by meteorological analysis. Additionally, we only investigated stack emissions impact, which could differ from area and volume emissions from greenhouse and outdoor cultivation or those emissions in indoor facilities with a leakier building envelope or inappropriate outdoor waste disposal practices.
4.2 Study limitations
In our attempt to extrapolate terpene emission rates to multiple facilities, there remains the limitation that facilities have different operating conditions. For instance, our facilities were well-established and had some emission controls in place (Tables S2–S5†), which may not be the case for emerging or small facilities. While regulations are still at the early stages, some facilities are already adopting Best Environmental Practices (BEP) and Best Available Technologies (BAT) and others are not. Furthermore, each strain cultivated in a facility has its own odor potential. Therefore, each facility has its own potential impact depending on what is cultivated, meteorology near site, and receptor distance (i.e., it is not just about size). Lastly, the industry is evolving according to the market needs and strains cultivated/processed in one year might not be the same in the next. All these points must be taken into consideration when using our results.Other limitations concern the scope of the samplings. For instance, many other VOCs such as butane7 and n-Heptanal,11 as well as Volatile Sulfur Compounds (VSC) such as 3- methyl-2-butene-1-thiol,18–20 which can have odor thresholds much lower than β-myrcene, may undergo similar temporal variation of terpenes.
Accounting for variations in each terpene, VOC, and VSC, can significantly increase the complexity and cost of a study. For instance, incorporating non-terpene chemicals would necessitate purchasing more standards and potentially using multiple instruments. Space constraints in the rooms present another challenge, as accommodating many instruments may be impractical in some cases due to room layouts with limited free area.
Future work characterizing VOCs and other species with the same high time resolution as a Photo-Ionization Detectors (PIDs) detector (i.e., real-time) could be achievable through PTR-ToF-MS91–95 also coupled with GC.96,97
Another limitation of this study concerns the assumptions made during the mass balance calculations. Specifically, using the mean partitioning coefficient of (+/–)-limonene obtained from Thevenet et al.73 for the deposition of all terpenes may not reflect true conditions, especially considering the range of chemical structures (e.g., number of cyclic carbons, and the number of unsaturated bonds).98 Similarly, we were not able to find information on the kinetic constants and yield for all terpene reactions with O3 and OH (see Section S1.9†). Thus, we had to assume values between terpenes with similar chemical structure.
Lastly, a recent communication99 outlined the development of a new trap for sampling indoors in cannabis facilities, and discussed the recovery efficiency of Tenax ®TA traps. We have not made corrections to the terpene values based on this communication, because both the analytical conditions (i.e., thermal desorber parameters and gas chromatograph parameters) and experiments differ from our approach. For instance, Brown99 sampled 10 L of air where we sampled 1.2 L maximum. We also performed multiple samples, baking the trap system between each sample. However, we wish to highlight Brown99 here as it could be useful for future studies.
4.3 Study contributions
We present a replicable methodology for continuous terpene measurements in cannabis cultivation and processing facility rooms. This approach provides a more detailed characterization than PIDs or similar techniques which measure total VOCs (or BVOCs) or techniques relying on collecting limited, isolated samples over time using sorbent tube extraction for profiling.The GC-FID approach also has less risk of loss or contamination during transport to and analysis in a laboratory, since the instruments stayed in the room where collection and analysis occur simultaneously. Through this method, we provide speciated and time-resolved emissions from each room sampled, covering most processes in the life-cycle of a cannabis plant, expanding the current literature database. This knowledge could assist in building emission inventories for the industry to support improved dispersion modeling of odorous emissions. The new GC-FID approach could also be incorporated to outdoor monitoring studies, which, currently, are very limited in number (e.g., Wang et al.).10
Apart from the scientific contribution of our methods, the results of this work could be used as guidance for regulators towards improved emission inventory assessments in the cannabis industry. For instance, we demonstrated that individual terpene and total terpene emissions vary by a number of factors, with the key three being: light cycle, plants stage of life, and work and no-work hours. The emission factors we derived also add to the existing, but limited, information available, and address almost the full life cycle of cannabis. For industrial stakeholders, our results will assist in addressing odorous emission control. For instance, for a facility with the same capacity as the CCF, the use of more than one charcoal filter in the room would be required to avoid the peak in terpene concentration during trimming.
Data availability
Data for this article, including rooms terpene concentration and chromatograms, calibration and blanks chromatograms, and post-processing Python scripts are available at Zenodo at https://zenodo.org/records/14606485 and https://zenodo.org/records/14606488.Author contributions
Davi de Ferreyro Monticelli conceptualization, data curation, investigation, methodology, formal analysis, visualization, writing – original draft. Cynthia Pham investigation, writing – review & editing Sahil Bhandari methodology, writing – review & editing. Amanda Giang conceptualization, funding acquisition, project administration, supervision, writing – review & editing. Nadine Borduas-Dedekind methodology, resources, writing – review & editing. Naomi Zimmerman conceptualization, funding acquisition, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the expertise contributions of WorkSafe BC. The authors would also like to acknowledge researchers from UBC Chemistry Department for the motivating discussions, in particular Paul Heine and Rickey Lee. Funding for this study was also provided by the New Frontiers in Research Fund Exploration Program [NFRFE-2019-00546] and WorkSafe BC. Davi de Ferreyro Monticelli was supported by the Vanier Canada Graduate Scholarship, Cynthia Pham was supported by the NSERC CGS-M Scholarship. This research was undertaken, in part, thanks to funding from the Canada Research Chairs Program. Lastly, cannabis icons used in this paper were made by: “Free Pick”, “POD Gladiator”, “Darius Dan”, “Eucalyp”, “AmethystDesign”, and “Vitaly Gorbachev” obtained at: https://www.flaticon.com/free-icons/cannabis.Notes and references
- Leafwell Team, Countries Where Weed is Legal, https://leafwell.com/blog/countries-where-weed-is-legal, Accessed: 2024-10-28 Search PubMed.
- K. Ashworth and W. Vizuete, High Time to Assess the Environmental Impacts of Cannabis Cultivation, Environ. Sci. Technol., 2017, 51, 2531–2533 CrossRef CAS PubMed.
- H. M. Summers, E. Sproul and J. C. Quinn, The greenhouse gas emissions of indoor cannabis production in the United States, Nat Sustainability, 2021, 1–7 Search PubMed.
- A. C. Wartenberg, P. A. Holden, H. Bodwitch, P. Parker-Shames, T. Novotny, T. C. Harmon, S. C. Hart, M. Beutel, M. Gilmore, E. Hoh and V. Butsic, Cannabis and the Environment: What Science Tells Us and What We Still Need to Know, Environ. Sci. Technol. Lett., 2021, 8, 98–107 CrossRef CAS.
- V. Desaulniers Brousseau, B. P. Goldstein, M. Lachapelle, I. Tazi and M. Lefsrud, Greener green: The environmental impacts of the Canadian cannabis industry, Resour., Conserv. Recycl., 2024, 208, 107737 CrossRef CAS.
- Z. Zheng, K. Fiddes and L. Yang, A narrative review on environmental impacts of cannabis cultivation, J. cannabis res., 2021, 1–10 Search PubMed.
- V. Samburova, M. McDaniel, D. Campbell, M. Wolf, W. R. Stockwell and A. Khlystov, Dominant volatile organic compounds (VOCs) measured at four Cannabis growing facilities: pilot study results, J. Air Waste Manage. Assoc., 2019, 69, 1267–1276 CrossRef CAS PubMed.
- C. T. Wang, C. Wiedinmyer, K. Ashworth, P. C. Harley, J. Ortega and W. Vizuete, Leaf enclosure measurements for determining volatile organic compound emission capacity from Cannabis spp., Atmos. Environ., 2019, 199, 80–87 CrossRef CAS.
- C. T. Wang, C. Wiedinmyer, K. Ashworth, P. C. Harley, J. Ortega, Q. Z. Rasool and W. Vizuete, Potential regional air quality impacts of cannabis cultivation facilities in Denver, Colorado (Poster No.16), Proceedings of the Air and Waste Management Association's Annual Conference and Exhibition, AWMA, 2019, pp. 13973–13987 Search PubMed.
- C. T. Wang, K. Ashworth, C. Wiedinmyer, J. Ortega, P. C. Harley, Q. Z. Rasool and W. Vizuete, Ambient measurements of monoterpenes near Cannabis cultivation facilities in Denver, Colorado, Atmos. Environ., 2020, 232, 117510 CrossRef CAS.
- R. L. Knights, The Cannabis Science Conference, Portland, 2017, pp. 1–6 Search PubMed.
- B. Silvey, E. Seto, A. Gipe, N. Ghodsian and C. D. Simpson, Occupational exposure to particulate matter and volatile organic compounds in two indoor cannabis production facilities, Ann. Work Exposures Health, 2020, 64, 715–727 CrossRef CAS PubMed.
- K. Urso, W. Vizuete, R. Moravec, A. Khlystov, A. Frazier and G. Morrison, Indoor monoterpene emission rates from commercial cannabis cultivation facilities in Colorado, J. Air Waste Manage. Assoc., 2023, 73, 321–332 CrossRef CAS PubMed.
- D. de Ferreyro Monticelli, S. Bhandari, A. Eykelbosh, S. B. Henderson, A. Giang and N. Zimmerman, Cannabis cultivation facilities: a review of their air quality impacts from the occupational to community scale, Environ. Sci. Technol., 2022, 56, 2880–2896 CrossRef CAS PubMed.
- R. Atkinson and J. Arey, Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review, Atmos. Environ., 2003, 37, 197–219 CrossRef.
- K. Urso, A. Frazier, S. Heald and A. Khlystov, Terpene Exhaust Emissions and Impact Ozone Modeling from Cannabis Plants at Commercial Indoor Cultivation Facilities in Colorado, J. Air Waste Manage. Assoc., 2022, 828–848 CrossRef CAS PubMed.
- N. Seltenrich, Odor Control in the Cannabis Industry: Lessons from the New Kid on the Block, Environ. Health Perspect., 2022, 130, 062001 CrossRef PubMed.
- S. Rice and J. A. Koziel, The relationship between chemical concentration and odor activity value explains the inconsistency in making a comprehensive surrogate scent training tool representative of illicit drugs, Forensic Sci. Int., 2015, 257, 257–270 CrossRef CAS PubMed.
- I. W. Oswald, M. A. Ojeda, R. J. Pobanz, K. A. Koby, A. J. Buchanan, J. Del Rosso, M. A. Guzman and T. J. Martin, Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography, ACS Omega, 2021, 6, 31667–31676 CrossRef CAS PubMed.
- I. W. Oswald, T. R. Paryani, M. E. Sosa, M. A. Ojeda, M. R. Altenbernd, J. J. Grandy, N. S. Shafer, K. Ngo, J. R. Peat, B. G. Melshenker, I. Skelly, K. A. Koby, M. F. Page and T. J. Martin, Minor, Nonterpenoid Volatile Compounds Drive the Aroma Differences of Exotic Cannabis, ACS Omega, 2023, 8, 39203–39216 CrossRef CAS PubMed.
- J. A. Koziel, A. Guenther, W. Vizuete, D. W. Wright and A. Iwasinska, “Skunky” Cannabis: Environmental Odor Troubleshooting and the ”need-for-Speed, ACS Omega, 2022, 7, 19043–19047 CrossRef CAS PubMed.
- C. D. Strunk, Yes to Cannabis ! Just Not in My Backyard an Analysis of Odor-Based, the Brief, 2020, vol. 49, pp. 32–37 Search PubMed.
- BCX Environmental Consulting, Odour Impact Assessment for a Medical Marihuana Grow Facility, BCX Environmental Consulting technical report, 2020 Search PubMed.
- BCX Environmental Consulting, Odour Impact Assessment for a Medical Marihuana Grow Facility, 199 Anglesea Street, BCX Environmental Consulting technical report, 2020 Search PubMed.
- RWDI AIR Inc., Wilmot Creek Secondary Plan - Municipality of Clarington, Ontario: Air Quality Feasibility Assessment, RWDI AIR Inc. Technical Report, 2018 Search PubMed.
- B. C. Stats, BC Population Projections - August 2024 Newsletter, 2024, https://www2.gov.bc.ca/assets/gov/data/statistics/people-population-community/population/newsletter_08_2024_bc_population_projections.pdf Search PubMed.
- M. Hedley and D. Singleton, Evaluation of an air quality simulation of the Lower Fraser Valley—I. Meteorology, Atmos. Environ., 1997, 31, 1605–1615 CrossRef CAS.
- K. Hayden, K. Anlauf, R. Hoff, J. Strapp, J. Bottenheim, H. Wiebe, F. Froude, J. Martin, D. Steyn and I. McKendry, The vertical chemical and meteorological structure of the boundary layer in the Lower Fraser Valley during Pacific’93, Atmos. Environ., 1997, 31, 2089–2105 CrossRef CAS.
- R. Moore, D. Spittlehouse, P. Whitfield and K. Stahl, Weather and Climate, Compendium of Forest Hydrology and Geomorphology in British Columbia, 2010, vol. 1, pp. 47–84 Search PubMed.
- C. Reuten, B. Ainslie, D. G. Steyn, P. L. Jackson and I. McKendry, Impact of climate change on ozone pollution in the Lower Fraser Valley, Canada, Atmos.-Ocean, 2012, 50, 42–53 CrossRef CAS.
- M. Brauer and J. R. Brook, Ozone personal exposures and health effects for selected groups residing in the Fraser Valley, Atmos. Environ., 1997, 31, 2113–2121 CrossRef CAS.
- R. Vingarzan and B. Taylor, Trend analysis of ground level ozone in the greater Vancouver/Fraser Valley area of British Columbia, Atmos. Environ., 2003, 37, 2159–2171 CrossRef CAS.
- D. G. Steyn, J. Bottenheim and R. Thomson, Overview of tropospheric ozone in the Lower Fraser Valley, and the Pacific’93 field study, Atmos. Environ., 1997, 31, 2025–2035 CrossRef CAS.
- B. Ainslie, D. Steyn, C. Reuten and P. Jackson, A retrospective analysis of ozone formation in the Lower Fraser Valley, British Columbia, Canada. Part II: influence of emissions reductions on ozone formation, Atmos.-Ocean, 2013, 51, 170–186 CrossRef CAS.
- J. Salmond and I. McKendry, Secondary ozone maxima in a very stable nocturnal boundary layer: observations from the Lower Fraser Valley, BC, Atmos. Environ., 2002, 36, 5771–5782 CrossRef CAS.
- I. McKendry, D. Steyn, J. Lundgren, R. Hoff, W. Strapp, K. Anlauf, F. Froude, J. Martin, R. Banta and L. Olivier, Elevated ozone layers and vertical down-mixing over the Lower Fraser Valley, BC, Atmos. Environ., 1997, 31, 2135–2146 CrossRef CAS.
- B. Ainslie, N. Moisseeva, R. Vingarzan, C. Schiller, D. Steyn and G. Doerksen, The Spatiotemporal Response of Summertime Tropospheric Ozone to Changes in Local Precursor Emissions in the Lower Fraser Valley, British Columbia, Atmos.-Ocean, 2018, 56, 303–321 CrossRef.
- B. Ainslie and D. G. Steyn, Spatiotemporal trends in episodic ozone pollution in the Lower Fraser Valley, British Columbia, in relation to mesoscale atmospheric circulation patterns and emissions, Appl. Meteorol. Climatol., 2007, 46, 1631–1644 CrossRef.
- S. Pryor, R. Barthelmie, R. Hoff, S. Sakiyama, R. Simpson and D. Steyn, REVEAL: characterizing fine aerosols in the Fraser Valley, BC, Atmos.-Ocean, 1997, 35, 209–227 CrossRef.
- K. Strawbridge and B. Snyder, Daytime and nighttime aircraft lidar measurements showing evidence of particulate matter transport into the Northeastern valleys of the Lower Fraser Valley, BC, Atmos. Environ., 2004, 38, 5873–5886 CrossRef CAS.
- M. Hedley, R. McLaren, W. Jiang and D. Singleton, Evaluation of an air quality simulation of the Lower Fraser Valley-II. Photochemistry, Atmos. Environ., 1997, 31, 1617–1630 CrossRef CAS.
- R. M. Healy, J. M. Wang, U. Sofowote, Y. Su, J. Debosz, M. Noble, A. Munoz, C.-H. Jeong, N. Hilker and G. J. Evans, et al., Black carbon in the Lower Fraser Valley, British Columbia: Impact of 2017 wildfires on local air quality and aerosol optical properties, Atmos. Environ., 2019, 217, 116976 CrossRef CAS.
- I. G. McKendry, PM10 levels in the Lower Fraser Valley, British Columbia, Canada: an overview of spatiotemporal variations and meteorological controls, J. Air Waste Manage. Assoc., 2000, 50, 443–452 CrossRef CAS PubMed.
- S.-M. Li, A concerted effort to understand the ambient particulate matter in the Lower Fraser Valley: the Pacific 2001 Air Quality Study, Atmos. Environ., 2004, 38, 5719–5731 CrossRef CAS.
- D. Yin, W. Jiang, H. Roth and É. Giroux, Improvement of biogenic emissions estimation in the Canadian Lower Fraser Valley and its impact on particulate matter modeling results, Atmos. Environ., 2004, 38, 507–521 CrossRef CAS.
- I. McKendry, J. Hacker, R. Stull, S. Sakiyama, D. Mignacca and K. Reid, Long-range transport of Asian dust to the lower Fraser Valley, British Columbia, Canada, J. Geophys. Res.:Atmos., 2001, 106, 18361–18370 CrossRef CAS.
- W. Jiang, D. L. Singleton, M. Hedley, R. McLaren, T. Dann and D. Wang, Comparison of organic compound compositions in the emissions inventory and ambient data for the Lower Fraser Valley, J. Air Waste Manage. Assoc., 1997, 47, 851–860 CrossRef CAS PubMed.
- W. Jiang, D. L. Singleton, M. Hedley and R. McLaren, Sensitivity of ozone concentrations to VOC and NOx emissions in the Canadian Lower Fraser Valley, Atmos. Environ., 1997, 31, 627–638 CrossRef CAS.
- G. Drewitt, K. Curren, D. Steyn, T. Gillespie and H. Niki, Measurement of biogenic hydrocarbon emissions from vegetation in the Lower Fraser Valley, British Columbia, Atmos. Environ., 1998, 32, 3457–3466 CrossRef CAS.
- K. Gurren, T. Gillespie, D. Steyn, T. Dann and D. Wang, Biogenic isoprene in the Lower Fraser Valley, British Columbia, J. Geophys. Res.:Atmos., 1998, 103, 25467–25477 CrossRef.
- Y. Xiong, M. A. Bari, Z. Xing and K. Du, Ambient volatile organic compounds (VOCs) in two coastal cities in western Canada: Spatiotemporal variation, source apportionment, and health risk assessment, Sci. Total Environ., 2020, 706, 135970 CrossRef CAS PubMed.
- Y. Xiong and K. Du, Source-resolved attribution of ground-level ozone formation potential from VOC emissions in Metropolitan Vancouver, BC, Sci. Total Environ., 2020, 721, 137698 CrossRef CAS PubMed.
- R. Maher, S. Bhandari, D. de Ferreyro Monticelli, A. Eykelbosh, S. B. Henderson, N. Zimmerman and A. Giang, ISES2021: Multisector Engagement for Addressing Emerging Environmental Exposures, 2021 Search PubMed.
- A. Eykelbosh, R. Maher, D. de Ferreyro Monticelli, A. Ramkairsingh, S. Henderson, A. Giang and N. Zimmerman, Elucidating the community health impacts of odours using citizen science and mobile monitoring, Environ. Health Rev., 2021, 64, 24–27 CrossRef.
- S. Bhandari, D. de Ferreyro Monticelli, K. Xie, A. Ramkairsingh, R. Maher, A. Eykelbosh, S. B. Henderson, N. Zimmerman and A. Giang, Odor, air quality, and well-being: understanding the urban smellscape using crowd-sourced science, Environ. Res. Health., 2024, 2, 035012 CrossRef.
- K. Chan, Metro Vancouver taking new steps to improve air quality even further, 2020, https://dailyhive.com/vancouver/metro-vancouver-air-quality-report Search PubMed.
- Health Canada, Types of cannabis and industrial hemp licences, 2025, https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/industry-licensees-applicants/applying-licence.html, Search PubMed.
- G. Micalizzi, F. Vento, F. Alibrando, D. Donnarumma, P. Dugo and L. Mondello, Cannabis Sativa L.: a comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization, J. Chromatogr. A, 2021, 1637, 461864 CrossRef CAS PubMed.
- J. V. Hinshaw, The flame ionization detector, LCGC North Am., 2005, 23, 1262–1272 CAS.
- D. A. Armbruster and T. Pry, Limit of blank, limit of detection and limit of quantitation, Clin. Biochem. Rev., 2008, 29(1), S49–S52 Search PubMed.
- S. Grossman, Warm Up Before You Run: Why conditioning your inlet parts after maintenance is good practice, 2024, https://www.restek.com/ca/en_CA/articles/warm-up-before-you-run Search PubMed.
- K. Dettmer-Wilde, W. Engewald, M. Adahchour, J. de Zeeuw, U. Albrecht, J. Andersson, H.-U. Baier, C. Bicchi, M. Biedermann, U. Brinkman, C. Cagliero, C. Cordero, L. Fragner, T. Furuhashi, M. Gruber, M. Jochmann, M. Juza, J. Laaks, E. Liberto, J. Mattusch, M. Moeder, S. Mothes, H. Ohtani, L. Ramos, S. Rohn, H. Rotzsche, P. Rubiolo, B. Sgorbini, T. Schmidt, P. Schultze, V. Schurig, J. Teske, O. Trapp, S. Tsuge and W. Weckwerth, Practical Gas Chromatography: A Comprehensive Reference, Springer, 2014, 11, pp. 206–221 Search PubMed.
- US EPA, Project Quality Assurance and Quality Control, US EPA Technical Report July, 2014 Search PubMed.
- U.S. EPA, Method TO-17: Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes, EPA Methods, 1999, 1–53 Search PubMed.
- U.S. EPA, Method TO-15: determination of volatile organic compounds in air collected in specially-prepared canisters ans analysed by gas chromatography/mass spectrometry (GC/MS), EPA Methods, 1999, 1–32 Search PubMed.
- NIOSH, NIOSH Manual of Analytical Methods (NMAM), TERPENES: METHOD 1552, National Institute for Occupational Safety and Health Technical, Terpenes 1552, 4th edn, 1996 Search PubMed.
- D. F. McGlynn, N. S. Panji, G. Frazier, C. Bi and G. Isaacman-VanWertz, An autonomous remotely operated gas chromatograph for chemically resolved monitoring of atmospheric volatile organic compounds, Environ. Sci.: Atmos., 2023, 3, 387–398 CAS.
- D. Pagonis, J. E. Krechmer, J. De Gouw, J. L. Jimenez and P. J. Ziemann, Effects of gas-wall partitioning in Teflon tubing and instrumentation on time-resolved measurements of gas-phase organic compounds, Atmos. Meas. Tech., 2017, 10, 4687–4696 Search PubMed.
- B. L. Deming, D. Pagonis, X. Liu, D. A. Day, R. Talukdar, J. E. Krechmer, J. A. De Gouw, J. L. Jimenez and P. J. Ziemann, Measurements of delays of gas-phase compounds in a wide variety of tubing materials due to gas-wall interactions, Atmos. Meas. Tech., 2019, 12, 3453–3461 CrossRef CAS.
- H. Rothweiler, P. A. Wäger and C. Schlatter, Comparison of Tenax Ta and Carbotrap for sampling and analysis of volatile organic compounds in air, Atmos. Environ. B, Urban Atmos., 1991, 25, 231–235 CrossRef.
- J. Hwan Lee, S. A. Batterman, C. Jia and S. Chernyak, Ozone artifacts and carbonyl measurements using tenax GR, tenax TA, carbopack B, and carbopack X adsorbents, J. Air Waste Manage. Assoc., 2006, 56, 1503–1517 CrossRef PubMed.
- G. MacLeod and J. M. Ames, Comparative assessment of the artefact background on thermal desorption of tenax GC and tenax TA, J. Chromatogr. A, 1986, 355, 393–398 CrossRef CAS.
- F. Thevenet, M. Verriele, P. Harb, S. Thlaijeh, R. Brun, M. Nicolas and S. Angulo-Milhem, The indoor fate of terpenes: Quantification of the limonene uptake by materials, Build. Sci., 2021, 188, 107433 Search PubMed.
- J. A. Manion, R. E. Huie, R. D. Levin, D. R. Burgess Jr, V. L. Orkin, W. Tsang, W. S. McGivern, J. W. Hudgens, V. D. Knyazev, D. B. Atkinson, E. Chai, A. M. Tereza, C.-Y. Lin, T. C. Allison, W. G. Mallard, F. Westley, J. T. Herron, R. F. Hampson and D. H. Frizzell, NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.6.8, Data version 2015.09, National Institute of Standards and Technology, Gaithersburg, Maryland, 20899-8320, https://kinetics.nist.gov/ Search PubMed.
- E. Gomez Alvarez, D. Amedro, C. Afif, S. Gligorovski, C. Schoemaecker, C. Fittschen, J.-F. Doussin and H. Wortham, Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 13294–13299 CrossRef PubMed.
- K. Emmerson and N. Carslaw, Night-time radical chemistry during the TORCH campaign, Atmos. Environ., 2009, 43, 3220–3226 CrossRef CAS.
- A. K. Lee, J. P. Abbatt, W. R. Leaitch, S.-M. Li, S. J. Sjostedt, J. J. Wentzell, J. Liggio and A. M. Macdonald, Substantial secondary organic aerosol formation in a coniferous forest: observations of both day-and nighttime chemistry, Atmos. Chem. Phys., 2016, 16, 6721–6733 CrossRef CAS.
- Y. Zhao, M. Saunois, P. Bousquet, X. Lin, A. Berchet, M. I. Hegglin, J. G. Canadell, R. B. Jackson, D. A. Hauglustaine and S. Szopa, et al., Inter-model comparison of global hydroxyl radical (OH) distributions and their impact on atmospheric methane over the 2000–2016 period, Atmos. Chem. Phys., 2019, 19, 13701–13723 CrossRef CAS.
- F. Pasquill, The estimation of the dispersion of windborne material, Meteorol. Mag., 1961, 90, 20–49 Search PubMed.
- Y. Nagata and N. Takeuchi, Measurement of odor threshold by triangle odor bag method, Odor measurement review, 2003, 118, 118–127 Search PubMed.
- S. Ahsan, M. Injamum-Ul-Hoque, S. Shaffique, A. Ayoobi, M. A. Rahman, M. M. Rahman and H. W. Choi, Illuminating Cannabis sativa L.: the power of light in enhancing C. sativa growth and secondary metabolite production, Plants, 2024, 13, 2774 CrossRef CAS PubMed.
- N. Dimopoulos, Q. Guo, S. J. Purdy, M. Nolan, R. A. Halimi, J. C. Mieog, B. J. Barkla and T. Kretzschmar, From dawn ‘til dusk: daytime progression regulates primary and secondary metabolism in Cannabis glandular trichomes, J. Exp. Bot., 2025, 76, 134–151 CrossRef PubMed.
- M. M. Holweg, E. Kaiser, I. F. Kappers, E. Heuvelink and L. F. Marcelis, The role of red and white light in optimizing growth and accumulation of plant specialized metabolites at two light intensities in medical cannabis (Cannabis sativa L.), Front. Plant Sci., 2024, 15, 1393803 CrossRef PubMed.
- P. Reichel, S. Munz, J. Hartung, S. Kotiranta and S. Graeff-Hönninger, Impacts of different light spectra on CBD, CBDA and terpene concentrations in relation to the flower positions of different Cannabis sativa L. strains, Plants, 2022, 11, 2695 CrossRef CAS PubMed.
- D. Jin, S. Jin and J. Chen, Cannabis Indoor Growing Conditions, Management Practices, and Post-Harvest Treatment: A Review, Am. J. Plant Sci., 2019, 10, 22 Search PubMed.
- N. Danziger and N. Bernstein, Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.), Ind. Crops Prod., 2021, 164, 113351 CrossRef CAS.
- J. K. Holopainen and J. Gershenzon, Multiple stress factors and the emission of plant VOCs, Trends Plant Sci., 2010, 15, 176–184 CrossRef CAS PubMed.
- F. Loreto and J.-P. Schnitzler, Abiotic stresses and induced BVOCs, Trends Plant Sci., 2010, 15, 154–166 CrossRef CAS PubMed.
- D. W. Wright, J. A. Koziel, F. W. Kuhrt, A. Iwasinska, D. K. Eaton and L. Wahe, Odor-Cued Grab Air Sampling for Improved Investigative Odorant Prioritization Assessment of Transient Downwind Environmental Odor Events, ACS Omega, 2024, 9, 29290–29299 CrossRef CAS PubMed.
- W. Cain, C. Shoaf, S. Velasquez, S. Selevan and W. Victery, Reference Guide to Odor Thresholds for Hazardous Air Pollutants Listed in the Clean Air Act Amendments of 1990, Environmental protection agency, washington, dc, 1992 Search PubMed.
- A. Jordan, S. Haidacher, G. Hanel, E. Hartungen, L. Märk, H. Seehauser, R. Schottkowsky, P. Sulzer and T. D. Märk, A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS), Int. J. Mass Spectrom., 2009, 286, 122–128 CrossRef CAS.
- D. Materić, M. Lanza, P. Sulzer, J. Herbig, D. Bruhn, V. Gauci, N. Mason and C. Turner, Selective reagent ion-time of flight-mass spectrometry study of six common monoterpenes, Int. J. Mass Spectrom., 2017, 421, 40–50 CrossRef.
- E. Pallozzi, G. Guidolotti, P. Ciccioli, F. Brilli, S. Feil and C. Calfapietra, Does the novel fast-GC coupled with PTR-TOF-MS allow a significant advancement in detecting VOC emissions from plants?, Agric. For. Meteorol., 2016, 216, 232–240 CrossRef.
- E. Kari, P. Miettinen, P. Yli-Pirilä, A. Virtanen and C. L. Faiola, PTR-ToF-MS product ion distributions and humidity-dependence of biogenic volatile organic compounds, Int. J. Mass Spectrom., 2018, 430, 87–97 CrossRef CAS.
- H. Li, M. Riva, P. Rantala, L. Heikkinen, K. Daellenbach, J. E. Krechmer, P. M. Flaud, D. Worsnop, M. Kulmala, E. Villenave, E. Perraudin, M. Ehn and F. Bianchi, Terpenes and their oxidation products in the French Landes forest: insights from Vocus PTR-TOF measurements, Atmos. Chem. Phys., 2020, 20, 1941–1959 CrossRef CAS.
- M. S. Claflin, D. Pagonis, Z. Finewax, A. V. Handschy, D. A. Day, W. L. Brown, J. T. Jayne, D. R. Worsnop, J. L. Jimenez and P. J. Ziemann, et al., An in situ gas chromatograph with automatic detector switching between PTR-and EI-TOF-MS: isomer-resolved measurements of indoor air, Atmos. Meas. Tech., 2021, 14, 133–152 CrossRef CAS.
- T. Majchrzak, W. Wojnowski, M. Lubinska-Szczygeł, A. Różańska, J. Namieśnik and T. Dymerski, PTR-MS and GC-MS as complementary techniques for analysis of volatiles: a tutorial review, Anal. Chim. Acta, 2018, 1035, 1–13 CrossRef CAS PubMed.
- F. Wania, Y. Lei, C. Wang, J. Abbatt and K.-U. Goss, Using the chemical equilibrium partitioning space to explore factors influencing the phase distribution of compounds involved in secondary organic aerosol formation, Atmos. Chem. Phys., 2015, 15, 3395–3412 CrossRef CAS.
- J. Brown, A New Thermal Desorption Tube for Sampling Terpenes in Air, Supelco Analytical Products, 2023, 6, 1–6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Methods: questionnaire; facilities information; GC-FID calibration data; discussion of sampling program; low-cost sensor data and calibration; light scanning; reactions kinetics data; dispersion modelling inputs. Results: time series plots for all rooms; tables with terpene variation per room. Terpenes correlation analysis. See DOI: https://doi.org/10.1039/d5em00253b |
| This journal is © The Royal Society of Chemistry 2025 |