Open Access Article
Open Access ArticleAnalytical techniques for studying cell aging in lithium–sulfur batteries
Ritu
Malik
,
Vijay K.
Tomer
 * and
Mohini
Sain
* and
Mohini
Sain
Department of Mechanical & Industrial Engineering, University of Toronto, Canada. E-mail: vj.kumar@utoronto.ca
First published on 14th January 2025
Abstract
Lithium–sulfur batteries (LiSBs) hold promise for future energy storage due to their high theoretical energy density, but practical use faces challenges like capacity loss, short cycle life, and poor rate performance, primarily due to sulfur's complex redox reactions and polysulfide dissolution. Despite these challenges affecting LiSBs’ lifespan and durability, post-mortem analysis of aged cells with physicochemical techniques is increasingly essential for studying batteries, identifying new species, and monitoring electrode health and energy stability. This review explores the literature on analyzing aged LiSBs, encompassing disassembly methodologies and techniques (microscopic, spectroscopic, and electrochemical) to characterize materials retrieved from aged batteries. This discussion explores how these techniques have been crucial in studying structural, morphological, and chemical changes in LiSBs during cycling, highlighting key findings and insights, while also addressing challenges and future directions in post-mortem analysis, emphasizing the need for advanced analytical methods and multi-modal approaches to unravel complex degradation mechanisms, ultimately advancing the LiSBs.
Broader contextReviewing post-mortem analytical techniques in lithium–sulfur batteries (LiSBs) provides a comprehensive understanding of the complex degradation mechanisms that limit their performance and longevity. By reviewing post-mortem analytical techniques, the most effective methods for studying LiSB degradation can be identified, including changes in structure, morphology, and chemistry. This knowledge can lead to the development of strategies to mitigate degradation, such as optimizing electrode/separator design, electrolyte composition, and cycling conditions. Additionally, the review provides insights into the broader field of battery research, highlighting the basic principles of analytical techniques and key insights in understanding battery degradation. This information can guide future research directions, such as the development of new analytical tools and techniques for studying other types of batteries. Overall, this comprehensive overview of the post-mortem analytical techniques in LiSBs offers valuable insights for researchers working to improve the performance and longevity of these promising energy storage devices with implications for improving the performance, safety, and sustainability of battery systems. |
1. Introduction
Batteries, which convert chemical energy into electricity, are crucial for devices like portable electronics, satellites, medical equipment, EVs, and renewable energy systems, and expanding their market requires improving electrochemical performance, lifespan, cost efficiency, and environmental impact.1–5 Lithium–sulfur batteries (LiSBs) stand out among current chemistries for their high potential in technological applications, offering exceptional specific capacity and cost-effective materials, with theoretical specific energy and volumetric energy densities around 2600 W h kg−1 and 2800 W h L−1—2 to 6 times greater than the limits of advanced commercial lithium-ion batteries (LIBs).6–9 Despite their high energy density, abundant precursors, and cost-effectiveness, LiSBs face commercialization challenges, including sulfur's poor conductivity and discharge species (lithium polysulfides, LiPS) leading to high electrochemical polarization and impedance, with LiPS diffusing into the electrolyte and reacting with the anode (shuttle effect), and sulfur cathode volume changes causing pulverization and detachment from conductive substrates.10–13 In addition, advancing LiSBs requires enhancing lifespan, fast charging, low-temperature performance, self-recovery, and safety while addressing issues like lithium plating, cathode pulverization, microstructural loss, and chemical degradation, making a thorough understanding of these mechanisms crucial for optimal design and safe operation.14,15The redox chemistry of LiSBs is more complex than LIBs, involving solid-state S8 and lower-ordered LiPSs (Li2S2/Li2S), while long-chain LiPSs dissolve in the electrolyte, causing the sulfur cathode to transition from solid to liquid and back during cycling, leading to significant challenges.16–18 Despite significant progress, gaps remain in fully understanding battery degradation, such as side reactions during cycling, aging, and material-level chemical breakdowns, leading to capacity loss and increased resistance, making it essential to grasp these mechanisms to enhance cell lifespan. Electrochemical methods such as cyclic voltammetry, galvanostatic cycling, EIS, and rate capability tests are used to explore reaction mechanisms and evaluate battery performance, linking macroscopic behavior with LiSBs’ internal design for optimal composition and structure; however, since these measurements alone can't fully uncover reaction pathways or microscopic electrode changes, turning the battery into a “black box”, in situ and operando techniques are employed to study real-time reaction mechanisms and sulfur cathode state changes.19–27 Compared to the operando methods, the postmortem analytical techniques offer distinct advantages over the operando methods for analyzing the operation and degradation of LiSBs, particularly after cycling or aging.28,29 LiSBs involve complex electrochemical processes, including the polysulfide shuttle, sulfur dissolution, and the formation of insulating Li2S during discharge. These processes contribute to capacity fading and induce structural and chemical transformations that are difficult to capture in real-time.30 Operando techniques, which provide valuable real-time insights into dynamic processes such as phase transitions, lithium-ion transport, and electrode–electrolyte interactions, often face limitations in spatial and temporal resolution due to the constraints of observing these changes while the battery is actively cycling.31–33 As a result, long-term degradation phenomena, such as irreversible sulfur species deposition or gradual electrode degradation, may go undetected. In contrast, postmortem analysis enables the detailed disassembly and examination of battery components after operation, providing a comprehensive understanding of cumulative degradation and failure modes. Because the battery is no longer operating, postmortem techniques can dismantle and closely examine battery components without concern for disrupting ongoing processes. Techniques such as scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and energy-dispersive X-ray spectroscopy (EDX) allow for the thorough investigation of electrode surfaces, residual sulfur species, and degradation products like LiPSs.34–38 Postmortem methods are highly effective at uncovering long-term degradation in lithium–sulfur batteries, revealing microstructural changes, electrolyte decomposition, and passivation layers that are not easily detected during operation. Postmortem analysis excels at identifying slower degradation processes like dendrite growth, particle cracking, and SEI evolution.28 By allowing battery disassembly, it provides a clearer understanding of failure modes such as material dissolution, lithium plating, inactive phase accumulation, and structural damage, making it more suitable for diagnosing root causes of failure after extended cycling.39,40 The increasing interest in examining aged LiSBs (Scheme 1) with various tools highlights the need for standardized procedures in cell opening, components (electrodes and separators, etc.) recovery, sample processing, and analysis to ensure accurate and reliable data while minimizing damage and contamination.
In this review, we cover advanced physicochemical methods for analyzing various components of aged LiSBs, addressing a gap in the literature where existing reviews on in situ and operando techniques for LiSBs have not concentrated on post-mortem analysis.41–43 As the first review in this direction, we outline the underlying principle for electrochemical approaches, microscopic, X-ray-based spectroscopic, and optical spectroscopic techniques (Scheme 2). These methods help understand degradation mechanisms in LiSBs and provide successful examples demonstrating the information derived from these techniques and their applications in the battery field (Table 1). We also discuss the latest methods which have been explored to analyze LIBs but not for LiSBs, highlighting the crucial gap in current post-mortem approach and suggesting future directions to enhance understanding of LiSBs.
| Method | Applications and benefits | Drawbacks | Equipment cost | Time consumed |
|---|---|---|---|---|
| Electrochemical impedance spectroscopy (EIS) | • EIS accurately assesses battery health through internal resistance measurements. | • Struggles to distinguish between overlapping processes like SEI formation and LIPSs migration. | * | ∼1 h |
| • It provides real-time monitoring of the state of charge and health. | • Limited frequency range reduces accuracy in detecting early dendrite growth. | |||
| • EIS identifies specific degradation mechanisms affecting battery performance. | • May not fully capture rapid electrode degradation or transient phenomena. | |||
| • It optimizes battery design by analyzing impedance contributions from components. | • Electrode polarization effects can obscure subtle impedance changes during cycling. | |||
| • EIS evaluates thermal stability, enhancing safety and preventing thermal runaway. | • Complex interpretation of overlapping signals from polysulfides and SEI layer. | |||
| • Less sensitive to detecting minor structural changes or local electrode defects. | ||||
| Cyclic voltammetry (CV) | • CV evaluates lithium intercalation and deintercalation reversibility in batteries. | • Lacks detailed insights into solid-state processes within the electrode bulk. | * | 1–2 h for a fast scan |
| • Measures peak currents and potentials to analyse electrochemical processes. | • Ineffective in detecting dendrite formation or early-stage lithium-metal deposition. | 2–3 days for a slow scan rate | ||
| • Provides insights into the stability of electrode materials during cycling. | • Insufficient for tracking high-rate or variable current behaviors during cycling. | |||
| • It assesses capacity retention and efficiency in fresh and cycled batteries. | • Limited sensitivity to SEI layer evolution, especially under dynamic cycling conditions. | |||
| • Aids in optimizing electrolyte formulations for enhanced performance. | • Poor resolution for distinguishing between polysulfide formation and side reactions. | |||
| • Does not provide comprehensive information on electrode degradation or microstructural defects. | ||||
| Scanning electron microscopy (SEM) | • Visualizes multi-dimensional structures in lithium-ion and lithium–sulfur batteries. | • Can damage e-beam-sensitive materials, such as the SEI layer. | ***** | 1–2 h for sample preparation and analysis |
| • Reveals details of crack formation in electrode materials during cycling. | • Limited in providing chemical composition details, crucial for analyzing polysulfide formation. | |||
| • Provides insights into morphological changes affecting battery performance over time. | • Inability to directly detect lithium ions or track dendrite growth beneath the surface. | |||
| • Enables assessment of surface features and porosity in electrode materials. | • Poor resolution for capturing dynamic electrode changes during cycling or side reactions. | |||
| • Helps evaluating the electrolyte interactions with electrodes under different conditions. | • Ineffective in visualizing early-stage electrode degradation or microstructural defects. | |||
| • Assists in understanding degradation mechanisms in fresh and cycled batteries | • Requires sample preparation that may alter the native morphology of sensitive materials | |||
| Transmission electron microscopy (TEM) | • Provides detailed insights into particle morphology in batteries. | • Only scans small sample areas, limiting analysis of larger electrode structures. | ******** | 1–2 h for sample preparation and analysis |
| • Analyzes crystallinity to assess material quality and stability. | • Sample preparation can alter the SEI layer and disrupt lithium-ion or polysulfide dynamics. | |||
| • Reveals stress distributions within electrode materials during battery operation. | • Less effective for detecting bulk dendrite formation or large-scale electrode degradation. | |||
| • Identifies magnetic domains, enhancing understanding of material properties. | • Difficult to capture real-time structural changes during cycling, especially for side reactions. | |||
| • Helps investigate phase changes in fresh and cycled batteries. | • High-vacuum conditions can induce material modifications, especially in sensitive sulfur or lithium components. | |||
| • Facilitates the study of nanoscale structural changes impacting battery performance | • Limited ability to provide chemical state information crucial for understanding electrode–electrolyte interactions. | |||
| Atomic force microscopy (AFM) | • Assesses surface roughness of active materials in batteries. | • Lacks real-time data on lithium-ion dynamics and electrolyte interactions during cycling. | **** | ∼1–2 h |
| • Provides particle size distribution for improved material characterization. | • Time-consuming process with limited ability to capture rapid electrode degradation or polysulfide formation. | |||
| • Reveals morphology changes of electrode materials during cycling processes. | • Inefficient for analyzing bulk properties like dendrite growth or large-scale electrode defects. | |||
| • Analyzes solid electrolyte interphase (SEI) deposition on lithium anodes. | • Provides no chemical composition information on SEI layer, polysulfides, or side reactions. | |||
| • Aids in understanding the relationship between structure and performance. | • Limited resolution for detecting nanoscale features such as lithium-ion transport pathways. | |||
| • Enhances insights into nanoscale features affecting battery efficiency and stability | • Cannot effectively measure internal structural changes or interactions within the battery electrodes. | |||
| Optical imaging | • Provides visual analysis of electrode degradation in batteries. | • Limited resolution prevents detailed visualization of SEI layer formation and degradation. | * | ∼0.5 h |
| • Captures color changes indicating chemical reactions during cycling. | • Cannot detect nanoscale defects like dendrite growth or subtle electrode surface changes. | |||
| • Allows for the examination of macroscopic defects in active materials. | • Inadequate for observing molecular-level interactions or side reactions, such as polysulfide shuttling. | |||
| • Optical images help assess the uniformity of electrode coatings. | • Unable to capture small cracks or morphological changes during lithium-ion cycling. | |||
| • Facilitates monitoring of interface interactions between electrodes and electrolytes. | • Lacks chemical information on lithium-ion migration or electrode composition. | |||
| • Aids in evaluating overall battery performance and stability over time. | • Ineffective for real-time analysis of rapid degradation processes in lithium–sulfur batteries. | |||
| X-ray photoelectron spectroscopy (XPS) | • Identifies surface compositions of electrode materials in batteries. | • Limited to surface analysis, providing no depth-resolved information on SEI or dendrites. | ******** | ∼3–4 h |
| • Determines oxidation states to reveal chemical changes during cycling. | • Cannot detect lithium-ion migration or polysulfide formation occurring beneath the surface. | |||
| • Analyzes electronic structures at the electrode/electrolyte interface for performance insights. | • Costly instrumentation and high-vacuum requirements complicate in situ or operando analysis. | |||
| • Characterizes SEI formation and its impact on cycling. | • Sensitivity to surface contamination may skew results in cycled lithium–sulfur batteries. | |||
| • Detects impurities that may adversely affect battery efficiency. | • Ineffective in capturing fast, dynamic changes like electrolyte degradation or side reactions. | |||
| • Provides information on surface functional groups influencing electrode behavior. | • Difficult to analyze materials with complex or mixed oxidation states due to overlapping signals. | |||
| • It helps monitor degradation processes in fresh and cycled batteries. | ||||
| X-ray diffraction (XRD) | • Tracks crystal changes in sulfur during battery cycling. | • Unable to detect soluble LiPSs during battery cycling or degradation. | **** | ∼1–2 h (slow scan) |
| • Identifies phase transitions in lithium polysulfides (LiPS) over time. | • Lacks sensitivity to amorphous SEI layer formation and dendrite initiation on the lithium anode. | |||
| • Analyzes the structural stability of electrode materials throughout cycling. | • Provides insufficient information on early-stage electrochemical changes in sulfur cathode. | |||
| • Provides insights into crystallinity changes affecting battery performance. | • Ineffective in analyzing side reactions or subtle structural defects in cycled electrodes. | |||
| • Helps determine the optimal composition of electrode materials. | • Limited to crystalline phases, missing vital information on electrolyte breakdown or degradation. | |||
| • Aids in understanding the effects of cycling on material properties. | • Fails to capture dynamic real-time changes during charge–discharge cycles in LiSBs. | |||
| X-ray absorption near edge spectroscopy (XANES) | • Reveals electronic structures of metallic ions. | • Limited spatial resolution hinders detailed analysis of local chemical environments in electrodes. | ******* | ∼2–3 h |
| • Analyzes the chemical states of metals in battery electrodes. | • Insufficient sensitivity to detect low-concentration species like polysulfides or lithium dendrites. | |||
| • Provides insights into lithium coordination environments and bonding characteristics. | • Unable to provide comprehensive insights into SEI layer characteristics and formation dynamics. | |||
| • Helps identify redox reactions occurring in electrodes during cycling. | • Challenges in differentiating between closely related oxidation states during electrode degradation. | |||
| • Aids in understanding degradation mechanisms affecting battery performance over time. | • Requires extensive sample preparation, potentially altering the native state of materials. | |||
| • Does not capture transient or dynamic processes during charge–discharge cycles effectively. | ||||
| X-ray fluorescence spectroscopy (XRF) | • Provides quantitative elemental analysis of battery materials. | • Limited sensitivity to low atomic number elements like ‘lithium’ hinders analysis. | *** | ∼1–1.5 h |
| • Analyses the distribution of elements within battery components. | • Complex sample preparation may alter material properties before analysis occurs. | |||
| • Helps identify impurities that may impact battery performance. | • Inability to provide detailed information on local chemical environments in electrodes. | |||
| • Evaluates the composition of electrode materials in fresh and cycled batteries. | • Does not detect soluble polysulfides or transient species during electrochemical processes. | |||
| • Assists in monitoring changes in elemental concentrations during cycling. | • May struggle to differentiate between overlapping spectral lines in complex mixtures. | |||
| • Supports the development of advanced materials by analyzing elemental ratios. | • Surface analysis only, potentially missing important information from the bulk material. | |||
| Raman spectroscopy | • Detects molecular vibrations of sulfur and polysulfides in batteries. | • Weak scattering signals from soluble polysulfides limit detection sensitivity and accuracy. | ** | ∼0.5 h |
| • Provides insights into the chemical bonding and structure changes. | • Cannot effectively identify structural changes occurring within the SEI layer. | |||
| • Helps monitor the formation and dissolution of polysulfides. | • May struggle to resolve overlapping peaks in complex material spectra. | |||
| • Identifies phase transitions in sulfur during cycling processes. | • High laser intensity can cause thermal degradation of sensitive battery materials. | |||
| • Enables real-time monitoring of electrochemical reactions in batteries. | • Limited spatial resolution may miss fine details of electrode degradation. | |||
| • Assists in evaluating the stability of electrode materials. | • Inability to analyze bulk properties of materials restricts comprehensive understanding. | |||
| Fourier Transformation Infra-red (FTIR) spectroscopy | • Detects surface functional groups in battery materials and electrodes. | • Inability to differentiate among various carbon species complicates analysis. | ** | ∼0.5 h |
| • Identifies fragmented molecules resulting from electrochemical reactions. | • Limited detection of low-concentration species may overlook critical reactions. | |||
| • Helps monitor changes in the SEI. | • Weak signals from solid-state materials can hinder accurate interpretation. | |||
| • Provides insights into electrolyte decomposition and stability during cycling. | • Cannot provide detailed information on three-dimensional electrode structures. | |||
| • Aids in understanding chemical interactions between electrode materials and electrolytes. | • Requires extensive sample preparation that may alter material properties. | |||
| • Assists in evaluating the effectiveness of additives in improving performance. | • Poor spatial resolution limits the analysis of localized degradation phenomena. | |||
| Energy dispersive X-ray spectroscopy (EDX) | • Verifies the elemental composition of active materials in batteries. | • Inability to detect lithium limits comprehensive analysis of battery materials. | *** | ∼1–2 h |
| • Detects additional phases, enhancing understanding of material interactions. | • Requires complementary techniques like SEM or TEM for effective operation. | |||
| • Facilitates spatial mapping of element distributions within electrode structures. | • Poor sensitivity to light elements complicates understanding of SEI layers. | |||
| • Provides quantitative analysis of elemental ratios critical for performance optimization. | • Limited spatial resolution restricts analysis of microstructural changes in electrodes. | |||
| • Assists in identifying contaminants that may degrade battery efficiency. | • Elemental mapping can be hindered by overlapping X-ray signals. | |||
| • Enables characterization of morphological changes in cycled battery components | • Surface contamination may significantly affect quantitative compositional analysis accuracy. | |||
| Inductive coupled plasma -atomic emission spectroscopy (ICP-AES) | • Detects new materials migrating from the cathode to the anode. | • Limited depth profiling restricts understanding of layer-specific composition changes. | ***** | ∼1–1.5 h |
| • Quantitatively analyzes trace elements in battery components. | • Lack of information on sample morphology complicates analysis of electrode defects. | |||
| • Provides insights into contamination sources affecting battery performance. | • Inability to detect soluble lithium polysulfides hinders comprehensive battery assessment. | |||
| • Characterizes ionic species in electrolytes during charge–discharge cycles. | • Cannot differentiate between oxidation states of transition metal ions present. | |||
| • Enables detailed analysis of elemental leaching processes in batteries. | • Requires extensive sample preparation, potentially altering the material's characteristics. | |||
| • Assists in evaluating the effectiveness of protective coatings on electrodes | • Sensitivity to volatile components may lead to inaccuracies in elemental quantification. | |||
| Electron paramagnetic resonance (EPR) | • Detects and quantifies paramagnetic species like radicals in battery systems. | • Limited sensitivity to paramagnetic species restricts detection of critical intermediates. | **** | ∼1–1.5 h |
| • Identifies transition metal ions influencing battery degradation mechanisms. | • Low operating temperatures fail to simulate actual battery operating conditions. | |||
| • Provides insights into reactive intermediates formed during electrochemical reactions. | • Inability to quantify species affects understanding of degradation mechanisms. | |||
| • Aids in characterizing the impact of impurities on battery performance. | • Difficulty in analyzing complex multi-species systems complicates interpretation of results. | |||
| • Analyzes spin states that correlate with charge transport processes. | • Time-consuming sample preparation may alter the state of the electrodes. | |||
| • Facilitates understanding of radical formation and stabilization in batteries | • Requires advanced equipment and expertise, increasing operational costs and complexity. | |||
| Secondary-ion mass spectrometry (SIMS) | • Provides detailed analysis of the SEI layer's elemental composition. | • Poor focusing capability leads to reduced spatial resolution in analyses. | ******** | ∼1–1.5 h |
| • Characterizes molecular species present on battery material surfaces. | • Difficult quantification limits accurate determination of elemental concentrations. | |||
| • Enables depth profiling to study SEI layer development over cycles. | • Sophisticated electronics increase complexity and maintenance costs of the system. | |||
| • Quantifies lithium distribution within electrodes, revealing interfacial dynamics. | • Sample damage during analysis may alter the state of electrodes. | |||
| • Assists in identifying degradation products influencing battery performance. | • Limited detection of lighter elements like lithium complicates comprehensive analysis. | |||
| • Facilitates spatial mapping of elemental compositions across surfaces | • Time-intensive sample preparation can hinder rapid assessments of battery performance. | |||
| Nuclear magnetic resonance (NMR) spectroscopy | • Elucidates the dynamics and diffusion properties of lithium ions. | • Expensive experimental setup limits accessibility for routine battery analysis. | ******** | ∼1–1.5 h |
| • Provides insights into the formation and composition of the SEI. | • Inability to capture dynamic processes during battery operation hinders insights. | |||
| • Characterizes the local environment of lithium in various phases. | • Limited sensitivity to sulfur species restricts analysis of key battery components. | |||
| • Allows for the monitoring of solvent interactions with lithium species. | • Low resolution may miss crucial details in electrode degradation phenomena. | |||
| • Enables the analysis of molecular mobility within electrode materials. | • Sample preparation can alter the state of materials, affecting results. | |||
| • Helps identify structural changes during battery cycling processes. | • Long acquisition times reduce throughput for analyzing multiple samples efficiently. | |||
| N2-sorption isotherms | • Provide quantitative data on the surface area of electrodes. | • Lack of electrochemical information limits understanding of ion transport dynamics. | ** | 6–8 h for degassing and 1–2 h for BET analysis |
| • Measures pore volume, critical for ion transport analysis. | • Does not provide insights into the stability of SEI layers formed. | |||
| • Elucidates the accessibility of electrolytes to electrode materials. | • Cannot directly correlate surface area with performance in cycling conditions. | |||
| • Enables characterization of porosity, influencing overall battery performance. | • Limited detection of microstructural defects affecting lithium-ion mobility. | |||
| • Assesses structural stability and changes during cycling. | • Does not account for changes in morphology during battery cycling. | |||
| • Helps optimize electrode design for enhanced lithium-ion diffusion. | • Inability to analyze real-time effects of polysulfides on electrode structure. | |||
2. Standard procedure for cell opening
Scheme 3 outlines the steps in post-use cell analysis, where non-destructive methods like CV and EIS provide initial insights into aging mechanisms before disassembly, but direct observation of chemical changes requires opening the cell (Scheme 4).19,44 Localized aging phenomena, often missed in averaged electrochemical measurements, necessitate crucial steps before cell opening, like charging or discharging to a specific state of charge (SOC).45–47 To ensure safety, deep discharge to 0 V is recommended, as it lowers energy content and minimizes the risk of thermal runaway in the event of a short circuit.48 Since some LiSB components react with O2 and H2O, a glove box with a high-purity Ar atmosphere (<0.1 ppm H2O and O2) is essential, and precautions must be taken during cell opening to prevent internal short circuits, which are most likely when cutting the cell housing or applying mechanical pressure to the electrode stack or jelly roll.49 After separating the cell components, electrolyte solvents like DME or DOL are used to clean to eliminate residual crystallized LiPSs or non-volatile solvents that could be confused with SEI elements or intercalated Li.493. Post-mortem characterization techniques
3.1. Electrochemical methods
Post-mortem characterization of LiSBs using electrochemical methods involves analyzing the electrochemical behavior of the cell components after cycling (before cell disintegration) to understand the behavioral changes and deviation from the fresh cells.20,22 Some key electrochemical methods for post-mortem characterization of LiSBs include: | ||
| Fig. 1 EIS variations in cells over 100 cycles in (a) 1 M and (b) 0.1 M electrolytes; reproduced with permission.50 Copyright 2020, American Chemical Society. (c) EIS plot of the integrated S-CNTs/Concan's/PVDF membrane after 200 and 400 cycles; reproduced with permission.51 Copyright 2019, Wiley-VCH. (d) EIS plots of Li symmetric cells after 10 and 100 cycles and (e) schematic of LiSB preparation using a 3D-printed N-pTi3C2Tx framework. Reproduced with permission.52 Copyright 2021, Elsevier. | ||
Similarly, Wang et al. utilized electrospinning and base-coating to create a flexible fibrous membrane that combines the cathode, interlayer, and separator into a single composite, improving polysulfide confinement, electron transfer, and Li+ diffusion while resolving interface issues.51 This LiSB starts with a capacity of 1501 mA h g−1 and retains 933 mA h g−1 after 400 cycles with minimal loss (0.069% per cycle), and demonstrates a consistent Rct reduction (Fig. 1c) from 16.86 Ω to 2.30 Ω, reflecting enhanced stability, better efficiency in electron and ion transfer, and improved sulfur redox kinetics. Wei et al.52 reported increased Rct after 100 cycles (Fig. 1d) and better Li+ conductivity using a 3D-printed N-doped Ti3C2 MXene framework (Fig. 1e), which acts as a sulfur host and lithium dendrite blocker. The 3D structure's porosity enhances charge transport and provides ample sulfur redox sites, while the N-pTi3C2Tx layer at the anode supports Li+ deposition, prevents dendrite formation, and ensures consistent Li stripping/plating due to enhanced lithiophilicity and stable SEI from fluorine groups. The composite cell shows reduced interfacial resistance and improved Li+ conductivity, with decreasing impedance over cycles, indicating better conductivity and SEI stability.
3.2. Microscopic techniques
Microscopy is pivotal in the post-mortem analysis of LiSBs, offering detailed insights into the structural and morphological alterations occurring during cycling.62 Techniques such as electron and optical microscopy enable the examination of electrode morphology, sulfur species distribution, and SEI layer formation.63,64 This detailed microscopic analysis is crucial for elucidating the degradation mechanisms responsible for capacity loss and cycling instability in LiSBs.64 Key microscopic methods include: | ||
| Fig. 3 SEM images and EDS spectra of CF@2H/1T MoS2, (a) before cycling, (b and c) after 50 cycles; reproduced with permission.68 Copyright 2021, American Chemical Society. Surface morphology of lithium metal anodes (d) before cycling and (e) after cycling, from the Li–S batteries using S@PNCS/NG cathode over 50 cycles at 1 C. Reproduced with permission.69 Copyright 2022, American Chemical Society. SEM image after 200 cycles for (f) CNF/CMPA/S (g) CNF/PANi/S, (h) CNF/S electrode (i) optical image of the fresh and cycled lithium. Reproduced with permission.70 Copyright 2022, Royal Society of Chemistry. (j–l) SEM images of electrodes containing different CMA contents and (m–o) folding fan structure. Reproduced with permission.71 Copyright 2023, Elsevier. (p) Cross-section SEM image of the Li anode-SPE interface in the S/SPE/Li cell (p-1) before discharge, (p-2) after the first cycle, (p-3) after ten cycles. Reproduced with permission.72 Copyright 2021, Wiley-VCH. (q) SEM images of Li2S cathodes after 5 cycles in electrolytes with/without TTCA-Li. Reproduced with permission.73 Copyright 2023, Wiley-VCH. | ||
Conducting polymers like polyaniline, known for their strong LiPS affinity and high conductivity, face challenges such as low polarity, instability, and limited surface area.74,75 To address these issues, Chen et al.70 developed a stable and 3D microporous conjugated microporous polyaniline (CMPA) with an extended p-conjugated system by combining the high surface area and N-rich properties of microporous polymers with the stability and conductivity of polyaniline. SEM analysis of cathodes post 200 cycles (Fig. 3f–h) reveals amorphous agglomerates of sulfur-modified materials and fused CNFs, leading to the collapse of the 3D CNF/S network. The optical image of the lithium anode (Fig. 3i) paired with the CNF/CMPA/S cathode shows minimal polysulfide presence, highlighting CMPA's superior LiPS trapping capability, which contributes to a high areal capacity of 7.42 mA h cm−2 and an energy density of 202.8 W h kg−1 cell, even at a sulfur loading of 8.72 mg cm−2.
The mixed-metal spinel oxide Co2Mn0.5Al0.5O4 (CMA) is an effective cathode material,76 with its cobalt and manganese oxides strongly interacting with sulfur species and its spinel structure—featuring tetrahedral and octahedral vacancies—actively trapping polysulfides and various LPS species. Santos et al.71 created a carbon-CMA composite for LiSBs, which showed folding-fan-like structures in SEM images (Fig. 3(j–l)), indicating effective LiPS trapping. The 90% CMA electrode (Fig. 3(m–o)) exhibited no needle-like structures, suggesting different SEI properties and demonstrating CMA's superior LiPS trapping, enhancing initial capacity to 1000 mA h g−1 and extending cyclability to about 360 cycles. In a significant study by Liu and colleagues,72 liquid electrolyte-based lithium–sulfur batteries (LELS) were shown to outperform solid electrolyte-based batteries. In LELS systems, only soluble polysulfides dissolve into the electrolyte and induce uniform corrosion of the anode. In contrast, in solid-state batteries using a PEO-based solid electrolyte, both polysulfides and sulfur dissolve, leading to uneven passivation on the anode surface. This uneven passivation results in non-uniform Li+ plating and stripping at the anode/solid electrolyte (SPE) interface (Fig. 3p). Over successive charge–discharge cycles, this inhomogeneity worsens, causing fluctuations in overpotential and ultimately resulting in cell failure. Similarly, Geng et al.73 developed a Li2S cathode coated in situ with a polymerizable electrolyte additive, trithiocyanuric acid trilithium salt (TTCA-Li), to reduce initial overpotential and limit lithium polysulfide shuttling. A full cell featuring the coated Li2S cathode paired with a lithium anode demonstrated coulombic efficiency exceeding 99.5%. The approach also proved effective in lithium-free cells, as shown in a high Li2S-loading pouch cell, and was extended to sulfur-based batteries using TTCA-Li additives. The SEM images (Fig. 3q) after five cycles revealed that the TTCA-Li-coated Li2S cathode maintained a smooth and stable surface, while the uncoated Li2S cathode exhibited significant surface roughness and structural fracture due to sulfur redistribution during cycling.
 | ||
| Fig. 4 (a-1) TEM image after 1000 cycles, (a-2) HRTEM image after discharging. Reproduced with permission.82 Copyright 2022, Springer Nature. TEM and EDX mapping of cathode after 200 cycles at 1 C (b and c) charged state and (d and e) discharged state; reproduced with permission.83 Copyright 2018, Wiley-VCH. TEM of the electrodes after 100 cycles and corresponding EDX mapping for (f) S-DIB-OLC-10; (g) S-DIB-OLC-10; (h) S-DIB-OLC-30. Reproduced with permission.84 Copyright 2017, Royal Society of Chemistry. | ||
The formation of inhomogeneous lithium deposition and rough SEI layers in lithium anodes leads to dendritic growth and the accumulation of “dead lithium”, resulting in reduced efficiency, capacity fading, and safety risks.90,91 Senthil et al. used AFM to analyze lithium anodes after 800 hours of cycling,92 finding that bare lithium anodes exhibited insufficient elasticity to accommodate strain during lithium expansion, as indicated by cantilever tip deflection (Fig. 5a). In contrast, graphene quantum dot (GQD)-modified anodes showed a more linear deflection pattern, suggesting elastic SEI restructuring, a smoother surface, and enhanced cycling stability (Fig. 5b). The lithium anode was recovered from an in situ restructured graphene quantum dots (GQDs) based separator, which not only formed an ultrasmooth and thin SEI by creating an intimate microstructure with the metallic lithium but also served as a physical barrier to prevent polysulfide diffusion and shuttle effects, thus reducing polysulfide deposition and lithium metal corrosion. To enhance SEI formation, Wang et al.93 developed a symmetrical polypropylene separator modified on both sides with VS2 nanotowers to maintain pore integrity. AFM images after 100 cycles (Fig. 5c) showed that while a standard PP separator developed rough dendritic surfaces, the VS2-modified separator remained smooth and effectively prevented dendrite growth. This separator exhibited a 16-fold increase in capacity (8.3 mA h cm−2) compared to a conventional PP separator (0.5 mA h cm−2) due to its “sulfiphilic” and “lithiophilic” properties, which mitigate LiPSs shuttle and support stable lithium growth.
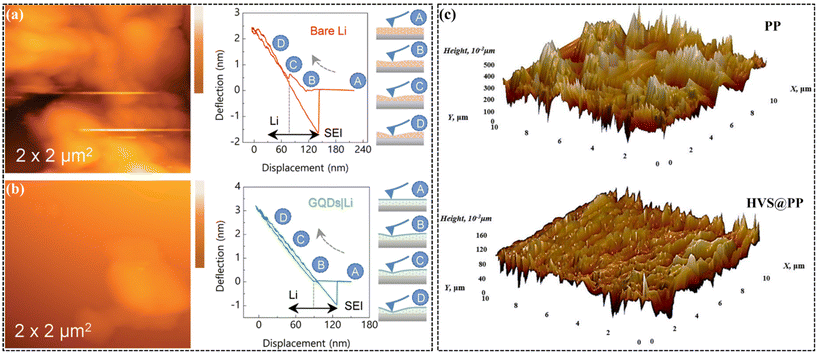 | ||
| Fig. 5 Images of Li anodes recovered from bare Li (a) and GQDs employed cell (b); reproduced with permission.92 Copyright 2022, Wiley-VCH. (c) AFM images of the surfaces of the lithium anode in the symmetric cells with different separators after 100 cycles. Reproduced with permission.93 Copyright 2020, American Chemical Society. | ||
In a notable example, Zeng et al.97 developed a metal–organic framework (MOF)-sulfur copolymer cathode to enhance redox kinetics and Li-ion transfer efficiency. After 100 cycles at a rate of 1 C, the CNT@UiO-66-V/S cathode exhibited the presence of notable yellow polysulfides (Fig. 6a) and corroded lithium metal anode, while the CNT@UiO-66-V-S anode (Fig. 6b) maintained a smoother surface with thinner dendrites and showed no detectable LiPSs. The CNT@UiO-66-V-S cathode delivered over a 100% improvement in discharge capacity at high sulfur loadings, demonstrating its superior electrochemical performance. In another study, Cheng et al.98 investigated 2D transition metal disulfides (VS2 and NbS2), finding that VS2 adsorbed polysulfides slightly better, enhancing catalytic activity. Incorporating 1/3 V into NbS2 slabs to form Nb3VS6 yielded high capacities of 1541 mA h g−1 at 0.1 C and retained 73.2% capacity post 1000 cycles. Optical images (Fig. 6c–f) showed that polyethylene separators with Nb3VS6 effectively suppressed shuttling and remained almost pristine.
 | ||
| Fig. 6 (a and b) Digital images of battery with different cathodes after 100 cycles; reproduced with permission.97 Copyright 2022, Wiley-VCH. (c) Optical images of Li metal and separator before cycling, post-mortem analyses of the (d) NbS2, (e) VS2, and (f) Nb3VS6 cells after 200 cycles at 1 C; reproduced with permission.98 Copyright 2023, American Chemical Society. (g) Optical images of the cathode, separator, and anode of the S@ResFArGO based pouch cell after cycling; reproduced with permission.99 Copyright 2022, Wiley-VCH. (h) Photographs of the anodes and separators of the V2C/S, V2C-140/S, V2C-160/S and V2C-180/S cells (from left to right) after 500 cycles at 0.5 C. Reproduced with permission.100 Copyright 2021, Royal Society of Chemistry. | ||
In another work, Carriazo et al.99 demonstrated the effectiveness of innovative porous carbon composites for sulfur cathodes by synthesizing two distinct types: one combining graphene oxide (GO) with coffee waste-derived carbon (rGOCaf) and another formed through resorcinol/formaldehyde condensation with GO (ResFArGO), with both composites characterized by the incorporation of graphene and small micropores, which together enhance electronic conductivity and efficiently trap LiPS. The LiSB with ResFArGO achieved over 1100 mA h g−1 capacity at high sulfur loadings (4 mg cm−2) and excellent C-rate performance. Post-mortem analysis (Fig. 6g) showed fractured electrodes with detached active material adhering to the separator. Also, Xu et al.100 developed a flexible MXene-based sulfur cathode featuring V2C/VO2 nanoribbons (VCOR) pillared between V2C/VO2 nanosheets (VCOS) during hydrothermal processing, creating a robust, lightweight sandwich architecture. Post-mortem analysis (Fig. 6h) revealed a clean, smooth surface on the separator and Li foil, indicating effective LiPS blocking by the V2C-160 host which enables the cell to realize a high areal capacity of 6.3 mA h cm−2 and exceptional capacity holding under bending conditions.
3.3. X-ray-based spectroscopic techniques
X-ray-based spectroscopic techniques are crucial for analyzing cycled batteries, offering detailed insights into their chemical composition, structural evolution, and surface chemistry.101–103 These methods enable the assessment of oxidation state changes, formation of the SEI layer, and surface reactions during battery operation. Additionally, they track alterations in crystal structure and phase composition, providing a deeper understanding of degradation mechanisms and failure modes in battery materials.Du et al.110 employed XPS to investigate a polyethylene (PE)-supported gel polymer electrolyte (GPE) incorporating ester-rich pentaerythritol tetraacrylate (PETEA) and divinyladipate, which achieved an ionic conductivity of 2.8 × 10−4 S cm−1. XPS analysis after 100 cycles (Fig. 7a) revealed the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak (288.7 eV) from the polymer matrix, indicating interaction with lithium polysulfides (LiPSs). This interaction contributed to the battery's ability to retain 70% of its capacity after 300 cycles at 0.5 C, a significant improvement over the 29% retention observed with a liquid electrolyte, highlighting the effectiveness of the ester groups in capturing polysulfides and enhancing cycling stability.
O peak (288.7 eV) from the polymer matrix, indicating interaction with lithium polysulfides (LiPSs). This interaction contributed to the battery's ability to retain 70% of its capacity after 300 cycles at 0.5 C, a significant improvement over the 29% retention observed with a liquid electrolyte, highlighting the effectiveness of the ester groups in capturing polysulfides and enhancing cycling stability.
 | ||
| Fig. 7 (a) C 1s XPS spectra of pristine and cycled PEGPE; reproduced with permission.110 Copyright 2018, Elsevier. (b) XPS spectra of the A-TS membrane after 200 cycles; reproduced with permission.111 Copyright 2022, Elsevier. XPS spectra of S 2p (c) and Li 1s (d) for the S-PPy electrode cycled in a ternary electrolyte; reproduced with permission.112 Copyright 2021, Elsevier. (e) XPS spectra of S 2p and Li 1s after 400 cycles; reproduced with permission.51 Copyright 2019, Wiley-VCH. (f) XPS spectra of Li 1s, Mo 3d, and Te 3d regions after 50 cycles. Reproduced with permission.113 Copyright 2022, Wiley-VCH. | ||
Carbon-based nanofibers, with high electrical conductivity, enhance Li storage in materials like hard carbon and graphite but often have lower Li affinity than metal oxides (e.g., ZnO, Al2O3, SiO2, TiO2), and metals (e.g., Ag, Si, Mg) with lower Li nucleation barriers.114,115 To address this, Wang et al. developed TiO2/SiO2 (A-TS) nanofiber membrane which promotes uniform charge distribution and fast Li+ diffusion.111 The XPS spectra of the cycled cell (Fig. 7b) provided critical insights into the electrochemical conversion and alloying processes within the battery, revealing the presence of various Li-containing compounds, including SiO2 (with peaks at 103.0 and 102.1 eV), Li4SiOx (at 101.1 eV), and Li–Si (at 98.1 eV), which indicate successful alloying with “dead” lithium. Additionally, the presence of a Li–F bond at 55.7 eV suggests the formation of the SEI layer or possible interactions between “dead” lithium and the electrolyte, further elucidating the chemical transformations occurring during cycling.40
Further to enhance SEI protection in LiSBs, Pathak et al.112 developed a worm-like sulfur-polypyrrole (S-PPy) cathode. This core–shell structure prevents polysulfide–electrolyte contact, reducing capacity fading and improving sulfur utilization. XPS analysis of cycled cells (Fig. 7c and d) reveals Li–S, Li2Se, and Li2Sx species at B.E. < 163 eV and SEI layer S–O species at B.E. > 164 eV. The Li 1s peak at 53.6 eV indicates a strong interaction between LiPS and the polymer matrix. Also, Wang et al. developed flexible, high-flux electrospun fibrous membranes that improve LiPSs trapping, electron transfer, and Li-ion diffusion.51 XPS analysis (Fig. 7e) revealed key insights into the chemical processes during prolonged cycling of batteries, showing S 2p peaks at 169.1 and 170.2 eV that indicate the oxidation of LiPSs to sulfate, likely catalyzed by Co nanoparticles. A weak peak at 163–164 eV suggests the presence of terminal and bridge sulfur atoms from newly adsorbed sulfides, while the Li 1s spectrum shows a 56.5 eV peak for Li–N bonds, confirming effective interactions between nitrogen dopants and LiPSs, highlighting the role of CoNCNFs in facilitating redox reactions and stabilizing LiPSs during battery operation. In another work, He et al. developed a MoTe2-CNT composite with 1T′-MoTe2 nanosheets that facilitate uniform lithium deposition and generate a thin SEI layer of lithium thiotellurate on the Li surface, stabilizing Li deposition, suppressing electrolyte decomposition, and reducing lithium loss to enhance cycle life.113 XPS spectra (Fig. 7f) confirmed the presence of a Li2TeS3 coating on the cycled MoTe2-CNT/Li anode, which maintains dense lithium layers, inhibits electrolyte degradation, and minimizes lithium depletion, ultimately extending the battery's operational life.
Yeon et al.120 utilized XRD to investigate a nanosulfur (nS) and reduced graphene oxide (rGO) composite, synthesized through spray-frozen assembly and ozonation, which resulted in a robust rGO/nS hybrid that demonstrated enhanced redox kinetics, improved sulfur utilization, and high-rate capacities. Post-mortem XRD analysis after 100 cycles at 0.1 C (Fig. 8a) revealed a prominent peak at 22.8°, associated with the (222) plane of crystalline sulfur, indicating reversible structural changes in the nS during cycling, with the rGO/nS hybrid achieving a significant capacity of 1269.1 mA h g−1, outperforming other rGO/sulfur hybrids. Yao et al. developed a twinborn ultrathin 2D graphene-based mesoporous SnO2/SnSe2 hybrid (G-mSnO2/SnSe2) as a polysulfide inhibitor, combining strong chemical affinity, high conductivity, and a dynamic intercalation–conversion mechanism.121 XRD patterns after 500 cycles (Fig. 8b) confirmed the stability of the crystalline structure and the reversible intercalation–conversion of SnSe2, maintaining structural integrity during cycling. When used as a separator in LiSBs, this hybrid material enabled high sulfur utilization, achieving 1544 mA h g−1 at 0.2 C, with a slow capacity decay rate of 0.0144% over 2000 cycles at 5 C.
 | ||
| Fig. 8 (a) XRD analysis of fresh and cycled cells; reproduced with permission.120 Copyright 2019, American Chemical Society. (b) XRD pattern of G-mSnO2/SnSe2 modified separator after 500 cycles at 0.5 C under full charge state; reproduced with permission.121 Copyright 2022, American Chemical Society. (c) XRD pattern of ZnS-SnS@NC modified separator before and after 300 cycles at 0.2 C; reproduced with permission.122 Copyright 2021, American Chemical Society. (d) XRD patterns for the ZIF-Co@NCS cathode before and after 600 cycles; reproduced with permission.123 Copyright 2023, American Chemical Society. (e) XRD pattern of the cycled Bi2Te3/S cathode; Reproduced with permission.124 Copyright 2022, Wiley-VCH. (f) XRD pattern of the cathode extracted from a cell at the end of 150th charge compared with the pristine electrode. Reproduced with permission.125 Copyright 2019, American Chemical Society. | ||
Transition metal sulfides (e.g. ZnS, SnS, SnS2etc.), known for strong catalytic activity towards LiPSs and high electrical conductivity, hold significant potential in LiSBs, with ZnS demonstrating robust LiPS catalytic ability and SnS offering superior conductivity due to its narrower band gap compared to SnS2.126,127 Capitalizing on these properties, Yao et al.122 developed a ZnS–SnS heterojunction coated with N-doped carbon shell (ZnS–SnS@NC) as a modification layer on the separator. Post-mortem X-ray diffraction (XRD) analysis (Fig. 8c) revealed that the ZnS–SnS@NC separator preserved its crystalline structure after cycling, indicating its stability and contributing to improved electrochemical performance by facilitating uniform lithium deposition. In a notable advance, metal–organic frameworks (MOFs) have emerged as powerful agents for trapping polysulfides through their chemical affinity with lithium polysulfides (LiPSs). Archana et al.123 demonstrated this potential by employing a ZIF-67-based MOF to engineer a cobalt@nitrogen-doped carbon–sulfur composite (ZIF-Co@NCS) as a cathode host for lithium–sulfur batteries. Post-mortem XRD analysis after 600 cycles (Fig. 8d) unveiled new peaks at 32.1 and 33.7 degrees, corresponding to Li2S, indicative of the discharge product. This finding underscores the remarkable stability and effective Li+ ion storage capabilities of the ZIF-Co@NCS cathode, affirming its role in enhancing battery performance and longevity through robust polysulfide immobilization.
Topological insulators (TIs) like Bi2Te3 are promising for sulfur electrochemistry due to their Dirac cone surface band structure, which offers excellent charge transport properties. Song et al.124 utilized Bi2Te3, selected for its ultrahigh Hall mobility (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm2 V−1 s−1) and simple one-step solvothermal preparation, to accelerate sulfur redox kinetics. Bi2Te3 effectively anchored soluble sulfur species, creating seamless electron transport pathways with adsorbed polysulfides. XRD analysis (Fig. 8e) confirmed the crystal structure's stability during cycling, demonstrating Bi2Te3's ability to enhance both sulfur reduction and reverse reactions. Bhargav et al.125 synthesized polyethylene hexasulfide (PEHS) via a simple condensation reaction and integrated it into a CNT network to create a composite cathode. Post-mortem XRD analysis after 150 cycles (Fig. 8f) revealed increased intensity of CNT peaks and a lack of distinct sulfur peaks, indicating possible delamination or inaccessibility of the active material, yet the lithium–sulfur battery still exhibited a high capacity of 1274 mA h g−1.
200 cm2 V−1 s−1) and simple one-step solvothermal preparation, to accelerate sulfur redox kinetics. Bi2Te3 effectively anchored soluble sulfur species, creating seamless electron transport pathways with adsorbed polysulfides. XRD analysis (Fig. 8e) confirmed the crystal structure's stability during cycling, demonstrating Bi2Te3's ability to enhance both sulfur reduction and reverse reactions. Bhargav et al.125 synthesized polyethylene hexasulfide (PEHS) via a simple condensation reaction and integrated it into a CNT network to create a composite cathode. Post-mortem XRD analysis after 150 cycles (Fig. 8f) revealed increased intensity of CNT peaks and a lack of distinct sulfur peaks, indicating possible delamination or inaccessibility of the active material, yet the lithium–sulfur battery still exhibited a high capacity of 1274 mA h g−1.
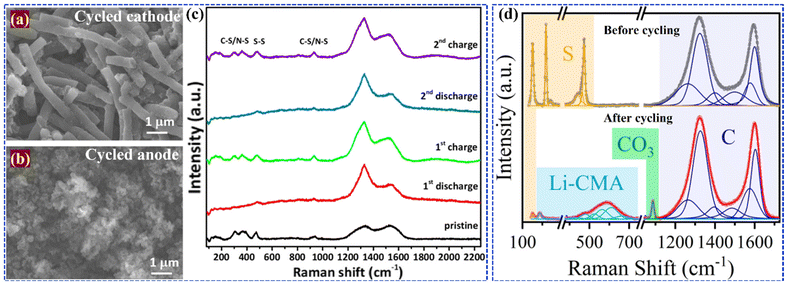
Zubair et al.132 developed free-standing cathodes by electrodepositing manganese oxide (MnOx) nanoflakes onto carbonized cellulose cloths, which enabled higher sulfur loadings with reduced electrolyte content. They achieved this by surface-engineering the MnOx nanoflakes through controlled annealing to obtain various oxidation states, resulting in defective interfaces that enhanced reaction activity, promoted polythionate formation, and improved LiPSs retention on the cathode surface. XANES analysis of the Mn K edge for the cycled electrodes (Fig. 9(a–c)) revealed that the MnOx/S cathodes processed in air at 300 °C and 400 °C exhibited a decrease in valence, while the MnOx/S cathode treated in argon at 400 °C showed a shift to a higher oxidation state due to incomplete delithiation, which enhanced structural stability. These oxygen-deficient manganese oxide nanoflake cathodes maintained a reversible capacity of 824 mA h g−1 at 0.5 C over 200 cycles.
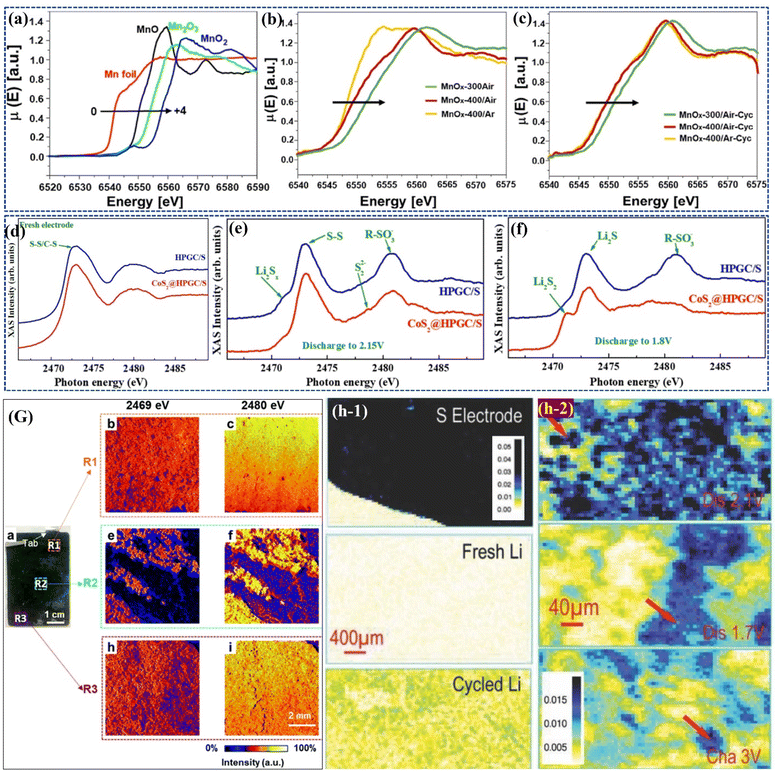 | ||
| Fig. 9 Mn K-edge XANES spectra for (a) Mn reference compounds, (b) fresh and (c) cycled electrodes. Reproduced with permission.132 Copyright 2023, Elsevier. TFY-NEXAFS spectra of the S K-edge (d) fresh electrode and cycled electrode discharged to (e) 2.15 V and (f) 1.8 V. Reproduced with permission.133 Copyright 2019, American Chemical Society. (G) Revealing the local chemical nature of cycled S cathode by combined XRF and XAS analysis. Reproduced with permission.134 Copyright 2020, Royal Society of Chemistry. (h-1) XRF images collected on sulfur/Ketjen black electrode, fresh Li foil, and cycled Li foil after one cycle,(h-2) XRF images collected on lithium anode electrodes harvested from the Li|Sulfur/Ketjen black cells at discharged 2.1 V, discharged 1.7 V, and re-charged 3 V states. Reproduced with permission.135 Copyright 2015, Wiley-VCH. | ||
In a different approach, Ai133in situ synthesized CoS2 nanocrystals within a 3D hierarchical porous graphitic carbon (HPGC), finding that the combination of microporous HPGC's strong physical absorption and polar nano-CoS2's chemical absorption effectively anchored polysulfides during charge/discharge. Ex situ NEXAFS spectra for fresh electrodes (Fig. 9d) displayed broad peaks around 2473 eV, corresponding to S–S and C–S combinations, and 2480 eV, due to multiple scattering effects and multielectron excitation. Post-cycling analysis of cathodes disassembled at 2.15 and 1.8 V during the first discharge (Fig. 9e and f) revealed similar peaks, with a distinct 2471.0 eV peak indicating differences in polysulfide species, where CoS2 nanocatalysts enhanced the conversion of LiPSs into short-chain Li2Sx.
In a classic work by Shi et al.134 on studying the failure mechanism of large-format LiS pouch cells, it was observed that uneven sulfur reactions cause sulfur loss, redistribution, and passivation, leading to morphological and compositional heterogeneities across the cathode. Cycling smooths the electrode surface due to sulfur deposition, while changes in morphology and increased tortuosity may hinder electrolyte flow, ultimately affecting cell performance. XRF imaging of the cycled sulfur cathode (Fig. 9G) reveals that the R1 region is predominantly covered by high-intensity sulfur species, represented in yellow, and randomly distributed low-intensity species in blue. When the cut-off energy is increased to 2480 eV, XRF detects a wider range of sulfur species, confirming uniform coverage in the R1 region. In contrast, the R2 region exhibits significant heterogeneity, as demonstrated by XRF mapping at both 2469 and 2480 eV, indicating more severe heterogeneous reactions. This analysis highlights the presence of varying sulfur oxidation states and different chemical compositions, suggesting that reaction non-uniformity exists across multiple length scales, particularly in the center region compared to the edges. A study by Yu and group135 suggested that polysulfide dissolution and redeposition lead to sulfur redistribution on the lithium anode, which was further analyzed using ex situ XRF on lithium foil anodes (Fig. 9h). The study revealed sulfur species deposited on the anode, contributing to low coulombic efficiency and poor cycle life. Long-chain polysulfides were found on the discharged anode, while insoluble short-chain polysulfides or Li2S appeared on fully discharged and charged anodes. These findings indicate that polysulfide deposition and anode corrosion significantly contribute to capacity fading in lithium–sulfur batteries, emphasizing the need for anode protection and the development of non-corrosive electrolytes alongside modifications to the sulfur cathode.
Utilizing biocarbon from avocado seeds as a source of activated carbon for LiSBs is a promising approach, especially given that avocado seeds, which account for about 20% of the fruit's weight, are a significant byproduct of the globally consumed 6 million tons of avocados annually, with a 3% growth rate.140,141 Morales et al. employed XRF analysis to derive activated carbon from these seeds and impregnated it with a dual copolymer of polypyrrole and polystyrene sulfonate to enhance electrode performance, particularly at low current densities.136 Analysis of sulfur content before and after cycling (Table 2) demonstrated that the polymer-impregnated electrode retained a greater amount of sulfur, highlighting its superior ability to absorb polysulfides and suppress the shuttle effect, thereby minimizing irreversible sulfur loss. This improvement led to the LiSB achieving a discharge capacity of approximately 1200 mA h g−1 after 250 cycles at 0.1 C.
3.4. Optical spectroscopic techniques
Optical spectroscopic techniques are versatile tools used in batteries for a range of purposes.142,143 They are employed in the analysis of electrode materials, providing insights into their composition, electronic structure, and properties, aiding in understanding their behavior, and facilitating the development of new materials.144 Additionally, optical spectroscopy is used to study electrolytes, analyzing their composition, conductivity, and interactions with electrodes, crucial for optimizing formulations and improving battery performance.145 These techniques also characterize interfaces within batteries, providing information about electrode–electrolyte and electrode–electrode interfaces, essential for enhancing stability and performance. Furthermore, optical spectroscopy not only detects degradation mechanisms like SEI layer formation, material dissolution, and dendrite growth, but also facilitates the development of strategies to mitigate these issues and extend battery lifespan. It also facilitates in situ and operando studies, enabling live visual and estimation of changes in battery properties during operation, which is crucial for understanding battery behavior and optimizing performance.146 Following are the important optical spectroscopic techniques reported thus far for LiSBs:Organosulfur compounds like sulfurized-polyacrylonitrile (SPAN) have gained attention for LiSBs due to their sustainability and lightweight properties, though their application is limited by low sulfur content, typically below 50 wt%.152 To address this, Weret et al. synthesized a fibrous sulfurized trithiocyanuric acid/polyacrylonitrile (STTCA@SPAN) composite via electrospinning TTCA with PAN, followed by inverse vulcanization, which leveraged TTCA's highly oxidizable thiol groups to increase the sulfur content to 58 wt%.147 The resulting fibrous cathodes achieved an initial discharge capacity of 1301 mA h g−1 and exhibited excellent cycling stability over 400 cycles, demonstrating the effectiveness of this approach in enhancing LiSB performance. The post-mortem analysis in Fig. 10a and b demonstrates that the cross-linked fibrous structure of the STTCA@SPAN cathode maintains its stability throughout extended charge–discharge cycles, as evidenced by the preservation of structural integrity. Ex situ Raman spectroscopy of the cycled cathodes in Fig. 10c reveals that while the intensities of the S–S, C–S, and S–N peaks diminish after complete discharge, they reappear upon full charging, indicating the reversibility of the electrochemical redox reactions, with a decrease in the ID/IG intensity ratio post-cycling further suggesting the involvement of conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in lithium storage. In another work, Santos et al.71 developed a spinel oxide Co2Mn0.5Al0.5O4 (CMA), demonstrating impressive performance in LiSBs with an initial capacity of 1000 mA h g−1 cm−2 and enhanced cyclability beyond 360 cycles. The Raman spectrum of the 50% CMA electrode (Fig. 10d), shows significant structural changes pre- and post-cycling, including the shift of elemental sulfur peaks and the emergence of a new peak at 609 cm−1, attributed to the M–O stretch mode of LiMO2 (M = Mn or Co), indicating that lithiation and redox mediator behavior critically contribute to CMA's superior performance in LiSBs.
C bonds in lithium storage. In another work, Santos et al.71 developed a spinel oxide Co2Mn0.5Al0.5O4 (CMA), demonstrating impressive performance in LiSBs with an initial capacity of 1000 mA h g−1 cm−2 and enhanced cyclability beyond 360 cycles. The Raman spectrum of the 50% CMA electrode (Fig. 10d), shows significant structural changes pre- and post-cycling, including the shift of elemental sulfur peaks and the emergence of a new peak at 609 cm−1, attributed to the M–O stretch mode of LiMO2 (M = Mn or Co), indicating that lithiation and redox mediator behavior critically contribute to CMA's superior performance in LiSBs.
Dillard et al.155 utilized FTIR post-mortem analysis to examine sulfur deposition onto electrospun carbon nanofibers (CNF), resulting in a binder-free, freestanding cathode that bypasses the need for slurry processing, insulating binders, toxic solvents, and heavy current collectors. The FTIR analysis of the S-CNF cathode (Fig. 11a) revealed a redshift in bands between 3100 and 600 cm−1, indicating weakened bonds within the CNF surface functional groups due to interactions with polysulfides (Table 3). This redshift suggests that polysulfides chemisorbed onto the CNF surface form covalent or weaker van der Waals/hydrogen bonds, redistributing electrons at ‘binding sites’ such as nitrogen or oxygen functionalities and leading to the weakening or stretching of local bonds; for instance, polysulfides interacting with pyridinic nitrogen cause adjacent C–N bonds to weaken as the nitrogen moves towards the polysulfide. In a notable study, Du et al.156 developed a polyethylene-supported gel polymer electrolyte (GPE) with ester groups by cross-linking pentaerythritol tetraacrylate (PETEA) with divinyladipate, and post-mortem FTIR analysis (Fig. 11b) revealed that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peak at 1739 cm−1 nearly vanished after 100 cycles, indicating significant chemical changes. This PEGPE demonstrated retained 70% of its capacity after 300 cycles at 0.5 C, compared to just 29% with a liquid electrolyte, due to the ester groups’ ability to trap polysulfides and the optimized SEI film on the Li metal anode.
O stretching peak at 1739 cm−1 nearly vanished after 100 cycles, indicating significant chemical changes. This PEGPE demonstrated retained 70% of its capacity after 300 cycles at 0.5 C, compared to just 29% with a liquid electrolyte, due to the ester groups’ ability to trap polysulfides and the optimized SEI film on the Li metal anode.
 | ||
| Fig. 11 (a) FT-IR absorption spectra of reference CNF, S-CNF, and cycled cathode; reproduced with permission.155 Copyright 2018, Elsevier. (b) FTIR spectra of pristine and cycled PEGPE. Reproduced with permission.156 Copyright 2018, Elsevier. | ||
| Assignment | Pristine CNF | Soaked S-CNF | Cycled S-CNF |
|---|---|---|---|
| N–H2 stretch | 3325 | 3489 | 3203 |
| CH/CH3 stretching | 2954 | 2947 | 2943 |
| CH/CH3 stretching | 2917 | 2917 | 2912 |
| CH/CH3 stretching | 2848 | 2846 | 2842 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in quinine or conjugated ketone group O in quinine or conjugated ketone group |
— | 1635 | 1621 |
| C–C/C–H bending | 1455 | 1456 | 1455 |
| C–H loop bending in aromatic carbon | 788 | 768 | 767 |
 | ||
| Fig. 12 (a) Schematic illustrations depicting the operational principles of flexible LiSBs, (b) S content in lithium metal anodes analyzed by ICP-AES after 400 cycles. Reproduced with permission.51 Copyright 2019, Wiley-VCH. (c) S standard curve derived from ICP-AES; (d) S content of the cycled lithium metal anodes. Reproduced with permission.157 Copyright 2021, Elsevier. | ||
Vacancy and interface engineering can significantly enhance the electronic structure and catalytic activity of metal chalcogenides, but their use in LiSBs is often limited by poor conductivity, loss of catalytic efficiency, and volumetric changes during cycling.14 To address these issues, Ye et al. developed bimetallic chalcogenide nanosheet arrays (CoZn-X, where X = S, Se, Te) with abundant vacancies and heterointerfaces, (Fig. 13a–c) which aimed to improve ion transport and stabilize CoZn–S during electrocatalysis.163 Post-mortem EPR spectra (Fig. 13d) revealed an increase in sulfur vacancies in CoZn–S after cycling, attributed to an induction period during initial sulfur conversion, enhancing catalytic activity. These nanosheet arrays exhibited a reversible capacity of 818 mA h g−1 over 110 cycles at 2 C with 90.9% retention, demonstrating that the engineered sulfur vacancies and interfacial fields in CoZn–S modulate the electronic structure and improve electron transfer rate.
 | ||
| Fig. 13 Schematic of the: (a) preparation process for the CoZn-X and (b) structure evaluation demonstrating catalyst, anion vacancies and heterogeneous interfaces, (c) the mechanism for sulfur conversion and (d) EPR spectra of fresh CoZn-S and the cycled CoZn-S. Reproduced with permission.163 Copyright 2022, Wiley-VCH. | ||
To address challenges such as polysulfide shuttle, sluggish reaction kinetics, and dendritic lithium growth, He et al. developed a dual-function, flexible framework combining catalytic 1T′-MoTe2 nanosheets with CNTs.113 This framework supports both sulfur and metallic lithium and facilitates the formation of a thin, tellurized SEI primarily composed of lithium thiotellurate (Li2TeS3) when paired with a sulfur/MoTe2-CNT cathode, thereby stabilizing lithium deposition and extending the battery cycle life. SIMS depth profiles after 50 cycles (Fig. 14) demonstrated that while the TeS– fragment from Li2TeS3 initially peaked sharply at 200 s and then decreased to 14% of its peak intensity by 8000 s, the LiTe– fragment from Li2Te peaked rapidly at 420 s and remained stable with depth, and the Li– fragment showed a trend similar to the C– fragment, confirming uniform lithium plating and stripping.
 | ||
| Fig. 14 After 50 cycles (a) depth profiles of various ions in MoTe2-CNT/Li and (b) 3D imagining of the MoTe2-CNT/Li. Reproduced with permission.113 Copyright 2022, Wiley-VCH. | ||
 | ||
| Fig. 15 1H NMR spectra after 100 cycles at 0.1 C. Reproduced with permission.84 Copyright 2017, Royal Society of Chemistry. | ||
3.5 N2 sorption isotherms
Nitrogen sorption isotherms are a fundamental technique for characterizing the pore structure and surface area of materials, achieved by exposing the sample to nitrogen gas under controlled pressures and temperatures, and subsequently measuring the quantity of gas adsorbed.172–174 The resulting isotherm describes how nitrogen adsorption varies with pressure, providing information about pore size, shape, and distribution. In battery research, N2 sorption isotherms characterize the porous structure of electrode materials, electrolytes, and separators.175 These factors influence battery performance, particularly in electrolyte infiltration, ion transport, and electrode–electrolyte interface formation.176For electrode materials, nitrogen sorption isotherms provide vital insights into the accessible surface area available for electrochemical reactions and electrolyte interaction, which are pivotal in understanding key performance metrics such as capacity, rate capability, and cycling stability.177 When applied to electrolytes and separators, these isotherms are instrumental in characterizing porosity and permeability, parameters that are critical for effective electrolyte retention, ion transport, and the overall performance of the battery.178 To elucidate the impact of binders on LiSB performance, Shafique et al. conducted a detailed study investigating the effects of different polymeric binders—polyethylene oxide (PEO), polyvinylidene difluoride (PVDF), and lithium polyacrylate (LiPAA)—each dispersed in specific solvents: PEO in acetonitrile (ACN), PVDF in N-methyl pyrrolidone (NMP), and LiPAA in an aqueous solution of water and alcohol.179 The study focused on correlating binder types with the electrochemical behavior and morphological stability of sulfur electrodes, particularly under a sulfur loading of approximately 4.0 mg cm−2, throughout the cycling process. N2-sorption isotherms of pristine and cycled cells (Fig. 16) demonstrated that while fresh electrodes had similar surface areas, the sulfur electrodes with PEO binder experienced a significant decrease in surface area and pore volume by an order of magnitude after cycling, whereas electrodes with LiPAA and PVDF binders showed minimal changes, with less than 10% variation as indicated in Table 4. These BET results aligned with electrochemical data, which demonstrated that LiSBs with LiPAA experienced the least capacity fading and the highest reproducibility, ranking the binders as LiPAA > PVDF ≫ PEO, and in terms of electrochemical kinetics during cycling, LiPAA also outperformed PVDF and PEO, ranking LiPAA > PEO ≫ PVDF, confirming LiPAA as the superior binder for maintaining cell capacity stability and enhancing reaction kinetics.
 | ||
| Fig. 16 Sorption isotherms of the pristine (solid lines) and cycled (2nd cycle, dashed lines) of different polymeric binder in LiSBs. Reproduced with permission.179 Copyright 2020, Elsevier. | ||
| Sample | Surface area (m2 g−1) | Correlation coefficient | Pore volume (cc g−1) | Pore size (nm) |
|---|---|---|---|---|
| Pristine LiPAA | 22.9 | 0.99942 | 0.032 | 3 |
| Pristine PVDF | 15.3 | 0.99948 | 0.045 | 1.5 |
| Pristine PEO | 23.8 | 0.99999 | 0.037 | 3.6 |
| Cycled LiPAA | 23.4 | 0.99997 | 0.062 | 3.1 |
| Cycled PVDF | 14.6 | 0.99999 | 0.016 | 1.5 |
| Cycled PEO | 2.6 | 0.99991 | 0.007 | 4.3 |
4. Conclusions and perspectives
Post-mortem analysis of cycled LiSBs is indispensable for a comprehensive understanding of redox processes, the formation of new compounds and interfacial layers, and the identification of aging mechanisms—factors that are critical for optimizing existing materials and pioneering new functional materials. Before initiating cell disassembly or conducting post-mortem analysis, non-destructive electrochemical techniques provide critical insights into the evolving electrochemical behavior of cycled cells, thus laying a robust foundation for subsequent structural and chemical investigations. Techniques such as EIS offer valuable insights into cell impedance across various frequencies, highlighting changes in electrode reactions and electrolyte properties,53 while CV provides information on redox reactions and reaction kinetics, helping to assess cell performance over cycles. The post-mortem analysis of LiSBs involves opening the cell and handling its air-sensitive components in an inert gas-filled glove box.71 To start with, washing of the recovered electrodes in a solvent like DME, an electrolyte component, is recommended to preserve sample quality, though its impact on SEI layers is unclear. Initially recording optical images of cycled electrodes and separators helps track changes in color, shape, thickness, or size compared to fresh components. Cost-effective digital photography documents degradation, aiding researchers in identifying trends and understanding mechanisms to develop mitigation strategies. Microscopic methods are essential for tracking changes in cycled cell components, offering high-resolution imaging that reveals structural and morphological alterations.86 SEM examines surface morphology changes, such as cracks, voids, or particle size variations, to identify degradation mechanisms and failure modes.63 TEM provides higher resolution images of electrode nanostructures and by-product formations like SEI layers or lithium dendrites, aiding in understanding their evolution and degradation trends.77 AFM analyzes electrode surface morphology at the nanoscale, offering insights into surface roughness and film formation, which impact cell performance.86 X-ray spectroscopy methods offer valuable insights into material chemical composition and electronic structure.102 For example, XPS is commonly used to analyze electrode material surface chemistry before and after cycling, identifying elements and their chemical states by measuring emitted electron binding energies. This reveals changes in composition and oxidation states.104,105 XRD provides information on phase changes, crystallographic defects, and new phase or compound formation in electrode material crystal structures during cycling. XANES gives insights into bonding and coordination environment changes of elements like sulfur, lithium, and transition metals.131 XRF provides spatial distribution information of elements (sulfur, lithium, etc.) in electrodes critical for battery performance.138 Optical spectroscopy methods offer detailed insights into the critical structural and chemical changes of electrode materials post-cycling. Raman spectra identify changes in chemical composition, phase transformation, and the formation of by-products like SEI layers or lithium dendrites.25 FTIR spectra detail functional groups, chemical bonds, alterations in bonding, oxidation states, and new compound formation.71 EDX offers distribution and concentration information of elements, revealing composition changes and by-product formation. ICP-AES determine elemental composition with high precision, crucial for studying active material and impurity concentration changes, providing insights into degradation mechanisms and material stability.180 NMR reveals changes in bonding and molecular structure by examining the local environment of atoms in molecules.170Looking forward, future research will likely prioritize improving the sensitivity, resolution, and efficiency of these analytical methods. As relatively new energy storage systems, lithium–sulfur batteries require further exploration of non-destructive techniques that are already well-established for LIBs. A few examples include:
4.1 Non-invasive current density imaging using magnetometry
Understanding current density is essential for analyzing SoC inhomogeneities, evaluating SEI thickness and formation, estimating heat generation, and addressing issues like uneven lithium-ion extraction and lithium plating. To advance this, Bason et al. introduced a non-invasive imaging technique to map intra-cell electrochemical activity using sensitive magnetometers, allowing measurement of local current densities by analyzing external magnetic fields and offering insights into cell performance and safety.181 Analyzing magnetic field maps of a lithium-ion battery under load (Fig. 17a) revealed current flow patterns influenced by overpotentials and impedance, enabling inference of critical cell properties such as lithiation state and diffusion coefficients from changes in magnetic field distribution. | ||
| Fig. 17 (a-1) Current flow within LIB and corresponding magnetic field, (a-2) reconstructed current density image corresponding to a single electrode pair, during a 10A discharge with the battery tabs located at x = 0; Reproduced with permission.181 Copyright 2022, Elsevier. (b) Schematic diagram of an ultrasonic scan of a LIB and analysis of SoC, reproduced with permission.183 Copyright 2023, American Chemical Society. (c) Ultrasonic Images of a LIB with electrolyte: (A) after wetting, (B) post formation, (C) post degas, (D) after 3000 cycles, and (E) capacity versus cycle number. Reproduced with permission.184 Copyright 2020, Elsevier. (d) First derivative CEPR signal, as measured in a field-swept EPR experiment, for metallic lithium with different morphologies. Reproduced with permission.185 Copyright 2018, Springer Nature. (e) Time-dependent temperature profiles for pristine (green) and aged cells (orange, pink, and blue) at different C rates. Reproduced with permission.187 Copyright 2025, Elsevier. (f) Full multi-scale comparison of polycrystalline NMC622/graphite pouch cell that was cycled for 2.5 years at C/5 from 3.0–4.1 V (bottom row) compared to control cell that was formation cycled only (top row). Reproduced with permission.188 Copyright 2023, IOP publishing. | ||
4.2 Computational modeling
The integration of advanced analytical techniques with computational modeling holds great promise for the future of cycled cell analysis.182 Computational models can simulate the complex electrochemical processes occurring within the cell, providing a theoretical framework for interpreting experimental data. By combining experimental results with computational simulations, researchers can gain deeper insights into the underlying mechanisms governing cell performance and degradation. This comprehensive strategy could result in the creation of predictive models to direct the design of advanced LiSBs with enhanced performance and durability.4.3 Ultrasonic signals to predict state-of-charge
To elucidate the distribution of SoC in lithium-ion batteries, Huang et al.183 employed an advanced acoustic method utilizing a focused ultrasound beam for cell scanning (Fig. 17b). They analyzed the transmitted ultrasonic waves through a deep learning algorithm based on a feedforward neural network (FNN). This integration of progressive scanning with focused ultrasound enabled SoC mapping with an impressive in-plane resolution of 1 mm, a critical advancement for understanding battery failure mechanisms and facilitating the development of high-performance rechargeable batteries.4.4 Ultrasonics to probe wetting of cells
Ultrasonic imaging technology, a non-destructive method for evaluating electrolytes and gases in pouch or prismatic cells, uses the fact that ultrasound attenuates more quickly in poorly wetted electrodes or separators to determine the minimum electrolyte injection volume and wetting time, optimizing the battery manufacturing process. It can also detect signs of electrolyte dry-out or unwetting in aged cells without disassembly, as gas can block ultrasound transmission. Deng and colleagues184 employed a focused ultrasound beam with a diameter under 1 mm and achieved sub-millimeter resolution and 0.2 mm positional accuracy (Fig. 17c) to assess electrolyte wetting, dry-out, and unwetting.4.5 Electron paramagnetic resonance (EPR) spectroscopy
EPR is a highly sensitive technique for detecting and characterizing lithium dendrites and plating, enabling the distinction between various lithium morphologies such as bulk, porous, and dendritic lithium. This is particularly useful for analyzing lithium metal in graphite anodes, where EPR can differentiate between active and “dead” lithium and provide quantitative insights into lithium deposition and lithiation processes.185 Conduction EPR (CEPR) further enhances this capability by detecting conduction electrons, observing mossy lithium, and quantifying lithium plating. Niemoller et al. observed that the EPR spectrum of metallic lithium is influenced by the skin effect, which limits the penetration depth of microwave radiation.185 This results in a Dysonian lineshape (Fig. 17d), with linewidth and intensity varying based on lithium morphology. Bulk lithium exhibits the broadest linewidth (∼0.15 mT) with a Dysonian lineshape, while porous and dendritic lithium have progressively narrower linewidths, with mossy lithium at ∼0.03 mT and dendritic lithium showing the narrowest linewidth (∼0.005 mT) and a Lorentzian lineshape due to its smaller particle size and surface characteristics.1864.6 Accelerating rate calorimeter (ARC)
The ARC is a thermal analysis tool that evaluates the thermal behavior and safety of lithium-ion batteries under conditions leading to thermal runaway or failure.189,190 By operating adiabatically, it minimizes heat loss, allowing accurate simulation of battery self-heating during exposure to rising temperatures and providing insights into exothermic reactions from short circuits, overcharging, or internal malfunctions.191 This capability identifies critical temperature thresholds for thermal runaway—an uncontrolled energy release that can cause fires or explosions—and facilitates safe stress testing of batteries. The ARC also generates data on heat capacity and thermal reaction rates, essential for optimizing battery design, improving thermal management systems, and preventing hazards associated with thermal runaway, thereby enhancing overall battery reliability.192 Manufacturers utilize ARC data to develop effective thermal management solutions and investigate failure modes linked to unwanted exothermic reactions. Huang et al.193 conducted a detailed analysis of the thermal runaway behavior in LiSBs, from the pouch cell to the electrode level, using an ARC. Their study revealed that unlike in LIBs, thermal runaway in Li–S cells is initially triggered by reactions between sulfur cathode derivatives and the electrolyte solvent, particularly 1,3-dioxolane (DOL), rather than by the lithium anode. Interactions between the lithium metal anode, electrolyte, and molten sulfur species further accelerate these reactions. Notably, polysulfides were found to reduce the rapid self-heating rate at high temperatures during thermal runaway. Solvent vaporization was identified as the primary driver of internal pressure buildup before thermal runaway, while heat-induced gas release, including methane (CH4) and ethylene (C2H4), intensified combustion in the later stages. The inherent thermal instability of the sulfur cathode and lithium anode, both prone to sublimation, melting, and cross-reactions at elevated temperatures, plays a critical role in determining the thermal runaway behavior. Even Li–S batteries with electrolytes of different thermal stabilities, including inorganic solid-state types, experienced rapid thermal runaway within a narrow temperature range due to inevitable melting-induced short circuits of the sulfur cathode and lithium anode. This in-depth understanding of thermal runaway mechanisms provides valuable insights for developing safer next-generation Li–S batteries. In a classic example in Fig. 17e, the time-dependent temperature profiles show that the A-Fr-ARC cell begins exothermic reactions at 92.04 °C and enters thermal runaway at 213.8 °C over 1317 minutes, ultimately reaching a maximum temperature of 308.0 °C.187 In contrast, the aged A-RT-4C cell starts to exotherm at 77.06 °C, approximately 15 °C lower than the A-Fr-ARC, indicating the detrimental impact of lithium plating on thermal stability. This cell enters thermal runaway at 213.6 °C in 1682 minutes, exhibiting a similar T2 value to the A-Fr-ARC but with a longer time interval due to its lower T1 and an accelerated reaction rate from lithium deposition. Furthermore, the maximum temperature for the A-RT-4C cell is 300.7 °C, likely due to lithium plating depleting most electrolytes and reducing heat release during chemical reactions.4.7 X-ray tomography
X-ray micro-computed tomography (micro-CT) is a high-resolution imaging technique that produces detailed three-dimensional representations of lithium-ion batteries at the microscale.194,195 By using X-ray radiation to capture multiple two-dimensional projections from various angles, micro-CT reconstructs these images into a 3D volumetric view, enabling visualization of internal battery components like electrodes, electrolytes, and separators at micrometer resolution.196 This technique leverages the differential attenuation of X-rays by different materials to identify variations in density and composition. In lithium-ion batteries, micro-CT is essential for studying structural evolution and degradation mechanisms during charge and discharge cycles, providing insights into phenomena such as lithium-ion transport, phase changes, and dendrite formation.197 For instance, Fig. 17f illustrates CT scans of deep-cycled and control cells, revealing pervasive cathode microcracking.188 This degradation process starts with microcracking, leading to particle swelling and a narrowing of the pore network, which increases tortuosity. The thickened cathode layer can push material beyond the aluminum current collector, expanding the jelly roll and damaging electrodes in confined spaces. Ultimately, this increases the pore volume of the cathode, which can result in electrolyte dry-out and rollover failure if it exceeds the available electrolyte.4.8 Operando analysis
In addition to ultrasonics and magnetometry, there is increasing interest in developing operando techniques that can monitor cycled cells under actual operating conditions.43,198 For example, in situ/operando techniques including FTIR, Raman, XRD, XPS, ICP-AES, SIMS, and EIS enable researchers to observe dynamic changes in cell properties during cycling, offering a more comprehensive understanding of degradation mechanisms. Some of these operando analyses have not been reported for LiSBs which opens avenues for researchers to observe and understand the live transition within LiSBs while in operation.28,199 Following are the various operando analytical techniques that have been used for batteries in general so far:As observed in the pie-chart in Scheme 1, although more research involving post-mortem analysis is being conceded, a huge number of important analytical techniques remain under-represented which need to be equally explored. In addition, we have observed in Table 5 that a single technique is not enough to gather all the important information required from the cycled cell. Thus, it becomes highly desirable to use a combination of these post-mortem techniques to analyze the cell from every perspective ranging from the structural, chemical, and electronic changes occurring within the cells. In addition, analyzing the cycled cells during post-mortem analysis offers invaluable benefits in understanding the degradation mechanisms and failure modes of battery materials. This understanding is vital for devising methods to boost battery efficiency, reliability, and longevity. Additionally, post-mortem analysis provides a thorough insight into the factors driving cell deterioration, promoting the advancement of more effective and resilient battery technologies for diverse applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank MITACS (Canada), Ms Denise Byrne at Powertrain Engineering Research & Development Centre (PERDC), and Ford Motor Company of Canada for providing financial and in-kind support for this project.References
- S. Kim, G. Park, S. J. Lee, S. Seo, K. Ryu, C. H. Kim and J. W. Choi, Adv. Mater., 2023, 35, 2206625 CrossRef PubMed.
- J. Kim, Y. Kim, J. Yoo, G. Kwon, Y. Ko and K. Kang, Nat. Rev. Mater., 2022, 8, 54–70 CrossRef.
- Z. P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler and Z. Chen, Nat. Energy, 2018, 3, 279–289 CrossRef.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 CrossRef.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 1–16 Search PubMed.
- G. Zhou, H. Chen and Y. Cui, Nat. Energy, 2022, 7, 312–319 CrossRef.
- H. Li, R. Meng, C. Ye, A. Tadich, W. Hua, Q. Gu, B. Johannessen, X. Chen, K. Davey and S. Z. Qiao, Nat. Nanotechnol., 2024, 2024, 1–8 Search PubMed.
- Y. Chen, T. Wang, H. Tian, D. Su, Q. Zhang and G. Wang, Adv. Mater., 2021, 33, 2003666 CrossRef PubMed.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1, 1–11 Search PubMed.
- M. Jana, R. Xu, X. B. Cheng, J. S. Yeon, J. M. Park, J. Q. Huang, Q. Zhang and H. S. Park, Energy Environ. Sci., 2020, 13, 1049–1075 RSC.
- B. Liu, H. Gu, J. F. Torres, Z. Yin and A. Tricoli, Energy Environ. Sci., 2024, 17, 1073–1082 RSC.
- Y. X. Song, Y. Shi, J. Wan, S. Y. Lang, X. C. Hu, H. J. Yan, B. Liu, Y. G. Guo, R. Wen and L. J. Wan, Energy Environ. Sci., 2019, 12, 2496–2506 RSC.
- Z. L. Xu, S. J. Kim, D. Chang, K. Y. Park, K. S. Dae, K. P. Dao, J. M. Yuk and K. Kang, Energy Environ. Sci., 2019, 12, 3144–3155 RSC.
- Y. Guo, Q. Niu, F. Pei, Q. Wang, Y. Zhang, L. Du, Y. Zhang, Y. Zhang, Y. Zhang, L. Fan, Q. Zhang, L. Yuan and Y. Huang, Energy Environ. Sci., 2024, 17, 1330–1367 RSC.
- M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescu and G. J. Offer, Energy Environ. Sci., 2015, 8, 3477–3494 RSC.
- G. Zhou, F. Li and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1307–1338 RSC.
- J. Guo, Q. Yang, Y. Dou, X. Ba, W. Wei and J. Liu, Energy Environ. Sci., 2024, 17(5), 1695–1724 RSC.
- V. K. Tomer, O. A. T. Dias, A. M. Gouda, R. Malik and M. Sain, Mater. Horiz., 2024, 11, 3090–3103 RSC.
- L. A. Middlemiss, A. J. R. Rennie, R. Sayers and A. R. West, Energy Rep., 2020, 6, 232–241 CrossRef.
- X. Yang and A. L. Rogach, Adv. Energy Mater., 2019, 9, 1900747 CrossRef.
- X. Li, S. Liu, J. Yang, Z. He, J. Zheng and Y. Li, Energy Storage Mater., 2023, 55, 606–630 CrossRef.
- H. A. Petersen, T. H. T. Myren, S. J. O'Sullivan and O. R. Luca, Mater. Adv., 2021, 2, 1113–1138 RSC.
- N. Meddings, M. Heinrich, F. Overney, J. S. Lee, V. Ruiz, E. Napolitano, S. Seitz, G. Hinds, R. Raccichini, M. Gaberšček and J. Park, J. Power Sources, 2020, 480, 228742 CrossRef.
- Y. H. Lin, S. J. Ruan, Y. X. Chen and Y. F. Li, Renewable Sustainable Energy Rev., 2023, 188, 113807 CrossRef.
- S. Lang, S. H. Yu, X. Feng, M. R. Krumov and H. D. Abruña, Nat. Commun., 2022, 13, 1–11 Search PubMed.
- S. Zhou, J. Shi, S. Liu, G. Li, F. Pei, Y. Chen, J. Deng, Q. Zheng, J. Li, C. Zhao, I. Hwang, C. J. Sun, Y. Liu, Y. Deng, L. Huang, Y. Qiao, G. L. Xu, J. F. Chen, K. Amine, S. G. Sun and H. G. Liao, Nature, 2023, 621, 75–81 CrossRef PubMed.
- S. H. Yu, X. Huang, K. Schwarz, R. Huang, T. A. Arias, J. D. Brock and H. D. Abruña, Energy Environ. Sci., 2018, 11, 202–210 RSC.
- F. Schomburg, B. Heidrich, S. Wennemar, R. Drees, T. Roth, M. Kurrat, H. Heimes, A. Jossen, M. Winter, J. Y. Cheong and F. Röder, Energy Environ. Sci., 2024, 17, 2686–2733 RSC.
- Y. Liu, H. Shi and Z. S. Wu, Energy Environ. Sci., 2023, 16, 4834–4871 RSC.
- A. Grant and C. O′dwyer, Appl. Phys. Rev., 2023, 10, 11312 CAS.
- A. M. Tripathi, W. N. Su and B. J. Hwang, Chem. Soc. Rev., 2018, 47, 736–851 RSC.
- J. Tan, D. Liu, X. Xu and L. Mai, Nanoscale, 2017, 9, 19001–19016 RSC.
- Y. Yang, J. Feijóo, V. Briega-Martos, Q. Li, M. Krumov, S. Merkens, G. De Salvo, A. Chuvilin, J. Jin, H. Huang, C. J. Pollock, M. B. Salmeron, C. Wang, D. A. Muller, H. D. Abruña and P. Yang, Curr. Opin. Electrochem., 2023, 42, 101403 CrossRef.
- J. Maibach, J. Rizell, A. Matic and N. Mozhzhukhina, ACS Mater. Lett., 2023, 5, 2431–2444 CrossRef CAS PubMed.
- H. Li, S. Guo and H. Zhou, J. Energy Chem., 2021, 59, 191–211 CrossRef CAS.
- S. Rehman, M. Pope, S. Tao and E. McCalla, Energy Environ. Sci., 2022, 15, 1423–1460 RSC.
- J. H. Tian, T. Jiang, M. Wang, Z. Hu, X. Zhu, L. Zhang, T. Qian and C. Yan, Small Methods, 2020, 4, 1900467 CrossRef CAS.
- T. J. Leckie, S. D. Robertson and E. Brightman, Energy Adv., 2024, 3(10), 2479–2502 RSC.
- M. Yousaf, U. Naseer, Y. Li, Z. Ali, N. Mahmood, L. Wang, P. Gao and S. Guo, Energy Environ. Sci., 2021, 14, 2670–2707 RSC.
- S. Rehman, M. Pope, S. Tao and E. McCalla, Energy Environ. Sci., 2022, 15, 1423–1460 RSC.
- Y. Ma, S. Li and B. Wei, Nanoscale, 2019, 11, 20429–20436 RSC.
- S. K. Park, W. M. Dose, D. Boruah, M. De Volder, S. K. Park, W. M. Dose, B. D. Boruah and M. De Volder, Adv. Mater. Technol., 2022, 7, 2100799 CrossRef.
- F. Strauss, D. Kitsche, Y. Ma, J. H. Teo, D. Goonetilleke, J. Janek, M. Bianchini and T. Brezesinski, Adv. Energy Sustainability Res., 2021, 2, 2100004 CrossRef.
- C. Pastor-Fernández, T. F. Yu, W. D. Widanage and J. Marco, Renewable Sustainable Energy Rev., 2019, 109, 138–159 CrossRef.
- A. Grant and C. O'dwyer, Appl. Phys. Rev., 2023, 10, 11312 Search PubMed.
- Y. Li, J. Guo, K. Pedersen, L. Gurevich and D. I. Stroe, Batteries, 2022, 8, 72 CrossRef.
- H. Park, O. Tamwattana, J. Kim, S. Buakeaw, R. Hongtong, B. Kim, P. Khomein, G. Liu, N. Meethong and K. Kang, Adv. Energy Mater., 2021, 11, 2003039 CrossRef.
- X. Lin, K. Khosravinia, X. Hu, J. Li and W. Lu, Prog. Energy Combust. Sci., 2021, 87, 100953 CrossRef.
- T. Waldmann, A. Iturrondobeitia, M. Kasper, N. Ghanbari, F. Aguesse, E. Bekaert, L. Daniel, S. Genies, I. J. Gordon, M. W. Löble, E. De Vito and M. Wohlfahrt-Mehrens, J. Electrochem. Soc., 2016, 163, A2149–A2164 CrossRef.
- F. Wu, F. Chu, G. A. Ferrero, M. Sevilla, A. B. Fuertes, O. Borodin, Y. Yu and G. Yushin, Nano Lett., 2020, 20, 5391–5399 CrossRef.
- J. Wang, G. Yang, J. Chen, Y. Liu, Y. Wang, C. Y. Lao, K. Xi, D. Yang, C. J. Harris, W. Yan, S. Ding and R. V. Kumar, Adv. Energy Mater., 2019, 9, 1902001 CrossRef.
- C. Wei, M. Tian, Z. Fan, L. Yu, Y. Song, X. Yang, Z. Shi, M. Wang, R. Yang and J. Sun, Energy Storage Mater., 2021, 41, 141–151 CrossRef.
- P. Vadhva, J. Hu, M. J. Johnson, R. Stocker, M. Braglia, D. J. L. Brett and A. J. E. Rettie, ChemElectroChem, 2021, 8, 1930–1947 CrossRef CAS.
- L. Stolz, M. Gaberšček, M. Winter and J. Kasnatscheew, Chem. Mater., 2022, 34, 10272–10278 CrossRef CAS.
- W. Choi, H. C. Shin, J. M. Kim, J. Y. Choi and W. S. Yoon, J. Electrochem. Sci. Technol., 2020, 11, 1–13 CrossRef CAS.
- O. A. T. Dias, F. Azarnia, K. Rathi, V. Pakharenko, V. K. Tomer and M. Sain, Nanoscale, 2024, 16(34), 16003–16014 RSC.
- X. Huang, B. Luo, R. Knibbe, H. Hu, M. Lyu, M. Xiao, D. Sun, S. Wang and L. Wang, Chem. – Eur. J., 2018, 24, 18544–18550 CrossRef CAS.
- Y. Huang, M. Shaibani, T. D. Gamot, M. Wang, P. Jovanović, M. C. Dilusha Cooray, M. S. Mirshekarloo, R. J. Mulder, N. V. Medhekar, M. R. Hill and M. Majumder, Nat. Commun., 2021, 12, 1–15 CrossRef.
- N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart and J. L. Dempsey, J. Chem. Educ., 2018, 95, 197–206 CrossRef CAS.
- X. Huang, Z. Wang, R. Knibbe, B. Luo, S. A. Ahad, D. Sun and L. Wang, Energy Technol., 2019, 7, 1801001 CrossRef.
- X. Huang, Z. Wang, R. Knibbe, B. Luo, S. A. Ahad, D. Sun and L. Wang, Energy Technol., 2019, 7, 1801001 CrossRef.
- J. Wu, M. Fenech, R. F. Webster, R. D. Tilley and N. Sharma, Sustainable Energy Fuels, 2019, 3, 1623–1646 RSC.
- E. Ventosa and W. Schuhmann, Phys. Chem. Chem. Phys., 2015, 17, 28441–28450 RSC.
- M. Zhang, M. Chouchane, S. A. Shojaee, B. Winiarski, Z. Liu, L. Li, R. Pelapur, A. Shodiev, W. Yao, J. M. Doux, S. Wang, Y. Li, C. Liu, H. Lemmens, A. A. Franco and Y. S. Meng, Joule, 2023, 7, 201–220 CrossRef.
- Z. Deng, X. Lin, Z. Huang, J. Meng, Y. Zhong, G. Ma, Y. Zhou, Y. Shen, H. Ding and Y. Huang, Adv. Energy Mater., 2021, 11, 2000806 CrossRef.
- M. Biton, V. Yufit, F. Tariq, M. Kishimoto and N. Brandon, J. Electrochem. Soc., 2017, 164, A6032–A6038 CrossRef.
- X. Liu, L. Zhang, H. Yu, J. Wang, J. Li, K. Yang, Y. Zhao, H. Wang, B. Wu, N. P. Brandon and S. Yang, Adv. Energy Mater., 2022, 12, 2200889 CrossRef.
- C. Tian, B. Li, X. Hu, J. Wu, P. Li, X. Xiang, X. Zu and S. Li, ACS Appl. Mater. Interfaces, 2021, 13, 6229–6240 CrossRef.
- Y. Song, X. Long, Z. Luo, C. Guo, C. N. Geng, Q. S. Ouyang, Z. Han, G. Zhou and J. J. Shao, ACS Appl. Mater. Interfaces, 2022, 14, 32183–32195 CrossRef PubMed.
- X. Chen, Y. Wang, J. Wang, J. Liu, S. Sun, L. Zhu, Q. Ma, N. Zhu, X. Wang, J. Chen and W. Yan, J. Mater. Chem. A, 2022, 10, 1359–1368 RSC.
- É. A. Santos, C. G. Anchieta, R. C. Fernandes, M. J. Pinzón, A. N. Miranda, I. Galantini, F. C. B. Maia, G. Doubek, C. B. Rodella, L. M. Da Silva and H. Zanin, Nano Energy, 2023, 116, 108809 CrossRef.
- Y. Liu, H. Liu, Y. Lin, Y. Zhao, H. Yuan, Y. Su, J. Zhang, S. Ren, H. Fan and Y. Zhang, Adv. Funct. Mater., 2021, 31, 2104863 CrossRef.
- C. Geng, W. Qu, Z. Han, L. Wang, W. Lv and Q. H. Yang, Adv. Energy Mater., 2023, 13, 2204246 CrossRef.
- P. Xiong, S. Zhang, R. Wang, L. Zhang, Q. Ma, X. Ren, Y. Gao, Z. Wang, Z. Guo and C. Zhang, Energy Environ. Sci., 2023, 16, 3181–3213 RSC.
- Z. J. Zheng, H. Ye and Z. P. Guo, Energy Environ. Sci., 2021, 14, 1835–1853 RSC.
- L. Tian, Z. Zhang, S. Liu, G. Li and X. Gao, Energy Environ. Mater., 2022, 5, 645–654 CrossRef.
- Y. Cheng, L. Zhang, Q. Zhang, J. Li, Y. Tang, C. Delmas, T. Zhu, M. Winter, M. S. Wang and J. Huang, Mater. Today, 2021, 42, 137–161 CrossRef.
- T. Shang, Y. Wen, D. Xiao, L. Gu, Y. S. Hu and H. Li, Adv. Energy Mater., 2017, 7, 1700709 CrossRef.
- Z. Fan, L. Zhang, D. Baumann, L. Mei, Y. Yao, X. Duan, Y. Shi, J. Huang, Y. Huang and X. Duan, Adv. Mater., 2019, 31, 1900608 CrossRef PubMed.
- X. Wu, S. Li, B. Yang and C. Wang, Electrochem. Energy Rev., 2019, 2, 467–491 CrossRef.
- M. Yousaf, U. Naseer, Y. Li, Z. Ali, N. Mahmood, L. Wang, P. Gao and S. Guo, Energy Environ. Sci., 2021, 14, 2670–2707 RSC.
- R. Pai, A. Singh, M. H. Tang and V. Kalra, Commun. Chem., 2022, 5, 1–11 CrossRef.
- J. Y. Hwang, H. M. Kim, S. Shin and Y. K. Sun, Adv. Funct. Mater., 2018, 28, 1704294 CrossRef.
- S. Choudhury, P. Srimuk, K. Raju, A. Tolosa, S. Fleischmann, M. Zeiger, K. I. Ozoemena, L. Borchardt and V. Presser, Sustainable Energy Fuels, 2017, 2, 133–146 RSC.
- S. Wang, Q. Liu, C. Zhao, F. Lv, X. Qin, H. Du, F. Kang and B. Li, Energy Environ. Mater., 2018, 1, 28–40 CrossRef.
- Z. Zhang, S. Said, K. Smith, R. Jervis, C. A. Howard, P. R. Shearing, D. J. L. Brett, T. S. Miller, Z. Zhang, S. Said, K. Smith, R. Jervis, P. R. Shearing, D. J. L. Brett and T. S. Miller, Adv. Energy Mater., 2021, 11, 2101518 CrossRef.
- M. Wang, Z. Song, J. Bi, H. Li, M. Xu, Y. Gong, Y. Zhou, Y. Zhao and K. Yang, Battery Energy, 2023, 2, 20230006 CrossRef.
- W. Chen, X. Chen, W. Chen and Z. Jiang, Energy Technol., 2023, 11, 2201372 CrossRef.
- Z. Deng, X. Lin, Z. Huang, J. Meng, Y. Zhong, G. Ma, Y. Zhou, Y. Shen, H. Ding and Y. Huang, Adv. Energy Mater., 2021, 11, 2000806 CrossRef.
- W. Liu, P. Liu and D. Mitlin, Adv. Energy Mater., 2020, 10, 2002297 CrossRef.
- W. Liu, P. Liu and D. Mitlin, Chem. Soc. Rev., 2020, 49, 7284–7300 RSC.
- C. Senthil, S. G. Kim, S. S. Kim, M. G. Hahm and H. Y. Jung, Small, 2022, 18, 2200919 CrossRef.
- J. Wang, S. Yi, J. Liu, S. Sun, Y. Liu, D. Yang, K. Xi, G. Gao, A. Abdelkader, W. Yan, S. Ding and R. V. Kumar, ACS Nano, 2020, 14, 9819–9831 CrossRef.
- F. Wu, J. Maier and Y. Yu, Chem. Soc. Rev., 2020, 49, 1569–1614 RSC.
- Z. Chang, Y. Qiao, H. Deng, H. Yang, P. He and H. Zhou, Energy Environ. Sci., 2020, 13, 1197–1204 RSC.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef.
- Q. Zeng, X. Li, W. Gong, S. Guo, Y. Ouyang, D. Li, Y. Xiao, C. Tan, L. Xie, H. Lu, Q. Zhang and S. Huang, Adv. Energy Mater., 2022, 12, 2104074 CrossRef.
- H. Cheng, Z. Shen, W. Liu, M. Luo, F. Huo, J. Hui, Q. Zhu and H. Zhang, ACS Nano, 2023, 17, 14695–14705 CrossRef.
- G. Jiménez-Martín, J. Castillo, X. Judez, J. L. Gómez-Urbano, G. Moreno-Fernández, A. Santiago, A. Saenz de Buruaga, I. Garbayo, J. A. Coca-Clemente, A. Villaverde, M. Armand, C. Li and D. Carriazo, Batteries Supercaps, 2022, 5, e202200167 CrossRef.
- M. Xu, T. Wu, J. Qi, D. Zhou and Z. Xiao, J. Mater. Chem. A, 2021, 9, 21429–21439 RSC.
- M. Fehse, A. Iadecola, L. Simonelli, A. Longo and L. Stievano, Phys. Chem. Chem. Phys., 2021, 23, 23445–23465 RSC.
- B. F. Baggio and Y. Grunder, Annu. Rev. Anal. Chem., 2021, 14, 87–107, DOI:10.1146/annurev-anchem-091020-100631.
- V. K. Tomer, R. L. Kumawat, O. A. Titton Dias, R. Malik, G. C. Schatz and M. Sain, J. Mater. Chem. A, 2024, 12, 15814–15828 RSC.
- V. Shutthanandan, M. Nandasiri, J. Zheng, M. H. Engelhard, W. Xu, S. Thevuthasan and V. Murugesan, J. Electron Spectrosc. Relat. Phenom., 2019, 231, 2–10 CrossRef.
- V. Shutthanandan, M. Nandasiri, J. Zheng, M. H. Engelhard, W. Xu, S. Thevuthasan and V. Murugesan, J. Electron Spectrosc. Relat. Phenom., 2019, 231, 2–10 CrossRef.
- F. Yang, X. Feng, Y. S. Liu, L. C. Kao, P. A. Glans, W. Yang and J. Guo, Energy Environ. Mater., 2021, 4, 139–157 CrossRef.
- I. Källquist, R. Le Ruyet, H. Liu, R. Mogensen, M. T. Lee, K. Edström and A. J. Naylor, J. Mater. Chem. A, 2022, 10, 19466–19505 RSC.
- S. Louisia, M. T. M. Koper and R. V. Mom, Curr. Opin. Electrochem., 2024, 101462 CrossRef.
- G. Greczynski, R. T. Haasch, N. Hellgren, E. Lewin and L. Hultman, Nat. Rev. Methods Primers, 2023, 3, 1–19 CrossRef.
- H. Du, S. Li, H. Qu, B. Lu, X. Wang, J. Chai, H. Zhang, J. Ma, Z. Zhang and G. Cui, J. Membr. Sci., 2018, 550, 399–406 CrossRef.
- J. Wang, Q. Ma, S. Sun, K. Yang, Q. Cai, E. Olsson, X. Chen, Z. Wang, A. M. Abdelkader, Y. Li, W. Yan, S. Ding and K. Xi, eScience, 2022, 2, 655–665 CrossRef.
- D. Dutta Pathak, B. P. Mandal and A. K. Tyagi, J. Power Sources, 2021, 488, 229456 CrossRef.
- J. He, A. Bhargav and A. Manthiram, Adv. Energy Mater., 2022, 12, 2103204 CrossRef.
- Y. Zhou, C. H. Wang, W. Lu and L. Dai, Adv. Mater., 2020, 32, 1902779 CrossRef PubMed.
- X. Zhou, B. Liu, Y. Chen, L. Guo and G. Wei, Mater. Adv., 2020, 1, 2163–2181 RSC.
- Y. C. Chien, A. S. Menon, W. R. Brant, M. J. Lacey and D. Brandell, J. Phys. Chem. C, 2022, 126, 2971–2979 CrossRef.
- G. Tonin, G. B. M. Vaughan, R. Bouchet, F. Alloin, M. Di Michiel and C. Barchasz, J. Power Sources, 2020, 468, 228287 CrossRef.
- S. Lang, X. Feng, J. Seok, Y. Yang, M. R. Krumov, A. Molina Villarino, M. A. Lowe, S. H. Yu and H. D. Abruña, Curr. Opin. Electrochem., 2021, 25, 100652 CrossRef.
- M. Xia, T. Liu, N. Peng, R. Zheng, X. Cheng, H. Zhu, H. Yu, M. Shui and J. Shu, Small Methods, 2019, 3, 1900119 CrossRef.
- J. S. Yeon, S. Yun, J. M. Park and H. S. Park, ACS Nano, 2019, 13, 5163–5171 CrossRef.
- W. Yao, J. Xu, Y. Cao, Y. Meng, Z. Wu, L. Zhan, Y. Wang, Y. Zhang, I. Manke, N. Chen, C. Yang and R. Chen, ACS Nano, 2022, 16, 10783–10797 CrossRef.
- W. Yao, W. Zheng, J. Xu, C. Tian, K. Han, W. Sun and S. Xiao, ACS Nano, 2021, 15, 7114–7130 CrossRef PubMed.
- S. Archana and P. Elumalai, Langmuir, 2023, 39, 17446–17457 CrossRef.
- X. Song, D. Tian, Y. Qiu, X. Sun, B. Jiang, C. Zhao, Y. Zhang, L. Fan and N. Zhang, Adv. Funct. Mater., 2022, 32, 2109413 CrossRef.
- A. Bhargav, C. H. Chang, Y. Fu and A. Manthiram, ACS Appl. Mater. Interfaces, 2019, 11, 6136–6142 CrossRef.
- L. Chen, G. Cao, Y. Li, G. Zu, R. Duan, Y. Bai, K. Xue, Y. Fu, Y. Xu, J. Wang and X. Li, Nano-Micro Lett., 2024, 16, 1–33 Search PubMed.
- H. Gamo, K. Hikima and A. Matsuda, ACS Omega, 2023, 8, 45557–45565 CrossRef.
- M. U. M. Patel, I. Arčon, G. Aquilanti, L. Stievano, G. Mali and R. Dominko, ChemPhysChem, 2014, 15, 894–904 CrossRef PubMed.
- M. Abe, F. Kaneko, N. Ishiguro, T. Kubo, F. Chujo, Y. Tamenori, H. Kishimoto and Y. Takahashi, J. Phys. Chem. C, 2022, 126, 14047–14057 CrossRef.
- R. Li, D. Rao, J. Zhou, G. Wu, G. Wang, Z. Zhu, X. Han, R. Sun, H. Li, C. Wang, W. Yan, X. Zheng, P. Cui, Y. Wu, G. Wang and X. Hong, Nat. Commun., 2021, 12, 1–8 CrossRef.
- Q. Guo, K. C. Lau and R. Pandey, Mater. Adv., 2021, 2, 6403–6410 RSC.
- U. Zubair, K. Jori, J. E. Thomas, J. Amici, C. Francia and S. Bodoardo, J. Power Sources, 2023, 580, 233457 CrossRef.
- G. Ai, Q. Hu, L. Zhang, K. Dai, J. Wang, Z. Xu, Y. Huang, B. Zhang, D. Li, T. Zhang, G. Liu and W. Mao, ACS Appl. Mater. Interfaces, 2019, 11, 33987–33999 CrossRef.
- L. Shi, S. M. Bak, Z. Shadike, C. Wang, C. Niu, P. Northrup, H. Lee, A. Y. Baranovskiy, C. S. Anderson, J. Qin, S. Feng, X. Ren, D. Liu, X. Q. Yang, F. Gao, D. Lu, J. Xiao and J. Liu, Energy Environ. Sci., 2020, 13, 3620–3632 RSC.
- X. Yu, H. Pan, Y. Zhou, P. Northrup, J. Xiao, S. Bak, M. Liu, K.-W. Nam, D. Qu, J. Liu, T. Wu, X.-Q. Yang, X. Yu, Y. Zhou, P. Northrup, S. Bak, M. Liu, X.-Q. Yang, H. Pan, J. Xiao, J. Liu, W. K. Nam, D. Qu and T. Wu, Adv. Energy Mater., 2015, 5, 1500072 CrossRef.
- F. Luna-Lama, A. Caballero and J. Morales, Sustainable Energy Fuels, 2022, 6, 1568–1586 RSC.
- L. Shi, S. M. Bak, Z. Shadike, C. Wang, C. Niu, P. Northrup, H. Lee, A. Y. Baranovskiy, C. S. Anderson, J. Qin, S. Feng, X. Ren, D. Liu, X. Q. Yang, F. Gao, D. Lu, J. Xiao and J. Liu, Energy Environ. Sci., 2020, 13, 3620–3632 RSC.
- M. Li, W. Liu, D. Luo, Z. Chen, K. Amine and J. Lu, ACS Energy Lett., 2022, 7, 577–582 CrossRef.
- C. Li, Y. Li, Y. Fan, F. Wang, T. Lei, W. Chen, A. Hu, L. Xue, J. Huang, C. Wu, C. Yang, Y. Hu and Y. Yan, Small, 2022, 18, 2106657 CrossRef.
- X. Guo, J. Zhang, L. Yuan, B. Xi, F. Gao, X. Zheng, R. Pan, L. Guo, X. An, T. Fan and S. Xiong, Adv. Energy Mater., 2023, 13, 2204376 CrossRef.
- V. K. Tomer, R. Malik, J. Tjong and M. Sain, Coord. Chem. Rev., 2023, 481, 215055 CrossRef.
- A. J. Cowan and L. J. Hardwick, Annu. Rev. Anal. Chem., 2019, 12, 323–346, DOI:10.1146/annurev-anchem-061318-115303.
- J. Lim, K. K. Lee, C. Liang, K. H. Park, M. Kim, K. Kwak and M. Cho, J. Phys. Chem. B, 2019, 123, 6651–6663 CrossRef PubMed.
- M. Weiling, F. Pfeiffer, M. Baghernejad, M. Weiling, F. Pfeiffer and M. Baghernejad, Adv. Energy Mater., 2022, 12, 2202504 CrossRef.
- S. Nowak and M. Winter, J. Anal. At. Spectrom., 2017, 32, 1833–1847 RSC.
- L. Meyer, N. Saqib and J. Porter, J. Electrochem. Soc., 2021, 168, 090561 CrossRef.
- M. A. Weret, C. F. J. Kuo, W. N. Su, T. S. Zeleke, C. J. Huang, N. A. Sahalie, T. A. Zegeye, Z. T. Wondimkun, F. W. Fenta, B. A. Jote, M. C. Tsai and B. J. Hwang, J. Power Sources, 2022, 541, 231693 CrossRef.
- L. Xue, Y. Li, A. Hu, M. Zhou, W. Chen, T. Lei, Y. Yan, J. Huang, C. Yang, X. Wang, Y. Hu and J. Xiong, Small Struct., 2022, 3, 2100170 CrossRef.
- R. Zhang, Y. Wu, Z. Chen, Y. Wang, J. Zhu and X. Zhuang, J. Mater. Chem. A, 2023, 11, 19195–19209 RSC.
- X. Q. Cheng, H. J. Li, Z. X. Zhao, Y. Z. Wang and X. M. Wang, New Carbon Mater., 2021, 36, 93–105 CrossRef.
- É. A. Santos, R. C. Fernandes, R. Vicentini, J. P. Aguiar, L. M. Da Silva and H. Zanin, J. Energy Storage, 2023, 71, 108203 CrossRef.
- Z. Pan, D. J. L. Brett, G. He, I. P. Parkin, Z. Pan, G. He, I. P. Parkin and D. J. L. Brett, Adv. Energy Mater., 2022, 12, 2103483 CrossRef CAS.
- L. Zhang, T. Qian, X. Zhu, Z. Hu, M. Wang, L. Zhang, T. Jiang, J. H. Tian and C. Yan, Chem. Soc. Rev., 2019, 48, 5432–5453 RSC.
- J. H. Tian, T. Jiang, M. Wang, Z. Hu, X. Zhu, L. Zhang, T. Qian and C. Yan, Small Methods, 2020, 4, 1900467 CrossRef CAS.
- C. Dillard, S. H. Chung, A. Singh, A. Manthiram and V. Kalra, Mater. Today Energy, 2018, 9, 336–344 CrossRef.
- H. Du, S. Li, H. Qu, B. Lu, X. Wang, J. Chai, H. Zhang, J. Ma, Z. Zhang and G. Cui, J. Membr. Sci., 2018, 550, 399–406 CrossRef.
- J. Liu, J. Wang, L. Zhu, X. Chen, Q. Ma, L. Wang, X. Wang and W. Yan, Chem. Eng. J., 2021, 411, 128540 CrossRef.
- Z. Liang, J. Shen, X. Xu, F. Li, J. Liu, B. Yuan, Y. Yu and M. Zhu, Adv. Mater., 2022, 34, 2200102 CrossRef.
- H. Li, C. Chen, Y. Yan, T. Yan, C. Cheng, D. Sun and L. Zhang, Adv. Mater., 2021, 33, 2105067 CrossRef.
- S. A. Bonke, T. Risse, A. Schnegg and A. Brückner, Nat. Rev. Methods Primers, 2021, 1, 1–20 CrossRef.
- L. Galazzo and E. Bordignon, Prog. Nucl. Magn. Reson. Spectrosc., 2023, 134–135, 1–19 CrossRef.
- T. Insinna, E. N. Bassey, K. Märker, A. Collauto, A. L. Barra and C. P. Grey, Chem. Mater., 2023, 35, 5497–5511 CrossRef.
- Z. Ye, Y. Jiang, L. Li, F. Wu and R. Chen, Adv. Mater., 2022, 34, 2109552 CrossRef.
- Y. Zhou, M. Su, X. Yu, Y. Zhang, J. G. Wang, X. Ren, R. Cao, W. Xu, D. R. Baer, Y. Du, O. Borodin, Y. Wang, X. L. Wang, K. Xu, Z. Xu, C. Wang and Z. Zhu, Nat. Nanotechnol., 2020, 15, 224–230 CrossRef PubMed.
- L. Cressa, Y. Sun, D. Andersen, M. Gerard, O. De Castro, D. Kopljar, M. Nojabaee, K. A. Friedrich, G. Schmitz, T. Wirtz and S. Eswara, Anal. Chem., 2023, 95, 9932–9939 CrossRef PubMed.
- K. Schaepe, H. Jungnickel, T. Heinrich, J. Tentschert, A. Luch and W. E. S. Unger, Characterization of Nanoparticles: Measurement Processes for Nanoparticles, 2020, pp. 481–509 Search PubMed.
- H. Mei, T. S. Laws, T. Terlier, R. Verduzco and G. E. Stein, J. Polym. Sci., 2022, 60, 1174–1198 CrossRef.
- K. Bagheri, M. Deschamps and E. Salager, Curr. Opin. Colloid Interface Sci., 2023, 64, 101675 CrossRef.
- X. Liu, Z. Liang, Y. Xiang, M. Lin, Q. Li, Z. Liu, G. Zhong, R. Fu and Y. Yang, Adv. Mater., 2021, 33, 2005878 CrossRef PubMed.
- N. Leifer, D. Aurbach and S. G. Greenbaum, Prog. Nucl. Magn. Reson. Spectrosc., 2024, 142–143, 1–54 CrossRef PubMed.
- J. B. Fritzke, S. Dey, C. A. O'Keefe and C. P. Grey, ECS Meeting Abstracts, 2023, MA2023-02, 2692.
- S. Duhan and V. K. Tomer, in Advanced Sensor and Detection Materials, ed. A. Tiwari and M. M. Demir, John Wiley and Sons Ltd, 2014, pp. 149–192 Search PubMed.
- V. K. Tomer, S. Duhan, P. V. Adhyapak and I. S. Mulla, J. Am. Ceram. Soc., 2015, 98, 741–747 CrossRef.
- V. K. Tomer and S. Duhan, Appl. Phys. Lett., 2015, 106, 063105 CrossRef.
- R. Malik, V. K. Tomer, M. Sain and Z. Chen, Renewable Sustainable Energy Rev., 2023, 181, 113348 CrossRef.
- B. Liu and V. S. Thoi, Chem. Commun., 2022, 58, 4005–4015 RSC.
- R. Yan, B. Mishra, M. Traxler, J. Roeser, N. Chaoui, B. Kumbhakar, J. Schmidt, S. Li, A. Thomas and P. Pachfule, Angew. Chem., Int. Ed., 2023, 62, e202302276 CrossRef PubMed.
- X. Li, K. Zhang, Z. Li, Y. Yan, Y. Yuan, L. Ma, K. Xie and K. Ping Loh, Angew. Chem., Int. Ed., 2023, 62, e202217869 CrossRef.
- A. Shafique, V. S. Rangasamy, A. Vanhulsel, M. Safari, M. K. Van Bael, A. Hardy and S. Sallard, Electrochim. Acta, 2020, 360, 136993 CrossRef.
- D. Hou, F. Bai, P. Dong, J. Chen, Y. Zhang, F. Meng, Z. Zhang, C. Zhang, Y. Zhang and J. Hu, J. Power Sources, 2023, 584, 233599 CrossRef.
- M. G. Bason, T. Coussens, M. Withers, C. Abel, G. Kendall and P. Krüger, J. Power Sources, 2022, 533, 231312 CrossRef.
- D. Atkins, E. Ayerbe, A. Benayad, F. G. Capone, E. Capria, I. E. Castelli, I. Cekic-Laskovic, R. Ciria, L. Dudy, K. Edström, M. R. Johnson, H. Li, J. M. G. Lastra, M. L. De Souza, V. Meunier, M. Morcrette, H. Reichert, P. Simon, J. P. Rueff, J. Sottmann, W. Wenzel and A. Grimaud, Adv. Energy Mater., 2022, 12, 2102687 CrossRef.
- Z. Huang, Y. Zhou, Z. Deng, K. Huang, M. Xu, Y. Shen and Y. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 8217–8223 CrossRef.
- Z. Deng, Z. Huang, Y. Shen, Y. Huang, H. Ding, A. Luscombe, M. Johnson, J. E. Harlow, R. Gauthier and J. R. Dahn, Joule, 2020, 4, 2017–2029 CrossRef.
- A. Niemöller, P. Jakes, R. A. Eichel and J. Granwehr, Sci. Rep., 2018, 8, 1–7 Search PubMed.
- H. Zhou, A. S. Alujjage, M. Terese, C. Fear, T. Joshi, V. R. Rikka, J. A. Jeevarajan and P. P. Mukherjee, Appl. Energy, 2025, 377, 124465 CrossRef.
- T. Bond, R. Gauthier, S. Gasilov and J. R. Dahn, J. Electrochem. Soc., 2022, 169, 080531 CrossRef.
- Y. J. Xu, B. Wang, Y. Wan, Y. Sun, W. L. Wang, K. Sun, L. J. Yang, H. Hu and M. B. Wu, New Carbon Mater., 2023, 38, 678–693 CrossRef.
- Y. S. Duh, Y. Sun, X. Lin, J. Zheng, M. Wang, Y. Wang, X. Lin, X. Jiang, Z. Zheng, S. Zheng and G. Yu, J. Energy Storage, 2021, 41, 102888 CrossRef.
- G. Xu, L. Huang, C. Lu, X. Zhou and G. Cui, Energy Storage Mater., 2020, 31, 72–86 CrossRef.
- Y. S. Duh, X. Liu, X. Jiang, C. S. Kao, L. Gong and R. Shi, J. Energy Storage, 2020, 30, 101422 CrossRef.
- D. Ouyang, M. Chen, J. Weng, K. Wang, J. Wang and Z. Wang, J. Energy Chem., 2023, 81, 543–573 CrossRef CAS.
- L. Huang, T. Lu, G. Xu, X. Zhang, Z. Jiang, Z. Zhang, Y. Wang, P. Han, G. Cui and L. Chen, Joule, 2022, 6, 906–922 CrossRef CAS.
- L. Vásárhelyi, Z. Kónya, A. Kukovecz and R. Vajtai, Mater. Today Adv., 2020, 8, 100084 CrossRef.
- Z. Yan, L. Wang, H. Zhang and X. He, Adv. Energy Mater., 2024, 14, 2303206 CrossRef CAS.
- X. Wan, X. Xu, F. Li, X. Song, C. Peng and J. Liu, Small Struct., 2024, 5, 2300196 CrossRef CAS.
- J. Le Houx and D. Kramer, Energy Rep., 2021, 7, 9–14 CrossRef.
- H. Li and W. Wang, Curr. Opin. Electrochem., 2023, 41, 101376 CrossRef.
- J. H. Um, S. J. Kim, J. H. Hyun, M. Kim, S. H. Lee and S. H. Yu, Acc. Chem. Res., 2023, 56, 440–451 CrossRef CAS PubMed.
- J. H. Tian, T. Jiang, M. Wang, Z. Hu, X. Zhu, L. Zhang, T. Qian and C. Yan, Small Methods, 2020, 4, 1900467 CrossRef CAS.
- D. Cao, Y. Zhang, T. Ji and H. Zhu, MRS Bull., 2023, 48, 1257–1268 CrossRef.
- H. Wang, D. Ning, L. Wang, H. Li, Q. Li, M. Ge, J. Zou, S. Chen, H. Shao, Y. Lai, Y. Zhang, G. Xing, W. K. Pang and Y. Tang, Small, 2022, 18, 2107491 CrossRef CAS.
- S. Zhou, K. Liu, Y. Ying, L. Chen, G. Meng, Q. Zheng, S. G. Sun and H. G. Liao, Curr. Opin. Electrochem., 2023, 41, 101374 CrossRef CAS.
- D. Yang, Y. X. A. Ng, K. Zhang, Q. Chang, J. Chen, T. Liang, S. Cheng, Y. Sun, W. Shen, E. H. Ang, H. Xiang and X. Song, ACS Appl. Mater. Interfaces, 2023, 15, 20583–20602 CrossRef CAS PubMed.
- H. Li and W. Wang, Curr. Opin. Electrochem., 2023, 41, 101376 CrossRef.
- R. Zhang, Y. Wu, Z. Chen, Y. Wang, J. Zhu and X. Zhuang, J. Mater. Chem. A, 2023, 11, 19195–19209 RSC.
- L. Xue, Y. Li, A. Hu, M. Zhou, W. Chen, T. Lei, Y. Yan, J. Huang, C. Yang, X. Wang, Y. Hu and J. Xiong, Small Struct., 2022, 3, 2100170 CrossRef.
- L. Xue, Y. Li, A. Hu, M. Zhou, W. Chen, T. Lei, Y. Yan, J. Huang, C. Yang, X. Wang, Y. Hu and J. Xiong, Small Struct., 2022, 3, 2100170 CrossRef.
- K. Bagheri, M. Deschamps and E. Salager, Curr. Opin. Colloid Interface Sci., 2023, 64, 101675 CrossRef.
- A. P. Black, A. Sorrentino, F. Fauth, I. Yousef, L. Simonelli, C. Frontera, A. Ponrouch, D. Tonti and M. R. Palacín, Chem. Sci., 2023, 14, 1641–1665 RSC.
- M. Pin, J. Choi, J. H. Chang, A. S. Schenk, J. Han, S. Wacławek, Y. Kim and J. Y. Cheong, Energy Storage Mater., 2024, 73, 103798 CrossRef.
- K. Wang, D. Luo, Q. Ma, X. Lai, L. He and Z. Chen, Energy Technol., 2024, 12, 2400199 CrossRef.
- M. M. Amaral, C. G. Real, V. Y. Yukuhiro, G. Doubek, P. S. Fernandez, G. Singh and H. Zanin, J. Energy Chem., 2023, 81, 472–491 CrossRef.
- Y. S. Byeon, W. Lee, S. Park, D. Kim, J. Jung, M. S. Park and W. S. Yoon, Small Sci., 2024, 2400165 CrossRef.
- É. A. Santos, M. M. Amaral, B. S. Damasceno, L. M. Da Silva, H. G. Zanin, J. N. Weker and C. B. Rodella, Nano Energy, 2024, 130, 110098 CrossRef.
- M. A. Bañares and M. Daturi, Catal. Today, 2023, 423, 114255 CrossRef.
- F. Strauss, D. Kitsche, Y. Ma, J. H. Teo, D. Goonetilleke, J. Janek, M. Bianchini and T. Brezesinski, Adv. Energy Sustainability Res., 2021, 2, 2100004 CrossRef.
- J. H. Um and S. H. Yu, Adv. Energy Mater., 2021, 11, 2003004 CrossRef.
- T. Zheng, M. Muneeswara, H. Bao, J. Huang, L. Zhang, D. S. Hall, S. T. Boles and W. Jin, ChemElectroChem, 2024, 11, e202400065 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |