Open Access Article
Open Access ArticleA comparative study of methods for calculating the oxidative potential (OP) of atmospheric particulate matter†
Eduardo José dos Santos
Souza
a,
Gaëlle
Uzu
*b,
Khanneh Wadinga
Fomba
 a,
Pamela A.
Dominutti
b,
Takoua
Mhadhbi
b,
Jean-Luc
Jaffrezo
b and
Hartmut
Herrmann
a,
Pamela A.
Dominutti
b,
Takoua
Mhadhbi
b,
Jean-Luc
Jaffrezo
b and
Hartmut
Herrmann
 *a
*a
aAtmospheric Chemistry Department (ACD) – Leibniz Institute for Tropospheric Research (TROPOS), Permoserstraße 15, 04318 Leipzig, Germany. E-mail: herrmann@tropos.de
bUniversité Grenoble Alpes (UGA), CNRS, IRD, Grenoble-INP, INRAE, UMR 5001, IGE, 38402, Grenoble, France. E-mail: gaelle.uzu@ird.fr
First published on 4th July 2025
Abstract
The oxidative potential (OP) of particulate matter (PM) is a pivotal metric to evaluate the potential health effects of air pollution. However, the variety of assays and protocols available to measure the OP poses a challenge for comparing one study with another. The present study aims to provide an analysis comparing four calculation methods for determining the OP. These methods include the use of calibration curves (CURVE), absorbance values (ABS), and two concentration-based (CC1 and CC2) methods. Two acellular assays, dithiothreitol (DTT; OPDTT) and ascorbic acid (AA; OPAA), were chosen to be examined. The application of these assays led to varying OP results depending on the applied calculation method. Regarding results, first of all, there is a notable agreement between the ABS and CC2 methods across both the DTT and AA assays. Second, however, for both assays, the CC1 method consistently leads to higher OP values, with OPDTT variations of up to 18% compared to ABS and CC2, and OPAA variations of up to 12%. Third, the CURVE method yields OPDTT and OPAA values that are up to 10% and 19% higher than those calculated by the ABS and the CC2 methods, respectively. Therefore, both the ABS and CC2 methods are recommended for calculating OP values, as they have shown better consistency across different PM samples. These findings underscore the importance of defining standardizing OP protocols which should explicitly include all needed calculation steps in order to further develop the OP metric into a comparable measure linking air quality and human health.
Environmental significanceAssessing the oxidative potential (OP) of particulate matter (PM) is crucial for understanding its role in air pollution-related health effects. However, variations in calculation methods can lead to discrepancies in reported OP values, limiting comparability across studies. This work highlights how different computational approaches influence OP estimates and identifies the methods that yield more consistent results. Establishing standardized OP calculation protocols is critical to enhancing the reliability of this metric, ultimately improving its applicability in air quality assessments and public health research. By refining OP quantification, this study contributes to a more robust framework for evaluating the oxidative stress burden associated with PM exposure. |
1. Introduction
Oxidative potential (OP), the ability of particulate matter (PM) to induce oxidation in the lung environment, is increasingly used as a metric to assess health effects of air pollution.1,2 Researchers worldwide have extensively explored methodologies for OP assays, recognizing them as more precise measures for predicting the toxicity of aerosol particles.3–7 So far, the capacity of aerosol species to catalyze redox reactions and influence the formation of reactive oxygen species (ROS) has been widely investigated in many studies using chemical acellular assays.8–13 The variability in OP measurements can be attributed to several factors, including the chemical composition of PM,14–16 emission sources,17–19 chemical interactions,20–22 size-segregated PM,23 reactant concentrations,24 and operating conditions,25 all of which have been investigated.The assessment of aerosol particle toxicity based on oxidative potential assays was initially conducted in the early 2000s.26,27 Since then, researchers worldwide have developed, optimized and applied a variety of methodologies to determine the OP of PM. Common acellular methods include the dithiothreitol (DTT) assay (OPDTT), the ascorbic acid (AA) assay (OPAA), the glutathione (GSH) assay (OPGSH) and other acellular assays.28,29 Due to the catalytic activity of redox-active PM species in the presence of antioxidants, redox reactions combined with absorbance measurement routines are used to determine the consumption rate of reducing agents, thereby measuring the dynamics of OP values based on air volume and sampled mass normalization.28,29 However, previous studies have not examined the mathematical approaches to derive OP values from measurements obtained with different assays, and apparently, there is no consensus on the optimal method to determine the OP associated with PM and redox-active species capable of depleting antioxidants. Many of the current protocols for measuring OPs involve the use of known concentrations of reducing agents, such as DTT or AA, incubated with PM extracts. OP values are then deduced from the consumption of the reagents over time, using various mathematical methods. These include calibration curves (CURVE),30–34 absorbance values linked to consumption rates (ABS)35–37 or concentration values associated with the decay kinetics of DTT or AA consumption (CC1 and CC2).16,38–41
Although previous studies have explored the uncertainties in OP assays by investigating the variability of OP values resulting from factors such as experimental repeatability, regression curves, and operational procedures,35 no studies have specifically addressed the variability in OP values resulting from different calculation methods. In this study, a critical comparison of different mathematical approaches for estimating the OP of PM is performed. A review of 130 publications resulted in identifying at least four distinct approaches. The methods are applied on specific data sets generated with both the OPDTT and OPAA assays, as these are the most prominently used assays in the domain. The primary objective of the present study is to assess the variability across different calculation methods for different assays, with the aim of establishing a standardized protocol for OP quantification as a common final step of all OP assays. This will help ensure more consistent and reliable measurements contributing to a better understanding of the toxic potency of aerosols. By promoting the adoption of uniform OP calculation methods, this research should enable more meaningful comparisons across global studies, fostering collaboration and advancing the field of environmental health research.
2. Materials and methods
2.1. Oxidative potential assays, a kinetic measurement with computational implications
Most oxidative potential measurements are based on the consumption of antioxidants or chemical surrogates (e.g., OPAA, OPDTT, and OPGSH) or the evaluation of ROS production (e.g., OPOH) by a PM sample. These analytical protocols involve multiple absorbance measurements over a period of 15 to 45 minutes to evaluate the kinetics of the reaction between the sample and the reactants. The rate of consumption of reducing agents, such as DTT and AA, is determined by applying linear regression to the absorbance data (which is proportional to the concentration loss of DTT or AA) as a function of incubation time. This analysis yields the regression slope and intercept, which are used to assess the reactivity of the PM samples. Thus, the slope is consistently used in various calculation methods to derive OP values, which are then normalized based on mass and volume. Specifically, the slope corresponds to the rate constant (k), which indicates the rate at which the concentrations of reductants (e.g., DTT and AA) decrease over time, in proportion to their respective concentrations, as shown in eqn (1). | (1) |
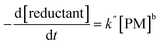 | (2) |
Hence, the rate law simplifies to a dependence solely on the concentration of PM, with the reaction order now being b. Notably, the presence of reactive PM species increases the overall oxidation of DTT and AA, reflected in a higher k. Over prolonged incubation periods, the consumption of DTT and AA may become more gradual, leading to a reduction in apparent k, which is better explained by a pseudo-first-order reaction.43–47 This has significant implications for routine OP assays, especially when very low concentrations of DTT and AA are used to incubate PM extracts, as it directly affects the reaction order of the OP assay. Another important consideration is the determination of OP values for PM samples with high reactivity, which, in turn, affects the apparent k and influences the linearity of DTT and AA consumption.
It should be noted that endpoint measurement protocols are also used to determine the oxidative potential based on the initial and final absorbance values.48,49 However, these protocols do not guarantee linear consumption of reducing agents, leading to significant variations in the measured OP since they typically involve long incubation periods. To address this, initial reaction rates are measured by collecting data at short intervals after the reaction begins to ensure linearity.13 Calibration curves and appropriate controls are used for accurate quantification of OPDTT and OPAA, along with critical monitoring of blank absorbance values, especially at time 0. Conditions are optimized to prevent rapid DTT and AA consumption, and automated systems are employed to improve precision and reproducibility.5
2.2. PM samples
The present study examined six PM10 samples (#A, #B, #C, #D, #E, and #F) to investigate the variability between different mathematical approaches for estimating OPDTT and OPAA values. Since this study focuses on method comparability by analyzing three distinct sources, the small sample size employed here is sufficient to ensure the variability in the OP values across different mathematical approaches. PM samples #A and #B were collected during Saharan dust events, samples #C and #D were collected under European urban conditions, and samples #E and #F were collected during winter in regions affected by biomass burning in Europe. Samples were collected using quartz fiber filters for 24 h of sampling (DA-80 devices, 720 m3).2.3. PM extraction and iso-concentration of particles
All PM samples were extracted at 37.4 °C using a combination of dipalmitoyl phosphatidylcholine (DPPC) and Gamble's solution to create a respiratory simulated lung fluid (RSLF).41 To ensure comparability of the experiments, extractions at iso-concentration of the particles were performed at 25 μg mL−1 and then incubated with DTT or AA.502.4. Dithiothreitol assay (OPDTT)
The OPDTT assay was investigated through TNB2− formation, which involved multiple absorbance measurements using a TECAN Infinite® M200 Pro spectrophotometer and 96-well CELLSTAR® Multiwell plates from Greiner Bio-One®. The reaction mixture contained 225 μL of phosphate buffer solution (PBS; Carl Roth GmbH + Co KG Karlsruhe, Germany), 50 μL of 250 μM DTT (CAS: 3483-12-3; Carl Roth GmbH + Co KG Karlsruhe, Germany) in PBS, and 20 μL of PM extraction. TNB2− formation was monitored at 412 nm with the addition of 50 μL of DTNB (CAS: 69-78-3; Carl Roth GmbH + Co KG Karlsruhe, Germany) at 0, 15, and 30 minutes of incubation. OPDTT measurements were carried out in triplicate. Blank measurements (n = 6) were carried out using the same protocol as previously described for PM samples. Positive controls consisted of monitoring the OP of 1,4-NQ (24.7 μM) to evaluate measurement quality and reproducibility. The coefficient of variation (COV; %) ranged between 2 and 8% (n = 8). Further details can be found in Calas et al.41 and Dominutti et al.182.5. Ascorbic acid assay (OPAA)
The depletion of AA was investigated using the same instrument as for the DTT assay. A redox reaction was performed by incubating 100 μL of 240 μM AA and 80 μL of the PM extract (25 μg mL−1) for 32 minutes. Absorbance measurements were taken every 4 minutes (at 0, 4, 8, 12, 16, 20, 24, 28, and 32 minutes) at 265 nm. The chemical controls involved monitoring and assessing the measurement quality and reproducibility using 1,4-NQ (24.7 μM). The COV ranged between 1 and 5% (n = 6). Additional information on the OPAA assay can be found in the studies by Marsal et al.3 and Borlaza et al.50 All OPAA measurements were carried out in triplicate.2.6. Mathematical approaches for quantifying OPDTT
The present study evaluates four mathematical approaches for assessing the OP of PM, identified through a comprehensive literature review (see Section 3.1: Calculation methods for quantifying oxidative potential). The OP values calculated using these different approaches—including the calibration curve (CURVE), absorbance-based (ABS), and two concentration-based methods (CC1 and CC2)—were initially assessed as the activity rate (nmol min−1). These values were then normalized further to account for 1 μg of incubated mass (nmol min−1 μg−1) and 1 m3 of air volume (nmol min−1 m−3).For all calculation methods, background absorbance values obtained from blank measurements were subtracted from the absorbance values of the PM samples prior to the calculation step. In addition, we carefully monitored the background absorbance and the autoxidation of DTT and AA in both OP assays, placing particular emphasis on accurately assessing these parameters to ensure data reliability.
Using the CURVE method, the OP is obtained as follows:
(i) An absorbance vs. (DTT/AA) concentration regression curve is established, and both the slope (m) and intercept (c) values are determined.
(ii) During the incubation of the samples with (DTT/AA) in the respective assays, the absorbance values at each incubation time point (At) are recorded.
(iii) The absorbance (At) is converted into a (DTT/AA) concentration using the calibration curve parameters obtained in (i) above.
(iv) The OP is determined as the rate of change of (DTT/AA) concentration over time.
(v) The value is multiplied by the solution volume (V), to obtain the OP values (nmol min−1).
The determination of OP values using the CURVE method can be represented as follows:
 | (3) |
Further features of the CURVE method are discussed in other studies.30–34
(i) Absorbance values related to DTT/AA consumption are recorded every 5, 10, or 15 min for the DTT assay and every 4 or 5 min for the AA assay.3,16
(ii) A calibration curve of absorbance values versus time is established, and both the slope and intercept are determined.
Accordingly, the OP (nmol min−1) using the ABS method is determined as follows (eqn (4)):
 | (4) |
(i) Replicate absorbance measurements of blank field filters are performed shortly after the addition of DTT and AA and represent the absorbance at time 0 (AB0).
(ii) Absorbance values are measured at equal time intervals after adding DTT or AA (At), during a controlled incubation time.
(iii) The OP values in nmol min−1 are determined according to eqn (5):
 | (5) |
(i) Absorbance values for the PM samples at a given incubation time (At), as well as the absorbance values for the PM samples at time 0 (APM0), are measured.
(ii) The consumption of DTT and AA is determined by measuring absorbance at specific time intervals during the incubation period. For the DTT assay, measurements are typically taken every 5, 10, or 15 minutes, while for the AA assay, intervals of 4 or 5 minutes are commonly used.3,16 These intervals are chosen to effectively monitor the reaction kinetics and accurately assess the consumption rates of DTT and AA.
For the OP determination (nmol min−1), as proposed by eqn (6), the [reductant]0 is the initial DTT or AA concentration (in mol L−1) and V is the solution volume (L).16,40,41,51
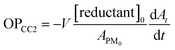 | (6) |
2.7. Statistical evaluation
A comprehensive statistical analysis was conducted to assess the variability in OP values derived from the four mathematical approaches (CURVE, ABS, CC1, and CC2). The COV was calculated to quantify the relative variability of OP values, providing a standardized measure of dispersion. Discrepancies between methods were quantified by comparing individual OP values to the overall OP mean. Analysis of variance (ANOVA) was used to determine statistically significant differences among methods, incorporating replicate measurements. Paired t-tests and associated p-values were employed to examine OP value variations across calibration curve ranges and to further investigate the differences between the calculation methods. The mean and median were also used to compare OP values obtained from each method.3. Results and discussion
3.1. Calculation methods for quantifying the oxidative potential
The mathematical approaches presented in the previous section are designed to investigate the linear consumption rate of DTT or AA during incubation with redox-active PM species. To fulfill linearity, the methods are applied for protocols with short incubation times, typically less than 45 minutes. However, some studies have used alternative assays that extend the incubation time, typically to four hours, and have used additional mathematical approaches to assess the OP of PM.42,43,52–55 These extended incubation methodologies are not discussed in the present study, as differences in incubation times potentially affect the dynamics of the redox mechanism and linearity. This issue alone might warrant a separate review-like treatment. Based on OP studies conducted over the past five years (2020–2024), the various mathematical approaches applied for determining OP are grouped to provide a comprehensive overview of the methodologies. Studies lacking sufficient information about their calculation methods are classified as “unknown.”A review of 130 peer-reviewed papers within this period revealed that the CC2 method (39 publications, 30%) and the ABS method (34 publications, 26.1%) were the most commonly employed approaches for assessing the oxidative potential of particulate matter (Fig. 1). Other OP studies used calibration curves to investigate the rate of DTT or AA consumption; however, this approach was less common, appearing in only 13.8% (18 publications) of the OP studies. Additionally, about 6% (8 publications) of the studies applied the CC1 method in DTT or AA assays. A substantial portion, 23.8% (31 publications), lacked sufficient information to be classified within the categories presented in the previous section.
 | ||
| Fig. 1 Number of peer-reviewed publications addressing different calculation methods used to estimate OP values in the scientific literature over the past 5 years (n = 130), including previous citations and others.56–171 | ||
3.2. Variability of OPDTT values across different mathematical methods
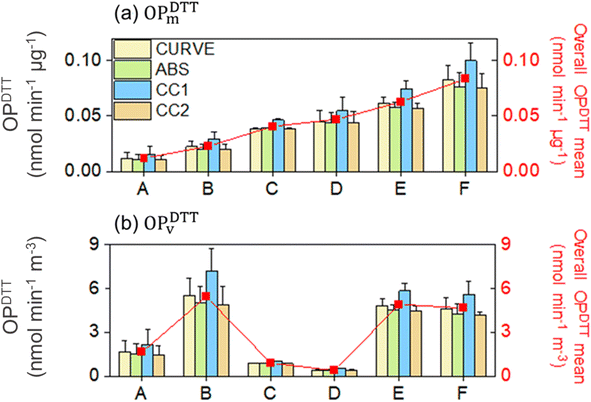
When comparing the OP values from different calculation methods, CC1 yields significantly higher results. For instance, in the case of sample #F, the OPDTTm values are roughly 25% higher (0.099 nmol min−1 μg−1) than those obtained using the ABS and CC2 methods. For OPDTTV, the ABS and CC2 methods yielded values of 4.24 and 4.19 nmol min−1 m−3, respectively, while CURVE and CC1 methods result in values of 4.59 and 5.57 nmol min−1 m−3, respectively. Therefore, both ABS and CC2 methods tend to produce comparable OPDTTm and OPDTTVvalues, whereas the CURVE and CC1 methods typically result in higher OPs. A detailed comparison of OPDTTm and OPDTTV values is presented in Table S1† for all the investigated samples, including information on their statistics, mean, median, and COV.
The ABS method leads to OPDTT values differing from the overall OP mean by 4.0% to 11%, while the CC2 method shows deviations of 4.5% to 14% (p > 0.05; Table S2†). Although both the ABS and CC2 methods exhibit similar profiles, the CC2 method reveals slightly higher variation compared to the ABS method. In contrast, the CURVE method exhibits the least variation, with the deviations in the OP values ranging between 1.1% and 5.7% relative to the overall OP mean.
The CC1 method displays the highest variability observed in this study, with variations of up to 27% (ranging from 14% to 27%). This indicates that CC1 provides OP values that significantly differ from those obtained through other mathematical approaches (p < 0.05; Table S2†). Notably, the parameters utilized for the OP determination in the CC1 method differ from the others, potentially exerting a substantial impact on the distribution of both OPDTTm and OPDTTv values.
The variability previously observed for OPDTTm remains for OPDTTv values, as shown in Fig. 2b, since both normalized OPs are primarily derived from the same OP values (nmol min−1). Moreover, the t-test reveals statistically significant differences for all methods except for the comparison between ABS and CC2, which yield similar OPDTTv and OPDTTm (Table S2†). To facilitate the interpretation and presentation of our findings, the following sections will focus on discussing the OPDTTm values.
In general, the descending order in both OPDTTm and OPDTTv values for the investigated methods is as follows: CC1 > CURVE > ABS ≈ CC2.
Although the differences in the mean values for these samples may be low, the relative variability of the results is higher for these PM samples. In other words, the COV for the CC2 method varies significantly between different PM samples, suggesting that its reliability is sample-dependent, as already observed for CC1. This implies that the variability observed in both the CC1 and CC2 methods may depend on the complex interplay of aerosol chemical species across different PM samples, or alternatively, may arise from the inherent effects of the variability caused by the distinct absorbance values at time zero, as incorporated within the framework of these methods.
The OPDTT values of PM samples #A, #B, #D, and #F are not significantly different, regardless of the calculation method used and replicate values (one-way ANOVA, p > 0.05 at the 0.05 level; Table S3†). This was different for the effects of replicate values on the OPDTT of PM samples #C and #E, which showed significant differences between the mathematical approaches. This aligns well with the previous observation, where the high COVs for the CC1 method indicates general variability issues, while the varying COVs suggest that each method's relative performance can be significantly different depending on the PM sample. A complete assessment of the statistical differences between the calculation methods with consideration of their replicated OP values, including ANOVA and p-values is provided in Table S3(a–f)† for the DTT assay. It should be noted that significant variation already exists in calculating the OP using different methods as previously described. However, the variability increases further due to differences between replicates for each PM sample, particularly for methods CC1 and CC2. In these cases, the variability, expressed here as the COV, is notably higher compared to those generated by other calculation methods.
A consistent pattern in the OP distribution emerges when varying the DTT concentration range between 0 to 100 μM (N = 5) and 0 to 140 μM (N = 6), as similar OPDTT values are obtained for both ranges, indicating consistency across these calibration intervals. However, across all PM samples, markedly lower OPDTTm values are observed when using the calibration curve in the lower range of 0–60 μM DTT (N = 4), as shown in Fig. 3. Thus, the OPDTTm values calculated with the CURVE method based on the 0–60 μM DTT calibration curve are, on average, up to 43% lower than the values derived either from 0 to 140 μM DTT (p < 0.05; Table S5a†) or from 0 to 100 μM DTT (p < 0.05; Table S5a†). This difference arises solely from the variation in calibration parameters (slope and intercepts) among the calibration curves used. As such, the slopes and intercepts play a key role in the observed differences in OP values when comparing different calibration curves. As evidenced in Table S4,† the differences in these parameters propagate through the calculation, ultimately resulting in substantial changes in the final OPDTT values. Similar trends are observed for OPDTTv, as both values are derived from non-normalized OPs (nmol min−1).
This disparity underscores the potential for significant deviations in OPDTT value determination when employing calibration curves of different concentration ranges. Specifically, variations in both slope and intercept values directly influence the baseline absorbance utilized in the CURVE method's calculation of DTT consumption.
This analysis supports the work of Molina et al.,34 where these authors demonstrated relative uncertainties in OPDTT values for both PM10 and PM2.5 samples, highlighting significant variation in linear regression analyses of PM samples. According to the authors, various operational conditions contribute to the variability of OP values in the DTT assay, including reproducibility factors associated with curve fitting analysis.34
Although significant variability exists between the mathematical approaches, the chosen concentration range of DTT for generating the calibration curves appears to significantly influence the assessment of OP. This suggests that a significant bias may arise from the concentration ranges used in the calibration curve, potentially due to a loss of linearity and issues related to DTT consumption at low concentrations, such as those observed at 0–60 μM. For routine OPDTT assays, the DTT concentration ranges between 0–140 μM and 0–100 μM, which provide results more consistent with those obtained using the ABS and CC2 methods, are thus preferred.
Fig. 4 shows that the OPDTT values exhibit substantial variability, primarily driven by changes in the AB0 values in the CC1 method, with OPDTT ranging from 0.046 to 0.070 nmol min−1. This variability corresponds to an additional variation of up to 34% in the OPs, as demonstrated by the cascading effect observed when varying AB0. The results suggest that larger differences between AB0 and At lead to lower OP values, while smaller variations among these parameters tend to yield higher OPs. Specifically, higher absorbance values at time 0 indicate reduced intrinsic reactivity of the PM samples, resulting in lower OP values that are more consistent with those obtained using the ABS and CC2 methods. Thus, the normalization with AB0 may explain the differences observed between CURVE, ABS, and CC2 methods. Trace levels of redox-active species and PM catalysts in the blank solution could also lead to increased consumption of DTT and AA, resulting in lower absorbance values at time 0. This, in turn, affects the results of the CC1 method.
Accordingly, minimizing variation in blank absorbances can enhance the reliability of OP values and reduce the variability between replicates. In summary, this section highlights the importance of using consistent absorbance values for blank measurements in routine OP assays, as this has direct implications for the accuracy of OP values. Future OP studies should consider comparing theoretical absorbance values, calculated based on the initial concentrations of DTT and AA, with the actual blank absorbance values obtained through instrumental measurements. Finally, it is crucial to carefully examine the intrinsic reactivity of blank samples in OP analysis, as this can contribute to more consistent OPs.
3.3. Contrasting OPDTT and OPAA outcomes for different methods
The observed variability in OPAA values across different methodologies mirrors previous findings for OPDTT (Fig. S3 and Table S6†). Notably, both CC1 and CURVE methods consistently produced higher OPAA values, while ABS and CC2 methods resulted in comparatively lower values. For OPAA, the CURVE method leads to the highest observed OPAA, followed by CC1, CC2 and ABS. This difference may be partially attributed to the effect of incubation time, as previously reported, or to the normalization approach used in the CC1 method. Specifically, variations in the absorbance values of blank samples at time 0 suggest substantial fluctuations in OPAA values when using the CC1 method, which could influence comparisons across studies.In terms of replicates, the CURVE and CC1 methods exhibit higher COVs for PM samples #A, #B, and #C. In contrast, the ABS and CC2 methods show higher COVs for samples #D, #E, and #F. Specifically, stronger AA depletion was associated with increased COVs for the ABS and CC2 methods, while weaker AA depletion corresponded to higher COVs for the CURVE and CC1 methods (Fig. S2†). Statistical analysis indicated significant differences among replicate measurements for PM samples #C, #D, #E, and #F (Table S3;† ANOVA, one way, p < 0.05). This finding is consistent with previous results from the DTT assay, highlighting the influence of replicate variability in OP assays and its dependence on the calculation methods employed, where the inherent variability in replicate measurements appears to be sample-dependent. Calibration curves with a narrower AA concentration range (0–144 μM) yielded lower OPAA values than those with wider ranges (0–192 μM and 0–240 μM; Fig. S3†). This trend is similar to that previously observed with the DTT assay, which indicates that higher DTT concentration ranges for the calibration curve result in increased OPDTT. This aligns with the findings of Lin et al.,12 who demonstrated that the initial concentration of DTT significantly affects OP values during incubation with PM.12 Specifically, the authors showed that lower DTT concentrations during incubation generally lead to lower OP values.
As a final comparison, the variability caused by the different calculation methods have a more pronounced effect on OPDTT compared to OPAA, as indicated by the greater variability observed in the OPDTT values (Fig. S1†). However, at higher consumption rates, as previously demonstrated for #D, both the ABS and CC2 methods have a greater influence on the AA compared to the DTT assay. For both assays, increasing the concentration ranges of DTT and AA used in calibration curves resulted in higher OP values compared to other calculation methods. While the CC1 method led to elevated OPs in both assays, its impact was particularly pronounced in the DTT assay.
4. Conclusion and recommended key features of a standardized OP determination
This study presents an in-depth analysis of the mathematical methodologies outlined in the literature for the determination of OP values. These approaches assess the consumption rates of DTT and AA in the presence of redox-active PM species and PM catalysts by utilizing relative absorbance values over time, which are subsequently extended to yield slope and rate constant values. The experimental results revealed distinct OP profiles, with varying calculation methods leading to divergent outcomes. These findings are best summarized as follows:(i) Both the ABS and CC2 methods exhibited significant similarities in both DTT and AA assays, demonstrating similar OP values and relative variation.
(ii) The CC1 method is prone to yielding higher OPDTT and OPAA values compared to the CURVE, ABS, and CC2 methods. The intrinsic variability of the CC1 method, as observed in measurement replicates, affects the precision and stability of OP values, which in turn influences comparisons across different studies. The blank assessment is the critical step within CC1 to obtain comparable OP values.
(iii) Variation in the concentration ranges of DTT and AA used in the CURVE method significantly influence the variability of OPDTT and OPAA values. Such variations carry important implications for the slope values associated with the rate constants of the redox and catalytic reactions involved in OP assays.
The findings of the present study highlight the importance of uniform OP protocols with guidelines on the methodological aspects of OP assays, including the adoption of more comparable mathematical approaches for measuring the OP and critical evaluation of blank solutions.
It is important to acknowledge the inherent limitations in the assumptions in the absorbance-to-concentration conversion methods. While the ‘CURVE’ method employs a more complex approach, other methods appear to utilize a simplified conversion factor ([reductant]0; p; AB0; APM0). This approach implicitly assumes ideal Beer–Lambert behavior and exclusive absorbance due to the reductant. However, as demonstrated in Table S4,† real-world measurements often exhibit a non-zero intercept, indicating deviations from ideality. This intercept suggests that background absorbance or other systematic effects contribute to the measured absorbance, rendering the simplified conversion factor potentially inaccurate. Therefore, for OP standardization, we must consider the limitations of these simplified methods. From an analytical chemistry standpoint, employing linear regression of absorbance versus concentration provides a more accurate and scientifically justified approach. Based on the findings of the present study and the observed discrepancies in the OP values obtained through each calculation method in comparison to the overall OP mean, both the ABS and CC2 methods exhibit greater consistency and are recommended for routine OPDTT and OPAA assays. While our analysis identifies statistical similarities among computational approaches, we acknowledge that numerical agreement alone does not imply scientific validity. Rather, our findings highlight the relative differences between methods across diverse PM sources, allowing us to identify approaches that yield more comparable results. These findings are intended as guidance for enhancing comparability across studies, rather than a definitive endorsement of any single method's absolute correctness.
To maintain the accuracy and consistency of measurements across all calculation methods, strict adherence to quality control protocols is essential. Experimental protocols should include an evaluation of the linearity of the regression analysis for both DTT and AA consumption, as well as a critical assessment of their concentration to ensure greater consistency across methods. Failure to account for potential deviations from linearity may lead to misinterpretations of the patterns observed in both the DTT and AA assays. Additionally, future studies could consider extracting OP values from different methods to enable a more detailed comparison across studies, improving the standardization and interpretability of OP assessments.
Overall, the present study contributes to the development of standardized protocols for the OP quantification stage, which will enhance consistency in predicting the toxic potency of aerosol particles and facilitate reliable comparisons across OP studies globally.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: ES and GU; methodology: ES and GU; formal analysis and OP measurements: TM; investigation: ES, GU, KWF and PD; resources: GU, KWF, JLJ and HH; data curation: ES; writing – original draft preparation: ES; writing – review and editing: ES, GU, KWF, PD, JLJ, and HH; supervision: GU, KWF, JLJ and HH; project administration: GU and KWF; funding acquisition: GU, KWF, JLJ, and HH.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Leibniz Collaborative Excellence Program for funding the Dustrisk project K225/2019 led by K. W. Fomba. We thank the PROCOPE program for awarding a grant to Eduardo Souza (0185-DEU-23-0008 LG1). We thank RI-Urbans, under grant agreement 101036245 (RI-Urbans), including the Post-doc grant of Pamela Dominutti. Analyses were funded through the University Grenoble Alpes grant ACME IDEX (ANR-15-IDEX-02) and the Air-O-Sol facility (equipment at Labex OSUG@2020 (ANR10 LABX56) and Predict'air project (grant FUGA-UGA 2022-16 and grant PR-PRE-2021 FUGA-Fondation Air Liquide).References
- D. Bhattu, S. N. Tripathi, H. S. Bhowmik, V. Moschos, C. P. Lee, M. Rauber, G. Salazar, G. Abbaszade, T. Cui, J. G. Slowik and P. Vats, Local incomplete combustion emissions define the PM2.5 oxidative potential in Northern India, Nat. Commun., 2024, 15(1), 3517, DOI:10.1038/s41467-024-47785-5.
- K. R. Daellenbach, G. Uzu, J. Jiang, L. E. Cassagnes, Z. Leni, A. Vlachou, G. Stefenelli, F. Canonaco, S. Weber, A. Segers and J. J. Kuenen, Sources of particulate-matter air pollution and its oxidative potential in Europe, Nature, 2020, 587(7834), 414–419, DOI:10.1038/s41586-020-2902-8.
- A. Marsal, J. J. Sauvain, A. Thomas, S. Lyon-Caen, L. J. Borlaza, C. Philippat, J. L. Jaffrezo, A. Boudier, S. Darfeuil, R. Elazzouzi and J. Lepeule, Effects of personal exposure to the oxidative potential of PM2. 5 on oxidative stress biomarkers in pregnant women, Sci. Total Environ., 2023, 168475, DOI:10.1016/j.scitotenv.2023.168475.
- L. J. S. Borlaza, S. Weber, G. Uzu, V. Jacob, T. Cañete, S. Micallef, C. Trébuchon, R. Slama, O. Favez and J. L. Jaffrezo, Disparities in particulate matter (PM 10) origins and oxidative potential at a city scale (Grenoble, France)–Part 1: Source apportionment at three neighbouring sites, Atmos. Chem. Phys., 2021, 21(7), 5415–5437, DOI:10.5194/acp-21-5415-2021.
- P. A. Dominutti, J.-L. Jaffrezo, A. Marsal, T. Mhadhbi, R. Elazzouzi, C. Rak, F. Cavalli, J.-P. Putaud, A. Bougiatioti, N. Mihalopoulos, D. Paraskevopoulou, I. S. Mudway, A. Nenes, K. R. Daellenbach, C. Banach, S. J. Campbell, H. Cigánková, D. Contini, G. Evans, M. Georgopoulou, M. Ghanem, D. A. Glencross, M. R. Guascito, H. Herrmann, S. Iram, M. Jovanović, M. Jovašević-Stojanović, M. Kalberer, I. M. Kooter, S. E. Paulson, A. Patel, E. Perdrix, M. C. Pietrogrande, P. Mikuška, J.-J. Sauvain, A. Seitanidi, P. Shahpoury, E. J. S. Souza, S. Steimer, S. Stevanovic, G. Suarez, P. S. G. Subramanian, B. Utinger, M. F. van Os, V. Verma, X. Wang, R. J. Weber, Y. Yang, X. Querol, G. Hoek, R. M. Harrison and G. Uzu, An interlaboratory comparison to quantify oxidative potential measurement in aerosol particles: challenges and recommendations for harmonisation, Atmos. Meas. Tech. Discuss., 2024, 18(1), 177–195, DOI:10.5194/amt-2024-107.
- S. J. Campbell, B. Utinger, A. Barth, S. E. Paulson and M. Kalberer, Iron and Copper Alter the Oxidative Potential of Secondary Organic Aerosol: Insights from Online Measurements and Model Development, Environ. Sci. Technol., 2023, 57, 13546–13558, DOI:10.1021/acs.est.3c01975.
- H. Jiang, C. S. Ahmed, Z. Zhao, J. Y. Chen, H. Zhang, A. Canchola and Y. H. Lin, Role of functional groups in reaction kinetics of dithiothreitol with secondary organic aerosols, Environ. Pollut., 2020, 263, 114402, DOI:10.1016/j.envpol.2020.114402.
- P. Shahpoury, S. Lelieveld, C. Johannessen, T. Berkemeier, V. Celo, E. Dabek-Zlotorzynska, T. Harner, G. Lammel and A. Nenes, Influence of aerosol acidity and organic ligands on transition metal solubility and oxidative potential of fine particulate matter in urban environments, Sci. Total Environ., 2024, 906, 167405, DOI:10.1016/j.scitotenv.2023.167405.
- R. Li, C. Yan, Q. Meng, Y. Yue, W. Jiang, L. Yang, Y. Zhu, L. Xue, S. Gao, W. Liu and T. Chen, Key toxic components and sources affecting oxidative potential of atmospheric particulate matter using interpretable machine learning: Insights from fog episodes, J. Hazard. Mater., 2024, 133175, DOI:10.1016/j.jhazmat.2023.133175.
- H. Yu, J. V. Puthussery and V. Verma, A semi-automated multi-endpoint reactive oxygen species activity analyzer (SAMERA) for measuring the oxidative potential of ambient PM2. 5 aqueous extracts, Aerosol Sci. Technol., 2020, 54(3), 304–320, DOI:10.1080/02786826.2019.1693492.
- H. Chen and J. Z. Yu, An online instrument for assessing oxidative potential of ambient particulate matter via dithiothreitol assay, using particle-into-liquid sampler (PILS) coupled with improved light absorption measurement, Atmos. Environ., 2025, 343, 120980, DOI:10.1016/j.atmosenv.2024.120980.
- M. Lin and J. Z. Yu, Dithiothreitol (DTT) concentration effect and its implications on the applicability of DTT assay to evaluate the oxidative potential of atmospheric aerosol samples, Environ. Pollut., 2019, 251, 938–944, DOI:10.1016/j.envpol.2019.05.074.
- M. Visentin, A. Pagnoni, E. Sarti and M. C. Pietrogrande, Urban PM2. 5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays, Environ. Pollut., 2016, 219, 72–79, DOI:10.1016/j.envpol.2016.09.047.
- M. C. Pietrogrande, C. Dalpiaz, R. Dell'Anna, P. Lazzeri, F. Manarini, M. Visentin and G. Tonidandel, Chemical composition and oxidative potential of atmospheric coarse particles at an industrial and urban background site in the alpine region of northern Italy, Atmos. Environ., 2018, 191, 340–350, DOI:10.1016/j.atmosenv.2018.08.022.
- J. G. Charrier, A. S. McFall, K. K. Vu, J. Baroi, C. Olea, A. Hasson and C. Anastasio, A bias in the “mass-normalized” DTT response–An effect of non-linear concentration-response curves for copper and manganese, Atmos. Environ., 2016, 144, 325–334, DOI:10.1016/j.atmosenv.2016.08.071.
- A. Calas, G. Uzu, F. J. Kelly, S. Houdier, J. M. Martins, F. Thomas, F. Molton, A. Charron, C. Dunster, A. Oliete, V. Jacob, J. Besombes, F. Chevrier and J. Jaffrezo, Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM 10 samples from the city of Chamonix (France), Atmos. Chem. Phys., 2018, 18(11), 7863–7875, DOI:10.5194/acp-18-7863-2018.
- V. D. Ngoc Thuy, J. L. Jaffrezo, I. Hough, P. A. Dominutti, G. Salque Moreton, G. Gille, F. Francony, A. Patron-Anquez, O. Favez and G. Uzu, Unveiling the optimal regression model for source apportionment of the oxidative potential of PM 10, Atmos. Chem. Phys., 2024, 24(12), 7261–7282, DOI:10.5194/acp-24-7261-2024.
- P. A. Dominutti, L. J. S. Borlaza, J. J. Sauvain, V. D. N. Thuy, S. Houdier, G. Suarez, J. L. Jaffrezo, S. Tobin, C. Trébuchon, S. Socquet and E. Moussu, Source apportionment of oxidative potential depends on the choice of the assay: insights into 5 protocols comparison and implications for mitigation measures, Environ. Sci.: Atmos., 2023, 3(10), 1497–1512, 10.1039/D3E000007A.
- J. Shen, S. Taghvaee, C. La, F. Oroumiyeh, J. Liu, M. Jerrett, S. Weichenthal, I. Del Rosario, M. M. Shafer, B. Ritz, Y. Zhu and S. Paulson, Aerosol oxidative potential in the greater Los Angeles area: source apportionment and associations with socioeconomic position, Environ. Sci.: Atmos., 2022, 56(24), 17795–17804 CrossRef CAS PubMed.
- E. J. S. Souza, K. W. Fomba, M. van Pinxteren, N. Deabji and H. Herrmann, Strong synergistic and antagonistic effects of quinones and metal ions in oxidative potential (OP) determination by ascorbic acid (AA) assays, J. Hazard. Mater., 2024, 135599, DOI:10.1016/j.jhazmat.2024.135599.
- H. Guo, H. Fu, L. Jin, S. Huang and X. Li, Quantification of synergistic, additive and antagonistic effects of aerosol components on total oxidative potential, Chemosphere, 2020, 252, 126573, DOI:10.1016/j.chemosphere.2020.126573.
- H. Yu, J. Wei, Y. Cheng, K. Subedi and V. Verma, Synergistic and antagonistic interactions among the particulate matter components in generating reactive oxygen species based on the dithiothreitol assay, Environ. Sci.: Atmos., 2018, 52(4), 2261–2270, DOI:10.1021/acs.est.7b04261.
- G. Simonetti, E. Conte, C. Perrino and S. Canepari, Oxidative potential of size-segregated PM in an urban and an industrial area of Italy, Atmos. Environ., 2018, 187, 292–300, DOI:10.1016/j.atmosenv.2018.05.051.
- M. C. Pietrogrande, I. Bertoli, F. Manarini and M. Russo, Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition, Atmos. Environ., 2019, 211, 103–112, DOI:10.1016/j.atmosenv.2019.05.012.
- M. A. Frezzini, N. De Francesco, L. Massimi and S. Canepari, Effects of operating conditions on PM oxidative potential assays, Atmos. Environ., 2022, 268, 118802, DOI:10.1016/j.atmosenv.2021.118802.
- Y. Kumagai, S. Koide, K. Taguchi, A. Endo, Y. Nakai, T. Yoshikawa and N. Shimojo, Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles, Chem. Res. Toxicol., 2002, 15(4), 483–489, DOI:10.1021/tx0100993.
- A. K. Cho, C. Sioutas, A. H. Miguel, Y. Kumagai, D. A. Schmitz, M. Singh, A. Eiguren-Fernandez and J. R. Froines, Redox activity of airborne particulate matter at different sites in the Los Angeles Basin, Environ. Res., 2005, 99(1), 40–47, DOI:10.1016/j.envres.2005.01.003.
- J. T. Bates, T. Fang, V. Verma, L. Zeng, R. J. Weber, P. E. Tolbert, J. Y. Abrams, S. E. Sarnat, M. Klein, J. A. Mulholland and A. G. Russell, Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects, Environ. Sci. Technol., 2019, 53(8), 4003–4019, DOI:10.1021/acs.est.8b03430.
- D. Gao, S. Ripley, S. Weichenthal and K. J. G. Pollitt, Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management, Free Radical Biol. Med., 2020, 151, 7–25, DOI:10.1016/j.freeradbiomed.2020.04.028.
- A. J. Kramer, W. Rattanavaraha, Z. Zhang, A. Gold, J. D. Surratt and Y.-H. Lin, Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol, Atmos. Environ., 2016, 130, 211–218, DOI:10.1016/j.atmosenv.2015.10.018.
- D. Gao, T. Fang, V. Verma, L. Zeng and R. J. Weber, “A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol (DTT) assay and comparisons between an urban and roadside site of water-soluble and total OP”, Atmos. Meas. Tech., 2017,(8), 2821–2835, DOI:10.5194/amt-10-2821-2017.
- S. Wang, J. Ye, R. Soong, B. Wu, L. Yu, A. J. Simpson and W. H. C. Arthur, “Relationship between chemical composition and oxidative potential of secondary organic aerosol from polycyclic aromatic hydrocarbons”, Atmos. Chem. Phys., 2018, 18(6), 3987–4003, DOI:10.5194/acp-18-3987-2018.
- K. E. Berg, K. M. Clark, X. Li, E. M. Carter, J. Volckens and C. S. Henry, High-throughput, semi-automated dithiothreitol (DTT) assays for oxidative potential of fine particulate matter, Atmos. Environ., 2020, 222, 117132, DOI:10.1016/j.atmosenv.2019.117132.
- C. Molina, C. Andrade, C. A. Manzano, A. Richard Toro, V. Verma and L.-G. M. A. Leiva-Guzmán, Dithiothreitol-based oxidative potential for airborne particulate matter: an estimation of the associated uncertainty, Environ. Sci. Pollut. Res., 2020, 27, 29672–29680, DOI:10.1007/s11356-020-09508-3.
- D. Cesari, E. Merico, F. M. Grasso, S. Decesari, F. Belosi, F. Manarini, P. De Nuntiis, M. Rinaldi, F. Volpi, A. Gambaro and E. Morabito, Source apportionment of PM2. 5 and of its oxidative potential in an industrial suburban site in South Italy, Atmosphere, 2019, 10(12), 758, DOI:10.3390/atmos10120758.
- D. Chirizzi, D. Cesari, M. R. Guascito, A. Dinoi, L. Giotta, A. Donateo and D. Contini, Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2. 5 and PM10, Atmos. Environ., 2017, 163, 1–8, DOI:10.1016/j.atmosenv.2017.05.021.
- T. Fang, V. Verma, J. T. Bates, A. Joseph, M. Klein, M. J. Strickland and S. E. Sarnat, et al., Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays, Atmos. Chem. Phys., 2016, 16(6), 3865–3879, DOI:10.5194/acp-16-3865-2016.
- E. Conte, S. Canepari, D. Frasca and G. Simonetti, Oxidative potential of selected PM components, Proceedings, 2017, 1(5), 108, DOI:10.3390/ecas2017-04131.
- L. Famiyeh, C. Jia, K. Chen, Y. T. Tang, D. Ji, J. He and Q. Guo, Size distribution and lung-deposition of ambient particulate matter oxidative potential: A contrast between dithiothreitol and ascorbic acid assays, Environ. Pollut., 2023, 336, 122437, DOI:10.1016/j.envpol.2023.122437.
- S. Weber, G. Uzu, A. Calas, F. Chevrier, J. L. Besombes, A. Charron, D. Salameh, I. Ježek, G. Močnik and J. L. Jaffrezo, An apportionment method for the oxidative potential of atmospheric particulate matter sources: application to a one-year study in Chamonix, France, Atmos. Chem. Phys., 2018, 18(13), 9617–9629, DOI:10.5194/acp-18-9617-2018.
- A. Calas, G. Uzu, J. M. Martins, D. Voisin, L. Spadini, T. Lacroix and J. L. Jaffrezo, The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter, Sci. Rep., 2017, 14(7(1)), 11617, DOI:10.1038/s41598-017-11979-3.
- P. R. Kim, S. W. Park, Y. J. Han, M. H. Lee, T. M. Holsen, C. H. Jeong and G. Evans, Variations of oxidative potential of PM2. 5 in a medium-sized residential city in South Korea measured using three different chemical assays, Sci. Total Environ., 2024, 920, 171053 CrossRef CAS PubMed.
- V. M. de Lagarde, T. Rogez-Florent, F. Cazier, D. Dewaele, F. Cazier-Dennin, A. Ollivier, M. Janona, S. Achard, V. Andre, C. Monteil and C. Corbiere, Oxidative potential and in vitro toxicity of particles generated by pyrotechnic smokes in human small airway epithelial cells, Ecotoxicol. Environ. Saf., 2022, 239, 113637, DOI:10.1016/j.ecoenv.2022.113637.
- A. Expósito, J. Maillo, I. Uriarte, M. Santibáñez and I. Fernandez-Olmo, Kinetics of ascorbate and dithiothreitol oxidation by soluble copper, iron, and manganese, and 1, 4-naphthoquinone: influence of the species concentration and the type of fluid, Chemosphere, 2024, 142435, DOI:10.1016/j.chemosphere.2024.142435.
- H. Jiang, C. S. Ahmed, A. Canchola, J. Y. Chen and Y. H. Lin, Use of dithiothreitol assay to evaluate the oxidative potential of atmospheric aerosols, Atmosphere, 2019, 10(10), 571, DOI:10.3390/atmos10100571.
- C. Li, Z. Fang, H. Czech, E. Schneider, C. P. Rüger, M. Pardo, R. Zimmermann, J. Chen, A. Laskin and Y. Rudich, pH modifies the oxidative potential and peroxide content of biomass burning HULIS under dark aging, Sci. Total Environ., 2022, 834, 155365, DOI:10.1016/j.scitotenv.2022.155365.
- T. Fang, V. Verma, H. Guo, L. E. King, E. S. Edgerton and R. J. Weber, “A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE)”, Atmos. Meas. Tech., 2015,(1), 471–482, DOI:10.5194/amt-8-471-2015.
- J. G. Charrier and C. Anastasio, On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals, Atmos. Chem. Phys., 2012, 12(5), 11317, DOI:10.5194/acp-12-9321-2012.
- G. R. Buettner and B. A. Jurkiewicz, Catalytic metals, ascorbate and free radicals: combinations to avoid, Radiat. Res., 1996, 145(5), 532–541, DOI:10.2307/3579271.
- L. J. S. Borlaza, G. Uzu, M. Ouidir, S. Lyon-Caen, A. Marsal, S. Weber, V. Siroux, J. Lepeule, A. Boudier, J. L. Jaffrezo and R. Slama, Personal exposure to PM2. 5 oxidative potential and its association to birth outcomes, J. Exposure Sci. Environ. Epidemiol., 2023, 33(3), 416–426, DOI:10.1038/s41370-022-00487-w.
- J. J. Sauvain, M. J. Rossi and M. Riediker, Comparison of three acellular tests for assessing the oxidation potential of nanomaterials, Aerosol Sci. Technol., 2012, 47(2), 218–227, DOI:10.1080/02786826.2012.742951.
- P. Trechera, T. Moreno, P. Córdoba, N. Moreno, F. Amato, J. Cortés, X. Zhuang, B. Li, J. Li, Y. Shangguan and A. O. Dominguez, Geochemistry and oxidative potential of the respirable fraction of powdered mined Chinese coals, Sci. Total Environ., 2022, 800, 149486, DOI:10.1016/j.scitotenv.2021.149486.
- S. Weichenthal, E. Lavigne, A. Traub, D. Umbrio, H. You, K. Pollitt, T. Shin, R. Kulka, D. M. Stieb, J. Korsiak and B. Jessiman, Association of sulfur, transition metals, and the oxidative potential of outdoor PM 2.5 with acute cardiovascular events: a case-crossover study of Canadian adults, Environ. Health Perspect., 2021, 129(10), 107005, DOI:10.1289/EHP9449.
- S. Ripley, D. Gao, K. J. G. Pollitt, P. S. Lakey, M. Shiraiwa, M. Hatzopoulou and S. Weichenthal, Within-city spatial variations in long-term average outdoor oxidant gas concentrations and cardiovascular mortality: Effect modification by oxidative potential in the Canadian Census Health and Environment Cohort, Environ. Epidemiol., 2023, 7(4), e257, DOI:10.1097/EE9.0000000000000257.
- C. H. Jeong, A. Traub, A. Huang, N. Hilker, J. M. Wang, D. Herod, E. Dabek-Zlotorzynska, V. Celo and G. J. Evans, Long-term analysis of PM2. 5 from 2004 to 2017 in Toronto: Composition, sources, and oxidative potential, Environ. Pollut., 2020, 263, 114652, DOI:10.1016/j.envpol.2020.114652.
- R. K. Cheung, L. Qi, M. I. Manousakas, J. V. Puthussery, Y. Zheng, T. K. Koenig, T. Cui, T. Wang, Y. Ge, G. Wei and Y. Kuang, Major source categories of PM2. 5 oxidative potential in wintertime Beijing and surroundings based on online dithiothreitol-based field measurements, Sci. Total Environ., 2024, 928, 172345, DOI:10.1016/j.scitotenv.2024.172345.
- Y. Kuang, Y. Guo, J. Chai, J. Shang, J. Zhu, S. Stevanovic and Z. Ristovski, Comparison of light absorption and oxidative potential of biodiesel/diesel and chemicals/diesel blends soot particles, J. Environ. Sci., 2020, 87, 184–193, DOI:10.1016/j.jes.2019.06.014.
- C. Li, H. Hakkim, V. Sinha, B. Sinha, M. Pardo, D. Cai, N. Reicher, J. Chen, K. Hao and Y. Rudich, Variation of PM2.5 Redox Potential and Toxicity During Monsoon in Delhi, India, ACS ES&T Air, 2024, 1(4), 316–329, DOI:10.1021/acsestair.3c00096.
- P. Ponsawansong, T. Prapamontol, K. Rerkasem, S. Chantara, K. Tantrakarnapa, S. Kawichai, G. Li, C. Fang, X. Pan and Y. Zhang, Sources of PM2. 5 oxidative potential during haze and non-haze seasons in Chiang Mai, Thailand, Aerosol Air Qual. Res., 2023, 23(10), 230030, DOI:10.4209/aaqr.230030.
- Y. Wang, J. V. Puthussery, H. Yu, Y. Liu, S. Salana and V. Verma, Sources of cellular oxidative potential of water-soluble fine ambient particulate matter in the Midwestern United States, J. Hazard. Mater., 2022, 425, 127777, DOI:10.1016/j.jhazmat.2024.134763.
- L. Moufarrej, D. Courcot and F. Ledoux, Assessment of the PM2. 5 oxidative potential in a coastal industrial city in Northern France: Relationships with chemical composition, local emissions and long-range sources, Sci. Total Environ., 2020, 748, 141448, DOI:10.1016/j.scitotenv.2020.141448.
- S. J. Campbell, K. Wolfer, B. Utinger, J. Westwood, Z. H. Zhang, N. Bukowiecki, S. S. Steimer, T. V. Vu, J. Xu, N. Straw and S. Thomson, Atmospheric conditions and composition that influence PM 2.5 oxidative potential in Beijing, China, ACP, Appl. Cardiopulm. Pathophysiol., 2021, 21(7), 5549–5573, DOI:10.5194/acp-21-5549-2021.
- A. Fushimi, D. Nakajima, A. Furuyama, G. Suzuki, T. Ito, K. Sato, Y. Fujitani, Y. Kondo, A. Yoshino, S. Ramasamy and J. J. Schauer, Source contributions to multiple toxic potentials of atmospheric organic aerosols, Sci. Total Environ., 2021, 773, 145614, DOI:10.1016/j.scitotenv.2021.145614.
- M. Ghanem, E. Perdrix, L. Y. Alleman, D. Rousset and P. Coddeville, Phosphate buffer solubility and oxidative potential of single metals or multielement particles of welding fumes, Atmosphere, 2020, 12(1), 30, DOI:10.3390/atmos12010030.
- Z. Li, D. Nie, M. Chen, P. Ge, Z. Liu, X. Ma, X. Ge and R. Gu, Seasonal variation of oxidative potential of water-soluble components in PM2. 5 and PM1 in the Yangtze River Delta, China, Air Qual. Atmos. Hlth., 2021, 14(11), 1825–1836, DOI:10.1007/s11869-021-01056-0.
- J. Wang, S. Zhao, H. Jiang, X. Geng, J. Li, S. Mao, S. Ma, S. Bualert, G. Zhong and G. Zhang, Oxidative potential of solvent-extractable organic matter of ambient total suspended particulate in Bangkok, Thailand, Environ. Sci.:Processes Impacts, 2022, 24(3), 400–413, 10.1039/d1em00414j.
- K. Baumann, M. Wietzoreck, P. Shahpoury, A. Filippi, S. Hildmann, S. Lelieveld, T. Berkemeier, H. Tong, U. Pöschl and G. Lammel, Is the oxidative potential of components of fine particulate matter surface-mediated?, Environ. Sci. Pollut. Res. Int., 2023, 30(6), 16749–16755, DOI:10.1007/s11356-022-24897-3.
- A. Carlino, M. P. Romano, M. G. Lionetto, D. Contini and M. R. Guascito, An overview of the automated and on-line systems to assess the oxidative potential of particulate matter, Atmosphere, 2023, 14(2), 256, DOI:10.3390/atmos14020256.
- C. Ran, C. Liu, C. Peng, X. Li, Y. Liu, Y. Li, W. Zhang, H. Cai and L. Wang, Oxidative potential of heavy-metal contaminated soil reflects its ecological risk on earthworm, Environ. Pollut., 2023, 323, 121275, DOI:10.1016/j.envpol.2023.121275.
- B. Utinger, S. J. Campbell, N. Bukowiecki, A. Barth, B. Gfeller, R. Freshwater, H. R. Rüegg and M. Kalberer, An automated online field instrument to quantify the oxidative potential of aerosol particles via ascorbic acid oxidation, Atmos. Meas. Tech., 2023, 16(10), 2641–2654, DOI:10.5194/amt-16-2641-2023.
- Y. Luo, X. Yang, D. Wang, H. Xu, H. Zhang, S. Huang, Q. Wang, N. Zhang, J. Cao and Z. Shen, Insights the dominant contribution of biomass burning to methanol-soluble PM2. 5 bounded oxidation potential based on multilayer perceptron neural network analysis in Xi'an, China, Sci. Total Environ., 2024, 908, 168273, DOI:10.1016/j.scitotenv.2023.168273.
- S. Romano, M. R. Perrone, S. Becagli, M. C. Pietrogrande, M. Russo, R. Caricato and M. G. Lionetto, Ecotoxicity, genotoxicity, and oxidative potential tests of atmospheric PM10 particles, Atmos. Environ., 2020, 221, 117085, DOI:10.1016/j.atmosenv.2019.117085.
- D. Ainur, Q. Chen, Y. Wang, H. Li, H. Lin, X. Ma and X. Xu, Pollution characteristics and sources of environmentally persistent free radicals and oxidation potential in fine particulate matter related to city lockdown (CLD) in Xi'an, China, Environ. Res., 2022, 210, 112899, DOI:10.1016/j.envres.2022.112899.
- A. Besis, M. P. Romano, E. Serafeim, A. Avgenikou, A. Kouras, M. G. Lionetto, M. R. Guascito, A. R. De Bartolomeo, M. E. Giordano, A. Mangone and D. Contini, Size-resolved redox activity and cytotoxicity of water-soluble urban atmospheric particulate matter: assessing contributions from chemical components, Toxics, 2023, 11(1), 59, DOI:10.3390/toxics11010059.
- N. Canha, S. Gonçalves, D. Sousa, C. Gamelas, S. Mendez, V. S. Cabo, S. M. Almeida, A. R. de Bartolomeo, M. R. Guascito, E. Merico and D. Contini, Pollution sources affecting the oxidative potential of fine aerosols in a Portuguese urban-industrial area-an exploratory study, Aerosol Air Qual. Res., 2024, 1, DOI:10.1007/s11869-024-01556-9.
- S. K. Dey, K. Sugur, V. G. Venkatareddy, P. Rajeev, T. Gupta and R. K. Thimmulappa, Lipid peroxidation index of particulate matter: Novel metric for quantifying intrinsic oxidative potential and predicting toxic responses, Redox Biol., 2021, 48, 102189, DOI:10.1016/j.redox.2021.102189.
- A. Dinoi, G. Pavese, M. Calvello, D. Chirizzi, A. Pennetta, G. E. De Benedetto, F. Esposito, C. Mapelli and D. Contini, Characterization of aerosol and its oxidative potential in a coastal semi-rural site of Southern Italy, Atmos. Environ., 2024, 14, 120656, DOI:10.1016/j.atmosenv.2024.120656.
- Z. Fang, A. Lai, D. Cai, C. Li, R. Carmieli, J. Chen, X. Wang and Y. Rudich, Secondary Organic Aerosol Generated from Biomass Burning Emitted Phenolic Compounds: Oxidative Potential, Reactive Oxygen Species, and Cytotoxicity, Environ. Sci. Technol., 2024, 58(19), 8194–8206, DOI:10.1021/acs.est.3c09903.
- L. Fusaro, E. Salvatori, A. Winkler, M. A. Frezzini, E. De Santis, L. Sagnotti, S. Canepari and F. Manes, Urban trees for biomonitoring atmospheric particulate matter: An integrated approach combining plant functional traits, magnetic and chemical properties, Ecol. Indic., 2021, 126, 107707, DOI:10.1016/j.ecolind.2021.107707.
- M. Gosselin and G. J. Zagury, Metal (loid) s inhalation bioaccessibility and oxidative potential of particulate matter from chromated copper arsenate (CCA)-contaminated soils, Chemosphere, 2020, 238, 124557, DOI:10.1016/j.chemosphere.2019.124557.
- M. R. Guascito, M. G. Lionetto, F. Mazzotta, M. Conte, M. E. Giordano, R. Caricato, A. R. De Bartolomeo, A. Dinoi, D. Cesari, E. Merico and L. Mazzotta, Characterisation of the correlations between oxidative potential and in vitro biological effects of PM10 at three sites in the central Mediterranean, J. Hazard. Mater., 2023, 448, 130872, DOI:10.1016/j.jhazmat.2023.130872.
- T. C. Hsiao, L. T. Chou, S. Y. Pan, L. H. Young, K. H. Chi and A. Y. Chen, Chemically and temporally resolved oxidative potential of urban fine particulate matter, Environ. Pollut., 2021, 291, 118206, DOI:10.1016/j.envpol.2021.118206.
- B. Hwang, T. Fang, R. Pham, J. Wei, S. Gronstal, B. Lopez, C. Frederickson, T. Galeazzo, X. Wang, H. Jung and M. Shiraiwa, Environmentally persistent free radicals, reactive oxygen species generation, and oxidative potential of highway PM2. 5, ACS Earth Space Chem., 2021, 5(8), 1865–1875, DOI:10.1021/acsearthspacechem.1c00135.
- D. Kahe, Z. Sabeti, P. Sarbakhsh, M. Shakerkhatibi, A. Gholampour, G. Goudarzi, J. Sharbafi, S. Dastgiri, A. Separham and E. Seyedrezazadeh, Effect of PM2. 5 exposure on adhesion molecules and systemic nitric oxide in healthy adults: The role of metals, PAHs, and oxidative potential, Chemosphere, 2024, 354, 141631, DOI:10.1016/j.chemosphere.2024.141631.
- Z. Khoshkam, M. Habibi-Rezaei, M. S. Hassanvand, Y. Aftabi, E. Seyedrezazadeh, A. Amiri-Sadeghan, H. Zarredar, L. Roshangar, A. Gholampour and A. A. Moosavi-Movahedi, The oxidative and neurotoxic potentials of the ambient PM2. 5 extracts: The efficient multi-solvent extraction method, Sci. Total Environ., 2022, 810, 152291, DOI:10.1016/j.scitotenv.2021.152291.
- M. G. Lionetto, M. R. Guascito, M. E. Giordano, R. Caricato, A. R. De Bartolomeo, M. P. Romano, M. Conte, A. Dinoi and D. Contini, Oxidative potential, cytotoxicity, and intracellular oxidative stress generating capacity of PM10: A case study in South of Italy, Atmosphere, 2021, 12(4), 464, DOI:10.3390/atmos12040464.
- S. Lu, J. Liu, G. Hou, J. Zhao, X. Liu, T. Xie, K. Xiao, S. Yonemochi, E. C. Ebere, W. Wang and Q. Wang, Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion, Toxics, 2023, 11(11), 921, DOI:10.3390/toxics11110921.
- Y. Luo, Y. Zeng, H. Xu, D. Li, T. Zhang, Y. Lei, S. Huang and Z. Shen, Connecting oxidative potential with organic carbon molecule composition and source-specific apportionment in PM2. 5 in Xi'an, China, Atmos. Environ., 2023, 306, 119808, DOI:10.1016/j.atmosenv.2023.119808.
- J. Ma, C. Liu, F. Liu, X. Zheng and H. Qian, Oxidation potential of PM2. 5 in a mechanical processing plant and its association with metal composition, Atmos. Environ., 2024, 319, 120318, DOI:10.1016/j.atmosenv.2023.120318.
- L. Massimi, M. Ristorini, G. Simonetti, M. A. Frezzini, M. L. Astolfi and S. Canepari, Spatial mapping and size distribution of oxidative potential of particulate matter released by spatially disaggregated sources, Environ. Pollut., 2020, 266, 115271, DOI:10.1016/j.envpol.2020.115271.
- Z. Ni, N. Gao, N. Chen, C. Zhang, Z. Liu, K. Zhu, V. K. Sharma and H. Jia, Particle-size distributions of environmentally persistent free radicals and oxidative potential of soils from a former gasworks site, Sci. Total Environ., 2023, 869, 161747, DOI:10.1016/j.scitotenv.2023.161747.
- N. Novo-Quiza, J. Sánchez-Piñero, J. Moreda-Piñeiro, I. Turnes-Carou, S. Muniategui-Lorenzo and P. López-Mahía, Oxidative potential of the inhalation bioaccessible fraction of PM10 and bioaccessible concentrations of polycyclic aromatic hydrocarbons and metal (oid) s in PM10, Environ. Sci. Pollut. Res. Int., 2024, 31(22), 31862–31877, DOI:10.1007/s11356-024-33331-9.
- S. H. Oh, M. Song, J. J. Schauer, Z. H. Shon and M. S. Bae, Assessment of long-range oriented source and oxidative potential on the South-west shoreline, Korea: Molecular marker receptor models during shipborne measurements, Environ. Pollut., 2021, 281, 116979, DOI:10.1016/j.envpol.2021.116979.
- M. Park, S. Lee, H. Lee, M. C. Denna, J. Jang, D. Oh, M. S. Bae, K. S. Jang and K. Park, New health index derived from oxidative potential and cell toxicity of fine particulate matter to assess its potential health effect, Heliyon, 2024, 10(3), e25310, DOI:10.1016/j.heliyon.2024.e25310.
- M. C. Pietrogrande, D. Bacco, A. Trentini and M. Russo, Effect of filter extraction solvents on the measurement of the oxidative potential of airborne PM 2.5, Environ. Sci. Pollut. Res. Int., 2021, 28, 29551–29563, DOI:10.1007/s11356-021-12604-7.
- J. V. Puthussery, A. Singh, P. Rai, D. Bhattu, V. Kumar, P. Vats, M. Furger, N. Rastogi, J. G. Slowik, D. Ganguly and A. S. Prevot, Real-time measurements of PM2. 5 oxidative potential using a dithiothreitol assay in Delhi, India, Environ. Technol. Lett., 2020, 7(7), 504–510, DOI:10.1021/acs.estlett.0c00342.
- N. Raparthi, S. Yadav, A. Khare, S. Dubey and H. C. Phuleria, Chemical and oxidative properties of fine particulate matter from near-road traffic sources, Environ. Pollut., 2023, 337, 122514, DOI:10.1016/j.envpol.2023.122514.
- E. Serafeim, A. Besis, A. Kouras, C. N. Farias, A. B. Yera, G. M. Pereira, C. Samara and P. de Castro Vasconcellos, Oxidative potential of ambient PM2. 5 from São Paulo, Brazil: variations, associations with chemical components and source apportionment, Atmos. Environ., 2023, 298, 119593, DOI:10.1016/j.atmosenv.2023.119593.
- B. Sharma, J. Mao, S. Jia, S. K. Sharma, T. K. Mandal, S. Bau and S. Sarkar, Size-distribution and driving factors of aerosol oxidative potential in rural kitchen microenvironments of northeastern India, Environ. Pollut., 2024, 343, 123246, DOI:10.1016/j.envpol.2023.123246.
- Y. C. Ting, P. K. Chang, P. C. Hung, C. C. Chou, K. H. Chi and T. C. Hsiao, Characterizing emission factors and oxidative potential of motorcycle emissions in a real-world tunnel environment, Environ. Res., 2023, 234, 116601, DOI:10.1016/j.envres.2023.116601.
- T. Xie, S. Lu, J. Zeng, L. Rao, X. Wang, M. S. Win, D. Zhang, H. Lu, X. Liu and Q. Wang, Soluble Fe release from iron-bearing clay mineral particles in acid environment and their oxidative potential, Sci. Total Environ., 2020, 726, 138650, DOI:10.1016/j.scitotenv.2020.138650.
- T. Xie, S. Lu, L. Rao, L. Zhang, X. Wang, W. Wang and Q. Wang, Dissolution factors and oxidative potential of acid soluble irons from chlorite mineral particles, Atmos. Environ., 2021, 255, 118436, DOI:10.1016/j.atmosenv.2021.118436.
- M. Xu, Z. Niu, C. Liu, J. Yan, B. Peng and Y. Yang, Oxidative potential of metal-containing nanoparticles in coal fly ash generated from coal-fired power plants in China, Environ. Health, 2023, 1(3), 180–190, DOI:10.1021/envhealth.3c00040.
- J. Zhu, M. Sheng, J. Shang, Y. Kuang, X. Shi and X. Qiu, Photocatalytic role of atmospheric soot particles under visible-light irradiation: reactive oxygen species generation, self-oxidation process, and induced higher oxidative potential and cytotoxicity, Environ. Sci. Technol., 2022, 56(12), 7668–7678, DOI:10.1021/acs.est.2c00420.
- X. Li, Y. Tao, L. Zhu, S. Ma, S. Luo, Z. Zhao, N. Sun, X. Ge and Z. Ye, Optical and chemical properties and oxidative potential of aqueous-phase products from OH and 3 C*-initiated photooxidation of eugenol, ACP, Appl. Cardiopulm. Pathophysiol., 2022, 22(11), 7793–7814, DOI:10.5194/acp-22-7793-2022.
- Y. Cheng, Y. Ma, B. Dong, X. Qiu and D. Hu, Pollutants from primary sources dominate the oxidative potential of water-soluble PM2. 5 in Hong Kong in terms of dithiothreitol (DTT) consumption and hydroxyl radical production, J. Hazard. Mater., 2023, 405, 124218, DOI:10.1016/j.jhazmat.2020.124218.
- K. Chen, J. Xu, L. Famiyeh, Y. Sun, D. Ji, H. Xu, C. Wang, S. E. Metcalfe, R. Betha, S. N. Behera and C. Jia, Chemical constituents, driving factors, and source apportionment of oxidative potential of ambient fine particulate matter in a Port City in East China, J. Hazard. Mater., 2022, 440, 129864 CrossRef CAS.
- J. M. Li, S. M. Zhao, S. H. Xiao, X. Li, S. P. Wu, J. Zhang and J. J. Schwab, Source apportionment of water-soluble oxidative potential of PM2. 5 in a port city of Xiamen, Southeast China, Atmos. Environ., 2023, 314, 120122, DOI:10.1016/j.atmosenv.2023.120122.
- J. M. Li, S. M. Zhao, S. P. Wu, B. Q. Jiang, Y. J. Liu, J. Zhang and J. J. Schwab, Size-segregated characteristics of water-soluble oxidative potential in urban Xiamen: Potential driving factors and implications for human health, Sci. Total Environ., 2024, 912, 168902, DOI:10.1016/j.scitotenv.2023.168902.
- R. Roy, R. Jan, R. Bhor, K. Pai and P. G. Satsangi, Size-fractionated ambient particulate matter induce toxicity: Oxidative potential, cytotoxic effect and inflammatory potential, Atmos. Environ., 2023, 312, 120032, DOI:10.1016/j.atmosenv.2023.120032.
- Y. Wang, Y. Zhang, J. J. Schauer, B. de Foy, T. Cai and Y. Zhang, Impacts of Sources on PM2. 5 Oxidation Potential during and after the Asia-Pacific Economic Cooperation Conference in Huairou, Beijing, Environ. Sci. Technol., 2020, 54(5), 2585–2594, DOI:10.1021/acs.est.9b05468.
- J. Li, C. Hua, L. Ma, K. Chen, F. Zheng, Q. Chen, X. Bao, J. Sun, R. Xie, F. Bianchi and V. M. Kerminen, Key drivers of the oxidative potential of PM2. 5 in Beijing in the context of air quality improvement from 2018 to 2022, Environ. Int., 2024, 187, 108724, DOI:10.1016/j.envint.2024.108724.
- A. Patel and N. Rastogi, Oxidative Potential of Atmospheric Aerosols over Different Regions of India and Surrounding Oceans, ACS Earth Space Chem., 2023, 7(12), 2582–2592, DOI:10.1021/acsearthspacechem.3c00250.
- H. Li, Q. Chen, C. Wang, R. Wang, T. Sha, X. Yang and D. Ainur, Pollution characteristics of environmental persistent free radicals (EPFRs) and their contribution to oxidation potential in road dust in a large city in northwest China, J. Hazard. Mater., 2022, 442, 130087, DOI:10.1016/j.jhazmat.2022.130087.
- M. Fadel, D. Courcot, G. Delmaire, G. Roussel, C. Afif and F. Ledoux, Source apportionment of PM2. 5 oxidative potential in an East Mediterranean site, Sci. Total Environ., 2023, 900, 165843, DOI:10.1016/j.scitotenv.2023.165843.
- I. Goyal, P. K. Verma, K. M. Kumari and A. Lakhani, Dynamic changes in the characteristics of fine particles and their oxidative potential in the city of Taj (Agra, India): the untold story of fireworks display, Air Qual. Atmos. Health, 2023, 16(11), 2193–2207, DOI:10.1007/s11869-023-01402-4.
- M. Vörösmarty, G. Uzu, J. L. Jaffrezo, P. Dominutti, Z. Kertész, E. Papp and I. Salma, Oxidative potential in rural, suburban and city centre atmospheric environments in central Europe, ACP, Appl. Cardiopulm. Pathophysiol., 2023, 23(22), 14255–14269, DOI:10.5194/acp-23-14255-2023.
- L. J. Borlaza, S. Weber, A. Marsal, G. Uzu, V. Jacob, J. L. Besombes, M. Chatain, S. Conil, and J. L. Jaffrezo, 9-Year Trends of PM 10 Sources and Oxidative Potential in a Rural Background Site in France, ACP, 2021, DOI:10.5194/acp-22-8701-2022.
- I. Seo, K. Lee, M. S. Bae, M. Park, S. Maskey, A. Seo, L. J. Borlaza, E. M. Cosep and K. Park, Comparison of physical and chemical characteristics and oxidative potential of fine particles emitted from rice straw and pine stem burning, Environ. Pollut., 2020, 267, 115599, DOI:10.1016/j.envpol.2020.115599.
- F. Barraza, G. Uzu, J. L. Jaffrezo, E. Schreck, H. Budzinski, K. Le Menach, M. H. Dévier, H. Guyard, A. Calas, M. I. Perez and L. A. Villacreces, Contrasts in chemical composition and oxidative potential in PM10 near flares in oil extraction and refining areas in Ecuador, Atmos. Environ., 2020, 223, 117302, DOI:10.1016/j.atmosenv.2020.117302.
- J. Wang, H. Jiang, H. Jiang, Y. Mo, X. Geng, J. Li, S. Mao, S. Bualert, S. Ma, J. Li and G. Zhang, Source apportionment of water-soluble oxidative potential in ambient total suspended particulate from Bangkok: Biomass burning versus fossil fuel combustion, Atmos. Environ., 2020, 235, 117624, DOI:10.1016/j.atmosenv.2020.117624.
- A. Ghosh, M. Dutta, S. K. Das, M. Sharma and A. Chatterjee, Acidity and oxidative potential of atmospheric aerosols over a remote mangrove ecosystem during the advection of anthropogenic plumes, Chemosphere, 2024, 352, 141316, DOI:10.1016/j.chemosphere.2024.141316.
- M. Ahmad, J. Chen, S. Panyametheekul, Q. Yu, A. Nawab, M. T. Khan, Y. Zhang, S. W. Ali and W. Phairuang, Fine particulate matter from brick kilns site and roadside in Lahore, Pakistan: Insight into chemical composition, oxidative potential, and health risk assessment, Heliyon, 2024, 10(4), e25884, DOI:10.1016/j.heliyon.2024.e25884.
- M. C. Pietrogrande, B. Biffi, C. Colombi, E. Cuccia, U. Dal Santo and L. Romanato, Contribution of chemical composition to oxidative potential of atmospheric particles at a rural and an urban site in the Po Valley: Influence of high ammonia agriculture emissions, Atmos. Environ., 2020, 318, 120203, DOI:10.1016/j.atmosenv.2023.120203.
- B. H. Isenor, J. P. Downey, S. A. Whidden, M. M. Fitzgerald and J. P. Wong, Oxidative potential of fine particulate matter emitted from traditional and improved biomass cookstoves, Environ. Sci.: Atmos., 2024, 4(2), 202–213, 10.1039/d3ea00135k.
- Y. Yang, M. A. Battaglia, M. K. Mohan, E. S. Robinson, P. F. DeCarlo, K. C. Edwards, T. Fang, S. Kapur, M. Shiraiwa, M. Cesler-Maloney and W. R. Simpson, Assessing the oxidative potential of outdoor PM2. 5 in wintertime Fairbanks, Alaska, ACS ES&T Air, 2024, 1(3), 175–187, DOI:10.1021/acsestair.3c00066.
- L. Borlaza-Lacoste, V. Mardoñez, A. Marsal, I. Hough, V. N. Dinh, P. Dominutti, J. L. Jaffrezo, A. Alastuey, J. L. Besombes, G. Močnik and I. Moreno, Oxidative potential of particulate matter and its association to respiratory health endpoints in high-altitude cities in Bolivia, Environ. Res., 2024, 255, 119179, DOI:10.1016/j.envres.2024.119179.
- S. Romano, S. Becagli, F. Lucarelli, M. Russo and M. C. Pietrogrande, Oxidative potential sensitivity to metals, Br, P, S, and Se in PM10 samples: New insights from a monitoring campaign in Southeastern Italy, Atmosphere, 2020, 11(4), 367, DOI:10.3390/atmos11040367.
- L. Zhang, X. Hu, S. Chen, Y. Chen and H. Z. Lian, Characterization and source apportionment of oxidative potential of ambient PM2. 5 in Nanjing, a megacity of Eastern China, Environ. Pollut. Bioavailability, 2023, 35(1), 2175728, DOI:10.1080/26395940.2023.2175728.
- W. Wang, G. Cao, J. Zhang, Z. Chen, C. Dong, J. Chen and Z. Cai, p-Phenylenediamine-derived quinones as new contributors to the oxidative potential of fine particulate matter, Environ. Sci. Technol. Lett., 2022, 9(9), 712–717, DOI:10.1021/acs.estlett.2c00484.
- S. J. Campbell, A. Barth, G. I. Chen, A. H. Tremper, M. Priestman, D. Ek, S. Gu, F. J. Kelly, M. Kalberer and D. C. Green, High time resolution quantification of PM2. 5 oxidative potential at a Central London roadside supersite, Environ. Int., 2024, 193, 109102, DOI:10.1016/j.envint.2024.109102.
- K. Glojek, V. D. Thuy, S. Weber, G. Uzu, M. Manousakas, R. Elazzouzi, K. Džepina, S. Darfeuil, P. Ginot, J. L. Jaffrezo and R. Žabkar, Annual variation of source contributions to PM10 and oxidative potential in a mountainous area with traffic, biomass burning, cement-plant and biogenic influences, Environ. Int., 2024, 108787, DOI:10.1016/j.envint.2024.108787.
- A. Singh, A. Patel, R. Satish, S. N. Tripathi and N. Rastogi, Wintertime oxidative potential of PM2. 5 over a big urban city in the central Indo-Gangetic Plain, Sci. Total Environ., 2024, 905, 167155, DOI:10.1016/j.scitotenv.2023.167155.
- M. In't Veld, M. Pandolfi, F. Amato, N. Pérez, C. Reche, P. Dominutti, J. Jaffrezo, A. Alastuey, X. Querol and G. Uzu, Discovering oxidative potential (OP) drivers of atmospheric PM10, PM2. 5, and PM1 simultaneously in North-Eastern Spain, Sci. Total Environ., 2023, 857, 159386, DOI:10.1016/j.scitotenv.2022.159386.
- L. J. Borlaza, V. D. Thuy, S. Grange, S. Socquet, E. Moussu, G. Mary, O. Favez, C. Hueglin, J. L. Jaffrezo and G. Uzu, Impact of COVID-19 lockdown on particulate matter oxidative potential at urban background versus traffic sites, Environ. Sci.: Atmos., 2023, 3(5), 942–953, 10.1039/D3EA00013C.
- Y. Shangguan, X. Zhuang, X. Querol, B. Li, N. Moreno, P. Trechera, P. C. Sola, G. Uzu and J. Li, Characterization of deposited dust and its respirable fractions in underground coal mines: Implications for oxidative potential-driving species and source apportionment, Int. J. Coal Geol., 2022, 258, 104017, DOI:10.1016/j.coal.2022.104017.
- S. K. Grange, G. Uzu, S. Weber, J. L. Jaffrezo and C. Hueglin, Linking Switzerland's PM 10 and PM 2.5 oxidative potential (OP) with emission sources, ACP, Appl. Cardiopulm. Pathophysiol., 2022, 22(10), 7029–7050, DOI:10.5194/acp-22-7029-2022.
- S. Weber, G. Uzu, O. Favez, L. J. Borlaza, A. Calas, D. Salameh, F. Chevrier, J. Allard, J. L. Besombes, A. Albinet and S. Pontet, Source apportionment of atmospheric PM 10 oxidative potential: synthesis of 15 year-round urban datasets in France, ACP, Appl. Cardiopulm. Pathophysiol., 2021, 21(14), 11353–11378, DOI:10.5194/acp-21-11353-2021.
- M. C. Pietrogrande, I. Bertoli, G. Clauser, C. Dalpiaz, R. Dell'Anna, P. Lazzeri, W. Lenzi and M. Russo, Chemical composition and oxidative potential of atmospheric particles heavily impacted by residential wood burning in the alpine region of northern Italy, Atmos. Environ., 2021, 253, 118360, DOI:10.1016/j.atmosenv.2021.118360.
- M. Ahmad, Q. Yu, J. Chen, S. Cheng, W. Qin and Y. Zhang, Chemical characteristics, oxidative potential, and sources of PM2. 5 in wintertime in Lahore and Peshawar, Pakistan, J. Environ. Sci., 2021, 102, 148–158, DOI:10.1016/j.jes.2020.09.0141001-0742.
- D. Gao, J. A. Mulholland, A. G. Russell and R. J. Weber, Characterization of water-insoluble oxidative potential of PM2. 5 using the dithiothreitol assay, Atmos. Environ., 2020, 224, 117327, DOI:10.1016/j.atmosenv.2020.117327.
- S. H. Oh, K. Park, M. Park, M. Song, K. S. Jang, J. J. Schauer, G. N. Bae and M. S. Bae, Comparison of the sources and oxidative potential of PM2. 5 during winter time in large cities in China and South Korea, Sci. Total Environ., 2023, 859, 160369, DOI:10.1016/j.scitotenv.2022.160369.
- W. Wang, Y. Zhang, G. Cao, Y. Song, J. Zhang, R. Li, L. Zhao, C. Dong and Z. Cai, Influence of COVID-19 lockdown on the variation of organic aerosols: Insight into its molecular composition and oxidative potential, Environ. Res., 2022, 206, 112597, DOI:10.1016/j.envres.2021.112597.
- A. Anand, S. Yadav and H. C. Phuleria, Chemical characteristics and oxidative potential of indoor and outdoor PM2. 5 in densely populated urban slums, Environ. Res., 2022, 212, 113562, DOI:10.1016/j.envres.2022.113562.
- Y. Fujitani, A. Furuyama, M. Hayashi, H. Hagino and M. Kajino, Assessing oxidative stress induction ability and oxidative potential of PM2. 5 in cities in eastern and western Japan, Chemosphere, 2023, 324, 138308, DOI:10.1016/j.chemosphere.2023.138308.
- Q. Liu, Z. Lu, Y. Xiong, F. Huang, J. Zhou and J. J. Schauer, Oxidative potential of ambient PM2. 5 in Wuhan and its comparisons with eight areas of China, Sci. Total Environ., 2020, 701, 134844, DOI:10.1016/j.scitotenv.2019.134844.
- C. Xing, Y. Wang, X. Yang, Y. Zeng, J. Zhai, B. Cai, A. Zhang, T. M. Fu, L. Zhu, Y. Li and X. Wang, Seasonal variation of driving factors of ambient PM2. 5 oxidative potential in Shenzhen, China, Sci. Total Environ., 2023, 862, 160771, DOI:10.1016/j.scitotenv.2022.160771.
- Y. Q. Yu and T. Zhu, Effects of endogenous and exogenous reductants in lung fluid on the bio accessible metal concentration and oxidative potential of ultrafine particles, Sci. Total Environ., 2023, 882, 163652, DOI:10.1016/j.scitotenv.2023.163652.
- Q. Meng, J. Liu, J. Shen, I. Del Rosario, P. S. Lakey, M. Shiraiwa, J. Su, S. Weichenthal, Y. Zhu, F. Oroumiyeh and S. E. Paulson, Fine particulate matter metal composition, oxidative potential, and adverse birth outcomes in Los Angeles, Environ. Health Perspect., 2023, 131(10), 107012, DOI:10.1289/EHP1219.
- J. Li, H. Chen, X. Li, M. Wang, X. Zhang, J. Cao, F. Shen, Y. Wu, S. Xu, H. Fan and G. Da, Differing toxicity of ambient particulate matter (PM) in global cities, Atmos. Environ., 2019, 212, 305–315, DOI:10.1016/j.atmosenv.2019.05.048.
- Y. Wang, M. Wang, S. Li, H. Sun, Z. Mu, L. Zhang, Y. Li and Q. Chen, Study on the oxidation potential of the water-soluble components of ambient PM2. 5 over Xi'an, China: Pollution levels, source apportionment and transport pathways, Environ. Int., 2020, 136, 105515, DOI:10.1016/j.envint.2020.105515.
- A. L. Kramer, S. Dorn, A. Perez, C. Roper, I. A. Titaley, K. Cayton, R. P. Cook, P. H. Cheong and S. L. Simonich, Assessing the oxidative potential of PAHs in ambient PM2. 5 using the DTT consumption assay, Environ. Pollut., 2021, 285, 117411, DOI:10.1016/j.envpol.2021.117411.
- Z. Zhao, X. S. Luo, Y. Jing, H. Li, Y. Pang, L. Wu, Q. Chen and L. Jin, In vitro assessments of bioaccessibility and bioavailability of PM2. 5 trace metals in respiratory and digestive systems and their oxidative potential, J. Environ. Sci., 2021, 409, 124638, DOI:10.1016/j.jhazmat.2020.124638.
- D. Paraskevopoulou, A. Bougiatioti, I. Stavroulas, T. Fang, M. Lianou, E. Liakakou, E. Gerasopoulos, R. Weber, A. Nenes and N. Mihalopoulos, Yearlong variability of oxidative potential of particulate matter in an urban Mediterranean environment, Atmos. Environ., 2019, 206, 183–196, DOI:10.1016/j.atmosenv.2019.02.027.
- M. Hakimzadeh, E. Soleimanian, A. Mousavi, A. Borgini, C. De Marco, A. A. Ruprecht and C. Sioutas, The impact of biomass burning on the oxidative potential of PM2. 5 in the metropolitan area of Milan, Atmos. Environ., 2020, 224, 117328, DOI:10.1016/j.atmosenv.2020.117328.
- H. Hu, C. Liu, F. Yang, H. Qian, A. Russell, A. Shahsavani and H. Kan, Mechanism changing iron solubility and oxidative potential associated with PM2. 5 during outdoor-to-indoor transport, Atmos. Environ., 2023, 308, 119879, DOI:10.1016/j.atmosenv.2023.119879.
- Z. Wang, X. Dong, M. He and J. Liu, Evaluation of indoor particulate matter control based on health risks and oxidative potential in a metro station, Atmos. Environ., 2024, 317, 120202, DOI:10.1016/j.atmosenv.2023.120202.
- S. Caumo, A. B. Yera, C. Alves, I. C. Rienda, N. Kováts, K. Hubai and P. de Castro Vasconcellos, Assessing the chemical composition, potential toxicity and cancer risk of airborne fine particulate matter (PM2. 5) near a petrochemical industrial area, Environ. Toxicol. Pharmacol., 2023, 101, 104170, DOI:10.1016/j.etap.2023.104170.
- F. Cervellati, M. Benedusi, F. Manarini, B. Woodby, M. Russo, G. Valacchi and M. C. Pietrogrande, Proinflammatory properties and oxidative effects of atmospheric particle components in human keratinocytes, Chemosphere, 2020, 240, 124746, DOI:10.1016/j.chemosphere.2019.124746.
- T. Fang, P. S. Lakey, R. J. Weber and M. Shiraiwa, Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid, Environ. Sci.: Atmos., 2020, 53(21), 12784–12792, DOI:10.1021/acs.est.9b03823.
- D. Ainur, Q. Chen, T. Sha, M. Zarak, Z. Dong, W. Guo, Z. Zhang, K. Dina and T. An, Outdoor health risk of atmospheric particulate matter at night in Xi'an, Northwestern China, Environ. Sci.: Atmos., 2023, 57(25), 9252–9265, DOI:10.1021/acs.est.3c02670.
- Y. Koike and T. Kameda, Effects of chemical reactions on the oxidative potential of humic acid, a model compound of atmospheric humic-like substances, Atmosphere, 2022, 13(6), 976, DOI:10.3390/atmos13060976.
- Y. Cui, L. Zhu, H. Wang, Z. Zhao, S. Ma and Z. Ye, Characteristics and Oxidative Potential of Ambient PM2. 5 in the Yangtze River Delta Region: Pollution Level and Source Apportionment, Atmosphere, 2023, 14(3), 425, DOI:10.3390/atmos14030425.
- W. Zhou, Y. Zhao, R. Li, H. Fu, Q. Li, L. Zhang and J. Chen, Metals, PAHs and oxidative potential of size-segregated particulate matter and inhalational carcinogenic risk of cooking at a typical university canteen in Shanghai, China, Atmos. Environ., 2022, 287, 119250, DOI:10.1016/j.atmosenv.2022.119250.
- T. Fang, B. C. Hwang, S. Kapur, K. S. Hopstock, J. Wei, V. Nguyen, S. A. Nizkorodov and M. Shiraiwa, Wildfire particulate matter as a source of environmentally persistent free radicals and reactive oxygen species, Environ. Sci.: Atmos., 2023, 3(3), 581–594, 10.1039/D2EA00170E.
- J. Yalamanchili, C. J. Hennigan and B. E. Reed, Precipitation of aqueous transition metals in particulate matter during the dithiothreitol (DTT) oxidative potential assay, Environ. Sci.:Processes Impacts, 2022, 24(5), 762–772, 10.1039/d2em00005a.
- N. Santiago-De La Rosa, V. Mugica-Álvarez, G. González-Cardoso, A. De Vizcaya-Ruiz, M. Uribe-Ramírez and B. L. Valle-Hernández, Emission factors of polycyclic aromatic hydrocarbons and oxidative potential of fine particles emitted from crop residues burning, Polycyclic Aromat. Compd., 2022, 42(8), 5123–5142, DOI:10.1080/10406638.2021.1924801.
- M. Mylonaki, M. Gini, M. Georgopoulou, M. Pilou, E. Chalvatzaki, S. Solomos, E. Diapouli, E. Giannakaki, M. Lazaridis, S. N. Pandis and A. Nenes, Wildfire and African dust aerosol oxidative potential, exposure and dose in the human respiratory tract, Sci. Total Environ., 2024, 913, 169683, DOI:10.1016/j.scitotenv.2023.169683.
- F. Costabile, M. Gualtieri, M. Rinaldi, S. Canepari, R. Vecchi, L. Massimi, G. Di Iulio, M. Paglione, L. Di Liberto, E. Corsini and M. C. Facchini, Exposure to urban nanoparticles at low PM 1 concentrations as a source of oxidative stress and inflammation, Sci. Rep., 2023, 13(1), 18616, DOI:10.1038/s41598-023-45230-z.
- R. Tang, J. Zhu and J. Shang, Effect of oxidation degree on photoactivity, physicochemical properties and oxidative potential changes of graphene-based materials under visible light irradiation, Carbon, 2023, 213, 118168, DOI:10.1016/j.carbon.2023.11816.
- S. Wang, M. Takhar, Y. Zhao, L. N. Al Rashdi and A. W. Chan, Dynamic oxidative potential of organic aerosol from heated cooking oil, ACS Earth Space Chem., 2021, 5(5), 1150–1162, DOI:10.1021/acsearthspacechem.1c00038.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ea00025d |
| This journal is © The Royal Society of Chemistry 2025 |