Open Access Article
Open Access ArticleCyanoHABs and CAPs: assessing community-based monitoring of PM2.5 with regional sources of pollution in rural, northeastern North Carolina†
Haley E.
Plaas‡
 *a,
Colleen
Karl
b,
Rachael
Cogbill
*a,
Colleen
Karl
b,
Rachael
Cogbill
 c,
Nicole
Rosales-Garcia
a,
Ashley H.
Stoop
d,
Lisa L.
Satterwhite
c,
Nicole
Rosales-Garcia
a,
Ashley H.
Stoop
d,
Lisa L.
Satterwhite
 e,
Martine E.
Mathieu-Campbell
e,
Martine E.
Mathieu-Campbell
 f,
Jennifer
Richmond-Bryant
f,
Jennifer
Richmond-Bryant
 fg,
Hans W.
Paerl
fg,
Hans W.
Paerl
 chi and
Douglas S.
Hamilton
chi and
Douglas S.
Hamilton
 a
a
aDepartment of Marine, Earth, and Atmospheric Sciences, North Carolina State University, 2800 Faucette Dr., 1142 Jordan Hall, Raleigh, NC 27695, USA. E-mail: heplaas@ncsu.edu; haley.e.plaas@columbia.edu
bChowan Edenton Environmental Group, Tyner, NC 27980, USA
cDepartment of Environmental Sciences and Engineering, UNC-Chapel Hill, Chapel Hill, NC 27599, USA
dAlbemarle Regional Health Services, Elizabeth City, NC 27909, USA
eDepartment of Civil and Environmental Engineering, Duke University, Durham, NC 27708, USA
fCenter for Geospatial Analytics, North Carolina State University, Raleigh, NC 27695, USA
gDepartment of Forestry and Environmental Resources, North Carolina State University, Raleigh, NC 27695, USA
hDepartment of Earth, Marine, and Environmental Sciences, UNC-Chapel Hill, Chapel Hill, NC 27599, USA
iInstitute of Marine Sciences, UNC-Chapel Hill, Morehead City, NC 28557, USA
First published on 15th April 2025
Abstract
Underserved rural communities in northeastern North Carolina (NC), surrounding the Albemarle Sound, have faced degraded environmental quality from various sources of air and water pollution. However, access to local air quality data is regionally scarce due to a lack of state-run monitoring stations, which has motivated local community science efforts. In January 2022, we co-developed a community-led study to investigate the relationship between fine particulate matter (PM2.5) and sources of regional air pollution, with a specific focus on previously identified emissions from cyanobacterial harmful algal blooms (CyanoHABs). Using low-cost PurpleAir air quality sensors to quantify PM2.5 mass, satellite-derived indicators of CyanoHABs, and other publicly available atmospheric and meteorological data, we assessed environmental drivers of PM2.5 mass in the airshed of the Albemarle Sound estuary during 2022–2023. We found that bias-corrected PurpleAir PM2.5 mass concentrations aligned with composite data from the three nearest federal reference equivalent measurements within 1 μg m−3 on average, and that the temporal variation in PM2.5 was most closely associated with changes in criteria air pollutants. Ultimately, satellite-based indicators of CyanoHABs (Microcystis spp. equivalent cell counts and bloom spatial extent) were not strongly associated with ambient/episodic increases in PurpleAir PM2.5 mass during our study period. For the first time, we provide local PM2.5 measurements to rural communities in northeastern NC with an assessment of environmental drivers of PM2.5 pollution events. Additional compositional analyses of PM2.5 are warranted to further inform respiratory risk assessments for this region of NC. Despite the lack of correlation between CyanoHABs and PM2.5 observed, this work serves to inform future studies that seek to employ widely available and low-cost approaches to monitor both CyanoHAB aerosol emissions and general air quality in rural coastal regions at high spatial and temporal resolutions.
Environmental significanceFine particulate matter (PM2.5) is demonstrated to increase in the airshed of blue-green algae blooms occurring in polluted waterbodies. Working with community scientists in rural, coastal North Carolina, USA, we examined regional variation in PM2.5 in association with localized air and water pollutants, with a focus on seasonal blue-green algae blooms. By leveraging publicly accessible databases with high spatial and temporal resolution, for the first time, we evaluated the use of low-cost air quality sensors (PurpleAir) and satellite-derived indicators of ocean color (CyAN) in the study of aerosol emissions from blue-green algae blooms. We also provide an assessment of PurpleAir derived PM2.5 for its use by local communities in environmental health research and/or decision-making in coastal North Carolina. |
1. Introduction
Fine particulate matter < 2.5 μm in diameter (PM2.5) is regulated as a criteria air pollutant (CAP) by the US-Environmental Protection Agency (EPA),1,2 and the World Health Organization (WHO) has also set guidelines for limiting PM2.5 exposures.3 Exposure to high concentrations of PM2.5 is associated with an increased risk of adverse child developmental outcomes, chronic respiratory illness, and cardiovascular related morbidity and mortality, especially in susceptible populations.4–10 In North Carolina (NC), USA, PM2.5 concentrations are primarily driven by industrial/vehicular sources in urban areas,11 but agricultural emissions in rural areas pose their own suite of inhalation risks.12,13 Moreover, sea spray in coastal zones can be a source of various aerosolized pathogens or biochemicals,14–16 suggesting that varied sources of aerosol influence air quality in the coastal plain of northeastern NC.The NC Department of Environmental Quality operates numerous environmental monitoring programs, including the maintenance of federal reference method air quality monitoring stations and a cyanobacterial harmful algal bloom (CyanoHAB) dashboard,17 where citizen volunteers can report real-time water quality events. These programs routinely measure and track changes to health-relevant pollutants in various environmental media, but are generally more limited in the rural, eastern part of the state compared to more densely populated and urban areas.18,19 Consequently, the data needed to inform localized environmental health-risk assessments and create adaptive policy in rural NC are often insufficient, as is true for many rural communities throughout the Southeastern (SE) United States.20,21
The Chowan River-Albemarle Sound is one such region in NC where historically poor water and air quality are compounded by a lack of resources allocated by research institutions. A key recreational, agricultural, fisheries, and residential region of coastal NC, the Albemarle Sound and its tributaries, span 5600 km2 of coastal plain with a residential population of 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in its surrounding towns and rural sprawl.22,23 Communities residing in this watershed experience some of the poorest respiratory health outcomes in the state;21,24,25 populations in Bertie, Chowan, and Hertford counties, which surround the Chowan River, experience among the highest asthma related emergency departments visits per year.24 In the 1970's, the Chowan River was declared “dead” due uncontrolled discharges from upstream paper mill plants resulting in CyanoHABs, and in the 1990's, a ten-year fish consumption advisory was put in place due to dioxin contamination from the mills.22,23 Targeted nutrient mitigation strategies successfully remediated CyanoHABs in the Albemarle Sound for the following decades, but in the late 2010's, toxin-producing CyanoHABs re-emerged seasonally with high occurrences of microcystin,26,27 a well characterized liver toxin produced by cyanobacteria.28
000 in its surrounding towns and rural sprawl.22,23 Communities residing in this watershed experience some of the poorest respiratory health outcomes in the state;21,24,25 populations in Bertie, Chowan, and Hertford counties, which surround the Chowan River, experience among the highest asthma related emergency departments visits per year.24 In the 1970's, the Chowan River was declared “dead” due uncontrolled discharges from upstream paper mill plants resulting in CyanoHABs, and in the 1990's, a ten-year fish consumption advisory was put in place due to dioxin contamination from the mills.22,23 Targeted nutrient mitigation strategies successfully remediated CyanoHABs in the Albemarle Sound for the following decades, but in the late 2010's, toxin-producing CyanoHABs re-emerged seasonally with high occurrences of microcystin,26,27 a well characterized liver toxin produced by cyanobacteria.28
In this study, conversations with community partners revealed a myriad of potential point sources of air and water pollutants in northeastern NC, but of particular concern were re-emergent CyanoHABs in the Chowan River and Albemarle Sound. While CyanoHABs have long been treated as a threat to water security across the globe,29 aerosol emissions bearing CyanoHAB cells and their toxins have only recently been recognized as a respiratory health threat.30,31 Many studies have quantified specific cyanobacterial biochemical compounds (e.g., microcystin) in aerosol,32–35 but such research relies on limited in situ monitoring campaigns with low spatiotemporal coverage.31 To better inform respiratory risk assessments related to CyanoHABs, there is a pressing need to understand the relationship between adverse water quality events like CyanoHABs and air quality at a larger spatiotemporal scale.
To address observational gaps in the fate and transport of environmental pollutants, many environmental monitoring programs rely on crowd-sourcing approaches to generate in situ data.36 Community-led research initiatives specifically utilize “community science” or “citizen science” participation not only to improve science literacy and outreach,37 but also, importantly, to increase data availability and researcher access to local knowledge.38–40 Since the early 2000s, community science efforts have gained reputability and expanded across multiple scientific disciplines.41 For air quality measurements, the use of low-cost PurpleAir sensors is one popular approach to increase public access to real-time information and improve the spatiotemporal resolution of air quality monitoring.42,43 In the past decade, numerous correction factors have been developed to improve the precision and accuracy of data provided by the PurpleAir network for use in scientific study and/or policymaking.44–47
Recent studies have demonstrated that CyanoHABs are correlated with increased PM2.5 concentrations in the immediate airshed of ongoing blooms, which is attributed to the enrichment of cyanobacterial metabolites in spray aerosol and the potential formation of secondary aerosol derived from volatile compounds produced by CyanoHABs.27,48–51 Given that real-time measurements of PM2.5 are widely accessible through the PurpleAir network and using the Albemarle Sound region of northeastern NC as a case study, we hypothesized that the PurpleAir sensor network could be leveraged to improve understanding of the link between CyanoHABs, air quality, and additional drivers of aerosol in northeastern NC and beyond.
Working directly with community scientists, we installed 13 PurpleAir PM2.5 sensors along the banks of the Albemarle Sound and in its surrounding townships. To understand regional variation in PM2.5 in relation to local sources of air and water pollution, we (1) corrected PurpleAir-derived PM2.5 data using established correction factors and compared the corrected-PM2.5 data with the nearest federal reference PM2.5 data, (2) examined PM2.5 mass as a function of satellite ocean color-derived CyanoHAB cell count and spatial extent, (3) assessed confounding sources of PM2.5 using CAP data from the nearest air quality system (AQS) station, and (4) placed the findings into environmental context using relevant meteorological data.
By leveraging publicly available air and water quality databases with high spatiotemporal resolution (i.e., PurpleAir and satellite ocean color), our study provides novel insight into the use of PurpleAir sensors to monitor aerosol emissions during CyanoHABs with implications for respiratory exposure guidelines. Our findings reveal previously unexplored connections between PM2.5 mass and satellite-derived indicators of CyanoHABs. For the first time, we also highlight the primary drivers of episodic/ambient PM2.5 mass in the airshed of the Albemarle Sound and evaluate PurpleAir derived PM2.5 data for its use by local communities and public health practitioners in environmental health research and/or decision-making.
2. Methods
2.1. Community-driven study development
From the inception of this study (proposal development) to its completion (interpretation and dissemination of findings), this research was a community-led effort that spanned May 2022 to December 2023. In January of 2022, co-authors were approached by a representative of the Chowan Edenton Environmental Group (co-author Karl), a nonprofit organization that promotes environmental research and education in the Albemarle region of northeastern NC (Fig. 1). Their organization sought to develop a community-led study investigating the relationship between fine particulate matter (i.e., PM2.5) and sources of regional air pollution.To actively engage community members from diverse backgrounds and lived experiences, new partnerships were developed and maintained between the Chowan Edenton Environmental Group and Albemarle Regional Health Services (local public health department), the Edenton Racial Reconciliation Group (local social injustice discussion group), Northampton First (regional environmental justice group), the town of Edenton (local government), and the Museum of the Albemarle (a local history museum). Community members raised concerns in various group forums that CyanoHABs, wood pellet plants, livestock and agricultural practices, and military operations are sources of CAPs of local concern. Based on these concerns, we identified the geographic location of CyanoHAB hotspots using the NC Department of Environmental Quality CyanoHAB dashboard,17 livestock operations using the NC Department of Environmental Quality's animal feeding operation data for the year 2023 (https://www.deq.nc.gov/water-resources/animalops/afo-permit-facilities-table-4-2023), the density of nearby cotton fields based on the US Department of Agriculture's cotton production estimates by county (https://www.nass.usda.gov/Statistics_by_State/North_Carolina/Publications/County_Estimates/Cotton.pdf), and wood pellet plant and military bombing range locations, which are publicly available (Fig. 1). Sites for air quality sensor placement were identified through community organizations, and regular email communications between co-authors and community partners were maintained to exchange research progress and findings. As such, two community members are included as co-authors on this study for their continued contributions (Karl and Stoops).
2.2. Particulate matter (PM2.5) quantification
Localized sources of PM2.5 in the Albemarle region are not currently represented in state and federal air quality monitoring networks, provided that the three nearest AQS PM2.5 sensors operate approximately 50 miles from the central Albemarle Sound in Greenville, NC (35.641, −77.360), Roanoke Rapids, NC (36.512, −77.655), and Hampton, Virginia (37.104, −76.387) (Fig. 1). Therefore, to quantify PM2.5, we installed 13 PurpleAir air quality sensors at the homes and workplaces of local volunteers between June 2022 and December 2023. PurpleAir sensors quantify PM2.5 using dual operating optical particle counters (#PMS5006, Plantower), labeled channels A and B. An atmospheric pressure, temperature, and humidity sensor (#BME280, BOSCH) is also included with PM2.5 readings. PurpleAir devices connect directly to WPA2 wifi networks and transmit air quality data to an open access map (https://map.purpleair.com/?mylocation) every 10 minutes.PurpleAir sensor placements were dependent on the availability of volunteer hosts with appropriate wireless capacity, but general locations were selected based on proximity to known CyanoHAB hotspots within the estuary (Fig. 1)—with three key hotspots being Arrowhead Beach, Edenton, and Elizabeth City (Fig. 2). To examine the reduction of PM2.5 with increasing distance from a CyanoHAB source, sensors were placed in approximate transects and categorized into sites <0.92 km and >0.92 km from the waterline. The value of 0.92 km was chosen to account for experimental observations of cyanotoxin degradation by atmospheric oxidants.52,53 Two sensors were strategically placed to provide terrestrial “control” sites with limited nearby aquatic and/or CyanoHAB influence (e.g., sensor 1562 and 1358; Fig. 2).
2.3. PurpleAir PM2.5 data bias-correction
Daily average raw PM2.5 data (‘pm2.5_cf_1’, for both channels A and B) were downloaded from http://develop.purpleair.com. While unprocessed PurpleAir PM2.5 data generally track changes to air quality accurately enough for risk assessments by the general public, for scientific analyses and to improve precision of measurements, a series of quality controls addressing calibration biases and particle hygroscopicity must first be applied.45,47 Published correction procedures generally follow the same steps to (1) remove values attributed to apparent measurement errors (e.g., humidity < 0%, PM2.5 < 0, extreme temperatures), (2) remove measurements where there is disagreement between dual channel readings, and (3) apply a correction factor based on humidity and/or temperature. The correction in step (3) is explicitly necessary for health-related research because dynamic mass concentrations of PM2.5 vary with weather conditions (i.e., condensation and evaporation of water vapor onto and off particle surfaces changes mass), but PM2.5 monitors maintained by federal and state agencies measure dry mass of the pollutants to comply with the US EPA National Ambient Air Quality Standards.1,2We tested two PM2.5 mass correction approaches to increase confidence in the accuracy and precision of our PurpleAir data, the first determined by Barkjohn et al. 2021.45 Their correction compared PurpleAir sensor PM2.5 data from across the US (US-wide) to collocated federal reference sensor PM2.5 data and adjusted PM2.5 mass based only on humidity measurements. The second correction we applied was recently developed by Mathieu-Campbell et al., 2024 (ref. 54) to address the high humidity conditions of southeastern US (SE-specific); their correction equation included adjustments for both humidity and temperature. The SE-specific correction also differed from the US-wide approach by applying a more stringent set of criteria for removing suspected erroneous readings.
To evaluate the efficacy of each correction factor, we used an identical input PM2.5 dataset that adhered to the removal criteria outlined in the SE-specific approach. When A/B channels disagreed by >5 μg m−3 and by >1 standard deviation, or a PM2.5 reading fell outside the range of 1.5–1000 μg m−3, the daily value was removed from the final dataset (n = 4257). As an additional data cleaning step to limit the influence of extreme aerosol pollution events in this study, the Fourth of July and the following three days were completely removed from analyses due to potential firework contamination. When PM2.5 measurements but internal PurpleAir data of temperature and relative humidity were not available for individual sensors, the temperature and relative humidity data were supplied from the next nearest PurpleAir device to apply the correction factor.
2.4. Water quality (CyanoHAB) measurements
CyanoHAB cell concentrations (bloom intensity) and spatial extent (bloom surface area) in the Albemarle Sound and its tributaries were retrieved from the multi-agency Cyanobacterial Assessment Network (CyAN) via the US National Aeronautics and Space Administration (NASA) ocean biology data archive center (https://oceancolor.gsfc.nasa.gov/about/projects/cyan/). We elected to utilize satellite-based estimates of CyanoHABs over in situ “grab” sampling methods due to their increased spatial and temporal coverage during our study period.CyAN utilizes imagery captured by the Ocean Land Color Instrument (OLCI) sensors aboard the Sentinel-3A/3B satellites. A cyanobacterial index (CIcyano) is calculated from three spectral bands using algorithms first developed by Wynne et al. in 2008 (ref. 55) and later updated by Matthews et al. in 2012 (ref. 56) and Lunetta et al. in 2015.57 The final CIcyano data product is corrected for upper atmospheric reflectance and is converted to Microcystis spp. equivalent cell counts as an indicator of CyanoHAB intensity.56–58 The lower detection limit of the OLCI sensor is somewhere between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 20
000 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL. Individual files retrieved from CyAN are provided at a daily, 0.3 km spatial resolution, but we composited daily values into weekly maximums to account for missing data (e.g., due to cloud cover preventing satellite images) and to average dynamic movements of CyanoHABs.59 Compositing weekly averages for CIcyano improved data availability for CyanoHAB indicators, with between 89 and 100% of satellite data pixels yielding a non-null value at the weekly level (Table S1†). Accordingly, all analyses involving CyanoHAB indicators were conducted at the weekly resolution.
000 cells per mL. Individual files retrieved from CyAN are provided at a daily, 0.3 km spatial resolution, but we composited daily values into weekly maximums to account for missing data (e.g., due to cloud cover preventing satellite images) and to average dynamic movements of CyanoHABs.59 Compositing weekly averages for CIcyano improved data availability for CyanoHAB indicators, with between 89 and 100% of satellite data pixels yielding a non-null value at the weekly level (Table S1†). Accordingly, all analyses involving CyanoHAB indicators were conducted at the weekly resolution.
CIcyano values were retrieved within three distances from each PurpleAir sensor (∼0.6 km, ∼1.2 km, and ∼2.4 km square pixel grid ‘radius’, with the distances extending at 90° [ESI Fig. S1†] from the center pixel). The three distances over which the CyanoHAB data were extracted were chosen to evaluate the extent of the ‘localized’ influence of CyanoHABs on PM2.5 concentrations. The three pixel grid areas were selected based on the minimum accurate resolution capacities as recommended by Coffer et al. (2021) (ref. 60) and Clark et al. (2017) (ref. 61) and to test cyanobacterial toxin aerosol decay kinetics in the atmosphere. As revealed in Zorbas et al. 2023,52 up to 25% of CyanoHAB derived microcystin is estimated to degrade within 0.92 km of its emission site, and therefore, we selected distances less than (0.6 km), just above (1.2 km), and significantly above (2.4 km) the 0.92 km threshold over which to extract CyanoHAB data.
2.5. Air quality system (AQS) criteria air pollutant (CAP) measurements
To contextualize PM2.5 mass as a function of CyanoHABs, we examined trends in additional air pollutants, primarily gas-phase CAPs (ozone [O3], carbon monoxide [CO], sulfur dioxide [SO2], nitrogen oxides [NOx]), and also particle-phase phosphate (PO4), in association with PM2.5. These data were accessed and downloaded from the US EPA's AQS Air Data network (https://epa.maps.arcgis.com/). Sensor locations with specific pollutant species accessed are provided in Fig. 1. Each pollutant was considered an approximate tracer (proxy) for community-identified key sources of PM2.5 in the region, with CO serving as an indicator of combustion, including wildfires and open agricultural burning.62 O3 serves as a proxy for volatile organic compounds (VOCs), as it is a product of photochemistry with industrial and biogenic VOCs.63 NOx serves as an additional proxy for anthropogenic/industrial emissions closely aligned with O3.64,65 SO2 acts as a proxy for fossil fuel burning and general combustion processes,66,67 and PO4 serves as a proxy for organophosphate pesticides common to this region and/or agricultural emissions. CO and O3 were measured every eight hours but converted into daily averages. All others, including PM2.5, are reported and retrieved as daily averages. For final analyses, data were composited into weeklong averages to facilitate direct comparison with CyanoHAB data (Section 2.4).2.6. Meteorological measurements
Daily weather data, including measurements of wind speed, wind direction, air temperature, relative humidity, and solar radiation, were accessed using the Visual Crossing weather query builder (https://www.visualcrossing.com/weather/weather-data-services). The Visual Crossing platform collates and triangulates weather data for specific latitude and longitude coordinates using the nearest National Weather Service stations—closer stations are weighted most strongly in the calculation. Daily averages were collated into weekly averages for analyses.To further assess potential point sources of PM2.5, we simulated backward air mass trajectories using the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model by the National Oceanic and Atmospheric Administration (NOAA) (https://www.ready.noaa.gov/HYSPLIT_traj.php). Backward trajectories were utilized to evaluate whether the identified point sources of pollution (Fig. 1) fell within the air mass trajectory prior to PM2.5 measurements by our PurpleAir sensor network. Using Edenton, NC or Arrowhead Beach, NC as a central reference point, we simulated 120-hour backward trajectories at the beginning and end of each week when average PM2.5 mass exceeded the seasonal average. HYSPLIT images are reported in the ESI (Fig. S2†).
2.7. Statistical analyses
To determine which PM2.5 correction factor best improved the accuracy and precision of our PurpleAir PM2.5 measurement, we compared both corrected and uncorrected daily PurpleAir PM2.5 to PM2.5 triangulated from the three nearest AQS stations (Fig. 1). Using one goodness of fit (coefficient of determination [R2]) and two error metric tests (restricted maximum likelihood [REML] and mean absolute error [MAE]), we evaluated the performance of each correction factor for PurpleAir PM2.5 in coastal NC.To evaluate variation in PM2.5 mass with respect to each sensor's location within the Albemarle estuary system, we calculated averages of PM2.5 for each sensor and compared means using a One-way Analysis of Variance (ANOVA) with Tukey multiple comparison to assess any systematic differences in measurements between sensors, which could have indicated sensor-bias or a local source of PM2.5 (ESI Fig. S3†). Averages were calculated over various temporal scales (seasonal, annual, and grouped by CyanoHAB conditions) for individual sensors and after compositing multiple sensors together, categorizing based on sensor proximity to the nearest shoreline (i.e., CyanoHAB aerosol source). To explicitly assess the impact of concentrated CyanoHAB events on localized PM2.5 mass, we compared PM2.5 concentrations measured by each sensor between bloom and non-bloom weeks, with a “bloom” week being defined by cell counts meeting the WHO's threshold for heightened health risk of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL.68 A Wilcoxon rank-sum test was then used to compare PM2.5 during bloom versus non-bloom per the imbalanced distribution of weeks when the bloom threshold was met.
000 cells per mL.68 A Wilcoxon rank-sum test was then used to compare PM2.5 during bloom versus non-bloom per the imbalanced distribution of weeks when the bloom threshold was met.
Weekly changes in PM2.5 mass were finally assessed as a function of multiple interacting environmental conditions using a series of multivariate generalized additive models (GAMs, R package ‘mgcv’). PM2.5 was modeled as the dependent variable with the following environmental variables as predictors: CyanoHAB indicators (spatial extent and cell counts), weather conditions (wind speed, wind direction, solar radiation, air temperature, and relative humidity), and CAP concentrations (CO, O3, SO2, PO4, and NOx).69 Models were fitted with increasing complexity, initially examining PM2.5 as a function of individual predictors (eqn (1)) and scaling up to multiple predictors assessing both additive and interactive effects (eqn (2)), where E indicates the modeled output value for PM2.5, Xn is the value for the environmental variable, β0 is the intercept, βn is the coefficient for categorical variables (e.g., sensor specific effects), sn indicates that the variable is treated with a smooth term, and tn indicates that the variables are treated with an interactive term. Specific combinations of interactions and additions between predictor variables are shown with model results in Table 1; further details on model configurations and results are found in the ESI.†
| E[PM2.5] = β0 + s(X) | (1) |
| E[PM2.5] = β0 + β1X1 + s2(X2) + t3,4(X3,X4) + … + βnXn + sn(Xn) + tn(Xn) | (2) |
| Variable | CO | O3 | SO2 | PO4 | NOx | CyanoHAB term | Wind speed | Solar radiation | Temp. | Relative humidity | AIC | BIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5∼ | t 1 | s 1 | t 1 | s 2 | s 3 | t 2 | t 2 | s 4 | t 3 | t 3 | 664 | 985 |
| PM2.5∼ | t 1 | s 1 | t 1 | s 2 | s 3 | — | s 4 | s 5 | t 2 | t 2 | 728 | 992 |
| PM2.5∼ | t 1 | s 1 | t 1 | s 2 | s 3 | t 2 | t 2 | s 4 | t 3 | t 3 | 731 | 998 |
| PM2.5∼ | t 1 | s 1 | t 1 | s 2 | s 3 | s 4 | — | — | — | — | 787 | 1028 |
| PM2.5∼ | — | — | — | — | — | t 1 | t 1 | s 2 | t 2 | t 2 | 1395 | 1476 |
Models were fitted using the Gaussian link function. REML was used to estimate smoothing parameters due to its performance in the nested assessment of nonlinearity between interactive predictors. To account for the zero-inflated distribution of the CyanoHAB data, using the ‘gamlss’ package, CyanoHAB indicators were transformed into a composite index reflecting their relationship with PM2.5 for use in the multivariate regressions.70 The fit of each model was compared using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and residual plots.
3. Results and discussion
3.1. Bias-correction of PurpleAir PM2.5
During the study period, daily averages for raw PM2.5 mass concentrations ranged between 1.5 and 78.0 μg m−3 for PurpleAir sensors and 1.5–67.0 μg m−3 for AQS stations, with the lower concentrations being bounded by our data cleaning procedures. These findings suggest that while uncorrected PurpleAir data do overpredict PM2.5 mass compared to gravimetric methods as frequently reported,44,47 the sensors tracked temporal variability in PM2.5 with nearest AQS sensor measurements as anticipated (Fig. 3).When we applied the US-wide correction factor to our PurpleAir PM2.5 data, daily PM2.5 mass was reduced by 4.8 μg m−3 on average (Fig. 3). Following the correction, the difference in annual average between PurpleAir and AQS PM2.5 fell below 1.0 μg m−3 (7.2 ± 6.7 and 7.9 ± 5.4 μg m−3, respectively), with corrected PurpleAir slightly exceeding the AQS measured PM2.5 mass. The SE-specific correction factor also increased the precision of the PurpleAir measurements when plotted as a function of AQS data, but in contrast to the US-wide correction, this approach resulted in PM2.5 mass that was slightly lower than the corresponding AQS data by ∼1 μg m−3 on average (8.8 ± 5.5 μg m−3) (Fig. 3).
We anticipated that the SE-specific correction factor would best improve the precision and accuracy of our PurpleAir PM2.5 readings, given that our sensor network fell within the geographic boundary over which this correction factor was derived, and furthermore, the average relative humidity in the airshed of the Albemarle was 78 ± 10%, exceeding the average ‘high’ humidity conditions under which the SE-specific correction factor was determined. Regression analyses of each corrected dataset revealed that both correction methods were comparable (Fig. 3), but ultimately the SE-specific correction factor more effectively improved the fit of the PurpleAir PM2.5 as a function of AQS measurements when compared to the US-wide correction factor (Fig. 3), as revealed by regression error metrics computed for daily measurements from each sensor (Fig. 3).
These findings suggested that the application of either correction factor would have been suitable for bias-correcting PurpleAir measured PM2.5 in northeastern NC. However, based on the comparison of correlation error metrics, for subsequent analyses of spatiotemporal variation in PM2.5 mass, we elected to utilize the SE-specific corrected PM2.5 values.
3.2. PM2.5 as a function of sensor locality
Sensors 1562, 5838, and 9875 reported the highest PM2.5 mass concentrations on average over the entire study (Fig. 3), but there was no apparent commonality between these sensors and nearby potential point-sources of pollution as identified by community scientists (Fig. 1 and 2). Generally, PM2.5 mass was highest at the sites further inland (Fig. 4), likely due to the dilution of PM2.5 at coastal sites from land-sea breezes, increasing coastal exchange at waterfront sites during diurnal cycles.71 Sensor 1562, located in the town of Murfreesboro, was one of two terrestrial control sites (with sensor 1358), with little suspected influence from CyanoHABs and aquatic aerosol. However, this sensor was located amid several other community-identified potential point sources of pollution including wood pellet plants, cotton fields, and confined animal feeding operations, suggesting possible confounding signals from these sources (Fig. 1). Sensor 5838, located in a residential area of downtown Edenton, was installed ∼1 km from the shoreline, and primary wind direction and speed indicated that aerosol formed in the northwestern basin of the Albemarle Sound likely influenced this site (Fig. 2). Similarly, sensor 9875 was located directly on the waterfront in Elizabeth City; however the primary wind direction did not flow across the river before reaching the sensor, suggesting that freshly emitted CyanoHAB aerosol did not consistently reach this sensor (Fig. 2).3.3. PM2.5 as a function of speciated CAPs
To isolate PM2.5 signals that could be more directly linked to CyanoHAB indicators, we assessed ambient and episodic variation in PM2.5 with AQS monitoring station CAPs as proxies for additional sources of PM2.5. We flagged episodic air pollution events during seven separate weeks when PM2.5 concentrations measured by two or more PurpleAir sensors exceeded the seasonal average by at least one standard deviation. All seven of these events occurred between April and November of 2023 (Fig. 4). In many cases, event-driven increases in PM2.5 were correlated with spikes in specific AQS CAPs (Fig. 4). Consequently, these weeks were removed from specific CyanoHAB and PM2.5 correlation analyses (as provided in Section 3.4).During air pollution events, HYSPLIT backward trajectory analyses provided insights into the general direction from which the pollution was potentially sourced, with particular attention given to the point sources as mapped in Fig. 1. In mid-to-late April 2023, a slight but statistically significant increase in PM2.5 was immediately preceded by a spike in CO levels. HYSPLIT analyses during this period suggested that PM2.5 was transported from the south-to-southeast, where a wildfire was impacting the Croatan National Forest ∼90 miles southeast of the Albemarle Sound (ESI Fig. S2†). Several weeks later, wildfire smoke again invaded the region and weekly averages for PM2.5 mass reached concentrations as high as 28 μg m−3 (Fig. 4). A significant spike in both CO and SO2 co-occurred with the PM2.5 spike in late June. HYSPLIT analyses suggest that the sharp increase in PM2.5 mass was likely driven by the extreme Canadian wildfires that burned 13 million acres in Quebec,72 transporting smoke across the Eastern seaboard of the US.
During one PM2.5 pollution event in late July of 2023, PO4 also exceeded its seasonal average (Fig. 4). Given that no wildfires were reported in the southeastern US during this period, we suspect that this signal was derived from an alternate source, possibly nearby agricultural operations. Both PM2.5 mass and O3 were elevated during the summer months as expected across the US due to higher temperature, sunlight, and increased emissions of biogenic VOCs.73,74
3.4. PM2.5 as a function of CyanoHABs
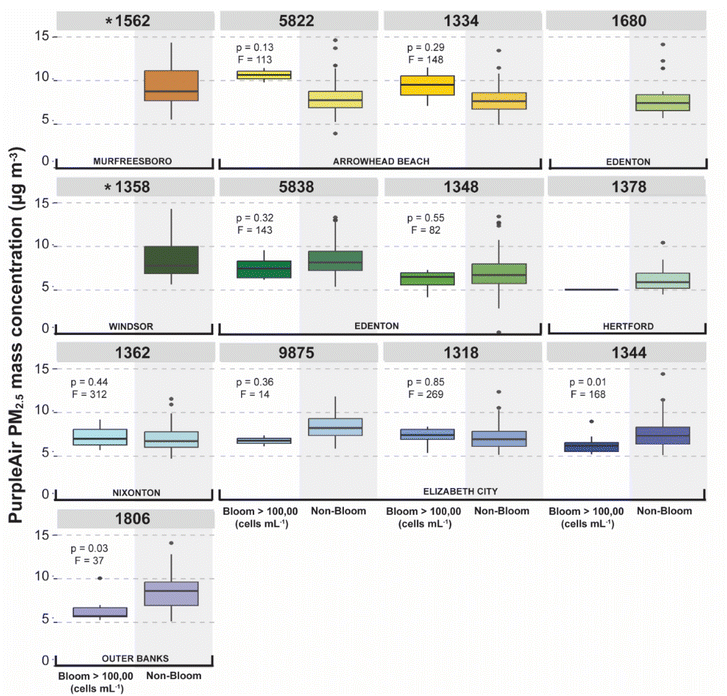
When examining PM2.5 only as a function of sensor proximity to CyanoHAB hotspots, there was no evidence to suggest that PM2.5 was elevated closer to the shoreline (ESI Fig. S3†). Apart from sensor 9875, PM2.5 mass generally increased with distance from the shoreline and increasing terrestrial influence (Fig. 2 and 3); this trend remained consistent despite seasonal and interannual variability.When both CyanoHAB indicators were treated as continuous variables, neither cell counts, nor bloom surface area were associated with changes in PM2.5 mass concentrations for any individual sensor or sensor grouping. However, during weeks when average CyanoHAB intensity exceeded the WHO's threshold for heightened health risk of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL68—we observed higher PM2.5 mass at sensors 5822 and 1334 compared to weeks when cell counts did not meet the WHO threshold (Fig. 5). However, this relationship was not statistically significant (p = 0.13, F = 113 and p = 0.29, F = 148, respectively). These two sensors were located on the northeast bank of the Chowan River near Arrowhead Beach, a known CyanoHAB hotspot and popular recreational area (Fig. 2). Satellite imagery identified a bloom occurring between July 2nd and July 16th, and HYSPLIT and wind direction analyses suggested that aerosol did blow onshore during this period. During the CyanoHAB, the median PM2.5 rose to 10.6 μg m−3 and 9.50 μg m−3 as measured by sensors 5822 and 1334, respectively. These values were between 2.0 and 3.0 μg m−3 higher than the average PM2.5 mass recorded at these locations when a high-risk bloom was not occurring (Fig. 5) as well as in the weeks immediately following the bloom.
000 cells per mL68—we observed higher PM2.5 mass at sensors 5822 and 1334 compared to weeks when cell counts did not meet the WHO threshold (Fig. 5). However, this relationship was not statistically significant (p = 0.13, F = 113 and p = 0.29, F = 148, respectively). These two sensors were located on the northeast bank of the Chowan River near Arrowhead Beach, a known CyanoHAB hotspot and popular recreational area (Fig. 2). Satellite imagery identified a bloom occurring between July 2nd and July 16th, and HYSPLIT and wind direction analyses suggested that aerosol did blow onshore during this period. During the CyanoHAB, the median PM2.5 rose to 10.6 μg m−3 and 9.50 μg m−3 as measured by sensors 5822 and 1334, respectively. These values were between 2.0 and 3.0 μg m−3 higher than the average PM2.5 mass recorded at these locations when a high-risk bloom was not occurring (Fig. 5) as well as in the weeks immediately following the bloom.
It is important to note that multiple other sensor locations also experienced CyanoHAB events with cell counts that exceeded the WHO threshold during our study period, but there were no measurable increases in PM2.5 in the immediate airshed of those blooms (Fig. 5). For example, sensor 1362 had the highest number of weeks when a nearby CyanoHAB exceeded the WHO threshold, but this site ultimately had the lowest average PM2.5 mass average for any sensor location in the entire study (Fig. 5).
3.5. PM2.5 as a function of interactive environmental conditions
Using GAMs to examine PM2.5 as a function of multiple environmental factors revealed that ambient changes in CAP concentrations explained the majority (up to 88%) of the temporal variation in PM2.5 mass. Out of all the AQS pollutants examined, SO2 was the most predictive of PM2.5 mass when considered individually, but the inclusion of more CAPs resulted in a model with the best fit. In this model, SO2 and CO were treated with an interactive term due to their co-emissions during combustion (anthropogenic activity and wildfire).67,75 Likewise, O3 and NOx were first tried as covariates due to their cyclic formation from VOCs,76,77 but we found that treating these CAPs separately led to a better fitting model, suggesting that O3 and NOx have separate (or mixed) precursors/emission sources in the case of NOx in this region. The inclusion of NOx and PO4 in the equation improved the fit but notably increased model complexity as indicated by the AIC and BIC (ESI Table S2†), suggesting that combustion aerosol and an atmospheric source of O3 most significantly contributed to PM2.5 mass in the Albemarle airshed during our study period.When considered independently of other environmental conditions, CyanoHAB indicators were not strong predictors of PM2.5 mass using GAMs. Treating CyanoHAB intensity and spatial extent individually, additively, and interactively within the model did not greatly change the model fit (ESI Table S4†). Furthermore, changes to the pixel-grid area (0.6, 1.2, and 2.4 km) over which CyanoHAB data were extracted did also not improve our ability to predict PM2.5 using the GAM.
Meteorological conditions explained up to 28% of variation in PM2.5 mass when treated independently from CAPs and CyanoHAB variables. Specifically, the model was best fitted only when temperature and humidity were treated with an interactive term. The inclusion of wind speed also improved the fit of the model, which suggests that wind-driven aerosolization of dust and river/sea spray is an important source of PM2.5 in this region. Interestingly, wind direction, which could have suggested directional point sources, did not have a significant impact on the model fit.
Despite variation in PM2.5 being mainly attributed to CAP concentrations, considering the additive effects of CAPs, weather, and CyanoHAB conditions ultimately resulted in the model with the best fit, explaining up to 98% of the variation in PM2.5 (Table 1). Specifically, solar radiation, temperature × humidity, O3, PO4, NOx, CO × SO2, and CyanoHAB × wind speed when modeled together best predicted changes in PM2.5 mass in the Albemarle airshed (with × indicating an interactive or covariate term; Table 1).
3.6. Discussion
By enhancing collaboration between local communities and scientific researchers, community science initiatives not only fill critical data gaps but also empower residents with the knowledge and tools to improve their own environmental health outcomes. Working with community scientist volunteers from nonprofit, healthcare, local government, and racial justice organizations, we sought to address and highlight this systemic issue in northeastern NC. In lieu of local, regulatory-grade air quality monitoring, we established a network of low-cost PurpleAir air quality sensors throughout the basin of the Chowan River-Albemarle Sound estuary for use by community members and researchers alike.During our study period (May 2022 to December 2023), and following the application of a correction factor, we determined that PM2.5 measured by our PurpleAir sensor network only deviated from the nearest regulatory-standard PM2.5 readings by 0.7 μg m−3 on average. Although no established criteria for sensor performance currently exist to qualify low-cost sensor generated data for technical analyses and regulatory decision-making, the US EPA recommends that such data correlate with nearest AQS readings within a RMSE of ≤7 μg m—3; our data met this criterion.43 While the utility of community-based data for scientific reporting, policy development, and/or formal risk assessment is not universally agreed upon,42 we argue that the <1 μg m−3 difference observed between our sensors and the nearest AQS sensors suggests that the bias-corrected PurpleAir PM2.5 data generated in this region can be used to inform future environmental health research and/or policymaking with relatively high accuracy and precision.
Furthermore, these <1 μg m−3 differences could be explained by geographic differences between our study region and the nearest AQS stations, which were ∼50 miles from our study region. As demonstrated by Mathieu-Campbell et al., 2024,54 the correlation of PM2.5 measurements from PurpleAir with AQS measurements decreases as a function of increasing distance between sensor locations. Our PurpleAir network was concentrated in a rural, coastal area with unique agricultural and aquatic aerosol sources. While specific correction factors for PurpleAir datasets affected by high humidity54 and wildfire smoke46 biases have been published, the systematic differences between PurpleAir and regulatory-standard measurements of PM2.5 mass in rural and/or coastal sites with agricultural and/or sea spray aerosol sources, remain less explored.
GAM findings suggest that complex interactions between environmental factors promote PM2.5 formation and atmospheric fate across the Albemarle Sound. PurpleAir sensors reveal local PM2.5 mass but do not provide compositional information, preventing specific source-apportionment. To address this, we retrieved CAP concentrations from the nearest AQS stations to use as proxies for well-established sources of aerosol.62,78,79 Proxies were assigned by referencing known relationships between emission sources and CAPs, but definitive tracing could not be achieved given that each air pollutant is associated with numerous emission sources.62 This study ultimately aimed to empower local communities to participate in ambient PM2.5 monitoring, with the high spatial and temporal resolution enabled by low-cost sensors being favored over compositional analyses.
Previous work by Plaas et al., 2022 (ref. 27) determined that PM2.5 mass increased by 3.6 μg m−3 during a three week long CyanoHAB in the Chowan River in summer 2020. This was based on in situ water sampling and nephelometer PM2.5 measurements from two waterfront locations. Herein, we examined satellite-derived indicators of CyanoHAB cell counts and spatial extent in association with PM2.5 mass, providing higher spatiotemporal resolution of both CyanoHAB and PM2.5 measurements than reported in previous work. However, we ultimately found that satellite-based indicators of CyanoHABs could not be associated with episodic increases in PM2.5 mass. At one site (Arrowhead Beach), when CyanoHAB cell counts met the WHO high risk bloom threshold of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL, PM2.5 mass was elevated. However, this difference in PM2.5 as a function of WHO bloom conditions was not observed for any other site. We speculate that this signal was distinguished at this site due to the (1) primary wind direction favoring onshore transport and (2) the long residence time (∼15 days) of the mid-upper Chowan River, leading to a persistent source of CyanoHAB aerosol. A combination of these conditions also likely concentrated the bloom along the coastline. Therefore, we conclude that regions with a fetch length that supports wave-driven aerosolization, and where wind and currents push blooms toward/onshore are the ones where CyanoHAB inhalation risks are the greatest.
000 cells per mL, PM2.5 mass was elevated. However, this difference in PM2.5 as a function of WHO bloom conditions was not observed for any other site. We speculate that this signal was distinguished at this site due to the (1) primary wind direction favoring onshore transport and (2) the long residence time (∼15 days) of the mid-upper Chowan River, leading to a persistent source of CyanoHAB aerosol. A combination of these conditions also likely concentrated the bloom along the coastline. Therefore, we conclude that regions with a fetch length that supports wave-driven aerosolization, and where wind and currents push blooms toward/onshore are the ones where CyanoHAB inhalation risks are the greatest.
Numerous studies have not found any apparent association between waterborne and airborne cyanotoxin concentrations in the field, suggesting that water conditions (i.e., ecological and physicochemical parameters) are not strong predictors of CyanoHAB aerosol formation.31,33 However, when we included a variable representing CyanoHAB–wind speed interactions, our multivariate regression model showed an improved fit, agreeing with previous findings that wind driven spray aerosol formation modulates CyanoHAB aerosol emissions.33,80
The area over which satellite-derived CyanoHAB data were composited did not significantly alter associated outcomes between CyanoHABs and PM2.5 mass. Using a kinetic decay model, Zorbas et al., 2023 estimated that up to 25% of microcystin in aerosol is degraded within 0.92 km of its point of emission during periods of high sunlight.52 Accordingly, we anticipated a decay in signal with increasing distance from the shoreline. Conversely, our observations indicated that average PM2.5 mass measured at our sensor locations increased with distance from the nearest shoreline. While microcystin suspended in spray aerosol may undergo chemically alteration over time and space in the atmosphere, particles with distinct physicochemical properties may persist over these distances and still pose an inhalation risk with unknown toxicological effects.
In this study, we did not explore the biochemical processes influencing CyanoHAB aerosol composition. Instead, we examined associations between indicators of CyanoHAB biomass and PM2.5, a widely monitored CAP, at a previously untested spatial and temporal resolution. However, we found no strong correlation between satellite-derived measures of CyanoHABs and episodic increases to PM2.5. A recent epidemiological study also reported no significant association between CyanoHABs and hospital visits pertaining to respiratory outcomes including asthma, wheezing, and allergic reactions.81 Thus, our findings, when considered with such perspectives, suggest that future CyanoHAB aerosol work should continue to explore the direct health effects of exposure to CyanoHAB biochemical tracers (e.g., cyanotoxins,33,35,82 lipopolysaccharides,34 and secondary aerosol from gas-phase CyanoHAB emissions51), as these studies may be more informative for inhalation risk assessments. Additional research examining chemical tracers associated with community-identified sources of air pollution, including CyanoHABs, is needed to better inform toxicological risk assessments for the Albemarle Sound region of northeastern NC.
4. Conclusion
To our knowledge, this community-led study provides the first spatiotemporal analysis of the drivers of PM2.5 mass in the airshed of the Albemarle Sound estuary, with a particular focus on CyanoHABs. For the first time, we examined satellite-derived CyanoHAB cell counts and spatial extent in association with localized PM2.5 mass. Neither was directly associated with episodic PM2.5 pollution events, suggesting that despite high spatiotemporal resolution and wide accessibility, satellite data products and low-cost sensor PurpleAir PM2.5 measurements are not likely to be useful indicators of aerosol emission rates from CyanoHABs when examined alone. Consideration of additional weather conditions, especially wind speed, is needed to accurately assess relative respiratory risks for those living near or recreating near CyanoHABs. We recommend that future research examining aerosol emissions from CyanoHABs prioritize assessing emission of and exposure to specific CyanoHAB biochemical tracers over correlations between blooms and PM2.5.Data availability
The programming scripts written to perform data retrieval, cleaning, statistical analyses, and preliminary visualizations are found on the first author's Github repositories dedicated to the project (Python: https://github.com/haleyplaas/CCRG, R: https://github.com/haleyplaas/CCRG_PurpleAir). Python libraries and R packages utilized are reported within each repository. ChatGPT v3.5 and v4.0 were used to iteratively write and correct scripts for both Python and R.83 Python code was primarily written and processed using Microsoft VScode v1.92.0 and R code was written using RStudio v2023.12.0. Cleaned data files are available in the Github repositories or are linked to in their README files. All data and programming scripts are labeled with Creative Commons Attribution 4.0 labeled for cited reuse.Author contributions
Conceptualization: H. E. P. and C. K., formal analysis: H. E. P., M. E. M. C., and J. R. B., investigation: H. E. P., C. K., R. C., and N. R. G., resources: H. W. P. and D. S. H., writing – original draft: H. E. P., writing – review & editing: H. E. P., C. K., R. C., N. R. G., A. H. S., L. L. S., M. E. M. C., J. R. B., H. W. P., and D. S. H., supervision: H. W. P. and D. S. H., visualization: H. E. P., project administration: H. E. P. and H. W. P., funding acquisition: H. E. P., C. K., H. W. P., and D. S. H.Conflicts of interest
The authors declare that they have no conflicts of interest related to this work to disclose.Acknowledgements
We thank the community volunteers who hosted and are hosting a PurpleAir air quality sensor: Cathy Woody, Anne Radke, Bo Dame, Sandra Ferebee, Colleen Nicholas, Steve Karl, Lynn Nash, Reba Wynns, Tom Stroud, Jon Morgan, Tommy Harrell, Missy and Chuck Shumer, Thomas Birckhead, Cheryl Messinger Lois Thompson, Terri Kirby Hathaway, Richie Harding, the towns of Windsor and Edenton, Albemarle Regional Health Services, Museum of the Albemarle, Northampton First, and partners with the Edenton Racial Reconciliation Group. This work was supported by the Community Collaborative Research Grant Program 21-22 (#2018-2951-05 22-CCRG-01), funded by the North Carolina Sea Grant, the NC Water Resources Research Institute, and the William R. Kenan Jr. Institute for Engineering, Technology, and Science. Additional support was provided by the National Science Foundation Graduate Research Program Fellowship awarded to H. E. P. (#2020295001). D. S. H. gratefully acknowledges support from NASA (Grant #80NSSC24K0446). J. R. B. gratefully acknowledges support from NIEHS (R21ES036500, P42ES013648, and P30ES025128).References
- EPA US, Final Rule to Strengthen the National Air Quality Health Standard for Particulate Matter Fact Sheet, 2024 Search PubMed.
- Reconsideration of the National Ambient Air Quality Standards for Particulate Matter, available from: https://www.federalregister.gov/d/2024-02637 Search PubMed.
- WHO, WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide, 2021, pp. 1–360 Search PubMed.
- A. Jbaily, X. Zhou, J. Liu, T. H. Lee, L. Kamareddine and S. Verguet, et al., Air pollution exposure disparities across US population and income groups, Nature, 2022, 601(7892), 228–233 Search PubMed , available from: https://www.nature.com/articles/s41586-021-04190-y.
- A. W. Correia, C. Arden Pope, D. W. Dockery, Y. Wang, M. Ezzati and F. Dominici, Effect of air pollution control on life expectancy in the United States: an analysis of 545 U.S. counties for the period from 2000 to 2007, Epidemiology, 2013, 24(1), 23–31 Search PubMed , available from: https://pmc/articles/PMC3521092/.
- A. Tomczak, A. B. Miller, S. A. Weichenthal, T. To, C. Wall and A. van Donkelaar, et al., Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study, Int. J. Cancer, 2016, 139(9), 1958–1966 Search PubMed , available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.30255.
- L. Gharibvand, D. Shavlik, M. Ghamsary, W. L. Beeson, S. Soret and R. Knutsen, et al., The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study, Environ. Health Perspect., 2017, 125(3), 378–384, DOI:10.1289/EHP124.
- F. Gilliland, E. Avol, P. Kinney, M. Jerrett, T. Dvonch and F. Lurmann, et al., Air Pollution Exposure Assessment for Epidemiologic Studies of Pregnant Women and Children: Lessons Learned from the Centers for Children's Environmental Health and Disease Prevention Research, Environ. Health Perspect., 2005, 113(10), 1447–1454 Search PubMed , available from: https://ehp.niehs.nih.gov/doi/10.1289/ehp.7673.
- C. W. Schmidt, Summertime Blues: Childhood Lead Exposure Peaks in Warm Months, Environ. Health Perspect., 2000, 108(2), A82 Search PubMed , available from: https://www.jstor.org/stable/3454501?origin=crossref.
- J. Seagrave, J. D. McDonald, E. Bedrick, E. S. Edgerton, A. P. Gigliotti and J. J. Jansen, et al., Lung Toxicity of Ambient Particulate Matter from Southeastern U.S. Sites with Different Contributing Sources: Relationships between Composition and Effects, Environ. Health Perspect., 2006, 114(9), 1387–1393 Search PubMed , available from: https://ehp.niehs.nih.gov/doi/10.1289/ehp.9234.
- B. Cheng and L. Wang-Li, Spatial and temporal variations of PM2.5 in North Carolina, Aerosol Air Qual. Res., 2019, 19(4), 698–710 Search PubMed , available from: https://aaqr.org/articles/aaqr-18-03-oa-0111.
- A. Mamane, I. Baldi, J. F. Tessier, C. Raherison and G. Bouvier, Occupational exposure to pesticides and respiratory health, Eur. Respir. Rev., 2015, 24(136), 306–319, DOI:10.1183/16000617.00006014.
- K. E. Wyer, D. B. Kelleghan, V. Blanes-Vidal, G. Schauberger and T. P. Curran, Ammonia emissions from agriculture and their contribution to fine particulate matter: a review of implications for human health, J. Environ. Manage., 2022, 323, 116285, DOI:10.1016/j.jenvman.2022.116285.
- M. A. Pendergraft, P. Belda-Ferre, D. Petras, C. K. Morris, B. A. Mitts and A. T. Aron, et al., Bacterial and Chemical Evidence of Coastal Water Pollution from the Tijuana River in Sea Spray Aerosol, Environ. Sci. Technol., 2023, 57(10), 4071–4081 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acs.est.2c02312.
- E. Van Acker, M. De Rijcke, Z. Liu, J. Asselman, K. A. C. De Schamphelaere and L. Vanhaecke, et al., Sea Spray Aerosols Contain the Major Component of Human Lung Surfactant, Environ. Sci. Technol., 2021, 55(23), 15989–16000 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acs.est.1c04075.
- J. M. Michaud, L. R. Thompson, D. Kaul, J. L. Espinoza, R. A. Richter and Z. Z. Xu, et al., Taxon-specific aerosolization of bacteria and viruses in an experimental ocean-atmosphere mesocosm, Nat. Commun., 2018, 9(1) DOI:10.1038/s41467-018-04409-z.
- Fish Kill & Algal Bloom Report Dashboard, North Carolina Department of Environmental Quality, Division of Water Resources, available from: https://www.arcgis.com/apps/dashboards/7543be4dc8194e6e9c215079d976e716 Search PubMed.
- N. V. Mohatt and D. Mohatt, Rural Prejudice-Urban Bias: The Stories and Structures That Oppress Rural Communities, in Prejudice, Stigma, Privilege, and Oppression, Springer, Cham, 2020, pp. 413–25, available from: https://link.springer.com/chapter/10.1007/978-3-030-35517-3_23 Search PubMed.
- J. Probst, J. M. Eberth and E. Crouch, Structural urbanism contributes to poorer health outcomes for rural america, Health Aff., 2019, 38(12), 1976–1984 Search PubMed.
- D. M. Purifoy, North Carolina [Un]incorporated: Place, Race, and Local Environmental Inequity, Am. Behav. Sci., 2021, 65(8), 1072–1103 Search PubMed.
- M. Lee, P. Koutrakis, B. Coull, I. Kloog and J. Schwartz, Acute effect of fine particulate matter on mortality in three Southeastern states from 2007-2011, J. Exposure Sci. Environ. Epidemiol., 2016, 26(2), 173–179 Search PubMed , available from: https://www.nature.com/jes.
- N. J. Craig and E. J. Kuenzler, Land use, nutrient yield, and eutrophication in the Chowan River Basin Dep. Environ. Sci. Eng., 1983, vol. 205 Search PubMed , available from: https://catalog.lib.unc.edu/catalog/UNCb1931981.
- Albemarle Resource Conservation and Development Council Inc., Albemarle RC&D 2020-2021 Annual Report, 2021 Search PubMed.
- H. Dieu, G. D. Kearney, H. Bian, K. Jones and A. Mohan, Asthma-Related Emergency Department Visits in North Carolina, 2010–2014, N. C. Med. J., 2018, 79(2), 81–87 Search PubMed , available from: https://www.ncmedicaljournal.com/content/79/2/81.
- T. Mulrooney, C. L. Liang, L. A. Kurkalova, C. McGinn and C. Okoli, Quantitatively defining and mapping rural: a case study of North Carolina, J. Rural Stud., 2023, 97, 47–56, DOI:10.1016/j.jrurstud.2022.11.011.
- M. Anderson, M. Valera and A. Schnetzer, Co-occurrence of freshwater and marine phycotoxins: A record of microcystins and domoic acid in Bogue Sound, North Carolina (2015 to 2020), Harmful Algae, 2023, 125, 102412 Search PubMed.
- H. E. Plaas, R. W. Paerl, K. Baumann, C. Karl, K. J. Popendorf and M. A. Barnard, et al., Harmful cyanobacterial aerosolization dynamics in the airshed of a eutrophic estuary, Sci. Total Environ., 2022, 852, 158383 Search PubMed.
- F. M. Buratti, M. Manganelli, S. Vichi, M. Stefanelli, S. Scardala and E. Testai, et al., Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation, Arch. Toxicol., 2017, 91(3), 1049–1130 Search PubMed , available from: https://link.springer.com/10.1007/s00204-016-1913-6.
- M. Burford, C. Carey, D. P. Hamilton, J. Huisman, H. Paerl and S. Wood, et al., Perspective: advancing the research agenda for improving understanding of cyanobacteria in a future of global change, Harmful Algae, 2020, 91, 101601 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S1568988319300514.
- L. C. Backer, S. V. McNeel, T. Barber, B. Kirkpatrick, C. Williams and M. Irvin, et al., Recreational exposure to microcystins during algal blooms in two California lakes, Toxicon, 2010, 55(5), 909–921 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S0041010109003481.
- H. E. Plaas and H. W. Paerl, Toxic Cyanobacteria: A Growing Threat to Water and Air Quality, Environ. Sci. Technol., 2021, 55(1), 44–64 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acs.est.0c06653.
- N. E. Olson, M. E. Cooke, J. H. Shi, J. A. Birbeck, J. A. Westrick and A. P. Ault, Harmful Algal Bloom Toxins in Aerosol Generated from Inland Lake Water, Environ. Sci. Technol., 2020, 54(8), 4769–4780 Search PubMed , available from: https://pubs.acs.org/doi/abs/10.1021/acs.est.9b07727.
- J. H. Shi, N. E. Olson, J. A. Birbeck, J. Pan, N. J. Peraino and A. L. Holen, et al., Aerosolized Cyanobacterial Harmful Algal Bloom Toxins: Microcystin Congeners Quantified in the Atmosphere, Environ. Sci. Technol., 2023, 57(51), 21801–21814 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acs.est.3c03297.
- P. Labohá, E. Sychrová, O. Brózman, I. Sovadinová, L. Bláhová and R. Prokeš, et al., Cyanobacteria, cyanotoxins and lipopolysaccharides in aerosols from inland freshwater bodies and their effects on human bronchial cells, Environ. Toxicol. Pharmacol., 2023, 98, 104073 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S1382668923000145.
- J. Sutherland, R. Turcotte, E. Molden, V. Moriarty, M. Kelly and M. Aubel, et al., The detection of airborne anatoxin-a (ATX) on glass fiber filters during a harmful algal bloom, Lake Reservoir Manage., 2021, 1–9 Search PubMed , available from: https://www.tandfonline.com/action/journalInformation?journalCode=ulrm20.
- D. Fraisl, G. Hager, B. Bedessem, M. Gold, P. Y. Hsing and F. Danielsen, et al., Citizen science in environmental and ecological sciences, Nat. Rev. Methods Primers, 2022, 2(1), 64 Search PubMed , available from: https://www.nature.com/articles/s43586-022-00144-4.
- S. Mahajan, P. Kumar, J. A. Pinto, A. Riccetti, K. Schaaf and G. Camprodon, et al., A citizen science approach for enhancing public understanding of air pollution, Sustain. Cities Soc., 2020, 52, 101800, DOI:10.1016/j.scs.2019.101800.
- J. E. Puma, E. S. Belansky, R. Garcia, S. Scarbro, D. Williford and J. A. Marshall, A Community-Engaged Approach to Collecting Rural Health Surveillance Data, J. Rural Health, 2017, 33(3), 257–265 Search PubMed , available from: https://onlinelibrary.wiley.com/doi/10.1111/jrh.12185.
- G. Raheja, L. Harper, A. Hoffman, Y. Gorby, L. Freese and B. O'Leary, et al., Community-based participatory research for low-cost air pollution monitoring in the wake of unconventional oil and gas development in the Ohio River Valley: empowering impacted residents through community science, Environ. Res. Lett., 2022, 17(6), 065006 Search PubMed , available from: https://iopscience.iop.org/article/10.1088/1748-9326/ac6ad6.
- A. Kaufman, R. Williams, T. Barzyk, M. Greenberg, M. O'Shea and P. Sheridan, et al., A Citizen Science and Government Collaboration: Developing Tools to Facilitate Community Air Monitoring, Environ. Justice, 2017, 10(2), 51–61 Search PubMed , available from: https://www.liebertpub.com/doi/10.1089/env.2016.0044.
- M. M. Haklay, S. Mazumdar and J. Wardlaw, Citizen Science for Observing and Understanding the Earth, Earth Obs. Open Sci. Innov., 2018, 69–88 Search PubMed , available from: https://link.springer.com/chapter/10.1007/978-3-319-65633-5_4.
- R. Williams, R. Duvall, V. Kilaru, G. Hagler, L. Hassinger and K. Benedict, et al., Deliberating performance targets workshop: potential paths for emerging PM2.5 and O3 air sensor progress, Atmos. Environ.: X, 2019, 2, 100031, DOI:10.1016/j.aeaoa.2019.100031.
- R. Duvall, A. Clements, G. Hagler, A. Kamal, V. Kilaru, L. Goodman, S. Frederick, K. Johnson Barkjohn, I. VonWald, D. Greene and T. Dye, Performance Testing Protocols, Metrics, and Target Values for Fine Particulate Matter Air Sensors: Use in Ambient, Outdoor, Fixed Site, Non-regulatory Supplemental and Informational Monitoring Applications, Washington, DC, 2021 Search PubMed.
- C. Malings, R. Tanzer, A. Hauryliuk, P. K. Saha, A. L. Robinson and A. A. Presto, et al., Fine particle mass monitoring with low-cost sensors: corrections and long-term performance evaluation, Aerosol Sci. Technol., 2020, 54(2), 160–174, DOI:10.1080/02786826.2019.1623863.
- K. K. Barkjohn, B. Gantt and A. L. Clements, Development and application of a United States-wide correction for PM2.5 data collected with the PurpleAir sensor, Atmos. Meas. Tech., 2021, 14(6), 4617–4637 Search PubMed , available from: https://amt.copernicus.org/articles/14/4617/2021/.
- K. K. Barkjohn, A. L. Holder, S. G. Frederick and A. L. Clements, Correction and Accuracy of PurpleAir PM2.5 Measurements for Extreme Wildfire Smoke, Sensors, 2022, 22(24), 9669 Search PubMed , available from: https://www.mdpi.com/1424-8220/22/24/9669.
- J. Tryner, C. L'Orange, J. Mehaffy, D. Miller-Lionberg, J. C. Hofstetter and A. Wilson, et al., Laboratory evaluation of low-cost PurpleAir PM monitors and in-field correction using co-located portable filter samplers, Atmos. Environ., 2020, 220, 117067 Search PubMed.
- R. C. Thakur, L. Dada, L. J. Beck, L. L. J. Quéléver, T. Chan and M. Marbouti, et al., An evaluation of new particle formation events in Helsinki during a Baltic Sea cyanobacterial summer bloom, Atmos. Chem. Phys., 2022, 22(9), 6365–6391 Search PubMed , available from: https://acp.copernicus.org/articles/22/6365/2022/.
- L. Bilyeu, B. Bloomfield, R. Hanlon, J. González-Rocha, S. J. Jacquemin and A. P. Ault, et al., Drone-based particle monitoring above two harmful algal blooms (HABs) in the USA, Environ. Sci.: Atmos., 2022, 2(6), 1351–1363 Search PubMed , available from: https://xlink.rsc.org/?DOI=D2EA00055E.
- L. Bilyeu, J. González-Rocha, R. Hanlon, N. AlAmiri, H. Foroutan and K. Alading, et al., Monitoring wind and particle concentrations near freshwater and marine harmful algal blooms (HABs), Environ. Sci.: Adv., 2025, 4, 279–291, 10.1039/d4va00172a.
- H. E. Plaas, J. Yan, C. Christensen, S. Chang, C. Cortez and S. Fern, et al., Secondary Organic Aerosol Formation from Cyanobacterial-Derived Volatile Organic Compounds, ACS Earth Space Chem., 2023, 7(9), 1798–1813 Search PubMed.
- V. Zorbas, M. Jang, B. Emam and J. Choi, Modeling of the Atmospheric Process of Cyanobacterial Toxins in Algal Aerosol, ACS Earth Space Chem., 2023, 7(5), 1141–1150 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acsearthspacechem.3c00050.
- M. Jang, D. E. Berthold, Z. Yu, C. Silva-Sanchez, H. D. Laughinghouse Iv and N. D. Denslow, et al., Atmospheric Progression of Microcystin-LR from Cyanobacterial Aerosols, Environ. Sci. Technol. Lett., 2020, 7(10), 740–745 Search PubMed , available from: https://pubs.acs.org/doi/abs/10.1021/acs.estlett.0c00464.
- M. E. Mathieu-Campbell, C. Guo, A. P. Grieshop and J. Richmond-Bryant, Calibration of PurpleAir Low-Cost Particulate Matter Sensors: Model Development for Air Quality under High Relative Humidity Conditions, Atmos. Meas. Tech., 2024, 17(22), 6735–6749 Search PubMed.
- T. T. Wynne, R. P. Stumpf, M. C. Tomlinson, R. A. Warner, P. A. Tester and J. Dyble, et al., Relating spectral shape to cyanobacterial blooms in the Laurentian Great Lakes, Int. J. Remote Sens., 2008, 29(12), 3665–3672 Search PubMed , available from: https://www.tandfonline.com/action/journalInformation?journalCode=tres20.
- M. W. Matthews, S. Bernard and L. Robertson, An algorithm for detecting trophic status (chlorophyll-a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters, Remote Sens. Environ., 2012, 124, 637–652, DOI:10.1016/j.rse.2012.05.032.
- R. S. Lunetta, B. A. Schaeffer, R. P. Stumpf, D. Keith, S. A. Jacobs and M. S. Murphy, Evaluation of cyanobacteria cell count detection derived from MERIS imagery across the eastern USA, Remote Sens. Environ., 2015, 157, 24–34, DOI:10.1016/j.rse.2014.06.008.
- S. Mishra, R. P. Stumpf, B. A. Schaeffer, P. J. Werdell, K. A. Loftin and A. Meredith, Measurement of Cyanobacterial Bloom Magnitude using Satellite Remote Sensing, Sci. Rep., 2019, 9(1), 1–17 Search PubMed , available from: https://www.nature.com/articles/s41598-019-54453-y.
- B. W. Ibelings, L. R. Mur and A. E. Walsby, Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes, J. Plankton Res., 1991, 13(2), 419–436 Search PubMed , available from: https://academic.oup.com/plankt/article/13/2/419/1500281.
- M. M. Coffer, B. A. Schaeffer, K. Foreman, A. Porteous, K. A. Loftin and R. P. Stumpf, et al., Assessing cyanobacterial frequency and abundance at surface waters near drinking water intakes across the United States, Water Res., 2021, 201, 117377 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S0043135421005753.
- J. M. Clark, B. A. Schaeffer, J. A. Darling, E. A. Urquhart, J. M. Johnston and A. R. Ignatius, et al., Satellite monitoring of cyanobacterial harmful algal bloom frequency in recreational waters and drinking water sources, Ecol. Indic., 2017, 84–95 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S1470160X17302194.
- H. R. Friedli, E. Atlas, V. R. Stroud, L. Giovanni, T. Campos and L. F. Radke, Volatile organic trace gases emitted from North American wildfires, Global Biogeochem. Cycles, 2001, 15(2), 435–452 Search PubMed.
- G. M. Hidy and C. L. Blanchard, Precursor reductions and ground-level ozone in the Continental United States, J. Air Waste Manage. Assoc., 2015, 65(10), 1261–1282 Search PubMed , available from: https://www.tandfonline.com/action/journalInformation?journalCode=uawm20.
- T. Butler, A. Lupascu and A. Nalam, Attribution of ground-level ozone to anthropogenic and natural sources of nitrogen oxides and reactive carbon in a global chemical transport model, Atmos. Chem. Phys., 2020, 20(17), 10707–10731, DOI:10.5194/acp-20-10707-2020.
- R. Delmas, D. Serça and C. Jambert, Global inventory of NOx sources, Nutr. Cycling Agroecosyst., 1997, 48(1–2), 51–60 Search PubMed.
- M. O. Andreae and P. Merlet, Emission of trace gases and aerosols from biomass burning, Global Biogeochem. Cycles, 2001, 15(4), 955–966 Search PubMed , available from: https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2000GB001382.
- S. J. Smith, J. Van Aardenne, Z. Klimont, R. J. Andres, A. Volke and S. Delgado Arias, Anthropogenic sulfur dioxide emissions: 1850-2005, Atmos. Chem. Phys., 2011, 11(3), 1101–1116 Search PubMed , available from: https://www.atmos-chem-phys.net/11/1101/2011/.
- WHO, Toxic Cyanobacteria in Water, Toxic Cyanobacteria in Water, 1999 Search PubMed.
- S. N. Wood, Generalized Additive Models, Chapman and Hall/CRC, 2017, available from: https://www.taylorfrancis.com/books/9781498728348 Search PubMed.
- R. A. Rigby and D. M. Stasinopoulos, Generalized Additive Models for Location, Scale and Shape, J. R. Stat. Soc. Ser. C Appl. Stat., 2005, 54(3), 507–554 Search PubMed , available from: https://academic.oup.com/jrsssc/article/54/3/507/7113027.
- H. H. Tsai, C. S. Yuan, C. H. Hung, C. Lin and Y. C. Lin, Influence of Sea-Land Breezes on the Tempospatial Distribution of Atmospheric Aerosols over Coastal Region, J. Air Waste Manage. Assoc., 2011, 61(4), 358–376 Search PubMed , available from: https://www.tandfonline.com/doi/full/10.3155/1047-3289.61.4.358.
- P. Jain, Q. E. Barber, S. W. Taylor, E. Whitman, D. C. Acuna and Y. Boulanger, et al., Drivers and Impacts of the Record-Breaking 2023 Wildfire Season in Canada, Nat. Commun., 2024, 15, 6764, DOI:10.1038/s41467-024-51154-7.
- L. Shen and L. J. Mickley, Seasonal prediction of US summertime ozone using statistical analysis of large scale climate patterns, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(10), 2491–2496 Search PubMed , available from: https://pnas.org/doi/full/10.1073/pnas.1610708114.
- K. Sindelarova, J. Markova, D. Simpson, P. Huszar, J. Karlicky and S. Darras, et al., High-resolution biogenic global emission inventory for the time period 2000-2019 for air quality modelling, Earth Syst. Sci. Data, 2022, 14(1), 251–270 Search PubMed.
- B. Friedman, P. Brophy, W. H. Brune and D. K. Farmer, Anthropogenic Sulfur Perturbations on Biogenic Oxidation: SO2 Additions Impact Gas-Phase OH Oxidation Products of α- and β-Pinene, Environ. Sci. Technol., 2016, 50(3), 1269–1279 Search PubMed , available from: https://pubs.acs.org/doi/10.1021/acs.est.5b05010.
- S. N. Pandis, R. A. Harley, G. R. Cass and J. H. Seinfeld, Secondary organic aerosol formation and transport, Atmos. Environ. A, Gen. Top., 1992, 26(13), 2269–2282 Search PubMed.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics: From Air Pollution to Climate Change Wiley, 2016 Search PubMed , available from: https://books.google.com/books?id=n_RmCgAAQBAJ.
- J. L. Jimenez, M. R. Canagaratna, N. M. Donahue, A. S. H. Prevot, Q. Zhang and J. H. Kroll, et al., Evolution of organic aerosols in the atmosphere, Science, 2009, 326(5959), 1525–1529 Search PubMed , available from: https://www.science.org/doi/abs/10.1126/science.1180353.
- J. H. Kroll and J. H. Seinfeld, Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere, Atmos. Environ., 2008, 42, 3593–3624 Search PubMed.
- M. Žilka, M. Tropeková, E. Zahradníková, Ľ. Kováčik and J. Ščevková, Temporal variation in the spectrum and concentration of airborne microalgae and cyanobacteria in the urban environments of inland temperate climate, Environ. Sci. Pollut. Res., 2023, 30(43), 97616–97628 Search PubMed.
- J. F. Murray, A. M. Lavery, B. A. Schaeffer, B. N. Seegers, A. F. Pennington and E. D. Hilborn, et al., Assessing the relationship between cyanobacterial blooms and respiratory-related hospital visits: Green bay, Wisconsin 2017–2019, Int. J. Hyg. Environ. Health, 2024, 255, 114272 Search PubMed , available from: https://linkinghub.elsevier.com/retrieve/pii/S1438463923001633.
- Y. S. Cheng, Characterization of Aerosols Containing Microcystin, Mar. Drugs, 2007, 5(4), 136–150 Search PubMed , available from: https://www.mdpi.org/marinedrugs/list07.htm#10.3390_md20070010.
- OpenAI, ChatGPT, OpenAI, 2023 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures are provided, including visualization of satellite-data retrieval, HYSPLIT model results, and regression analyses. Tables containing descriptive data for each air quality sensor and multivariate generalized additive model (GAM) details are also provided. See DOI: https://doi.org/10.1039/d5ea00020c |
| ‡ Current institution: Center for Climate Systems Research, Columbia University, NASA Goddard Institute for Space Studies, New York, NY, USA |
| This journal is © The Royal Society of Chemistry 2025 |