Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal–organic frameworks (MOFs) toward SO2 detection
Juan L.
Obeso
 ab,
Catalina V.
Flores
ab,
Catalina V.
Flores
 ab,
Ricardo A.
Peralta
ab,
Ricardo A.
Peralta
 c,
Margarita
Viniegra
c,
Margarita
Viniegra
 c,
N.
Martín-Guaregua
c,
Michael T.
Huxley
*d,
Diego
Solis-Ibarra
c,
N.
Martín-Guaregua
c,
Michael T.
Huxley
*d,
Diego
Solis-Ibarra
 *a,
Ilich A.
Ibarra
*a,
Ilich A.
Ibarra
 *a and
Christoph
Janiak
*a and
Christoph
Janiak
 *e
*e
aLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Coyoacán, 04510 Ciudad de México, Mexico. E-mail: diego.solis@unam.mx; argel@unam.mx
bInstituto Politécnico Nacional, Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada, Legaria 694, Col. Irrigación, Miguel Hidalgo, 11500 Ciudad de México, Mexico
cDepartamento de Química, División de Ciencias Básicas e Ingeniería. Universidad Autónoma Metropolitana (UAM-I), 09340, Mexico
dSchool of Physics, Chemistry and Earth Sciences, Faculty of Sciences, Engineering and Technology, The University of Adelaide, Adelaide, South Australia 5005, Australia. E-mail: michael.huxley@adelaide.edu.au
eInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de
First published on 10th March 2025
Abstract
Developing technology that can precisely monitor specific air pollutants in diverse settings is essential to control emissions and ensure safe exposure limits are not exceeded. Metal–organic frameworks (MOFs) are crystalline organic–inorganic hybrid materials, which are promising candidates for SO2 detection. Their chemically mutable periodic structure confers outstanding surface area, thermal stability, and a well-defined pore distribution. Moreover, MOFs have exhibited extraordinary performance for SO2 capture. Therefore, research has focused on their possible applications for SO2 sequestration due to the selective and robust chemical and physical interactions of SO2 molecules within MOFs. The variable SO2 affinity presented by MOFs enables the adsorption mechanism and preferential adsorption sites to be resolved. However, for MOF-based SO2 detection, selective SO2 capture at shallow partial pressure (0.01–0.1 bar) is required. Thus, capturing SO2 at low concentration is crucial for SO2 detection, where textural properties of MOFs, mainly the pore-limiting diameter, are essential to achieve selective detection. In this review, we discuss the fundamental aspects of SO2 detection in MOFs, providing a step-by-step methodology for SO2 detection in MOFs. We hope this review can provide valuable background around SO2 detection in MOFs and inspire further research within this new and exciting field.
1. Introduction
The environmental and health implications of volatile pollutants pose major technological and economic challenges to modern society. The role of anthropogenic CO2 and methane emissions in promoting an enhanced greenhouse effect is no doubt the most publicized example, yet lesser known pollutants such as carbon monoxide (CO), tropospheric ozone (O3), ammonia (NH3), volatile organic compounds, hydrogen sulfide (H2S), and sulfur dioxide (SO2) are harmful and prevalent in their own right.1 These toxic gases contribute to poor health outcomes, crop damage, acidification of soils and waters, and the loss of biodiversity.2,3 Therefore, targeting emissions and remediating contaminated areas remains a principal goal of governments worldwide.The developed world's accelerating demand for energy,4 which is still predominantly satisfied by fossil fuels, represents the major anthropogenic source of volatile pollutants.5 Natural sources, such as volcanic activity, are a further contributing factor.6 For example, México hosts several of the world's largest and most frequently active volcanoes. Volcanic gas emissions from these and other volcanoes are damaging to both the environment and human health in localized areas.7,8
Of the pollutants identified above, SO2 is particularly hazardous due to a combination of toxicity and its ubiquity in flu gas emissions and various industrial settings. SO2 is a colourless, irritating, and non-flammable gas with a strong odor that can be absorbed through the respiratory system or dermal contact.9 It is classified as one of the most hazardous gases: exposure can to concentrations exceeding 100 ppm can be fatal in minutes.10 However, even at lower concentrations, inhalation can cause severe respiratory complications.11,12 The maximum daily average concentration for human exposure to SO2 is 20 μg m−3 (8 ppb). Therefore, based on environmental and human health considerations, it is necessary to enforce stringent SO2 emission regulations and prioritize the detection of SO2 in both ecological and workplace settings.13
1.1 Physicochemical properties of SO2
To understand the challenges associated with the capture and detection of SO2, the chemical and physical properties of the compound must be considered.14,15 Valence shell electron pair repulsion theory predicts SO2 to possess a bent geometry with an approximately 120° angle between the central sulfur and peripheral oxygen atoms (Fig. 1a). The bonding in SO2 can be described with mesomeric bonds: a covalent S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond and an ionic S+–O− bond (Fig. 1b). The molecule is polar (dipole moment 1.63305 D or 5.4473 × 10−30 C m) and is therefore soluble in water (Fig. 1c).16 The S–O bond length in SO2 is 1.43 Å, commensurate with the bonding models described above.17
O double bond and an ionic S+–O− bond (Fig. 1b). The molecule is polar (dipole moment 1.63305 D or 5.4473 × 10−30 C m) and is therefore soluble in water (Fig. 1c).16 The S–O bond length in SO2 is 1.43 Å, commensurate with the bonding models described above.17
 | ||
| Fig. 1 (a) Valence bond resonance SO2 structure, (b) scheme displaying the molecular orbital bonding model for SO2, (c) SO2 dipole moment. | ||
1.2 Sources of SO2 pollution
The SO2 pollution is directly related to industrial activities associated with burning fossil fuels and biomass by power plants and chemical industries. This can include metal extraction from mines, locomotives, vehicles, and volcanos.18 In general, power plants generate electricity via combustion, which releases SO2 because the feedstocks contain sulfur compounds.Therefore, industrial cities are confronted with an SO2 pollution problem. Jion et al.19 reported that 27.6% of SO2 emissions in Asian countries arise from coal burning, while industry accounts for 20.7%, fossil fuel and biomass burning 13.8%, power plants and brick kilns 10.3%, and domestic production 3.4%. The increase in SO2 pollution is related to industrialization, urbanization, and economic development. Specifically, the SO2 concentration observed in several Asian countries is relatively high. For example, at Langkawi Island, Malaysia, the concentration is 14 ppb (data from 1999–2011)20 while in Lahore City, Pakistan, it is 19.11 ± 6.18 ppb.21 For Longfengshan, Shangdianzi, Houma, Huaian, Lin'an, kaili, Chenzhou, Meixian, Dianbai of China is 23.59 ± 23.97 ppb (data from 2010).22 In rural sites in China is 21.06 ± 9.23 ppb (data from 2007–2008).23 Furthermore, Mousavi et al.24 reported an analysis of the SO2 concentration arising from flares at the Maroon gas refinery located in the suburb of Ahvaz, Iran. It was found that the SO2 concentration rises to 82.1 ppb during the cold season.
1.3 Industrial uses for SO2 and existing capture technologies
SO2 finds multifarious industrial uses. For example, the remarkable antiseptic and antioxidant properties of SO2 have led to its frequent use as a food and beverage preservative,25 particularly in winemaking, where it acts as an antimicrobial agent during the aging and storage of wine.26 In the chemical industry, SO2 is an intermediate in the production of sulfuric acid (H2SO4). The industrial synthesis of H2SO4 takes place by first transforming sulfur into SO2 using O2 as an oxidant, followed by the conversion of SO2 into sulfur trioxide (SO3) using vanadium and alkali oxides.27 The resulting SO3 is dissolved in 98 wt% H2SO4 solution to generate a 99.7 vol% H2SO4 solution.28 SO2 is also employed as a bleaching agent29 and was a first-generation refrigerant due to its high heat of evaporation.30Indeed, industrial demand for SO2 and inadvertent emission from coal-fired power stations necessitates strict control for safety and environmental reasons. Considering the need to limit anthropogenic SO2 emissions, significant investment has been expended toward SO2 capture at point sources such as coal-fired power stations. The first SO2 capture system, the spiral-tile packed tower, was developed in the early 1930s31–33 but is highly inefficient due to the consumption of vast quantities of water during its operation. The process also produces large quantities of sulfuric acid contaminated water.34
SO2 scrubbing, also known as flue-gas desulfurization (FGD).35 FGD is employed using either a once-through or regenerative process. In the former, the spent sorbent (which is calcium sulfate) can be used in the construction industry or otherwise disposed. The regenerative process is more desirable because the sorbent is re-activated, and SO2 is recovered for use in chemical industries. Despite this process being widely applied and largely successful in mitigating the worst impacts of acid rain, FGD systems still release significant quantities of SO2 into the atmosphere.36 Therefore, interest has been garnered by alternative processes such as the use of ceramic hollow fiber membranes filled with various aqueous solutions to capture SO2.37–40 Finally, various ‘wet-sulfuric acid’ processes have been used extensively for sulfur removal since the 1980s,41 motivated in part by the generation of valuable byproducts.42
1.4 Emerging technologies for SO2 capture
The aforementioned processes generate large quantities of wastewater, corrode pipelines, impose significant economic costs, and leave residual traces of SO2.43 Thus, as an alternative, solid-state adsorbents have received growing interest. For example, zeolites and metal oxides have been investigated for SO2 uptake.44,45 Zeolites are widely used as adsorptive materials, ion exchangers, and catalysts.46 Zeolites present attractive qualities in adsorption applications, including low-cost synthesis, relatively high surface area, microporosity, and thermal and mechanical stability.47 However, zeolites exhibit drawbacks associated with their regeneration process. In some cases, the strong host–guest interaction between a zeolite and gas molecule invokes chemical bonding,48 necessitating thermal activation (200 °C) under vacuum to regenerate the adsorbent and increasing operating costs.49 Similarly, metal-oxides offer advantageous properties for adsorption applications but often form non-reversible interactions with gases of interest.50Therefore, new porous materials have been investigated with a focus on sustainable development and real-world applications.51,52 This includes a new generation of organic or hybrid organic–inorganic adsorbent materials such as metal–organic cages (MOCs),53 porous organic cages (POCs),54 and metal–organic frameworks (MOFs).55 The latter are crystalline, typically microporous materials constructed from metal ions interconnected by organic linkers, forming two or three-dimensional coordination networks.56,57 MOFs feature tunable physicochemical properties due to reticular design principles, narrow pore size distributions, high surface areas, and in some instances, chemical and thermal stability.58,59 Their metal and linker building blocks allow the design of a tremendous range of different MOFs which can be tuned via reticular synthesis to suite specific applications. These properties have conferred significant advantages in adsorption,60–63 catalysis,64–66 drug delivery,67,68 separation,69,70 and proton conductivity71,72 applications.
Only a limited number of chemically stable MOFs have so-far exhibited promising SO2 adsorption properties. This paucity reflects the often-poor stability of coordination clusters – central to the structural integrity of MOFs – towards SO2 exposure.73 During adsorption, SO2 molecules interact with MOFs via chemical or physical adsorption, depending on the nature of the binding sites available in the framework. The stability of MOFs towards SO2 is dependent on the strength of the metal–ligand coordination bond (ranging between 300 kJ mol−1 to 600 kJ mol−1 for carboxylate linkers) and coordination number of the metal node.74,75 Displacement of metal-linker bonds by SO2 leads to decomposition of the MOF sorbent. Since linkers are classified as electron-donating species and metal ions are electron-accepting species,76 Pearson's hard-soft acid–base (HSAB) concept provides a rationale for the stability of MOFs. Hard bases establish stronger bonds with hard acids and soft acids with soft bases.77
Based on these principles, a range of chemically stable MOFs have been synthesized and found to exhibit high SO2 uptakes.78 Chemical stability is, however, only one of the challenges facing chemists as they work to establish an industrial role for MOFs. Criticism frequently centers around the high cost of MOF linkers as well as the scalability of MOF synthesis, leading to questions about the economic feasibility of industrial-scale SO2 capture (and that of other gases such as CO2) using MOFs.79 Indeed, the feasibility of adsorptive SO2 capture with MOFs at scale remains uncertain. However, laboratory scale results for MOF-based SO2 removal suggest that other applications that require smaller quantities of adsorbent, particularly SO2 detection rather than capture, are promising avenues for MOF research.
1.5 Principals for SO2 detection
Detecting a specific molecule relies on stimulating a specific response in the sensor, which, when measured, gives either a quantitative or qualitative measure of the concentration (or presence) of the analyte.80,81 Since MOFs are naturally suited to sensing applications due to their intrinsic porosity and functional versatility, a wide range of MOF based sensing techniques have been envisaged.82 These include MOF-based chemiresistive sensors,83 luminescent sensors,84 colourimetric sensors,85 and magnetic sensors.86 MOF-based chemo resistive materials are based on the change in resistance in response to a chemical surface reaction or adsorption of a guest molecule.87 Sensors based on luminescence response employ the change in luminescence properties of certain MOFs, which generate a turn-on or, more often, turn-off fluorescence response.88,89 Furthermore, some MOFs exhibit a characteristic shift in their emission wavelength(s) when exposed to specific molecules such as ammonia.90 Colourimetric detection is used for simplicity and can be performed via visual analysis.91 Additionally, the spin-crossover (SCO) effect has gained interest in the scientific community for its applications in magnetic sensors. In MOFs for instance, exposure to external stimuli such as temperature, pressure or magnetic field can induce measurable changes in the spin state of framework metal ions (typically Fe(II) framework nodes).92,93 However, guest molecules can also induce a spin transition, which can be exploited for the purpose of detection.94 These techniques have been combined to detect various small molecules, including organic solvents,95 aqueous pollutants,96 greenhouse gases,97 and acidic solvents.98 However, SO2 detection has received limited attention.Presently available SO2 detectors employ an electrochemical system based on a solid polymer, usually polycarbonate. In such devices, an electrochemical reaction occurs, generating an electron in the working electrode, which produces an electrical current that is proportional to the SO2 concentration. The SO2 detection range is from 0 to 20 ppm with a response time of 30 s.99,100 Such devices are frequently used in coal mines and the petroleum and chemical industries where SO2 is encountered. However, drawbacks associated with existing SO2 detectors, including interference from other gases, and sensitivity towards temperature and humidity fluctuations which lead to low sensitivity and accuracy.101–103
SO2 detectors can be improved by introducing new solid-state materials with increased selectivity towards SO2. Therefore, considering the promising SO2 adsorption properties of MOFs, SO2 detection is a logical next step. SO2 tolerant MOFs have shown moderate to high SO2 uptake. Intuitively, materials with a high SO2 affinity – interpreted as evidence for an enhanced interaction between SO2 and the MOF framework – could be promising candidates for detection applications.104 To exploit this potential, it is necessary to understand the fundamental interactions between SO2 molecules and the MOF. By transforming these host–guest interactions into measurable signals, the presence and, in some cases, concentration of SO2 can be reliably determined. To meet this goal, researchers must draw on the vast wealth of research which has characterized the structure–property relationships of MOFs and optimized their mechanical and chemical stability – both crucial properties for real-world applications where MOFs are incorporated into functional devices. The accelerated development of MOFs to improve their properties for gas detection is crucial for building functional devices.
Thus, this review provides a comprehensive summary and analysis of MOF-based SO2 detection strategies. To provide a suitable background, seminal examples of MOF-based detection of sulfur compounds other than SO2 (and also in solution) are also provided. We emphasize the relationship between specific characteristics of porous materials (i.e., surface area, pore volume, pore diameter, and functionalisation), which combine with the molecular properties of SO2 to provide a means for reliable detection. The primary techniques with which SO2 detection is studied in MOFs are discussed in detail. We aim to encourage further investigation into the exciting field of MOF-based environmental remediation and sensing applications.
2. MOFs for SO2 capture
One of the primary purposes of this review is to explore existing – and postulate promising – MOF candidates for detecting SO2. Therefore, the characteristic properties shared by MOFs that exhibit a high affinity towards SO2 must be examined so that these desirable properties can be refined for SO2 detection applications.2.1 Main interactions of the SO2 molecule within MOFs
The host–guest interaction between SO2 molecules and MOFs provides a fundamental basis for understanding the application of MOFs in SO2 detection. Considering the chemical diversity of MOF pores, it is necessary to establish the potential modes by which SO2 can interact with adsorbents.The adsorption of gases on surfaces is divided into two limiting processes: (i) physisorption, that is, physical adsorption, which displays weak gas–sorbent interactions comprising van der Waals forces, reversibility and a low heat of adsorption (<50 kJ mol−1); and (ii) chemisorption, that is, chemical adsorption, which exhibits comparatively strong interactions characteristic of chemical bonding, a high heat of adsorption (>50 kJ mol−1), and less facile reversibility.105 From this point of view, SO2 adsorption processes are governed by the chemistry of available adsorption sites within a MOF, which determines the type and strength of interactions.
Preferential adsorption sites within MOF structures (Fig. 2a) can include hydroxyl/amino groups, open metal sites (including defects and missing linkers), and halogen/methyl groups.106 Thus, the extraordinary chemical diversity available in MOFs gives rise to a range of possible interactions with polar SO2 molecules (Fig. 2b), including hydrogen bonding, direct coordination to framework metal ions, sulfur–halogen bonding, S–π interactions, and other electrostatic interactions.107–109
 | ||
| Fig. 2 (a) Main adsorption sites in MOF, (b) summary of possible SO2–MOF interactions, and (c) metal bonding modes of the SO2 molecule depicted schematically. Based on ref. 106,110. | ||
When coordinating to metal centres, such as open metal sites in MOFs, an SO2 molecule can exhibit multiple binding modes that employ both oxygen and sulfur donors. Typical SO2 coordination modes are summarized in Fig. 2c and include (i) η1-SO2, planar and S-bonded, (ii) η1-SO2, pyramidal and S-bonded, (iii) η2-SO2, both S and O-bonded, and (iv) η1-SO2, O-bonded.111 These metal–SO2 coordination modes have been exploited to improve SO2 adsorption in MOFs at open metal centres.
Metal centres do not comprise the only sites with which SO2 can interact within MOFs. Hydrogen bond donors are a common preferential adsorption site in MOFs, particularly in the form of hydroxyl and amine moieties. The hydroxido, hydroxo, or hydroxy group is an intrinsic characteristic of numerous MOFs bearing cluster-based SBUs, where, for instance, the μ-OH moieties bridge two or three metal centers.112 Amino groups on the other hand are provided via suitably functionalized organic linkers.113 The interaction between SO2 molecules and hydroxy sites in a MOF was first directly identified in 2012 by Yang et al.114 in NOTT-300(Al) (later renamed MFM-300(Al), linker BPTC)115 using in situ powder X-ray diffraction (PXRD), inelastic neutron scattering, and grand canonical Monte Carlo (GCMC) simulations. The NOT-300(Al) structure features μ-OH groups, which bridge between Al(III) ions to form infinite 1D chains that extend along the MOF pores and are bridged by BPTC moieties. Comprehensive analysis revealed that SO2 molecules engage in hydrogen bonds (SO2(O)⋯H(OH) = 2.376(13) Å) with μ-OH sites (Fig. 3a), supported by complementary interactions with aromatic C–H sites of adjacent linkers. Five hydrogen bond interactions were observed between the host framework and bound SO2. Furthermore, the SO2 molecules bound to the framework interact via dipole–dipole S⋯O interactions (S⋯O = 3.34(7) Å) with secondary SO2 molecules located within the MOF pore (Fig. 3b). A follow-up study published in 2020 established the long-term stability of NOT-300(Al) towards SO2, NH3, and NO2. This study highlighted the capacity of diffraction techniques to precisely elucidate the interaction mechanisms behind SO2 adsorption in robust, crystalline adsorbents.
 | ||
| Fig. 3 (a) View of the crystal structure of NOTT-300·4.0-SO2 obtained from Rietveld refinement of data on SO2-loaded material at 1.0 bar. The adsorbed SO2 molecules in the void are highlighted using a ball-and-stick represention. The sulfur atom of the second site of SO2 is highlighted in blue. (b) Detailed view of the OH and CH contact with SO2 molecules in a distorted pocket-like cavity. (Aluminum, green; carbon, grey; oxygen, red; hydrogen, white; sulfur, yellow.) (Reprinted (adapted) with permission from ref. 114 Copyright (2012) Springer Nature). | ||
MFM-300(Sc), which is isostructural to MFM-300(Al) (previously named NOT-300(Al) as described above), exhibits infinite 1D [Sc2(μ-OH)] chains interconnected by BPTC moieties. SO2 interactions were elucidated using GCMC simulations, which revealed that SO2 molecules engage in hydrogen bonding with μ-OH sites situated along the inorganic node.116 The indium analog MFM-300(In) displayed high selectivity for SO2 over N2, CH4, and CO2. In situ PXRD revealed similar behavior to that observed in MFM-300(Al): SO2 occupies two adsorption sites. One molecule interacts with a bridging hydroxyl group [SO2(O)⋯H(OH) = 3.17 Å] while at the same time, a second SO2 molecule is supported in the MOF pore via dipole–dipole S⋯O interactions with the bound SO2 molecule. Inelastic neutron scattering experiments probed the interaction between N2, CO2, and SO2 gas molecules and μ-OH sites. A substantial shift in signals associated with wagging/bending modes of aromatic C–H bonds and bridging μ-OH sites was observed upon exposure to SO2. A less significant shift was observed upon CO2 adsorption, confirming that SO2 adsorption is associated with stronger hydrogen bonding interactions with these framework sites.117 Further spectroscopic evidence for the hydrogen bonding interaction was provided by monitoring the ν(OH) band at 3657 cm−1. These studies validate the role of hydrogen bonding between SO2 and inorganic hydroxyl sites and intermolecular SO2–SO2 interactions in stabilizing adsorbed SO2 in robust MOFs.
Similar interactions have been described for various μ-OH bearing MOFs. For example, rigid MIL-53(Al)-TDC (TDC = 2,5-thiophenedicarboxylate) and the flexible MIL-53(Al)-BDC displayed this characteristic interaction.118 DFT simulations were employed to probe the SO2/MOF host–guest chemistry. SO2 was observed to interact through hydrogen bonding with the μ-OH group of both MIL-53(Al)-TDC and MIL-53(Al)-BDC (with a mean SO2(O)⋯H(OH) separation distance of 2.05 Å and 1.78 Å, respectively). The shorter hydrogen bonding interactions observed in the more flexible framework were related to adsorption-induced decrease in pore size in the flexible framework, facilitating stronger hydrogen bonding interactions. Multiple steps in the SO2 adsorption isotherm supported this flexible behavior. Furthermore, the strong affinity for SO2 molecules at the μ-OH site leads to a remarkable selectivity over a wide range of gases. Another framework bearing bridging μ-OH groups, DUT-4119 (with the linker NDC), displays relatively high SO2 adsorption (13.6 mmol g−1 – compared to 8.9 mmol g−1 for MIL-53(Al)-TDC and 0.8 mmol g−1 for MIL-53(Al)-BDC) at 298 K and 1 bar).118 DFT studies show that SO2 interacts with the μ-OH group and the linker (distance of 2.9 and 2.7 Å, respectively). The affinity towards the μ-OH group contributed to selective adsorption of SO2 over CH4. Furthermore, the μ-OH bearing framework, Mn-CUK with the linker PDCA = 2,4-pyridinedicarboxylate, contains a [Mn3(μ3-OH)2] cluster and displays moderate SO2 adsorption capacity (5.51 mmol g−1) at 298 K and 1 bar.120 Variable-temperature SCXRD studies suggested that SO2 binds via hydrogen bonding with the μ3-OH sites.
MIL-160 (with the linker FDCA = 2,5-furandicarboxylate) is a furan-based MOF with a moderate SO2 uptake (7.2 mmol g−1) at 293 K and 0.97 bar.121 However, the framework displays high selectivity towards SO2 over CO2, CH4, N2, and H2. The feasible binding sites for SO2 in MIL-160 were identified by DFT calculations using geometry optimization of SO2 within the pores (Fig. 4a–c). Three main interactions were found to occur between MIL-60 and SO2: dipole–dipole bonding at furan oxygen sites (SO2(S)⋯O(furan) distance 3.27 Å), hydrogen bonding at μ-OH (SO2(O)⋯H(OH) distance 2.10 Å), and finally, dipole–dipole bonding between SO2 and two furan units (distances of 3.15 and 3.36 Å). The short SO2(O)⋯H(OH) hydrogen bond contact implies a high affinity between SO2 and the hydroxyl sites which contributes to the outstanding selectivity toward SO2.
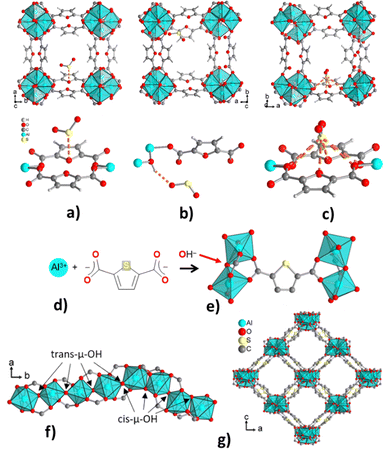 | ||
| Fig. 4 DFT-simulated binding sites of SO2 in MIL-160. (a) Ofuran⋯SSO2 interaction, (b) OHAl-chain⋯OSO2 interaction, and (c) Ofuran/carboxylate⋯SSO2 interaction. (Reprinted with permission from ref. 121 Copyright (2019) American Chemical Society). Crystal structure of CAU-23: (d) Al3+, 2,5-thiophenedicarboxylate (TDC2−) and hydroxide ions as building blocks, (e) TDC2− linker coordination to {AlO6} octahedra, (f) chains composed of alternating segments of four helical cis- and four trans-corner sharing {AlO6} octahedra, (g) section of the packing diagram with the {AlO6} chains connected by the TDC2− linkers to yield square-shaped channels. (Reprinted with permission from ref. 122 Copyright (2022) with permission from John Wiley & Sons). | ||
Similarly, CAU-23 (with the linker TDC) displays cis and trans-μ-OH sites in the inorganic building unit (Fig. 4d–g) and has been evaluated for gas sorption properties.122 CAU-23 shows a relatively high SO2 adsorption capacity (8.4 mmol g−1, at 1 bar and 293 K) and low CO2 and CH4 adsorption capacity (3.97 mmol g−1 and 0.89 mmol g−1, respectively, all at 1 bar and 293 K). Moreover, the presence of cis and trans-μ-OH groups imparts a high affinity towards polar SO2 molecules over CO2, H2, and CH4. Further to the behavior described above, adsorbed SO2 can also interact favorably with the π-system and S atom from the linker.
Coordinatively unsaturated sites can be generated in MOFs at the framework nodes when coordinated solvent (i.e., water) is dissociated during thermal activation, leaving behind an accessible Lewis acidic metal site.123,124 This attribute has drawn considerable interest in the adsorption and catalysis fields.125,126 M-MOF-74 (with the linker DHTP = 2, 5-dihydroxyterephthalate) (M = Zn and Mg) is one such material and displays strong interactions between adsorbed SO2 and open metal sites generated during activation.127 Using in situ infrared spectroscopy and ab initio DFT calculations, the first preferential adsorption site was identified as a direct SO2(O)–M interaction. Another MOF, MFM-170, features well-defined Cu(II) sites which also interact directly with SO2. MFM-70 consists of a [Cu2(O2CR)4] (O2CR = 4′,4′′′-(pyridine-3,5-diyl)bis([1,1′-biphenyl]-3,5-dicarboxylate) dimer with four linker carboxylate moieties occupying the equatorial sites and one linker N-pyridyl donor coordinating to one of the two axial sites of the dimer (the second being available for guest coordination).128 This available Cu(II) coordination site facilitates reversible SO2 capture, while the structure remains stabile even towards exposure to wet SO2. Using in situ SCXRD, FTIR microspectroscopy, and inelastic neutron scattering, the open Cu(II) sites were confirmed to act as SO2(O)–Cu adsorption sites. The Cu(II) framework, MFM-190 (linker: 5,5′-(pyridine-2,5-diyl)diisophthalate), also exhibits open Cu2+ sites which form the primary adsorption site for SO2.129 Furthermore, an S–π interaction was observed between SO2 and delocalized π systems of the two neighboring phenyl rings. In situ neutron powder diffraction, inelastic neutron scattering, and synchrotron infrared microspectroscopy studies revealed the location of host–guest binding. The MOF MIL-101(Cr)-4F(1%) is a partially fluorinated MOF from the MIL-101(Cr) family. This Cr(III)-based MOF was synthesised by mixing BDC and 2,3,5,6-tetrafluoro-1,4-benzenedicarboxylate (BDC-4F), thereby doping the structure with fluorine (MIL-101(Cr)-4F(1%) = [Cr3O(BDC)2.91(BDC-F4)0.09]Cl).130 The presence of fluorine modulates the pore-surface electron density leading to considerably improved SO2 capture due to the enhanced dipole–dipole interactions with the pore surface.
Defect sites in MOFs – such as missing linker or missing cluster defects, which are prominent in Zr(IV) frameworks, among many others131,132 – are correlated with a decrease in the chemical stability of the framework but provide new interaction sites for adsorbate molecules, including SO2.133 The MOF [Ni8(OH)4(H2O)2(BDP_X)6],134 (where H2BDP_X = 1,4-bis(pyrazol-4-yl)benzene-X with X = H (1), OH (2), NH2 (3)) (Fig. 5a), was post-synthetically modified by placing the material in ethanolic solutions of potassium hydroxide to generate the defect rich frameworks K[Ni8(OH)3(EtO)3(BDP_X)5.5] (1@KOH, 3@KOH) and K3[Ni8(OH)3(EtO)(BDP_O)5] (2@KOH). The defective frameworks were soaked in aqueous Ba(NO3)2, leading to exchange of extra-framework potassium ions for Ba(II), giving Ba0.5[Ni8(OH)3(EtO)3(BDP_X)5.5] (1@Ba(OH)2, X = H; 3@Ba(OH)2, X = NH2), and Ba1.5[Ni8(OH)3(EtO)(BDP_O)5] (2@Ba(OH)2). The logical basis for this extensive post-synthetic modification was to imbue the defective frameworks with a greater capacity to interact with SO2. Possible SO2 interactions were evaluated by DFT calculations (Fig. 5b–e). The preferential SO2 adsorption sites in 1@Ba(OH)2 are the crystal defects where SO2 coordinates in a bidentate fashion with Ba(II) ions. This is contrasted with 1@KOH wherein SO2 coordinates through a less favourable monodentate mode with potassium ions. Ba(II) ions are therefore associated with enhanced interactions between SO2 and the framework. The formation of missing linker defects, where hydroxide displaces framework linkers, also contributes since the hydroxyl moieties interact favorably with SO2. Thus, this novel defect engineering methodology facilitated improved adsorption performance by producing defect sites with a high affinity towards SO2 and improving the accessibility of the framework to sorbate due to the presence of missing linker defects.134,135
 | ||
| Fig. 5 (a) Schematic representation of the successive post-synthetic modifications, from pristine nickel pyrazolate [Ni8(OH)4(H2O)2(BDP_X)6] (H2BDP_X = 1,4-bis(pyrazol-4-yl)benzene-4-X with X = H (1), OH (2), NH2 (3)) frameworks to yield the missing linker defective K[Ni8(OH)3(EtO)3(BDP_X)5.5] (1@KOH, 3@KOH) and K3[Ni8(OH)3(EtO)(BDP_O)5] (2@KOH) and subsequently, the ion-exchanged Ba0.5[Ni8(OH)3(EtO)3(BDP_X)5.5] (1@Ba(OH)2, X = H; 3@Ba(OH)2, X = NH2), and Ba1.5[Ni8(OH)3(EtO)(BDP_O)5] (2@Ba(OH)2) materials. Organic linker (grey bar), potassium (purple), barium (cyan). Sulfur dioxide interaction with crystal defect sites. DFT structure minimization of the molecular configuration of one (b) and (c) and two (d) and (e) adsorbed SO2 molecules on 1@KOH (left) and 1@Ba(OH)2 (right) materials. (Reprinted (adapted) with permission from ref. 134 Copyright (2017) Springer Nature under a Creative Commons CC BY license). | ||
Finally, the installation of halogen atoms on organic linkers can enhance the gas capture performance of MOFs. For example, the HHU-2-X (X = Cl, I, and Br) family are halogen functionalized MOF-801 derivatives, which are composed of halofumarate linkers which bridge 12-connected [Zr6O4(OH)4] clusters.136 These materials display moderate SO2 uptake compared to pristine MOF-801 which shares the same fcu topology but an unfunctionalized fumarate linker. HHU-2-Cl for instance displayed an SO2 adsorption capacity of 9.69 mmol g−1 at 296 K and 1 bar, while MOF-801 reaches only 8.00 mmol g−1 at 296 K and 1 bar. Halogen functionalisation increases the polarity of the MOF pores, improving the affinity towards polar SO2 molecules over CO2.
Thus, it is evident that the chemical functionality of MOFs directly affects their SO2 affinity by modulating the SO2 interaction mechanism. Preferential SO2 adsorption sites range from μ-OH moieties involved in hydrogen bonding to coordinatively unsaturated metal centres where coordination chemistry can take place.117,120 The studies highlighted so far have focused on SO2 adsorption at relatively high pressure (1 bar). However, systems that detect SO2 must possess strong and selective affinity towards the gas at much lower pressures.
2.2 Selective capture of SO2 in MOFs
An important consideration for the effective MOF-based detection of SO2 is high selectivity. The modular nature of MOFs provides opportunities to tune their frameworks via the incorporation of specific functional groups that preferentially interact with SO2 over other molecules. One of the earliest investigations into selective SO2 adsorption in MOFs was reported in 2008 by Britt et al.137 Using kinetic breakthrough measurements the authors calculated the dynamic SO2 adsorption capacity of MOF-5, IRMOF-3, MOF-74, MOF-177, MOF-199, and IRMOF-62. Remarkably, the pore functionality (i.e., unsaturated metal sites and amino functionality) was found to play a dominant role in determining the dynamic SO2 adsorption performance. Later, it was reported that the incorporation of urea within Zn(II)-based MOFs (achieved using the linker 6-oxo-6,7-dihydro-5H-dibenzo[d,f][1,3]diazepine-3,9-dicarboxylate),138 provided enhanced hydrogen-bonding interactions with SO2 over other gas molecules such as CO2.Savage et al.117 demonstrated the utility of the hydroxo functional group (–OH) in promoting high SO2 selectivity in MFM-300(In). The material exhibited remarkable selectivity (SO2/CO2 60, SO2/CH4 425, and SO2/N2 5000) under ambient conditions (i.e., 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture at 1 bar and 298 K). The origin of this behavior was investigated by combining crystallographic and spectroscopic techniques including inelastic neutron scattering; which revealed that enhanced supramolecular binding interactions – especially hydrogen bonding by the –OH functional group – are directly responsible for observed affinity towards SO2. Using in situ synchrotron X-ray diffraction experiments, the same authors established the role of μ3-O and μ3-OH functional groups in the remarkable SO2/CO2 and SO2/N2 selectivity observed in MFM-601 (with the linker PPTA = 4,4′,4′′,4′′′-(1,4-phenylenebis(pyridine-4,2,6-triyl))tetrabenzoate).139 The dipole moment of SO2 interacts favorably with the μ3-O and μ3-OH groups within the pores of MFM-601, which explains the affinity between MFM-601 and polar SO2 over non-polar CO2 or N2. MIL-160 is an Al(III)-based MOF which also exhibits high SO2 uptakes at low pressures (p < 0.01 bar) and a remarkable selectivity towards SO2 over CO2 due to the presence of furan moieties which provide preferential binding sites for SO2(O(furan)⋯S(SO2)).121 Recently, the SO2/CO2 selectivity of NH2-MIL-101(Cr), Basolite F300 (Fe-1,3,5-BTC), HKUST-1, ZIF-8 and ZIF-67 was evaluated in comparison to non-MOF adsorbents Zeolite Y, SAPO-34, silica gel 60 and CTF-1,140 concluding that Zeolite Y and CTF-1(600) showed the most promising SO2/CO2 selectivity results with an ideal adsorbed solution theory selectivity in the range of 265–149 and 63–43 with a mole fraction of 0.01–0.5 SO2 at 293 K and 1 bar.
50 mixture at 1 bar and 298 K). The origin of this behavior was investigated by combining crystallographic and spectroscopic techniques including inelastic neutron scattering; which revealed that enhanced supramolecular binding interactions – especially hydrogen bonding by the –OH functional group – are directly responsible for observed affinity towards SO2. Using in situ synchrotron X-ray diffraction experiments, the same authors established the role of μ3-O and μ3-OH functional groups in the remarkable SO2/CO2 and SO2/N2 selectivity observed in MFM-601 (with the linker PPTA = 4,4′,4′′,4′′′-(1,4-phenylenebis(pyridine-4,2,6-triyl))tetrabenzoate).139 The dipole moment of SO2 interacts favorably with the μ3-O and μ3-OH groups within the pores of MFM-601, which explains the affinity between MFM-601 and polar SO2 over non-polar CO2 or N2. MIL-160 is an Al(III)-based MOF which also exhibits high SO2 uptakes at low pressures (p < 0.01 bar) and a remarkable selectivity towards SO2 over CO2 due to the presence of furan moieties which provide preferential binding sites for SO2(O(furan)⋯S(SO2)).121 Recently, the SO2/CO2 selectivity of NH2-MIL-101(Cr), Basolite F300 (Fe-1,3,5-BTC), HKUST-1, ZIF-8 and ZIF-67 was evaluated in comparison to non-MOF adsorbents Zeolite Y, SAPO-34, silica gel 60 and CTF-1,140 concluding that Zeolite Y and CTF-1(600) showed the most promising SO2/CO2 selectivity results with an ideal adsorbed solution theory selectivity in the range of 265–149 and 63–43 with a mole fraction of 0.01–0.5 SO2 at 293 K and 1 bar.
Using solid-state cationexchange, Mon et al.141 post-synthetically modified a Ni(II)-based MOF (with the linker MPBA = N,N′-2,4,6-trimethyl-1,3-phenylenebis(oxamate)) to increase its N2/SO2 selectivity considerably. By soaking the MOF crystals in a saturated aqueous solution of Ba(NO3)2 for 48 hours, Ni(II) ions hosted within the framework were exchanged for hydrated Ba(II) ions. Using X-ray crystallography and theoretical calculations the authors identified that the hydrated barium cations act as preferential adsorption sites for SO2. Then, Chen et al.142 observed high SO2/CO2 selectivity (325) and ultrahigh selectivities for SO2/N2 (>1.0 × 104) and SO2/CH4 (>1.0 × 104) in M-gallate MOFs, which was attributed to particularly favourable pore apertures and chemical functionality. In a similar vein, excellent SO2/CO2 selectivities have been achieved by optimising the pore aperture to approximate the size of SO2. For instance, by modulating methyl group densities at the benzenedicarboxylate linker in [Ni2(BDC-X)2DABCO] (BDC-X = mono-, di-, and tetramethyl-1,4-benzene-dicarboxylate/terephthalate; DABCO = 1,4-diazabicyclo[2,2,2]octane) the pore size can be precisely tuned.143 Indeed, the highly selective SO2 adsorption by these methyl-functionalized DMOFs was accredited to the numerous non-covalent interactions between the small methyl-functionalized pore and SO2 molecules, which was revealed by DFT calculations (this work is described in further detail below). This strategy was also investigated in ECUT-77, a Co(II)-based MOF composed of 4-(4H-1,2,4-triazol-4-yl)benzoate linkers, which exhibits a SO2/CO2 selectivity of 44 due to its small pore aperture (approximately 3 Å).144
Thus, as outlined above, by tuning the MOF pore aperture and allocating appropriate chemical functionality to the molecular components,145 high SO2 selectivities can be achieved.146 Indeed, SO2 adsorption based applications benefit significantly from the modular and chemically mutable nature of MOFs.147
2.3 Low-pressure capture of SO2 in MOFs
Considering that concentration intervals for SO2 detection are at the ppm level (or sometimes even the ppb level depending on the application), it is SO2 adsorption in the low-partial pressure range that is of interest. Thus, total SO2 uptake at ambient pressure becomes irrelevant. Instead, the most important metric for MOFs intended for SO2 detection applications is SO2 adsorption capacity at low pressure (p ≪ 0.1 bar). For example, after scrubbing, SO2 concentrations in flu gas lie between 150–450 ppm, corresponding to a shallow partial pressure (0.0005 bar)148 and trace concentrations in the atmosphere can be considered to be under 1000 ppm. That is, SO2 exerts a partial pressure of around 0.001 bar.149 Ideally, a MOF should exhibit high SO2 adsorption and affinity in a pressure range from 0.001 to 0.05 bar to be considered a candidate for SO2 detection. Furthermore, high selectivity towards SO2 over other atmospheric gases such as O2, NOx, CH4, and CO2 is vital. This low-pressure range could be ideal for SO2 detection since only a few SO2 molecules interact with the adsorption sites within the material.60Some specific factors which influence SO2 uptake in MOFs at low pressure include the SO2 interaction mechanism and affinity (as described above) and the physical properties of MOFs, particularly the pore diameter. Indeed, the pore limiting diameter (PLD), the smallest diameter of a pore or window present in a framework, pore volume, and chemical functionalization thereof can directly influence the low-pressure SO2 adsorption capacity. These effects can be elucidated experimentally by comparing the adsorption behavior of MOFs with diverse physicochemical properties.
In a comparative study the MOF-based (NH2-MIL-101(Cr), Basolite F300(Fe-1,3,5-BTC), HKUST-1, ZIF-8 and ZIF-67) non-MOF-based adsorbents (Zeolite Y, SAPO-34, silica gel 60 and CTF-1, and Basolite F300) were investigated on account of their small pore diameters.140 The prototypical MOFs listed above possess a robust structure and high chemical stability, which make them feasible for real-world applications, including gas adsorption/detection. However, ZIF-8 and ZIF-67 show low SO2 adsorption capacity under the same conditions, which was attributed to their pore window diameter (3.4 Å) being smaller than the kinetic diameter of SO2 (4.1 Å).140 Thus, below the gate-opening pressure (0.3 bar), SO2 cannot enter the pore, which significantly retards the low-pressure adsorption capacity. At 0.01 bar, the highest uptakes were 5.0 mmol g−1 for Zeolite Y, 2.2 mmol g−1 for CTF-1(400), 2.0 mmol g−1 for HKUST-1, and 1.9 mmol g−1 for SAPO-34. HKUST-1 displays the highest SO2 adsorption at 0.1 bar among these materials (10.1 mmol g−1 at 293 K).140 The outstanding performance of HKUST-1 is attributed to the presence of open metal sites in combination with an optimal PLD (5–11 Å).123,150 The highest affinity towards and uptake of SO2 at low partial pressures (0.01–0.1 bar) were registered for materials featuring pore diameters of ≈4–8 Å (Fig. 6) and aromatic nitrogen atoms (i.e., CTF frameworks).140
 | ||
| Fig. 6 Surface specific SO2 at 0.01 bar (squares) and 0.1 bar (circles) vs. the pore limiting diameter. For silica gel 60, CTF-1, and Ketjenblack, only the smallest pore diameter is indicated, and these materials have a broad pore size distribution. (Reprinted with permission from the author of ref. 140 Copyright (2021) John Wiley & Sons under the Creative Commons CC-BY-NC-ND license). | ||
Dispersion forces between a gas molecule and the pore surface are optimized when the pore diameter (defined by the Connolly surface, which is the accessible surface for a probe molecule of given size) approximates the length of the gas molecule. As alluded to above, an optimal pore aperture for SO2 at low pressure is in a range from ≈4–8 Å. The upper limit of this range (∼8 Å = 2 × 4 Å) is approximately double the length of an SO2 molecule and arises due to favorable dipole–dipole interactions between two SO2 molecules bound to adjacent pore walls.151
Data presented in Table 1 substantiates these points. These findings support the prioritization of frameworks that feature an optimal PLD (4–8 Å), which can significantly improve SO2 uptake at low pressure range pertinent to detection applications.
| Material | BET SA (m2 g−1) | V p (cm3 g−1) | Pore diameter (Å) | SO2 uptake (mmol g−1) at different pressure (bar) | T (K) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.05 | 0.1 | 1 | ||||||
| BET SA: BET surface area, Vp: pore volume, T: temperature, +taken from isotherm *structure collapse after SO2 uptake. | |||||||||
| NH2-MIL-101(Cr) | 2290 | 1.16 | 15.4 | 1.2 | 2.9+ | 4.1 | 16.7 | 293 | 140 |
| Fe(BTC) | 1070 | 0.49 | 18 | 0.6 | 1.5+ | 2.4 | 9.5 | 293 | |
| ZIF-8 | 1820 | 0.80 | 3.4 | 0.1 | 0.4+ | 0.7 | 8.2 | 293 | |
| ZIF-67 | 1980 | 0.69 | 3.4 | 0.1 | 0.5+ | 0.9 | 11.0 | 293 | |
| HKUST-1 | 1490 | 0.61 | 5 | 2.0 | 7.2+ | 10.1 | 13.8 | 293 | |
| HKUST-1 | 1400 | 3.86 | 8.4 | 298 | 152 | ||||
| YIL0.5@HKUST-1 | 5.10 | 7.54 | 298 | ||||||
| PIL0.5@HKUST-1 | 5.15 | 7.73 | 298 | ||||||
| HIL0.5@HKUST-1 | 5.45 | 8.06 | 298 | ||||||
| HIL1@HKUST-1 | 600 | 5.71 | 8.33 | 298 | |||||
| MOF-177 | 4100 | 1.51 | 10.6 | 0.3 | 0.5+ | 1.0 | 25.7* | 293 | 121 |
| NH2-MIL-125(Ti) | 1560 | 0.651 | 5 | 3.0 | 4.95+ | 7.9 | 10.8 | 293 | |
| MIL-160 | 1170 | 0.460 | 5 | 4.2 | 4.8+ | 5.5 | 7.2 | 293 | |
| Zr-Fum | 600 | 0.290 | 4.8 | 1.2 | 2.4+ | 3.1 | 4.9 | 293 | 151 |
| MOF-808 | 1990 | 0.749 | 4.8 | 2.1 | 2.9+ | 3.6 | 14.6 | 293 | |
| DUT-67(Zr) | 1260 | 0.544 | 8.8 | 0.7 | 1.55+ | 2.3 | 9.0 | 293 | |
| NH2-MIL-53(Al) | 620 | 0.358 | 7.3 | 2.0 | 3.7+ | 4.3 | 8.0 | 293 | |
| Al-Fum | 970 | 0.447 | 5.8 | 1.0 | 3.1+ | 4.1 | 7.5 | 293 | |
| CAU-10-H | 600 | 0.258 | 6 | 1.2 | 3.1+ | 3.7 | 4.8 | 293 | |
| MIL-96(Al) | 530 | 0.237 | 1.2 | 2.2+ | 3.7 | 6.5 | 293 | ||
| MIL-100(Al) | 1890 | 0.824 | 25 | 0.4 | 1.4+ | 2.5 | 16.3 | 293 | |
| NH2-MIL-101(Al) | 1770 | 1.001 | 25 | 1.5 | 2.7+ | 3.6 | 17.3 | 293 | |
| NU-1000 | 1740 | 1.196 | 12 | 0.6 | 1.5+ | 2.6 | 12.2 | 293 | |
| NU-1000 | 1970 | 2.1 | 10.9 | 298 | 153 | ||||
| [Ir]@NU-1000. | 1842 | 2.4 | 10.6 | 298 | |||||
| [RuGa]@NU-1000 | 1796 | 0.5 | 2.2 | 7.5 | 298 | 154 | |||
| MIL-53(Al)-BDC | 1450 | 0.706 | 8.5 | 0.4 | 2.45+ | 3.3 | 10.5 | 293 | 151 |
| MIL-53(Al)-BDC | 1210 | 0.51 | 8.5 | 0.65 | 0.95 | 10.8 | 298 | 118 | |
| MIL-53(Al)-TDC | 1000 | 0.415 | 8 | 0.6 | 3.6+ | 5.0 | 6.9 | 293 | 151 |
| MIL-53(Al)-TDC | 1260 | 0.45 | 8 | 4.7 | 8.9 | 298 | 118 | ||
| DUT-67-HCl | 1349 | 0.509 | 6 | 3.0+ | 9.3 | 298 | 155 | ||
| DMOF | 1956 | 0.76 | 7 | 0.25 | 0.9+ | 7.21 | 13.09 | 293 | 143 |
| DMOF-M | 1557 | 0.63 | 7 | 0.46 | 1.8+ | 6.40 | 12.15 | 293 | |
| DMOF-DM | 1343 | 0.52 | 7 | 1.0 | 3.0+ | 5.70 | 10.40 | 293 | |
| DMOF-TM | 900 | 0.43 | 6 | 3.79 | 5.1+ | 6.43 | 9.68 | 293 | |
| HHU-2-Cl | 852 | 0.41 | 2.9+ | 3.6+ | 4.5+ | 9.69 | 293 | 136 | |
| HHU-2-Br | 620 | 0.31 | 1.7+ | 2.3+ | 3.0+ | 6.07 | 293 | ||
| MOF-801 | 939 | 0.43 | 2.1+ | 2.9+ | 3.9+ | 8.00 | 293 | ||
| nanoCB6-H | 441 | 0.22 | 6 | 2.3+ | 2.9+ | 3.4+ | 4.98 | 293 | 156 |
| MIL-101 | 3217 | 1.54 | 29 | 0.6 | 1.5+ | 4.4+ | 24.4 | 298 | 157 |
| CB6@MIL-101-Cl | 2077 | 1.0 | 2.0 | 3.0+ | 5.2+ | 17.0 | 298 | ||
| UR1-MIL-101(Cr) | 1700 | 0.98 | 0.9+ | 1.8+ | 2.7+ | 8.2 | 293 | 158 | |
| UR2-MIL-101(Cr) | 1360 | 0.82 | 1.3+ | 1.7+ | 2.4+ | 6.9 | 293 | ||
| UR3-MIL-101(Cr) | 1900 | 0.96 | 1.8+ | 2.9+ | 4.0+ | 13.9 | 293 | ||
| UR4-MIL-101(Cr) | 1340 | 0.68 | 1.3+ | 2.4+ | 3.3+ | 11.0 | 293 | ||
| CAU-23 | 1176 | 0.51 | 7.6 | 0.9+ | 4.5+ | 6.0+ | 8.4 | 293 | 122 |
| CCIQS-1 | 398 | 4.2 | 1.3 | 298 | 159 | ||||
| Bz@InOF-1 | 5.4 | 6.3 | 298 | 160 | |||||
| CAU-10 | 630 | 0.25 | 7 | 3.9 | 4.47 | 298 | 161 | ||
| Co-URJC-5 | 233 | 8.9 | 0.8 | 1.48* | 298 | 162 | |||
| DUT-4 | 1348 | 0.71 | 8 | 2.4 | 5.1 | 13.6 | 298 | 119 | |
| SU-101 | 412 | 6.8 | 2.2 | 298 | 163 | ||||
| MFM-300(Sc) | 1360 | 0.56 | 8.1 | 7.0 | 9.4 | 298 | 116 | ||
| UNAM-1 | 522 | 7.3 | 1.1 | 3.5 | 298 | 164 | |||
| MIL-101(Cr)-4F(1%) | 2176 | 1.19 | 4.6 | 18.4 | 298 | 130 | |||
| NiBDP | 1220 | 9 | 1.52 | 8.48 | 298 | 165 | |||
| IL/MIL-0.7 | 3 | 0.14 | 1.68 | 4.87 | 13.17 | 298 | 166 | ||
| HBU-23 | 384.2 | 6.8 | 2.42 | 298 | 167 | ||||
| HBU-20 | 1551.1 | 7.0 | 6.71 | 298 | 145 | ||||
| ECUT-100 | 688 | 0.27 | 5.5 | 4.95 | 298 | 168 | |||
| DUT-5 | 1611 | 0.9 | 11 | 2.17 | 298 | 169 | |||
| PCN-250 (Fe) | 1495 | 0.48 | 7.93 | 11.21 | 298 | 170 | |||
| PCN-250 (Fe2Co) | 1583 | 0.51 | 8.06 | 11.92 | 298 | ||||
| PCN-250 (Fe2Ni) | 1619 | 0.52 | 8.64 | 12.44 | 298 | ||||
| PCN-250 (Fe2Mn) | 1483 | 0.47 | 7.70 | 11.14 | 298 | ||||
| PCN-250 (Fe2Zn) | 1560 | 0.50 | 8.21 | 12.11 | 298 | ||||
| Zr-bptc | 960 | 0.34 | 4.5 | 2.5+ | 5.1+ | 6.2 | 7.8 | 298 | 171 |
| UiO-66-Cu(II) | 1068 | 0.54 | 7.3 | 0.6+ | 2.1+ | 3.0 | 8.2 | 298 | |
| UiO-66-NH2 | 1037 | 0.52 | 7.3 | 0.8+ | 2.9+ | 3.7 | 8.8 | 298 | |
| Zr-DMTDC | 1345 | 0.68 | 7.3 | 0.8+ | 2.4+ | 3.1 | 9.6 | 298 | |
| UiO-66 | 1221 | 0.55 | 7.3 | 0.3+ | 1.7+ | 2.1 | 8.6 | 298 | |
| MFM-133 | 2156 | 0.96 | 10.4 | 0.1+ | 0.8+ | 1.2 | 8.9 | 298 | |
| MFM-422 | 3296 | 7.7 | 0.2+ | 1.0+ | 1.8 | 13.6 | 298 | ||
| MFM-190(F) | 2538 | 1.041 | 11 | 1.6+ | 3.4+ | 6.0+ | 18.3 | 298 | 129 |
| MFM-190(NO2) | 2304 | 0.962 | 11 | 1.8+ | 7.1+ | 10.0+ | 12.7 | 298 | |
| MFM-190(CH3) | 2550 | 1.011 | 11 | 0.6+ | 3.1+ | 6.9+ | 15.9* | 298 | |
| MFM-100 | 1445 | 0.68 | 6 | 1.0+ | 2.8+ | 4.5+ | 7.6* | 298 | |
| MFM-101 | 2300 | 0.885 | 11 | 2.4+ | 3.1+ | 8.1+ | 18.7 | 298 | |
| MFM-102 | 2873 | 1.138 | 15 | 1.0+ | 2.2+ | 3.8+ | 12.1* | 298 | |
| MFM-126 | 965 | 0.47 | 12 | 2.0+ | 4.8+ | 5.3+ | 7.3 | 298 | |
| MFM-300(Cr) | 1360 | 7.0 | 7.9 | 298 | 172 | ||||
| MFM-300(Al0.67Cr0.33) | 1305 | 8.5 | 9.5 | 298 | |||||
| MFM-170 | 2408 | 0.87 | 15.9 | 4.9+ | 6.2+ | 17.5 | 298 | 128 | |
| MFM-305 | 779 | 0.373 | 6.2 | 6.99 | 298 | 173 | |||
| MFM-305-CH3 | 256 | 0.181 | 5.2 | 5.16 | 298 | ||||
| MFM-600 | 2281 | 9 | 3.0 | 5.0 | 298 | 139 | |||
| MFM-601 | 3644 | 12 | 7.9 | 12.3 | 298 | ||||
| MFM-300(In) | 1071 | 0.419 | 7.5 | 5.9 | 7.1+ | 8.28 | 298 | 117 | |
| MFM-300(Al) | 1370 | 0.375 | 6.5 | 4.65 | 7.03 | 7.69 | 293 | 114 | |
| Ni-gallate | 455 | 0.154 | 4.85 | 3.37 | 3.79 | 4.49 | 298 | 142 | |
| Co-gallate | 494 | 0.186 | 4.85 | 4.16 | 4.51 | 5.30 | 298 | ||
| Mg-gallate | 576 | 0.213 | 4.85 | 4.87 | 5.19 | 5.81 | 298 | ||
| SIFSIX-1-Cu | 1178 | 8.0 | 3.43 | 8.74 | 11.1 | 298 | 174 | ||
| SIFSIX-2-Cu-i | 503 | 5.2 | 4.16 | 6.01 | 6.90 | 298 | |||
| SIFSIX-3-Zn | 250 | 4.2 | 1.68 | 1.89 | 2.10 | 298 | |||
| SIFSIX-3-Ni | 368 | 4.2 | 2.43 | 2.55 | 2.74 | 298 | |||
| SNFSIX-Cu-TPA | 1169 | 3.33 | 8.09 | 298 | 175 | ||||
| MAF-66 | 1226 | 6 | 308 | 176 | |||||
| F-Ce-MOF-SC-18.1@1.0PA | 52.1 | 0.11 | 8.9 | 15.3 | 298 | 177 | |||
| NbOFFIVECu-TPA | 1179 | 0.50 | 2.0 | 3.8 | 6.3 | 298 | 178 | ||
| TaOFFIVECu-TPA | 1041 | 0.43 | 1.43 | 3.5 | 6.0 | 298 | |||
| ELM-12 | 706 | 0.26 | 4.3 | 0.72 | 1.95 | 2.73 | 298 | 146 | |
| CPL-1 | 335 | 0.125 | 4.1 | 0.47 | 1.06 | 2.0 | 298 | 179 | |
| Zr-TPA-HAc | 2150 | 19.6 | 298 | 180 | |||||
| Zr-TPA-FA | 2190 | 22.7 | 298 | ||||||
| men-MIL-101(Cr) | 2377 | 1.2 | 2.1 | 3.0 | 298 | 181 | |||
| 18-UiO-66-cyanoacetic acid | 1375 | 0.76 | 11.91 | 298 | 182 | ||||
| Ni(BDC)(TED)0.5 | 1783 | 0.74 | 7.8 | 4.54 | 9.97 | 298 | 183 | ||
| Zn(BDC)(TED)0.5 | 1888 | 0.84 | 7.8 | 4.41 | 298 | ||||
| DZU-17 | 1307.9 | 0.68 | 4 | 14.11 | 298 | 184 | |||
| Co6-MOF-3 | 1905.4 | 0.99 | 5 | 16.40 | 298 | ||||
| CPL-11 | 1182 | 6.7 | 5.29 | 298 | 185 | ||||
| BUT-78 | 2031 | 15 | 13.8 | 298 | 186 | ||||
The family of isostructural M-gallate MOFs (M = Mg, Co, and Ni) exhibit record SO2 adsorption at low pressure (0.002 bar).142 The pore structure within these MOFs displays three-dimensional interconnected zigzag channels with a size again approximating the kinetic diameter of SO2 (Fig. 7a–g), leading to solid confinement of SO2. The Co, Mg, and Ni derivatives exhibit SO2 adsorption capacities of 3.99, 4.65, and 2.67 mmol g−1, respectively, at 0.002 bar and 298 K. DFT calculations indicate that the synergistic combination of hydrogen bonding interactions involving SO2 and the unique microstructure of the MOF pores directly contribute to the high SO2 uptake observed at low pressure.
 | ||
| Fig. 7 (a) Illustration of the preparation process and local coordination environments of metal atoms and the ligands. (b) The structure along the c axis displaying the main channels and the periodic branched channels leaning against the main channels. (c) Accessible Connolly surface determined by using a probe with a radius of 1.0 Å. (d) Molecular size of the sulfur dioxide molecule. (e) 3.58 × 4.85 Å2 for Mg-gallate, (f) 3.68 × 4.95 Å2 for Co-gallate, and (g) 3.52 × 4.85 Å2. (Reprinted with permission from ref. 142 Copyright (2021) American Chemical Society). | ||
Based on the idea that an ideal PLD can significantly enhance low-pressure SO2 capture, the pore environment of a Ni(II)-based MOF, Ni2(BDC-X)2DABCO (X = mono-, di- and tetramethyl) was systematically modified via methylation to modulate the low-pressure SO2 adsorption properties (Fig. 8).143 In this case, four homologous MOFs were compared, where different methyl functionalization was introduced: the parent MOF (DMOF) as well as reticular frameworks composed of BDC based linkers substituted with one (M), two (DM) or four (TM) methyl groups. The BDC-TM framework (DMOF-TM) displayed the greatest low pressure SO2 uptake (3.79 mmol g−1 at 293 K and 0.01 bar). This was attributed to increased steric hindrance and hydrophobicity arising from the extensive methyl substitution, leading to changes in the physicochemical properties of the framework, particularly the pore aperture.143 Notably, the SO2 capacity at 0.97 bar decreased with greater methyl substitution due to the systematic decrease in pore volume and BET (Brunauer–Emmett–Teller) surface area. The excellent low-pressure SO2 adsorption capacity conforms to the expected relationship between PLD and low-pressure adsorption capacity since DMOF-TM exhibits a PLD value of ≈4.5 Å (close to the kinetic diameter of SO2) and high uptake at low pressure (in contrast to the other methyl-DMOFs). When confined within pores that approximate the SO2 kinetic diameter, the SO2 molecules engage in extensive dispersion interactions with the pore surface, leading to enhanced uptake.187,188
 | ||
| Fig. 8 Top row: Sections of the packing diagram of DMOF showing the channel structures along the b- (and identical a-) axis and along the c-axis. Bottom row: The building blocks of the Ni2 cluster, DABCO, and BDC/BDC-X in DMOF/DMOF-X. X represents the monomethyl (M), 2,5 dimethyl (DM), or 2,3,5,6 tetramethyl (TM) substituents. (Reprinted from ref. 143 Copyright 2021 with permission from John Wiley & Sons under the Creative Commons CC-BY-NC-ND license). | ||
2.4 Relationship between low and high-pressure SO2 adsorption and in the textual properties of MOFs
As mentioned above, different framework properties influence SO2 capture at low and high pressures. The results described so far indicate that BET surface area and the pore volume are the main factors contributing to high SO2 adsorption capacity at high pressure. Fig. 9 presents the relationship between BET surface area (Fig. 9a) and pore volume (Fig. 9b) with total SO2 uptake at 1 bar. The data indicates that MOF-808, MIL-100(Al), and NH2-MIL-101(Al) display high SO2 uptakes in this pressure regime due to their high surface areas. This effect is related to their micro and mesopore distribution, which improves the SO2 uptake for MIL-100(Al) and NH2-MIL-101(Al), associated with the large BET surface area.151 The framework NU-1000 exhibits a mixture of micro and mesopores (∼12 and ∼29 Å) and is an outlier in the surface area/pore volume relationship observed in other frameworks (Fig. 9b). It is known that saturation is not achieved under these experimental conditions (at 1 bar and room temperature).154 The pore volume represents a limit for the maximum SO2 capacity for a MOF.189 Zr-fum and NH2-MIL-53(Al) show a low SO2 uptake associated with the low surface area and pore volume. These results clearly illustrate the effect of surface area and pore volume on SO2 uptake at higher pressures. | ||
| Fig. 9 SO2 uptake (1 bar, 293 K) vs. (a) BET-surface area and (b) total pore volume. The dashed line is a trend line as a guide to the eye, (c) surface-specific SO2 uptake at 0.1 bar (293 K), which is the uptake at this pressure divided by the BET-surface area vs. the pore limiting diameter (PLD). (Reprinted with permission from ref. 151 Copyright (2021) American Chemical Society). | ||
However, unlike high-pressure SO2 adsorption, SO2 uptake within the low-pressure range is unrelated to surface area and pore volume. Instead, the uptake at low pressure correlates with the affinity between SO2 and the MOF pore surface. This can be mediated by chemical functionalization and/or by tuning the pore diameter using reticular synthesis techniques. Pore diameters only slightly larger than the 4.1 Å kinetic diameter of the SO2 molecule afford high-affinity interactions at low pressure. A clear correlation can be observed by plotting the surface-specific uptake at 0.1 bar divided by the BET surface area against PLD (Fig. 9c).190 As discussed above, pore diameter in the ∼4 and 8 Å range is optimal for high SO2 uptake at low pressure, which correlates well with the SO2 kinetic diameter (4.1 Å) and is supported by GCMC simulations. A PLD size within this range optimizes dispersive interactions between adsorbed SO2 molecule and the pore surface.
To supplement this discussion, SO2 adsorption capacities at pressure increments between 0.01 and 1 bar are summarized in Table 1 in conjunction with crucial framework metrics, including surface area, pore diameter, and pore volume. As expected from the points elaborated on above, this data confirms a relationship between the physical metrics of MOF pores and the observed SO2 uptake. For example, as the BET surface area (Fig. 10a) and pore volume (Fig. 10b) increase, so does SO2 adsorption capacity at 1 bar. For instance, MFM-101 exhibits a high BET surface area (2300 m2 g−1) and an outstanding adsorption capacity (18.7 mmol g−1) at 1 bar and 298 K.129 UR3-MIL-101(Cr) shows a BET surface area of 1900 m2 g−1 and SO2 capture of 13.9 mmol g−1 at 1 bar and 293 K.158 MFM-422 shows a BET surface area of 3296 m2 g−1 and SO2 capture of 13.6 mmol g−1 at 1 bar and 298 K.171 Ni(BDC)(TED)0.5 displays a BET surface area of 1783 m2 g−1 and SO2 capture of 9.97 mmol g−1 at 1 bar and 293 K.183 In the case of pore volume, CB6@MIL-101-Cl displays a high pore volume of 1.0 cm3 g−1 with the uptake of 17.0 mmol g−1 at 1 bar and 298 K.157 MIL-53(Al) with a high volume of 0.706 cm3 g−1 and uptake of 10.5 mmol g−1 at 1 bar, and 293 K.151 DUT-4 shows a high pore volume of 0.71 cm3 g−1 with the uptake of 13.6 mmol g−1 at 1 bar and 298 K.119 MFM-133 shows a high pore volume of 0.96 cm3 g−1 with an uptake of 8.9 mmol g−1 at 1 bar and 298 K.171
 | ||
| Fig. 10 Relation between SO2 uptake at 1 bar and 298 K and (a) BET surface area and (b) pore volume. For references to the individual MOFs, see Table 1. | ||
We note that for studies whose sole ambition is to contend the MOF SO2 adsorption record, a high BET surface area and high pore volume is optimal. However, such characteristics are largely irrelevant to detecting low concentrations of SO2. Instead, selectivity and adsorption capacity at low pressure must be prioritised.
When optimizing the low-pressure SO2 adsorption capacity, the pore diameter becomes arguably the most essential property of MOF. At 0.01 bar, high SO2 adsorption (3–5 mmol g−1) is strongly correlated to a pore diameter between 4 to 10 Å (Fig. 11), which is in good agreement with the above discussion. For example, SIFSIX-2-Cu-I with the linker 4,4′-dipyridylacetylene possesses a narrow pore diameter (5.2 Å) and a high SO2 adsorption (4.16 mmol g−1) at 0.01 bar and 298 K.174 This is because the kinetic diameter of the SO2 molecule (4.1 Å) is close to the pore diameter, thereby maximizing dispersion forces between SO2 and the pore walls. In the case of SO2 adsorption experiments, to increase the intermolecular interactions, the adequate diffusion of the SO2 gas through the MOF pores is necessary to achieve adsorption successfully.191
 | ||
| Fig. 11 Relation between SO2 uptake at 0.01 bar with PLD. For references to the individual MOFs, see Table 1. | ||
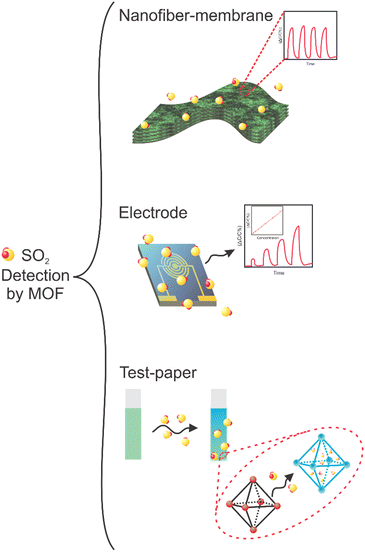
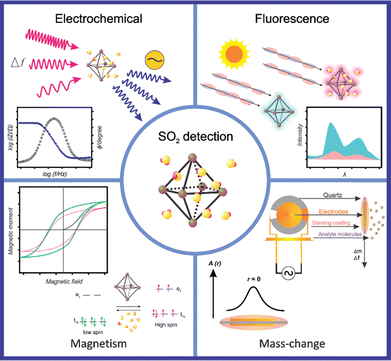
3. MOFs applied in SO2 detection
Although the detection of SO2 using MOFs remains poorly explored, various techniques that leverage the advantageous features of MOFs are currently under investigation for this purpose. In principle, the presence of an analyte can be confirmed by monitoring characteristic MOF properties that are altered after an external stimulus (in this case, the SO2 interaction). This is the fundamental principle upon which MOF-based sensors are premised. The response to the host–guest interaction provides a probe for qualitative and quantitative sensing or detection applications. Only a few MOFs have been employed for SO2 detection and these have been based on (i) analyte-induced changes in their luminescent properties (generating an energy transfer), (ii) changes in the electrochemical properties (changes in electrical resistance), (iii) changes in spin-crossover (SCO) behavior (change in the spin state), and (iv) a change in the sample mass. To supplement this discussion, the MOF-based materials applied for SO2 detection are summarized in Table 2 in conjunction with crucial parameters, including sensing technique, sensitivity, and selectivity.| Material | Method | Matrix | Selectivity | SO2 concentration range | SO2 detection level | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Eu-BDC-NH2 film | Luminescence | MOF film | Over N2, CO2, O2, NH3, HCHO, H2O, and H2S | 0–200 ppm | 0.65 ppm | Turn-off effect by energy transfer | 192 |
| MOF-303 | Luminescence | Solid state (powder) | Over CO2, CH4, and H2O | Up to 0.1 bar | Turn-on effect | 193 | |
| CYCU-3 | Luminescence | Solid state (powder) | Over CO2, and H2O | Up to 0.1 bar | Turn-on effect by energy transfer | 194 | |
| Ce–PA–Tb | Luminescence | Solid state (powder) | 0–70.4 ppm | 0.093 μM | Turn-on effect by energy transfer | 195 | |
| DNA–Tb-MOF | Luminescence | Test paper | 0.2–1.6 ppm | 0.02 ppm | Turn-on effect by energy transfer | 196 | |
| MOP-CDC | Luminescence | Solid state (powder) | Up to 0.1 bar | Turn-off effect | 197 | ||
| Mg2DOBPDC | Luminescence | Solid state (powder) | Up to 0.1 bar | Turn-on effect | 198 | ||
| Ni2(dobpdc) | Luminescence | Solid state (powder) | Over CO2, and H2O | Up to 0.1 bar | Turn-on effect | 199 | |
| MIL-53(Cr)-Br | Luminescence | Solid state (powder) | Over CO2, and H2O | Up to 0.1 bar | Turn-on effect | 200 | |
| MUF-16 | Luminescence | Solid state (powder) | Over NO2, CO2, H2O, H2S, O2, N2, and CH4 | Up to 0.1 bar | Turn-on effect | 201 | |
| THF suspension | 1–250 mM | 80.72 ppm | |||||
| MOF-5-NH2 | Luminescence | Test paper | Over NO2, NH3, N2, CO2, H2S, and CS2 | 0–3 ppm | 0.05 ppm | Turn-on effect | 202 |
| UTSA-16(Zn) | Luminescence | THF suspension | 1–5 mM | 114.6 ppm | Turn-off effect | 203 | |
| Ni3BTC2/OH-SWNTs | Electrochemical | Microelectrode | Over NO2, CH4, CO, and C2H2 | 4–20 ppm | 4 ppm | Electron transfer | 204 |
| CoZn-NCNTs | Electrochemical | Solid state (powder) | Over NO2, MeOH, acetone, NH3, CO, H2, and EtOH | 0.5–30 ppm | 0.5 ppm | Increase of hole density | 205 |
| Ni-MOF/-OH-SWNTs | Electrochemical | Solid state (powder) | Over NO2, NH3, and CO | 0.5–15 ppm | 0.5 ppm | Electron transfer | 206 |
| UiO-66-NH2/PVDF NM | Electrochemical | Nanofibers membrane | 1–150 ppm | Interaction with NH2 groups | 207 | ||
| PAN@UiO-66-NH2 NM | Electrochemical | Nanofibers membrane | Over CO, CH4O, C2H6O, C3H8O, and C3H6O | 1–125 ppm | Interaction with NH2 groups | 208 | |
| UiO-66-THB/PAN-based | Electrochemical | Electrode | Over CO2, H2S, NO2, NO, CO, NH3, C3H6O, and C2H6O | 1–125 ppm | 0.1 ppm | Hydrogen bonding | 209 |
| TM-Ag@NU-901 | Electrochemical | MOF film | 10–200 μM | 0.1 ppm | Interaction with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups C groups |
210 | |
| UiO-66-NH2 | Electrochemical | Solid state (powder) | 1–10 ppm | 1 ppm | Formation of a charge-transfer complex | 211 | |
| MFM-300(In) | Electrochemical | Electrode | Over CH4, H2, CO2, C3H8, C7H8, and NO2 | 75–1000 ppb | 75 ppb | Capacitance | 212 |
| Fe(PZ)[Pt(CN)4] | Magnetism | Solid state (powder) | Over CO2, and CS2 | Stabilization of the LS state | 213 | ||
| KAUST-7 | Gravimetric | QCM | Over H2O | 0–500 ppm | 5 ppm | Mass change | 214 |
3.1 MOFs for luminescent SO2 detection
Luminescence behavior has been extensively studied in the MOF field.215 Generally, such materials are called luminescent metal–organic frameworks (LMOFs) and have been used in optical, medical, and detection applications.216–218 Different strategies have been developed to construct luminescent MOFs, which are based on the “signal-off” or “signal-on” response strategies (in other words, the so-called turn-on and turn-off effect).219 The emission centers in such materials may constitute the metal ions, organic linkers, and guest species. The organic linkers typically present π-conjugated systems, facilitating a fluorescence response due to accessible π–π* transitions.220 In the case of metal centers, the lanthanide family – particularly Tb3+ and Eu3+ – are frequently employed due to the accessible transitions between 5D0–7Fj states.221 Considering these properties, MOFs are excellent candidates for the detection of not only SO2 but also multiple analytes using luminescent properties.96 Notably, the rational construction of LMOFs that exhibit energy transfer properties can tune the luminescence.222,223LMOFs can be synthesized with a tremendous diversity of organic linkers and metal clusters (including pristine MOFs or with linker modifications), providing a wide range of energy transfer LMOFs (ET-LMOFs),224 affording multiple detection options depending on the target analyte.225 Additionally, chromophores can be regularly aligned and carefully ordered inside the crystalline LMOF lattice, providing a basis for understanding the short- and long-distance energy transfer mechanisms.226 The high crystallinity and periodicity of MOFs are advantageous for computational models and calculations that aim to elucidate the luminescence mechanism of LMOFs.227,228
LMOFs have been intensely studied for solar cells,229 photocatalysis,230 scintillators,231 X-ray and NMR imaging,232 and for detecting analytes pertinent to gas pollution.84 The possible luminescent centers and charge transfer processes in LMOFs (Fig. 12) are classified as (Fig. 12a) linker-centered emission, guest-centered emission, and metal-centered emission, and (Fig. 12b) linker-to-linker, metal-to-metal, metal-to-linker, the linker-to-metal, guest to host, and host to guest.233 Herein, we will not specifically discuss each case since this would constitute a significant departure from the stated aim of this contribution. However, we provide a brief description when necessary and encourage readers to consider several relevant contributions.234,235 Aside from possessing suitable luminescent behavior, the first essential requirement for an LMOF to be considered for SO2 detection applications is demonstrable chemical stability towards SO2 under ambient conditions (including humidity), as previously mentioned (vide supra).
 | ||
| Fig. 12 Schematization of (a) possible luminesce centers and (b) charge transfer processes. Based on ref. 233. | ||
For example, Chen and Wang reported a Ce4+/Tb3+ MOF, Ce–PA–Tb MOF, with the linker PA = m-phthalate, with promising attributes for SO2 detection.195 The design of this novel MOF was inspired by the advantages of lanthanide luminescent properties, which include a long luminescence lifetime.236 The MOF is a bimetallic material with Ce4+ and Tb3+ centers coordinated with PA linkers. To assess the detection prowess of the material, the authors generated SO2 gas in situ using ‘Kipp's device’ – a chamber wherein sodium sulfite (Na2SO3) is combined with sulfuric acid (H2SO4) under a N2 atmosphere to generate the SO2 gas (Fig. 13). Samples containing SO2 were analyzed using three separate methods: Ce–PA–Tb MOF probed by luminescence, Ce–PA–Tb MOF incorporated into a test strip, and formaldehyde absorbing pararosaniline spectrophotometry (FAPA). The limit of detection (LOD) was found to be 0.006 μg mL−1 (0.093 μM), 0.5 μg mL−1 (7.8 μM), and 0.05 μg mL−1 (0.78 μM) for the respective detection methods. Notably, the luminescence-based measurement is ten times more sensitive to SO2 than the Ce–PA–Tb-MOF test strip method or FAPA. The mechanism involves the SO2-induced reduction of Ce4+ to Ce3+; subsequent irradiation with 250 nm photons induces an energy transfer from Ce3+ to the adjacent Tb3+ ion. An electronic transition within the Tb3+ ion leads to emission at 545 nm, which is measured. Crucially, the energy transfer does not occur from Ce4+ to Tb3+. The presence of Ce3+ was confirmed using XPS spectroscopy. It was not stated if the sensor is re-usable.
 | ||
| Fig. 13 Determination of SO2 gas by the methods of standard formaldehyde absorbing pararosaniline (FAPA) spectrophotometry, Ce–PA–Tb luminescence and Ce–PA–Tb test strip with a 254 nm UV lamp. (Reprinted from ref. 195 Copyright 2020 with permission from Royal Society of Chemistry). | ||
The use of luminescent MOF-based SO2 sensors was recently expanded with the development of a DNA-based Tb-MOF composite for SO2 detection.196 Briefly, single-stranded DNA (ssDNA) was combined with Tb3+ to form ssDNA-Tb3+ which was combined with IR-MOF-3 MOF in an ethanol suspension to form a composite. A test strip was fabricated using the DNA-based Tb-MOF composite in this case. The authors used Kipp's device to generate SO2 for the purpose of assessing the performance of the composite sensor. The results indicate a LOD value of 0.02 ppm of SO2, a low value which confirms that the material provides a promising platform for SO2 detection. The DNA-based Tb-MOF composite exhibits a weak PL emission and displays an apparent turn-on effect after interaction with SO2 and analogues thereof. The authors suggested that the material operates via a charge transfer mechanism: the amino groups present in DNA–Tb-MOF function as electron-donors from the perspective of the Tb3+ ions. When SO2 and its analogues such as HSO3− interact with the amino group, it negates the typical energy transfer between the amino group and Tb3+ ions, generating a PL turn-on effect at 491, 546, 585, and 620 nm upon irradiation at 290 nm. These investigations confirm that Tb-MOFs exhibit luminescent properties which form a promising basis for SO2 detection.
Interestingly, apart from the mechanisms already discussed, changes in luminescence may also be induced by the interaction between SO2 and the structural linkers. A Cu(II)–metal–organic polyhedron (MOP-CDC, CDC = 9H-carbazole-3,6-dicarboxylate) displays a turn-off effect in its fluorescence after SO2 adsorption.197 At low pressure (0.05 bar), MOP-CDC exhibits an SO2 uptake of 1.0 mmol g−1 at 298 K. Under 440 nm excitation, MOP-CDC exhibits strong fluorescence emission at 540 and 639 nm. After the SO2 exposure, these bands are quenched, providing a convenient probe for the presence of SO2. DFT calculations demonstrate that the SO2 molecule interacts with the carbazole NH site through hydrogen-bonding [N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]. Due to this strong host–guest interaction, SO2 adsorption induces fluorescence quenching. Notably, CO2 adsorption (a potential interfering gas) had no apparent effect on fluorescence intensity.
O]. Due to this strong host–guest interaction, SO2 adsorption induces fluorescence quenching. Notably, CO2 adsorption (a potential interfering gas) had no apparent effect on fluorescence intensity.
However, in some cases, energy transfer processes involving the organic linker result in a turn-on effect. For instance, Mg2DOBPDC (DOBPDC = 4,4-dioxidobiphenyl-3,3-dicarboxylate), which shows high SO2 adsorption at low pressure (0.05 bar, 6 mmol g−1 at 298 K).198 At an even lower pressure of 0.002 bar, the material displays an SO2 uptake of approximately 2.4 mmol g−1. This value is comparable to record low pressure SO2 adsorption exhibited by M-gallate MOFs142 and several frameworks listed in Table 1. GCMC simulations revealed that SO2 preferentially adsorbs at open Mg2+ coordination sites in a monodentate fashion (SO2(O)–Mg = 2.17 Å). Nevertheless, the coordinated SO2 also engages in hydrogen bonding with the adjacent DOBPDC linker, thereby modulating the luminescent properties of the material. Thus, during SO2 exposure under 320 nm irradiation, the broad photoluminescence band at 437 nm shifts to 461 nm, concomitantly increasing the band's intensity (Fig. 14).
 | ||
| Fig. 14 Emission spectra of Mg2(dobpdc) before (blue) and after (pink) exposure to SO2. Both samples were excited with 320 nm UV light. (Reproduced from ref. 198 Copyright 2022 with permission from Royal Society of Chemistry). | ||
In addition, the isostructural framework Ni2(DOBPDC) was investigated for application in SO2 detection.199 Under 350 nm irradiation, Ni2(DOBPDC) exhibits a broad emission band at 450 nm. After the sample is exposed to SO2, the emission peak shifts to 405 nm with a 61% increase in emission intensity. This behavior was observed even at low SO2 pressure (0.1 bar). To investigate the luminescent mechanism, a time-resolved photoluminescence (TRPL) experiment was performed using a 340 nm picosecond-pulsed LED as the excitation source. The results revealed that the average decay lifetime increases from 2.14 to 2.47 ns upon SO2 exposure. This suggests that interaction between the SO2 and Ni2+ centers within the framework nullifies the organic linker's molecular motion, minimizing the non-radiative decay pathways available and thereby causing the fluorescence lifetime to increase.
MOF-303 is composed of Al(III) centers which are interconnected by PZDC linkers (PZDC = 1H-pyrazole-3,5-dicarboxylate) and was recently evaluated for SO2 detection.193 MOF-303 displays one of the highest low pressure SO2 adsorption capacities so-far reported (6.21 mmol g−1 at 298 K and 0.1 bar). At 298 K, the first adsorption step occurs at 0.05 bar and corresponds to 5.44 mmol g−1 of SO2, confirming a high affinity between SO2 and MOF-303. In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) experiments revealed the preferential adsorption sites to be μ2-OH and linker N–H sites, which interact with SO2 through hydrogen bonding. In this material, a hydrogen-bonded dimer forms via adjacent pyrazole groups within the pore, generating hydrophilic pockets that bind small molecules, here SO2. Considering the fluorescent properties of the PZDC linker in several coordination compounds, the luminescent properties of MOF-303 were investigated. However, in MOF-303, the linker fluorescence is quenched because the absorbed energy is released through non-radiative pathways. However, exposure to SO2 under 248 nm irradiation resulted in a fluorescence turn-on effect with emission at 299 nm (Fig. 15). This represents an approximately 125 nm shift in emission relative to that of the linker. No apparent change in emission was observed in the presence of the common interfering gases CH4 or CO2. The authors suggest that the physisorption of SO2 within MOF-303 leads to a rigidification of the structure which suppresses non-radiative decay pathways, thereby intensifying emission.
 | ||
| Fig. 15 Solid-state emission spectra of PZDC linker (black line), activated MOF-303 (green line), after exposure to: CH4 (red line); CO2 (orange line); H2O (purple line); and SO2 (blue line). The excitation wavelength was set at 248 nm. (Reproduced from ref. 193 Copyright 2022 with permission from American Chemical Society). | ||
Similarly, CYCU-3, also an Al(III)-based MOF but composed with SDC linkers (SDC = 4,4′-stilbenedicarboxylate), was assessed for SO2 detection and capture applications.194 CYCU-3 shows a total uptake of 11.03 mmol g−1 at 1 bar and 298 K. The interaction between SO2 and the pore surface was elucidated using in situ DRIFTS experiments and theoretical calculations. Bridging hydroxyl moieties within the inorganic cluster were identified as preferential interaction sites for SO2. The fluorescence spectra of both CYCU-3 and solid H2SDC were recorded. Under 343 nm irradiation, H2SDC produces a fluorescence emission peak at 450 nm. However, the fluorescent emission from CYCU-3 is blue shifted and less intense than that of the free ligand due to charge transfer between the organic linker and Al(III) centres. After the sample is exposed to SO2 under irradiation at 343 nm, the emission at 450 nm increased in intensity. This performance was attributed to an enhanced ligand-centered π* → π electronic transition.
Cr(III)-MOFs have also been applied for SO2 adsorption and detection, including MIL-53(Cr) (linker: BDC) and the novel reticular analogs MIL-53(Cr)-Br and MIL-53(Cr)-NO2 with the linkers BDC-Br and BDC-NO2 respectively.200 In the presence of SO2, these MOFs show a turn-off effect under irradiation at 300, 360, and 350 nm, respectively, corresponding to a decrease in the emission intensity at 415, 420, and 507 nm, respectively. The intensity decrease was associated with a charge transfer process involving the organic linker. MIL-53(Cr) displays a slight red shift, suggesting metal-to-linker charge transfer while MIL-53(Cr)-Br shows a change in the emission peak from 450 to 436 nm.
MUF-16 is a Co(II) based framework composed of 5-aminoisophthalate (AIP) linkers, formula [Co(AIP)2], which was explored for the selective detection and capture of SO2.201 The SO2 adsorption isotherm shows an uptake of 2.2 mmol g−1 at 298 K and 1 bar. Employing FTIR, DFT calculations, and GCMC simulations, SO2 was found to engage in favorable hydrogen bonding interactions with the amino groups which decorate the framework. An increased fluorescence response is observed in the presence of SO2 compared to the other common gases such as CO2, NO2, N2, O2, CH4, and water vapor (Fig. 16a and b). The LOD was calculated using a THF solution of SO2 and was found to be 1.26 mM (∼81 ppm). A fluorescence mechanism was proposed using TRPL analysis.201
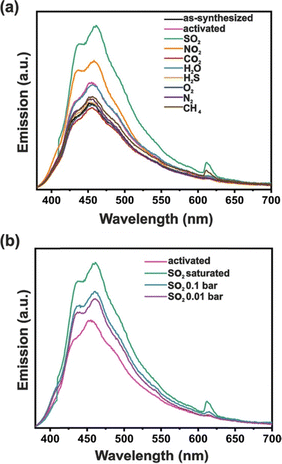 | ||
| Fig. 16 (a) Comparison of solid-state emission spectra of MUF-16 exposed to different gases, and (b) comparison of solid-state emission spectra of MUF-16 exposed to different SO2 pressures (Reproduced from ref. 201 Copyright 2024 with permission from American Chemical Society Under CC-BT 4.0 license). | ||
The amino-functionalized derivative of MOF-5, IR-MOF-3, was incorporated into a test strip for rapid and selective sensing of SO2 and its derivatives via a luminescence enhancement turn-on effect.202 The test strip offers real-time detection of SO2 with a detection limit of 0.05 ppm. Within IR-MOF-3 the amino groups donate electron density to the metal centres which quenches the luminescence. However, when SO2 (or HSO32−) interacts with the amino group, a complex is formed which disrupts the linker-to-metal charge transfer process, turning on the characteristic luminescence of the linker. XPS spectroscopy confirms the formation of N–S interactions between amino groups within IR-MOF-3 and SO32−. Test strips containing MOF-5 and IR-MOF-3 were exposed to SO2 gas generated using a Kipp apparatus (Fig. 17a–c). Notably, unfunctionalized MOF-5 exhibits no response to SO2. The test strip impregnated with MOF-5-NH2 was found to be stable after exposure to SO2, suggesting that the system is reusable for detecting SO2 with a particularly short 15-second response time. The LOD was calculated to be 0.05 ppm for the test paper. It is worth mentioning that the chemical stability of MOF-5 should be considered when evaluating its suitability for SO2 detection. The material has for instance proven unstable to water.237
 | ||
| Fig. 17 (a) and (b) Schematic diagrams of the device for detecting SO2 gas using MOF-5 and MOF-5-NH2 luminescent test paper, respectively. (c) Luminescence response photographs of MOF-5-NH2 luminescent test paper after exposure to various gas species under a 365 nm UV lamp. The final concentrations of SO2, NO2, NH3, N2, CO2, and H2S were 2 ppm, while CS2 gas was saturated vapor of liquid-state CS2. (Reproduced from ref. 202 Copyright 2018 with permission from American Chemical Society). | ||
A technique, named in situ secondary growth, allows MOFs to be deposited on membranes. Qian et al.192 reported a MOF film based on a Eu(III) MOF with BDC-NH2 linkers. First, the authors prepared a hydroxyl functionalized glass surface using ‘piranha’ solution (H2SO4/H2O2 solution). Then, UiO-66-NH2 was synthesized in situ on the functionalized glass. Subsequently, the Eu-MOF was grown by solvothermal synthesis to form a layer which acts as a fluorescence probe for SO2. Exposure to SO2 leads to quenching of the fluorescent solid emission due to the 5D0 → 7F2 transition of Eu3+. The decay curves for N2 and SO2 indicate a reduced emission lifetime of 381.8 μs in 1% SO2, suggesting the involvement of a charge transfer process between the linker and SO2 molecules. The LOD value was reported to be 0.65 ppm with a response time of as short as 6 s.
3.2 MOFs for electrochemical SO2 detection
Besides the luminescence-based sensors described above, electrochemical processes have also been widely used for small molecule detection and quantification. The operation of electrochemical sensors depends on electron transfer events that occur during interactions between the surface of the material and analyte gas molecules.238 The transfer of electrons accompanying analyte interactions leads to a change in resistance, which can be measured. This change in resistance, and therefore the sensor's sensitivity, depends to a considerable extent on the nature of the material with which the analyte interacts.239 Materials commonly employed in electrochemical sensors include metal oxides, carbons, nitrides, sulfides, and – to a growing extent – MOFs. The most important parameters to consider in achieving optimal performance are selectivity, response and recovery speed, and stability.240 The surface area and the reactivity of the surface towards the analyte strongly influence the response. A high response factor can be accompanied by a low LOD, the minimum analyte concentration to which the sensor is sensitive. Various interfering species may be present in the environment besides the analyte of interest. Thus, selectivity is crucial for accurate and reliable detection and is usually evaluated by cross-sensitivity comparison wherein the senor is exposed to various interfering species at fixed concentrations. Selectivity is affected by many factors related to the environment, such as humidity and temperature, the nature and composition of the sensor, the affinity between the gas molecules, and the properties of the sensor material.241Electrochemical techniques are now being implemented for SO2 detection using MOFs. For example, a composite based on nickel benzene-tricarboxylate (Ni3BTC2) and OH-functionalized single-walled carbon nanotubes (OH-SWNTs) was investigated for this purpose.204 After the composite was exposed to SO2, the measured change in voltage was successfully related to the SO2 concentration. A response time of 4.59 s with a recovery time of 11.04 s was achieved with a low SO2 concentration (15 ppm). This behavior was attributed to an electron transfer from the composite to the SO2 molecule. In this case, the composite is a p-type material, where a transfer of electrons from the composite to the SO2 molecule (an electron acceptor) occurs. The selectivity of the composite sensor is maintained in the presence of NO2, CH4, CO, and C2H2, typical interfering gases in nature.
Moreover, the relative change in electrical resistance can also be leveraged for small molecule sensing. For example, in 2018 Li et al.205 reported a composite material derived from pyrolysis of Zn/Co bimetallic ZIF-67 which undergoes a 53% change in resistance in the presence of SO2 (100 ppm). A cross-selectivity test was performed using NO2, MeOH, acetone, NH3, CO, H2, and EtOH vapor. The material shows high selectivity over these gases even at low SO2 concentrations (30 ppm). The response and recovery times are reportedly 88 and 900 s, respectively, with a limit of detection for SO2 equal to 0.5 ppm.
The changes in the electrical resistance of a Ni-MOF composite (Ni-MOF/-OH-SWNTs) allowed a rapid response time of 10 s with a fast recovery time of 30 s for SO2 (1 ppm).206 This function is maintained even in the presence of NO2, NH3, and CO. It is known that holes form the major charge carrier within the Ni-MOF composite in the absence of SO2. However, since SO2 acts as an electron donor it acts to reduce the population of holes via recombination. Because holes are the major carrier within the composite the presence of SO2 leads to a quantifiable increase in resistance.
Building on these developments, Zhang et al.207 reported a capacitive sensor composed of UiO-66-NH2 incorporated into a nanofiber membrane composed of polyvinylidene fluoride and carbon nanotubes. The composite material was employed as a sensing layer for real-time monitoring of SO2. The amine functional groups interact strongly with SO2 inside the sensor, leading to a change in conductivity (Fig. 18a–d). The detection response time was reportedly 435 s and 185 s towards 150 ppm and 1 ppm SO2, respectively. Importantly, the SO2 concentration and change in capacitance are strongly correlated, which was attributed to the adsorption capacity of UiO-66-NH2. The sensor also shows high reproducibility for 100 ppm SO2 over ten consecutive cycles. A long-term study was conducted over the course of 20 days in which 10 and 50 ppm SO2 samples were measured, the change in conductivity was retained ∼89% of its original value over this time. The SO2 sensing performance is stable towards moderate temperature changes, dropping only 22% in going from 30 to 70 °C.
 | ||
| Fig. 18 (a) Detection of SO2 in the range of 1 to 150 ppm concentration and linear response for the testing range of the inset; (b) reproducibility for the detection of 100 ppm SO2; (c) response ability for the sensor at 10 and 50 ppm SO2 within 20 days; (d) temperature influence on the sensor performance. (Reproduced from ref. 207 Copyright 2021 with permission from John Wiley & Sons). | ||
To improve the response time of the nanofiber membrane, the authors also designed a new flexible gas sensor in which UiO-66-NH2 was incorporated into electrospun polyacrylonitrile (PAN) nanofibers.208 The device was equipped with carbon nanotube electrodes. The high surface area and porosity of UiO-66-NH2 make it particularly useful in an electrochemical detection device since the analyte can rapidly diffuse into the MOF. Crucially, the well-established flexibility of the membrane provides exceptional long-term stability.242 The sensor reportedly to operates with a 1 ppm LOD for SO2, and the porous MOF platform facilitates rapid SO2 diffusion within the material with a fast response time of 255 s.
In a separate investigation from the same research group, the MOF UiO-66-NH2 was incorporated into a nanofiber membrane which was modified to improve SO2 adsorption and thereby improve the limit of detection.209 UiO-66-NH2 was loaded onto a PAN nanofiber membrane and modified with 2,3,4-trihydroxybenzaldehyde (THBA). The composite was synthesized by using imine condensation to cross-link the amine and aldehyde groups to form a Schiff base and obtain a UiO-66-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-THB/PAN-based capacitive gas sensor. This design achieved a lower SO2 detection limit of 0.1 ppm. Based on DFT calculations, hydrogen bonding between SO2 and the THB hydroxyl groups resulted in a high adsorption affinity. Considering the potential of MOF-based membranes in SO2 detection applications, NU-901 (with the linker TBAPy = 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl)tetrabenzoate) was embedded in a silica film.210 This film was modified with thiol-magenta (TM) and Ag nanoparticles (TM-Ag@NU-901). SO2 was detected by surface-enhanced Raman scattering, a new alternative strategy for detecting SO2.
C-THB/PAN-based capacitive gas sensor. This design achieved a lower SO2 detection limit of 0.1 ppm. Based on DFT calculations, hydrogen bonding between SO2 and the THB hydroxyl groups resulted in a high adsorption affinity. Considering the potential of MOF-based membranes in SO2 detection applications, NU-901 (with the linker TBAPy = 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl)tetrabenzoate) was embedded in a silica film.210 This film was modified with thiol-magenta (TM) and Ag nanoparticles (TM-Ag@NU-901). SO2 was detected by surface-enhanced Raman scattering, a new alternative strategy for detecting SO2.
The UiO-66 analogs UiO-66-NH2 and UiO-66-OH were employed as chemoresistive sensors for SO2, NO2, and CO2.211 Archetypal UiO-66 does not exhibit a change in resistance after exposure to any of the acidic gases listed above. However, UiO-66-NH2 responds with a 22 ± 3% change in resistance to the presence of 10 ppm SO2, with a 1 ppm LOD (corresponding to a 3.2 ± 0.2% response). This performance was attributed to the formation of a charge-transfer complex when SO2 interacts with the amine-functionalized linker.
As discussed already, MFM-300(In) exhibits outstanding properties for SO2 sorption and sensing applications due to a high SO2 uptake at low pressure and excellent stability. Building on previous work, MFM-300(In) was applied as an electrode for SO2 detection.212 The In(III)-based MOF was coated on interdigitated electrodes, and the capacitance changes that occur in response to SO2 were measured. This sensor displays one of the highest sensitivities to SO2 and excellent selectivity over interfering gases such as methane, hydrogen, carbon dioxide, nitrogen dioxide, propane, and toluene at 1000 ppb. SO2 concentration was successfully measured from 75 to 1000 ppb with a detection limit of 5 ppb. The electrochemical response was attributed to the interaction between SO2 and the μ2-OH groups in the MOF node (through hydrogen bonds), with further dipole–dipole interactions between adsorbed SO2 molecules. The resulting electrostatic changes perturb the capacitance of the electrode.
3.3 Other detection techniques
In addition, MOFs have been employed in alternative SO2 detection systems that use magnetic and mass change sensors. These provide an opportunity to exploit the diverse physicochemical properties of MOFs that do not find utility in the sensing techniques explored so-far. However, only a few examples have been reported, and we therefore emphasize the opportunity these methodologies present for future sensing applications.In general, for magnetic gas sensors involve analyte-induced changes to the magnetic properties of the sensing material. Such changes can be measured through a range of sophisticated techniques that are beyond the scope of this review.243 Magnetic gas sensors offer advantages over other gas sensors; for example, they can be designed to operate in a wide temperature range, do not require an electrical current source (therefore, the risk of explosion or fire is reduced), and the response time is much reduced compared to chemosensitivity sensors.244,245 Various materials are employed as sensing materials in magnetic gas sensors, recently this has included MOFs.246,247
Spin-crossover has emerged as an essential chemical phenomenon upon which magnetic gas sensors can be designed. Recently, MOFs that exhibit spin-crossover behavior have been studied. These typically exhibit structural nodes with 3d4–3d7 transition metals in an octahedral coordination geometry. The spin-crossover phenomenon involves stimuli-induced switching between a low-spin and high-spin electronic configuration.248,249 Of relevance in gas sensors, this change can be induced by the interaction between an analyte gas and the sensing material.
For example, Pham et al.213 undertook a highly explorative study to demonstrate in principal that spin-crossover (SCO) behavior in a MOF can be exploited for SO2 detection. {Fe(PZ)[Pt(CN)4]} (PZ = pyrazine) was used to explore how adsorption of SO2 affects the population of high and low spin states. Differences between the SCO properties of {Fe(PZ)[Pt(CN)4]} during the adsorption of various gases point to specific guest–framework interactions, which appear to be sensitive to the physicochemical properties of the guest molecule. In this case, the gas molecules stabilized the LS state of the framework. The material was exposed to CO2, SO2, and CS2 during the heating process in both experimental and simulated settings (Fig. 19). The SO2 molecule was found to stabilize the LS state, leading to a 20 K shift in temperature caused by changes in the Fe–N bonds within the framework.
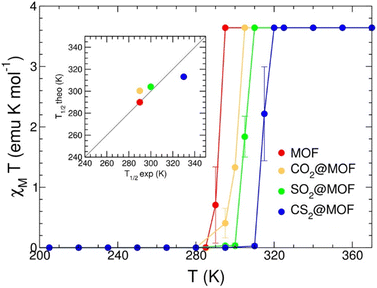 | ||
| Fig. 19 Temperature dependence of χMT calculated from MC/MD simulations of {Fe(PZ)[Pt(CN)4]} with no adsorbed guest molecules (MOF) as well as upon adsorption of CO2 (CO2@MOF), SO2 (SO2@MOF), and CS2 (CS2@MOF). The theoretical values of the SCO temperature (T1/2) are compared in the inset with the experimental values. (Reproduced from ref. 213 Copyright 2018 with permission from American Chemical Society). | ||
Mass change gas sensors which employ a quartz crystal microbalance (QCM) are popular and widely used in industry. QCM sensors exploit the quantitative relationship between the change in frequency of a quartz crystal resonator and the mass change resulting from the adsorption of analyte gas molecules on the QCM.250 Crucially, the quartz surface can be coated with an appropriate film to enhance the sensitivity and selectivity of the sensor.251,252 The advantage of QCM sensors is that they are susceptible to mass changes in the nano-gram range. However, fragility can present challenges.253 Porous materials such as silicas and MOFs have been used as coatings on the quartz surface to improve the performance of QCM sensors.234 However, it is worth mentioning that gravimetric detection exhibits drawbacks related to low selectivity.
For example, the isostructural fluorinated MOFs KAUST-7 ([Ni(NbOF5)(pyrazine)2]·2H2O) and KAUST-8 ([Ni(AlF5(OH2))(pyrazine)2]·2H2O) were employed as coatings on QCM based SO2 sensors.214 The difference between these materials is the presence of (NbOF5)2−versus (AlF5(OH2))2− within the framework. The authors noted that KAUST-7 exhibits a high affinity for SO2, SCXRD confirms that SO2 interacts with two electronegative fluorine atoms of the adjacent (NbOF5)2− moiety via the electropositive sulfur atom, while four C–H⋯O contacts stabilize the interaction. Meanwhile, in KAUST-8, the SO2 molecule only interacts with four C–H⋯O from two neighboring pyrazines. Based on these properties, the materials were studied for SO2 detection in the presence and absence of humidity to mimic atmospheric conditions. Following the change in frequency of the quartz crystal resonator, SO2 was successfully detected at concentrations between 0 and 500 ppm in balance with nitrogen. The system exhibited with high stability and reproducibility. Both MOF-coated materials show a nonlinear decrease in sensitivity with the increased SO2 concentration. The lowest detection limit was estimated to be about 100 ppb with noise drift in the resonance frequency of ±1.5 Hz. However, the experimental lowest detection limit was 5 ppm.
4. Overview of SO2 detection methodology
Considering the discussion above, it is evident that metal–organic frameworks and coordination polymers are well suited for application in gas sensors and detectors. Notably, and unlike many other applications proposed for MOFs, high surface area or elevated gas uptake is not imperative for sensing. Thus, materials deemed unsuitable for “traditional” adsorption applications may find utility in sensing and detection processes where chemical robustness and functionality are prized over uptake capacity. As discussed in this review, the ideal MOF for sensing is stable under relevant working conditions and exhibits a precise and reproducible physical response upon interaction with the analyte at environmental concentrations. Considering these metrics, various devices designed for SO2 detection were discussed (Fig. 20). These were primarily based on (i) nanofiber membranes, (ii) electrodes, and (iii) test strips.Below (Fig. 21), the most relevant characterization techniques are evaluated for their potential in gas sensing applications.
(a) Fluorescence measurements: given the broad applicability, high selectivity, and potential for use in super-resolution experiments, fluorescence is one of the most commonly used chemo-sensing techniques.254 Some fluorescence measurement techniques which are frequently encountered include:
(i) Fluorescence spectroscopy: this technique involves measuring the emission spectrum of a MOF before and after gas adsorption. The change in fluorescence intensity or wavelength can be used to detect and quantify the presence of gas molecules and determine the selectivity of the MOF towards the analyte of interest.255
(ii) Time-resolved fluorescence spectroscopy: this technique involves measuring the decay time of the fluorescence emission of a MOF after excitation. The change in decay time upon gas adsorption can be used to detect and quantify the presence of gas molecules and determine the selectivity of the MOF towards the analyte of interest.196
(b) Electrochemical measurements: another possible physical response that can be used to sense or detect gaseous molecules is the change in the material's conductivity (or resistivity). The sorption of gas molecules can alter the electrical conductivity of MOFs, which can be measured to detect (and even quantify the concentration of) specific gas molecules. MOFs provide an ideal platform for gas sensing and detection using this technique.256 However, it must be noted that most MOFs and coordination polymers have very high resistivity and, thus, are not amenable to this kind of measurement. Some of the commonly used conductivity measurements are:
(i) Two and four-point probe measurements: this technique involves applying a voltage to the MOF and measuring the current flowing through it. Thus, the resistivity and conductivity of the MOF can be calculated from the measured values. The measurement can be performed in single crystals, films, or pellets of polycrystalline materials. However, special care must be taken to ensure that the contacts do not interfere with gas sorption and that a reproducible contact is made between the sample and electrodes.257,258
(ii) Impedance spectroscopy: this technique involves applying an AC voltage to the MOF and measuring the impedance of the MOF as a function of frequency. The frequency-dependent impedance can provide information about the charge transport properties of the MOF. Similarly, the measurement can be performed in single crystals, thin films, or press pellets. Once again, changes in these properties can be induced by the sorption of the analyte, providing a probe for the detection and quantification of analyte gases.259,260
(iii) Field effect transistors (FETs): when a MOF is used as the active material in a FET, changes in the conductivity of the framework upon gas adsorption can be measured using the FET. However, unlike the previous techniques, this measurement is not amenable to polycrystalline samples; instead, MOF single crystals or films are required, which, depending on the material, could pose a synthetic bottleneck.261,262
(c) Magnetism measurements: this technique is premised on the fact that the magnetic properties of certain materials will change during sorption of analyte molecules. Changes in magnetic properties can be measured using a variety of sophisticated techniques and related to the concentration of analyte gas.263,264
(d) Mass-change: the change of mass that a material, such as a MOF, experiences after the adsorption of a specific gas can be used to evaluate the presence and/or concentration of that gas.
(e) Other techniques: other techniques are used case-by-case to evaluate, study, and apply MOFs as sensors. Some examples are UV-vis absorption, calorimetry, and many others.265,266
A comprehensive understanding of these techniques is essential to designing and optimizing MOF-based gas sensors and detectors.
Conclusions
A select class of Metal–organic Frameworks possess high surface area, well-defined pore distribution, and high thermal and chemical stability. In light of these properties, it is not surprising that MOFs have recently garnered significant interest in detection and sensing research. Of particular interest is the detection of SO2, a hazardous gas to which several chemically stable MOFs have demonstrated promising compatibility. Most SO2 related MOF research has concentrated on SO2 adsorption capacity, emphasizing the highest uptake capacity. This is typically reported in conjunction with comprehensive computational and experimental studies that aim to elucidate the specific chemical and physical interactions between SO2 and the framework with a view to identifying the preferential adsorption sites. This approach has been successfully used for a wide range of materials, yielding valuable insight into the nature of SO2 adsorption in porous materials. However, in the context of SO2 detection, both the SO2 affinity of the framework and the SO2 adsorption capacity at low pressure must be considered – rather than overall uptake at high pressure. Thus, it is necessary to explore characteristics of MOFs pertinent to SO2 detection, which typically diverge from those that promote large SO2 uptake at high pressure. Higher SO2 uptake at low pressure reflects stronger SO2 interactions within the framework; this is critical to the operation of SO2 detectors under environmental conditions since relatively low SO2 concentrations of ≤5000 ppm are typically relevant for sensing. Analysis of the most effective MOFs for SO2 capture has demonstrated a clear correlation between SO2 capture at low pressure (0.01 bar) and the pore-limiting diameter.We have provided an overview of techniques used to perform SO2 detection in MOFs and evaluated which MOF candidates are likely to perform best. In addition to a high adsorption capacity at low pressure and requisite chemical stability, MOFs require distinct characteristics to selectively detect specific analytes such as SO2. MOF-based analyte detection is predicated on quantitative (or, in some cases, qualitative) measurement of the response to a particular environmental stimulus (i.e., SO2 adsorption). As we have outlined, the response typically consists of changes in luminescence, electrochemical properties, or magnetism. Examples of MOF based SO2 detection using these methodologies have been reported and outlined in detail in the main text. Cruicially, advancement in materials processing combined with excellent chemical stability allow select MOFs to be incorporated into detectors based on nanofiber membranes, electrodes, and test strips.
This review provides a broad overview of the significant role that chemically stable MOFs will play in the expanding field of SO2 detection. The extraordinary diversity of physiochemical properties displayed by MOFs provides space for chemists to further refine MOF-SO2 interactions, guided by new characterisation techniques and supported by advanced computational tools. The insights garnered from this process will inform the design of future MOF-based detectors for SO2 and other volatile compounds.
List of abbreviations
| AIP | 5-Aminoisophthalate |
| ATT | 3-Amino-1,2,4-triazole-5-thiol |
| ADC | Acetylenedicarboxylate |
| BET | Brunauer–Emmett–Teller |
| BTC | Benzene-1,3,5-tricarboxylate, trimesate |
| BDC | 1,4-Benzenedicarboxylate, terephthalate |
| BDC-Br | 2-Bromoterephthalate |
| BDC-NH2 | 2-Aminoterephthalate |
BDC-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 | 2-Vinylterephthalate |
| BDC-NO2 | 2-Nitroterephthalate |
| BDC-4F | 2,3,5,6-Tetrafluoro-1,4-benzenedicarboxylate |
| BPDC | 4,4′-Biphenyldicarboxylate |
| BPTC | Biphenyl-3,3′,5,5′-tetracarboxylate |
| BTEC | 1,2,4,5-Benzenetetracarboxylate |
| BDP | 1,4-Bis(4-pyrazolyl)benzene |
| CPE | Carbon paste electrode |
| CDC | 9H-Carbazole-3,6-dicarboxylate |
| CD | Carbon dot |
| CYCU | Chung-Yuan Christian University |
| CAU | Christian-Albrechts-University |
| CUK | Cambridge-University-KRICT |
| CNT | Carbon nanotubes |
| DFT | Density functional theory |
| DUT | Dresden University of Technology |
| DOBPDC | 4,4-Dioxidobiphenyl-3,3-dicarboxylate |
| DABCO | 1,4-Diazabicyclo[2,2,2]octane |
| DHTP | 2,5-Dihydroxyterephthalate |
| DRIFTS | Diffuse reflectance infrared Fourier transform spectroscopy |
| ETTA | 4,4′,4′′,4′′′-(Ethene-1,1,2,2-tetrayl)tetrabenzoicate |
| ET-LMOF | Energy transfer LMOFs |
| GCMC | Grand canonical Monte Carlo |
| GAL | Gallate |
| FTIR | Fourier transform infrared |
| FDCA | 2,5-Furandicarboxylate |
| Fum | Fumarate |
| FGD | Flue-gas desulfurization |
| HS | High-spin |
| HSAB | Pearson's hard-soft acid–base theory |
| HHTP | 2,3,5,6,10,11-Hexahydroxytriphenylene |
| HATP | 2,3,6,7,10,11-Hexaaminotriphenylene |
| HKUST | Hong Kong University of Science and Technology |
| LMOFs | Luminescent metal–organic frameworks |
| LOD | Limit of detection |
| NDC-(NO2)2 | 4,8-Dinitronaphthalene-2,6-dicarboxylate |
| NDC | 1,4-Naphthalenedicarboxylate |
| NOTT | Nottingham University |
| NU | Northwestern University |
| IRMOF | Isoreticular metal–organic framework |
| MOCs | Metal–organic cages |
| MOFs | Metal–organic frameworks |
| MIL | Matériaux de l’ Institut Lavoisier |
| MFM | Manchester framework material |
| MUF | Massey University Framework |
| MPBA | N,N′-2,4,6-Trimethyl-1,3-phenylenebis(oxamate) |
| OTf | Trifuoromethanesulfonate |
| THBA | 2,3,4-Trihydroxybenzaldehyde |
| TBAPy | 4,4′,4′′,4′′′-(Pyrene-1,3,6,8-tetrayl)tetrabenzoate |
| TCPP | meso-Tetrakis(4-carboxylphenyl)porphyrin |
| TDC | 2,5-Thiophenedicarboxylate |
| TBA | 4-(4H-1,2,4-Triazol-4-yl)benzoate |
| TRPL | Time-resolved photoluminescence |
| LS | Low-spin |
| LOD | Limit of detection |
| TATB | 2,4,6-Tris(4-carboxyphenyl)-1,3,5-triazine |
| TDC | 2,5-Thiophenedicarboxylate |
| SDC | 4,4′-Stilbenedicarboxylate |
| SCO | Spin-crossover |
| ppm | Parts per million |
| ppb | Parts per billion |
| POCs | Porous organic cages |
| PAN | Polyacrylonitrile |
| PAC | meso-Tetrakis(4-carboxylphenyl)porphyrin |
| PA | m-Phthalate |
| PDDB | 4,4′-(Pyridine-2,6-diyl)dibenzoate |
| PHEN | 1,10-Phenanthroline |
| PBTA | 4,4′4′′,4′′′-(4,4′-(1,4-Phenylene)bis (pyridine-6,4,2-triyl))-tetrabenzoate |
| PCN | Porous coordination network |
| PPTA | 4,4′,4′′,4′′′-(1,4-Phenylenebis(pyridine-4,2,6-triyl))tetrabenzoate |
| PXRD | Powder X-ray diffraction |
| PDCA | 2,4-Pyridinedicarboxylate |
| PLD | Pore limiting diameter |
| PVDF | Polyvinylidene fluoride |
| PTBA | 4,4′,4′′,4′′′-(1,4-Phenylenebis(azanetriyl))tetrabenzoate |
| PZDC | 1H-Pyrazole-3,5-dicarboxylate |
| UiO | Universitetet i Oslo |
| SCXRD | Single-crystal X-ray diffraction |
| ZIF | Zeolitic imidazolate framework |
Data availability
All data is available in the main text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. L. O. and C. V. F. thanks CONAHCYT for the PhD fellowship (1003953 and 1040318). I. A. I. thanks PAPIIT-UNAM (IN201123), México, for financial support. We thank U. Winnberg (Euro Farmacos EIR) for scientific discussions and G. Ibarra-Winnberg for scientific encouragement. C. J. thanks the DFG for funding within the Priority Program SPP 1928/2 COORNETs (grant Ja466/43-1).References
- L. Bai, J. Wang, X. Ma and H. Lu, Int. J. Environ. Res. Public Health, 2018, 15, 780 CrossRef PubMed.
- M. Höök and X. Tang, Energy Policy, 2013, 52, 797–809 CrossRef.
- B. Zhang and G. Chen, Process Saf. Environ. Prot., 2010, 88, 253–262 CrossRef CAS.
- N. Abas, A. Kalair and N. Khan, Futures, 2015, 69, 31–49 CrossRef.
- F. Perera, Int. J. Environ. Res. Public Health, 2017, 15, 16 CrossRef PubMed.
- M. E. Quesada-Valverde and A. Quesada-Román, Geosciences, 2023, 13, 39 CrossRef.
- A. N. Torres, A. L. M. Del Pozzo, G. Groppelli and M. Del Carmen Jaimes Viera, J. Volcanol. Geotherm. Res., 2023, 433, 107733 CrossRef CAS.
- S. Valade, D. Coppola, R. Campion, A. Ley, T. Boulesteix, N. Taquet, D. Legrand, M. Laiolo, T. R. Walter and S. De La Cruz-Reyna, Nat. Commun., 2023, 14, 3254 CrossRef CAS PubMed.
- B. Divol, M. Du Toit and E. Duckitt, Appl. Microbiol. Biotechnol., 2012, 95, 601–613 CrossRef CAS PubMed.
- E. Calabrese, C. Sacco, G. Moore and S. DiNardi, Med. Hypotheses, 1981, 7, 133–145 CrossRef CAS PubMed.
- P. Amoatey, H. Omidvarborna, M. S. Baawain and A. Al-Mamun, Process Saf. Environ. Prot., 2019, 123, 215–228 Search PubMed.
- R. J. Melia, C. D. Florey and A. V. Swan, J. Epidemiol. Community Health, 1981, 35, 161–167 CrossRef CAS PubMed.
- W. Van Roy, B. Van Roozendael, L. Vigin, A. Van Nieuwenhove, K. Scheldeman, J.-B. Merveille, A. Weigelt, J. Mellqvist, J. Van Vliet, D. Van Dinther, J. Beecken, F. Tack, N. Theys and F. Maes, Commun. Earth Environ., 2023, 4, 391 CrossRef.
- V. Jonas, G. Frenking and M. T. Reetz, J. Am. Chem. Soc., 1994, 116, 8741–8753 CrossRef CAS.
- T. P. Cunningham, D. L. Cooper, J. Gerratt, P. B. Karadakov and M. Raimondi, J. Chem. Soc., Faraday Trans., 1997, 93, 2247–2254 RSC.
- D. Patel, D. Margolese and T. R. Dykea, J. Chem. Phys., 1979, 70, 2740–2747 CrossRef CAS.
- I. Jana, S. Naskar and D. Nandi, J. Phys.:Conf. Ser., 2020, 1412, 052005 CrossRef CAS.
- Y. Qu, J. An, Y. He and J. Zheng, J. Environ. Sci., 2016, 44, 13–25 CrossRef CAS PubMed.
- M. M. M. F. Jion, J. N. Jannat, M. Y. Mia, M. A. Ali, M. S. Islam, S. M. Ibrahim, S. C. Pal, A. Islam, A. Sarker, G. Malafaia, M. Bilal and A. R. M. T. Islam, Sci. Total Environ, 2023, 876, 162851 CrossRef CAS PubMed.
- N. D. Abdul Halim, M. T. Latif, F. Ahamad, D. Dominick, J. X. Chung, L. Juneng and M. F. Khan, Heliyon, 2018, 4, e01054 Search PubMed.
- M. Ali and M. Athar, Environ. Monit. Assess., 2010, 171, 353–363 CrossRef CAS PubMed.
- Z.-Y. Meng, X.-B. Xu, T. Wang, X.-Y. Zhang, X.-L. Yu, S.-F. Wang, W.-L. Lin, Y.-Z. Chen, Y.-A. Jiang and X.-Q. An, Atmos. Environ., 2010, 44, 2625–2631 CrossRef CAS.
- C. Mallik and S. Lal, Environ. Monit. Assess., 2014, 186, 1295–1310 CrossRef CAS PubMed.
- S. S. Mousavi, G. Goudarzi, S. Sabzalipour, M. M. Rouzbahani and E. Mobarak Hassan, Environ. Sci. Pollut. Res., 2021, 28, 56996–57008 CrossRef CAS PubMed.
- B. J. Freedman, Br. J. Dis. Chest, 1980, 74, 128–134 CrossRef CAS PubMed.
- S. Giacosa, S. Río Segade, E. Cagnasso, A. Caudana, L. Rolle and V. Gerbi, Red Wine Technology, Elsevier, 2019, pp. 309–321 Search PubMed.
- P. Mars, J. Catal., 1968, 10, 1–12 CrossRef CAS.
- F. García-Labiano, L. F. De Diego, A. Cabello, P. Gayán, A. Abad, J. Adánez and G. Sprachmann, Appl. Energy, 2016, 178, 736–745 CrossRef.
- A. S. Milev, G. S. K. Kannangara and M. A. Wilson, in Kirk-Othmer Encyclopedia of Chemical Technology, ed. Kirk-Othmer, Wiley, 1st edn, 2005 Search PubMed.
- R. Saidur, M. Sattar, H. H. Masjuki and M. Y. Jamaluddin, Energy Environ., 2009, 20, 533–551 CrossRef CAS.
- S. Piché, F. Larachi and B. P. A. Grandjean, Ind. Eng. Chem. Res., 2001, 40, 476–487 CrossRef.
- F. W. Adams, Ind. Eng. Chem., 1933, 25, 424–428 CrossRef CAS.
- B. Hanley and C. Chen, AIChE J., 2012, 58, 132–152 CrossRef CAS.
- M. Reda and G. R. Carmichael, Atmos. Environ., 1982, 16, 151–159 CrossRef CAS.
- R. K. Srivastava, W. Jozewicz and C. Singer, Environ. Prog., 2001, 20, 219–228 CrossRef CAS.
- D. B. Gingerich, E. Grol and M. S. Mauter, Environ. Sci.: Water Res. Technol., 2018, 4, 909–925 RSC.
- P. Luis, A. Garea and A. Irabien, J. Membr. Sci., 2009, 330, 80–89 CrossRef CAS.
- Y. Sun, J. Xie, W. Huang, G. Li, S. Li, P. Cui, H. Xu, Z. Qu and N. Yan, Fuel, 2021, 288, 119714 CrossRef CAS.
- Q. Xin, C. Zhang, Y. Zhang, Q. Liang, L. Zhang, S. Wang, H. Ye, X. Ding and Y. Zhang, Sep. Purif. Technol., 2021, 259, 118222 CrossRef CAS.
- P. Luis, A. Garea and A. Irabien, J. Chem. Technol. Biotechnol., 2008, 83, 1570–1577 CrossRef CAS.
- H.-K. Lee, B. R. Deshwal and K.-S. Yoo, Korean J. Chem. Eng., 2005, 22, 208–213 CrossRef CAS.
- Y. A. Husnil, R. Andika and M. Lee, J. Process Control, 2019, 74, 147–159 CrossRef CAS.
- W. Liu, R. D. Vidic and T. D. Brown, Environ. Sci. Technol., 2000, 34, 154–159 CrossRef CAS.
- H. Yi, H. Deng, X. Tang, Q. Yu, X. Zhou and H. Liu, J. Hazard. Mater., 2012, 203–204, 111–117 CrossRef CAS PubMed.
- H. Fu, X. Wang, H. Wu, Y. Yin and J. Chen, J. Phys. Chem. C, 2007, 111, 6077–6085 CrossRef CAS.
- Y. Li, L. Li and J. Yu, Chem, 2017, 3, 928–949 CAS.
- S. Beyaz Kayiran and F. Lamari Darkrim, Surf. Interface Anal., 2002, 34, 100–104 CrossRef.
- E. Pérez-Botella, S. Valencia and F. Rey, Chem. Rev., 2022, 122, 17647–17695 CrossRef PubMed.
- Y. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896–910 CrossRef CAS PubMed.
- N. K. Gupta, E. J. Kim, S. Baek, J. Bae and K. S. Kim, Sci. Rep., 2022, 12, 15387 CrossRef CAS PubMed.
- C. Petit, Curr. Opin. Chem. Eng., 2018, 20, 132–142 CrossRef.
- J. L. Obeso, D. R. Amaro, C. V. Flores, A. Gutiérrez-Alejandre, R. A. Peralta, C. Leyva and I. A. Ibarra, Coord. Chem. Rev., 2023, 485, 215135 Search PubMed.
- A. Carné-Sánchez, J. Martínez-Esaín, T. Rookard, C. J. Flood, J. Faraudo, K. C. Stylianou and D. Maspoch, ACS Appl. Mater. Interfaces, 2023, 15, 6747–6754 CrossRef PubMed.
- E. Martínez-Ahumada, D. He, V. Berryman, A. López-Olvera, M. Hernandez, V. Jancik, V. Martis, M. A. Vera, E. Lima, D. J. Parker, A. I. Cooper, I. A. Ibarra and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 17556–17563 CrossRef PubMed.
- J. L. Obeso, A. López-Olvera, C. V. Flores, E. Martínez-Ahumada, R. Paz, H. Viltres, A. Islas-Jácome, E. González-Zamora, J. Balmaseda, S. López-Morales, M. A. Vera, E. Lima, I. A. Ibarra and C. Leyva, J. Mol. Liq., 2022, 368, 120758 CrossRef CAS.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 Search PubMed.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. P. Suh and J. Reedijk, CrystEngComm, 2012, 14, 3001 Search PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276 Search PubMed.
- H.-C. Joe Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- N. K. Gupta, A. López-Olvera, E. González-Zamora, E. Martínez-Ahumada and I. A. Ibarra, ChemPlusChem, 2022, 87, e202200006 CrossRef CAS PubMed.
- J. L. Obeso, H. Viltres, C. V. Flores, A. López-Olvera, A. R. Rajabzadeh, S. Srinivasan, I. A. Ibarra and C. Leyva, J. Environ. Chem. Eng., 2023, 11, 109872 CrossRef CAS.
- J. L. Obeso, V. B. López-Cervantes, C. V. Flores, H. Viltres, C. Serrano-Fuentes, L. Herrera-Zuñiga, N. S. Portillo-Vélez, R. A. Peralta, D. Solis-Ibarra, I. A. Ibarra and C. Leyva, ACS Sustainable Resour. Manage., 2024, 1, 661–669 CrossRef CAS.
- C. V. Flores, J. L. Obeso, H. Viltres, R. A. Peralta, I. A. Ibarra and C. Leyva, Environ. Sci.: Water Res. Technol., 2024, 10, 2142–2147 Search PubMed.
- J. L. Obeso, J. G. Flores, C. V. Flores, V. B. López-Cervantes, V. Martínez-Jiménez, J. A. De Los Reyes, E. Lima, D. Solis-Ibarra, I. A. Ibarra, C. Leyva and R. A. Peralta, Dalton Trans., 2023, 52, 12490–12495 Search PubMed.
- J. G. Flores, J. L. Obeso, V. Martínez-Jiménez, N. Martín-Guaregua, A. Islas-Jácome, E. González-Zamora, H. Serrano-Espejel, B. Mondragón-Rodríguez, C. Leyva, D. A. Solís-Casados, I. A. Ibarra, R. A. Peralta, J. Aguilar-Pliego and J. Antonio De Los Reyes, RSC Adv., 2023, 13, 27174–27179 RSC.
- V. Pascanu, G. González Miera, A. K. Inge and B. Martín-Matute, J. Am. Chem. Soc., 2019, 141, 7223–7234 Search PubMed.
- S. Rojas, I. Colinet, D. Cunha, T. Hidalgo, F. Salles, C. Serre, N. Guillou and P. Horcajada, ACS Omega, 2018, 3, 2994–3003 Search PubMed.
- E. Medel, J. L. Obeso, C. Serrano-Fuentes, J. Garza, I. A. Ibarra, C. Leyva, A. K. Inge, A. Martínez and R. Vargas, Chem. Commun., 2023, 59, 8684–8687 RSC.
- S. Bügel, Q.-D. Hoang, A. Spieß, Y. Sun, S. Xing and C. Janiak, Membranes, 2021, 11, 795 CrossRef PubMed.
- H. B. Tanh Jeazet, C. Staudt and C. Janiak, Dalton Trans., 2012, 41, 14003 Search PubMed.
- B. Gil-Hernández, S. Millan, I. Gruber, M. Quirós, D. Marrero-López, C. Janiak and J. Sanchiz, Inorg. Chem., 2022, 61, 11651–11666 Search PubMed.
- B. Gil-Hernández, P. Gili, J. K. Vieth, C. Janiak and J. Sanchiz, Inorg. Chem., 2010, 49, 7478–7490 CrossRef PubMed.
- X. Han, S. Yang and M. Schröder, Nat. Rev. Chem., 2019, 3, 108–118 CrossRef CAS.
- S. Han, Y. Huang, T. Watanabe, S. Nair, K. S. Walton, D. S. Sholl and J. Carson Meredith, Microporous Mesoporous Mater., 2013, 173, 86–91 Search PubMed.
- G. Mouchaham, S. Wang and C. Serre, in Metal-Organic Frameworks, ed. H. García and S. Navalón, Wiley, 1st edn, 2018, pp. 1–28 Search PubMed.
- K. O. Kirlikovali, S. L. Hanna, F. A. Son and O. K. Farha, ACS Nanosci. Au, 2023, 3, 37–45 CrossRef CAS PubMed.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- Y. Jin, H. Liu, M. Feng, Q. Ma and B. Wang, Adv. Funct. Mater., 2023, 2304773 Search PubMed.
- I. Senkovska and S. Kaskel, Chem. Commun., 2014, 50, 7089 RSC.
- J. Valdes-García, L. D. Rosales-Vázquez, I. J. Bazany-Rodríguez and A. Dorazco-González, Chem. – Asian J., 2020, 15, 2925–2938 CrossRef PubMed.
- C. Bargossi, M. C. Fiorini, M. Montalti, L. Prodi and N. Zaccheroni, Coord. Chem. Rev., 2000, 208, 17–32 CrossRef CAS.
- H.-Y. Li, S.-N. Zhao, S.-Q. Zang and J. Li, Chem. Soc. Rev., 2020, 49, 6364–6401 Search PubMed.
- Y. Jian, W. Hu, Z. Zhao, P. Cheng, H. Haick, M. Yao and W. Wu, Nano-Micro Lett., 2020, 12, 71 Search PubMed.
- G. Yang, X. Jiang, H. Xu and B. Zhao, Small, 2021, 17, 2005327 Search PubMed.
- Y. Wang, Y. Zhu, A. Binyam, M. Liu, Y. Wu and F. Li, Biosens. Bioelectron., 2016, 86, 432–438 CrossRef CAS PubMed.
- Y. T. Azar, M. S. Lakmehsari, S. M. Kazem Manzoorolajdad, V. Sokhanvaran, Z. Ahadi, A. Shahsavani and P. K. Hopke, Mater. Chem. Phys., 2020, 239, 122105 CrossRef CAS.
- W.-T. Koo, J.-S. Jang and I.-D. Kim, Chem, 2019, 5, 1938–1963 Search PubMed.
- Y. Li, S. Zhang and D. Song, Angew. Chem., Int. Ed., 2013, 52, 710–713 CrossRef CAS PubMed.
- R. Lin, S. Liu, J. Ye, X. Li and J. Zhang, Adv. Sci., 2016, 3, 1500434 CrossRef PubMed.
- N. B. Shustova, A. F. Cozzolino, S. Reineke, M. Baldo and M. Dincă, J. Am. Chem. Soc., 2013, 135, 13326–13329 CrossRef CAS PubMed.
- X. Li, B. Liu, K. Ye, L. Ni, X. Xu, F. Qiu, J. Pan and X. Niu, Sens. Actuators, B, 2019, 297, 126822 CrossRef CAS.
- Z.-P. Ni, J.-L. Liu, Md. N. Hoque, W. Liu, J.-Y. Li, Y.-C. Chen and M.-L. Tong, Coord. Chem. Rev., 2017, 335, 28–43 CrossRef CAS.
- A. Tissot, X. Kesse, S. Giannopoulou, I. Stenger, L. Binet, E. Rivière and C. Serre, Chem. Commun., 2019, 55, 194–197 RSC.
- P. D. Southon, L. Liu, E. A. Fellows, D. J. Price, G. J. Halder, K. W. Chapman, B. Moubaraki, K. S. Murray, J.-F. Létard and C. J. Kepert, J. Am. Chem. Soc., 2009, 131, 10998–11009 CrossRef CAS PubMed.
- N. Kau, G. Jindal, R. Kaur and S. Rana, Results Chem., 2022, 4, 100678 CrossRef CAS.
- P. Samanta, S. Let, W. Mandal, S. Dutta and S. K. Ghosh, Inorg. Chem. Front., 2020, 7, 1801–1821 RSC.
- H. Zhou, X. Hui, D. Li, D. Hu, X. Chen, X. He, L. Gao, H. Huang, C. Lee and X. Mu, Adv. Sci., 2020, 7, 2001173 CrossRef CAS PubMed.
- G. Ji, T. Zheng, X. Gao and Z. Liu, Sens. Actuators, B, 2019, 284, 91–95 CrossRef CAS.
- W.-J. Ju, L.-M. Fu, R.-J. Yang and C.-L. Lee, Lab Chip, 2012, 12, 622–626 RSC.
- T. R. Khan and J. C. Meranger, Environ. Int., 1983, 9, 195–206 Search PubMed.
- P. Popp and G. Oppermann, J. Chromatogr. A, 1981, 207, 131–137 Search PubMed.
- W. L. Crider, Anal. Chem., 1965, 37, 1770–1773 CrossRef CAS.
- M. S. Yogendra Kumar, M. D. Gowtham, Mahadevaiah and G. Nagendrappa, Anal. Sci., 2006, 22, 757–761 CrossRef CAS PubMed.
- Y. Wen, P. Zhang, V. K. Sharma, X. Ma and H.-C. Zhou, Cell Rep., 2021, 2, 100348 Search PubMed.
- O. D. Agboola and N. U. Benson, Front. Environ. Sci., 2021, 9, 678574 CrossRef.
- E. Martínez-Ahumada, M. L. Díaz-Ramírez, M. D. J. Velásquez-Hernández, V. Jancik and I. A. Ibarra, Chem. Sci., 2021, 12, 6772–6799 RSC.
- A. J. Rieth, A. M. Wright and M. Dincă, Nat. Rev. Mater., 2019, 4, 708–725 CrossRef CAS.
- Q. Zhang, H. Yang, T. Zhou, X. Chen, W. Li and H. Pang, Adv. Sci., 2022, 9, 2204141 CrossRef CAS PubMed.
- X.-D. Song, S. Wang, C. Hao and J.-S. Qiu, Inorg. Chem. Commun., 2014, 46, 277–281 Search PubMed.
- E. Martínez-Ahumada, A. López-Olvera, V. Jancik, J. E. Sánchez-Bautista, E. González-Zamora, V. Martis, D. R. Williams and I. A. Ibarra, Organometallics, 2020, 39, 883–915 CrossRef.
- G. J. Kubas, Inorg. Chem., 1979, 18, 182–188 CrossRef CAS.
- D. Kim, M. Kang, H. Ha, C. S. Hong and M. Kim, Coord. Chem. Rev., 2021, 438, 213892 CrossRef CAS.
- Y. Lin, C. Kong and L. Chen, RSC Adv., 2016, 6, 32598–32614 RSC.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- J. H. Carter, C. G. Morris, H. G. W. Godfrey, S. J. Day, J. Potter, S. P. Thompson, C. C. Tang, S. Yang and M. Schröder, ACS Appl. Mater. Interfaces, 2020, 12, 42949–42954 CrossRef CAS PubMed.
- J. A. Zárate, E. Sánchez-González, D. R. Williams, E. González-Zamora, V. Martis, A. Martínez, J. Balmaseda, G. Maurin and I. A. Ibarra, J. Mater. Chem. A, 2019, 7, 15580–15584 RSC.
- M. Savage, Y. Cheng, T. L. Easun, J. E. Eyley, S. P. Argent, M. R. Warren, W. Lewis, C. Murray, C. C. Tang, M. D. Frogley, G. Cinque, J. Sun, S. Rudić, R. T. Murden, M. J. Benham, A. N. Fitch, A. J. Blake, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Adv. Mater., 2016, 28, 8705–8711 Search PubMed.
- A. López-Olvera, J. A. Zárate, E. Martínez-Ahumada, D. Fan, M. L. Díaz-Ramírez, P. A. Sáenz-Cavazos, V. Martis, D. R. Williams, E. Sánchez-González, G. Maurin and I. A. Ibarra, ACS Appl. Mater. Interfaces, 2021, 13, 39363–39370 CrossRef PubMed.
- A. López-Olvera, S. Pioquinto-García, J. Antonio Zárate, G. Diaz, E. Martínez-Ahumada, J. L. Obeso, V. Martis, D. R. Williams, H. A. Lara-García, C. Leyva, C. V. Soares, G. Maurin, I. A. Ibarra and N. E. Dávila-Guzmán, Fuel, 2022, 322, 124213 CrossRef.
- S. G. Dunning, N. K. Gupta, J. E. Reynolds, M. Sagastuy-Breña, J. G. Flores, E. Martínez-Ahumada, E. Sánchez-González, V. M. Lynch, A. Gutiérrez-Alejandre, J. Aguilar-Pliego, K.-S. Kim, I. A. Ibarra and S. M. Humphrey, Inorg. Chem., 2022, 61, 15037–15044 CrossRef CAS PubMed.
- P. Brandt, A. Nuhnen, M. Lange, J. Möllmer, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2019, 11, 17350–17358 CrossRef CAS PubMed.
- C. Jansen, N. Tannert, D. Lenzen, M. Bengsch, S. Millan, A. Goldman, D. N. Jordan, L. Sondermann, N. Stock and C. Janiak, Z. Anorg. Allg. Chem., 2022, 648, e202200170 CrossRef CAS.
- Ü. Kökçam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC.
- L. Tao, C.-Y. Lin, S. Dou, S. Feng, D. Chen, D. Liu, J. Huo, Z. Xia and S. Wang, Nano Energy, 2017, 41, 417–425 CrossRef CAS.
- A. Yadav, S. Kumari, P. Yadav, A. Hazra, A. Chakraborty and P. Kanoo, Dalton Trans., 2022, 51, 15496–15506 RSC.
- J. H. Choe, H. Kim and C. S. Hong, Mater. Chem. Front., 2021, 5, 5172–5185 RSC.
- K. Tan, S. Zuluaga, H. Wang, P. Canepa, K. Soliman, J. Cure, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2017, 29, 4227–4235 CrossRef CAS.
- G. L. Smith, J. E. Eyley, X. Han, X. Zhang, J. Li, N. M. Jacques, H. G. W. Godfrey, S. P. Argent, L. J. McCormick McPherson, S. J. Teat, Y. Cheng, M. D. Frogley, G. Cinque, S. J. Day, C. C. Tang, T. L. Easun, S. Rudić, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Nat. Mater., 2019, 18, 1358–1365 Search PubMed.
- W. Li, J. Li, T. D. Duong, S. A. Sapchenko, X. Han, J. D. Humby, G. F. S. Whitehead, I. J. Victórica-Yrezábal, I. Da Silva, P. Manuel, M. D. Frogley, G. Cinque, M. Schröder and S. Yang, J. Am. Chem. Soc., 2022, 144, 13196–13204 Search PubMed.
- E. Martínez-Ahumada, M. L. Díaz-Ramírez, H. A. Lara-García, D. R. Williams, V. Martis, V. Jancik, E. Lima and I. A. Ibarra, J. Mater. Chem. A, 2020, 8, 11515–11520 Search PubMed.
- O. V. Gutov, M. G. Hevia, E. C. Escudero-Adán and A. Shafir, Inorg. Chem., 2015, 54, 8396–8400 CrossRef CAS PubMed.
- M. Szufla, J. A. R. Navarro, K. Góra-Marek and D. Matoga, ACS Appl. Mater. Interfaces, 2023, 15, 28184–28192 CrossRef CAS PubMed.
- W. Xiang, Y. Zhang, Y. Chen, C. Liu and X. Tu, J. Mater. Chem. A, 2020, 8, 21526–21546 RSC.
- L. M. Rodríguez-Albelo, E. López-Maya, S. Hamad, A. R. Ruiz-Salvador, S. Calero and J. A. R. Navarro, Nat. Commun., 2017, 8, 14457 CrossRef PubMed.
- S. Li, W. Han, Q. An, K. Yong and M. Yin, Adv. Funct. Mater., 2023, 33, 2303447 CrossRef CAS.
- T. Matemb Ma Ntep, H. Breitzke, L. Schmolke, C. Schlüsener, B. Moll, S. Millan, N. Tannert, I. El Aita, G. Buntkowsky and C. Janiak, Chem. Mater., 2019, 31, 8629–8638 CrossRef CAS.
- D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 Search PubMed.
- S. Glomb, D. Woschko, G. Makhloufi and C. Janiak, ACS Appl. Mater. Interfaces, 2017, 9, 37419–37434 CrossRef CAS PubMed.
- J. H. Carter, X. Han, F. Y. Moreau, I. Da Silva, A. Nevin, H. G. W. Godfrey, C. C. Tang, S. Yang and M. Schröder, J. Am. Chem. Soc., 2018, 140, 15564–15567 CrossRef CAS PubMed.
- P. Brandt, A. Nuhnen, S. Öztürk, G. Kurt, J. Liang and C. Janiak, Adv. Sustainable Syst., 2021, 5, 2000285 CrossRef CAS.
- M. Mon, E. Tiburcio, J. Ferrando-Soria, R. Gil San Millán, J. A. R. Navarro, D. Armentano and E. Pardo, Chem. Commun., 2018, 54, 9063–9066 Search PubMed.
- F. Chen, D. Lai, L. Guo, J. Wang, P. Zhang, K. Wu, Z. Zhang, Q. Yang, Y. Yang, B. Chen, Q. Ren and Z. Bao, J. Am. Chem. Soc., 2021, 143, 9040–9047 CrossRef CAS PubMed.
- S. Xing, J. Liang, P. Brandt, F. Schäfer, A. Nuhnen, T. Heinen, I. Boldog, J. Möllmer, M. Lange, O. Weingart and C. Janiak, Angew. Chem., Int. Ed., 2021, 60, 17998–18005 Search PubMed.
- Y. L. Fan, H. P. Zhang, M. J. Yin, R. Krishna, X. F. Feng, L. Wang, M. B. Luo and F. Luo, Inorg. Chem., 2021, 60, 4–8 CrossRef CAS PubMed.
- Y.-B. Ren, H.-Y. Xu, S.-Q. Gang, Y.-J. Gao, X. Jing and J.-L. Du, Chem. Eng. J., 2022, 431, 134057 Search PubMed.
- Y. Zhang, P. Zhang, W. Yu, J. Zhang, J. Huang, J. Wang, M. Xu, Q. Deng, Z. Zeng and S. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 10680–10688 CrossRef CAS PubMed.
- K. Vellingiri, A. Deep and K.-H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 29835–29857 Search PubMed.
- J.-Y. Lee, T. C. Keener and Y. J. Yang, J. Air Waste Manage. Assoc., 2009, 59, 725–732 Search PubMed.
- Y. Igarashi, Y. Sawa, K. Yoshioka, H. Matsueda, K. Fujii and Y. Dokiya, J. Geophys. Res., 2004, 109, 2003JD004428 Search PubMed.
- S. D. Worrall, M. A. Bissett, P. I. Hill, A. P. Rooney, S. J. Haigh, M. P. Attfield and R. A. W. Dryfe, Electrochim. Acta, 2016, 222, 361–369 CrossRef CAS.
- P. Brandt, S.-H. Xing, J. Liang, G. Kurt, A. Nuhnen, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2021, 13, 29137–29149 CrossRef CAS PubMed.
- P. Liu, K. Cai, K. Wang, T. Zhao and D.-J. Tao, Green Energy Environ., 2023, S2468025723001309 Search PubMed.
- S. Gorla, M. L. Díaz-Ramírez, N. S. Abeynayake, D. M. Kaphan, D. R. Williams, V. Martis, H. A. Lara-García, B. Donnadieu, N. Lopez, I. A. Ibarra and V. Montiel-Palma, ACS Appl. Mater. Interfaces, 2020, 12, 41758–41764 CrossRef CAS PubMed.
- J. García Ponce, M. L. Díaz-Ramírez, S. Gorla, C. Navarathna, G. Sanchez-Lecuona, B. Donnadieu, I. A. Ibarra and V. Montiel-Palma, CrystEngComm, 2021, 23, 7479–7484 Search PubMed.
- X.-H. Xiong, Z.-W. Wei, W. Wang, L.-L. Meng and C.-Y. Su, J. Am. Chem. Soc., 2023, 145, 14354–14364 Search PubMed.
- J. Liang, S. Xing, P. Brandt, A. Nuhnen, C. Schlüsener, Y. Sun and C. Janiak, J. Mater. Chem. A, 2020, 8, 19799–19804 RSC.
- Y. Sun, J. Liang, P. Brandt, A. Spieß, S. Öztürk and C. Janiak, Nanoscale, 2021, 13, 15952–15962 RSC.
- N. Tannert, Y. Sun, E. Hastürk, S. Nießing and C. Janiak, Z. Anorg. Allg. Chem., 2021, 647, 1124–1130 CrossRef CAS.
- M. D. J. Velásquez-Hernández, V. B. López-Cervantes, E. Martínez-Ahumada, M. Tu, U. Hernández-Balderas, D. Martínez-Otero, D. R. Williams, V. Martis, E. Sánchez-González, J.-S. Chang, J. S. Lee, J. Balmaseda, R. Ameloot, I. A. Ibarra and V. Jancik, Chem. Mater., 2022, 34, 669–677 CrossRef.
- L. J. Barrios-Vargas, J. G. Ruiz-Montoya, B. Landeros-Rivera, J. R. Álvarez, D. Alvarado-Alvarado, R. Vargas, A. Martínez, E. González-Zamora, L. M. Cáceres, J. C. Morales and I. A. Ibarra, Dalton Trans., 2020, 49, 2786–2793 RSC.
- J. A. Zárate, E. Domínguez-Ojeda, E. Sánchez-González, E. Martínez-Ahumada, V. B. López-Cervantes, D. R. Williams, V. Martis, I. A. Ibarra and J. Alejandre, Dalton Trans., 2020, 49, 9203–9207 Search PubMed.
- A. López-Olvera, H. Montes-Andrés, E. Martínez-Ahumada, V. B. López-Cervantes, R. D. Martínez-Serrano, E. González-Zamora, A. Martínez, P. Leo, C. Martos, I. A. Ibarra and G. Orcajo, Eur. J. Inorg. Chem., 2021, 4458–4462 CrossRef.
- E. S. Grape, J. G. Flores, T. Hidalgo, E. Martínez-Ahumada, A. Gutiérrez-Alejandre, A. Hautier, D. R. Williams, M. O’Keeffe, L. Öhrström, T. Willhammar, P. Horcajada, I. A. Ibarra and A. K. Inge, J. Am. Chem. Soc., 2020, 142, 16795–16804 CrossRef CAS PubMed.
- R. Domínguez-González, I. Rojas-León, E. Martínez-Ahumada, D. Martínez-Otero, H. A. Lara-García, J. Balmaseda-Era, I. A. Ibarra, E. G. Percástegui and V. Jancik, J. Mater. Chem. A, 2019, 7, 26812–26817 Search PubMed.
- J. L. Obeso, K. Gopalsamy, M. Wahiduzzaman, E. Martínez-Ahumada, D. Fan, H. A. Lara-García, F. J. Carmona, G. Maurin, I. A. Ibarra and J. A. R. Navarro, J. Mater. Chem. A, 2024, 12, 10157–10165 Search PubMed.
- H. Zhao, X. Gu, T. Yan, G. Han and D. Liu, AIChE J., 2023, 69, e17942 CrossRef CAS.
- S.-Q. Gang, Z.-Y. Liu, Y.-N. Bian, R. Wang and J.-L. Du, Sep. Purif. Technol., 2024, 335, 126153 CrossRef CAS.
- L. J. Guo, X. F. Feng, Z. Gao, R. Krishna and F. Luo, Inorg. Chem., 2021, 60, 1310–1314 CrossRef CAS PubMed.
- A. López-Olvera, J. A. Zárate, J. L. Obeso, E. Sánchez-González, J. A. De Los Reyes, R. A. Peralta, E. González-Zamora and I. A. Ibarra, Inorg. Chem., 2023, 62, 20901–20905 Search PubMed.
- J. Yao, Z. Zhao, L. Yu, J. Huang, S. Shen, S. Zhao, Y. Wu, X. Tian, J. Wang and Q. Xia, J. Mater. Chem. A, 2023, 11, 14728–14737 Search PubMed.
- J. Li, G. L. Smith, Y. Chen, Y. Ma, M. Kippax-Jones, M. Fan, W. Lu, M. D. Frogley, G. Cinque, S. J. Day, S. P. Thompson, Y. Cheng, L. L. Daemen, A. J. Ramirez-Cuesta, M. Schröder and S. Yang, Angew. Chem., Int. Ed., 2022, 61, e202207259 CrossRef CAS PubMed.
- L. Briggs, R. Newby, X. Han, C. G. Morris, M. Savage, C. P. Krap, T. L. Easun, M. D. Frogley, G. Cinque, C. A. Murray, C. C. Tang, J. Sun, S. Yang and M. Schröder, J. Mater. Chem. A, 2021, 9, 7190–7197 RSC.
- L. Li, I. Da Silva, D. I. Kolokolov, X. Han, J. Li, G. Smith, Y. Cheng, L. L. Daemen, C. G. Morris, H. G. W. Godfrey, N. M. Jacques, X. Zhang, P. Manuel, M. D. Frogley, C. A. Murray, A. J. Ramirez-Cuesta, G. Cinque, C. C. Tang, A. G. Stepanov, S. Yang and M. Schroder, Chem. Sci., 2019, 10, 1472–1482 RSC.
- X. Cui, Q. Yang, L. Yang, R. Krishna, Z. Zhang, Z. Bao, H. Wu, Q. Ren, W. Zhou, B. Chen and H. Xing, Adv. Mater., 2017, 29, 1606929 CrossRef PubMed.
- W. Li, C. Cheng, G. Gao, H. Xu, W. Huang, Z. Qu and N. Yan, Mater. Horiz., 2024, 11, 1889–1898 RSC.
- N. Aboosedgh and S. Fatemi, J. Environ. Chem. Eng., 2024, 12, 112042 CrossRef CAS.
- X. Kan, G. Zhang, J. Ma, F. Liu, Y. Tang, F. Liu, X. Yi, Y. Liu, A. Zheng, L. Jiang, F. Xiao and S. Dai, Adv. Funct. Mater., 2024, 34, 2312044 Search PubMed.
- W. Xu, L. Li, M. Guo, F. Zhang, P. Dai, X. Gu, D. Liu, T. Liu, K. Zhang, T. Xing, M. Wang, Z. Li and M. Wu, Angew. Chem., Int. Ed., 2023, 62, e202312029 CrossRef CAS PubMed.
- Y. Zhang, Z. Chen, X. Liu, Z. Dong, P. Zhang, J. Wang, Q. Deng, Z. Zeng, S. Zhang and S. Deng, Ind. Eng. Chem. Res., 2020, 59, 874–882 CrossRef CAS.
- W. Gong, Y. Xie, A. Yamano, S. Ito, X. Tang, E. W. Reinheimer, C. D. Malliakas, J. Dong, Y. Cui and O. K. Farha, J. Am. Chem. Soc., 2023, 145, 26890–26899 CrossRef CAS PubMed.
- Z. Zhang, B. Yang and H. Ma, Sep. Purif. Technol., 2021, 259, 118164 CrossRef CAS.
- Y. Ma, A. Li and C. Wang, Chem. Eng. J., 2023, 455, 140687 Search PubMed.
- K. Tan, P. Canepa, Q. Gong, J. Liu, D. H. Johnson, A. Dyevoich, P. K. Thallapally, T. Thonhauser, J. Li and Y. J. Chabal, Chem. Mater., 2013, 25, 4653–4662 CrossRef CAS.
- X.-D. Zhang, N. Wang, Y. Liu, M.-K. Yang, W. Gao, Y.-Z. Zhang, L. Geng, D.-S. Zhang, S. Zhuang and X. Zhang, Inorg. Chem. Commun., 2024, 170, 113174 Search PubMed.
- L.-Z. Yang, W. Xie, L. Yan, Q. Fu, X. Yuan, Q. Zheng and X. Zhao, Sep. Purif. Technol., 2024, 346, 127513 CrossRef CAS.
- G.-R. Si, X.-J. Kong, T. He, Z. Zhang and J.-R. Li, Nat. Commun., 2024, 15, 7220 Search PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 Search PubMed.
- R. Vaidhyanathan, S. S. Iremonger, K. W. Dawson and G. K. H. Shimizu, Chem. Commun., 2009, 5230 Search PubMed.
- W. Liu, Y. Zhang, S. Wang, L. Bai, Y. Deng and J. Tao, Molecules, 2021, 26, 5267 CrossRef CAS PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 Search PubMed.
- F. Elwinger, P. Pourmand and I. Furó, J. Phys. Chem. C, 2017, 121, 13757–13764 Search PubMed.
- J. Zhang, T. Xia, D. Zhao, Y. Cui, Y. Yang and G. Qian, Sens. Actuators, B, 2018, 260, 63–69 CrossRef CAS.
- J. L. Obeso, E. Martínez-Ahumada, A. López-Olvera, J. Ortiz-Landeros, H. A. Lara-García, J. Balmaseda, S. López-Morales, E. Sánchez-González, D. Solis-Ibarra, C. Leyva and I. A. Ibarra, ACS Appl. Energy Mater., 2023, 6, 9084–9091 CrossRef CAS.
- J. L. Obeso, V. B. López-Cervantes, C. V. Flores, A. Martínez, Y. A. Amador-Sánchez, N. S. Portillo-Velez, H. A. Lara-García, C. Leyva, D. Solis-Ibarra and R. A. Peralta, Dalton Trans., 2024, 53, 4790–4796 Search PubMed.
- L. Wang and Y. Chen, Chem. Commun., 2020, 56, 6965–6968 RSC.
- Y. Xie, H. Ma, F. L. He, J. Chen, Y. Ji, S. Han and D. Zhu, Analyst, 2020, 145, 4772–4776 RSC.
- E. Martínez-Ahumada, A. López-Olvera, P. Carmona-Monroy, H. Díaz-Salazar, M. H. Garduño-Castro, J. L. Obeso, C. Leyva, A. Martínez, M. Hernández-Rodríguez, D. Solis-Ibarra and I. A. Ibarra, Dalton Trans., 2022, 51, 18368–18372 RSC.
- E. Martínez-Ahumada, D. W. Kim, M. Wahiduzzaman, P. Carmona-Monroy, A. López-Olvera, D. R. Williams, V. Martis, H. A. Lara-García, S. López-Morales, D. Solis-Ibarra, G. Maurin, I. A. Ibarra and C. S. Hong, J. Mater. Chem. A, 2022, 10, 18636–18643 RSC.
- V. B. López-Cervantes, D. W. Kim, J. L. Obeso, E. Martínez-Ahumada, Y. A. Amador-Sánchez, E. Sánchez-González, C. Leyva, C. S. Hong, I. A. Ibarra and D. Solis-Ibarra, Nanoscale, 2023, 15, 12471–12475 RSC.
- V. B. López-Cervantes, D. Bara, A. Yañez-Aulestia, E. Martínez-Ahumada, A. López-Olvera, Y. A. Amador-Sánchez, D. Solis-Ibarra, E. Sánchez-González, I. A. Ibarra and R. S. Forgan, Chem. Commun., 2023, 59, 8115–8118 RSC.
- V. B. López-Cervantes, A. López-Olvera, J. L. Obeso, I. K. Torres, E. Martínez-Ahumada, P. Carmona-Monroy, E. Sánchez-González, D. Solís-Ibarra, E. Lima, E. Jangodaz, R. Babarao, I. A. Ibarra and S. G. Telfer, Chem. Mater., 2024, 36, 2735–2742 CrossRef.
- M. Wang, L. Guo and D. Cao, Anal. Chem., 2018, 90, 3608–3614 CrossRef CAS PubMed.
- V. B. López-Cervantes, M. L. Martínez, J. L. Obeso, C. García-Carvajal, N. S. Portillo-Vélez, A. Guzmán-Vargas, R. A. Peralta, E. González-Zamora, I. A. Ibarra, D. Solis-Ibarra, J. L. Woodliffe and Y. A. Amador-Sánchez, Dalton Trans., 2025, 54, 1646–1654 RSC.
- N. Ingle, S. Mane, P. Sayyad, G. Bodkhe, T. AL-Gahouari, M. Mahadik, S. Shirsat and M. D. Shirsat, Front. Mater., 2020, 7, 93 CrossRef.
- Q. Li, J. Wu, L. Huang, J. Gao, H. Zhou, Y. Shi, Q. Pan, G. Zhang, Y. Du and W. Liang, J. Mater. Chem. A, 2018, 6, 12115–12124 RSC.
- N. Ingle, P. Sayyad, M. Deshmukh, G. Bodkhe, M. Mahadik, T. Al-Gahouari, S. Shirsat and M. D. Shirsat, Appl. Phys. A: Mater. Sci. Process., 2021, 127, 157 CrossRef CAS.
- X. Zhang, Z. Zhai, J. Wang, X. Hao, Y. Sun, S. Yu, X. Lin, Y. Qin and C. Li, ChemNanoMat, 2021, 7, 1117–1124 CrossRef CAS.
- Z. Zhai, X. Zhang, J. Wang, H. Li, Y. Sun, X. Hao, Y. Qin, B. Niu and C. Li, Chem. Eng. J., 2022, 428, 131720 CrossRef CAS.
- Z. Zhai, J. Wang, Y. Sun, X. Hao, B. Niu, H. Xie and C. Li, Appl. Surf. Sci., 2023, 613, 155772 CrossRef CAS.
- N. Huo, D. Li, S. Zheng and W. Deng, Chem. Eng. J., 2022, 432, 134317 CrossRef CAS.
- M. E. DMello, N. G. Sundaram, A. Singh, A. K. Singh and S. B. Kalidindi, Chem. Commun., 2019, 55, 349–352 RSC.
- V. Chernikova, O. Yassine, O. Shekhah, M. Eddaoudi and K. N. Salama, J. Mater. Chem. A, 2018, 6, 5550–5554 RSC.
- C. H. Pham and F. Paesani, Inorg. Chem., 2018, 57, 9839–9843 CrossRef CAS PubMed.
- M. R. Tchalala, P. M. Bhatt, K. N. Chappanda, S. R. Tavares, K. Adil, Y. Belmabkhout, A. Shkurenko, A. Cadiau, N. Heymans, G. De Weireld, G. Maurin, K. N. Salama and M. Eddaoudi, Nat. Commun., 2019, 10, 1328 CrossRef CAS PubMed.
- M. Pamei and A. Puzari, Nano-Struct. Nano-Objects, 2019, 19, 100364 CrossRef CAS.
- Z. Liao, T. Xia, E. Yu and Y. Cui, Crystals, 2018, 8, 338 CrossRef.
- M. Runowski, D. Marcinkowski, K. Soler-Carracedo, A. Gorczyński, E. Ewert, P. Woźny and I. R. Martín, ACS Appl. Mater. Interfaces, 2023, 15, 3244–3252 CrossRef CAS PubMed.
- W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242–3285 RSC.
- Y. Liu, X.-Y. Xie, C. Cheng, Z.-S. Shao and H.-S. Wang, J. Mater. Chem. C, 2019, 7, 10743–10763 RSC.
- C. Yuan, S. Saito, C. Camacho, S. Irle, I. Hisaki and S. Yamaguchi, J. Am. Chem. Soc., 2013, 135, 8842–8845 CrossRef CAS PubMed.
- Y. Zheng, Y. Zhou, J. Yu, Y. Yu, H. Zhang and W. P. Gillin, J. Phys. D: Appl. Phys., 2004, 37, 531–534 CrossRef CAS.
- D. E. Williams and N. B. Shustova, Chem. – Eur. J., 2015, 21, 15474–15479 CrossRef CAS PubMed.
- S. Xing and C. Janiak, Chem. Commun., 2020, 56, 12290–12306 RSC.
- M. Zeng, A. Ren, W. Wu, Y. Zhao, C. Zhan and J. Yao, Chem. Sci., 2020, 11, 9154–9161 RSC.
- L. Chen, Y. Song, W. Liu, H. Dong, D. Wang, J. Liu, Q. Liu and X. Chen, J. Alloys Compd., 2022, 893, 162322 CrossRef CAS.
- L. Yu, H. Wang, W. Liu, S. J. Teat and J. Li, Cryst. Growth Des., 2019, 19, 6850–6854 CrossRef CAS.
- J. L. Mancuso, A. M. Mroz, K. N. Le and C. H. Hendon, Chem. Rev., 2020, 120, 8641–8715 CrossRef CAS PubMed.
- M. Ji, X. Lan, Z. Han, C. Hao and J. Qiu, Inorg. Chem., 2012, 51, 12389–12394 CrossRef CAS PubMed.
- M. Shen, Y. Zhang, H. Xu and H. Ma, iScience, 2021, 24, 103069 CrossRef CAS PubMed.
- F. Ahmadijokani, H. Molavi, S. Tajahmadi, M. Rezakazemi, M. Amini, M. Kamkar, O. J. Rojas and M. Arjmand, Coord. Chem. Rev., 2022, 464, 214562 CrossRef CAS.
- J. Perego, I. Villa, A. Pedrini, E. C. Padovani, R. Crapanzano, A. Vedda, C. Dujardin, C. X. Bezuidenhout, S. Bracco, P. E. Sozzani, A. Comotti, L. Gironi, M. Beretta, M. Salomoni, N. Kratochwil, S. Gundacker, E. Auffray, F. Meinardi and A. Monguzzi, Nat. Photonics, 2021, 15, 393–400 CrossRef CAS.
- M. A. Chowdhury, ChemBioEng Rev., 2017, 4, 225–239 CrossRef CAS.
- J.-X. Wang, J. Yin, O. Shekhah, O. M. Bakr, M. Eddaoudi and O. F. Mohammed, ACS Appl. Mater. Interfaces, 2022, 14, 9970–9986 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- A. Mallick, A. M. El-Zohry, O. Shekhah, J. Yin, J. Jia, H. Aggarwal, A.-H. Emwas, O. F. Mohammed and M. Eddaoudi, J. Am. Chem. Soc., 2019, 141, 7245–7249 CrossRef CAS PubMed.
- K. Miyata, T. Nakagawa, R. Kawakami, Y. Kita, K. Sugimoto, T. Nakashima, T. Harada, T. Kawai and Y. Hasegawa, Chem. – Eur. J., 2011, 17, 521–528 CrossRef CAS PubMed.
- Y. Ming, N. Kumar and D. J. Siegel, ACS Omega, 2017, 2, 4921–4928 CrossRef CAS PubMed.
- I.-D. Kim, A. Rothschild and H. L. Tuller, Acta Mater., 2013, 61, 974–1000 Search PubMed.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincă, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CrossRef CAS PubMed.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Chem. Rev., 2019, 119, 599–663 CrossRef CAS PubMed.
- L. Torsi, M. Magliulo, K. Manoli and G. Palazzo, Chem. Soc. Rev., 2013, 42, 8612 RSC.
- Z. Hu, J. Miu, X. Zhang, M. Jia and J. Yao, J. Appl. Polym. Sci., 2022, 139, e52810 CrossRef CAS.
- P. V. Shinde and C. S. Rout, Nanoscale Adv., 2021, 3, 1551–1568 RSC.
- A. Punnoose, K. M. Reddy, A. Thurber, J. Hays and M. H. Engelhard, Nanotechnology, 2007, 18, 165502 CrossRef.
- A. Punnoose, K. M. Reddy, J. Hays, A. Thurber and M. H. Engelhard, Appl. Phys. Lett., 2006, 89, 112509 CrossRef.
- A. A. Bagade, V. V. Ganbavle, S. V. Mohite, T. D. Dongale, B. B. Sinha and K. Y. Rajpure, J. Colloid Interface Sci., 2017, 497, 181–192 CrossRef CAS PubMed.
- H. Goesmann and C. Feldmann, Angew. Chem., Int. Ed., 2010, 49, 1362–1395 CrossRef CAS PubMed.
- O. Kahn and C. J. Martinez, Science, 1998, 279, 44–48 CrossRef CAS.
- C. Bartual-Murgui, A. Akou, C. Thibault, G. Molnár, C. Vieu, L. Salmon and A. Bousseksou, J. Mater. Chem. C, 2015, 3, 1277–1285 RSC.
- L. Wang, Z. Wang, Q. Xiang, Y. Chen, Z. Duan and J. Xu, Sens. Actuators, B, 2017, 248, 820–828 CrossRef CAS.
- R. Paolesse, C. Di Natale, A. Macagnano, F. Davide, T. Boschi and A. D’Amico, Sens. Actuators, B, 1998, 47, 70–76 CrossRef CAS.
- L. Zhang, Q. Yang and Z. Zhu, Foods, 2024, 13, 1936 Search PubMed.
- X. Wang, J. Zhang, Z. Zhu and J. Zhu, Appl. Surf. Sci., 2007, 253, 3168–3173 Search PubMed.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- C. Wu, H. Xu, Y. Li, R. Xie, P. Li, X. Pang, Z. Zhou, B. Gu, H. Li and Y. Zhang, Talanta, 2019, 200, 78–83 CrossRef CAS PubMed.
- M.-S. Yao, W.-H. Li and G. Xu, Coord. Chem. Rev., 2021, 426, 213479 CrossRef CAS.
- R.-M. Neubieser, J.-L. Wree, J. Jagosz, M. Becher, A. Ostendorf, A. Devi, C. Bock, M. Michel and A. Grabmaier, Micro Nano Eng., 2022, 15, 100126 CrossRef CAS.
- M. Gupta, H. Hawari, P. Kumar and Z. Burhanudin, Crystals, 2022, 12, 264 CrossRef CAS.
- H. S. Magar, R. Y. A. Hassan and A. Mulchandani, Sensors, 2021, 21, 6578 Search PubMed.
- V. Balasubramani, S. Sureshkumar, T. S. Rao and T. M. Sridhar, ACS Omega, 2019, 4, 9976–9982 CrossRef CAS PubMed.
- B. Zong, Q. Li, X. Chen, C. Liu, L. Li, J. Ruan and S. Mao, ACS Appl. Mater. Interfaces, 2020, 12, 50610–50618 CrossRef CAS PubMed.
- A. Paghi, S. Mariani and G. Barillaro, Small, 2023, 19, 2206100 CrossRef CAS PubMed.
- J. Park, H. Kim and Y. Jung, J. Phys. Chem. Lett., 2013, 4, 2530–2534 CrossRef CAS.
- M. Mukoyoshi, M. Maesato, S. Kawaguchi, Y. Kubota, K. Cho, Y. Kitagawa and H. Kitagawa, Inorg. Chem., 2022, 61, 7226–7230 CrossRef CAS PubMed.
- C. Zhai, Q. Zhao, K. Gu, D. Xing and M. Zhang, J. Alloys Compd., 2019, 784, 660–667 CrossRef CAS.
- H. Yuan, J. Tao, N. Li, A. Karmakar, C. Tang, H. Cai, S. J. Pennycook, N. Singh and D. Zhao, Angew. Chem., Int. Ed., 2019, 58, 14089–14094 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |