Open Access Article
Open Access ArticleChiral nanographenes exhibiting circularly polarized luminescence
Viksit
Kumar
,
José L.
Páez
 ,
Sandra
Míguez-Lago
,
Sandra
Míguez-Lago
 ,
Juan M.
Cuerva
,
Juan M.
Cuerva
 ,
Carlos M.
Cruz
,
Carlos M.
Cruz
 * and
Araceli G.
Campaña
* and
Araceli G.
Campaña
 *
*
Department of Organic Chemistry, Unidad de Excelencia de Química (UEQ), Faculty of Sciences, University of Granada. Avda. Fuente Nueva s/n, 18071 Granada, Spain. E-mail: cmorenoc@ugr.es; araceligc@ugr.es
First published on 10th April 2025
Abstract
Chiral nanographenes constitute an unconventional material class that deviates from planar graphene cutouts. They have gained considerable attention for their ability to exhibit circularly polarized luminescence (CPL), which offers new opportunities in chiral optoelectronics. Their unique π-conjugated architectures, coupled with the ability to introduce chirality at the molecular level, have made them powerful contenders in developing next-generation optoelectronic devices. This review thoroughly explores recent advances in the synthesis, structural design, and CPL performance of chiral nanographenes. We delve into diverse strategies for inducing chirality, including covalent functionalization, helically twisted frameworks, and heteroatom doping, each of which unlocks distinct CPL behaviors. In addition, we discuss the mechanistic principles governing CPL and future directions in chiral nanographenes to achieve high dissymmetry factors (glum) and tunable emission properties. We also discuss the key challenges in this evolving field, including designing robust chiral frameworks, optimizing CPL efficiency, and scaling up real-world applications. Through this review, we aim to shed light on recent developments in the bottom-up synthesis of structurally precise chiral nanographenes and evaluate their impact on the growing domain of circularly polarized luminescent materials.
1. Introduction
Carbon-based materials are fundamental to modern science and technology due to their exceptional properties.1 The carbon atom can form extended networks held together by covalent bonds in diverse orbital hybridizations, resulting in a wide range of materials with unique mechanical, electrical, and chemical characteristics. The versatility and abundance of carbon make it ideal for developing next-generation materials addressing key challenges in energy, sustainability, and high-performance electronics.2–4 Two naturally occurring allotropes of carbon are diamond, solely containing sp3-hybridized carbon atoms, and graphite, which is only formed by sp2-hybridized carbon atoms, both in their pristine forms. Besides, other carbon allotropes such as fullerenes, carbon nanotubes, and graphene were all discovered in quick succession in the late 20th and early 21st centuries.5 The story of graphene dates back to 1947 when Wallace began his theoretical investigation on single-layer graphene.6 However, it was not until 2004 that Novoselov and Geim isolated and identified it for the first time, for which they got the 2010 Nobel Prize in Physics.7 Each carbon atom in graphene is bonded to its three nearest neighbours by hybridization of the 2s, 2px, and 2py atomic orbitals into sp2 molecular orbitals, while the 2pz orbitals create delocalized π and π* bands that are perpendicular to the graphene plane. This seemingly simple structure results in a unique combination of physical properties8 and countless possible applications.9 From its high conductivity and resistance to its flexibility and transparency, graphene brings together a whole range of uses in sensors, nano-electronic devices, spin devices, catalysis, and energy storage and it has thus been the subject of extensive experimental and theoretical research10 over the last twenty years.11Concerning its optical properties, graphene absorbs a considerable fraction of incident white light (2.3%) despite being only one-atom thick.12 Photoluminescence in graphene, on the other hand, requires opening a bandgap in its electronic structure, which can be reached by the application of strain, an electric field or by reducing its size to the nanometric scale. In this sense, bottom-up synthesis comes into play, enabling the synthesis of small graphene cutouts with structural precision.13,14 Nanographenes (NGs), a large category of polycyclic conjugated hydrocarbon (PCH) structures, are roughly described as finite graphenic units made of fused six-membered rings with sp2 hybridized carbon atoms. NGs themselves have been the subject of substantial research, hand in hand with the goal of discovering bottom-up synthetic pathways to manufacture defect-free single-layered graphene sheets. Following the classification made by Müllen and co-workers, NGs are considered graphene fragments between 1 and 100 nm (Fig. 1),15,16 including graphene molecules (fragments between 1 and 5 nm in diameter), graphene nanoribbons (fragments between 5 and 10 nm in length), and graphene quantum dots for fragments below 100 nm. Since the seminal work in the late 1950s by Clar17,18 and Halleux,19 with the synthesis of the flagship NG, hexa-peri-hexabenzocoronene (HBC), much work has been done on the bottom-up synthesis of well-defined NGs, following in the footsteps of pioneers such as Müllen.20,21
More recently, designing non-planar, twisted, or contorted objects with inherent chirality has received major attention in the search of their intriguing properties. These chiral, non-planar NGs have been synthesized using a variety of methodologies like Scholl cyclodehydrogenation and alkyne-based approaches.22–24 In the context of creating twisted structures, Miao and co-workers recent review of Scholl chemistry highlights its extensive use in producing chiral NGs.25 Chirality can be introduced in NGs through various strategies,26 being one of the most widely employed the inclusion of twisted or helical motifs in its structure.27,28 Another approach involves the incorporation of chiral auxiliaries or substituents into NG frameworks, leading to asymmetry. In addition, axial or point chirality can be introduced by chemical modification of the edges or peripheries28 of NGs with chiral groups or ligands.29–32 In parallel, researchers have also explored the construction of twisted or saddle-shaped NGs containing non-benzenoid rings and heteroatoms, which exhibit intrinsic chirality due to the breaking of their symmetry.20
Chiral NGs allow the differential interaction of each enantiomer with circularly polarized light, through which their chiroptical properties can be explored. Among them, circularly polarized luminescence (CPL) represents a promising property, with applications in sensing, organic electronics, bioimaging, encryption, etc.33–37
At the electronic level, the coupling between electronic transitions and chiral geometry can result in large chiroptical responses, enhancing the degree of circular polarization. This interplay between structure and CPL performance is a key area of investigation as researchers seek to design NGs that optimize luminescence efficiency and CPL intensity.
Picking up on the variety of chiroptical properties it is necessary to describe the light-matter interplay. At the very moment polarized light penetrates the sample and interacts with the chiral NGs, such light can be either right or left circularly polarized, depending on whether the accompanying electromagnetic vector is a clockwise or counterclockwise spiral. To this regard, the enantiopure NGs show differential absorption of left and right circularly polarized light, which can usually be used to gain information on the chirality of the ground state. In the same manner, this can be applied to the study of aggregates, supramolecular systems and intramolecular interactions.38 Mathematically, CD can be defined by eqn (1.1), being AL and AR the absorption of left and right circularly polarized light, respectively. We refer to either electronic CD (ECD) when we measure it in the ultraviolet-visible region or to vibrational CD (VCD) when it appears in the infrared region.
| CD = AL − AR | (1.1) |
Circular dichroism can turn into a value independent of the sample concentration, when expressed in extinction coefficient units (ε), analogously to the Lambert–Beer law, (eqn (1.2)) being c the concentration and b the optical pathlength.
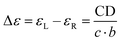 | (1.2) |
Nevertheless, in order to be able to compare the CD response of compounds with different ε, a dimensionless magnitude, named the absorption dissymmetry factor gabs, is employed (eqn (1.3)):
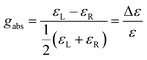 | (1.3) |
In chiral NGs, different enantiomers emit left-handed and right-handed circularly polarized light with varying intensities under incident light, which reflects the chirality of the NGs in the excited state (Fig. 2). Generally, chirality and fluorescence are two mandatory elements for achieving intrinsic CPL. Parallel to absorption, in emission, the first criterion for evaluating the quality of CPL is the luminescence dissymmetry factor (glum) (eqn (1.4)).
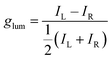 | (1.4) |
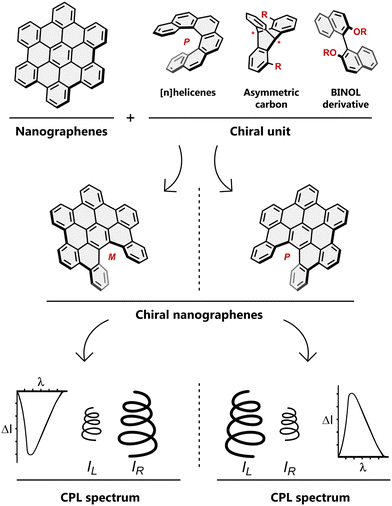 | ||
| Fig. 2 Structural motifs that furnish chiral nanographenes, schematic representation of a pair of enantiomeric chiral nanographenes (seco-HBC) and their opposite CPL spectra. | ||
This factor depends on the molecular symmetry, the momenta components of the electronic transitions involved, and the strength of the coupling between them, being IL and IR the left and right circularly polarized fluorescence intensities, respectively. Both gabs and glum values should fall between −2 and 2 by definition, while g = 0 indicates null discrimination in terms of circular polarization of the absorbed/emitted light. In addition, both g factors can also be predicted theoretically. Whilst for gabs, it can be calculated for each S0 → Sn transition, for glum, one should consider that in emission, usually solely the transition from the first excited state to the ground state of a molecule (S1 → S0) is computed. Besides, both g factors can be calculated from the associated rotatory (R) and dipolar strengths (D) of a certain transition, or, in other words, from the dipole magnetic (m) and electric (μ) moments for a given transition, along with the angle between them (θ) (eqn (1.5)).
 | (1.5) |
Thus, careful examination of eqn (1.5) hints at the enormous influence of the dipole moments on the chiroptical response. Specially for small organic molecules, μ is usually larger than m; therefore, increasing m directly impacts both CD and CPL responses. Dissymmetry factors are, besides, dependent on an appropriate alignment of the moments, which should orient with each other with an angle θ close to either 0° or 180°. Nevertheless, large glum values are not always synonymous with straightforward applicability of the compound in case the emitter presents a small fluorescence quantum yield. An example of that are lanthanide and d-block metal complexes in which transitions are metal-centered and electric dipole forbidden.39,40
To understand overall CPL efficiency, extra parameters other than glum are explained in literature. Example of that is the report of Mori and co-workers in 2020 were circular polarization luminosity (ΛCPL) per single chiral molecule (eqn (1.6)) was proposed to get an intrinsic index for CPL efficiency.41
 | (1.6) |
 | (1.7) |
In here, ελ is the extinction coefficient at the excitation wavelength, and ΦF the fluorescence quantum yield.
In chiral NGs, CPL efficiency can be modulated by several factors, including the extent of π-conjugation, the rigidity of the molecular framework, and the degree of electronic coupling between chromophores.43 For example, helical NGs with extended conjugation tend to exhibit higher glum values in comparison with their non-extended helical counterparts (simple helicenes) due to the strong interaction between the delocalized π-electron system and the chiral environment. Additionally, introducing functional groups that enhance electronic asymmetry can further improve the CPL performance, though fostering luminescence (typically by μ enhancement). Thus, what sets chiral NGs apart is their ability to combine the intrinsic optoelectronic properties of NGs with the chiroptical activity of chiral molecules, by introducing chirality into the planar or quasi-planar NG structure. By virtue of OLED display technologies raising, among others, CPL materials have become a research hotspot in the field of chiral materials and luminescent materials. Thus, the non-compiled outstanding achievements concerning NG-based CPL materials in the past years, claim for a review article, which is expected to promote further progress in this unique research area. To the best of our knowledge, though some general reviews focusing on the synthesis and applications of CPL emitters have been reported in the literature,28,44–46 there is no report that primarily focuses on the chiral NGs as CPL emitters. This review summarizes the recent advances in the synthesis of chiral NGs-based CPL emitters, prepared through bottom-up synthesis. Reviews focusing on graphene quantum dots mostly prepared by top-down methodologies lacking well defined structures will be deliberately set aside, given their deficiency in understanding the pursued structure–property relationships at molecular level.47–49 As NGs, we have chosen the aforementioned 1 × 1 nm size criteria from among the vast array of chiral polycyclic conjugated hydrocarbons. Furthermore, only chiral NG examples that have been described and reported their CPL emission have been included in this review. More importantly, this review will provide a detailed analysis of the influence of structural design on chiroptical properties under each section.
To understand the structure–property relationship in NGs as chiral emitters, we have included a final summary table with the most relevant optical and chiroptical data. Furthermore, the opportunities and challenges for achieving high-performance CPL emitters are also presented and discussed. This review will contribute to guide researchers in the design of new chiral NG-based CPL emitters for functional applications.
2. NGs as CPL emitters
2.1. Design and synthetic strategies
The synthesis of chiral NGs requires precise control over their π-conjugated core and the introduction of chirality.50 Several synthetic strategies have been developed to achieve this goal, including the bottom-up synthesis of NG cutouts. In order to achieve that, there are several well-established synthetic approaches to prepare chiral NGs, including cyclodehydrogenation (mostly under Scholl conditions) of polycyclic conjugated hydrocarbons into larger NG structures. This reaction can be adapted to form twisted or helically chiral NGs by carefully choosing sterically hindered precursors and reaction conditions.51–54Cross-coupling reactions (e.g. Suzuki, Sonogashira, Stille, Kumada) are commonly used for the introduction of various functional groups at specific positions on the NG core, allowing to generate prochirality into the structure. Recent advances in synthetic chemistry have also enabled the introduction of heteroatoms (such as nitrogen, sulphur, or boron) into the NG framework, which can further modulate the chiral and photophysical properties.55,56 Due to the altered electronic structure, these heteroatom-doped chiral NGs exhibit enhanced luminescence and unique CPL characteristics. Moreover, researchers have explored the blend of NGs with inherently chiral scaffolds, such as chiral polymers and other dopants, to further enhance their CPL activity. Here we classify all different non-planar NGs into benzenoid, non-benzenoid and heteroatom-containing families.
To conclude, the design of chiral NGs as CPL emitters hinges on creating a balance between structural chirality and electronic properties. Strategies such as introducing helical or twisted geometries, covalently attaching chiral groups, or using self-assembly techniques can lead to efficient CPL emission. Fine control over molecular size, edge structure, heteroatom doping, and functionalization enables the tuning of CPL properties, making NGs promising candidates for applications in chiral optoelectronics and sensing.
2.2. NGs bearing benzenoid rings
This category of NGs comprises helically twisted π-extended NGs containing ortho-fused benzene ring systems, [n]helicenes, where n represents the number of the rings forming the helix. Chiral NGs bearing benzenoid rings represent a unique class of materials that combine the structural rigidity and extended π-conjugation of fused benzene rings with chirality. These NGs are not only defined by their polycyclic conjugated hydrocarbon frameworks but also by the introduction of chirality through helical structures and how all together assemble. According to our stablished criteria, seco-HBC, which is an HBC in which a benzenoid ring is cleaved to form a carbo[5]helicene, can be regarded as the simplest chiral NG (compound 1, Fig. 3). Jux and co-workers reported its synthesis,64 although an alternative synthesis and its chiroptical properties were recently investigated by Tanaka and co-workers.65 Compound (P)-1 was prepared via enantioselective synthesis using a combination of a Ni(cod)2 catalysed [2+2+2] cycloaddition followed by a Scholl reaction. Compound (P)-1 exhibits a ΦF of 0.11 and a glum of 1 × 10−3 (BCPL = 5 M−1 cm−1). Structurally related with seco-HBC, there are examples of larger carbo[n]helicenes embedded into the structure of HBC with CPL responses. In this sense, Narita and co-workers reported the synthesis of compounds 2 and 3 (Fig. 3) by regioselective oxidative cyclodehydrogenation.43 They represent chiral HBC analogues bearing a carbo[7] and carbo[9]helicene, respectively. While 2 shows a |glum| of 0.77 × 10−3 and a ΦF of 0.25, 3 shows an improvement of one order of magnitude in the chiroptical response (|glum| = 7.4 × 10−3, ΦF = 0.41, and BCPL = 12.6 M−1 cm−1) because of embedding a longer helicene in the structure which result in a larger internal area for the case of 3. On the other hand, the higher ΦF values of 2 and 3 compared with pristine [7]helicene and [9]helicene, whose ΦF values account to 0.021 and 0.014, respectively,66 clearly evidences the impact of the π-extension of the system in their emission performance.Selected partially π-extended double helicenes 4 to 6 (Fig. 3) are included for comparison as they could be considered as double helical analogues of 1. That is the case of tetrabenzoperopyrene (4, Fig. 3) as double carbo[5]helicene whose synthesis was reported by Clar and co-workers in 1959,18 however, its CPL response was studied in 2024 by Hu and co-workers.67 Compound 4 exhibits a high ΦF of 0.81 (pristine peropyrene, ΦF = 0.9) and the measured |glum| value of their isolated enantiomers reached a value of 0.53 × 10−3. In 2018, Tanaka reported the synthesis of 5 (Fig. 3), as a partially π-extended double carbo[6]helicene.68 It was synthesized by Rh(I)/binap-catalysed enantioselective intramolecular [2+2+2] cycloaddition of 2-phenylnaphthalene-linked triynes followed by posterior aromatization by Scholl oxidation. Compound 5 displayed a low |glum| value of 0.75 × 10−3 and a remarkable ΦF = 0.75, which could be due to the location of the HOMO and LUMO orbitals in the central peropyrene core without the influence of the lateral helicenes. In fact, this g factor is very similar to the one previously reported for chiral peropyrenes with twisted backbones based on the steric congestion of bay regions and the inclusion of octagonal rings, synthetized by the groups of Chalifoux and Juríček (|glum| = 0.7 × 10−3 and 0.2 × 10−3, respectively), respectively.69,70 In 2017, Narita and co-workers reported the first X-shaped double carbo[7]helicene (compound 6, Fig. 3) as a novel structural motif obtained by regioselective cyclodehydrogenation. The molecule showed promising |gabs| of 1.5 × 10−2 and high ΦF = 0.8, though its CPL property was not studied at that moment.52 However, later on, in 2022 they reported a |glum| of 0.07 × 10−3 and compared it with other N-doped X-shaped double [7]helicenes with different terminal heterocycles as compound 60 (Section 2.4.1, Fig. 15).61,71 A comparison of the chiroptical responses of 1vs.4 or 2vs.6, bearing one or two carbohelicenes of the same size, shows again how the inclusion of multiple helicene can result in a considerable decrease in the dissymmetry emission, in one order of magnitude (|glum| = 1 × 10−3 and 0.53 × 10−3 for 1 and 4, respectively; |glum| = 0.77 × 10−3 and 0.07 × 10−3 for 2 and 6, respectively). These examples reveal again that not always more helical moieties result in enhanced chiroptical responses and bring out attention to the fine design required for a better performance in well-defined NGs.
By combination of three pentahelicene units, Mori and co-workers synthesized hexabenzotriphenylene (compound 7, Fig. 3). Compound 7 could resemble as a triple seco-HBC, where three benzenoid rings are cleaved.72 The D3-symmetry propeller-like structure was isolated, with the three carbo[5]helicenes in the same configuration. This compound was found stable towards undesirable racemization and degradation relative to pH change. Its ΦF in CH2Cl2 is 0.018 and shows a gabs (S0 → S1 transition) and glum values of −1.8 × 10−3 and −1.3 × 10−3, respectively, for (P,P,P)-7. It resulted that merging three pentahelicenes in a single structure gave significantly lower chiroptical response in comparison with pristine pentahelicene (−7.6 × 10−3 and −2.7 × 10−3, for gabs and glum, respectively) although with better photostability and lower excited-state relaxation.72 This example reveals that special attention has to be paid to increase the chiroptical response by merging more than one helicene, required for a better performance.73
Moving from peropyrene to teropyrene-cored NGs, Chaolumen and co-workers described the preparation of compound 8 (Fig. 3).74 The enantiopure samples of 8 were isolated, exhibiting the same configuration in all the [6]helicenes. Remarkably, the frontier molecular orbitals resemble those of pyrene, although with a lower HOMO–LUMO gap. Compound 8 exhibit a ΦF = 0.58 with an emission centred at 612 nm. The |glum| value was evaluated as 0.78 × 10−3 with a BCPL = 6.8 M−1 cm−1.
Using a perylene core, Wang and co-workers reported the synthesis of a chiral NG with luminescent properties by incorporating two lateral dibenzo[6]helicene fragments (compound 9, Fig. 4).75 In this case, a high ΦF is expected due to the distribution of the frontier molecular orbitals in the central part of the perylene core and together with a certain degree of orbital delocalization on the helicenes, which justifies the chiroptical activity. This compound exhibits a remarkably high ΦF value of 0.93 among the previously reported chiral NGs and an excellent BCPL of 32 M−1 cm−1. In addition, the two enantiomers showed maximum |gabs| of 7.0 × 10−3 in the ECD spectrum and CPL with a |glum| of 0.8 × 10−3. As π-extended analogues of 9, one can find other two examples bearing double carbo[6]helicenes as chiral NGs. Compounds 10 and 11 (Fig. 4), reported by Qiu and co-workers in 2022, reach a polyaromatic framework of 29 conjugated benzenoid rings.76 In this case, an overcrowded perylene-cored oligophenylene is created by sequential regioselective Scholl reaction in the peri- and bay regions, forming the double carbo[6]helicene motif. The larger π-extension demonstrated a great effect in decreasing HOMO–LUMO gaps, and quasi-panchromatic absorption, along with intense, red-shifted emission was observed. Besides, CPL signal is centred at 625 nm with |glum| values estimated to be 1.5 × 10−3, and notably reaching 800 nm. Single crystals of 10 and 11 were grown in pentane/CS2 mixtures, packing in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Crystals of 10 consisted of intercalated layers of (P,P) and (M,M) enantiomers (Fig. 4). Compound 10 has a smaller helix pitch and interplanar angles (3.11–3.18 Å, 44.3–50.6°) compared to 11 (3.22–3.31 Å, 55.6–64.4°).
space group. Crystals of 10 consisted of intercalated layers of (P,P) and (M,M) enantiomers (Fig. 4). Compound 10 has a smaller helix pitch and interplanar angles (3.11–3.18 Å, 44.3–50.6°) compared to 11 (3.22–3.31 Å, 55.6–64.4°).
 | ||
| Fig. 4 Chiral perylene-cored nanographene 9 and its extended analogues 10 and 11. Crystal packing of compound 10 (bottom). | ||
The perylene core has been also used as initial scaffold to construct perylene-HBC hybrids. Wu and co-workers reported the synthesis of a series of helical NGs (12, 13, 14 and 15, Fig. 5), where perylene is fused with one to four HBC subunits via Diels–Alder cycloaddition followed by Scholl reaction. X-Ray crystallographic analysis confirmed their structures, revealing helicene moieties integrated into a highly contorted framework and demonstrating respectable ΦF values of 0.319, 0.150, 0.137, and 0.065, respectively, with emission maxima reaching 1010 nm. Besides, they evaluated the |gabs|max at 292 nm (3.0 × 10−3), 492 nm (8.5 × 10−3), 454 nm (3.0 × 10−3), and 604 nm (1.2 × 10−3) for 12, 13, 14, and 15, respectively. Furthermore, the CPL spectra of enantiopure 12, 13, and 14 were also measured in toluene, displaying mirror images consistent with their emission profiles, and maximum |glum| values of 4.5 × 10−3 for 12, 1.3 × 10−3 for 13, and 1.4 × 10−3 for 14. However, they did not observe CPL signal in the NIR-II region for 15.77
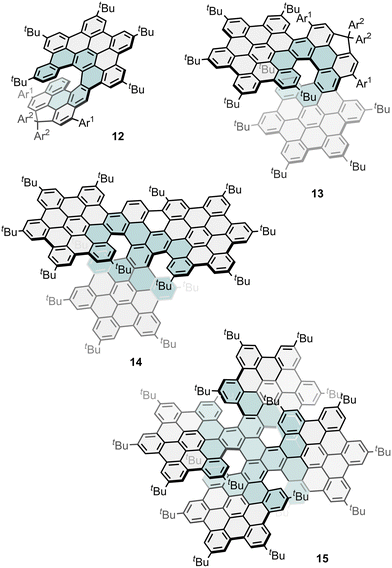 | ||
| Fig. 5 Examples of chiral perylene-cored nanographenes. Ar1 = (4-tert-butyl)phenyl; Ar2 = (3,5-di-tert-butyl)phenyl. | ||
Belonging to this family of chiral NGs exhibiting multiple HBC units we can find the link of two (or more) HBCs by chiral moieties, leading to the family of bi or multilayer NGs (with simple carbohelicenes as hinge) or fully helical ribbon-shaped or propeller-shaped NGs (with π-extended helicenes as link) some of which bearing heteroatoms or non-benzenoid rings will be mentioned in the following sections.
In the case of all-carbon bilayer NGs, they have shown to significantly change their electronic properties depending on the interplanar distance and rotation angle of the NGs. Martín and co-workers reported, at the beginning of 2018, an all-carbon fully fused bilayer NGs with a central [10]helicene,78 and it was later in 2023 when they presented the synthesis and full chiroptical characterization including CPL of three derivatives constituted by two HBCs linked by a [9], [10] or [11]helicene (17, 18, and 19, Fig. 6).79 Ma, Feng and co-workers complemented this family with the [7]helicene analogue (16, Fig. 6).80 Curiously, in this family, the larger conjugation (from [9] to [11]helicene) resulted in a hypsochromic shift of the emission (λem = 575, 543 and 528 nm, respectively) and in a decrease in both the dissymmetry factor (|glum| = 3.6 × 10−2, 1.0 × 10−2 and 0.9 × 10−2, respectively) and the intensity (ΦF = 0.22, 0.10 and 0.11, respectively) of the emission. A careful structural comparison of this family showed that the size of the carbohelicene link has great impact on the overlapping of the bilayer π-system, being maximized in 17, with the shorter carbo[9]helicene. In a related example, Aratani and co-workers reported in 2021 a bilayer compound by bridging two coronenes with a 1,8-naphthalene hinge.81 X-Ray crystallographic analysis revealed that the anti-form is a chiral twisted bilayer coronene type, with an internal bilayer distance of 2.914 Å. Therefore, it was optically resolved and characterized by ECD and CPL spectroscopy. The absolute dissymmetry factor values were moderate reaching 1.5 × 10−3 for |gabs| at 436 nm and 2.0 × 10−3 for |glum| at 435 nm.
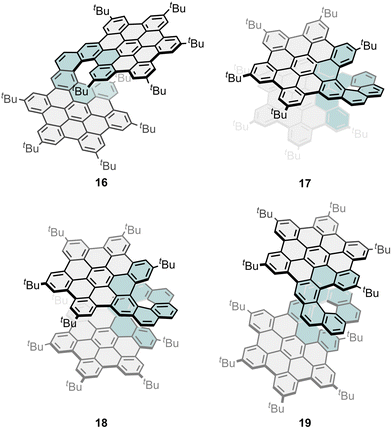 | ||
| Fig. 6 Examples of bilayer nanographenes connecting two HBC units through a [7], [9], [10] and [11]helicene. | ||
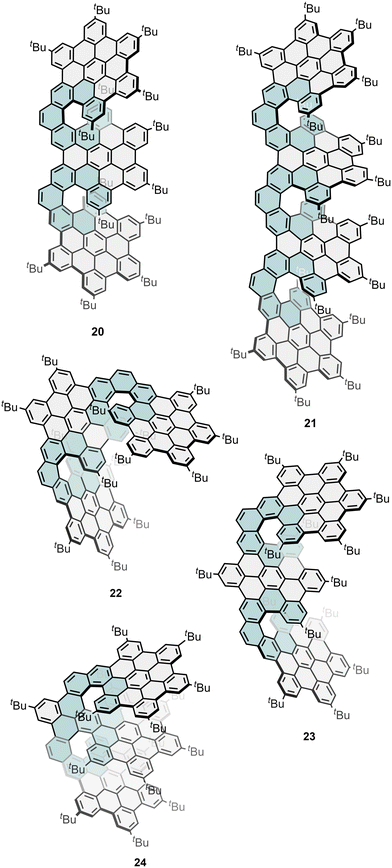
Ma, Feng and co-workers have presented a modular synthetic strategy to construct a series of multilayered helical NGs (16, 20, and 21, Fig. 6 and 7) through a consecutive Diels–Alder reaction and regioselective cyclodehydrogenation from phenanthrene-based precursors substituted with ethynyl groups.80 In this report, they highlighted the change in the photophysical properties of these helical NGs with respect to the degree of π-extension, which varies with the number of layers, leading to obvious redshifted absorption, a fast-rising ε, and markedly boosted ΦF. Moreover, the embedded [7]helicene subunits in these NGs result in configurational stability, enabling both chiral resolution and exploration of their layer-dependent chiroptical properties. For that, they investigated the CPL spectra of the isolated enantiomers of 16, 20 and 21. Every pair of NG enantiomers showed mirror-like CPL spectra, revealing a gradually declined |glum| of 7.9 × 10−3 for 16 at 563 nm, 2.7 × 10−3 for 20 at 575 nm, and 1.5 × 10−3 for 21 at 595 nm, respectively, despite the increased number of helicenes and larger conjugation. These trends closely aligned with the observed tendency of |gabs|max which also decreased in the larger analogues. These results again show that increasing the structural complexity and number of helicenes does not always result in improved chiroptical response. Despite the modest |glum| values found for these layered NGs, the intense ε and high ΦF ensure high BCPL, whose values were estimated as 168, 112, and 106 M−1 cm−1 for multilayer NGs 16, 20 and 21, respectively. In a subsequent study, Ma, Niu and co-workers also reported the influence of the geometric arrangement of three units of HBC linked by double [7]helicenes. To this end, they compared the chiroptical response of above-mentioned compound 20 (Fig. 6), where the two [7]helicene units are fused at the ortho position of the central HBC moiety with two other analogues that are linked in the para- (22, Fig. 7) and meta-position (23, Fig. 7).82 To explain the changes in dissymmetry factors and establish the relationship between the geometry of double [7]helicenes and their chiroptical properties, TD-DFT calculations were performed to check μ and m. In the geometric engineering of two [7]helicene units from ortho-, meta- to para-positions, in this order, the θ and |μ| gradually decrease, while |m| remains at a constant level. This leads to a simultaneous increase in cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ and |m|/|μ|, consequently resulting in a significant boost of |gabs| of 3.9 × 10−3 for 20, 10 × 10−3 for 22 and 18 × 10−3 for 23. Continuing, they did TD-DFT calculations on their excited state to understand the origin of the increased glum values. A comparison with 20 reveals a simultaneous increase in cos
θ and |m|/|μ|, consequently resulting in a significant boost of |gabs| of 3.9 × 10−3 for 20, 10 × 10−3 for 22 and 18 × 10−3 for 23. Continuing, they did TD-DFT calculations on their excited state to understand the origin of the increased glum values. A comparison with 20 reveals a simultaneous increase in cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ and |m|/|μ| for 22 and 23, resulting in increased glum values of 2.7 × 10−3 for 20, 8.7 × 10−3 for 23, and 13.5 × 10−3 for 22 together with BCPL values of 112, 143 and 176 M−1 cm−1, respectively. Tan and co-workers reported another trilayer NG (24, Fig. 7) with a double helicene, together with its O-doped analogue (see Section 2.4.4).83 The two enantiomers of 24 showed a series of mirror-image Cotton effects extending to 610 nm, indicating a chirality transfer throughout the molecule and showing a maximum |gabs| of 4.2 × 10−3 at 472 nm and a |glum| value of approximately 1 × 10−3. Related to 24, Shen and co-workers reported a flag-hinge-like NG with a central peropyrene unit and two lateral HBCs.84 This NG exhibited a |glum| = 5.0 × 10−3 with an excellent BCPL of 305 M−1 cm−1 thanks to its good ΦF of 0.52.
θ and |m|/|μ| for 22 and 23, resulting in increased glum values of 2.7 × 10−3 for 20, 8.7 × 10−3 for 23, and 13.5 × 10−3 for 22 together with BCPL values of 112, 143 and 176 M−1 cm−1, respectively. Tan and co-workers reported another trilayer NG (24, Fig. 7) with a double helicene, together with its O-doped analogue (see Section 2.4.4).83 The two enantiomers of 24 showed a series of mirror-image Cotton effects extending to 610 nm, indicating a chirality transfer throughout the molecule and showing a maximum |gabs| of 4.2 × 10−3 at 472 nm and a |glum| value of approximately 1 × 10−3. Related to 24, Shen and co-workers reported a flag-hinge-like NG with a central peropyrene unit and two lateral HBCs.84 This NG exhibited a |glum| = 5.0 × 10−3 with an excellent BCPL of 305 M−1 cm−1 thanks to its good ΦF of 0.52.
As mentioned at the beginning of the section, together with the synthesis of seco-HBC 1, the group of Tanaka reported chiral NGs bearing longer π-extended carbohelicenes.65 They reported the enantioselective synthesis (up to 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 enantiomeric ratio) of partially π-extended carbo[11] (25, Fig. 8) and carbo[13]helicene (26, Fig. 8) finding them to be good CPL emitters with |glum| values of 4.0 × 10−2 and 3.4 × 10−2 and a very high BCPL of 490 and 287 M−1 cm−1, respectively. These π-extended helicenes are synthesized via an enantioselective triple [2+2+2] cycloaddition to construct low-distortion expanded carbo[13] and carbo[15]helicene skeletons, followed by subsequent π-extension by means of the Scholl reaction.
13 enantiomeric ratio) of partially π-extended carbo[11] (25, Fig. 8) and carbo[13]helicene (26, Fig. 8) finding them to be good CPL emitters with |glum| values of 4.0 × 10−2 and 3.4 × 10−2 and a very high BCPL of 490 and 287 M−1 cm−1, respectively. These π-extended helicenes are synthesized via an enantioselective triple [2+2+2] cycloaddition to construct low-distortion expanded carbo[13] and carbo[15]helicene skeletons, followed by subsequent π-extension by means of the Scholl reaction.
These last examples show structures where the HBC units are linked through [n]helicenes. However, HBC moieties can be arranged to create fully extended [n]helicenes. Thus, the simplest example could be the arrangement of two HBCs into an extended carbo[5]helicene, however, this structure remains unexplored and only a heptagon-containing analogue has been reported by our group (Section 2.3.2, Fig. 12, compound 38). Conversely, its double carbo[5]helicene analogue has been reported by Wang and co-workers,85 however, its chiroptical properties remain unexplored. Other impressive examples with even larger structures, bearing multiple helical motifs have been reported by the same group.86,87 Nevertheless, CPL was not determined for any of these compounds. Regarding this family (often called superhelicenes), a ribbon-shaped NG constituted by four HBC units arranged around a central fully π-extended carbo[9]helicene was presented by Gong and co-workers in 2024 (27, Fig. 9).88 The large conjugated framework resulted in an emission in the NIR (600–900 nm) and the helical shaped resulted in a considerable |glum| value of 4.50 × 10−2, and a BCPL as large as 304 M−1 cm−1, both higher than the values reported for bilayer 17 bearing a central carbo[9]helicene moiety as well. Those results aim again to the lateral π-extension and elongation of chiral NG to enhance their emissive and chiroptical properties. The same group reported two analogues of 27 containing pore defects at one or the two edge HBC units.89 Remarkably, these pore-containing analogues exhibited lower |gabs| and |glum| than 27 in solution. Additionally, in polymethyl methacrylate (PMMA) and powder films, both analogues show lower |gabs| and |glum|. The presence of these pore defects led to a blue shift in the ECD and CPL spectra. Later, the same group presented a larger example constituted by five HBC units arranged in a W-shape bearing two fully π-extended [7]helicenes (28, Fig. 9) displaying fluorescence emission (λem = 636 nm) with a ΦF = 0.10.90 In this case, despite the larger π-system, it exhibits lower |glum| of 4 × 10−3, and a BCPL of 42 M−1 cm−1 in one order of magnitude compared with the previous smaller member of the family, pointing out the importance of the mutual arrangement when two carbohelicenes are present. Single crystals of 28 revealed that the racemic mixture crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, with each enantiomer featuring two identical carbo[7]helicene units. The enantiomers pair into dimers, stabilized by π–π and CH–π interactions between neighboring HBC units. These dimers self-assemble into a one-dimensional architecture, extending to a three-dimensional network via CH–π interactions, showcasing a remarkable hierarchical organization (Fig. 9).
space group, with each enantiomer featuring two identical carbo[7]helicene units. The enantiomers pair into dimers, stabilized by π–π and CH–π interactions between neighboring HBC units. These dimers self-assemble into a one-dimensional architecture, extending to a three-dimensional network via CH–π interactions, showcasing a remarkable hierarchical organization (Fig. 9).
 | ||
| Fig. 9 Examples of nanographenes as fused HBC units arranged around [n]helicenes. Crystal packing of 28 (bottom). | ||
Another example of a compound bearing double π-extended helicenes was reported by Hu, Narita and co-workers, which presented a π-extended double [9]helicene with remarkable NIR emission. However, they did not report its CPL response, whereas its side product (29, Fig. 9) shows a |gabs| value of 0.008 at 582 nm. In addition, the CPL of 29 was also measured, showing a |glum| of 4 × 10−3.91
Recently, Ravat and co-workers reported helically twisted chiral nanoribbons featuring a central pyrene core, or fused pyrenes, anchored at the terminal K regions through one or two [7]helicenes (compounds 30–33Fig. 10) via stereospecific APEX reactions.92,93 The [7]helicene influences on the conformational stabilization according to the DFT and single crystal structure analyses. The experimentally obtained |glum| of 30 and 31 is 1.27 × 10−3 and 0.63 × 10−3, respectively, evidencing the negative effect of fusing additional pyrene units. Conversely, the |glum| of 32 and 33 were evaluated as 0.54 × 10−3 and 1.54 × 10−3, respectively, which points to the additional [7]helicene as a key factor to enhance the CPL response. This fact was further supported by TD-DFT, which predicted a larger |m| and higher cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ values for the S1 → S0 transition in 33 compared to those in 32 and 30.
θ values for the S1 → S0 transition in 33 compared to those in 32 and 30.
 | ||
| Fig. 10 Chiral nanographenes combining helicene(s) and pyrene units (30–33), triptycene-fused double HBC-based nanographene (34) and triindane-fused double seco-HBC nanographene (35) as CPL emitters. | ||
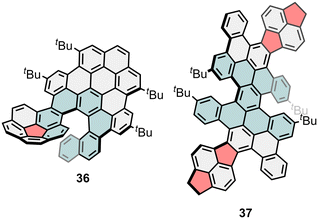
In addition to the inclusion of helicenes as intrinsic chirality providers in NGs, less common chiral scaffolds bearing asymmetric carbon atoms are also of great interest. Such is the case of triptycene, which has been used as efficient solubilizing group of large PAHs,94 three-dimensional scaffold,95,96 and can also bridge two HBC units to create a chiral NG as shown by Ikay group (34, Fig. 10).97 In this case, the chirality is provided by the two asymmetric sp3 carbon atoms present in the triptycene moiety. Its forbidden conformational interconversion allowed its resolution by HPLC, as well as the crystallization of one of the enantiomers, after which the evaluation of the CPL resulted in |glum| values of 1.0 × 10−3. Another interesting example is the triindane moiety, which has been used by Martín and co-workers as a chiral linker between two seco-HBC (35, Fig. 10).98 The synthesis of 35 is performed through an enantiospecific Scholl reaction, with the triindane acting as chiral auxiliary unit. Compound 35 possess three asymmetric carbon atoms and two carbo[5]helicenes, being possible to conduct its racemic resolution. CPL response of 35 was evaluated with a |glum| = 1.9 × 10−3 and a BCPL = 16.7 M−1 cm−1. Despite the generalized lack of asymmetric sp3 carbon atoms in extended polycyclic conjugated hydrocarbons (PCHs) and NGs, it is possible to find new CPL-active carbon-based structures containing tetragonal stereogenic centres, which originates novel architectures from interesting scaffolds. Recently, Du and co-workers reported a cycloparaphenylene-HBC (CPP-HBC) hybrid as CPL emitter.99 The synthesis was based on the preparation of a cycloparaphenylene unit functionalized with two triflate moieties over the same benzene ring. Thus, a further Suzuki coupling with two equivalents of a boronic ester HBC derivative yielded the target NG. The corresponding enantiomers were separated by chiral stationary phase HPLC, where the size of the HBCs prevented its racemization. This CPP-HBC hybrid showed ΦF = 0.35 with a CPL centred at 469 nm with |glum| = 1.10 × 10−3.
2.3. NGs bearing non-benzenoid rings
As mentioned in Section 2.2, inducing chirality in NGs is mainly tackled by attaching inherently chiral motifs within the π-conjugated scaffold. Helicenes are one of the most commonly implemented chiral elements since they are complemented by a delocalized electronic structure. The combination of helicenes and non-benzenoid rings is a recurrent strategy to alter both the distortion and chiroptical properties of NGs. Therefore, this section covers those NGs bearing non-benzenoid rings in their structure and displaying CPL. Introduction of non-benzenoid rings with different sizes can generate many types of curvatures, for example, including pentagonal rings in the π-conjugated core generates a positive curvature while insertion of heptagonal rings generates a negative curvature, both having an influence in the (chir)optical properties of the resulting molecules. Thus, reported examples of NGs containing five-, seven-, eight- and nine-membered rings, where the helicene insertion strategy is used, are reviewed in this section. This section is organized according to the sizes of the non-benzenoid ring and, specifically the seven-membered ring section, according to the location of this non-benzenoid ring (either in the chiral unit, in the helicene, or in the NG core). | ||
| Fig. 12 Saddle-helix hybrid nanographenes with CPL response, prepared by Campaña and co-workers. Crystal packing of 38 (bottom). | ||
 | ||
| Fig. 14 Saddle-helix hybrid nanographenes exhibiting an octagon (45 and 46) and nonagon (47) ring, prepared by Campaña and co-workers. | ||
In view of the above-presented results, introducing non-benzenoid rings in the NG scaffolds evidences a great influence in the structure of the resulting NG. It enhances racemization barriers and improves configurational stability, avoiding the use of bulky substituents in the fjord region. Therefore, it leads to a higher distortion when embedded into the structure of a helicene compared to its fully hexagonal analogue.
2.4. NGs bearing heteroatoms
 | ||
| Fig. 15 CPL-active nanographenes containing an azocine moiety (48 and 49) and related amines and lactams (50–53). | ||
In 2023, Qiu and co-workers reported a curved NG bearing a [1,4]diazocine and octagon-pentagon fused rings (54, Fig. 16) reaching a glum value of 0.26 × 10−3 for (P)-54 at λem = 450 nm.114 The glum values were reported in solution, in contrast to their aggregates, which resulted to be CPL-silent. Despite that, in aggregate state these species can form a charge transfer complex with non-chiral acceptors, such as tetrafluoroterephthalonitrile (TFTPM) and 1,2,4,5-tetracyanobenzene (TCNB), and activate the CPL response. The former showed yellow emission reaching glum values of +0.41 × 10−3 for (M)-54:TFTPM at 587 nm. The latter showed red emission reaching glum values of −1.4 × 10−3 for (P)-54:TCNB at 655 nm. The same group reported another related analogue based on a carbazole-built π-extended diaza[7]helicene with double negatively curved heptagons generated by Pd-catalysed intramolecular C–H arylations (55, Fig. 16).115 Compound 55 exhibited intense green fluorescence with λem = 517 nm and their enantiopure samples showed a bisignated CPL spectra with glum = 2.0 × 10−3 at 545 nm.
Recently, Casado, Liu and co-workers presented the development of a non-alternant NG containing a N-centred cyclopenta[ef]heptalene and an aza[7]helicene units.116 These strained moieties represent a heteroatom-doped member of the saddle-helix hybrid NG family with CPL response. Compounds 56 and 57 (Fig. 16) were synthesised to evaluate the relevance of the π-extension of the cyclopenta[ef]heptalene in the chiroptical properties of the helical NGs. Single crystal structure of 57 show multiple CH–π contacts allowing the formation of 1D columns with close packing (Fig. 17). They reported |glum| values of 7.0 × 10−3 for 57 that are significantly larger than the |glum| values for the non-extended one (56, |glum| = 2.4 × 10−3). These compounds also showed intense fluorescence and phosphorescence emission, specifically, 57 showed a narrow red emission centred at 588 nm with a ΦF = 0.32. Two new carbazole-centred extended helically NGs, (58 and 59, Fig. 16), were reported by the groups of Babu and Gong, respectively.117,118 The structural difference between compounds 58 and 59 lies in the substituent of the carbazole moiety, bearing a hexyl or HBC unit, respectively. These compounds exhibit an orange/red fluorescence in solution (λem = 542 and 595 nm for 58 and 59, respectively) with a high ΦF of 0.75 and 0.40. The substitution of the carbazole unit by incorporation of an hexyl or HBC units affords additional chemical and configurational stability, compared to the unsubstituted one, reported by Jux and co-workers.119 In the study of 59, the authors observed that the BCPL depends on the solvent, achieving a lower value in CH2Cl2 (glum = 0.43 × 10−3 for (P)-enantiomer and BCPL = 9.89 M−1 cm−1). While solutions in THF (glum = 1.06 × 10−3 and BCPL = 28.57 M−1 cm−1) and MeCN (glum = 1.30 × 10−3 and BCPL = 19.43 M−1 cm−1) exhibited higher glum and BCPL values. On the other hand, the |glum| for compound 58 in 2-methyl-THF was evaluated as 1.1 × 10−3, reaching a BCPL of 45.77 M−1 cm−1 for (M)-58.
Hu and co-workers reported a double hetero[7]helicene fused with four triazole rings (60, Fig. 16) in 2022.120 The N-doping proved to have a great impact on the luminescence intensity, reaching a ΦF = 0.96 as well as in the glum, which was significantly enhanced in almost one order of magnitude with respect to its full-carbon analogue (Section 2.2, Fig. 3, compound 6) reaching a value of 9.1 × 10−4 for (P,P)-60. The terminal triazole units provide the compound with an ambipolar redox behaviour, where stable radical cations were formed by both chemical and electrochemical methods. This double terminal triazole helicenes constitute an interesting platform to modulate emissive, chiroptical and electrochemical properties. The same group also presented a doble diaza[7]helicene (61, Fig. 16) as an intermediate in the synthesis of a negatively curved aza-NG bearing two octagonal rings, with |glum| values of 2.2 × 10−3.121 Curiously, the final curved saddle-shape NG showed an electron-rich nature that leads to an interesting association with fullerenes (Ka = 9.5 × 103 M−1 with C60 and Ka = 3.7 × 104 M−1 with C70).
Recently, Babu and co-workers reported the synthesis of a three π-extended helical NG family.122 These helical NGs are constituted by a phenazine-embedded bilayer NG (62, Fig. 18) with |glum| = 0.73 × 10−3, and two [1,4]-diazocine-embedded helicenes (63 and 64, Fig. 18) with |glum| = 0.89 × 10−3 and |glum| = 5.5 × 10−3, respectively, for the P-enantiomers. Specifically, the insertion of a [1,4]-diazocine-helical moiety in 64 shows an enhancement of the chiroptical properties. The incorporation of this helical moiety was useful in engineering θ. In addition, this work shows that the aromaticity character of the diazocine unit takes relevance in the determination of both types of transition dipole moments. Due to the antiaromatic character of the diazocine unit, 63 and 64 show an enhance of their m values which contribute to get the |glum| values of the reported π-extended helical NG 64. These compounds can be also compared to the bilayer NG 16 (Section 2.2, Fig. 6), to understand the effect of embedding a phenazine or a [1,4]-diazocine into its structure. Remarkably, the pristine bilayer NG 16 exhibited a |glum| of 7.9 × 10−3, which represents a higher value compared to 62, 63 and 64.
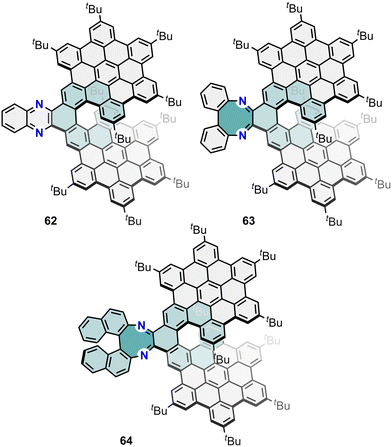 | ||
| Fig. 18 Phenazine- (62) and [1,4]diazocine-embedded (63 and 64) bilayer nanographenes reported by Babu and co-workers. | ||
Finally, within the context of N-doped NGs, it should be noted that we have considered chiral perylene diimides (PDIs) or naphthalene diimides (NDIs) reported as CPL emitters as out of the scope of this review centred in NGs, due to their special structures and particularities.123–127
Recently, a family of NGs based on 2,6-bis(3,6-di-tert-butyl-9H-carbazol-9-yl)boron (DtBuCzB) was reported by Zhang and co-workers.132 The authors reported the synthesis of five chiral NGs (70–74, Fig. 20), which can be divided in two groups: the ones having a double helical motif (70, 71 and 72) and the ones embedding seven-membered rings in its structure (73 and 74). All the family shows thermally activated delayed fluorescence (TADF) and the authors highlighted that the presence of heptagonal rings decreased ΦF. Contrariwise, these heptagonal rings increased the chiroptical response, producing higher |glum| values (2.9 × 10−3 for 73, and 5.0 × 10−3 for 74) with respect to those ones with only double helical motifs (2.5 × 10−3 for 70, 2.7 × 10−3 for 71, and 2.7 × 10−3 for 72). A similar structure, featuring a fused double DtBuCzB has been studied by Wang and co-workers in 2021 (75–77, Fig. 20).133 This family of double hetero[7]helicenes also exhibited TADF, with λem centred at 660, 684 and 696 nm, respectively, and extraordinary ΦF of 1.00, 0.99 and 0.90, for 75, 76 and 77, respectively. Mirror-image CPL spectra with |glum| up to 2 × 10−3 for 75. The BCPL values of 75–77 were determined as 28.5, 37.1, and 40.0 M−1 cm−1, respectively. In 2023, Ravat and co-workers also reported the study of two helicene-fused DtBuCzB analogues.134 However, their π-extended analogues will be discussed in this review (78–80, Fig. 21).135 The authors reported that the rigidification of the NG core with azaborine units led to an ultra-narrow fluorescence emission, with full width at half maximum (FWHM) of 17.5, 16 and 17 nm for 78–80, respectively, in toluene. The CPL spectra also show extraordinary FWHM of 18, 19 and 18 nm for 78–80, respectively, in toluene. Besides, the increase of azaborine units resulted in a blue shift in the absorption and emission bands, with reported |glum| values of 2.3 × 10−3 for 80, 0.75 × 10−3 for 79, and 1.9 × 10−3 for 78.
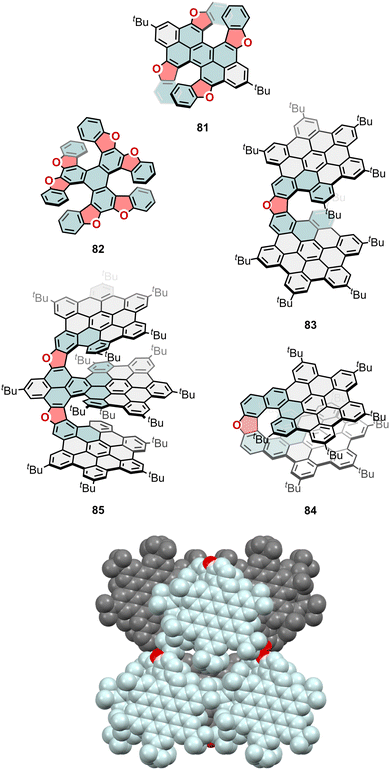 | ||
| Fig. 22 Furan-containing chiral nanographenes as part of peropyrene (81), triphenylene (82), and bilayer (83 and 84) or trilayer (85) nanographenes. Crystal packing of (M)-84 (bottom). | ||
Further, more extended architectures containing furan units were envisioned and reported, as it is the case of bi and trilayer structures, where furan units take relevance due to the modulation of their interlayer overlap and π-conjugation. Such is the case of a bilayer NG linked by a furan unit reported by Jux and co-workers in 2018.138 Although in this publication the racemic resolution and chiroptical properties were not included, this was later realized in collaboration with Fuchter's group,139 being the |glum| value of 83 equal to 0.3 × 10−3 at 540 nm. This dissymmetric emission was amplified by 500 times when embedded into an achiral polydioctylfluorene (PFO) matrix (|glum| of PFO:83 blends ≈ 0.15), due to a Forster resonance energy transfer (FRET) phenomenon between the electric and magnetic dipole moments of the PFO (donor) and 83 (acceptor).
Also, by the end of 2024, Martín and co-workers reported a new example of enantioselective strategy to obtain enantiomerically pure helical bilayer NGs.140 The prepared BINOL-based polyarene precursor was used in a monoesterification with enantiopure 1R-10-camphorsulfonyl chloride to afford two diastereoisomers easily isolable by flash column chromatography. Once the two enantiomers were obtained, the authors performed a Scholl oxidation to obtain the corresponding enantiomers of 84 (Fig. 22), as oxa[9]helicene-embedded bilayer NGs. Both enantiomers crystallize in the C2221 space group (Fig. 22), with the molecules located in sheets parallel to the (110). Huge spaces could be found between the layers, containing high disordered solvent molecules. The chiroptical properties of 84 were studied, reaching |glum| values of 2.6 × 10−3 for (M)-84. As previously observed in their full-carbon analogues (Section 2.2, Fig. 6, compounds 16 and 17), compounds 83 and 84 evidence the effect of the overlapping of the bilayer π-system and the size of the helicene. Thus, 84 exhibits an increased CPL response compared to 83 (2.6 × 10−3vs. 0.3 × 10−3). In addition, compared with their full-carbon analogues, compounds 83 and 84 exhibit lower glum values than compounds 16 and 17. This fact suggests that the inclusion of furane rings into bilayer NG structures is detrimental to the CPL response. In 2023, Tan and collaborators reported the CPL studies on a trilayer NG bearing two oxa[8]helicene units (85, Fig. 22) and compared them with its full-carbon analogue (Section 2.2, Fig. 7, compound 24).83 The CPL spectra of 85 showed a |glum| value of 0.3 × 10−3, which is lower than the 1 × 10−3 reported for 24. Remarkably, the interlayer overlap is higher in 24, which affects the photoluminescence lifetime, among other properties.
Besides furan units, O-doped heptagonal rings have been reported in literature. The synthesis of a BINOL-like atropisomeric chiral NG (86, Fig. 23) was described by An et al. in 2022.141 The CPL of the pair of atropisomers displayed opposite signals with a |glum| of 0.24 × 10−3 at 480 nm. Regarding NGs that combine O-doped and medium-sized rings, Tan and co-workers, and Miao and co-workers recently reported examples including (oxa)pentagon–heptagon (87–91, Fig. 23) and (oxa)pentagon–nonagon pairs, respectively.142,143 The family reported by Tan and co-workers was composed by five members: (i) an extended HBC with two furan moieties embedded into two helicenes (87); (ii) its one pentagon–heptagon pair (88); (iii) an extended HBC with four furan moieties embedded into three helicenes (89); (iv) its one pentagon–heptagon derivative (90); and (v) its two pentagon–heptagon pairs and an oxa[9]helicene derivative (91). The reported |glum| values were evaluated as 0.6 × 10−3, 1.5 × 10−3, 0.5 × 10−3 1.6 × 10−3 and 2.1 × 10−3, respectively. Therefore, the authors conclude that increasing the pentagon–heptagon pairs in the structure will enhance the CPL response. In Miao's examples, the same trend is observed.
3. Future perspectives
3.1. Enantioselective synthesis
Achieving precise control over the chirality of NGs during synthesis is challenging and usually relies on the racemic synthesis followed by a tedious racemic resolution. Chirality induction often relies on enantioselective synthesis or post-functionalization, which can be complex, expensive, and low yielding. On the one hand, producing large quantities of chiral NGs with consistent optical properties is difficult. On the other hand, scaling up from laboratory synthesis to industrial production while maintaining purity and enantiomeric excess remains a significant hurdle.New synthetic strategies involving enantioselective catalysis or chiral templates could offer better control over chirality while increasing yields and reducing costs.65,98,140 Post-synthetic functionalization strategies could allow for fine-tuning of optical and electronic properties, enhancing the performance of chiral NG-based emitters.
3.2. Boosting chiroptical properties
While NG structures can be tailored for specific chiroptical properties, the vast diversity of possible structural modifications creates challenges in systematically understanding the relationships between structure, chirality, and optical behaviour. On the other hand, chiral NGs often exhibit relatively low ΦF. This fact limits their potential in applications such as display technologies or light-emitting devices,147 where high brightness and efficiency are crucial. Non-radiative decay channels in these molecules reduce the efficiency of light emission. Thus, finding ways to suppress such losses while maintaining chirality is still an open challenge.148More critical is the fact that chiral NGs usually exhibit low g in their CPL emission, limiting the detection of the circular polarization excess. As commented before (eqn (1.5)), glum value is controlled mainly by a couple of dipole transition vectors, μ and m, which depends on non-obvious quantum mechanics.149,150
In the literature, some examples have been described with some success. In 2021, Narita and co-workers reported π-extended [7] and [9]helicenes (2 and 3, Fig. 3), revealing that the slight variation in helical length from [7] to [9] can cause an approximately 10-fold increase in the dissymmetry factors. To explain this, they studied the molecules theoretically and calculated transition dipole moments using TD-DFT. The higher |m|, lower |μ|, and larger cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ of 3 than of 2 all lead to an increase in the calculated dissymmetry factor by a factor of 10 with respect to that of 2, consistent with the trend observed experimentally.43 Later, in 2022, following the strategy reported by Mori and co-workers for carbohelicenes,73 Hu and co-workers also studied X-shaped double carbo[7]helicene when they exchanged terminal benzene rings with triazole, resulting in 12.8 fold increase in glum and a significant increase in ΦF in comparison to double carbo[7]helicene.61 Since these compounds share comparable |μ| and cos
θ of 3 than of 2 all lead to an increase in the calculated dissymmetry factor by a factor of 10 with respect to that of 2, consistent with the trend observed experimentally.43 Later, in 2022, following the strategy reported by Mori and co-workers for carbohelicenes,73 Hu and co-workers also studied X-shaped double carbo[7]helicene when they exchanged terminal benzene rings with triazole, resulting in 12.8 fold increase in glum and a significant increase in ΦF in comparison to double carbo[7]helicene.61 Since these compounds share comparable |μ| and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ values, the boost in glum can be mainly attributed to the increased |m| value. Later, in 2023, Martín and co-workers reported a series of helical bilayer NGs and studied the impact of the central helicene size on μ and m. When going from [11] to [9]helicene, a simultaneous increase in cos
θ values, the boost in glum can be mainly attributed to the increased |m| value. Later, in 2023, Martín and co-workers reported a series of helical bilayer NGs and studied the impact of the central helicene size on μ and m. When going from [11] to [9]helicene, a simultaneous increase in cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ and |m|/|μ| consequently resulting in very high dissymmetry factors for [9]helicene bilayer NG in comparison to [11]helicene bilayer NG. Also related is the study presented by Feng and co-workers establishing the relationship between the geometry of HBC units linked by double [7]helicenes and their chiroptical properties.82 Again, geometric engineering showed the role played by the relative position on the θ, μ and m values, and therefore on the final glum values.
θ and |m|/|μ| consequently resulting in very high dissymmetry factors for [9]helicene bilayer NG in comparison to [11]helicene bilayer NG. Also related is the study presented by Feng and co-workers establishing the relationship between the geometry of HBC units linked by double [7]helicenes and their chiroptical properties.82 Again, geometric engineering showed the role played by the relative position on the θ, μ and m values, and therefore on the final glum values.
Nevertheless, human limitations to infer the frontier molecular orbitals with precision have hampered the deep understanding on how the molecular structure influences CD and CPL performance, a future evolving field. While exploiting forbidden transitions allows for high dissymmetry factors due to an increased contribution of |m| and very small |μ| this strategy often comes at the cost of lowering the absorption/emission strength, |μ|2, and consequently the photoluminescence quantum yield. An alternative approach is to engineer the molecule structure to balance the contributions of |m| and |μ| and to optimize the angle between them. In a very naïve view, optimization of CPL performance at small molecule level then depends on two critical factors: (i) the molecule must be emissive, that is, dipole strength |μ|2 must be reasonably high; and (ii) the magnetic dipole transition moment |m| must be also high to maintain a suitable ratio. Roughly, and considering a parallel alignment, a |μ| value of 400 × 10−20 esu cm, requires an |m| value of 100 × 10−20 erg G−1 to achieve an impressive glum value of 1. For a figure of merit of 0.1 a |m| value of 25 × 10−20 erg G−1 is still required. Owing to usual values for |m| in single organic molecules are around 1 × 10−20 erg G−1, guidelines to design molecules with such large |m| values are then indispensable. Within this context our group recently managed to present a rational relationship between the magnitude of |m| and the chemical structure with the aid of machine learning approaches.151,152 In this way, it was shown that the best |m| values occur for those transitions whose orbitals are fully delocalized around a helical movement. Remarkably, the magnitude of m is directly related with the surrounding area of this electron movement during the transition which, from the structure point of view, is directly related to either the number of turns or the size of the turn in helical conjugated structures.151,153 It is also worth noting that cylindrical geometries of π-conjugated frameworks have proved to be effective strategies for increasing |m| values that should be also considered when designing CPL active NGs.154,155 Despite of these powerful structure–property correlations, in terms of chiral emission, it is the lowest-energy electronic transition S1 → S0 the one that must be magnetically allowed without compromising the |μ|. Although the introduction of substituents in selected positions with large orbital coefficients have proven to be a suitable strategy for changing orbital energy,156 thus optimizing the symmetry and magnitude of their lowest-energy electronic transitions, further development of the corresponding general rules are still lacking. However, considering the large number of both possible substituents and substitution positions in a particular conjugated core, machine learning techniques become again the approach of choice to tackle those problems.152 In fact, an optimization of chiroptical properties of polysubstituted [6]helicenes has demonstrated that trained neural networks are suitable tools in this field, and probably they are going to be of special focus in the near future.
3.3. Combining/hybridizing chiral NGs with other materials
Combining/hybridizing chiral NGs with other materials (e.g., metal–organic frameworks, quantum dots, polymers) could also boost dissymmetry factors and enhance CPL emission. This is the case of the presence of chiral near fields in the surroundings of chiral plasmonic nanoparticles. The coupling of such dense chiral fields promotes an increased chiral light–matter interaction boosting the chiroptical responses.157 In a similar way, when chiral NGs are embedded in an achiral conjugated polymer matrix a remarkable phenomenon appears. In 2020, Fuchter and co-workers demonstrated a 500-fold amplification of the |glum| of 82,158 previously reported by Jux and co-workers.159 They rationalized that the amplification arises through electrodynamic coupling between the polymer donor's electric and magnetic transition dipoles and superhelicene acceptor, resulting in CPL resonance energy transfer. Considering the delocalized exciton in the polymer as a kind of chiral near field, an alternative mechanism may emerge which could be more broadly adopted to enhance the dissymmetry factors of NGs.160,161 Other interesting approach is the spin-OLEDs162–164 in which the chiral matter polarizes the electron/hole transport within the material producing a spin dependent recombination and CPL emission even in achiral emissive layers. In that case, the chiral NGs would be the media in which the so-called chiral induced spin selectivity (CISS) effect takes place.3.4. Responsive systems
On the other hand, creating NGs that change their chiroptical properties in response to external stimuli (such as light, temperature, or pH) could lead to new classes of smart, dynamic materials for adaptive optics and sensing.37,113,165–167 This is especially appealing as probes for the so-called CPL microscopies,168,169 taking circular polarization as an additional information channel.In summary, the meticulous design of the molecular structure can give optimum |μ|, |m| and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ values, which result in high g. However, the increased contribution of |m| may result in low absorption/emission strengths if |μ| is not considered, which is why smart synthetic designs are required. Hence, tunable molecular designs supported by artificial intelligence and efficient computation, can be adopted to boost dissymmetry factors in chiral NGs. Other than that, the chiroptical activity of NGs has been enhanced through aggregation and assembly in polymer matrix, although the limits of this approach have not been defined to date.
θ values, which result in high g. However, the increased contribution of |m| may result in low absorption/emission strengths if |μ| is not considered, which is why smart synthetic designs are required. Hence, tunable molecular designs supported by artificial intelligence and efficient computation, can be adopted to boost dissymmetry factors in chiral NGs. Other than that, the chiroptical activity of NGs has been enhanced through aggregation and assembly in polymer matrix, although the limits of this approach have not been defined to date.
Conclusions
In this review, we have summarised recent advancements in the bottom-up synthesis of chiral NGs, including various benzenoid, non-benzenoid rings, and heteroatom-containing systems. The synthesis and study of chiral NGs have advanced at an exponential rate. In addition to the natural interest in chirality as the foundation of the phenomena of life, there is a growing interest in creating novel exotic carbon structures with various structural defects, which allows modulation of their molecular orbital structure and, thus, their photophysics. Next, Tables 1–3 summarizes the main photophysical properties reported for CPL-active NGs in this review. Despite significant efforts, finding a direct relationship between the structure of chiral NGs and their CPL emission does not seem obvious. It became clear that the random inclusion of more chiral motifs might not necessarily result in better CPL performance. Π-extension and elongation of helical motifs seem to have a more significant effect. It is still difficult to modulate the luminescence dissymmetry factor and quantum yield efficiently by adjusting the electric and magnetic transition dipole moment vectors of the S1 → S0 transition. The balance between these two parameters is especially crucial, and to keep that in mind, designing chiral NGs remains unsolved and would require a deep rational design based on orbital engineering. The key thing here is to create delocalized HOMO/LUMO levels including the helical part of the molecule, thus ensuring that the electronic circulation takes place in the S1 to S0 states. This can be done placing suitable donor/acceptor groups in critical positions or incorporation electron rich motives as alkynes as part of the helical structure.170–172 On the other hand, intense chiroptical responses can be attributed to the nature of the helical system. Higher the enclosed area of a transition, higher the strength of the corresponding signal. Combining the above-mentioned advice the designing chiral NGs would become more rational, and the actual limits could be surpassed. When looking for enhanced chiroptical response, the focus must be in a deep rational design based on orbital engineering instead of usual criteria of preparing magnificent chemical structures. In fact, very simple rationally designed structures could lead to impressive responses. Nevertheless, in any case, the generation of unprecedented complicated conjugated structures would always remain as a fascinating challenge for organic chemists. In this sense, and despite significant progress in synthesizing NGs, developing enantioselective synthetic techniques for synthesizing chiral NGs constitutes a specially challenging topic.Moving forward, interdisciplinary approaches that combine molecular design, advanced chiral characterization, artificial intelligence (AI) methods, and computational modelling are likely to propel this field toward practical applications.151,173 With continued research, chiral NGs could be the foundation for next-generation CPL-active materials, opening exciting possibilities in optoelectronics, bioimaging, and sensing.
Author contributions
V. K., J. L. P., S. M.-L, J. M. C., C. M. C. and A. G. C.: writing – original draft, writing – review & editing.Data availability
Data for this article, including Chemdraw files of the reported structures, are available at Zenodo at https://doi.org/10.5281/zenodo.14649011.Conflicts of interest
There are no conflicts to declare.Appendix
| Compound |
λ
abs,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
ε max/103 (M−1 cm−1) |
λ
em,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
Φ F | |gabs|/10−3 (nm) | |glum|/10−3 | B CPL (M−1 cm−1) | |μ|/10−20 (esu cm−1) | |m|/10−20 (erg G−1) | θ (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| a From S0 → S1 transition. b From S1 → S0 transition. | ||||||||||
| 1 67 | 420 | 89.59 | 467 | 0.11 | 1 (423) | 1 | 5 | — | — | — |
| 2 43 | 441 | 11.2 | 495 | 0.250 | 1.24 (446) | 0.77 | 1.1 | 469.2a | 0.81a | 84.1a |
| 3 43 | 452 | 7.1 | 528 | 0.41 | 10.58 (471) | 7.44 | 12.6 | 407.0a | 2.24a | 69.6a |
| 4 67 | 464 | — | 504 | 0.81 | 6.6 | 0.53 | — | 650b | 0.048b | 29.8b |
| 5 68 | 491 | — | 529 | 0.75 | 0.93 (490) | 0.75 | — | — | — | 81.7 |
| 6 61 | 375 | 42 | 572 | 0.80 | 0.48 (514) | 0.07 | 1.2 | 571b | 0.097b | 19.94b |
| 7 72 | 427 | — | 483 | 0.018 | 1.80 (427) | 1.30 | — | — | — | 0 |
| 8 74 | 592 | — | 612 | 0.58 | 0.69 (592) | 0.78 | 6.8 | 910a | 0.132a | 5.78a |
| 9 75 | 538 | 85 | 562 | 0.930 | 7.00 (360) | 0.80 | 32 | 1000b | 0.54b | 0b |
| 10 76 | 477 | 350 | 625 | 0.110 | 1.57 (550) | 1.50 | — | — | — | — |
| 11 76 | 479 | 240 | 625 | 0.082 | 1.88 (450) | 1.50 | — | — | — | — |
| 12 77 | 349 | — | 654 | 0.32 | 3.0 (292) | 4.5 | — | 474.39a | 1.99a | 107.2a |
| 13 77 | 359 | — | 812 | 0.15 | 8.5 (492) | 1.3 | — | 913.48a | 2.45a | 87.4a |
| 14 77 | 366 | — | 817 | 0.14 | 3.0 (454) | 1.4 | — | 830.88a | 1.76a | 95.5a |
| 15 77 | 359 | — | 1010 | 0.07 | 1.2 (604) | — | — | 916.85a | 0.14a | 107a |
| 16 80 | 518 | 16.8 | 562 | 0.45 | 8.5 (545) | 7.9 | 168 | 474a | 3.7a | 82.47a |
| 17 79 | 373 | 136 | 575 | 0.22 | 36 | 36 | 81 | 101.6b | 0.2627b | 3.4b |
| 18 79 | 377 | 103.9 | 543 | 0.10 | 16 | 11 | 21 | 94.5b | 0.1401b | 17.78b |
| 19 79 | 377 | 125 | 528 | 0.11 | 10 | 8.9 | 27 | 83.8b | 0.1749b | 63.51b |
| 20 80 | 554 | 26.7 | 577 | 0.74 | 3.9 (560) | 2.7 | 112 | 857a | 5.5a | 87.04a |
| 21 80 | 570 | 72 | 585 | 0.91 | 2.4 (580) | 1.5 | 106 | 1146a | 7.1a | 88.23a |
| 22 82 | 533 | — | 562 | 0.44 | 18 (540) | 13.2 | 176 | 748b | 5.05b | 80b |
| 23 82 | 543 | — | 564 | 0.41 | 10 (546) | 8.7 | 143 | 735b | 5.17b | 82b |
| 24 83 | 376 | — | 616 | 0.24 | 4.2 (472) | 1 | — | — | — | — |
| 25 65 | 544 | 80 | 612 | 0.31 | 30 (559) | 40 | 490 | 230a | 2.26a | 5.19a |
| 26 65 | 552 | 73.6 | 607 | 0.23 | 25 (567) | 34 | 287 | 218a | 2.25a | 8.74a |
| 27 88 | 472 | 135 | 684 | 0.1 | 27.6 (640) | 45 | 304 | 251a | 3.32a | 38.73a |
| 292b | 3.60b | 40.54b | ||||||||
| 28 90 | 496 | 210 | 636 | 0.1 | 8.6 (592) | 4 | 42 | 31.4b | 0.153b | 2.56b |
| 29 91 | 297 | 174.3 | 635 | 0.063 | 8 (582) | 4 | — | 383.8a | 2.83a | 78.9a |
| 30 92 | — | — | — | 0.04 | 2.5 (421) | 1.27 | 0.28 | 363.86a | 1.32a | 83.68a |
| 31 93 | 426 | 13.39 | 493 | 0.19 | 2.43 | 0.54 | — | 331.29b | 0.08b | 50.95b |
| 32 93 | 431 | 7.32 | 473 | 0.05 | 2.64 | 0.63 | — | 461.35b | 0.31b | 71.9b |
| 33 93 | 425 | 12.53 | 492 | 0.15 | 4.05 | 1.54 | — | 395.89b | 0.80b | 19.95b |
| 34 97 | 363 | — | 365 | 0.04 | — | 1.00 | — | — | — | — |
| 35 98 | 361 | 202.214 | 469 | — | 6.3 (294) | 1.9 | 16.7 | — | — | — |
| Compound |
λ
abs,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
ε max/103 (M−1 cm−1) |
λ
em,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
Φ F | |gabs|/10−3 (nm) | |glum|/10−3 | B CPL (M−1 cm−1) | |μ|/10−20 (esu cm−1) | |m|/10−20 (erg G−1) | θ (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| a From S0 → S1 transition. | ||||||||||
| 36 100 | 398 | — | 508 | 0.22 | — | 0.43 | — | — | — | — |
| 37 101 | 554 | — | 569 | 0.43 | 3.26 | 1 | — | — | — | — |
| 38 51 | 444 | 66 | 560 | 0.13 | 2.7 (370) | 0.23 | — | — | — | — |
| 39 53 | 472 | 160 | 610 | 0.098 | 2.5 (580) | 2 | 12.3 | — | — | — |
| (P,P,P/M,M,M)-40102 | 517 | 297 | 643 | 0.28 | 8.2 | 0.3 | 40 | — | — | — |
| (P,P,M/M,M,P)-40102 | 522 | 412 | 650 | 0.28 | 0.88 | 0.2 | 62 | — | — | — |
| 41 104 | 365 | 145 | 697 | 0.04 | 3.0 (520–675) | 3.0 | 90.5 | — | — | — |
| 42 105 | 496 | 1.21 | 537 | 0.02 | ≈4.7 (307) | 0.95 | — | — | — | — |
| 43 106 | 509 | — | 542 | 0.47 | 3.5 | 0.94 | — | — | — | — |
| 44 107 | 512 | 10.4 | 602 | 0.03 | 6.6 (553) | 1.3 | — | — | — | — |
| 45 110 | 356 | 71 | 480 | 0.13 | 1.3 | 0.4 | — | — | — | — |
| 46 110 | 342 | 53 | 554 | 0.08 | 1.5 (294) | 0.7 | 1.30 | — | — | — |
| 47 111 | 355 | 80 | 456 | 0.11 | 3.9 (281) | — | — | 113a | 0.0360a | 118.69a |
| Compound |
λ
abs,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
ε max/103 (M−1 cm−1) |
λ
em,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max (nm) max (nm) |
Φ F | |gabs|/10−3 (nm) | |glum|/10−3 | B CPL (M−1 cm−1) | |μ|/10−20 (esu cm−1) | |m|/10−20 (erg G−1) | θ (°) |
|---|---|---|---|---|---|---|---|---|---|---|
| a From S0 → S1 transition. b From S1 → S0 transition. | ||||||||||
| 48 113 | 351 | 85 | 457 | 0.04 | 0.65 (352) | 0.8 | 1.40 | — | — | — |
| 49 106 | 513 | — | 552 | 0.12 | 1.9 | 16 | — | — | — | — |
| 50 106 | 510 | — | 536 | 0.48 | 2 | 9.1 | — | — | — | — |
| 51 106 | 547 | — | 609 | 0.56 | 2.4 | 6 | — | — | — | — |
| 52 106 | 510 | — | 539 | 0.52 | 3 | 6 | — | — | — | — |
| 53 106 | 510 | — | 543 | 0.51 | 2.1 | 2.4 | — | — | — | — |
| 54 114 | 350 | — | 450 | — | — | ≈0.23 | — | — | — | — |
| 55 115 | 331 | 109.65 | 517 | — | 3.1 (432) | 2 | — | — | — | — |
| 56 116 | 452 | 11 | 503 | 0.33 | 6.7 | 2.4 | 9.1 | — | — | — |
| 57 116 | 580 | 16 | 588 | 0.32 | 10 | 7.0 | 95.2 | — | — | — |
| 58 117 | 382 | 108.4 | 542 | 0.75 | 3.91 | 1.12 | 45.77 | 245.73b | 0.362b | 166.56b |
| 59 118 | 360 | 113 | 595 | 0.40 | 2.98 (469) | 1.30 | 28.57 | 327b | 0.186b | 178.87b |
| 60 120 | 368 | 69 | 553 | 0.96 | 1.3 (511) | 0.91 | 30.1 | 57b | 0.097b | 20b |
| 61 121 | 420 | 3.52 | 531 | 0.45 | 44.6 | 2.2 | 173 | 548.02a | 0.707a | 158.83a |
| 62 122 | — | 88.0 | 646 | 0.44 | 1.2 | 0.73 | 14.13 | 345.49b | 0.40b | 87.29b |
| 63 122 | — | 81.4 | 582 | 0.43 | 1.4 | 0.89 | 15.57 | 150.42b | 0.06b | 60.94b |
| 64 122 | — | 67.2 | 576 | 0.79 | 2.9 | 5.5 | 145.9 | 237.77b | 0.82b | 52.12b |
| 65 129 | 489 | 19.5 | 528 | 0.80 | — | 0.75 | — | — | — | — |
| 66 130 | 490 | 20 | 526 | 0.64 | 1.9 (496) | 1.1 | — | — | — | — |
| 67 131 | 504 | 36 | 522 | 0.65 | 2.5 (492) | 0.90 | 14.6 | — | — | — |
| 68 131 | 524 | — | 567 | 0.99 | 8.5 (368) | 1 | — | — | — | — |
| 69 131 | 518 | — | 541 | 0.90 | 6.3 (368) | 1 | — | — | — | — |
| 70 132 | 545 | — | 585 | 0.66 | 8.6 | 2.5 | 45.8 | 689.3b | 0.80b | 24.4b |
| 71 132 | 548 | — | 595 | 0.68 | 7.4 | 2.7 | 41.5 | 755.9b | 0.30b | 136.8b |
| 72 132 | 553 | — | 598 | 0.64 | 3.1 | 2.7 | 58.7 | 682.5b | 1.5b | 48.7b |
| 73 132 | 622 | — | 675 | 0.11 | 4.7 | 2.9 | 2.0 | 464.6b | 4.6b | 80.0b |
| 74 132 | 595 | — | 641 | 0.01 | 6.6 | 5.0 | 4.0 | 352.2b | 4.3b | 79.2b |
| 75 133 | 627 | — | 660 | 1.00 | 3.3 (502) | 2.00 | 28.5 | 552a | 0.46a | 0a |
| 76 133 | 650 | — | 684 | 0.99 | 3.1 (518) | 2.00 | 37.1 | 617a | 0.41a | 0a |
| 77 133 | 662 | — | 696 | 0.90 | 2.6 (526) | 2.00 | 40.0 | 736a | 0.41a | 0a |
| 78 135 | 495 | 56 | 498 | 0.83 | 2.7 | 1.9 | — | 376.1b | 1.23b | 84.26b |
| 79 135 | 493 | 57 | 500 | 0.85 | 1.0 | 0.75 | — | 691.3b | 1.45b | 83.68b |
| 80 135 | 479 | 24 | 485 | 0.59 | 4.1 | 2.3 | — | 229.4b | 1.85b | 81.95b |
| 81 136 | 375 | — | 511 | 0.71 | — | 0.75 | — | — | — | — |
| 82 137 | 262 | — | 492 | 0.04 | ≈5.95 (314) | ≈1.7 | — | 0.63b | 0.00053b | 45b |
| 83 139 | 525 | — | 620 | 0.85 | 2.00 (509) | 0.30 | — | — | — | — |
| 84 140 | 371 | 146.9 | 500 | 0.30 | 2.7 (452) | 2.6 | — | — | — | — |
| 85 83 | 364 | — | 565 | 0.37 | 2.7 (378) | 0.3 | — | — | — | — |
| 86 141 | 360 | 170 | 475 | 0.02 | — | 0.24 | — | — | — | — |
| 87 142 | 503 | — | 535 | 0.49 | 5.2 | 0.6 | 10–8 | — | — | — |
| 88 142 | 542 | — | 622 | 0.03 | 3.3 | 1.5 | 1.7 | — | — | — |
| 89 142 | 575 | — | 611 | 0.54 | 4.6 | 0.5 | 13.9 | — | — | — |
| 90 142 | 590 | — | 639 | 0.26 | 4.0 | 1.6 | 14.7 | — | — | — |
| 91 142 | 594 | — | 644 | 0.10 | 3.6 | 2.1 | 7.1 | — | — | — |
| 92 144 | 400 | — | 546 | 0.50 | — | ≈2.4 | ≈31 | 412b | 0.34b | 175.34b |
| 93 62 | 351 | 130 | 475 | 0.18 | — | 1.1 | 13 | — | — | — |
| 94 62 | 353 | 140 | 473 | 0.30 | — | 0.56 | 12 | — | — | — |
| 95 145 | 387 | 61 | 517 | 0.03 | ≈14.5 (352) | ≈0.55 | — | — | — | — |
| 96 67 | 392 | 930 | 569 | 0.83 | 4 (310) | 2.2 | 84.9 | 499b | 0.456b | 2.4b |
| 97 67 | 397 | 680 | 583 | 0.91 | 5.3 (365) | 2.1 | 65 | 435b | 0.632b | 54b |
| 98 146 | 387 | — | 558 | 0.43 | 14 (327) | 1.2 | — | 782.69b | 0.254b | 12.04b |
| 99 146 | 420 | — | 614 | 0.31 | 7.2 (323) | 1.1 | — | 824.73b | 0.275b | 21.87b |
Acknowledgements
This work has received funding from: Grant PID2021-127521NB-I00 and grant PID2022-137403NA-I00 funded by MICIU/AEI/10.13039/501100011033 and by ERDF/EU. C. M. C. acknowledges grant RYC2023-044652-I funded by MICIU/AEI/10.13039/501100011033 and by ESF+. J. L. P. acknowledges Grant PRE2022-102320 funded by MICIU/AEI/10.13039/501100011033 and by ESF+.Notes and references
- J. X. Flores-Lasluisa, M. Navlani-García, Á. Berenguer-Murcia, E. Morallón and D. Cazorla-Amorós, Front. Mater., 2024, 11, 1381363 CrossRef
.
- D. R. Lobato-Peralta, P. U. Okoye and C. Alegre, J. Power Sources, 2024, 617, 235140 CrossRef CAS
.
- Q. Zheng, Z. Hu, L. Liu, H. Lu, X. Wang, Y. Lei, C. Han and W. Li, J. Mater. Chem. A, 2024, 12, 21531–21552 RSC
.
- V. Meunier, G. Bepete, M.-S. Cao, Y. Chen, C. de Tomas, J. Di, C. Ewels, N. Koratkar, Q. Li, C. Liu, N. Sheremetyeva and M. Terrones, Carbon, 2024, 229, 119488 CrossRef CAS
.
- V. Georgakilas, J. A. Perman, J. Tucek and R. Zboril, Chem. Rev., 2015, 115, 4744–4822 CrossRef CAS PubMed
.
- P. R. Wallace, Phys. Rev., 1947, 71, 622–634 CrossRef CAS
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS
.
-
E. L. Wolf, Applications of Graphene: An Overview, Springer, New York, 2014 Search PubMed
.
- L. Chen, Y. Hernandez, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2012, 51, 7640–7654 CrossRef CAS
.
- P. Izquierdo-García, J. M. Fernández-García and N. Martín, J. Am. Chem. Soc., 2024, 146, 32222–32234 CrossRef PubMed
.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Science, 2008, 320, 1308 CrossRef CAS PubMed
.
- G. M. Paternò, Goudappagouda, Q. Chen, G. Lanzani, F. Scotognella and A. Narita, Adv. Opt. Mater., 2021, 9, 2100508 CrossRef
.
- Y. Gu, Z. Qiu and K. Müllen, J. Am. Chem. Soc., 2022, 144, 11499–11524 CrossRef CAS
.
- L. Chen, Y. Hernandez, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2012, 51, 7640–7654 CrossRef CAS PubMed
.
- R. Sekiya and T. Haino, Chem. – Eur. J., 2021, 27, 187–199 CrossRef
.
- E. Clar and C. T. Ironside, Proc. Chem. Soc., 1958, 125–156 Search PubMed
.
- E. Clar, C. T. Ironside and M. Zander, J. Chem. Soc., 1959, 142–147 RSC
.
- A. Halleux, R. H. Martin and G. S. D. King, Helv. Chim. Acta, 1958, 41, 1177–1183 CrossRef CAS
.
- Y. Segawa, H. Ito and K. Itami, Nat. Rev. Mater., 2016, 1, 1–14 Search PubMed
.
- A. Narita, X. Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC
.
- A. V. Gulevskaya and D. I. Tonkoglazova, Adv. Synth. Catal., 2022, 364, 2502–2539 CrossRef CAS
.
- K. M. Magiera, V. Aryal and W. A. Chalifoux, Org. Biomol. Chem., 2020, 18, 2372–2386 RSC
.
- M. Ball, Y. Zhong, Y. Wu, C. Schenck, F. Ng, M. Steigerwald, S. Xiao and C. Nuckolls, Acc. Chem. Res., 2015, 48, 267–276 CrossRef CAS PubMed
.
- Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554–14593 CrossRef CAS PubMed
.
- R. Sekiya, S. Arimura, H. Moriguchi and T. Haino, Nanoscale, 2025, 17, 774–787 RSC
.
-
S. Míguez-Lago, J. P. Mora-Fuentes, C. M. Cruz and A. G. Campaña, in Helicenes: Synthesis, Properties and Applications, ed. J. Crassous, I. G. Stará and I. Starý, Wiley-VCH, Weinheim, 2022, vol. 9, pp. 283–328 Search PubMed
.
- H. V. Anderson, N. D. Gois and W. A. Chalifoux, Org. Chem. Front., 2023, 10, 4167–4197 RSC
.
- L. Qin, J. Xie, B. Wu, H. Hong, S. Yang, Z. Ma, C. Li, G. Zhang, X. S. Zhang, K. Liu and D. Zhang, J. Am. Chem. Soc., 2024, 146, 12206–12214 CrossRef CAS PubMed
.
- P. Izquierdo-García, J. M. Fernández-García, I. Fernández, J. Perles and N. Martín, J. Am. Chem. Soc., 2021, 143, 11864–11870 CrossRef PubMed
.
- P. Izquierdo-García, J. M. Fernández-García, J. Perles, I. Fernández and N. Martín, Angew. Chem., Int. Ed., 2023, 62, e202215655 CrossRef PubMed
.
- S. Li, R. Li, Y. K. Zhang, S. Wang, B. Ma, B. Zhang and P. An, Chem. Sci., 2023, 14, 3286–3292 RSC
.
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 76 CrossRef CAS PubMed
.
- Y. Xuefeng, G. Xiaoqing, Z. You-Xuan, K. Hua, C. Chuan-Feng, L. Minghua, D. Pengfei and T. Zhiyong, CCS Chem., 2023, 5, 2760–2789 CrossRef
.
- M. Saqlain, H. M. Zohaib, S. Qamar, H. Malik and H. Li, Coord. Chem. Rev., 2024, 501, 215559 CrossRef CAS
.
- J. Liu, Z.-P. Song, L.-Y. Sun, B.-X. Li, Y.-Q. Lu and Q. Li, Responsive Mater., 2023, 1, e20230005 Search PubMed
.
- K. Takaishi, C. Maeda and T. Ema, Chirality, 2023, 35, 92–103 CrossRef CAS PubMed
.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef CAS PubMed
.
- Y. Zhong, Z. Wu, Y. Zhang, B. Dong and X. Bai, InfoMat, 2023, 5, e12392 CrossRef CAS
.
- O. G. Willis, F. Zinna and L. Di Bari, Angew. Chem., Int. Ed., 2023, 62, e202302358 CrossRef CAS
.
- Y. Nagata and T. Mori, Front. Chem., 2020, 8, 448 CrossRef CAS
.
- L. Arrico, L. Di Bari and F. Zinna, Chem. – Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed
.
- Z. Qiu, C. W. Ju, L. Frédéric, Y. Hu, D. Schollmeyer, G. Pieters, K. Müllen and A. Narita, J. Am. Chem. Soc., 2021, 143, 4661–4667 CrossRef CAS PubMed
.
- R. Li, D. Wang, S. Li and P. An, Beilstein J. Org. Chem., 2023, 19, 736–751 CrossRef CAS
.
- H. Luo and J. Liu, Angew. Chem., Int. Ed., 2024, 63, e202410759 CAS
.
- Y. Zhu and J. Wang, Acc. Chem. Res., 2023, 56, 363–373 CrossRef CAS PubMed
.
- Y. Liu, X. Gao, B. Zhao and J. Deng, Nanoscale, 2024, 16, 6853–6875 RSC
.
- X. Yan, H. Zhao, K. Zhang, Z. Zhang, Y. Chen and L. Feng, ChemPlusChem, 2023, 88, e202200428 CrossRef CAS PubMed
.
- N. Suzuki, Y. Wang, P. Elvati, Z.-B. Qu, K. Kim, S. Jiang, E. Baumeister, J. Lee, B. Yeom, J. H. Bahng, J. Lee, A. Violi and N. A. Kotov, ACS Nano, 2016, 10, 1744–1755 CrossRef CAS PubMed
.
- H. V. Anderson, N. D. Gois and W. A. Chalifoux, Org. Chem. Front., 2023, 10, 4167–4197 RSC
.
- C. M. Cruz, I. R. Márquez, I. F. A. Mariz, V. Blanco, C. Sánchez-Sánchez, J. M. Sobrado, J. A. Martín-Gago, J. M. Cuerva, E. Maçôas and A. G. Campaña, Chem. Sci., 2018, 9, 3917–3924 RSC
.
- I. R. Márquez, S. Castro-Fernández, A. Millán and A. G. Campaña, Chem. Commun., 2018, 54, 6705–6718 RSC
.
- C. M. Cruz, S. Castro-Fernández, E. Maçôas, J. M. Cuerva and A. G. Campaña, Angew. Chem., Int. Ed., 2018, 57, 14782–14786 CrossRef CAS PubMed
.
- Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554–14593 CrossRef CAS PubMed
.
- M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed
.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W. S. Wong, M. Żyła-Karwowska and M. Stȩpień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed
.
- M. M. Martin, F. Hampel and N. Jux, Chem. – Eur. J., 2020, 26, 10210–10212 CrossRef CAS PubMed
.
- Y. Shen and C. F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed
.
- H. Luo and J. Liu, Angew. Chem., Int. Ed., 2024, 63, e202410759 CAS
.
- Chaolumen, I. A. Stepek, K. E. Yamada, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2021, 60, 23508–23532 CrossRef CAS PubMed
.
- J. Hong, X. Xiao, H. Liu, E. Dmitrieva, A. A. Popov, Z. Yu, M.-D. Li, T. Ohto, J. Liu, A. Narita, P. Liu, H. Tada, X.-Y. Cao, X.-Y. Wang, Y. Zou, K. Müllen and Y. Hu, Chem. – Eur. J., 2022, 28, e202202243 CrossRef CAS PubMed
.
- R. Li, B. Ma, S. Li, C. Lu and P. An, Chem. Sci., 2023, 14, 8905–8913 RSC
.
- V. Kumar, S. D. Dongre, G. Venugopal, A. Narayanan and S. S. Babu, Chem. Commun., 2024, 60, 11944–11947 RSC
.
- M. M. Martin, F. Hampel and N. Jux, Chem. – Eur. J., 2020, 26, 10210–10212 CrossRef CAS PubMed
.
- F. Morita, Y. Kishida, Y. Sato, H. Sugiyama, M. Abekura, J. Nogami, N. Toriumi, Y. Nagashima, T. Kinoshita, G. Fukuhara, M. Uchiyama, H. Uekusa and K. Tanaka, Nat. Synth., 2024, 3, 774–786 CrossRef CAS
.
- J. B. Birks, D. J. S. Birch, E. Cordemans and E. Vander Donckt, Chem. Phys. Lett., 1976, 43, 33–36 CrossRef CAS
.
- Y. Ma, L. Zhou, J. Tan, W. Sun, Y. Zou and Y. Hu, Adv. Opt. Mater., 2025, 13, 2402446 CrossRef CAS
.
- R. Yamano, Y. Shibata and K. Tanaka, Chem. – Eur. J., 2018, 24, 6364–6370 CrossRef CAS PubMed
.
- W. Yang, G. Longhi, S. Abbate, A. Lucotti, M. Tommasini, C. Villani, V. J. Catalano, A. O. Lykhin, S. A. Varganov and W. A. Chalifoux, J. Am. Chem. Soc., 2017, 139, 13102–13109 CrossRef CAS PubMed
.
- A. Bernhardt, D. Čavlović, M. Mayländer, O. Blacque, C. M. Cruz, S. Richert and M. Juríček, Angew. Chem., Int. Ed., 2024, 63, e202318254 CrossRef CAS PubMed
.
- Y. Hu, X.-Y. Wang, P.-X. Peng, X.-C. Wang, X.-Y. Cao, X. Feng, K. Müllen and A. Narita, Angew. Chem., Int. Ed., 2017, 56, 3374–3378 CrossRef CAS PubMed
.
- H. Tanaka, Y. Kato, M. Fujiki, Y. Inoue and T. Mori, J. Phys. Chem. A, 2018, 122, 7378–7384 CrossRef CAS PubMed
.
- H. Tanaka, M. Ikenosako, Y. Kato, M. Fujiki, Y. Inoue and T. Mori, Commun. Chem., 2018, 1, 38 CrossRef
.
- Y. Dong, Z. Zhang, Y. Hashikawa, H. Meng, F. Bai, K. Itami and Chaolumen, Angew. Chem., Int. Ed., 2024, 63, e202406927 CrossRef CAS PubMed
.
- J. K. Li, X. Y. Chen, W. L. Zhao, Y. L. Guo, Y. Zhang, X. C. Wang, A. C. H. Sue, X. Y. Cao, M. Li, C. F. Chen and X. Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202215367 CrossRef CAS PubMed
.
- J. Wang, C. Shen, G. Zhang, F. Gan, Y. Ding and H. Qiu, Angew. Chem., Int. Ed., 2022, 61, e202115979 CrossRef CAS PubMed
.
- G. Huo, W. Xu, J. Hu, Y. Han, W. Fan, W. Wang, Z. Sun, H. Yang and J. Wu, Angew. Chem., Int. Ed., 2025, 64, e202416707 CrossRef CAS PubMed
.
- P. J. Evans, J. Ouyang, L. Favereau, J. Crassous, I. Fernández, J. Perles and N. Martín, Angew. Chem., Int. Ed., 2018, 57, 6774–6779 CrossRef CAS PubMed
.
- P. Izquierdo-García, J. M. Fernández-García, S. Medina Rivero, M. Šámal, J. Rybáček, L. Bednárová, S. Ramírez-Barroso, F. J. Ramírez, R. Rodríguez, J. Perles, D. García-Fresnadillo, J. Crassous, J. Casado, I. G. Stará and N. Martín, J. Am. Chem. Soc., 2023, 145, 11599–11610 CrossRef PubMed
.
- W. Niu, Y. Fu, Z.-L. Qiu, C. J. Schürmann, S. Obermann, F. Liu, A. A. Popov, H. Komber, J. Ma and X. Feng, J. Am. Chem. Soc., 2023, 145, 26824–26832 CrossRef CAS PubMed
.
- K. Uehara, H. Kano, K. Matsuo, H. Hayashi, M. Fujiki, H. Yamada and N. Aratani, ChemPhotoChem, 2021, 5, 974–978 CrossRef CAS
.
- W. Niu, Y. Fu, Q. Deng, Z. L. Qiu, F. Liu, A. A. Popov, H. Komber, J. Ma and X. Feng, Angew. Chem., Int. Ed., 2024, 63, e202319874 CrossRef CAS PubMed
.
- Y. Y. Ju, L. Chai, K. Li, J. F. Xing, X. H. Ma, Z. L. Qiu, X. J. Zhao, J. Zhu and Y. Z. Tan, J. Am. Chem. Soc., 2023, 145, 2815–2821 CrossRef CAS PubMed
.
- W. Cui, Z. Jin, W. Fu and C. Shen, Chin. Chem. Lett., 2024, 35, 109667 CrossRef CAS
.
- S. Ma, J. Gu, C. Lin, Z. Luo, Y. Zhu and J. Wang, J. Am. Chem. Soc., 2020, 142, 16887–16893 CrossRef CAS PubMed
.
- Y. Zhu, X. Guo, Y. Li and J. Wang, J. Am. Chem. Soc., 2019, 141, 5511–5517 CrossRef CAS PubMed
.
- Y. Chen, C. Lin, Z. Luo, Z. Yin, H. Shi, Y. Zhu and J. Wang, Angew. Chem., Int. Ed., 2021, 60, 7796–7801 CrossRef CAS PubMed
.
- Y.-J. Shen, N.-T. Yao, L.-N. Diao, Y. Yang, X.-L. Chen and H.-Y. Gong, Angew. Chem., Int. Ed., 2023, 62, e202300840 CrossRef CAS PubMed
.
- K. Zhu, Z. Li, J. Liang, K. Zou, Y. Shen and H. Gong, Angew. Chem., Int. Ed., 2024, 63, e202409713 CrossRef CAS PubMed
.
- Y.-J. Shen, L.-J. Peng, L.-N. Diao, N.-T. Yao, W.-K. Chen, Y. Yang, M. Qiu, W.-X. Zhu, X. Li, X.-Y. Wang and H.-Y. Gong, Org. Lett., 2024, 26, 7279–7284 CrossRef CAS PubMed
.
- J. Tan, X. Xu, J. Liu, S. Vasylevskyi, Z. Lin, R. Kabe, Y. Zou, K. Müllen, A. Narita and Y. Hu, Angew. Chem., Int. Ed., 2023, 62, e202218494 CrossRef CAS PubMed
.
- A. K. Swain, K. Radacki, H. Braunschweig and P. Ravat, J. Org. Chem., 2022, 87, 993–1000 CrossRef CAS PubMed
.
- A. Swain, K. Radacki, H. Braunschweig and P. Ravat, Chem. Sci., 2024, 15, 11737–11747 RSC
.
- B. P. Benke, L. Hertwig, X. Yang, F. Rominger and M. Mastalerz, Eur. J. Org. Chem., 2021, 72–76 CrossRef CAS PubMed
.
- P.-C. Zhu, Y. Liu, L.-H. Peng and C. Zhang, Tetrahedron Lett., 2014, 55, 521–524 CrossRef CAS
.
- C. Tang, H. Han, R. Zhang, L. S. de Moraes, Y. Qi, G. Wu, C. G. Jones, I. H. Rodriguez, Y. Jiao, W. Liu, X. Li, H. Chen, L. Bancroft, X. Zhao, C. L. Stern, Q.-H. Guo, M. D. Krzyaniak, M. R. Wasielewski, H. M. Nelson, P. Li and J. F. Stoddart, J. Am. Chem. Soc., 2024, 146, 20158–20167 CrossRef CAS PubMed
.
- Y. Wada, K. Shinohara and T. Ikai, Chem. Commun., 2019, 55, 11386–11389 RSC
.
- M. Buendía, J. M. Fernández-García, J. Perles, S. Filippone and N. Martín, Nat. Synth., 2024, 3, 545–553 CrossRef
.
- Y. Zhou, X. Zhang, B. Yuan, D. Lu, G.-L. Zhuang and P. Du, Org. Lett., 2024, 26, 5635–5639 CrossRef CAS PubMed
.
- Q. Xu, C. Wang, J. He, X. Li, Y. Wang, X. Chen, D. Sun and H. Jiang, Org. Chem. Front., 2021, 8, 2970–2976 RSC
.
- J. Bergner, J. Borstelmann, L. M. Cavinato, J. P. Fuenzalida-Werner, C. Walla, H. Hinrichs, P. Schulze, F. Rominger, R. D. Costa, A. Dreuw and M. Kivala, Chem. – Eur. J., 2024, 30, e202303336 CrossRef CAS PubMed
.
- C. M. Cruz, I. R. Márquez, S. Castro-Fernández, J. M. Cuerva, E. Maçôas and A. G. Campaña, Angew. Chem., Int. Ed., 2019, 58, 8068–8072 CrossRef CAS PubMed
.
- S. Castro-Fernández, C. M. Cruz, I. F. A. Mariz, I. R. Márquez, V. G. Jiménez, L. Palomino-Ruiz, J. M. Cuerva, E. Maçôas and A. G. Campaña, Angew. Chem., Int. Ed., 2020, 59, 7139–7145 CrossRef PubMed
.
- S. Míguez-Lago, I. F. A. Mariz, M. A. Medel, J. M. Cuerva, E. Maçôas, C. M. Cruz and A. G. Campaña, Chem. Sci., 2022, 13, 10267–10272 RSC
.
- Z. Qi, H. Shang, B. Ji, Y. Shi, T. Ye, Y. Li and J. Xiao, J. Org. Chem., 2023, 88, 14550–14558 CrossRef CAS PubMed
.
- J. Borstelmann, L. Schneider, F. Rominger, F. Deschler and M. Kivala, Angew. Chem., Int. Ed., 2024, 63, e202405570 CrossRef CAS PubMed
.
- L. Yang, Y. Y. Ju, M. A. Medel, Y. Fu, H. Komber, E. Dmitrieva, J. J. Zhang, S. Obermann, A. G. Campaña, J. Ma and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202216193 CrossRef CAS PubMed
.
- G. González Miera, S. Matsubara, H. Kono, K. Murakami and K. Itami, Chem. Sci., 2022, 13, 1848–1868 RSC
.
- J. Urieta-Mora, M. Krug, W. Alex, J. Perles, I. Fernández, A. Molina-Ontoria, D. M. Guldi and N. Martín, J. Am. Chem. Soc., 2020, 142, 4162–4172 CrossRef CAS PubMed
.
- M. A. Medel, R. Tapia, V. Blanco, D. Miguel, S. P. Morcillo and A. G. Campaña, Angew. Chem., Int. Ed., 2021, 60, 6094–6100 CrossRef CAS PubMed
.
- M. A. Medel, C. M. Cruz, D. Miguel, V. Blanco, S. P. Morcillo and A. G. Campaña, Angew. Chem., Int. Ed., 2021, 60, 22051–22056 CrossRef CAS PubMed
.
- P. An, R. Li, B. Ma, R.-Y. He, Y.-K. Zhang, M.-J. Xiao and B. Zhang, Angew. Chem., Int. Ed., 2021, 60, 24478–24483 CrossRef CAS PubMed
.
- M. A. Medel, L. Hortigüela, V. Lloveras, J. Catalán-Toledo, D. Miguel, A. J. Mota, N. Crivillers, A. G. Campaña and S. P. Morcillo, ChemistryEurope, 2023, 1, e202300021 CrossRef
.
- F. Gan, C. Shen, W. Cui and H. Qiu, J. Am. Chem. Soc., 2023, 145, 5952–5959 CrossRef CAS PubMed
.
- F. Gan, G. Zhang, J. Liang, C. Shen and H. Qiu, Angew. Chem., Int. Ed., 2024, 63, e202320076 CrossRef PubMed
.
- S. Qiu, A. C. Valdivia, W. Zhuang, F. F. Hung, C. M. Che, J. Casado and J. Liu, J. Am. Chem. Soc., 2024, 146, 16161–16172 CrossRef PubMed
.
- V. Kumar, G. Venugopal, A. B. Jadhav, S. D. Dongre, R. Gonnade, J. Kumar, P. C. Ruer, B. Hupp, A. Steffen and S. S. Babu, Angew. Chem., Int. Ed., 2025, 64, e202422125 CrossRef PubMed
.
- X. Wang, J. Bai, Y. Shen, Z. Li and H. Gong, Angew. Chem., Int. Ed., 2025, 64, e202417745 CrossRef PubMed
.
- D. Reger, P. Haines, K. Y. Amsharov, J. A. Schmidt, T. Ullrich, S. Bönisch, F. Hampel, A. Görling, J. Nelson, K. E. Jelfs, D. M. Guldi and N. Jux, Angew. Chem., Int. Ed., 2021, 60, 18073–18081 CrossRef PubMed
.
- J. Hong, X. Xiao, H. Liu, E. Dmitrieva, A. A. Popov, Z. Yu, M. Li, T. Ohto, J. Liu, A. Narita, P. Liu, H. Tada, X. Cao, X. Wang, Y. Zou, K. Müllen and Y. Hu, Chem. – Eur. J., 2022, 28, e202202243 CrossRef PubMed
.
- J. Liu, J. Hong, Z. Liao, J. Tan, H. Liu, E. Dmitrieva, L. Zhou, J. Ren, X. Y. Cao, A. A. Popov, Y. Zou, A. Narita and Y. Hu, Angew. Chem., Int. Ed., 2024, 63, e202400172 CrossRef PubMed
.
- S. D. Dongre, G. Venugopal, V. Kumar, A. B. Jadhav, J. Kumar and S. S. Babu, Angew. Chem., Int. Ed., 2024, e202420767 Search PubMed
.
- T. Ikeda, T. Masuda, T. Hirao, J. Yuasa, H. Tsumatori, T. Kawai and T. Haino, Chem. Commun., 2012, 48, 6025 RSC
.
- J. Kumar, T. Nakashima, H. Tsumatori, M. Mori, M. Naito and T. Kawai, Chem. – Eur. J., 2013, 19, 14090–14097 CrossRef PubMed
.
- B. Liu, M. Böckmann, W. Jiang, N. L. Doltsinis and Z. Wang, J. Am. Chem. Soc., 2020, 142, 7092–7099 CrossRef PubMed
.
- X. Tian, K. Shoyama, B. Mahlmeister, F. Brust, M. Stolte and F. Würthner, J. Am. Chem. Soc., 2023, 145, 9886–9894 CrossRef PubMed
.
- S. E. Penty, G. R. F. Orton, D. J. Black, R. Pal, M. A. Zwijnenburg and T. A. Barendt, J. Am. Chem. Soc., 2024, 146, 5470–5479 CrossRef CAS PubMed
.
- Y. Yu, C. Wang, F.-F. Hung, C. Chen, D. Pan, C.-M. Che and J. Liu, J. Am. Chem. Soc., 2024, 146, 22600–22611 CrossRef CAS PubMed
.
- Z. Sun, C. Yi, Q. Liang, C. Bingi, W. Zhu, P. Qiang, D. Wu and F. Zhang, Org. Lett., 2020, 22, 209–213 CrossRef CAS PubMed
.
- Z. Sun, C. Yu, N. Zhang, L. Li, Y. Jiao, P. Thiruvengadam, D. Wu and F. Zhang, Org. Lett., 2022, 24, 6670–6675 CrossRef CAS PubMed
.
- D. Tan, J. Dong, T. Ma, Q. Feng, S. Wang and D. Yang, Angew. Chem., Int. Ed., 2023, 62, e202304711 CrossRef CAS PubMed
.
- Y.-Y. Ju, L.-E. Xie, J.-F. Xing, Q.-S. Deng, X.-W. Chen, L.-X. Huang, G.-H. Nie, Y.-Z. Tan and B. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202414383 CrossRef CAS PubMed
.
- J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C.-H. Sue, X.-Y. Cao and X.-Y. Wang, J. Am. Chem. Soc., 2021, 143, 17958–17963 CrossRef CAS PubMed
.
- F. Zhang, F. Rauch, A. Swain, T. B. Marder and P. Ravat, Angew. Chem., Int. Ed., 2023, 62, e202218965 CrossRef CAS PubMed
.
- F. Zhang, V. Brancaccio, F. Saal, U. Deori, K. Radacki, H. Braunschweig, P. Rajamalli and P. Ravat, J. Am. Chem. Soc., 2024, 146, 29782–29791 CrossRef CAS PubMed
.
- H. Chang, H. Liu, E. Dmitrieva, Q. Chen, J. Ma, P. He, P. Liu, A. A. Popov, X.-Y. Cao, X.-Y. Wang, Y. Zou, A. Narita, K. Müllen, H. Peng and Y. Hu, Chem. Commun., 2020, 56, 15181–15184 RSC
.
- F. Zhou, Z. Huang, Z. Huang, R. Cheng, Y. Yang and J. You, Org. Lett., 2021, 23, 4559–4563 CrossRef CAS PubMed
.
- D. Reger, P. Haines, F. W. Heinemann, D. M. Guldi and N. Jux, Angew. Chem., Int. Ed., 2018, 57, 5938–5942 CrossRef CAS PubMed
.
- J. Wade, J. R. Brandt, D. Reger, F. Zinna, K. Y. Amsharov, N. Jux, D. L. Andrews and M. J. Fuchter, Angew. Chem., Int. Ed., 2021, 60, 222–227 CrossRef CAS PubMed
.
- P. Izquierdo-García, J. M. Fernández-García, J. Perles and N. Martín, J. Am. Chem. Soc., 2024, 146, 34943–34949 CrossRef PubMed
.
- S. Li, R. Li, Y.-K. Zhang, S. Wang, B. Ma, B. Zhang and P. An, Chem. Sci., 2023, 14, 3286–3292 RSC
.
- Y. Ju, H. Luo, Z. Li, B. Zheng, J. Xing, X. Chen, L. Huang, G. Nie, B. Zhang, J. Liu and Y. Tan, Angew. Chem., Int. Ed., 2024, 63, e202402621 CrossRef CAS PubMed
.
- D. Yang, K. M. Cheung, Q. Gong, L. Zhang, L. Qiao, X. Chen, Z. Huang and Q. Miao, Angew. Chem., Int. Ed., 2024, 63, e202402756 CrossRef CAS PubMed
.
- G. Venugopal, V. Kumar, A. Badrinarayan Jadhav, S. D. Dongre, A. Khan, R. Gonnade, J. Kumar and S. Santhosh Babu, Chem. – Eur. J., 2024, 30, e202304169 CrossRef CAS PubMed
.
- W. Wang, X. Li, Z. Qi, B. Ji, Z. Wang, S. Wang and J. Xiao, Org. Lett., 2023, 25, 1343–1347 CrossRef CAS PubMed
.
- L. Zhou, H. Liu, J. Tan, C. Liu, X. Cao, A. Narita and Y. Hu, Chem. – Asian J., 2022, 17, e202200336 CrossRef CAS PubMed
.
- L. Wan, J. Wade, X. Shi, S. Xu, M. J. Fuchter and A. J. Campbell, ACS Appl. Mater. Interfaces, 2020, 12, 39471–39478 CrossRef CAS PubMed
.
- U. Deori, G. P. Nanda, C. Murawski and P. Rajamalli, Chem. Sci., 2024, 15, 17739–17759 RSC
.
- J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1–16 CrossRef CAS
.
- G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Chirality, 2016, 28, 696–707 CrossRef CAS PubMed
.
- R. G. Uceda, C. M. Cruz, S. Míguez-Lago, L. Á. de Cienfuegos, G. Longhi, D. A. Pelta, P. Novoa, A. J. Mota, J. M. Cuerva and D. Miguel, Angew. Chem., Int. Ed., 2024, 63, e202316696 CrossRef CAS PubMed
.
- R. G. Uceda, A. Gijón, S. Míguez-Lago, C. M. Cruz, V. Blanco, F. Fernández-Álvarez, L. Á. de Cienfuegos, M. Molina-Solana, J. Gómez-Romero, D. Miguel, A. J. Mota and J. M. Cuerva, Angew. Chem., Int. Ed., 2024, 63, e202409998 CrossRef CAS PubMed
.
- A. M. Ortuño, P. Reiné, S. Resa, L. Álvarez De Cienfuegos, V. Blanco, J. M. Paredes, A. J. Mota, G. Mazzeo, S. Abbate, J. M. Ugalde, V. Mujica, G. Longhi, D. Miguel and J. M. Cuerva, Org. Chem. Front., 2021, 8, 5071–5086 RSC
.
- T. M. Fukunaga, C. Sawabe, T. Matsuno, J. Takeya, T. Okamoto and H. Isobe, Angew. Chem., Int. Ed., 2021, 60, 19097–19101 CrossRef CAS PubMed
.
- S. Sato, A. Yoshii, S. Takahashi, S. Furumi, M. Takeuchi and H. Isobe, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 13097–13101 CrossRef CAS PubMed
.
- H. Kubo, T. Hirose, T. Nakashima, T. Kawai, J. Y. Hasegawa and K. Matsuda, J. Phys. Chem. Lett., 2021, 12, 686–695 CrossRef CAS PubMed
.
- D. Meng, T. Zhao, D. Yang, X. Jin and P. Duan, Mater. Chem. Front., 2023, 7, 3259–3277 RSC
.
- J. Wade, J. R. Brandt, D. Reger, F. Zinna, K. Y. Amsharov, N. Jux, D. L. Andrews and M. J. Fuchter, Angew. Chem., Int. Ed., 2021, 60, 222–227 CrossRef CAS PubMed
.
- D. Reger, P. Haines, F. W. Heinemann, D. M. Guldi and N. Jux, Angew. Chem., Int. Ed., 2018, 57, 5938–5942 CrossRef PubMed
.
- J. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold and M. J. Fuchter, Chem. Sci., 2021, 12, 8589–8602 RSC
.
- J. M. Moreno-Naranjo, F. Furlan, J. Wang, S. T. J. Ryan, T. Matulaitis, Z. Xu, Q. Zhang, L. Minion, M. Di Girolamo, T. Jávorfi, G. Siligardi, J. Wade, N. Gasparini, E. Zysman-Colman and M. J. Fuchter, Adv. Mater., 2024, 36, 2402194 CrossRef CAS PubMed
.
- Q. Wang, H. Zhu, Y. Tan, J. Hao, T. Ye, H. Tang, Z. Wang, J. Ma, J. Sun, T. Zhang, F. Zheng, W. Zhang, W. Choi, W. C. H. Choy, D. Wu, X. W. Sun, K. Wang, Q. Wang, H. Zhu, Y. Tan, J. Hao, T. Ye, H. Tang, Z. Wang, J. Ma, J. Sun, T. Zhang, F. Zheng, W. Zhang, X. W. Sun, K. Wang, H. W. Choi, W. C. H. Choy and D. Wu, Adv. Mater., 2024, 36, 2305604 CrossRef CAS PubMed
.
- Y. H. Kim, Y. Zhai, H. Lu, X. Pan, C. Xiao, E. A. Gaulding, S. P. Harvey, J. J. Berry, Z. V. Vardeny, J. M. Luther and M. C. Beard, Science, 2021, 371, 1129–1133 CrossRef CAS PubMed
.
- M. P. Hautzinger, X. Pan, S. C. Hayden, J. Y. Ye, Q. Jiang, M. J. Wilson, A. J. Phillips, Y. Dong, E. K. Raulerson, I. A. Leahy, C. S. Jiang, J. L. Blackburn, J. M. Luther, Y. Lu, K. Jungjohann, Z. V. Vardeny, J. J. Berry, K. Alberi and M. C. Beard, Nature, 2024, 631, 307–312 CrossRef PubMed
.
- L. Zhang, H. X. Wang, S. Li and M. Liu, Chem. Soc. Rev., 2020, 49, 9095–9120 RSC
.
- Y. Gao, C. Ren, X. Lin and T. He, Front. Chem., 2020, 8, 541567 Search PubMed
.
- J. L. Ma, Q. Peng and C. H. Zhao, Chem. – Eur. J., 2019, 25, 15441–15454 CrossRef CAS PubMed
.
- G. Albano, A. Taddeucci, G. Pescitelli and L. Di Bari, Chem. – Eur. J., 2023, 29, e202301982 CrossRef CAS PubMed
.
- D. F. De Rosa, P. Stachelek, D. J. Black and R. Pal, Nat. Commun., 2023, 14, 1–11 Search PubMed
.
- S. P. Morcillo, D. Miguel, L. Álvarez de Cienfuegos, J. Justicia, S. Abbate, E. Castiglioni, C. Bour, M. Ribagorda, D. J. Cárdenas, J. M. Paredes, L. Crovetto, D. Choquesillo-Lazarte, A. J. Mota, M. C. Carreño, G. Longhi and J. M. Cuerva, Chem. Sci., 2016, 7, 5663–5670 RSC
.
- P. Reine, A. G. Campaña, L. Álvarez de Cienfuegos, V. Blanco, S. Abbate, A. J. Mota, G. Longhi, D. Miguel and J. M. Cuerva, Chem. Commun., 2019, 55, 10685–10688 RSC
.
- I. López.Sicilia, A. M. Ortuño, P. Reine, D. Otero, M. R. Martín-Romero, L. Camacho, L. Álvarez de Cienfuegos, A. Orte, J. J. Giner-Casares, D. Miguel and J. M. Cuerva, J. Mater. Chem. C, 2023, 11, 2591–2599 RSC
.
- L. Bustillo, T. Laino and T. Rodrigues, Chem. Sci., 2023, 14, 10378–10384 RSC
.
| This journal is © The Royal Society of Chemistry 2025 |