 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Common mechanism of dual emission in linearly-linked donor–acceptor-type thermally activated delayed fluorescence molecules†
Tomohiro
Ryu
a,
Arvydas
Ruseckas
b,
Masaki
Saigo
a,
Kiyoshi
Miyata
a,
Youichi
Tsuchiya
 c,
Hajime
Nakanotani
c,
Hajime
Nakanotani
 c,
Chihaya
Adachi
c,
Ifor D. W.
Samuel
b and
Ken
Onda
c,
Chihaya
Adachi
c,
Ifor D. W.
Samuel
b and
Ken
Onda
 *a
*a
aDepartment of Chemistry, Faculty of Science, Kyushu University, 744 Motooka, Nishi, Fukuoka 819-0395, Japan. E-mail: konda@chem.kyushu-univ.jp
bOrganic Semiconductor Centre, School of Physics and Astronomy, University of St Andrews, North Haugh, St Andrews, Fife, KY16 9SS, UK
cCenter for Organic Photonics and Electronics Research, Kyushu University, 744 Motooka, Nishi, Fukuoka 819-0395, Japan
First published on 3rd June 2025
Abstract
Linearly-linked donor–acceptor-type (D–A) thermally activated delayed fluorescence molecules have been expected to be used as efficient emitters in organic light emitting diodes. Despite their simple molecular structures, some of these molecules exhibit a complex dual emission mechanism due to their two conformers: quasi-coplanar (q-copl.) and perpendicular (perp.) conformers. We have investigated three molecules of this type: phenothiazine–triphenyltriazine, 9,9-dimethyl-9,10-dihydroacridine–triphenyltriazine, and phenoxazine–triphenyltriazine using picosecond time-resolved photoluminescence and femtosecond transient absorption spectroscopy measurements. We have revealed the dual emission mechanism common to the three molecules: after photoexcitation, in the q-copl. conformer, the second singlet excited state with local excitation character emits strong fluorescence, which decays in 3–7 ps as it relaxes to the lowest singlet excited state with charge transfer (CT) character. The CT state exhibits relatively weak fluorescence with a lifetime of tens to hundreds of picoseconds. In the perp. conformer, the excited state shows a pronounced CT character with a weaker oscillator strength reduced by two orders of magnitude, structural relaxation in about 4 ps and a slow decay in >1 ns. The dual emission intensity ratio is determined by the population ratio between the q-copl. and perp. conformers in the ground state. The difference in this intensity ratio between the three molecules is ascribed to the difference in relative energetic stability between the two conformers in the ground state. The emission mechanism common to the linearly-linked D–A molecules deepens the understanding of their photophysical properties and opens new pathways for the development of advanced photofunctional materials.
Introduction
Organic molecules that possess charge transfer (CT) excited states have recently attracted attention as promising materials for organic electronic devices. In these molecules, charge distribution within or between molecules is significantly localized by electronic transitions and the spatially separated transition orbitals lead to various functions. For example, thermally activated delayed fluorescence (TADF) arising from the small energy gap between singlet and triplet states in the CT excited states is used in organic light-emitting diodes (OLEDs).1–4 Near-infrared emission from the CT excited state with a large Stokes shift is used in bioimaging.5–7 Intermolecular CT excited states are used in organic photovoltaics.8,9 The simplest strategy to create molecules possessing CT excited states is to link electron donor and acceptor molecules by carbon single bonds, enabling intramolecular CT in the excited states, referred to as linearly-linked D–A molecules. The advantage of this type of molecule is that diverse functions can be easily achieved by appropriate selection of donor and acceptor molecules.Some linearly-linked D–A molecules exhibit dual emission caused by two emissive conformers in the excited state.10–12 Typical examples are a series of molecules bearing a phenothiazine (PTZ) as a donor.13–16 Tanaka et al. first reported a PTZ-donor D–A molecule exhibiting dual emission, using triphenyltriazine (TRZ) as the acceptor, named PTZ–TRZ (Fig. 1a).17 This molecule has two types of conformers in the excited state, a quasi-coplanar (q-copl.) or quasi-axial conformer (Fig. 1d), in which the bent donor and acceptor are arranged horizontally, and a perpendicular (perp.) or quasi-equatorial conformer (Fig. 1e), in which the planner donor and planar acceptor are arranged perpendicularly. This is attributed to the bending of the PTZ plane and the flexible dihedral angle between the donor and acceptor. They found that each emission originates from a different CT excited state and has different photophysical properties: only the perp. conformer exhibits TADF. D–A molecules analogous to PTZ–TRZ also exhibit dual emission from two conformers. Stavrou et al. demonstrated that 9,9-dimethyl-9,10-dihydroacridine–TRZ (DMAC–TRZ, Fig. 1b), which had not been previously discussed in the context of dual emission, exhibits dual emission and similar emission properties to PTZ–TRZ through a comprehensive investigation.18
Linearly-linked D–A molecules exhibiting dual emission have complex excited-state dynamics due to their two flexible conformers. Understanding the complex dynamics arising from the simple structure is important not only for their applications but also for fundamental photophysics. For PTZ–TRZ, Chen et al. investigated the conformational change between the two conformers from the time profile of each emission on the picosecond timescale and determined its time constant to be 1.8 ps in cyclohexane solution.19 We investigated the excited state dynamics of PTZ–TRZ and their solvent dependence by time-resolved measurements.20 The time-resolved photoluminescence (TR-PL) measurements revealed that the q-copl. conformer exhibited emission from a higher singlet excited state. The measurements also revealed that the photophysical properties of the two structures respond differently to the polarity of the solvent: a shorter emission lifetime and no TADF in high dielectric permittivity solution in the perp. conformer, and no significant solvent dependence in the q-copl. conformer. The visible transient absorption (TA) measurements revealed that the structural change from the q-copl. to perp. conformers occurs with the time constant of approximately 8 ps. For DMAC–TRZ, Franca et al. demonstrated the picosecond behavior of the excited state and its solvent dependence.21 The TA measurements revealed that the structural change from the q-copl. conformer to the perp. conformer occurs within 20 ps in cyclohexane solution, while solvation occurs within 17 ps in toluene solution. These previous studies suggest that the excited-state dynamics of linearly-linked D–A molecules involve complex picosecond dynamics; however, no spectra of each emission have been observed on the picosecond timescale, leading to an incomplete understanding of the detailed dynamics. Moreover, these investigations focused on specific molecules; thus, the comprehensive dynamics common to more D–A molecules remain unexplored.
In order to explore the common excited-state dynamics in linearly-linked D–A molecules on the picosecond timescale, we have investigated three prototypical molecules: PTZ–TRZ and DMAC–TRZ, which are typical molecules exhibiting dual emission, and phenoxazine–TRZ (PXZ–TRZ, Fig. 1c) as an analogue using picosecond TR-PL and femtosecond TA spectroscopies. We found that all three molecules exhibit dual emission originating from the two conformers, q-copl. and perp. After photoexcitation, in the q-copl. conformer, the second singlet excited (S2) state with localized excitation (LE) character emits strong fluorescence with a lifetime of 3–7 ps and simultaneously relaxes to the lowest singlet excited state (S1) with CT character, which exhibits weak fluorescence with a lifetime of tens to hundreds of picoseconds. In the perp. conformer, the structural relaxation in the S1 state with CT character occurs with a lifetime of 4 ps and then the S1 state after the relaxation exhibits long-lived fluorescence. The relative emission intensity between the q-copl. and perp. conformers is determined by the stability of the q-copl. conformer in the ground (S0) state. The results propose a new energy diagram and emission mechanism that would be common to similar linearly-linked D–A molecules.
Experimental
Sample preparation
PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ were synthesized as described in the previous study.17,22,23Steady-state PL spectroscopy and UV-vis absorption spectroscopy
Steady-state photoluminescence (PL) spectra were measured using a spectrofluorometer (JASCO FP-8300). UV-vis absorption spectra were obtained with a spectrophotometer (JASCO V-780). The samples for the steady-state PL and UV-vis absorption measurements were prepared using 0.1 mM and 0.01 mM toluene solution, respectively.TR-PL spectroscopy
The TR-PL spectra were measured using a streak camera system (Hamamatsu C6860, response function: 2 ps). The samples were excited by a third harmonic (343 nm) of the output of a Yb:KGW regenerative amplifier (Light Conversion PHAROS, central wavelength: 1030 nm, pulse duration: 200 fs) at a pulse repetition rate of 100 kHz. The optical path length of the solution cell was 1 cm. The measurements were conducted while stirring the solution to avoid damage to the sample. All samples were prepared using 0.1 mM toluene or dichloromethane (DCM) solution. The obtained TR-PL spectra were analyzed by the global analysis using Glotaran.24Femtosecond TA spectroscopy
The femtosecond TA measurements were performed using home-built pump–probe setups. The Yb:KGW regenerative amplifier at a pulse repetition rate of 1 kHz (pulse energy: 1 mJ per pulse) was used as the light source. The output pulse was split into two optical paths using a beam splitter. One of the split outputs was fed into an optical parametric amplifier (OPA, Light Conversion ORPHEUS), where the output wavelength was tuned to 740 nm, 720 nm, and 690 nm. Each light beam was converted using a β-BaB2O4 (BBO) crystal to its second harmonic at 370 nm (for DMAC–TRZ), 360 nm (for PTZ–TRZ), and 345 nm (for PXZ–TRZ), respectively, and used as pump pulses. The other output was focused on a sapphire crystal (3 mm in thickness), generating white light from 500 to 950 nm, which was used as the probe pulse. The magic angle (∼54.7°) was adopted between the pump and probe polarizations. The probe pulse that passed through the sample was dispersed by a polychromator (JASCO CT-10, 300 grooves/500 nm) and detected using a multichannel CMOS sensor-based system (UNISOKU USP-PSMM-NP). The pump pulse fluence at the sample position was 0.31 mJ cm−2 for PTZ–TRZ, 0.23 mJ cm−2 for DMAC–TRZ, and 0.20 mJ cm−2 for PXZ–TRZ. All samples were prepared using 0.1 mM toluene solution in a 1 mm cell. The obtained TA spectra were analyzed by the global analysis using Glotaran.24Quantum chemical calculations
Quantum chemical calculations were performed using the Gaussian 16 C.01 package.25 The S0 state was calculated based on density functional theory (DFT), and the singlet excited states were calculated based on time-dependent (TD)-DFT. The solvent effect was examined by using the polarizable continuum model of toluene solution (dielectric constant: 2.38). We employed the 6-31G(d,p) basis set and B3LYP functional.Results and discussion
Steady-state photophysical properties
Fig. 2a shows the UV-vis absorption and PL spectra of PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ in toluene solution. The extinction coefficient of PTZ–TRZ in the low energy absorption band with a peak at 350 nm is about twice higher than those in the other two molecules. All the PL spectra show dual emission at 409 nm and 580 nm for PTZ–TRZ, at 413 nm and 500 nm for DMAC–TRZ, and at 409 nm and 560 nm for PXZ–TRZ (Fig. 2b). The PL quantum yields in toluene solution for PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ are 20%, 79%, and 30%, respectively.20,22,26The dual emissions observed for PTZ–TRZ and DMAC–TRZ are consistent with the previous reports, whereas that for PXZ–TRZ is newly observed. To confirm this, we conducted concentration-dependent steady-state PL measurements for the three D–A molecules in 0.01 mM, 0.1 mM, and 1 mM toluene solutions (Fig. S1–S3, ESI†). Note that the decrease of the relative intensity of the peak in the short-wavelength range with increasing concentration for DMAC–TRZ and PXZ–TRZ is attributed to self-absorption owing to a large overlap between the absorption and emission spectra in the short-wavelength range as shown in Fig. 2a.
According to the previous studies on PTZ–TRZ and DMAC–TRZ, the emission in the short-wavelength range is assigned to the emission from the q-copl. conformer.17–21 Thus, this result indicates that all three molecules have both q-copl. and perp. conformers in the excited state.
TR-PL spectra in the picosecond range
To explore the emission mechanism at the earliest stage, we conducted TR-PL measurements with a time resolution of ∼1 ps. Fig. 3a–c shows the TR-PL spectra of PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ, respectively. In this time region, all the molecules clearly exhibit dual emission at 410 nm and 557 nm in PTZ–TRZ, at 406 nm and 497 nm in DMAC–TRZ, and at 400 nm and 543 nm in PXZ–TRZ. Because the shorter wavelength emission peaks in the spectra at 0.1 ps and 150 ps for all the molecules resemble those in the steady-state PL spectra (a comparison of the normalized spectra is shown in Fig. S4, ESI†), we assigned these peaks to the emission from the q-copl. conformer. Based on our previous report, the slight red shift from 0.1 ps to 150 ps (Fig. S5, ESI†) is attributed to the internal conversion from a higher singlet excited state to the S1 state of the q-copl. conformers associated with vibrational relaxation and solvation. In the emission of the perp. conformer, the red shift was observed in PXZ–TRZ, while it was not observed in the other molecules because the emission is overlapped by the shoulder of the emission from the q-copl. conformer. Unlike the emissions from the q-copl. conformers, the changes in the emission intensity of the perp. conformers were small. In addition, the finding that the maximum intensities of emission appear immediately after photoexcitation (at 0.1 ps) for both conformers indicates that the two conformers are excited simultaneously for all the molecules.Origin of the emission immediately after photoexcitation
To clarify the origin of the emission immediately after photoexcitation, accompanied by a red shift in both conformers, we calculated the oscillator strengths between the S1 to S5 states and the S0 state in the q-copl. and perp. conformers for the three molecules using the optimized structure of the S1 state and the values are summarized in Table 1. In the q-copl. conformer, the oscillator strengths of the S1 state for the three molecules were almost zero, while those of the S2 state were 0.74, 0.78, and 0.67 for PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ, respectively. Thus, the strong emission from the q-copl. conformer immediately after photoexcitation is attributed to the emission from the S2 state. To estimate the absorption spectra, the oscillator strengths of the same transitions were calculated using the optimized structure of the S0 state (Table S1, ESI†). As a result, the oscillator strengths of the S1 state were almost zero, while those of the S2 state were approximately 0.9 for the three molecules, indicating that the absorption peak of the q-copl. conformer is assigned to the transition to the S2 state for each molecule.| f (S1 state) | S1 | S2 | S3 | S4 | S5 | |
|---|---|---|---|---|---|---|
| PTZ–TRZ | q-copl. | 0 | 0.74 | <0.01 | 0 | <0.01 |
| perp. | 0 | 0 | <0.01 | 0 | 0 | |
| DMAC–TRZ | q-copl. | 0 | 0.78 | 0 | 0.10 | 0.25 |
| perp. | 0 | 0 | <0.01 | <0.01 | 0 | |
| PXZ–TRZ | q-copl. | 0 | 0.67 | <0.01 | <0.01 | 0.21 |
| perp. | 0 | 0 | <0.01 | <0.01 | 0 | |
Based on the calculated NTOs (natural transition orbitals) shown in Fig. S8, S10 and S12 (ESI†), the large oscillator strength in the S2 state arises from the large overlap between hole and electron orbitals associated with the transition. This means that the S2 state is dominated by LE character (1LEq-copl. state). In contrast, the S1 state has a prominent CT character (1CTq-copl. state) as indicated by the small overlap between hole and electron orbitals. Because the energies of the S2 and S1 states are close as shown in Fig. S6 (ESI†), these singlet excited states are expected to acquire mixed LE–CT character. This is consistent with the quantum chemical calculations presented by Stavrou et al. for DMAC–TRZ.18 In the perp. conformer, the oscillator strengths of the three molecules were almost zero primarily due to the small overlap of its transition orbitals (Fig. S9, S11, and S13, ESI†). We concluded from these results that the initial emission from the perp. conformer is assigned to the S1 state dominated by CT character (1CTperp. state). The redshift of this emission, which appears prominently for PXZ–TRZ, is presumably ascribed to the structural relaxation in the 1CTperp. state. Note that the emission from the perp. conformer is attributed to the emission from a slightly twisted structure caused by fluctuations in the dihedral angle between the donor–acceptor, as previously reported for DMAC–TRZ.26
Time constants of the relaxation processes
We estimated the time constants of the fast processes in the emission mechanism using the global analysis for the TR-PL spectra. The global analysis was performed in the wavelength region corresponding to the emission of the q-copl. conformer within the dual emission: 370–480 nm in PTZ–TRZ, 395–450 nm in DMAC–TRZ, and 398–460 nm in PXZ–TRZ. Note that, in the case of DMAC–TRZ and PXZ–TRZ, the wavelength region of Raman scattering was not included in the analysis: 370–395 nm in DMAC–TRZ and 370–398 nm in PXZ–TRZ. We performed the analysis using a sequential two-state model for the emission of the q-copl. conformer assuming that two evolution associated spectra (EAS1 and EAS2) are assigned to the 1LEq-copl. and 1CTq-copl. states, respectively (Fig. S14, ESI†). These results are shown in Fig. 4 and the values are summarized in Table S2 (ESI†). In PTZ–TRZ, the time constant of EAS1 (τEAS1) was estimated to be 5.2 ps (Fig. 4a and b). Considering that the 1LEq-copl. state exhibits strong emission, this time constant is attributed to a relaxation process from the 1LEq-copl. state to the 1CTq-copl. state including vibration relaxation and solvation. For DMAC–TRZ and PXZ–TRZ, the τEAS1 values were estimated to be 6.8 ps (Fig. 4c and d) and 3.4 ps (Fig. 4e and f), respectively. These results indicate that the q-copl. conformer for all three molecules has the same emission mechanism: immediately after photoexcitation, the 1LEq-copl. state emits strong fluorescence, which decays in 3–7 ps as it acquires CT character. It should be noted that since the emission from the q-copl. conformer had not fully decayed within the experimental time window up to 150 ps, the estimated time constants of EAS2 (τEAS2) do not accurately represent the lifetime of the emission from the 1CTq-copl. state.The global analysis was also performed for the emission of PXZ–TRZ in the wavelength region corresponding to the emission of the perp. conformer within the dual emission (480–652 nm). We performed the analysis using the same model of the emission of the q-copl. conformer assuming that EAS1 and EAS2 are assigned to the 1CTq-copl. states before and after structural relaxation, respectively (Fig. S14, ESI†). The results are shown in Fig. S15 and Table S2 (ESI†). The τEAS1 was estimated to be 4.0 ps, indicating that the structural relaxation in the 1CTperp. state occurs at 4 ps. The τEAS2 was longer than the time window, which was assigned to the fluorescence of the 1CTperp. state after relaxation. For the other molecules, the global analysis for the emission of the perp. conformer was not performed because the emission of the perp. conformer immediately after photoexcitation was largely overlapped by the shoulder of the emission of the q-copl. conformer. However, the structural relaxation with a time constant similar to that of PXZ–TRZ may also occur in the other molecules.
Since the fluorescence of CT states is highly sensitive to the polarity of the solvent, TR-PL measurements of the q-copl. conformer were conducted in DCM, which has a much higher dielectric permittivity (ε = 9.1) compared to toluene (ε = 2.38) (Fig. S16, ESI†). Global analysis of the TR-PL spectra in DCM was performed in the same manner as in toluene solution and the fast decay component was determined to be 2–3 ps for all three molecules. This result indicates that the relaxation process from the LE state to the CT state in the q-copl. conformer is accelerated in the solvent of higher dielectric permittivity. A similar trend has been observed in molecules with twisted intramolecular charge transfer (TICT) in the excited state and is reported to be due to the stabilization of the CT state, which makes the potential energy surface steeper and the activation barrier from the LE to CT state smaller.27
Intensity ratios of the dual emission
Although the three molecules have almost the same time constant for their respective emissions, the intensity ratios and the time evolutions of the dual emission ratio are significantly different. In PTZ–TRZ, the emission ratio of the q-copl. conformer to the perp. conformer, IE,q-copl./IE,perp., was estimated to be approximately 141 immediately after photoexcitation and it decreased to 2.8 at 150 ps. In DMAC–TRZ, IE,q-copl./IE,perp. was estimated to be 13.9 immediately after photoexcitation and it decreased to 0.81 at 150 ps. In PXZ–TRZ, IE,q-copl./IE,perp. was estimated to be 1.5 immediately after photoexcitation and it decreased to 0.18 at 150 ps. These values of IE,q-copl./IE,perp. immediately after photoexcitation are summarized in Table 2.| I E,q-copl./IE,perp. | N q-copl./Nperp. | f q-copl./fperp. | |
|---|---|---|---|
| PTZ–TRZ | 141 | 2.9 | 47.9 |
| DMAC–TRZ | 13.9 | 0.085 | 164 |
| PXZ–TRZ | 1.5 | 0.012 | 123 |
Given that the emission mechanism is the same for the three molecules, the emission intensity immediately after photoexcitation should be determined by the population in the S0 state before photoexcitation. To estimate the population in the S0 state, we first calculated the S0 energies of the q-copl. conformer with respect to that of the perp. conformer (ΔE) for the three molecules by DFT calculations and the values are summarized in Fig. 5. The values of ΔE were −28 meV, 64 meV, and 114 meV in PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ, respectively. Note that we confirmed that these values do not largely depend on the functional in the calculations (Table S3, ESI†). Because the less the energy of a state is, the more the state is populated in thermal equilibrium, this order of ΔE is consistent with the order of magnitude of the q-copl./perp. emission ratio immediately after photoexcitation in the dual emission. To confirm whether the two structures are in thermal equilibrium in the S0 state, the potential energy surfaces along the D–A dihedral rotation of the three molecules in the S0 state were calculated (Fig. S17, ESI†). The activation energy from the q-copl. to perp. conformer was 44–128 meV and that from the perp. to q-copl. conformer was 109–165 meV (Table S4, ESI†). These results indicate that the structural change over the activation energy can occur at room temperature. The stability of the q-copl. conformer is influenced by the fold angle of the donor plane and the bent angle of the donor with respect to the acceptor within the q-copl. conformer. Further details are provided in the ESI,† SI-8.
 | ||
| Fig. 5 The calculated S0 energies of the q-copl. conformer with respect to that of the perp. conformer for PTZ–TRZ, DMAC–TRZ and PXZ–TRZ. | ||
From the values of ΔE, we calculated the population ratio of the q-copl. and perp. conformers of the three molecules in the S0 state. Based on the Boltzmann distribution, the ratio of their populations, Nq-copl./Nperp., can be expressed using the following equation:28
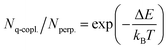 | (1) |
TA spectra and their analyses
To confirm the early emission dynamics derived from the TR-PL spectra, the TA spectra of PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ were measured in toluene solution, as shown in Fig. 6a, d and g, respectively. A common feature of the TA spectra of the three molecules is that the spectral intensity slightly increases as a function of delay time. Simultaneously, the spectral shape varies but the degree of variation depends on the molecule. PTZ–TRZ, with a higher population of the q-copl. conformer in the S0 state, showed a significant change from the broad featureless spectrum to that with vibronic progression. DMAC–TRZ showed a similar trend but to a lesser extent, while PXZ–TRZ showed only minor changes. These trends can be more obviously seen in the normalized TA spectra shown in Fig. S19 (ESI†).These results indicate that the higher the population of the q-copl. conformer in the S0 state is, the more pronounced the spectral changes are. According to the emission mechanism proposed above, immediately after photoexcitation, the 1LEq-copl. and 1CTperp. states are simultaneously populated. Subsequently the 1LEq-copl. state undergoes relaxation to the 1CTq-copl. state with a time constant of 3–7 ps and structural relaxation to the 1CTperp. state. Based on this mechanism, the broad spectra immediately after photoexcitation in the TA spectra are attributed to the coexistence of the 1LEq-copl. and 1CTperp. states, while the subsequent spectra with vibrational progression correspond to the coexistence of the 1CTq-copl. and 1CTperp. states. Furthermore, in all three molecules, the total area of each spectrum does not decrease up to 1 ns, indicating that the excited state decay is negligible on the sub-nanosecond timescale. This finding agrees well with the above conclusion that the fast decay of fluorescence for the q-copl. conformer is attributed to the decay from the high oscillator strength state (1LEq-copl.) to the almost zero oscillator strength state (1CTperp.) with respect to the S0 state.
We conducted the global analysis of the time evolutions of the TA spectra up to 100 ps using a sequential two-state model based on the results of TR-PL and TA spectroscopy shown in Fig. S20 (ESI†). In this model, EAS1 was assigned to the coexistence state of the 1LEq-copl. and 1CTperp. states before structural relaxation, and EAS2 was assigned to the coexistence state of the 1CTq-copl. and 1CTperp. states after structural relaxation. The results of PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ are shown in Fig. 6b, c, e, f, h and i, respectively. The spectral change from EAS1 to EAS2 was pronounced for PTZ–TRZ and DMAC–TRZ, and only slightly for PXZ–TRZ. The τEAS1 values were 6.6 ps, 5.2 ps, and 6.9 ps for PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ, respectively, and the τEAS2 values were longer than 100 ps in all three molecules because the spectra had not fully decayed within the time window up to 100 ps. These results indicate that the time constant at which the dominant transient species transitions from the 1LEq-copl. and the 1CTperp. state before structural relaxation to the 1CTq-copl. and the 1CTperp. state after structural relaxation is 5–7 ps. These time constants are consistent with those estimated by TR-PL, corresponding to the relaxation from the 1LEq-copl. to the 1CTq-copl. state and the structural relaxation in the 1CTperp. state.
Dual emission mechanism common to the three molecules
The common dual emission mechanism we proposed here for all three molecules is depicted in Fig. 7. In the S0 state, both q-copl. and perp. conformers coexist, and the ratio of their presence depends on the energetic stability of the q-copl. conformer. Upon UV irradiation, both conformers are excited to the higher excited state ((i) in Fig. 7). After that, in the q-copl. conformer, the 1LEq-copl. state (S2 state) with high oscillator strength from the S0 state is created and emits strong fluorescence centered at approximately 400 nm (ii). Simultaneously, internal conversion occurs to the 1CTq-copl. state (S1 state) associated with vibrational relaxation and solvation with a time constant of 3–7 ps (iii). Subsequently, the 1CTq-copl. state emits weak fluorescence (iv). Fast PL decay within a few picoseconds indicates electronic and structural relaxation of the excited state as it acquires some CT character and its oscillator strength decreases. In the perp. conformer, after photoexcitation, the structural relaxation in the 1CTperp. state (S1 state) occurs with a time constant of 4 ps (v). Then the 1CTperp. state with low oscillator strength emits long-lifetime fluorescence (vi). The emission intensity ratio between the q-copl. and perp. conformers is determined by the stability of the q-copl. conformer in the S0 state. The structural change from the q-copl. conformer to the perp. conformer, which is reported in the previous studies, could not be clearly observed in our measurements. However, we cannot rule out the possibility that the structural change occurs along with the excited-state processes shown here. | ||
| Fig. 7 The schematic of the proposed common emission mechanism for the three linearly-linked D–A molecules exhibiting dual emission: PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ. | ||
Conclusions
We have investigated the dual emission mechanism in the three linearly-linked D–A type TADF molecules PTZ–TRZ, DMAC–TRZ, and PXZ–TRZ using picosecond TR-PL spectroscopy, femtosecond TA spectroscopy, and quantum chemical calculations. We found that not only PTZ–TRZ and DMAC–TRZ but also PXZ–TRZ exhibit dual emission in the steady-state PL spectra. The TR-PL spectra revealed that all three molecules more clearly exhibit dual emission in the picosecond region. In the q-copl. conformer, the emission exhibits a red shift as a function of delay time in all three molecules, which is attributed to the relaxation from the higher singlet excited state to the S1 state. In contrast, in the perp. conformer, a red shift in the emission was observed only in PXZ–TRZ, which is attributed to the structural relaxation of the S1 state. The quantum chemical calculations revealed that the higher excited state exhibiting fluorescence in the q-copl. conformer of each molecule was the 1LEq-copl. state (S2 state). The time constant of the relaxation to the 1CTq-copl. state (S1 state) in the q-copl. conformer is 3–7 ps and that of structural relaxation in the 1CTperp. state (S1 state) in the perp. conformer is 4 ps. The emission intensity of the q-copl. conformer immediately after photoexcitation relative to that of the perp. conformer is determined by the population ratio of the two conformers in the S0 state. The TA spectra of the three molecules changed with a time constant of 5–7 ps, accompanied by varying degrees of change for each molecule. These changes are assigned to the relaxation from the mixed state of the 1LEq-copl. and 1CTperp. states to the mixed state of the 1CTq-copl. and 1CTperp. states. The dual-emission mechanism we proposed here is presumably applicable to other linearly-linked D–A type TADF molecules. These studies have revealed the previously overlooked photophysical properties of D–A molecules through a detailed analysis of their physical properties using spectroscopic techniques. Our findings provide a more profound comprehension of the photophysical properties of D–A molecules and underscore the significance of meticulous spectroscopic analysis in elucidating intricate excited-state dynamics. These insights pave the way for the development of new types of photofunctional materials.Author contributions
K. O. conceived and supervised the project. T. R. contributed to conceptualization, methodology, investigation, and writing the original draft. M. S. investigated the data. K. M. contributed to conceptualization, methodology, and investigation. A. R. and I. S. provided instruments and contributed to conceptualization and investigation. Y. T., H. N. and C. A. prepared all molecules. All authors contributed to the review and editing of the manuscript and critically commented on the project.Data availability
The data supporting this article have been included as part of the ESI and are also available at the link given in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Numbers JP23H01977, JP23H04631, JP23K20039, and JP24KJ1791. The work at the University of St Andrews was supported by the Engineering and Physical Sciences Research Council of the UK through grant ER/R035164/1. We acknowledge Prof. Nobuhiro Yanai at Kyushu University for the measurement of UV-vis absorption spectra.References
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- V. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias and A. P. Monkman, Highly efficient TADF OLEDs: How the emitter–host interaction controls both the excited state species and electrical properties of the devices to achieve near 100% triplet harvesting and high efficiency, Adv. Funct. Mater., 2014, 24, 6178–6186 CrossRef CAS.
- Y. Kitamoto, T. Namikawa, D. Ikemizu, Y. Miyata, T. Suzuki, H. Kita, T. Sato and S. Oi, Light blue and green thermally activated delayed fluorescence from 10 H -phenoxaborin-derivatives and their application to organic light-emitting diodes, J. Mater. Chem. C, 2015, 3, 9122–9130 RSC.
- H. Peng, Y. Xu, C. Zhou, R. Pei, J. Miao, H. Liu and C. Yang, Donor extension on Spiro-acridine enables highly efficient TADF-OLEDs with relieved efficiency roll-off, Adv. Funct. Mater., 2023, 33, 2211696 CrossRef CAS.
- W. Chen, S. Xu, J. J. Day, D. Wang and M. Xian, A general strategy for development of near-infrared fluorescent probes for bioimaging, Angew. Chem., 2017, 129, 16838–16842 CrossRef.
- X. Zhang, T. Ren, F. Yang and L. Yuan, Rational design of far red to near-infrared rhodamine analogues with huge Stokes shifts for single-laser excitation multicolor imaging, Chin. Chem. Lett., 2021, 32, 3890–3894 CrossRef CAS.
- H. Li, Y. Shen, Z. Dong, W. Li and L. Yuan, Rational design of tunable near-Infrared oxazine probe with large Stokes shift for leucine aminopeptidase detection and imaging, Chem. – Asian J., 2023, 18, e202300701 CrossRef CAS PubMed.
- H. Hu, P. C. Y. Chow, G. Zhang, T. Ma, J. Liu, G. Yang and H. Yan, Design of Donor Polymers with Strong Temperature-Dependent Aggregation Property for Efficient Organic Photovoltaics, Acc. Chem. Res., 2017, 50, 2519–2528 CrossRef CAS PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- K. Wang, C.-J. Zheng, W. Liu, K. Liang, Y.-Z. Shi, S.-L. Tao, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Avoiding energy loss on TADF emitters: Controlling the dual conformations of D–A structure molecules based on the pseudoplanar segments, Adv. Mater., 2017, 29, 1701476 CrossRef PubMed.
- W. Li, X. Cai, B. Li, L. Gan, Y. He, K. Liu, D. Chen, Y.-C. Wu and S.-J. Su, Adamantane-substituted acridine donor for blue dual fluorescence and efficient organic light-emitting diodes, Angew. Chem., 2019, 131, 592–596 CrossRef.
- D. de Sa Pereira, D. R. Lee, N. A. Kukhta, K. H. Lee, C. L. Kim, A. S. Batsanov, J. Y. Lee and A. P. Monkman, The effect of a heavy atom on the radiative pathways of an emitter with dual conformation, thermally-activated delayed fluorescence and room temperature phosphorescence, J. Mater. Chem. C, 2019, 7, 10481–10490 RSC.
- K. Wang, Y.-Z. Shi, C.-J. Zheng, W. Liu, K. Liang, X. Li, M. Zhang, H. Lin, S.-L. Tao, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Control of Dual Conformations: Developing Thermally Activated Delayed Fluorescence Emitters for Highly Efficient Single-Emitter White Organic Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2018, 10, 31515–31525 CrossRef CAS PubMed.
- S. Xiang, R. Guo, Z. Huang, X. Lv, S. Sun, H. Chen, Q. Zhang and L. Wang, Highly efficient yellow nondoped thermally activated delayed fluorescence OLEDs by utilizing energy transfer between dual conformations based on phenothiazine derivatives, Dyes Pigm., 2019, 170, 107636 CrossRef CAS.
- X. Song, S. Shen, M. Lu, Y. Wang and Y. Zhang, Trifluoromethyl enable high-performance single-emitter white organic light-emitting devices based on quinazoline acceptor, Chin. Chem. Lett., 2024, 35, 109118 CrossRef CAS.
- X. Tian, S. Xiao, J. Sun, J. Yan, G. Li, B. Zhao, Y. Miao, L. Wang, H. Wang and D. Ma, Dual fluorescence induced thermally activated delayed fluorescence materials based on 2,4-Diphenylthieno[3,2-d]pyrimidine with an efficient single-molecular white electroluminescence, Chem. Eng. J., 2024, 485, 149692 CrossRef CAS.
- H. Tanaka, K. Shizu, H. Nakanotani and C. Adachi, Dual Intramolecular Charge-Transfer Fluorescence Derived from a Phenothiazine–Triphenyltriazine Derivative, J. Phys. Chem. C, 2014, 118, 15985–15994 CrossRef CAS.
- K. Stavrou, L. G. Franca, T. Böhmer, L. M. Duben, C. M. Marian and A. P. Monkman, Unexpected quasi-axial conformer in thermally activated delayed fluorescence DMAC-TRZ, pushing green OLEDs to blue, Adv. Funct. Mater., 2023, 33, 202300910 CrossRef.
- D.-G. Chen, T.-C. Lin, Y.-A. Chen, Y.-H. Chen, T.-C. Lin, Y.-T. Chen and P.-T. Chou, Revisiting Dual Intramolecular Charge-Transfer Fluorescence of Phenothiazine–triphenyltriazine Derivatives, J. Phys. Chem. C, 2018, 122, 12215–12221 CrossRef CAS.
- T. Ryu, K. Miyata, M. Saigo, Y. Shimoda, Y. Tsuchiya, H. Nakanotani, C. Adachi and K. Onda, Solvent-dependent dual emission processes in charge-transfer excited states of phenothiazine–triphenyltriazine conformers, Chem. Phys. Lett., 2022, 809, 140155 CrossRef CAS.
- L. G. Franca, A. Danos, R. Saxena, S. Kuila, K. Stavrou, C. Li, S. Wedler, A. Köhler and A. P. Monkman, Exploring the Early Time Behavior of the Excited States of an Archetype Thermally Activated Delayed Fluorescence Molecule, J. Phys. Chem. Lett., 2024, 15, 1734–1740 CrossRef CAS PubMed.
- H. Tanaka, K. Shizu, H. Miyazaki and C. Adachi, Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative, Chem. Commun., 2012, 48, 11392–11394 RSC.
- W.-L. Tsai, M.-H. Huang, W.-K. Lee, Y.-J. Hsu, K.-C. Pan, Y.-H. Huang, H.-C. Ting, M. Sarma, Y.-Y. Ho, H.-C. Hu, C.-C. Chen, M.-T. Lee, K.-T. Wong and C.-C. Wu, A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices, Chem. Commun., 2015, 51, 13662–13665 RSC.
- J. J. Snellenburg, S. Laptenok, R. Seger, K. M. Mullen and I. H. M. van Stokkum, Glotaran: A Java-Based Graphical User Interface for the R Package TIMP, J. Stat. Softw., 2012, 49, 1–22 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsujiet al., Gaussian 16, Revision C.01, 2016, Gaussian Inc., Wallingford CT Search PubMed.
- Y. Shimoda, K. Miyata, M. Saigo, Y. Tsuchiya, C. Adachi and K. Onda, Intramolecular-rotation driven triplet-to-singlet upconversion and fluctuation induced fluorescence activation in linearly connected donor-acceptor molecules, J. Chem. Phys., 2020, 153, 204702 CrossRef CAS PubMed.
- P. Changenet, P. Plaza, M. M. Martin and Y. H. Meyer, Role of intramolecular torsion and solvent dynamics in the charge-transfer kinetics in triphenylphosphine oxide derivatives and DMABN, J. Phys. Chem. A, 1997, 101, 8186–8194 CrossRef CAS.
- S. A. C. McDowell, A simple derivation of the Boltzmann distribution, J. Chem. Educ., 1999, 76, 1393 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp01226k |
| This journal is © the Owner Societies 2025 |





