Open Access Article
Open Access ArticleTheoretical understanding and prediction of metal-doped CeO2 catalysts for ammonia dissociation†
Yongjie
Shen
 a,
Wongsathorn
Kaewraung
b and
Min
Gao
*a
a,
Wongsathorn
Kaewraung
b and
Min
Gao
*a
aInstitute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Sapporo 001-0021, Japan. E-mail: gaomin@icredd.hokudai.ac.jp
bGraduate School of Chemical Sciences and Engineering, Hokkaido University, Sapporo 060-8628, Japan
First published on 25th February 2025
Abstract
Ammonia plays a critical role in energy and environmental catalysis, particularly in ammonia dissociation reactions. Understanding the adsorption and dissociation of ammonia-related species on catalysts is essential for the development of new chemical reactions and high-performance catalysts. However, establishing the relationship between catalyst properties and the adsorption of dissociated species remains challenging, particularly for metal oxide catalysts. This study employs density functional theory calculations to investigate the adsorption properties of ammonia and dissociated intermediate species on metal-doped CeO2. Through a feature correlation heat map, certain descriptors, such as single atom formation energy, gaseous atom formation heat, valence band maximum, and work function, were determined to exhibit a strong linear relationship with the adsorption properties of NHx species. As deduced from the density of states properties and orbital theory, it is also found that the energy difference between the lowest unoccupied orbital of the metal and the highest occupied orbital of ammonia, has a good relationship with the adsorption energy of NH3.
1. Introduction
Ammonia (NH3) has attracted considerable attention in the fields of energy and environmental catalysis owing to its high hydrogen content and energy density, making it a promising renewable energy source.1–4 The synthesis of NH3 from N2 and H2 has been extensively studied for a century, tracing back to the development of the Haber–Bosch reaction.5–7 Additionally, recent advancements in NH3 decomposition technology for hydrogen production have demonstrated significant potential for hydrogen energy storage and transportation.8–10 In the field of environmental catalysis, NH3 selective catalytic reduction (NH3-SCR)11–13 and NH3 selective catalytic oxidation (NH3-SCO)14–16 technologies are widely employed as key measures to address exhaust emissions from industrial and mobile sources. In response to the escalating energy crisis and environmental challenges, researchers have explored the electrochemical reduction of nitrate (NO3−) in solution17–19 to produce NH3, as well as the electrochemical coupling of C–N bonds in CO2 and NH3 to synthesize chemicals with industrial value.20–22 These technologies involve NH3 adsorption and dissociation reactions (NH3 ↔ NH2–H ↔ NH–2H ↔ N–3H) on catalysts, which are crucial in determining the reaction rate and have become the focus research in this field.Analysis of the adsorption and dissociation properties of catalysts for NH3-related species was conducted using temperature-programmed desorption (TPD),23in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS),24 and adsorption penetration25 tests for NH3. Hahn et al. demonstrated that Fe species enhanced the adsorption of NH3 over H+-exchanged beta zeolite molecular sieves through TPD experiments.26 Zhang et al. used in situ DRIFTS to show that Cu sites on TiO2 promoted the conversion of NH3 into NO2 species, which is an important participant in the fast SCR reaction.27 They also proved that S doping enhances the NH3 adsorption capacity of the Fe2O3/TiO2 catalyst through adsorption penetration testing.28 Although various characterization methods exist, most of them primarily examine the NH3 adsorption properties of the catalyst, making it challenging to clarify the adsorption effects of intermediate reaction species on the catalyst surface. Additionally, experimental screening of catalysts for high activity is typically costly and time-consuming. Conversely, screening the adsorption properties of catalysts and reaction intermediates with theoretical calculations can greatly reduce these costs.
Theoretical calculations are commonly used to complement experimental findings in NH3 catalytic reactions.29–34 Zhang et al. performed theoretical calculations to compare the adsorption sites of NH3 on Ag/CeSnOx catalysts and discovered that NH3 is more readily adsorbed on Ag as a Lewis acid site.35 Using theoretical calculation, Jiang et al. demonstrated that K species reduce the adsorption energy of NH3 on the catalyst surface.36 Liu et al. compared the reaction paths of NH3 decomposing into N2 and H2 on Ni(111) and Ni/CeO2 catalysts and confirmed that CeO2 enhances the dispersion of Ni clusters, thereby reducing the reaction activation energy.37 With the advancement of computational power, theoretical calculations are increasingly utilized to screen catalysts and identify factors that influence catalytic efficiency.38–44 Hansgen et al. screened bimetallic alloy catalysts using the adsorption energy of N atoms and alloys as descriptors. They predicted that catalysts with Ni–Pt–Pt surface chemistry exhibit excellent catalytic performance for hydrogen production by NH3 decompostion.45 Similarly, Yang et al. employed high-throughput theoretical calculations to screen metal catalysts for high activity in the NH3-SCO reaction. They found that the binding energy of N and O on metals serves as a descriptor for catalytic performance, and predicted that Cu(100) and Pt(111) catalysts possess the highest activity.46 Zheng et al. investigated the factors influencing the activation energy of transition metal-doped TiO2 catalysts to dissociate NH3 into NH2 and H species, as well as the subsequent activation energy of reaction with NO to form N2O, and found that the activity of surface oxygen has a significant impact.47 Currently, establishing the relationship between catalyst properties and dissociated species adsorption through a single descriptor is challenging for low-cost and readily available metal oxide catalysts in NH3 dissociation reactions. Therefore, exploring the factors influencing the interaction between metal oxide catalysts and NH3-related species is crucial to facilitate more effective catalyst screening.
In this study, the effects of transition metal doping on cerium dioxide (CeO2) and on NH3 dissociation in gas–solid interface thermal catalytic reaction were theoretically investigated. CeO2 is commonly used in NH3 catalytic reactions owing to its affordability, widespread availability, excellent dispersibility of active sites, and high oxygen storage capacity.48–50 The impact of metal doping on the local distortion and chemical properties of the CeO2 structure was explored. The adsorption positions and energies of species that participate in NH3 dissociation on the catalyst surface were examined. A feature correlation heat map was employed to scrutinize the relationship between the adsorption energies of NH3-related species and the physicochemical properties of the catalyst. The projected density of states (PDOS) was used to further analyze the electronic interactions between the adsorption sites on the catalyst surface and the NHx species. This work will offer valuable insights for the design and comprehension of metal oxide catalysts in NH3 dissociation reactions.
2. Computational methods
Density functional theory (DFT) calculations were performed using the Vienna ab initio simulation package (VASP 6.4.2).51 The projector augmented wave method was used to describe the electronic structure of atoms.52 The exchange correlation energy functional is described by the Perdew–Burke–Ernzerhof (PBE) functional in generalized gradient approximation.53 Hubbard U was used to rationalize the Coulomb repulsion between electrons in local orbitals. Therefore, PBE+U was adopted to improve the processing of strongly correlated electron systems.54 The U(Ueff) was used to represent the U–J parameters. All metallic elements containing d and f orbitals were assigned Ueff values (Table S1, ESI†). The plane wave cutoff energy was set to 500 eV. The force convergence criterion for the structure was −0.02 eV Å−1. The DFT-D3 method was used to improve the treatment of van der Waals interactions.55 The K-point convergence tests were conducted (Fig. S1, ESI†). The energies of the structures converged by (1 × 1 × 1), (2 × 2 × 1), and (4 × 4 × 1) k-point meshes were compared. All variations are very small and do not affect the conclusions. Hence, the Brillouin zone integration56 was performed using a k-point grid (1 × 1 × 1). All electronic structure calculations were performed using Monkhorst–Pack (3 × 3 × 1).The lattice parameter of the optimized CeO2 bulk structure was 5.47 Å, which is close to the experimental value of 5.41 Å.57 The unit cell of the CeO2(111) plane was used as the surface model as this plane is the most thermodynamically stable surface. The surface structure of CeO2(111) and metal-doped CeO2(111) was composed of a supercell (3 × 2) with 9 atomic layers and surface terminals of O–Ce–O (Fig. 1). The replacement of a surface Ce atom by another metal atom constitutes a doped structure. The adsorption of NH3, NH2–H, NH–2H, and N–3H species was performed on the surface model. During structural optimization, the six atomic layers at the bottom of the supercell were fixed.
The energy of a single metal atom (Esingle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal
metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) atom) was calculated using the following formula:
atom) was calculated using the following formula:
Esingle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) atom = EM-bulk/n atom = EM-bulk/n | (1) |
The formation energy (Edoped) of metal-doped CeO2 was calculated by the following formula:
| Edoped = EM–CeO2 − ECeO2 − (EM-bulk/n) + (ECe-bulk/n) | (2) |
The oxygen vacancy formation energies (EOv) were obtained by the following calculation formula:
| EOv = EM–CeO2–Ov − EM–CeO2 + EO2/2 | (3) |
The adsorption energies (Eads(species)) of NH3, NH2–H, NH–2H, and N–3H species are calculated using the following formula:
| Eads(species) = Especies/surface − Esurface − ENH3 | (4) |
The adsorption energy of a single H atom (Eads(H)) was calculated using the following formula:
| Eads(H) = EH/surface − Esurface − (EH2/2) | (5) |
The d-band center (ξd) of metal doped CeO2 was calculated by the following formula:58
 | (6) |
The valence band maximum (VBM), conduction band minimum (CBM), band gap, d-band center and work function were obtained through the calculation of the density of states and the work function.
3. Result and discussion
3.1. Structure filtered by distortion
In the dissociation reaction of NH3, the presence of active surface oxygen species and oxygen vacancies plays a crucial role in facilitating hydrogen extraction. Metal doping can modulate the local lattice distortion in metal oxides, thereby affecting the presence of active surface oxygen species and oxygen vacancies.59 Metal oxides with higher lattice distortions exhibit higher concentrations of surface oxygen species and oxygen vacancies. By combining the structure and distance between the metal dopant and oxygen (Fig. S2 and Table S2, ESI†), the local lattice distortion58 (Δ) of CeO2 doped with various metals was calculated using the following formula: | (7) |
 | ||
| Fig. 2 (a) Scatter plot of local structural distortion of Ce and metal dopant sites. Schematic of structures that are (b) prone to structural distortion and (c) not prone to structural distortion. | ||
As depicted in Fig. 2b and c, the structures of metal-doped CeO2 can be divided into two categories based on the lattice distortion. One category exhibits a higher tendency for local structural distortion (Δ > 0.01), which promotes the formation of active surface oxygen species and oxygen vacancies. The other category exhibits a lower tendency for local structural distortion (Δ < 0.01), rendering these structures more stable and less likely to generate active surface oxygen species and oxygen vacancies. The structures that were susceptible to distortion were selected for further adsorption of species in the NH3 dissociation reaction.
3.2. Adsorption of reaction species
The adsorption strength of reactants and intermediates significantly impacts reaction activity and selectivity. The NH3 dissociation reaction primarily follows the following steps:| NH3 + * → NH3* | (8) |
| NH3* + * → NH2* + H* | (9) |
| NH2* + H* + * → NH* + 2H* | (10) |
| NH* + 2H* + * → N* + 3H* | (11) |
The adsorption properties of the above species were studied on all metal-doped CeO2 surfaces (*). As illustrated in Fig. 3 and Fig. S3, S4 in the ESI,† NH3 adsorbs onto the catalyst surface, where the N lone pairs of electrons interact and bonds with the empty orbitals of the metal dopant. The H atoms of NH3 undergo hydrogen bonding interactions with neighboring surface lattice oxygen. Through interactions between the metal dopant and the adjacent surface lattice oxygen, NH3 progressively dissociates as the N–H bonds break and the three H atoms are adsorbed onto the adjacent lattice oxygen. The metal dopant not only directly influences the adsorption energies of NH3 and intermediate species, but also indirectly impacts the chemical activity of the neighboring O atoms, thus affecting the adsorption energy of H atoms. Therefore, it is essential to investigate the correlation between the chemical properties of metal dopants and the adsorption energies of dissociated species.
 | ||
| Fig. 3 Adsorption structure diagram of NH3 and its dissociated intermediate species on the catalysts. | ||
3.3. Descriptor screening for elucidating adsorption
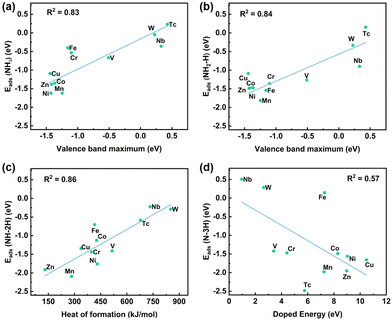
The feature correlation heat map depicts the strength of the correlation between the chemical properties of metal dopants and the adsorption energies of species. Fig. 4 and Tables S3, S4 in the ESI,† present various chemical properties of the metal dopants. The Pearson correlation coefficient was employed as a statistical measure to analyze the relationship between two variables. The value in each cell of the figure represents the correlation strength between each pair of variables, which ranges from −1 to 1. Positive values indicate a positive correlation between the variables, while negative values indicate a negative correlation. The darkness of the cell's color signifies the strength of the correlation, with darker colors indicating stronger correlations. Evidently, the analysis shows that the VBM (0.83), CBM (0.83), and work function (−0.81) of metal dopants are strongly correlated with the adsorption energies of NH3. Additionally, the VBM (0.84), work function (−0.76), and heat of formation (0.76) are strongly correlated with the adsorption energies of NH2–H species. Furthermore, the heat of formation (0.86), VBM (0.79), and work function (−0.75) are strongly correlated with the adsorption energies of NH–2H species. Lastly, the doping formation energy (−0.57), heat of formation (0.55), and covalent radius (0.53) exhibit some correlation with the adsorption energy of N–3H species.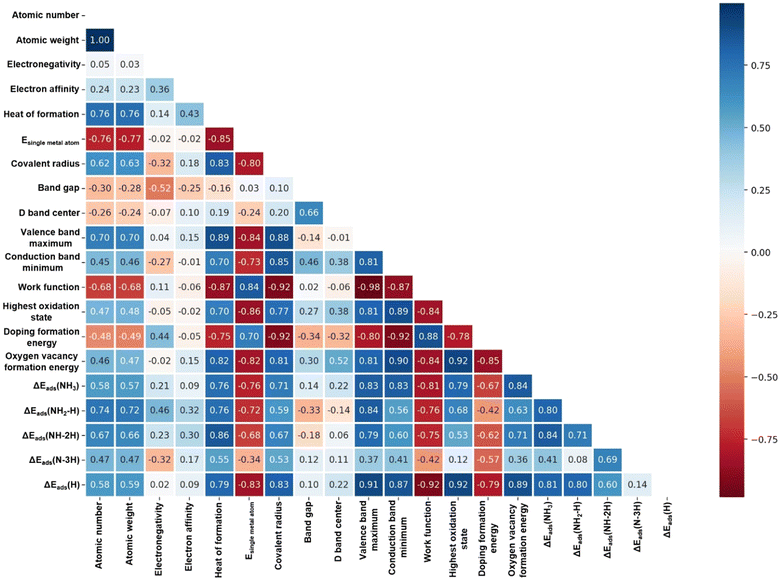 | ||
| Fig. 4 Feature correlation heat map. The correlation between individual descriptors and adsorption energies of species in NH3 dissociation. (Correlation coefficient is Pearson). | ||
Plots exhibiting the strongest linear correlation with the adsorption of the four intermediate species are presented in Fig. 5, along with Fig. S5 in the ESI,† which demonstrate that the adsorption energies of the NH3, NH2–H, and NH–2H species on various metal-doped catalysts are all highly linearly correlated with the VBM. The dissociation of a single H atom has a minimal impact on the adsorption energy and linearity, suggesting that the adsorption intensity is primarily governed by the interaction between the N atoms and the metal dopants. As further dissociation of H atoms occurs, the overall adsorption energy of the species is influenced by the adsorption of both N and H atoms on the metal dopant and adjacent O atoms, respectively. Additionally, the VBM has a good linear relationship with the heat of formation of the metal (0.89), the energy of a single metal atom (−0.84), and the atomic radius (0.88). However, the linear correlation between the above three metal properties and the adsorption energies of NH3-related species is not as strong as the VBM, except for the heat of formation and NH–2H. This may be because the three metal properties jointly affect the VBM, and further affect the adsorption energies of NH3-related species. Additionally, Ni-doped CeO2 exhibits strong adsorption for NH3-related species, aligning with the excellent performance of Ni-based catalysts in NH3 dissociation reaction experiments.60–63 Meanwhile, Zn-, Cu-, Co-, and Mn-doped CeO2 catalysts also demonstrate strong adsorption of NH3-related species, warranting further study.
Oxygen vacancies play an important role in the reaction process. The linear correlation between the adsorption energies (Eads) of NH3-related species and the oxygen vacancy formation energies (EOv) is shown in Fig. S6 (ESI†). The linear correlation between Eads and EOv of NH3, NH2–H, NH–2H, and N–3H species is 0.84, 0.63, 0.71, and 0.36 respectively. The linearity (0.84) between Eads and EOv of NH3 shows that the lower the formation energies of the oxygen vacancy, the more favorable it is for the oxygen atom (adsorbed on the oxygen vacancy) to bind to the first H atom of NH3. The oxygen atom that has already bound to the first H atom has little effect on the binding of the remaining H atoms with other oxygen atoms, so the linear correlation between oxygen vacancy formation energies and NH3 adsorption energies is high, and other correlations are low.
Meanwhile, the activation energies of the gradual dissociation of NH3 on Ni, V, and Nb doped CeO2 surfaces were also obtained by DFT calculation, and its relationship with the valence band maximum of the catalyst is shown in Fig. S7 (ESI†). There is no obvious linear relationship between the valence band maximum and the activation energies of NH dissociation. This may be because the activation energy of NH dissociation is affected by both the properties of the doped metal and the adsorption ability of H by different surrounding oxygen atoms.
The density of states (Fig. 6) of Ni-, V-, and Nb-doped catalysts with NH3 adsorbed on the surface were studied to further elucidate the effect of metal dopants on adsorption species from the electronic structure level. As shown in Fig. 6a, d, and g, the valence band of the catalyst is primarily composed of atomic orbital energy levels occupied by O and Ce electrons. Owing to the presence of only one metal dopant atom, its contribution to the valence band is relatively small. Nevertheless, the VBM values of Ce, O, and the metal dopant are all equal. This equality arises from the strong interaction between the atomic orbitals of the metal dopant and the surrounding O atoms, resulting in the hybridization of atomic orbital and subsequently impacting the overall valence band. Consequently, the adsorption of NH3 on the catalyst largely originates the interaction between N atoms and metal dopant atoms. Therefore, the adsorption effect of NH3 can be elucidated by studying the orbital properties of metal dopant and N orbitals.
Before NH3 adsorption, based on Frontier molecular orbital theory,64 the energy difference (ΔELUMO–HOMO) between the LUMO of the metal atom at the adsorption site and the HOMO of the N atom in NH3 can be used to predict the ease of adsorption (Scheme 1 and Fig. S8, ESI†). A smaller ΔELUMO–HOMO indicates that adsorption occurs more easily. As shown in Fig. 6b, e, and h, the density of state for the metal dopant is predominantly contributed by the d orbital, while the density of state for N is predominantly contributed by the p orbital. Therefore, the minimum value of the metal d orbital energy level above the Fermi level is regarded as ELUMO, and the maximum value of the N p orbital energy level below the Fermi level is regarded as EHOMO. It was found that ΔELUMO–HOMO(Ni) = 0.18 eV < ΔELUMO–HOMO(V) = 0.53 eV < ΔELUMO–HOMO(Nb) = 1.43 eV. This suggests that NH3 is easily adsorbed on Ni-doped CeO2, but less readily adsorbed onto Nb-doped CeO2. This is consistent with the conclusion in Fig. 5a that the adsorption of NH3 on Ni doped CeO2 is stronger than that of NH3 on Nb-doped CeO2. Moreover, ΔELUMO–HOMO has a good linear correlation (R2 = 0.85) with the NH3 adsorption energy (Fig. S9, ESI†). From the density of states after NH3 adsorption, it can be observed that for Ni-doped CeO2, the Ni d orbitals and N p orbitals exhibit significant overlap both above and below the Fermi level. In contrast, for V- and Nb-doped CeO2, the overlap between the metal (V, Nb) d orbitals and N p orbitals is primarily below the Fermi level, with only a slight overlap occurring above the Fermi level. This indicates that the interaction strength between Ni and N is higher than that of the other two, which also indicates that the adsorption of NH3 on Ni-doped CeO2 is stronger than on the other two metal dopants. Therefore, the interaction between metal orbitals and N orbitals can also be analyzed by the HOMO and LUMO energy difference.
3.4. Discussion
In Fig. 4 and Fig. S10 in the ESI,† the work function, as the VBM, has a good linear relationship with the adsorption energies of NH3, NH2–H and NH–2H species, but the linearity is opposite. The work function is strongly linearly correlated (−0.98) with the VBM (Fig. S11, ESI†). The work function is equal to the difference between the vacuum level and the Fermi level of the catalyst. The vacuum energy levels of the catalysts are nearly equal, and the transition metal-doped CeO2 predominantly exhibits acceptor-type doping, which shifts the Fermi level closer to the valence band. Therefore, the correlation between work function and adsorption energy are affected by the position of the valence band. Additionally, the adsorption energy of a single H atom has a good linear correlation with various properties of the catalyst (Fig. S12, ESI†). This can be attributed to the fact that the adsorption sites of H atoms are surface lattice O atoms adjacent to the metal dopant. The chemical properties of the adjacent O atom are directly affected by the doping metal.The reason for the good linear correlation shown in Fig. 5c between the heat of formation and the adsorption energy of NH–2H species remains unclear. The heat of formation data is obtained from the enthalpy of formation of gaseous atoms for each element. The formation enthalpy of gaseous atoms is significantly influenced by the atomic radius and the energy of a single metal atom. As shown in Fig. 4, the heat of formation has a good linear relationship with the radius of the metal atom and the energy of a single metal atom, at 0.83 and −0.85, respectively. Therefore, the relationship between the heat of formation and the adsorption energy of NH–2H species might be associated with the atomic radius and the energy of a single metal atom. Additionally, a weak linear correlation exists between the adsorption energy of N–3H species and the catalyst properties, with only a low correlation coefficient of 0.57 observed with the doping formation energy (as shown in Fig. 5d). This may be because the adsorption of species by the catalyst during the NH3 dissociation process is influenced not only by the adsorption of N by metal dopant sites, but also by the adsorption of three H atoms by three different surface lattice oxygens surrounding the metal. In general, the process of complete dissociation of NH3 is not considered, because in related reactions involving NH3 (such as NH3-SCO and NH3-SCR), the dissociation of NH3 typically does not progress to form a co-adsorbed N and three H atoms.
4. Conclusions
In summary, density functional theory calculations were used to study the chemical properties and adsorption of NH3-related species on different metal-doped CeO2 catalysts. According to the degree of local distortion, the metal-doped CeO2 structures are divided into two categories: structures that are easily distorted and those that are not easily distorted. For catalysts whose structures are easily distorted, the VBM, work function, heat of formation, and metal atom energy can be used as descriptors with good linear relationships with the adsorption energies of NH3, NH2–H and NH–2H species in the feature correlation heat map. Ni-, Zn-, Cu-, Mn-, and Co-doped CeO2 catalysts all strongly adsorb NH3-related species. Through the HOMO and LUMO theories, it is found that NH3 adsorption is also affected by the difference between the LUMO of the metal dopant d orbital and the HOMO of the N p orbital in NH3. Although some well-correlated relationships cannot be explained at the chemical level, the phenomena and conclusions of this study offer valuable insights for designing metal oxide catalysts for NH3 dissociation reactions.Author contributions
M. G. and Y. S. designed, performed, and conceived this study. S. Y., M. G., and K. W. analyzed the data. All authors read and agreed to the published version of the manuscript.Data availability
The data supporting this article has been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP24H02219 (M. G.) in Transformative Research Areas (A) JP24A202 Integrated Science of Synthesis by Chemical Structure Reprogramming (SReP). The results were computed at the Computer Center of Kyoto University and the Research Center for Computational Science, Okazaki, Japan (Project Number 22-IMS-C002).References
- K. Nakajima, H. Toda, K. Sakata and Y. Nishibayashi, Nat. Chem., 2019, 11, 702–709 Search PubMed.
- P. L. Dunn, B. J. Cook, S. I. Johnson, A. M. Appel and R. M. Bullock, J. Am. Chem. Soc., 2020, 142, 17845–17858 Search PubMed.
- Q. Li, G.-h Liu, S. Chen, L. Qi and H. Wang, Chem. Eng. J., 2023, 473, 145120 Search PubMed.
- S. Li, Y. Zhou, X. Fu, J. B. Pedersen, M. Saccoccio, S. Z. Andersen, K. Enemark-Rasmussen, P. J. Kempen, C. D. Damsgaard, A. Xu, R. Sazinas, J. B. V. Mygind, N. H. Deissler, J. Kibsgaard, P. C. K. Vesborg, J. K. Norskov and I. Chorkendorff, Nature, 2024, 629, 92–97 Search PubMed.
- M. Kitano, J. Kujirai, K. Ogasawara, S. Matsuishi, T. Tada, H. Abe, Y. Niwa and H. Hosono, J. Am. Chem. Soc., 2019, 141, 20344–20353 CrossRef CAS PubMed.
- S. Feng, W. Gao, Q. Wang, Y. Guan, H. Yan, H. Wu, H. Cao, J. Guo and P. Chen, J. Mater. Chem. A, 2021, 9, 1039–1047 RSC.
- T. Wu, B. Chang, Y. Li, X. Zhang, X. Zhao, Z. Liu, G. Zhang, X. Liu, L. Zhao, Y. Zhang, H. Zhang, H. Liu and W. Zhou, Nano Energy, 2023, 116, 108855 CrossRef CAS.
- K. Nagaoka, T. Eboshi, Y. Takeishi, R. Tasaki, K. Honda, K. Imamura and K. Sato, Sci. Adv., 2017, 3, e1602747 CrossRef.
- H. Fang, S. Wu, T. Ayvali, J. Zheng, J. Fellowes, P. L. Ho, K. C. Leung, A. Large, G. Held, R. Kato, K. Suenaga, Y. I. A. Reyes, H. V. Thang, H. T. Chen and S. C. E. Tsang, Nat. Commun., 2023, 14, 647 CrossRef.
- M. Asif, S. Sidra Bibi, S. Ahmed, M. Irshad, M. Shakir Hussain, H. Zeb, M. Kashif Khan and J. Kim, Chem. Eng. J., 2023, 473, 145381 CrossRef.
- G. Yang, J. Ran, X. Du, X. Wang, Z. Ran, Y. Chen, L. Zhang and J. Crittenden, Phys. Chem. Chem. Phys., 2021, 23, 4700–4710 RSC.
- Y. Shan, G. He, J. Du, Y. Sun, Z. Liu, Y. Fu, F. Liu, X. Shi, Y. Yu and H. He, Nat. Commun., 2022, 13, 4606 CrossRef PubMed.
- Y. Fu, W. Ding, H. Lei, Y. Sun, J. Du, Y. Yu, U. Simon, P. Chen, Y. Shan, G. He and H. He, J. Am. Chem. Soc., 2024, 146, 11141–11151 CrossRef PubMed.
- G. Jiang, F. Zhang, Z. Wei, Z. Wang, Y. Sun, Y. Zhang, C. Lin, X. Zhang and Z. Hao, Catal. Sci. Technol., 2020, 10, 1477–1491 RSC.
- Y. Guo, L. Ma, Z. Li, Z. Liu, H. Chang, X. Zhao and N. Yan, Catal. Sci. Technol., 2022, 12, 6507–6517 RSC.
- H. S. Pillai, Y. Li, S. H. Wang, N. Omidvar, Q. Mu, L. E. K. Achenie, F. Abild-Pedersen, J. Yang, G. Wu and H. Xin, Nat. Commun., 2023, 14, 792 CrossRef.
- J. Hu, A. Ma, X. Wu, Y. Yin, D. Liu, A. T. Kuvarega, B. B. Mamba and J. Gui, Chem. Commun., 2023, 59, 7232–7235 RSC.
- F. Zhao, G. Li, Q. Hua, J. Cao, J. Song, L. Gao, T. Ma, X. Ren and A. Liu, Catal. Sci. Technol., 2023, 13, 5543–5548 RSC.
- K. Liu, H. Li, M. Xie, P. Wang, Z. Jin, Y. Liu, M. Zhou, P. Li and G. Yu, J. Am. Chem. Soc., 2024, 146, 7779–7790 CrossRef PubMed.
- H. Wang, Y. Dong, C. Zheng, C. A. Sandoval, X. Wang, M. Makha and Y. Li, Chemistry, 2018, 4, 2883–2893 CrossRef.
- R. Wu, F. Li, X. Cui, Z. Li, C. Ma, H. Jiang, L. Zhang, Y. P. J. Zhang, T. Zhao, Y. Zhang, Y. Li, H. Chen and Z. Zhu, Angew. Chem., Int. Ed., 2023, 62, e202218387 CrossRef.
- P. Li, Z. Zhang, X. Yang, Y. Zhu, Z. Zhou, X. Jiang, Q. Wang, X. Gao, X. Yang, Y. Shen and M. Wang, ChemCatChem, 2023, 16, e202301302 CrossRef.
- K. T. Wang, S. Nachimuthu and J. C. Jiang, Phys. Chem. Chem. Phys., 2018, 20, 24201–24209 RSC.
- W. Yang, H. He, Q. Ma, J. Ma, Y. Liu, P. Liu and Y. Mu, Phys. Chem. Chem. Phys., 2016, 18, 956–964 RSC.
- J. W. Lee, G. Barin, G. W. Peterson, J. Xu, K. A. Colwell and J. R. Long, ACS Appl. Mater. Interfaces, 2017, 9, 33504–33510 CrossRef.
- C. Hahn, J. Seidel, F. Mertens and S. Kureti, Phys. Chem. Chem. Phys., 2022, 24, 7493–7504 RSC.
- T. Lan, M. Gao, J.-Y. Hasegawa, Y. Shen, W. Qu, Q. Hu, J. Deng, D. Cheng and D. Zhang, ACS Catal., 2023, 13, 14070–14079 CrossRef.
- X. Liu, P. Wang, Y. Shen, L. Zheng, L. Han, J. Deng, J. Zhang, A. Wang, W. Ren, F. Gao and D. Zhang, Environ. Sci. Technol., 2022, 56, 11646–11656 CrossRef PubMed.
- L. Huang, K. Zha, S. Namuangruk, A. Junkaew, X. Zhao, H. Li, L. Shi and D. Zhang, Catal. Sci. Technol., 2016, 6, 8516–8524 RSC.
- H. Zheng, W. Song, Y. Zhou, S. Ma, J. Deng, Y. Li, J. Liu and Z. Zhao, J. Phys. Chem. C, 2017, 121, 19859–19871 CrossRef.
- H. Ma and W. F. Schneider, J. Catal., 2020, 383, 322–330 CrossRef.
- H. Liu, C. You and H. Wang, Chem. Eng. J., 2020, 382, 122756 CrossRef CAS.
- D. Fang, S. Hou, Y. Ye, Q. Jin, F. He and J. Xie, Appl. Surf. Sci., 2022, 599, 153998 CrossRef CAS.
- L. Yang, J. Fan and W. Zhu, Phys. Chem. Chem. Phys., 2023, 25, 26659–26665 RSC.
- Y. Zhang, M. Zhang, Y. Zang, H. Wang, C. Liu, L. Wei, Y. Wang, L. He, W. Wang, Z. Zhang, R. Han, N. Ji, C. Song, X. Lu, D. Ma, Y. Sun and Q. Liu, ACS Catal., 2023, 13, 1449–1461 CrossRef CAS.
- Y. Jiang, T. Liu, C. Lai, Z. Yang, R. Lin, X. Wang and X. Zhu, Appl. Surf. Sci., 2021, 547, 149196 CrossRef CAS.
- H. Liu, Y. Zhang, S. Liu, S. Li and G. Liu, Chem. Eng. J., 2023, 473, 145371 CrossRef CAS.
- Q. An, Y. Shen, A. Fortunelli and W. A. Goddard III, J. Am. Chem. Soc., 2018, 140, 17702–17710 CrossRef CAS PubMed.
- A. R. Singh, J. H. Montoya, B. A. Rohr, C. Tsai, A. Vojvodic and J. K. Nørskov, ACS Catal., 2018, 8, 4017–4024 CrossRef CAS.
- H. Zhang, S. Wang, H. Wang, B. Huang, S. Dong, Y. Dai and W. Wei, Nanoscale, 2021, 13, 17331–17339 RSC.
- Y. Li, G. Zheng, L. Li, X. Zhang, Z. Tian and L. Chen, Phys. Chem. Chem. Phys., 2022, 24, 29112–29119 RSC.
- Y. Wang and M. Shao, ACS Catal., 2022, 12, 5407–5415 CrossRef CAS.
- Z. Li, R. Dong, X. Liu, C. Lin, Y. Li, X. Feng, Z. W. Seh and Q. Zhang, Surf. Sci., 2023, 730, 122238 Search PubMed.
- M. Chen, Z. Zhu, J. Chen, L. Xia, L. Gan and Y. Zhou, J. Mater. Chem. A, 2024, 12, 14035–14044 RSC.
- D. A. Hansgen, D. G. Vlachos and J. G. Chen, Nat. Chem., 2010, 2, 484–489 Search PubMed.
- J. Yang, X. Chen, Z. Liu, Q. Wang, Y. Wen, A. Zhang, R. Chen and B. Shan, J. Mater. Chem. A, 2022, 10, 12447–12457 RSC.
- H. Zheng, R. Li, C. Zhong, Z. Li, Y. Kang, J. Deng, W. Song and Z. Zhao, Catal. Sci. Technol., 2022, 12, 1429–1440 RSC.
- I. Lucentini, G. García Colli, C. D. Luzi, I. Serrano, O. M. Martínez and J. Llorca, Appl. Catal., B, 2021, 286, 119896 CrossRef CAS.
- J. Zhang, Y. Fan, L. Chen, L. Yang, L. Zhou, X. Luo, J. Zou and W. Dai, Chem. Eng. J., 2023, 473, 145272 CrossRef CAS.
- H. Sun, H. Wang and Z. Qu, ACS Catal., 2023, 13, 1077–1088 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 Search PubMed.
- A. I. Liechtenstein, V. V. Anisimov and J. Zaanen, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, R5467–R5470 Search PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- S. Colis, A. Bouaine, G. Schmerber, C. Ulhaq-Bouillet, A. Dinia, S. Choua and P. Turek, Phys. Chem. Chem. Phys., 2012, 14, 7256–7263 Search PubMed.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 CAS.
- X. Zhang, C. Pei, X. Chang, S. Chen, R. Liu, Z. J. Zhao, R. Mu and J. Gong, J. Am. Chem. Soc., 2020, 142, 11540–11549 CrossRef CAS.
- J.-L. Cao, Z.-L. Yan, Q.-F. Deng, Z.-Y. Yuan, Y. Wang, G. Sun, X.-D. Wang, B. Hari and Z.-Y. Zhang, Catal. Sci. Technol., 2014, 4, 361–368 RSC.
- R. Wang, H. Liu, K. Zhang, G. Zhang, H. Lan and J. Qu, Chem. Eng. J., 2021, 404, 126795 CrossRef CAS.
- Y. Jin, Y. Liu, R. Wu and J. Wang, Chem. Commun., 2024, 60, 1104–1107 RSC.
- I. Nakamura and T. Fujitani, Appl. Catal., A, 2016, 524, 45–49 CrossRef CAS.
- K. Fukui, T. Yonezawa and H. Shingu, J. Chem. Phys., 1952, 20, 722–725 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp00430f |
| This journal is © the Owner Societies 2025 |