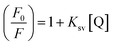Hg(II) causes photoluminescence quenching of pyrene inside a blue emitting ionic liquid-derived crystalline nanoball†
Najmin
Tohora
a,
Rajkumar
Sahoo
b,
Sabbir
Ahamed
a,
Jyoti
Chourasia
a,
Shubham
Lama
a,
Manas
Mahato
a,
Shreya
Ali
a and
Sudhir
Kumar Das
 *a
*a
aDepartment of Chemistry, University of North Bengal, Raja Rammohunpur, Darjeeling, West Bengal 734013, India. E-mail: sudhirkumardas@nbu.ac.in
bDepartment of Chemistry, Indian Institute of Technology, Kharagpur, West Bengal 721302, India
First published on 8th April 2025
Abstract
This report presents the self-assembly of a blue-emitting ionic liquid (IL), NTIL, prepared by combining pyrene butyrate with a quaternary phosphonium ionic liquid (IL) through a straightforward ion exchange method. Water-dispersible crystalline nanoparticles, referred to as nNTIL, were developed using a reprecipitation technique. The nanocrystalline and molecular-level organization of pyrene moieties within these nanoparticles was validated using various spectroscopic, microscopic, and calorimetric analyses. Pyrene counterparts in the nanocrystalline nanomaterials demonstrate a strong tendency to self-associate when excited. The self-aggregation of pyrene moieties in their electronic excited state is found to be pronounced and beyond the simple excimeric dimerization process. Hg2+ ions cause strong photoluminosity quenching, which is found to be pronounced owing to the strong π–π stacking interactions among the pyrene moieties inside the crystalline nanoball due to the strong electrostatic interaction between pyrene butyrate and Hg2+ ions, causing further clotting of water dispersed nanoparticles. The induction of further coagulation of nNTIL by Hg2+ ions was validated through scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) analysis. Analysis of the quenching of photoluminosity through photoluminescence lifetime decay analysis revealed that the process is dynamic. The practical applications of nNTIL were illustrated through analyses of water and soil samples, paving the way for applications in diverse fields. Furthermore, we investigated the sensor's effectiveness in detecting Hg2+ ions using affordable test strips. The present report introduces the fabrication and implications of metal-sensitive IL-based low-dimensional materials exhibiting remarkable photophysical properties compared with traditional ones.
1. Introduction
Ionic liquids (ILs) are a distinctive class of organic salts that have melting points below 100 °C. Unlike conventional ionic salts, the propensity for crystal formation in ILs is substantially diminished because of sterically incompatible ions, which leads to a substantial reduction in their melting points.1–3 They are recognized for their relatively low toxicity and biocompatibility, as well as their antibacterial properties and selective catalytic capabilities. They can be tailored to meet specific requirements by altering their constituent ions and incorporating various functional groups into their structures.3 This versatility allows for a wide range of applications, such as extraction and separation processes, the formulation and delivery of active pharmaceutical ingredients (APIs), biocatalysis, sensor development, gel and molecular probe synthesis, electrochemistry, energy efficiency enhancements, and the design of microemulsions.4,5 ILs are being increasingly utilized to replace conventional solvents and electrolytes, evolving into distinct functional media, fluids, and materials. This shift marks a new trend to explore photonic materials that have gained attention in recent years.6–10 The integration of ILs with photonic materials has notably enhanced their applicability across a range of optoelectronic applications. In these contexts, ILs act as versatile building blocks, forming the basis for innovative photonic materials.11 These new materials tend to offer significant advantages over traditional molecular counterparts. Because of the high modularity of ILs, specific functionalization tailored for particular optoelectronic applications can be achieved. This is often accomplished through ion-exchange metathesis reactions or by introducing specific functional groups into ILs’ structure, resulting in the formation of novel ion-containing materials. Such photonic materials maintain the essential properties of their original counterparts while incorporating the beneficial characteristics of ILs, leading to the development of advanced photo-functional materials. However, much of the existing literature focuses on the role of ILs as solvents and electrolytes, along with the advancement of high-performance functional materials.12,13 Research exploring the application of ILs in the design and development of photonic materials for various optoelectronic functions, such as photon upconversion, white light generation, and FRET-mediated down conversion, remains relatively scarce. Furthermore, nanoparticles can be synthesized through straightforward methods by adjusting the length of the aliphatic side chains within the ILs. These nanoparticles hold promise for applications in sensing, biomedical fields, and energy conversion.14–18In today's world, nanoparticles play an integral role in various aspects of life primarily because of their distinctive intrinsic properties, such as enhanced surface-to-volume reactivity relative to bulk materials, distinct atomic and electronic structures, and notable morphological characteristics.19,20 Recently, nanomaterials have emerged as a focal point in biomedical research, finding applications in therapeutics, cellular imaging, drug delivery systems, and biological sensing.21,22 Ionic liquids (ILs) have increasingly been recognized for their role in the synthesis of these biologically active nanoparticles, serving as effective templates or solvents.23 Their ability to form cage-like hydrogen bonds enhances self-organization and molecular aggregation.24 Nanomaterials derived from ILs present a range of promising applications across the biomedical landscape, as well as in sensing and energy conversion technologies.25,26
Pyrene and its derivatives are exceptional aromatic hydrocarbons known for their unique properties and broad range of applications. These include aggregation-induced fluorescence characteristics, significant delocalization of π electrons, excimer formation in the excited state, strong UV-visible absorption and emission characteristics, high quantum yield, and prolonged photoluminescence lifespan.27–31 Additionally, the monomer and excimer exhibit distinct responses when interacting with various metal ions, dye molecules, and biomolecules.27,32–34 These properties enable their use as core components in diverse photoelectronic devices, including excimer lamps, lasers, and photovoltaics.35 Hence, modulating the photophysical properties of pyrene moieties inside the core of ILs would be more beneficial for fabricating IL-based photonic materials and for multifaceted optoelectronic applications.
The investigation of heavy and transition-metal ions for detection and quantification has gained attention because of their significant harmful effects on the environment and living organisms.36 Among these metals, mercury stands out as the most toxic, associated with numerous serious health issues such as cardiovascular diseases, kidney toxicity, neurotoxic effects, cancer, immune system impairment, and adverse reproductive and developmental outcomes.37–41 Mercury poses risks in all its oxidation states (0, +1, and +237), despite variations in their solubility and potential for redox reactions. Its chemical forms primarily determine the ecological and toxicological impacts of mercury, as various natural processes in aquatic environments convert mercury into different inorganic and organic compounds. Notably, certain lower organisms can convert inorganic mercury into methylmercury, a compound known for its neurotoxic properties.42–44 Given that the maximum allowable concentration in food and drinking water is around 2 ppb,45 there is a strong interest in developing effective sensors for mercury detection. Various types of sensors have been developed that utilize electrochemical responses and conductivity changes,46,47 as well as variations in color and fluorescence.45,48–57 However, many of these sensors are limited to nonaqueous environments, and the direct detection of elemental mercury has primarily been conducted in its vapor form.58,59 Therefore, sensors that can function in aqueous environments are of significant practical importance.
In our efforts to promote secure and healthy surroundings, we have concentrated on exploring straightforward methods for detecting and eliminating toxic substances from commonly affected areas. Various techniques have been utilized for the identification of Hg2+, such as inductively coupled plasma mass spectrometry, surface-enhanced Raman spectroscopy, and atomic absorption spectrometry. However, these methods tend to be expensive, require extensive pre-treatment, and necessitate skilled personnel for operation.60,61 Recently, there has been a surge of interest in colorimetric and fluorometric sensors for Hg2+ ion detection, primarily due to their potential for miniaturization and suitability for field applications.62–65 In this regard, fluorescent nanomaterial-based colorimetric sensors utilizing diverse functionalization techniques have significantly advanced the detection of Hg2+ ions.66–70 Nevertheless, most research has focused on detecting mercury ions in liquid solutions. At the same time, solid-state sensing methods are favored for practical, real-time applications due to their ease of handling, operational simplicity, stability, and portability.
Considering all relevant factors, we introduce an IL and IL-based organic nanosensor that incorporates the pyrene moiety. Through the reprecipitation technique, we developed water-dispersible crystalline nanoparticles that possess various important properties. Moreover, introducing Hg2+ into the nNTIL solution results in a bathochromic shift of 2 nm (from 344 nm to 346 nm) and is accompanied by prominent Mie-scattering, inferring further coagulation of nNTIL. nNTIL demonstrated a substantial increase in fluorescence (ϕ = 0.37) when exposed to 365 nm UV light, making it readily observable compared with the NTIL solution. When mercury(II) ions were added to the nNTIL solution, a decrease in fluorescence was noticed at 478 nm (ϕ = 0.035). The quenching process is dynamic and is evident from the photoluminescence titration spectral and excited state lifetime decay analysis. The present report discusses the fabrication and implications of metal-sensitive IL-based nanoparticles that exhibit remarkable properties compared with traditional ones.
2. Experimental section
Information regarding the synthesis methods for NTIL and nNTIL, general protocols for conducting UV-visible and fluorescence analysis, determination of photoluminescence quantum yields, and various interactive plots are given in the ESI.†2.1 Materials and instrumentations
For synthesizing pyrene butyrate anion-based NTIL, pyrene butyric acid (PBA), and trihexyltetradecylphosphonium chloride (P66614Cl) (>95%) were purchased from Sigma-Aldrich, India. All the solvents used for the experiments were of analytical grade and obtained from Sigma-Aldrich, India, and TCI, India. All the metal chloride salts and Hg(II) anions used in the present study were purchased from Sigma-Aldrich, India, and TCI, India, respectively. All the chemicals were utilized without additional purification. 1H and 31P NMR spectra were recorded on a Bruker 400 MHz spectrometer in CDCl3 with chemical shifts (δ) in ppm units. 13C NMR spectra were recorded on a Bruker 100 MHz spectrometer in CDCl3. FT-IR spectra were measured on a Bruker Optik GMBH FTIR spectrometer (Germany). High-resolution mass spectra (HRMS) were recorded on an Agilent 6545XT AdvanceBio LC/Q-TOF spectrometer. UV-visible spectral analysis was recorded on a Hitachi U-2910 instrument, and fluorometric experiments were performed using a Hitachi F-7100 fluorimeter. Throughout the steady state fluorometric experiment, excitation and emission wavelengths were kept at 330 nm and 340–650 nm, respectively, with 5 nm excitation and emission slit under ambient conditions and processed using Origin software.The size and shape of nNTIL were estimated using field emission scanning electron microscopy (SEM) (ZEISS) employing an operating voltage of 50 kV by drop casting the required amounts of nNTIL (20 μg mL−1) on the carbon-coated copper grids. The average particle size was determined considering the size of more than 100 particles. Transmission electron microscopy (JEOL TEM-2010) was used for determining the morphology of the nanoparticles with an operating voltage of 200 kV. The average particle size was obtained by measuring the size of more than 100 particles. The hydrodynamic radius of nNTIL was measured using the dynamic light scattering (DLS) technique (Malvern Zeta Sizer Nano) using a 4 mW He–Ne laser (λ = 632.8 nm). Differential scanning calorimetry (DSC) experiment was carried out between 25 °C to 200 °C using a PerkinElmer aluminum sample pan kit in a PerkinElmer (DSC Q20) instrument at a scan rate of 2 °C min−1 under a nitrogen atmosphere. Time-resolved fluorescence decays were obtained by using a picosecond time-correlated single-photon counting Life Spec II spectrophotometer (Edinburgh Instruments, UK). The decay profile was analyzed using F900 software from Edinburgh Instruments. For measuring the photoluminescence lifetime of nNTIL, samples were excited with 375 nm picosecond laser diodes (EPL-375) having an instrument response function (IRF) of ∼260 ps. The signals were collected at a magic angle of 54.7°, monitoring the emission maxima at 480 nm. All of the fluorescence decays were fitted with a multiexponential function corresponding to a χ2 value close to 1, indicating a good fit. After deconvoluting the IRF, each decay curve was fitted with the help of the exponential decay function: 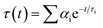 , where αi and τi values are the experimentally obtained pre-exponential factors having corresponding lifetimes. Experimentally as-obtained pre-exponential factors were then normalized by the equation
, where αi and τi values are the experimentally obtained pre-exponential factors having corresponding lifetimes. Experimentally as-obtained pre-exponential factors were then normalized by the equation 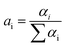 to achieve actual pre-exponential factors. The average photoluminescence lifetime was estimated utilizing the equation
to achieve actual pre-exponential factors. The average photoluminescence lifetime was estimated utilizing the equation 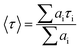 and percentage corresponding component were determined by employing ai × 100.
and percentage corresponding component were determined by employing ai × 100.
3. Results and discussion
3.1 Characterization of NTIL and nNTIL
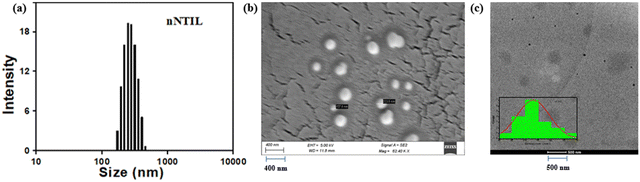
Various spectroscopic techniques were employed to characterize the prepared NTIL. The results from 1H, 13C, and 31P NMR studies confirm that the ion-exchange reaction effectively yields NTIL (Fig. S1–S3, ESI†). In the high-resolution mass spectrometry (HRMS) analysis, a peak at 483.544 (m/z) corresponds to the cationic phosphonium moiety. In contrast, a peak at 287.104 (m/z) indicates the presence of the anionic pyrene butyrate moiety (Fig. S4, ESI†). The molecular weight of NTIL was calculated to be 770.648 Da from the equimolar mixing of the two oppositely charged components, and further confirms the synthesis of NTIL. The FT-IR spectrum revealed two bands associated with C–H stretching vibrations at 2927 and 2853 cm−1, further supporting the development of NTIL (Fig. S5, ESI†). NTIL was observed to be a viscous solid at ambient temperature. To determine if the synthesized ionic salt can be classified as an ionic liquid, we carried out differential scanning calorimetry (DSC) analysis, which indicated a melting point of 69 °C (Fig. S6, ESI†), inferring that the synthesized organic salt belongs to the class of ionic liquids.The UV-visible absorption spectrum of nNTIL displays a structured band that is notably broad and exhibits a bathochromic shift of approximately 6 nm relative to pyrene butyrate in solution (Fig. S7, ESI†). This redshift, along with significant Mie-scattering, represents the aggregation of pyrene moieties in the ground electronic state of water-suspended nNTIL. The presence of strong Mie-scattering suggests that the self-association of pyrene butyrate has led to the formation of water-suspended nanoparticles (Fig. 1(a)). nNTIL suspended in water displays a structured monomeric emission along with a broad, excimer-like emission characterized by high emission intensity displaying a cyan color photoluminosity. The cyan colored fluorogenicity from the water-suspended nanoparticles was observable even with minimal amounts of NTIL (Fig. 1(b)). The CIE diagrams for NTIL (x = 0.1617, y = 0.0368) and nNTIL (x = 0.1403, y = 0.2478) infer that these color co-ordinates values fall within the color gamut of blue and cyan color which are also visible to the naked eye under the exposure of a 365 nm UV lamp (Fig. 1(c)). The observation of a notably intense excimer-like emission implies that the pyrene molecules are closely stacked, resulting from substantial van der Waals interactions, leading to their pre-aggregation within the nanostructure. The presence of nanoparticles is validated by high-resolution dynamic light scattering (DLS) (Fig. 2(a)), SEM (Fig. 2(b)), transmission electron microscopy (HR-TEM) (Fig. 2(c)) analysis and energy dispersive X-ray spectroscopy (EDS) (Fig. S8, ESI†) and time-resolved photoluminescence decay analysis (Fig. 1(d)). The hydrodynamic radius was measured to be 410 ± 10 nm according to the DLS spectrum which was confirmed by SEM image analysis. The nNTIL nanoparticles suspended in water exhibit a spherical shape, suggesting the development of nanoballs. HR-TEM images also reveal that the nanoaggregates have a nearly spherical morphology, with an average size of 56 ± 5 nm. The melting point of the prepared nanoparticles was determined to be 153 °C, which was revealed from the observation of an exothermic peak during the heating process and associated with the melting temperature of ionic crystalline nNTIL as obtained from the differential scanning calorimetry (DSC) analysis (Fig. S9, ESI†). However, no exothermic peak was found in the cooling phase because of crystallization.
The excited-state photophysics of pyrene units within the nanoparticles was explored through time-resolved photoluminescence studies. In Birks’ mechanism, the decay profile of the excimer is characterized by two components when preaggregation is absent.71 The first is a rising component associated with the formation of the excited state dimer, which has a negative prefactor (−a1). The second is a decaying component with a positive prefactor (a2), which is linked to the deactivation process of the excimer. However, this mechanism may not be suitable for pre-aggregated systems, where there is a significant likelihood of associative pyrene dimer formation in the ground state. In these systems, a substantial portion of the excimer originates from the pre-aggregated dimers. This means that the rising component should be detectable on a very short timescale, specifically within 1 nanosecond.72 Time-resolved fluorescence measurements reveal that the decay profile of the excited species of nNTIL observed at the excimer-like emission exhibits a very short rise time accompanied by long decay components. The rising component has a lifetime of less than 1 nanosecond, which is significantly faster than that of pyrene excimers in bulk solvent. These results strongly suggest that the pyrene moieties are situated in an environment that enhances their association in the excited electronic state, leading to a much quicker formation of the excited state complex compared with what is observed in bulk solvents. In contrast to the concept of excited state dimerization, one could contend that a significant association of multiple pyrene moieties occurs in the excited state rather than solely the formation of dimers within the synthesized nanoparticle system. For NTIL in THF solution, time-resolved photoluminescence decay shows three exponential profiles, with lifetimes of τ1 = 0.555 ns (36%), τ2 = 2.819 ns (60%), and τ3 = 35.567 ns (4%) inferring that the faster, moderate and long lifetime components are likely excimeric, pre-aggregated and aggregated pyrene moieties ones. The excited state lifetime decay profile of nNTIL is characterized by a triexponential profile, with lifetimes of τ1 = 1.160 ns (−29%), τ2 = 10.006 ns (41%), and τ3 = 35.567 ns (88%), inferring that rise, moderate and long lifetime components are for the excimeric, pre-aggregated and aggregated pyrene moieties, respectively, and most of the pyrene moieties are stacked in aggregated states. The average fluorescence lifetime (〈τ〉) yields a lifetime of 35.284 ns for the aggregated pyrene (88%) in water-suspended nanoparticles, while the average photoluminescence lifetime of NTIL in THF is 2.682 ns, with only 4% pyrene moieties in the aggregated state (Fig. 1(d) and Table 1).
| a 1 | τ 1 (ns) | a 2 | τ 2 (ns) | a 3 | τ 3 (ns) | 〈τ〉c (ns) |
χ
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
|
|---|---|---|---|---|---|---|---|---|
| a Denotes the pre-exponential factors. b τ 1, τ2, and τ3 are in ns. c Indicates average fluorescence lifetime. d Represents the parameters indicating the goodness of fit. | ||||||||
| NTIL | 0.36 | 0.555 | 0.60 | 2.819 | 0.04 | 21.135 | 2.682 | 1.15 |
| nNTIL | −0.29 | 1.160 | 0.41 | 10.006 | 0.88 | 35.567 | 35.284 | 1.08 |
3.2 Spectroscopic response of nNTIL towards metal ions
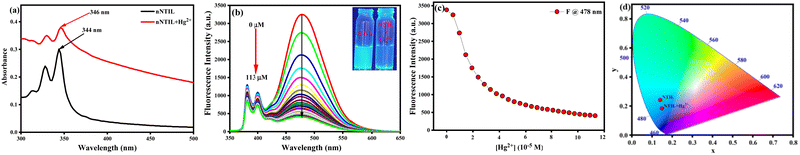
The absorption characteristics of nNTIL were evaluated using UV-visible spectroscopy in an aqueous environment. The UV-visible absorption spectrum of nNTIL displays a structured band that is notably broad and exhibits a bathochromic shift of approximately 6 nm relative to pyrene butyrate in solution (Fig. S7, ESI†). This redshift, along with significant Mie-scattering, represents the aggregation of pyrene moieties in the ground electronic state of the water-suspended nNTIL. The presence of strong Mie-scattering suggests that the self-association of pyrene butyrate has led to the formation of nanoparticles that are suspended in water. Furthermore, the UV-visible spectra of the synthesized nNTIL nanoparticles show a marked change in absorption upon the addition of Hg2+ ions (Fig. 3(a)). Specifically, introducing Hg2+ into the nNTIL solution results in a bathochromic shift of 2 nm (from 344 nm to 346 nm) and is accompanied by prominent Mie-scattering. The long-wavelength tail observed in the absorption spectrum of the nanoparticle suspension is attributed to λ−4 Rayleigh-type scattering.73 The observation of a greater bathochromic shift and prominent Mie-scattering in the presence of Hg2+ ions indicate the further association of pyrene moieties inside the crystalline nanoball due to the strong electrostatic interaction among pyrene butyrate and Hg2+ ions. However, we have found no change in the absorption spectra with the addition of Hg2+ in NTIL solution (Fig. S10, ESI†). Furthermore, titration of aqueous Hg2+ solution with nNTIL at different concentrations showed significant Mie-scattering (Fig. S11, ESI†).To better understand the binding characteristics of nNTIL towards Hg2+ ions, fluorescence titration experiments were performed. nNTIL exhibited remarkable sensitivity to Hg2+ ions among the various metal ions examined. The probe demonstrated a significantly enhanced cyan color fluorescence (ϕ = 0.37) under 365 nm UV light, which is easily observable to the naked eye. Upon the addition of Hg2+ ions to the probe solution, cyan color fluorescence quenching was observed at 478 nm (ϕ = 0.035) (Fig. 3(b) and (c)), which is also evident from the CIE diagram (Fig. 3(d)). The coordinate values for nNTIL are x = 0.1402 and y = 0.2416. In the case of nNTIL-Hg2+, the coordinate values are x = 0.1485 and y = 0.1839. Thus, nNTIL acts as an effective “turn-off” sensor for the detection of Hg2+ ions. However, there is no obvious change in fluorescence intensity with the addition of Hg2+ in NTIL solution (Fig. S12, ESI†). The quenching effect of the probe increased progressively with rising concentrations of Hg2+ ions from 0 to 113 μM. Utilizing the fluorometric titration data and applying the 3σ/K and 10σ/K methods, we estimated the limit of detection (LOD) and quantification (LOQ) for Hg2+ ions.74 In this context, σ denotes the standard deviation of the blank, while K represents the slope of the calibration curve. A robust linear correlation between fluorescence intensity and Hg2+ ions concentration was established in two ranges: 5 μM to 30 μM and 60 μM to 120 μM, yielding R2 values of 0.96 and 0.98, respectively. The LOD and LOQ for Hg2+ were estimated to be 17 nM and 57 nM, respectively (Fig. S13a, ESI†), which is considerably lower than those reported in earlier studies (Table S2, ESI†). Hg2+ ions are inclined to bind with the carboxyl groups of nNTIL; however, this interaction is expected to be weak, as ‘soft’ Hg2+ ions favor a ‘soft’ coordination environment. These results suggest that the quenching response of the sensor is primarily due to the strong π–π stacking interactions among the pyrene moieties inside the crystalline nanoball owing to the pronounced electrostatic interaction among pyrene butyrate and Hg2+ ions. The association constant is determined to be 0.55 × 105 M−1 using the Benesi–Hildebrand equation75 (Fig. S13b, ESI†).
To validate the suggested mechanism, the size distribution of particles with the addition of Hg2+ ions is measured by DLS (Fig. S14, ESI†). The DLS spectrum of free nNTIL shows that the hydrodynamic diameter of the nanoparticles suspended in water falls within the range of 410 ± 10 nm, but the hydrodynamic diameter of the aqueous suspended nanoparticles is in the range of 320 ± 10 nm after the addition of Hg2+. The decrease in hydrodynamic radius might be due to the removal of bonded water from the core of nNTIL due to the further Hg2+-induced clotting of NTIL. Also, the DLS spectrum of NTIL-Hg2+ lies in a broader size range than NTIL. Therefore, there is coagulation of nano-particles after the addition of Hg2+. From the SEM images of nNTIL-Hg2+ (Fig. S15, ESI†), we can conclude that more aggregation is pronounced when Hg2+ is present inside the crystalline nanoball, which is also observed in the UV-visible spectral studies (Fig. 3(a)). Also, the presence of Hg2+ is evident from the EDS analysis. EDS results show that only three components are present in nNTIL (C, O, and P) (Fig. S8, ESI†), while four components are present in nNTIL-Hg2+: C, O, P, and Hg (Fig. S16, ESI†).
To assess the selectivity of nNTIL for Hg2+ and to explore the mechanistic details of its detection, time-resolved photoluminescence decay analysis was conducted. Fig. 4 shows the excited-state photoluminescence lifetime decay of nNTIL with and without Hg2+ (various Hg2+ ion concentrations at a 375 nm excitation wavelength). Remarkably, the average fluorescence lifetime (〈τ〉) of nNTIL is exceptionally diminished with the inclusion of Hg2+ ions, and the average lifetime diminished from 35.284 ns to 11.117 ns at λexc = 375 nm when Hg2+ is present (Table 2).
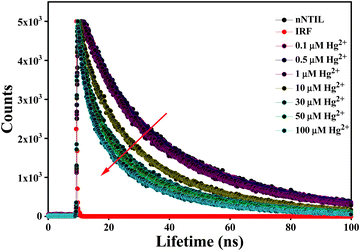 | ||
| Fig. 4 Photoluminescence lifetime decays of nNTIL in water with and without Hg2+ ions at various concentrations. λexc. = 375 nm. | ||
| [Hg2+] (μM) | a 1 | τ 1 (ns) | a 2 | τ 2 (ns) | a 3 | τ 3 (ns) | 〈τ〉c (ns) |
χ
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
|---|---|---|---|---|---|---|---|---|
| a Denotes the pre-exponential factors. b τ 1, τ2, and τ3 are in ns. c Indicates average fluorescence lifetime. d Represents the parameters indicating the goodness of fit. | ||||||||
| 0 | −0.29 | 1.160 | 0.41 | 10.006 | 0.88 | 35.567 | 35.284 | 1.08 |
| 0.1 | −0.21 | 1.441 | 0.39 | 10.237 | 0.82 | 35.444 | 32.663 | 1.03 |
| 0.5 | −0.14 | 1.519 | 0.37 | 10.693 | 0.77 | 35.381 | 31.128 | 1.04 |
| 1 | −0.14 | 1.408 | 0.38 | 9.940 | 0.76 | 34.173 | 29.619 | 1.04 |
| 10 | 0.14 | 1.424 | 0.35 | 9.469 | 0.51 | 29.921 | 18.721 | 1.15 |
| 30 | 0.26 | 1.301 | 0.35 | 8.313 | 0.39 | 27.914 | 14.136 | 1.08 |
| 50 | 0.33 | 1.710 | 0.34 | 9.163 | 0.33 | 27.812 | 12.942 | 1.16 |
| 100 | 0.37 | 1.439 | 0.34 | 8.428 | 0.29 | 26.818 | 11.117 | 1.1 |
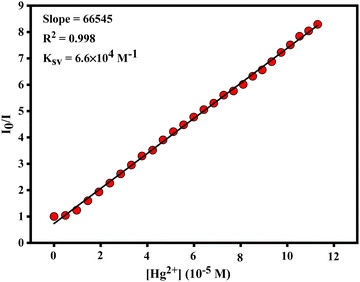
The fluorescence emission of nNTIL exhibits quenching behavior in response to varying concentrations of Hg2+ ions, adhering to the Stern–Volmer relationship:
3.4 Fluorescence behavior of nNTIL in response to various cations and anions
A key aspect in assessing the performance of a chemical sensor is its high selectivity for the probe nNTIL compared with other competing species present in the sample. We investigated the selectivity of nNTIL towards different cations: Hg2+, Sn2+, Zn2+, Cu2+, Mn2+, Ni2+, K+, Na+, Pb2+, Al3+, Ba2+, Ca2+ and Fe3+. The nNTIL probe solution exhibits cyan fluorescence and the presence of these metal ions did not affect the fluorescence properties of the probe (Fig. 6(c)). However, Hg2+ ions quench the cyan fluorescence when examined under a UV lamp with an excitation wavelength of 365 nm. Additionally, we assessed the selectivity of the probe against a range of anions: AcO−, Br−, Cl−, CN−, F−, HSO4−, I−, NO3−, PO43−, S2−, and SO42−. The minimal interference from different competing metal ions and anions highlights the exceptional selectivity of the sensor toward Hg2+ ions in aqueous solutions.To confirm the strong selectivity for Hg2+ amidst a complex mixture of various cations in an aqueous medium, we examined the fluorescence intensity quenching of nNTIL in the presence of Hg2+ ions when different competing cations and anions are present. Hg2+ at 113 μM was introduced into the probe solution alongside various metal ions and anions. The quenching of photoluminescence intensity due to the presence of Hg2+ ions remained consistent, even after the inclusion of various cations and anions (Fig. 6(a) and (b)). Notably, the added cations and anions did not affect the detection of Hg2+ ions when equivalent amounts were present in the probe solution. This indicates that the nNTIL probe is likely to function effectively in vivo, despite the presence of other competing ions. Overall, these findings demonstrate that the specified ions do not notably hinder the identification of Hg2+ ions by nNTIL.
4. Analysis in real water and soil samples
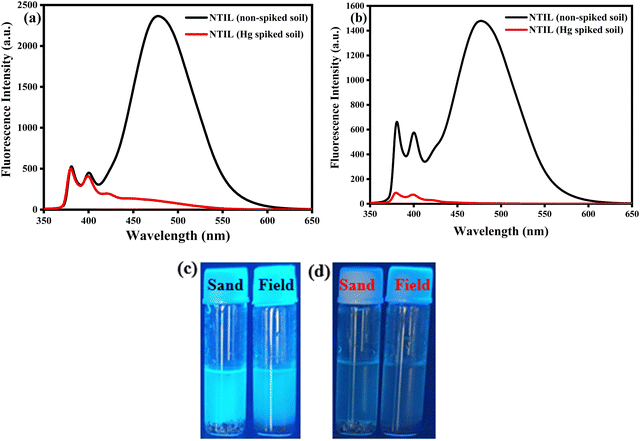
Sensing systems that facilitate the detection of trace metal ions in both real and spiked samples have gained considerable interest.49,50 To assess the practicality of the proposed method, a study was conducted to detect Hg2+ ions in actual water samples collected from Siliguri, West Bengal. All samples were spiked with Hg2+ ions. The quantitative recovery results were obtained from these spiked samples using a calibration curve for the calculations. The recovery rates for Hg2+ in the water samples were between 98% and 99% (Table S1, ESI†). These findings demonstrate that the nNTIL probe is capable of accurately and precisely detecting Hg2+ in environmental analyses.Mercury is notoriously resistant to degradation and tends to accumulate in crops and soil, making its quantitative detection in soil crucial. In this investigation, we employed the nNTIL probe to measure mercury levels in soil samples sourced from Siliguri, West Bengal. Each sample was initially weighed at 0.5 g. The fluorescent sensor was first used to analyze the unspiked samples. Following this, all samples were spiked with Hg2+ and re-evaluated using the developed fluorescent sensor. The results indicated that the unspiked sandy soil produced a strong cyan fluorescence, while the field soil showed a lower intensity of fluorescence under UV light (365 nm). After spiking with Hg2+, both soil types exhibited a significant reduction in cyan fluorescence (Fig. 7(a)–(d)). These results demonstrate the potential of the nNTIL probe for effectively detecting mercury in environmental materials, especially in water and soil samples.
5. Sensing with test strips
To assess the sensing characteristics of the probe for mercury(II) ions in aqueous solutions, we performed experiments using test strips for practical applications. These strips were prepared by soaking them in an nNTIL solution, followed by the addition of different metal ion solutions. Afterward, the strips were allowed to dry at room temperature. The results indicated that the cyan fluorescence of the test strip only diminished in the presence of mercury when irradiated at 365 nm (Fig. 8(a) and (b)). Furthermore, we conducted a real sample analysis using the paper strip method to confirm its applicability in real-time settings. In this process, the nNTIL-coated paper strip was dipped into various water samples containing Hg2+ ions, including municipal and tap water from Siliguri, West Bengal, and subsequently dried in an oven. Notably, the dried strips exhibited a quenching of cyan fluorescence, indicating the presence of Hg2+ ions (Fig. 9(a) and (b)). We conducted a detailed investigation into the concentration-dependent response of nNTIL-coated paper strips to Hg2+ ions, aiming to illustrate their ability to quantify Hg2+ ions. The experiment was carried out across a range of Hg2+ ions concentrations, from 10−1 to 10−9. The cyan fluorescence of the nNTIL-coated paper strips was quenched (Fig. 8(c)). The effective use of affordable test strip techniques and their application in different water samples demonstrate the probe's potential for detecting and quantifying mercury(II) ions in real sample analysis.6. Utilization of neat NTIL for the identification of Hg2+ ions
Neat NTIL displays a broad fluorescence band at 478 nm, producing a bright cyan fluorescence when illuminated with a 365 nm UV lamp. This luminescence nearly disappears upon the introduction of mercury, with the intensity at 478 nm almost decreasing, indicating its capability to detect trace levels of Hg2+ ions. The fluorescence characteristics of the NTIL-loaded filter paper and its quenching effect upon the addition of Hg2+ ions in solution under 365 nm UV light are illustrated in Fig. 10(a) and (b). A crucial feature of any detection system is its ability to restore functionality after use. To address this, we aimed to recover the cyan fluorescence of NTIL-loaded filter paper. After washing the NTIL-loaded filter paper with DCM and drying it under vacuum conditions, there was a partial recovery of fluorescence (Fig. 10(a) and (b)).7. Conclusion
The present report introduces a pyrene butyrate-based ionic liquid (NTIL) and its low dimensional material, nNTIL, for the selective and sensitive identification of Hg2+ ions. In an aqueous environment, NTIL generates nanoparticles that possess various important photophysical properties. Within these nanomaterials (referred to as nanoballs), the pyrene units are arranged in a crystalline lattice structure. The nanocrystallinity within the nanoparticles was investigated using steady-state and time-resolved spectroscopy, microscopy, and calorimetric techniques. These nanoparticles demonstrate a unique excited state association of luminogens, where the chromophore units further aggregate in their first excited electronic state, exhibiting behavior that goes beyond mere dimerization. Moreover, introducing Hg2+ into the nNTIL solution results in a bathochromic shift of 2 nm (from 344 nm to 346 nm). It is accompanied by prominent Mie-scattering, inferring further aggregation due to the strong electrostatic interaction between pyrene butyrate and Hg2+ ions, resulting in a remarkable quenching of photoluminosity of nNTIL. The quenching process is dynamic due to strong π–π stacking interactions among the pyrene moieties due to enhanced electrostatic interactions between pyrene butyrate and Hg2+ ions. The enhanced electrostatic interaction between pyrene butyrate and Hg2+ ions causes the congealing of nanoparticles, which is revealed from SEM analysis. The EDS results further corroborate the presence of Hg2+ ions within the coagulated crystalline nanomaterials. The quenching process is also evident from the photoluminescence lifetime decay analysis. The practical applications of this sensor were illustrated through analysing water and soil samples, paving the way for further applications in diverse fields. Furthermore, we investigated the sensor's effectiveness in detecting mercury(II) ions using affordable test strips. Also, neat NTIL can identify Hg2+ ions and restore the cyan fluorescence of NTIL by washing with DCM using these cost-effective strips.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the Anusandhan National Research Foundation (ANRF) erstwhile Science and Engineering Research Board (SERB), New Delhi, Govt. of India, (Grant No: EEQ/2023/000048) for the financial support; the Ministry of Minority Affairs for the Maulana Azad National Fellowship awarded to NT; and the Govt. of West Bengal, India, for the Swami Vivekananda Merit Cum Means Scholarship provided to SA, JC, and MM, respectively. We are indebted to Prof. Mintu Halder, Indian Institute of Technology, Kharagpur, for allowing us to record time-resolved fluorescence decay profiles in their TCSPC set-up.References
- J. Burke, A taste of the future, Nat. Mater., 2003, 2, 363–365 CrossRef PubMed.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Applications of ionic liquids in the chemical industry, Chem. Soc. Rev., 2007, 37, 123–150 RSC.
- P. Kipkorir and S. Magut, Ionic Liquids and GUMBOS for Biomedical and Sensing Applications, PhD thesis, Louisiana State University, 2014 Search PubMed.
- D. R. Macfarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy applications of ionic liquids, Energy Environ. Sci., 2013, 7, 232–250 RSC.
- M. Smiglak, A. Metlen and R. D. Rogers, The second evolution of ionic liquids: from solvents and separations to advanced materials–energetic examples from the ionic liquid cookbook, Acc. Chem. Res., 2007, 40, 1182–1192 CrossRef CAS PubMed.
- M. Smiglak, J. M. Pringle, X. Lu, L. Han, S. Zhang, H. Gao, D. R. Mac Farlane and R. D. Rogers, Ionic liquids for energy, materials, and medicine, Chem. Commun., 2014, 50, 9228–9250 RSC.
- S. Zhang, K. Dokko and M. Watanabe, Porous ionic liquids: synthesis and application, Chem. Sci., 2015, 6, 3684–3691 RSC.
- S. Zhang, K. Dokko and M. Watanabe, Carbon materialization of ionic liquids: from solvents to materials, Mater. Horiz., 2015, 2, 168–197 RSC.
- S. Zhang, Q. Zhang, Y. Zhang, Z. Chen, M. Watanabe and Y. Deng, Beyond solvents and electrolytes: Ionic liquids-based advanced functional materials, Prog. Mater. Sci., 2016, 77, 80–124 CrossRef CAS.
- J. Liang, M. Chu, Z. Zhou, Y. Yan and Y. S. Zhao, Optically Pumped Lasing in Microscale Light-Emitting Electrochemical Cell Arrays for Multicolor Displays, Nano Lett., 2020, 20, 7116–7122 CrossRef CAS PubMed.
- J. Cui, Y. Li, D. Chen, T.-G. Zhan, K.-D. Zhang, J. Cui, Y. Li, T.-G. K. Zhan, D. Zhang and D. Chen, Ionic Liquid-Based Stimuli-Responsive Functional Materials, Adv. Funct. Mater., 2020, 30, 2005522 CrossRef CAS.
- Y. Yoshida and H. Kitagawa, Chromic Ionic Liquids, ACS Appl. Electron. Mater., 2021, 3, 2468–2482 CrossRef CAS.
- W. I. S. Galpothdeniya, F. R. Fronczek, M. Cong, N. Bhattarai, N. Siraj and I. M. Warner, Tunable GUMBOS-based sensor array for label-free detection and discrimination of proteins, J. Mater. Chem. B, 2016, 4, 1414–1422 RSC.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- Z. Rahman and S. K. Das, Ionic Liquids based Acid-base Indicators for Aqueous to the Non-Aqueous Medium: An Overview, ChemistrySelect, 2021, 6, 9164–9174 CrossRef CAS.
- Z. Rahman and S. K. Das, Ionic-Liquid-Based, Sustainable Wavelength-Shifting Materials for Energy Conversion: A Minireview, ChemistrySelect, 2022, 7, e202103898 CrossRef CAS.
- M. Mahato, Y. Murakami and S. K. Das, Recent advances and applications of ionic liquids-based photonic materials, Appl. Mater. Today, 2023, 32, 101808 CrossRef.
- C. N. R. Rao and A. K. Cheetham, Science and technology of nanomaterials: current status and future prospects, J. Mater. Chem., 2001, 11, 2887–2894 RSC.
- S. Kayal, A. Mandal, P. Pramanik and M. Halder, Hypothesizing the applicability of the principle of linear combination in predicting sensing behaviors of quantum dots: A deeper understanding of the precise tuning of quantum dot properties with capping composition, J. Photochem. Photobiol., A, 2019, 373, 182–190 CrossRef CAS.
- G. Sahay, J. O. Kim, A. V. Kabanov and T. K. Bronich, The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents, Biomaterials, 2010, 31, 923–933 CrossRef CAS PubMed.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- M. Galluzzi, S. Bovio, P. Milani and A. Podestà, Surface Confinement Induces the Formation of Solid-Like Insulating Ionic Liquid Nanostructures, J. Phys. Chem. C, 2018, 122, 7934–7944 CrossRef CAS.
- M. Antonietti, D. Kuang, B. Smarsly and Y. Zhou, Ionic Liquids for the Convenient Synthesis of Functional Nanoparticles and Other Inorganic Nanostructures, Angew. Chem., Int. Ed., 2004, 43, 4988–4992 CrossRef CAS PubMed.
- J. Z. Du, X. J. Du, C. Q. Mao and J. Wang, Tailor-Made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- H. Kasai, T. Murakami, Y. Ikuta, Y. Koseki, K. Baba, H. Oikawa, H. Nakanishi, M. Okada, M. Shoji, M. Ueda, H. Imahori and M. Hashida, Creation of Pure Nanodrugs and Their Anticancer Properties, Angew. Chem., Int. Ed., 2012, 51, 10315–10318 CrossRef CAS PubMed.
- F. Winnik, Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media, Chem. Rev., 1993, 93, 587–614 CrossRef CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Principles of Fluorescence Spectroscopy, 2006, pp. 1–954 Search PubMed.
- Q. Dai, W. Liu, X. Zhuang, J. Wu, H. Zhang and P. Wang, Ratiometric fluorescence sensor based on a pyrene derivative and quantification detection of heparin in aqueous solution and serum, Anal. Chem., 2011, 83, 6559–6564 CrossRef CAS PubMed.
- G. Sivaraman, T. Anand and D. Chellappa, Development of a pyrene based “ turn on” fluorescent chemosensor for Hg2+, RSC Adv., 2012, 2, 10605–10609 RSC.
- H. Tong, Y. Hong, Y. Dong, M. Häussler, Z. Li, J. W. Y. Lam, Y. Dong, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Protein detection and quantitation by tetraphenylethene-based fluorescent probes with aggregation-induced emission characteristics, J. Phys. Chem. B, 2007, 111, 11817–11823 CrossRef CAS PubMed.
- J. R. Lakowicz, Instrumentation for Fluorescence Spectroscopy, Principles of Fluorescence Spectroscopy, 2006, pp. 27–61 Search PubMed.
- S. V. Wegner, A. Okesli, P. Chen and C. He, Design of an emission ratiometric biosensor from MerR family proteins: A sensitive and selective sensor for Hg2+, J. Am. Chem. Soc., 2007, 129, 3474–3475 CrossRef CAS PubMed.
- R. Häner, S. M. Biner, S. M. Langenegger, T. Meng and V. L. Malinovskii, A Highly Sensitive, Excimer-Controlled Molecular Beacon, Angew. Chem., Int. Ed., 2010, 122, 1249–1252 CrossRef.
- P. Pramanik, S. K. Das and M. Halder, FRET-Selective and Ion-Exchange Responsive Smart Nano-GUMBOS from Functionalized Pyrene: First Observation of Excited State Aggregation (Exciaggremer) Inside Crystalline Nanoball, J. Phys. Chem. C, 2020, 124, 4791–4801 CrossRef CAS.
- A. Kumar, M. Dubey, R. Pandey, R. K. Gupta, A. Kumar, A. C. Kalita and D. S. Pandey, A Schiff base and its copper(II) complex as a highly selective chemodosimeter for mercury(II) involving preferential hydrolysis of aldimine over an ester group, Inorg. Chem., 2014, 53, 4944–4955 CrossRef CAS PubMed.
- P. Holmes, K. A. F. James and L. S. Levy, Is low-level environmental mercury exposure of concern to human health?, Sci. Total Environ., 2009, 408, 171–182 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Review: Environmental exposure to mercury and its toxicopathologic implications for public health, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- R. A. Bernhoft, Mercury Toxicity and Treatment: A Review of the Literature, J. Environ. Public Health, 2012, 2012, 460508 Search PubMed.
- C. T. Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob and N. Pirrone, Mercury as a global pollutant: Sources, pathways, and effects, Environ. Sci. Technol., 2013, 47, 4967–4983 CrossRef CAS PubMed.
- G. Genchi, M. S. Sinicropi, A. Carocci, G. Lauria and A. Catalano, Mercury Exposure and Heart Diseases, Int. J. Environ. Res. Public Health, 2017, 14, 74 CrossRef PubMed.
- B. Gworek, O. Bemowska-Kałabun, M. Kijeńska and J. Wrzosek-Jakubowska, Mercury in Marine and Oceanic Waters—a Review, Water, Air, Soil Pollut., 2016, 227, 1–19 CrossRef CAS PubMed.
- S. M. Ullrich, T. W. Tanton and S. A. Abdrashitova, Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation, Crit. Rev. Environ. Sci. Technol., 2001, 31, 241–293 CrossRef CAS.
- A. J. Poulain, E. Garcia, M. Amyot, P. G. C. Campbell, F. Raofie and P. A. Ariya, Biological and chemical redox transformations of mercury in fresh and salt waters of the high arctic during spring and summer, Environ. Sci. Technol., 2007, 41, 1883–1888 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Tools and tactics for the optical detection of mercuric ion, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- J. Wang, B. Tian, J. Lu, J. Wang, D. Luo and D. MacDonald, Remote Electrochemical Sensor for Monitoring Trace Mercury, Electroanalysis, 1998, 10, 399–402 CrossRef CAS.
- G. Mor-Piperberg, R. Tel-Vered, J. Elbaz and I. Willner, Nanoengineered electrically contacted enzymes on DNA scaffolds: Functional assemblies for the selective analysis of Hg2+ Ions, J. Am. Chem. Soc., 2010, 132, 6878–6879 CrossRef CAS PubMed.
- A. Ono, H. Togashi, A. Ono and H. Togashi, Highly Selective Oligonucleotide-Based Sensor for Mercury(II) in Aqueous Solutions, Angew. Chem., Int. Ed., 2004, 116, 4400–4402 CrossRef.
- M. Dong, Y. W. Wang and Y. Peng, Highly selective ratiometric fluorescent sensing for Hg2+ and Au3+, respectively, in aqueous media, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed.
- X. J. Zhu, S. T. Fu, W. K. Wong, J. P. Guo and W. Y. Wong, A near-infrared-fluorescent chemodosimeter for mercuric ion based on an expanded porphyrin, Angew. Chem., Int. Ed., 2006, 45, 3150–3154 CrossRef CAS PubMed.
- I. B. Kim and U. H. F. Bunz, Modulating the sensory response of a conjugated polymer by proteins: An agglutination assay for mercury ions in water, J. Am. Chem. Soc., 2006, 128, 2818–2819 CrossRef CAS PubMed.
- Y. Zhao and Z. Zhong, Tuning the sensitivity of a foldamer-based mercury sensor by its folding energy, J. Am. Chem. Soc., 2006, 128, 9988–9989 CrossRef CAS PubMed.
- J. Liu and Y. Lu, Rational Design of “Turn-On” Allosteric DNAzyme Catalytic Beacons for Aqueous Mercury Ions with Ultrahigh Sensitivity and Selectivity, Angew. Chem., Int. Ed., 2007, 46, 7587–7590 CrossRef CAS PubMed.
- C. K. Chiang, C. C. Huang, C. W. Liu and H. T. Chang, Oligonucleotide-based fluorescence probe for sensitive and selective detection of mercury(II) in aqueous solution, Anal. Chem., 2008, 80, 3716–3721 CrossRef CAS PubMed.
- F. Loe-Mie, G. Marchand, J. Berthier, N. Sarrut, M. Pucheault, M. Blanchard-Desce, F. Vinet, M. Vaultier, F. Loe-Mie, G. Marchand, J. Berthier, N. Sarrut, F. Vinet, M. Pucheault, M. Blanchard-Desce and M. Vaultier, Towards an Efficient Microsystem for the Real-Time Detection and Quantification of Mercury in Water Based on a Specifically Designed Fluorogenic Binary Task-Specific Ionic Liquid, Angew. Chem., Int. Ed., 2010, 49, 424–427 CrossRef CAS PubMed.
- W. Lin, X. Cao, Y. Ding, L. Yuan and L. Long, A highly selective and sensitive fluorescent probe for Hg2+ imaging in live cells based on a rhodamine – thioamide – alkyne scaffold, Chem. Commun., 2010, 46, 3529–3531 RSC.
- N. Dave, M. Y. Chan, P. J. J. Huang, B. D. Smith and J. Liu, Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water, J. Am. Chem. Soc., 2010, 132, 12668–12673 CrossRef CAS PubMed.
- J. J. Mcnerney, P. R. Buseck and R. C. Hanson, Mercury Detection by Means of Thin Gold Films, Science, 1972, 178, 611–612 CrossRef CAS PubMed.
- J. Drelich, C. L. White and Z. Xu, Laboratory Tests on Mercury Emission Monitoring with Resonating Gold-coated Silicon Cantilevers, Environ. Sci. Technol., 2008, 42, 2072–2078 CrossRef CAS PubMed.
- M. Amde, Y. Yin, D. Zhang and J. Liu, Methods and recent advances in speciation analysis of mercury chemical species in environmental samples: a review, Chem. Speciation Bioavailability, 2016, 28, 51–65 CrossRef CAS.
- D. K. Sarfo, A. Sivanesan, E. L. Izake and G. A. Ayoko, Rapid detection of mercury contamination in water by surface enhanced Raman spectroscopy, RSC Adv., 2017, 7, 21567–21575 RSC.
- T. Sultana, M. Mahato, S. Ahamed, N. Tohora, J. Chourasia, S. Ghanta and S. Kumar Das, A ‘Turn-on’ fluorogenic probe for selective and specific detection of Hg(II) ions, J. Photochem. Photobiol., A, 2025, 459, 116028 CrossRef CAS.
- M. Oguz, D. Aydin, S. Malkondu and S. Erdemir, Specific and low-level detection of Hg2+ and CN− in aqueous solution by a new fluorescent probe: Its real sample applications including cell, soil, water, and food, Sens. Actuators, B, 2025, 433, 137527 CrossRef CAS.
- A. Aswathy, P. K. Vineetha, V. Kandathil, J. Jose, S. G. Bhat and N. Manoj, A Simple Live Cell Imaging “Turn–On” Fluorescence Probe for the Selective and Sensitive Detection of Aqueous Hg2+ Ions, J. Fluoresc., 2024, 34, 1671–1682 CrossRef CAS PubMed.
- H. Li, J. Li, Z. Pan, T. Zheng, Y. Song, J. Zhang and Z. Xiao, Highly selective and sensitive detection of Hg2+ by a novel fluorescent probe with dual recognition sites, Spectrochim. Acta, Part A, 2023, 291, 122379 CrossRef CAS PubMed.
- W. Chansuvarn, T. Tuntulani and A. Imyim, Colorimetric detection of mercury(II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials, TrAC, Trends Anal. Chem., 2015, 65, 83–96 CrossRef CAS.
- J. Du, L. Jiang, Q. Shao, X. Liu, R. S. Marks, J. Ma and X. Chen, Colorimetric Detection of Mercury Ions Based on Plasmonic Nanoparticles, Small, 2013, 9, 1467–1481 CrossRef CAS PubMed.
- X. Xu, Y. F. Li, J. Zhao, Y. Li, J. Lin, B. Li, Y. Gao and C. Chen, Nanomaterial-based approaches for the detection and speciation of mercury, Analyst, 2015, 140, 7841–7853 RSC.
- G. Chen, Z. Guo, G. Zeng and L. Tang, Fluorescent and colorimetric sensors for environmental mercury detection, Analyst, 2015, 140, 5400–5443 RSC.
- L. R. Pokhrel, N. Ettore, Z. L. Jacobs, A. Zarr, M. H. Weir, P. R. Scheuerman, S. R. Kanel and B. Dubey, Novel carbon nanotube (CNT)-based ultrasensitive sensors for trace mercury(II) detection in water: A review, Sci. Total Environ., 2017, 574, 1379–1388 CrossRef CAS PubMed.
- J. B. Birks, D. J. Dyson and I. Munro, ‘Excimer’ fluorescence II. Lifetime studies of pyrene solutions, Proc. R. Soc. London, Ser. A, 1963, 275, 575–588 CAS.
- F. Winnik, Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media, Chem. Rev., 1993, 93, 587–614 CrossRef CAS.
- R. D. Pensack, R. J. Ashmore, A. L. Paoletta and G. D. Scholes, The Nature of Excimer Formation in Crystalline Pyrene Nanoparticles, J. Phys. Chem. C, 2018, 122, 21004–21017 CrossRef CAS.
- A. Shrivastava and V. Gupta, Methods for the determination of limit of detection and limit of quantitation of the analytical methods, Chron. Young Sci., 2011, 2, 21 CrossRef.
- H. A. Benesi and J. H. Hildebrand, A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- D. Ghosh, N. Nandi and N. Chattopadhyay, Differential Förster resonance energy transfer from the excimers of poly(N-vinylcarbazole) to coumarin 153, J. Phys. Chem. B, 2012, 116, 4693–4701 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available See DOI: https://doi.org/10.1039/d4cp04660a |
| This journal is © the Owner Societies 2025 |