Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Kinetic processes of interfacial transport of reactive species across plasma–water interfaces: the effect of temperature†
Frederick J
Green
and
Mohammad I
Hasan
 *
*
Department of Electrical Engineering and Electronics, The University of Liverpool, Brownlow Hill, L69 3GJ, UK. E-mail: mihasan@liverpool.ac.uk
First published on 6th January 2025
Abstract
This work quantifies, through use of molecular dynamics (MD) simulations, the kinetic rates of physical surface processes occurring at a plasma–water interface. The probabilities of adsorption, absorption, desorption and scattering were computed for O3, N2O, NO2, NO, OH, H2O2, HNO2, HNO3, and N2O5 as they interact with the interface at three water temperatures: 298 K, 323 K, and 348 K. Species are categorised into the short-residence group (O3, N2O, NO2, and NO) and the long-residence group (OH, H2O2, HNO2, HNO3, and N2O5) based on their mean surface residence time. It is reported that the most probable process for the short-residence group is desorption, which limits their characteristic residence time at the interface to less than 100 ps, while the long-residence species experience a mixture of absorption and desorption, with a characteristic residence time exceeding 200 ps for many species in this group. With increasing water temperature, a universal decline in characteristic surface residence time is observed. It is found that the short-residence group experience a reduction in probability of desorption in favour of scattering, whereas the long-residence group experience a reduction in probability of adsorption in favour of absorption and desorption. The data reported in this work facilitate the development of a basic surface kinetic model, which was used to find that tuning the plasma toward the production of HNO3 will result in an increase in the rate of uptake of reactive nitrogen species by a factor of 250%.
1. Introduction
The question of how plasma-generated reactive species are transported across a plasma–water interface is crucial, with broad implications for various scientific and technological fields where plasma activates water.1–4 Understanding the behaviour of these reactive species at a plasma–water interface holds significance for applications such as environmental remediation,5,6 materials science7 and biomedicine.3,8–10 Plasma-generated reactive species, particularly H2O2,11,12 are known for their strong antimicrobial properties13–15 and ability to treat toxic organic compounds in wastewater,16,17 occupying key roles in plasma-based sterilization methods.18–20A key step to investigate the transport of plasma-generated reactive species across a plasma–water interface is to describe how a plasma–water interface differs from a gas–water interface. Since theory and measurements for the multiphase transport of reactive species across a gas–water interface are widely available,21–24 it is reasonable to question how much of that knowledge is applicable to a plasma–water interface. The presence of plasma at the surface of water drives multiple processes that are unique to it; these include the application of an electric field, enhanced chemical reactivity due to the presence of reactive species, mechanical convection, and heat transfer. Multiple studies have investigated the electric field at such interfaces, reporting a typical strength in the order of 104 to 105 V m−1.25,26 Such values are much lower than 109 V m−1, the strength at which the electric field induces significant structural and behavioural changes in water.27–29 Chemically, the typical plasma densities in atmospheric pressure plasmas, along with the densities of generated reactive species (assuming no significant accumulation) are on the order of 1021 m−3,30 which is seven orders of magnitudes lower than the density of liquid water at ambient conditions (around 1028 m−3). When converted to surface densities, this means that for every molecule of a reactive species at the interface, there are approximately 50,000 surface water molecules. This suggests that the characteristics of water at the interface are minimally impacted by the presence of reactive species as their influence is negligible. Mechanically, despite the very dilute nature of the plasma, it has been shown in multiple studies that the interaction at the interface is significant enough to drive a flow in the bulk of the water.26,31–33 In addition to the observed macroscopic flow, smaller and faster perturbations exist, such as the Kelvin–Helmholtz instability.31 Thermally, most experiments where the plasma interacts directly with water report a significant increase in the temperature of the bulk water.34–36 Based on this discussion, it is reasonable to describe the plasma–liquid interface as a special case of the gas–liquid interface where the water experiences a shear turbulent flow at an elevated temperature, as a first approximation.
Most studies on the transport of gaseous species across a gas–water interface were conducted under equilibrium conditions, under which the interfacial transport can be described by Henry's law.37 Indeed, this assumption is often made even in explicit treatment of a plasma–water interface.17 Given that nonthermal plasmas are far from equilibrium, the applicability of Henry's law is questionable, and a kinetic description is needed. In such a description, a molecule of a reactive species arriving from the plasma phase to the interface may experience a chemical process such as a chemical reaction, or may experience a physical process such as thermal or mass accommodation. In this work, we take thermal accommodation to mean that the radical arriving to the interface has had enough time interacting with water molecules such that its kinetic energy is equilibrated with that of the water molecules,38 and mass accommodation is defined as the probability that a molecule residing at the interface will be absorbed into the bulk phase.39,40 Therefore, quantifying the characteristic timescales and probabilities of such processes is vital for constructing a detailed model of the surface in plasma liquid interactions.41 Indeed, in a comprehensive 2016 review of plasma–liquid interactions, Bruggeman et al. concluded that understanding the transport and chemistry of reactive species at the plasma–liquid interface is a key challenge in the field.42
To build a kinetic description of the interfacial transport of reactive species at the gas–water interface, previous work has been undertaken focusing on the thermal and mass accommodation of a variety of reactive species in the context of atmospheric chemistry. One approach was to compute the free energy of solvation for a variety of reactive species (notably O3, OH and H2O2) via molecular dynamics (MD) simulations, subsequently computing the probability of surface effects such as adsorption, absorption and desorption.38,43 However, this approach has proven challenging as the calculated probabilities depend upon how they are derived from the solvation energy. An alternative, more intuitive approach has since emerged, which has been applied to a wider range of species including N2O5, NO2, HO2 and CH3NH2.4,38,44–47 In this approach, a molecule is bombarded against a slab of water and its fate; namely whether it is scattered, absorbed, adsorbed or desorbed, is determined within a timeframe. By repeating this process over sufficiently many runs it is possible to quantify the probability of each individual process.48 Notably, work by Vieceli et al.4 using this approach applies it to O3 and OH, reporting that an O3 molecule has an approximate desorption probability of 65% on the water surface, an adsorption probability of 20%, and an absorption probability of 4%. The corresponding figures for OH were found to be 5%, 57% and 33% respectively. This work aims to investigate and quantify the mass accommodation processes of reactive species typically generated in air plasmas, these are H2O2, O3, OH, N2O, N2O5, HNO2, HNO3, NO and NO2, as the water temperature is varied. Processes investigated include absorption, adsorption, scattering and desorption, as well as quantifying the characteristic residence time of a given species on the interface as function of temperature. The data reported in this work paves the way for the construction of detailed surface kinetic models of the reactive species at the plasma–water interface, capable of capturing the non-equilibrium nature of the transport of reactive species across the plasma–water interface. Mathematically, the processes analysed in this work can be represented as processes 1 to 3, which are desorption, adsorption and absorption respectively.
 | (1) |
 | (2) |
 | (3) |
2. Methods
The MD simulations in this work were conducted using LAMMPS (version 8 Feb 2023).49 The reactive species investigated were H2O2, OH, O3, N2O, N2O5, HNO2, HNO3, NO and NO2 as these are the most frequently reported-on species in the context of air plasmas. The molecular geometries used for the simulated molecules were obtained from the Automated Topology Builder (ATB) repository,50 which uses the GROMOS 54A7 force field.51 The repository utilises GAMESS-US, wherein the first stage the molecule is optimised at HF/STO-3G level of theory, followed by refinement stage where it is further optimised at B3LYP/6-31G level of theory. This is done assuming an implicit polarisable continuum model (PCM). The optimization process yields the geometry as well as the partial charges assigned to each atom in the molecule.50 For water, the SPC/E model was used as this combination of force field is widely used.52–55 Both force fields are nonreactive force fields, which restricts the investigated surface processes to physical processes as chemical reactions cannot be directly captured. Despite the existence of reactive force fields such as ReaxFF56 that explicitly model bond formation, the size of the timesteps required to resolve these dynamics appropriately is too small to capture the timescale of the physical processes studied in this article. The size of the simulation domain was 31 Å × 31 Å × 150 Å. A slab of water consisting of 1000 molecules was created in the region of 0 < Z < 31 Å, leaving enough space for the studied molecule to be introduced into the simulation domain far from the surface of the water slab. These parameters were chosen to obtain a water density of approximately 1 g cm−3. To further verify the density, a hyperbolic tangent function was fit to the Z axis profile of the water slab. Selecting a cutoff density threshold of one-half the bulk maximum, a value of 30.75 Å was obtained. Taking the X and Y axes to both equal 31 Å according to spatial restriction due to the application of a periodic boundary condition, we found that the density of the slab to be 1.01 g cm−3. The Ewald summation method was used to calculate long-range coulombic interactions.57 The Nosé–Hoover thermostat was used to control the system temperature,58 which was set to three values for each species, being 298 K, 323 K and 348 K. The SHAKE algorithm was employed to keep the water molecules rigid.59 The simulation box as well as the procedure used for generating the results are summarised in Fig. 1. The procedure for generating the reported data consisted of 3 stages: the equilibration phase, production phase, and post-processing analysis.In the water equilibration phase, the water slab was equilibrated for 2 nanoseconds in the canonical (NVT) ensemble with a timestep of 2 femtoseconds, for the three investigated temperatures. To prevent the slab from drifting in the box, an invisible atom of large mass was attached to the bulk. A snapshot of the system at the end of equilibration for each temperature was then used as the starting point for the subsequent bombardment simulations to circumvent the need to equilibrate the system for every run. In the production phase, a molecule was introduced into the simulation box at a distance, calculated from the centres of mass of the molecules, of 30 Å above the surface and assigned an initial velocity that directed it toward the water slab. Its interaction with the slab was quantified by recording the Z coordinate of the centre of mass of the molecule as function of time, and outputting that into a trajectory file. Note that the positions of all molecules are recorded using the centre of mass of the molecule. For each trajectory, the file begins recording this data as soon as the molecule's distance from the surface is equal to or less than 9 Å as this is the cut off used for the force field, defining the threshold at which the molecule starts to interact with the surface. If the molecule's Z coordinate is higher than 75 Å, which corresponds to the molecule leaving the surface after having interacted with it, the simulation was terminated to prevent the molecule from crossing the periodic boundary condition. The orientation of the molecule with respect to the surface, and its X and Y coordinates in the plane where it is introduced, were randomly assigned for each trajectory. The molecule is assigned an initial thermal velocity in the negative Z direction, calculated using eqn (4), which moves it toward the surface of the water. The molecule at this stage is excluded from the NVT ensemble until it reaches the surface.
 | (4) |
 | ||
| Fig. 2 Illustration of the classification criteria used to quantify the probability of the individual surface processes and explaining the procedure followed for the quantification. | ||
Scattering: a species is considered scattered if it resides on the water surface for less time than required to reach thermal equilibrium with the water slab. This definition renders scattering phenomenologically distinct from desorption, given that there is not sufficient contact with the surface to facilitate significant energy exchange. This value was chosen to be 2 picoseconds before being ejected back into the gas phase, based on previous work.4,38,60 precisely, that is ZSmin < Zmolecule < ZSmax for less than 2 ps. Notably, there is some contention in the literature regarding the thermal equilibration time, with some works reporting that it can take an interval on the order of 10 ps or longer for the kinetic energy of some of the investigated molecules to equilibrate with that of the bulk.61 However, an analysis of time-dependent kinetic energy fluctuation with respect to ensemble average by Vieceli et al. yields the general result that 2 ps is sufficient time for a molecule to dissipate its excess kinetic energy.4
Desorption: if the species is not on or below the surface at the end of the simulation, but was not scattered, then it is considered desorbed, that is: Zmolecule > ZSmax at the end of the trajectory file. Absorption: if the species is below the surface at the end of the simulation, then it is considered absorbed, that is: Zmolecule < ZSmin. Adsorption: if the species is on the surface at the end of the simulation, then it is considered adsorbed, that is: ZSmin < Zmolecule < ZSmax. It is worth noting that the investigated species, during the simulation, may undergo one or several of these processes during its trajectory, but only its state at the end of the trajectory is recorded. For example, a molecule residing on the water surface may become absorbed by entering the bulk but return to the surface for the remaining duration of the simulation. In this case, it is considered absorbed while under the surface, but will cease to be considered absorbed after returning to the surface. Ultimately, the classification for that trajectory will be adsorption.
3. Results and discussion
3.1. Validation of the simulation procedure
To verify that the results obtained in this work are physical, two validation approaches were followed. First, a comparison of the data obtained in this work to that already reported for similar conditions, which validates the simulation procedure. Second, considering that the temperature variance is a novel aspect of this work, the suitability of the employed force field to capture temperature variation is discussed. Most of the previous works focused on the processes that O3 and OH experience at ambient conditions on the surface of water,4,38 as such conditions are the most common in the context of atmospheric chemistry. O3 and OH are chosen as representatives for hydrophobic and hydrophilic species, respectively. Fig. 3 shows the normalised time-dependent surface population of both species for the 298 K case as computed in this work in comparison to those reported in the literature. It should be noted that t = 0 in this figure is the time of arrival of the molecule to the interface from the gas phase for all trajectories. As time progresses, some trajectories leave the surface by either desorption or absorption, therefore the fraction shown on the Y axis of this figure indicates the ratio of the resident molecules at a given time to the overall number of molecules at the surface. A clear qualitative agreement exists between the presented results in this work and the reported results in the literature, showing the tendency of O3 to completely leave the surface within 100 ps, where it is mostly desorbed due to its hydrophobic nature. The figure shows a similar agreement for OH, which stays for an extended period on the surface and is mostly lost to absorption. This is a reflection of its solvation energy, which has a pronounced minimum at the surface.38 Quantitively, there are noticeable differences between the results reported here and those reported in the literature, particularly for OH. These stem largely from differences in the method used to classify whether the molecule is in the gas, interfacial or bulk phase. Some work has employed the solvent accessible surface area (SASA) technique, where the surface area of a molecule that is available to a solvent is calculated, and its phase inferred.38 This approach precludes the requirement for an interface region, though at increased computational cost. Another reason is the difference in some of the force field parameters used. In this work we opted for consistency by using the GROMOS force field for all molecules. In the referred works, the force field parameters were collected from diverse sources, potentially arbitrarily. Despite the differences, the overall behaviour is similar and therefore the method followed in this work is valid for generating a similar type of data. It is worth noting that while the classical force field effectively models hydrogen bonding and other non-reactive interactions, and allows for a direction comparison to the literature in this case, it does not capture potential reactive pathways, such as OH recombination or reaction with the water surface. | ||
| Fig. 3 The time-dependent surface populations of O3 and OH as computed in this work in comparison to those reported by Roselová1et al. and Vieceli et al.4 | ||
The validation of the temperature trend is more challenging, as data on surface processes as a function of temperature for different molecules is scarce. However, there are multiple works reporting on the GROMOS force field's ability to capture the effect of different temperatures in the solvent.51,62,63 Furthermore, considering that the decrease of solubility of gases in water with increasing temperature is a well-established phenomenon in physical chemistry, a simple test was conducted, computing the radial distribution function (RDF) of O3 and HNO3 molecules at the three investigated temperatures in this work. The RDF was computed and averaged over a trajectory of 1 ns in the bulk of the water slab shown in Fig. 1. The RDFs of O3 and HNO3 are shown in Fig. 4, which demonstrates a reduction in the peak of the RDF as the temperature of the solvent is increased, representing a weaker hydration shell which indirectly corresponds to lower solubility. While the decrease in this peak seems marginal, it is consistent in its amplitude to that reported for the RDF of water at different temperatures.64 Quantitively, the RDF and the solubility of gases are fundamentally correlated, but their correlation is complex and far beyond the scope of this work.
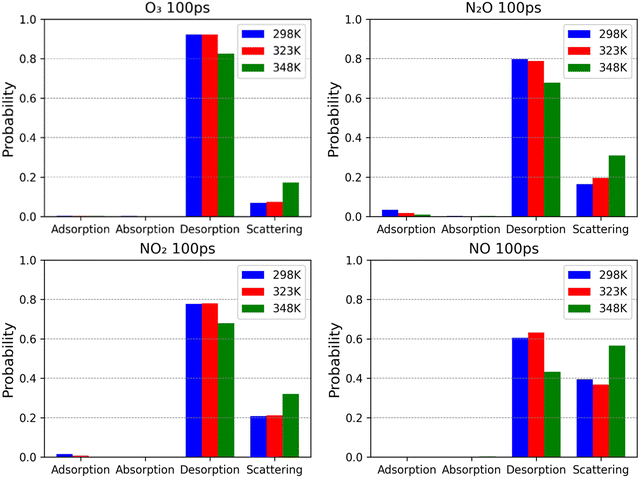
3.2. Probabilities of surface processes
As explained in Section 2, the outcome of each trajectory was categorised at two timeframes into adsorption, desorption, absorption and scattering, then the number of trajectories in each category was normalised by the overall number of trajectories to give the probability of a specific process. Based on the preliminary analysis of the results it was found that the species can be split into two groups with distinctive behaviours, differing mainly in surface residence time and absorption probability. The first group will be referred to as the short-residence group, that includes O3, N2O, NO and NO2. The second is the long-residence group that consists of OH, H2O2, HNO2, HNO3, and N2O5. Fig. 5 shows the probability of these surface processes at 100 ps for the short-residence group at the three temperatures, while Fig. 6 shows the same figure for the long-residence group at two timeframes (100 ps and 200 ps). To give an example of how these figures can be interpreted; a species the probability of adsorption after 100 ps of arrival to the surface is 40%.Another more general description of interfacial transport is given by the free energy of solvation or the potential of mean force (PMF) corresponding to the solvation process,43 which can be computed using umbrella sampling.67 This description encapsulates the same information contained in Henry's coefficient in addition to providing information on the likelihood of the species residing on the surface. Examining the PMF reported in the literature for most species analysed in this work, a common feature is the existence of a free energy minimum at the interface, indicating a preference for adsorption over immediate absorption (that is molecules residing on the surface for some time before being absorbed). This applies to O3,43 OH,43 H2O2,43 N2O568 and NO2.69 We could not find the PMF of NO, HNO2 or HNO3, however, a MD study has shown a strong preference for HNO3 to reside on the surface as well,70 indicating the presence of a minimum of the PMF there. Since the PMF describes the Gibbs free energy of solvation, it can be converted into a relative probability using the Boltzmann factor, providing information on the relative probability of adsorption and desorption, which makes a direct comparison between the PMF and the absolute probabilities presented in Fig. 5 and 6 impossible. Moreover, the relative probabilities described by the PMF represent steady state behaviour, providing further difficulty in making a direct comparison between the PMF and the probabilities reported in Fig. 5 and 6.
A third description of the interfacial transport of reactive species in water is given by the mass accommodation coefficient (α) which, unlike the earlier descriptions, provides kinetic information on the interfacial transport process; that is the fraction of the gaseous molecules being taken into the water bulk following thermal accommodation. Experimental determination of the mass accommodation coefficient of a species is challenging, and as a result their exact values vary widely.71 Even from the perspective of an atomistic simulation, inconsistency still exists due to the variety of the force fields used in the simulations. Nonetheless, the range of values reported for a given species is relatively consistent. For example, α of NO and NO2 in water are reported to be less than 10−3,72 for O3 and HNO2 it is in the order of 10−2,4,38,73,74 for OH, H2O2, and HNO3 it is in the order of 10−1.38,40,73,75 The literature varies widely on the mass accommodation coefficient of N2O5, ranging from values close to 10−3 to values approaching 0.5.73,76 We could not find α for N2O reported anywhere in the literature. The mass accommodation coefficient is the closest parameter to be compared to the absorption probability of any species shown in Fig. 5 and 6. However, a small difference is that the mass accommodation coefficients describe the overall probability of absorption, which includes the direct absorption from the gas phase as well as the adsorption-mediated absorption,43 which are dealt with as two separate processes in Fig. 5 and 6. Therefore, these figures paint the most comprehensive picture of the molecular processes occurring on the surface among all reported works in the literature.
As time progresses, the difference in the behaviour of the two groups becomes clearer. Within the first 100 ps, desorption has the highest probability among the short-residence group, with values ranging from 60% for NO to 92% for O3. The absence of data for absorption in O3 and NO may suggest that no absorption occurs. However, considering the finite number of trajectories used to generate these figures, the absence of a process merely indicates that its probability is less than the threshold detectable probability of 0.25%. Comparing this number to the values of α reported earlier for O3 shows that this work slightly underestimates the probability of absorption. The probability of absorption as described in Fig. 5 for O3 at a temperature of 298 K has already been reported for two other works.4,38 Comparing our value to those, it is perfectly in line with O3 behaviour reported by Roeselová et al.,38 while it is underestimated in comparison to that reported by Vieceli et al.4 Since our values and those of Roeselová et al. are much closer to the experimental mass accommodation coefficient of O3, we believe our estimate is closer to the real value than that reported by Vieceli et al. The behaviour of N2O, NO2 and NO is similar to that of O3, as Fig. 5 shows. This demonstrates a perfect overlap between the short-residence group and the poorly soluble species as defined by Henry's coefficient. It is also quite consistent with the very low mass accommodation coefficient of NO2 reported earlier. The long-residence group (OH, H2O2, HNO2, HNO3 and N2O5) is characterised by spending considerable periods of time on the surface compared to the short-residence group. Over time, their adsorption probabilities decline as the probabilities of absorption and desorption increase as Fig. 6 shows. A common feature among these 5 species is the non-negligible desorption probability, in the range of 5–10% in the 100 ps timeframe. This explains why highly soluble species such as H2O2, HNO2, and HNO3, with Hcc approaching 106 have mass accommodation coefficients in the order of 10−2 to 10−1; desorption in this case works as a limiting process, setting the characteristic time needed for the solvated concentration to reach its steady state value. A distinctive feature among these species is how much of the absorbed species end up desorbing or absorbing. On one end there is N2O5, for which the desorption probability increases from 19% at 100 ps to 57% in 200 ps, while the absorption probability increases from 1% to 4% in the same time interval. This indicates that the reported results are more consistent with the lower estimate of the mass accommodation coefficient of N2O5 reported in the literature. On the other end there is HNO3, for which there is an increase in the probability of absorption from 3% at 100 ps to 5% at 200 ps. For its probability of desorption, there is a decrease from 8% to 4% in the same interval. Other species such as OH, H2O2, and HNO2, show an intermediate behaviour in which the increase in the desorption rate is more significant than that of absorption. However, the increase in the desorption rate of OH and H2O2, at ∼15% each remains higher than or roughly equal to that of adsorption. In general, the overall behaviour of the long-residence group is consistent with its solubility as described by Henry's coefficients and the reported mass accommodation coefficients.
The response to the increase in the temperature of the short-residence group, namely O3, N2O, NO and NO2, is different from the long-residence group, as the competition here is between desorption and scattering. An increase in temperature shows a shift from desorption to scattering, with the exception of NO, which is discussed below. Fundamentally, the result of both processes is the same in that the molecule is ejected from the surface, while the core difference is the interaction time with the surface. In that sense, the shift toward scattering as the temperature increases is a result of a reduced average residence time on the surface.
The increase in the temperature of the water from 298 K to 323 K appears to affect NO differently, wherein scattering probability decreases slightly, with a complementary increase in desorption probability. From 323 K to 348 K however, there is a sharp increase in scattering probability. This is seemingly anomalous, but in fact is due to choice of scattering threshold. While our selection of 2 ps is supported by previous works, it is possible that it is not appropriate for all species given the range of factors, such as mass of the investigated species,79 that may impact thermal equilibration time. Fig. 7 can be used to illustrate this point. The surface residence probabilities for each temperature for NO transiently form the expected temperature trend at around 20 ps. Therefore, a scattering threshold set at 20 ps would reflect this temperature trend, though would deviate from the consensus of previous works. However, it is still favourable to use this threshold rather than calculate such a threshold for each species in this work, given the large range of species included.
3.3. The time-dependent surface population
As discussed in Section 3.2, all species experience surface interaction, however briefly. Since adsorption is the most common and best statistically sampled process and, given also that adsorption quantifies the residence time on the surface, it defines a time window in which chemical reactions may occur. In this section the trajectories are analysed based on the time they spent on the surface. That is, the number of trajectories on the surface for each timestep is computed, omitting all scattered trajectories. When a trajectory leaves the surface at a given time, the value of the population of the surface is decreased by 1 at that time. All plots are normalised to 1 at t = 0 (the moment of arrival to the surface) such that scattered trajectories are omitted. Fig. 7 shows the time-dependent surface residence probabilities for all species at all temperatures. Clearly, surface populations decrease exponentially with time, indicating first order kinetics. It should be noted that the decay in these plots is a result of a combination of desorption and absorption. The relative contribution of each process can be inferred from Fig. 5 and 6. Fig. 7 is an illustration of the basis on which the species were split into the two groups; the short residence group (O3, N2O, NO2, and NO) leaves the surface within the first 100 ps, while the long-residence group exhibits variable behaviour. For example, OH, HNO2, N2O5 and H2O2 seem to leave the surface at comparable rates. HNO3 is the slowest at leaving the surface, almost exhibiting zeroth-order kinetics, as it has a strong preference to stay on the surface as discussed in Section 3.2. To provide a better overview of the characteristic residence times, the data shown in Fig. 7 were fit to a single decaying exponential and presented in Table 1.| Group | Species | 298 K | 323 K | 348 K |
|---|---|---|---|---|
| Short residence | O3 | 20.5 | 17.5 | 14.1 |
| N2O | 40.8 | 27.6 | 21.6 | |
| NO2 | 23.9 | 20.2 | 16.8 | |
| NO | 12.1 | 9.7 | 8.9 | |
| Long residence | OH | 597.4 | 251.8 | 242.4 |
| H2O2 | 296.3 | 136.3 | 132.6 | |
| HNO2 | 561.3 | 454 | 208.1 | |
| HNO3 | 669.3 | 550.5 | 289.8 | |
| N2O5 | 292.5 | 116.7 | 96.1 |
The main influence of temperature on the characteristic residence time of the species is to cause a decrease in the residence time for all species. The most interesting aspect of this behaviour is N2O5, which experiences a reduction in characteristic residence time from 292.5 ps at 298 K to 96.1 ps at 348 K. As such, it is possible to postulate that N2O5 at elevated temperature ‘switches groups’ and acts as a short-residence species. The remaining species appear to stay in their corresponding groups. The species least affected by the temperature increase is N2O, which shows an average decrease in the characteristic residence time of 10% per 25 K increase. On the other end, HNO3 shows a decrease of 36% per 25 K increase. The rate of the decrease is nonlinear in most of the long-residence group, while it is linear in the short-residence group. Species such as HNO3 and N2O5 show a noticeable change in the rate of variation in the curve, particularly at 348 K.
An interesting feature observed in Fig. 7 is the presence of small-scale fluctuations in the time-dependent surface populations for long-residence species such as HNO3 and H2O2, and its complete absence in short-residence species such as O3 and N2O. These small-scale fluctuations are a result of trajectories that switch between adsorbed and absorbed states during the simulation. Similarly, some trajectories switch between desorbed and adsorbed states, albeit this occurs to a smaller extent in comparison to adsorption–absorption fluctuations. While the existence of these fluctuations may introduce a source of noise to the probabilities depicted in Fig. 5 and 6, their small amplitude in comparison to the overall trend supports its validity. The origin of these fluctuations stems from the previously discussed solvation free energy minimum present at the interface for the investigated species. It follows from this that after absorption, assuming the molecule stays close to the surface for some time, there should be a greater probability of returning to the surface than remaining in the bulk, and that this should be more pronounced for species with a greater surface free energy minimum, which is reflected in the literature.43 Considering that only a small fraction of trajectories experiences these rapid fluctuations, proper quantification for this phenomenon requires much larger statistical sampling to provide accurate quantitative measures of their importance, thus it is beyond the scope of this work.
3.4. Implications on the plasma–water interfacial transport processes
Considering that the aim of the work presented here is to build a detailed picture of processes occurring at the plasma–water interface, it is important to put the results into perspective. At the plasma–water interface, it is possible to describe the surface population of a reactive species by eqn (5), which assumes that the surface is far from saturation, which is a reasonable condition considering the dilute nature of low temperature plasmas: | (5) |
In eqn (5), ns is the surface density of the reactive species, γ is the sticking coefficient, which is defined from Fig. 5 and 6 as 1 minus the scattering probability. ng is the density of the species in the plasma phase, vth is its thermal velocity. The first term describes the flux from the plasma phase to the interface. In the second and the third terms, kdes is the rate constant of the desorption process as described by eqn (1) and kabs is the rate constant of the absorption in the bulk as described by eqn (2). The second and the third term on the right-hand side of eqn (5) can be summed into one term that describes the departure of the surface species from the interface, that is kdep = kabs + kdes. The departure rate can be directly estimated as 1/τ where τ is the characteristic residence time given in Table 1. The split of kdep into kabs and kdes is done based on the probabilities of desorption and absorption shown in Fig. 5 and 6, according to eqn (6).
 | (6) |
The same procedure was applied to all species and processed, and the corresponding rate constants are reported in Table 2. It should be noted that eqn (5) only describes the physical processes, implicitly lacking a description of chemical kinetics at the interface, as well as the processes of resurfacing and hopping along the interface, for which work on extending the description of eqn (5) is ongoing. Table 3 reports the sticking coefficients of the different species as a function of temperature.
| Group | Species | k des (s−1) | k abs (s−1) | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 323 K | 348 K | 298 K | 323 K | 348 K | ||
| Short residence | O3 | 4.9 × 1010 | 5.7 × 1010 | 7.1 × 1010 | <2.7 × 108 | <3 × 108 | <4.3 × 108 |
| N2O | 2.5 × 1010 | 3.6 × 1010 | 4.6 × 1010 | <1.4 × 108 | <1.9 × 108 | <2.8 × 108 | |
| NO2 | 4.2 × 1010 | 5 × 1010 | 6 × 1010 | <2.3 × 108 | <2.6 × 108 | <3.5 × 108 | |
| NO | 8.3 × 1010 | 1.0 × 1011 | 1.1 × 1011 | <4.9 × 108 | <5.8 × 108 | <8.6 × 108 | |
| Long residence | OH | 7 × 108 | 2.0 × 109 | 2.1 × 109 | 9.8 × 108 | 1.9 × 109 | 2 × 109 |
| H2O2 | 1.5 × 109 | 3.8 × 109 | 5 × 109 | 1.9 × 109 | 3.6 × 109 | 2.5 × 109 | |
| HNO2 | 1.7 × 109 | 2.0 × 109 | 4.2 × 109 | 4.6 × 107 | 1.8 × 108 | 6.2 × 108 | |
| HNO3 | 3.9 × 108 | 3.2 × 108 | 1.3 × 109 | 1.1 × 109 | 1.5 × 109 | 2.1 × 109 | |
| N2O5 | 3.3 × 109 | 8.4 × 109 | 1 × 1010 | 1.5 × 108 | 1.7 × 108 | <6 × 107 | |
| Group | Species | γ | ||
|---|---|---|---|---|
| 298 K | 323 K | 348 K | ||
| Short-residence | O3 | 0.915 | 0.95 | 0.815 |
| N2O | 0.920 | 0.96 | 0.810 | |
| NO2 | 0.935 | 0.96 | 0.855 | |
| NO | 0.845 | 0.885 | 0.760 | |
| Long-residence | OH | 0.985 | 0.995 | 0.975 |
| H2O2 | 0.995 | 0.995 | 0.995 | |
| HNO2 | 0.995 | 1 | 0.970 | |
| HNO3 | 0.995 | 1 | 0.985 | |
| N2O5 | 0.975 | 0.970 | 0.940 | |
Using eqn (5) and the data presented in Tables 2 and 3, it becomes clear that the uptake of short-residence reactive species into the water is a slow process. Among the long residence group on the other hand there is a noticeable variation in the rate of the uptake of the different species. An apt comparison is between HNO2 and HNO3. Hydrolysis of these species leads to the formation of nitrates and nitrites in the water, which is vital for many emerging applications relying on plasma activation of water. At room temperature, and assuming an equal density of 1021 m−3 for both species in the gas phase, the rate of bulk uptake, defined as the third term in the right-hand side of eqn (5) of HNO2 is 2.54 × 1021 m−2 s−1, while that of HNO3 is 6.32 × 1022 m−2 s−1. This shows that the flux of HNO3 into the bulk is 25 times larger than that of HNO2 assuming the same temperature and densities in the gas phase. Combining that with the characteristic time of hydrolysis (dissociation into H+ and an anion) of HNO3 being in the order of 3 ps and that of HNO2 in the order of 10 ns,80 shows that the rate of activation by the plasma can be faster by 4 to 5 orders of magnitude if the formation of HNO3 is promoted in the plasma. Therefore, tuning the plasma chemistry to maximise the formation of HNO3 will lead to significantly faster activation rate.
4. Conclusions
This work presents one of the most comprehensive studies on the physical processes occurring at a plasma–liquid interface for 9 of the most widely encountered reactive species in the context of air plasmas. The study investigated adsorption, desorption, absorption and scattering probabilities for the investigated species on a longer timescale than reported for any species for which similar studies were conducted, to the best of the authors’ knowledge. The results reported in this work were found to be consistent with experimentally reported mass accommodation coefficients, as well as with the findings of other studies. In comparison to widely reported parameters describing the interfacial transport processes of gaseous species such as Henry's coefficients, the probabilities presented in this work are absolute probabilities that describe the kinetic rate of these processes, paving the way for a new generation of plasma–water interaction models where the chemical kinetics of the surface can be explicitly modelled.The 9 species investigated were O3, N2O, NO, NO2, OH, H2O2, HNO2, HNO3, and N2O5. It was found that these species can be split into two groups based on their characteristic residence times on the surface; a short-residence group consisting of O3, N2O, NO, and NO2, while the rest fall into the long-residence group. A discussion of surface process probability at a constant temperature of 298 K finds that the short-residence group has a probability of desorption in the order of 95% within the first 100 ps of residence on the surface, followed by scattering as the second most probable process. Absorption has a probability of less than 0.5%. The behaviour of this group is fully consistent with its Henry's coefficient being less than 1, reflecting their poor solubility in water. It is also consistent with their mass accommodation coefficients being in the order of 10−3 to 10−2.
The long-residence group on the other hand, shows that the most probable surface process in the first 100 ps is adsorption, which ranges from a minimum of ∼78% for N2O5 and H2O2 to a maximum of 90% for HNO3. Noticeably, all the species in this group have a relatively large desorption probability of around 10–20%, which explains the low values of the mass accommodation coefficients, in the range of 10−2 to 10−1 despite their high Henry's coefficients that exceed 1000 for the majority of them. This indicates that a molecule of these species experiences multiple absorption and adsorption events before ultimately being desorbed. On a longer timeframe of 200 ps, the probability of adsorption decreases, with the sharpest decrease occurring in N2O5, dropping from 78% at 100 ps to 37% at 200 ps. In contrast, HNO3 experienced the lowest decrease, remaining at ∼90% for both timeframes. The decrease in the adsorption probability manifests foremost as an increase in the probability of desorption, followed by absorption.
The influence of the temperature of water was also investigated in this work, since the plasma–water interface was modelled as hot water as a first approximation. For the short-residence group, the increase in the temperature manifested as a reduction in the desorption probability and an increase in the scattering probability. For the long-residence group, the temperature increase shifted the probability from adsorption and absorption to desorption. The residence time of all species decreased as the temperature increased, the most notable behaviour is that of N2O5, which experiences a significant reduction in its residence time to the point that it can be counted as a short-residence species at elevated water temperature.
Given that the scope of this work lies within the study of strictly physical processes at the plasma–water interface, it follows that a major limitation is not accounting for the chemical reactions that many of these reactive species may experience at the surface. Our choice of the force field was made so that it provides a broad coverage of as many species that can be encountered in plasma conditions as possible. While this approach offers a flexible “recipe” extendable to other plasma types, fully capturing reactivity requires a reactive force field (such as ReaxFF) equipped with plasma-optimised parameters, which are not yet available. Addressing this limitation is a work in progress. Nonetheless, a basic kinetic model describing the uptake of HNO2 and HNO3 into the water was built using the data computed in this work. It was found that the uptake flux, the rate at which the species is solvated, of reactive nitrogen species at the plasma–liquid interface can be 250% times faster if the plasma chemistry is tuned to maximise the production of HNO3.
Data availability
The data supporting this article has been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge support of Engineering and Physical Sciences Research Council (EPSRC) Grant No. EP/T000104/1. We would like to acknowledge Dr Martin Stroet from the University of Queensland for his assistance with open-shell molecular models.References
- M. Roeselová, J. Vieceli, L. X. Dang, B. C. Garrett and D. J. Tobias, J. Am. Chem. Soc., 2004, 126, 16308–16309 CrossRef PubMed.
- P. Vanraes and A. Bogaerts, Appl. Phys. Rev., 2018, 5(3), 031103 Search PubMed.
- R. Zhou, R. Zhou, P. Wang, Y. Xian, A. Mai-Prochnow, X. Lu, P. J. Cullen, K. Ostrikov and K. Bazaka, J. Phys. D: Appl. Phys., 2020, 53, 303001 CrossRef CAS.
- J. Vieceli, M. Roeselová, N. Potter, L. X. Dang, B. C. Garrett and D. J. Tobias, J. Phys. Chem. B, 2005, 109, 15876–15892 CrossRef CAS PubMed.
- Y. Y. Zhao, T. Wang, M. P. Wilson, S. J. MacGregor, I. V. Timoshkin and Q. C. Ren, IEEE Trans. Plasma Sci., 2016, 44, 2084–2091 CAS.
- D. Gimžauskaitė, A. Tamošiūnas, M. Aikas and R. Uscila, Environ. Res., 2023, 229, 115976 CrossRef PubMed.
- K. Takenaka and Y. Setsuhara, Jpn. J. Appl. Phys., 2013, 52, 11NE04 CrossRef.
- S. Kim and C.-H. Kim, Biomedicines, 2021, 9(11), 1700 CrossRef CAS PubMed.
- H.-R. Metelmann, T. Von Woedtke and K.-D. Weltmann, Comprehensive clinical plasma medicine: cold physical plasma for medical application, Springer, 2018 Search PubMed.
- N. K. Kaushik, B. Ghimire, Y. Li, M. Adhikari, M. Veerana, N. Kaushik, N. Jha, B. Adhikari, S.-J. Lee and K. Masur, Biol. Chem., 2019, 400, 39–62 CrossRef CAS PubMed.
- M. N. Bathina, S. Mickelsen, C. Brooks, J. Jaramillo, T. Hepton and F. M. Kusumoto, J. Am. Coll. Cardiol., 1998, 32, 1384–1388 CrossRef CAS PubMed.
- M. Krebs, P. Bécasse, D. Verjat and J. Darbord, Int. J. Pharm., 1998, 160, 75–81 CrossRef CAS.
- K. S. Wong, N. S. L. Chew, M. Low and M. K. Tan, Processes, 2023, 11(7), 2213 CrossRef CAS.
- B. J. Juven and M. D. Pierson, J. Food Prot., 1996, 59, 1233–1241 CrossRef CAS PubMed.
- E. Linley, S. P. Denyer, G. McDonnell, C. Simons and J.-Y. Maillard, J. Antimicrob. Chemother., 2012, 67, 1589–1596 CrossRef CAS PubMed.
- O. Lesage, L. Falk, M. Tatoulian, D. Mantovani and S. Ognier, Chem. Eng. Process., 2013, 72, 82–89 CrossRef CAS.
- J. Foster, Phys. Plasmas, 2017, 24(5), 055501 CrossRef.
- A. Sakudo, Y. Yagyu and T. Onodera, Int. J. Mol. Sci., 2019, 20, 5216 CrossRef CAS PubMed.
- T. G. Klämpfl, G. Isbary, T. Shimizu, Y.-F. Li, J. L. Zimmermann, W. Stolz, J. Schlegel, G. E. Morfill and H.-U. Schmidt, Appl. Environ. Microbiol., 2012, 78, 5077–5082 CrossRef PubMed.
- H. Rauscher, O. Kylián, J. Benedikt, A. von Keudell and F. Rossi, ChemPhysChem, 2010, 11, 1382–1389 CrossRef CAS PubMed.
- T. Kamidollayev and J. P. Trelles, J. Phys. D: Appl. Phys., 2023, 56, 505203 CrossRef CAS.
- G. Uchida, A. Nakajima, K. Takenaka, K. Koga, M. Shiratani and Y. Setsuhara, IEEE Trans. Plasma Sci., 2015, 43, 4081–4087 CAS.
- T. Brubaker, K. Ishikawa, H. Kondo, T. Tsutsumi, H. Hashizume, H. Tanaka, S. Knecht, S. Bilén and M. Hori, J. Phys. D: Appl. Phys., 2018, 52, 075203 CrossRef.
- B. C. Garrett, G. K. Schenter and A. Morita, Chem. Rev., 2006, 106, 1355–1374 CrossRef CAS PubMed.
- P. Rumbach, J. P. Clarke and D. B. Go, Phys. Rev. E, 2017, 95, 053203 CrossRef PubMed.
- A. Dickenson, J. Walsh and M. Hasan, J. Appl. Phys., 2021, 129(21), 213301 CrossRef CAS.
- M. Prasad, N. J. English and S. N. Chakraborty, J. Chem. Phys., 2023, 159(5), 054504 CrossRef CAS PubMed.
- S. V. Shevkunov and A. Vegiri, J. Mol. Struct. THEOCHEM, 2002, 593, 19–32 CrossRef CAS.
- A. Vegiri, J. Chem. Phys., 2002, 116, 8786–8798 CrossRef CAS.
- A. A. Fridman, M. Keidar and E. H. Choi, Plasma Biosciences and Medicine, Springer, 2023, pp. 1–34 Search PubMed.
- J. Lai, V. Petrov and J. E. Foster, IEEE Trans. Plasma Sci., 2018, 46, 875–881 CAS.
- F. Mitsugi, S. Kusumegi, K. Nishida and T. Kawasaki, IEEE Trans. Plasma Sci., 2020, 49, 9–14 Search PubMed.
- C. T. Ryan, A. A. Darhuber, R. P. Kunnen, H. Gelderblom and A. Sobota, Sci. Rep., 2024, 14, 17152 CrossRef CAS PubMed.
- W. Hoeben, P. Van Ooij, D. Schram, T. Huiskamp, A. Pemen and P. Lukeš, Plasma Chem. Plasma Process., 2019, 39, 597–626 CrossRef CAS.
- F. Judée, S. Simon, C. Bailly and T. Dufour, Water Res., 2018, 133, 47–59 CrossRef PubMed.
- J. Silsby, A. Dickenson, J. Walsh and M. Hasan, Front. Phys., 2022, 10, 1045196 CrossRef.
- R. Sander, Atmos. Chem. Phys., 2015, 15, 4399–4981 CrossRef CAS.
- M. Roeselová, P. Jungwirth, D. J. Tobias and R. B. Gerber, J. Phys. Chem. B, 2003, 107, 12690–12699 CrossRef.
- C. Kolb, P. Davidovits, J. Jayne, Q. Shi and D. Worsnop, Prog. React. Kinet. Mech., 2002, 27, 1–46 CrossRef CAS.
- J. Ponche, C. George and P. Mirabel, J. Atmos. Chem., 1993, 16, 1–21 CrossRef CAS.
- P. Bruggeman, A. Bogaerts, J. Pouvesle, E. Robert and E. Szili, J. Appl. Phys., 2021, 130(20), 200401 CrossRef CAS.
- P. J. Bruggeman, M. J. Kushner, B. R. Locke, J. G. E. Gardeniers, W. G. Graham, D. B. Graves, R. C. H. M. Hofman-Caris, D. Maric, J. P. Reid, E. Ceriani, D. Fernandez Rivas, J. E. Foster, S. C. Garrick, Y. Gorbanev, S. Hamaguchi, F. Iza, H. Jablonowski, E. Klimova, J. Kolb, F. Krcma, P. Lukes, Z. Machala, I. Marinov, D. Mariotti, S. Mededovic Thagard, D. Minakata, E. C. Neyts, J. Pawlat, Z. L. Petrovic, R. Pflieger, S. Reuter, D. C. Schram, S. Schröter, M. Shiraiwa, B. Tarabová, P. A. Tsai, J. R. R. Verlet, T. von Woedtke, K. R. Wilson, K. Yasui and G. Zvereva, Plasma Sources Sci. Technol., 2016, 25, 053002 CrossRef.
- P. S. Robert Vácha, Martin Mucha, Barbara J. Finlayson-Pitts and Pavel Jungwirth, J. Phys. Chem. A, 2004, 108, 11573–11579 CrossRef.
- R. D. Hoehn, M. A. Carignano, S. Kais, C. Zhu, J. Zhong, X. C. Zeng, J. S. Francisco and I. Gladich, J. Chem. Phys., 2016, 144(21), 214701 CrossRef PubMed.
- S. D. Belair, H. Hernandez and J. S. Francisco, J. Am. Chem. Soc., 2004, 126, 3024–3025 CrossRef CAS PubMed.
- B. Hirshberg, E. R. Molina, A. W. Götz, A. D. Hammerich, G. M. Nathanson, T. H. Bertram, M. A. Johnson and R. B. Gerber, Phys. Chem. Chem. Phys., 2018, 20, 17961–17976 RSC.
- G. Murdachaew, M. E. Varner, L. F. Phillips, B. J. Finlayson-Pitts and R. B. Gerber, Phys. Chem. Chem. Phys., 2013, 15, 204–212 RSC.
- R. J. Kee, M. E. Coltrin, P. Glarborg and H. Zhu, Chemically reacting flow: theory, modeling, and simulation, John Wiley & Sons, 2017 Search PubMed.
- A. P. Thompson, H. M. Aktulga, R. Berger, D. S. Bolintineanu, W. M. Brown, P. S. Crozier, P. J. In't Veld, A. Kohlmeyer, S. G. Moore and T. D. Nguyen, Comput. Phys. Commun., 2022, 271, 108171 CrossRef CAS.
- A. K. Malde, L. Zuo, M. Breeze, M. Stroet, D. Poger, P. C. Nair, C. Oostenbrink and A. E. Mark, J. Chem. Theory Comput., 2011, 7, 4026–4037 CrossRef CAS PubMed.
- N. Schmid, A. P. Eichenberger, A. Choutko, S. Riniker, M. Winger, A. E. Mark and W. F. van Gunsteren, Eur. Biophys. J., 2011, 40, 843–856 CrossRef CAS PubMed.
- G. D. Barbosa and C. H. Turner, J. Mol. Liq., 2023, 389, 122826 CrossRef CAS.
- V. Sundaram, A. V. Lyulin and B. R. Baumeier, J. Phys. Chem. B, 2020, 124, 11030–11039 CrossRef CAS PubMed.
- M. M. Reif, M. Winger and C. Oostenbrink, J. Chem. Theory Comput., 2013, 9, 1247–1264 CrossRef CAS PubMed.
- J. Razzokov, S. Fazliev, A. Kodirov, P. AttrI, Z. Chen and M. Shiratani, Int. J. Mol. Sci., 2022, 23, 6330 CrossRef CAS PubMed.
- T. P. Senftle, S. Hong, M. M. Islam, S. B. Kylasa, Y. Zheng, Y. K. Shin, C. Junkermeier, R. Engel-Herbert, M. J. Janik, H. M. Aktulga, T. Verstraelen, A. Grama and A. C. T. van Duin, npj Comput. Mater., 2016, 2(1), 1–14 CrossRef.
- B. A. Wells and A. L. Chaffee, J. Chem. Theory Comput., 2015, 11, 3684–3695 CrossRef CAS PubMed.
- H. A. Posch, W. G. Hoover and F. J. Vesely, Phys. Rev. A:At., Mol., Opt. Phys., 1986, 33, 4253 CrossRef PubMed.
- J.-P. Ryckaert, G. Ciccotti and H. J. Berendsen, J. Comput. Phys., 1977, 23, 327–341 CrossRef CAS.
- A. Rajabpour, R. Seif, S. Arabha, M. M. Heyhat, S. Merabia and A. Hassanali, J. Chem. Phys., 2019, 150(11), 114701 CrossRef PubMed.
- M. A. Wilson and A. Pohorille, J. Phys. Chem. B, 1997, 101, 3130–3135 CrossRef CAS PubMed.
- M. Stroet, M. Setz, T. Lee, A. K. Malde, G. van den Bergen, P. Sykacek, C. Oostenbrink and A. E. Mark, J. Phys. Chem. B, 2024, 128, 4602–4620 CrossRef CAS PubMed.
- Z. Gattin, S. Riniker, P. J. Hore, K. H. Mok and W. F. van Gunsteren, Protein Sci., 2009, 18, 2090–2099 CrossRef CAS PubMed.
- A. Soper, Chem. Phys., 2000, 258, 121–137 CrossRef CAS.
- R. Sander, Atmos. Chem. Phys., 2023, 23, 10901–12440 CrossRef CAS.
- D. V. Schroeder, An introduction to thermal physics, Oxford University Press, 2020 Search PubMed.
- J. Kästner, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 932–942 Search PubMed.
- V. W. D. Cruzeiro, M. Galib, D. T. Limmer and A. W. Götz, Nat. Commun., 2022, 13, 1266 CrossRef CAS PubMed.
- M. T. Martins-Costa, J. M. Anglada, J. S. Francisco and M. F. Ruiz-López, Chem. - Eur. J., 2019, 25, 13899–13904 CrossRef CAS PubMed.
- J. M. Anglada, M. T. Martins-Costa, J. S. Francisco and M. F. Ruiz-Lopez, J. Am. Chem. Soc., 2020, 143, 453–462 CrossRef PubMed.
- M. J. Gleichweit, M. A. Mohajer, D. P. B. Dit Avocat, M. E. Divéky, G. David and R. Signorell, Phys. Chem. Chem. Phys., 2024, 26, 16296–16308 RSC.
- J. Lee and I. Tang, Atmos. Environ., 1988, 22, 1147–1151 CrossRef CAS.
- M. Leriche, L. Deguillaume and N. Chaumerliac, J. Geophys. Res.:Atmos., 2003, 108(14), 4433 Search PubMed.
- S. Mertes and A. Wahner, J. Phys. Chem., 1995, 99, 14000–14006 CrossRef CAS.
- M. Barth, S. W. Kim, W. Skamarock, A. L. Stuart, K. Pickering and L. Ott, Atmos. Chem. Phys., 2007, 7(18), 4709–4731 CrossRef CAS.
- G. Gržinić, T. Bartels-Rausch, A. Türler and M. Ammann, Atmos. Chem. Phys., 2017, 17, 6493–6502 CrossRef.
- D. R. Lide, CRC handbook of chemistry and physics, CRC Press, 2004 Search PubMed.
- J. Y. Park and Y. N. Lee, J. Phys. Chem., 1988, 92, 6294–6302 CrossRef CAS.
- Y. Sheng, Y. Hu, Z. Fan and H. Bao, Phys. Rev. B, 2022, 105, 075301 CrossRef CAS.
- J. Silsby, S. Simon, J. Walsh and M. Hasan, Plasma Chem. Plasma Process., 2021, 41, 1363–1380 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp04272g |
| This journal is © the Owner Societies 2025 |