Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Salt formation of cabozantinib with hydrochloric and hydrobromic acids – influence on the in vitro dissolution behavior and food effect†
Sreela
Ramesh
ac,
Eliška
Zmeškalová
ab,
Monika
Kučeráková
b,
Vít
Zvoníček
c and
Miroslav
Šoóš
 *a
*a
aDepartment of Chemical Engineering, University of Chemistry and Technology, Technická 3, 166 28 Prague 6 – Dejvice, Czech Republic. E-mail: miroslav.soos@vscht.cz
bDepartment of Structure Analysis, Institute of Physics of the Czech Academy of Sciences, Na Slovance 1999/2, 182 00 Praha 8, Czech Republic
cZentiva, k.s, U Kabelovny 130, 10237 Prague 10, Czech Republic
First published on 14th January 2025
Abstract
The aim of this study was to prepare stable novel crystalline salts of cabozantinib, a tyrosine kinase inhibitor used to treat medullary thyroid cancer, to improve its dissolution properties and thereby reduce the positive food effect associated with its marketed salt form (L-malate). Five novel multi-component solid forms were obtained. In addition to the detailed physicochemical characterization, we report the crystal structures of the saccharinate, hydrochloride, and hydrobromide salts. The hydrobromide and hydrochloride salts of cabozantinib were chosen for further studies based on promising preliminary results from dissolution rate measurements. The potential of the hydrochloride and hydrobromide salts to be used for drug formulation was determined by testing their physical stability using hygroscopicity tests and by observing their melting properties. To investigate the impact of these salts on reducing the patient-relevant food effect, in vitro dissolution studies in stimulated fed and fasted states using biorelevant media were performed and compared to the marketed form of the drug.
1. Introduction
New molecular entities (NMEs) today are frequently large-molecular-weight, lipophilic, and poorly water-soluble compounds that most often fall into BCS Class II.1 BCS class II drugs characterized by low solubility and high permeability have a tendency to display a positive food effect (increased solubility in the fed state) associated with them.2 The U.S. FDA issued a guidance in December 2002 entitled “Food-Effect Bioavailability and Fed Bioequivalence Studies”.3 High-fat meal studies are recommended by the FDA, since such meal conditions are expected to provide the greatest effects on gastrointestinal physiology so that systemic drug availability is maximally affected. The common belief is that food effects result from changes in drug solubility and other factors as listed by the FDA that food may: “delay gastric emptying; stimulate bile flow; change the gastrointestinal pH; increase splanchnic blood flow; change the luminal metabolism of a drug substance, and physically or chemically interact with a dosage form or a drug substance.”Cabozantinib, a BCS class II drug, is a small-molecule multi-target tyrosine kinase inhibitor (smTKI).4 The U.S. Food and Drug Administration has approved cabozantinib to be used in the treatment of renal cell carcinoma and progressive metastatic medullary thyroid cancer.5 Cabozantinib is marketed under the trade names Cabometyx and Cometriq6,7 in the form of tablets and capsules, respectively. It is marketed in the form of a salt with L-malic acid in both formulations.8 Cabozantinib is found to have a positive food effect associated with it, and hence, it is not advised to be taken along with food.9 smTKIs like cabozantinib have low solubility in vivo, which limits absorption and hence gives way to undesirable pharmacokinetic changes in the presence of food.10 Clinically, such food–drug interactions for smTKIs are predicted to occur due to metabolism by cytochrome P-450 isoenzymes hepatically.11 The critical importance of thoroughly investigating the influence of food on the bioavailability of these drugs cannot be overstated and such insights must be explicitly addressed in the labeling of these marketed drugs to ensure that patients and healthcare providers have the necessary guidance for appropriate administration.12 A recent report13 shows how oral absorption of cabozantinib was improved approximately 2-fold in rats by converting it to a lipid-based formulation containing its docusate salt. Since the absorption of cabozantinib is limited by its solubility, discovering new formulations that can dissolve better can possibly help eliminate the food effect associated with the drug.
One of the best and easiest ways to improve the solubility of ionizable drugs is through salt formation.14 For instance, by sulfonate salt formation, up to 200-times faster dissolution of an antidepressant agomelatine was achieved.15 A previous study conducted by our group to improve the dissolution properties of an anti-psychotic drug cyamemazine showed how salt formation with dicarboxylic acids helped achieve better solubility.16 In a study conducted by Mannava et al., a 25-fold increase in the dissolution properties and subsequent enhancement of the bioavailability of the antituberculosis drug ethionamide was obtained by salt formation with oxalic acid.17
The basic nitrogen of cabozantinib has a pKa of 5.9,18 and hence for salt formation, the acid should have a pKa value such that the pKa difference between the API and the salt-forming co-former is 3 or more.19 After an initial round of extensive screening, salts were formed with tartaric acid, cyclamic acid, saccharin, hydrobromic acid, and hydrochloric acid. The pKa values of these acids20–22 are listed in Table 1 and were found to be in agreement with the general delta pKa ≥ 3 rule. The preparation and preliminary analysis (X-ray powder diffraction (XRPD) patterns and solution 1H nuclear magnetic resonance (NMR) spectra) of these salts are described in detail in the coming sections. The crystal structures of the saccharinate, hydrochloride, and hydrobromide salts were solved from single-crystal X-ray diffraction. In vitro dissolution measurements allowed for the identification of the best candidates (hydrochloride and hydrobromide salts) to undergo thermal analysis and hygroscopicity tests. Finally, the capability of hydrobromide and hydrochloride salts to reduce the interaction with food was studied by in vitro dissolution tests in biorelevant media.
| Acid | pKa |
|---|---|
| Saccharin | 1.6 |
| Cyclamic acid | 1.71 |
| Tartaric acid | 2.72 |
| Hydrochloric acid | −6 |
| Hydrobromic acid | −9 |
2. Experimental
2.1 Materials
Cabozantinib (CBZ) was kindly provided by Zentiva, k.s. solvents and acids of analytical purity were obtained from various commercial suppliers and were used as received.2.2 Solution-mediated salt preparation
CBZ (20 mg) and the acids (tartaric acid, saccharin, cyclamic acid, hydrochloric acid and hydrobromic acid) were added in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in 500 μL of acetone into glass vials. Salt formation happened through slurry conversion, and the contents of the vial were filtered through a laboratory glass funnel (frit S3) under a vacuum. The filtered powders were dried in a vacuum oven at 40 °C. The CBZ L-malate salt was prepared by the evaporation method. CBZ (20 mg) and L-malic acid were added in a 1
1 molar ratio in 500 μL of acetone into glass vials. Salt formation happened through slurry conversion, and the contents of the vial were filtered through a laboratory glass funnel (frit S3) under a vacuum. The filtered powders were dried in a vacuum oven at 40 °C. The CBZ L-malate salt was prepared by the evaporation method. CBZ (20 mg) and L-malic acid were added in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to 500 μL of tetrahydrofuran (THF) into a glass vial and heated until completely dissolved. The vial was left in the hood for the solvent to evaporate leaving behind the crystalline salt. The salt was dried overnight in a vacuum oven at 35 °C.
1 ratio to 500 μL of tetrahydrofuran (THF) into a glass vial and heated until completely dissolved. The vial was left in the hood for the solvent to evaporate leaving behind the crystalline salt. The salt was dried overnight in a vacuum oven at 35 °C.
2.3 Single-crystal preparation
Single crystals of CBZ·HCl, CBZ·HBr and CBZ saccharinate salts were obtained by dissolving 2 mg of the salts in 150 μL of methanol, 150 μL of ethanol and a mixture of 100 μL of methanol and 100 μL of ethanol, respectively, in 1.5 ml glass vials. The lids were perforated with a needle to allow very slow solvent evaporation, resulting in single crystals.2.4 Analytical methods
3. Results and discussion
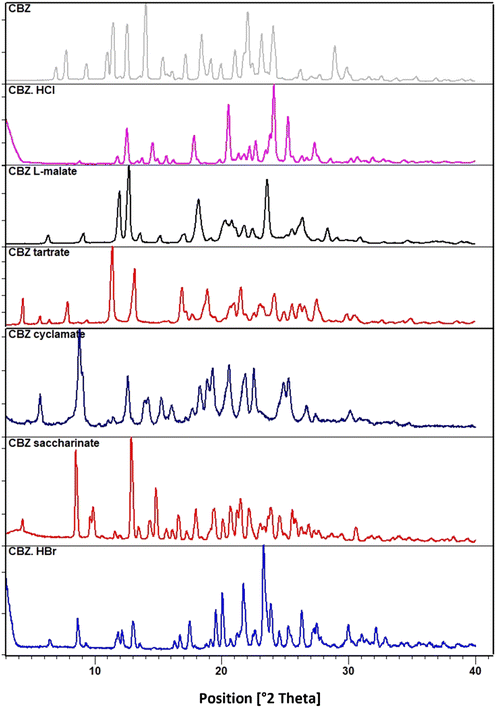
3.1 Crystalline salts of CBZ
Solid forms obtained with saccharin, cyclamic acid, tartaric acid, hydrochloric acid and hydrobromic acid using the methods described in section 2.2 were characterized using XRPD analysis (Fig. 1). All forms were found to be novel crystalline solids as their XRD patterns were different from those of the starting materials. Even though CBZ·HBr, CBZ·HCl and CBZ saccharinate were confirmed to be salts from their crystal structure data, single crystals of CBZ tartrate and CBZ cyclamate could not be obtained. But, the IR spectra (Fig. S1†) of these compounds reveal a characteristic broad stretch in the region 2300 to 2700 cm−1, indicative of protonation of the basic tertiary nitrogen in cabozantinib since absorption in this area in IR spectroscopy is associated with the NH+ stretch. Moreover, a lack of absorption in this area for pure cabozantinib even further confirms protonation in the salts. Solution 1H NMR (Fig. S2†) suggested that the salts with cyclamic acid, saccharin, and tartaric acid were indeed 1:1 salts. Due to the broadness of the peaks, this could not be determined for the CBZ·HCl and CBZ·HBr salts from solution NMR data, but their solved crystal structures prove the ratio to be also1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The CBZ L-malate salt prepared according to the method in section 2.2 was analyzed using XRPD (Fig. S3†) and used as a reference for the marketed form of the drug for dissolution studies.
1. The CBZ L-malate salt prepared according to the method in section 2.2 was analyzed using XRPD (Fig. S3†) and used as a reference for the marketed form of the drug for dissolution studies.
3.2 Preliminary intrinsic dissolution rate measurement
Since the study's main aim was to find solid forms with higher dissolution rates than the marketed L-malate salt, the IDRs (Fig. 2) of all the salts were measured. The rates were measured at a neutral pH of 6.8 in phosphate buffer with 0.1% SDS to mimic the pH range of the fasted state of the human small intestine. CBZ saccharinate and CBZ tartrate salts dissolved slower than the CBZ L-malate salt, whereas the CBZ cyclamate salt had a slightly higher rate compared to the CBZ L-malate salt. CBZ·HBr and CBZ·HCl salts were found to dissolve at least two times faster than the marketed CBZ L-malate salt.The significantly faster dissolution of CBZ·HCl and CB·HBr compared to other salts maybe attributed to the high strength of these acids, which in turn implies a high ionizing power and dissociation. The solubility of the drug cations in aqueous environments is hence higher contributing to faster dissolution. The smaller anion sizes for Cl− and Br− compared to the organic, larger anions like tartrate or saccharinate also probably allows for simpler packing of the salts and higher hydration energies aiding improved solubility.
3.3 Crystal structures
To get an insight into the crystal packing and interactions of CBZ with the counterions, we attempted to prepare their single crystals and solve their structures. We were successful in the preparation of the CBZ·HCl, CBZ·HBr and CBZ saccharinate salts. The data confirmed the protonation of the aromatic quinoline nitrogen of cabozantinib and, therefore, confirmed that these solid forms are salts and not co-crystals. The selected structural parameters are shown in Table 2. All other details about the measurement and refinement, as well as the geometry and H-bonding tables, can be found in the ESI† (Tables S1 and S2). The figures depicting the asymmetric unit with the thermal ellipsoids, the unit cell with highlighted anions, and H-bonding patterns are shown in Fig. 3.| CBZ·HCl | CBZ·HBr | CBZ saccharinate | |
|---|---|---|---|
| Formula | C28H25Cl1F1N3O5 | C28H25Br1F1N3O5 | C35H29FN4O8S |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/c | P21/c | Pbca |
| a (Å) | 8.2418 | 14.1241 | 8.4541 |
| b (Å) | 15.0392 | 8.8108 | 18.1835 |
| c (Å) | 19.989 | 21.0359 | 41.4054 |
| α (deg) | 90 | 90 | 90 |
| β (deg) | 91.61 | 105.123 | 90 |
| γ (deg) | 90 | 90 | 90 |
| V (Å3) | 2476.66 | 2527.15 | 6365.05 |
| CCDC number | 2303274 | 2303273 | 2347514 |
CBZ·HCl crystallizes in the monoclinic system in the space group P21/c. There is one molecule of the CBZ cation and one chloride anion in the asymmetric unit and four of each in the unit cell, thus confirming the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The H-bonds run along the b direction, making an infinite chain of –CBZ–Cl–CBZ–, connected by quinoline N(3)H⋯Cl−⋯HN(2) amidic H-bonds. In addition, there is also an intramolecular hydrogen bond between the adjacent amidic groups of CBZ N(1)H⋯O(6). The crystal packing is made possible by the short-range interaction between the oxygen atoms of the methoxy groups and hydrogen atoms on the fluorine-substituted aromatic rings.
1 ratio. The H-bonds run along the b direction, making an infinite chain of –CBZ–Cl–CBZ–, connected by quinoline N(3)H⋯Cl−⋯HN(2) amidic H-bonds. In addition, there is also an intramolecular hydrogen bond between the adjacent amidic groups of CBZ N(1)H⋯O(6). The crystal packing is made possible by the short-range interaction between the oxygen atoms of the methoxy groups and hydrogen atoms on the fluorine-substituted aromatic rings.
CBZ·HBr crystallizes in the monoclinic system in the space group P21/c. There is one molecule of the CBZ cation and one chloride anion in the asymmetric unit and four of each in the unit cell, thus confirming the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. H-bonding is close to identical as in the CBZ·HCl. The packing in the crystal is made of anti-parallel chains of salt units running both horizontally and vertically.
1 ratio. H-bonding is close to identical as in the CBZ·HCl. The packing in the crystal is made of anti-parallel chains of salt units running both horizontally and vertically.
CBZ saccharinate crystallizes in the orthorhombic system in the space group Pbca. There is one molecule of the CBZ cation and one chloride anion in the asymmetric unit and eight of each in the unit cell, thus confirming the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The main H-bonding pattern is an infinite chain of –CBZ–CBZ– molecules running along the a direction connected by ladder-like H-bonds formed by two self-complimentary amidic groups of CBZ, N(18)H⋯O(25) and N(26)H⋯O(20). This chain is decorated by hanging saccharinate anions connected by the quinoline N(35)H⋯ HN(6)amidic H-bonds.
1 ratio. The main H-bonding pattern is an infinite chain of –CBZ–CBZ– molecules running along the a direction connected by ladder-like H-bonds formed by two self-complimentary amidic groups of CBZ, N(18)H⋯O(25) and N(26)H⋯O(20). This chain is decorated by hanging saccharinate anions connected by the quinoline N(35)H⋯ HN(6)amidic H-bonds.
3.4 Thermal analysis and hygroscopicity
In order to confirm the thermal stability and purity of the prepared CBZ·HCl and CBZ·HBr salts, DSC and TGA measurements were performed. DSC thermograms (Fig. 4) of both these salts in our study showed single, sharp melting endotherms, thus confirming the purity of the samples prepared. Their melting points were higher (at least by 40 °C) than the marketed CBZ L-malate salt that had a peak melting temperature of 181.6 °C (Fig. S4†). This observation could be due to the presence of stronger ionic interactions arising from the fact that HCl and HBr are some of the strongest acids known. The high melting points indicate the high thermal stability of the salts thereby making them suitable for pharmaceutical development purposes. Furthermore, TGA (Fig. S5†) revealed no significant loss of the sample weight for either salt, confirming that the samples are pure, crystalline, and non-solvated.Inorganic acids, such as HCl or HBr, are known for their strong polarity, which contributes to the formation of salts that are more susceptible to water uptake.27 Hygroscopicity is a critical factor in the pharmaceutical industry, as excessive moisture absorption can compromise the physical stability of solid dosage forms during storage. Hence, the hygroscopicity of the CBZ·HBr and CBZ·HCl salts were checked using DVS analysis (section S4†). In both these cases, it was found that there was no significant water uptake, thereby proving their physical stability against humidity.
3.5 Biorelevant dissolution for food effect studies
The change in the dissolution behaviour of the CBZ·HCl and CBZ·HBr salts compared to the marketed form, the CBZ L-malate salt, in the fed and fasted states was predicted by recording intrinsic dissolution rates of these salts in biorelevant dissolution media (FaSSIF and FeSSIF). These media have been found to model the composition of the intestinal contents before and after a meal intake.28 Dissolution tests conducted in biorelevant media are extensively utilized to predict the in vivo behavior of drugs, including their food-effect profiles.29 Even though the CBZ·HCl salt had the highest dissolution rate in the fasted state (Fig. 5), the variability in the dissolution rates between the fasted and fed states was approximately the same as in the CBZ L-malate salt. While the CBZ L-malate salt showed a 2.5- fold increase in the dissolution rate in FeSSIF compared to FaSSIF (Fig. 5), for the CBZ·HCl salt, the increase was ∼3- fold. The CBZ·HBr salt was found to dissolve two times faster than the CBZ L-malate salt in the fasted state (Fig. 5). In FeSSIF, the increase in the dissolution rate for the CBZ·HBr salt was found to be 1.7 times that of the rate in FaSSIF, which suggests persistence of a positive food effect but less variability than the marketed L-malate salt and also the CBZ·HCl salt. | ||
| Fig. 5 Log IDR (IDR originally measured in units μg cm−2 min) for CBZ L-malate, CBZ·HCl and CBZ·HBr salts in FaSSIF and FeSSIF. | ||
4. Conclusions
In this study, the preparation of five crystalline salts (with hydrochloric acid, hydrobromic acid, cyclamic acid, saccharin, and tartaric acid) of cabozantinib is described. The hydrobromide and hydrochloride salts were chosen for further characterization because their dissolution rates were higher than the marketed L-malate salt compared to the other novel salts. Both these salts displayed higher thermal stability with melting points at least 40 °C higher than the L-malate salt. These salts were found to be non-hygroscopic as well, making them suitable candidates for formulation purposes. The variability in the dissolution rates of these salts in biorelevant media mimicking the fasted and fed conditions of the human intestine was used to determine the potential of the novel salts to diminish the undesired food effect of the marketed form. Even though the variability between the fed and fasted states was observed for all salts, the hydrobromide salt offers a ∼30% improvement over the marketed form of L-malate. The food effect, even though still expected to happen, occurs to a lesser extent than the marketed form when the hydrobromide salt is used. Further in vivo studies are required to conclude the potential of these salts to be used in humans.Data availability
Crystallographic data for the solid forms of cabozantinib (salts of cabozantinib with HCl, HBr and saccharine) have been deposited at the CCDC under 2303274, 2303273 and 2347514 and can be obtained from https://www.ccdc.cam.ac.uk/structures/?.The data supporting this article have been included as part of the ESI.† This contains IR spectra for prepared solid forms, solution 1H NMR spectra, XRPD diffractograms, IDR profiles, DSC signals from the CBZ L-malate salt, TGA curves for CBZ·HBr, CBZ·HCl and CBZ, and DVS curves for CBZ·HCl and CBZ·HBr as well as full crystallographic information about the prepared new solid forms.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the Solid-State Department of Zentiva, k.s. for the materials and all the help provided. Our great thanks go to Ondřej Dammer, Lukáš Krejčík, and Marcela Tkadlecova. This work received financial support from the project New Technologies for Translational Research in Pharmaceutical Sciences (NETPHARM), project ID CZ.02.01.01/00/22_008/0004607, co-funded by the European Union, and specific university research grants (A1_FCHI_2022_006, A2_FCHI_2021_016) and by the Czech Science Foundation project 24-10558S. This work was also supported by the CzechNanoLab project LM2018110 funded by MEYS CR, which is gratefully acknowledged for the financial support of the measurements/sample fabrication at the LNSM Research Infrastructure. We would also like to thank the Pharmaceutical Applied Research Center (PARC) for support in various parts of this project.References
- C. Y. Wu and L. Z. Benet, Pharm. Res., 2005, 22, 11–23 CrossRef CAS PubMed.
- K. A. Lentz, AAPS J., 2008, 10(2), 282–288 CrossRef CAS PubMed.
- https://www.fda.gov/files/drugs/published/Food-Effect-Bioavailability-and-Fed-Bioequivalence-Studies.pdf (accessed on 27 November, 2023).
- F. M. Yakes, J. Chen, J. Tan, K. Yamaguchi, Y. Shi, P. Yu, F. Qian, F. Chu, F. Bentzien, B. Cancilla and J. Orf, Mol. Cancer Ther., 2011, 10(12), 2298–2308 CrossRef CAS PubMed.
- T. K. Choueiri, B. Escudier, T. Powles, P. N. Mainwaring, B. I. Rini, F. Donskov, H. Hammers, T. E. Hutson, J. L. Lee, K. Peltola and B. J. Roth, N. Engl. J. Med., 2015, 373(19), 1814–1823 CrossRef CAS PubMed.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203756lbl.pdf (accessed on 27 November, 2023).
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208692s010lbl.pdf (accessed on 27 November, 2023).
- Cabozantinib s-malate https://pubchem.ncbi.nlm.nih.gov/compound/Cabozantinib-S-malate (accessed on 27 November, 2023).
- L. Nguyen, J. Holland, R. Mamelok, M. K. Laberge, J. Grenier, D. Swearingen, D. Armas and S. Lacy, J. Clin. Pharmacol., 2015, 55(11), 1293–1302 CrossRef CAS PubMed.
- M. Herbrink, B. Nuijen, J. H. Schellens and J. H. Beijnen, Cancer Treat. Rev., 2015, 41(5), 412–422 CrossRef CAS PubMed.
- M. Veerman, K. Hussaarts, F. Jansman, S. Koolen, R. Leeuwen and R. Mathijssen, Lancet Oncol., 2020, 21(5), e265–e279 CrossRef PubMed.
- R. Z. Szmulewitz and M. J. Ratain, Clin. Pharmacol. Ther., 2013, 93(3), 242–244 CrossRef CAS PubMed.
- H. D. Williams, L. Ford, S. Han, K. J. Tangso, S. Lim, D. M. Shackleford, D. T. Vodak, H. Benameur, C. W. Pouton, P. J. Scammells and C. J. Porter, Mol. Pharmaceutics, 2018, 15(12), 5678–5696 CrossRef CAS PubMed.
- S. M. Berge, L. D. Bighley and D. C. Monkhouse, J. Pharm. Sci., 1977, 66, 1–19 CrossRef CAS PubMed.
- E. Skorepová, D. Bím, M. Hušák, J. Klimes, A. Chatziadi, L. Ridvan, T. Boleslavská, J. Beránek, P. Šebek and L. Rulísek, Cryst. Growth Des., 2017, 17, 5283–5294 CrossRef.
- S. Ramesh, E. Skořepová, V. Eigner, V. C. Paingad, V. Zvoníček, P. Kužel and M. ŠoóŠ, Cryst. Growth Des., 2022, 23(1), 168–179 CrossRef.
- K. C. Mannava, K. Suresh and A. Nangia, Cryst. Growth Des., 2016, 16, 1591–1598 CrossRef.
- https://go.drugbank.com/drugs/DB08875 (accessed on 27 November 2023).
- S. L. Childs, G. P. Stahly and A. Park, Mol. Pharmaceutics, 2007, 4, 323–338 CrossRef CAS PubMed.
- B. Kapadiya Dhartiben and K. D. Aparnathi, Int. J. Curr. Microbiol. Appl. Sci., 2017, 6(6), 1283–1296 CrossRef.
- Tartaric acid https://go.drugbank.com/drugs/DB09459 (accessed on 1 December, 2023).
- T. Munegumi, World J. Chem. Educ., 2013, 1(1), 12–16 Search PubMed.
- CrysAlisPRO, Oxford Diffraction/Agilent Technologies UK Ltd, Yarnton, England Search PubMed.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr. - Cryst. Mater., 2014, 229, 345–352 CrossRef.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786–790 CrossRef CAS.
- J. Rohlíček and M. Hušák, J. Appl. Crystallogr., 2007, 40, 600–601 CrossRef.
- D. Gupta, D. Bhatia, V. Dave, V. Sutariya and S. Varghese Gupta, Molecules, 2018, 23(7), 1719 CrossRef PubMed.
- J. B. Dressman and C. Reppas, Eur. J. Pharm. Sci., 2000, 11, S73–S80 CrossRef CAS PubMed.
- S. Klein, AAPS J., 2010, 12(3), 397–406 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Solution 1H NMR spectra, additional XRPD patterns, additional crystallographic data, IDR curves from experimental data and DSC curves, CCDC: 2303273, 2303274 and 2347514. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ce01278j |
| This journal is © The Royal Society of Chemistry 2025 |