DOI:
10.1039/D4CE00760C
(Paper)
CrystEngComm, 2025,
27, 55-63
Flexible hybrid capacitors based on NiMoS@NiCo-LDH composites under variable work conditions†
Received
31st July 2024
, Accepted 15th November 2024
First published on 15th November 2024
Abstract
It is well known that the morphology and structure of electrode materials seriously affect the whole performance of devices. Therefore, transition metal sulfides are desirable cathode materials for supercapacitors due to their high conductivity and rich redox active sites. However, the low energy density restricts their large-scale application. Herein, we design NiMoS@NiCo-LDH core–shell structures through facile synthesis routes. The unique structures relieve volume expansion of the electrode materials during charging/discharging and promote the redox reaction. The as-fabricated products deliver a specific capacity of 1456 C g−1 at 1 A g−1. A flexible device based on the obtained cathode provides an energy density of 80.21 W h kg−1 at a power density of 2698.65 W kg−1. It can maintain 85% of its initial capacity after cycling 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. Furthermore, they still work stably at extreme temperatures ranging from 25 to −20 °C. The asymmetric supercapacitor (ASC) also presents excellent mechanical durability and stability at different bending angles.
000 times. Furthermore, they still work stably at extreme temperatures ranging from 25 to −20 °C. The asymmetric supercapacitor (ASC) also presents excellent mechanical durability and stability at different bending angles.
1. Introduction
Global environmental pollution and energy scarcity have prompted the development of energy storage and conversion systems.1–4 The applications of lithium-ion batteries (LIBs) and other new secondary batteries are restricted by their poor safety, low temperature resistance and complicated preparation processes.5–7 However, supercapacitors are thought to be suitable devices because of their fast charge/discharge rates, long-term cycling life and environmental friendliness.8–12 Unsatisfactorily, their energy density is significantly behind practical applications. Therefore, it remains a challenge to design superior electrode materials with desirable performance.13,14
Transition metal oxide material NiMoO4 is widely investigated due to its higher theoretical capacity than NiO and MoO3.15–17 For example, Shim's group synthesized NiMoO4 nanoflakes with a specific capacity of 590 C g−1 at 1 A g−1 using an electrodeposition approach.18 Nevertheless, single-component structures present low conductivity and inhibit the rapid transfer of electrons, which leads to their poor electrochemical performance.19 Transition metal sulfides (TMSs) attract wide attention because they have higher conductivity and richer redox active sites than the corresponding oxides.20–22 Du et al. fabricated NiMoS4 electrode materials with a specific capacity of 313 C g−1 at 1 A g−1via a chemical co-precipitation process.23 Our group reported NiMo2S4 microspheres grown on nickel foam (NF) through a two-step hydrothermal route.24 The as-assembled NiMo2S4 nanosheets delivered a specific capacity of 700 C g−1 at 1 A g−1.25 Although a lot of progress has been made, it is still a need to achieve high-capacitance energy storage devices in large-scale applications.26
The fabrication of composite electrodes is considered an efficient approach to increase the specific capacity by the synergistic effects between different electrode materials.27–29 Nickel-cobalt-based layered double hydroxides (LDHs) usually present a satisfactory specific surface area and exposure of many reactive sites due to their two-dimensional layered structure.30–32 In the literature, Wang and co-workers prepared NiCo-LDH@NiMoO4 structures with a specific capacity of 1186 C g−1 at 1 A g−1.33 NiCo-LDH/Co3S4 composites for supercapacitors showed a specific capacity of 728.1 C g−1 at 1 A g−1.34 Herein, we report Ni-based sulfides/NiCo-based layered double hydroxides via a facile hydrothermal route. The construction of core–shell structures can effectively increase the specific surface area of the prepared samples. The as-fabricated NiMoS@NiCo-LDH sample possesses a specific capacity of 1456 C g−1 at 1 A g−1 and retains a capacity of 77% after cycling 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. In addition, the assembled NiMoS@NiCo-LDH//AC device shows an energy density of 80.21 W h kg−1 at a power density of 2699 W kg−1. Moreover, the ASC also demonstrates superior mechanical stability at various bending angles and can work stably from 0 °C to −20 °C.
000 times. In addition, the assembled NiMoS@NiCo-LDH//AC device shows an energy density of 80.21 W h kg−1 at a power density of 2699 W kg−1. Moreover, the ASC also demonstrates superior mechanical stability at various bending angles and can work stably from 0 °C to −20 °C.
2. Experimental
2.1. Synthesis of NiMoS microspheres
In a typical procedure, a piece of NF (3 × 3 cm2) was put into a 1.0 M HCl solution for 1 h. Afterward, the NF was washed several times by ethanol and deionized water and then vacuum dried at 60 °C overnight. Then, 0.5816 g Ni(NO3)2·6H2O and 0.4839 g Na2MoO4·2H2O were added into 60 mL deionized (DI) water and stirred for 30 min. Subsequently, the pretreated NF was put into the above solution and kept at 160 °C for 6 h. Then, the sample was calcined at 350 °C for 2 h with a heating speed of 5 °C min−1 in air. Finally, 0.4375 g Na2S was dissolved in 50 mL DI water. The as-fabricated precursor and solution were mixed and heated at 120 °C for 4 h. After washing, it was dried for 12 h at 60 °C.
2.2. Synthesis of NiMoS@NiCo-LDH samples
0.5816 g Ni(NO3)2·6H2O, 0.437 g Co(NO3)2·6H2O and 1 g CTAB were immersed into 45 mL methyl alcohol and 15 mL DI water. Afterward, the green solution was stirred for 1 h. NiMoS was dissolved into the above solution and then transferred into a 100 mL autoclave and heated at 120 °C for 4 h. After being cooled to room temperature, the mixture was washed several times with DI water and ethanol and dried at 60 °C. Finally, the as-fabricated products were named NiMoS@NiCo-LDH. Their average mass loadings are 1, 1.3, 1.8 and 2.4 mg cm−2, respectively. The corresponding samples were denoted as NiMoO4, NiMoS, NiCo-LDH and NiMoS@NiCo-LDH.
2.3. Preparation of activated carbon (AC) anode material
Activated carbon, acetylene black, and polyvinylidene fluoride (PVDF) were mixed with a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then N-methylpyrrolidone (NMP) organic solvent was dropped into the mixture to obtain a slurry. Then it was spread evenly over the pretreated NF and then vacuum dried at 60 °C for 12 h.
1. Then N-methylpyrrolidone (NMP) organic solvent was dropped into the mixture to obtain a slurry. Then it was spread evenly over the pretreated NF and then vacuum dried at 60 °C for 12 h.
2.4. Characterization of materials
The crystal structures and elemental composition of the as-synthesized samples were determined by X-ray diffraction (XRD, 7000, Shimadzu, 40 kV, λ = 0.1541 nm) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha). The morphology of the as-obtained samples was observed with a scanning electron microscope (SEM, Gemini 300-71-31). N2 absorption and desorption isotherms (JW-TB200) were conducted to investigate their specific surface area through the Brunauer–Emmett–Teller (BET) measurement.
2.5. Electrochemical characterization
The electrochemical performances of several samples were evaluated using an electrochemical workstation (CHI660E) in a three-electrode system. The as-synthesized NiMoS@NiCo-LDH samples were used as the working electrode, Hg/HgO as the reference electrode and Pt foil as the counter electrode. The specific capacitance (C g−1) was determined as:where Δt is the discharge time (s), I is the discharge current (A) and m is the mass of the active materials (g).
The ASCs were composed of the cathode (NiMoS@NiCo-LDH), anode (AC), separator (cellulose paper) and electrolyte (1.5 M PVA-KOH gel). In order to balance the charge conservation (Q+ = Q−), the optimum mass ratio of cathode and anode electrodes was calculated by the following equation:
| | | M+/m− = Cm−(ΔV)−/Cm+(ΔV)+ | (4) |
where
Q,
C, and Δ
V represent the charge storage capacity, specific capacitance (C g
−1) and voltage window (V), respectively.
The energy density (E) and power density (P) were calculated according to the following equations:
3. Results and discussion
Fig. 1 shows the synthesis schematic of the NiMoS@NiCo-LDH materials. Firstly, NiMoO4 precursors are grown on the NF surface via a hydrothermal route. Secondly, NiMoS microspheres are fabricated after vulcanization at 120 °C for 4 h. Finally, a layer of NiCo-LDH nanosheets is formed on the surface of NiMoS microspheres. XRD is first conducted to investigate the crystal structure of the as-prepared product. From Fig. S1,† the diffraction peaks of the samples belong to the NiMoO4 phase (JCPDs No. 13-0128).35 From Fig. 2a, the three strongest diffraction peaks at 44.4°, 51.7° and 76.3° are indexed to the NF substrate (JCPDS No. 20-0781).36 The NiMoS@NiCo-LDH composite presents the characteristic diffraction peaks of two crystalline phases. The peaks with “♦” are assigned to the Ni3S2 phase (JCPDs No. 73-0698). Sulfurization of the sample results in low Mo content, which is consistent with a previous report.37 The other peaks marked with “ ” are located at 2θ = 11.59°, 23.14°, 34.95°, 39.4° and 60.85°, which can be indexed as the hydrotalcite-like Ni0.75Co0.25(CO3)0.125(OH)2·0.38 H2O phase (JCPDs No. 40-0216).38
” are located at 2θ = 11.59°, 23.14°, 34.95°, 39.4° and 60.85°, which can be indexed as the hydrotalcite-like Ni0.75Co0.25(CO3)0.125(OH)2·0.38 H2O phase (JCPDs No. 40-0216).38
 |
| | Fig. 1 Preparation route schematic diagram of the NiMoS@NiCo-LDH heterostructure. | |
 |
| | Fig. 2 Structural characterization of the as-prepared samples: (a) XRD patterns, (b) XPS survey spectra of the NiMoS@NiCo-LDH samples, (c) XPS survey spectra of the NiMoO4 samples, (d) Ni 2p, (e) Mo 3d, (f) S 2p, (g) Co 2p, and (h) O 1s spectra and (i) BET of the three products. | |
XPS is then used to study the surface chemical states and composition of the samples. The full spectra (Fig. 2b and c) prove the existence of the Ni, Co, O, S and Mo elements. As seen in Fig. 2d, the Ni 2p spectra of NiMoO4 and NiMoS@NiCo-LDH materials can be divided into four peaks, which contain two spin–orbit doublets with characteristics of Ni 2p3/2 and Ni 2p1/2 orbitals and two satellites.39 The spin–orbit demonstrates coexistence of Ni2+ and Ni3+. Compared with the NiMoO4 product, the Ni 2p peaks of NiMoS@NiCo-LDH electrodes shift toward a high binding energy, which is conducive to the electrochemical performance of NiMoS@NiCo-LDH samples.40 In the Mo 3d spectra (Fig. 2e), the peaks of the composites with binding energies at 232.1 and 234.8 eV are ascribed to Mo 3d5/2 and Mo 3d3/2 orbitals. In addition, Mo 3d peaks overlap with the S 2s region.41 From Fig. 2f, the S 2p spectra at binding energies of 161.2 and 163.1 eV are assigned to S 2p3/2 and S 2p1/2 orbitals and another satellite peak can be seen at 168.6 eV.42Fig. 2g shows the Co 2p spectra with Co 2p1/2 and Co 2p3/2 orbitals. The peaks at 781.2 and 797.4 eV correspond to Co2+ and those at 783.5 and 800.7 eV belong to Co3+.43 Moreover, two satellite peaks can be seen at 786.8 and 804.2 eV, respectively. In Fig. 2h, the signal peaks of the O 1s spectra of the NiMoS@NiCo-LDH sample at 532.9, 531.7 and 530.8 eV are deconvoluted into physicochemical water on the surface, oxygen ions and metal oxygen bonds, respectively.44Fig. 2i presents the N2 adsorption–desorption isotherms of the three samples. They reveal typical IV hysteresis loops in a relative pressure range of 0 and 1, demonstrating their mesoporous feature. The composite possesses a specific surface area of 79.958 m2 g−1, which is larger than that of NiMoS (17.832 m2 g−1) and NiMoO4 (16.139 m2 g−1). The average pore volume (0.36 cm3 g−1) and pore size (18.133 nm) of the NiMoS@NiCo-LDH sample are larger than those of NiMoS (0.030 cm3 g−1, 6.822 nm) and NiMoO4 (0.039 cm3 g−1, 9.585 nm), respectively. The results show that the composite possesses many active sites, which is beneficial to enhancing the electrochemical performance of the supercapacitor.
After that, we observe the morphologies of the as-fabricated samples by SEM. In Fig. S2a,† NiMoO4 materials possess sphere-like shapes connected with each other. After sulfurization, the overall morphology of the NiMoS microspheres remains almost unchanged but the surface roughness is significantly increased (Fig. S2b†). As seen in Fig. S2c,† NiCo-LDH products present a nanosheet array structure with a thickness of 50 nm. Fig. 3a shows that NiMoS@NiCo-LDH composites are vertically grown on the surface of the NF. From Fig. 3b and c, the NiCo-LDH materials are evenly coated on the surface of NiMoS microspheres. The unique core-shell structure protects NiMoS products from dissolution and improves their specific surface area. This also maintains the stability of the structure and relieves volume expansion during charging and discharging. The energy-dispersive X-ray (EDX) spectra (Fig. S2d†) reveal that the materials are composed of Ni, Mo, S and Co elements. The corresponding elemental mappings (Fig. 3d) indicate that the above elements are uniformly distributed on the surface of the NiMoS@NiCo-LDH composites.
 |
| | Fig. 3 Morphology characterization: (a–c) SEM images of the NiMoS@NiCo-LDH sample and (d) elemental mapping images of the NiMoS@NiCo-LDH product. | |
Subsequently, we explore the electrochemical performance of the as-prepared products in a standard three-electrode system. Fig. 4a illustrates the CV curves of several electrodes at 50 mV s −1. It is found that NiMoS@NiCo-LDH samples possess a larger curve area than other materials, demonstrating their excellent capacitance. Fig. S3a–c† shows the CV curves of the NiMoO4, NiMoS and NiCo-LDH samples at different scan rates, revealing that they possess distinct faradaic redox peaks. Fig. 4b illustrates the GCD curves of four materials at a current density of 1 A g−1. The NiMoS@NiCo-LDH composite shows the longest charge and discharge times compared to the other samples, which matches well with the corresponding CV curves. And it is seen that the NiMoS@NiCo-LDH composite possesses two charging and discharging platforms compared to the three single materials with a charge and discharge platform. This demonstrates that it combines the electrochemical characteristics of single materials and shows the advantages of composites. Fig. 4c shows the CV curves of the NiMoS@NiCo-LDH samples. As the scanning rate increases, the area of the CV curve increases relatively, and the redox peaks are shifted in the cathode and anode directions. Its shape remains almost unchanged, revealing the satisfactory reversibility of the electrode and fast ion transfer kinetics.
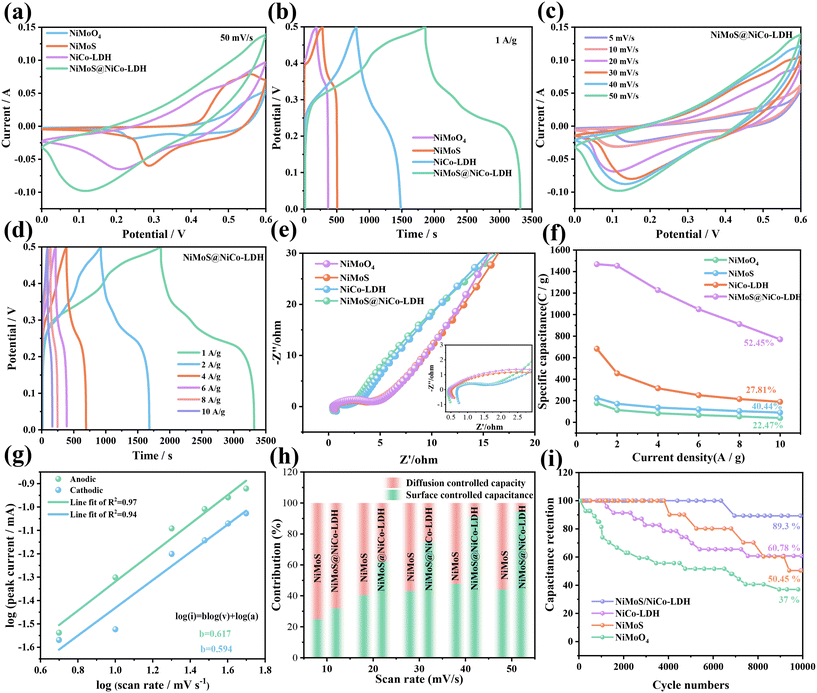 |
| | Fig. 4 Electrochemical performance of the electrode materials: (a) comparison of CV curves, (b) comparison of GCD curves, (c) CV curves of the NiMoS@NiCo-LDH sample at different scan rates, (d) GCD curves of the NiMoS@NiCo-LDH sample at different current densities, (e) Nyquist plots, (f) specific capacitance, and (g) b value of NiMoS@NiCo-LDH, (h) contribution ratio between capacitance and the diffusion-limited one, and (i) cycling performance at 2 A g−1. | |
The electrochemical mechanism of the NiMoS@NiCo-LDH electrode materials is represented by the following equations:45,46
| | | NiS + OH− ↔ NiS(OH) + e− | (7) |
| | | MoS + OH− ↔ MoS(OH) + e− | (8) |
| | | Ni(OH)2 + OH− ↔ NiOOH + e− | (9) |
| | | Co(OH)2 + OH− ↔ CoOOH + e− | (10) |
| | | CoOOH + OH− ↔ CoO2 + H2O + 3e− | (11) |
The GCD curves of the NiMoS@NiCo-LDH composites present two obvious charge and discharge platforms (Fig. 4d). This suggests that they possess excellent pseudo-capacitance performance and electrochemical reversibility. Besides, the charging time of the materials reduces gradually at high current densities. It is due to the reduced interaction between the active substance and electrolyte ions. The product delivers specific capacity values of 1462, 757, 322, 179, 117 and 76 C g−1 at 1, 2, 4, 6, 8 and 10 A g−1, respectively. In comparison, they are higher than those of other electrodes (Table S1†).
The as-fabricated materials are further investigated using Nyquist curve measurements (Fig. 4e). The corresponding equivalent circuit is depicted in Table 1. NiMoS@NiCo-LDH composites (0.41 Ω, 1.34 Ω) show superior Rs and Rct compared to NiMoO4 (0.45 Ω, 3.24 Ω), NiMoS (0.52 Ω, 2.62 Ω) and NiCo-LDH (0.68 Ω, 1.16 Ω) samples. They also present the maximum slope compared with other materials, which indicates that the hybrid structure exhibits outstanding kinetic behavior and ion diffusion rate. From Fig. 4f, the specific capacitance of the NiMoS@NiCo-LDH electrode is 1462 C g−1 at 1 A g−1. It is 52.45% of the initial capacitance at 10 A g−1. When the current density is the same, NiMoO4, NiMoS and NiCo-LDH materials maintain 22.47%, 40.44% and 27.81% capacitive retention, respectively. This confirms the excellent rate performance of the NiMoS@NiCo-LDH electrode (Table 2).
Table 1 Electrochemical impedance analysis
| Samples |
R
s
|
R
ct
|
CPE-T (F cm−2) |
CPE-P |
| NiMoO4 |
0.45 |
3.24 |
0.00298 |
0.79 |
| NiMoS |
0.52 |
2.62 |
0.00257 |
0.80 |
| NiCo-LDH |
0.66 |
1.16 |
0.00164 |
0.81 |
| NiMoS@NiCo-LDH |
0.41 |
1.34 |
0.00228 |
0.76 |
Table 2 Electrochemical performance of the electrode materials
| Composite materials |
Specific capacity (F g−1) |
Energy density (W h kg−1) |
Power density (W kg−1) |
Cycle number |
Capacity retention rate |
Refs. |
| Ni0.7Co0.3MoS4 |
1019.6 |
28.9 |
968.3 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
92.4% |
43
|
| NiCo-LDH@NiOOH |
2622 |
51.7 |
599 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
88.5% |
44
|
| RGO@NiCo2S4@NiMo-LDH |
1346 |
59.38 |
808.19 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
80% |
45
|
| Mo–NiS2@NiCo-LDH |
2604.8 |
38.1 |
800 |
— |
— |
46
|
| This work |
2912 |
80.21 |
2698.65 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
89% |
— |
CV curves are employed to investigate the reaction kinetics of the as-fabricated materials. The equation is shown below:47
where
a and
b correspond to constants,
i is the peak current,
ν shows the scan rate, and the
b value can be calculated using the log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i
i and log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v slopes.
When b = 0.5, the energy storage of the material is mainly controlled by diffusion behavior. This shows battery material characteristics by the embedding and detachment of ions. When the b value is 1, the energy storage mechanism of the product is carried out through the electro-sorption of ions. It is manifested as a capacitive behavior of the product. Fig. 4g provides the curves after fitting the anodic and cathodic peaks of the NiMoS@NiCo-LDH samples. They correspond to 0.617 and 0.594, respectively.
To differentiate between diffusion capacitance and surface capacitance, the following equation is used:48
where
i,
k1 and
k2, and
ν denote the current, constants and scan rate, respectively.
As shown in Fig. 4h, the capacitive contribution is calculated at various scan rates. The surface capacitance contribution gradually dominates, which is attributed to the decreasing of the diffusion-controlled ion insertion process leading to a decrease in the contribution of the capacitance. Fig. 4i presents the capacitance retention of four samples after cycling 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times at 5 A g−1. The NiMoS@NiCo-LDH composite has a capacitance retention of 89.3%, which is higher than that of the NiMoO4 (37%), NiMoS (50.45%) and NiCo-LDH (60.78%) electrode materials. In the process of charge and discharge, with the increase of the number of cycles, the internal microstructure of the electrode material slightly collapses, resulting in a decrease of the specific capacity.
000 times at 5 A g−1. The NiMoS@NiCo-LDH composite has a capacitance retention of 89.3%, which is higher than that of the NiMoO4 (37%), NiMoS (50.45%) and NiCo-LDH (60.78%) electrode materials. In the process of charge and discharge, with the increase of the number of cycles, the internal microstructure of the electrode material slightly collapses, resulting in a decrease of the specific capacity.
Then, we construct several ASCs with NiMoS@NiCo-LDH samples as cathodes and AC as anodes (Fig. 5a). After being divided by the PVA-KOH gel electrolyte, they are wrapped in an Al-plastic film. Fig. 5b shows the CV curves of the NiMoS@NiCo-LDH electrodes and AC in the range of 0 to 0.6 V and −1 to 0 V at 100 mV s−1. A theoretical potential of 1.6 V can be obtained for the flexible device from the individual potential windows of the two materials. From Fig. 5c, the CV curve reveals an approximate rectangular shape, indicating that the ASC possesses both a bilayer capacitance contribution and a pseudo-capacitance contribution. In Fig. 5d, due to the addition of the AC anode, the shape of the GCD curve is changed from circular to linear. This demonstrates the pseudo-capacitive contribution of the device in charging and discharging processes. It delivers the capacitances of 142, 106, 78, 69, 60 and 56 C g−1 at 0.5, 1, 2, 4, 6 and 8 A g−1, respectively, which are superior to those of the other samples. The CV and GCD curves of NiMoO4, NiMoS and NiCo-LDH materials are shown in Fig. S4.†Fig. 5e presents the Nyquist curves of the electrode in the 100 kHz–0.01 Hz range. The matching circuit is shown in the inset. It is clearly found that NiMoS@NiCo-LDH//AC products possess a low internal resistance. After five EIS tests on the same sample, the standardized deviations are calculated to be Rs (AVG = 1.66, R.S.D. = 1.2%), Rct (AVG = 2.05, R.S.D. = 5.18%) and CPE-P (AVG = 0.82, R.S.D. = 0.74%). The result shows no remarkable range variations in the samples, confirming its excellent repeatability. From Fig. 5f, the device achieves an energy density of 80.21 W h kg−1 at a power density of 2699 W kg−1. The electrochemical performance is visually revealed through an enclosed area using power density, energy density, specific capacity, current density, number of cycles and capacity retention as parameters. The area of the integration curve under the NiMoS@NiCo-LDH composites is larger than those under other reported materials, indicating the dominance of our assembled device.49–52
 |
| | Fig. 5 Electrochemical performance of the ASC: (a) schematic of the ASC device, (b) CV curves of the NiMoS@NiCo-LDH sample and active carbon, (c) CV curves of the NiMoS@NiCo-LDH//AC sample at different scan rates, (d) GCD curves of the NiMoS@NiCo-LDH//AC sample at different current densities, (e) Nyquist plots, and (f) radar plots. | |
Finally, we investigate the practical application of NiMoS@NiCo-LDH//AC. Fig. 6a illustrates the CV curves of the ASC at different folding angles. After folding 500 times, we still observe that the curves almost completely overlap at different angles. From Fig. 6b, the values of Rct progressively rise as the folding angles vary. It goes back to its original state after the process of consecutive bending, demonstrating its excellent flexibility and mechanical stability. A blue LED is lit for 9 min by connecting three NiMoS@NiCo-LDH//AC devices in series, as shown in Fig. 6c. This demonstrates potential applications for portable devices. Fig. 6d shows the cycling curves of the products at different temperatures. After cycling 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, the flexible device retains 85%, 72%, 71% and 58% of the initial capacitance at 25, 0, −10 and −20 °C, respectively. The SEM image after cycling is shown in the inset, demonstrating that the shape of the NiMoS@NiCo-LDH materials is retained well.
000 times, the flexible device retains 85%, 72%, 71% and 58% of the initial capacitance at 25, 0, −10 and −20 °C, respectively. The SEM image after cycling is shown in the inset, demonstrating that the shape of the NiMoS@NiCo-LDH materials is retained well.
 |
| | Fig. 6 (a) CV curves in multiple states, (b) Nyquist plots in multiple states, (c) digital photos of the blue LED, and (d) cycling performance at different temperatures. | |
4. Conclusions
In summary, we report a novel NiMoS@NiCo-LDH core–shell structure grown on NF. The prepared NiMoS microspheres as the substrate improves the electrochemical conductivity and provides rich redox active sites. Meanwhile, the obtained product shows outstanding specific capacity and cycle performance owing to the synergistic effect between the electrodes. In addition, the fabricated ASC achieves favorable mechanical stability at various bending angles and stable cycle ability at extreme temperatures (−20 °C). This work provides a strategy for the development of novel electrodes for high-performance flexible supercapacitors.
Data availability
The data that supports the findings of this study is available from the corresponding authors upon reasonable request.
Author contributions
Qi He: methodology, conceptualization, software, data curation, and writing – original draft preparation. Wei Jia: visualization and software. Xiang Wu and Jinghai Liu: supervision, writing – reviewing and editing.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The work is supported by the National Natural Science Foundation of China (No. 52172218) and the open research funding of the Inner Mongolia Engineering Research Center of Lithium–Sulfur Battery Energy Storage (MDK2023084).
References
- Y. Liu and X. Wu, J. Energy Chem., 2023, 87, 334–341 CrossRef CAS
 .
.
- M. Arif, J. Riaz, A. Bibi, H. R. Yang and T. Zhu, APL Mater., 2024, 12, 071119 CrossRef CAS
 .
.
- Y. Liu, Y. Liu, X. Wu and Y. R. Cho, Enhanced electrochemical performance of Zn/VOx batteries by a carbon encapsulation strategy, ACS Appl. Mater. Interfaces, 2022, 14, 11654–11662 CrossRef CAS PubMed
 .
.
- Y. C. Chen, Y. L. Li, Y. X. Dong, D. H. Li, S. J. Shen, J. D. Hu, Y. J. Fu, D. Y. He and J. H. Li, Sci. China Mater., 2024, 67, 816–823 CrossRef CAS
 .
.
- Y. Liu and X. Wu, Nano Energy, 2024, 127, 109809 CrossRef CAS
 .
.
- K. L. Li, C. Q. Yin, X. J. Dai, J. Y. Zhang, S. Yi, J. S. Rao and Y. X. Zhang, J. Energy Storage, 2022, 55, 105722 CrossRef
 .
.
- K. L. Li, H. Teng, X. J. Dai, Y. Wang, D. S. Wang, X. F. Zhang, Y. J. Yao, X. Y. Liu, L. Feng, J. S. Rao and Y. X. Zhang, CrystEngComm, 2022, 24, 2081–2088 RSC
 .
.
- J. J. Zhang and X. Wu, Batteries Supercaps, 2024, 7, e202400205 CrossRef CAS
 .
.
- Y. F. Ren, Z. L. He, H. Z. Zhao and T. Zhu, Rare Met., 2022, 41, 830–835 CrossRef CAS
 .
.
- J. Y. Liang, S. Qin, S. Luo, D. Pan, P. F. Xu and J. Li, J. Colloid Interface Sci., 2024, 653, 504–516 CrossRef CAS PubMed
 .
.
- J. J. Zhang and X. Wu, Int. J. Miner., Metall. Mater., 2024, 31(1), 179–185 CrossRef CAS
 .
.
- T. Shu, X. Yang, Z. Huang, M. Qiao, J. Y. Ning, K. L. Li, Y. X. Zhang, L. Li, X. Liu and K. X. Yao, J. Mater. Chem. A, 2024, 12, 4666–4677 RSC
 .
.
- T. Zhu, J. Pan, Z. An, R. Zhe, Q. Ou and H. E. Wang, J. Mater. Chem. A, 2022, 10, 20375–20385 RSC
 .
.
- D. Y. Jiang, Y. Q. Wang, C. Du, M. J. Xie, J. Chen, Y. Zhang and L. Wan, J. Alloys Compd., 2024, 970, 172685 CrossRef CAS
 .
.
- X. Y. Huai, J. X. Liu and X. Wu, Chin. J. Struct. Chem., 2023, 42(10), 100158 CrossRef CAS
 .
.
- F. Shi, S. Zhao, J. Yang, Y. Tong, J. Li, S. Zhai, X. Zhao, S. Wu, H. Li, Q. An and K. Wang, J. Mater. Chem. A, 2022, 10, 12679–12691 RSC
 .
.
- Y. B. Chen, J. J. You, Y. H. Chen, L. A. Ma, H. X. Chen, Z. H. Wei, X. Y. Ye and L. Zhang, CrystEngComm, 2022, 24(29), 5238–5250 RSC
 .
.
- V. S. Kumbhar, V. Q. Nguyen, Y. R. Lee, C. D. Lokhande, D.-H. Kim and J.-J. Shim, New J. Chem., 2018, 42(18), 14805–14816 RSC
 .
.
- G. G. Wang, Q. Y. Chen, J. Zhang, X. G. An, Q. Liu, L. S. Xie, W. T. Yao, X. P. Sun and Q. Q. Kong, J. Colloid Interface Sci., 2023, 647, 73–80 CrossRef CAS PubMed
 .
.
- X. W. Wang, Y. C. Sun, W. C. Zhang and X. Wu, Chin. Chem. Lett., 2023, 34(3), 107593 CrossRef CAS
 .
.
- Y. T. Wang, X. F. He, G. Y. He, C. Meng, X.-M. Chen, F.-T. Li and Y. Zhou, Crit. Rev. Solid State Mater. Sci., 2022, 48(4), 502–518 CrossRef
 .
.
- J. Khan, N. Shakeel, S. Alam, F. Alam and A. Dahshan, Batteries Supercaps, 2023, 7(1), e202300366 CrossRef
 .
.
- D. W. Du, R. Lan, J. Humphreys, W. Xu, K. Xie, H. Wang and S. W. Tao, J. Electrochem. Soc., 2017, 164(12), A2881–A2888 CrossRef CAS
 .
.
- X. Y. Huai, J. X. Liu and X. Wu, CrystEngComm, 2023, 25(37), 5310–5315 RSC
 .
.
- A. Ali, I. Hussain, I. Hameed, M. A. Khan and J. J. Shim, J. Alloys Compd., 2024, 1003, 175568 CrossRef CAS
 .
.
- Q. Sun, Z. Y. Guo, T. Shu, Y. F. Li, K. L. Li, Y. X. Zhang, L. Li, J. Y. Ning and K. X. Yao, ACS Appl. Mater. Interfaces, 2024, 16(10), 12781–12792 CrossRef CAS PubMed
 .
.
- K. Zhang, X. Gao, F. Yao, Y. Q. Xie, H. Bai, Y. J. Sun, R. G. Liu and H. Y. Yue, J. Colloid Interface Sci., 2023, 650, 105–111 CrossRef CAS PubMed
 .
.
- A. Kandula, K. R. Shrestha, G. Rajeshkhanna, N. H. Kim and J. H. Lee, ACS Appl. Mater. Interfaces, 2019, 11(12), 11555–11567 CrossRef
 .
.
- L. An, J. R. Feng, Y. Zhang, R. Wang, H. W. Liu, G. C. Wang, F. G. Cheng and P. X. Xi, Adv. Funct. Mater., 2018, 29(1), 1805298 CrossRef
 .
.
- X. Li, J. Ren, D. Sridhar, B. B. Xu, H. Algadi, Z. M. El, Y. Bahy, T. Li Ma and Z. Guo, Mater. Chem. Front., 2023, 7(8), 1520–1561 RSC
 .
.
- Y. Q. Guo, X. F. Hong, Y. Wang, Q. Li, J. S. Meng, R. T. Dai, X. Liu, L. He and L. Q. Mai, Adv. Funct. Mater., 2019, 29(24), 1809004 CrossRef
 .
.
- Y. C. Wang, M. Zhang, Y. Y. Liu, Z. K. Zheng, B. Y. Liu, M. Chen, G. Q. Guan and K. Yan, Adv. Sci., 2023, 10(13), 2207519 CrossRef CAS
 .
.
- S. Wang, C. H. Hu, J. H. Wang, M. Z. Xie, S. Y. Wu, L. H. Zhao, J. X. Gong and Y. T. Dai, J. Energy Storage, 2023, 73, 109034 CrossRef
 .
.
- C. Y. Yang, F. X. Song and Q. L. Chen, ACS Appl. Nano Mater., 2023, 6(12), 10804–10816 CrossRef CAS
 .
.
- Y. C. Sun, X. W. Wang, A. Umar and X. Wu, RSC Adv., 2022, 12(23), 14858–14864 RSC
 .
.
- D. Zhao, X. Liu, W. C. Zhang and X. Wu, Small Methods, 2023, 8, 2301474 CrossRef PubMed
 .
.
- J. X. Liu, S. Q. Zhao and X. Wu, Chin. Chem. Lett., 2024, 35(7), 109059 CrossRef CAS
 .
.
- M. D. Wang, X. Y. Liu, H. Q. Liu, D. P. Zhao and X. Wu, J. Alloys Compd., 2022, 903, 163926 CrossRef CAS
 .
.
- G. Yu, J. Zhou, Q. Chen, Z. Huang, K. Tao and L. Han, Mater. Today Chem., 2023, 30, 101601 CrossRef CAS
 .
.
- H. Q. Liu, D. P. Zhao, M. Z. Dai, X. F. Zhu, F. Y. Qu, A. Umar and X. Wu, Chem. Eng. J., 2022, 428, 131183 CrossRef CAS
 .
.
- J. X. Li, J. Zhao, L. W. Qin, Q. Zhang, X. L. Tang and Y. Y. Xu, RSC Adv., 2020, 10(38), 22606–22615 RSC
 .
.
- C. Germán-Santiana Espín, W. M. B. Morocho, A. Á. S. Cordero, S. Chandra, P. Bansal, H. Kaur, M. A. Mustafa and S. Islam, J. Electroanal. Chem., 2024, 960, 118163 CrossRef
 .
.
- J. Y. Ma, Q. Sun, C. Jing, F. L. Ling, X. Tang, Y. H. Li, Y. J. Wang, S. Jiang, K. X. Yao and X. J. Zhou, CrystEngComm, 2023, 25, 3066–3078 RSC
 .
.
- Q. He and X. Wu, Batteries, 2024, 10(7), 230 CrossRef CAS
 .
.
- R. Manikandan, C. J. Raj, G. Nagaraju, M. Pyo and B. C. Kim, J. Mater. Chem. A, 2019, 7(44), 25467–25480 RSC
 .
.
- S. Z. Cui, Q. Z. Hu, K. J. Sun, X. Wang, F. Q. Wang, H. A. Hamouda, H. Peng and G. F. Ma, ACS Appl. Nano Mater., 2022, 5(5), 6181–6191 CrossRef CAS
 .
.
- M. Z. Dai, D. P. Zhao, H. Q. Liu, X. F. Zhu, X. Wu and B. Wang, ACS Appl. Energy Mater., 2021, 4(3), 2637–2643 CrossRef CAS
 .
.
- M. Wei, X. D. Wu, Y. B. Yao, S. H. Yu, R. Sun and C. P. Wong, ACS Sustainable Chem. Eng., 2019, 7(24), 19779–19786 CrossRef CAS
 .
.
- Y. C. Lu, B. G. Huang, J. G. Yuan, Y. F. Qiao, W. Y. Zhang, G. Y. He and H. Q. Chen, Dalton Trans., 2022, 51(46), 17820–17826 RSC
 .
.
- H. Y. Liang, J. H. Lin, H. N. Jia, S. L. Chen, J. L. Qi, J. Cao, T. S. Lin, W. D. Fei and J. C. Feng, J. Power Sources, 2018, 378, 248–254 CrossRef CAS
 .
.
- C. Cheng, Y. J. Zou, F. Xu, C. L. Xiang, Q. L. Sui, J. Zhang, L. X. Sun and Z. M. Chen, J. Energy Storage, 2022, 52, 230 Search PubMed
 .
.
- M. M. Shi, M. S. Zhao, L. D. Jiao, Z. Su, M. Li and X. P. Song, J. Power Sources, 2021, 509, 230333 CrossRef CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a and
Jinghai
Liu
*a and
Jinghai
Liu
 *b
*b
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. Furthermore, they still work stably at extreme temperatures ranging from 25 to −20 °C. The asymmetric supercapacitor (ASC) also presents excellent mechanical durability and stability at different bending angles.
000 times. Furthermore, they still work stably at extreme temperatures ranging from 25 to −20 °C. The asymmetric supercapacitor (ASC) also presents excellent mechanical durability and stability at different bending angles.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. In addition, the assembled NiMoS@NiCo-LDH//AC device shows an energy density of 80.21 W h kg−1 at a power density of 2699 W kg−1. Moreover, the ASC also demonstrates superior mechanical stability at various bending angles and can work stably from 0 °C to −20 °C.
000 times. In addition, the assembled NiMoS@NiCo-LDH//AC device shows an energy density of 80.21 W h kg−1 at a power density of 2699 W kg−1. Moreover, the ASC also demonstrates superior mechanical stability at various bending angles and can work stably from 0 °C to −20 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then N-methylpyrrolidone (NMP) organic solvent was dropped into the mixture to obtain a slurry. Then it was spread evenly over the pretreated NF and then vacuum dried at 60 °C for 12 h.
1. Then N-methylpyrrolidone (NMP) organic solvent was dropped into the mixture to obtain a slurry. Then it was spread evenly over the pretreated NF and then vacuum dried at 60 °C for 12 h.
 ” are located at 2θ = 11.59°, 23.14°, 34.95°, 39.4° and 60.85°, which can be indexed as the hydrotalcite-like Ni0.75Co0.25(CO3)0.125(OH)2·0.38 H2O phase (JCPDs No. 40-0216).38
” are located at 2θ = 11.59°, 23.14°, 34.95°, 39.4° and 60.85°, which can be indexed as the hydrotalcite-like Ni0.75Co0.25(CO3)0.125(OH)2·0.38 H2O phase (JCPDs No. 40-0216).38

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = b
i = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log
v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i and log
i and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v slopes.
v slopes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times at 5 A g−1. The NiMoS@NiCo-LDH composite has a capacitance retention of 89.3%, which is higher than that of the NiMoO4 (37%), NiMoS (50.45%) and NiCo-LDH (60.78%) electrode materials. In the process of charge and discharge, with the increase of the number of cycles, the internal microstructure of the electrode material slightly collapses, resulting in a decrease of the specific capacity.
000 times at 5 A g−1. The NiMoS@NiCo-LDH composite has a capacitance retention of 89.3%, which is higher than that of the NiMoO4 (37%), NiMoS (50.45%) and NiCo-LDH (60.78%) electrode materials. In the process of charge and discharge, with the increase of the number of cycles, the internal microstructure of the electrode material slightly collapses, resulting in a decrease of the specific capacity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, the flexible device retains 85%, 72%, 71% and 58% of the initial capacitance at 25, 0, −10 and −20 °C, respectively. The SEM image after cycling is shown in the inset, demonstrating that the shape of the NiMoS@NiCo-LDH materials is retained well.
000 times, the flexible device retains 85%, 72%, 71% and 58% of the initial capacitance at 25, 0, −10 and −20 °C, respectively. The SEM image after cycling is shown in the inset, demonstrating that the shape of the NiMoS@NiCo-LDH materials is retained well.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.




