Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design and synthesis of ternary multi-component crystals by sublimation
Matthew C.
Scheepers
 * and
Delia A.
Haynes
* and
Delia A.
Haynes

Department of Chemistry & Polymer Science, Stellenbosch University, P. Bag X1, Stellenbosch, 7599, South Africa. E-mail: mcscheepers@sun.ac.za
First published on 28th October 2025
Abstract
Three ternary multi-component crystals have been prepared by crystallisation from the gas phase (sublimation); two co-crystal salts and one neutral co-crystal. One of the co-crystal salts is reported here for the first time. Control of the product obtained from sublimation is attained by controlling sublimation temperature or stoichiometry of the co-formers.
Crystal engineering is the design and synthesis of solid materials with new or improved properties from existing materials.1 One approach to accomplish this is the preparation of multi-component crystals such as co-crystals and salts.2,3 Co-crystals are crystals which contain two or more neutral compounds in a stoichiometric ratio, while molecular salts often result from proton transfer between two molecules. There are cases where the crystal contains both neutral molecules and ions, and a more hierarchical approach to describing these crystals is required.2,4
One of the greatest issues with the development of multi-component crystals is their design and subsequent preparation. The general approach involves choosing co-formers which contain structural features or functional groups that result in the formation of strong, directional intermolecular interactions between co-formers.5,6 Generally, this is easier to accomplish for binary multi-component crystals, but for ternary and higher multi-component crystals (crystals containing three or more molecular components), this proves to be more challenging.7,8 Not only is it necessary to overcome crystallisation of the individual components, but the crystallisation of binary co-crystals must also be prevented.7 Even with a strong rationale for the choice of particular co-formers, a variety of factors involved in the crystallisation process may prohibit the formation of a potential ternary multi-component crystal.
Many molecular crystals are crystallised from solution, usually yielding crystals appropriate for single-crystal X-ray diffraction (SC-XRD) experiments. One of the key problems with crystallisation from solution is finding suitable solvents and/or solvent mixtures that can sufficiently dissolve the material of interest. This becomes even more challenging with multi-component crystals, where the co-formers can have vastly different solubilities, which may prohibit dissolving all the components in the same solvent system. An alternate method for synthesising multi-component crystals is mechanochemistry, which allows co-formers with vastly different solubilities to interact.9 An important disadvantage of mechanochemistry is that the resulting product is a powder, which may make it more difficult to determine the crystal structure of the resulting product. Sublimation, or crystallisation from the gas phase, is a potentially useful method for producing SC-XRD-quality multi-component crystals in a relatively short time frame, while minimising or removing the use of solvents. The sublimation technique usually involves heating the solid materials under vacuum until they enter the gas phase. This gas then moves to a colder region of the container, where it desublimes, forming crystals on the surface of the container. This technique can be used to form co-crystals, molecular salts and even hydrates.10–13
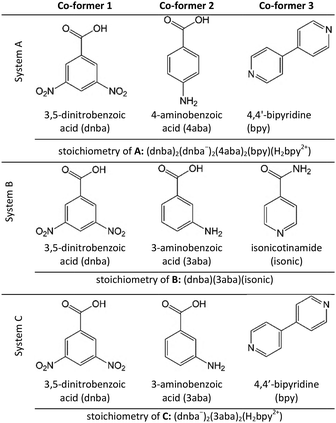
Most of the previous work involving the preparation of multi-component crystals using sublimation involves binary systems, and there is only one report of the synthesis of a ternary (or higher) multi-component crystal using this method.14 Therefore, the aim of this work was to investigate the use of sublimation as a means to synthesise ternary multi-component crystals. Two previously reported ternary multi-component crystals were identified for investigation (Table 1). The first, A, is formed from 3,5-dinitrobenzoic acid (dnba), 4-aminobenzoic acid (4aba) and 4,4′-bipyridine (bpy) (system A).8 The asymmetric unit of this multi-component crystal (Cambridge Structural Database15 (CSD) refcode: BEYZIQ8) contains two molecules of 4aba, four molecules of bpy (one fully protonated, one neutral). It can thus be described as a co-crystal salt.4 The second ternary multi-component crystal we investigated, B (refcode BIZTIP16), contains dnba, 3-aminobenzoic acid (3aba) and isonicotinamide (isonic) (system B), and is a neutral co-crystal. Finally, we also report a new ternary multi-component crystal, C, which is formed from dnba, 3aba and bpy (system C), and is also a co-crystal salt. This material has been prepared for the first time by sublimation.
In this study, crystallisation from the gas phase was carried out in sublimation tubes (thin Schlenk tubes). A mixture of the co-formers (0.24 mmol each) was added to the bottom of a Schlenk tube (length = 220 mm, diameter = 10 mm). The tube was placed under static vacuum (p = 0.86 mbar) and submerged in an oil bath pre-heated to a selected temperature. After some time, the tube was removed from the oil bath, and crystals were observed on the inside of the tube in the cooler regions. Most crystals were observed to form after 5–60 min. Products were identified using PXRD, and confirmed by unit cell analysis for all single-component and binary sublimations, as well as ternary sublimations wherever possible. Unless explicitly stated otherwise, all samples sublimed completely. Several sublimation experiments were repeated, and in all cases the results were reproducible. Details, including PXRD of products, are given in the SI.
We first established the sublimation temperatures of the individual co-formers. Table S2 reports the temperature range in which each co-former is observed to start subliming in our sublimation apparatus, as well as the solid-state phases of the starting material and the sublimed product. With the exception of bpy, the co-formers used in this work are polymorphic. Both isonic and 3aba changed polymorphic form on sublimation. The change of 3aba from Form III to Form IV from sublimation has been previously reported.17 The change of isonic from Form I to Form II on sublimation was unexpected, as previous work by Aakeröy et al. reported that Form I is obtained from sublimation.18 It is plausible that both polymorphs could crystallise from sublimation, but under different sublimation conditions (the experimental conditions used by Aakeröy et al. were not reported).
Table S3 lists the binary multi-component crystals formed from the co-formers in this study that have been previously reported in the CSD. Generally, sublimation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of co-formers gave the same binary multi-component crystals as those reported in the CSD for a particular conformer combination (Table S4). For the combination of dnba + isonic, previous work has reported that the solvent system determines whether the 1
1 molar ratio of co-formers gave the same binary multi-component crystals as those reported in the CSD for a particular conformer combination (Table S4). For the combination of dnba + isonic, previous work has reported that the solvent system determines whether the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dnba
2 dnba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isonic form is obtained from solution, regardless of the ratio of the starting materials.19 From our 1
isonic form is obtained from solution, regardless of the ratio of the starting materials.19 From our 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sublimation experiment we obtained the 1
1 sublimation experiment we obtained the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 crystal, as well as a potential new crystal form that we were unable to identify. For the 3aba + bpy combination, we obtained a concomitant mixture of products from sublimation; the stoichiomorphs have previously been reported to crystallise from different solvents.20 It is interesting that removal of the matrix in the sublimation experiment also removes the selectivity for a particular form, and both forms crystallise concomitantly. We have noted this effect in our previous work on competition between hydrogen and halogen bonds.13
2 crystal, as well as a potential new crystal form that we were unable to identify. For the 3aba + bpy combination, we obtained a concomitant mixture of products from sublimation; the stoichiomorphs have previously been reported to crystallise from different solvents.20 It is interesting that removal of the matrix in the sublimation experiment also removes the selectivity for a particular form, and both forms crystallise concomitantly. We have noted this effect in our previous work on competition between hydrogen and halogen bonds.13
The sublimation of dnba + 3aba yielded a new crystal form, Form II (we will label the form reported by Lynch et al.21 Form I), which consists of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of dnba
1 ratio of dnba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3aba. The PXRD results obtained from the sublimation experiments show that only this new Form II is formed from sublimation. Form II features no proton transfer between dnba and 3aba, making this a rare example of what has been called co-crystal-salt polymorphism.22
3aba. The PXRD results obtained from the sublimation experiments show that only this new Form II is formed from sublimation. Form II features no proton transfer between dnba and 3aba, making this a rare example of what has been called co-crystal-salt polymorphism.22
Having confirmed the behaviour of the single-component and binary systems in sublimation experiments, we turned to ternary systems. First, we wanted to determine if it was possible to synthesise a known ternary multi-component crystal by sublimation. One reason for choosing system A is that the ternary multi-component crystals A are orange,8 which makes it easy to confirm whether the sublimation has successfully formed the ternary crystal (the other possible single or binary co-crystals that can form are either yellow or white/colourless). In our initial sublimation experiment, we used a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molecular ratio of dnba
1 molecular ratio of dnba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aba
4aba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
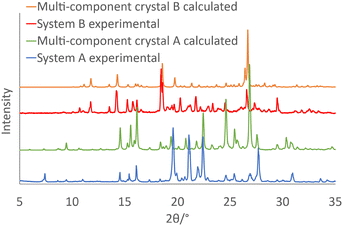
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy. This mixture was heated under vacuum at 180 °C, such that dnba would sublime at a rate that is comparable with the other components.† This experiment resulted in the formation of a single band of orange crystals, indicating the successful formation of the ternary multi-component crystal A. The PXRD pattern of these orange crystals was found to match with the calculated pattern of the structure reported by Seaton et al. (refcode BEYZIQ8), confirming the successful synthesis of A, as indicated in Fig. 1. Although the molecular ratio in the reported crystal structure is 2
bpy. This mixture was heated under vacuum at 180 °C, such that dnba would sublime at a rate that is comparable with the other components.† This experiment resulted in the formation of a single band of orange crystals, indicating the successful formation of the ternary multi-component crystal A. The PXRD pattern of these orange crystals was found to match with the calculated pattern of the structure reported by Seaton et al. (refcode BEYZIQ8), confirming the successful synthesis of A, as indicated in Fig. 1. Although the molecular ratio in the reported crystal structure is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dnba
1 dnba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aba
4aba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy, using an equimolar ratio of co-formers in the sublimation still yielded the ternary product. Before subliming, the mixture of co-formers dnba + 4aba + bpy was observed to change from a colourless/white and yellow powder to an orange powder. PXRD patterns for this orange powder confirmed that it is ternary multi-component crystal A, i.e. simply heating the mixture of co-formers yields A, which then sublimes.
bpy, using an equimolar ratio of co-formers in the sublimation still yielded the ternary product. Before subliming, the mixture of co-formers dnba + 4aba + bpy was observed to change from a colourless/white and yellow powder to an orange powder. PXRD patterns for this orange powder confirmed that it is ternary multi-component crystal A, i.e. simply heating the mixture of co-formers yields A, which then sublimes.
We carried out a similar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sublimation at 180 °C for system B, in which we obtained yellow crystals, shown by PXRD and single-crystal X-ray diffraction to be the ternary multicomponent material B (Fig. 1).
1 sublimation at 180 °C for system B, in which we obtained yellow crystals, shown by PXRD and single-crystal X-ray diffraction to be the ternary multicomponent material B (Fig. 1).
To assess the effect of sublimation temperature on the products obtained, we carried out three-component sublimations at 10 °C intervals in the range of 140–180 °C. Temperatures above 180 °C resulted in significant decomposition of the co-formers, and were not explored further. For system A, different results were observed at different temperatures, with the resulting PXRD patterns given in the SI. At 140 °C only the (4aba)2(bpy) co-crystal was observed. In this same experiment, orange crystals of the ternary multi-component crystal A were observed to form at the bottom of the tube. In sublimations carried out between 150–170 °C, two distinct bands of crystals were observed. In each case the bottom band was the ternary multi-component crystal, while the top band was the binary co-crystal (4aba)2(bpy). This is consistent with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of co-formers used, which would leave an excess of 4aba and bpy after formation of the 2
1 ratio of co-formers used, which would leave an excess of 4aba and bpy after formation of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dnba
1 dnba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aba
4aba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy ternary crystal. As mentioned above, at 180 °C, only crystals of the ternary material A formed by sublimation. In system A, crystallisation of (4aba)2(bpy) or the ternary co-crystal A can be controlled by changing the sublimation temperature.
bpy ternary crystal. As mentioned above, at 180 °C, only crystals of the ternary material A formed by sublimation. In system A, crystallisation of (4aba)2(bpy) or the ternary co-crystal A can be controlled by changing the sublimation temperature.
For system B, sublimations were also carried out at 140–180 °C (Fig. S19). Generally, PXRD shows that a mixture of 3aba Form IV and the binary co-crystal (3aba)(isonic)2 formed in these experiments. This indicates that, although dnba begins to sublime in the temperature range 140–170 °C, its sublimation is too slow compared to 3aba and isonic, resulting in the formation of products without dnba. The PXRD pattern for the sample collected from the sublimation at 180 °C indicates that the desired ternary co-crystal B formed as the major product.
Different stoichiometric ratios of the starting materials were also explored. In these experiments, the stoichiometry of either one or two components was doubled, and the sublimations were carried out at 180 °C. The results of these experiments are summarised in Tables S5 and S6. When the amount of only one of the co-formers was doubled for system A (Table S5), the ternary multi-component crystal A sublimed: only one band of orange crystals was observed in each case. It should be noted that after sublimation a black amorphous solid was observed at the bottom of the tubes, indicating that the some of the starting material decomposed before it sublimed. When the amount of two co-formers was doubled, crystals of A formed, as well as an additional product. In each case, the additional product involved the more volatile conformer that was present in excess.
Similar experiments were carried out for system B (Table S6). A single band of crystals formed in each case, although usually this contained a mixture of products. Generally, the co-crystal (3aba)(isonic)2 was observed where there was an excess of either 3aba or isonic. The formation of the binary co-crystal in preference to the ternary co-crystal is most likely due to the faster sublimation rate of 3aba and isonic compared to dnba. There was also evidence of crystallisation of single-component crystals in these experiments. One of the most prominent of these is the Form IV of 3aba.
From the experiments on system A, it is apparent that some of the starting materials interact upon heating and form a ternary multi-component crystal before subliming. It is thus not clear if the formation of the ternary multi-component crystal occurs from the gas phase, or whether it is simply the sublimation of the ternary multi-component crystal occurring. A series of experiments was therefore carried out where the individual solid co-formers were physically separated, so that co-formers could only come into contact with one another once they had entered the gas phase. Each co-former was placed in the bottom of a cut glass sample vial which were placed together in a larger glass apparatus (see Fig. S1). This system was then heated under vacuum at 180 °C. For system A the PXRD indicates that A did form from the sublimation, although there are peaks indicating that the binary co-crystal (4aba)2(bpy) also crystallised. B was also successfully formed from sublimation in this way, although the peaks in the PXRD tend to be broader, indicating reduced crystallinity in this case. Both experiments indicate that the ternary multi-component crystals can form from the gas phase, whether there is physical contact between the starting materials before sublimation or not.
To confirm that ternary multi-component crystals can readily be obtained via sublimation, we targeted a third ternary crystal, using the co-formers in system C. We successfully obtained a new ternary multi-component crystal from sublimation, C, which was shown by SC-XRD to contain a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of dnba−
1 ratio of dnba−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3aba
3aba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
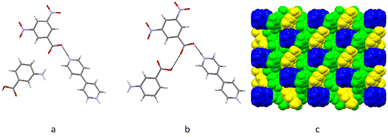
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy2+, i.e. it is a co-crystal salt. This ternary multi-component crystal, which is also orange, was obtained by sublimation using the same conditions described for systems A and B above. It should be noted that there was some degree of decomposition of the starting material, which remained at the bottom of the tube as a black amorphous material. The same orange crystals could also be obtained at 170 °C, with a smaller degree of decomposition of the starting material. The asymmetric unit of C contains one molecular ion of dnba, one molecule of 3aba and half of the molecular ion of bpy (Fig. 2). The 3aba molecule is disordered, and has been modelled in two different positions, which have been shown separately in Fig. 2a and b. Proton transfer between dnba and bpy has occurred, which forms a charge-assisted hydrogen bond between the two molecular ions. A hydrogen bond forms between the carboxylate of dnba and the carboxylic group of 3aba (O7–H7⋯O1). The packing consists of alternating stacks of each of the different molecules and ions of this co-crystal (Fig. 2c).
bpy2+, i.e. it is a co-crystal salt. This ternary multi-component crystal, which is also orange, was obtained by sublimation using the same conditions described for systems A and B above. It should be noted that there was some degree of decomposition of the starting material, which remained at the bottom of the tube as a black amorphous material. The same orange crystals could also be obtained at 170 °C, with a smaller degree of decomposition of the starting material. The asymmetric unit of C contains one molecular ion of dnba, one molecule of 3aba and half of the molecular ion of bpy (Fig. 2). The 3aba molecule is disordered, and has been modelled in two different positions, which have been shown separately in Fig. 2a and b. Proton transfer between dnba and bpy has occurred, which forms a charge-assisted hydrogen bond between the two molecular ions. A hydrogen bond forms between the carboxylate of dnba and the carboxylic group of 3aba (O7–H7⋯O1). The packing consists of alternating stacks of each of the different molecules and ions of this co-crystal (Fig. 2c).
It is clear from our results that it is possible to not only obtain previously reported ternary multi-component crystals from sublimation, but it is also straightforward to obtain new ternary multi-component crystals from sublimation. We have also shown that ternary multi-component crystals can be formed directly from the gas phase, i.e. without physical contact between the co-formers before sublimation. Careful choice of the sublimation temperature can give excellent control over which multi-component crystal is produced from a particular sublimation process. Varying the stoichiometry of a sublimation can also be used to control the product obtained from a sublimation. These results confirm that sublimation is a powerful technique for ternary co-crystal screening, and offers excellent control over the product of a crystallisation experiment.
Both authors were responsible for conceptualisation as well as writing (reviewing and editing). MS was responsible for investigation and writing the original draft. DAH was responsible for supervision and funding acquisition.
This work is based on the research supported in part by the National Research Foundation of South Africa (SRUG2204224316). We also thank Stellenbosch University for funding.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: Experimental details, tables of sublimation results, PXRD patterns of products. See DOI: https://doi.org/10.1039/d5cc04584c.CCDC 2479206–2479211 and 2493319 contain the supplementary crystallographic data for this paper.23a–g
Notes and references
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef PubMed.
- S. Aitipamula, R. Banerjee, A. K. Bansal, K. Biradha, M. L. Cheney, A. R. Choudhury, G. R. Desiraju, A. G. Dikundwar, R. Dubey, N. Duggirala, P. P. Ghogale, S. Ghosh, P. K. Goswami, N. R. Goud, R. R. K. R. Jetti, P. Karpinski, P. Kaushik, D. Kumar, V. Kumar, B. Moulton, A. Mukherjee, G. Mukherjee, A. S. Myerson, V. Puri, A. Ramanan, T. Rajamannar, C. M. Reddy, N. Rodriguez-Hornedo, R. D. Rogers, T. N. G. Row, P. Sanphui, N. Shan, G. Shete, A. Singh, C. C. Sun, J. A. Swift, R. Thaimattam, T. S. Thakur, R. Kumar Thaper, S. P. Thomas, S. Tothadi, V. R. Vangala, N. Variankaval, P. Vishweshwar, D. R. Weyna and M. J. Zaworotko, Cryst. Growth Des., 2012, 12, 2147–2152 CrossRef.
- M. Karimi-Jafari, L. Padrela, G. M. Walker and D. M. Croker, Cryst. Growth Des., 2018, 18, 6370–6387 CrossRef.
- E. Grothe, H. Meekes, E. Vlieg, J. H. Ter Horst and R. De Gelder, Cryst. Growth Des., 2016, 16, 3237–3243 CrossRef.
- D. E. Lynch, S. Chatwin and S. Parsons, Cryst. Eng., 1999, 2, 137–144 CrossRef.
- C. B. Aakeröy and K. R. Seddon, Chem. Soc. Rev., 1993, 22, 397–407 RSC.
- S. Tothadi and G. R. Desiraju, Chem. Commun., 2013, 49, 7791–7793 RSC.
- C. C. Seaton, N. Blagden, T. Munshi and I. J. Scowen, Chem. – Eur. J., 2013, 19, 10663–10671 CrossRef PubMed.
- A. Delori, T. Friščić and W. Jones, CrystEngComm, 2012, 14, 2350–2362 RSC.
- A. L. Volkwyn and D. A. Haynes, CrystEngComm, 2023, 25, 5887–5892 RSC.
- P. McArdle and A. Erxleben, CrystEngComm, 2021, 23, 5965–5975 RSC.
- P. Harsha, M. Khan, R. Thakuria and D. Das, Cryst. Growth Des., 2024, 24, 3109–3113 CrossRef.
- J. Lombard, T. Le Roex and D. A. Haynes, Cryst. Growth Des., 2020, 20, 7384–7391 CrossRef.
- C. O’Malley, C. Bouchet, G. Manyara, N. Walsh, P. McArdle and A. Erxleben, Cryst. Growth Des., 2021, 21, 314–324 CrossRef.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef PubMed.
- C. C. Seaton, CrystEngComm, 2014, 16, 5878–5886 RSC.
- P. A. Williams, C. E. Hughes, G. K. Lim, B. M. Kariuki and K. D. M. Harris, Cryst. Growth Des., 2012, 12, 3104–3113 CrossRef.
- C. B. Aakeröy, A. M. Beatty, B. A. Helfrich and M. Nieuwenhuyzen, Cryst. Growth Des., 2003, 3, 159–165 CrossRef.
- S. Tothadi and G. R. Desiraju, Philos. Trans. R. Soc., A, 2012, 370, 2900–2915 CrossRef.
- D. E. Lynch, S. Chatwin and S. Parsons, Cryst. Eng., 1999, 2, 137–144 CrossRef.
- D. Lynch, G. Smith, K. Byriel and C. Kennard, Aust. J. Chem., 1994, 47, 1789–1798 CrossRef.
- D. Bernasconi, S. Bordignon, F. Rossi, E. Priola, C. Nervi, R. Gobetto, D. Voinovich, D. Hasa, N. T. Duong, Y. Nishiyama and M. R. Chierotti, Cryst. Growth Des., 2020, 20, 906–915 CrossRef.
- (a) CCDC 2479206: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6td4; (b) CCDC 2479207: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6tf5; (c) CCDC 2479208: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6tg6; (d) CCDC 2479209: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6th7; (e) CCDC 2479210: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6tj8; (f) CCDC 2479211: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p6tk9; (g) CCDC 2493319: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pphnk.
Footnote |
| † One key aspect to consider in co-sublimation experiments is that all components should be in the gas phase simultaneously. In each of the crystal systems studied here, dnba sublimes at the highest temperature, so multi-component sublimations were carried out at temperatures higher than the sublimation temperature of dnba. |
| This journal is © The Royal Society of Chemistry 2025 |