Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Encasing the paramagnetic copper(II)-ion by the ring-contracted corrin ligand of vitamin B12†‡
Christoph
Kieninger
 ab,
Klaus
Wurst
ab,
Klaus
Wurst
 c,
Daniel
Leitner
c,
Luis P.
Peters
bc,
Dennis F.
Dinu
c,
Daniel
Leitner
c,
Luis P.
Peters
bc,
Dennis F.
Dinu
 bc,
Markus
Wiedemair
bc,
Markus
Wiedemair
 ab,
Marc-Kevin
Zaretzke
d,
Martin
Bröring
ab,
Marc-Kevin
Zaretzke
d,
Martin
Bröring
 d,
Stephan
Hohloch
d,
Stephan
Hohloch
 c,
Klaus R.
Liedl
c,
Klaus R.
Liedl
 bc and
Bernhard
Kräutler
bc and
Bernhard
Kräutler
 *ab
*ab
aInstitute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck, Austria. E-mail: bernhard.kraeutler@uibk.ac.at
bCenter for Molecular Biosciences (CMBI), University of Innsbruck, 6020 Innsbruck, Austria
cInstitute of General, Inorganic & Theoretical Chemistry, University of Innsbruck, 6020 Innsbruck, Austria
dInstitute for Inorganic and Analytical Chemistry, TU Braunschweig, 38106 Braunschweig, Germany
First published on 9th June 2025
Abstract
The d9-Cu(II)-corrin cupribyrate (Cuby) was synthesized in 93% crystalline yield by rapid chelation of Cu2+-ions by the metal-free corrin-ligand of vitamin B12. Single crystals of the EPR-active Cuby allowed for the first X-ray structure determination of a Cu-corrin. SCF-calculations provided insights complementary to the experimental data of Cuby and indicated an out-of-plane displacement of the reduced d10-Cu(I)-ion, consistent with the observed reductive activation of Cuby towards loss of its Cu-center.
The ring-contracted natural corrin ligand of the B12-derivatives is a uniquely skewed, helical environment1,2 that binds cobalt-ions very tightly.3,4 This biosynthetically costly ligand for cobalt5,6 represents a precisely evolved entatic state module,2 giving B12-cofactors the unique capacity for their exceptional bio-organometallic catalysis.7–9 The complementary fundamental question, why cobalt? in B12-cofactors,1,3,9,10 has generated the long-standing experimental quest for non-cobalt analogues of the B12-derivatives,11,12 a challenge met by newly developed synthetic approaches.2,13 We have, thus, prepared Rh(III)-,13–17 Ni(II)-18 and Zn(II)-complexes19 of natural corrin ligands for studies of their structures and reactivity. Here, we report on cupribyrate (Cuby) (Scheme 1), the Cu(II)-complex of hydrogenobyric acid (Hby),2 including the first Cu-corrin X-ray crystal structure.
 | ||
| Scheme 1 Structure-based outline of the synthesis of cupribyrate (Cuby) from hydrogenobyric acid (Hby) (see the ESI‡) and the structural formula of Coαcyano, Coβaquo-cobyric acid (Coby). | ||
The complexation of metal-free Hby with Cu(II)-ions occurred readily at room temperature (RT) in a 0.25 M aqueous solution of Cu(II)-acetate at pH 6 and was practically quantitative within 90 min (see the ESI‡). It did not require the reported strong heating (‘brief boiling’).11,20 Crystallization of the raw Cuby-isolate from water/acetonitrile mixtures furnished Cuby in >93% yield.
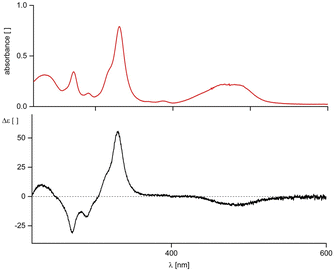
The UV/Vis-spectrum (Fig. 1) of an aqueous solution of Cuby exhibits a corrin-type and is comparable to the earlier reported spectra of partially characterized Cu(II)-corrins.20,21 UV/Vis- and CD-spectra of Cuby show remarkably similar features to the corresponding spectra of the Zn(II)-complex19 of Hby, consistent with the dominating role of the corrin chromophore for the spectral signature in the UV- and Vis-range. A HR-ESI mass spectrum of Cuby confirmed the calculated molecular formula of C45H64CuN10O8 (see the ESI,‡ Fig. S1).
Glassy frozen solutions of the paramagnetic Cu(II)-corrin Cuby in 20% glycerol in H2O showed the typical EPR-signature (see Fig. 2, for a spectrum at T = 148 K) of a roughly square-planar 4-coordinate Cu(II)-N4-complex with an index22,23 gII/AII = 98.2 cm, assigning an exceptionally low value to the encasement of the Cu(II)-ion by the corrin ligand (see the ESI‡ for further details).
 | ||
Fig. 2 EPR-spectrum of a 1.34 mM frozen solution (at T = 148 K) of Cuby in H2O:glycerol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and its simulation with key parameters obtained by least square fitting (for details see the ESI,‡ Table S1). The spectra exhibited a significant T-dependence, with a maximum signal intensity of around 200 K and continuous decrease at lower temperatures (see the ESI,‡ Fig. S4). 1) and its simulation with key parameters obtained by least square fitting (for details see the ESI,‡ Table S1). The spectra exhibited a significant T-dependence, with a maximum signal intensity of around 200 K and continuous decrease at lower temperatures (see the ESI,‡ Fig. S4). | ||
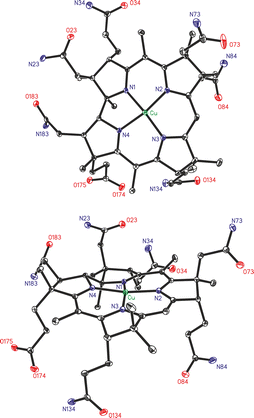
The neutral cupribyrate Cuby crystallized from an aqueous solution upon addition of acetonitrile. The monoclinic crystals (space group P21) contain two Cuby molecules per unit cell, as well as molecules of water and acetonitrile (ordered near the Cuby-carboxylate). The Cu(II)-center of the Cuby molecule sits only +0.033 Å above the mean plane of the four ‘inner’ corrin N-atoms, which span an unsymmetrical and nearly planar coordination pattern (see Fig. 3), as reflected by the value of the geometry index τ4 = 0.17.24 However, Cuby exhibits a less planar arrangement around its 4-coordinate d9 Cu(II)-center, than in the Ni(II)-corrin nibyrate (Niby),18 which experiences a better fit of its 4-coordinate low-spin d8 Ni(II)-ion (see the ESI,‡ Table S3). The average Cu–N distance in Cuby amounts to 1.91 Å, merely 0.05 Å longer than in Niby, in which the 0.08 Å smaller low spin d8-ion Ni(II)25 induced a slight contraction.18 In fact, binding of the d9 Cu(II)-ion largely retains the architecture of the coordination hole of the metal-free corrin ligand Hby, expanded by two ‘inner’ protons.2 In Cuby, the critical angle parameters corrin-fold26 (10.0°) and corrin helicity2 (12.4°) are also similar to those of the ligand Hby,2 but remarkably larger than in Niby. Likewise, the angle between the planes N1–Cu–N2 and N3–Cu–N4 (roughly 13.6°) relating to the inner coordination-sphere around the Cu(II)-center (see the ESI,‡ Table S3) is close to the value derived for Hby.2 Interestingly, the N1–M–N3 pseudo-diagonal in Cuby was roughly 0.07 Å shorter than the N2–M–N4 counterpart, thus displaying a larger difference of the distances across these pseudo-diagonals than in Niby. This desymmetrization of the corrin core in Cuby also goes along the one observed in Hby and its Zn(II)-complex,19 but is insignificant in the Co(II)-corrins Co(II)-cobalamin (CblII)27 and cob(II)ester28 and in typical Co(III)-corrins, such as coenzyme B12 (AdoCbl)29 and vitamin B12.30,31
Our self-consistent field (SCF) in the gas-phase calculation of cupribyric acid (HCuby+), the cationic carboxylate-protonated form of Cuby, used the atomic coordinates of the Cuby crystal structure. In this model, large artefactual electron density contributions of the carboxylate function of Cuby to occupied MOs were lacking, consistent with the experimental absence of such interactions. The so-derived computational insights into the bonding interactions of a d9-Cu(II)-corrin were fully consistent with separately located calculated frontier molecular orbitals, either as π-type corrin ligand MOs or as the singly occupied dx2–y2-type orbital on the Cu(II)-center (see Fig. 4 and ESI‡ Fig. S13). We also tested computed models of cuprobyric acid (Cu(I)by) to shed light on the difficult32 one-electron reduction to a d10-Cu(I)-corrin (for details, see the ESI,‡ Fig. S14). The calculations suggest a large upper axial out of plane movement of the Cu(I)-ion, comparable to the position of the rather weakly bound Zn(II)-center in zincobyric acid (Znby).19 Indeed, the iso-electronic nature of the closed shell d10-ions Cu(I) and Zn(II) suggested the likelihood of the complete removal of a Cu(I)-ion from reduced Cuby in a weakly acidic aqueous medium. In an exploratory experiment, Cuby was treated with Zn-powder in an aqueous NH4Cl solution, leading to the effective replacement of the Cu-center of Cuby by Zn(II), furnishing Znby,19 and its tentatively (by mass- and UV/Vis-spectroscopy) characterized dihydro-form H2-Znby, an unprecedented ring-reduced yellow corrinoid33,34 (see ESI,‡ Scheme S1). We ascribe the observed formation of Znby from Cuby to a transient generation of an exchange-labile d10-Cu(I)-center by the Zn-reduction, thus strategically circumventing Eschenmoser's postulate that a B-type transition metal could not be removed without destruction of the corrin-ligand.35
The replacement, by copper, of the biologically selected cobalt-center of a corrinoid B12-derivative1,36,37 erases its fundamental organometallic redox-reactivity.7 The single unpaired electron of the paramagnetic Cuby does not contribute any (cobalt-mimetic) radical reactivity, but is ‘buried’ in a dx2–y2 orbital of its d9 Cu(II)-center. Consistent with the EPR-spectral fingerprint of Cuby and its large 14N-hfcs with the four inner corrin N-atoms, in particular, the unpaired spin is located in a dx2–y2 orbital of an effectively antibonding type with respect to the coordinating corrin N-atoms (Fig. 4). Compared to the d8 Ni(II)-ion in Niby, the Cu(II)–N bonds in Cuby are, indeed, longer. Copper complexes of the superficially similar corroles represent a remarkably more complex situation:38,39 there ‘non-innocence’ of the corrole ligand is caused by its extended π-system, assisting an intramolecular electron-shift and stabilizing the copper center in a higher oxidation state.40
The chelation of the fluorescent metal-free corrin Hby2,41 by Cu(II)-ions in aqueous solution occurs cleanly at ambient temperature at pH 5. The Cu(II)-ions chelate Hby with a rate kCu(II) = 0.54 ± 0.04 L mol−1 min−1, remarkably quicker by about 2 × 102 times than the binding of the biologically crucial Co(II)-ions (kCo(II) of about 3 × 10−3 L mol−1 min−1), and five times faster than Zn(II)-ions (kZn(II) = 0.111 ± 0.002 L mol−1 min−1, see the ESI‡). The chelation rates of these metal ions follow the trend established with Eschenmoser's model corrin35 and with a water-soluble tetra-mesopyridyl-porphyrin.42
Obviously, the biological roles of Co7,8,43 and Cu44–47 do not match. However, the 4-coordinate Cu(II)-complexes of natural corrin ligands may serve as structural mimics of reduced B12-derivatives. In concert with the divergent reactivity profiles of copper- and cobalt-corrins, biologically interesting applications are likely. Cuby is structured similar to the corrin-core of enzyme-activated 4-coordinate Co(II)-cobamides, first characterized in an ATP:Co(I)-corrinoid adenosyltransferase that generates AdoCbl from 4-coordinate Co(II)-Cbl.48 With their largely inert 4-coordinate d9- and d8-metal-centers, respectively, Cu(II)- and Ni(II)-corrins18 may effectively mimic the structures of the highly activated 4-coordinate Co(II)- and Co(I)-corrins. Indeed, nibalamin (Nibl), the diamagnetic Ni(II)-analogue of ‘base-off’ Co(II)-Cbl, was shown to be an effective inhibitor of the corrinoid adenosyltransferase BtuR from Brucella melitensis.18 The crystal structure of Cuby qualifies Cu(II)-containing B12-derivatives, such as cupribalamin (Cubl), for similar inhibitory effects.
Transition metal analogues of vitamin B12 and other cobalamins (Cbls), also classified as metbalamins (Metbls),49,50 lack the precise cobalt-dependent reactivity of Cbls8,43 and, when mimicking Cbl-structures, may represent genuine antivitamins B12.50,51 This is the case for rhodibalamins (Rhbls), the Rh(III)-homologues of Cbls. Surprisingly, their Rh(III)-center has even been revealed to experience a slightly better fit to the corrin ligand than the naturally selected Co(III)-ions.13–15 Whereas uptake and physiological activity of Metbls with stable 4-coordinate corrin-bound metal centers are still unknown in humans and animals, microorganisms are typically more structure-promiscuous for B12-import, satisfying their supply with cobamides by de novo biosynthesis5 or by partial assembly from salvaged natural corrinoids.52,53 As deduced for some Rhbls13,15 and for Nibl,18 transition metal-based structural mimics of B12-cofactors or of corrinoid B12-biosynthesis intermediates17 may selectively inhibit bacterial growth. As mimics of enzyme-bound Cbl-structures in B12-dependent enzymes at intermediate stages of catalysis, Metbls may specifically act as very effective enzyme inhibitors. The Cu(II)-analogues of natural B12-derivatives are, hence, EPR-active candidates for their applications as B12-antimetabolites for B12-dependent microorganisms, an expansion of the toolbox of Cu-coordinating natural products47 as antimicrobial agents.
Synthetic, analytical and spectroscopic work: C. K. and M. W.; crystallography: C. K. and K. W.; theoretical and computational study: L. P. P., D. F. D., and K. R. L.; EPR-spectroscopy – data acquisition, supervision and data curation: D. L., M.-K. Z., M. B., and S. H.; and research conceptualization and conduction and original draft: B. K.; all authors have reviewed and contributed to the final draft.
We are particularly grateful to Evelyne Deery and Martin Warren for a generous supply of hydrogenobyric acid. This work was supported by the Austrian Science Fund (FWF projects P-28892 and P-33059 to BK and P-34626 to SH and DL).
Conflicts of interest
There are no conflicts to declare.Data availability
See the ESI.‡ Crystallographic data for cupribyrate (Cuby) have been deposited at the Cambridge Crystallographic Data Center (CCDC) and are available under accession number CCDC-2402239.Notes and references
- A. Eschenmoser, Angew. Chem., Int. Ed. Engl., 1988, 27, 5–39 CrossRef.
- C. Kieninger, E. Deery, A. D. Lawrence, M. Podewitz, K. Wurst, E. Nemoto-Smith, F. J. Widner, J. A. Baker, S. Jockusch, C. R. Kreutz, K. R. Liedl, K. Gruber, M. J. Warren and B. Kräutler, Angew. Chem., Int. Ed., 2019, 58, 10756–10760 CrossRef CAS PubMed.
- J. M. Pratt, Inorganic Chemistry of Vitamin B12, Academic Press, New York, 1972 Search PubMed.
- N. J. Lewis, R. Nussberger, B. Kräutler and A. Eschenmoser, Angew. Chem., Int. Ed. Engl., 1983, 22, 736–737 CrossRef.
- D. A. Bryant, C. N. Hunter and M. J. Warren, J. Biol. Chem., 2020, 295, 6888–6925 CrossRef CAS PubMed.
- E. Raux, H. L. Schubert and M. J. Warren, Cell. Mol. Life Sci., 2000, 57, 1880–1893 CrossRef CAS PubMed.
- B. Kräutler, in Adv. Bioorganomet. Chem., ed. T. Hirao and T. Moriuchi, Elsevier, Cambridge, USA, 2019, pp. 399–429 Search PubMed.
- K. L. Brown, Chem. Rev., 2005, 105, 2075–2149 CrossRef CAS PubMed.
- H. M. Marques, J. Coord. Chem., 2024, 77, 1161–1210 CrossRef CAS.
- E.-I. Ochiai, J. Chem. Educ., 1978, 55, 631 CrossRef CAS.
- V. B. Koppenhagen, in B12, ed. D. Dolphin, John Wiley & Sons, 1982, vol. 2, pp. 105–150 Search PubMed.
- G. Holze and H. H. Inhoffen, Angew. Chem., Int. Ed. Engl., 1985, 24, 867–869 CrossRef.
- F. J. Widner, A. D. Lawrence, E. Deery, D. Heldt, S. Frank, K. Gruber, K. Wurst, M. J. Warren and B. Kräutler, Angew. Chem., Int. Ed., 2016, 55, 11281–11286 CrossRef CAS PubMed.
- F. J. Widner, C. Kieninger, K. Wurst, E. Deery, M. J. Warren and B. Kräutler, Synthesis, 2021, 332–337 CAS.
- M. Wiedemair, C. Kieninger, K. Wurst, M. Podewitz, E. Deery, M. D. Paxhia, M. J. Warren and B. Kräutler, Helv. Chim. Acta, 2023, 106, e202200158 CrossRef CAS.
- M. Ruetz, R. Mascarenhas, F. Widner, C. Kieninger, M. Koutmos, B. Kräutler and R. Banerjee, Biochemistry, 2024, 63, 1955–1962 CrossRef CAS PubMed.
- F. J. Widner, C. Kieninger and B. Kraeutler, J. Porph. Phthal., 2025, 29, 408–417 CrossRef CAS.
- C. Kieninger, K. Wurst, M. Podewitz, M. Stanley, E. Deery, A. D. Lawrence, K. R. Liedl, M. J. Warren and B. Kräutler, Angew. Chem., Int. Ed., 2020, 59, 20129–20136 CrossRef CAS PubMed.
- C. Kieninger, J. A. Baker, M. Podewitz, K. Wurst, S. Jockusch, A. D. Lawrence, E. Deery, K. Gruber, K. R. Liedl, M. J. Warren and B. Kräutler, Angew. Chem., Int. Ed., 2019, 58, 14568–14572 CrossRef CAS PubMed.
- V. B. Koppenhagen and J. J. Pfiffner, J. Biol. Chem., 1970, 245, 5865–5867 CrossRef CAS PubMed.
- V. B. Koppenhagen and J. J. Pfiffner, J. Biol. Chem., 1971, 246, 3075–3077 CrossRef CAS PubMed.
- M. Ray, R. Mukherjee, J. F. Richardson, M. S. Mashuta and R. M. Buchanan, J. Chem. Soc., Dalton Trans., 1994, 965–969 RSC.
- U. Sakaguchi and A. W. Addison, J. Chem. Soc., Dalton Trans., 1979, 600–608 RSC.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- B. Cordero, V. Gomez, A. E. Platero-Prats, M. Reves, J. Echeverria, E. Cremades, F. Barragan and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC.
- V. B. Pett, M. N. Liebman, P. Murray-Rust, K. Prasad and J. P. Glusker, J. Am. Chem. Soc., 1987, 109, 3207–3215 CrossRef CAS.
- B. Kräutler, W. Keller and C. Kratky, J. Am. Chem. Soc., 1989, 111, 8936–8938 CrossRef.
- B. Kräutler, W. Keller, M. Hughes, C. Caderas and C. Kratky, J. Chem. Soc., Chem. Commun., 1987, 1678–1680 RSC.
- L. Ouyang, P. Rulis, W. Y. Ching, G. Nardin and L. Randaccio, Inorg. Chem., 2004, 43, 1235–1241 CrossRef CAS PubMed.
- C. Kratky and B. Kräutler, in Chemistry and Biochemistry of B12, ed. R. Banerjee, John Wiley & Sons, New York, Chichester, 1999, pp. 9–41 Search PubMed.
- B. Kräutler, R. Konrat, E. Stupperich, G. Färber, K. Gruber and C. Kratky, Inorg. Chem., 1994, 33, 4128–4139 CrossRef.
- K. A. Rubinson, J. Caja, R. W. Hurst, E. Itabashi, T. M. Kenyhercz, W. R. Heineman and H. B. Mark, J. Chem. Soc., Chem. Commun., 1980, 47–48 RSC.
- G. Schlingmann and V. B. Koppenhagen, Zürich Switzerland, 1979.
- G. Schlingmann, B. Dresow, L. Ernst and V. B. Koppenhagen, Liebigs Ann. Chem., 1981, 2061–2066 CrossRef CAS.
- H. U. Blaser, E. L. Winnacker, A. Fischli, B. Hardegger, D. Bormann, N. Hashimoto, J. Schossig, R. Keese and A. Eschenmoser, Helv. Chim. Acta, 2015, 98, 1845–1920 CrossRef CAS.
- G. A. Holliday, J. A. Thornton, A. Marquet, A. G. Smith, F. Fabrice Rebeille, R. Mendel, H. L. Schubert, A. D. Lawrence and M. J. Warren, Nat. Prod. Rep., 2007, 24, 972–1087 RSC.
- A. Eschenmoser, Angew. Chem., Int. Ed., 2011, 50, 12412–12472 CrossRef CAS PubMed.
- A. Ghosh, Chem. Rev., 2017, 117, 3798–3881 CrossRef CAS PubMed.
- M. Bröring, F. Brégier, E. C. Tejero, C. Hell and M. C. Holthausen, Angew. Chem., Int. Ed., 2007, 46, 445–448 CrossRef PubMed.
- C. M. Lemon, M. Huynh, A. G. Maher, B. L. Anderson, E. D. Bloch, D. C. Powers and D. G. Nocera, Angew. Chem., Int. Ed., 2016, 55, 2176–2180 CrossRef CAS PubMed.
- S. Jockusch and B. Kräutler, Chem. Commun., 2025, 61, 3904–3907 RSC.
- P. Hambright, in The Porphyrin Handbook, Academic Press, 2000, vol. 3, pp. 129–210 Search PubMed.
- B. Kräutler, in Compreh. Organomet. Chem. IV, ed. G. Parkin, K. Meyer and D. O’Hare, Elsevier, Oxford, 2022, pp. 73–95 Search PubMed.
- E. I. Solomon, R. K. Szilagyi, S. D. George and L. Basumallick, Chem. Rev., 2004, 104, 419–458 CrossRef CAS PubMed.
- E. Kim, E. E. Chufan, K. Kamaraj and K. D. Karlin, Chem. Rev., 2004, 104, 1077–1133 CrossRef CAS PubMed.
- G. E. Kenney and A. C. Rosenzweig, Ann. Rev. Biochem., 2018, 87, 645–676 CrossRef CAS PubMed.
- O. M. Manley and A. C. Rosenzweig, J. Biol. Inorg. Chem., 2025, 30, 111–124 CrossRef CAS PubMed.
- M. S. S. Maurice, P. Mera, K. Park, T. C. Brunold, J. C. Escalante-Semerena and I. Rayment, Biochemistry, 2008, 47, 5755–5766 CrossRef PubMed.
- F. Zelder, M. Sonnay and L. Prieto, ChemBioChem, 2015, 16, 1264–1278 CrossRef CAS PubMed.
- B. Kräutler, Chem. – Eur. J., 2020, 26, 15438–15445 CrossRef PubMed.
- B. Kräutler, Chem. Eur. J., 2015, 21, 11280–11287 CrossRef PubMed.
- S. Gude, G. J. Pherribo and M. E. Taga, Msystems, 2022, 7, 00288 CrossRef PubMed.
- J. C. Escalante-Semerena, J. Bacteriol., 2007, 189, 4555–4560 CrossRef PubMed.
Footnotes |
| † Dedicated to the memory of Albert Eschenmoser on the occasion of his 100th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2402239. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc02129d |
| This journal is © The Royal Society of Chemistry 2025 |