Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of electronically tuneable N-heterocyclic borates†
I. J.
Huerfano‡
 ,
Evgueni
Gorobets‡
,
Evgueni
Gorobets‡
 ,
Wen
Zhou
,
Wen
Zhou
 ,
Warren E.
Piers
,
Warren E.
Piers
 ,
Jeffrey F.
Van Humbeck
,
Jeffrey F.
Van Humbeck
 * and
Darren J.
Derksen
* and
Darren J.
Derksen
 *
*
Department of Chemistry, University of Calgary, Calgary, AB T2N 1N4, Canada. E-mail: jeffrey.vanhumbec1@ucalgary.ca; dderksen@ucalgary.ca
First published on 18th June 2025
Abstract
In this contribution, we describe the synthesis and characterization of novel borates constructed with N-heterocyclic arene substituents. Beyond providing comparable electronic and redox properties to their fluoroarene analogues, the use of N-heterocycles allows for facile protonation of aromatic nitrogen atoms, thus providing a framework for tuneable reagents with varying Lewis basicity. The synthesized protonated borates were further examined as Brønstead–Lowry acids through pKa bracketing and evaluation of the kinetic accessibility of their protons by variable temperature NMR. Finally, we report the synthesis of onium and alkali metal salts of these borates through deprotonation by a variety of bases providing useful starting points for future applications.
Given the utility of electron poor borates as weakly coordinating anions,1–3 as activators in polymer catalysis,4,5 and as electrolytes in energy-related applications,6 we recognized that novel borates retaining the properties of polyfluorinated borates would be valuable to the chemical community. Combined with the desire to reduce the use of organofluorine compounds,7–9 we explored the synthesis and properties of tunable N-heterocyclic borates. Based on available Hammett parameters,10 4-pyridyl and 5-pyrimidyl have similarities to well-studied pentafluorophenyl substituents but have available sites for further functionalization (Fig. 1).
 | ||
| Fig. 1 Comparison of Hammett parameters of aryl and heteroaryl substituents.10 | ||
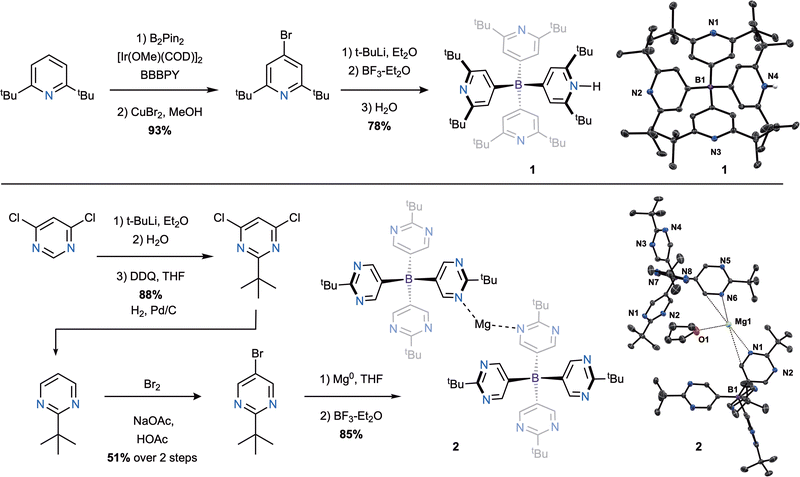
To sterically shield the Lewis basic sites of the N-heterocycles, we selected tert-butyl substituents to flank the nitrogen atoms. 2,6-Di-tert-butylpyridine was functionalized with a boronic pinacol ester using Ir catalysis in the presence of 4,4′-di-tert-butyl-2,2′-dipyridyl (BBBPY, Scheme 1).11 Conversion to the corresponding 4-bromopyridine derivative proceeds in 93% yield over 2 steps, which is then lithiated and reacted with boron trifluoride diethyl etherate to produce HB(Py)4 (1) upon aqueous workup. 1H- and 13C-NMR spectra of 1 display two sets of tert-butyl and pyridyl signals in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as well as a broad signal integrating to a single proton at >9 ppm in the 1H-NMR spectrum, consistent with a three-fold symmetric borate with one of the four pyridyl groups protonated. This assignment is confirmed by structural characterization using single-crystal X-ray diffraction where no cation is observed and the pyridinium proton is seen substitutionally disordered on the four pyridyl arms. Bond lengths and angles are typical of a tetrahedral aryl borate with a τ4 value12 of 0.937 confirming the successful synthesis of 1. Compound 1 exhibits good solubility in both arene and halogenated solvents as well as polar organic solvents such as tetrahydrofuran (THF).
1 ratio as well as a broad signal integrating to a single proton at >9 ppm in the 1H-NMR spectrum, consistent with a three-fold symmetric borate with one of the four pyridyl groups protonated. This assignment is confirmed by structural characterization using single-crystal X-ray diffraction where no cation is observed and the pyridinium proton is seen substitutionally disordered on the four pyridyl arms. Bond lengths and angles are typical of a tetrahedral aryl borate with a τ4 value12 of 0.937 confirming the successful synthesis of 1. Compound 1 exhibits good solubility in both arene and halogenated solvents as well as polar organic solvents such as tetrahydrofuran (THF).
4,6-Dichloropyrimidine was combined with tert-butyl lithium, followed by aqueous workup and oxidation with DDQ. Hydrogenation of the resulting 2-tert-butyl pyrimidine, followed by bromination, yielded the 5-bromo pyrimidine amenable to formation of a Grignard reagent that reacted efficiently with boron trifluoride diethyl etherate to produce Mg[B(Pm)4]2 (2). While 2 can be isolated as the magnesium borate salt, demonstrating remarkable stability in air and water, the pyridyl borate, 1, is isolated in its protonated form, rather than the lithium salt after aqueous workup.
1H- and 13C-NMR spectra of the magnesium pyrimidyl borate, 2, display only a single set of signals, initially suggesting a symmetric borate in solution. Single crystals suitable for X-ray diffraction were grown from a concentrated THF solution layered with pentane and cooled to 5 °C, revealing a solid-state structure containing a magnesium counterion interacting with two pyrimidyl borates through adjacent nitrogen and carbon atoms of one of the pyrimidyl substituents as well as a single THF molecule (Scheme 1, bottom right). The Mg–N distances (2.689 and 2.847 Å) are significantly longer than typical magnesium–nitrogen bonds, falling outside of the sum of the covalent radii,13 suggesting strong interactions rather than formal bonds. The Mg–O distance of the interacting THF molecule is similarly long (2.773 Å). Long atomic distances may be indicative of lability in the magnesium-borate interactions allowing for dynamic behavior in solution that accounts for the observed symmetric NMR spectra. Similar to 1, the borate anion of 2 contains bond lengths and angles typical of tetrahedral aryl borates with a τ4 value of 0.976. For direct comparison to 1, compound 2 was reacted with 1 equivalent of 1 M HCl/Et2O targeting the analogous protonated pyrimidyl borate, HB(Pm)4 (3; Scheme 2). The 1H-NMR spectrum of 3 exhibits a single set of tert-butyl and pyrimidyl peaks slightly shifted from the starting material, suggesting successful protonation of the borate. The observed 4-fold symmetry and absence of an observable pyrimidinium proton indicates that this acidic proton is highly fluxional at room temperature (vide infra). In contrast to 1, compound 3 shows sparing solubility in arene solvents, but is highly soluble in halogenated solvents and moderately soluble in THF.
Compound 3 can be further reacted with an additional equivalent of HCl/Et2O (Scheme 2) to form the doubly protonated chloride salt, [H2B(Pm)4]Cl (4). Single crystals of 4 suitable for X-ray diffraction were grown from a concentrated methylene chloride solution layered with pentane and cooled to 5 °C. The solid-state structure shows a borate with protonated pyrimidyl substituents where the two protons are substitutionally disordered across all four arms, analogous to 1 (Scheme 2). Further, the structure contains a chloride anion with a close contact of 2.961(3) Å with one of the protonated nitrogen atoms indicating a hydrogen bonding interaction14 and confirming the cationic character of the doubly protonated borate. Bond lengths and angles are typical of a tetrahedral aryl borate with a τ4 value of 0.955. As expected for chloride salts, 4 shows drastically decreased solubility in organic solvents with only modest solubility in methylene chloride (DCM) but can be dissolved in highly polar solvents including dimethyl sulfoxide (DMSO). Like 3, the 1H- and 13C-NMR spectra show only a single set of peaks indicating fluxional acidic protons. Due to the unique ability of the synthesized pyridyl and pyrimidyl borates to be protonated at their ring nitrogens, we sought to probe their Brønstead–Lowry acid character. Many borate-based acids, most notably Brookhart's Acid, have seen applications in synthesis for the delivery of a proton and weakly-coordinating anion,15 but most often in their aqueous (hydronium cation) or ethereal (secondary oxonium) forms.
As the formation of water or ethers can be counterproductive to stable cation formation upon proton transfer, a directly protonated borate-acid may serve as a better alternative for some applications. Further, the lack of separate acidic cations can provide a more atom economic reaction overall. The Brønstead–Lowry acidic character of 1 and 3 were evaluated through pKa bracketing experiments with a variety of neutral organic bases. Through these experiments it was determined that the deprotonated pyridyl borate is moderately basic as 1 shows no reactivity with tertiary amines, but can be deprotonated by stronger organic bases with a pKa (MeCN scale) between 18.8 (N,N-diisopropylethylamine) and 21.7 ([−]-spartein).16 For comparison, 1 is less acidic than lutidinium (a common proton source in catalysis; pKa = 14.2 MeCN scale) suggesting a possible coulombic component in addition to the effect of an electron donating borate substituent in the para-position.17 As expected, the pyrimidyl borate, 3, shows greater acidity relative to its pyridyl congener with a pKa bracketed between 15.07 (imidazole) and 18.8 (triethyl amine). The increased acidity results from the greater nitrogen content of the aromatic ring as well meta-positioning of the nitrogen atom relative to the carbon-boron bond.18 As pKa is a thermodynamic property, we also sought to evaluate the kinetic accessibility of the proton on the pyridyl and pyrimidyl borates through variable-temperature 1H-NMR experiments using dynamic self-exchange processes as an evaluator of proton mobility (see Fig. S33 and S34, ESI†). The pyridyl borate, 1, shows no coalescence up to 100 °C in toluene maintaining its two sets of peaks in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, indicating there is a high barrier to proton transfer between two separate borates. This observation likely results from the two large ortho tert-butyl groups. In contrast, 3 exhibits fast exchange at room temperature (vide supra) which can be de-coalesced at low temperature to give two sets of peaks analogous to 1. The coalescence point for 3 was determined to be at −57 °C in DCM indicating a low barrier for proton transfer. Having evaluated the Brønstead–Lowry acidic character of 1 and 3, we next synthesized onium and alkali metal salts of the pyridyl and pyrimidyl borates. Based on the bracketed pKa values and steric protection around the pyridinium proton, 1, was reacted with equimolar tris(tert-butyl)phosphinimine (HNPtBu3) and allowed to stir at room temperature in toluene for 18 hours (Scheme 2, right). In this time, precipitation of colorless crystals is observed. Upon removal of solvent in vacuo, [H2NP(tBu)3][BPy4] (5) is isolated as a white microcrystalline powder in quantitative yield. 1H- and 13C-NMR spectra of the isolated solid show that the product contains a completely symmetric pyridyl borate in solution with only one set of signals. Further, 1H- and 31P-NMR confirm the presence of the phosphiniminium counter cation. Single crystals of 5 suitable for X-ray diffraction were grown from a concentrated toluene solution layered with pentane and confirms the successful synthesis of the targeted phosphiniminium borate (Scheme 2). The bond lengths and angles of 5 are all consistent with a tetrahedral borate anion with a τ4 value of 0.946. Notably, the acidic protons of the phosphiniminium cation do not hydrogen bond with the pyridyl nitrogen atoms of the borate but instead are directed towards the borate center itself. As 3 can be deprotonated by tertiary amines, it was instead reacted with one equivalent of a hydrophobic amine, containing two long aliphatic (C16–C18) chains, in benzene at room temperature for 6 hours (Scheme 2, left). Over the course of the reaction, the mixture changed from a light orange suspension, due to the low solubility of 3 in aromatic solvents, to a homogeneous off-white solution. The final ammonium borate product, 6, was isolated as a tacky off-white solid after removing the solvent by lyophilization.
1 ratio, indicating there is a high barrier to proton transfer between two separate borates. This observation likely results from the two large ortho tert-butyl groups. In contrast, 3 exhibits fast exchange at room temperature (vide supra) which can be de-coalesced at low temperature to give two sets of peaks analogous to 1. The coalescence point for 3 was determined to be at −57 °C in DCM indicating a low barrier for proton transfer. Having evaluated the Brønstead–Lowry acidic character of 1 and 3, we next synthesized onium and alkali metal salts of the pyridyl and pyrimidyl borates. Based on the bracketed pKa values and steric protection around the pyridinium proton, 1, was reacted with equimolar tris(tert-butyl)phosphinimine (HNPtBu3) and allowed to stir at room temperature in toluene for 18 hours (Scheme 2, right). In this time, precipitation of colorless crystals is observed. Upon removal of solvent in vacuo, [H2NP(tBu)3][BPy4] (5) is isolated as a white microcrystalline powder in quantitative yield. 1H- and 13C-NMR spectra of the isolated solid show that the product contains a completely symmetric pyridyl borate in solution with only one set of signals. Further, 1H- and 31P-NMR confirm the presence of the phosphiniminium counter cation. Single crystals of 5 suitable for X-ray diffraction were grown from a concentrated toluene solution layered with pentane and confirms the successful synthesis of the targeted phosphiniminium borate (Scheme 2). The bond lengths and angles of 5 are all consistent with a tetrahedral borate anion with a τ4 value of 0.946. Notably, the acidic protons of the phosphiniminium cation do not hydrogen bond with the pyridyl nitrogen atoms of the borate but instead are directed towards the borate center itself. As 3 can be deprotonated by tertiary amines, it was instead reacted with one equivalent of a hydrophobic amine, containing two long aliphatic (C16–C18) chains, in benzene at room temperature for 6 hours (Scheme 2, left). Over the course of the reaction, the mixture changed from a light orange suspension, due to the low solubility of 3 in aromatic solvents, to a homogeneous off-white solution. The final ammonium borate product, 6, was isolated as a tacky off-white solid after removing the solvent by lyophilization.
The 1H-NMR spectrum of 6 is consistent with a completely symmetric borate containing a tertiary ammonium cation. Compared to the starting amine, the α-methyl/methylene protons are now further split into doublets due to coupling with the acidic proton bound to the nitrogen center and supporting the assigned structure (see Fig. S22, ESI†). Similar to the discussed onium salts, alkali metal salts of the pyridyl and pyrimidyl borates are also accessible through use of an appropriate strong base. Compound 1 was deprotonated using benzyl potassium (KBn) in THF or dimethoxyethane (DME) to give KBPy4, 7, as white powder after removal of solvents in vacuo (Scheme 2, right). Compound 7 is only soluble in ethereal solvents (diethyl ether, THF, and DME) but dissolves in arene or halogenated solvents with addition of a small amount of ethereal solvent additive. Additionally, 7 can be recrystallized from DME to give the highly soluble K(DME)nBPy4 (7·DMEn) where n = 1–4 and must be quantified by 1H-NMR. The 1H- and 13C-NMR spectra of 7·DMEn confirm their identity as separated ion pairs showing only one set of borate signals, regardless of the number of DME ligands. Due to the poor solubility of 3 in non-halogenated solvents, its deprotonation by strong alkali metal bases leads to inconsistent results. As these bases are incompatible with DCM or chloroform, 3 was first converted to 6in situ then reacted with sodium hexamethylsilazide (NaHMDS) in toluene to give the corresponding sodium salt, 8 (Scheme 2, left). The 1H- and 13C-NMR spectra of 8 are very similar to that of 2 showing only a single set of borate peaks suggesting weak sodium association to the borate and fluxional behavior in solution. The redox properties of these anions were evaluated through cyclic voltammetry (CV) to determine their resistance to oxidation by possible redox-active cations. CV of 7 in THF shows a single irreversible oxidation process with a peak potential approaching the solvent window of THF around 1.8 V vs. Fc/Fc+. This oxidation potential is comparable to the redox potential of B(C6F5)4− and B[C6H3(CF3)2]4− suggesting the pyridyl borate may be useful as a counteranion from a redox perspective.19–21 Similarly, the CV of 2 in DCM shows a quasi-reversible oxidation occurring at 1.7 V vs. Fc/Fc+ also demonstrating the pyrimidyl borates possible utility as a redox-resistant counteranion.
We have demonstrated that borates with heterocyclic substituents are synthetically accessible on gram scale. These compounds have tuneable properties compared to pentafluorophenyl substituents with respect to basicity and electrochemistry. Given the broad applications of borates, we anticipate that this novel family of compounds will be useful to the chemical community.
This work was conceptualized by DJD, JFVH and WEP. Synthesis and characterization were completed by IJH and EG. Crystallography was completed by WZ. Writing of original draft was completed by DJD and IJH with reviewing and editing by all authors.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 1, 2, 4, and 5 has been deposited at the CCDC under 2431948–2431951.Notes and references
- S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169 CrossRef CAS PubMed.
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982 CrossRef CAS PubMed.
- S. H. Strauss, Chem. Rev., 1993, 93, 927 CrossRef CAS.
- J. F. Van Humbeck, M. L. Aubrey, A. Alsbaiee, R. Ameloot, G. W. Coates, W. R. Dichtel and J. R. Long, Chem. Sci., 2015, 6, 5499 RSC.
- S. Fischer, J. Schmidt, P. Strauch and A. Thomas, Angew. Chem., Int. Ed., 2013, 52, 12174 CrossRef CAS PubMed.
- Z. G. Huang, S. N. Wang, R. D. Dewhurst, N. V. Ignat’ev, M. Finze and H. Braunschweig, Angew. Chem., Int. Ed., 2020, 59, 8800 CrossRef CAS PubMed.
- M. G. Evich, M. J. B. Davis, J. P. McCord, B. Acrey, J. A. Awkerman, D. R. U. Knappe, A. B. Lindstrom, T. F. Speth, C. Tebes-Stevens, M. J. Strynar, Z. Wang, E. J. Weber, W. M. Henderson and J. W. Washington, Science, 2022, 375, eabg9065 CrossRef CAS PubMed.
- I. T. Cousins, J. C. Dewitt, J. Glüge, G. Goldenman, D. Herzke, R. Lohmann, M. Miller, C. A. Ng, M. Scheringer, L. Vierke and Z. Wang, Environ. Sci.: Processes Impacts, 2020, 22, 1444 RSC.
- J. Scholl, J. Lisec, H. Haase and M. Koch, Anal. Bioanal. Chem., 2024, 416, 6405 CrossRef CAS PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- L. Zeqing, L. Zhibin, W. Yuqiang and Y. Pei, Res. Chem. Intermed., 2013, 39, 1917 CrossRef.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955 RSC.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832 RSC.
- T. Steiner, Acta Crystallogr., Sect. B, 1998, 54, 456 CrossRef.
- M. Brookhart, B. Grant and A. F. Volpe Jr, Organometallics, 1992, 11, 3920 CrossRef CAS.
- S. Tshepelevitsh, A. Kütt, M. Lõkov, I. Kaljurand, J. Saame, A. Heering, P. G. Plieger, R. Vianello and I. Leito, Eur. J. Org. Chem., 2019, 6735 CrossRef CAS.
- T. Taniguchi, Chem. – Eur. J., 2022, 28, e202104333 CrossRef CAS PubMed.
- J. Spanget-Larsen, J. Chem. Soc., Perkin Trans. 2, 1985, 417 RSC.
- S. B. Beil, S. Möhle, P. Enders and S. R. Waldvogel, Chem. Commun., 2018, 54, 6128 RSC.
- W. E. Geiger and F. Barrière, Acc. Chem. Res., 2010, 43, 1030 CrossRef CAS PubMed.
- S. H. Dempsey, A. Lovstedt and S. R. Kass, J. Org. Chem., 2023, 88, 10525 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2431948–2431951. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01578b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |