 Open Access Article
Open Access Article“Click” disaggregation-induced emission of a fluorescent dye†
Kaleena
Basran
a and
Nathan W.
Luedtke
 *ab
*ab
aDepartment of Chemistry, McGill University, Montreal, Quebec H3A 0B8, Canada
bDepartment of Pharmacology and Therapeutics, McGill University, Montreal, Quebec H3G 1Y6, Canada
First published on 10th February 2025
Abstract
Here we demonstrate a new approach to fluorogenic labelling, where a cationic hemicyanine (CHyC) exhibits disaggregation-induced emission (DIE) upon undergoing an azide–alkyne “click” reaction. CHyC self-associates and is self-quenched in aqueous buffer over a low micromolar concentration range. When an azido nucleoside (AmdU) or azide-containing cellular DNA is added to CHyC in the presence of Cu(I), a copper-catalysed azide–alkyne cycloaddition drives dye disaggregation, significantly increasing the fluorescence intensity of the probe upon its covalent attachment to modified biomolecules.
Fluorogenic bioorthogonal “click” chemical reactions can enable convenient, no-wash cellular imaging.1 In the context of nucleic acids,2 click reactions with fluorescent probes provide powerful tools for characterizing DNA/RNA metabolism, cell cycle progression, viral entry, and therapeutic mechanisms of known and new drug candidates.3 Classical fluorophores like rhodamines, cyanines, coumarins, and others4 are now widely available with clickable handles—such as tetrazines, azides, and alkynes—to facilitate conjugation reactions such as copper-catalysed azide–alkyne cycloadditions (CuAAC).5 Increasing the fluorescence intensity of the labelled biomolecule as compared to the unreacted dye is an important and challenging goal in wash-free imaging applications.6
Cyanine dyes are a diverse family of fluorophores which are classified by the number of methine “bridge” units and terminal heterocycles present.7 Styryl hemicyanines containing two methine carbons have been used in three-way junction DNA aptamers,8 fluorescent oligonucleotide probes,9 and for non-covalent binding of DNA.10 Moreover, the metabolic modification of nucleic acids with alkene groups followed by treatment with tetrazine-substituted styryl hemicyanines enabled inverse electron-demand Diels–Alder (IEDDA) reactions on cellular DNA.11 Indeed, tetrazines are well established to quench fluorophores,12 allowing for wash-free imaging of metabolically labelled DNA in live cells.6b
Azides groups are invaluable in chemical biology and drug development due to their small size and bioorthogonal reactivity.13 Despite their widespread applications,3f,14 a general “turn-on” strategy for azide-reactive dyes remains elusive. Azide–alkyne cycloadditions are not inherently fluorogenic, although triazole formation has been shown to result in increased in emissions of highly tailored systems.15 Exploring innovative turn-on mechanisms for azide-modified nucleic acids, such as disaggregation-induced emission (DIE) where fluorescence is triggered by the disaggregation of aggregated probes is a promising new approach (Scheme 1).16 Non-covalent DIE reactions have previously been used for detecting small molecules,17 monitoring the equilibrium of G-quadruplexes,18 and probing cellular membranes and proteins.19 Herein, we designed a cationic hemicyanine (CHyC) that exhibits DIE upon reacting with an azide-containing nucleoside, 5-(azidomethyl)-2′-deoxyuridine (AmdU),14dvia CuAAC reaction. The irreversible covalent chemical reaction shifts the dye self-association equilibrium towards disaggregation, resulting in enhanced fluorescence emission.
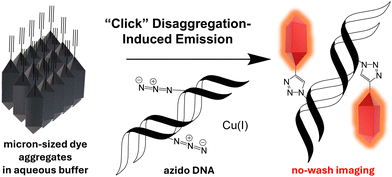 | ||
| Scheme 1 A quenched and aggregated alkyne-containing fluorescent dye undergoes disaggregation and enhanced fluorescence upon CuAAC reaction with azido DNA. | ||
To synthesize CHyC, 6-methoxy-2-naphthaldehyde 1 was transformed into benzoindole 2 through a base-promoted Knoevenagel condensation and Hemetsberger indolization (Scheme 2).20 First, ethyl-2-azidoacetate 1a was synthesized in a 98% yield from ethyl-2-bromoacetate.21 6-Methoxy-2-naphthaldehyde 1 and azidoacetate 1a were dissolved in ethanol along with a sacrificial electrophile, ethyl trifluoroacetate. 20% sodium ethoxide in ethanol was added at 0 °C and the reaction was stirred overnight yielding the α-azido-β-arylacrylate 1b. Thermolysis of intermediate 1b gave the benzo[g]indole 2 as the only regioisomeric indole in a 62% yield over two-steps. The propargyl group was introduced by treating 2 with sodium hydride followed by the dropwise addition of propargyl bromide to give the desired product 3 in an 83% yield. 3 was then reduced to the corresponding aldehyde 4 in two consecutive steps in a 79% yield. 4 and 1,2,3,3-tetramethyl-3H-indol-1-ium iodide were heated to 70 °C overnight in ethanol to yield CHyC 5 as a dark purple solid with low water solubility in 91% isolated yield (Scheme 2). The probe and all relevant intermediates were fully characterized by 1H NMR, 13C NMR, and high-resolution ESI MS (see ESI†). Stock solutions of CHyC for photophysical and biological studies were prepared in DMSO and diluted into the indicated solvents (0.5% DMSO unless stated otherwise) prior to analysis.
 | ||
| Scheme 2 Synthesis of CHyC (5) and all relevant intermediates where EtOH = ethanol, DMF = N,N-dimethylformamide, LAH = lithium aluminium hydride, THF = tetrahydrofuran, and ACN = acetonitrile. See the ESI† for the synthesis and characterization of these compounds. | ||
The photophysical properties of CHyC 5 were evaluated at various concentrations upon dilution into 1× PBS buffer, pH = 7.4 (Fig. 1a). The aqueous samples displayed a linear relationship between absorbance (λmax = 520 nm) and CHyC concentration over the range 0.2–12.4 μM (ESI,† Fig. S1, ε520 = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1 M−1). In contrast, non-linear concentration-dependent effects were observed in the fluorescence emission intensities of the same samples (ESI,† Fig. S1), giving lower quantum yield values (Φ = 1.1–0.063%) with increasing concentration (Fig. 1b). Microscopic evaluation of the samples prepared at 2–10 μM in PBS revealed the presence of purple, non-fluorescent particles with diameters ranging from roughly 2–8 μm (ESI,† Fig. S2). In contrast, CHyC samples prepared entirely in DMSO exhibited better solubility, a higher measured extinction coefficient (ε545 = 41
300 cm−1 M−1). In contrast, non-linear concentration-dependent effects were observed in the fluorescence emission intensities of the same samples (ESI,† Fig. S1), giving lower quantum yield values (Φ = 1.1–0.063%) with increasing concentration (Fig. 1b). Microscopic evaluation of the samples prepared at 2–10 μM in PBS revealed the presence of purple, non-fluorescent particles with diameters ranging from roughly 2–8 μm (ESI,† Fig. S2). In contrast, CHyC samples prepared entirely in DMSO exhibited better solubility, a higher measured extinction coefficient (ε545 = 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 cm−1 M−1) and concentration-independent quantum yield (Φ = 5.4%). In DMSO, CHyC exhibited a red-shifted absorbance (λmax = 540 nm) and emission (λmax = 625 nm) as compared to 1× PBS. The absorbance spectrum of CHyC in acetonitrile (ACN) closely resembled that of DMSO. However, in methanol (MeOH), additional solvent effects led to a further redshift of CHyC, albeit with a lower quantum yield (Φ = 1.0%) than the 5.4% for DMSO (Fig. 1c and ESI,† Table S1). Together these results suggest that the micro-aggregated form(s) of CHyC in PBS have some twisting about the styryl bridge and/or self-assembly into H-type aggregates.22 The dynamic, self-quenching and self-association behaviour of CHyC over the low μM concentration range suggested that it may exhibit “turn-on” fluorescence behaviour upon chemical reaction with groups that would endow enhanced solubility properties of the product in water.
900 cm−1 M−1) and concentration-independent quantum yield (Φ = 5.4%). In DMSO, CHyC exhibited a red-shifted absorbance (λmax = 540 nm) and emission (λmax = 625 nm) as compared to 1× PBS. The absorbance spectrum of CHyC in acetonitrile (ACN) closely resembled that of DMSO. However, in methanol (MeOH), additional solvent effects led to a further redshift of CHyC, albeit with a lower quantum yield (Φ = 1.0%) than the 5.4% for DMSO (Fig. 1c and ESI,† Table S1). Together these results suggest that the micro-aggregated form(s) of CHyC in PBS have some twisting about the styryl bridge and/or self-assembly into H-type aggregates.22 The dynamic, self-quenching and self-association behaviour of CHyC over the low μM concentration range suggested that it may exhibit “turn-on” fluorescence behaviour upon chemical reaction with groups that would endow enhanced solubility properties of the product in water.
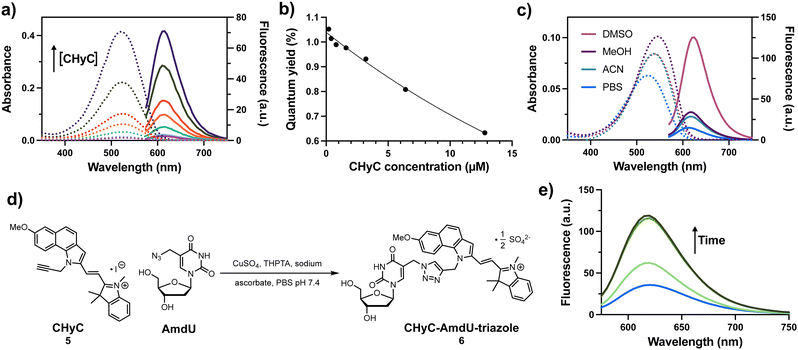 | ||
| Fig. 1 (a) Absorbance (dashed) and fluorescence (solid) spectra of 0.2–12.4 μM solutions of CHyC 5 in 1× PBS (pH 7.4, 2% EtOH). (b) Calculated quantum yields verses CHyC concentrations in 1× PBS. (c) Absorbance (dashed) and fluorescence (solid) spectra of a 2 μM solution of CHyC 5 in various solvents and 1× PBS (pH 7.4, 2% EtOH). (d) CuAAC reaction of CHyC 5 and AmdU where THPTA = tris(benzyltriazolylmethyl)amine. (e) Fluorescence spectrum of a 100 μM solution of CHyC, 1 mM CuSO4, 2 mM THPTA, 1 mM AmdU, and 10 mM sodium ascorbate in PBS pH 7.4 (1.3% DMSO) at time = 0 min, 20 min, 40 min, and 60 min into the reaction. For all fluorescence: ex: 546 nm, em: 570–750 nm. See the ESI† for the characterization of CHyC-AmdU-triazole 6. | ||
To evaluate if a click reaction involving a partially soluble dye can induce disaggregation-induced emission (DIE), a 100 μM solution of CHyC 5 was subjected to standard CuAAC conditions with a 10-fold excess of AmdU in 1× PBS containing 1% DMSO (Fig. 1d). The reaction was monitored by fluorescence (Fig. 1e) as well as high performance liquid chromatography (ESI,† Fig. S3). Both analyses indicated complete consumption of CHyC 5 in less than one hour. Remarkably, the fluorescence intensity of the solution showed a ∼3-fold increase; reminiscent of the changes observed in DMSO (Fig. 1c). The CHyC-AmdU-triazole reaction product 6 was isolated in a 70% yield and was characterized to confirm its identity (see ESI†). These results demonstrate that DIE during a bioorthogonal chemical reaction can be used to track reaction progress in real time.
To evaluate the potential utility of DIE of CHyC in no-wash cellular staining and imaging, HeLa cell cultures were treated with 100 μM of an AmdU monophosphate derivative bearing two 5′-pivaloyloxymethyl masking groups “POM-AmdU”,23 for 17 hours prior to fixation and staining with 10 μM CHyC in 1× PBS containing 1% DMSO and Cu(I). The cells were imaged while still in the staining solution, revealing large fluorescence enhancements of the nuclei in cells pre-treated with POM-AmdU as compared to those receiving vehicle only. As a control, we compared the performance of CHyC with a commercially available Cy5 alkyne derivative “Alexa Fluor™ 647 Alkyne” that was also found to be compatible with no-wash imaging, but it displayed little or no selectivity for the cellular nuclei of cells that had been pre-treated with POM-AmdU (ESI,† Fig. S4). To evaluate the DNA selectivity of CHyC staining in POM-AmdU treated cells, the CHyC staining solutions were removed by aspiration, and a second solution containing the non-covalent DNA stain Hoechst 33342 was added to the cells and imaged without washing (Fig. 2). Only cells receiving POM-AmdU exhibited CHyC “turn-on” fluorescence that co-localized with Hoechst staining with a Pearson correlation coefficient (PCC) of 0.76 ± 0.03 as compared to a PCC = 0.31 ± 0.08 for the control cells not pre-treated with POM. A perfect correlation of 1.0 was not expected because only a fraction of the cells had passed though S-phase during the 17-hour incubation with POM-AmdU.
In summary, CHyC is a novel cationic hemicyanine dye that undergoes disaggregation-induced emission (DIE) after CuAAC click reactions. In the current example, DNA is targeted by virtue of AmdU incorporation into cellular DNA. In theory, RNA could be targeted by CHyC by using appropriate metabolic labels such as N6-ethylazido-adenosine or 2′-azidoadenosine.14f While fast, the CuAAC reaction is limited to fixed cells due to its toxicity,24 and hence catalyst-free DIE reactions based on SPAAC25 or vinyl-tetrazine ligation6b could provide future access to wash-free imaging of live cells.
Funding was provided by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 2020-05048), the Canada Foundation for Innovation (JELF), and the Canada First Research Excellence Fund (D2R).
Data availability
The data supporting this article have been included in the main article and as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Chen, H. Jiang, T. Hao, N. Zhang, M. Li, X. Wang, X. Wang, W. Wei and J. Zhao, Chem. Biomed. Imaging, 2023, 1(7), 590–619 CrossRef CAS PubMed.
- (a) D. Ganz, D. Harijan and H.-A. Wagenknecht, RSC Chem. Biol., 2020, 1(3), 86–97 RSC; (b) N. Z. Fantoni, A. H. El-Sagheer and T. Brown, Chem. Rev., 2021, 121(12), 7122–7154 CrossRef CAS PubMed; (c) J. I. H. Knaack and C. Meier, ChemMedChem, 2024, 19(15), e202400160 CrossRef CAS PubMed.
- (a) S. Ding, X. Qiao, J. Suryadi, G. S. Marrs, G. L. Kucera and U. Bierbach, Angew. Chem., Int. Ed., 2013, 52(12), 3350–3354 CrossRef CAS PubMed; (b) A. B. Neef, L. Pernot, V. N. Schreier, L. Scapozza and N. W. Luedtke, Angew. Chem., Int. Ed., 2015, 54(27), 7911–7914 CrossRef CAS PubMed; (c) T. Triemer, A. Messikommer, S. M. K. Glasauer, J. Alzeer, M. H. Paulisch and N. W. Luedtke, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(7), E1366–E1373 Search PubMed; (d) M. Tera, Z. Harati Taji and N. W. Luedtke, Angew. Chem., 2018, 130(47), 15631–15635 CrossRef; (e) M. Kubota, S. Nainar, S. M. Parker, W. England, F. Furche and R. C. Spitale, ACS Chem. Biol., 2019, 14(8), 1698–1707 CrossRef CAS PubMed; (f) A. Messikommer, K. Seipel, S. Byrne, P. J. M. Valk, T. Pabst and N. W. Luedtke, ACS Pharmacol. Transl. Sci., 2020, 3(6), 1225–1232 CrossRef CAS PubMed; (g) Y. Li, Y. Ling, M. O. Loehr, S. Chaabane, O. W. Cheng, K. Zhao, C. Wu, M. Buscher, J. Weber, D. Stomakhine, M. Munker, R. Pientka, S. B. Christ, M. Dobbelstein and N. W. Luedtke, Life Sci., 2023, 330, 122000 CrossRef CAS PubMed.
- N. Klöcker, F. P. Weissenboeck and A. Rentmeister, Chem. Soc. Rev., 2020, 49(23), 8749–8773 RSC.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. Am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Nat. Rev. Methods Primers, 2021, 1, 30 CrossRef CAS PubMed.
- (a) J. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno, R. H. Singer, T. Lionnet and L. D. Lavis, Nat. Methods, 2015, 12(3), 244–250 Search PubMed; (b) M. O. Loehr and N. W. Luedtke, Angew. Chem., Int. Ed., 2022, e202112931 Search PubMed; (c) A. Spampinato, E. Kuzmova, R. Pohl, V. Sykorova, M. Vrabel, T. Kraus and M. Hocek, Bioconjugate Chem., 2023, 34(4), 772–780 Search PubMed; (d) V. T. Sterrenberg, D. Stalling, J. I. H. Knaack, T. K. Soh, J. B. Bosse and C. Meier, Angew. Chem., Int. Ed., 2023, 62(38), e202308271 Search PubMed; (e) M. Kuba, P. Khoroshyy, M. Lepsik, E. Kuzmova, D. Kodr, T. Kraus and M. Hocek, Angew. Chem., Int. Ed., 2023, 62(38), e202307548 Search PubMed; (f) A. Martin and P. Rivera-Fuentes, Nat. Chem., 2024, 16(1), 28–35 Search PubMed.
- (a) A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100(6), 1973–2011 Search PubMed; (b) G. S. Gopika, P. M. H. Prasad, A. G. Lekshmi, S. Lekshmypriya, S. Sreesaila, C. Arunima, M. S. Kumar, A. Anil, A. Sreekumar and Z. S. Pillai, Mater. Today Proc., 2021, 46, 3102–3108 Search PubMed.
- (a) A. J. Van Riesen, J. Le, S. Slavkovic, Z. R. Churcher, A. A. Shoara, P. E. Johnson and R. A. Manderville, ACS Appl. Bio Mater., 2021, 4(9), 6732–6741 CrossRef CAS PubMed; (b) A. J. Van Riesen, B. Kalnitsky, A. A. Shoara, S. Slavkovic, Z. R. Churcher, P. E. Johnson and R. A. Manderville, Dyes Pigm., 2023, 209 Search PubMed.
- (a) M. M. Rubner, C. Holzhauser, P. R. Bohländer and H. A. Wagenknecht, Chem. – Eur. J., 2012, 18(5), 1299–1302 Search PubMed; (b) S. Arndt, H.-K. Walter and H.-A. Wagenknecht, J. Vis. Exp., 2016, 113, e54121 Search PubMed; (c) J. Steinmeyer, F. Rönicke, U. Schepers and H. A. Wagenknecht, ChemistryOpen, 2017, 6(4), 514–518 Search PubMed; (d) B. Ditmangklo, J. Taechalertpaisarn, K. Siriwong and T. Vilaivan, Org. Biomol. Chem., 2019, 17(45), 9712–9725 Search PubMed; (e) J. Gebhard, L. Hirsch, C. Schwechheimer and H. A. Wagenknecht, Bioconjugate Chem., 2022, 33(9), 1634–1642 CrossRef CAS PubMed; (f) P. Geng, E. List, F. Rönicke and H. A. Wagenknecht, Chem. – Eur. J., 2023, 29(8), e202203156 CrossRef CAS PubMed.
- (a) P. R. Bohländer and H.-A. Wagenknecht, Org. Biomol. Chem., 2013, 11(43), 7458 RSC; (b) K. Supabowornsathit, K. Faikhruea, B. Ditmangklo, T. Jaroenchuensiri, S. Wongsuwan, S. Junpra-Ob, I. Choopara, T. Palaga, C. Aonbangkhen, N. Somboonna, J. Taechalertpaisarn and T. Vilaivan, Sci. Rep., 2022, 12, 14250 CrossRef CAS PubMed; (c) S. Wangngae, U. Ngivprom, T. Khrootkaew, S. Worakaensai, R.-Y. Lai and A. Kamkaew, RSC Adv., 2023, 13(3), 2115–2122 RSC.
- (a) D. Ganz, P. Geng and H. A. Wagenknecht, ACS Chem. Biol., 2023, 18(5), 1054–1059 CrossRef CAS PubMed; (b) B. Pfeuffer, P. Geng and H. A. Wagenknecht, ChemBioChem, 2024, 25(4), e202300739 Search PubMed; (c) N. Seul, D. Lamade, P. Stoychev, M. Mijic, R. T. Michenfelder, L. Rieger, P. Geng and H. A. Wagenknecht, Angew. Chem., Int. Ed., 2024, 63(22), e202403044 CrossRef CAS PubMed.
- H. Wu, J. Yang, J. Seckute and N. K. Devaraj, Angew. Chem., Int. Ed., 2014, 53(23), 5805–5809 CrossRef CAS PubMed.
- F. Müggenburg and S. Müller, Chem. Rec., 2022, 22(5), e202100322 CrossRef PubMed.
- (a) S. Broder, Antivir. Res., 2010, 85(1), 1–18 Search PubMed; (b) M. Aigner, M. Hartl, K. Fauster, J. Steger, K. Bister and R. Micura, ChemBioChem, 2011, 12(1), 47–51 CrossRef CAS PubMed; (c) A. H. El-Sagheer and T. Brown, Chem. Commun., 2011, 47(44), 12057 Search PubMed; (d) A. B. Neef and N. W. Luedtke, ChemBioChem, 2014, 15(6), 789–793 Search PubMed; (e) J. M. Holstein, D. Schulz and A. Rentmeister, Chem. Commun., 2014, 50(34), 4478–4481 RSC; (f) S. Nainar, S. Beasley, M. Fazio, M. Kubota, N. Dai, I. R. Corrêa and R. C. Spitale, ChemBioChem, 2016, 17(22), 2149–2152 CrossRef CAS PubMed; (g) M. K. Yates and K. L. Seley-Radtke, Antiviral Res., 2019, 162, 5–21 CrossRef CAS PubMed; (h) L. Taemaitree, A. Shivalingam, A. H. El-Sagheer and T. Brown, Nat. Commun., 2019, 10, 1610 CrossRef PubMed; (i) D. Wang, Y. Zhang and R. E. Kleiner, J. Am. Chem. Soc., 2020, 142(34), 14417–14421 CrossRef CAS PubMed; (j) S. Moreno, J. M. Ramos Pittol, M. Hartl and R. Micura, Org. Biomol. Chem., 2022, 20(39), 7845–7850 RSC.
- (a) Z. Zhou and C. J. Fahrni, J. Am. Chem. Soc., 2004, 126(29), 8862 CrossRef CAS PubMed; (b) J. C. Jewett and C. R. Bertozzi, Org. Lett., 2011, 13(22), 5937–5939 CrossRef CAS PubMed; (c) P. Shieh, M. J. Hangauer and C. R. Bertozzi, J. Am. Chem. Soc., 2012, 134(42), 17428–17431 CrossRef CAS PubMed; (d) F. Friscourt, C. J. Fahrni and G. J. Boons, J. Am. Chem. Soc., 2012, 134(45), 18809–18815 CrossRef CAS PubMed.
- (a) D. Zhai, W. Xu, L. Zhang and Y.-T. Chang, Chem. Soc. Rev., 2014, 43(8), 2402 RSC; (b) K. Saczuk, M. Dudek, K. Matczyszyn and M. Deiana, Nanoscale Horiz., 2024, 9, 1390–1416 Search PubMed.
- (a) L. K. Kumawat, A. A. Abogunrin, M. Kickham, J. Pardeshi, O. Fenelon, M. Schroeder and R. B. P. Elmes, Front. Chem., 2019, 7 Search PubMed; (b) P. Zhang, M. S. Zhu, H. Luo, Q. Zhang, L. E. Guo, Z. Li and Y. B. Jiang, Anal. Chem., 2017, 89(11), 6210–6215 CrossRef CAS PubMed; (c) J. J. Gao, X. X. Lang, Q. Q. Yu, H. Y. Li, H. J. Wang and M. Q. Wang, Spectrochim. Acta, Part A, 2021, 252, 119492 CrossRef CAS PubMed; (d) L. Liu, C. Liu, L. Wang, X.-C. Shen and H. Chen, Sens. Actuators, B, 2022, 371, 132542 Search PubMed.
- (a) M. Deiana, K. Chand, J. Jamroskovic, I. Obi, E. Chorell and N. Sabouri, Angew. Chem., Int. Ed., 2020, 59(2), 896–902 CrossRef CAS PubMed; (b) M. Deiana, K. Chand, J. Jamroskovic, R. N. Das, I. Obi, E. Chorell and N. Sabouri, Nanoscale, 2020, 12(24), 12950–12957 RSC; (c) S. Liu, L. Bu, Y. Zhang, J. Yan, L. Li, G. Li, Z. Song and J. Huang, Anal. Chem., 2021, 93(12), 5267–5276 Search PubMed; (d) G.-F. Liu, Y.-S. Chen, Z.-L. Wang, D. Gu and M.-Q. Wang, Dyes Pigm., 2024, 225, 112107 CrossRef CAS.
- (a) K. Mizusawa, Y. Ishida, Y. Takaoka, M. Miyagawa, S. Tsukiji and I. Hamachi, J. Am. Chem. Soc., 2010, 132(21), 7291–7293 CrossRef CAS PubMed; (b) T.-C. Hou, Y.-Y. Wu, P.-Y. Chiang and K.-T. Tan, Chem. Sci., 2015, 6(8), 4643–4649 RSC; (c) D. Wu, S. Cheung, G. Sampedro, Z. L. Chen, R. A. Cahill and D. F. O'Shea, Biochim. Biophys. Acta, Biomembr., 2018, 1860(11), 2272–2280 CrossRef CAS PubMed; (d) J.-Z. Li, H.-L. Lin, H.-Y. Li, H.-W. Cao, X.-X. Lang, Y.-S. Chen, H.-W. Chen and M.-Q. Wang, Dyes Pigm., 2023, 216, 111357 Search PubMed.
- W. L. Heaner Iv, C. S. Gelbaum, L. Gelbaum, P. Pollet, K. W. Richman, W. Dubay, J. D. Butler, G. Wells and C. L. Liotta, RSC Adv., 2013, 3(32), 13232 RSC.
- F. Shi, J. P. Waldo, Y. Chen and R. C. Larock, Org. Lett., 2008, 10(12), 2409–2412 CrossRef CAS PubMed.
- (a) M. Kasha, Radiat. Res., 1963, 20(1), 55–70 CrossRef CAS PubMed; (b) A. S. Klymchenko, J. Nanosci. Lett., 2013, 3(21), 1–8 Search PubMed.
- M. Tera, S. M. K. Glasauer and N. W. Luedtke, ChemBioChem, 2018, 19(18), 1939–1943 CrossRef CAS PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(43), 16793–16797 CrossRef CAS PubMed.
- M. Tera and N. W. Luedtke, Methods Enzymol., 2020, 641, 433–457 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05916f |
| This journal is © The Royal Society of Chemistry 2025 |

