Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in lanthanide cyclononatetraenyl chemistry
Xiaofei
Sun
 *a,
Grégory
Nocton
*a,
Grégory
Nocton
 b and
Peter W.
Roesky
b and
Peter W.
Roesky
 *ac
*ac
aInstitute of Inorganic Chemistry (AOC), Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131, Karlsruhe, Germany. E-mail: xiaofei.sun@kit.edu; roesky@kit.edu
bLCM, CNRS, Ecole Polytechnique, Institut Polytechnique de Paris, 91120 Palaiseau, France
cInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131, Karlsruhe, Germany
First published on 23rd December 2024
Abstract
Cyclononatetraenyl (Cnt) is a nine-membered monoanionic aromatic ligand. Despite its early discovery in 1963, it has been rarely utilised in coordination chemistry, which is mainly due to its large diameter and easy skeletal rearrangement. Only in 2017, the first lanthanide Cnt complex was synthesised, marking the beginning of a new era in organolanthanide chemistry. Since then, the chemistry displayed by Cnt has expanded rapidly in lanthanide chemistry. Due to the ligand flexibility, both classical planar η9-Cnt ligands and heavily bent ones with lower hapticity were found in Ln coordination complexes. These novel structure motifs exhibit reactivity which differs significantly from traditional cyclopentadienyl (Cp) and cyclooctatetraenediide (Cot) complexes. Some of the obtained compounds also show interesting photoluminescence and magnetic properties. This review presents an overview of the synthesis, structure and reactivity of the growing family of lanthanide Cnt compounds.
Introduction
Since Faraday isolated benzene in 1825,1 aromaticity has become a fundamental concept in chemistry. Although defining aromaticity can be challenging today, with various criteria and definitions being developed,2 the classical way to define aromatic compounds relies on Hückel's (4n + 2)π electron rule.3 In general, aromatic C-based monocyclic ring systems are frequently used as ligands in coordination chemistry (Fig. 1). The discovery of the first metallocene (ferrocene) in 1951 by Kealy and Pauson,4 and its subsequent structural elucidation5 was a milestone in modern chemistry. In ferrocene, the iron centre is sandwiched between two aromatic monoanionic cyclopentadienyl (Cp) ligands. Shortly after the discovery of ferrocene, the Cp ligand was also introduced in organolanthanide chemistry. In 1954, Wilkinson and Birmingham reported the first organolanthanide compounds, namely, the tris(cyclopentadienyl)lanthanides [(Cp)3LnIII].6 Since then, Cp-type ligands have played a dominating role in lanthanide chemistry. | ||
| Fig. 1 Aromatic four- to nine-membered carbocycles used as ligands in lanthanide chemistry. For the four-membered ring only a substituted derivate was used as ligand. | ||
Following the widespread use of Cp ligands, the 10π-aromatic cyclooctatetraenediide (Cot) dianion has also played a crucial role in the chemistry of f-elements for more than five decades.7,8 Lanthanide complexes featuring Cp or Cot ligands have become important not only due to their unique structures but also their potential applications in catalysis, electrochemistry, and material science. Beyond the ubiquitous Cp and Cot rings in organolanthanide chemistry, other aromatic carbocycles have also been employed as ligands. A significant step was made by Cloke et al. in 1987 when they synthesized several lanthanide bis(arene) sandwich compounds with bulky benzene derivatives using electron-beam vaporization techniques.9 Very recently, the first examples of lanthanide complexes comprising the four-membered substituted cyclobutadienyl dianion have also been disclosed.10,11
It is well-established that the dianionic Cot ligand can serve as a bridging unit to form lanthanide multidecker species.12–14 In addition, the purely carbon-based seven-membered cycloheptatrienyl trianion has also been employed in organolanthanide chemistry, acting as bridging ligand for inverse sandwich and multidecker compounds.15–17
All the carbocycles with ring sizes from four to eight have been utilised in organolanthanide chemistry for over 20 years. The next larger aromatic carbocycle is the nine-membered 10π-electron cyclononatetraenyl (Cnt) monoanion, which was only introduced in organolanthanide chemistry in 2017.18
The Cnt anion has been known since the 1960s with the corresponding alkali metal salts (Li(Cnt) or K(Cnt)) being synthesized from Cot in a two-step procedure.19,20 On the basis of their NMR data and the strong UV absorption, their aromatic character has been confirmed. However, synthesising stable complexes with Cnt ligands were found to be extremely challenging due to the large ring size and tendency of skeletal rearragement.21,22 Still, Cnt remains attractive as a planar π-ligand in coordination chemistry, especially for generating novel sandwich complexes with ligands beyond the classical smaller ring systems. Early studies on the coordination chemistry of Cnt ligands with transition metal precursors were reported already in the 1970s. The two examples are the synthesis of the heteroleptic compounds, [(Cp)TiII(Cnt)]23 and [(Cp)2NbIII(Cnt)],24 where the spectroscopic observations revealed η3- and η7-coordination modes for the Ti and Nb compounds, respectively. More recently, in 2009, a tetrapalladium Cnt-based sheet sandwich-type complex was reported and the molecular structure was disclosed.25
The Cnt anion can exist as two different isomers, Cnt-trans and Cnt-cis (Fig. 2). Only recently, the molecular structure of the potassium salt of the Cnt ligand (K(Cnt)) was reported by Nocton26 and Roesky27 independently. Depending on the reaction and crystallisation conditions, the outcome of the formation of K(Cnt) slightly differs.28 Nocton et al. reported that recrystallisation of K(Cnt) from diethyl ether, led to single crystals with disorder for the C1 atom (Fig. 3, right), which is linked to the formation of the cis–cis–cis–cis isomer (Cnt-cis) and the cis–cis–cis–trans isomer (Cnt-trans) forming a structure resembling a pac-man. Both isomers are present in solution, as evidenced by NMR spectroscopy. For the cis-isomer, the monoanionic charge is delocalized over the entire ring, as shown in the fully planar structure and the nearly equivalent C–C bond distances, resulting a D9h symmetry. In contrast, for the trans analogue, one of the carbon atoms is inside the ring, and the negative charge is mainly localised over there. Therefore, C2v symmetry is assumed in first approximation. Roesky et al. obtained single crystals of K(Cnt) from dimethoxyethane and the structure showed only the Cnt-cis form, with the potassium ion exhibiting complete η9-coordination towards the ring (Fig. 3, left).
 | ||
| Fig. 3 The molecular structures of KCnt·(dme)2 (left) and KCnt·Et2O (right) in the solid state with thermal ellipsoids at 40% probability.† | ||
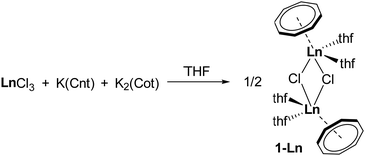
Already in 1973, more than half a century ago, Steitwieser et al. attempted to incorporate the Cnt ligand in organolanthanide complexes.29 They aimed to synthesise the heteroleptic sandwich complex [(Cnt)LnIII(Cot)] (Ln = Ce, Pr, Nd and Sm). However, instead of the expected product, the one-pot reaction between LnCl3, K2(Cot), and K(Cnt) in THF led to the formation of a new class of mono(cyclooctatetraenediide) compounds, the dimeric chlorides [LnIII(Cot)(thf)2Cl]2 (1-Ln, Ln = Ce, Pr and Sm) (Scheme 1).
The large diameter (approximately 4.1 Å) of the Cnt ligand appears to be particularly well-suited for metal centres with large ionic radii such as heavy main group metals like Ba, Pb, Bi, and the f-elements. In 2005, Sitzmann et al. reported the reaction between K(Cnt) and BaI2 in THF, which resulted in the formation of the homoleptic complex [Ba(η9-Cnt)2] (2) (Scheme 2).30 Although no single crystals could be obtained for structure determination, DFT calculations proposed the sandwich-type structure of 2. Further spectroscopic methods (NMR spectroscopy and EI MS) indicated the successful isolation of 2.
Lanthanidocenes: divalent bis(cyclononatetraenyl) lanthanide sandwich compounds
In 2017, Nakajima and co-workers made a significant advancement by introducing the Cnt ligand into the coordination sphere of a divalent Ln ion for the first time.18 They synthesised the homoleptic EuII sandwich complex [EuII(η9-Cnt)]2 (3-Eu) via salt elimination from K(Cnt) and EuI2 in THF (Scheme 3) in extremely low yield (4%). The structure of 3-Eu was elucidated using single crystal X-ray diffraction (SCXRD) analysis, revealing that the EuII ion is sandwiched between the two planar Cnt rings (Fig. 4). The 10π-aromatic nature of the Cnt ring is retained as evidenced by the sum of the internal angles (1260°) and the nearly equivalent C–C bond lengths (1.35–1.45 Å, Table 1). The EuII ion is located at the centre of inversion, which results in a completely linear structure with the Ct–Eu–Ct angle being 180°. The high symmetry was further corroborated by Raman spectroscopy. Complex 3-Eu shows blue-green emission at 516 nm in toluene at room temperature and at 77 K. This emission is significantly blue-shifted compared to other well-known organoeuropium(II) sandwich complexes since typically, EuII sandwich complexes with Cp or Cot ligands show red photoluminescence emission at around 600 nm.31–33 According to DFT calculations, the blue-shift emission of 3-Eu originates from the energy lowering of the 5d-6s hybridised orbitals and the weak electrostatic potential induced by the Cnt ligands. | ||
| Fig. 4 Molecular structures of [LnII(η9-Cnt)2] (3-Ln, Ln = Sm, Eu, Tm, Yb) in the solid state with thermal ellipsoids at 40% probability.† | ||
| 3-Sm | 3-Eu | 3-Tm | 3-Yb | |
|---|---|---|---|---|
| Distances are given in Å and angles are given in deg (°). | ||||
| C–C | 1.33(1)–1.47(2) | 1.353(15)–1.448(13) | 1.32(1)–1.47(2) | 1.33(3)–1.43(3) |
| C–C (av.) | 1.39(4) | 1.39(2) | 1.39(4) | 1.39(4) |
| M–C | 2.833(8)–2.958(6) | 2.796(13)–2.957 | 2.736(8)–2.760(8) | 2.74(2)–2.86(2) |
| M–C (av.) | 2.88(3) | 2.87(4) | 2.75(1) | 2.79(3) |
| M–CntCt | 2.061 | 2.065 | 1.91 | 1.932 |
| CntCt–CntCt | 4.122 | 4.131 | 3.82 | 3.865 |
| Ring size (max C⋯C–C) | 4.05 | 4.02 | 4.02 | 4.06 |
| CntCt–Ln–CntCt | 180 | 180 | 180 | 180 |
In addition to the experimental findings on 3-Eu, the electronic structure and photoluminescence properties of 3-Eu were further analysed via quantum computational methods.34 Ligand-field density functional theory (LFDFT), a DFT-based model incorporating the configuration interaction algorithm through a model Hamiltonian, was employed for the calculations. In the optimized structure of 3-Eu, both the eclipsed D9h and the staggered D9d conformations, were found to be nearly degenerate, suggesting that the Cnt ligand can freely rotate in the gas phase. The calculated molecular orbital diagram reveals that the Eu 4f orbitals contribute only weakly to the predominantly ionic chemical bonding. Time-dependent DFT (TD-DFT) and LFDFT calculations explained the photoluminescence emission as electric dipole-allowed Eu 4f-5d transitions between the lowest excited 4f65d1 state of the EuII ion and the 4f75d0 ground state. The low quantum yield in toluene solution at room temperature may be indicative of non-radiative decay caused by strong interactions with solvent molecules.
In 2018, the group of Nocton extended the bis(Cnt) chemistry to other divalent lanthanides, Sm, Tm and Yb.26 They discovered that both the starting material and the solvent are crucial for the high yield synthesis of [LnII(η9-Cnt)2]. The trans/cis isomer mixture of K(Cnt) was necessary for the successful high yield synthesis and when the reactions between K(Cnt) and LnI2 were performed in coordinating solvents (THF, Et2O), the yields remained very low. In contrast, reacting K(Cnt) with LnI2 (Ln = Sm, Eu, Tm, Yb) in toluene with a few drops of THF, crystals of 3-Ln were obtained in good yields (33–62%) from the toluene filtrate (Scheme 4). 1H NMR spectroscopy of the diamagnetic 3-Yb and paramagnetic 3-Sm both indicated the existence of three isomers [LnII(Cnt-cis)2], [LnII(Cnt-trans)2] and [LnII(Cnt-cis)(Cnt-trans)] in solution. Complete isomerisation to the [LnII(Cnt-cis)2] isomers took a few days and the yields were extremely low, which suggested that a better isomerisation solvent is required.
 | ||
| Scheme 4 Synthesis of the homoleptic Ln bis(Cnt) complexes as isomer mixture and the further isomerisation to the [LnII(Cnt-cis)2] complexes 3-Ln. | ||
Dissolving the crystals of 3-Yb in THF led to the formation of the ion pair [YbII(Cnt)(thf)4][Cnt] (4-Yb) with one coordinated and one uncoordinated Cnt ligand (Scheme 4). SCXRD analysis revealed that the coordinated one exhibits a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio disorder between the trans and cis forms, while the uncoordinated one consists entirely of the cis isomer. Treating the crystals of 3-Yb with acetonitrile resulted in the full decoordination of the two Cnt ligands from the YbII centre and the formation of the ion pair [YbII(CH3CN)7][Cnt]2 (5-Yb). The YbII ion exhibits a distorted pentagonal bipyramidal geometry and the two Cnt anions are in cis configuration. A pivotal step to get access to pure [YbII(Cnt-cis)2] is to dry the crystals of 5-Yb under 10−3 mbar vacuum for 8 h (Scheme 4). Applying similar procedures for the other divalent Ln complexes also led to the clean formation of [LnII(Cnt-cis)2] (3-Ln, Ln = Sm, Eu and Tm).
1 ratio disorder between the trans and cis forms, while the uncoordinated one consists entirely of the cis isomer. Treating the crystals of 3-Yb with acetonitrile resulted in the full decoordination of the two Cnt ligands from the YbII centre and the formation of the ion pair [YbII(CH3CN)7][Cnt]2 (5-Yb). The YbII ion exhibits a distorted pentagonal bipyramidal geometry and the two Cnt anions are in cis configuration. A pivotal step to get access to pure [YbII(Cnt-cis)2] is to dry the crystals of 5-Yb under 10−3 mbar vacuum for 8 h (Scheme 4). Applying similar procedures for the other divalent Ln complexes also led to the clean formation of [LnII(Cnt-cis)2] (3-Ln, Ln = Sm, Eu and Tm).
3-Sm, 3-Tm and 3-Yb are isomorphous to the previously reported 3-Eu. The high symmetry of the sandwich structures can be reflected from their crystallographic features as the LnII atoms are positioned at the centre of inversion, leading to rigorously linear sandwich motifs. Additionally, the Cnt rings exhibit disorder with altering stacked D9h and eclipsed D9d symmetry, which could potentially result in interesting spectroscopic properties. For example, very recently, Vitova et al. investigated the electronic structure of [SmII(η9-Cnt)2] (3-Sm) by valence band high-energy resolution resonant inelastic X-ray scattering (VB-RIXS), high-resolution X-ray absorption near-edge structure (HR-XANES) and quantum chemical computations. The results demonstrated that the ionic bond of 3-Sm can be photo-modulated and the Sm–C bond covalency increases by transfer of the SmII 4f electrons to the 5d orbitals.35 This change leads to a reconfiguration of 3-Sm with a detectable contraction in Sm–C bond lengths and increased disorder in the local SmII coordination environment.
Super sandwich complexes: heteroleptic trivalent lanthanide complexes
Soon after the report on the homoleptic bis(Cnt) lanthanidocenes by Nocton,26 Roesky et al. introduced a new class of super sandwich complexes, which are heteroleptic sandwich compounds comprising both the Cot and Cnt ligands, [(Cnt)LnIII(η8-Cot)].27 While the majority of the previously reported classical sandwich compounds consists the small five-membered Cp ring, the term super sandwich compounds is introduced to define sandwich complexes in which the central metal atom is coordinated by ligands with more than 16 carbon atoms in total. As already mentioned, the synthesis of [(Cnt)LnIII(η8-Cot)] was initially attempted by Streitwieser et al. in 1973 using a one-pot procedure from the chloride precursors (Scheme 1),36 but only the Cot-functionalised lanthanide chlorides were isolated at that time. In 2019, Roesky et al. reported the successful synthesis of these heteroleptic sandwich complexes using iodide-based Ln compounds as starting materials.The synthesis began with the preparation of the half-sandwich compounds [(η8-Cot)LnIIII(thf)n)] (Ln = Nd, Sm, Dy and Er; n = 2 for Sm, Dy, Er, n = 3 for Nd) from cyclooctatetraene, Ln metal and iodine, following a slightly modified procedure by Mashima et al.37 Refluxing K(Cnt) with [(η8-Cot)LnIIII(thf)n)] in toluene yielded the super sandwich complexes 6-Ln (Ln = Nd, Sm, Dy and Er) in moderate yields of 31–36% after crystallisation from toluene (Scheme 5). Despite some disorder in the crystal structures, the 6-Nd and 6-Sm complexes exhibit perfect sandwich-type structure motifs with η8-coordinated Cot and η9-coordinated Cnt rings in nearly ideal coplanar arrangement (Fig. 5). Notably, the smaller Cot ligand is much closer to the LnIII centre compared to the larger Cnt ligand, which is mostly attributed to the dianionic charge of Cot which results in a stronger electrostatic attraction. Greater disorder was found in the crystal structures of the smaller lanthanides, 6-Dy and 6-Er, and detailed investigation of the structures were published later.38
 | ||
| Scheme 5 Synthesis of the heteroleptic sandwich complexes [(Cnt)LnIII(η8-Cot)] (6-Ln, Ln = Nd, Sm, Dy and Er) from [(η8-Cot)LnIIII(thf)n)] and K(Cnt). | ||
 | ||
| Fig. 5 Molecular structures of 6-Nd (left) and 6-Sm in the solid state with thermal ellipsoids at 40% probability.† | ||
It is known that equatorial ligands are beneficial for enhancing the magnetic anisotropy of ErIII due to the prolate nature of its highest mJ state. 6-Er exhibited single molecule magnet (SMM) behaviour, characterised by an open butterfly-like magnetic hysteresis loop up to 10 K, which was well-reproduced by CASSCF calculations. The magnetic properties of 6-Er indicate that the combination of the Cot/Cnt ligands induces a strong equatorial ligand field. Conversely, 6-Dy showed less promising magnetic properties since the highest mJ state with oblate shape is best to be stabilised by axial ligands, instead of the equatorial Cot/Cnt ligands.
Roesky and Nocton further expanded the chemistry of the super sandwich complexes to additional late lanthanides, Tb, Ho, Tm and Lu.38 In an alternative synthesis to the one described earlier (Scheme 5), the reactions between the half-sandwich starting material [(η8-Cot)LnIIII(thf)n)] and K(Cnt) were performed in acetonitrile/toluene mixture at room temperature (Scheme 6). For the smallest element Lu, the synthesis was optimised by refluxing the borohydride precursor [(η8-Cot)LuIII(BH4)(thf)2)] and K(Cnt) in toluene (Scheme 6). The analogues YbIII sandwich complex was not formed due to reduction by the Cot ligand. Sufficient drying before recrystallisation from toluene is crucial in order to remove the coordinating solvent molecules (THF or acetonitrile), as incomplete removal results in crystal structures with partially coordinated solvent molecules and lower hapticities than η9 for the Cnt ligands.
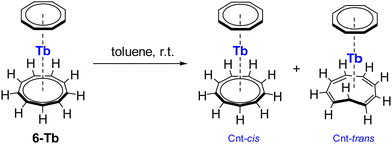
The properties of 6-Ln were analysed in toluene-d8 solution using 1H NMR spectroscopy. Depending on the nature of the LnIII, the spectra showed either two broad or well-defined sharp singlet signals for the Cnt and Cot protons at room temperature. Complex 6-Lu exhibited no fluxional behaviour from −80 °C to 80 °C. Interestingly, for the 6-Tb complex, the Cnt ligand consisted initially of purely cis-configuration, but it partially isomerised to 6-Tb′ over the course of several days (Scheme 7). This isomerisation process was followed by NMR spectroscopy and the amount of 6-Tb′ increased over the time. 6-Tb′ consists of an η8-coordinating Cot ring and the Cnt-trans ligand, in which one of the carbon atoms is inside of the ring. This configuration has been known since the 1960s and was recently discussed for the divalent lanthanidocene complexes 3-Ln.26,28,39,40 Complex 6-Tb′ with Cnt-trans was also observed in the solid-state structure. Depending on the reaction temperature and crystallisation method, single crystals of the TbIII sandwich complex were found to contain either only the Cnt-cis isomer or a disorder of Cnt-cis/Cnt-trans isomers (Fig. 6). When the reaction was performed in toluene/acetonitrile mixture at room temperature, the isolated crystals from toluene consist of solely the Cnt-cis isomer.
 | ||
| Fig. 6 Molecular structures of [(Cnt)TbIII(η8-Cot)] with purely Cnt-cis (left) and Cnt-cis/Cnt-trans disorder (right) in the solid state with thermal ellipsoids at 40% probability.† | ||
Synthesising the TbIII species in hot toluene resulted in a mixture of sandwich compounds with Cnt-cis and Cnt-trans isomers. Among all 6-Ln complexes, this isomerisation has only been detected for the TbIII species.
Molecular structures of all 6-Ln complexes were studied by SCXRD analyses. Among the late lanthanides (Ln = Tb, Dy, Ho, Er, Tm and Lu) (Fig. 6), only the TbIII complex 6-Tb displayed two almost coplanar rings with the Cnt ligand in the η9-coordination mode (Fig. 6 left). As for the smaller lanthanides, DyIII to LuIII, the solid-state structures exhibit significant disorder, with the structure of 6-Lu being the most disordered one (Fig. 7). The disorder in the structures posed challenges in the refinement. The Ln is positioned close to the centre of inversion with 0.5 occupancy, and a 0.5/0.5 disorder of both the Cot/Cnt rings were found. The optimal approach to model this disorder problem is to maintain the Cot ring relatively planar, which is consistent with its typical planarity reported in the literature. The CtCot–LnIII distances nicely correlate to the lanthanide contraction, gradually decreasing from CtCot–TbIII (1.804 Å) to CtCot–LuIII (1.653 Å). In contrast, the Cnt exhibited some degree of deformation from planarity, which is indicative for a lower hapticity. A careful evaluation of the structural metrics revealed that from TbIII to LuIII, the lanthanide contraction results in a transition of the hapticity of the Cnt ligand from η9 (Tb) to η6 (Lu) and meanwhile the Cnt ring shows greater deviation of planarity for the smaller LnIII ions (Fig. 6 and 7). Furthermore, different measurement temperatures also have slight effect on the coordination mode.
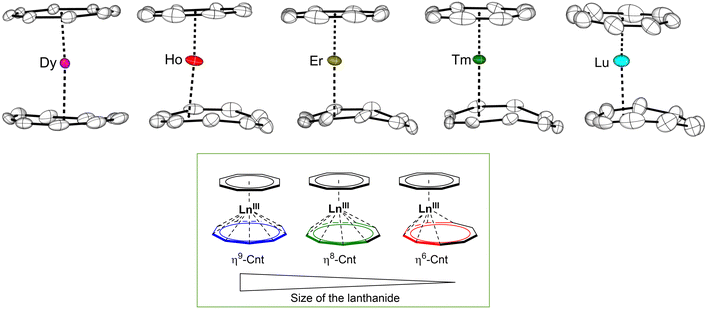 | ||
| Fig. 7 Top: Molecular structures of [(Cnt)LnIII(η8-Cot)] (6-Ln, Ln = Dy, Ho, Er, Tm and Lu) in the solid state with thermal ellipsoids at 40% probability.† Bottom: Representation of size-dependent coordination modes of the Cnt ligand in the [(Cnt)LnIII(η8-Cot)] (6-Ln) sandwich complexes. | ||
Magnetic measurements of 6-Tb, 6-Dy, 6-Ho and 6-Tm showed that the large aromatic Cnt ligand is well suited for the prolate ErIII and TmIII ions. However, none of the four compounds behave as SMMs, which is different compared to the erbium congener 6-Er. Ab initio computational studies indicated that the deviation from planarity and the reduced hapticity of the Cnt do not significantly impact the overall anisotropy in the case for the prolate ions but influence the composition and ratio of mixed mJ states in non-adapted oblate ions.
Molecular lanthanide switches based on the lability of the cyclononatetraenyl ligand
Based on the lability of Cnt ligands in the presence of coordinating solvents, Roesky et al. reported that the heteroleptic sandwich complexes [(Cnt)LnIII(η8-Cot)] (6-Ln) can be used as tools for reversible manipulation of magnetic and photoluminescent properties.41 Upon addition of THF to the solid samples of [(Cnt)LnIII(η8-Cot)] (6-Ln, Ln = La, Ce, Nd, Tb and Er), a colour change occurred immediately, indicating changes in chemical environments of the LnIII metals (Scheme 8). Crystallisation from hot THF solution yielded single crystals for all the five complexes. Depending on the size of the LnIII metal, the obtained products could be classified into three different categories. For the larger LaIII and CeIII metals, coordination of THF led to the formation of the neutral complexes [(η4-Cnt)LnIII(thf)2(η8-Cot)] (7-Ln, Ln = La and Ce, category I). These neutral species feature a planar η8-Cot ligand and an η4-coordinated, heavily bent Cnt ligand. In addition, two THF molecules are coordinated to the central metal ions. For the large and medium-sized LnIII ions, Ce, Nd and Tb, the ionic products [LnIII(thf)n(η8-Cot)][Cnt] (8-Ln, Ln = Ce, Nd and Tb, category II) were obtained. The Cnt ligand is completely replaced by four THF molecules and act as non-coordinating anion. Interestingly, for cerium, THF coordination can lead to formation of both the neutral species 7-Ce (category I) and the ionic species 8-Ce (category II), depending on the crystallisation temperature. For the smallest ErIII ion, the ionic complex [ErIII(thf)3(η8-Cot)][Cnt] (9-Er, category III) with three coordinated THF molecules was formed. The lower coordination number is likely due to the smaller ionic radius of ErIII in comparison to the other lanthanides (CeIII, NdIII and TbIII). This solvation process was entirely reversible, drying the products 7-Ln–9-Ln under dynamic vacuum gave the corresponding starting materials [(Cnt)LnIII(η8-Cot)] (6-Ln, Scheme 8). This reversible process was repeatable and can be controlled by external stimuli such as pressure or temperature, enabling selective modification of the ligand sphere through solid-to-solid transformation. This solvation/desolvation process was nicely demonstrated by Raman spectroscopy in an inert vessel using the CeIII complex [(Cnt)CeIII(η8-Cot)] (6-Ce) as a model system. A Nd:YAG laser was used as an external heating source. The laser beam was capable of removing THF from the sample in a spatially resolved manner and upon cooling the process was reverted.Utilising these reversible properties, the CeIII and TbIII complexes 6-Ln can act as switchable luminophores. Significant differences were observed in the photoluminescence properties of the solvent-free neutral sandwich compounds [(Cnt)LnIII(η8-Cot)] (6-Ln) compared to the solvated ionic analogues [LnIII(thf)4(η8-Cot)][Cnt] (8-Ln). While 6-Ln did not show significant light emission, the ionic versions 8-Ln exhibited intense photoluminescence upon UV-light irradiation. The two forms were easily switched by adding THF to 6-Ln and applying vacuum to 8-Ln. While the emission properties changed, the colours of the respective crystalline materials also changed, which clearly indicated the successful solvation and desolvation.
Besides acting as switchable luminophores, the magnetic properties of the ErIII and NdIII complexes 6-Er and 6-Nd showed significant different SMM behaviours after THF addition. For instance, the neutral Er complex [(Cnt)ErIII(η8-Cot)] (6-Er) exhibits good SMM behaviour with high energy barriers, whereas the THF-solvated analogue 9-Er showed faster relaxation and lower barriers. In contrast to 6-Er and 9-Er, the Nd system showed more distinct behaviour. While [(Cnt)NdIII(η8-Cot)] (6-Nd) behaved as an SMM, the SMM character was entirely shut off upon solvation and formation of 8-Nd. Theoretical calculations further provided explanation of the different magnetic behaviour of these compounds. The almost pure character mJ ground states of 6-Nd accounted for its SMM character. Computational analysis also explained the lack of SMM character of 8-Nd, which is attributed to the pseudo-planar anisotropy of its ground doublet state with highly mixed mJ values.
Photo-isomerisation of the cyclononatetraenyl ligand and its rare-earth complexes
The Cnt ligand can exist in two isomeric forms (Fig. 2), Cnt-cis and Cnt-trans. Both isomers are known to exist as free ligands and in some lanthanide complexes. According to the original ligand synthesis reported by Katz et al. in 1963, the cis-isomer is formed when treating the dipotassium cyclooctatetraenediide with either dichloromethane or dichloromethylmethylether.19 In an alternative synthetic pathway reported by Lalancette et al. in the same year, the trans-isomer is favoured.20 Boche's studies in 1978 detailed this isomerisation,28 highlighting the trans-to-cis isomerisation and indicating that the cis-form is the thermodynamically more stable isomer. Besides the early reports on the Cnt ligand, the different isomers of the Cnt ligand have also been observed during the synthesis of some divalent and trivalent rare-earth compounds (vide supra).26,38 The serendipitous observation of slow cis-to-trans isomerisation of Cnt ligand in [(Cnt)TbIII(η8-Cot)] (6-Tb) reported by Nocton and Roesky,38 contradicts the classical thermodynamics for the pure ligand isomerisation reported by Boche. Boche's pioneering work also briefly suggested that light might induce the isomerisation of the Cnt ligand.28 This led to an in-depth study of this phenomenon for the Cnt ligand and in related rare-earth complexes, which was very recently reported by Nocton.42Spontaneous isomerisation of Cnt-trans to Cnt-cis occurs at room temperature, but by maintaining the temperature at 233 K, a trans:cis ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 can be achieved. By performing the synthesis under light exclusion, this ratio can be increased to 95
20 can be achieved. By performing the synthesis under light exclusion, this ratio can be increased to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Instead of the mixture of different isomers (Scheme 4), by having the Cnt-trans ligand (K(Cnt-trans)) in high purity in hand, the reaction between SmI2 and K(Cnt-trans) was performed under light protection in toluene, resulting in the formation of pure [Sm(Cnt-trans)2] (3-Sm-trans, Scheme 9). Although pure 3-Sm-trans was obtained according to NMR spectroscopy, crystallisation always led to a mixture of different isomers, likely due to the unavoidable slow isomerisation during the crystallisation process and the solubility differences between the trans and cis isomers.
5. Instead of the mixture of different isomers (Scheme 4), by having the Cnt-trans ligand (K(Cnt-trans)) in high purity in hand, the reaction between SmI2 and K(Cnt-trans) was performed under light protection in toluene, resulting in the formation of pure [Sm(Cnt-trans)2] (3-Sm-trans, Scheme 9). Although pure 3-Sm-trans was obtained according to NMR spectroscopy, crystallisation always led to a mixture of different isomers, likely due to the unavoidable slow isomerisation during the crystallisation process and the solubility differences between the trans and cis isomers.
Furthermore, to deepen the understanding of how light and solvent affect the outcome of the synthesis of [(Cnt)LnIII(η8-Cot)] (6-Ln) and to obtain a series of heteroleptic sandwich complexes analogues to 6-Ln with the Cnt either in purely cis or trans form, the synthesis was carefully performed under different conditions. Analogous to the synthesis described earlier (Scheme 6), when reacting K(Cnt) and [(η8-Cot)LnIIII(thf)n)] (Ln = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho; n = 2, 3) in a toluene/acetonitrile mixture under ambient light, 6-Ln complexes with pure Cnt-cis were obtained. Alternatively, performing the reaction in non-coordinating solvent (toluene) under light protection using K(Cnt-trans) and [(η8-Cot)LnIIII(thf)n)] (Ln = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho; n = 2, 3), crystalline products with a high ratio of [(Cnt-trans)LnIII(8-Cot)] (6-Ln-trans) were obtained from concentrated toluene solutions in all cases (Fig. 8). The trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratios were analysed using 1H NMR spectroscopy and SCXRD analysis. For the obtained crystals of 6-Ln-trans, the trans
cis ratios were analysed using 1H NMR spectroscopy and SCXRD analysis. For the obtained crystals of 6-Ln-trans, the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio varied depending on the rare-earth metal ion. Crystals of the Tb, Dy and Ho complexes consisted of 100% of the trans isomer, while the ratios for the other rare-earth metals were 71% (Gd), 69% (Sm), 58% (Nd), 55% (Pr), 50% (Ce), 25% (La) and 73% (Y), respectively. The Ln–C distances for both the Cot and Cnt rings, as well as the Ln–C1 distance decrease along the lanthanide series, with a notable exception in 6-Ho, due to changes in the coordination mode (Table 2).38 Solid structures only represent the portion of the product mixture that crystallised. Since Cnt-trans is more soluble than Cnt-cis, the observed ratios according to XRD analyses are not accurate. Therefore, further solution analysis was performed. Complex 3-Sm-trans showed five signals in 2
cis ratio varied depending on the rare-earth metal ion. Crystals of the Tb, Dy and Ho complexes consisted of 100% of the trans isomer, while the ratios for the other rare-earth metals were 71% (Gd), 69% (Sm), 58% (Nd), 55% (Pr), 50% (Ce), 25% (La) and 73% (Y), respectively. The Ln–C distances for both the Cot and Cnt rings, as well as the Ln–C1 distance decrease along the lanthanide series, with a notable exception in 6-Ho, due to changes in the coordination mode (Table 2).38 Solid structures only represent the portion of the product mixture that crystallised. Since Cnt-trans is more soluble than Cnt-cis, the observed ratios according to XRD analyses are not accurate. Therefore, further solution analysis was performed. Complex 3-Sm-trans showed five signals in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the 1H NMR spectrum, consistent with the Cnt-trans having C2v symmetry in solution. For the series of 6-Ln-trans complexes, both isomers were identified according to the 1H NMR spectra. Using that method allowed the correct identification of both isomers in solution.
1 molar ratio in the 1H NMR spectrum, consistent with the Cnt-trans having C2v symmetry in solution. For the series of 6-Ln-trans complexes, both isomers were identified according to the 1H NMR spectra. Using that method allowed the correct identification of both isomers in solution.
| 6-Y-trans | 6-La-trans | 6-Ce-trans | 6-Pr-trans | 6-Nd-trans | 6-Sm-trans | 6-Gd-trans | 6-Tb-trans | 6-Dy-trans | 6-Ho-trans | |
|---|---|---|---|---|---|---|---|---|---|---|
| Distances are given in Å. | ||||||||||
| Ln–CCot (av.) | 2.542(8) | 2.694(4) | 2.54(4) | 2.636(6) | 2.625(9) | 2.595(6) | 2.570(7) | 2.552(10) | 2.541(5) | 2.478(7) |
| Ln–CCnt-C8 (av.) | 2.806(9) | 2.905(8) | 2.881(15) | 2.86(2) | 2.87(2) | 2.833(8) | 2.826(9) | 2.818(11) | 2.802(8) | 2.792(9) |
| Ln–C1A | 2.657(10) | 2.851(16) | 2.750(12) | 2.749(15) | 2.79(2) | 2.687(13) | 2.703(17) | 2.664(16) | 2.660(9) | 2.673(11) |
| Ln–CCnt (av.) | 2.776(9) | 2.894(10) | 2.85(1) | 2.84(2) | 2.85(2) | 2.804(9) | 2.801(11) | 2.787(13) | 2.774(8) | 2.768(9) |
 | ||
| Fig. 8 Molecular structures of [(Cnt-trans)LnIII(η8-Cot)] (6-Ln-trans, Ln = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho) in the solid state with thermal ellipsoids at 40% probability.† | ||
The photo-isomerisation of the Cnt ligand, as well as complex 3-Sm and 6-Ln, were studied in detail. The solutions were directly irradiated in NMR tubes at different wavelengths, and the resulting isomer ratios were quantified using 1H NMR spectroscopy. The isomerisation of the Cnt ligand from Cnt-trans to Cnt-cis was monitored using 427 nm irradiation, showing a pseudo-first-order reaction rate, with complete conversion achieved after 5 min. Under 427 nm irradiation, complex 3-Sm-trans isomerised via the intermediate 3-Sm-trans–cis to 3-Sm within 30 min, with both steps showing pseudo-first-order reaction rates. However, complete photochemical analysis was not conducted for the isomerisation of the Cnt ligand and the 3-Sm complex.
The photo-isomerisation of the heteroleptic complexes 6-Ln and further analysis are more challenging due to strong paramagnetic nature of some lanthanides and overlapping signals of the isomers in the 1H NMR spectra. For the compounds where integration was possible, irradiation at different wavelengths produced different trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratios (Table 3). Kinetic studies on complex 6-Sm showed that the photo-stationary state (PSS) was reached after 30 min of irradiation, meaning the trans:cis ratio no longer changed. This behaviour was studied for all the 6-Ln (Ln = Y, La, Ce, Sm, Tb, Dy and Lu) complexes, and it varied depending on the irradiation wavelengths and metal ion. For the early lanthanides La and Ce, as well as Y, different wavelengths showed minor changes in the PSS ratios, consistently favouring the trans isomers (Table 3). In contrast, for the later lanthanides, Sm, Tb, Dy and Lu, the trans ratios showed significant differences depending on the irradiation wavelengths. Under high-energy irradiation, the trans isomers were favoured while under low energy irradiation, the cis isomers were preferentially formed. These variations might be explained by differences in the energy barriers and absorption cross-sections of the isomers. The photo-isomerisation process of these systems is complex, DFT and TD-DFT were performed to offer a preliminary orbital explanation.
cis ratios (Table 3). Kinetic studies on complex 6-Sm showed that the photo-stationary state (PSS) was reached after 30 min of irradiation, meaning the trans:cis ratio no longer changed. This behaviour was studied for all the 6-Ln (Ln = Y, La, Ce, Sm, Tb, Dy and Lu) complexes, and it varied depending on the irradiation wavelengths and metal ion. For the early lanthanides La and Ce, as well as Y, different wavelengths showed minor changes in the PSS ratios, consistently favouring the trans isomers (Table 3). In contrast, for the later lanthanides, Sm, Tb, Dy and Lu, the trans ratios showed significant differences depending on the irradiation wavelengths. Under high-energy irradiation, the trans isomers were favoured while under low energy irradiation, the cis isomers were preferentially formed. These variations might be explained by differences in the energy barriers and absorption cross-sections of the isomers. The photo-isomerisation process of these systems is complex, DFT and TD-DFT were performed to offer a preliminary orbital explanation.
| 6-Y-trans (%) | 6-La-trans (%) | 6-Ce-trans (%) | 6-Sm-trans (%) | 6-Tb-trans (%) | 6-Dy-trans (%) | 6-Lu-trans (%) | |
|---|---|---|---|---|---|---|---|
| NMR | 61 | 55 | 79 | 89 | 87 | 75 | 0 |
| XRD | 73 | 25 | 50 | 69 | 100 | 100 | 0 |
| PSS 370 nm | 96 | 93 | 97 | 64 | 78 | 76 | 69 |
| PSS 370 nm | 99 | 98 | 100 | 52 | 79 | 75 | 71 |
| PSS 427 nm | 97 | 99 | 94 | 28 | 50 | 48 | 29 |
| PSS 440 nm | 95 | 99 | 86 | 23 | 39 | — | 20 |
| PSS 450 nm | — | — | — | — | — | 33 | — |
| PSS 467 nm | 92 | 99 | 64 | 13 | 28 | — | 11 |
| PSS 525 nm | 90 | 98 | 88 | 12 | 1 | 6 | 9 |
Mono(cyclononatetraenyl) half-sandwich complexes
Very recently, Roesky et al. reported the first examples of trivalent lanthanide half-sandwich complexes comprising the planar Cnt ligand.43 Reaction of the well-soluble tris(borohydride) precursors [LnIII(BH4)3(thf)3] (Ln = La, Ce) and K(Cnt) in equimolar ratio at 60 °C in toluene led to the formation of the half-sandwich compounds 10-Ln (Ln = La, Ce; Scheme 10). This procedure only succeeded in the isolation of the respective compounds with the early and large lanthanides, LaIII and CeIII. Attempts to synthesise the corresponding SmIII complex using [SmIII(BH4)3(thf)3] and K(Cnt) yielded the divalent complex [SmII(η9-Cnt)2] (3-Sm) along with unidentifiable side products. Further attempts to achieve the mono(Cnt) compounds with the even smaller lanthanides DyIII and ErIII were also unsuccessful. Such different outcomes may be explained by the different ionic radii of the respective lanthanides. | ||
| Scheme 10 Synthesis of the half sandwich complexes 10-Ln and reaction between [SmIII(BH4)3(thf)3] and K(Cnt). | ||
Single crystals of 10-Ln were obtained from the concentrated toluene solutions at −10 °C in 66% and 72% yields for 10-La and 10-Ce. The two isostructural complexes with the general formula of [(η9-Cnt)LnIII(BH4)3(thf)] exhibit three-legged piano-stool-type structure motifs (Fig. 9). In these structures, the LnIII-ions are being η9-coordinated by the planar Cnt ring, two terminal BH4 ligands and one THF molecule. The molecular structures resemble similarities to that of the LnIII half sandwich borohydride compounds with the sterically-demanding silyl-functionalized Cot ligand.44 The binding mode of the Cnt ring was further confirmed by Raman spectroscopy, which show clearly assignable characteristic ring vibrations (νasym(η9-Cnt) = 1520 cm−1 (10-La), 1522 cm−1 (10-Ce); νsym(η9-Cnt) = 678 cm−1 (10-La), 679 cm−1 (10-Ce)) and (ν(Ln-Cnt) = 145 cm−1 (10-La), 140 cm−1 (10-Ce)). In the 1H NMR spectra, a singlet signal was detected for the Cnt protons at 7.01 ppm for the diamagnetic 10-La and at 5.41 ppm for the paramagnetic 10-Ce. The BH4 proton signals were found at 1.26–0.61 ppm for 10-La and at 35.2 ppm for 10-Ce.
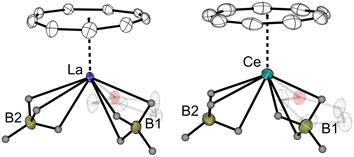 | ||
| Fig. 9 Molecular structures of [(η9-Cnt)LnIII(BH4)3(thf)] (10-Ln, Ln = La, Ce) in the solid state with thermal ellipsoids at 40% probability.† | ||
Solvation experiments revealed that dissolving 10-La and 10-Ce in warm THF led to the formation of the ionic species [LnIII(BH4)2(thf)5][Cnt] (11-Ln) (Ln = La, Ce, Scheme 11), in which the Cnt ligands decoordinate from the LnIII ions. Instead, five THF molecules are coordinated to the metal centres in their equatorial positions. The two terminal BH4 anions coordinate to the LnIII centres in their axial positions, resulting in cationic parts that exhibit pentagonal bipyramidal geometries. A similar displacement of the Cnt ligand by THF was also found for the super sandwich complexes [(Cnt)LnIII(η8-Cot)] (6-Ln).41 These results clearly demonstrate the labile coordination between the LnIII ions and the Cnt ligand, in comparison to the strong coordination with the hard THF donor. Additionally, the Cnt is also weaker bound to the LnIII ions compared to the smaller Cp ligand, as these compounds do not dissociate in THF. Interestingly, the displacement of Cnt by THF from 10-Ln to 11-Ln is fully reversible as the half-sandwich compounds 10-Ln were formed again after drying the crystals of 11-Ln under vacuum. This was proven by Raman spectroscopy of the dried crystals, indicating their potential use for switchable materials.
 | ||
| Scheme 11 Top left: Equilibrium between complexes 10-Ln and 11-Ln upon reaction with THF or drying under vacuum. Right: Polymeric complex 12-La formed after drying 10-La at 60 °C under vacuum and crystallisation from toluene. Bottom: Molecular structures of 11-La and 12-La in the solid state with thermal ellipsoids at 40% probability.† | ||
Furthermore, the THF in complex 10-La was fully removed after drying it under high vacuum at 60 °C for several days, which produced the poorly soluble polymeric species [LaIII(μ-η2:η2-BH4)2(η3-BH4)(η9-Cnt)] (12-La) in extremely low yield, as only a few single crystals were obtained from toluene (Scheme 10). The polymeric structure is best described as a trigonal-prismatic coordination polymer, in which each LaIII ion is coordinated by one Cnt ring (η9), one terminal BH4 ligand (η3), and two bridging BH4 (μ-η2:η2) ligands. NMR spectroscopy showed a single peak for the Cnt both in the 1H (δ 7.44 ppm) and 13C NMR (δ 113.4 ppm) spectra and confirmed the presence of the BH4 groups.
Tris(cyclononatetraenyl) rare-earth complexes
In 2022, Nocton et al. reported the reactions between the potassium salt K(Cnt) and LnI3 in toluene at room temperature, which led to the formation of a series of tris(Cnt) complexes 13-Ln (Ln = Y, Gd, Tb, Dy, Ho, Er, and Tm) with the medium-sized to smaller lanthanides (Scheme 12).45 A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio reaction is optimal, but 2
1 ratio reaction is optimal, but 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio reactions also gave the [LnIII(Cnt)3] products in lower yields. The reaction time and yield varied with the ionic radii of the lanthanides: for Y, Gd, Tb and Dy, the reaction takes three to four days with moderate yields of 33–37%. For Ho and Er, the reaction required two to three weeks, and the isolated yield is 21% for 13-Ho and 23% for 13-Er. In contrast, for the smaller Tm, the reaction proceeded for six weeks and the obtained yield of 13-Tm was only 15%. When SmI3(thf)4 or YbI3(thf)3 were employed in the reaction with K(Cnt), spontaneous reduction occurred and the bis(Cnt) complexes [LnII(Cnt)2] (3-Ln) were formed (Scheme 12). Attempts to use oxidizing agents (AgI or FeCl3) to obtain the desired [LnIII(Cnt)3] (Ln = Sm, Eu) were unsuccessful. Steric factors might partly explain this reduction, but the higher redox potential of [LnII(Cnt)2] compared to [LnII(Cp*)2] might also be a reason that facilitates the reduction. Moreover, electronic contributions should also not be neglected, as the net valence of the Ln metal in [Sm(Cp)3] and [Yb(Cp)3] is not an integer but consists of multiconfigurational intermediate states.
1 ratio reactions also gave the [LnIII(Cnt)3] products in lower yields. The reaction time and yield varied with the ionic radii of the lanthanides: for Y, Gd, Tb and Dy, the reaction takes three to four days with moderate yields of 33–37%. For Ho and Er, the reaction required two to three weeks, and the isolated yield is 21% for 13-Ho and 23% for 13-Er. In contrast, for the smaller Tm, the reaction proceeded for six weeks and the obtained yield of 13-Tm was only 15%. When SmI3(thf)4 or YbI3(thf)3 were employed in the reaction with K(Cnt), spontaneous reduction occurred and the bis(Cnt) complexes [LnII(Cnt)2] (3-Ln) were formed (Scheme 12). Attempts to use oxidizing agents (AgI or FeCl3) to obtain the desired [LnIII(Cnt)3] (Ln = Sm, Eu) were unsuccessful. Steric factors might partly explain this reduction, but the higher redox potential of [LnII(Cnt)2] compared to [LnII(Cp*)2] might also be a reason that facilitates the reduction. Moreover, electronic contributions should also not be neglected, as the net valence of the Ln metal in [Sm(Cp)3] and [Yb(Cp)3] is not an integer but consists of multiconfigurational intermediate states.
 | ||
| Scheme 12 Synthesis of the tris(Cnt) complexes 13-Ln and reaction between K(Cnt) and LnI3 (Ln = Sm, Yb), leading to [LnII(Cnt)2] (3-Sm and 3-Yb). | ||
SCXRD analysis revealed that all the complexes 13-Ln (Y-Er) are isostructural (Fig. 10). Instead of the typical η9-coordination modes in the bis(Cnt) sandwich type complexes 3-Ln, all the Cnt ligands are consistently η4-coordinating to the LnIII centres. Despite significant bending of the Cnt ligands around the LnIII ions, their aromaticity remains intact, without significant changes in C–C bond distances or C(sp2) hybridisation. There are interesting trends which nicely correlate with the ionic radii along the lanthanide series (Table 4): The shortest Ln–C bond was found to be 2.63(2) Å for the gadolinium complex 13-Gd and 2.54(1) Å for the thulium complex 13-Tm. A similar trend was observed for the average Ln–C4 distance, reflecting the lanthanide contraction along the series. Overall, the average Ln–C9 distance does not show any obvious trend and is rather similar for all seven compounds, suggesting a dynamic coordination behaviour of the Cnt ligand. An increase in LnIII size pushes the closest C atoms away but brings the farthest ones closer.
 | ||
| Fig. 10 Molecular structures of [LnIII(Cnt)3] (13-Ln, Ln = Y, Gd, Tb, Dy, Er, Ho, Tm) in the solid state with thermal ellipsoids at 40% probability.† | ||
| 13-Y | 13-Gd | 13-Tb | 13-Dy | 13-Ho | 13-Er | 13-Tm | |
|---|---|---|---|---|---|---|---|
| Distances are given in Å.a Average of the nine C atoms in the Cnt ring.b Average of the four closest C atoms in the Cnt ring. | |||||||
| Ln–C (closest) | 2.58(1) | 2.63(2) | 2.61(2) | 2.58(3) | 2.59(2) | 2.56(2) | 2.54(1) |
| Ln–C (farthest) | 4.40(18) | 4.41(18) | 4.40(19) | 4.41(18) | 4.42(18) | 4.43(18) | 4.48(3) |
| Ln–C9 (av.)a | 3.35(7) | 3.38(9) | 3.37(10) | 3.37(10) | 3.37(11) | 3.37(10) | 3.40(3) |
| Ln–C4 (av.)b | 2.69(14) | 2.73(12) | 2.71(13) | 2.70(14) | 2.69(14) | 2.69(15) | 2.57(16) |
Due to the lability of the Cnt ligand in THF, 1H NMR spectra were recorded for all 13-Ln complexes in toluene-d8 to ensure their stability. For the Gd complex 13-Gd, the 1H NMR spectrum remained silent. For the diamagnetic Y complex 13-Y, only one singlet signal for the Cnt protons was found at δ 6.27 ppm. For all the other paramagnetic Ln species, this Cnt proton signal appeared as broad singlet at δ 295.5 ppm (13-Tb), δ 287.5 ppm (13-Dy), δ 273.0 ppm (13-Ho), δ 226.5 ppm (13-Er) and δ 264.3 ppm (13-Tm), showing a clear trend along the Ln series. The coordination environment is not temperature dependent, as the VT 1H NMR of 13-Y showed little change from −80 °C to 80 °C. According to UV-vis spectroscopy, typical ligand-based π to π* transitions were detected for all complexes in the visible region, with 13-Er and 13-Tm exhibiting hypersensitive f–f transitions. An additional charge-transfer band was observed for 13-Tm. Magnetic susceptibility measurements for all paramagnetic complexes 13-Ln matched well with the nature of the trivalent LnIII ions. None of the tris(Cnt) complexes displayed single molecule magnet behaviour. Computational studies further interpreted the electronic structures and the magnetic behaviour, suggesting that the Cnt ligands in 13-Ln provide a spherical crystal field.
Summary and outlook
Compared to the classical smaller aromatic carbocyclic ligands, the 10π-electron cyclononatetraenyl (Cnt) monoanion has been part of the organolanthanide chemistry for less than ten years. Since its first coordination towards Ln in 2017, the chemistry of Cnt has expanded rapidly in the area of the lanthanides. Whilst classical homoleptic and heteroleptic sandwich-type complexes can be obtained using Cnt ligands, the large diameter and low charge density make the Cnt ligand more flexible and labile in coordination chemistry. For instance, the hapticity is often lower than η9, or the Cnt ligand can be easily displaced from the metal centre using coordinating solvents. This feature initially seems disadvantageous but opens the door for applications as switchable materials (e.g., luminophores, SMMs). The successful integration of the Cnt ligands with divalent and trivalent lanthanides open new avenues in lanthanide chemistry, the reported works clearly showcase that the Cnt ligand provides structures and reactivities which differ significantly from those of the classical Cp and Cot ligands in lanthanide chemistry. Currently, only the unsubstituted Cnt ligand has been utilised. One possible future direction is to introduce different substituents on the ring to enhance the solubility and modify the electronic and steric properties, similar as described for the well-known Cot ligand.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through the Collaborative Research Centre “4f for Future” (CRC 1573 project number 471424360, project C1).Notes and references
- M. Faraday, Philos. Trans. R. Soc., 1825, 115, 440–466 CrossRef.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS.
- E. Hückel, Z. Phys., 1931, 70, 204–286 CrossRef.
- T. J. Kealy and P. L. Pauson, Nature, 1951, 168, 1039–1040 CrossRef CAS.
- G. Wilkinson, M. Rosenblum, M. C. Whiting and R. B. Woodward, J. Am. Chem. Soc., 1952, 74, 2125–2126 CrossRef CAS.
- G. Wilkinson and J. M. Birmingham, J. Am. Chem. Soc., 1954, 76, 6210 CrossRef CAS.
- A. Streitwieser and U. Mueller-Westerhoff, J. Am. Chem. Soc., 1968, 90, 7364 CrossRef CAS.
- F. Mares, K. Hodgson and A. Streitwieser, J. Organomet. Chem., 1970, 24, C68–C70 CrossRef CAS.
- J. G. Brennan, F. G. N. Cloke, A. A. Sameh and A. Zalkin, J. Chem. Soc., Chem. Commun., 1987, 1668–1669 RSC.
- J. P. Durrant, B. M. Day, J. Tang, A. Mansikkamäki and R. A. Layfield, Angew. Chem., Int. Ed., 2022, 61, e202200525 CrossRef CAS PubMed.
- B. M. Day, F.-S. Guo, S. R. Giblin, A. Sekiguchi, A. Mansikkamäki and R. A. Layfield, Chem. Eur. J., 2018, 24, 16779–16782 CrossRef CAS PubMed.
- L. Münzfeld, A. Hauser, P. Hädinger, F. Weigend and P. W. Roesky, Angew. Chem., Int. Ed., 2021, 60, 24493–24499 CrossRef.
- L. Münzfeld, S. Gillhuber, A. Hauser, S. Lebedkin, P. Hädinger, N. D. Knöfel, C. Zovko, M. T. Gamer, F. Weigend, M. M. Kappes and P. W. Roesky, Nature, 2023, 620, 92–96 CrossRef PubMed.
- F. T. Edelmann, New J. Chem., 2011, 35, 517–528 RSC.
- K. L. M. Harriman, J. J. Le Roy, L. Ungur, R. J. Holmberg, I. Korobkov and M. Murugesu, Chem. Sci., 2017, 8, 231–240 RSC.
- A. Hauser, L. Münzfeld, S. Schlittenhardt, C. Uhlmann, L. Leyen, E. Moreno-Pineda, M. Ruben and P. W. Roesky, J. Am. Chem. Soc., 2024, 146, 13760–13769 CrossRef CAS.
- T. Arliguie, M. Lance, M. Nierlich and M. Ephritikhine, J. Chem. Soc., Dalton Trans., 1997, 2501–2504 RSC.
- K. Kawasaki, R. Sugiyama, T. Tsuji, T. Iwasa, H. Tsunoyama, Y. Mizuhata, N. Tokitoh and A. Nakajima, Chem. Commun., 2017, 53, 6557–6560 RSC.
- T. J. Katz and P. J. Garratt, J. Am. Chem. Soc., 1963, 85, 2852–2853 CrossRef CAS.
- E. A. Lalancette and R. E. Benson, J. Am. Chem. Soc., 1963, 85, 2853 CrossRef CAS.
- P. Radlick and G. Alford, J. Am. Chem. Soc., 1969, 91, 6529–6530 CrossRef CAS.
- G. Boche and F. Heidenhain, J. Organomet. Chem., 1976, 121, C49–C51 CrossRef CAS.
- H. T. Verkouw, M. E. E. Veldman, C. J. Groenenboom, H. O. Van Oven and H. J. De Leifde Meijer, J. Organomet. Chem., 1975, 102, 49–56 CrossRef CAS.
- A. Westerhof and H. J. De Liefde Meijer, J. Organomet. Chem., 1978, 149, 321–325 CrossRef CAS.
- T. Murahashi, R. Inoue, K. Usui and S. Ogoshi, J. Am. Chem. Soc., 2009, 131, 9888–9889 CrossRef CAS.
- M. Xémard, S. Zimmer, M. Cordier, V. Goudy, L. Ricard, C. Clavaguéra and G. Nocton, J. Am. Chem. Soc., 2018, 140, 14433–14439 CrossRef.
- L. Münzfeld, C. Schoo, S. Bestgen, E. Moreno-Pineda, R. Köppe, M. Ruben and P. W. Roesky, Nat. Commun., 2019, 10, 3135 CrossRef.
- G. Boche and A. Bieberbach, Chem. Ber., 1978, 111, 2850–2858 CrossRef CAS.
- F. Mares, K. O. Hodgson and A. Streitwieser, J. Organomet. Chem., 1971, 28, C24–C26 CrossRef CAS.
- M. D. Walter, G. Wolmershäuser and H. Sitzmann, J. Am. Chem. Soc., 2005, 127, 17494–17503 CrossRef CAS.
- S. Harder, D. Naglav, C. Ruspic, C. Wickleder, M. Adlung, W. Hermes, M. Eul, R. Pöttgen, D. B. Rego, F. Poineau, K. R. Czerwinski, R. H. Herber and I. Nowik, Chem. Eur. J., 2013, 19, 12272–12280 CrossRef CAS.
- R. P. Kelly, T. D. M. Bell, R. P. Cox, D. P. Daniels, G. B. Deacon, F. Jaroschik, P. C. Junk, X. F. Le Goff, G. Lemercier, A. Martinez, J. Wang and D. Werner, Organometallics, 2015, 34, 5624–5636 CrossRef CAS.
- T. Tsuji, S. Fukazawa, R. Sugiyama, K. Kawasaki, T. Iwasa, H. Tsunoyama, N. Tokitoh and A. Nakajima, Chem. Phys. Lett., 2014, 595–596, 144–150 CrossRef CAS.
- H. Ramanantoanina, L. Merzoud, J. T. Muya, H. Chermette and C. Daul, J. Phys. Chem. A, 2020, 124, 152–164 CrossRef CAS.
- T. Vitova, H. Ramanantoanina, B. Schacherl, L. Münzfeld, A. Hauser, R. S. K. Ekanayake, C. Y. Reitz, T. Prüßmann, T. S. Neill, J. Göttlicher, R. Steininger, V. A. Saveleva, M. W. Haverkort and P. W. Roesky, J. Am. Chem. Soc., 2024, 146, 20577–20583 CrossRef CAS PubMed.
- K. O. Hodgson, F. Mares, D. F. Starks and A. Streitwieser, J. Am. Chem. Soc., 1973, 95, 8650–8658 CrossRef CAS.
- K. Mashima, Y. Nakayama, A. Nakamura, N. Kanehisa, Y. Kai and H. Takaya, J. Organomet. Chem., 1994, 473, 85–91 CrossRef CAS.
- M. Tricoire, L. Münzfeld, J. Moutet, N. Mahieu, L. La Droitte, E. Moreno-Pineda, F. Gendron, J. D. Hilgar, J. D. Rinehart, M. Ruben, B. Le Guennic, O. Cador, P. W. Roesky and G. Nocton, Chem. – Eur. J., 2021, 27, 13558–13567 CrossRef CAS.
- G. Boche, D. Martens and W. Danzer, Angew. Chem., Int. Ed. Engl., 1969, 8, 984 CrossRef CAS.
- G. Boche, H. Weber, D. Martens and A. Bieberbach, Chem. Ber., 1978, 111, 2480–2496 CrossRef CAS.
- L. Münzfeld, M. Dahlen, A. Hauser, N. Mahieu, S. K. Kuppusamy, J. Moutet, M. Tricoire, R. Köppe, L. La Droitte, O. Cador, B. Le Guennic, G. Nocton, E. Moreno-Pineda, M. Ruben and P. W. Roesky, Angew. Chem., Int. Ed., 2023, 62, e202218107 CrossRef.
- L. Pedussaut, N. Mahieu, C. Chartier, T. Rajeshkumar, I. Douair, N. Casaretto, L. Maron, G. Danoun and G. Nocton, Chem. Sci., 2024, 15, 19273–19282 RSC.
- L. Münzfeld, A. Hauser, M. T. Gamer and P. W. Roesky, Chem. Commun., 2023, 59, 9070–9073 RSC.
- L. Münzfeld, X. Sun, S. Schlittenhardt, C. Schoo, A. Hauser, S. Gillhuber, F. Weigend, M. Ruben and P. W. Roesky, Chem. Sci., 2022, 13, 945–954 RSC.
- O. Stetsiuk, L. La Droitte, V. Goudy, B. Le Guennic, O. Cador and G. Nocton, Organometallics, 2022, 41, 133–140 CrossRef CAS.
Footnote |
| † Reproduced from the CIF file having CCDC numbers of 1861445 (KCnt·Et2O), 1894445 (KCnt·(dme)2), 1861447 (3-Sm), 1544973 (3-Eu), 1861450 (3-Tm), 1861446 (3-Tm), 1894447 (6-Nd), 1894448 (6-Sm), 2073514, 2073526 (6-Tb), 2073516 (6-Dy), 2073518 (6-Ho), 2073520 (6-Er), 2073522 (6-Tm), 2073525 (6-Lu), 2125024 (7-La), 2125026 (8-Ce), 2125029 (9-Er), 2267628 (10-La), 2267629 (10-Ce), 2267630 (11-La), 2267631 (11-Ce), 2267632 (12-La), 2113686 (13-Y), 2113687 (13-Gd), 2113688 (13-Tb), 2113689 (13-Dy), 2113691 (13-Er), 2113690 (13-Ho), 2113692 (13-Tm), 2370453 (6-Y-trans), 2370455 (6-La-trans), 2370457 (6-Ce-trans), 2370459 (6-Pr-trans), 2370461 (6-Nd-trans), 2370463 (6-Sm-trans), 2370465 (6-Gd-trans), 2370466 (6-Tb-trans), 2370467 (6-Dy-trans), 2370468 (6-Ho-trans). |
| This journal is © The Royal Society of Chemistry 2025 |