 Open Access Article
Open Access ArticleThermal management challenges in lithium-ion batteries: understanding heat generation mechanisms†
Kenza
Maher
 *a,
Ameni
Boumaiza
a and
Ruhul
Amin
*a,
Ameni
Boumaiza
a and
Ruhul
Amin
 *b
*b
aQatar Environment and Energy Research Institute (QEERI), Hamad Bin Khalifa University (HBKU), Qatar Foundation (QF), Doha, Qatar. E-mail: kmaher@hbku.edu.qa
bEnergy and Transportation Science Division, Oak Ridge National Laboratory, Oak Ridge, TN, USA. E-mail: aminr@ornl.gov
First published on 7th February 2025
Abstract
This paper investigates heat generation in commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium-ion battery cells and the thermal management challenges from their high energy density and electrochemical processes. Thermal effects can degrade performance, accelerate aging, and increase thermal runaway risk. Using isothermal calorimetry and EIS, the study emphasizes optimizing thermal behavior to improve battery efficiency, safety, and durability.
650 lithium-ion battery cells and the thermal management challenges from their high energy density and electrochemical processes. Thermal effects can degrade performance, accelerate aging, and increase thermal runaway risk. Using isothermal calorimetry and EIS, the study emphasizes optimizing thermal behavior to improve battery efficiency, safety, and durability.
As the demand for high-performance lithium-ion batteries (LIBs) continues to rise, particularly in electric vehicles (EVs), electric vertical takeoff and landing (EVTOL) vehicles, and large-scale energy storage systems, managing thermal behavior has become a critical challenge.1–3 LIBs are valued for their high energy density, long lifespan, and efficiency, making them the dominant energy storage technology in modern applications.3,4 However, their performance, safety, and longevity are directly influenced by their ability to handle the heat generated during the charge–discharge process and rest cycles.5–8 Improper thermal management can lead to capacity degradation, reduced efficiency, accelerated aging, and, in extreme cases, catastrophic safety hazards such as thermal runaway.9–12 Addressing these thermal challenges is essential to ensure the safe and reliable operation of LIBs across a wide range of demanding applications.13–16
Lithium-ion batteries’ thermal behavior is influenced by internal and external factors, such as ambient temperature, charge and discharge rates, and the state of charge (SOC).17 Elevated temperatures can significantly degrade battery performance, reduce capacity, and compromise the battery's overall lifespan.18,19 Proper thermal management is crucial in mitigating these effects and ensuring the long-term efficiency and safety of lithium-ion battery systems, especially in high-temperature environments or during intense operational conditions.20,21
Heat generation in lithium-ion batteries is a complex phenomenon involving various electrochemical, physical, and chemical processes, which can be categorized into reversible and irreversible heat generation. Reversible heat is linked to entropy changes during charge and discharge cycles.22 In contrast, irreversible heat arises from overpotential, including ohmic losses, charge transfer resistances at the interface, and mass transfer limitations.23 Chemical degradation also contributes to heat generation. Repeated cycling causes the electrodes and electrolyte to degrade, leading to side reactions like electrolyte breakdown or gas formation, which increase internal resistance and heat. These effects are more pronounced at high charge/discharge rates or elevated temperatures, accelerating material degradation and worsening thermal management. As side reactions intensify, they heighten internal resistance, raising heat generation and safety risks. Understanding these heat generation mechanisms, both electrochemical and chemical, is essential for developing effective thermal management strategies that enhance battery performance and safety.8,24–26
This paper investigates the key factors contributing to heat generation in lithium-ion batteries, including charge and discharge rates, operating temperatures, and state of charge/discharge. By employing a combination of analytical methods such as isothermal calorimetry and electrochemical impedance spectroscopy (EIS), we seek to provide a deeper insight into the understanding of optimizing thermal performance and mitigating the risks of thermal runaway and other safety concerns associated with lithium-ion batteries.
Fig. 1 presents the voltage and heat generation profiles of commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 LIB cells at a rate of 0.05C and a temperature of 30 °C during charge and discharge. The voltage and heat flow are measured over time using an isothermal calorimeter. This figure shows the heat generation and cell voltage as a function of time, with heat generation depicted on the left axis and voltage on the right axis. Table S1 (ESI†) summarizes the specifications and characteristics of these cells, as outlined in their technical data sheets.
650 LIB cells at a rate of 0.05C and a temperature of 30 °C during charge and discharge. The voltage and heat flow are measured over time using an isothermal calorimeter. This figure shows the heat generation and cell voltage as a function of time, with heat generation depicted on the left axis and voltage on the right axis. Table S1 (ESI†) summarizes the specifications and characteristics of these cells, as outlined in their technical data sheets.
 | ||
Fig. 1 Voltage and heat generation of commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium-ion battery cell at 0.05C and a temperature of 30 °C during charge and discharge. 650 lithium-ion battery cell at 0.05C and a temperature of 30 °C during charge and discharge. | ||
The heat generation fluctuates between negative and positive values, beginning with an initial decline and a steady rise. Starting at approximately 3.2 V, the voltage curve gradually increases as the cell progresses through the charge cycle. The variations in heat generation throughout the cycle reflect the combined effects of reversible and irreversible processes based on (1).
| Qt = Qr + Qirr | (1) |
At the beginning of the cycle, the negative heat generation profile shows negative values due to endothermic reactions, primarily from entropy changes during initial lithium-ion interaction into the anode. As the cycle progresses, heat generation turns positive, reflecting exothermic processes like Joule heating, charge transfer resistance, and other losses. The interplay of endothermic and exothermic reactions highlights the complex thermal behavior of the lithium-ion battery cells during cycling, influenced by both reversible and irreversible thermal effects. At a certain SOC/SOD, the cell resistance and entropy changes happened dynamically. The cell resistance and entropy alternation have different impacts on heat generation (reversible and irreversible heat).
The difference in heat generation characteristics between charge and discharge can be explained by the interaction between irreversible heat and reversible heat from entropy changes. Irreversible heat, caused by resistive losses, cell polarization, and overpotential, becomes more significant toward the end of the discharge, where polarization is at its peak. On the other hand, reversible heat is associated with entropy changes during lithium-ion intercalation and deintercalation, especially in the mid-state of charge. These entropy changes can either absorb or release heat, depending on the reaction direction, resulting in shifts in the sign of heat generation. The phenomenon has also been reported in our previous paper.8,14 The balance between irreversible and reversible heat is key to understanding the thermal behavior of lithium-ion cells.
Fig. 2 compares lithium-ion battery cell heat generation profiles as a function of time during charge and discharge cycles at three different temperatures: 20 °C, 30 °C, and 40 °C.
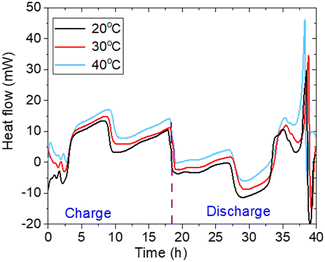 | ||
| Fig. 2 Comparison of heat generation profiles at 0.05C rate and three different temperatures (20 °C, 30 °C, 40 °C) as a function of time. | ||
At 20 °C, the heat generation starts with a slight endothermic reaction (negative heat generation) during the initial phase of charge, followed by an increase in exothermic heat generation as the cycle progresses. The heat generation then becomes positive, reaching a peak and maintaining a consistent exothermic pattern throughout the rest of the charge cycle.
At 30 °C, the heat generation profile follows a similar pattern to that at 20 °C, but the magnitude of heat generation is slightly higher. This increase in heat generation is due to the elevated temperature, which enhances the exothermic reactions within the battery. As the temperature rises, the thermal activity within the battery becomes more pronounced.
At 40 °C, heat generation shows a more pronounced exothermic behavior during both charge and discharge cycles. The transition from endothermic to exothermic heat generation during charging occurs more rapidly than at lower temperatures. The overall heat generation rate is higher throughout the entire cycle, reflecting the impact of the elevated temperature on the battery's thermal dynamics.
The discharge process also exhibits increased heat generation, reflecting more significant thermal losses and resistive heating effects at this elevated temperature.
Towards the end of the discharge cycle, heat generation increases sharply at all temperatures due to the dominance of irreversible heat. This significant rise occurs primarily because irreversible heat generation surpasses reversible heat generation,27,28 leading to a considerable thermal increase.
The differences in heat generation profiles at various temperatures highlight the critical role of thermal conditions on the battery's thermal management and overall performance. As the temperature increases, the heat generation during charge and discharge becomes more pronounced, influencing the battery's efficiency, longevity, and safety.
Fig. 3 compares heat generation profiles for lithium-ion batteries operating at two charge rates, 0.5C and 1C, measured at 30 °C.
The maximum heat generation rates are recorded at 325.2 mW for the 0.5C rate and 365.4 mW for the 1C rate, indicating a significant increase in heat generation with higher charge rates. Both curves exhibit a peak in heat generation around 30% SOC, highlighting phase changes during the charging process where thermodynamic changes intensify, predominantly due to entropy changes associated with electrochemical reactions. The results demonstrate that irreversible heat generation becomes the dominant factor at higher C-rates, driven by non-equilibrium processes that lead to increased ohmic losses and other irreversible contributions.8 This is particularly relevant at the midpoint of the charge process, where the maximum entropy change occurs, correlating with a notable rise in heat generation. Overall, the total heat generation results from reversible and irreversible processes. Understanding the balance between reversible and irreversible heat generation is essential for optimizing thermal management in lithium-ion batteries, ensuring their performance, safety, and longevity. These findings highlight the importance of effective thermal management strategies to prevent thermal-related issues in real-world applications.
To address this, advanced thermal management systems, such as phase change materials, liquid cooling, and high-performance heat sinks,29 can be implemented to dissipate excess heat efficiently. In addition, battery designs that promote uniform temperature distribution and use materials with higher thermal stability can help reduce thermal risks.
Further enhancing safety involves integrating thermal runaway prevention mechanisms, such as shutdown separators, flame-retardant additives, and advanced battery management systems (BMS) with real-time monitoring and predictive algorithms.30
Research into innovative materials, including solid-state electrolytes and thermally stable cathode materials,31 combined with proper usage guidelines and safety protocols, can significantly mitigate thermal runaway risks, ensuring lithium-ion batteries’ long-term safety, reliability, and performance.
EIS measurements are performed to analyze the dynamic characteristics of commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium-ion cells. The impedance spectra are shown in Fig. S1 (ESI†).
650 lithium-ion cells. The impedance spectra are shown in Fig. S1 (ESI†).
Fig. 4 illustrates the behavior of different resistance components, including Ohmic resistance (R1) and charge transfer resistances (R2 and R3) as functions of SOC and DOD.
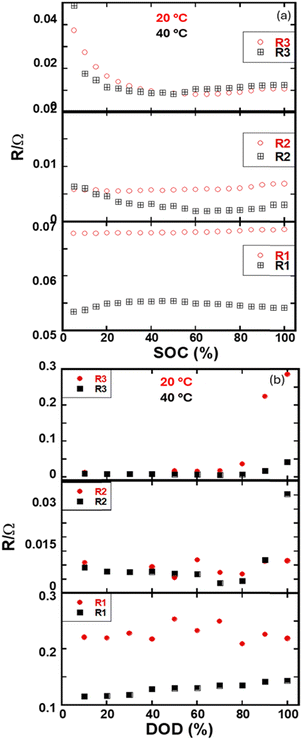 | ||
| Fig. 4 Individual resistance components at two different temperatures, 20 °C and 40 °C, as a function of (a) SOC and (b) DOD. | ||
These resistance elements are deconvoluted using the equivalent circuit model in Fig. S2 (ESI†).
R1 represents electrolyte resistance and remains stable across SOC and DOD but is lower at 40 °C than at 20 °C due to increased ionic mobility and reduced electrolyte viscosity at higher temperatures. Reduced thermal energy and a thicker SEI layer hinder ion transport at lower temperatures, causing higher resistance.
R2, associated with electrochemical reactions at the electrode–electrolyte interface, shows a slight increase at 20 °C due to slower reaction kinetics and reduced ionic mobility, which leads to less effective charge transfer. At 40 °C, enhanced thermal energy improves ionic mobility and reaction rates, lowering R2. This resistance is mainly related to the electrolyte-graphite interface, with better performance observed at higher temperatures.
As the cathode is less electronically conductive, the graphite anode capacitance value is higher at this interface, and the lower frequency impedance spectra are related to R3. It can be seen from Fig. 4 that the R3 value gradually decreases at the beginning of charging the battery, and then it is leveled up. At the beginning of the charge process, the electronic conductivity of active materials increases.32,33 The flux of charge premises at the contact surface is reduced, and interfacial resistance is decreased. After that, it remains constant and further increases the state of change or discharge. However, the R3 value does not vary significantly between the two measured temperatures (20 °C and 40 °C). However, at lower SOC values and higher DOD, R3 increases due to the slower diffusion of ions, particularly at 20 °C.
This study highlights the critical importance of thermal management in lithium-ion batteries, focusing on heat generation mechanisms in commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium-ion battery cells. It shows that reversible heat from entropy changes irreversible heat from ohmic losses, and charge transfer resistance significantly affects battery performance, safety, and lifespan. Elevated temperatures increase heat generation, accelerating capacity degradation and aging, with thermal runaway risks. Isothermal calorimetry and impedance spectroscopy findings reveal temperature's impact on resistance components and battery efficiency. Addressing these thermal challenges is essential for improving battery safety and reliability, with implications for the future of lithium-ion technology in high-demand applications.
650 lithium-ion battery cells. It shows that reversible heat from entropy changes irreversible heat from ohmic losses, and charge transfer resistance significantly affects battery performance, safety, and lifespan. Elevated temperatures increase heat generation, accelerating capacity degradation and aging, with thermal runaway risks. Isothermal calorimetry and impedance spectroscopy findings reveal temperature's impact on resistance components and battery efficiency. Addressing these thermal challenges is essential for improving battery safety and reliability, with implications for the future of lithium-ion technology in high-demand applications.
K. Maher: led the manuscript writing, conducted the experimental heat generation and data analysis work, and provided critical revisions. A. Boumaiza: participated in analyzing the thermal data and contributed to developing and writing the manuscript sections related to heat generation mechanisms. R. Amin: provided expertise in Electrochemical Impedance Spectroscopy (EIS), conducted the experimental work on EIS, and participated in interpreting the EIS results and manuscript writing.
Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- D. H. Doughty, P. C. Butler, R. G. Jungst and E. P. Roth, J. Power Sources, 2002, 110, 357–363 CrossRef CAS.
- S. S. Zhang, J. Power Sources, 2006, 161, 1385–1391 CrossRef CAS.
- F. M. Nizam Uddin Khan, M. G. Rasul, A. S. M. Sayem and N. K. Mandal, J. Energy Storage, 2023, 71, 108033 CrossRef.
- Z. Lin, D. Li and Y. Zou, J. Energy Storage, 2023, 74, 109386 CrossRef.
- A. M. Divakaran, D. Hamilton, K. N. Manjunatha and M. Minakshi, Energies, 2020, 13, 1477 CrossRef CAS.
- S. Durgam, P. Datir, O. Tawase, D. Savant, G. Tapkir, R. M. Warkhedkar and N. M. Gawai, IOP Conf. Ser.: Mater. Sci. Eng., 2021, 1126, 012072 CAS.
- A. Gharehghani, M. Rabiei, S. Mehranfar, S. Saeedipour, A. M. Andwari, A. García and C. M. Reche, Renewable Sustainable Energy Rev., 2024, 202, 114654 CrossRef.
- K. Maher, A. Boumaiza and R. Amin, J. Power Sources, 2024, 623, 235504 CrossRef CAS.
- J. Zhang, L. Zhang, F. Sun and Z. Wang, IEEE Access, 2018, 6, 23848–23863 Search PubMed.
- S. S. Madani, C. Ziebert and M. Marzband, Symmetry, 2023, 15, 1925 CrossRef CAS.
- D. Ouyang, M. Chen, Q. Huang, J. Weng, Z. Wang and J. Wang, Appl. Sci., 2019, 9, 2483 CrossRef CAS.
- P. M. Sutheesh, A. P. Atul, R. B. Nichit and R. Bandaru, Int. Commun. Heat Mass Transfer, 2024, 159, 107983 CrossRef CAS.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li and N. Tavajohi, J. Energy Chem., 2021, 59, 83–99 CrossRef CAS.
- K. Maher, 2023 6th Int. Conf. Renewable Energy Power Eng., 2023, pp. 156–162.
- J. V. Rao Vajja, A. Serov, M. Sudarshan, M. Singh and V. Tomar, Batteries, 2024, 10, 355 CrossRef.
- K. Maher and A. Boumaiza, 2024 7th International Conference on Renewable Energy and Power Engineering, 2024 DOI:10.1109/REPE62578.2024.10810068.
- Y. Huang, Y. Zhao, W. Bai, Y. Cao, W. Xu, X. Shen and Z. Wang, Process Saf. Environ. Prot., 2024, 191, 1483–1494 CrossRef CAS.
- S. Ma, M. Jiang, P. Tao, C. Song, J. Wu, J. Wang, T. Deng and W. Shang, Prog. Nat. Sci.: Mater. Int., 2018, 28, 653–666 CrossRef CAS.
- K. Maher, P. Kubiak and Z. Cen, 2023 6th Int. Conf. Renewable Energy Power Eng., 2023, 300–304.
- A. Gharehghani, M. Rabiei, S. Mehranfar, S. Saeedipour, A. M. Andwari, A. García and C. M. Reche, Renewable Sustainable Energy Rev., 2024, 202, 114654 CrossRef.
- S. Chavan, B. Venkateswarlu, M. Salman, J. Liu, P. Pawar, S. W. Joo, G. S. Choi and S. C. Kim, Int. J. Heat Mass Transfer, 2024, 232, 125918 CrossRef CAS.
- K. E. Thomas, J. Newman and R. M. Darling, Advances in Lithium-Ion Batteries, 2007, pp. 345–392 Search PubMed.
- D. Bernardi, E. Pawlikowski and J. Newman, J. Electrochem. Soc., 1985, 132, 5 CrossRef CAS.
- E. Schuster, C. Ziebert, A. Melcher, M. Rohde and H. J. Seifert, J. Power Sources, 2015, 286, 580–589 CrossRef CAS.
- L. Kraft, A. Hoefling, T. Zünd, A. Kunz, M. Steinhardt, J. Tübke and A. Jossen, J. Electrochem. Soc., 2021, 168, 053505 CrossRef CAS.
- S. Oh, S. Park and K. Yoo, J. Energy Storage, 2024, 75, 109678 CrossRef.
- Y. Saito, M. Shikano and H. Kobayashi, J. Power Sources, 2013, 244, 294–299 CrossRef CAS.
- M. Balasundaram, V. Ramar, C. Yap, L. Li, A. A. O. Tay and P. Balaya, J. Power Sources, 2016, 328, 413–421 CrossRef CAS.
- Z. Maqbool, M. Hanief and M. Parveez, J. Energy Storage, 2023, 60, 106591 CrossRef.
- L. Kong, Y. Li and W. Feng, Electrochem. Energy Rev., 2021, 4, 633–679 Search PubMed.
- H. Pourzolfaghar, P. Y. Wang, X. Y. Jiang, S. Kositsarakhom, W. Jirasupcharoen, C. Suwantri, D. Jyothi, K. Prabhakaran and Y. Y. Li, Chem. Eng. J., 2024, 500, 157394 Search PubMed.
- R. Amin and I. Belharouk, J. Power Sources, 2017, 348, 311–317 CrossRef CAS.
- P. R. Kumar, A. Kheireddine, U. Nisar, R. A. Shakoor, R. Essehli, R. Amin and I. Belharouak, J. Power Sources, 2019, 429, 149–155 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05619a |
| This journal is © The Royal Society of Chemistry 2025 |

