Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pressure regulated CO2 electrolysis on two-dimensional Bi2O2Se†
Ruofan
Sun‡
,
Jiwu
Zhao‡
,
Hang
Liu
 ,
Yanrong
Xue
and
Xu
Lu
,
Yanrong
Xue
and
Xu
Lu
 *
*
Division of Physical Science and Engineering (PSE), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Kingdom of Saudi Arabia
First published on 2nd January 2025
Abstract
The electrochemical reduction of carbon dioxide (CO2RR) offers potential for sustainable production and greenhouse gas mitigation, particularly with renewable energy integration. However, its widespread application is hindered by expensive catalysts, low selectivity, and limited current density. This study addresses these challenges by developing a low-mass-loading two-dimensional (2D) Bi2O2Se catalyst via chemical vapor deposition (CVD). The catalyst achieves a formate faradaic efficiency (FE) of 47.1% with a high current density of 4649 mA mg−1 at −1.15 V (vs. RHE), significantly outperforming bulk Bi2O2Se. Pressurizing CO2, a condition commonly encountered in industrial processes, further enhances formate selectivity and current density, increasing from 2189 mA mg−1 at ambient pressure (1.01 bar) to 7457 mA mg−1 at 40 bar. In situ Raman spectroscopy and DFT calculations reveal the intermediates and pathways involved, underscoring the critical role of pressure in regulating CO2RR pathways. These findings highlight the potential of 2D catalysts for sustainable and industrially relevant CO2 conversion under high pressure.
Production of value-added chemicals via renewable-driven electrochemical reduction of CO2 (CO2RR) holds promise to deal with the pressing global warming and energy crisis.1–3 To date, the predominant focus of CO2RR research lies in examining its performance under ambient pressure conditions, while industrial CO2 is typically pressurized during capture, transport, and storage. Coincidentally, CO2RR benefits from high pressure in aqueous solutions because low CO2 solubility under ambient pressure usually leads to the formation of unfavorable active carbon species, and subsequently causes diminished current density and reduced selectivity.4–6 We have reported that higher CO2 pressure can significantly improve the formate selectivity during aqueous-based CO2RR over commonly used catalysts such as Cu, Au, Ag and Sn.7 Cu2O@Cu catalysts with a hollow sphere morphology were also found to produce ethanol when operating under pressure.8 These studies pointed out the important role of high pressure in regulating the CO2RR pathways.
Bismuth-based catalysts have garnered considerable interest in the CO2RR field because of their high selectivity toward formate, cost effectiveness and low toxicity, rendering them a capable material for large-scale applications.3,9 Two-dimensional (2D) bismuth materials have aroused increasing attention in light of their high specific surface area, large atomic exposure rate and tunable electronic states.10 Han et al. showcased the effectiveness of ultrathin BiNS catalysts, achieving superior formate selectivity (>90% FE), current density (24 mA cm−2), and durability.11 Peng et al. synthesized ultrathin bismuth nanosheets (1.02 nm thick) with enhanced intrinsic activity and abundant active sites.12,13 Recently, 2D Bi2O2Se has emerged as a robust semiconducting material with ultra-high electron mobility and quantum oscillations,14 and we believe that 2D Bi2O2Se may hold the potential to catalyze the CO2RR effectively. Moreover, 2D Bi2O2Se may be highly cost-effective: on the one hand, Bi is a kind of non-noble metal and on the other hand, 2D materials exhibit low mass loading.
Here we report the pressure-regulated CO2RR to formate as catalyzed by 2D Bi2O2Se. The 2D Bi2O2Se catalyst with a layered “2D Zipper” structure is synthesized by a chemical vapor deposition (CVD) method on glassy carbon. While the catalyst loading is as low as 5.2 μg cm−2, our 2D Bi2O2Se manifests a CO2RR current density as high as 4649 mA mg−1 with a formate FE of 47%, surpassing reported Bi based 2D catalysts and the Bi2O2Se bulk counterparts. By pressurizing CO2 from ambient pressure (1.01 bar) to 40 bar, we steer the CO2RR pathway toward formate, achieving a record-high current density of 7084 mA mg−1 with a formate FE of 78%. In situ Raman spectroscopy and density functional theory (DFT) calculations reveal the mechanism of CO2RR to formate under high pressure, evidencing a stronger CO2 adsorption, enhanced *OCHO intermediate formation and more favorable pathway.
The 2D Bi2O2Se was synthesized through a two-step process: (i) preparation of bismuth oxide on glassy carbon via E-beam evaporation and (ii) selenization by the CVD method (Fig. 1a). Using glassy carbon as the substrate allowed the in situ synthesis of 2D Bi2O2Se, enabling its direct use as a working electrode in H-cell setups without the need for 2D material transfer. Fig. 1b illustrates the atomic structure of Bi2O2Se, which comprises alternating layers of bismuth atoms in hexagonal lattices, oxygen atoms positioned between these layers, and selenium atoms filling the interlayer gaps.15 As documented in the literature, the Raman peak at the A1g mode (159 cm−1) indicates the symmetric stretching vibration mode of the Bi–O bonds in the Bi2O2 layer (Fig. S1, ESI†).16 The lattice structure of Bi2O2Se was confirmed using high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), which aligns with the proposed “2D Zipper” model (Fig. S2, ESI†).17 This model suggests that the chemical bonds near the surface of Bi2O2Se are strengthened, enhancing the stability of the few-layer structures. Additionally, electron energy loss spectroscopy (EELS) mapping of Bi2O2Se revealed a uniform distribution of Bi, O, and Se elements across the entire area (Fig. S3, ESI†). Atomic force microscopy (AFM) characterization on Bi2O2Se confirmed an average thickness of 10.49 nm (Fig. S4, ESI†), revealing 17 layers of 2D monolayer.14 This thickness suggests a low catalyst mass loading, which was further validated by inductively coupled plasma mass spectrometry (ICP-MS), revealing a catalyst loading of 5.2 μg cm−2. X-ray photoelectron spectroscopy (XPS) was used to verify the presence of Bi, O, and Se (Fig. S5 and S6, ESI†). The spectra revealed two oxidation states of the Bi atom, represented by Bi 4f7/2 and Bi 4f5/2 at binding energies of 159 and 164 eV, respectively, as well as O 1s states at 530 eV and Se 3d states at 53 eV, consistent with previous reports.18 These observations authenticated the successful synthesis of 2D Bi2O2Se and motivated us to conduct the subsequent electrochemical experiments.
In contrast to wet chemical reduction,19 galvanic replacement reaction,20 hydrothermal reaction,21 and electrochemical conversion22 methods, the direct CVD growth of 2D Bi-based catalysts on glassy carbon substrates offers low mass loading by depositing only a few layers of catalyst (Fig. S4, ESI†). This may also enhance electron transport from the conductive substrate to the 2D catalyst. The CO2RR activity of 2D Bi2O2Se was firstly assessed in 0.5 M KHCO3 electrolyte by linear sweep voltammetry (LSV) at ambient pressure (1.01 bar), as shown in Fig. S7 (ESI†). In a CO2 saturated electrolyte, the 2D Bi2O2Se demonstrates a higher current density compared to an N2 saturated electrolyte at −0.7 V vs. reversible hydrogen electrode (RHE), indicating a greater favorability for CO2RR over the hydrogen evolution reaction (HER).
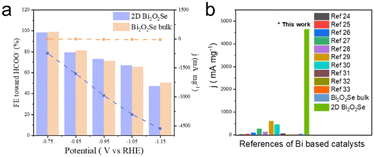
To assess the activity in terms of current density per unit mass (mA mg−1) and faradaic efficiency (FE), we compared the synthesized 2D Bi2O2Se with Bi2O2Se bulk prepared by the spin coating method with catalyst loading of 0.5 mg cm−2 (Fig. 2a). As shown in Fig. S8 (ESI†), 1H nuclear magnetic resonance (NMR) analysis identified formate as the only liquid product from the CO2RR, while gas chromatography (GC) detected H2 and CH4 as the gas products. It was observed that 2D Bi2O2Se exhibits significantly higher current density per unit mass, reaching 4649 mA mg−1 at −1.15 V (vs. RHE), which is 2 orders of magnitude greater than that of the bulk counterpart. However, the FE toward formate for both 2D Bi2O2Se and Bi2O2Se bulk remained at the same level and decreased with increased potential. This was attributed to the stronger dependence of the Volmer step potential on the overall potential compared to CO2 adsorption, leading to a higher preference for the HER at more negative potentials (Fig. S8, ESI†).23 To understand the specific surface area properties of 2D Bi2O2Se and Bi2O2Se bulk, electrochemically active surface area (ECSA) calculation (Fig. S9 and S10, ESI†) and non-mass-normalized LSV (Fig. S7, ESI†) were performed. Both 2D Bi2O2Se and Bi2O2Se bulk exhibit similar surface area and non-mass-normalized current density, indicating minimal effect of ECSA when comparing the mass-normalized CO2RR current density. More than that, we found that both Bi2O2Se and Bi2O3 were firstly reduced to the Bi metal state and it is Bi (012) that acts as the main facet during the CO2RR (Fig. S11, ESI†). These findings suggested that 2D Bi2O2Se growth on glassy carbon enabled more effective utilization of the catalyst in light of the low mass loading, while maintaining comparable CO2RR activity compared to bulk Bi2O2Se. A similar trend can be found when comparing 2D Bi2O3 and Bi2O3Se bulk (Fig. S12, ESI†). Furthermore, the CVD grown 2D Bi2O2Se catalyst greatly outperforms the benchmarks in the literature, including mesoporous Bi nanosheets,24 free-standing 2D bismuth nanosheets,25 layered Bi nanosheets,26 electron-rich Bi nanosheets,27 3D network of interconnected 2D bismuthene arrays,28 atomically thin bismuthene with rich defects,29 heterostructured bismuth-based catalysts,30 Bi2O2CO3 nanosheets31 and Bi-MOF32,33 (Fig. 2b).
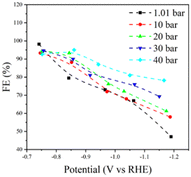
Pressurization of CO2 has been reported to steer the selectivity34,35 and boost the CO2RR current density.4,36 To further enhance the CO2RR performance of our 2D Bi2O2Se catalyst, we subjected the system to elevated CO2 pressures ranging from 1.01 to 40 bar. In general, the FE towards formate increased as the CO2 pressure increased, rising from 79.5% at 1.01 bar to 95% at 40 bar at −0.85 V (vs. RHE) (Fig. S13, ESI†). This demonstrates the capability of pressurized CO2 to regulate the selectivity towards formate on 2D Bi2O2Se, consistent with previous study.7 The corresponding total and formate partial current densities at various CO2 pressures are plotted in Fig. S14 (ESI†) and Fig. 3. In particular, the formate partial current density was improved from 2146 to 7308 mA mg−1 when the pressure increased from 1.01 to 40 bar at −1.15 V, indicating a greatly promoted production rate of formate. To compare the 2D Bi2O2Se with Bi2O2Se bulk under high CO2 pressure, we plotted current densities with corresponding formate FEs (Fig. 2a and Fig. S15, ESI†). The results align with prior research, underscoring the efficacy of our 2D Bi2O2Se catalyst with minimal mass loading in sustaining high current densities. As depicted in Fig. S16 (ESI†), Nyquist plots for CO2RR over the 2D Bi2O2Se catalyst showed a decrease in charge transfer resistance with increased CO2 pressures. This indicates that a significantly accelerated electron transfer with higher CO2 pressure as enhanced CO2 solubility in electrolytes promoted reactant transportation to the electrodes, that means, despite HER being more favorable under more negative potentials according to previous reported works,37,38 the elevated CO2 pressure can still suppress the HER (Fig. S8 and S17, ESI†).
These findings motivated us to investigate the mechanism of the CO2RR to formate under high CO2. In general, the CO2RR to formate pathway follows 4 steps:
| CO2 + * → *CO2 | (1) |
| *CO2 + H+ + e− → *OCHO | (2) |
| *OCHO + H+ + e− → *HCOOH | (3) |
| *HCOOH → * + HCOOH | (4) |
In order to track reaction intermediates during CO2RR over 2D Bi2O2Se, in situ Raman spectroscopy measurements were performed in ambient CO2 pressure (1.01 bar) and pressurized CO2 (20 bar) (Fig. 4a and Fig. S18, ESI†). In the scan of applied potential from −0.75 to −1.2 V vs. RHE over the 2D Bi2O2Se catalyst, two intrinsic Raman peaks were detected. The Raman peaks at 1052 cm−1 were attributed to HCO3−, respectively, indicating a CO2 saturated environment during the CO2RR.39,40 In addition, the peak at 2898 cm−1 was ascribed to the C–H stretching of *OCHO radicals.41 This revealed the favored *CO2− formation to activate CO2 molecules and enhanced adsorption strength of *OCHO intermediates, leading to superior activity and selectivity toward formate. Due to the low catalyst loading and strong Raman signal from glassy carbon, the D band and G band of glassy carbon were observed at 1346 cm−1 and 1572 cm−1. To gain a deep understanding on high pressure CO2RR, the DFT and Ab initio molecular dynamic (AIMD) calculations were performed in the Vienna Ab initio Simulation Package (VASP). In light of the pressure dependent CO2 solubility, we sought to explore the influence of CO2 coverage from 1/8 monolayer (ML) to 3/8 ML on the Bi (012) facet, the dominant facet of the prepared catalyst, confirmed by X-ray diffraction (XRD) measurement, as shown in Fig. S11 (ESI†).
The optimized structures and free energy diagram of adsorbed CO2RR to formate intermediates are depicted in Fig. S19 (ESI†) and Fig. 4b. In the initial step (CO2 + * → *CO2), a smaller free energy difference for 3/8 ML CO2 coverage (0.1 eV) indicated a stronger CO2 adsorption when compared with that of 1/8 ML CO2 coverage (0.32 eV). The first proton-coupled electron transfer process (*CO2 + H+ + e− → *OCHO) was defined as the rate-determine-step (RDS) of the CO2RR to formate with 1/8 ML CO2 coverage due to the large uphill energy. Moreover, in this step, the required free energy for 3/8 ML coverage is 0.1 eV, notably lower than that for 1/8 ML coverage (0.25 eV). That is, CO2RR under high pressure exhibits higher activity for *OCHO formation due to the lower reaction energy barrier. For the CO2RR to formate with 3/8 ML CO2 coverage, the second proton-coupled electron transfer process (*OCHO + H+ + e− → *HCOOH) was defined as the RDS. This proton-coupled electron transfer process (*OCHO + H+ + e− → *HCOOH) and the desorption process (*HCOOH → * + HCOOH) contributed to the final formate production. The free energy difference of these two steps with 3/8 ML CO2 coverage is lower, revealing a more favorable pathway. DFT calculations on the HER under pressurized CO2 were also conducted, as shown in Fig. S20 (ESI†). HER exhibited decreased energy barrier for RDS from 1 V to 0.92 V with enhanced CO2 coverage from 1/8 ML to 3/8 ML, whereas the CO2RR has an RDS energy barrier of only 0.13 V under high pressure. As a result, CO2RR is preferred compared to HER as the pressure increases, aligning with the experimental data (Fig. S8 and S17, ESI†).
In conclusion, we devise a low-mass-loading 2D Bi2O2Se catalyst and leverage the pressure to enhance CO2RR for formate production. The 2D catalyst exhibited a significantly higher current density of 4649 mA mg−1 with 47.1% FE to formate at −1.15 V (vs. RHE) compared to the bulk phase, indicating its cost-effectiveness. High CO2 pressure, commonly encountered in industrial processes involving CO2 capture, transport, and storage, significantly enhances formate selectivity and current density during CO2RR. Under high pressure, the partial current density increases from 2146 mA mg−1 at 1.01 bar to 7308 mA mg−1 at 40 bar at −1.15 V. Moreover, in situ Raman measurements and DFT calculations under pressurized CO2RR revealed the intermediates and pathways involved. These findings highlight the potential of 2D catalysts for sustainable CO2 conversion at high pressure with industrial relevance and underscore the importance of pressure in regulating the CO2RR pathways.
This work was financially supported by the Baseline Fund (BAS/1/1413-01-01) to X. L. from King Abdullah University of Science and Technology (KAUST).
Data availability
The data supporting the findings of this study are available within the article and its ESI.† Additional raw data and experimental details can be provided by the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Notes and references
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, Joule, 2018, 2, 825–832 CrossRef CAS
.
- S. Yoshida, M. Sampei, N. Todoroki, E. Hisamura, K. Nakao, K. Albrecht and T. Wadayama, Chem. Commun., 2023, 59, 3459–3462 RSC
.
- B.-Q. Miao, W.-S. Fang, B. Sun, F.-M. Li, X.-C. Wang, B.-Y. Xia and Y. Chen, Chin. J. Struct. Chem., 2023, 42, 100095 CrossRef
.
- J. Li, Y. Kuang, Y. Meng, X. Tian, W.-H. Hung, X. Zhang, A. Li, M. Xu, W. Zhou and C.-S. Ku, J. Am. Chem. Soc., 2020, 142, 7276–7282 CrossRef CAS PubMed
.
- J. Li, J. Guo and H. Dai, Sci. Adv., 2022, 8, eabo0399 CrossRef CAS PubMed
.
- S. Ruan, B. Zhang, J. Zou, W. Zhong, X. He, J. Lu, Q. Zhang, Y. Wang and S. Xie, Chin. J. Catal., 2022, 43, 3161–3169 CrossRef CAS
.
- L. Huang, G. Gao, C. Yang, X.-Y. Li, R. K. Miao, Y. Xue, K. Xie, P. Ou, C. T. Yavuz and Y. Han, Nat. Commun., 2023, 14, 2958 CrossRef CAS PubMed
.
- R. Qiu, J. Jia, L. Peng, R. Li, S. Yan, J. Li, J. Zhang, D. T. Sun, Z. Lan and T. Xue, Green Chem., 2023, 25, 684–691 RSC
.
- B. Ávila-Bolívar, V. Montiel and J. Solla-Gullón, ChemElectroChem, 2022, 9, e202200272 CrossRef
.
- S. Lu, F. Lou and Z. Yu, Catalysts, 2022, 12, 228 CrossRef CAS
.
- N. Han, Y. Wang, H. Yang, J. Deng, J. Wu, Y. Li and Y. Li, Nat. Commun., 2018, 9, 1320 CrossRef PubMed
.
- C. J. Peng, X. T. Wu, G. Zeng and Q. L. Zhu, Chem. – Asian J., 2021, 16, 1539–1544 CrossRef CAS PubMed
.
- S. Liu, Y. Fan, Y. Wang, S. Jin, M. Hou, W. Zeng, K. Li, T. Jiang, L. Qin and Z. Yan, Nano Lett., 2022, 22, 9107–9114 CrossRef CAS PubMed
.
- J. Wu, H. Yuan, M. Meng, C. Chen, Y. Sun, Z. Chen, W. Dang, C. Tan, Y. Liu and J. Yin, Nat. Nanotechnol., 2017, 12, 530–534 CrossRef CAS PubMed
.
- H. Zhou, X. Zhu, W. Liu, S. Liu, Y. Ding, Q. Zhang, Z. Zhang and R. Zhang, Appl. Surf. Sci., 2024, 672, 160851 CrossRef CAS
.
- W. Chen, U. Khan, S. Feng, B. Ding, X. Xu and B. Liu, Adv. Funct. Mater., 2020, 30, 2004960 CrossRef CAS
.
- Q. Wei, R. Li, C. Lin, A. Han, A. Nie, Y. Li, L.-J. Li, Y. Cheng and W. Huang, ACS Nano, 2019, 13, 13439–13444 CrossRef CAS PubMed
.
- M. T. Hossain and P. Giri, J. Appl. Phys., 2021, 129, 17 Search PubMed
.
- H. Xie, T. Zhang, R. Xie, Z. Hou, X. Ji, Y. Pang, S. Chen, M. M. Titirici, H. Weng and G. Chai, Adv. Mater., 2021, 33, 2008373 CrossRef CAS PubMed
.
- J. Fan, X. Zhao, X. Mao, J. Xu, N. Han, H. Yang, B. Pan, Y. Li, L. Wang and Y. Li, Adv. Mater., 2021, 33, 2100910 CrossRef CAS PubMed
.
- J. Ma, J. Yan, J. Xu, J. Ni, R. Li, L. Li and L. Lu, Chem. – Eur. J., 2022, 28, e202201747 CrossRef CAS PubMed
.
- P. Liu, H. Liu, S. Zhang, J. Wang and C. Wang, J. Colloid Interface Sci., 2021, 602, 740–747 CrossRef CAS PubMed
.
- P. D. Pedersen, M. M. Melander, T. Bligaard, T. Vegge, K. Honkala and H. A. Hansen, J. Phys. Chem. C, 2023, 127, 18855–18864 CrossRef CAS
.
- H. Yang, N. Han, J. Deng, J. Wu, Y. Wang, Y. Hu, P. Ding, Y. Li, Y. Li and J. Lu, Adv. Energy Mater., 2018, 8, 1801536 CrossRef
.
- F. Yang, A. O. Elnabawy, R. Schimmenti, P. Song, J. Wang, Z. Peng, S. Yao, R. Deng, S. Song and Y. Lin, Nat. Commun., 2020, 11, 1088 CrossRef CAS PubMed
.
- Y. Qiao, W. Lai, K. Huang, T. Yu, Q. Wang, L. Gao, Z. Yang, Z. Ma, T. Sun and M. Liu, ACS Catal., 2022, 12, 2357–2364 CrossRef CAS
.
- Z. Li, B. Sun, D. Xiao, Z. Wang, Y. Liu, Z. Zheng, P. Wang, Y. Dai, H. Cheng and B. Huang, Angew. Chem., Int. Ed., 2023, 62, e202217569 CrossRef CAS PubMed
.
- Y. C. He, D. D. Ma, S. H. Zhou, M. Zhang, J. J. Tian and Q. L. Zhu, Small, 2022, 18, 2105246 CrossRef CAS PubMed
.
- M. Zhang, W. Wei, S. Zhou, D.-D. Ma, A. Cao, X.-T. Wu and Q.-L. Zhu, Energy Environ. Sci., 2021, 14, 4998–5008 RSC
.
- P. F. Sui, C. Xu, M. N. Zhu, S. Liu, Q. Liu and J. L. Luo, Small, 2022, 18, 2105682 CrossRef CAS PubMed
.
- T. Fan, W. Ma, M. Xie, H. Liu, J. Zhang, S. Yang, P. Huang, Y. Dong, Z. Chen and X. Yi, Cell Rep. Phys. Sci., 2021, 2, 3 Search PubMed
.
- Z. W. Yang, J. M. Chen, Z. L. Liang, W. J. Xie, B. Zhao and L. N. He, ChemCatChem, 2023, 15, e202201321 CrossRef CAS
.
- Q. Huang, X. Sha, R. Yang, H. Li and J. Peng, ACS Appl. Mater. Interfaces, 2024, 16, 13882–13892 CrossRef CAS PubMed
.
- C. M. Gabardo, A. Seifitokaldani, J. P. Edwards, C.-T. Dinh, T. Burdyny, M. G. Kibria, C. P. O’Brien, E. H. Sargent and D. Sinton, Energy Environ. Sci., 2018, 11, 2531–2539 RSC
.
- K. Hara, A. Kudo and T. Sakata, J. Electroanal. Chem., 1995, 391, 141–147 CrossRef
.
- E. J. Dufek, T. E. Lister, S. G. Stone and M. E. McIlwain, J. Electrochem. Soc., 2012, 159, F514 CrossRef CAS
.
- X.-Z. Wang, S. Liu, Q. Liu and J.-L. Luo, Electrochem. Commun., 2019, 107, 106531 CrossRef CAS
.
- R. Sun, J. Zhao and X. Lu, J. Mater. Chem. A, 2024, 12, 8429–8437 RSC
.
- A. Dutta, I. n Zelocualtecatl Montiel, K. Kiran, A. Rieder, V. Grozovski, L. Gut and P. Broekmann, ACS Catal., 2021, 11, 4988–5003 CrossRef CAS
.
- H. Li, P. Wei, D. Gao and G. Wang, Curr. Opin. Green Sustainable Chem., 2022, 34, 100589 CrossRef CAS
.
- S. Jiang, K. Klingan, C. Pasquini and H. Dau, J. Chem. Phys., 2019, 150, 4 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05357e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |