Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A general and accessible approach to enrichment and characterisation of natural anti-Neu5Gc antibodies from human samples†
Esme
Hutton
 ab,
Yumiko
Uno
ab,
Yumiko
Uno
 ab,
Emma
Scott
ab,
Emma
Scott
 c,
Craig
Robson
c,
Craig
Robson
 c,
Martin A.
Fascione
c,
Martin A.
Fascione
 *a and
Nathalie
Signoret
*a and
Nathalie
Signoret
 *b
*b
aDepartment of Chemistry, University of York, York, UK. E-mail: martin.fascione@york.ac.uk
bHull York Medical School, University of York, York, UK. E-mail: nathalie.signoret@york.ac.uk
cNewcastle University, Centre for Cancer, Newcastle, UK
First published on 15th May 2025
Abstract
N-Glycolylneuraminic acid (Neu5Gc) is a non-human sialic acid which is presented on the surface of human cells following uptake from dietary sources. Antibodies against Neu5Gc have implications for many aspects of human health such as inflammation, xenograft rejection and cancer. However, current methods to detect and study anti-Neu5Gc antibodies require complex synthesis of glycan structures, animal handling expertise, or access to expensive reagents and equipment. Here, we outline a simple workflow to enrich and detect anti-Neu5Gc antibodies from small volume human serological samples. This strategy involves a micro-scale affinity purification step, followed by an indirect ELISA detection step which uses CMAH-transfected human cells as a source of Neu5Gc-containing human glycans in their native context. Parental wild type cells are also used as a paired Neu5Gc-negative control. Using this workflow, Neu5Gc-specific antibodies could be enriched from intravenous immunoglobulin (IVIG) and individual plasma specimens from ten healthy donors. Anti-Neu5Gc antibodies were detected in all donors, regardless of age or sex. The lysate ELISA assay was also sufficiently sensitive to observe reproducible individual differences in the anti-Neu5Gc reactivity of each donor specimen. Importantly, despite this individual variation, enriched antibodies from all donor specimens bound effectively to Neu5Gc-containing glycans presented on the surface of whole human cells, highlighting the potential physiological relevance of these antibodies.
Introduction
Sialic acids are 9-carbon nonulosonic acid sugars predominantly found on the terminal end of cell surface glycans. This position at the outermost limits of the glycocalyx makes sialic acids key ligands for many glycan-binding proteins, such as viral lectins, bacterial toxins and inhibitory siglecs on immune cells.1,2 As such sialylation is implicated in many biological processes, in both health and disease.3 Mammals produce two main sialic acid species: N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc)4 (Fig. 1A). However, conserved loss-of-function mutations in the enzyme cytidine-monophospho-N-acetylneuraminic acid hydroylase (CMAH), which is essential for Neu5Gc synthesis, have led to an absence of endogenous Neu5Gc production in humans.5Despite no evidence for an alternative biosynthetic pathway,6 Neu5Gc can still be detected at low levels in some human tissues, particularly those with high metabolic activity and sialic acid turnover such as the epithelia, foetal tissues, and cancer cells.7 This is due to uptake and incorporation of Neu5Gc from exogenous sources, such as red meat or dairy in the diet.8,9 Presentation of Neu5Gc on human sialoglycans is hypothesised to trigger the production of anti-Neu5Gc antibodies.10,11 These Neu5Gc-directed antibodies arise in early life,12 and are proposed as a driving factor in many inflammation-associated conditions such as atherosclerosis, xenograft rejection and cancer.13–15
Despite wide exploration of their role in human health, there is a lack of agreement surrounding the extent to which anti-Neu5Gc antibodies are present in the human population.16 This variation in the reported prevalence of anti-Neu5Gc antibodies may be linked to the absence of a general method for their detection and study. Enzyme-linked immunosorbent assays (ELISAs) detecting antibody binding to homogenous synthetic glycans have high sensitivity, but presenting a single glycan structure is not representative of the complex mixtures of linkage types and branches found in natural glycans.17 Glycan microarrays may overcome this problem by displaying Neu5Gc conjugated to a range of underlying glycan structures, but these platforms often do not accommodate the multivalent presentation of glycans in biological contexts.18 Flow cytometry has also been used to investigate anti-Neu5Gc binding to cell surface glycans, mostly with the aim of understanding xenograft rejection. These studies frequently use porcine cells which express different glycan profiles to those found in humans.19,20 Another key issue with many of these methods is that they require specialist equipment or expertise, including chemical/chemoenzymatic synthesis of complex glycan structures, animal handling, or microarray fabrication and derivatisation apparatus. As a result, study of anti-Neu5Gc antibodies is often not accessible to biomedical research groups.
To address these issues, we have developed a simple strategy to enrich anti-Neu5Gc antibodies out of small-volume serological specimens, and an ELISA method, which can be used to characterise the ability of these antibodies to bind to human Neu5Gc-containing glycans. This strategy utilises commercially available reagents, and use of cell lysates rather than whole cells may also extend its accessibility even to laboratories without access to cell culture facilities. It is anticipated that this workflow will broaden the accessibility of research into the anti-Neu5Gc antibody response, furthering understanding of the complex role these antibodies play in human health.
Results and discussion
Background and method design
Chimpanzee serum (CS) glycoproteins are similar in structure and composition to human serum (HS) glycoproteins.21 Unlike humans, however, chimpanzees express functional CMAH and produce a wide range of Neu5Gc-containing glycans. This was shown experimentally by adsorbing CS and HS onto a 96-well ELISA plate and assessing overall Neu5Gc content using a commercial polyclonal chicken anti-Neu5Gc IgY antibody (Fig. 1B). Enrichment of anti-Neu5Gc antibodies from serological samples has been reported previously using an affinity purification process which exploits this, using HS and CS as a paired system of Neu5Gc-negative and Neu5Gc-positive matrices. The affinity column system has been used to enrich anti-Neu5Gc antibodies from IVIG and pooled human serum. These antibodies were tested on sialoglycan microarrays,22,23 not accounting for glycan presentation in a natural context. This approach also uses large sample volumes not compatible with routine serological specimens, and therefore requires adaptations for micro-scale purification.A key advance in the detection of anti-Neu5Gc antibodies was the development of a CMAH knockout mouse model that does not endogenously synthesize Neu5Gc.24 This model was then used to develop an ELISA protocol where wild-type (WT) mouse serum was used as a source of Neu5Gc-containing glycans, and CMAH knock-out (CMAH KO) mouse serum was used as a paired Neu5Gc-negative control.25 While effective, this strategy requires access to animal handling expertise, and mouse glycan structures may not represent the full range of Neu5Gc-containing glycans possibly found in human tissues.26 A more physiologically relevant alternative is to re-introduce CMAH activity in human cells, an approach already shown to enable binding of human Neu5Gc-reactive IgGs to transfected cells in flow cytometry experiments.27,28 However, studies investigating neuraminidase cleavage of surface sialic acids in various cell lines reported that up to a third of total sialic acid content may be intracellular, for example as part of the sialoglycoconjugates undergoing processing in the golgi before presentation on the cell surface.29–31 Flow cytometry experiments only account for cell surface glycans, and therefore may not detect the full range of Neu5Gc-reactive antibodies produced by humans. This presents the need for a parallel strategy which accounts for total cellular Neu5Gc content.
Based on this knowledge, we proposed that paired Neu5Gc-positive lysates from CMAH-transfected HEK 293 cells (mCMAH-HEK) and Neu5Gc-negative lysates from parental cells (WT-HEK) could be used on an ELISA platform for detection of specific antibody binding to human Neu5Gc-containing glycans. This strategy uses physiologically relevant glycan structures, accounts for total cell sialic acid content, and can be applied to multiple samples in a high-throughput manner. We also aimed to explore whether the pair of WT-HEK and mCMAH-HEK lysates could represent a more cost-effective alternative to using HS and CS for the affinity column enrichment of human anti-Neu5Gc antibodies from serological specimens.
mCMAH-HEK development
HEK 293 cells were chosen as a model to develop a paired system of Neu5Gc-positive CMAH-transfected cells. Neu5Gc-negative parental HEK cells were used as a control for background non-Neu5Gc antibody binding. HEK 293 cells were used due to their ability to express recombinant proteins,32 alongside published reports that HEK cells express many of the human glycosylation genes, leading to production of a diverse range of sialoglycan structures.33,34 HEK 293 cells were transfected with a plasmid encoding murine CMAH (mCMAH) using a lipid-based transfection reagent. Transfected cells were maintained under selection antibiotic to produce a heterogeneous mix of stable mCMAH-expressing cells (Fig. 2A). Using a non-clonal pool of cells with different levels of CMAH expression ensures that a wide range of Neu5Gc-containing glycoconjugates are represented.Incorporation of Neu5Gc into cell-surface glycoproteins of mCMAH-HEK cells was validated by flow cytometry using a commercial polyclonal chicken IgY anti-Neu5Gc antibody (Fig. 2B). Neu5Gc presentation on the surface of mCMAH-HEK was also shown by immunostaining with the same anti-Neu5Gc antibody (Fig. S1A, ESI†). The efficient conversion of Neu5Ac into Neu5Gc upon expression of the mCMAH gene was further validated by western blotting (Fig. 2C). Trypsin cleavage of cell surface proteins led to a reduction in total Neu5Gc content of mCMAH-HEK lysates, which confirmed the successful incorporation of synthesised Neu5Gc into glycoproteins (Fig. S1B, ESI†).
Importantly, while stable mCMAH-HEK were used for all subsequent experiments as a matter of convenience, HEK 293 cells transiently transfected with the same murine CMAH construct show no significant difference in Neu5Gc production compared to stably transfected cells (Fig. S1C and D, ESI†), meaning this paired system does not require establishment of a stable CMAH-expressing cell line to be effective.
Establishing a lysate ELISA method to characterize anti-Neu5Gc antibodies
We next investigated whether lysates from mCMAH- and WT-HEK could be immobilised on a solid support and used as a system to interrogate binding of anti-Neu5Gc antibodies. To achieve this, an indirect ELISA strategy was developed, which is outlined in Fig. 2D. To perform this ‘lysate ELISA’, whole lysates from mCMAH-HEK or WT-HEK cells were directly adsorbed onto 96-well ELISA plates. Plates were blocked with fish gelatin (FG) which does not present Neu5Gc unlike standard blocking agents such as BSA35 which show binding to the commercial anti-Neu5Gc IgY (Fig. S2A, ESI†). Anti-Neu5Gc antibodies were added, and binding was detected using an HRP-conjugated secondary antibody. Normalised Neu5Gc-specific signal was calculated as described in the methods section.Proof of concept experiments with the polyclonal chicken IgY anti-Neu5Gc antibody showed specific IgY binding to mCMAH-HEK lysates (Fig. 2E). We also performed titration experiments using serial dilutions of anti-Neu5Gc IgY spiked into human serum (Fig. 2F). These experiments confirmed the specific detection of anti-Neu5Gc IgY binding even in the presence of a complex matrix such as human serum. The lysate ELISA was also shown to be sensitive, detecting anti-Neu5Gc IgY concentrations as low as 50 ng mL−1.
Affinity purification of anti-Neu5Gc antibodies
While a number of studies have been published using CMAH-positive cells to detect human anti-Neu5Gc antibodies in unprocessed serological samples,27,36,37 we were unable to replicate these findings for whole plasma or IVIG using either our lysate ELISA (Fig. S2B, ESI†) or flow cytometry-based approaches (Fig. S2C and D, ESI†). This may be due to high background from the wide range of non-Neu5Gc-containing antigens in the CMAH-HEK and WT-HEK lysates, which could mask the comparatively small pool of these antibodies which are Neu5Gc-reactive. This reinforces the importance of including appropriate controls to identify this background reactivity for each serological specimen being tested, such as paired CMAH-negative cells which will show similar levels of non-Neu5Gc-related antibody binding. As the lysate ELISA and flow cytometry were not able to identify anti-Neu5Gc antibodies in unprocessed plasma, we decided to enrich anti-Neu5Gc antibodies from the serological samples.Using the strategy outlined in Fig. 2G, serological samples were first pre-cleared on a Neu5Gc-negative column containing HS coupled to resin beads. Flowthrough from the HS column was then loaded onto a Neu5Gc-positive CS column. Increasing concentrations of a simple Neu5Gc α-methyl glycoside (GcOMe) produced in-house, but also commercially available, was then used to elute bound Neu5Gc-specific antibodies. Anti-Neu5Gc antibodies have higher affinity for the α-linked GcOMe than unconjugated Neu5Gc, which exists predominantly in the β-configuration.38 However, at concentrations of above 1 mM, unconjugated Neu5Gc also competed for anti-Neu5Gc binding to some extent (Fig. S3, ESI†). It may therefore be possible to use Neu5Gc to elute the antibodies if synthesis or purchase of the methyl glycoside is not accessible.
This purification process was initially trialled on Immunovenin Intact (BulBio NCIPD Ltd, Bulgaria), an IVIG preparation pooled from thousands of healthy donors, which was previously reported to be anti-Neu5Gc-positive on microarray studies.23 Fractions of 1 mL were collected, and immunoglobulin-positive fractions were confirmed by western blotting (Fig. S4, ESI†) and pooled. Enriched anti-Neu5Gc antibodies from IVIG (IV-Gc) were tested using the lysate ELISA, but this time with an HRP-conjugated anti-human IgG for detection. Neu5Gc-specific antibody binding was calculated as described in the methods section. IV-Gc preferentially bound to mCMAH-HEK lysates (Fig. 2H, CMAH-WT > 0), which was not observed in the unprocessed IVIG preparation (Fig. S2A, ESI†). In titration experiments, IV-Gc bound to mCMAH-HEK lysates across a range of concentrations (Fig. 2I). Importantly, the interaction between IV-Gc and mCMAH-HEK lysates was shown to be Neu5Gc-dependent, as binding returned to levels of non-transfected cells (CMAH-WT = 0) after co-incubation with a competing dose of GcOMe (Fig. 2J). IV-Gc was also used to adjust the lysate ELISA conditions for efficient detection of enriched human anti-Neu5Gc antibodies, identifying the optimum binding time and plate coating concentration (Fig. S5A and B, ESI†), although this may need to be optimised separately if cells other than HEK cells are used. Specific binding of enriched IV-Gc to mCMAH-HEK was also detectable by flow cytometry and western blotting (Fig. S5C and D, ESI†).
Interestingly, substituting foetal bovine serum (FBS) for CS as a source of Neu5Gc-containing glycans did not allow for the enrichment of Neu5Gc-specific human antibodies (Fig. S6A, ESI†). This may indicate that the similarity of chimpanzee and human glycan profiles21 is important to the success of the affinity purification process, or reflect the slightly lower abundance of Neu5Gc in CS compared to FBS (Fig. S6B, ESI†).
While this large scale affinity purification method effectively enriched Neu5Gc-specific antibodies from IVIG and pooled donor plasma (Fig. S7, ESI†), it required large sample volumes (2–5 mL) and could only process one sample at a time, which is not compatible with enrichment of anti-Neu5Gc antibodies from small volumes of single-donor serological specimens. To address this, the purification process was scaled down to spin columns loaded with HS- and CS-coupled resin, which could process samples of less than 500 μL. IV-Gc prepared using the micro-scale columns showed no significant loss of anti-Neu5Gc reactivity compared to the large-scale columns, highlighting this as a viable scale-down strategy (Fig. 3A).
Applicability of micro-scale anti-Neu5Gc purification
This approach was then applied to plasma fractions from blood of ten healthy donors of various ages and sexes, to explore whether the affinity purification and lysate ELISA strategy could be utilised to assess individual levels of circulating natural anti-Neu5Gc antibodies. Plasma anti-Neu5Gc antibodies (Pl-Gc) were enriched and tested on the lysate ELISA. Neu5Gc-specific binding was calculated as described in the methods section. This revealed notable differences in Pl-Gc binding to Neu5Gc-containing glycans between the donors (Fig. 3B).Similar donor variation was detected when measuring Pl-Gc binding to the surface of intact mCMAH-HEK or WT-HEK cells by flow cytometry (Fig. 3C), validating the findings from the lysate ELISA. These experiments using Pl-Gc established the presence of natural human anti-Neu5Gc antibodies in all donors, albeit at low levels in some, and their ability to recognise Neu5Gc-glycoconjugates in their native context on human cells.
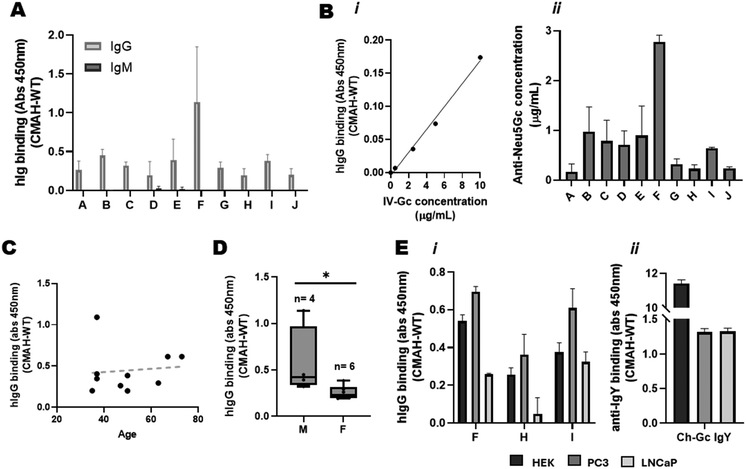
Enrichment of anti-Neu5Gc antibodies from donor specimens also presented the opportunity to further dissect different components of the human anti-Neu5Gc antibody response. Use of isotype-specific detection antibodies revealed that the natural anti-Neu5Gc antibody response is predominantly comprised of IgG antibodies, with little to no detection of Neu5Gc-specific IgM antibodies (Fig. 4A), agreeing with previous reports.27 This meant that, after assessing IV-Gc concentration using a BCA assay, a titration of IV-Gc could be included as a standard for the lysate ELISA to perform more quantitative analyses (Fig. 4B). This allowed us to estimate the concentration of natural Neu5Gc-specific antibodies enriched from donor specimens at between 0.1 and 2.8 μg mL−1.
Individual variation in the anti-Neu5Gc antibody response was not significantly associated with donor age (Fig. 4C). Higher anti-Neu5Gc reactivity was observed in samples from male donors compared to female donors (Fig. 4D). However this is a very small set of samples, and a wider cohort would be needed to ascertain a difference that may be influenced by diet39 or immune function40 amongst other unaccounted for factors, such as previous infections, or the composition of the microbiome.22,41
Additionally, the paired CMAH and WT lysate strategy was not limited to HEK 293 cells. PC3 and LNCaP prostate cancer cell lines could also be transiently transfected with the mCMAH gene and lysates used as the coating antigen for the lysate ELISA. This showed Pl-Gc binding with similar donor variability across the cell lines (Fig. 4Ei). Note that the extent of single donor Pl-Gc binding to lysates from the different cell types did not mirror the binding of the commercial anti-Neu5Gc IgY (Fig. 4Eii). These observations may reflect the variation in glycosylation patterns between the PC3 and LNCaP cell lines,42 or recognition of different Neu5Gc-containing glycan structures by antibodies from different donor specimens.
Exploiting mCMAH-HEK and WT-HEK lysates for micro-scale affinity purification of anti-Neu5Gc antibodies
Our overall aim was to develop an accessible method to enrich anti-Neu5Gc antibodies from serological specimens which can process many samples in parallel. A key limitation to our existing strategy was the cost and amount of human and chimpanzee sera required for preparation of multiple columns. To address this, we explored whether mCMAH-HEK and WT-HEK cell lysates could be used as the paired Neu5Gc-positive and Neu5Gc-negative solid phases micro-scale affinity purification (Fig. 5A). Effective coupling of lysates and presence of Neu5Gc on the resin was confirmed using on-resin BCA and ELISA assays, respectively (Fig. S8A and B, ESI†). Initial tests of this strategy were carried out using IVIG, with anti-Neu5Gc enriched fractions collected from lysate loaded columns (Fig. S8C, ESI†) and tested using the lysate ELISA. We found no significant difference in the detection of Neu5Gc-specific binding for IV-Gc enriched using the lysate or serum affinity columns (Fig. 5B), and a competitive dose of GcOMe equally displaced the binding of IV-Gc enriched from each column system (Fig. 5C). Similarity in Neu5Gc-specific enrichment was also validated by flow cytometry (Fig. 5D). As mentioned previously, using FBS as a source of Neu5Gc-containing glycans instead of CS or mCMAH-HEK lysates was less efficient, suggesting that enriched anti-Neu5Gc antibodies preferentially bind to human or human-like glycan structures, or reflecting a lower concentration of Neu5Gc in FBS (Fig. S8E, ESI†).This approach was then applied for enrichment of pooled donor plasma specimens. Fractions eluted following the lysate columns (Fig. S8D, ESI†) were similarly enriched in anti-Neu5Gc antibodies than those prepared using the sera columns, detected by both ELISA (Fig. 5E) and flow cytometry (Fig. 5F). Finally, we investigated if this method was sensitive enough to enrich Neu5Gc-specific antibodies from individual donor specimens. Pl-Gc from donors H and I were previously determined to have low and high Neu5Gc-specific binding, respectively. Individual donor (H or I) Pl-Gc enriched using each column system showed similar donor variability when tested by the lysate ELISA (Fig. 5G) and flow cytometry (Fig. 5H). This indicated that the lysate columns are as effective to isolate anti-Neu5Gc antibodies from small volume individual specimens. Importantly, these results also validated the reproducibility of the enrichment and detection strategy, with independent purifications yielding the same Neu5Gc-specific signal for the selected donor specimens.
Conclusions
Overall, we have optimised a simple method to enrich anti-Neu5Gc antibodies from human serological specimens. This uses a paired column system, with a Neu5Gc-negative ‘pre-cleaning’ column and a Neu5Gc-positive ‘capturing’ column. We have also designed an ELISA method to detect and characterise the binding of resulting enriched anti-Neu5Gc antibodies. This strategy can be applied to small-volume (<500 μL) serological samples, making it compatible with the enrichment of anti-Neu5Gc antibodies from individual donor specimens obtained during routine serological testing. Furthermore, this approach presents Neu5Gc in the context of native human glycan structures without requiring complex chemical or chemoenzymatic synthesis, and can be performed using commercially available reagents. Use of cell lysates from WT and CMAH-transfected human cells also avoids the expense of large volumes of sera for column preparation, further increasing its accessibility.Additionally, the lysate ELISA detection approach could be modified for use on various human cell lines. This presents the valuable opportunity to explore the anti-Neu5Gc antibody response against specific tissue types. Considering biological context is important when studying anti-Neu5Gc antibodies, given the marked variation in glycosylation patterns between different human tissues and disease states,43 and the potential that different individuals may express anti-Neu5Gc antibodies against a different profile of glycan structures.44 We also anticipate that this method may be adapted to enrich antibodies from individual donor specimens against other immunogenic glycans, for example αGal, which has been implicated in xenograft rejection and red meat allergy.45,46
Finally, by applying this enrichment and detection workflow to healthy donor plasma specimens, we revealed that anti-Neu5Gc antibodies could be reproducibly detected across a group of ten healthy donors of different ages and sexes. Anti-Neu5Gc antibodies were detectable in all donors regardless of age or sex, and exhibited individual differences which may be influenced by factors such as diet or past infections.9,12,47 Importantly, even in donor specimens with relatively low anti-Neu5Gc reactivity, these antibodies could bind to Neu5Gc-containing glycans on the surface of intact human cells, supporting the hypothesis that they may be relevant to human health.
Materials and methods
Reagents and antibodies
All materials and reagents were purchased from Thermo Fisher Scientific and chemicals sourced from Merck, unless otherwise stated. The purified chicken polyclonal IgY anti-Neu5Gc antibody (Poly21469) was purchased from BioLegend, secondary anti-chicken or anti-human antibodies (HRP- or Alexa-488) were purchased from Invitrogen. Commercial human serum was purchased from Sigma Aldrich.Cell culture
HEK 293 cells were grown in Dulbecco's modified Eagle medium supplemented with 10% FBS, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per ml penicillin, 10
000 units per ml penicillin, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg ml−1 streptomycin and 2 mM L-glutamine. HEK 293 stable transfectants were maintained in this same medium with addition of 125 μg mL−1 hygromycin. All cells were maintained in T75 tissue culture flasks at 37 °C under 5% CO2 and were passaged upon reaching confluence. Cells were detached using trypsin/EDTA or 10 mM EDTA-PBS for passage or experiments, respectively.
000 μg ml−1 streptomycin and 2 mM L-glutamine. HEK 293 stable transfectants were maintained in this same medium with addition of 125 μg mL−1 hygromycin. All cells were maintained in T75 tissue culture flasks at 37 °C under 5% CO2 and were passaged upon reaching confluence. Cells were detached using trypsin/EDTA or 10 mM EDTA-PBS for passage or experiments, respectively.
Stable transfection with mCMAH cDNA
Mouse CMAH (mCMAH) cDNA in a pCMV3 expression vector was purchased from Sino Biological (#NM_001111110.2). HEK 293 cells were transfected with 1 μg mL−1 cDNA using the XtremeGENE HP transfection reagent (Roche, #6366244001). Stably transfected cells were selected using 125 μg mL−1 hygromycin and maintained as a heterogeneous pool. LNCaP and PC3 cells were transiently transfected with 2 μg mL−1 mCMAH cDNA using the same transfection reagent. Transiently transfected cells were harvested 48 h after transfection.IVIG and plasma samples
ImmunoVenin Intact IVIG was a generous gift from BulBio NCIPD Ltd, Bulgaria. Frozen plasma fractions used were isolated from blood samples of healthy volunteers collected following written informed consent, according to a blood donation protocol from the University of York approved by the Biology department Ethics Committee (BEC: PBMC donation protocol Version 3; November 2019) and delivered in compliance with ICH GCP, the Data Protection Act, and other regulatory requirements, as appropriate. Additional ethical approval was received from the Health Research Authority through the Yorkshire and Humber research ethics committee (REC reference 19/YH/0394, for IRAS project ID: 269597).48Preparation of cell lysates
Cells were harvested using 10 mM EDTA in PBS. Lysis was carried out using 1% Triton X-100 lysis buffer (0.1 M Tris pH 8, 0.1 M NaCl, 1 mM CaCl2, 1% Triton X-100) supplemented with a protease inhibitor cocktail (Roche, #11697498001) for 15 min. Lysates were clarified by centrifugation for 30 min, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g at 4 °C. Protein concentration was determined via BCA assay.
000×g at 4 °C. Protein concentration was determined via BCA assay.
SDS-PAGE and western blotting
Samples were boiled at 95 °C in SDS sample buffer (2% SDS, 2 mM β-mercaptoethanol, 4% glycerol, 0.01% bromophenol blue, 0.04 M Tris-HCl pH 6.8) for 5 min, then loaded onto 10% acrylamide SDS-PAGE gels. Gels were run at 150 V until fully resolved. Gels were stained with Coomassie blue, destained, and imaged using an iBright 1500 imaging system (Invitrogen, #A44114). For western blotting, proteins were transferred onto nitrocellulose using the Trans-Blot Turbo transfer system (Bio-Rad, #1704150). Membranes were blocked in 1% fish gelatin in PBS plus 0.05% TWEEN-20 (FG/PBST). Chicken anti-Neu5Gc IgY (Biolegend, #146903) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 dilution was added in FG/PBST overnight at 4 °C. HRP anti-chicken IgY (Invitrogen, #A16054) secondary antibody was added at 1
4000 dilution was added in FG/PBST overnight at 4 °C. HRP anti-chicken IgY (Invitrogen, #A16054) secondary antibody was added at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in FG/PBST for 1 h at room temperature. Membranes were developed using BM Chemiluminescence western blotting substrate (Roche, #11500708001) and images were acquired using the iBright 1500 imaging system.
500 in FG/PBST for 1 h at room temperature. Membranes were developed using BM Chemiluminescence western blotting substrate (Roche, #11500708001) and images were acquired using the iBright 1500 imaging system.
Lysate ELISA
Flat bottom NUNC Maxisorp 96-well plates were coated overnight with 1 μg mL−1 mCMAH-HEK or WT-HEK lysates in 0.05 M carbonate coating buffer (pH 9.6). Wells were blocked with 1% fish gelatin in PBS (FG/PBS) for 2 h at room temperature. Anti-chicken IgY was added at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 in FG/PBS and incubated for 1 h at room temperature. Affinity purified antibodies were added 1
5000 in FG/PBS and incubated for 1 h at room temperature. Affinity purified antibodies were added 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 in FG/PBS and incubated for 3 h at room temperature. Wells were washed 3× with 0.05% PBST and HRP anti-chicken IgY (Invitrogen, #A16054, 1
25 in FG/PBS and incubated for 3 h at room temperature. Wells were washed 3× with 0.05% PBST and HRP anti-chicken IgY (Invitrogen, #A16054, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), HRP-anti-human IgG (FineTest, #FNSA-0105, 1
1000), HRP-anti-human IgG (FineTest, #FNSA-0105, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) or HRP-anti-human IgM (FineTest, #FNSA-0103, 1
5000) or HRP-anti-human IgM (FineTest, #FNSA-0103, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) was added in PBS and incubated for 1 h at RT. Plates were washed 3× with PBST and developed using TMB high sensitivity substrate (BioLegend, #421501). The reaction was stopped using 1 M H2SO4 and absorbance was read at 450 nm.
500) was added in PBS and incubated for 1 h at RT. Plates were washed 3× with PBST and developed using TMB high sensitivity substrate (BioLegend, #421501). The reaction was stopped using 1 M H2SO4 and absorbance was read at 450 nm.
Date were normalised by dividing all raw Abs 450 nm values by the Abs 450 nm value for the secondary antibody only, in order to account for differences in overall absorbance reading between experiments. To calculate Neu5Gc-specific antibody binding, normalised Abs 450 nm values for WT-HEK Neu5Gc-negative were subtracted from normalised Abs 450 nm values for mCMAH-HEK Neu5Gc-positive wells.
| Neu5Gc specific Abs 450 nm = (mCMAH Abs 450 nm/2° only Abs 450 nm) − (WT Abs 450 nm/2° only Abs 450 nm) |
ELISA detection of Neu5Gc content in human and chimpanzee sera
Flat bottom NUNC Maxisorp 96-well plates were coated overnight with pooled human serum (Sigma, #H6914) or chimpanzee serum (https://Antibodies.com, #A116435) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution in 0.05 M carbonate coating buffer (pH 9.6). Wells were blocked with 1% fish gelatin in PBS (FG/PBS) for 2 h at room temperature. HRP-conjugated anti-chicken IgY was added at 1
100 dilution in 0.05 M carbonate coating buffer (pH 9.6). Wells were blocked with 1% fish gelatin in PBS (FG/PBS) for 2 h at room temperature. HRP-conjugated anti-chicken IgY was added at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in FG/PBS and incubated for 1 h at room temperature. Wells were washed 3× with 0.05% PBST and HRP anti-chicken IgY (Invitrogen, #A16054, 1
1000 in FG/PBS and incubated for 1 h at room temperature. Wells were washed 3× with 0.05% PBST and HRP anti-chicken IgY (Invitrogen, #A16054, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) was added in PBS and incubated for 1 h at RT. Plates were washed 3× with PBST and developed using TMB high sensitivity substrate (BioLegend, #421501). The reaction was stopped using 1 M H2SO4 and absorbance was read at 450 nm.
1000) was added in PBS and incubated for 1 h at RT. Plates were washed 3× with PBST and developed using TMB high sensitivity substrate (BioLegend, #421501). The reaction was stopped using 1 M H2SO4 and absorbance was read at 450 nm.
Flow cytometry assays
Cells detached in PBS/EDTA were harvested and resuspended in FACS buffer (PBS, 1% FBS, 0.05% sodium azide). Cells were transferred to 96-well round bottom plates (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) and kept on ice. Plated cells were washed through addition of ice-cold FACS buffer followed by a 5 min 1500×g spin at 4 °C. After three washes, Fc receptors were blocked using 30 μg mL−1 rat IgG in FACS buffer for 30 min, and then incubated with polyclonal chicken anti-Neu5Gc IgY (1
000 cells per well) and kept on ice. Plated cells were washed through addition of ice-cold FACS buffer followed by a 5 min 1500×g spin at 4 °C. After three washes, Fc receptors were blocked using 30 μg mL−1 rat IgG in FACS buffer for 30 min, and then incubated with polyclonal chicken anti-Neu5Gc IgY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) or affinity purified human anti-Neu5Gc fractions (1
1000) or affinity purified human anti-Neu5Gc fractions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) in FACS buffer for 4 h under agitation at 4 °C. After three more washes, Alexa-488 anti-chicken IgY (Invitrogen, #A-11039, 1
20) in FACS buffer for 4 h under agitation at 4 °C. After three more washes, Alexa-488 anti-chicken IgY (Invitrogen, #A-11039, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) or FITC anti-human IgG/A/M (Invitrogen, #15440814, 1
200) or FITC anti-human IgG/A/M (Invitrogen, #15440814, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) secondary antibodies diluted in FACS buffer were added for 1 h. Cells were washed three times and fixed in 1% formaldehyde containing FACS buffer overnight at 4 °C. Washed, fixed cells were resuspended in FACS buffer and samples run on a CytoFLEX S Flow cytometer (Beckman Coulter) recording readings of Alexa-488 mean fluorescence intensity (MFI).
200) secondary antibodies diluted in FACS buffer were added for 1 h. Cells were washed three times and fixed in 1% formaldehyde containing FACS buffer overnight at 4 °C. Washed, fixed cells were resuspended in FACS buffer and samples run on a CytoFLEX S Flow cytometer (Beckman Coulter) recording readings of Alexa-488 mean fluorescence intensity (MFI).
Normalised MFI was calculated by subtracting cell-only MFI values. Specific MFI was then calculated by dividing normalised MFI for specimen anti-Neu5Gc antibodies by secondary only MFI values to account for differences in overall MFI value between experiments. Neu5Gc-specific MFI was then calculated as WT-HEK MFI values subtracted from mCMAH-HEK MFI values.
| MFI = Sample mean fluorescence − Cell only mean fluorescence |
| Neu5Gc-specific MFI = (mCMAH MFI/2° only MFI) − (WT MFI/2° only MFI) |
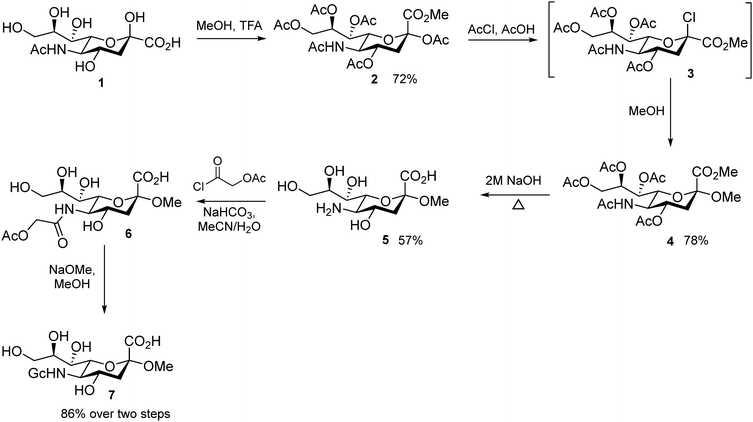
Neu5Gc-α-methyl glycoside synthesis
Neu5Gc α-methyl glycoside (GcOMe) was prepared according to published protocols.23,44,49 Detailed experimental information can be found in Scheme S1 (ESI†).
Micro-scale affinity purification of anti-Neu5Gc antibodies
To prepare serum-loaded columns, heat inactivated HS and CS in 0.1 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (pH 7.5) were coupled to Affi-gel 15 resin (Bio-Rad, #153-6052) according to the manufacturer's protocol. Beads were loaded with a final concentration of 1 mg serum per 1 mL resin. To prepare lysate-loaded columns, lysates were prepared as described above and coupled to Affi-gel 15 resin at 250 μg lysate per mL of resin in MOPS buffer. Coupling was carried out overnight at 4 °C.Active esters in the resin were neutralised with 0.1 M ethanolamine-HCl for 1 h. Into each empty mini Bio-spin column (BioRad, #7326207) 1 mL resin was loaded to form Neu5Gc-negative (HS or WT-HEK lysate) columns and Neu5Gc-positive (CS or mCMAH-HEK lysate) columns. To prime the columns, 3× washes with 50 mM sodium acetate (pH 5.5), 3× washes with PBS (pH 7.4), 3× washes with 0.1 M citric acid (pH 3.0) and 3× washes with PBS were carried out sequentially, each for 1 min at 500 rpm. Columns were stored in PBS containing 0.5% sodium azide.
Plasma samples were heat inactivated for 30 minutes at 56 °C and diluted in PBS to a final volume of 500 μL. Plasma was loaded onto the primed HS columns and incubated for 5 min, before centrifuging for 3 min at 500 rpm. The flowthrough was loaded back onto the HS column for a total of 4 rounds of pre-clearing. The resulting flowthrough was then loaded onto the CS column and incubated for 3 min, followed by 3 min centrifugation. The flowthrough was re-loaded through the column for a total of 5 passes. To elute non-specifically bound antibodies, the Neu5Gc-positive columns were washed with 5 mM glucuronic acid for 1 min at 500 rpm, then washed with PBS. To elute anti-Neu5Gc antibodies, 400 μL 0.5 mM Neu5Gc α-methyl glycoside (GcOMe) in PBS was added to the columns and incubated for 6 min, followed by 3 min centrifugation at 500 rpm. The previous step was repeated with 400 μL 2.5 mM GcOMe. To elute additional antibodies, the columns were washed with citric acid for 1 min at 500 rpm and the flowthrough was neutralised with Tris-HCl (pH 9). Antibodies were dialysed against PBS overnight through a 3500 MWCO membrane and stored at −20 °C. For experiments, the 0.5 mM and 2.5 mM elutions were pooled 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by an MRC DiMeN Doctoral Training Partnership PhD award to EH, and an Horizon Europe Guarantee Consolidator award to MAF (selected by the ERC, funded by UKRI; EP/X023680/1).References
- R. Schauer, Sialic acids as regulators of molecular and cellular interactions, Curr. Opin. Struct. Biol., 2009, 19(5), 507–514 CrossRef CAS PubMed.
- A. Varki and P. Gagneux, Multifarious roles of sialic acids in immunity, Ann. N. Y. Acad. Sci., 2012, 1253(1), 16–36 CrossRef CAS PubMed.
- C. Reily, T. J. Stewart, M. B. Renfrow and J. Novak, Glycosylation in health and disease, Nat. Rev. Nephrol., 2019, 15(6), 346–366 CrossRef PubMed.
- A. Paul and V. Padler-Karavani, Evolution of sialic acids: Implications in xenotransplant biology, Xenotransplantation, 2018, 25(6), e12424 CrossRef PubMed.
- C. Dhar, A. Sasmal and A. Varki, From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc, Front. Immunol., 2019, 10, 807 CrossRef CAS PubMed.
- L. Hof and H. Faillard, The serum dependence of the occurrence of N-glycolylneuraminic acid in HeLa cells, Biochim. Biophys. Acta, 1973, 297(2), 561–563 CrossRef CAS PubMed.
- E. Rodrigues and M. S. Macauley, Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities, Cancers, 2018, 10(6), 207 CrossRef PubMed.
- K. Banda, C. J. Gregg, R. Chow, N. M. Varki and A. Varki, Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid, J. Biol. Chem., 2012, 287(34), 28852–28864 CrossRef CAS PubMed.
- P. Tangvoranuntakul, P. Gagneux, S. Diaz, M. Bardor, N. Varki, A. Varki and E. Muchmore, Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(21), 12045–12050 CrossRef CAS PubMed.
- A. N. Samraj, K. A. Bertrand, R. Luben, Z. Khedri, H. Yu and D. Nguyen, et al., Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk, PLoS One, 2018, 13(6), e0197464 CrossRef PubMed.
- M. O. Altman and P. Gagneux, Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective, Front. Immunol., 2019, 10, 789 CrossRef CAS PubMed.
- R. E. Taylor, C. J. Gregg, V. Padler-Karavani, D. Ghaderi, H. Yu and S. Huang, et al., Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid, J. Exp. Med., 2010, 207(8), 1637–1646 CrossRef CAS PubMed.
- K. Kawanishi, C. Dhar, R. Do, N. Varki, P. Gordts and A. Varki, Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(32), 16036–16045 CrossRef CAS PubMed.
- E. M. Reuven, S. Leviatan Ben-Arye, T. Marshanski, M. E. Breimer, H. Yu and I. Fellah-Hebia, et al., Characterization of immunogenic Neu5Gc in bioprosthetic heart valves, Xenotransplantation, 2016, 23(5), 381–392 CrossRef PubMed.
- A. N. Samraj, H. Laubli, N. Varki and A. Varki, Involvement of a non-human sialic Acid in human cancer, Front. Oncol., 2014, 4, 33 Search PubMed.
- E. Hutton, E. Scott, C. N. Robson, N. Signoret and M. A. Fascione, A systematic review reveals conflicting evidence for the prevalence of antibodies against the sialic acid ‘xenoautoantigen’ Neu5Gc in humans and the need for a standardised approach to quantification, Front. Mol. Biosci., 2024, 11, 1390711 CrossRef CAS PubMed.
- B. A. H. Smith and C. R. Bertozzi, The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans, Nat. Rev. Drug Discovery, 2021, 20(3), 217–243 CrossRef CAS PubMed.
- C. Gao, M. Wei, T. R. McKitrick, A. M. McQuillan, J. Heimburg-Molinaro and R. D. Cummings, Glycan Microarrays as Chemical Tools for Identifying Glycan Recognition by Immune Proteins, Front. Chem., 2019, 7, 833 CrossRef CAS PubMed.
- A. Zhu and R. Hurst, Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum, Xenotransplantation, 2002, 9(6), 376–381 CrossRef PubMed.
- M. Kim, C. S. Park, C. Moon, J. Kim, S. Yang and L. Jang, et al., Structural and quantitative comparison of viral infection-associated N-glycans in plasma from humans, pigs, and chickens: Greater similarity between humans and chickens than pigs, Antiviral Res., 2024, 231, 106009 CrossRef CAS PubMed.
- P. Gagneux, B. Amess, S. Diaz, S. Moore, T. Patel and W. Dillmann, et al., Proteomic comparison of human and great ape blood plasma reveals conserved glycosylation and differences in thyroid hormone metabolism, Am. J. Phys. Anthropol., 2001, 115(2), 99–109 CrossRef CAS PubMed.
- S. Bashir, L. K. Fezeu, S. Leviatan Ben-Arye, S. Yehuda, E. M. Reuven and F. Szabo de Edelenyi, et al., Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Sante study, BMC Med., 2020, 18(1), 262 CrossRef CAS PubMed.
- S. Leviatan Ben-Arye, C. Schneider, H. Yu, S. Bashir, X. Chen, S. von Gunten and V. Padler-Karavani, Differential Recognition of Diet-Derived Neu5Gc-Neoantigens on Glycan Microarrays by Carbohydrate-Specific Pooled Human IgG and IgA Antibodies, Bioconjugate Chem., 2019, 30(5), 1565–1574 CrossRef CAS PubMed.
- M. Hedlund, P. Tangvoranuntakul, H. Takematsu, J. M. Long, G. D. Housley and Y. Kozutsumi, et al., N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution, Mol. Cell. Biol., 2007, 27(12), 4340–4346 CrossRef CAS PubMed.
- V. Padler-Karavani, A. H. Tremoulet, H. Yu, X. Chen, J. C. Burns and A. Varki, A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease, PLoS One, 2013, 8(3), e58443 CrossRef CAS PubMed.
- J. P. Soulillou, E. Cozzi, C. Galli and J. M. Bach, Can we extrapolate from a Cmah (-/-) Ldlr (-/-) mouse model a susceptibility for atherosclerosis in humans?, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(4), 1845–1846 CrossRef CAS PubMed.
- S. Hurh, B. Kang, I. Choi, B. Cho, E. M. Lee and H. Kim, et al., Human antibody reactivity against xenogeneic N-glycolylneuraminic acid and galactose-alpha-1,3-galactose antigen, Xenotransplantation, 2016, 23(4), 279–292 CrossRef PubMed.
- D. Dorvignit, K. F. Boligan, E. Relova-Hernández, M. Clavell, A. López and M. Labrada, et al., Antitumor effects of the GM3(Neu5Gc) ganglioside-specific humanized antibody 14F7hT against Cmah-transfected cancer cells, Sci. Rep., 2019, 9(1), 9921 CrossRef PubMed.
- P. M. Kraemer, Sialic acid of mammalian cell lines, J. Cell. Physiol., 1966, 67(1), 23–34 CrossRef CAS PubMed.
- S. A. Rosenberg and A. B. Einstein, Sialic acids on the plasma membrane of cultured human lymphoid cells. Chemical aspects and biosynthesis, J. Cell Biol., 1972, 53(2), 466–473 CrossRef CAS PubMed.
- G. P. Bhide and K. J. Colley, Sialylation of N-glycans: mechanism, cellular compartmentalization and function, Histochem. Cell Biol., 2017, 147(2), 149–174 CrossRef CAS PubMed.
- P. Thomas and T. G. Smart, HEK293 cell line: A vehicle for the expression of recombinant proteins, J. Pharmacol. Toxicol. Methods, 2005, 51(3), 187–200 CrossRef CAS PubMed.
- Y. Narimatsu, H. J. Joshi, R. Nason, J. Van Coillie, R. Karlsson and L. Sun, et al., An Atlas of Human Glycosylation Pathways Enables Display of the Human Glycome by Gene Engineered Cells, Mol. Cell, 2019, 75(2), 394–407.e5 CrossRef CAS PubMed.
- X. Yang, S. Tao, R. Orlando, I. Brockhausen and F. W. K. Kan, Structures and biosynthesis of the N- and O-glycans of recombinant human oviduct-specific glycoprotein expressed in human embryonic kidney cells, Carbohydr. Res., 2012, 358, 47–55 CrossRef CAS PubMed.
- K. Garapati, A. Jain, B. J. Madden, D.-G. Mun, J. Sharma, R. Budhraja and A. Pandey, Defining albumin as a glycoprotein with multiple N-linked glycosylation sites, J. Transl. Med., 2024, 22(1), 454 CrossRef CAS PubMed.
- K. H. Song, Y. J. Kang, U. H. Jin, Y. I. Park, S. M. Kim and H. H. Seong, et al., Cloning and functional characterization of pig CMP-N-acetylneuraminic acid hydroxylase for the synthesis of N-glycolylneuraminic acid as the xenoantigenic determinant in pig-human xenotransplantation, Biochem. J., 2010, 427, 179–188 CrossRef CAS PubMed.
- A. M. Hernández, N. Rodríguez, J. E. González, E. Reyes, T. Rondón and T. Griñán, et al., Anti-NeuGcGM3 Antibodies, Actively Elicited by Idiotypic Vaccination in Nonsmall Cell Lung Cancer Patients, Induce Tumor Cell Death by an Oncosis-Like Mechanism, J. Immunol., 2011, 186(6), 3735–3744 CrossRef PubMed.
- E. Severi, A. Müller, J. R. Potts, A. Leech, D. Williamson, K. S. Wilson and G. H. Thomas, Sialic Acid Mutarotation Is Catalyzed by the Escherichia coli β-Propeller Protein YjhT*, J. Biol. Chem., 2008, 283(8), 4841–4849 CrossRef CAS PubMed.
- S. Bashir, L. K. Fezeu, S. L. Ben-Arye, S. Yehuda, E. M. Reuven and F. S. de Edelenyi, et al., Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Sante study, BMC Med., 2020, 18(1), 262 CrossRef CAS PubMed.
- N. M. Wilkinson, H.-C. Chen, M. G. Lechner and M. A. Su, Sex Differences in Immunity, Annu. Rev. Immunol., 2022, 40, 75–94 CrossRef CAS PubMed.
- L. S. Zaramela, C. Martino, F. Alisson-Silva, S. D. Rees, S. L. Diaz and L. Chuzel, et al., Gut bacteria responding to dietary change encode sialidases that exhibit preference for red meat-associated carbohydrates, Nat. Microbiol., 2019, 4(12), 2082–2089 CrossRef PubMed.
- P. Shah, X. Wang, W. Yang, S. Toghi Eshghi, S. Sun and N. Hoti, et al., Integrated Proteomic and Glycoproteomic Analyses of Prostate Cancer Cells Reveal Glycoprotein Alteration in Protein Abundance and Glycosylation, Mol. Cell. Proteomics, 2015, 14(10), 2753–2763 CrossRef CAS PubMed.
- M. He, X. Zhou and X. Wang, Glycosylation: mechanisms, biological functions and clinical implications, Signal Transduction Targeted Ther., 2024, 9(1), 194 CrossRef PubMed.
- V. Padler-Karavani, H. Yu, H. Cao, H. Chokhawala, F. Karp and N. Varki, et al., Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease, Glycobiology, 2008, 18(10), 818–830 CrossRef CAS PubMed.
- R. Buonomano, C. Tinguely, R. Rieben, P. J. Mohacsi and U. E. Nydegger, Quantitation and characterization of anti-Galalpha1-3Gal antibodies in sera of 200 healthy persons, Xenotransplantation, 1999, 6(3), 173–180 CrossRef CAS PubMed.
- T. Senage, A. Paul, T. Le Tourneau, I. Fellah-Hebia, M. Vadori and S. Bashir, et al., The role of antibody responses against glycans in bioprosthetic heart valve calcification and deterioration, Nat. Med., 2022, 28(2), 283–294 CrossRef CAS PubMed.
- M. Bardor, D. H. Nguyen, S. Diaz and A. Varki, Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells, J. Biol. Chem., 2005, 280(6), 4228–4237 CrossRef CAS PubMed.
- D. Kealy, J. Wilson, T. Jaconelli, K. Hogg, R. Coop and G. Forshaw, et al., Blood immune profiles reveal a CXCR3/CCR5 axis of dysregulation in early sepsis, J. Leukocyte Biol., 2024, 117(2), 204 CrossRef PubMed.
- T. Q. Gregar and J. Gervay-Hague, Synthesis of oligomers derived from amide-linked neuraminic acid analogues, J. Org. Chem., 2004, 69(4), 1001–1009 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cb00073d |
| This journal is © The Royal Society of Chemistry 2025 |