A rhodamine-conjugated fluorescent and colorimetric receptor for the detection of Cu2+ ions: environmental utility and smartphone integration†
Karuppaiyan
Kaviya‡
 ab,
Ramar
Rajamanikandan
ab,
Ramar
Rajamanikandan
 *c,
Madhappan
Santhamoorthy‡
*c,
Madhappan
Santhamoorthy‡
 de,
Mohammad Abul
Farah
f and
Kailasam Saravana
Mani
de,
Mohammad Abul
Farah
f and
Kailasam Saravana
Mani
 *ab
*ab
aDepartment of Chemistry, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu, India. E-mail: chemsaran.mani@gmail.com
bCentre for Material Chemistry, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu, India
cDepartment of Physics, Gachon University, Seongnam-si, 13120, Republic of Korea. E-mail: chemistrmkd@gmail.com
dDepartment of Physiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science (SIMATS), Saveetha University, Chennai, 600077, India
eSchool of Chemical Engineering, Yeungnam University, Gyeongsan 38544, Republic of Korea
fDepartment of Zoology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
First published on 8th January 2025
Abstract
A new rhodamine based turn on florescent probe (Z)-3′,6′-bis(ethylamino)-2-(((6-methoxy-2-oxo-1,2-dihydroquinolin-3-yl)methylene)amino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one (RME) was efficiently synthesized through a simple condensation reaction of 2-amino-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one and 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde. The receptor RME is highly non-fluorescent and when copper ions (Cu2+ ions) are added in DMF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium, the receptor RME exhibits a specific “turn-on” colorimetric and fluorometric response. Moreover, RME binding with Cu2+ ions produced a remarkable color variation that was perceptible to the human eye, changing from colorless to pink. The binding stoichiometry was 1
2, v/v) medium, the receptor RME exhibits a specific “turn-on” colorimetric and fluorometric response. Moreover, RME binding with Cu2+ ions produced a remarkable color variation that was perceptible to the human eye, changing from colorless to pink. The binding stoichiometry was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, confirmed through theoretical DFT, Job's plot, and mass spectral analysis. RME showed good sensing capability for Cu2+ ions, with LOD values of 11.25 nM for ratiometric colorimetry, 1.42 nM for fluorometry, and 107 nM for smartphone-based detection. These LOD values are significantly lower than WHO's acceptable threshold of 2 μM. Additionally, developing portable techniques based on paper-based test strips and smartphone assistance allowed for qualitative and quantitative detection of Cu2+ ions. These portable and economical methods eliminate the need for expensive laboratory apparatus, enabling precise and affordable on-site assessments. In addition, the developed sensing protocol for the detection of Cu2+ ions was extended to real water samples such as tap, dam, and drinking water samples. These results demonstrated the effectiveness of RME in detecting Cu2+ ions in complex matrices, making it a promising tool for environmental monitoring and water quality assessment.
1, confirmed through theoretical DFT, Job's plot, and mass spectral analysis. RME showed good sensing capability for Cu2+ ions, with LOD values of 11.25 nM for ratiometric colorimetry, 1.42 nM for fluorometry, and 107 nM for smartphone-based detection. These LOD values are significantly lower than WHO's acceptable threshold of 2 μM. Additionally, developing portable techniques based on paper-based test strips and smartphone assistance allowed for qualitative and quantitative detection of Cu2+ ions. These portable and economical methods eliminate the need for expensive laboratory apparatus, enabling precise and affordable on-site assessments. In addition, the developed sensing protocol for the detection of Cu2+ ions was extended to real water samples such as tap, dam, and drinking water samples. These results demonstrated the effectiveness of RME in detecting Cu2+ ions in complex matrices, making it a promising tool for environmental monitoring and water quality assessment.
1. Introduction
Industrial chemical waste streams often contain hazardous waterborne pollutants, including heavy metal ions, bacteria, viruses, and organic solvents. Because of their incapacity to break down in biological systems, heavy metals are among the substances that pose the greatest hazard to all living things, including humans. Heavy metals can therefore accumulate in tissues and cause a variety of health concerns.1,2 It is now urgently necessary to purify contaminated water due to the increasing lack of clean water. After iron and zinc, copper is the third most abundant essential metal in the human body, playing a critical role as a nutrient in various biological systems. However, at elevated levels, it becomes toxic, making its detection crucial in chemical, biological, and environmental studies. Furthermore, plants, animals, and humans all require Cu2+ ions as a trace element.3 However, the absence or excess copper amounts may result in diseases like kidney and liver damage and4 neurotoxicity5 and also cause Wilson's disease, gastrointestinal disorders, hypoglycemia, liver disease, and dyslexia in babies.6–10 Owing to the concentration-dependent nature of Cu2+ ions, guidelines have been released by the World Health Organization to guarantee that the concentration of Cu2+ ions in drinking water is kept below 2 ppm.11 Anthropogenic activities such as mining, industrial processes, and agricultural practices emit Cu2+ ions into the air, soil, and water, increasing their concentration in our surroundings.12–14 Cu2+ ions are one of the main elements required for the normal functioning of living organisms. They catalyze reactions and help transfer electrons within DNA and RNA.15–19 It is extremely desirable and in great demand to counteract this concentration-dependent variable behavior of Cu2+ ions with something simple, selective, sensitive, and especially below the allowable limit.Until now, Cu2+ ions had only been applied in a few widely used, very expensive instrumental methods, like atomic absorption spectroscopy (AAS),20 inductively coupled plasma atomic emission spectroscopy (ICP-AES),21 potentiometry,22 anodic stripping voltammetry,23 spectrophotometry, and spectrofluorimetry.24 Of the methods mentioned above, spectrophotometric and fluorescence chemosensors have garnered the most interest from research groups working in a variety of fields.25 For example, appropriate chemosensors for detecting heavy metal ions are critical due to the direct benefits applicable to essential areas such as the environment, industry, biology, and human health. Optical chemosensors are popular due to their great sensitivity, selectivity, and ease of fabrication. They also display metal-ion-specific fluorescence. In the past few years, fluorescent compounds with heavy metal detection capabilities have emerged as the most fascinating field of study.26 Scientists and researchers primarily use colorimetric or fluorescence methods to determine the presence of heavy metal ions in their work; however, the latter have more affordable and rapid procedures that are easier to apply. In analytical chemistry, creating innovative Cu2+ sensors with less expensive equipment has become popular.27 Because of its quick response time, good spectral resolution, and simple sample preparation, fluorescence is one of the most popular technologies.28 It is noted that multiple events demonstrate how the coordination of Cu2+ affects the photophysical behavior of the receptor. In particular, the main reason why Cu2+ can be detected via CHEQ or CHEF methods is electronic changes based on charge transfer from the ligand to metal (LMCT – ligand-to-metal charge transfer).29 Turn-on fluorescent sensors are favored over turn-off fluorescein-based chemosensors because low sensitivity and turn-off detection are caused by background fluorescence.30
Rhodamine is the fluorophore moiety of a variety of fluorescent probes that have the most potential in creating fluorescence sensors for ions and molecules. Rhodamine conjugates exhibit a visual color shift in the presence of analytes, which allows the analytes to be quickly detected.31–34 This “off–on” process of accuracy and efficiency of rhodamine makes it feasible for researchers to develop a large number of rhodamine derivatives for rapid recognition and visual determination of metal ions with the naked eye.35,36 This occurs since rhodamine and rhodamine derivatives have a spirocyclic ring, which results in ring opening, causing a notable color change as well as absorbance or fluorescence alterations.37,38 Rhodamine dyes with improved emission maxima, visible excitation, photostability,39 and excellent spectroscopic properties are used as biomarkers, in industrial coloring, and as sensing probes. The rhodamine derivatives we obtained which had an open spirolactam ring exhibit a bright pink color and luminous emission due to metal ion chelation.40 In particular, a chemosensor that possesses twin signalling capabilities that is, one that can both generate a discernible shift and increase fluorescence emission will achieve the detection objective. While the majority of described receptors show fluorescence quenching upon interaction with copper ions, only a limited number of sensors for Cu2+ have been shown to exhibit increased fluorescence.41–43 The successful utilization of our compound's fluorescence in response to Cu2+ ions was effectively applied for live cell imaging.44
Herein, we describe a rhodamine-based chemosensor RME that operates in the longer wavelength range with excitation and emission both as a colorimetric and a turn-on fluorescence chemosensor upon binding Cu(II) ions in the DMF/water medium. The sensing mechanism has been examined by systematic spectral and theoretical studies. The notable colorimetric changes observed in the current work were further extended to smartphone-based detection of Cu2+ ions. As expected, RME can specifically recognize and quantify Cu2+ ions from potentially interfering metal ions, with the lowest detection limit as low as 11.25 nM. Furthermore, photometric titrations verified the complexation ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as per JOB's working diagram and the fluorescence enhancement of RME by Cu2+ ions. A reversible Cu2+ detection device was developed with the aid of EDTA, and an implication logic gate was also constructed for Cu2+ detection. Finally, RME was effectively applied to determine Cu2+ ions in real water samples and paper strip applications.
1 as per JOB's working diagram and the fluorescence enhancement of RME by Cu2+ ions. A reversible Cu2+ detection device was developed with the aid of EDTA, and an implication logic gate was also constructed for Cu2+ detection. Finally, RME was effectively applied to determine Cu2+ ions in real water samples and paper strip applications.
2. Experimental section
2.1. Materials and instrumentation
All the reagents and solvents were purchased from commercial sources. Rhodamine 6G and hydrazine hydrate 80% were sourced from Sigma Aldrich. FT IR spectra were measured using the BRUKER Alpha II model in ATR mode. 1H and 13C NMR spectra were recorded on a Bruker Avance Neo (400 MHz) in DMSO-d6 solvent. Alumina-coated F254 Merck plates were used in TLC to monitor the reaction progress. ESI-MS spectra were recorded on a Thermo Scientific High-Resolution Magnetic Sector MS DFS by the positive ion electrospray ionization (ESI) method. UV-visible absorbance spectra were recorded using a JASCO V-750 spectrophotometer. Fluorescence spectrum experiments are conducted using a Horiba Fluoromax Plus spectrofluorometer. Tap and drinking water samples were taken from our university campus (Karpagam Academy of Higher Education) and dam water samples were collected from Siruvani Dam, Coimbatore. Without any pretreatment, collected water samples were utilized for real sample investigations.2.2. Synthesis of RME
The synthesis of 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde was carried out by employing the Vilsmeier–Haack formylation procedure by using a mixture of p-anisidine and acetic anhydride to get N-(4-methoxyphenyl)acetamide which undergoes formylation to afford 2-chloro-6-methoxy quinoline-3-carbaldehyde; furthermore, this aldehyde was hydrolyzed to get 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde by following the previous work.45 The synthesis of Rhodamine 6G hydrazide was carried out by using Rhodamine 6G and hydrazine hydrate 80% in ethanol medium. The reaction mixture was stirred and refluxed at 90 °C for 6 h. The resulting precipitate was filtered, washed with distilled water, and left to dry. The crude product was crystallized with ethanol. The rhodamine 6G hydrazide was obtained as a pink solid.46The probe RME was synthesized by adding an equimolar mixture of synthesized 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde (0.09 g, 1 mmol) and rhodamine 6G hydrazide (0.2 g, 1 mmol) in 10 mL of ethanol, and the reaction mixture was allowed to reflux at 90 °C in an oil bath. The reaction process was monitored by TLC, and the obtained precipitate was filtered and then completely washed with cold ethanol. The product was vacuum dried and taken for further steps without any purification.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2965 (–NH), 1646 (CH
O), 2965 (–NH), 1646 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (400 MHz, DMSO-d6) δppm: 11.74 (s, 1H), 8.61 (s, 1H), 8.13 (s, 1H), 7.93 (d, J = 4.0 Hz, 1H), 7.58–7.53 (m, 2H), 7.26–7.20 (m, 2H), 7.13 (s, 1H), 6.99 (d, J = 4.0 Hz, 1H), 6.35 (s, 2H), 6.22 (s, 2H), 5.06 (s, 2H), 4.36 (s, 1H), 3.76 (s, 3H), 3.15 (t, J = 8.0 Hz, 4H), 1.86 (s, 6H), 1.23 (t, J = 8.0 Hz, 6H); 13C NMR (100.1 MHz) δppm: 164.63, 160.68, 154.82, 152.87, 151.14, 148.24, 141.08, 134.46, 134.16, 134.12, 129.02, 127.94, 126.96, 126.42, 124.02, 123.58, 121.27, 119.78, 118.72, 116.81, 110.21, 104.97, 96.54, 65.66, 55.89, 19.02, 17.46, 14.63, 37.94. MS (ESI – M + Na) m/z 636.6. Anal. calcd For C37H35N5O4; C, 72.41; H, 5.75; N, 11.41; Found; C, 72.45; H, 5.71; N, 11.42.
N); 1H NMR (400 MHz, DMSO-d6) δppm: 11.74 (s, 1H), 8.61 (s, 1H), 8.13 (s, 1H), 7.93 (d, J = 4.0 Hz, 1H), 7.58–7.53 (m, 2H), 7.26–7.20 (m, 2H), 7.13 (s, 1H), 6.99 (d, J = 4.0 Hz, 1H), 6.35 (s, 2H), 6.22 (s, 2H), 5.06 (s, 2H), 4.36 (s, 1H), 3.76 (s, 3H), 3.15 (t, J = 8.0 Hz, 4H), 1.86 (s, 6H), 1.23 (t, J = 8.0 Hz, 6H); 13C NMR (100.1 MHz) δppm: 164.63, 160.68, 154.82, 152.87, 151.14, 148.24, 141.08, 134.46, 134.16, 134.12, 129.02, 127.94, 126.96, 126.42, 124.02, 123.58, 121.27, 119.78, 118.72, 116.81, 110.21, 104.97, 96.54, 65.66, 55.89, 19.02, 17.46, 14.63, 37.94. MS (ESI – M + Na) m/z 636.6. Anal. calcd For C37H35N5O4; C, 72.41; H, 5.75; N, 11.41; Found; C, 72.45; H, 5.71; N, 11.42.
2.3. General procedure for absorption and fluorescence measurements
RME stock solution (10 mM) was prepared by adding 6.4 mg in 10 mL of DMF solution and the stock solution (1 mM) of Cu2+ ions was prepared in double distilled water. For photometric titration experiments, a cuvette was filled with 10 μM RME dissolved in 2.5 mL of DMF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). Subsequently, appropriate amounts of Cu2+ stock solution were blended with the reaction mixture and shaken well. After a 2 min incubation period, the UV-vis absorbance spectra were recorded in the range of 600 nm to 200 nm. A similar protocol was followed for fluorescence spectral investigations. The excitation wavelength for the fluorescence spectra was set at 415 nm, and spectrum analysis was carried out after 2 min with a 2/2 nm slit emission/excitation slid width. Absorbance/emission spectrum investigations were used in a photometric titration based technique, and specificity measurements were carried out in place of Cu2+ ions and other potentially interfering metal ions mixed with the reaction mixture. All the sensing-related measurements were performed a minimum of three times.
3, v/v). Subsequently, appropriate amounts of Cu2+ stock solution were blended with the reaction mixture and shaken well. After a 2 min incubation period, the UV-vis absorbance spectra were recorded in the range of 600 nm to 200 nm. A similar protocol was followed for fluorescence spectral investigations. The excitation wavelength for the fluorescence spectra was set at 415 nm, and spectrum analysis was carried out after 2 min with a 2/2 nm slit emission/excitation slid width. Absorbance/emission spectrum investigations were used in a photometric titration based technique, and specificity measurements were carried out in place of Cu2+ ions and other potentially interfering metal ions mixed with the reaction mixture. All the sensing-related measurements were performed a minimum of three times.
2.4. Smartphone assisted Cu2+ recognition
To identify Cu2+ in DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) solution, a smartphone-based color assist application (color Grap) was applied to digitalize the color shift of RME with Cu2+ that can be seen with the naked eye. In a glass tube, different quantities of Cu2+ ions were mixed in RME (10 μM) in a DMF/water medium. Color variations were captured on the back camera of a Vivo V23 Pro smartphone. Lastly, the Color Grab app, a freely available mobile application, digitalized the red, green, and blue (RGB) values of the photos.
3 v/v) solution, a smartphone-based color assist application (color Grap) was applied to digitalize the color shift of RME with Cu2+ that can be seen with the naked eye. In a glass tube, different quantities of Cu2+ ions were mixed in RME (10 μM) in a DMF/water medium. Color variations were captured on the back camera of a Vivo V23 Pro smartphone. Lastly, the Color Grab app, a freely available mobile application, digitalized the red, green, and blue (RGB) values of the photos.
2.5. Paper strip application
The first identical-size Whatman filter paper was developed to create the paper-based test strips. The Whatman filter paper was soaked in an RME-dissolved DMF/water stock solution to make RME sensor test strips and dried in air. Subsequently, RME-coated paper test strips were spiked into standard solutions of Cu2+ ions and dried in air. Finally, colorimetric images of the paper-based test strips were taken using a Vivo V23 Pro (108 MP camera resolution) under ambient conditions (without flash).3. Results and discussion
3.1. Design and synthesis of the probe
The synthesis of the fluorescent receptor RME was explored and is outlined in Scheme 1via the well-known Schiff-base condensation reaction of compound 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde and rhodamine 6G hydrazide under reflux conditions at 90 °C in ethanol medium. The rhodamine 6G hydrazide and the compound 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde were synthesized by using the previously described technique.45,46 The structure of the synthesized receptor, RME was attested through spectroscopic techniques like FTIR, 1H and 13C NMR, and ESI-MS spectral analysis.In the IR spectrum of probe RME, the characteristic peaks at 1711 cm−1, 2965 cm−1, and 1646 cm−1 were attributed to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NH –C
O, –NH –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and functional groups respectively (Fig. S1†) The 1H NMR spectrum of the receptor RME (Fig. S2†), the –NH proton appearing at δ 11.78 ppm as a singlet corresponds to the quinoline core, OCH3 appeared at δ 3.76 ppm as a singlet, azomethine proton CH
N, and functional groups respectively (Fig. S1†) The 1H NMR spectrum of the receptor RME (Fig. S2†), the –NH proton appearing at δ 11.78 ppm as a singlet corresponds to the quinoline core, OCH3 appeared at δ 3.76 ppm as a singlet, azomethine proton CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N appeared at δ 8.6 ppm as a singlet, two –NH protons present in the rhodamine core appeared at δ 6.22 ppm as singlets, –CH2 protons in the ethyl group appeared as a triplet at δ 3.15 ppm and –CH3 protons in the ethyl group appeared as a triplet at δ 1.25 ppm of the rhodamine core. In the 13C NMR spectrum (Fig. S3†), the peak at δ 56.5 ppm was due to OCH3 and the peak at δ 164.3 ppm was due to –NHC
N appeared at δ 8.6 ppm as a singlet, two –NH protons present in the rhodamine core appeared at δ 6.22 ppm as singlets, –CH2 protons in the ethyl group appeared as a triplet at δ 3.15 ppm and –CH3 protons in the ethyl group appeared as a triplet at δ 1.25 ppm of the rhodamine core. In the 13C NMR spectrum (Fig. S3†), the peak at δ 56.5 ppm was due to OCH3 and the peak at δ 164.3 ppm was due to –NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the quinoline core. The –C
O in the quinoline core. The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon in the indole core appeared at δ 160.6 ppm and the two –CH3 carbons appeared at δ 14.6 ppm and –CH2CH3 carbons appeared at δ 37.9, 17.4 and 19.0 ppm respectively. Furthermore, the molecular ion peak at m/z 614.6 (M + H)+ (ESI-MS) (Fig. S4†) confirms the structure of RME as (Z)-3′,6′-bis(ethylamino)-2-(((6-methoxy-2-oxo-1,2-dihydroquinolin-3-yl)methylene)amino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one.
O carbon in the indole core appeared at δ 160.6 ppm and the two –CH3 carbons appeared at δ 14.6 ppm and –CH2CH3 carbons appeared at δ 37.9, 17.4 and 19.0 ppm respectively. Furthermore, the molecular ion peak at m/z 614.6 (M + H)+ (ESI-MS) (Fig. S4†) confirms the structure of RME as (Z)-3′,6′-bis(ethylamino)-2-(((6-methoxy-2-oxo-1,2-dihydroquinolin-3-yl)methylene)amino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one.
3.2. Spectrophotometric features of ratiometric/colorimetric selectivity of the receptor RME
UV-vis absorption spectrum investigations were performed to track the photophysical action of RME beforehand and following the different additions of Cu2+ ions. The related results are shown in Fig. 1a. In the absence of Cu2+ ions, the receptor RME shows three absorbance bands at 306 nm, 337 nm, and 410 nm, respectively, in the DMF/water medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium, which corresponds to the π–π* and n–π* transitions, respectively.47 It can be seen from Fig. 3 that after the addition of Cu2+ ions to the receptor RME, the peak at 306 nm starts decreasing and the peak around 337 nm starts increasing; simultaneously there is a new absorption peak emerging at 525 nm in the visible region. RME with Cu2+ ions has a significant color change from yellow to pink. As a result, a noticeable color variation from yellow to pink is visible to the naked eye. It is evident that the addition of additional competing anions and cations (Mg2+, Na+, Zn2+, Ni2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Fe2+, Fe3+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+) to the appropriate medium (RME) does not affect the color or spectral features of the RME receptor (inset of Fig. 1b). Additionally, ratiometric (A525/A410) investigation for Cu2+ may be accomplished utilizing these two wavelengths; other interfering anions and cations including Mg2+, Na+, Zn2+, Ni2+, Cu2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Fe2+, Fe3+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+ lack this capability, making RME-based ratiometric/colorimetric chemosensors extremely specific for Cu2+ ions (Fig. 1b).
2, v/v) medium, which corresponds to the π–π* and n–π* transitions, respectively.47 It can be seen from Fig. 3 that after the addition of Cu2+ ions to the receptor RME, the peak at 306 nm starts decreasing and the peak around 337 nm starts increasing; simultaneously there is a new absorption peak emerging at 525 nm in the visible region. RME with Cu2+ ions has a significant color change from yellow to pink. As a result, a noticeable color variation from yellow to pink is visible to the naked eye. It is evident that the addition of additional competing anions and cations (Mg2+, Na+, Zn2+, Ni2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Fe2+, Fe3+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+) to the appropriate medium (RME) does not affect the color or spectral features of the RME receptor (inset of Fig. 1b). Additionally, ratiometric (A525/A410) investigation for Cu2+ may be accomplished utilizing these two wavelengths; other interfering anions and cations including Mg2+, Na+, Zn2+, Ni2+, Cu2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Fe2+, Fe3+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+ lack this capability, making RME-based ratiometric/colorimetric chemosensors extremely specific for Cu2+ ions (Fig. 1b).
3.3. Spectrophotometric titration experiments for RME with Cu2+ ions
At the same time, absorbance spectral changes of RME with incremental amounts of Cu2+ ions were monitored in DMF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium, and the typical equilibrium process is depicted in Fig. 2. After measuring the spectra, it was discovered that the isosbestic point was in the 442 nm region, signifying the beginning of the formation of a stable complex between the Cu2+ ions and the receptor RME where the continuous addition of Cu2+ towards the receptor manifests a clear color shift, and the translucent solution turns pink. This event unequivocally demonstrates that Cu2+ binding causes the RME ring-opening structure. The notable spectral changes, colorimetric alterations, and a new absorbance peak that appeared at 525 nm should be attributed to the characteristic peak for the Cu2+ ion induced by the open ring-closed spirolactam ring in the receptor RME.48 According to colorimetric measurements, the aforementioned findings show that the receptor RME recognizes Cu2+ ions highly preferentially. To calculate the lowest limit of detection (LOD) of the ratiometric detection tactic, the calibration curve was plotted between the (A525/A410) and the different concentrations of Cu2+ ions. In the concentration range of 10 μM to 100 μM, good linear behavior was attained with a correlation coefficient of 0.99. The LOD was estimated to be 11.25 nM by using the formula LOD = 3σ/S, σ is the standard deviation of five measurements taken in the blank (0.0015) and S shows the calibration curve's slope (Fig. S5†). The determined LOD is significantly less than the maximum allowable level of Cu2+ ions in drinking water that has been approved by the WHO.
2, v/v) medium, and the typical equilibrium process is depicted in Fig. 2. After measuring the spectra, it was discovered that the isosbestic point was in the 442 nm region, signifying the beginning of the formation of a stable complex between the Cu2+ ions and the receptor RME where the continuous addition of Cu2+ towards the receptor manifests a clear color shift, and the translucent solution turns pink. This event unequivocally demonstrates that Cu2+ binding causes the RME ring-opening structure. The notable spectral changes, colorimetric alterations, and a new absorbance peak that appeared at 525 nm should be attributed to the characteristic peak for the Cu2+ ion induced by the open ring-closed spirolactam ring in the receptor RME.48 According to colorimetric measurements, the aforementioned findings show that the receptor RME recognizes Cu2+ ions highly preferentially. To calculate the lowest limit of detection (LOD) of the ratiometric detection tactic, the calibration curve was plotted between the (A525/A410) and the different concentrations of Cu2+ ions. In the concentration range of 10 μM to 100 μM, good linear behavior was attained with a correlation coefficient of 0.99. The LOD was estimated to be 11.25 nM by using the formula LOD = 3σ/S, σ is the standard deviation of five measurements taken in the blank (0.0015) and S shows the calibration curve's slope (Fig. S5†). The determined LOD is significantly less than the maximum allowable level of Cu2+ ions in drinking water that has been approved by the WHO.
 | ||
Fig. 2 UV-visible absorption spectra of RME (10 μM) upon adding Cu2+ ions (0–100 μM) into DMF/aqueous (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium and the inset shows the magnified view of the newly appeared peak at 525 nm. 2, v/v) medium and the inset shows the magnified view of the newly appeared peak at 525 nm. | ||
3.4. Fluorescence emission spectroscopic studies of receptor RME with Cu2+ ions
To further verify the binding ability of RME with Cu2+ ions, emission spectral studies were carried out. The fluorescence emission titration experiment was carried out with the incremental addition of Cu2+ ions to the receptor RME in DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). Upon the addition of Cu2+ ions to the receptor RME, the emission intensity (550 nm) starts to gradually increase (approx. 14-fold) (Fig. 3) with a strong red shift in the wavelength. Due to the fluorescence resonance energy transfer (FRET) method, the probe RME showed a rise in fluorescence in the DMF/water medium when stimulated at 415 nm as the concentration of Cu2+ increased.49 Under an UV lamp, when Cu2+ was added to the RME chemosensor, no notable color change was noticed. The paramagnetic couple Cu2+ interact in many ways would make sense if the resultant strong fluorescent complex between RME and Cu2+ ions. This strong interaction could induce the spirolactam ring of (RME) and subsequently increase the fluorescence of the xanthenyl moiety.50 Based on the emission enhancement, the concentrations of Cu2+ ions were quantified. Then, the LOD value was determined by using the equation, LOD = 3σ/S, which was applied to calculate the limit of detection (LOD) of RME for Cu2+ ions, which is defined as a signal-to-noise ratio (S/N) of 3.51–53 From the calibration plot, the LOD value was calculated to be 1.42 nM, which is much lower than the WHO criteria (2 ppm). Therefore, the proposed work is more appropriate for the detection of Cu2+ ions in drinking water using the RME receptor. Furthermore, the effect of pH on RME and RME with Cu2+ ions was investigated by emission spectral studies. A pH of 4–10 was accomplished by mixing NaOH or HCl with 0.01 M phosphate buffer saline (PBS). The developed receptor RME and RME with Cu2+ ions display the maximum emission intensity in neutral pH around 6 to 8 (Fig. S6†). Thus, we have kept the pH at 7 for all sensing experiments.
2, v/v). Upon the addition of Cu2+ ions to the receptor RME, the emission intensity (550 nm) starts to gradually increase (approx. 14-fold) (Fig. 3) with a strong red shift in the wavelength. Due to the fluorescence resonance energy transfer (FRET) method, the probe RME showed a rise in fluorescence in the DMF/water medium when stimulated at 415 nm as the concentration of Cu2+ increased.49 Under an UV lamp, when Cu2+ was added to the RME chemosensor, no notable color change was noticed. The paramagnetic couple Cu2+ interact in many ways would make sense if the resultant strong fluorescent complex between RME and Cu2+ ions. This strong interaction could induce the spirolactam ring of (RME) and subsequently increase the fluorescence of the xanthenyl moiety.50 Based on the emission enhancement, the concentrations of Cu2+ ions were quantified. Then, the LOD value was determined by using the equation, LOD = 3σ/S, which was applied to calculate the limit of detection (LOD) of RME for Cu2+ ions, which is defined as a signal-to-noise ratio (S/N) of 3.51–53 From the calibration plot, the LOD value was calculated to be 1.42 nM, which is much lower than the WHO criteria (2 ppm). Therefore, the proposed work is more appropriate for the detection of Cu2+ ions in drinking water using the RME receptor. Furthermore, the effect of pH on RME and RME with Cu2+ ions was investigated by emission spectral studies. A pH of 4–10 was accomplished by mixing NaOH or HCl with 0.01 M phosphate buffer saline (PBS). The developed receptor RME and RME with Cu2+ ions display the maximum emission intensity in neutral pH around 6 to 8 (Fig. S6†). Thus, we have kept the pH at 7 for all sensing experiments.
 | ||
Fig. 3 Fluorescence spectra of RME solutions in the presence of Cu2+ ions in DMF/aqueous (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. 2, v/v) medium. | ||
3.5. Density functional theory calculations
A molecular-level investigation was conducted into the process of complex formation between the RME and Cu2+ ions using a density functional theory-based computational technique which was optimized at the B3LYP/M06-2X functional and LANL2DZ basis set and the quantum chemical calculations were done using the Gaussian 16 software package. The Cu2+ complex of RME, together with its optimal geometry and highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO), is displayed in Fig. 4. It is evidenced from Fig. 4 that the HOMO was dispersed across the rhodamine core and the LUMO was dispersed in the quinoline moiety. After the coordination of RME with Cu2+ ions, the HOMO and LUMO were dispersed in the rhodamine core. | ||
| Fig. 4 Frontier molecular orbital energy levels of the RME–Cu2+ complex calculated using the B3LYP/M06-2X functional and LANL2DZ basis set. | ||
The DFT-optimized structures of the receptor RME and RME–Cu2+ ensemble are disclosed in Fig. S7.† From Fig. S7,† the geometry of RME–Cu2+ is trigonal bipyramidal, where the receptor RME coordinates with Cu2+ through quinoline (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), imine (–CH
O), imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and ring-opened (–C–O) with calculated bond distances of 2.001 Å, 1.998 Å and 2.029 Å respectively (Fig. S7†). The SO42− formed a coordination with Cu2+ with bond distances of 2.413 Å and 1.979 Å respectively. The HOMO–LUMO energy level gap for the complex is 4.437 eV, while the energy level gap for the HOMO and LUMO of the probe RME is 5.528 eV. Based on the previously described results, the RME–Cu2+ complex showed a smaller frontier orbital gap than the receptor RME, which made it more reactive and polarizable.
N) and ring-opened (–C–O) with calculated bond distances of 2.001 Å, 1.998 Å and 2.029 Å respectively (Fig. S7†). The SO42− formed a coordination with Cu2+ with bond distances of 2.413 Å and 1.979 Å respectively. The HOMO–LUMO energy level gap for the complex is 4.437 eV, while the energy level gap for the HOMO and LUMO of the probe RME is 5.528 eV. Based on the previously described results, the RME–Cu2+ complex showed a smaller frontier orbital gap than the receptor RME, which made it more reactive and polarizable.
3.6. Reversibility experiments with RME and EDTA
Reversibility research is crucial for the growth of chemo biosensors with high degrees of specificity and sensitivity for components required for accurate and efficient analyte detection in real-world applications. Using EDTA, a chelating agent, as a coordinating receptor to ascertain the probe's reusable feature in the absorbance detection process allowed for the determination of the probe RME's reversibility towards Cu2+ ions. The Cu2+ analyte and the probe RME were first coupled experimentally to generate a complex at 550 nm in DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
After adding EDTA, the RME–Cu2+ complex was found to have returned to its original state (Scheme 2). When the EDTA–Cu2+ complex formed, the pink hue of the RME–Cu2+ complex returned to the pale-yellow color of the free receptor RME. A series of investigations were conducted to test the receptor reversibility, and the results established that the probe can detect Cu2+ ions four times, as shown in (Fig. 5a and b). This reaction has shown a decrease in emission intensity when the Cu2+ ions are added to the RME + Cu2+ ions + EDTA combination. Nearly ∼80% of the emission intensity was stored at the end of the fourth cycle (Fig. 5b). This investigation was conducted to evaluate the sensor's regularity and dependability in identifying certain analytes. Thus, it has been demonstrated that adding an EDTA solution can reverse the RME's reaction to the Cu2+ ion. The receptor RME is reusable because this cycle can potentially be repeated at least four times.
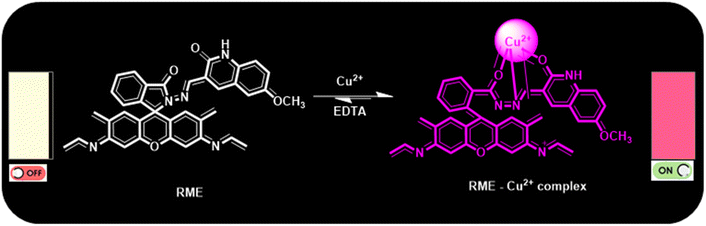 | ||
| Scheme 2 Plausible mechanism for the formation of the RME–Cu2+ complex and reversibility with a chelating agent, EDTA. | ||
 | ||
| Fig. 5 Reversibility spectra (a) and cycle (b) of RME towards Cu2+ (100 μM) along with EDTA (100 μM). | ||
3.7. Logic gate behavior
Using the sequence-dependent fluorescence output between EDTA and Cu2+ ions as chemical inputs, we have studied the fluorescence “turn on” process of the probe RME to build the molecular logic gate behavior of the current system.44,45 In this instance, an INHIBIT logic gate behavior corresponds quite well with this receptor RME. The given scenario uses the INHIBIT logic gate belongings of the probe RME, Cu2+ ions, and EDTA as inputs (1 & 2) and the fluorescence peak intensity at 525 nm as the output (Fig. 6a). The logic gate is ON if the output is “1”, and this behavior can only be seen when Cu2+ ions are present. In three distinct scenarios, the existence of EDTA, the occurrence of both EDTA and Cu2+ ions, and without mixing of both EDTA and Cu2+ ions if the output is “0”, it indicates that the logic gate is OFF. These results implied that the behavior of the receptor RME is that of an INHIBIT logic gate.45 There are probably several interactions between the paramagnetic Cu2+ ions, which results in the production of a highly fluorescent complex with RME. The spirolactam ring in RME may open as a result of this interaction, increasing the fluorescence of the xanthenyl moiety. The INHIBIT logic gate was the subject of another logic gate application carried out for the study (Fig. 6b–d). Herein, Cu2+ and EDTA signify input values 1 and 2, while color change (Fig. 6b), and/or A525/A410 (Fig. 6c), the outputs. In these systems, when both inputs are absent (0, 0), both inputs are present (1, 1), or input 2 is present but input 1 is absent (0, 1), the output of these systems is 0. In contrast, the result is 1 when input 1 is present and input 2 is not (1, 0) (Fig. 6d). This action, which results in a color shift from yellow to pink, is explained by the Cu2+ ions producing an enhanced absorbance band that is dependent on the concentration at 525 nm.3.8. Paper strip based applications
Because they are inexpensive to prepare, paper-strip-based chemosensors can be used in a wide range of settings, including those with limited resources and on-site monitoring applications. Numerous chemosensors based on paper offer prompt data, enabling prompt executive processing. For instance, certain organic molecules can yield outcomes in a couple of minutes, which is crucial in situations like ecological tracking or food-related investigations where speed is of the essence. In this work, Whatman filter paper was soaked in an RME-dissolved DMF/water mixture to develop paper-based sensor test strips which contain 2.5 mL of the receptor RME dissolved DMF/water mixture, and kept soaked for more than 5 min. Before applying the Cu2+ ion sensor, the paper-based test strips were allowed to air dry for almost eight hours. RME-deposited paper strips with incremental quantities of Cu2+ ions were investigated and the corresponding colorimetric photographs are illustrated in Fig. 7. Before the spiking of Cu2+ ions, paper strips were slight yellow in color. As the concentration of metal ions increased, the test strip's pink color got more vibrant, and as illustrated in Fig. 7, it was able to detect Cu2+ at concentrations as low as 20 μM using receptor RME. Additionally, the RME–Cu2+ complex has been applied as an organic ink. Initially, Whatman filter paper was dipped in the receptor RME (100 μM) for 5 min and then it was dried under air. This paper is yellow in color. Subsequently, a butterfly was sketched and inscribed using Cu2+ ions (50 μM) on the RME-coated paper strips; both the written word and the butterfly are readily visible to the naked eye. These findings demonstrate that the RME–Cu2+ complex was applied for (ink or marker) writing purposes on the filter paper.3.9. Integration of the smartphone with the present colorimetric method
A straightforward facile to use smartphone testing may additionally validate the color variation for RME in the presence of Cu2+ ions in DMF/water medium, as it yields more accurate and sensitive results than optical detection. Here, the various quantities of Cu2+ ions are mixed distinctly with the standard solution of the probe RME (100 μM) in DMF/water medium and the corresponding color variation photograph is illustrated in the inset of Fig. 8. An existing smartphone color app (color picker) was used to resolve the red, green, and blue (RGB) primary color intensity values. This smartphone-based sensing technique primarily interprets digital photos of glass vials containing colloidal solutions using RGB values, which cover from 0 to 255. These systems are capable of translating color into RGB values using values like [0, 0, 0] for pure black and [255, 255, 255] for white.53 Descriptive photos of each set of the probe RME solution with varying concentrations of Cu2+ ions were taken using the smartphone. By keeping a constant distance from the smartphone, the receptor RME with and without Cu2+ ion solutions was captured in a picture against a white background. The receptor RME solution's color gradually changed from slightly yellow to pink as the concentration of Cu2+ ions raised from 10 to 70 μM. The mean RGB values and Cu2+ ion concentrations between 10 and 70 μM were regressed linearly using the equation y = 193.35 + 1.61 [Cu2+ ions] with R2 = 0.978. A calibration curve can be constructed using the average RGB value's linear increase with the quantities of Cu2+ ions as displayed in Fig. 8. From the slope of the linear calibration curve, the value of LOD is calculated to be 107 nM, which is quite lower than the tolerable limits by authorities' reports. As a result, a quick, easy, on-site, and reasonably priced method of detecting Cu2+ ions based on the receptor RME's color fluctuation is made possible via smartphone applications. Table S1† summarizes the collected analytical parameters of the current approach and compares them with those of previously published methods of Cu2+ ion detection using relevant chemical compounds. Table S1† illustrates the three distinct methods for detecting Cu2+ ions, which can be two to three orders extended or have comparable LOD values. It may contribute to the RME sensing probe's excellent qualities, such as its quick reaction time, lowest detection limit, selectivity, and so forth (RME). | ||
Fig. 8 The calibration curve of the RGB color response using a smartphone and the inset shows a color variance of RME with different amounts of Cu2+ ions in DMF/aqueous (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. 2, v/v) medium. | ||
3.10. Selectivity of RME towards various metal ions
Selectivity is a crucial factor for the chemosensors. The fluorescence selectivity experiments of the synthesized receptor, RME towards potentially interfering metal cations anions including Mg2+, Na+, Zn2+, Ni2+, Cu2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Co2+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, Cr3+ and Cu2+ were tracked in DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium and the results are outlined in Fig. 9. As evidenced from Fig. 9, with the addition of 5-fold increasing amounts of different metal ions to the RME solution, only the Cu2+ ions induced a complete fluorescence enhancement of the receptor, RME (Fig. 9). The other abovementioned metal cations and anions such as Mg2+, Na+, Zn2+, Ni2+, Cu2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Co2+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+ could not induce any change in the emission intensity and colorimetric nature under daylight. These findings suggested that the receptor RME might function as a colorimetric and fluorescence chemosensor that is selective for Cu2+ ions. To understand the binding nature of Cu2+ with RME, the continuous variation method (Job's plot) was applied, in which the intensity of the band was plotted against the molar fraction of RME (Fig. S8†). The observed results suggested the formation of 1
1, v/v) medium and the results are outlined in Fig. 9. As evidenced from Fig. 9, with the addition of 5-fold increasing amounts of different metal ions to the RME solution, only the Cu2+ ions induced a complete fluorescence enhancement of the receptor, RME (Fig. 9). The other abovementioned metal cations and anions such as Mg2+, Na+, Zn2+, Ni2+, Cu2+, Ce3+, Cd2+, Bi2+, K+, Co2+, Ca2+, Co2+, HSO3−, Ag+, Pb2+, Cl−, Br−, Ba2+, I−, NO3−, and Cr3+ could not induce any change in the emission intensity and colorimetric nature under daylight. These findings suggested that the receptor RME might function as a colorimetric and fluorescence chemosensor that is selective for Cu2+ ions. To understand the binding nature of Cu2+ with RME, the continuous variation method (Job's plot) was applied, in which the intensity of the band was plotted against the molar fraction of RME (Fig. S8†). The observed results suggested the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between RME and Cu2+ during their complexation. Furthermore, the 1
1 stoichiometry between RME and Cu2+ during their complexation. Furthermore, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was confirmed through the mass spectroscopy m/z value of 774.34 (Fig. S9†).
1 binding stoichiometry was confirmed through the mass spectroscopy m/z value of 774.34 (Fig. S9†).
3.11. Practical applications
The amounts of Cu2+ ions in tap, dam, and drinking water samples were successfully measured using the probe RME. To find the Cu2+ ions in actual water samples, a conventional addition technique was used.52 In the drinking, dam, and tap water samples, Cu2+ ions were first not found. To account for a notable rise in emission intensity for triplicate observations, Cu2+ ions were mixed into environmental water samples. Table S2† displays the actual water research observations that were gathered. When Cu2+ ions were introduced into tap, dam, and drinking water samples, there was an outstanding recovery from 91.75 to 96.82%. The current method's great accuracy was suggested by its lowest relative standard deviation value (>2.21%).4. Conclusion
In conclusion, a new rhodamine and quinoline-based simple naked eye and switch-on luminescent receptor (RME) has been efficaciously synthesized for Cu2+ ion recognition in DMF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. The spirolactam compound demonstrated outstanding specificity towards Cu2+ ions over other typically occurring cations in conventional DMF/H2O medium. Upon the interaction of Cu2+ ions, the receptor color varied from slightly yellow to pink in color which was easily viewed by the naked eye whereas strong spectral fluctuations were noticed in absorbance and emission studies. RME showed a good sensing performance for Cu2+ ions with LOD 11.25 nM for ratiometric colorimetry, 1.42 nM for fluorometry, and 107 nM for the smartphone-based detection strategy, significantly lower than the tolerable limits (20 μM) by WHO. The mass spectral analysis, Job's plot, and DFT method were employed to reveal the probable 1
2, v/v) medium. The spirolactam compound demonstrated outstanding specificity towards Cu2+ ions over other typically occurring cations in conventional DMF/H2O medium. Upon the interaction of Cu2+ ions, the receptor color varied from slightly yellow to pink in color which was easily viewed by the naked eye whereas strong spectral fluctuations were noticed in absorbance and emission studies. RME showed a good sensing performance for Cu2+ ions with LOD 11.25 nM for ratiometric colorimetry, 1.42 nM for fluorometry, and 107 nM for the smartphone-based detection strategy, significantly lower than the tolerable limits (20 μM) by WHO. The mass spectral analysis, Job's plot, and DFT method were employed to reveal the probable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation mode between Cu2+ ions and the receptor RME. Furthermore, the developed fluorescence sensor was applied for the detection of Cu2+ ions in environmental water samples such as tap, dam, and drinking water samples. Additionally, practical smartphone and paper-based test strip applications were utilized to fruitfully accomplish both qualitative and quantitative Cu2+ ion quantification, avoiding the need for sophisticated equipment and enabling accurate and reasonably priced on-site assessment of potential Cu2+ ions.
1 stoichiometric complexation mode between Cu2+ ions and the receptor RME. Furthermore, the developed fluorescence sensor was applied for the detection of Cu2+ ions in environmental water samples such as tap, dam, and drinking water samples. Additionally, practical smartphone and paper-based test strip applications were utilized to fruitfully accomplish both qualitative and quantitative Cu2+ ion quantification, avoiding the need for sophisticated equipment and enabling accurate and reasonably priced on-site assessment of potential Cu2+ ions.
Data availability
The authors ensure that all the data mentioned in this paper are available on request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research work has been supported financially by the Department of Science and Technology (DST/TDT/TC/RARE/2022/13) dated 27.03.2023. The author also acknowledges NMR facility, CIC, Bharathiar University supported by DST (PURSE Phase II programme), New Delhi. The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSPD2025R694), King Saud University, Riyadh, Saudi Arabia.References
- M. R. Awual, I. M. M. Rahman, T. Yaita, M. A. Khaleque and M. Ferdows, pH dependent Cu(II) and Pd(II) ions detection and removal from aqueous media by an efficient mesoporous adsorbent, Chem. Eng. J., 2014, 236, 100–109 CrossRef CAS.
- E. Fan, H. Guo, T. Hao, R. Zhao, P. Zhang, Y. Feng, Y. Liu and K. Deng, Morpholine-modified polyacrylamides with polymerization-induced emission and its specific detection to Cu2+ ions, Spectrochim. Acta, Part A, 2024, 309, 123782 Search PubMed.
- D. G. J. Barceloux, Clin. Toxicol., 1999, 37, 217 CAS.
- V. Kumar, J. Kalita, H. K. Bora and U. K. Misra, Temporal kinetics of organ damage in copper toxicity: A histopathological correlation in rat model, Regul. Toxicol. Pharmacol., 2016, 81, 372–380 CrossRef CAS.
- M. S. Moorthy, H. J. Cho, E. J. Yu, Y. S. Jung and C. S. Ha, A modified mesoporous silica optical nanosensor for selective monitoring of multiple analytes in water, Chem. Commun., 2013, 49, 8758–8760 RSC.
- R. Chandra, A. Ghorai and G. K. Patra, A simple benzildihydrazone derived colorimetric and fluorescent ‘on–off–on’ sensor for sequential detection of copper(II) and cyanide ions in aqueous solution, Sens. Actuators, B, 2018, 255, 701–711 CrossRef CAS.
- H. S. Jung, M. Park, D. Y. Han, E. Kim, C. Lee, S. Ham and J. S. Kim, Cu2+ Ion-Induced Self-Assembly of Pyrenylquinoline with a Pyrenyl Excimer Formation, Org. Lett., 2009, 11, 3378–3381 Search PubMed.
- Y. Zhou, F. Wang, Y. Kim, S. J. Kim and J. Yoon, Cu2+-Selective Ratiometric and “Off-On” Sensor Based on the Rhodamine Derivative Bearing Pyrene Group, Org. Lett., 2009, 11, 4442–4445 CrossRef CAS.
- M. S. Moorthy, M. J. Kim, J. H. Bae, S. S. Park, N. Saravanana, S. H. Kim and C. S. Ha, Multifunctional periodic mesoporous organosilicas for biomolecule recognition, biomedical applications in cancer therapy, and metal adsorption, Eur. J. Inorg. Chem., 2013, 2013, 3028–3038 CrossRef CAS.
- D. H. Joo, J. S. Mok, G. H. Bae, S. E. Oh, J. H. Kang and C. Kim, Colorimetric Detection of Cu2+ and Fluorescent Detection of PO43− and S2− by a Multifunctional Chemosensor, Ind. Eng. Chem. Res., 2017, 56, 8399–8407 CrossRef CAS.
- WHO, Guidelines for Drinking-Water Quality, World Health Organization (WHO), Geneva, 2011 Search PubMed.
- S. C. Chibueze Izah, N. Chakrabarty and A. L. Srivastsav, A Review on Heavy Metal Concentration in Potable Water Sources in Nigeria: Human Health Effects and Mitigating Measures, Expo, Health, 2016, 8, 285–304 CrossRef.
- M. S. Moorthy, H. B. Kim, A. R. Sung, J. H. Bae, S. H. Kim and C. S. Ha, Fluorescent mesoporous organosilicas for selective monitoring of Hg2+ and Fe3+ ions in water and living cells, Microporous Mesoporous Mater., 2014, 194, 219–228 CrossRef CAS.
- S. J. Kulkarni, Heavy metal pollution: sources, effects, and control methods, in Hazardous Waste Management and Health Risks, 2020, pp. 97–112 Search PubMed.
- A. Senthil Murugan, E. R. Abel Neolson and J. Annaraj, Solvent dependent colorimetric, ratiometric dual sensor for copper and fluoride ions: Real sample analysis, cytotoxicity and computational studies, Inorg. Chim. Acta., 2016, 450, 131–139 CrossRef CAS.
- G. Liao, C. Zheng, D. Xue, C. Fan, G. Liu and S. Pu, A diarylethene-based fluorescent chemosensor for the sequential recognition of Fe3+ and cysteine, RSC Adv., 2016, 6, 34748–34753 RSC.
- L. Jin, C. Liu, N. An, Q. Zhang, J. Wang, L. Zhao and Y. Lu, Fluorescence turn-on detection of Fe3+ in pure water based on a cationic poly(perylene diimide) derivative, RSC Adv., 2016, 6, 58394–58400 RSC.
- Y. S. Kim, J. J. Lee, S. Y. Lee, T. G. Jo and C. Kim, A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(II) and Fe(III) and the fluorometric detection of Zn(II) in aqueous media, RSC Adv., 2016, 6, 61505–61515 RSC.
- A. Luo, H. Wang, Y. Wang, Q. Huang and Q. Zhang, A novel colorimetric and turn-on fluorescent chemosensor for Iron(III) ion detection and its application to cellular imaging, Spectrochim. Acta: Mol. Biomol. Spectrosc., 2016, 168, 37–44 CrossRef CAS.
- M. Mirzaei, M. Behzadi, N. M. Abadi and A. Beizaei, Simultaneous separation/preconcentration of ultra-trace heavy metals in industrial wastewaters by dispersive liquid−liquid microextraction based on solidification of floating organic drop prior to determination by graphite furnace atomic absorption spectrometry, J. Hazard Mater., 2011, 186, 1739–1743 CrossRef CAS PubMed.
- R. Sitko, P. Janik, B. Zawisza, E. Talik, E. Margui and I. Queralt, Green Approach for Ultratrace Determination of Divalent Metal Ions and Arsenic Species Using Total-Reflection X-ray Fluorescence Spectrometry and Mercapto-Modified Graphene Oxide Nanosheets as a Novel Adsorbent, Anal. Chem., 2015, 87(6), 3535–3542 CrossRef PubMed.
- M. S. Moorthy, Y. Oh, S. Bharathiraja, P. Manivasagan, T. Rajarathinam, B. Jang, T. T. V. Phan, H. Jang and J. Oh, Synthesis of amine-polyglycidol functionalised Fe3O4@SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and bioimaging applications, RSC Adv., 2016, 6, 110444–110453 RSC.
- S. Petrović, V. Guzsvány, N. Ranković, J. Beljin, S. Rončević, B. Dalmacija, A. M. Ashrafi, Z. Kónya, I. Švancara and K. Vytřas, Trace level voltammetric determination of Zn(II) in selected nutrition related samples by bismuth-oxychloride-multiwalled carbon nanotube composite based electrode, Microchem. J., 2019, 146, 179–186 Search PubMed.
- I. P. Oliveri, G. Munzi and S. D. Bella, A simple approach based on transmetalation for the selective and sensitive colorimetric/fluorometric detection of copper(II) ions in drinking water, New J. Chem., 2022, 46, 18018–18024 RSC.
- M. S. Moorthy, H. J. Song, J. H. bae, S. H. Kim and C. S. Ha, Red fluorescent hybrid mesoporous organosilicas for simultaneous cell imaging and anticancer drug delivery, RSC Adv., 2014, 4, 43342–43345 RSC.
- G. Sivaraman, V. Sathiyaraja and D. Chellappa, Turn-on fluorogenic and chromogenic detection of Fe(III) and its application in living cell imaging, J. Lumin., 2014, 145, 480–485 CrossRef.
- S. Shyamsivappan, A. Saravanan, N. Vandana, T. Suresh, S. Suresh, R. Nandhakumar and P. S. Mohan, Novel quinoline-based thiazole derivatives for selective detection of Fe3+, Fe2+, and Cu2+ ions, ACS Omega, 2020, 42, 27245–27253 CrossRef.
- M. E. Germain and M. J. Knapp, Optical explosives detection: from color changes to fluorescence turn-on, Chem. Soc. Rev., 2009, 28, 2543–2555 RSC.
- K. Saravana Mani, R. Rajamanikandan, B. Murugesapandian, R. Shankar, G. Sivaraman, M. Ilanchelian and S. P. Rajendran, Coumarin based hydrazone as an ICT-based fluorescence chemosensor for the detection of Cu2+ ions and the application in HeLa cells, Spectrochim. Acta, Part A, 2019, 214, 170–176 CrossRef PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic, New York, 1999 Search PubMed.
- M. Santhamoorthy, K. Thirupathi, T. Periyasamy, D. Thirumalai, V. Ramkumar and S. C. Kim, Ethidium bromide-bridged mesoporous silica hybrid nanocarriers for fluorescence cell imaging and drug delivery applications, New J. Chem., 2021, 45, 20641–20648 RSC.
- M. Shellaiah, P. Venkatesan, N. Thirumalaivasan, S. P. Wu and K. W. Sun, Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging, Chemosensors, 2023, 11(2), 115 CrossRef.
- H. F. Xie, C. J. Yu, Y. L. Huang, H. Xu, Q. L. Zhang, X. H. Sun, X. Feng and C. Redshaw, A turn-off fluorescent probe for the detection of Cu2+ based on a tetraphenylethylene-functionalized salicylaldehyde Schiff-base, Mater. Chem. Front., 2020, 4, 1500–1506 RSC.
- T. Simon, M. Shellaiah, V. Srinavasadesikan, C. C. Lin, F. H. Ka, K. W. Sun and M. C. Lin, Novel anthracene- and pyridine-containing Schiff base probe for selective “off–on” fluorescent determination of Cu2+ ions towards live cell application, New J. Chem., 2016, 40, 61010–6108 Search PubMed.
- S. K. Mishra, S. Dehuri and B. Bag, Effect of n-alkyl substitution on Cu(II)-selective chemosensing of rhodamine B derivatives, Org. Biomol. Chem., 2020, 18, 316–332 RSC.
- R. Manjunath and P. Kannan, Highly selective rhodamine-based fluorescence turn-on chemosensor for Al3+ ion, Opt. Mater., 2018, 79, 38–44 CrossRef.
- A. Pipattanawarothai and T. Trakulsujaritchok, Hybrid polymeric chemosensor bearing rhodamine derivative prepared by sol-gel technique for selective detection of Fe3+ ion, Dyes Pigments, 2020, 173, 107946 CrossRef.
- L. L. Chang, Q. Gao, S. Liu, C. C. Hu, W. J. Zhou and M. M. Zheng, Selective and differential detection of Hg2+ and Cu2+ with use of a single rhodamine hydrazone-type probe in the absence and presence of UV irradiation, Dyes Pigments, 2018, 153, 117–124 CrossRef.
- M. Iniya, D. Jeyanthi, K. Krishnaveni and D. Chellappa, Vitamin B6 cofactor based fluorescent probe for sensing an anion (F−) and cation (Co2+) independently in a pure aqueous medium, RSC Adv., 2014, 4, 25393–35397 RSC.
- V. Dujols, F. Ford and A. W. Czarnik, A Long-Wavelength Fluorescent Chemodosimeter Selective for Cu(II) Ion in Water, J. Am. Chem. Soc., 1997, 119(31), 7386–7387 CrossRef.
- W. Breuer, S. Epsztejn, P. Millgram and I. Z. Cabantchik, Transport of iron and other transition metals into cells as revealed by a fluorescent probe, Am. J. Physiol.: Cell Physiol., 1995, 268, 1354–1361 CrossRef.
- K. A. McCall and C. A. Fierke, Colorimetric and Fluorimetric Assays to Quantitate Micromolar Concentrations of Transition Metals, Anal. Biochem., 2000, 284(2), 307–315 CrossRef.
- P. C. Crooker, N. Garrido and G. A. Ahearn, Copper transport by lobster hepatopancreatic epithelial cells separated by centrifugal elutriation: measurements with the fluorescent dye Phen Green, J. Exp. Biol., 2001, 204, 1433 CrossRef PubMed.
- A. Senthil Murgan, N. Vidhyalakshmi, U. Ramesh and J. Annaraj, In vivo bio-imaging studies of highly selective, sensitive rhodamine based fluorescent chemosensor for the detection of Cu2+/Fe3+ ions, Sens. Actuators, B, 2018, 274, 22–29 CrossRef.
- S. Kaleeswaran, D. S. Prabakaran, M. Santhamoorthy, S. Ansar, M. A. Farah, R. Rajamanikandan and K. S. Mani, Novel quinoline-based chemosensor as specific fluorescence sensing of copper ions in cancer cells and for organelle-specific imaging application, Inorg. Chim. Acta, 2025, 575, 122440 CrossRef.
- X. Xie, M. Pan, L. Hong, K. Liu, J. Yang, S. Wang and S. Wang, An “Off–On” Rhodamine 6G Hydrazide-Based Output Platform for Fluorescence and Visual Dual-Mode Detection of Lead(II), J. Agric. Food Chem., 2021, 69, 7209–7217 CrossRef.
- B. S. Chauhan, A. Rai, A. K. Sonkar, K. Tripathi, S. Upadhyay, L. Mishra and S. Srikrishna, Neuroprotective Activity of a Novel Synthetic Rhodamine-Based Hydrazone against Cu2+-Induced Alzheimer's Disease in Drosophila, ACS Chem. Neurosci., 2022, 13(10), 1566–1579 CrossRef.
- A. Senthil Murugan, M. Kiruthika, E. R. Abel Noelson, P. Yogapandi, G. Gnana Kumar and J. Annaraj, Fluorescent sensor for in-vivo bio-imaging, precise tracking of Fe3+ ions in Zebrafish embryos and visual measuring of Cu2+ ions in pico-molar level, Arab. J. Chem., 2021, 14(1), 102910 CrossRef.
- Y. Wang, H. Q. Chang, W. N. Wu, W. B. Peng, Y. F. Yan, C. M. He, T. T. Chen, X. L. Zhao and Z. Q. Xu, Rhodamine 6G hydrazone bearing pyrrole unit: Ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches, Sens. Actuators, B, 2016, 228, 395–400 CrossRef.
- X. Q. Xu, X. J. Mao, Y. Wang, W. N. Wu, P. D. Mao, X. L. Zhao, Y. C. Fan and H. J. Li, Rhodamine 6G hydrazone with coumarin unit: a novel single-molecule multianalyte (Cu2+ and Hg2+) sensor at different pH value, RSC Adv., 2017, 7, 42312–42319 RSC.
- K. Wechakorn, U. Eiamprasert, J. Masoongnoen, A. Tantipanjaporn, P. Surawatanawong, P. Kanjanasirirat, Y. Pewkliang, S. Borwornpinyo, P. Kongsaeree and C. Pitsanuwong, A highly sensitive and selective rhodamine-semicarbazide based fluorescent sensor for Cu2+ detection in real water samples and fluorescence bioimaging in HepG2 cells, Talanta, 2024, 270, 125530 CrossRef.
- K. S. Mani, R. Rajamanikandan, G. Ravikumar, B. Vijaya pandian, P. Kolandaivel, M. Ilanchelian and S. P. Rajendran, Highly Sensitive Coumarin–Pyrazolone Probe for the Detection of Cr3+ and the Application in Living Cells, ACS Omega, 2018, 3(12), 17212–17219 CrossRef.
- K. S. Mani, R. Rajamanikandan, M. Ilanchelian, N. Muralidharan, M. Jothi and S. P. Rajendran, Smart phone assisted quinoline-hemicyanine based fluorescent probe for the selective detection of glutathione and the application in living cells, Spectrochim. Acta, Part A, 2020, 243, 118809 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: The NMR spectra, mass spectra, DFT theoretical data, and additional data. See DOI: https://doi.org/10.1039/d4ay01925c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |





