 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iron complexation by biomass model compounds†
Anurag S.
Mandalika
 a and
Troy M.
Runge
a and
Troy M.
Runge
 *b
*b
aCenter for Energy Studies, Louisiana State University, 93 S Quad Drive, Baton Rouge, LA 70806, USA
bBiological Systems Engineering, University of Wisconsin–Madison, 460 Henry Mall, Madison, WI 53706, USA. E-mail: trunge@wisc.edu
First published on 25th September 2024
Abstract
Iron chelating agents have important roles to play, both in human physiology and in the environment. In the latter case, persistence in the environment has given cause for concern in the case of synthetic iron chelating agents such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA), which do not readily biodegrade. Due to their long lifespan in the environment synthetic iron chelators can also participate in mobilization reactions, particularly with radionuclides such as 60Co. There is an eminent need to explore alternative iron chelating compounds, preferably, renewable in origin, to overcome the drawbacks of synthetic compounds, making plant biomass a potential source of iron chelating agents. Twelve biomass model compounds, representative of the biomass constituents, cellulose, hemicellulose, lignin and extractives (tannins), were tested for their iron complexation ability by measurement of the binding strengths with Fe(II) and Fe(III) in dimethylsulfoxide (DMSO), to ensure solubility, using spectrophotometric titration. The flavonols, kaempferol, quercetin and myricetin displayed the strongest binding affinity to Fe(II) and Fe(III) along with the greatest positive cooperativity as determined by the calculation of Hill coefficients. The lignin-representative compound, p-coumaric acid, showed the highest binding affinity to Fe(II) only. Carbohydrate model compounds did not show any evidence of binding to iron, despite some contrary evidence in literature about their ability to do so. This study points to the potential role that the flavonols class of compounds, and therefore by extension, plant tissues that are rich in extractives, may play in the exploration of biomass-derived iron chelants.
Environmental significanceThe present work details binding constants and cooperativity of several biomass model compounds towards Fe(II) and Fe(III) in the neutral solvent, dimethylsulfoxide. We address the issues faced with occurrence of iron in waters (for human consumption) and some of the drawbacks associated with common chelants such as ethylenediamminetetraoixde (EDTA) by proposing research into naturally-occurring, renewable, and biodegradable source of sorbents for Fe abatement. While experiments were conducted in DMSO to ensure solubility of all the model compounds studied, we believe these can inform further research into sourcing biomass types that are abundant in certain phenolic groups for the purpose of iron chelation from water. |
Introduction
Chelation of metal ions, such as Fe(II) and Fe(III), by the biomass tissues of certain plants is a well-known phenomenon that has sparked investigations into their health benefits. Iron absorption in the human body and its bioavailability have been topics of interest to researchers due to the importance of the metal in human physiology. On the one hand, the low bioavailability of iron from some foods has encouraged research into exploring how iron absorption may be augmented. This has been a cause for concern as it is estimated that around 30% of the global population is anemic, and cultural values often dictate diets containing foods with low iron bioavailability. For example, one of the strategies to address iron deficient anemia (IDA) has been to fortify foods with iron, which has led to investigations into the bioavailability of iron in vegetables and their extracts.1 The discoloration of food due to the complexation of catechol groups in food phenolics with iron (and the consequent reduction in iron bioavailability) was investigated.2On the other hand, excess iron in the body can pose problems, as well. In vivo, higher concentrations of iron (even those below 10−18 M) have been toxic as iron, in the presence of hydrogen peroxide, is responsible for catalyzing Fenton-type reactions which release hydroxyl radicals that cause damage to cells.3,4 The ability of iron to undergo redox reactions, while essential for physiological function, is also responsible in generating free radicals which can oxidize a wide variety of organic substrates.3 These Fenton-type reactions are thought to play a role in causing age-related degenerative effects to the human body, and are even implicated in diseases such as Alzheimer's and Parkinson's.5 Here, accumulation of iron in the brain is thought to promote these diseases through oxidative stress and the generation of α-synuclein and β-amyloid peptides, the toxic redox coupling of iron and dopamine,6 requiring the use of iron chelators that are permeable through barriers in the brain.7 This has driven interest in discovering iron chelators which can quench the free radical formation activity of iron in the human body.
Some of the most widely-researched synthetic iron chelators are compounds that prevent Fenton-type reactions by stabilizing iron and preventing either its oxidation by hydrogen peroxide, or reduction. Iron-transport compounds such as transferritin and lactoferrin prevent iron reduction by reducing agents, whereas 1,10-phenanthroline and bipyridine prevent iron oxidation by hydrogen peroxide.3 These chelators, however, can be indiscriminate in chelating iron from labile cellular pools, and also adversely impacting the levels of metals such as copper, zinc and calcium.8 Other popular metal chelators such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA) were shown to have either a prooxidant, or anti-oxidant effect depending on the ligand–metal ratio employed, which can lead to unwanted adverse outcomes unless carefully monitored.5
Besides human health effects, chelation of iron from the environment can have applications in drinking water treatment by removal of excess iron. Excess iron concentrations present in water, a common occurrence in aquifers in several regions of the world such as India, Bangladesh, Cambodia, Pakistan, etc., can have adverse effects on taste and cause the clogging of pipes and irrigation systems.9 The permissible level of iron in water is 0.3 mg L−1, according to standards from the World Health Organization (WHO),10 and the EPA regards the secondary maximum contaminant load (SCML) for iron as 0.3 mg L−1, with noticeable impacts above this concentration including brown color, sediment, metallic taste, and reddish staining.11,12 There are several ways of abating high quantities of iron from drinking water over a range of expenses, including filtration, aeration, ion-exchange, etc.11 Water softening applications have typically employed chelants such as citric acid or EDTA to remove metals that adversely impact water quality. Excess iron present in drinking water impacts its potability and would need to be removed using cost-effective means. EDTA, in particular, has been regarded as a strong chelant that can form octahedral complexes with metals, and prevent the metal ligands from being available for other chemistries, as evidenced by the chelation of manganese in the bleaching of pulp. Here, EDTA is used to encapsulate Mn and prevent its interference with peroxide in bleaching operations.
Even though EDTA is a very effective metal chelant, its high stability at natural conditions has led to concerns regarding its environmental persistence,13–18 and therefore, sustainability. EDTA is primarily degraded by photolytic means, but this is nearly impossible when dealing with subsoil or subsurface regimes. Additionally, EDTA displays high stability in environmental systems, as evidenced by thermal stability up to 250 °C,19 and low rates of biodegradation to 14CO2.20,21 Biodegradation of EDTA under laboratory conditions showed that 60–72% (light vs. dark) of the original chelant persisted over a 173 days period.22 Persistence of EDTA in the environment has been documented for several decades now,15 and it is important to find alternate metal chelants that have a limited lifespan in the environment once their role is performed. Compounds that originate from plants can serve as iron chelators that will naturally biodegrade and not remain an environmental threat that persist on decadal timescales. Persistence of chelates in the environment is not a trivial issue, and has been associated with unintentional migration of toxic metals, such as the leaching of the radionuclide 60Co at Oak Ridge, TN, USA.23 Other strong iron complexation agents include synthetic chelators such as ferrozine,24–26 organic ligands such as siderophores,27–29 and organic carbon,30 have been studied both for applications and better understanding of iron cycling in the environment, although these can be expensive and/or hard to produce; underutilized biomass residues can offer the potential to produce effective iron chelators for a diverse array of applications.
With the goal of finding natural iron chelators that are derived from plants, the scope of this study is invested in detecting plant compounds that are able to strongly complex with iron, with an emphasis on compiling the chemical functionality that allows for chelation by studying pertinent model compounds pertaining to individual biomass constituents.
Lignocellulosic biomass in iron complexation
It is estimated that annual terrestrial biomass production accounts for ∼100 billion tons with another ∼50 billion tons produced aquatically.31 The amount of aboveground biomass in forests is estimated to be ∼420 (109) tons; in forestry operations, it is estimated that about 34% on a volumetric basis is left as unused residues on the forest floor.32 Additionally, forest thinning operations such as precommercial thinning (PCT) have been proposed to reduce overstocked forests and mitigate wildfire risks.33,34 In the United States alone, it is predicted that by the year 2030, ∼102 and ∼265 million dry tons of forest and agricultural waste biomass and waste potential will be available annually.35 This sizeable availability, coupled with the renewable nature of the resource, make biomass resources an ideal candidate for consideration towards applications as iron chelating agents.Lignocellulosic biomass is broadly comprised of cellulose, hemicellulose, lignin, extractives (tannins and other polyphenols), and other minor constituents. All constituents play vital roles in the growth and development of the plant: cellulose is a structural polymer accounting for the majority of the lignocellulosic plant material (40–45% by dry weight in the secondary cell walls), hemicellulose aids in structure and promotes cross-linking of cellulose microfibrils, lignin acts as the hydrophobic matrix to hold the cell wall together and repel water (∼15–36% of dry weight of wood), extractives and plant polyphenols are present in small amounts, and primarily have roles in plant defense against microbial pests. The subsequent sections review the research undertaken in iron chelation using various biomass constituents.
Polyphenols
Plant polyphenols are broadly considered for their antioxidant (and prooxidant) capabilities, and one way of categorizing these is by virtue of their interactions with iron.36 Iron oxides interact with plant polyphenols, particularly those found in the constituent referred to as tannins. Tannins have been known to play a vital role in plant defense against microbial toxicity by complexation with metals such as Fe(III), thus depriving microorganisms of this necessary element. The presence of two o-dihydroxyphenol groups in most tannins is postulated to chelate metals such as cupric and ferric ions.37 Plant polyphenols such as tannins have been known to chelate metal cations (such as ions of Ca, Mg, Mn, Fe, and Cu), the nature of the chelation being typified by the presence of adjacent hydroxy groups on the phenyl ring.38 In particular, the presence of o-dihydroxyphenyl chelating functional groups in most tannin molecules enables the formation of stable complexes with metal ions. This property enables the uptake of important micro- and macronutrients that plants need from the soil. The complexation between Fe(III) and tannins forms the basis for tanning leather, clothes, hair, etc., and also as one of the primary components in writing inks.39 Studying the iron-binding ability of several isolated polyphenolics, researchers have determined that the presence of an o-catechol moiety (3′,4′-dihydroxy group on the flavonoid ring B) was necessary for iron-binding, whereas the presence of a galloyl group (3′,4′,5′ trihydroxy group on rings B or C) was detrimental for iron binding.40Alternately, Fazary, et al.41 have reported on the complexation ability of gallic acid to form binary complexes with Fe(III) and tertiary complexes with Fe(III) and glycine in a stepwise manner. Khokhar and Owusu Apenten40 studied the interaction of Fe(III) with catechol and galloyl groups by interacting model compounds with ferric ammonium sulfate (FAS) reagent and measuring the absorbance at 587 and 680 nm. Andjelković, et al.,42 studied the iron-chelating properties of seven phenolic acids and hydroxytyrosol in Tris buffer by measuring the complex formation using absorbance.
Plant polyphenols, particularly flavonoids, have been studied for their antioxidant properties in vivo. Besides playing a role in scavenging free radicals, they are also known to chelate transition metals such as iron and copper,43 which are centrally implicated in reducing dioxygen to generate oxygen radicals which are then able to attack biomolecules such as DNA, proteins and phospholipids in the body.44 El Hajji, et al.45 have studied the binding and subsequent autoxidation of quercetin in the presence of metal ions such as Cu(I), Cu(II), Fe(II) and Fe(III). It was observed that free quercetin underwent slow autoxidation at neutral pH, which accelerated in the presence of Cu ions. Autoxidation was, however, inhibited in the presence of both Fe(II) and Fe(III) ions, with the former ion being rapidly oxidized in the presence of quercetin.
Carbohydrates
Stable carbohydrate iron complexes are known to form in aqueous solutions using soluble carbohydrate species.46 In alkaline solutions, it has been shown that materials such as cotton, viscose, modal and lyocell show a tendency to complex ferric ions by ligand–exchange reactions when treated with ferric-sugar acid complexes such as Fe(III)–D-gluconic acid (Fe(III)–DGL) and Fe(III)–hepta-D-gluconic acid (Fe(III)–HDGL).47 The iron–sugar acid complexes were employed as the dissolved iron system from which iron complexation with the insoluble, or partially soluble ligands was studied.There have long been contributions to the literature pertaining to the complexation of carbohydrates and their derivatives with ferric ions.48 The carbohydrate–metal complexes are not very stable in neutral or acidic solutions, but are most stable in alkaline systems due to deprotonation of the alcoholic hydroxy groups and substitution of the water molecules in the first coordination sphere of the metal cations by the electron-donating oxygen atoms. Study of the complex formation phenomenon is compounded by the existence of multiple carbohydrate isomers in solution and the varying interactions of these with the metal ions. Fe(III)–carbohydrate complexes that were precipitated from alkaline solutions were obtained as brownish amorphous solids, with the ferric ion in a coordination number of 6, and the average Fe–O distance of 195 pm as determined by EXAFS, which is consistent with octahedral oxygen coordination of Fe(III) irrespective of the ligand involved.48
Study of this interaction is important from several perspectives. From a clinical standpoint, binding of iron to water-soluble carbohydrates has been investigated as the basis for potential treatment strategies to treat either iron deficiency or iron overload in the human body. Examples of such complexes used in treatment applications are the Fe(III)–D-sorbitol–D-gluconic acid mixed ligand complex and the Fe(III)–lactobionic acid–acetate–cyclodextrin complex. A commercial variant titled ‘Nifrex’, which is a ferric chloride–D-glucose complex, has been used in the treatment of anemia.
Lignin
Interactions of metal ions, particularly Fe, with lignin has been studied from the perspective of organic matter transformation and the fate and mobility of metals in soils. Although biomass lignin does not persist as long as it was previously thought to, there is still scope for interactions between metals and lignin structures in the soil which impacts the bioavailability and persistence of either component.The ability of lignin model compounds to form complexes with metal cations, in particular iron, has been studied for both monomeric49 and polymeric lignins.50 Merdy, et al.49 report on the complexation of Fe(III), Cu(II) and Mn(II) by coniferyl alcohol, sinapic acid, ferulic acid and p-coumaric acid between pH 5–9. These investigations were extended to a dimeric and a polymeric lignin model compound prepared by condensation of lignin monomers.50
Choice of model compounds
The biomass-origin model compounds used in this study were selected for their chemical functionality as a determinant of their iron complexation ability, but only if they were ubiquitously found in lignocellulosic biomass sources; in cases where their occurrence was rare, selection in this study was justified based on literature on the use of the particular model compound(s) for binding iron.To measure the strength of binding, it is imperative to estimate the binding constant for the complexation reaction between the model compound (receptor) and Fe(II) or Fe(III) (ligand). A simple method for studying the complexation of iron to the model compounds is by determining the equilibrium constant by measuring the ultraviolet absorbance for the model compound by titrating with iron and observing the red shift caused as a result in comparison to the unbound molecule.51 This approach is used in this study to estimate the binding constants for complex formation between the ligand and the receptor, and is described in the Methods section.
No effort has yet been made to quantify and compare the iron-complexation ability of lignocellulosic biomass model compounds. While phenolic acids and their derivatives have received the most attention in light of the interest in the antioxidant properties of polyphenols, other moieties such as carbohydrates are also known to chelate iron in solution. This study aims to develop a comprehensive understanding of the iron-chelating ability of biomass model compounds and inform the science on biomass chemical functionality as it relates to iron complexation.
Materials and methods
Quercetin and kaempferol were purchased from Santa Cruz Biotechnology (Dallas, TX). Myricetin glucose, fructose, arabinose, xylose, p-coumaric acid, tannic acid, caffeic acid, vanillic acid and syringic acid were purchased from Millipore Sigma (St. Louis, MI). Iron(II) chloride tetrahydrate and iron(III) chloride hexahydrate (Millipore Sigma) were used as the Fe(II) and Fe(III) sources, respectively to interact with the biomass model compounds (also purchased from Sigma-Aldrich, St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO). The structures for the model compounds considered in this study are depicted in Fig. 1–4. | ||
| Fig. 1 Examples of polyphenolic model compounds containing the catechol and galloyl moieties (quercetin and myricetin, respectively) and not containing either moiety (kaempferol) used in this study. | ||
 | ||
| Fig. 3 Lignin representative compounds of the C6-C1 type, gallic acid, syringic acid, and vanillic acid. | ||
Model compound chelation titrations
Chelation experiments with model compounds and Fe(II) and Fe(III) were performed in DMSO at a model compound–ligand molar ratio of at least 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Fe(II) was prepared in DMSO using FeCl2·4H2O and Fe(III) using FeCl3·6H2O. Increasing amounts of 0.2 mM Fe(II) or Fe(III) were added to the model compound and the subsequent absorbance spectrum was obtained (see Fig. 5 as example for quercetin). Evidence of complexation was determined by observing bathochromic shifts in the absorbance spectra with increasing ligand concentration. When complexation did not occur, these shifts were not observed, and were determined as non-evidence of binding.
10. Fe(II) was prepared in DMSO using FeCl2·4H2O and Fe(III) using FeCl3·6H2O. Increasing amounts of 0.2 mM Fe(II) or Fe(III) were added to the model compound and the subsequent absorbance spectrum was obtained (see Fig. 5 as example for quercetin). Evidence of complexation was determined by observing bathochromic shifts in the absorbance spectra with increasing ligand concentration. When complexation did not occur, these shifts were not observed, and were determined as non-evidence of binding.
Analytical
| L + R ⇌ LR | (1) |
The equilibrium dissociation constant, Kd, is given by
 | (2) |
The fractional occupancy of the receptor (Y) may be defined as:
 | (3) |
 | (4) |
At Y = 0.5, eqn (4) yields Kd = [L], which may be obtained graphically; this enables the determination of Kd as the ligand concentration when fractional occupancy of the receptor is 0.5.
Absorbance of the ligand–receptor complex may be used to calculate the fractional yield, Y:
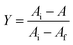 | (5) |
Lastly, the equilibrium binding constant is obtained as the inverse of the dissociation constant.
| Kb = 1/Kd | (6) |
To ascertain the complexation cooperativity, the Hill–Langmuir treatment was employed, and the Hill coefficient was determined using eqn (7):
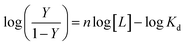 | (7) |
Upon rearrangement, a log–log plot between  and [L] linearizes the Hill equation and the slope of the curve yields the Hill coefficient, n (see Fig. 5D and 6).
and [L] linearizes the Hill equation and the slope of the curve yields the Hill coefficient, n (see Fig. 5D and 6).
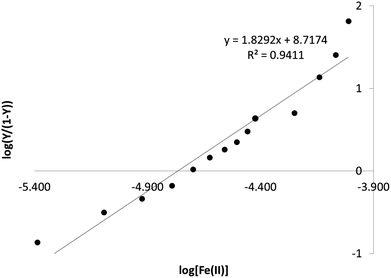 | ||
| Fig. 6 Hill plot for Fe(II) complexation with quercetin showing calculation of the Hill coefficient by determining the slope of the relationship between log[L] and log(Y/1 − Y). | ||
Where n denotes the Hill coefficient which describes the cooperativity of the complexation for the following scenarios:
For n = 1, the binding is non-cooperative, where the bound ligands do not impact further binding of ligand molecules to the receptor.
For n < 1, the binding is negatively-cooperative, where bound ligand molecules reduce the receptor's affinity for further binding, and.
When n > 1, the binding is positively-cooperative, where bound ligands increase the receptor's affinity for further binding.
Despite its relative simplicity and widespread appeal, the Hill equation may not be reliably employed to estimate the number of ligands bound to a receptor molecule, and the Hill coefficient is best thought of as an interaction coefficient, particularly in positively-cooperative systems.52 With this caveat, the Hill coefficient is used in this study to glean into the cooperativity of ligand binding to the model compound receptors, and not necessarily as a representation of the number of ligands that could be bound to the receptor molecule.
Results and discussion
Model compound binding constants with Fe(II) and Fe(III)
Binding constants of the model compounds with Fe(II) and Fe(III) were calculated and are listed in Table 1; in general, the model compounds showed stronger binding to Fe(II) than Fe(III). The highest binding constant was measured for p-coumaric acid with Fe(II) at ∼0.25 M−1, followed by kaempferol at ∼0.18 M−1 (Fig. 7). The latter compound also showed a high binding affinity for Fe(III) at ∼0.1 M−1 compared to the other flavonols, myricetin and quercetin, which were measured at ∼0.05 and ∼0.03 M−1 for Fe(II) and Fe(III), respectively.| Model compound | 0.2 mM Fe(II) | 0.2 mM Fe(III) | ||
|---|---|---|---|---|
| Average Kb (M−1) | Average Hill n | Average Kb (M−1) | Average Hill n | |
| Myricetin | 0.055 ± 0.01 | 2.61 ± 0.32 | 0.037 ± 0.01 | 2.09 ± 0.31 |
| Quercetin | 0.050 ± 0 | 1.83 ± 0.21 | 0.047 ± 0.02 | 2.23 ± 0.45 |
| Kaempferol | 0.160 ± 0.03 | 2.97 ± 0.72 | 0.103 ± 0.02 | 1.77 ± 0.14 |
| Syringic acid | 0 | N/A | 0.018 ± 0 | 1.68 ± 0.11 |
| Gallic acid | 0 | N/A | 0 | N/A |
| Vanillic acid | 0 | N/A | 0 | N/A |
| Caffeic acid | 0.050 ± 0.01 | 1.43 ± 0.15 | 0 | N/A |
| p-Coumaric acid | 0.253 ± 0.04 | 2.68 ± 0.33 | 0 | N/A |
| Fructose | 0 | N/A | 0 | N/A |
| Arabinose | 0 | N/A | 0 | N/A |
| Xylose | 0 | N/A | 0 | N/A |
| Glucose | 0 | N/A | 0 | N/A |
 | ||
| Fig. 7 Calculated binding constant values for the model compounds chosen (with error bars showing standard deviation for triplicate experiments). | ||
It is counter-intuitive to note that the C6-C3 type lignin representative model compound p-coumaric acid, with just one benzylic hydroxyl group showed stronger binding to Fe(II) than caffeic acid (∼0.05 M−1), with two such hydroxyl groups. Neither compound showed any evidence of complexation with Fe(III), however. A similar trend is reflected in the case of the flavonols, where kaempferol, with just one hydroxyl group on the B ring, showed stronger binding to Fe(II) and Fe(III) than quercetin and myricetin, with two and three hydroxyl groups, respectively.
Single-factor Analysis of variance (ANOVA) was performed in MS Excel to test for significance. This analysis led to a computation of the p-value for the binding constants for Fe(II) as 1.71 × 10−16 and for Fe(III) as 1.19 × 10−14, indicating high confidence that the null hypothesis was rejected.
Model compound binding cooperativity with Fe(II) and Fe(III)
Estimated Hill coefficients for the binding of the model compounds with Fe(II) and Fe(III) are shown in Fig. 8 (and Table 1). All model compounds that could form iron complexes showed positive cooperativity in binding with iron, with kaempferol showing the largest Hill coefficient with Fe(II) at 2.9 ± 0.72 (and also for Fe(III) at 0.103 ± 0.02). Caffeic acid showed the smallest Hill coefficient for binding with Fe(II) at 1.43 ± 0.15. | ||
| Fig. 8 Averages (with standard deviation for triplicate estimations) of the Hill coefficients for model compounds that showed binding to Fe(II) and/or Fe(III). | ||
Single-factor ANOVA performed on interaction of model compounds with Fe(II) and Fe(III) resulted in p-values of 4.96 × 10−11 and 1.11 × 10−14, respectively, indicating that the calculated results are statistically significant.
Influence of structure on iron complexation
In a study exploring the binding of several phenolic acids with Fe(III), it was reported42 that vanillic acid and syringic acid did not exhibit complexation, whereas gallic acid and caffeic acid did show complexation, among others. Our results show some divergence from this set of data wherein gallic acid showed no complexation with either Fe species and caffeic acid could form complexes with Fe(II) and syringic acid with Fe(III). The complexation and analytical methods used in this study were similar to what were used in the current study, although the authors report their results in Tris buffer, which could have led to some of the differences observed.For the lignin representative model compounds, both the C6-C3 type compounds showed complexation with Fe(II), whereas none of the C6-C1 type compounds did; only syringic acid evidenced complexation with Fe(III) at the lowest binding strength recorded. This observation leads to the notion that for the smaller phenolic acids (in comparison to the flavonols), binding is not merely a product of the positioning of the phenolic groups, or the presence or absence thereof.
Fe(II) and Fe(III) complexation
For the model compounds that showed complexation with both Fe(II) and Fe(III), the calculated binding constants were greater with Fe(II) than with Fe(III). This is possibly due to the fact that complexation between the ligands and the iron ions occurs via coordination and formation of coordinate bonds is easier with Fe2+ than with Fe3+ due to the fewer amounts of electrons required.Much of the literature surveyed has focused on the complexation of catechol moieties with Fe(III), and this has been attributed to the formation of coordination complexes between the ions and the catechol and substituted catechol ligands (including flavonols and flavonoids).43,53–55 This is due to the insolubility of Fe(III) in biological environments, and the interest in studying the interactions with classes of compounds such as siderophores which can make iron biologically available. There has been much less focus on Fe(II) complexation. In this study, it was shown that while compounds such as p-coumaric acid and caffeic acid show strong complexation with Fe(II), the same is not seen for their interaction with Fe(III). Daugherty, et al.56 report that the majority of Fe(II) was bound by carboxyl groups in reduced natural organic matter (NOM) at neutral pH, so this is potentially a factor that is in play with the C6-C3 compounds tested in the current study.
As mentioned earlier, the Hill coefficient is a measure of the cooperativity of the complexation, with positive values indicating cooperative binding, i.e., the promotion of multimeric complexation. All of the model compounds studied showed a positive binding coefficient to the respective iron species in DMSO. In similar work Davies, et al.57 report a Hill coefficient of 1.86 for the binding of uric acid with Fe(III), which is along similar lines to what was found in the present study.
Implications for biomass constituents and bioprocessing
From the model compound analysis, it is clear that the model compounds that showed strongest complexation with either form of iron were the flavonols myricetin, quercetin and kaempferol (and the lignin model compound p-coumaric acid, with Fe(II) only). Syringic acid and caffeic acid showed complexation with Fe(III) and Fe(II), respectively, but with lower binding constants, particularly for the former. From these observations, the obvious choice of biomass constituent to explore for iron binding are the tannins fraction, and tannin-rich biomass feedstock would make ideal iron-complexation candidates and may be considered being employed in environmental remediation applications. Consequently, since the carbohydrate model compounds did not show any evidence for Fe complexation, it precludes the biomass constituents of cellulose and hemicellulose from being suitable candidates as iron-chelating entities.Additionally, identification of the role played by hydroxyl groups in iron binding can expand the scope for implementation of this strategy towards other biomass constituents, particularly the lignin fraction. The amount of catechol groups in lignin can be increased, which portends to be a potential route that can be pursued using technical lignins for iron complexation. For example, the catalytic hydrogenation depolymerization of ethanol/methanol organosolv lignin using Cu-doped metal catalyst58 can be utilized for the generation of catechol products, which can serve as a precursor for complexation with iron. Demethylation of lignin aromatic methoxy groups (in native wood59 or in technical lignins60) via fungal activity is another strategy of generating catechol moieties in the lignin structure. Use of lignin in this scheme vastly expands the raw material available in terms of renewable and biodegradable iron chelants, owing to the vastly greater quantities of technical lignins generated, their inexpensiveness and under-utilized stature.
From a biorefining perspective, this analysis suggests the use of tannin-rich biomass wastes generated as byproducts from biomass processing applications where the primary components utilized arise from the polysaccharide fractions. Bark tissue wastes generated in lumber manufacture and wood pulping that are rich in tannins, may be employed in iron complexation schema. Similarly, regiospecific agricultural operations such as viniculture and polyphenol-rich fruit production, e.g., cranberries, could be considered as potential sources for tannin-rich feedstock to aid in the environmental remediation of iron. As biomass processing continues to be explored for meeting the needs of society in more sustainable ways, all components of lignocellulosic biomass will need to be valorized to maximize resource utilization, and the application of tannins and lignin for iron complexation is one step in this direction.
Data availability
The data supporting this article have been included as part of ESI† in the form of figures used to collect the raw data and perform the analysis described.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based upon work supported by the United States Department of Agriculture National Institute of Food and Agriculture, under ID number WIS02075. The authors declare no conflicts of interest to the best of their knowledge.References
- I. Rodriguez-Ramiro, C. Dell'Aquila, J. L. Ward, A. L. Neal, S. F. A. Bruggraber, P. R. Shewry and S. Fairweather-Tait, Estimation of the iron bioavailability in green vegetables using an in vitro digestion/Caco-2 cell model, Food Chem., 2019, 301, 125292, DOI:10.1016/j.foodchem.2019.125292.
- J. Bijlsma, W. J. C. de Bruijn, J. A. Hageman, P. Goos, K. P. Velikov and J.-P. Vincken, Revealing the main factors and two-way interactions contributing to food discolouration caused by iron-catechol complexation, Sci. Rep., 2020, 10(1), 8288, DOI:10.1038/s41598-020-65171-1.
- C. C. Winterbourn, Toxicity of iron and hydrogen peroxide: the Fenton reaction, Toxicol. Lett., 1995, 82–83, 969–974, DOI:10.1016/0378-4274(95)03532-X.
- M. Guo, C. Perez, Y. Wei, E. Rapoza, G. Su, F. Bou-Abdallah and N. D. Chasteen, Iron-binding properties of plant phenolics and cranberry's bio-effects, Dalton Trans., 2007,(43), 4951–4961 RSC.
- M. D. Engelmann, R. T. Bobier, T. Hiatt and I. F. Cheng, Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron, BioMetals, 2003, 16(4), 519–527, DOI:10.1023/A:1023480617038.
- D. J. Hare and K. L. Double, Iron and dopamine: a toxic couple, Brain, 2016, 139(4), 1026–1035, DOI:10.1093/brain/aww022.
- S. Mandel, O. Weinreb, L. Reznichenko, L. Kalfon and T. Amit, Green tea catechins as brain-permeable, non toxic iron chelators to “iron out iron” from the brain, In Oxidative Stress and Neuroprotection, ed. H. Parvez and P. Riederer, Springer Vienna, 2006, pp. 249–257 Search PubMed.
- Y. Wei and M. Guo, Hydrogen Peroxide Triggered Prochelator Activation, Subsequent Metal Chelation, and Attenuation of the Fenton Reaction, Angew. Chem., Int. Ed., 2007, 46(25), 4722–4725, DOI:10.1002/anie.200604859.
- Y. Liu, T. Ma, J. Chen, C. Xiao, R. Liu, Y. Du and S. Fendorf, Contribution of clay-aquitard to aquifer iron concentrations and water quality, Sci. Total Environ., 2020, 741, 140061, DOI:10.1016/j.scitotenv.2020.140061.
- N. Khatri, S. Tyagi and D. Rawtani, Recent strategies for the removal of iron from water: A review, J. Water Process Eng., 2017, 19, 291–304, DOI:10.1016/j.jwpe.2017.08.015.
- A. Colter and R. L. Mahler, Iron in Drinking Water, University of Idaho, Moscow, 2006 Search PubMed.
- EPA, Secondary Drinking Water Standards: Guidance for Nuisance Chemicals, US Environmental Protection Agency, 2023, https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals Search PubMed.
- S. Gluhar, A. Kaurin and D. Lestan, Soil washing with biodegradable chelating agents and EDTA: Technological feasibility, remediation efficiency and environmental sustainability, Chemosphere, 2020, 257, 127226, DOI:10.1016/j.chemosphere.2020.127226.
- M. Shahid, A. Austruy, G. Echevarria, M. Arshad, M. Sanaullah, M. Aslam, M. Nadeem, W. Nasim and C. Dumat, EDTA-Enhanced Phytoremediation of Heavy Metals: A Review, Soil Sediment Contam.: Int. J., 2014, 23(4), 389–416, DOI:10.1080/15320383.2014.831029.
- C. Oviedo and J. Rodríguez, EDTA: the chelating agent under environmental scrutiny, Quim. Nova, 2003, 26(6), 901–905 CrossRef CAS.
- M. Bucheli-Witschel and T. Egli, Environmental fate and microbial degradation of aminopolycarboxylic acids, FEMS Microbiol. Rev., 2001, 25(1), 69–106, DOI:10.1111/j.1574-6976.2001.tb00572.x.
- F. G. Kari and W. Giger, Modeling the photochemical degradation of ethylenediaminetetraacetate in the River Glatt, Environ. Sci. Technol., 1995, 29(11), 2814–2827 CrossRef CAS PubMed.
- E. Meers, A. Ruttens, M. J. Hopgood, D. Samson and F. M. G. Tack, Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals, Chemosphere, 2005, 58(8), 1011–1022, DOI:10.1016/j.chemosphere.2004.09.047.
- A. E. Martell, R. J. Motekaitis, A. R. Fried, J. S. Wilson and D. T. MacMillan, Thermal Decomposition of EDTA, NTA, and Nitrilotrimethylenephosphonic Acid in Aqueous Solution, Can. J. Chem., 1975, 53(22), 3471–3476, DOI:10.1139/v75-498.
- J. M. Tiedje, Microbial Degradation of Ethylenediaminetetraacetate in Soils and Sediments, Appl. Microbiol., 1975, 30(2), 327 CrossRef CAS PubMed.
- J. M. Tiedje, Influence of Environmental Parameters on EDTA Biodegradation in Soils and Sediments, J. Environ. Qual., 1977, 6(1), 21–26, DOI:10.2134/jeq1977.00472425000600010006x.
- J. L. Means, T. Kucak and D. A. Crerar, Relative degradation rates of NTA, EDTA and DTPA and environmental implications, Environ. Pollut., Ser. B, 1980, 1(1), 45–60, DOI:10.1016/0143-148X(80)90020-8.
- J. L. Means, D. A. Crerar and J. O. Duguid, Migration of Radioactive Wastes: Radionuclide Mobilization by Complexing Agents, Science, 1978, 200(4349), 1477, DOI:10.1126/science.200.4349.1477.
- G. L. Smith, A. A. Reutovich, A. K. Srivastava, R. E. Reichard, C. H. Welsh, A. Melman and F. Bou-Abdallah, Complexation of ferrous ions by ferrozine, 2,2′-bipyridine and 1,10-phenanthroline: Implication for the quantification of iron in biological systems, J. Inorg. Biochem., 2021, 220, 111460, DOI:10.1016/j.jinorgbio.2021.111460.
- M. R. Jones and K. Lee, Sequential injection analysis Ferrozine spectrophotometry versus Luminol chemiluminescence for continuous online monitoring of the concentration and speciation of iron, Microchem. J., 2020, 157, 104881, DOI:10.1016/j.microc.2020.104881.
- K. Kang, W. D. C. Schenkeveld, G. Weber and S. M. Kraemer, Stability of Coumarins and Determination of the Net Iron Oxidation State of Iron–Coumarin Complexes: Implications for Examining Plant Iron Acquisition Mechanisms, ACS Earth Space Chem., 2023, 7(12), 2339–2352, DOI:10.1021/acsearthspacechem.3c00199.
- L. E. Moore, M. I. Heller, K. A. Barbeau, J. W. Moffett and R. M. Bundy, Organic complexation of iron by strong ligands and siderophores in the eastern tropical North Pacific oxygen deficient zone, Mar. Chem., 2021, 236, 104021, DOI:10.1016/j.marchem.2021.104021.
- A. F. R. Gomes, M. C. Almeida, E. Sousa and D. I. S. P. Resende, Siderophores and metallophores: Metal complexation weapons to fight environmental pollution, Sci. Total Environ., 2024, 932, 173044, DOI:10.1016/j.scitotenv.2024.173044.
- L. E. Manck, J. Park, B. J. Tully, A. M. Poire, R. M. Bundy, C. L. Dupont and K. A. Barbeau, Petrobactin, a siderophore produced by Alteromonas, mediates community iron acquisition in the global ocean, ISME J., 2022, 16(2), 358–369, DOI:10.1038/s41396-021-01065-y.
- A. Joshani, Y. Mirzaei, A. Barber, K. Balind, C. Gobeil and Y. Gélinas, Organic matter preservation through complexation with iron minerals in two basins of a dimictic boreal lake with contrasting deep water redox regimes, Sci. Total Environ., 2024, 925, 171776, DOI:10.1016/j.scitotenv.2024.171776.
- K. Srirangan, L. Akawi, M. Moo-Young and C. P. Chou, Towards sustainable production of clean energy carriers from biomass resources, Appl. Energy, 2012, 100, 172–186, DOI:10.1016/j.apenergy.2012.05.012.
- M. Parikka, Global biomass fuel resources, Biomass Bioenergy, 2004, 27(6), 613–620, DOI:10.1016/j.biombioe.2003.07.005.
- B. L. Polagye, K. T. Hodgson and P. C. Malte, An economic analysis of bio-energy options using thinnings from overstocked forests, Biomass Bioenergy, 2007, 31(2–3), 105–125, DOI:10.1016/j.biombioe.2006.02.005.
- C. X. Grano, Precommercial Thinning of Loblolly Pine, J. For., 1969, 67(11), 825–827 Search PubMed.
- R. Perlack and S. U. S. BJ, Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry. Energy, Oak Ridge National Laboratory, Oak Ridge, TN, 2011, p. 227 Search PubMed.
- N. R. Perron and J. L. Brumaghim, A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding, Cell Biochem. Biophys., 2009, 53(2), 75–100, DOI:10.1007/s12013-009-9043-x.
- A. Scalbert, Antimicrobial properties of tannins, Phytochemistry, 1991, 30(12), 3875–3883, DOI:10.1016/0031-9422(91)83426-L.
- S. Quideau, D. Deffieux, C. Douat-Casassus and L. Pouységu, Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis, Angew. Chem., Int. Ed., 2011, 50(3), 586–621, DOI:10.1002/anie.201000044.
- M. McDonald, I. Mila and A. Scalbert, Precipitation of Metal Ions by Plant Polyphenols: Optimal Conditions and Origin of Precipitation, J. Agric. Food Chem., 1996, 44(2), 599–606, DOI:10.1021/jf950459q.
- S. Khokhar and R. K. Owusu Apenten, Iron binding characteristics of phenolic compounds: some tentative structure–activity relations, Food Chem., 2003, 81(1), 133–140, DOI:10.1016/S0308-8146(02)00394-1.
- A. E. Fazary, M. Taha and Y.-H. Ju, Iron Complexation Studies of Gallic Acid, J. Chem. Eng. Data, 2009, 54(1), 35–42, DOI:10.1021/je800441u.
- M. Andjelković, J. Van Camp, B. De Meulenaer, G. Depaemelaere, C. Socaciu, M. Verloo and R. Verhe, Iron-chelation properties of phenolic acids bearing catechol and galloyl groups, Food Chem., 2006, 98(1), 23–31, DOI:10.1016/j.foodchem.2005.05.044.
- L. Mira, M. Tereza Fernandez, M. Santos, R. Rocha, M. Helena Florêncio and K. R. Jennings, Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity, Free Radical Res., 2002, 36(11), 1199–1208, DOI:10.1080/1071576021000016463.
- S. D. Aust, L. A. Morehouse and C. E. Thomas, Role of metals in oxygen radical reactions, J. Free Radicals Biol. Med., 1985, 1(1), 3–25, DOI:10.1016/0748-5514(85)90025-X.
- H. E. Hajji, E. Nkhili, V. Tomao and O. Dangles, Interactions of quercetin with iron and copper ions: Complexation and autoxidation, Free Radical Res., 2006, 40(3), 303–320, DOI:10.1080/10715760500484351.
- P. J. Charley, B. Sarkar, C. F. Stitt and P. Saltman, Chelation of iron by sugars, Biochim. Biophys. Acta, 1963, 69, 313–321, DOI:10.1016/0006-3002(63)91264-2.
- A. Kongdee and T. Bechtold, The complexation of Fe(III)-ions in cellulose fibres: a fundamental property, Carbohydr. Polym., 2004, 56(1), 47–53, DOI:10.1016/j.carbpol.2003.12.001.
- B. Gyurcsik and L. Nagy, Carbohydrates as ligands: coordination equilibria and structure of the metal complexes, Coord. Chem. Rev., 2000, 203(1), 81–149, DOI:10.1016/S0010-8545(99)00183-6.
- P. Merdy, E. Guillon, M. Aplincourt and J. Duomonceau, Interaction of metallic cations with lignins. Part 1: Stability of iron (III), manganese (II) and copper (II) complexes with phenolic lignin model compounds: coumaric, ferulic and sinapic acids and coniferyl alcohol, J. Chem. Res., 2000, 2000(2), 76–77 CrossRef.
- E. Guillon, P. Merdy, M. Aplincourt, J. Dumonceau and H. Vezin, Structural Characterization and Iron(III) Binding Ability of Dimeric and Polymeric Lignin Models, J. Colloid Interface Sci., 2001, 239(1), 39–48, DOI:10.1006/jcis.2001.7535.
- J. Ren, S. Meng, C. E. Lekka and E. Kaxiras, Complexation of Flavonoids with Iron: Structure and Optical Signatures, J. Phys. Chem. B, 2008, 112(6), 1845–1850, DOI:10.1021/jp076881e.
- J. N. Weiss, The Hill equation revisited: uses and misuses, Faseb. J., 1997, 11(11), 835–841, DOI:10.1096/fasebj.11.11.9285481.
- A. Avdeef, S. R. Sofen, T. L. Bregante and K. N. Raymond, Coordination chemistry of microbial iron transport compounds. 9. Stability constants for catechol models of enterobactin, J. Am. Chem. Soc., 1978, 100(17), 5362–5370, DOI:10.1021/ja00485a018.
- M. Elhabiri, C. Carrër, F. Marmolle and H. Traboulsi, Complexation of iron(III) by catecholate-type polyphenols, Inorg. Chim. Acta, 2007, 360(1), 353–359, DOI:10.1016/j.ica.2006.07.110.
- M. D. Engelmann, R. Hutcheson and I. F. Cheng, Stability of Ferric Complexes with 3-Hydroxyflavone (Flavonol), 5,7-Dihydroxyflavone (Chrysin), and 3‘,4‘-Dihydroxyflavone, J. Agric. Food Chem., 2005, 53(8), 2953–2960, DOI:10.1021/jf048298q.
- E. E. Daugherty, B. Gilbert, P. S. Nico and T. Borch, Complexation and Redox Buffering of Iron(II) by Dissolved Organic Matter, Environ. Sci. Technol., 2017, 51(19), 11096–11104, DOI:10.1021/acs.est.7b03152.
- K. J. A. Davies, A. Sevanian, S. F. Muakkassah-Kelly and P. Hochstein, Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid, Biochem. J., 1986, 235(3), 747–754, DOI:10.1042/bj2350747.
- M. Montazeri and M. J. Eckelman, Life Cycle Assessment of Catechols from Lignin Depolymerization, ACS Sustain. Chem. Eng., 2016, 4(3), 708–718, DOI:10.1021/acssuschemeng.5b00550.
- T. R. Filley, G. D. Cody, B. Goodell, J. Jellison, C. Noser and A. Ostrofsky, Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown rot fungi, Org. Geochem., 2002, 33(2), 111–124, DOI:10.1016/S0146-6380(01)00144-9.
- L. Zou, B. M. Ross, L. J. Hutchison, L. P. Christopher, R. F. H. Dekker and L. Malek, Fungal demethylation of Kraft lignin, Enzyme Microb. Technol., 2015, 73–74, 44–50, DOI:10.1016/j.enzmictec.2015.04.001.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00383c |
| This journal is © The Royal Society of Chemistry 2024 |



