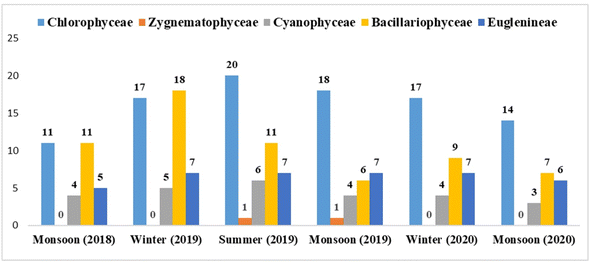Open Access Article
Open Access ArticleIntensive aquaculture affects lake's trophic status and aquatic floral diversity†
Divya
Dubey
 *a,
Kiran
Toppo
*a,
Kiran
Toppo
 b,
Saroj
Kumar
b,
Saroj
Kumar
 a and
Venkatesh
Dutta
a and
Venkatesh
Dutta
 *a
*a
aRiver Systems and Aquatic Ecology Lab, Department of Environmental Science, Babasaheb Bhimrao Ambedkar University, Lucknow, India. E-mail: divyadubey.sai21@gmail.com; dvenks@gmail.com; sarojbhargava73@gmail.com; Tel: +91-9918466778
bAlgology Laboratory, CSIR-National Botanical Research Institute, Rana Pratap Marg, Lucknow-226001, India. E-mail: toppokiran@yahoo.co.in
First published on 27th September 2024
Abstract
This study aims to assess the impact of intensive aquaculture on a lake that has experienced significant anthropogenic impacts. Specifically, it investigates the consequences of aquaculture activities, such as Trapa cultivation (water chestnut) and fish rearing, on the lake's water quality, trophic state, and floristic diversity, with a primary emphasis on algae and macrophytes. Satellite imageries spanning the last five decades, from 1976 to 2022, were analyzed to understand the impact of urbanization and changes in land use and land cover within the lake's catchment. The study found that aquaculture activities negatively impacted algae and macrophytes' diversity, dominance, and community structure in the freshwater lake. The study reported a total of 61 algal species from five families during both sampling phases. Dominant species belonged to the Chlorophyceae and Euglenophyceae families, alongside several diatom species. Notably, the reported algal species served as bioindicators of organic pollution, as assessed by the algae pollution index. During the second year of sampling, intensive fish-rearing activities disrupted the macrophytic diversity, which was replaced by the proliferated growth of planktonic algae, resulting in the biotic shift of the lake's floristic diversity. The study provides valuable insights into the effective management of lakes impacted by intensive aquaculture, shedding light on the intricate relationships between aquaculture practices and the ecological dynamics of freshwater ecosystems in developing countries.
Environmental significanceIn many developing countries, there is no strict legislation or guidelines regarding aquaculture activities practiced in freshwater lakes. This study provides valuable insights into the effective management of lakes impacted by intensive aquaculture, shedding light on the intricate relationships between aquaculture practices and the ecological dynamics of freshwater ecosystems. Previously, no such studies have been reported for the selected geographical region. This paper will serve as a stepping stone for understanding the impacts of aquaculture on the ecological functions of lakes. |
Introduction
The biotic and abiotic features of freshwater lake ecosystems are constantly evolving due to the various human activities globally. Short-term lake management strategies typically prioritize economic and social development over the conservation of the freshwater environment, which may impact the long-term sustainability of freshwater resources.1,2 Despite serving many important ecosystem services and being a crucial part of the biosphere, freshwater ecosystems are increasingly exploited as economic growth resources mainly for aquaculture activities.3,4 The biosphere and freshwater environments have coevolved with humankind in a robust form, and this trend will continue, provided the necessary equilibrium is maintained.5,6 Cultural eutrophication due to aquaculture activities is currently one of the main threats that freshwater lakes are facing.7,8 As aquaculture activities continue to increase, they deteriorate the normal lake ecosystem functioning.9Aquaculture activities affect aquatic biodiversity by introducing non-native species and increasing the trophic status using herbicides, and pesticides10 as well as nutrients and feed during fish rearing.11,12 The inadvertent and/or intentional introduction of exotic or alien aquatic species is known to have a detrimental ecological impact on native species.13 Monoculture growth of Trapa natans as well as shrimp and salmon production are at the forefront of the rapidly expanding fish-rearing aquaculture-based sector.14 However, the production cycles of such aquaculture operations rely heavily on external inputs and generate huge organic waste.2
Unregulated aquaculture is a major contributor to water pollution in South Asia.15 Aquaculture practices discharge untreated wastewater containing organic matter, inorganic chemicals, algae, antimicrobial agents, and antibiotics, leading to oxygen deficiency, algae blooms, and several environmental and health risks.16 Intensive aquaculture generates polluted organic effluents including biochemical oxygen demand (BOD), total ammonia, alkalinity, calcium nitrates, calcium, potassium, iron, chlorides, and sodium.17 Aquaculture ecosystems are distinct freshwater systems that resemble both human-managed land agroecosystems and aquatic ecosystems. They differ from natural aquatic ecosystems in four ways. First, some of the energy and material input into the system is managed by humans and is not dependent on external variables like solar radiation, precipitation, and runoff.18 Secondly, to maximize production, the biodiversity of the freshwater lake is disrupted and gradually reduced. Furthermore, the biological communities in the system are not naturally occurring, and the dominant organisms are usually artificially introduced. Aquaculture in freshwater ecosystems may have negative impacts as it results in the organic and nutrient enrichment in the lakes.
Various studies have reported the assessment of the ecological risk of the antibiotics released during aquaculture production,19 further affecting phytoplankton functional groups.20 Studies have been conducted on the impact of residual feed from aquaculture and sediment that threaten the freshwater ecosystem and their interaction among algae, heavy metals, and nutrients.21 Several studies have been done on the effects of aquaculture on water quality,22 phytoplankton diversity,23 bacterial community,24 and fish communities.25 At the same time, fewer studies are reported on the effects of aquaculture activities on the tropical aquatic ecosystem of the central Gangetic plain.
Thus, this study mainly focuses on the assessment of alterations in the trophic status of the lake and changes in aquatic biodiversity, especially the algae and macrophytes brought about by intensive aquaculture activities. The major challenge faced during the data collection was the shift in the aquaculture activities, i.e. from the Trapa cultivation in the first years to the fish rearing activities in the second year resulting in cultural eutrophication. The findings from this study will enhance understanding of the impacts of human intervention on the natural diversity of freshwater lakes. The results will be also useful in developing and implementing strategies to mitigate the detrimental impacts of aquaculture on aquatic flora. This, in turn, will improve the monitoring and management frameworks of urban lakes that are subject to a wide range of anthropogenic and urban pressures.
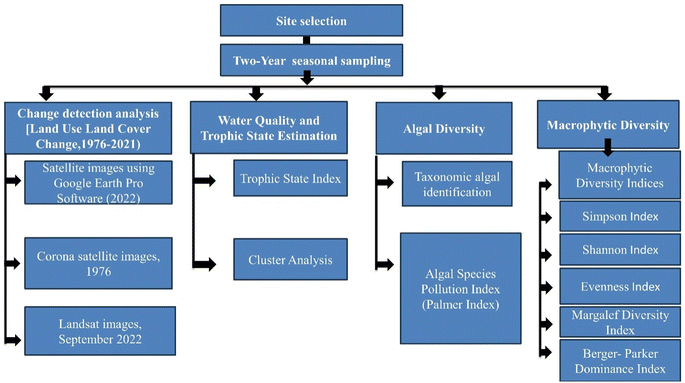
Methodology
Study area
The Kathauta lake selected for the study is in Gomti Nagar, Lucknow, Uttar Pradesh extending between 81.03° E longitude and 26.86° N latitude (Fig. 1). The main water source for this lake is the Sharda Canal originating from the Sharda River, a tributary of the Ganges. This lake is fragmented into two segments presently; a larger cemented segment of the lake is used for water supply to the neighbouring colonies of Lucknow, and it has permanent fenced boundaries with an area of 59.09 acres and a perimeter of 2.79 km. The other small, isolated segment of this lake is in natural condition which has an area of 34.33 acres and a perimeter of 1.14 km. The lake was divided into six sampling zones. In each zone, observations were made by boat and by diving at random spots, from shorelines to deep pools. The depth of the pool selected for the study varied from 10–11 feet. This section of the lake supports several species of aquatic flora and fauna. During the time of the first year of the sampling, the lake was temporarily fragmented and was used for both the Trapa cultivation and fish rearing by the local Trapa cultivators and fisherman but during the second phase of the sampling the whole lake was used for the fish rearing activity. This was done by local fisherman and Trapa cultivators without any influence of the researchers.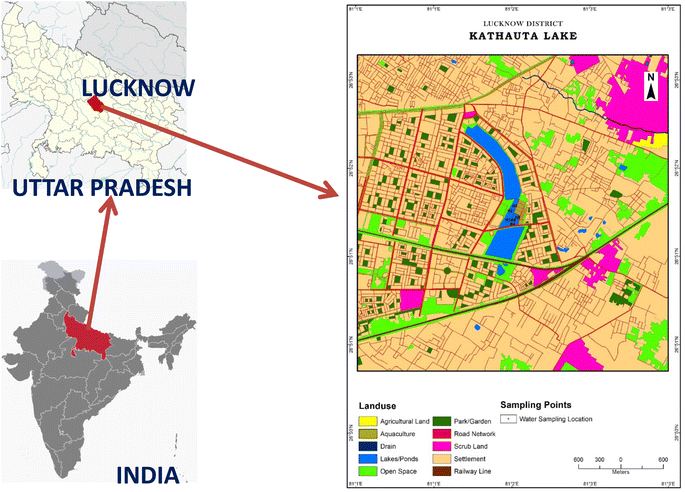 | ||
| Fig. 1 Sampling location of the selected lake situated in the Central Gangetic Plain of Northern India. | ||
Estimation of temporal changes in the lake's catchment
To evaluate the anthropogenic factors causing temporal changes in the lake area and size, lake morphometry was assessed using older and recent satellite images. Changes in the size and area of the lake along with the changes in the catchment's land use and land were assessed during the last five decades. A land use and land cover change map was prepared with the help of ArcGIS 10.7 using satellite data over the 46 year gap (1976–2022). First, cloud-free and high-quality data were screened and acquired from the current Google satellite image of September 2022 and an older 1976 image from the Corona satellite (specifically, Kathauta Lake on 2 January 1976) obtained from the Earth Explorer of the United States Geological Survey (USGS) website. Subsequently, the downloaded satellite images underwent georeferencing and rectification. A 2 km square area was selected from the lake catchment for focused analysis. The downloaded images were then digitized and extracted to identify various land use categories. A key was prepared based on visual interpretation of the images, leading to the final creation of land use land cover maps. The last steps involved generating statistics for the different land use categories, providing comprehensive insights into the study area.An overview of the methodology is given in Fig. 2 below.
The study was conducted during a three-year period between 2018 – 2020. During the first year, sampling was conducted in all three seasons namely, immediately after monsoon (October 2018), winter (January 2019), and summer (May 2019). During the second year, monsoon (October 2019) and winter (January 2020) sampling was conducted but sampling in summer (May 2020) could not be done due to the COVID lockdown in India. Further sampling was resumed in monsoon (October 2020) after the COVID lockdown. Lake was in varying states during the three sampling periods, depending on various factors including the invasion of macrophytes and management strategies. In Table 1, various anthropogenic stressors in the lake as witnessed during the two years of the study are summarized.
| Major factors observed | First year of sampling (2018–19) | Second year of sampling (2019–20) |
|---|---|---|
| Lake fragmentation | In the monsoon and winter seasons, the lake was fully fragmented whereas in the winter season, it was partially fragmented | Absent |
| Active aquaculture activities | In the monsoon season aquaculture was reported whereas in the winter and summer seasons, it was not reported | Only fishing was reported |
| Macrophytic condition | -Monsoon-Trapa natans were dominated in the Trapa cultivation segment | Macrophytes in the open area were uprooted for fishing, and few were reported in the shoreline area |
| -Winter- fishing segment is dominated by the free-floating minute macrophytes i.e. Lemna sp., Wolffia sp., and many more | ||
| -Summer- fishing segment is dominated by Pistia stratiotes, Eichhornia crassipes, and many species of Lemna sp. | ||
| Anthropogenic activities | Cloth washing by local washermen reported during the time of the study | Cloth washing reported |
| Transparency | Moderate transparent water | Moderate transparent water |
| Wastewater discharge | Wastewater from nearby urban settlements was discharged | Wastewater from nearby urban settlements was discharged |
| Planktonic algae (suspended) | -Monsoon- dominant in the fishing section | Excessive growth of algae Euglena sp. due to fishing |
| -Winter and summer- moderate in both the sections | ||
| Addition of pesticides and feed in the lake for Trapa cultivation and fish rearing | Pesticides were added for Trapa cultivation | Fish feed was added to support fish-rearing |
Water quality analysis
The physicochemical characteristics of water quality were observed on a seasonal basis during the sampling of the lake water. Water samples were collected from the epilimnion layer of the lake (at the depth of 0.3 to 0.6 m) from the selected six sites as shown in Fig. 1 between 10.00 and 12.00 hours in one-liter plastic bottles. The parameters of the water quality were monitored and calculated using standard procedures.26 Total thirteen water quality parameters viz. pH, temp, total dissolved solids (TDS), electrical conductivity (EC), dissolved oxygen (DO), biochemical oxygen demand (BOD), total suspended solids (TSS), alkalinity, nitrate, nitrite, total phosphorous (TP), chlorophyll a (Chl a) and chlorophyll b (Chl b) were estimated.27 Parameters that were monitored on the spot at the time of sampling include transparency, depth, temperature, pH, and EC while the rest of the parameters were analyzed in the university laboratory within 24 hours of sampling.Estimation of Carlson trophic state index
The Trophic State Index (TSI) is a robust classification system that rigorously rates freshwater lakes based on the amount of biological productivity they support.28,29 It is an invaluable measure of water quality that is instrumental in helping water managers understand and enhance water quality, as well as in the development of effective management and restoration plans. This index is an essential baseline for measuring biological integrity, identifying human-induced interferences, and developing rehabilitation and restoration strategies in aquatic ecosystems. The complex nature of eutrophication is influenced by both anthropogenic and natural factors. TSI values help water managers assess water body quality over time. For example, increasing TSI values over several years could indicate declining health of the lake.30 Secchi depth was observed by using a Secchi disk having a diameter of 20 cm. Reading was monitored at the maximum depth at which the Secchi disk can be seen when lowered into the lake. TP was estimated by the method prescribed in APHA 2017. Chlorophyll a was assessed by the acetone method.31 Water was filtered through GF/F filters, and thereafter; the filter was pulverized with 90% acetone in a rotor. The resulting mixture was centrifuged for 20 min at 4000 rpm. The optical density of the algae supernatant was measured at 630, 647, 664, and 750 nm (ref. 32) using a spectrophotometer (Model—Systronics-2203). The amount of chlorophyll a was assessed using the absorption coefficient. The trophic state was calculated using the formula given by Carlson.28 Based on the TSI value, the lake was further classified as an oligotrophic, mesotrophic, and eutrophic lake.Statistical analysis
The statistical mean along with the standard deviation (X ± SD) of all the physicochemical parameters of the lake water samples collected from each sampling site was calculated using the statistical analysis tool SPSS (version 20.0) and MS Office Excel. One-way ANOVA (p < 0.05) from each sampling site of the selected lake was carried out for the estimation of variance. This was done to compare the mean among several physicochemical parameters concerning different sampling locations. Cluster Analysis (CA) of the mean of the water quality categorized by seasons was performed using PAST 3.0 software. CA employed Euclidean distance and non-weighted clustering using the arithmetic mean (UPGMA) for various water quality parameters.Collection and identification of the algal species
Algal samples were collected at the same time at which water samples were collected. A total of 18 algal samples were collected during both the years of sampling. Algal samples were kept in 50 ml pre-rinsed sterilized specimen bottles and transported to the laboratory in an ice box for microphotography and morphological analysis. The algal samples were preserved in 4% formalin (aqueous solution of formaldehyde) and stored in polythene bottles. Microphotography and microscopic observation of algal samples were carried out by a Leica DM 500 research microscope connected with a computer having a digital image analyzer and software LAS EZ 1.8.0 taken with attached camera LEICA EC-3 from CSIR-NBRI Algology laboratory. The identification of green algae was authenticated based upon standard keys33–38 and taxonomy was updated using the online database called Algae Base (World-wide electronic publication available at http://www.algaebase.org/). The tolerance to organic pollution in the selected freshwater lake was calculated using the Algal Species Pollution Index following the methodology provided.39,40Collection and identification of the aquatic macrophytes
Macrophytes were identified using prominent taxonomic features. Data was categorized based on macrophytic species, family, and type of macrophytes; detailed table is given in the ESI Table 12.† Sites were selected at equal distances to cover the whole lake area so that the most precise and accurate results could be obtained for the macrophytes present in the lake. Macrophytes that were easily accessible were handpicked and those in deep pools were collected with the help of local fishermen. The lake was divided into six sampling zones. In each zone, observations were made by boat and by diving at random spots, from shorelines to deep pools. The directory on ‘Biodiversity of the aquatic and semi-aquatic plant of Uttar Pradesh’ was used to identify macrophytes.41Estimation of macrophytic diversity
The quantitative analysis of aquatic macrophytes was done following the standard methodology.42,43 A floating quadrant of 1 m × 1 m was used for the estimation of macrophytic diversity which was used for counting the number of the macrophytes of different species present in the quadrant. The importance value index (IVI) (summation of relative frequency, relative density, and relative abundance) of the macrophytes at different selected sites in the lake was also used for the estimation of the macrophytic diversity. This was calculated for each species after assessing their relative density, frequency, and dominance.9Results
Temporal changes in the lake catchment
Satellite images were retrieved for the past five decades from the year 1976 to 2022 to assess the changes in the lake area, size, morphometric changes, and fragmentation of the lake due to urbanization along with land use and land cover changes in its catchment. Clear changes are visible between the images of the year 1976 and December 2003. The overall nine satellite images from 1976 to 2022 clearly show the changes that the lake underwent during 46 years including the changes in morphometry and water level status. During this duration lake had gone through major anthropogenic stresses resulting in its fragmentation, encroachment, and drying of water or reduction in the water level during December 2003. The extent of water spread in the lake was poor in January 2006, October 2008, and April 2009. The larger segment of the lake was used for water storage which helped in supplying water to the newer settlements of Lucknow as seen in the satellite image of June 2011 (ESI Fig. 3†).Land use and land cover change in the lake's catchment
Based on the satellite images as shown in Fig. 2[A] and [B], various attributes of the lake catchment have been studied in the years 1976 and 2022 respectively which clearly shows the changes in the catchment area of the lakes in 46 years almost close to a half-century. Various changes in the catchment's attributes of the lake are observed in Fig. 3[A] of the year 1976 and Fig. 3[B] of the year 2022. The area of the agricultural and scrubland was comparatively high in 1976 whereas the year 2022 shows a major decrease in the agricultural area in the lake's catchment. In the year 2022, a major catchment area was covered with human settlements which are mainly caused due to increase in urban sprawl and other anthropogenic activities. | ||
| Fig. 3 (A) Land use and land cover map of the lake in 1976 (source: Corona image of January 1976) (B) Land use and land cover map of the lake in September 2022. | ||
Change in land use classes
Percentage change in various attributes shows a decrease in the agricultural land, lake/ponds area, scrubland, and waterlogged area whereas an increase in the aquaculture, open space area, construction of the railway lines and road networks along with the abrupt increase in settlements around the lake catchment from 1976 to 2022. This was mainly due to an increase in the anthropogenic activities in the lake catchment caused due to urbanization in the lake catchment as it is situated in Lucknow, the urban metropolitan city of Uttar Pradesh. Table 2 summarizes the overall percentage change in the various land use classes in the 2 km catchment area of the lake.| Kathauta lake | |||
|---|---|---|---|
| Land use class | Area (ha) | ||
| 1976 | 2022 | % Change | |
| Agricultural land | 1445.96 | 6.82 | −99.52 |
| Aquaculture | 0.00 | 2.93 | 2.93 |
| River/drain | 9.80 | 1.91 | −80.51 |
| Lakes/ponds | 81.24 | 48.57 | −40.21 |
| Open space | 0.00 | 198.71 | 198.71 |
| Park/garden | 0.00 | 63.88 | 63.88 |
| Railway line | 19.30 | 19.81 | 2.64 |
| Road network | 34.58 | 164.94 | 376.98 |
| Scrub land | 226.83 | 134.61 | −40.65 |
| Settlement | 60.65 | 1295.89 | 2036.66 |
| Waterlogged | 59.70 | 0.00 | −100.00 |
| Grand total | 1938.07 | 1938.07 | |
Water quality analysis
Physicochemical analysis of the 15 water quality parameters was analyzed from the selected six sites in all three seasons during the first year of the sampling. K1, K2, and K3 sites selected are from the region where Trapa cultivation was done and K4, K5, and K6 sites are from the region where fish rearing was done. The average of all 6 sites' physicochemical parameters of all three seasons depicts maximum temperature during the summer season, TDS was maximum during the winter season, and minimum DO, BOD, and TSS were reported during the winter season, the main reason being the seasonal variation. Hardness was minimum during the monsoon season and alkalinity was minimum during the summer season. Maximum nitrate, nitrite, and total phosphorous were reported during the winter season. Chl a and Chl b were maximum during monsoon season due to the active aquaculture activity in the lake as seen in the percentile graph during the first year of the sampling shown in Fig. 4.In the second year of sampling, the pH levels remained relatively stable across all three selected seasons. Notably, higher values of total dissolved solids (TDS) were reported during the winter season, followed by the monsoon (2020) season. A noteworthy decrease in alkalinity was observed during the winter (2020) season as compared to monsoon seasons of both the years. Nitrate levels demonstrated minimal fluctuations among the selected three seasons and the winter (2020) season recorded the lowest nitrite levels and the highest total phosphorous concentrations. Additionally, Chl a and Chl b concentrations were lower during the monsoon season due to less conducive growth conditions for algae compared to the winter (2020) to monsoon (2020) periods. A comprehensive overview of these water quality variations across the three seasons has been depicted in Fig. 4. This graphical representation provides a visual representation of the fluctuations in the lake's water quality parameters, offering valuable insights into the seasonal dynamics of the ecosystem. The weather data related to temperature, sunshine hours and rainfall for the year 2018, 2019, and 2020 are provided in the ESI (Fig. 4A, B, 5A and B†).
Trophic state index of the lake
The trophic state index was calculated using the formulae given by Carlson.28 The trophic state in the monsoon, winter, and summer seasons was found to be in mesotrophic condition. In all three seasons during the first year, the trophic state of the lakes remains stable i.e. in mesotrophic conditions. During the second year of the study, trophic state of the lake in monsoon, and winter season (2020) was found to be in mesotrophic condition caused due to cultural eutrophication due to aquaculture in the lake, as shown in Fig. 5.Multivariate cluster analysis of the physicochemical water parameters
Cluster analysis using Euclidean distance and non-weighted clustering using the arithmetic mean (UPGMA) was carried out by PAST 3.0 software. The mean of 14 physicochemical parameters of the selected three seasons during the two-year study after the exclusion of variables with commonality values lower than 0.6 was evaluated. The phenogram obtained showed two separate groups; one showing the monsoon season and the other summer and winter. Thus, the phenetic analysis divided the groups based on seasonal changes along with the impacts of the aquaculture activities on the lake ecosystem. The phenogram obtained for the first year of the sampling using PAST software is given in Fig. 6[A]. In the second year of study, the phenogram in the cluster analysis (Fig. 6[B]) displayed two distinct divisions. One division includes the monsoon seasons of 2019 and 2020. This grouping is primarily due to the correlated physicochemical values of the water quality, influenced by similar weather and seasonal conditions, as well as active fish rearing in the lake during the monsoon seasons. The other division represents the winter season. The variations in water quality during this period are attributed to the low-temperature conditions, which lead to increased dissolved oxygen levels. Additionally, the favorable conditions in winter promote algal growth in the lake water. These factors, combined with the ongoing fish rearing activities, result in distinct water quality characteristics during the winter season. Cluster analysis phenogram during the second year of the sampling is shown in Fig. 6[B].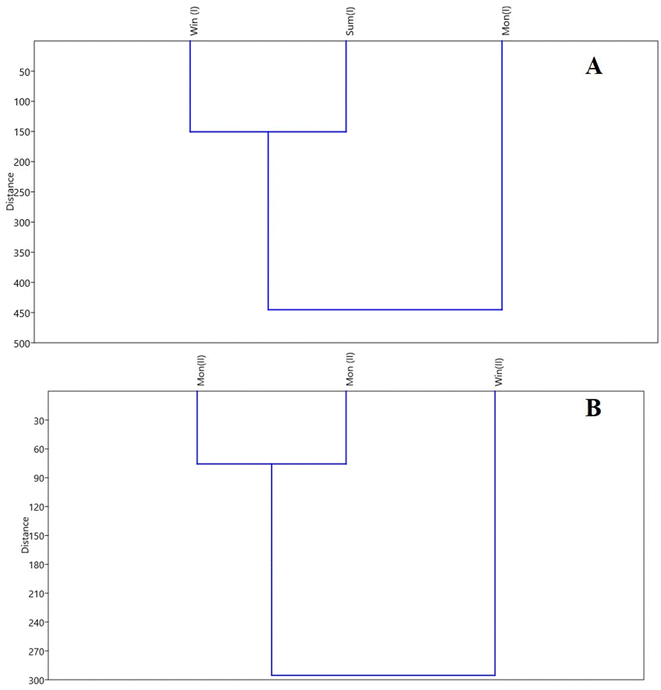 | ||
| Fig. 6 Multivariate statistical analysis [A] cluster analysis during the first year of the sampling [B] cluster analysis during the second year of the sampling. | ||
Algal diversity during both years of the study
Overall 61 algal species were reported belonging to five families namely Chlorophyceae, Cyanophyceae, Bacillariophyceae, Zygnematophyceae, and Euglenaceae in the two-year study. During the first year of the lake sampling, a total of 20 algal species belonging to Chlorophyceae were reported, with 11 being reported during the monsoon season, 17 during the winter season, and 20 during the summer season. Only one algal species was documented in the Zygnematophyceae class, and it was only in the summer. From the Cyanophyceae family total of 6 algal species were recorded, the maximum was reported in the summer season followed by the winter and monsoon seasons. In the Bacillariophyceae family total of 11 algae species were reported, the maximum was reported in the winter season whereas the summer and monsoon seasons reported the same number of species. The Euglenaceae family reported overall 7 species of algae. Maximum and nearly equal algal species were reported in winter and summer seasons mostly from the genera Phacus spp. and Euglena spp., and minimum species were reported in the monsoon season as shown in Fig. 6 main reason being the active aquaculture activities in this season.During the second year of the sampling, a total of 20 Chlorophyceae algae were reported of which a maximum (18) were in the monsoon season followed by the winter (17) season (2020), and a minimum (14) were reported in the monsoon season (2020). Only one algal species was reported in the Zygnematophyceae class that too in the monsoon season only. In Cyanophyceae, a total of 4 algal species were reported of which the maximum was reported in the monsoon and winter (2020) season and the minimum in monsoon (2019) season, and in Bacillariophyceae, a total of 9 algal species were reported of which the maximum was reported in the winter (2020) season followed by the monsoon (2020) season, and the minimum in the monsoon season. A total of 7 species of algae are reported in the Euglenaceae. A maximum and equal number of species were reported in the monsoon and in winter (2020) and minimum algal species were recorded in the monsoon (2020) season as shown in Fig. 7. Images of the major algae reported at the time of sampling are shown in Fig. 8 of the algae image plates.
Algal species pollution index
An ‘Algal Species Pollution Index’ was developed to evaluate the algal species' tolerance to organic pollution which is helpful for rating water quality.39 A pollution score having a value >20 means high organic pollution, a pollution value between 15 – 19 means probable evidence of organic pollution, and the results of the pollution index calculated by using algal species identified in the selected lake are given in Table 11 in the ESI.† The pollution-tolerant status of genera Euglena spp. and Oscillatoria sp. are used as indicators of eutrophication of the aquatic ecosystem. The presence of the genus Scenedesmus indicates the eutrophic nature of the water body.40 Algal genera viz. Chlorella, Scenedesmus, Pediastrum, Oscillatoria, Melosira, Navicula, Nitzschia, Gomphonema, and Euglena indicate organic pollution of water bodies.44,45Values of the Palmer index in the selected two years of the sampling show the high organic pollution in the lakes as the values exceed the permissible value of 20 as given in Fig. 9 in all the seasons of the selected two phases of the sampling. During the first phase of the sampling highest Palmer index value was reported in the summer season (81) followed by winter (66) and monsoon season. The main reason is the increase in water pollution in summer along with the increase in the temperature which favors the growth of many algal species. During the second year, the maximum palmer index was reported in the winter (2020) followed by monsoon season and monsoon (2020). Values of the Palmer index during both years of the study are given in Fig. 9.
Macrophytic diversity of the lake
During the first year of the study, a total of 37 macrophytes, including free-floating, submerged, and emergent macrophytes, as well as sedges and littoral grasses were reported on the lake's shores. Maximum macrophytes are reported in the summer season followed by the winter season and minimum in the monsoon season as shown in Fig. 10[A]. The major reason for minimum macrophytes in the monsoon season was due to the active Trapa cultivation that results in its dominance in the lake. Maximum free-floating macrophytes are reported in the summer season because excessive free-floating macrophytic growth was favored due to nutrient enrichment in the lake because of post-aquaculture conditions, and fewer but same number of macrophytes in the winter and monsoon seasons. Maximum species of submerged macrophytes are reported in the winter season followed by monsoon and minimum in the summer season, this confirms that the excessive proliferation of free-floating macrophytes results in a decrease in the number of submerged macrophytes in the summer season. Maximum emergent macrophytes are reported in the winter and monsoon and minimum in the summer season. Maximum sedges and littoral grasses are reported in the winter season followed by monsoon and minimum in the summer season as shown in Fig. 10[A]. | ||
| Fig. 10 Macrophytic diversity of the lake [A] number of macrophytes reported [B] macrophytic diversity indices. | ||
During the second year of the sampling, a total of 36 macrophytes were reported in this lake. Maximum and equal numbers of macrophytes are reported in the winter (2020) and monsoon (2020) samples and minimum macrophytes are reported in the monsoon season (2019). The number of free-floating macrophytes remains constant in all the three seasons i.e. monsoon (2019), winter (2020), and monsoon (2020). Submerged macrophytes were not reported in the monsoon season of both 2019 and 2020 and only one species was reported in the winter (2020) sampling. Nearly fewer submerged macrophytes were reported mainly due to the fish-rearing activity during this phase of the sampling because of which submerged macrophytes were uprooted by the fishermen. Maximum emergent macrophytes are reported in the monsoon (2020) sampling followed by winter (2020) sampling and minimum in the monsoon (2019) sampling. Maximum sedges and littoral grasses were reported in the winter (2020) and monsoon (2020) sampling and a minimum in the monsoon (2019) season. Overall 37 macrophytes were reported during this two-year study and in the second year a high reduction in the macrophytes was reported due to fish-rearing activity in the lake in comparison to the first year of the sampling as shown in Fig. 10[A].
Macrophytic diversity indices
During the first year of sampling, macrophytic diversity was evaluated for all the three seasons. The findings reveal the highest Simpson and Shannon indices during the summer season. This is attributed to the post-aquaculture effects of summer, which promote the proliferation of diverse macrophytic species within the lake. Subsequently, the monsoon season shows the next highest indices, followed by the winter season. The peak evenness was observed during the summer season, primarily due to the robust proliferation of Eichhornia and Pistia species within this period. In contrast, both the monsoon and winter seasons exhibit nearly identical evenness indices. Margalef diversity index of monsoon and summer are approximately the same whereas winter has a minimum index. Berger–Parker dominance index was maximum in the winter season followed by monsoon and minimum was found in the summer season. During the second year of sampling, macrophytic diversity was found to be zero as there were no macrophytic populations in the open area of the lake. Simpson index, Shannon index, Evenness, Margalef diversity index, and Berger Parker dominance index values are in binary numbers i.e. diversity shows a value of 0 and evenness shows a value of 1 as given in Fig. 10[B].Discussion
a. Land use and land cover change in the lake catchment
The selected lake faces severe problems of land use change and fragmentation due to an increase in the human population and increased anthropogenic activities. Presently a major portion or maximum portion of the lake is used for water storage and supply to the nearby urban settlements. Only a small segment of the lake is present in the natural conditions which are utilized for cloth washing by washermen, Trapa cultivation, and for fish rearing by local fishermen. Land use change results depict the extreme anthropogenic stress lakes have faced in the last five decades. The decrease in the agricultural area and increase in the urban area are mainly caused by increase in urbanization and irrational anthropogenic activities. Hence it resulted in a decrease in the lake area, catchment area and fragmentation of the lake which adversely affects the water quality, quantity, and aquatic biodiversity and poses challenges in the lake management.b. Water quality analysis
During the first year of the sampling DO, BOD, and TSS showed the variations were mainly caused due to the aquaculture and seasonal changes whereas maximum nitrate, nitrite, and total phosphorous were reported in the winter season (post-aquaculture condition) when the lake was left as it is and resulted in the biological degradation of the matured Trapa macrophytes resulting in the cultural nutrient enrichment of the lake. Chl a and Chl b were maximum during monsoon season due to the active aquaculture activity during this season which increased the organic pollution and resulted in increased algal biomass in lake. The trophic state of the lake during the first year of the study was found to be in mesotrophic condition. The anthropogenic activities (Trapa cultivation, fish rearing, and cloth washing by local washermen) are the major factors resulting in the mesotrophic condition of the lake caused due to nutrient enrichment resulting from these activities. During the second year of the sampling, seasonal changes and aquaculture resulted in changes in the lake's water quality. Due to the fish rearing during this phase Chl a and Chl b concentrations were reported significantly higher in the winter (2020) to monsoon (2020) due to an increase in the algal growth, as the fish-rearing activities resulting in an increase in nutrient enrichment and organic pollution favors the growth of the algae mainly of Chlorophyceae family in the lake.During the second year of the study lake was again reported to be in a mesotrophic condition. The fish-rearing activities during this phase are the major factors resulting in the higher trophic state with an increased TSI value resulting in the lake's mesotrophic condition. In the first-year study cluster analysis phenogram obtained showed two separate groups, one showing the monsoon season and the other connected phenogram of summer and winter seasons. This clearly shows that active aquaculture activities in the monsoon season (2018) cause significant changes in the water quality due to an increase in nutrients and organic load in the lake, whereas their consequences are more evident throughout the winter and summer seasons, as in this two seasons active aquaculture activities were absent as reflected in the cluster analysis. Thus, the phenetic analysis divided the groups based on changes caused due to aquaculture activities in lake water quality. During the second year of the study, the phenogram obtained showed two distinct and connected groups of monsoon seasons of 2019 and 2020. The monsoon seasons of 2019 and 2020 are connected due to the similarity in the physicochemical values of the water quality parameter during these two seasons. The winter (2020) season is separated from these two distinct monsoon seasons mainly because of the aquaculture activities and seasonal variations. Seasonal changes along with the aquaculture activities resulted in the input of fish feed, and fish excreta thereby increasing the nutrient and organic load in the lake. In the second-year study, the fish-rearing activities were actively conducted during all the three seasons.
Both natural processes like precipitation, the geography of the watershed, atmospheric condition; and the anthropogenic activities like wastewater discharge from industries and domestic sources, aquaculture activities in the lakes and agriculture run-off determine the chemical, physical and biological composition of the freshwater lakes. As in this study, we reported that Trapa cultivation in the lake results in a decrease in TDS and EC, whereas DO was observed to be comparatively better as opposed to fishing season. Other parameters such as hardness, nitrate, nitrite, and total phosphorus were significantly lower in comparison to those observed during the fishing season. The main reason behind this is the phytoremediation potential of the Trapa natans that improve the water quality but along with it their excessive growth on the water surface chokes the life of the aquatic biota present in the pelagic region of the lake. During the fishing activity in the lake, increase in pH, EC, TDS, TSS, nitrite, and total phosphorus and a decrease in Secchi depth was reported. Hence the aquaculture activities influence the geochemical characteristics of the lakes.
c. Effect of trophic state and seasons on changes in algal diversity
The major dominant algal family reported was of Euglenaceae as they indicated high organic pollution in the lake owing to fishing activities. During the initial survey in October 2018, water samples from the Trapa region did not record any algal species, only diatoms were reported. This was due to the antagonistic activity between algae and macrophytes in the lakes, whereas the diverse algal species are documented in the fishing segment of the lake. The active human activities by local washermen, Trapa cultivation, and fishing in the lake over the selected three seasons resulted in fewer filamentous algae and more planktonic algae. The monsoon season saw a decrease in algal species owing to active Trapa cultivation and fish rearing, however, in the summer season an increase in diversity and abundance of algae and diatoms was observed due to the synergistic impact of increasing temperature and nutrient enrichment. The other reason is the unrestricted discharge of sewage from the surrounding residential areas into the lake. Therefore, the anthropogenic stress resulted in the polluted water leading to the mesotrophic condition of the lake.Algae such as Navicula spp. and Nitzschia spp. were reported in abundance due to their possession of a frustule and their ability to migrate vertically in the lake sediment through the secretion of a mucous substance for ease of movement and protection.46,47Navicula spp. is mainly reported in polluted freshwater bodies. Variation in the diversity of algal species in the lake was mainly caused due to the changes that occurred in the freshwater environment and water quality due to the changes in trophic state and pollution level in the lake. It resulted in qualitative and quantitative variations in freshwater lacustrine biology, including macrophytes and algae leading to the disappearance of certain species and the emergence of other species causing a floristic biotic shift, which has been also observed by other researchers.48,49
d. Bioindicator algal species reported
Many freshwater algae species have been identified as indicators of water quality and trophic conditions by numerous researchers and scientists.50 Several different types of algae, including Chlorophyceae, Cyanophyceae, Bacillariophyceae, and Euglenophyceae, were identified as indicators of water contamination in research conducted on the Kadra reservoir in Karnataka.51As far as aquaculture is concerned, research on the amount, diversity, and location of algae in aquatic ecosystems, especially freshwater ecosystems, is gaining momentum.52 Water temperature, transparency, nitrate, nitrite, and total hardness represent the most important environmental factors influencing the structure of the algal communities including their preference for suitable environmental factors. The highest dominance of Bacillariophyta was encountered mostly in winter. The dominance of Chlorophyta during the winter season is mainly caused by the proliferation of the filamentous algae, the dominance of large green algae is not frequent in winter, but they show some common adaptations that allow them to grow in low values of light and temperature.53
e. Organic pollution status calculated based on algal species pollution index
The study shows instances of high organic pollution in all the samping seasons main reason being the aquaculture activities in the lake. During the first year of the sampling, highest palmer index value was reported in summer season due to increase in nutrient enrichment and the increase in the atmospheric temperature which favors the growth of several algal species. During the second year, the highest palmer index was reported in the winter (2020) followed by monsoon of 2019 and 2020 which is comparatively lower in comparison to the first year study. This is due to the active fish-rearing activities in the lake which cause excessive growth of the algae of the Euglenaceae family thereby reducing the diversity of the algal species present in the first year study. Eight pollution-tolerant genera viz., Euglena, Scenedesmus, Phacus, Lepocinclis, Micractinium, Pandorina, Synedra, and Nitzschia were found from the list of 20 pollution-tolerant genera of Palmer's algal genus index. Among them, eight genera are organic pollution tolerant viz., Euglena, Scenedesmus, Phacus, Micractinium, Pandorina, Synedra, and Nitzschia. It was found that the most pollution-tolerant genus Euglena was recorded in both phases of the study due to increase in the organic pollution in the lake caused due to aquaculture activities. The proliferation of cyanophyceae due to aquaculture activities further exacerbates eutrophication, which changes the species composition and overall floristic diversity. It is seen that when macrophytes were manually uprooted, the planktonic algae took over the lake environment due to the aquaculture activities. The high dominance of diatoms during winter can be interpreted as a net growth of phytoplankton that is possibly favored by low predation and high nutrient availability. Consequently, changes in the biodiversity composition of the lake environment as well as shifts in the algae and macrophytic diversity were reported.f. Macrophytic diversity variation along with changing trophic state
Lake having numerous species of littoral grasses, herbaceous plants, and sedges, as well as free-floating, submerged, and emergent macrophytes have been documented during the study period. During the first year of study, lesser diversity of macrophytes is reported in the monsoon season. The major reason being the active Trapa cultivation that results in its dominance in the lake. A higher diversity of free-floating macrophytes in the summer season are observed due to nutrient enrichment post aquaculture activities. Consequently, lesser number of submerged macrophytes in this season is observed due to inhibitions by the free-floating macrophytes. This confirms that the excessive proliferation of free-floating macrophytes results in a decrease in the number of submerged macrophytes.54 During the monsoon season, half of the area 17.17 acres of the lake was used for Trapa cultivation. Trapa cultivation has negative effects on freshwater lakes during the growing and declining seasons. In the growing season, Trapa can reach a high biomass of about 504 ± 91 gm dry weight per m2.54,55 Minimum number of emergent macrophytes, sedges, and littoral grasses are reported along the shoreline in the summer season as high temperatures and scarcity of water do not favor their growth, the water level in the lake also declines considerably.The findings reveal the highest Simpson and Shannon indices during the summer season of first year samping. This is attributed to the post-aquaculture effects caused during summer, which promote the proliferation of diverse macrophytic species due to nutrient enrichment within the lake. The maximum evenness was also observed during the summer season, caused due to the robust proliferation of Eichhornia and Pistia species during this period. In the monsoon and winter seasons, evenness values was less in comparison to summer season main reason being the seasonal factors and active aquaculture activities.
During the second year of the sampling, free-floating macrophytes remained almost constant in all the three seasons. Submerged macrophytes were not reported in the monsoon of both 2019 and 2020 sampling and only one species was reported in the winter (2020) sampling. Nearly fewer submerged macrophytes were observed due to the fish-rearing activity during this phase of the sampling, as they were uprooted by fishermen to provide proper habitat for the fish to survive and thrive. The evenness index was 1 in the open area of the lake due to lack of submerged and free-floating macrophytes. The rapid decline in the diversity of the macrophytes is brought by anthropogenic interference mainly fish-rearing activities in the lake.
Conclusion
Freshwater lakes are threatened by land use change, fragmentation, cultural eutrophication, and biotic shift due to increased nutrients. This study has documented the impact of intensive aquaculture on a lake that has undergone substantial human-induced changes. It specifically examines how aquaculture activities, including the cultivation of water chestnut Trapa and fish farming, affect the lake's overall water quality, trophic state and floristic diversity, with a particular focus on algae and macrophytes. To evaluate the anthropogenic factors driving temporal changes in the lake's area and size, historical and recent satellite images spanning five decades (1976–2022) were analyzed. The findings show that significant changes have occurred in the land use patterns of the lake's immediate catchments. These changes, driven by human activities, have altered the natural landscape, affecting the lake's hydrology and ecology. During this period, the lake experienced significant anthropogenic pressures, leading to its fragmentation, encroachment, and periodic drying up of water. As in the developing country, there is no strict legislation regarding aquaculture activities in the freshwater lakes.The study identifies multiple sources of contaminants entering the lake, including organic pollution from fish feed and the cultivation of Trapa. Aquaculture activities increased the lake's organic pollution which adversely affects the diversity and evenness of the algae and macrophytes. Maximum species of submerged macrophytes are reported in the winter season followed by monsoon and minimum in the summer season, the excessive proliferation of free-floating macrophytes such as Eichhornia and Pistia results in a decrease in the number of submerged macrophytes in the summer season. The findings suggest that aquaculture activities are linked to decreased native floristic diversity.
The study reported a total of 61 algal species from five families during both sampling phases. The dominant species belonged to the Chlorophyceae and Euglenophyceae families, alongside several diatom species. An ‘Algal Species Pollution Index’ was used to assess the tolerance of algal species to organic pollution, which is useful for judging water quality. Values of the Palmer index in the selected two years of the sampling show the high organic pollution in the lakes as the values exceed the permissible limit. The pollution-tolerant status of genera Euglena spp. and Oscillatoria sp. are used as indicators of eutrophication of the aquatic ecosystem. The presence of the genus Scenedesmus indicates the eutrophic nature of the water body. Algal genera Chlorella, Scenedesmus, Pediastrum, Oscillatoria, Melosira, Navicula, Nitzschia, Gomphonema, and Euglena indicate organic pollution of water bodies. The ongoing human activities, including those of local washermen, Trapa cultivation, and fishing led to a decrease in filamentous algae and an increase in planktonic algae in the lake over the three selected seasons. During the second year of sampling, intensive fish-rearing activities disrupted the macrophytic diversity which was replaced by the proliferated growth of planktonic algae, resulting in the biotic shift of the lake's floristic diversity. This shift is largely due to nutrient enrichment, which favors the growth of species that thrive in nutrient-rich conditions, altering the lake's ecological balance.
The study found that aquaculture activities and land use changes had a negative impact on the diversity, dominance, and community structure of algae and macrophytes in the freshwater lake. Therefore, strict regulatory measures must be implemented to closely supervise activities and ensure economic growth while safeguarding the native aquatic biodiversity of the lake ecosystem. Overall, the study is important for understanding the impact of human activities on fragile aquatic ecosystems and for promoting sustainable development practices in urban areas while preserving the native freshwater biodiversity.
Ethical approval
Participation of human subjects did not occur in this study.Consent for publication
This manuscript was approved for publication by all the authors.Data availability
The data supporting this article have been included as part of the ESI.† The data collected are the property of our department but will be made available by the authors when requested.Author contributions
Divya Dubey-project administration, conceptualization, methodology, data curation, microscopy of the algal sample, writing-review and editing of original draft. Kiran Toppo-algae identification, review, validation Saroj Kumar-review, formal analysis. Venkatesh Dutta-conceptualization, supervision, methodology, validation, editing, and finalization of the manuscript.Conflicts of interest
The author(s) declare no potential conflicts of interest concerning the authorship, research, and publication of this article.Acknowledgements
The authors gratefully acknowledge the support provided by the Department of Environmental Science, Babasaheb Bhimrao Ambedkar University (BBAU), Lucknow to carry out this work. Doctoral research grant under the Junior and Senior Research Fellowship (JRF and SRF Award: 190520171221) to the first author by the University Grants Commission (UGC) is greatly acknowledged. The authors also thank the two anonymous reviewers and the handling editor for helpful comments on the earlier draft.References
- J. Heino, J. Alahuhta, L. M. Bini, Y. Cai, A. S. Heiskanen, S. Hellsten, P. Kortelainen, N. Kotamäki, K. T. Tolonen, P. Vihervaara and A. Vilmi, Lakes in the era of global change: moving beyond single-lake thinking in maintaining biodiversity and ecosystem services, Biol. Rev., 2021, 96(1), 89–106 CrossRef PubMed.
- A. Behroozi, Promoting Pro-Environmental Behavior for Sustainable Water Resource Management: A Social Exchange Perspective, Qeios, 2023 Search PubMed.
- S. Parveen, Sustainable Development and Ecological Issues, Indian J. Polit. Sci., 2021, 55(1–2), 76–88 Search PubMed.
- G. Upreti, Valuation of Biodiversity, Ecosystem Services, and Natural Capital, in Ecosociocentrism: the Earth First Paradigm for Sustainable Living, Springer Nature Switzerland, Cham, 2023, pp. 163–188 Search PubMed.
- W. Steffen, J. Rockström, K. Richardson, T. M. Lenton, C. Folke, D. Liverman, C. P. Summerhayes, A. D. Barnosky, S. E. Cornell, M. Crucifix and J. F. Donges, Trajectories of the Earth System in the Anthropocene, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(33), 8252–8259 CrossRef CAS PubMed.
- I. A. Sheergojri, I. Rashid, S. Aneaus, I. Rashid, A. A. Qureshi and I. U. Rehman, Enhancing the social-ecological resilience of an urban lake for sustainable management, Environ. Dev. Sustain, 2023, 16, 1–26 Search PubMed.
- J. Jones and M. T. Brett, Lake nutrients, eutrophication, and climate change, Glob. Environ. Change, 2014, 273–279 Search PubMed.
- C. E. Boyd, C. Tucker, A. McNevin, K. Bostick and J. Clay, Indicators of resource use efficiency and environmental performance in fish and crustacean aquaculture, Rev. Fish. Sci., 2007, 15(4), 327–360 CrossRef.
- D. Dubey, S. Kumar and V. Dutta, Impact of nutrient enrichment on habitat heterogeneity and species richness of aquatic macrophytes: evidence from freshwater tropical lakes of Central Ganga Plain, India, Int. J. Environ. Sci. Technol., 2022, 19(6), 5529–5546 CrossRef CAS.
- M. Chagnon, D. Kreutzweiser, E. A. Mitchell, C. A. Morrissey, D. A. Noome and J. P. Van der Sluijs, Risks of large-scale use of systemic insecticides to ecosystem functioning and services, Environ. Sci. Pollut. Res., 2015, 22, 119–134 CrossRef CAS PubMed.
- H. S. Ayele and M. Atlabachew, Review of characterization, factors, impacts, and solutions of Lake eutrophication: lesson for lake Tana, Ethiopia, Environ. Sci. Pollut. Res., 2021, 28(12), 14233–14252 CrossRef CAS PubMed.
- S. Lin, M. Milardi, Y. Gao and M. H. Wong, Sustainable management of non-native grass carp as a protein source, weed-control agent and sport fish, Aquacult. Res., 2022, 53(17), 5809–5824 CrossRef CAS.
- F. Teletchea, J. N. Beisel and R. Sajal, Alien fish species in France with emphasis on the recent invasion of gobies, Biol. Resour. Water, 2018, 25, 75–92 Search PubMed.
- V. A. Zamlynskyi, V. M. Kondratyuk, A. I. Livinskyi, A. V. Naida and I. S. Naida, Priority tasks and marine aquaculture development strategy, in AIP Conference Proceedings, AIP Publishing, 2022, vol. 2413, 1 Search PubMed.
- T. V. Nagaraju, G. S. Bala, S. Bonthu and S. Mantena, Modelling biochemical oxygen demand in a large inland aquaculture zone of India: Implications and insights, Sci. Total Environ., 2024, 906, 167386 CrossRef CAS PubMed.
- T. V. Nagaraju, S. B. Malegole, B. Chaudhary and G. Ravindran, Assessment of environmental impact of aquaculture ponds in the western delta region of Andhra Pradesh, Sustainability, 2022, 14(20), 13035 CrossRef.
- T. V. Nagaraju, B. M. Sunil, B. Chaudhary, C. D. Prasad and R. Gobinath, Prediction of ammonia contaminants in the aquaculture ponds using soft computing coupled with wavelet analysis, Environ. Pollut., 2023, 331, 121924 CrossRef CAS PubMed.
- S. L. Dong, Q. F. Gao and L. Li, Aquaculture Ecosystem. InAquaculture Ecology, Springer Nature Singapore, Singapore, 2023 Apr vol. 5, pp. 33–91 Search PubMed.
- L. Zhang, H. Wei, C. Wang, Y. Cheng, Y. Li and Z. Wang, Distribution and ecological risk assessment of antibiotics in different freshwater aquaculture ponds in a typical agricultural plain, China, Chemosphere, 2024, 31, 142498 CrossRef PubMed.
- Y. Ge, X. Gu, Z. Mao, H. Chen, Q. Zeng and H. Yang, How does aquaculture activity affect phytoplankton functional groups in Gaoyou Lake, China, J. Freshwater Ecol., 2023, 38(1), 2159554 CrossRef.
- W. Kong, Q. Xu, H. Lyu, J. Kong, X. Wang, B. Shen and Y. Bi, Sediment and residual feed from aquaculture water bodies threaten aquatic environmental ecosystem: Interactions among algae, heavy metals, and nutrients, J. Environ. Manage., 2023, 326, 116735 CrossRef CAS PubMed.
- B. L. Querijero and A. L. Mercurio, Water quality in aquaculture and non-aquaculture sites in Taal lake, Batangas, Philippines, J. Exp. Biol., 2016, 4, 1 Search PubMed.
- H. Xu, D. Zhao, J. Zeng, Z. Mao, X. Gu and Q. L. Wu, Evaluating the effects of aquaculture on the freshwater lake from the perspective of plankton communities: The diversity, co-occurrence patterns and their underlying mechanisms, Environ. Pollut., 2022, 309, 119741 CrossRef CAS PubMed.
- J. Zeng, Y. Lin, D. Zhao, R. Huang, H. Xu and C. Jiao, Seasonality overwhelms aquacultural activity in determining the composition and assembly of the bacterial community in Lake Taihu, China, Sci. Total Environ., 2019, 683, 427–435 CrossRef CAS PubMed.
- M. D. Rennie, P. J. Kennedy, K. H. Mills, C. M. Rodgers, C. Charles, L. E. Hrenchuk, S. Chalanchuk, P. J. Blanchfield, M. J. Paterson and C. L. Podemski, Impacts of freshwater aquaculture on fish communities: A whole-ecosystem experimental approach, Freshwater Biol., 2019, 64(5), 870–885 CrossRef.
- American Public Health Association, APHA Standards Methods for Examination of Water and Wastewater, American Water Work Association, Water Environment Federation, Washington, DC, 23rd edn. 2017 Search PubMed.
- A. Lumb, T. C. Sharma and J. F. Bibeault, A review of genesis and evolution of water quality index (WQI) and some future directions, Water Qual., Exposure Health, 2011, 3, 11–24 CrossRef.
- R. E. Carlson, A trophic state index for lakes 1, Limnol. Oceanogr., 1977, 22(2), 361–369 CrossRef CAS.
- S. P. Partha, V. Bharathidasan, P. Damotharan, P. Selvaraj, P. Murugesan, S. Sivaraj, A. Syed and A. M. Elgorban, Assessment of ecological status of Uppanar and Vellar estuaries through multivariate pollution indices, Mar. Pollut. Bull., 2024, 203, 116390 CrossRef PubMed.
- C. Acuña-Alonso, X. Álvarez, O. Lorenzo, Á. Cancela, E. Valero and Á. Sánchez, Assessment of water quality in eutrophized water bodies through the application of indexes and toxicity, Sci. Total Environ., 2020, 728, 138775 CrossRef PubMed.
- A. F. Marker, The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin, Freshwater Biol., 1972, 2(4), 361–385 CrossRef.
- S. T. Jeffrey and G. F. Humphrey, New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton, Biochem. Physiol. Pflanz., 1975, 167(2), 191–194 CrossRef CAS.
- J. B. Komárek, Das Phytoplankton des SuBwassers. 7. Teil. 1. Halfte. Chlorophyceae (Grunalgen) Ordnung: Chlorococcales. Die Binnengewasser, 1983 Search PubMed.
- K. Anagnostidis, Modern approach to the classification system of cyanophytes. 3. Oscillatoriales, Arch. Hydrobiol., Suppl., 1988, 80, 327–472 Search PubMed.
- J. Komárek, K. Anagnostidis and I. Cyanoprokaryota, Teil: Chroococcales, Süßwasserflora von Mitteleuropa. vol. 19/1, Spektrum, Heidelberg, Berlin, 1998 Search PubMed.
- J. Komárek and K. Anagnostidis. Süßwasserflora von Mitteleuropa, bd. 19/2: Cyanoprokaryota: Oscillatoriales, 2005 Search PubMed.
- J. Komárek, B. Büdel, G. Gärtner and L. Krienitz, Cyanoprokaryota 3rd Part: Heterocytous heterocytous genera, Süsswasserflora von Mitteleuropa. 2013, vol. 19, 3 Search PubMed.
- B. Karthick, P. B. Hamilton and J. P. Kociolek. An Illustrated Guide to Common Diatoms of Peninsular India. Gubbi labs, 2013 Search PubMed.
- C. M. Palmer, A composite rating of algae tolerating organic pollution 2, J. Phycol., 1969, 5(1), 78–82 CrossRef CAS PubMed.
- C. M. Palmer, Algae and Water Pollution, Castle House Pub. Ltd, New York, 1980, vol. 110 Search PubMed.
- D. C. Saini, S. K. Singh, K. Rai and S. K. Singh, Biodiversity of Aquatic and Semi-aquatic Plants of Uttar Pradesh:(with Special Reference to Eastern Uttar Pradesh), Uttar Pradesh State Biodiversity Board, 2010 Search PubMed.
- K. Biswa and L. C. Calder, Handbook of Common Water and Marsh Plants of India and Burma, Bisher, 1984, 2nd edn. p 216.Hlth. Bull. Calcutta No. 24 Search PubMed.
- K. C. Mishra. Manual of Plant Ecology. Oxford and IBH Publishing Co, New Delhi. 1980, p. 491 Search PubMed.
- A. D. Kshirsagar, M. L. Ahire and V. R. Gunale, Phytoplankton diversity related to pollution from Mula River at Pune City, Terr. Aquatic Environ. Toxicol., 2012, 6(2), 136–142 Search PubMed.
- S. D. Noel and M. R. Rajan, Evaluation of organic pollution by Palmer's algal genus index and physico-chemical analysis of Vaigai river at Madurai, India, Nat. Resour. Conserv. Res., 2015, 3(1), 7–10 Search PubMed.
- A. Poulíčková, P. Hašler, M. Lysáková and B. Spears, The ecology of freshwater epipelic algae: an update, Phycologia, 2008, 47(5), 437–450 CrossRef.
- J. Z. Andrejić, J. Krizmanić and M. Cvijan, Diatom species composition of the Nišava river and its tributaries Jerma and Temska rivers (Southern Serbia), Arch. Biol. Sci., 2012, 64(3), 1127–1140 CrossRef.
- I. K. A. H. Al-Daraji, The use of phytoplankton biomarkers to assess the impact of human activities on the water quality of Beit Zuweina River, Diyala Governorate, MSc. Thesis, College of Education for Pure Sciences, (Ibn Al-Haitham) University of Baghdad, 2015 Search PubMed.
- S. O. Obaje and E. A. Okosun, Taxonomic Notes on Marker Planktonic Foraminifera of Tomboy Field, Offshore Western Niger Delta, Nigeria, Int. J. Sci. Technol., 2013, 2(9), 622–627 Search PubMed.
- S. N. Nandan and N. H. Aher, Algal community used for assessment of water quality of Haranbaree dam and Mosam river of Maharashtra, J. Environ. Biol., 2005, 26(2), 223–227 CAS.
- S. Zargar and T. K. Ghosh, Influence of cooling water discharges from Kaiga nuclear power plant on selected indices applied to plankton population of Kadra reservoir, J. Environ. Biol., 2006, 27(2), 191–198 Search PubMed.
- N. Manickam, P. S. Bhavan, P. Vijayan and G. Sumathi, Phytoplankton species diversity in the parambikulam-aliyar irrigational canals (Tamil Nadu, India), Int. J. Pharma Bio Sci., 2012, 3(3), 289–300 Search PubMed.
- D. M. McKnight, B. L. Howes, C. D. Taylor and D. D. Goehringer, Phytoplankton dynamics in a stably stratified Antarctic lake during winter darkness, J. Phycol., 2000, 36(5), 852–861 CrossRef CAS.
- C. Yuan, X. Bai, T. Zhu, Z. Wen, T. Cao, X. Zhang and L. Ni, Long-term effects of the harvesting of Trapa natans on local water quality and aquatic macrophyte community in Lake Erhai, China, Front. Environ. Sci., 2021, 9, 706746 CrossRef.
- R. Bolpagni, E. Pierobon, D. Longhi, D. Nizzoli, M. Bartoli, M. Tomaselli and P. Viaroli, Diurnal exchanges of CO2 and CH4 across the water–atmosphere interface in a water chestnut meadow (Trapa natans L.), Aquat. Bot., 2007, 87(1), 43–48 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00038b |
| This journal is © The Royal Society of Chemistry 2024 |