Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sandwiching high energy frameworks by taking advantage of π-philic molecular recognition†
Jatinder
Singh
 a,
Richard J.
Staples
a,
Richard J.
Staples
 b,
Magdalena
Fabin
b,
Magdalena
Fabin
 ac and
Jean'ne M.
Shreeve
ac and
Jean'ne M.
Shreeve
 *a
*a
aDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu; Fax: (+1) 208-885-5173
bDepartment of Chemistry, Michigan State University, East Lansing, Michigan 48824, USA
cDepartment of Physical Chemistry and Technology of Polymers, Silesian University of Technology, 44-100 Gliwice, Poland
First published on 13th June 2024
Abstract
Development of thermally stable, insensitive materials with attractive physiochemical properties continues to be heavily pursued in the field of energetic materials. High-energy-density compounds assembled by the combination of polynitro, nitroamino or azo groups and nitrogen-rich frameworks are often thermally unstable and sensitive to impact and friction. This conflicting nature of energy and stability strongly encourages designers of molecules to study existing structures at the molecular level in order to develop new methodologies for construction of potential energetic materials. In this work, a robust strategy which takes advantage of π-philic molecular recognition between planar energetic anions and cations is pursued. Our work not only generates insights for the design and synthesis of new energetic sandwich salts with good thermal stability and low sensitivity but also inspires the enrichment of a variety of new excellent performing high-energy materials.
1 Introduction
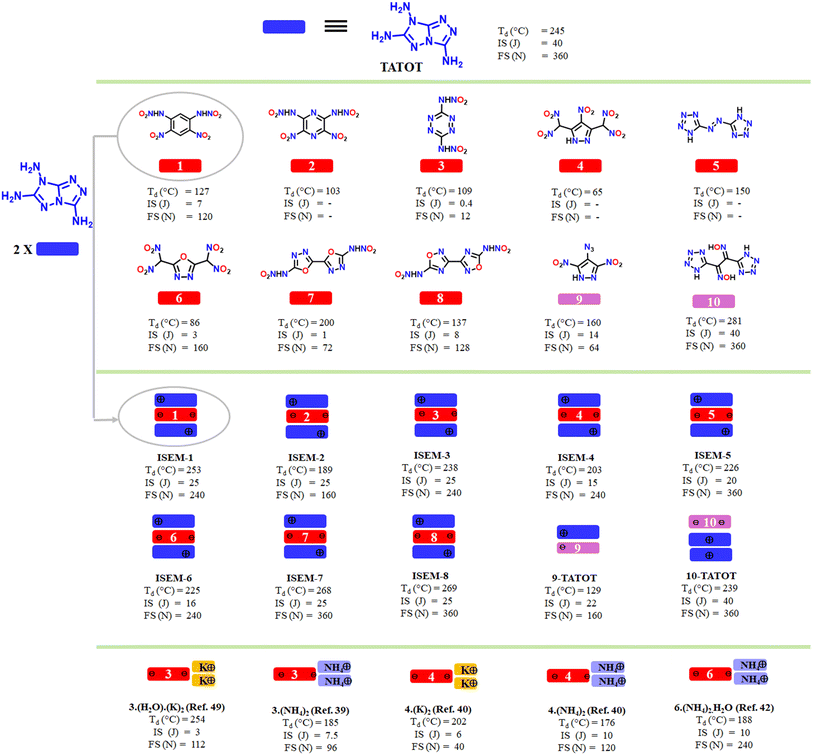
Noncovalent attractions are associations within and between molecules that influence shape and structural stability.1,2 Nearly all organic materials involve these attractions, which influence physical and chemical properties markedly.3,4 π-Philic molecular recognition between planar aromatic organic rings (often known as weak π–π interactions) play an important role in the field of chemistry and biology.5 These attractions have also been the subject of extensive computational and experimental studies since they play a complex function prior to or at the transition state in chemical reactions and catalytic processes; for example, they are central to understanding the stability of DNA, protein folding and drug binding.6 Molecular recognition between π-systems of planar aromatic organic rings often leads to the formation of stacked structures (Fig. 1B).7 In supramolecular chemistry, π-philic molecular recognition offers potential opportunities for tailoring structure and properties to various functions and applications (Fig. 1C).8,9 Recently, supramolecules as π-philic molecular sensors were applied in the detection of nitroaromatics.10 Among energetic materials, 1,3,5-triamino 2,4,6-trinitrobenzene (TATB) is a well-known highly stable compound owing to the synergetic effect of various noncovalent attractions such as inter/intra-molecular H-bond and π-stacking (Fig. 1E). A better understanding of weak attractions would be helpful in crystal engineering, molecular recognition, and in the design of future energetic materials.11–14Energetic salt formation is an efficient strategy to maximize weak noncovalent interactions.15–20 Energetic salts also generate a single multifunctional product efficiently by incorporating two or more reactants and thus is regarded as a highly useful strategy to expand molecular diversity and complexity.21 This simple conventional methodology also showcases the art of holding two or more units together by weak interactions and is regarded as the most useful in producing thermally stable energetic materials. For instance, dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) possesses higher thermal stability and better detonation performance than its precursors (Fig. 2A), suggesting that salt formation is an effective method to access stable energetic materials.22–25
Despite the effectiveness of salt formation strategy, energetic salts produced from N-rich cations and sensitive anions are often unable to overcome their inherent sensitivity to external stimuli.26–28 One other unresolved issue in the development of energetic salts is their high solubility in water, which raises concerns and difficulties associated with their use.29,30 Therefore, it is important for synthetic and theoretical researchers to resolve these issues by developing new methods. Now designers of molecules have turned their attention increasingly to the development of approaches to maximize the stabilizing interaction by producing planar energetic metal–organic frameworks, hydrogen-bonded organic frameworks, polymers, co-crystals, etc. (Fig. 2B–D).31–35
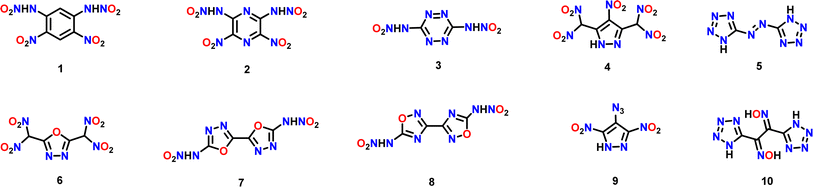
In all the above techniques, weak attractions play a central role in overcoming the issues related to thermal stability, sensitivity, acidity and solubility in water. In 2015, Klapötke and coworkers demonstrated the utility of a planar 3,6,7-triamino-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazolium (TATOT) cation in making thermally stable energetic nitrogen-rich cationic salts.36 Recently, we reported that salt formation between a planar dinitramide and TATOT results in better thermal stability and insensitivity in comparison to other N-rich salts.37 Now we have analyzed the structure at the molecular level and extended the observation to other thermally unstable and sensitive frameworks. We anticipated that salt formation between similar planar frameworks and TATOT could lead to π-philic molecular recognition and sandwich type structures, which would be useful in stabilizing high-energy-high-sensitive compounds. The thermally unstable nitroamine, polynitro and azo compounds shown in Fig. 3 were chosen for reactions with TATOT.
2 Results and discussion
2.1 Synthesis and characterization
Compounds 1–8 and their corresponding silver or ammonium salts were prepared following the literature procedures {see (ESI)†}.37–44 The planar 3,6,7-triamino-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazolium (TATOT) cation was selected for molecular recognition studies with various energetic anions because of its planar geometry. The silver salts or ammonium salts of compounds 1–8 (Fig. 3) were reacted with the hydrochloride salt of 3,6,7-triamino-[1,2,4]triazolo[4,3-b][1,2,4]triazole (TATOT.HCl) in aqueous solution to obtain insensitive sandwich energetic materials ISEMs 1–8 (Scheme 1).In ISEM-1, the π–π interaction brought about by stacking of a dianion and two mono cations is visualized by plotting non-covalent interactions (NCI plots)45,46 (Fig. 4M). Single crystals of ISEM-2 suitable for X-ray analysis are obtained by slow evaporation from water. It crystallizes in a monoclinic (P21/c) space group with one dinitramide anion, two TATOT cations and two water molecules to give C10H18N24O10, with a density of 1.758 g cm−3 at 100 K (See ESI, Section S2†).
 | ||
| Fig. 4 Molecular structures (A) ISEM-1 (ref. 37). (B) ISEM-2. (C) ISEM-3. (D) ISEM-4. (E) ISEM-5. (F) ISEM-6. Packing diagrams. (G) ISEM-1 (ref. 37). (H) ISEM-2. (I) ISEM-3. (J) ISEM-4. (K) ISEM-5. (L) ISEM-6. π-Philic molecular recognition between the ring visualized through NCI plots (M) ISEM-1. (N) ISEM-2. (O) ISEM-3. (P) ISEM-4. (Q) ISEM-5. (R) ISEM-6. | ||
In ISEM-2, each dinitramide anion is sandwiched between TATOT cations through π-philic molecular recognition interactions (Fig. 4B and N). The eight O atoms from the two nitroamino groups are fixed by H-bonds from the surrounding TATOT cations and water molecules, with lengths ranging from 1.80 Å to 2.56 Å. Single crystals of ISEM-3 suitable for X-ray analysis were obtained by slow evaporation from dimethyl sulfoxide. It crystallizes in a monoclinic (P21/n) space group with one dinitramide anion, and two TATOT cations to give C8H14N24O4, with a density of 1.808 g cm−3 at 100 K (ESI, Section S2†). In ISEM-3, each dinitramide anion is sandwiched between TATOT cations through π-philic molecular recognition (Fig. 4C and O). The four O atoms from two nitroamino groups are fixed by H bonds from the surrounding TATOT cations, with lengths ranging from 1.88 Å to 2.57 Å. The π–π interactions aroused by stacking of the dianion and two mono cations are visualized by plotting non-covalent interactions plots. In comparison with ISEM-1, the rings in ISEM-3 are found to be more displaced.
Single crystals of ISEM-4 and ISEM-5 are obtained by slow evaporation from H2O and methanol co-solvent mixtures. ISEM-4 crystallizes in a monoclinic (Pc) space group to give a formula C11H14N23O10, with a density of 1.860 g cm−3 at 100 K and ISEM-5 crystallizes in a triclinic (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) space group to give a formula C8H12N26, with a density of 1.674 g cm−3 at 100 K. In both compounds, each dianion is sandwiched between TATOT cations (Fig. 4D–E, 4P and 4Q). In ISEM-4, the ten O atoms from five nitro groups are fixed by H bonds from the surrounding TATOT cations, with lengths ranging between 1.87 Å to 2.62 Å, In ISEM-5, the length of H-bonds range from 1.98 Å to 2.29 Å. The length of π–π stacking interactions is found in the range of 3.35 Å to 3.37 Å. For ISEM-6, single crystals were also obtained by slow evaporation from H2O and methanol co-solvent mixtures. ISEM-6 crystallizes in an orthorhombic (Pca21) space group to give a formula C10H14N22O9, with a density of 1.855 g cm−3 at 100 K. The sandwich structure is shown in Fig. 4F and R.
) space group to give a formula C8H12N26, with a density of 1.674 g cm−3 at 100 K. In both compounds, each dianion is sandwiched between TATOT cations (Fig. 4D–E, 4P and 4Q). In ISEM-4, the ten O atoms from five nitro groups are fixed by H bonds from the surrounding TATOT cations, with lengths ranging between 1.87 Å to 2.62 Å, In ISEM-5, the length of H-bonds range from 1.98 Å to 2.29 Å. The length of π–π stacking interactions is found in the range of 3.35 Å to 3.37 Å. For ISEM-6, single crystals were also obtained by slow evaporation from H2O and methanol co-solvent mixtures. ISEM-6 crystallizes in an orthorhombic (Pca21) space group to give a formula C10H14N22O9, with a density of 1.855 g cm−3 at 100 K. The sandwich structure is shown in Fig. 4F and R.
The effect of solvent on the packing of these sandwich molecules was observed. For ISEMs 1, 3, 4, 5, and 6 the single crystals suitable for X-ray analysis were obtained from water:methanol co-solvent mixtures. Suitable single crystals for ISEM-2 only grew in water due to slight solubility. Similar to ISEM-2, single crystals for slightly water soluble ISEM-3 and ISEM-6 were obtained to learn if the H2O would disrupt the π-philic molecular recognition between the rings. From H2O, ISEM-3 crystallizes in a monoclinic (P21/n) space group with one H2O molecule, one dinitramide anion, and two TATOT cations to give a formula C8H14N24O4·H2O with a density of 1.759 g cm−3 at 100 K. It is seen that the H2O molecule in the lattice does not disrupt the π-philic molecular recognition between the rings (See ESI, Section 2†). For ISEM-5, the single crystals obtained from H2O show different cell parameters. From H2O, ISEM-5 crystallizes in a monoclinic space group P21/c with two molecular moieties per unit cell.36 From H2O, ISEM-6 crystallizes in a monoclinic (Pna21) space group with one H2O molecule, one dinitramide anion, and two TATOT cations to give a formula C10H14N22O9·H2O with a density of 1.792 g cm−3 at 100 K. It is seen that the H2O molecule in the lattice slightly disrupts the π-philic molecular recognition between the rings (See ESI, Section 2†). The water:methanol co-solvent mixture is found to be the best combination to grow crystals for these sandwich molecules.
To determine the importance of π-philic molecular recognition between two TATOT cations and a planar anion ring, the reactions of a non-planar anions with TATOT were examined. The silver salt of 4-azido-3,5-dinitropyrazole47 was reacted with TATOT. HCl in aqueous solution to obtain 9. Since these molecules lack the π-philic molecular recognition sites between the two TATOT cations and anion, sandwich-type packing is not observed (Fig. 5A). Another such example from the literature which includes a non-planar dianion is 10-TATOT. It also lacks the π-philic molecular recognition with TATOT due to its non-planar framework (Fig. 5B).48
 | ||
| Fig. 5 Reaction of TATOT with monoanions. (A) 9-TATOT. (B) 10-TATOT (ref. 48). | ||
2.2 Thermal and sensitivity properties
With a heating rate of 5 °C min−1, the thermal decomposition temperatures of ISEM-1, ISEM-2 and ISEM-3 occurred at 253 °C, 189 °C, and 238 °C, respectively, which are significantly higher than their nitroamine precursors 1, 2 and 3, respectively (Fig. 6); decomposition of ISEM-4 and ISEM-5 occurred at 203 °C and 226 °C, respectively; decomposition of ISEMs 6–8 at 225 °C, 268 °C and 269 °C, respectively. The thermal and sensitivity properties of ISEMs 6–8 are also compared with typical metal salts and non-metallic salts (Fig. 6) to highlight the advantages of this structure.39,40,42,49 For compounds 9-TATOT and 10-TATOT, the thermal decomposition temperatures are found to be lower than those for the neutral precursors 9 and 10, respectively (Fig. 6).To better understand the relationship between molecular structures and their physical characteristics, Hirshfeld surfaces and 2D fingerprints were examined (Fig. 7A–F).50,51 In ISEMs 1–4 and 6, a large sum of stabilizing interactions such as O–H⋯N and N–H⋯N, enhances molecular stability (Fig. 7A–D, F). In ISEMs 1–4 and 6 the total number of O–H⋯N and N–H⋯N interactions is around 60% or above. In ISEM-5, a large number of N–H⋯N interactions (65%) contribute towards molecular stability (Fig. 7E). For ISEMs 1–6, the contributions of N⋯N, and N⋯C interactions which denote p–p stacking are found in the range of 7–17%.
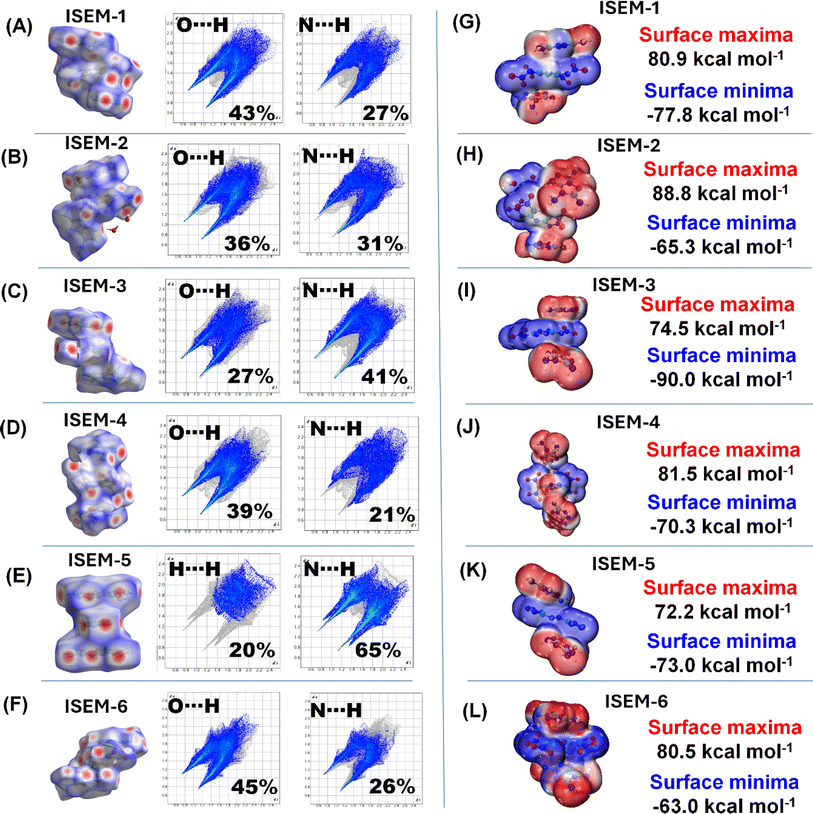 | ||
| Fig. 7 (A–F) Hirshfeld surfaces and 2D fingerprint plots. (G–L) Electrostatic potential (ESP) surfaces. | ||
For ISEMs 1–8 sensitivities to impact and friction were measured by using BAM standard methods,52,53 and are given in Fig. 6. These compounds show very low sensitivity towards impact in comparison with their neutral precursor and silver salts. The low sensitivities of compounds are further explained from both the crystal structure and at the molecular level. At the molecular level, sensitivities of materials toward impact are very much related to their electrostatic surface potentials (ESPs). The electrostatic potentials (ESP) of ISEMs 1–6 were calculated by using the B3LYP/6-311G(d, p) method with optimized structures.45 The calculated ESPs mapped surfaces of ISEM 1–6 are shown in Fig. 7G–L. It is seen that for ISEMs 1–6, the positive ESP concentrates on the TATOT cation, while the negative ESP distributes on the dianion.
2.3 Detonation properties
To calculate the detonation properties, room temperature densities for ISEMs 1–8 were measured using a gas pycnometer and are in the range of 1.63–1.80 g cm−3, respectively (Table 1). The enthalpies of formation (ΔHf) for compounds ISEMs 1–8 are given in Table 1.54 All compounds have positive enthalpies of formation in the range of 903.9 kJ mol−1 and 1811.8 kJ mol−1, respectively. With experimental densities and calculated enthalpies of formation, the detonation properties of compounds were calculated using EXPLO5 (v7.01.01).55 The calculated detonation velocities are in the range of 8054–8752 m s−1, and detonation pressures fall between 22.9–29.2 GPa, respectively. The detonation properties of ISEM-3 are found to be comparable with RDX.| Compound | P (g cm−3) | ΔHfb kJmol−1 | P (GPa) | D v (m s−1) |
|---|---|---|---|---|
| a Density at 25 °C using gas pycnometer. b Molar enthalpy of formation (calculated using isodesmic reactions with the Gaussian 03 suite of programs (revision D.01)). c Pressure. d Velocity (calculated using EXPLO5 version 7.01.01). e Ref 37. f Ref 36. g Ref 56. | ||||
| ISEM-1 | 1.76e | 1277.9e | 26.2 | 8245 |
| ISEM-2 | 1.72 | 1381.3 | 26.5 | 8267 |
| ISEM-3 | 1.75 | 1699.8 | 29.2 | 8752 |
| ISEM-4 | 1.80 | 986.0 | 28.2 | 8436 |
| ISEM-5 | 1.63 | 1811.8f | 22.9 | 8054 |
| ISEM-6 | 1.80 | 903.9 | 27.9 | 8435 |
| ISEM-7 | 1.76 | 1313.6 | 26.0 | 8305 |
| ISEM-8 | 1.75 | 1200.1 | 25.1 | 8185 |
| RDX | 1.80 | 92.6 | 34.2 | 8836 |
3 Conclusions
In conclusion, π-philic molecular recognition was employed to design and synthesize a new class of energetic sandwich materials. Molecular insights into structure–property relationships are provided through X-ray and theoretical studies, which highlight the role of weak but pivotal non-covalent interactions. In addition to improved thermal stability and insensitivity, these materials exhibit acceptable physiochemical properties and very low solubilities in water. We believe these insights will enable further advances toward constructing energetic sandwich materials suitable for practical applications.Data availability
The data underlying this study are available in the published article and its online ESI.†Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.Acknowledgements
The diffractometer (Rigaku Synergy S) for SC-XRD was purchased with support from the National Science Foundation (MRI program) under grant no.1919565. We are grateful to the Fluorine-19 fund for support. MF – National Science Centre's PRELUDIUM-BIS grant no. 2020/39/O/ST5/03293; MF – Polish National Agency for Academic Exchange grant no. BPN/PRE/2022/1/00043.References
- K. E. Riley and P. Hobza, Acc. Chem. Res., 2013, 46, 927–936 CrossRef CAS PubMed
.
- M. J. Plevin, D. L. Bryce and J. Boisbouvier, Nat. Chem., 2010, 2, 466–471 CrossRef CAS PubMed
.
- A. S. Mahadevi and G. N. Sastry, Chem. Rev., 2016, 116, 2775–2825 CrossRef CAS PubMed
.
- P. Kollman, New Compr. Biochem., 1984, 6, 55–71 CAS
.
- C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191–2201 RSC
.
- G. B. McGaughey, M. Gagné and A. K. Rappé, J. Biol. Chem., 1998, 273, 15458–15463 CrossRef CAS PubMed
.
- B. J. J. Timmer and T. J. Mooibroek, J. Chem. Educ., 2021, 98, 540–545 CrossRef CAS PubMed
.
- G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, Angew. Chem., Int. Ed., 2015, 54, 6110–6150 CrossRef PubMed
.
- J. Singh, H. Kim and K. W. Chi, Chem. Rec., 2021, 21, 574–593 CrossRef CAS PubMed
.
- S. Shanmugaraju and P. S. Mukherjee, Chem. Commun., 2015, 51, 16014–16032 RSC
.
- D. S. Kretić, J. I. Radovanović and D. Z. Veljković, Phys. Chem. Chem. Phys., 2021, 23, 7472–7479 RSC
.
- J. Singh, R. J. Staples and J. M. Shreeve, Sci. Adv., 2023, 9, eadk375 Search PubMed
.
- G. Liu, S. H. Wei and C. Zhang, Cryst. Growth Des., 2020, 20, 7065–7079 CrossRef CAS
.
- O. Bolton and A. J. Matzger, Angew. Chem., Int. Ed., 2011, 50, 8960–8963 CrossRef CAS PubMed
.
- J. Zhang, Q. Zhang, T. T. Vo, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS PubMed
.
- S. Banik, P. Kumar, V. D. Ghule, S. Khanna, D. Allimuthu and S. Dharavath, J. Mater. Chem. A, 2022, 10, 22803–22811 RSC
.
- T. M. Klapötke, J. Stierstorfer and A. U. Wallek, Chem. Mater., 2008, 20, 4519–4530 CrossRef
.
- A. A. Larin, A. N. Pivkina, I. V. Ananyev, D. V. Khakimov and L. L. Fershtat, Front. Chem., 2022, 10, 1012605 CrossRef CAS PubMed
.
- P. Bhatia, K. Pandey, B. Avasthi, P. Das, V. D. Ghule and D. Kumar, J. Org. Chem., 2023, 88, 15085–15096 CrossRef CAS PubMed
.
- W. Hu, J. Tang, X. Ju, Z. Yi, H. Yang, C. Xiao and G. Cheng, ACS Cent. Sci., 2023, 9, 742–747 CrossRef CAS PubMed
.
- Q. Lang, Q. Sun, Q. Wang, Q. Lin and M. Lu, J. Mater. Chem. A, 2020, 8, 11752–11760 RSC
.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418 RSC
.
- T. M. Klapötke and M. Suceska, Z. Anorg. Allg. Chem., 2021, 647, 572–574 CrossRef
.
- J. Tang, H. Xiong, G. Zhang, Y. Tang, H. Yang and G. Cheng, Chem. Commun., 2022, 58, 11847–11850 RSC
.
- D. Fischer, J. Ennifer, L. Gottfried, T. M. Klapötke, K. Karaghiosoff, J. Stierstorfer and T. G. Witkowski, Angew. Chem., Int. Ed., 2016, 128, 16366–16369 CrossRef
.
- M. Benz, T. M. Klapötke, J. Stierstorfer and M. Voggenreiter, ACS Appl. Eng. Mater., 2022, 1, 3–6 CrossRef
.
- Y. Zhou, H. Gao and J. M. Shreeve, Energ. Mater. Front., 2020, 1, 2–15 CrossRef
.
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed
.
-
T. M. Klapötke, Chemistry of High-Energy Materials, De Gruyter, 2022, vol. 6 Search PubMed
.
-
J. P. Agrawal, High Energy Materials, Wiley, 2010 Search PubMed
.
- A. J. Bennett, L. M. Foroughi and A. J. Matzger, J. Am. Chem. Soc., 2024, 146, 1771–1775 CrossRef CAS PubMed
.
- J. Zhang, Y. Feng, Y. Bo, A. K. Chinnam, J. Singh, R. J. Staples, X. He, K. Wang, J. Zhang and J. M. Shreeve, Chem, 2022, 8, 2678–2687 CAS
.
- J. Zhang, Z. Zhu, M. Zhou, J. Zhang, J. P. Hooper and J. M. Shreeve, ACS Appl. Mater. Interfaces, 2020, 12, 40541–40547 CrossRef CAS PubMed
.
- J. Zhang, Y. Feng, R. J. Staples, J. Zhang and J. M. Shreeve, Nat. Commun., 2021, 12, 1–7 CrossRef CAS PubMed
.
- V. A. Kuehl, A. M. Schmalzer, C. J. Snyder and D. E. Chavez, ACS Omega, 2023, 8, 38879–38884 CrossRef CAS PubMed
.
- T. M. Klapötke, P. C. Schmid, S. Schnell and J. Stierstorfer, Chem.–Eur. J., 2015, 21, 9219–9228 CrossRef PubMed
.
- J. Singh, S. Lal, R. J. Staples and J. M. Shreeve, Mater. Chem. Front., 2022, 6, 933–938 RSC
.
- J. Singh, A. K. Chinnam, R. J. Staples and J. M. Shreeve, Inorg. Chem., 2022, 61, 16493–16500 CrossRef CAS PubMed
.
- H. Li, T. Zhang, Z. Li, Y. Wang, J. Ren and T. Zhang, J. Energ. Mater., 2022, 40, 15–33 CrossRef CAS
.
- J. Singh, R. J. Staples and J. M. Shreeve, ACS Appl. Mater. Interfaces, 2021, 13, 61357–61364 CrossRef CAS PubMed
.
- M. M. Williams, W. S. Mcewan and R. A. Henry, J. Phys. Chem., 1957, 61, 261–267 CrossRef CAS
.
- Q. Yu, P. Yin, J. Zhang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 8816–8819 CrossRef CAS PubMed
.
- T. S. Hermann, K. Karaghiosoff, T. M. Klapötke and J. Stierstorfer, Chem.–Eur. J., 2017, 23, 12087 CrossRef CAS PubMed
.
- Y. Cao, H. Huang, X. Lin, J. Yang and X. Gong, New J. Chem., 2018, 42, 11390–11395 RSC
.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed
.
- X. Y. Zhang, X. Y. Lin, B. Y. Guo, C. Tan and Y. Han, J. Mol. Struct., 2022, 1267, 133526 CrossRef CAS
.
- J. Singh, R.
J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2023, 11, 12896–12901 RSC
.
- T. Zhang, J. Du, Z. Li, X. Lin, L. Wang, L. Yang and T. Zhang, CrystEngComm, 2019, 21, 765–772 RSC
.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC
.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC
.
- NATO, Standardization Agreement 4489 (STANAG4489), Explosives, Impact Sensitivity Tests, 1999.
- NATO, Standardization Agreement 4487 (STANAG 4487), Explosives, Friction Sensitivity Tests, 2002.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel and G. E. Scuseria, et al., Revision D.01, Gaussian Inc., Wallingford CT, 2003 Search PubMed
.
-
M. Sućeska, EXPLO5, Version 6.01, Brodarski Institute, 2019 Search PubMed
.
-
R. Mayer, J. Köhler and A. Homburg, Explosives, Wiley-VCH, 2007 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compounds, isodesmic reactions, characterization data. CCDC 2100988 (ISEM-1); 2345095 (ISEM-2); 2345096 (ISEM-3); 2345097 (ISEM-4); 2345098 (ISEM-5); 2345099 (9-TATOT); 2346398 (ISEM-3-H2O); 2350606 (ISEM-6); 2350679 (ISEM-6-H2O). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta02521k |
| This journal is © The Royal Society of Chemistry 2024 |