Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Introduction of the first commercial biobased benzoxazines for the manufacturing of fibre reinforced polymers†
Gideon
Abels
 a,
Katharina
Koschek
a,
Katharina
Koschek
 a,
Paul
Jones
a,
Paul
Jones
 b and
Wendy
Howarth
b and
Wendy
Howarth
 *b
*b
aFraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM, Wiener Strasse12, 28359 Bremen, Germany
bBitrez Group Ltd, Bradley Halll Trading Estate, Bradley Lane, Standish, Wigan WN6 0XQ, UK. E-mail: wendy.howarth@bitrez.co.uk
First published on 22nd August 2024
Abstract
Benzoxazines are a promising material class due to their flexible molecular design, high thermomechanical properties, and inherent flame retardancy. Especially the latter makes them interesting for all kinds of applications, for example lightweight constructions in the transportation sector. The first commercial benzoxazines were based on bisphenol and aniline, petrochemical resources produced from crude oil. However, due to the growing demand for more sustainable materials the use of biobased resources for benzoxazine synthesis has been thoroughly investigated in the past years. In this work, we present the first commercial benzoxazines that consist of biobased compounds. After analysing the curing behaviour of these new resins using thermal analysis, polymers are manufactured and characterised from them. Finally, the resins were used for manufacturing fibre-reinforced polymers (FRP) for flame-retardant lightweight applications. Thermomechanical and combustion analysis showed that the polymers achieve high flexural moduli up to 2.8 MPa and glass transition temperatures of 100 °C and 141 °C. In addition, the biobased benzoxazines have promising flame-retardancy due to intumescence, resulting in high LOI values of 31.4 ± 0.2 and 33.3 ± 0.1%
Sustainability spotlightPolybenzoxazines offer many advantageous properties, for example their inherent flame-retardant properties and flexible molecular design and will be used in a broad range of applications in the upcoming years. Especially polybenzoxazine-based flame-retardant FRPs could boost the development of various electric vehicles, greatly increasing the use of renewable energies in the transportation sector. Consequently, large amounts of benzoxazine must be produced to meet the industrial needs. Because of this the use of waste materials and renewable biobased materials for benzoxazine syntheses instead of petrochemical resources on an industrial level is of utmost importance. Our work emphasizes the importance of the following UN sustainable development goals: affordable and clean energy (SDG 7), industry, innovation, and infrastructure (SDG 9), climate action (SDG 13). |
1 Introduction
Fibre-reinforced polymers (FRP) are widely applied lightweight materials offering a number of advantages compared to metals, including high mechanical properties, low weight and excellent corrosion resistance.1,2 However, the organic polymer matrix of FRPs is often combustible, so these materials have a low fire safety which has to be improved for many applications.3,4 One possibility is the use of new polymers. Benzoxazines are a class of thermosetting polymers that have an inherent flame retardancy5 as well as high glass transition temperatures,6 near zero shrinkage7 and high mechanical properties.8 Therefore, benzoxazine matrices allow the manufacturing of FRPs with high fire safety.9,10 This is not only interesting for existing fields of application but could also open new possibilities for FRPs in applications with high flame-retardancy requirements, for example in the railway4 and the shipbuilding industry.11Traditionally, benzoxazines are synthesised by reactions of (i) a phenolic compound, for example bisphenol F and A,8 (ii) formaldehyde or paraformaldehyde as aldehyde compound,8,12 and (iii) a primary amine like aniline that contains one or multiple primary amine groups.13 After many years of research, the first commercial benzoxazines have been introduced to the market, for example the MT35500 and MT35700 resins from Huntsman. However, these resins consist solely of petrochemical compounds like bisphenols and aniline.10,14 Due to the limited availability of crude oil for petrochemical production and the growing demand for sustainable materials, numerous partial and fully biobased benzoxazines have been developed in recent years, synthesised with phenols, amines and aldehydes from renewable resources.15,16 For example, guaiacol,17 sesamol,18 cardanol19 and vanillin20 have been used as phenolic compounds and furfurylamine,21 ethylamine,22 diaminohexane23 and stearyl amine17 as amine compounds. In terms of aldehydes, formaldehyde has been successfully substituted by benzaldehyde,24 pentanal25 and furfural.26
Although many bio-based components have been described in the literature so far, no resulting bio-based benzoxazine has achieved commercial acceptance and scalability until now. There are two main reasons for this. The first problem is the difficulty of finding suitable combinations of biobased phenols, aldehydes and amines that give a benzoxazine good processability like a low viscosity and sufficient processing times, as well as good thermomechanical properties of the resulting polybenzoxazine at the same time. The second problem is that the feedstocks for these biobased compounds must be large enough for commercial production.27 Until now, no fully or partially bio-based benzoxazine has fulfilled both conditions and achieved market availability.
However, in this work the first commercial, partially bio-based benzoxazines BDP4740 and FB601 are introduced that utilise the renewable compounds furfural and cardanol. Furfural is derived from the dehydration of sugars, and occurs in a variety of agricultural by-products, including corncobs, oats, and wheat bran.28 Cardanol is a biobased phenol compound that is produced from cashew nut shells.27 Both are inexpensive and renewable raw materials from naturally occurring feedstocks and thus perfectly suitable for benzoxazine syntheses. These new benzoxazines mark the first step towards bio-based benzoxazines industrialisation achieving a higher sustainability. The goal of this work was the characterisation of these new resins and the resulting polymers, as well as an evaluation of their potential use for fire resistant FRPs using a purely petrochemically based benzoxazine as reference. All benzoxazine monomers and polymers in this study were analysed by DSC, TGA, rheological tests and DMA to obtain processing parameters and their thermomechanical properties. With this information, FRPs were manufactured using a prepreg approach, their fibre mass content determined, and their mechanical properties analysed via ILSS. Furthermore, the combustion behaviour of all polymers and FRPs was characterised using MCC measurements, LOI and UL-94 tests. All results were compared with those of a petrochemical benzoxazine based on bisphenol F, formaldehyde and aniline.
2 Experimental section
2.1 Materials
Bisphenol-F and aniline based benzoxazine (BF-a) Araldite MT35700 from by Huntsman Advanced Materials GmbH (Basel, Switzerland) and two biobased benzoxazines FUROX BDP4740 and FUROX FB601 with bio contents of 59% and 45% from Bitrez Ltd (Standish, United Kingdom) were used. For FRP manufacturing, two kinds of glass fibres from Saertex (Saerbeck, Germany) were used as received. The first is an E-Glass bidirectional 0/90° non-crimp fabric 600 tex with an aerial weight of 625 g m−2, and the second is an E-Glass bidirectional ±45° non-crimp fabric 300 tex fixed with threads of 0/90° made as well of E-Glass with 68 tex and an aerial weight of 610 g m−2.2.2 Methods
FUROX BDP4740 and FUROX FB601 were processed in the same ovens in a similar way but at different temperatures. The resins were heated to 120 °C, degassed for 10 minutes, poured in a preheated silicone or metal mould and degassed for another 5 minutes. The curing steps were performed at 15 bar in an autoclave (Scholz Maschinenbau, Coesfeld, Germany) as follows: heating to 180 °C in 15 minutes, maintaining at 180 °C for 2 h, increasing the temperature to 200 °C in 15 minutes, maintaining at 200 °C for 4 h.
The MT35700 resin was molten at 140 °C in a vacuum oven and degassed for 10 minutes. Vacuum infusion was performed in a convection oven at a temperature of 140 °C. After complete wetting of the fibres, the temperature was immediately increased in order to start the curing process. The latter was the same as for the neat monomer was used.
For the biobased resins, a similar procedure was used. However, the resin was heated up to and degassed at 120 °C in a vacuum oven for 10 minutes and infusion took place at 100 °C in a convection oven. The curing process consisted of the same steps as for the neat biobased resins.
Samples of all FRP materials for further analysis were cut out using a water-cooled circular saw.
2.3 Material characterization
Differential scanning analysis (DSC) was performed using a Discovery DSC (TA Instruments, Hüllhorst, Germany) from 20 °C to 270 °C with a heating rate of 10 K min−1. Thermogravimetric analysis (TGA) was conducted on a TGA Q5000 (TA Instruments, Hüllhorst, Germany) from 30 °C to 800 °C in a nitrogen atmosphere. The heating rate was 10 K min−1.Rheological analyses were performed on an ARES rheometer (TA Instruments, Hüllhorst, Germany). The temperature-dependent viscosity was determined in oscillatory mode with a heating rate of 2 K min−1 and an oscillation frequency of 1 Hz in the temperature range from 50 to 220 °C. Isothermal measurements with a plate-to-plate geometry at chosen temperatures were used to determine the pot-life and were carried out in continuous mode at 140 °C for MT35700 and at 100 °C for both BDP4740 and FB601.
Dynamical mechanical analysis (DMA) was performed using a DMA Q800 (TA Instruments, Hüllhorst, Germany) from 20 °C to 250 °C with a heating rate of 2 K min−1 and a frequency of 1 Hz. Tests were carried out in twofold determination for each material.
UL94 tests were performed according to DIN EN 60695-11-10 in vertical alignment and the determination of the Limiting Oxygen Index (LOI) was conducted according to DIN EN ISO 4589 using an LOI device by Fire Testing Technology. Samples for both tests had a thickness of 3 mm. Micro calorimetric (MCC) measurements were performed according to ASTM D7309 on a FAA Micro Calorimeter (Fire Testing Technologies, East Grinstead, United Kingdom) between 80 °C and 950 °C in threefold determination and the data was fitted with the MCC Curve Fit Software from the same manufacturer. For all obtained parameters, averages and standard deviation were calculated.29
Fibre volume content (FVC) of the FRPs was determined in a loss on ignition process according to DIN ISO 1172. Measurements of the interlaminar shear strength (ILSS) of the FRPs were conducted according to DIN EN ISO 14130 using a 10 kN Zwick machine at a speed of 1 mm min−1. Six FRP samples with a size of 40 × 20 mm and a thickness of 3 mm were tested for each matrix polymer.
3 Results and discussion
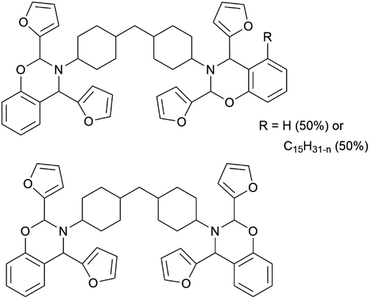
Fig. 1 shows the structures of BDP4740 and FB601. Both monomers utilize furfural as aldehyde component. In addition, in the BDP4740 25% of the phenol units are substituted by cardanol. The resulting bio content is 45% for FB601 and 59% for BDP4740.3.1 Thermal analysis of the monomers
The thermal behaviour of the biobased resins was analysed using DSC and TGA to identify suitable curing temperatures. For this, BDP4740 and FB601 were kept in vacuum at 120 °C for 15 minutes to remove air from the resins as well as residual volatile compounds like solvents from the manufacturing process. Only degassed samples were analysed in the following. The petrochemical reference MT35700 was also degassed for 15 minutes at 140 °C to remove residual air from the resin.3.2 DSC measurements
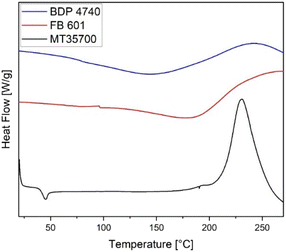
The DSC curve of MT35700 revealed an endothermic peak at 45 °C, as well as a strong exothermic peak at 230 °C (Fig. 2). The first peak was assigned to the melting of the material and the second to the benzoxazine polymerisation. In contrast, BDP4740 and FB601 showed no endothermic melting peak since they were already viscous at room temperature. Their polymerisation peaks were at 242 °C and 267 °C and thus at higher temperatures than for MT35700 (Table 1), while the onset temperatures of their polymerisation were at much lower temperatures of 170 °C and 190 °C, compared to the 212 °C measured for MT35700. Therefore, the polymerisation signals of the two biobased resins were much broader and less intense compared to the MT35700. This indicated that the biobased resins might require higher curing temperatures or longer curing times than the MT35700.| Monomer | T onset [°C] | T peak [°C] | T 5% [°C] |
|---|---|---|---|
| BDP4740 | 170 | 242 | 91 |
| FB601 | 190 | 267 | 127 |
| MT35700 | 212 | 230 | 207 |
3.3 TGA measurements
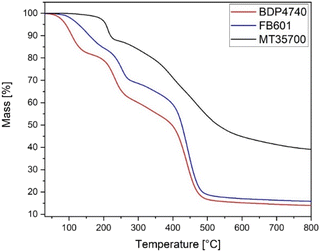
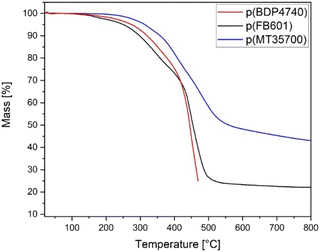
TGA analysis of the FB601 and BDP4740 showed three mass losses of 20%, 10% and 50%. The first was between 100 °C and 200 °C, the second between 200 °C and 280 °C, and the third between 380 °C and 500 °C. In contrast, MT35700 showed one mass loss of 10% around 200 °C followed by a slow but continuous mass loss of 50% till the end of the measurement (Fig. 3). This revealed that the biobased resins had not only degraded earlier but also exhibited higher mass losses than the MT35700. Especially the first mass loss below 200 °C was of relevance with regard to the manufacturing. The T5% temperatures of FB601 and BDP4740 were 60 °C to 80 °C below the onset temperature (Table 1), indicating strong pore formation within the polymer during curing. The reason for this is probably volatile compounds in the neat resins that have not been fully removed during the initial degassing step. However, longer degassing times were not feasible because the viscosity of the resin increased noticeably after a total of 30 min in the vacuum oven for heating and degassing. This made the following FRP manufacturing extremely difficult. Similar observations were made for higher degassing temperatures. The TGA results further showed that although these volatile compounds should have evaporated around 200 °C, there is still a mass loss, indicating that the monomer itself is thermally not stable and decomposes partially. So even with longer degassing steps, pore formation in the polymer during curing was expected. Therefore, the curing of FB601 and BDP4740 had to take place in an autoclave to manufacture porous free samples for further analysis. In contrast, MT35700 samples could be cured porous-free in the convection oven without additional overpressure.3.4 Rheological analyses
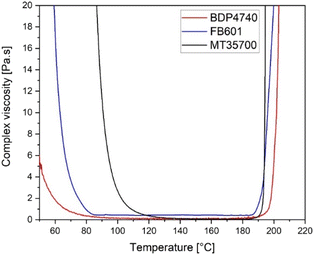
Having assessed the thermal behaviour of the resins, rheological measurements were performed to determine the temperature and time dependent viscosity of the resins at specific temperatures.Temperature-dependent rheological analysis was used to determine the minimum processing temperature of the three resins. For FRP manufacturing a resin viscosity below 1 Pa s was necessary to ensure a sufficient fibre impregnation. The results in Fig. 4 show that the viscosity falls below 1 Pa s at 66 °C for BDP4740, at 80 °C for FB601 and at 109 °C for MT35700. The lower viscosities of the biobased resins result from the fact that these are more aliphatic and have no melting point, compared to the MT35700 (Table 1). In addition, the lower viscosity of BDP4740 compared to FB601 is probably caused by the long aliphatic side chain in the cardanol unit, which increases the monomer's flexibility and decreases its rigid aromatic character.
Although 66 °C and 80 °C are the lowest processing temperatures FB601 and BDP4740 can be processed at, for practical applications it was decided to include a temperature buffer to consider possible processing steps with insufficient heating. At the same time, the processing temperatures should be as low as possible to avoid monomer decomposition. Therefore, the processing temperature was set to 100 °C for both FB601 and BDP4704. This temperature marked the begin of a plateau with the lowest viscosity of both resins. For MT35700, a processing temperature of 140 °C was used in the following steps as recommended by the supplier.
After defining suitable manufacturing temperatures for each resin, time-dependent rheology measurements were performed for each resin to determine the available processing time for FRP manufacturing for each resin, typically known as pot time. It was defined as the time from the beginning of the measurement until the viscosity exceeded 1 Pa s. The results in Fig. 5 show that BDP4740 and FB601 have pot times of at least 120 minutes. This is not only sufficient even for complex manufacturing processes but also longer than for MT35700, which has only a manufacturing window of 90 minutes. These higher pot times likely result from the 40 °C lower processing temperature of the biobased resins.
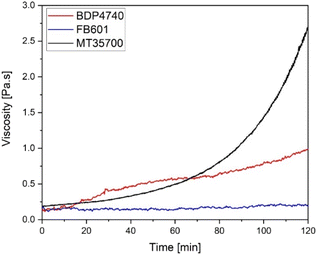 | ||
| Fig. 5 Time-dependent viscosity curves of BDP4740 and FB01 at 100 °C as well as of MT3700 at 140 °C. | ||
Combining the obtained data from DSC, TGA and rheological analysis with the curing parameters of MT35700, a process for the manufacturing of bulk samples from BDP4740 and FB601 for thermomechanical analysis and flammability tests was defined. The respective biobased resin was first heated up to 120 °C and then degassed for 10 minutes by applying a vacuum to remove air left in the molten resin. Afterwards, the resin was poured in preheated silicone or metal moulds, degassed again for 5 minutes and cured for 2 h at 180 °C and 4 h at 200 °C in an autoclave. This procedure resulted in the formation of porous-free bulk samples of BDP4740 and FB601 for DMA, UL94 and LOI measurements. For MT35700, the same processing steps were used, but with a melting and degassing temperature of 140 °C and the curing program of 3 h at 170 °C, 2 h at 180 °C and 4 h at 200 °C.
DSC analysis of all samples confirmed that they were fully cured. The obtained polymers were denoted as p(BDP4740), p(FB601) and p(MT35700).
3.5 DMA measurements
For all polymers, the storage modulus at room temperature as well as the glass transition temperature (Tg) based on the maximum of the loss modulus were characterised via DMA. The data in Table 2 showed that both biobased polymers had storage a modulus of more than 2 MPa at room temperatures and glass transitions above 100 °C, whereas p(FB601) displayed higher values. The lower storage modulus and glass transition temperature of p(BDP4740) polymer resulted from the aliphatic side chain of the cardanol unit. It might have thermally decomposed during the curing, but the side chain probably reduced the crosslinking density of the curing p(BDP4740), causing the polymer to be more flexible than the FB601 network. Compared to the p(MT3570), the p(FB601) exhibited a slightly lower storage modulus and Tg while the p(BDP4740) showed significantly lower values. Nonetheless, both biobased benzoxazines still offered good thermomechanical properties, making them suitable for practical applications.| Polymer | Storage modulus (20 °C) [MPa] | T g [°C] | T 5% [°C] |
|---|---|---|---|
| p(BDP4740) | 2.1 | 100 | 274 |
| p(FB601) | 2.8 | 141 | 251 |
| p(MT35700) | 3.3 | 162 | 314 |
3.6 Thermal analysis of the polymers
The polymers were further analysed by TGA to assess their thermal stability. With T5% of 251 °C and 274 °C, the biobased polymers show high thermal stability, although these values are still lower than for the p(MT35700) which displayed a T5% of 314 °C (Table 2). However, the samples of the biobased resins also showed a strong intumescence which the p(MT35700) did not. Especially the p(BDP4740) had such a strong intumescence that the sample cup was lifted from the holder, causing the TGA measurement to stop around 460 °C. This phenomenon took place with different TGA devices and sample cups, so the TGA curve of p(BDP4740) in Fig. 6 is incomplete. Compared to p(MT35700), the biobased polymers have a higher aliphatic content, especially the p(BDP4740) due to the side chain of the cardanol unit. It is likely that during the decomposition of these aliphatic groups, large amounts of volatile compounds are formed which ultimately bloat the charred benzoxazine layers formed by the aromatic groups. In contrast, the p(MT35700) has almost no aliphatic groups so it can only form charr and shows no noticeable intumescence.3.7 Burning tests of the polymers
In addition to the thermal stability, the flame-retardant properties of the benzoxazine polymers were evaluated using MCC, LOI analysis and UL-94 tests under standardised procedures. The three methods comprise various parameters and properties and give a good overview of the material's combustion behaviour. Specimen for all tests were prepared according to the respective standards.Micro combustion calorimeter (MCC) was used to investigate the temperature-dependent heat release of the materials. With this method, the peak heat release (PHRR) and its corresponding temperature (TPHRR) as well as the total heat release (THR) of the benzoxazine samples were recorded. Furthermore, the charr yield was determined by weighing the sample masses before and after the MCC measurement. The results in Table 3 showed that the PHRR of the two biobased benzoxazine polymers were about three times higher and the TPHRR was 40 °C lower than for p(MT35700). In addition, THR values were 50% higher and the charr yields less than half for the biobased polymers. These results indicated that p(BDP4740) and p(FB601) burned hotter at slightly lower temperatures and more complete than p(MT35700). This can be explained by the higher degree of aliphatic moieties in the p(FB601) and especially the p(BDP4740) compared to the almost entirely aromatic p(MT35700).
| Polymer | PHRR [W g−1] | T PHRR [°C] | THR [kJ g−1] | Charr yield [%] | LOI [%] | UL-94 classification |
|---|---|---|---|---|---|---|
| p(BDP4740) | 257.6 ± 7.8 | 459 ± 3 | 24.6 ± 0.5 | 19 ± 1 | 31.4 ± 0.2 | n.c. |
| p(FB601) | 220.6 ± 4.3 | 453 ± 1 | 24.3 ± 0.2 | 18 ± 1 | 33.3 ± 0.1 | n.c. |
| p(MT35700) | 80.4 ± 6.2 | 498 ± 3 | 16.6 ± 0.7 | 45 ± 2 | 26.8 ± 0.4 | V-1 |
Since MCC measurements cover only the chemical decomposition and combustion behaviour of materials due to the small sample size, LOI and UL-94 tests were performed next. With these methods, the influence of physical phenomena on the combustion behaviour can be investigated as well, for example a barrier formation due to intumescence. The results are summarised in Table 3. Both p(BDP4740) and p(FB601) showed high LOI values of more than 30%. These values were not only much higher than the average oxygen content in the air, classifying the polymers as hardly or even non-flammable, they were also 4.6% and 6.5% higher than for p(MT35700) making the biobased resins less flammable than their fossil analogue. This increase was probably caused by the strong intumescence of the biobased polymers (see also Fig. S1, ESI†). The tested LOI specimens clearly showed the formation of a foam-like structure at the burned surface.
Surprisingly, the LOI value of p(BDP4740) was lower than for p(FB601) although the former had shown a stronger intumescence in the TGA. It is possible that due to the stronger decomposition, the foam was less compact and subsequently less insulating than for p(FB601).
Despite their intumescence both p(BDP4740) and p(FB601) showed only a low self-extinguishment capability in normal air during the UL-94 test and were not classifiable according to DIN EN 60695-11-10 (n.c., Table 3). This seemingly contradicts the LOI values but can be explained by the different burning condition situations in both tests. While the sample gets ignited from above during the LOI test until it burns, it is not only ignited from below during the UL-94 test but also kept in the flame for 10 seconds. Thus, the specimens are much stronger exposed to the flame during the UL-94 test. Under these circumstances, the insulating effect of the intumescent layer might not be strong enough or the aromatic content of the polymer might be too low to induce self-extinguishment. In addition, it is also possible that decomposed species have been entrapped in the polymer structure during the curing at 15 bar. These are then released and combust during the UL-94 test, prolonging the material's afterburning time. The p(MT35700) on the other hand showed good self-extinguishing properties due to its high aromatic content and was classified as V-1. The fact that p(MT35700) also did not achieve V-0 classification indicated that there were also volatile components left in the polymer. It was probably residual monomer that could not polymerise even at high temperatures due to the high rigidity of the already formed polymer.
All in all, the results of the combustion behaviour analysis indicated that the biobased benzoxazines combust more intense than p(MT35700). This is a typical phenomenon for biobased materials due to their higher content of aliphatic groups.30 Despite this, the strong intumescence observed for p(BDP4740) and p(FB601) is very promising to obtain a good fire safety for these materials. For further improvement, the use of flame-retardant additives will be investigated in the future.5
3.8 ILSS determination
In the next step, FB601 and BDP4740 were used to manufacture FRPs with glass fibre fabrics via vacuum infusion. The curing programs were the same as for the neat polymers. The resulting FRPs showed high fibre mass contents of 76.5% for BDP4740-FRP and 78.2% for FB601-FRP (Table 4). These values were slightly higher than for the MT35700-FRP, probably because the latter was not manufactured in an autoclave and did not thermally degrade as much as the biobased benzoxazines.| FRP material | Fibre mass content [%] | Apparent interlaminar shear strength [MPa] |
|---|---|---|
| BDP4740-FRP | 76.5 ± 0.4 | 10.18 ± 1.1 |
| FB601-FRP | 78.2 ± 0.4 | 5.6 ± 0.5 |
| MT35700-FRP | 72.2 ± 0.5 | 41.1 ± 1.4 |
The mechanical properties of the FRPs were evaluated by interlaminar shear strength tests. Here, the BDP4740-FRP and FB601-FRP showed apparent interlaminar shear strengths of 10.2 MPa and 5.6 MPa. These were only 25% and 13% of the petrochemical reference indicating only weak fibre–matrix interactions in the biobased FRPs (Table 4). Since the same fibres were used for all FRPs, this difference was caused by the different benzoxazine chemistries. It is likely that during the curing process gaseous products from the biobased monomer's thermal decomposition gathered between polymer and fibre and the resulting vacancies lead to a barrier effect impeding the formation of strong fibre–matrix interactions. Therefore, cross-sectional analysis of the samples under a light microscope with 50× magnification was performed to investigate this hypothesis (Tables S7–S9, ESI†).
However, no vacancies along the fibres were found, though it is possible that they were too small for observation with the chosen device. Instead, the microscope pictures revealed isolated pores embedded in local resin accumulations between the fibres bundles which probably also had a negative impact the interlaminar shear strength of the FRPs. A potential solution for low fibre–matrix interactions is the use of higher pressure for curing. A promising possibility is the compression moulding process which allows the application of both high temperatures and very high pressures. For this study, the available autoclave could not apply more than 15 bar so this hypothesis remains to be tested.
In addition, the BDP4740-FRP showed higher apparent interlaminar shear strengths than the FB601-FRP. Due to the stronger formation of volatile thermal decomposition products of BDP4740 lower shear strengths would have been expected. This indicates that there was a second counteracting mechanism to the gas barrier. The most likely explanation for this is that the phenolic unit of the BDP4740 was more reactive due to its decomposing side chain and could form covalent bonds between matrix and fibres more easily.
3.9 Burning tests of the FRPs
The results of the FRP burning tests are summarised in Table 5. The PHRR of BDP4740-FRP and FB601-FRP were about two times higher and their THR values 50% higher than for MT35700-FRP. At the same time, the biobased FRPs achieved LOI values of 46.5% and 51.2% which were slightly lower than for the reference material.| FRP material | PHRR [W g−1] | T PHRR [°C] | THR [kJ g−1] | Charr yield [%] | LOI [%] | UL-94 classification |
|---|---|---|---|---|---|---|
| BDP4740-FRP | 44.8 ± 3.3 | 468 ± 3 | 5.8 ± 0.3 | 80 ± 1 | 51.2 ± 0.1 | n.c. |
| FB601-FRP | 45.4 ± 6.0 | 476 ± 1 | 6.0 ± 1.3 | 80 ± 3 | 46.5 ± 0.5 | n.c. |
| MT35700-FRP | 21.5 ± 1.1 | 434 ± 3 | 3.9 ± 0.3 | 84.3 | 53.9 ± 0.3 | V-1 |
Compared to the neat resins, the PHRR and THR values were much lower and the LOI values significantly higher (Table 3). This resulted from the high FMGs of the FRPs since glass fibres are not combustible. However, in contrast to the neat polymers the LOI values of the biobased FRPs were not higher anymore compared to the samples with MT35700 but instead slightly lower. This indicated that the effect of the biobased polymer's protective intumescence was strongly reduced in the FRPs. It is likely that due to the high FMC no intumescent layer could form. This was supported by the observation that none of the LOI samples showed any signs of intumescence or delamination after the tests. Without an intumescent protective layer, the more aliphatic biobased benzoxazine combusted more intense than the highly aromatic MT35700 resulting in the lower LOI values of the FRPs. However, the absent delamination was also a beneficial effect since the composite did not lose its integrity while burning.
Another difference to the neat polymers was the observation that the BDP4740-FRP had higher LOI value than the FB601-FRP whereas for the neat polymers it was the other way around. There are different possible explanations for this. On the one hand, the presence of the glass fibres in combination with the different intensity of the thermal decomposition product formation might have influenced the respective formed polymer networks. On the other hand, it is possible that the phenolic unit of the BDP4740 induces additional reactions due to its decomposing side chain as already discussed for the ILSS results. However, such reactions are not limited to fibre–matrix interactions but could also affect the reactions within the matrix, resulting in a better cross-linked and a more stable polymer network than for the FB601.
The presence of the incombustible glass fibres not only improved the LOI values but also significantly reduced the burning times of BDP4740-FRP and FB601-FRP in the UL-94 test compared to the neat polymer (see UL94 data in the appendix). However, even in the FRP their afterburning times were still too long for any UL-94 classification.
In contrast, the MT35700-FRP reached a V-1 classification like the neat resin. The afterburning times were only marginally shorter by 1–2 seconds in the FRP. Thus, despite the addition of the incombustible glass fibres this material surprisingly did not achieve a V-0 classification. This result indicated that there was still some unreacted monomer left, as discussed before for the neat polymer. It is likely that the presence of the glass fibres has hindered the polymerisation, causing potentially even more monomer to be left unreacted than for the neat polymer. This would have cancelled out the positive effect of the glass fibres on the flame-retardancy that was observed for the biobased systems and explained the V-1 classification for the MMT35700-FRP.
All in all, the results of the burning tests of the FRPs clearly showed that the FRPs with biobased benzoxazines combusted more intense than the MT35700 FRPs. Since all FRPs had similar FMCs, these results are ascribed to the different matrix properties, in particular their respective aromaticity and combustibility. To achieve similar or better flame-retardant properties as the fossil-aromatic benzoxazine, additional measures are necessary for the FUROX FB601 and BDP4740, for example the use of flame-retardant additives. For the MT35700 FRPs, the addition of FeCl3 could prove beneficial to fully cure and further cross-link the polymer, resulting in an improved thermal stability and potentially a V-0 classification.31
4 Conclusions
This study introduced the first commercial biobased benzoxazines FUROX BDP4740 and Furox FB601 and characterised resins, polymers and FRPs thereof. The new benzoxazines utilised furfural and cardanol as biobased compounds, resulting in bio contents of 45% and 59%.DSC, TGA and rheological analysis were used to identify a suitable curing program for the biobased monomers. Due to their thermal decomposition, all curing steps had to be performed under overpressure in an autoclave. The biobased benzoxazine resins could be cured in similar temperature ranges as the reference material.
The obtained biobased polymers were porous-free, had high flexural moduli over more than 2 MPa and glass transition temperatures of 100 °C and 141 °C. In addition, thermal and combustion analysis revealed a strong intumescence resulting from the mixture of aliphatic and aromatic groups in the biobased monomers. These intumescent layers formed a protective barrier leading to higher LOI values compared to a nearly completely aromatic benzoxazine. At the same time, the higher aliphatic content also led to stronger combustions in the MCC and UL-94 tests.
For FRP manufacturing, the more aliphatic biobased resins required a lower infusion temperature than the aromatic reference benzoxazine which facilitated the manufacturing. ILSS measurements revealed only weak interactions between the biobased polymer matrix and the fibres as well as some pores, however, probably due to the thermal decomposition of the matrix monomers during the curing process. LOI tests showed that the FRPs achieved high LOI values around 48%. MCC analysis revealed that the biobased FRPs combusted stronger and released more heat than the petrochemical reference and were not classifiable in the UL-94 tests.
All in all, the new biobased benzoxazines FUROX BDP4740 and FUROX FB601 are polymers that show promising thermomechanical and flame-retardant behaviour for different applications. However, for the manufacturing of fire resistant FRPs more work needs to be done to improve the fibre–matrix interactions and the flame-retardant properties of the FRPs and to avoid any porosity.
Data availability
Datasets used in this work have been uploaded as part of the manuscript or the ESI.† In addition, only public literature has been used in this work and is cited at the end of the manuscript.Author contributions
Gideon Abels: investigation, visualization, writing – original draft. Katharina Koschek: conceptualization, supervision, project administration, writing – review & editing. Paul Jones: resources, validation, writing – review & editing. Wendy Howarth: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of the Federal Ministry for Economic Affairs and Climate Action (GreenLight, 03SX515E) as well as the material supply from Huntsmann and Saertex.Notes and references
- I. Shakir Abbood, S. A. Odaa, K. F. Hasan and M. A. Jasim, Properties evaluation of fiber reinforced polymers and their constituent materials used in structures – A review, Mater. Today: Proc., 2021, 43, 1003–1008, DOI:10.1016/j.matpr.2020.07.636.
- D. K. Rajak, D. D. Pagar, P. L. Menezes and E. Linul, Fiber-Reinforced Polymer Composites, Polymers, 2019, 11, 1667, DOI:10.3390/polym11101667.
- X.-H. Shi, X.-L. Li, Y.-M. Li, Z. Li and D.-Y. Wang, Flame-retardant strategy and mechanism of fiber reinforced polymeric composite, Composites, Part B, 2022, 233, 109663, DOI:10.1016/j.compositesb.2022.109663.
- M. Häublein, M. Demleitner and V. Altstädt, in Composite Materials, ed. Y. D. I. Low, Elsevier, 2021, pp. 383–417 Search PubMed.
- I. Machado, C. Shaer, K. Hurdle, V. Calado and H. Ishida, Towards the Development of Green Flame Retardancy by Polybenzoxazines, Prog. Polym. Sci., 2021, 121, 101435, DOI:10.1016/j.progpolymsci.2021.101435.
- S. Ren, X. Yang, X. Zhao and W. H. Y. Zhang, An m-phenylenediamine-based benzoxazine with favorable processability and its high-performance thermoset, J. Appl. Polym. Sci., 2016, 133, 43368, DOI:10.1002/app.43368.
- H. Ishida and D. J. Allen, Physical and mechanical characterization of near-zero shrinkage polybenzoxazines, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 1019–1030, DOI:10.1002/(SICI)1099-0488(19960430)34:6<1019::AID-POLB1>3.0.CO;2-T.
- X. Ning and H. Ishida, Phenolic materials via ring-opening polymerization of benzoxazines, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 921–927, DOI:10.1002/polb.1994.090320515.
- N. Wolter, V. C. Beber, T. Haubold, A. Sandinge, P. Blomqvist, F. Goethals, M. Van Hove, E. Jubete, B. Mayer and K. Koschek, Effects of flame-retardant additives on the manufacturing, mechanical, and fire properties of basalt fiber-reinforced polybenzoxazine, Polym. Eng. Sci., 2021, 61, 551–561, DOI:10.1002/pen.25599.
- N. Wolter, V. C. Beber, A. Sandinge, P. Blomqvist, F. Goethals, M. van Hove, E. Jubete, B. Mayer and K. Koschek, Carbon, Glass and Basalt Fiber Reinforced Polybenzoxazine, Polymers, 2020, 12, 2379, DOI:10.3390/polym12102379.
- S. Backens, J. Unseld, N. Glück and A. Wolter, Incombustible, Inorganic Fiber-reinforced Composites for Shipbuilding, Lightweight Des. Worldwide, 2019, 12, 38–43, DOI:10.1007/s41777-019-0059-7.
- A. Minigher, E. Benedetti, O. De Giacomo, P. Campaner and V. Aroulmoji, Synthesis and Characterization of Novel Cardanol Based Benzoxazines, Nat. Prod. Commun., 2009, 4, 1934578X0900400416, DOI:10.1177/1934578X0900400416.
- H. Ishida and H. Y. Low, A Study on the Volumetric Expansion of Benzoxazine-Based Phenolic Resin, Macromolecules, 1997, 30, 1099–1106, DOI:10.1021/ma960539a.
- L. Pursche, A. Wolf, T. Urbaniak and K. Koschek, Benzoxazine/amine-based polymer networks featuring stress-relaxation and reprocessability, Front. Soft Matter, 2023, 3, 1197868, DOI:10.3389/frsfm.2023.1197868.
- A. Trejo-Machin, L. Puchot and P. Verge, in Paint and Coatings Industry, ed. F. Yilmaz, IntechOpen, London, UK, 2019, pp. 53–69 Search PubMed.
- Y. Lyu and H. Ishida, Natural-sourced benzoxazine resins, homopolymers, blends and composites, Prog. Polym. Sci., 2019, 99, 101168, DOI:10.1016/j.progpolymsci.2019.101168.
- C. F. Wang, J. Q. Sun, X. D. Liu, A. Sudo and T. Endo, Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine, Green Chem., 2012, 14, 2799–2806, 10.1039/C2GC35796H.
- M. L. Salum, D. Iguchi, C. R. Arza, L. Han, H. Ishida and P. Froimowicz, Making Benzoxazines Greener: Design, Synthesis, and Polymerization of a Biobased Benzoxazine Fulfilling Two Principles of Green Chemistry, ACS Sustain. Chem. Eng., 2018, 6, 13096–13106 CrossRef CAS.
- B. S. Rao and A. Palanisamy, Monofunctional benzoxazine from cardanol for bio-composite applications, React. Funct. Polym., 2011, 71, 148–154, DOI:10.1016/j.reactfunctpolym.2010.11.025.
- N. K. Sini, J. Bijwe and I. K. Varma, Renewable benzoxazine monomer from Vanillin, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 7–11, DOI:10.1002/pola.26981.
- I. Machado, I. Hsieh, E. Rachita, M. L. Salum, D. Iguchi, N. Pogharian, A. Pellot, P. Froimowicz, V. Calado and H. Ishida, A truly bio-based benzoxazine derived from three natural reactants obtained under environmentally friendly conditions and its polymer properties, Green Chem., 2021, 23, 4051–4064 RSC.
- Y. E. Dogan, B. Satilmis and T. Uyar, Synthesis and characterization of bio-based benzoxazines derived from thymol, J. Appl. Polym. Sci., 2019, 136, 47371, DOI:10.1002/app.47371.
- M. I. Necolau, I. E. Biru, J. Ghiţman, C. Stavarache and H. Iovu, Insightful characterization of sesamol-based polybenzoxazines: Effect of phenol and amine chain type on physical and nanomechanical properties, Polym. Test., 2022, 110, 107578 CrossRef.
- S. Mukherjee, N. Amarnath and B. Lochab, Oxazine Ring-Substituted 4th Generation Benzoxazine Monomers & Polymers: Stereoelectronic Effect of Phenyl Substituents on Thermal Properties, Macromolecules, 2021, 54, 10001–10016, DOI:10.1021/acs.macromol.1c01582.
- R. C. S. Pereira, L. R. V. Kotzebue, D. Zampieri, G. Mele, S. E. Mazzetto and D. Lomonaco, Influence of natural substituents in the polymerization behavior of novel bio-based benzoxazines, Mater. Today Commun., 2019, 21, 100629, DOI:10.1016/j.mtcomm.2019.100629.
- W. J. Grigsby and C. MacIntosh, Novel reactive benzoxazine resins using renewables: Leveraging inherent chemistries to produce novel thermoset materials, Ind. Crops Prod., 2022, 189, 115541, DOI:10.1016/j.indcrop.2022.115541.
- S.-M. Zhang, J.-Q. Zhao, Y. Liu, Y.-X. Liu and C.-M. Liu, Renewable furan-derived diamine as primary amine source to prepare fully bio-based bis-benzoxazine monomer under solvent-free condition, React. Funct. Polym., 2021, 165, 104957, DOI:10.1016/j.reactfunctpolym.2021.104957.
- K. J. Yong, T. Y. Wu, C. B. T. L. Lee, Z. J. Lee, Q. Liu, J. M. Jahim, Q. Zhou and L. Zhang, Furfural production from biomass residues: Current technologies, challenges and future prospects, Biomass Bioenergy, 2022, 161, 106458 CrossRef CAS.
- A. N. Shankar, A. N. Netravali, R. A. Mensah and O. Das, Microscale combustion calorimetry assessment of green composites made with chicken feather-modified soy protein resins and jute fabric, Compos., Part C: Open Access, 2023, 12, 100394 CAS.
- H. Ding, X. Wang, L. Song and Y. Hu, Recent Advances in Flame Retardant Bio-Based Benzoxazine Resins, J. Renewable Mater., 2022, 10, 871 CAS.
- Q.-C. Ran, D.-X. Zhang, R.-Q. Zhu and Y. Gu, The structural transformation during polymerization of benzoxazine/FeCl3 and the effect on the thermal stability, Polymer, 2012, 53, 4119–4127, DOI:10.1016/j.polymer.2012.07.033.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00192c |
| This journal is © The Royal Society of Chemistry 2024 |